Abstract
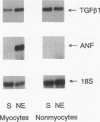
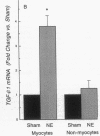
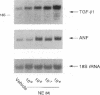
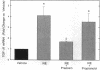
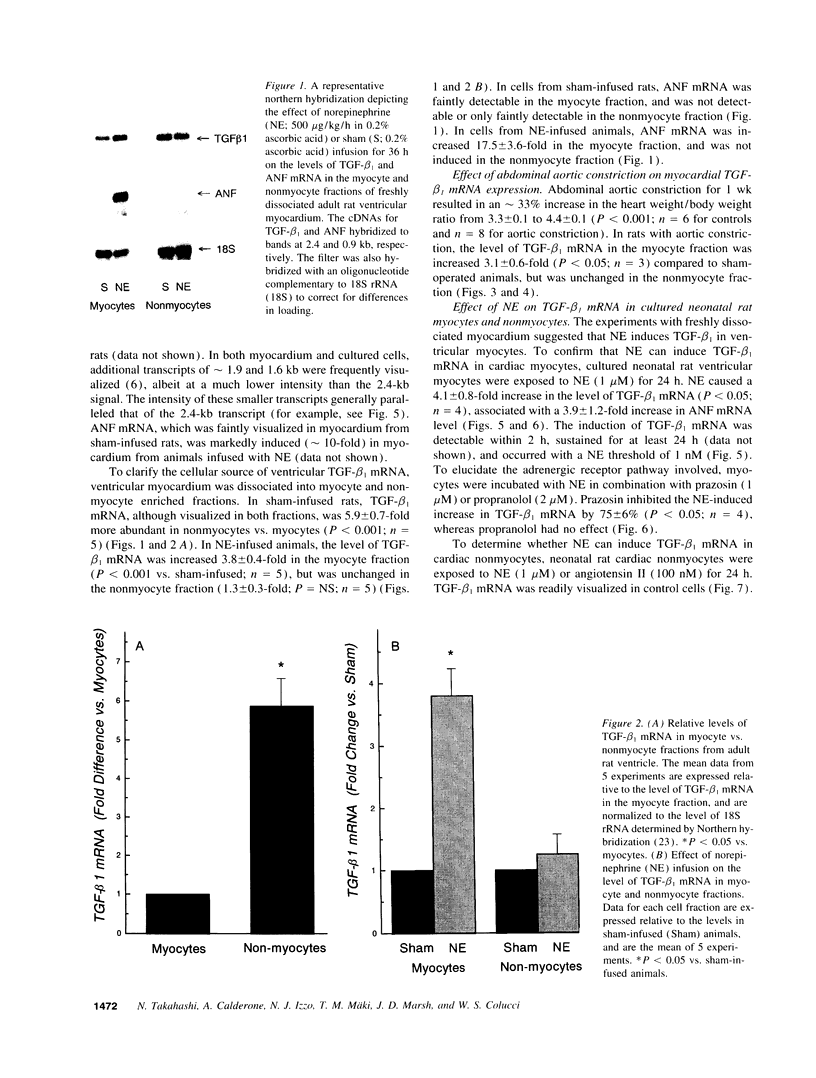
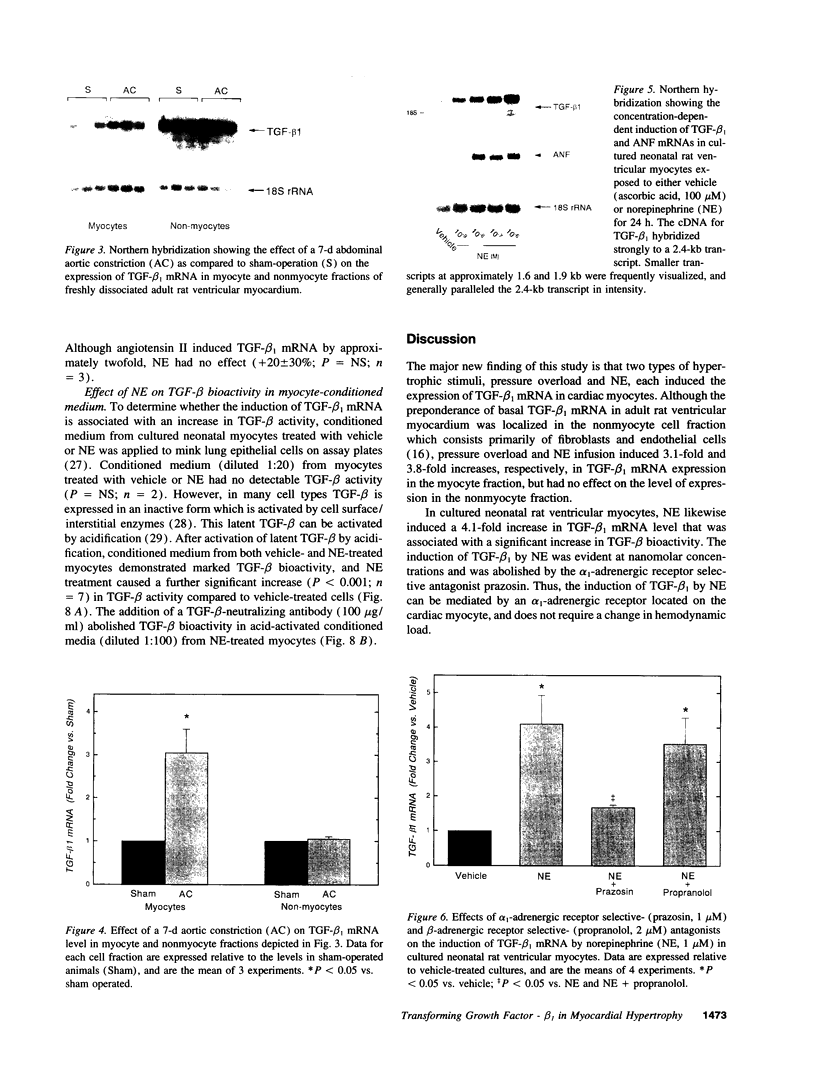
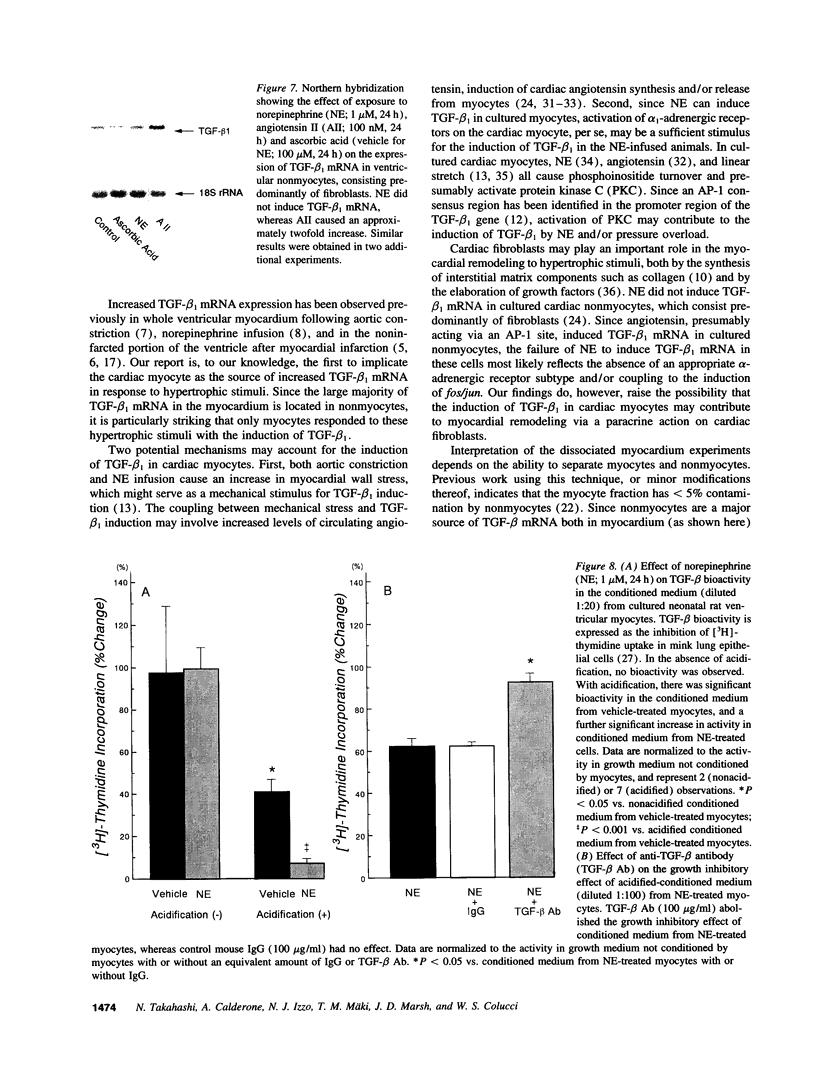
Transforming growth factor-beta 1 (TGF-beta 1) is a peptide growth factor that may play a role in the myocardial response to hypertrophic stimuli. However, the cellular distribution, mechanism of induction, and source of increased TGF-beta 1 in response to hypertrophic stimuli are not known. We tested the hypothesis that the cardiac myocyte responds to hypertrophic stimuli with the increased expression of TGF-beta 1. In adult rat ventricular myocardium freshly dissociated into myocyte and nonmyocyte cellular fractions, the preponderance of TGF-beta 1 mRNA visualized by Northern hybridization was in the nonmyocyte fraction. Abdominal aortic constriction (7 d) and subcutaneous norepinephrine infusion (36 h) each caused ventricular hypertrophy associated with 3.1-fold and 3.8-fold increases, respectively, in TGF-beta 1 mRNA in the myocyte fraction, but had no effect on the level of TGF-beta 1 mRNA in the nonmyocyte fraction. In ventricular myocytes, norepinephrine likewise caused a 4.1-fold increase in TGF-beta 1 mRNA associated with an increase in TGF-beta bioactivity. This induction of TGF-beta 1 mRNA occurred at norepinephrine concentrations as low as 1 nM and was blocked by prazosin, but not propranolol. NE did not increase the TGF-beta 1 mRNA level in nonmyocytes, primarily fibroblasts, cultured from neonatal rat ventricle. Thus, the cardiac myocyte responds to two hypertrophic stimuli, pressure overload and norepinephrine, with the induction of TGF-beta 1. These data support the view that TGF-beta 1, released by myocytes and acting in an autocrine and/or paracrine manner, is involved in myocardial remodeling by hypertrophic stimuli.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker K. M., Chernin M. I., Wixson S. K., Aceto J. F. Renin-angiotensin system involvement in pressure-overload cardiac hypertrophy in rats. Am J Physiol. 1990 Aug;259(2 Pt 2):H324–H332. doi: 10.1152/ajpheart.1990.259.2.H324. [DOI] [PubMed] [Google Scholar]
- Barnard J. A., Lyons R. M., Moses H. L. The cell biology of transforming growth factor beta. Biochim Biophys Acta. 1990 Jun 1;1032(1):79–87. doi: 10.1016/0304-419x(90)90013-q. [DOI] [PubMed] [Google Scholar]
- Bhambi B., Eghbali M. Effect of norepinephrine on myocardial collagen gene expression and response of cardiac fibroblasts after norepinephrine treatment. Am J Pathol. 1991 Nov;139(5):1131–1142. [PMC free article] [PubMed] [Google Scholar]
- Black F. M., Packer S. E., Parker T. G., Michael L. H., Roberts R., Schwartz R. J., Schneider M. D. The vascular smooth muscle alpha-actin gene is reactivated during cardiac hypertrophy provoked by load. J Clin Invest. 1991 Nov;88(5):1581–1588. doi: 10.1172/JCI115470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. H., Buxton I. L., Brunton L. L. Alpha 1-adrenergic and muscarinic cholinergic stimulation of phosphoinositide hydrolysis in adult rat cardiomyocytes. Circ Res. 1985 Oct;57(4):532–537. doi: 10.1161/01.res.57.4.532. [DOI] [PubMed] [Google Scholar]
- Casscells W., Bazoberry F., Speir E., Thompson N., Flanders K., Kondaiah P., Ferrans V. J., Epstein S. E., Sporn M. Transforming growth factor-beta 1 in normal heart and in myocardial infarction. Ann N Y Acad Sci. 1990;593:148–160. doi: 10.1111/j.1749-6632.1990.tb16107.x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Claycomb W. C., Lanson N. A., Jr Proto-oncogene expression in proliferating and differentiating cardiac and skeletal muscle. Biochem J. 1987 Nov 1;247(3):701–706. doi: 10.1042/bj2470701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb W. C., Palazzo M. C. Culture of the terminally differentiated adult cardiac muscle cell: a light and scanning electron microscope study. Dev Biol. 1980 Dec;80(2):466–482. doi: 10.1016/0012-1606(80)90419-4. [DOI] [PubMed] [Google Scholar]
- Danielpour D., Dart L. L., Flanders K. C., Roberts A. B., Sporn M. B. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989 Jan;138(1):79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Eghbali M. Cellular origin and distribution of transforming growth factor-beta in the normal rat myocardium. Cell Tissue Res. 1989 Jun;256(3):553–558. doi: 10.1007/BF00225603. [DOI] [PubMed] [Google Scholar]
- Eghbali M., Tomek R., Sukhatme V. P., Woods C., Bhambi B. Differential effects of transforming growth factor-beta 1 and phorbol myristate acetate on cardiac fibroblasts. Regulation of fibrillar collagen mRNAs and expression of early transcription factors. Circ Res. 1991 Aug;69(2):483–490. doi: 10.1161/01.res.69.2.483. [DOI] [PubMed] [Google Scholar]
- Gibbons G. H., Pratt R. E., Dzau V. J. Vascular smooth muscle cell hypertrophy vs. hyperplasia. Autocrine transforming growth factor-beta 1 expression determines growth response to angiotensin II. J Clin Invest. 1992 Aug;90(2):456–461. doi: 10.1172/JCI115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki K., Sukhatme V. P., Shubeita H. E., Chien K. R. Alpha- and beta-adrenergic stimulation induces distinct patterns of immediate early gene expression in neonatal rat myocardial cells. fos/jun expression is associated with sarcomere assembly; Egr-1 induction is primarily an alpha 1-mediated response. J Biol Chem. 1990 Aug 15;265(23):13809–13817. [PubMed] [Google Scholar]
- Izzo N. J., Jr, Seidman C. E., Collins S., Colucci W. S. Alpha 1-adrenergic receptor mRNA level is regulated by norepinephrine in rabbit aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6268–6271. doi: 10.1073/pnas.87.16.6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. D., Grignolo A., Kuhn C. M., Schanberg S. M. Hypertension and cardiovascular hypertrophy during chronic catecholamine infusion in rats. Life Sci. 1983 Jul 11;33(2):169–180. doi: 10.1016/0024-3205(83)90410-1. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Angel P., Lafyatis R., Hattori K., Kim K. Y., Sporn M. B., Karin M., Roberts A. B. Autoinduction of transforming growth factor beta 1 is mediated by the AP-1 complex. Mol Cell Biol. 1990 Apr;10(4):1492–1497. doi: 10.1128/mcb.10.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro I., Katoh Y., Kaida T., Shibazaki Y., Kurabayashi M., Hoh E., Takaku F., Yazaki Y. Mechanical loading stimulates cell hypertrophy and specific gene expression in cultured rat cardiac myocytes. Possible role of protein kinase C activation. J Biol Chem. 1991 Jan 15;266(2):1265–1268. [PubMed] [Google Scholar]
- Kurihara H., Yoshizumi M., Sugiyama T., Takaku F., Yanagisawa M., Masaki T., Hamaoki M., Kato H., Yazaki Y. Transforming growth factor-beta stimulates the expression of endothelin mRNA by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1435–1440. doi: 10.1016/0006-291x(89)92270-5. [DOI] [PubMed] [Google Scholar]
- Long C. S., Henrich C. J., Simpson P. C. A growth factor for cardiac myocytes is produced by cardiac nonmyocytes. Cell Regul. 1991 Dec;2(12):1081–1095. doi: 10.1091/mbc.2.12.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R. M., Keski-Oja J., Moses H. L. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988 May;106(5):1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R. M., Keski-Oja J., Moses H. L. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988 May;106(5):1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLellan W. R., Brand T., Schneider M. D. Transforming growth factor-beta in cardiac ontogeny and adaptation. Circ Res. 1993 Nov;73(5):783–791. doi: 10.1161/01.res.73.5.783. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Yuki K., Takaku F., Wernstedt C., Kanzaki T., Olofsson A., Hellman U., Heldin C. H. Latent forms of TGF-beta: structure and biology. Ann N Y Acad Sci. 1990;593:51–58. doi: 10.1111/j.1749-6632.1990.tb16099.x. [DOI] [PubMed] [Google Scholar]
- Morgan H. E., Baker K. M. Cardiac hypertrophy. Mechanical, neural, and endocrine dependence. Circulation. 1991 Jan;83(1):13–25. doi: 10.1161/01.cir.83.1.13. [DOI] [PubMed] [Google Scholar]
- Parker T. G., Packer S. E., Schneider M. D. Peptide growth factors can provoke "fetal" contractile protein gene expression in rat cardiac myocytes. J Clin Invest. 1990 Feb;85(2):507–514. doi: 10.1172/JCI114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton R. W., Saxena B., Jones M., Moses H. L., Gold L. I. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991 Nov;115(4):1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S. W., Kondaiah P., Casscells W., Roberts A. B., Sporn M. B. A second messenger RNA species of transforming growth factor beta 1 in infarcted rat heart. Cell Regul. 1991 Mar;2(3):241–249. doi: 10.1091/mbc.2.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J., Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J. 1993 Apr;12(4):1681–1692. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J., Izumo S. Molecular characterization of angiotensin II--induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993 Sep;73(3):413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- Sadoshima J., Xu Y., Slayter H. S., Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993 Dec 3;75(5):977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- Schunkert H., Dzau V. J., Tang S. S., Hirsch A. T., Apstein C. S., Lorell B. H. Increased rat cardiac angiotensin converting enzyme activity and mRNA expression in pressure overload left ventricular hypertrophy. Effects on coronary resistance, contractility, and relaxation. J Clin Invest. 1990 Dec;86(6):1913–1920. doi: 10.1172/JCI114924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. Stimulation of hypertrophy of cultured neonatal rat heart cells through an alpha 1-adrenergic receptor and induction of beating through an alpha 1- and beta 1-adrenergic receptor interaction. Evidence for independent regulation of growth and beating. Circ Res. 1985 Jun;56(6):884–894. doi: 10.1161/01.res.56.6.884. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992 Dec;119(5):1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springhorn J. P., Ellingsen O., Berger H. J., Kelly R. A., Smith T. W. Transcriptional regulation in cardiac muscle. Coordinate expression of Id with a neonatal phenotype during development and following a hypertrophic stimulus in adult rat ventricular myocytes in vitro. J Biol Chem. 1992 Jul 15;267(20):14360–14365. [PubMed] [Google Scholar]
- Thompson N. L., Bazoberry F., Speir E. H., Casscells W., Ferrans V. J., Flanders K. C., Kondaiah P., Geiser A. G., Sporn M. B. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors. 1988;1(1):91–99. doi: 10.3109/08977198809000251. [DOI] [PubMed] [Google Scholar]
- Thompson N. L., Flanders K. C., Smith J. M., Ellingsworth L. R., Roberts A. B., Sporn M. B. Expression of transforming growth factor-beta 1 in specific cells and tissues of adult and neonatal mice. J Cell Biol. 1989 Feb;108(2):661–669. doi: 10.1083/jcb.108.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal F. J., Dillmann W. H. Cardiac hypertrophy-induced changes in mRNA levels for TGF-beta 1, fibronectin, and collagen. Am J Physiol. 1992 Jun;262(6 Pt 2):H1861–H1866. doi: 10.1152/ajpheart.1992.262.6.H1861. [DOI] [PubMed] [Google Scholar]