Abstract
Mutations in SOX9, a gene essential for chondrocyte differentiation cause the human disease campomelic dysplasia (CD). To understand how SOX9 activates transcription, we characterized the DNA binding and cell-free transcription ability of wild-type SOX9 and a dimerization domain SOX9 mutant. Whereas formation of monomeric mutant SOX9–DNA complex increased linearly with increasing SOX9 concentrations, formation of a wild-type SOX9–DNA dimeric complex increased more slowly suggesting a more sigmoidal-type progression. Stability of SOX9–DNA complexes, however, was unaffected by the dimerization mutation. Both wild-type and mutant SOX9 activated transcription of a naked Col2a1 DNA template. However, after nucleosomal assembly, only wild-type and not the mutant was able to remodel chromatin and activate transcription of this template. Using a cell line, in which the Col2a1 vector was stably integrated, no differences were seen in the interactions of wild-type and mutant SOX9 with the chromatin of the Col2a1 vector using ChIP. However, the mutant was unable to activate transcription in agreement with in vitro results. We hypothesize that the SOX9 dimerization domain is necessary to remodel the Col2a1 chromatin in order to allow transcription to take place. These results further clarify the mechanism that accounts for CD in patients harboring SOX9 dimerization domain mutations.
INTRODUCTION
Most skeletal elements are formed by endochondral ossification involving a cartilage intermediate. In the multistep process of chondrogenesis, committed mesenchymal cells first condense and then differentiate into chondrocytes to form cartilage. Differentiation into chondrocytes is associated with activation of a repertoire of cartilage-specific extracellular matrix genes such as collagen types II, IX and XI, CD-Rap, aggrecan, matrilin1 and others. The collagen type X gene is expressed later, when chondrocytes become hypertrophic (1–3).
The role of SOX9 in chondrogenesis was suggested first by the identification of heterozygous mutations in and around the SOX9 gene in the human genetic disease campomelic dysplasia (CD), a generalized disease of cartilage causing severe skeletal malformations often associated with XY male-to-female sex reversal (4,5). SOX9 belongs to the Sox family of transcriptional regulators characterized by a high-mobility group (HMG) box DNA binding domain first identified in the sex-determining factor SRY. In mouse embryos, Sox9 is expressed in all chondroprogenitor cells and all chondrocytes, but its expression is abolished in hypertrophic chondrocytes and there is no expression of Sox9 in osteoblasts. SOX9 is also expressed in and required for the differentiation of a discrete number of other cell types (6–8). Heterozygous Sox9 mouse mutants duplicate the skeletal manifestations of human CD (9). Several lines of evidence demonstrated that SOX9 was required for cartilage formation. Indeed, in mouse chimeras, Sox9−/− cells were excluded from chondrogenic mesenchymal condensations, but were present as juxtaposed mesenchyme that did not express chondrocyte-specific markers (10). Moreover, no cartilage developed in teratomas derived from Sox9−/− embryonic stem (ES) cells (10). Experiments using the Cre/loxP recombination system to generate mouse embryos, in which Sox9 was either ablated in undifferentiated mesenchymal cells of limb buds or inactivated after chondrogenic mesenchymal condensations formed, demonstrated that Sox9 was required during sequential steps of the chondrocyte differentiation pathway (11).
A common feature in chondrocyte-specific enhancers of cartilage genes such as those of collagen types II, IX and XI, CD-Rap, aggrecan and matrilin1 is the presence of pairs of SOX9 binding sequences. These enhancers are activated by SOX9 in transfection experiments, and mutations in these enhancers that prevent SOX9 binding abolish their chondrocyte-specific activity in DNA transfections and in transgenic mice (12–21). A 7-bp consensus DNA element (A/T)(A/T)CAA (A/T)G has been identified as a recognition element for SOX proteins, which interact with the minor groove of DNA. DNA binding of SOX proteins is accompanied by a bend in the DNA. SOX9 also harbors a transcription activation domain located at the C-terminus of the protein. In several transcriptional enhancers found in genes involved in chondrogenesis, SOX9 forms a dimer with pairs of sites arranged in an inverted repeat configuration (16,18). In several of these enhancers, mutations in one or the other site of the pair abolished activity of the enhancer (16,18,22).
SOX9 mutations have been identified in patients with CD in a conserved region preceding the HMG DNA binding domain (23,24). In contrast to wild-type SOX9, which forms a dimeric SOX9–DNA complex with pairs of binding sites in chondrocyte enhancers, mutant SOX9 binds to these DNAs mainly as a monomer. In transient expression experiments, activation of these enhancers in the genes for collagen types II, IX and XI and CD-Rap was much lower with the SOX9 mutants than with wild-type SOX9. However, activation of reporters containing the promoters of the SF1 and Amh genes, which are expressed in Sertoli cells of male gonads, was not affected by the mutant SOX9, suggesting that SOX9 dimerization is required for chondrogenesis but not for sex determination (23,24).
To improve our understanding of the role of SOX9 in transcription, we used wild-type and dimerization mutant recombinant SOX9 polypeptides to further characterize their binding to DNA and to examine their ability to activate transcription in vitro in a cell-free system with both naked DNA and chromatin-assembled templates. Wild-type recombinant SOX9 activated a Col2a1 promoter–enhancer template both as naked DNA and after assembly of this DNA with nucleosomes. In contrast, the dimerization domain SOX9 mutant activated the naked Col2a1 DNA template but unlike wild-type SOX9 was unable to activate this template when assembled into chromatin.
MATERIALS AND METHODS
Plasmid construction
The p89/4 × 48 bp Col2a1 luciferase construction and its mutant MA6 were described previously (13). Plasmid p120 containing a segment of the mouse α1(III) collagen gene promoter was used as internal control in transcription assays (25). Wild-type and dimerization-deletion mutant human SOX9 coding sequences, linked to a Flag-tag at their N-terminus, were cloned into the pBACgus-2cp between NheI and Not1 sites and then transferred into baculovirus DNA (Figure 1A). The 10-amino acid deletion (Δ66–75) was introduced into the construct using PCR. These Flag-tagged SOX9 were also linked to 3 × HA tag at the C-terminus and cloned into EcoRI and BamHI sites of modified pEGFP-C1 mammalian expression vector (Clontech) that was deleted of the EGFP sequence. The sequences of all constructs were verified by DNA sequencing.
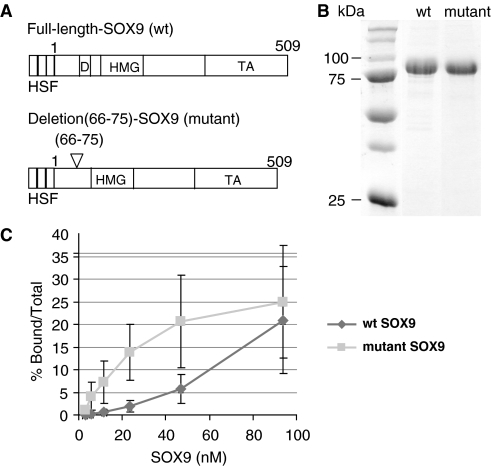
Figure 1.
Comparison of DNA binding of purified wild-type and mutant SOX9 proteins to the Col2a1 enhancer. (A) Schematic representation of the proteins used in the study: wild-type SOX9 and dimerization mutant SOX9. (H, His-tag; S, S-tag; F, FLAG-tag; D, dimerization domain; HMG, HMG box; TA, transcription activation domain). (B) SDS–PAGE analysis of purified recombinant SOX9 polypeptides. (C) Amounts of DNA–protein complexes computed using a Phosphoimager from four sets of EMSAs with a 31-bp oligonucleotide probe corresponding to the 3′-end of the 48-bp Col2a1 intron 1 enhancer and increasing amounts of wild-type (wt) or mutant SOX9 proteins.
Generation of recombinant proteins
Wild-type and mutant SOX9 polypeptides were generated in Sf9 cells using a baculovirus expression system (Novagen). Sf9 cells were grown in suspension to a density of 2 × 106 cells per ml and were infected with wt-SOX9 or mutant-SOX9 virus at 5 pfu per cell. After 60 h, cells were collected by centrifugation and resuspended in a buffer containing 10 mM Tris–HCl, pH 7.9, 0.5 M NaCl, 0.1% NP-40, 5 mM 2β-ME and 1 mM phenylmethylsulfonyl fluoride (PMSF).
The cells were sonicated on ice for four times for a duration of 15 s with 45 s interruption. After centrifugation, the supernatant was loaded on a nickel-nitrotriacetic acid agarose column (Qiagen Inc.,Valencia, CA, USA) previously equilibrated in the buffer described above. The resin was washed with a buffer containing 20 mM Tris–HCl, pH 7.9, 10 mM KCl, 5 mM 2β-ME and 0.5 mM PMSF. Twenty millimolar imidazole was added to the wash buffer and used for additional wash of the resin. The proteins were eluted with the wash buffer containing 200 mM imidazole. The fractions were collected and the purity of proteins was verified by Coomassie blue staining after SDS gel electrophoresis (Figure 1B).
Nuclear extracts
HeLa cells were grown in suspension in Joklik medium supplemented with 5% calf serum at a density of 0.8 × 106 cells/ml. Nuclear extracts were prepared as described (26).
Electrophoretic Mobility Shift Assay
Electrophoretic mobility shift assays (EMSAs) were performed using a Col2a1 enhancer probe containing either a wild-type or mutated SOX9 binding site. The upper strands were as follows: Wild-type (5′-GGCGCTTGAGAAAAGCCCCATTCATGAGAGG-3′); Site 1 mutant (5′-GGCGCTTGAGATTAGCCCCATTCATGAGAGG-3′); and Site 2 mutant (5′-GGCGCTTGAGAAAAGCCCGAATCCTGAGAGG-3′). The binding reactions were carried out with 5 fmol of 32P-end-labeled probe in a buffer containing 20 mM HEPES (pH 7.9), 50 mM KCl, 10% glycerol, 0.1% (vol/vol) Nonidet P-40, 0.5 mM EDTA, 4 mM dithiothreitol (DTT), 1 mM PMSF and 30 µg of bovine serum albumin. Purified proteins were assayed in the presence of 20 ng of poly(dG–dC). Reaction mixtures (25 µl) were incubated for various times at room temperature and fractionated on a 5% polyacrylamide gel in a 0.5× TGE buffer at 150 V. All experiments were performed three times providing very similar results.
Chromatin assembly
Chromatin assembly reactions were performed as described previously (27,28). In a typical 60-µl assembly reaction, 30 μl of Drosophila embryo S190 extract was incubated with 1.6 μg of purified core histones in RO buffer (10 mM HEPES, pH 7.5, 10 mM KCl, 0.5 mM EGTA, 10% glycerol, 10 mM B-glycerophosphate, 1 mM DTT, 0.2 mM PMSF) at room temperature for 30 min. To this sample 10× ATP mix (300 mM creatine phosphate, 30 mM ATP, 1μg of creatine phosphokinase and 26 mM MgCl2) was added and incubated for 5 min on ice. One microgram of plasmid DNA (preincubated or not with SOX9 for 20 min on ice) was then added, and chromatin assembly was allowed to proceed for 4.5 h at 27°C. To assay the quality of chromatin, a sample chromatin was digested with micrococcal nuclease (Mnase) and the DNA was subjected to electrophoresis on a 1.5% agarose gel.
In vitro transcription
Col2a1 promoter constructs, either as naked plasmid DNA or as chromatin-reconstituted DNA were used. An aliquot of chromatin assembly reaction containing 175 ng of DNA (not preincubated with SOX9) or 300 ng of naked DNA were incubated with SOX9 for 20 min on ice prior to transcription. Aliquots of chromatin assembly reaction containing 175 ng of DNA (incubated with SOX9 prior to chromatin assembly) were used directly for transcription. In a typical 25 µl transcription reaction, DNA was incubated with transcription mix (1% polyvinyl alcohol, 1% polyethylene glycol, 1 mM NTPs, 5 mM MgCl2 final concentration), HeLa nuclear extracts and 200 ng of naked plasmid DNA p120 as an internal control template. Incubations were carried out at 27°C for 1 h and reactions were stopped by adding 75 µl of a solution containing 0.4 M NaOAc, pH 5.2 and 1% SDS. RNAs were purified by phenol/chloroform extraction, chloroform extraction and ethanol precipitation with glycogen as carrier. RNAs were subjected to primer extension using Avian Myeloblastosis Virus (AMV) reverse transcriptase in presence of 32P-labeled primers corresponding to a segment of the luciferase reporter gene and to a sequence in the chloramphenicol acetyltransferase gene. Primer extension products were fractionated by electrophoresis in 7 M urea, 8% polyacrylamide gel in 1× TBE buffer, analyzed by autoradiography and quantified with Phosphorimager. All experiments were repeated at least three times and provided similar results.
Mnase digestion
Immediately after chromatin assembly, an aliquot of the chromatin assembly reaction (containing 175 ng of DNA) was digested with Mnase (0.12 U/ml final concentration) in presence of 3 mM of CaCl2 for 20 min at room temperature. The digestion was stopped by adding stop buffer (EDTA 175 mM, RNAse A-DNAse-free 0.05 mg/ml in TE) for 15 min at 37°C. This was followed by proteinase K treatment (50 µg/ml final concentration) for 15 min at 37°C. The DNA was purified by phenol–chloroform extraction, chloroform extraction and ammonium acetate–ethanol precipitation with glycogen as carrier. DNA was subjected to electrophoresis on a 1.5% agarose gel.
SOX9 interaction with chromatin DNA
After incubation of chromatin DNA with recombinant SOX9, Mnase digestion, and DNA extraction, the DNA corresponding to the Col2a1 promoter/enhancer was subjected to linear amplification using a 32P-labeled primer corresponding to a segment of the luciferase reporter gene. DNA (100 ng), the reaction buffer and Vent (exo−) polymerase (New England Biolabs) were added to a mixture containing the labeled oligonucleotide primer. The extension program was run first for 4 min at 95°C, followed by eight cycles of 1 min at 94°C, 3 min of annealing at 45°C, 30 s at 74°C and hold at 18°C. After NaOAc–EtOH precipitation, the DNA was analyzed on a 6% sequencing gel using a NaOAc gradient (top buffer: 0.5× TBE; bottom buffer: 1× TBE/1 M NaOAc).
Establishment of stable cell lines
The tandemly ligated Col2a1 48-bp enhancer and Col2a1 minimum promoter were inserted 5′ to the firefly luciferase gene of pGL4.18 plasmid (Promega). Then the puromycin-resistant gene was inserted into a site located 5′ to the multimerized Col2a1 enhancer to generate Col2a1-F.Luc-puror. The HEK293T cells (293T), derived from human embryonic kidney cells, were transfected with the Col2a1-F.Luc-puror plasmid by use of Fugene 6 (Roche) according to the manufacturer’s protocol, and the stably transfected cells were selected by use of 2 μg/ml puromycin hydrochloride. Four colonies among the puromycin-resistant colonies were picked up and expanded. The clone that had the highest luciferase activity in the presence of ectopic SOX9 was used in the experiment of Figure 7.
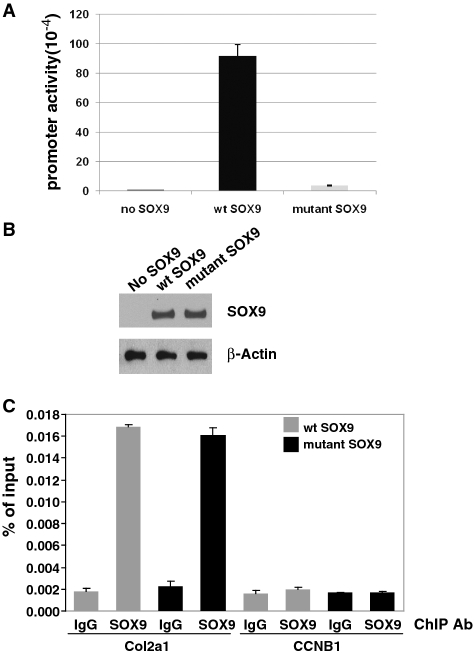
Figure 7.
ChIP analysis of the occupancy by SOX9 of a stably integrated Col2a1 reporter in 293T cells. (A) The stably transfected Col2a1 reporter is strongly activated by wild-type (wt) SOX9. Promoter activities are measured as luciferase units (× 104) and are given as average values ±SD for triplicate transfections of one representative experiment. No significant activation was observed with mutant SOX9 or in the control with no SOX9. (B) In this cell line, the protein levels of SOX9 were similar 40 h after transient transfection with wild-type (wt) SOX9 or mutant SOX9 as shown in a western blot probed with SOX9 antibody. (C) ChIP–qPCR analysis. The 293T cells in which the SOX9-dependent Col2a1 reporter construct was stably integrated were transfected with wild-type or mutant SOX9 and ChIP–qPCR analysis was performed as described in ‘Material and Methods’ section. The data represent the ratio of target fragment to input DNA. Probes corresponding to the 5′ part of the luciferase gene, located at a distance of approximately 200 to 300 bp from the SOX9 binding sites were used for qPCR. The DNA fragment size used for ChIP was about 500 bp. The cylin B1 gene was used as a ChIP–qPCR negative control. In cells transfected with both wild-type and mutant SOX9, the amount of specific DNA precipitated by SOX9 antibodies was more than 8-fold higher than that precipitated by IgG.
Reporter assay
The 1.5 × 105 293T cells, stably tranfected with Col2a1-F.Luc-puror plasmid, were inoculated into each well of 12-well plates. After 24-h incubation, the cells were transfected with 0.25 μg/well of a vector expressing wild-type SOX9 or mutant SOX9 by use of Fugene 6. After 40-h incubation, the firefly luciferase activities were measured by the luciferase assay system (Promega).
Chromatin immunoprecipitation and quantitative PCR
The 293T cells that had been stably transfected with the Col2a1-F.Luc-puror plasmid (described above) were used in the chromatin immunoprecipitation experiment (ChIP). ChIP was performed according to ChIP assay kit (Millipore Co., Ltd). Approximately 1 × 107 cells were transfected with 5 μg of empty vector, wild-type SOX9 or dimerless mutant SOX9 plasmid by use of Fugene 6 (Roche Diagnostics Co., Ltd) and cultured for 40 h. Subconfluent cells were treated with 1% formaldehyde in serum-free medium for 20 min followed by a treatment with 125 mM glycine. Cells were washed with cold PBS, resuspended with 10 mM Tris–HCl, pH 7.4, 3 mM MgCl2, 0.1% Nonidet P40, 1 mM PMSF and EDTA-free protease inhibitor cocktail (Roche Diagnostics Co., Ltd) and prepared by centrifugation for nuclei isolation. Nuclear extracts were sonicated to shear the DNA to an average length of about 500 bp and diluted to the final concentration of 0.05% SDS. To reduce non-specific background, sheared chromatin was precleared with 30 μl of protein G magnetic beads (Cell signaling Co., Ltd) in the presence of 5 μg rabbit IgG for 1 h at 4°C with agitation and then was incubated with 5 μg antibodies specific to SOX9 (Millipore, AB5809) or rabbit IgG for control overnight at 4°C with rotation. The immunocomplexes were absorbed with 30 μl of protein G magnetic beads and washed with the buffers as described in manufacturer’s protocol (Millipore Co., Ltd). After reverse cross-linking, ChIP DNA was purifed by phenol/chloroform extraction followed by ethanol precipitation according to the manufacturer’s protocol. The ChIP–quantitative PCR (qPCR) experiments were carried out by SYBR Green PCR Master Mix and ABI7900HT (Applied Biosystems) using ChIP DNA as a template. The data was computed as percent antibody bound per input. The primers specific for the 5′-end of the luciferase gene (5′-TACGACGGTGGGCTGGCTGAT-3′ and 5′-GTGTTGGGTGCCCTGTTCATC-3′) and the primers specific for the cyclin B1 gene (5′-AATCTGAGGCTAGGCTGGCTCTT-3′ and 5′-CGACCAGCCAAGGACCTACA-3′) were used for the qPCR. The results represent the average of three independent experiments. The similarity of the expression levels between wild-type and dimerless mutant SOX9 was verified by western blotting using anti-SOX9 antibody. β-actin was used as loading control.
RESULTS
Binding of recombinant full-length and mutant SOX9 to the Col2a1 enhancer
An in-frame deletion of amino acid residues 66–75 in a conserved region preceding the HMG domain of SOX9 (Figure 1A), which was identified in a patient presenting with CD (23), was previously shown to inhibit formation of a SOX9 dimer on its target DNA, although the mutant SOX9 bound this DNA as a monomer. We compared the concentration-dependent binding of purified recombinant wild-type SOX9 and the dimerization domain in-frame deletion mutant to a probe containing pairs of SOX9 binding sites. This DNA probe is located within a previously characterized chondrocyte-specific enhancer in intron 1 of Col2a1. In EMSA with wild-type SOX9, a slow-migrating complex was observed, corresponding to a DNA-bound dimer (Figure 1C), whereas the dimerization mutant formed a faster moving complex, corresponding to a DNA-bound SOX9 monomer. While formation of the monomer complex increased linearly at least at the lower concentrations of mutant SOX9, formation of the dimer complex increased more slowly with increasing concentrations of wild-type SOX9 suggesting a more sigmoidal-type progression (Figure 1D).
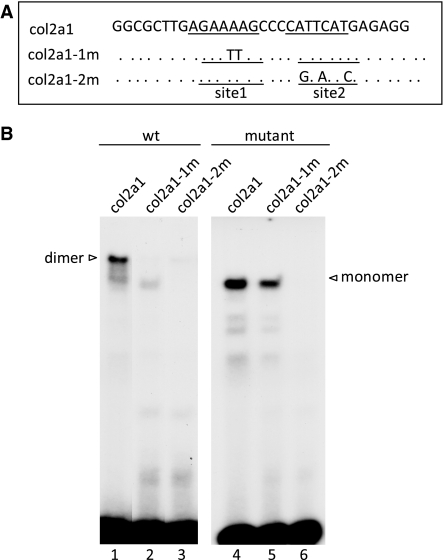
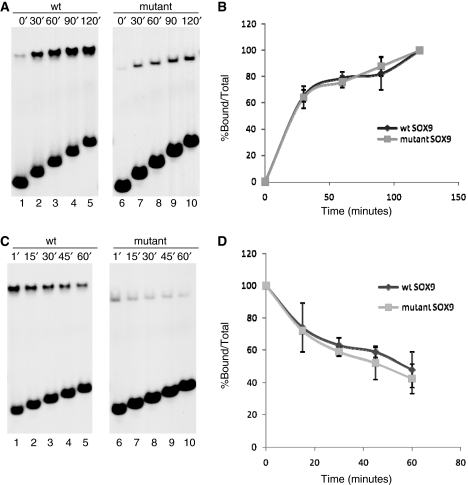
The DNA probe used in EMSA contained a pair of potential SOX9 binding sites, designated sites 1 and 2, in the Col2a1 enhancer (Figure 2A). Site 2 diverges from the consensus SOX recognition sequence (A/T)(A/T)CAA(A/T)G at a single position and Site 1 at two positions. A probe with mutations in Site 1 altered the binding of wild-type SOX9 from dimeric to monomeric binding with a concomitant reduction of binding to the probe (Figure 2B, lane 2), whereas a probe with mutations in Site 2 essentially abolished dimeric binding and also showed no momomeric binding (Figure 2B, lane 3). These experiments suggested that Site 1 is a low-affinity site and Site 2 is a high-affinity monomeric binding site. This finding was confirmed by the observation that mutant SOX9 did not bind to the probe with a mutated Site 2, but bound as a monomer to the probe with a mutated Site 1 (Figure 2B, lanes 5 and 6). To identify differences in the kinetics of SOX9–DNA complex formation between the wild-type and mutant SOX9, the recombinant polypeptides were incubated with the probe for increasing periods of time (Figure 3A, left and right panels). When the formation of DNA–protein complexes in EMSA was computed, no difference in the on-rate of the two proteins was observed when sufficient amounts of SOX9 were added to form a single dimeric–DNA complex with wild-type SOX9 and a monomer complex with mutant SOX9 (Figure 3B). We also compared the stability of wild-type and mutant SOX9 protein–DNA complexes in EMSA (Figure 3C). When SOX9–DNA complexes were allowed to form for 2 h and were then challenged by a 25-fold excess of the non-radiolabeled probe, about 50% of the original complexes were still present 60 min later (Figure 3D). These results showed that both wild-type and mutant SOX9 proteins have similar off-rates under these conditions.
Figure 2.
Comparison of DNA binding of purified wild-type and mutant SOX9 proteins to the Col2a1 enhancer harboring mutations in SOX9 binding Sites 1 and 2. (A) Wild-type and mutant Col2a1 probes for which the upper strand of the wild-type sequence is shown. Sites 1 and 2 correspond to 7-bp HMG-like binding sites. Mutated nucleotides in Sites 1 and 2 are indicated. (B) EMSA with wild-type and mutant probes using recombinant wild-type and dimerization mutant SOX9.
Figure 3.
Comparison of on-rates and DNA binding stability of wild-type and mutant SOX9 proteins. (A) Comparison of the on-rate of wild-type and mutant SOX9 binding. Wild-type and mutant SOX9 proteins were incubated for indicated lengths of time with the 31-bp Col2a1 probe. Aliquots were removed from the reaction at the indicated times and analyzed in EMSA. (B) Amounts of protein–DNA complexes present at each timepoint were measured using a Phosphoimager and plotted as percentage of the complexes found at the end of the 2-h incubation. Similar results were obtained in three different experiments. (C) Comparison of the stability of wild-type and mutant SOX9–DNA complexes. SOX9–DNA complexes were allowed to form for 2 h and challenged by a 25-fold excess amount of cold competitor oligonucleotides for indicated lengths of time. Aliquots were removed from the reaction at the indicated times and analyzed in EMSA. (D) Amount of protein–DNA complex present at each timepoint was determined using a Phosphoimager and computed as a percentage of the amount at the time the competitor was added.
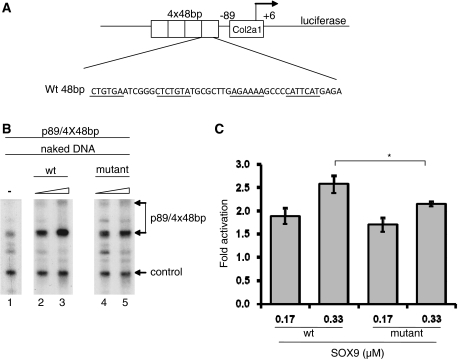
Activation of a Col2a1 promoter/enhancer by recombinant SOX9 in an in vitro transcription system
Previous transient transfection experiments have shown that Col2a1 promoter/enhancer constructions were activated in BALB3T3 and other fibroblast lines when cotransfected with a SOX9 expression vector (12). To learn more about how SOX9 activates transcription, we determined whether recombinant SOX9 could activate transcription in a cell-free system. In vitro transcription experiments were performed by adding HeLa nuclear extracts to a naked DNA template, p89/(4 × 48 bp), which consisted of a minimal mouse Col2a1 promoter (−89 to +6) preceded by a 4-fold tandem repeat of a 48-bp segment in intron 1 of Col2a1 (Figure 4A). A control Col3a1 promoter that is not responsive to SOX9 was also added to the reaction for normalization. In transfection experiments, the activity of the Col2a1 vector was strongly enhanced by cotransfection with SOX9 (12). A vector, in which four tandem copies of the 48-bp Col2a1 enhancer in intron 1, placed 3′ to a Col2a1 fragment containing 309 bp of the promoter, 237 bp of exon 1, and 70 bp of the intron 1 linked to the lacZ gene, showed chondrocyte-specific activity in transgenic mice (13). A mutation in the 48-bp sequence of this transgene abolished SOX9 binding and also abolished chondrocyte-specific activity in transgenic mice. Both wild-type and mutant recombinant SOX9 polypeptides activated transcription of the p89/4 × 48 bp Col2a1 template. Note that the level of transcription with wild-type SOX9 compared to mutant SOX9 was very similar at the lower concentration and slightly greater (1.2-fold) at the highest concentration (Figure 4B and C).
Figure 4.
Transactivation of naked Col2a1 template by SOX9 proteins in an in vitro transcription system. (A) Schematic of the p89/4 × 48 bp Col2a1 template. Four tandem copies of the 48-bp Col2a1 intron 1 enhancer were cloned upstream of a 95-bp minimal Col2a1 promoter (−89/+6) in a luciferase reporter plasmid. (B) The p89/4 × 48 bp Col2a1 promoter was transcribed in an in vitro transcription experiment as naked plasmid DNA. The DNA was added to the transcription mix together with HeLa nuclear extract. Increasing amounts of recombinant wild-type (wt) SOX9 or mutant SOX9 proteins were added as indicated above the lanes. The Col3a1 promoter was used as an internal control. RNAs were analyzed by primer extension. The two transcription start sites of p89/4 × 48 bp are indicated. Similar results were obtained in three different experiments. (C) Amount of cDNA transcripts obtained after primer extension was determined using a Phosphoimager, and the values were corrected for the control DNA Col3a1 and compared to the sample with no SOX9 (*P ≤ 0.05, t-test). Both wild-type and mutant SOX9 activated transcription. At the concentration of 0.33 µM, the level of transcription was slightly greater with wild-type SOX9 than with mutant SOX9.
The dimerization domain of SOX 9 is required for SOX9 to activate chromatin-mediated transcription of Col2a1
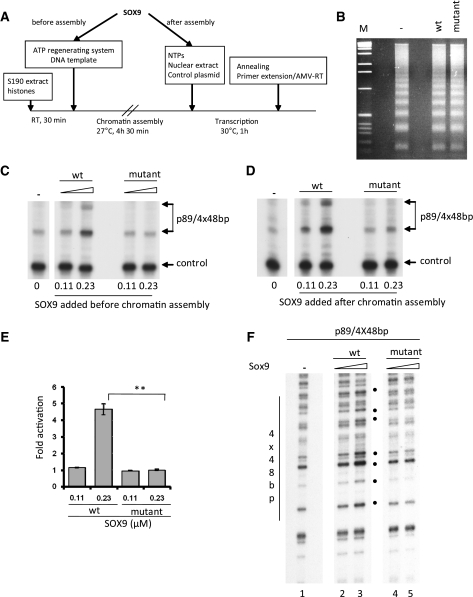
To determine whether SOX9 could overcome the repressive effect of nucleosomes in a chromatin template, the p89/(4 × 48 bp) Col2a1 DNA was assembled into nucleosomes by mixing the DNA with histones and Drosophila embryo S190 extract (Figure 5A). The formation of nucleosomes was verified by Mnase digestion of the chromatin template, which produced a DNA ladder of multiples of 146 bp, indicating that a regularly spaced nucleosomal array had formed on the DNA similar to what is found in vivo (Figure 5B). Addition of wild-type SOX9 either before or after assembly of nucleosomes stimulated transcription between 4- and 5-folds. In contrast, mutant SOX9 did not activate transcription of the chromatin DNA template. This strongly suggested that in the context of chromatin DNA only SOX9 polypeptides that form dimers on DNA could activate transcription. The same result was obtained whether mutant SOX9 was added before or after chromatin assembly (Figure 5C, D and E).
Figure 5.
Transactivation of chromatin Col2a1 template by SOX9 proteins in an in vitro transcription system. (A) Sequential steps for in vitro chromatin assembly and in vitro transcription. (B) Nucleosomal DNA templates were digested with Mnase. The DNA fragments were isolated and separated on a 1.5% agarose gel and visualized by ethidium bromide staining. A 1 kb ladder was used as a molecular weight marker (M). (C, D) The p89/4 × 48 bp Col2a1 promoter was transcribed in an in vitro transcription system after assembly in a nucleosomal DNA template. Nucleosomal DNA was added to the transcription mix together with HeLa nuclear extracts. Increasing amounts of recombinant wild-type (wt) or mutant SOX9 proteins were added prior to or after chromatin assembly, as indicated. A plasmid containing the Col3a1 promoter used as an internal control was added after nucleosomal assembly and hence transcribed as naked DNA. RNAs were analyzed by primer extension. The two transcription start sites of p89/4 × 48 bp are indicated. Similar results were obtained in three different experiments. (E) Amount of DNA obtained after primer extension was determined using a Phosphoimager, and the values were corrected for the control DNA Col3a1 and compared to the sample with no SOX9 (**P ≤ 0.01, t-test). Wild-type SOX9 at the concentration of 0.23 µM clearly activated transcription, but no activation occurs with mutant SOX9. (F) The dimerization domain of SOX9 is needed to disrupt chromatin Col2a1 DNA. Sox9 proteins were added to the DNA before chromatin assembly. Nucleosomal DNA templates were then digested with Mnase. The digested DNA was subjected to linear amplification and analyzed on a 6% sequencing gel. Location of the four copies of the 48-bp enhancer is indicated. Filled dots represent hypersensitive bands generated by interactions of SOX9 with the enhancer.
The dimerization domain of SOX9 is required for SOX9 to remodel chromatin
To determine whether SOX9 was able to remodel chromatin, we employed a partial Mnase assay using p89/4 × 48 bp nucleosomal DNA in presence of SOX9. The Mnase-digested DNA was then analyzed by linear amplification using a radiolabeled primer that hybridized to the luciferase reporter gene in the template. Upon addition of wild-type SOX9 to the chromatin template, increased Mnase cleavage was observed at several discrete sites in the multimerized Col2a1 enhancer region, suggesting that the nucleosomal structure was disrupted by the binding of SOX9 to the enhancer (Figure 5F, lanes 2 and 3). In contrast, addition of mutant SOX9 did not increase Mnase digestion at these specific sites, suggesting that mutant SOX9 did not disrupt the chromatin structure (Figure 5F, lanes 4 and 5).
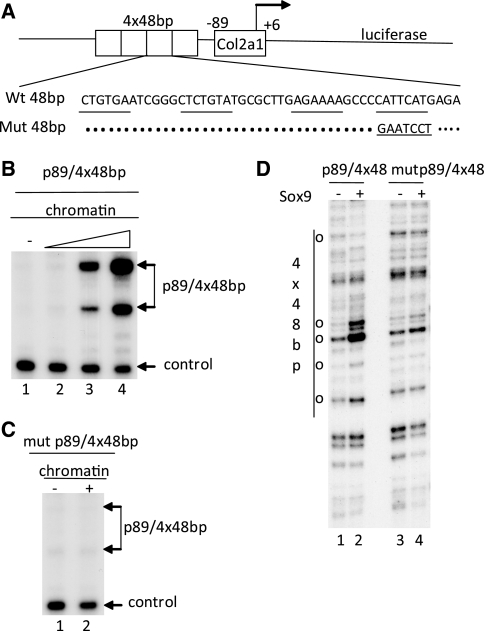
In a control experiment, we tested the transactivation potential of wild-type SOX9 in an in vitro transcription system, in which the chromatin DNA template carried mutations in the SOX9 binding site (Figure 6A), mutations that have been shown previously to completely inhibit DNA binding of SOX9. With the nucleosomal p89/4 × 48 bp Col2a1 DNA template carrying these mutations both activation of transcription and disruption of the chromatin template by wild-type SOX9, at the highest concentration used in Figure 6B, were abolished (Figure 6C, lane 2 and Figure 6D, lane 4).
Figure 6.
Mutations in Site 2 of the Col2a1 enhancer abolish transactivation by SOX9. (A) Schematic representation of Col2a1 construct, p89/4 × 48 bp and mutant p89/4 × 48 bp. Nucleotides corresponding to the four potential SOX9 binding sites are underlined. Mutations that abolish SOX9 binding, are indicated. (B) The wild-type p89/4 × 48 bp Col2a1 promoter was transcribed in an in vitro transcription experiment as chromatin-assembled DNA. The DNA was added to the transcription mix together with HeLa nuclear extract. The plasmid with the Col3a1 control promoter was added after nucleosomal assembly and transcribed as naked DNA. RNAs were analyzed by primer extension. SOX9 was added to DNA prior to chromatin assembly. The two transcription start sites of p89/4 × 48 bp are indicated. (C) The mutant p89/4 × 48 bp Col2a1 template was transcribed as chromatin-assembled DNA. Recombinant SOX9 was added to DNA prior to chromatin assembly. (D) SOX9 was added to the DNA before chromatin assembly. Nucleosomal DNA templates were then digested with Mnase and the DNA subjected to linear amplification and analyzed on a 6% sequencing gel. Location of the four copies of the 48-bp enhancer is indicated. Open dots represent hypersensitive bands generated by interaction of SOX9 with the enhancer.
In parallel experiments, we generated cell lines in which a similar Col2a1 vector as the one used for our in vitro experiments was stably transfected in the genome of 293T cells. This should produce a situation somewhat analogous to the one produced by the nucleosomal template in vitro. Transient transfection with increasing amounts of wild-type SOX9 strongly stimulated the activity of the Col2a1 promoter/enhancer-luciferase vector. In contrast, stimulation by the mutant SOX9 was markedly reduced (Figure 7A). We then performed a chromatin IP experiment using SOX9 antibodies to test whether there was a difference in the levels of interaction of SOX9 with the chromatin of the stably integrated Col2a1 promoter/enhancer vector in the same cells transfected with either wild-type or mutant SOX9. Our results indicated that the degree of occupancy of either wild-type or mutant SOX9 in the chromatin of the stably integrated Col2a1 promoter/enhancer were very similar. Thus in the cells in which wild-type SOX9 caused a much stronger activation of the Col2a1 promoter/enhancer-luciferase than the mutant SOX9, interactions of the wild-type and mutant SOX9 with the chromatin of this Col2a1 promoter/enhancer were not different.
DISCUSSION
SOX9 heterozygous mutations that cause CD include nonsense mutations that truncate the polypeptide, missense mutations in the DNA binding domain that prevent SOX9 binding, mutations in RNA splice sites, chromosomal translocations in the upstream regulatory region that inhibit the level of expression of SOX9 (4,5), a mutation in a nuclear localization signal, and missense and in-frame deletion mutations in a dimerization domain of SOX9 (23,24). This dimerization domain is highly conserved among the three members of the SOX E subgroup, SOX8, -9 and -10, and was first identified by deletion experiments in SOX10 (29). To better understand the mechanisms by which the dimerization mutations inhibit the activity of SOX9, we used purified recombinant wild-type and dimerization mutant SOX9 to further characterize the DNA binding of wild-type and SOX9 mutants and to identify the role of these proteins in the activation of a SOX9-dependent promoter in cell-free transcription systems.
The concentration dependence of DNA binding of wild-type and mutant SOX9 to a previously characterized SOX9-responsive chondrocyte enhancer sequence was compared. While binding of mutant SOX9 increased linearly with concentration, formation of a dimeric wild-type SOX9–DNA complex increased more slowly suggesting a more sigmoidal pattern, consistent with the hypothesis that binding of wild-type SOX9 to its recognition sites in the Col2a1 intron 1 enhancer might be a more cooperative process. In previous experiments, which also compared concentration-dependent binding of wild-type and mutant SOX9 harboring a point mutation in the dimerization domain to a Col11a2 dimeric binding site, the difference in the pattern of concentration-dependent binding was not as striking (24). Several factors could account for this discrepancy. Our experiments were performed with purified recombinant SOX9, whereas the previous experiments used SOX9 molecules generated by in vitro transcription–translation. Hence, these SOX9 preparations contained components of reticulocyte lysates, which might have influenced binding. Moreover, the difference in the sequences of the SOX9 binding sites in Col11a2 and Col2a1 may also have affected the concentration-dependent binding.
Analysis of binding of wild-type and mutant SOX9 to Col2a1 DNA probes containing mutations in one or the other half of the dimeric binding site suggests that Site 2 is a higher affinity monomeric binding site than Site 1. In addition, higher concentrations of wild-type SOX9 are needed for stable binding than are needed for the mutant.
The on-rate of binding at concentrations of the protein at which the wild-type SOX9 bound almost exclusively as a dimer and the stability of the DNA–SOX9 complexes were very similar for the wild-type and mutant proteins. Previous experiments with SOX10 proteins compared the on-rate of binding of a truncated SOX10 that contained both the DNA binding and the dimerization domains of SOX10 and that of a SOX10 protein containing only the HMG DNA binding domain (29). In these experiments, the on-rate reached a maximum within 1 min with both proteins, whereas in our experiment binding with either full-length wild-type or mutant SOX9 had not yet reached maximal binding even after 2 h. This difference might be attributable to the differences in experimental conditions.
To reproduce the activation of chondrocyte-specific genes by SOX9 in a cell-free system, we used recombinant SOX9, a Col2a1 template consisting of multimerized SOX9 binding sites and a HeLa cell nuclear extract that did not contain SOX9. When a naked DNA template was used, somewhat unexpectedly both wild-type and mutant SOX9 stimulated transcription, indicating that the dimerization domain is not needed to activate transcription. However, when the transcriptional activities of wild-type and mutant SOX9 were compared using the same template assembled into nucleosomes only the wild-type SOX9 activated transcription, whereas the mutant SOX9 was completely inactive. We propose that the reason for this inactivity is that, unlike wild-type SOX9, the mutant SOX9 was unable to efficiently disrupt the chromatin, which is likely to be needed for transcription once challenged by the assembly of nucleosomes on the template. This inability to disrupt chromatin occurred despite the ability of mutant SOX9 to stably bind to naked DNA, when this complex was challenged with excess DNA containing a SOX9 binding site.
In previous transient expression experiments reported by others, the ability of mutant SOX9 with mutations in the dimerization domain to activate chondrocyte-specific reporters was markedly reduced (23,24). Our experiments with a cell line in which the Col2a1 vector was stably integrated confirmed these results and showed that the mutant SOX9 was unable to activate transcription. In contrast, in ChIP experiments using the same cell line, no differences were seen in the interaction of wild-type and mutant SOX9 with the chromatin of the stably integrated Col2a1 promoter/enhancer vector. Thus, we speculate that despite a similar degree of occupancy of wild-type and mutant SOX9 on the chromatin of the Col2a1 template in vivo, other chromatin changes need to occur for transcription activation. We hypothesize that the differences that were observed in the Mnase assay between wild-type and mutant SOX9 in vitro correspond to the inability of mutant SOX9 to bring about these changes.
Finally, since the SOX9 dimerization mutant was able to activate transcription of reporters containing promoters that are targets of SOX9 in Sertoli cells (23,24), one can speculate that in these promoters the other proteins present in Sertoli cells are required to assist SOX9 in producing changes in chromatin.
In summary, our experiments suggest new insights into the molecular mechanisms, which are responsible for the skeletal symptoms of the disease CD caused by mutations in the dimerization domain of SOX9.
FUNDING
National Institutes of Health (AR042919 and AR053568 to B.dC.); postdoctoral fellowships from the Arthritis Foundation (to C.-d.O.). Funding for open access charge: National Institutes of Health.
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
We are grateful to Janie Finch and Karen Clayton for editorial assistance.
REFERENCES
- 1.Johnson RL, Tabin CJ. Molecular models for vertebrate limb development. Cell. 1997;90:979–990. doi: 10.1016/s0092-8674(00)80364-5. [DOI] [PubMed] [Google Scholar]
- 2.de Crombrugghe B, Lefebvre V, Nakashima K. Regulatory mechanisms in the pathways of cartilage and bone formation. Curr. Opin. Cell Biol. 2001;13:721–727. doi: 10.1016/s0955-0674(00)00276-3. [DOI] [PubMed] [Google Scholar]
- 3.Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res. C Embryo Today. 2005;75:200–212. doi: 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- 4.Foster JW, Dominguez-Steglich MA, Guioli S, Kowk G, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 5.Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 6.Stolt CC, Lommes P, Sock E, Chaboissier MC, Schedl A, Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17:1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vidal VP, Chaboissier MC, Lützkendorf S, Cotsarelis G, Mill P, Hui CC, Ortonne N, Ortonne JP, Schedl A. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr. Biol. 2005;15:1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 8.Mori-Akiyama Y, van den Born M, van Es JH, Hamilton SR, Adams HP, Zhang J, Clevers H, de Crombrugghe B. SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology. 2007;133:539–546. doi: 10.1053/j.gastro.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Bi W, Huang W, Whitworth DJ, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc. Natl Acad. Sci. USA. 2001;98:6698–6703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat. Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 11.Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol. Cell. Biol. 1997;17:2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou G, Lefebvre V, Zhang Z, Eberspaecher H, de Crombrugghe B. Three high mobility group-like sequences within a 48-base pair enhancer of the Col2a1 gene are required for cartilage-specific expression in vivo. J. Biol. Chem. 1998;273:14989–14997. doi: 10.1074/jbc.273.24.14989. [DOI] [PubMed] [Google Scholar]
- 14.Bell DM, Leung KK, Wheatley SC, Ng LJ, Zhou S, Ling KW, Sham MH, Koopman P, Tam PP, Cheah KS. SOX9 directly regulates the type-II collagen gene. Nat. Genet. 1997;16:174–178. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- 15.Ng LJ, Wheatley S, Muscat GE, Conway-Campbell J, Bowles J, Wright E, Bell DM, Tam PP, Cheah KS, Koopman P. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev. Biol. 1997;183:108–121. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- 16.Genzer MA, Bridgewater LC. A Col9a1 enhancer element activated by two interdependent SOX9 dimers. Nucleic Acids Res. 2007;35:1178–1186. doi: 10.1093/nar/gkm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang P, Jimenez SA, Stokes DG. Regulation of human COL9A1 gene expression. Activation of the proximal promoter region by SOX9. J. Biol. Chem. 2003;278:117–123. doi: 10.1074/jbc.M208049200. [DOI] [PubMed] [Google Scholar]
- 18.Bridgewater LC, Walker MD, Miller GC, Ellison TA, Holsinger LD, Potter JL, Jackson TL, Chen RK, Winkel VL, Zhang Z, et al. Adjacent DNA sequences modulate Sox9 transcriptional activation at paired Sox sites in three chondrocyte-specific enhancer elements. Nucleic Acids Res. 2003;31:1541–1553. doi: 10.1093/nar/gkg230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie WF, Zhang X, Sakano S, Lefebvre V, Sandell LJ. Trans-activation of the mouse cartilage-derived retinoic acid-sensitive protein gene by Sox9. J. Bone Miner. Res. 1999;14:757–763. doi: 10.1359/jbmr.1999.14.5.757. [DOI] [PubMed] [Google Scholar]
- 20.Sekiya I, Tsuji K, Koopman P, Watanabe H, Yamada Y, Shinomiya K, Nifuji A, Noda M. SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J. Biol. Chem. 2000;275:10738–10744. doi: 10.1074/jbc.275.15.10738. [DOI] [PubMed] [Google Scholar]
- 21.Rentsendorj O, Nagy A, Sinko I, Daraba A, Barta E, Kiss I. Highly conserved proximal promoter element harbouring paired Sox9-binding sites contributes to the tissue- and developmental stage-specific activity of the matrilin-1 gene. Biochem. J. 2005;389:705–716. doi: 10.1042/BJ20050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins E, Moss JB, Pace JM, Bridgewater LC. The new collagen gene COL27A1 contains SOX9-responsive enhancer elements. Matrix Biol. 2005;24:177–184. doi: 10.1016/j.matbio.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sock E, Pagon RA, Keymolen K, Lissens W, Wegner M, Scherer G. Loss of DNA-dependent dimerization of the transcription factor SOX9 as a cause for campomelic dysplasia. Hum. Mol. Genet. 2003;12:1439–1447. doi: 10.1093/hmg/ddg158. [DOI] [PubMed] [Google Scholar]
- 24.Bernard P, Tang P, Liu S, Dewing P, Harley VR, Vilain E. Dimerization of SOX9 is required for chondrogenesis, but not for sex determination. Hum. Mol. Genet. 2003;12:1755–1765. doi: 10.1093/hmg/ddg182. [DOI] [PubMed] [Google Scholar]
- 25.Coustry F, Hu Q, de Crombrugghe B, Maity SN. CBF/NF-Y functions both in nucleosomal disruption and transcription activation of the chromatin-assembled topoisomerase IIalpha promoter. Transcription activation by CBF/NF-Y in chromatin is dependent on the promoter structure. J. Biol. Chem. 2001;276:40621–40630. doi: 10.1074/jbc.M106918200. [DOI] [PubMed] [Google Scholar]
- 26.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamakaka RT, Bulger M, Kadonaga JT. Potentiation of RNA polymerase II transcription by Gal4-VP16 during but not after DNA replication and chromatin assembly. Genes Dev. 1993;7:1779–1795. doi: 10.1101/gad.7.9.1779. [DOI] [PubMed] [Google Scholar]
- 28.Kamakaka RT, Kadonaga JT. The soluble nuclear fraction, a highly efficient transcription extract from Drosophila embryos. Methods Cell. Biol. 1994;44:225–235. doi: 10.1016/s0091-679x(08)60916-4. [DOI] [PubMed] [Google Scholar]
- 29.Peirano RI, Wegner M. The glial transcription factor Sox10 binds to DNA both as monomer and dimer with different functional consequences. Nucleic Acids Res. 2000;28:3047–3055. doi: 10.1093/nar/28.16.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]