Summary
In this study, we imaged the differentiation and migratory behavior of nascent plasma cells (PCs) in mouse lymph nodes by intravital microscopy.. Pre-PCs exhibited a unique migration pattern characterized by long, linear paths that were randomly oriented. Although chemotaxis via Gαi coupled-receptors has been implicated in PC migration, treatment with Pertussis toxin (Ptx), which ablates these signals, did not prevent movement of pre-PCs while it arrested other lymphocytes. In vitro, pre-PCs displayed processive amoeboid locomotion on surfaces coated with integrin ligand, while fully-differentiated PC moved slowly or were arrested. Both PC arrest and differentiation occurred in the medullary cords. Ptx treatment before PC differentiation blocked their accumulation in the medullary cords but pre-PCs still differentiated in other lymph node regions. Taken together, we suggest pre-PCs undergo a persistent random walk to find the medullary cords, where localized chemokines help retain these cells until they undergo differentiation and arrest in situ.
Introduction
Plasma cells (PCs) play important roles in the acute response to infection and the long-term protection of the host by acting as antibody factories (Calame et al., 2003; Tarlinton et al., 2008). These terminally differentiated B cells can be divided into two subsets. Short-lived PCs are produced early in the immune responses, starting on day 3 after immunization providing a first wave of lower affinity antibodies, but die shortly thereafter (Ho et al., 1986) . Meanwhile, long-lived late PCs are produced by T cell-dependent germinal centers (GCs) that coalesce around day 6 post immunization and can continue to generate PCs for weeks. These late PCs generate higher affinity antibodies through affinity maturation (Radbruch et al., 2006). Acute ablation of the germinal center by anti-CD40 treatment halts generation of new PCs and prevents further improvements of antibody affinity (Takahashi et al., 1998).
PCs are identified by syndecan-1 surface expression, have an extensive rough endoplasmic reticulum (ER), and are enriched within the red pulp of the spleen, medullary cords of lymph nodes, and in the bone marrow (Smith et al., 1996). The precursors of PCs (pre-PCs, also called plasmablasts), are dividing cells that are found in B cell follicles in addition to red pulp and medullary cords, but not in the bone marrow. Plasma cells and pre-PC are collectively referred to as antibody secreting cells (ASCs) or antibody forming cells (AFCs) based on their ability to secrete antibody, which is often class-switched.
PC differentiation is dependent on a key transcriptional repressor, Blimp-1 (Calame et al., 2003), which inhibits many B-cell lineage (Pax5) and GC-specific (Aicda, Bcl6) genes (Shaffer et al., 2002). By repressing Pax5, Blimp-1 allows expression of XBP1, a transcription factor essential to survive ER stress associated with massive antibody secretion. Nutt and colleagues inserted an internal ribosome entry site, green-fluorescent protein (GFP) cDNA construct into exon 6 of the Prdm1 locus to generate a Blimp-1 reporter mouse (Blimp-1-GFP) and showed Blimp-1 expression as an early marker of PC development (Kallies et al., 2004). Pre-PCs expressed lower levels of Blimp-1-GFP than fully differentiated PCs, and were heterogeneous for syndecan-1 expression (Kabashima et al., 2006; Kallies et al., 2004).
Within secondary lymphoid organs, both short and long-lived PCs localize to medullary cords in lymph nodes and red-pulp regions of the spleen where they are thought to secrete antibody (Smith et al., 1996). Within these anatomic locations, PCs are largely sessile (Allen et al., 2007; Schwickert et al., 2007). PC migration to these regions has never been visualized directly, but chemokine receptors are thought to play a role because expression of CXCR4, CCR6 and EBI2 increases while CXCR5 is reduced during PC differentiation (Hargreaves et al., 2001; Pereira et al., 2009; Wehrli et al., 2001). In addition, in vitro transwell assays have shown chemotaxis of spleen red pulp PCs to the chemokines S1p and CXCL12, which are ligands of S1p1/S1p3 and CXCR4 receptors, respectively (Hargreaves et al., 2001; Kabashima et al., 2006). Consistent with this idea, CXCL12 is expressed in red pulp and medullary cords, and genetic ablation experiments showed that CXCR4-deficient PCs failed to accumulate in red pulp or bone marrow, but were enriched in blood and normal in lymph nodes compared to CXCR4-sufficent cells (Hargreaves et al., 2001). These results suggest a role for CXCL12 in PC homing (Hargreaves et al., 2001; Wehrli et al., 2001).
Long-lived PC egress from lymph nodes and homing to the bone marrow is critical for their long-term survival. In both s1p1- and β2 integrin-deficient conditions (Kabashima et al., 2006; Pabst et al., 2005), PCs are unable to exit the lymph nodes. S1p expression is high in blood and low in secondary lymphoid organs providing a gradient that may be used for egress (Rosen and Goetzl, 2005; Schwab and Cyster, 2007). Intercellular adhesion molecule-1 (ICAM-1) is highly expressed in medullary cords, which are the exit sites of PCs from lymph nodes.
From these reports and others, an image of newly-minted pre-PCs leaving the germinal center to the medullary cords along a chemokine highway has emerged. However, the current model of pre-PC chemotaxis to the medullary cords poses a few conceptual challenges. While CXCL12 is a candidate for attracting PCs to the medullary cords, it is not clear how pre-PCs would first escape from the GC, where CXCL12 is also used to partition GC B cells between the light and dark zones (Allen et al., 2004). After exit from the GC, a long and stable chemokine gradient would have to be produced to attract cells to structures that may lie millimeters away. Finally, for many existing paradigms for chemokine function (Allen et al., 2004; Bajenoff et al., 2006), regions of abundant cognate chemokines often lead to high cell motility, while in the case of plasma cells, their localization in the medullary cords seems to correlate with an arrested phenotype.
We used direct intravital imaging of pre-PCs using two-photon laser scanning microscopy (TPLSM) to understand their migration. Surprisingly, pre-PCs exhibit a randomly oriented highly linear migration within the follicle and T zone that does not depend on Gαi signaling. Processive amoeboid locomotion with rare direction changes provides an efficient means for pre-PCs to reach the medulla without a need to evolve a distinct chemotactic system. Upon reaching the medullary cords, chemokine and adhesive contacts may serve to confine and retard PCs within the medullary cords while they mature and arrest.
Results
Generation and Characterization of Blimp-1YFP mice
To study plasma cells in vivo, we used bacterial artificial chromosome transgenic mice (Rutishauser et al., 2009) that express yellow fluorescent protein (YFP) under the control of regulatory sequences from the Prdm1 gene (Turner et al., 1994), which encodes the Blimp-1 protein. These Blimp-1YFP mice showed normal B cell development in the spleen, bone marrow and peritoneum when compared to wild type B6 mice (Figure S1A). Consistent with the Blimp-1-GFP reporter mouse (Kallies et al., 2004), analysis of lymphoid organs showed that only a small number of B cells (which varied 0.05 to 0.5% in a given mouse) expressed the Blimp-1YFP transgene in the spleen or bone marrow in the steady state, and these cells expressed Syndecan-1+ , CD19low, B220low, CD43+, CXCR5− , CXCR4+ surface profiles consistent with plasma cells (Figure S1B–C). As reported by others (Kallies et al., 2004) some ASCs were Syndecan-1+ Blimp-1YFP−(Figure S1B), possibly due to transgene variegation effects (Williams et al., 2008).
To examine the development of antigen specific plasma cells in vivo we produced Blimp-1YFP mice that also carry the B1.8high immunoglobulin (Ig) heavy chain allele (Blimp-1YFP B1.8high mice), which encodes a nitrophenyl (NP) hapten-specific B cell receptor when combined with Igλ (Shih et al., 2002). Naive (CD45.2+BlimpYFPB1.8high) B cells were adoptively transferred into congenic (CD45.1+) B6 recipients, boosted with NP-OVA or without (control), and analyzed for short or long-lived PC production at early (day 4) or late (day 10), times respectively, as well as an in between (day 7) timepoint during the response. Transferred B cells produced a small subset of YFP+ cells that accumulated in draining lymph nodes, peaking at day 7 (Figure 1A). Although both YFPhigh and YFPlow populations were detectable at all three timepoints, the YFPhigh cells increased after day 4. On day 7, within the GC compartment of the transferred population (2% of total), a rare fraction (0.09%) of YFP+ cells could be detected (Figure 1B), which were YFPlow cells. Transferred B cells were subdivided into naive, GC, YFPhigh and YFPlow populations and analyzed for surface phenotypes (Figure 1C). The YFPhigh cells were consistent with a PC surface phenotype, namely Syndecan-1+ CD19low CXCR5low, while half of YFPlow cells were Syndecan-1+. These surface phenotypes for the characterized subsets were consistent with analyses on day 10. On day 4, YFPhigh cells showed no CD19 downregulation (Figure 1C), consistent with a short-lived plasma cell phenotype (Kallies et al., 2004). Heterogeneous expression of Syndecan-1 by the Blimp-1-YFPlow cells is consistent with these cells containing transitional pre-PCs (Kallies et al., 2004).
Figure 1. Characterization of Blimp-1-YFP+ Cells.
Purified, naïve B1.8high Blimp-1-YFP+ B cells were transferred into immunized congenic CD45.1+ C57BL/6 recipients. Mice were boosted subcutaneously with NP-OVA or without (control) one day post transfer. Lymphocytes were analyzed 4, 7, 10 days after boost by flow cytometry. Numbers reflect the relative percentages of cells within the gates. (A) Transferred cells (CD45.1−) generated rare Blimp-YFP+ cells on day 4, 7 and 10 but not in control mice. (B) Day 7 transferred cells were subdivided into YFPhigh, YFPlow, and YFP− populations and the GC compartment (Fas+GL7+) was assessed. Some YFPlow cells were GC+ but none that were YFPhigh. (C) Transferred populations were subdivided into naive B cells (gate I: YFP− Fas− GL7−), GC B cells (gate II: YFP− Fas+GL7+), YFPlow (gate III), and YFPhigh (gate IV) and analyzed for surface expression of CD19, CD138, CXCR4, CXCR5. For comparison, CD19 and CD138 profiles are shown for Day 4 and Day 10 populations. Flow cytometry analysis shown are samples pooled from duplicate mice in a single experiment, but is representative of at least three independent experiments. [Where is the explanation for Panel D?]
To characterize nascent pre-PCs, we sorted Blimp-1-YFP+, Fas+, GL7+ cells and compared their cellular structures to sorted populations of naïve, GC B cells, and PCs using transmission electron microscopy. The cellular morphology of the pre-PC was more consistent with GC cells than mature PCs (Figure 1D), although they showed some expansion in their ER, consistent with early stages of plasma cell differentiation (Geuze and Slot, 1980).
Blimp-1-YFP+ Distribution in the Lymph Node
We characterized PC development during an immune response by determining the distribution and expression of Blimp-1-YFP+ cells within the lymph node. Using TPLSM on days 4, 7 and 10, we obtained 3D-tiled stacks from both the follicular and medullary sides of fixed popliteal lymph nodes, which represented 50–75% of the total lymph node volume. We identified Blimp-1YFPB1.8high (green) distribution within the medullary cords (cyan, labeling various macrophages by non-specific uptake of quantum dots), follicular dendritic cells (FDC) network of the GCs (red labeling by NP-Tomato surface adhesion), and the remaining B cell follicles and T cell zone, collectively called BT Zones. We found Blimp-1-YFP+ cells concentrated in the medulla but present throughout the BT zones as well, with a few cells within GCs on day 7 and 10 (Figure 2A–B), in contrast to naïve B cells (blue) expressing cyan fluorescent protein (CFP) constitutively were more commonly distributed within B cell follicles than in the medulla. The few rare Blimp-1-YFP+ cells found in GCs appeared to be positioned along the periphery of the structure (Movie S1, Figure S2). We compared pre-PC distribution with respect to the FDC network of the GC and found them to be more peripherally localized than naïve B cells. In a previous study where Blimp-1 expression was identified in the GC, there was no mention of a peripheral localization (Angelin-Duclos et al., 2000). However, differences in the techniques from these two studies could account for the discrepancy.
Figure 2. Blimp-1-YFP+ Distribution in the Lymph Node.
Recipient mice were prepared for imaging as in Figure 1 to induce B1.8high Blimp-1-YFP cell in vivo (green). Before imaging, mice received IV transfers of naive CFP+ B cells (blue) as controls, and subcutaneous injection of QDot705 (that label phagocytes concentrated in the medullary cords, in cyan) plus NP-tomato (to label FDCs in GC light zones in red). (A) Examples of flattened 3D stacks taken by TPLSM of the follicle and medulla sides of whole fixed popliteal lymph nodes from days 4, 7, 10 are shown. Images were analyzed to determine Blimp-1-YFP cell distributions within medullary cords, GC, and other regions (collectively referred to as BT zones) per lymph node and summarized in (B) as percent total. The same analysis was made for naive CFP B cells for comparison. At least four lymph nodes were pooled per condition. Error bars represent standard error of the mean (SEM). Scale bars are 200µm in all images.
Dynamics of Blimp-1+ Cell Migration
We imaged the migratory behavior of Blimp-1-YFP+ cells in the lymph node by intravital time-lapse TPLSM. In vivo, Blimp-1-YFP+ cells in the follicle were similar in size and shape to blasting GC B cells, which are larger and more polarized than naïve B cells (Schwickert et al., 2007) (Movie S2). In general, Blimp-1-YFP+ were highly motile in the follicle and largely sessile in the medullary cords (Figure 3A). Blimp-1-YFP+ cell migration seemed to be similar on days 4, 7, and 10 (Movie S2, Figure 3B), with cells in the medullary cords slightly more motile on day 4 than on day 7 or 10, which correlated with their transition from pre-PC to PC in the medulla. Outside the medullary cords, Blimp-1-YFP+ cell average track velocity was lower than naïve cells (Figure 3B) although some cells reached similar speeds.
Figure 3. Dynamics of Blimp-1-YFP+ Cell Migration.
Imaging conditions as described in Figure 2 were used to image Blimp-1-YFP migration at days 4, 7 and 10. (A) Images of movies with sample cell tracks that were segmented by region as medullary cords (red) or BT zones (yellow) on various days. Naive B cells (in blue) sample tracks are in gray. Scale bars are 110 µm. (B) Average track velocities from populations from several imaging experiments, from various days, segmented by region and pooled, were tallied and plotted with t-tests comparing regions with p-values abbreviated as ** (p<0.001) and *** (p<0.0001). (C) Hypothetical plots of the mean square displacement (MSD) over time for a random Brownian walk, confined migration, and a random walk with persistence (or flow migration) as reference for experimental measurements. (D) MSD plots from the populations in B for naive B cells (black), Blimp-1-YFP+ cells within BT zones (green) or medullary cords (red) from all three time-points. Error bars reflect SEM. (E) The x,y,z displacements from Blimp-1-YFP+ (green) and naive B cell (blue) tracks are plotted for three sample movies from each timepoint. Means and standard deviation (SD) are marked. T-tests were performed between naive and Blimp-1+ components for each movie, but none reached a 95% significance.
Pre-PC migration was highly linear over long distances and less confined as compared with naïve B cells, which migrate randomly in the follicle. To determine if pre-PCs were more processive than naïve B cells, we calculated the average mean squared displacement (MSD) for cell tracks over time. As modeled in Figure 3C, the MSD for random walk scales linearly with time, while the MSD for confined motion reached a plateau. Random walks with long free path lengths or persistent random walks generate exponential MSD plots that accelerated with increased path lengths (see (Codling et al., 2008; Zygourakis, 1996) for further discussion). We calculated the MSD for Blimp-1-YFP+ cells, segmented by day and region, and compared them to control naïve B cells in those fields. The MSD plots for naïve cells had initial slopes that were linear which reached a maximum displacement after ~30 minutes consistent with limited follicle size (Figure 3D). Non-medullary Blimp-1-YFP+ cells showed higher displacements than naïve B cells, despite having slower speeds (Figure 3B). Based on fitting the average MSD plots to an equation for 3D persistent random walk (see methods), pre-PCs showed an average 3 minute persistence time, compared to 1 minute for naïve cells. Confinement of Blimp-1-YFP+ cells in the medullary cords increased at later time points (Figure 3D).
Despite a high degree of linearity in pre-PC movement, analysis of the track displacement showed no overall directionality at any timepoint (Figure 3E). We could not detect a bias in pre-PC displacements along any axis, or when compared to naïve B cells in the same fields. In movies that contained regions of medulla or GC, pre-PCs showed no bias towards or away from these elements (Movies S2). Moreover, we anecdotally observed Blimp-1-YFP+ pre-PCs crawling linearly until reaching the capsule of the lymph node, changing direction and continuing in a new direction, in what appeared to be a blind search for the medulla (Movie S3).
In vivo Requirements for Blimp-1+ pre-PC Migration
Despite the absence of directional movement by pre-PCs, changes in CXCR4 and CXCR5 chemokine receptor expression on PCs and reports of chemotaxis in vitro suggested that chemokinesis might have a role in coordinating pre-PC motility in the lymph node (Hargreaves et al., 2001; Wehrli et al., 2001). To determine whether G-protein coupled receptor (GPCR) signaling might be involved in pre-PC motility in vivo, mice were treated with a high dose of Pertussis toxin (Ptx), which ablates Gαi signaling and most chemokine directed movement (Kaslow and Burns, 1992) in B cells. Treatment with Ptx completely stopped naive B cell migration (Figure 4B), which served as a positive control; cells extended small filopodial projections (Movie S4,5) but were completely confined based on their MSD plots (Figure 4C). In contrast, most Blimp-1-YFP+ cells continued crawling and in a highly linear fashion based on the MSD plots (Figure 4D), but with reduced average track velocity (4.8 to 3.9 µm/sec2, Fig 4B). One explanation for this small reduction may be the presence of large numbers of arrested lymphocytes creating barriers and added resistance to Blimp-1 cell migration. We saw similar trends in migration at all three timepoints. Medullary Blimp-1-YFP+ cells at later timepoints that were already arrested remained arrested. We confirmed that Blimp-1-YFP+ cells were capable of chemotaxis in response to CXCL12, as had been previously reported for pre-PCs. Both Blimp-1-YFP+ cells and naive cells migrated towards CXCL12 in an in vitro transwell migration assay (Figure S3A). Pre-treatment of these cells with Ptx completely blocked transwell migration.
Figure 4. Ptx Blocks Migration of Naïve B Cells but not pre-PC Migration.
Blimp-1-YFP+ and naïve B cells were imaged on day 4, 7, and 10 as described in Figure 3. Mice were treated or not with Ptx one day prior to imaging. (A) Sample images from 3D time-lapse movies on days 4 and 10 from Ptx-treated mice (see Figure 3 legend). Scale bars are 110 µm. (B) Quantification of average track velocity for naïve cells, and Blimp-1-YFP+ cells on days 4, and 10 segmented by regions. T-tests were performed between conditions, *** indicate p<0.001. (C) MSD plots comparing conditions in B with (red) or without (black) Ptx treatment. Error bars reflect SEM.
S1p chemokine signaling has also been implicated in PC chemotaxis and migration from the medulla to the bone marrow. S1p signals through S1p1 and S1p3 GPCRs, which use the Ptx sensitive Gαi for signaling. However, S1p3 can also utilize the Ptx insensitive Gα12,13 and Gαq for signaling (Spiegel and Milstien, 2003). To block these additional pathways, Ptx-treated mice were also given FTY720, which down-regulates S1p1 and S1p3 receptors and prevents signaling. Ptx and FTY720 treated mice showed no significant differences in pre-PC movement when compared to mice treated with Ptx (Figure S3B). These experiments indicate that although Blimp-1+ cells are capable of (in vitro) chemotaxis, their migration in vivo is only partially dependent on chemokine cues.
Pre-PC cell Migration is Adhesion-Mediated
We conducted in vitro live cell imaging studies to determine what factors mitigate Blimp-1-YFP+ cell motility to allow arrest in the medulla. Glass substrates were coated with the adhesion molecule, ICAM-1, and with or without chemokine ligands, CXCL12 or CXCL13. We focused on ICAM-1 because of the established role of β2 integrins in PC egress from lymph nodes and the presence of high levels of ICAM-1 on many lymph nodes cells (Pabst et al., 2005). In vitro activated Blimp-1-YFP+ cells were imaged in combination with naive dsRed+ B cells as controls. We used interference reflection microscopy (IRM) to image the cell contacting the substrate in order to measure adhesion and differentiate crawling versus fluid flow movement. Both cell types were capable of migrating on these substrates (Figure 5A, Movies S6–7). Naive cells migrated rapidly in response to either chemokine, but also on ICAM-1 alone if BSA was present (Figure 5C), and chemokines were required for naive cell migration in the presence of fatty acid free BSA (Figure S5). Ptx treatment blocked naive cell migration (Figure 5B, 5D) and also reduced their ability to adhere to the substrate (Figure 5E). In contrast, Blimp-1-YFP+ cells, which migrated slower than naive cells (Figure 5C), only required ICAM-1 for migration (Figure S5). Blimp-1-YFP+ cell adhesion and migration on ICAM-1 substrates were unaffected by Ptx (Figure 5B, 5D, 5E, Movie S8).
Figure 5. Migration of Blimp-1+ Cells is Adhesion Mediated.
In A–B, examples of in vitro time-lapse movies of ICAM-1 coated slides with Blimp-YFP+ cells (green) and naive B cells (red) that were pretreated with Ptx (B, Movie S8) or not (A, Movie S7). Tracks are overlaid, with colors respective to the cell type. Naive and Blimp-1+ cell migration was plotted as average track velocity (C) or displacement velocity (D). Ptx-treated naive cells showed decreases in migration (D) and adhesion (E) to the substrate, as compared to Blimp-1+ cells. In F, examples of IRM contact area of naive and Blimp-1+ B cells compared to their cell body (or fluorescent) area (see movie S9). The mean ratio of IRM area to cell area was computed (G) for both cell populations was calculated and were different (p = 0.004). Bars represent mean, with SD errors shown. Scale bars are 70µm.
Based on the IRM image captured during cell migration, we could visualize the cell footprint, which informs on the binding to ICAM-1, as well as the mode of locomotion. Blimp-1+ cells, which are larger cells, had a larger contact surface than naive B cells, even when normalized to the cell size (Figure 5F–G). In time-lapse movies, we observed that (Movie S9) naive B cells had a dynamic and transient IRM image, with frequent rounds of detachment and reattachment to the substrate. In contrast, Blimp-1+ cells showed a stable IRM pattern, which spread evenly as they migrated in a processive amoeboid fashion.
Blimp-1+ cell velocity was heterogeneous in vitro (Figure 5C) and in vivo (Figure 3B). However, the cell speed was inversely related to YFP+ expression, a measure of their maturation state (Kallies et al., 2004). YFPlow cells were highly motile, while YFPhigh cells appeared slow or arrested (Figure 6A). This relationship was also detected in in vivo track analysis (Figure 6B). Since this behavior is seen on ICAM-1 alone, it suggests PC arrest is cell-autonomous.
Figure 6. Blimp-1+ cells arrest with differentiation.
Blimp-1 cell track velocity on ICAM-1 was plotted as a function of YFP intensity (A). Analysis of in vivo tracks taken on day 7 showed a similar relationship (B).
Proper PC Localization in Medulla Requires Chemokine
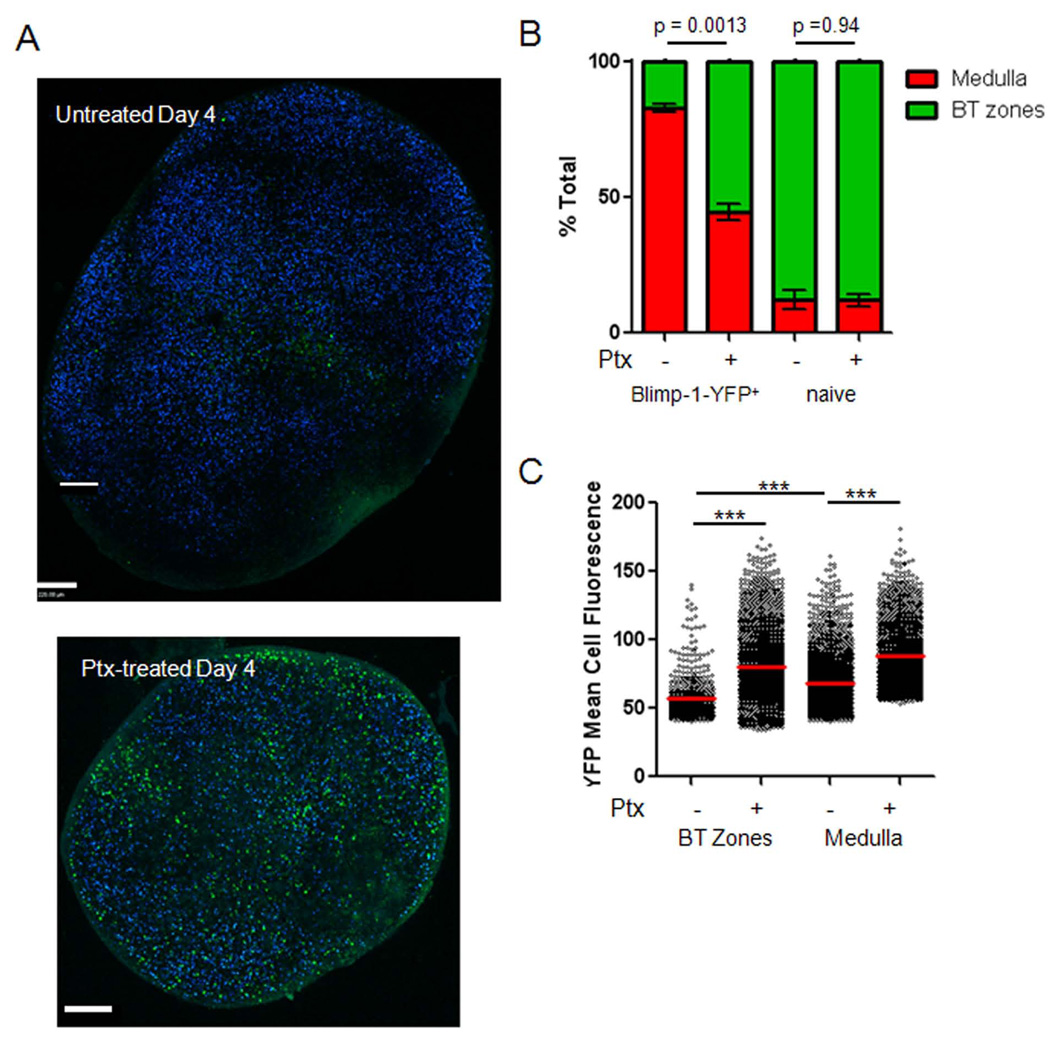
On day 4, after immunization large numbers of early pre-PCs develop, migrate, with ~80% accumulation in the medullary cords as compared to 10% of naïve B cells. However, Ptx treatment (24 hours before visualization) reduced PC accumulation in the medulla to 40% while leaving naïve B-cells distributed normally (Figure 7A–B, Movie S5). BT zones normally contain YFPlow cells consistent with pre-PC, however after treatment, BT zones were enriched in YFPhigh cells consistent with PCs (Figure 7C). At later timepoints, most PCs were already arrested in the medullary cords, and Ptx treatment had no effect on their distribution (data not shown). We conclude that chemokine signaling is required for Blimp-1+ cell accumulation in the medulla.
Figure 7. Ptx Blocks PC Accumulation in Medullary Cords but not Blimp-1+ Expression.
(A) As in Figure 2, TPLSM 3D tiled stacks from the follicle and medulla sides of whole fixed popliteal lymph nodes from day 4 with or without Ptx treatment. (B) The distribution of Blimp-1-YFP and naïve B cells were isolated, and segmented into medullary cords, GC, and other regions (collectively referred to as BT zones) and plotted as the total YFP distribution per lymph node (using both sides of the lymph node). At least four lymph nodes were pooled per condition. Error bars represent SEM. Scale bars are 200µm in all images. (C) The average YFP intensity for all Blimp-1-YFP cells collected was calculated and plotted by region and Ptx condition. T-tests comparison of the conditions were made with p-values labeled above or with *** (p<0.001).
Discussion
PC retention in secondary lymphoid organs and bone marrow is critical for their long-term survival. Using a newly generated Blimp-1-YFP mouse, we were able to image pre-PCs as they differentiate and migrate to become PCs. To understand their migration, we compared their behavior to naive B cells, which are found throughout the lymph node. Naive B cells migrate rapidly in the B cell follicle in random directions, with short linear excursions punctuated by frequent turns (Miller et al., 2002). This frequent random turning is thought to be mediated by chemokine signals, particularly CXCL13, presented on surfaces of stromal FDC networks, such that the scale for the free path between turns is set by the spacing of natural branch points in the stromal network (Bajenoff et al., 2006; Okada and Cyster, 2006). The exploration of this elaborate stromal network allows for a high-density search inside the B cell follicle (Miller et al., 2002). Consistent with the proposed role of chemokines in naïve B cell movement in lymph nodes, Ptx treatment caused them to arrest.
In vivo, pre-PCs displayed a greater displacement velocity than naïve B cells and their pattern of migration was similar to a random walk but with a very long free path length between turns. We describe this as a "random sprint". Pre-PCs showed no directional bias away from the GC or follicle or towards the medullary cords, as might be expected for chemotaxis. This is consistent with the minor effect on migration by blocking chemokine signals with Ptx and FTY720 treatment. The pre-PCs only appear to turn when they encounter obstacles like the sub-capsular sinus (Movie S4), suggesting that their migration to the medulla may be stochastic. The long, linear paths followed by pre-PC are shared by positively selected thymocytes moving toward the thymic medulla (Witt et al., 2005). However, the movement of the positively selected thymocytes is highly directed and chemotactic, whereas this is not the case for the movement of pre-PCs. Since the motility coefficient for a random walk process scales linearly with the free path length or time persistence (Zygourakis, 1996), random motility with a long free path is an ideal strategy to search for the medullary cords, a compartment that may be hundreds of microns from the GC (Friedrich, 2008).
In vitro migration of naive and Blimp-1 cells revealed a more mechanistic explanation for the in vivo observations. Naive B cell contacts with the ICAM-1 surface were highly dynamic, and rapidly contracted. Their migration and even adhesion to ICAM-1 was Ptx-sensitive, suggesting a requirement for Gαι signaling. In contrast, Blimp-1+ cells adhered to ICAM-1 and displayed slow and steady migration in the presence of Ptx and in the absence of chemokine signals. Both of these two modes of migration have recently been described for naive T cells by Krummel and colleagues (Jacobelli et al., 2009). T cells can migrate using either an intermittent amoeboid locomotion dependent upon myosin IIa contraction or with a processive amoeboid "sliding" mode involving continuous ICAM-1 adhesion linked to retrograde actin flow. Naive B cells primarily use the intermittent, low adhesion mode allowing for frequent direction changes, while pre-PCs use the processive adhesion dependent mode, leading to persistence of direction over 10’s of microns. This search strategy is effective because the long free path length is well matched to the distances that the pre-PC need to travel to reach the medullary cords. Pre-PCs may use FRC and FDC branched networks as scaffolds for adhesion and migration. However their long free paths suggests pre-PCs do not utilize these networks exclusively. ICAM-1 is probably not the only adhesion molecule that pre-PC can use for migration in lymph nodes. In the absence of β2 integrins, PCs still accumulate in the medullary cords but are unable to exit the tissue for the bone marrow (Pabst et al., 2005). We would propose that the ICAM-1 dependent arrest of PC allowed maturation in the medullary cords prior to a slower, integrin dependent egress process that allows trafficking to the bone marrow.
The random sprint serves pre-PCs by facilitating their rapid exit from the GC. Pre-PCs might encounter a number of compartments on these random sprints including subcapsular region, T cell zone, other follicles, cortical sinusoids and the medullary cords. Movement between compartments in tissues has typically been guided by chemokine gradients with CCR7 for T cell zones and thymocyte movement to the medulla (Nitta et al., 2008), CXCR5 for the follicle and S1p for the lymph and blood. While CXCR4 has a role in accumulation of PC in the medullary cords, this does not appear to be the product of chemotaxis and may play additional roles in bone marrow homing (Hauser et al., 2002).
As pre-PCs mature, their motility arrests in vivo as well as in vitro. We can observe this phenomenon on ICAM-1 alone, indicating that no extrinsic cues are required, aside from maturation signals. These Blimp-1-YFPhigh cells correspond to arrested plasma cells in the LN. Indeed Blimp-1-YFPhigh cells are also less chemotactic than Blimp-1-YFPlow cells in transwell assays with S1p or CXCL12 (Kabashima et al., 2006; Wehrli et al., 2001). PC arrest and differentiation are coordinated in the medullary cords through a Ptx-sensitive pathway, but at short distances. In previous reports, CXCR4−/− ASCs were able to exit white pulp, and were more abundant in the blood than in the red pulp, consistent with a retention defect rather than a failure to migrate. Furthermore, inhibition of S1p affected bone marrow homing, but showed normal ASC distribution in the spleen (Kabashima et al., 2006). Chemotaxis of ASCs to CXCL12 and S1p in in-vitro transwell assays may be modeling the in vivo retention of these cells in the medulla and red pulp rather than their directed migration to these regions. Indeed, Ptx treatment inhibited accumulation but not migration to the medulla, suggesting retention in the medullary cords is chemokine-dependent. PC arrest and differentiation were mis-localized but not inhibited by Ptx, confirming that in vivo, these processes are also cell-autonomous. Myeloid cells in the medullary cords may provide survival signals such as BAFF and April (Belnoue et al., 2008; Mohr et al., 2009) but only for PCs that manage to find the medullary cords before they arrest. Further work to is needed to determine if cell-cell interactions contribute to PC migration and arrest as well.
Experimental Procedures
Animals, Immunization, Transfer
For adoptive transfer experiments, wild-type C57BL/6, B6.SJL (CD45.1+), CFP+, and dsRed+ mice (Jackson Laboratories) between 7–12 weeks of age were used with protocols approved by Institutional Animal Care and Use Committee. CD45.1+ hosts were immunized with an intrapertioneal (IP) immunization of 50µg/mouse of OVA (Sigma) emulsified in Alum (Pierce), to enrich T-cell help two weeks prior to cell transfer. B1.8high Blimp-1-YFP mice (with or without CFP+ transgene) were used for donor cells. Splenic naive B-cells were purified by CD43-depletion using MACS® beads (Miltenyi Biotec) and transferred intravenously (IV) to hosts (5–10×106/mouse). After 24 hours, hosts received a subcutaneous (S/C) boost in the footpads with 100 µg of NP(15)-OVA (Biosearch). Lymphocytes were harvested and analyzed 3 to 10 days later by flow cytometry on a LSR II (BD Bioscences).
For imaging experiments, hosts were imaged on day 4, 7 and 10 post-boost with NP-OVA. One day prior to imaging, naïve control B-cells purified from CFP+ B6 mice were adoptively transferred into the mouse recipient as motility controls. To label medullary cord macrophages, 1pmole of QTracker© 705 non-targeted quantum dot (Invitrogen) was injected in the hind footpad. In addition, 2.5µg of NP(11)-Tomato or NP- Phycoerythrin (PE) (Biosearch) was injected in the hind footpad to label the light zone of the germinal center structures. NP-tomato was made in house, as a tomato-GST construct, expressed in e coli, purified by Glutathione column (Amersham), and conjugated with NP-SE (Biosearch). For in vivo experiments, Pertussis toxin (Ptx, 400 µg/kg Calbiochem) was administered IV, FTY720 (1 mg/kg) was delivered IP 12–24h prior to imaging (Huang et al., 2007).
In vitro Imaging and Transwell
Blimp-YFP+ naive B cells were purified and stimulated with LPS (25ng/mL), IL-4 (5ng/mL), IL-5 (15ng/mL), and BAFF (10ng/mL) for 3–4 days. Naive dsRed+ B cells were purified and incubated in RPMI+10% FBS overnight before imaging or transwell experiments. For transwell experiments, cells were incubated in upper chamber of 5µm Costar transwells for two hours with CXCL12 (1µg/mL, R&D Systems) and were counted on a FACScalibur. For imaging, cells were imaged in Lab-Tek ™ chamber slides systems (Nunc) using an adapted protocol as kindly provided from the laboratory of Ronen Alon at the Weizmann Institute of Science in Rehovot, IL. Chemokines were spotted on clean glass surface for 2h at 37°C, washed, and then spotted with 5 µL of transmembrane ICAM-1 at 400 molecules/µm2 for 1hr at room temperature. The glass surface was blocked with 10% bovine serum albumin (BSA) or 10% Fatty acid free (FAF)-BSA for 1hr, washed, and then used for imaging. Prior to imaging, cells were incubated in RPMI+1% BSA (Regular or FAF, Sigma) with or without Ptx (50ng/mL) for two hours. Cells were resuspended in HBSS supplemented with 10mM HEPES, 2mg/mL BSA (FAF or Regular), and imaged at 37°C using a Zeiss LSM710 using standard confocal settings for YFP and dsRed fluorophores.
TPLSM Imaging and Analysis
To prepare for imaging, mice were anesthetized using KXA (Ketamine, Xyalzine, Acepromazine) solution (4µl/g). We exposed the popliteal lymph node by shaving the leg, dissecting the skin and fatpad in an aseptic manner. A metal stabilization plate and coverslip were used to immobilize the lymph node. The mouse was comforted with regular injections of KXA, temperature control stage, and nose-cone with O2 supplement.
For intravital TPLSM, an upright BioRad microscope was used as before (Schwickert et al., 2007) using a 40X Nikon objective and also repeated on a Zeiss LSM-710 inverted microscope with Mai-Tai Sapphire Laser for two-photon excitation using a 25X objective. Time lapse z-stacks were taken at 30 sec intervals on either system, with similar measurements for cell speeds and migratory behavior. Tiled 3D stacks of the lymph node were collected on the Zeiss system.
Collected imaging series were analyzed for cell position and dynamics using Volocity 4.2 (Improvision), Excel 2003 (Microsoft) and GraphPad 4.0 (Prism). Movies were annotated using Adobe Affect Effects. Cell migrations were tracked automatically using Volocity, annotated for region and pruned manually. Only continuous tracks that persisted at least 6 minutes in the field were used for analysis. For cell distributions within a lymph node, cell bodies were identified by RGB thresholding to cells. Rather than segmenting individual cells, which frequently clustered particularly in the medullary cords, total cell pixel volumes were determined, subdivided by region in the lymph node, and presented as a percentage found in the total lymph node. Both sides of the lymph node were totaled for each measurement and repeated for 4–6 lymph nodes per condition. For MSD measurements, sampling was rounded to the nearest minute. The initial 30 minutes of the MSD plots were fit to a 3D persistent random walk (Equation 1) as
| (Equation 1) |
described (Zygourakis, 1996) using non-linear least squares (Origin 7.0) and solved for persistence time (P) using the average constant speed from Figure 3B as speed (S).
Cell Staining, Flow Cytometry
Flow cytometry measurements were conducted on a BD LSR II and analyzed using FlowJo 7.2. Cell populations were stained using BD Pharmagen antibodies unless otherwise stated: CD19, B220, CD138, CXCR5, CXCR4, Fas, GL7, and CD45.1.
Electron Microscopy Preparation and Imaging
Cells were washed with serum-free media or appropriate buffer then fixed with a modified Karmovsky's fix of 2.5% glutaraldehyde, 4% paraformaldehyde and 0.02% picric acid in 0.1M sodium caocdylate buffer at pH 7.2. Following a secondary fixation in 1% osmium tetroxide, 1.5% potassium ferricyanide, samples were dehydrated through a graded ethanol series, cleared with acetonitrile then infiltrated and embedded in an epon analog resin. Ultrathin sections were cut using a Diatome diamond knife (Diatome) on a Leica Ultracut S ultramicrotome (Leica). Sections were collected on copper grids, further contrasted with lead citrate, and viewed on a JSM 100 CX-II electron microscope (JEOL, USA, Inc., Peabody, MA) operated at 80 kV. Images were recorded on Kodak 4489 Electron Image film then digitized on an Epson Expression1600 Pro scanner.
Supplementary Material
Figure 8.
Figure 9.
Figure 10.
Figure 11.
Acknowledgements
We would like to thank E. Meffre for Blimp-1 YFP mice, T. Starr for making ICAM-1 reagents, and the EM facility at Rockefeller University for help preparing and imaging cells. This work was supported by NIH grants R01AI072529 (MCN and MLD), R56AI44931 (MLD), CA009161-34 (DRF), and Schering fellowship (TS).
References
- Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- Angelin-Duclos C, Cattoretti G, Lin KI, Calame K. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J Immunol. 2000;165:5462–5471. doi: 10.4049/jimmunol.165.10.5462. [DOI] [PubMed] [Google Scholar]
- Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belnoue E, Pihlgren M, McGaha TL, Tougne C, Rochat AF, Bossen C, Schneider P, Huard B, Lambert PH, Siegrist CA. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 2008;111:2755–2764. doi: 10.1182/blood-2007-09-110858. [DOI] [PubMed] [Google Scholar]
- Calame KL, Lin KI, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Annu Rev Immunol. 2003;21:205–230. doi: 10.1146/annurev.immunol.21.120601.141138. [DOI] [PubMed] [Google Scholar]
- Codling EA, Plank MJ, Benhamou S. Random walk models in biology. J R Soc Interface. 2008;5:813–834. doi: 10.1098/rsif.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich BM. Search along persistent random walks. Phys Biol. 2008;5:026007. doi: 10.1088/1478-3975/5/2/026007. [DOI] [PubMed] [Google Scholar]
- Geuze HJ, Slot JW. The subcellular localization of immunoglobulin in mouse plasma cells, as studied with immunoferritin cytochemistry on ultrathin frozen sections. Am J Anat. 1980;158:161–169. doi: 10.1002/aja.1001580206. [DOI] [PubMed] [Google Scholar]
- Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou YR, Littman DR, Cyster JG. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194:45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser AE, Debes GF, Arce S, Cassese G, Hamann A, Radbruch A, Manz RA. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J Immunol. 2002;169:1277–1282. doi: 10.4049/jimmunol.169.3.1277. [DOI] [PubMed] [Google Scholar]
- Ho F, Lortan JE, MacLennan IC, Khan M. Distinct short-lived and long-lived antibody-producing cell populations. Eur J Immunol. 1986;16:1297–1301. doi: 10.1002/eji.1830161018. [DOI] [PubMed] [Google Scholar]
- Huang JH, Cardenas-Navia LI, Caldwell CC, Plumb TJ, Radu CG, Rocha PN, Wilder T, Bromberg JS, Cronstein BN, Sitkovsky M, et al. Requirements for T lymphocyte migration in explanted lymph nodes. J Immunol. 2007;178:7747–7755. doi: 10.4049/jimmunol.178.12.7747. [DOI] [PubMed] [Google Scholar]
- Jacobelli J, Bennett FC, Pandurangi P, Tooley AJ, Krummel MF. Myosin-IIA and ICAM-1 regulate the interchange between two distinct modes of T cell migration. J Immunol. 2009;182:2041–2050. doi: 10.4049/jimmunol.0803267. [DOI] [PubMed] [Google Scholar]
- Kabashima K, Haynes NM, Xu Y, Nutt SL, Allende ML, Proia RL, Cyster JG. Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J Exp Med. 2006;203:2683–2690. doi: 10.1084/jem.20061289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallies A, Hasbold J, Tarlinton DM, Dietrich W, Corcoran LM, Hodgkin PD, Nutt SL. Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. J Exp Med. 2004;200:967–977. doi: 10.1084/jem.20040973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslow HR, Burns DL. Pertussis toxin and target eukaryotic cells: binding, entry, and activation. FASEB J. 1992;6:2684–2690. doi: 10.1096/fasebj.6.9.1612292. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- Mohr E, Serre K, Manz RA, Cunningham AF, Khan M, Hardie DL, Bird R, MacLennan IC. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J Immunol. 2009;182:2113–2123. doi: 10.4049/jimmunol.0802771. [DOI] [PubMed] [Google Scholar]
- Nitta T, Murata S, Ueno T, Tanaka K, Takahama Y. Thymic microenvironments for T-cell repertoire formation. Adv Immunol. 2008;99:59–94. doi: 10.1016/S0065-2776(08)00603-2. [DOI] [PubMed] [Google Scholar]
- Okada T, Cyster JG. B cell migration and interactions in the early phase of antibody responses. Curr Opin Immunol. 2006;18:278–285. doi: 10.1016/j.coi.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Pabst O, Peters T, Czeloth N, Bernhardt G, Scharffetter-Kochanek K, Forster R. Cutting edge: egress of newly generated plasma cells from peripheral lymph nodes depends on beta 2 integrin. J Immunol. 2005;174:7492–7495. doi: 10.4049/jimmunol.174.12.7492. [DOI] [PubMed] [Google Scholar]
- Pereira JP, Kelly LM, Xu Y, Cyster JG. EBI2 mediates B cell segregation between the outer and centre follicle. Nature. 2009 doi: 10.1038/nature08226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- Schwickert TA, Lindquist RL, Shakhar G, Livshits G, Skokos D, Kosco-Vilbois MH, Dustin ML, Nussenzweig MC. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007;446:83–87. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, Staudt LM. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- Shih TA, Roederer M, Nussenzweig MC. Role of antigen receptor affinity in T cell-independent antibody responses in vivo. Nat Immunol. 2002;3:399–406. doi: 10.1038/ni776. [DOI] [PubMed] [Google Scholar]
- Smith KG, Hewitson TD, Nossal GJ, Tarlinton DM. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur J Immunol. 1996;26:444–448. doi: 10.1002/eji.1830260226. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Dutta PR, Cerasoli DM, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J Exp Med. 1998;187:885–895. doi: 10.1084/jem.187.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlinton D, Radbruch A, Hiepe F, Dorner T. Plasma cell differentiation and survival. Curr Opin Immunol. 2008;20:162–169. doi: 10.1016/j.coi.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Turner CA, Jr, Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- Wehrli N, Legler DF, Finke D, Toellner KM, Loetscher P, Baggiolini M, MacLennan IC, Acha-Orbea H. Changing responsiveness to chemokines allows medullary plasmablasts to leave lymph nodes. Eur J Immunol. 2001;31:609–616. doi: 10.1002/1521-4141(200102)31:2<609::aid-immu609>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Williams A, Harker N, Ktistaki E, Veiga-Fernandes H, Roderick K, Tolaini M, Norton T, Williams K, Kioussis D. Position effect variegation and imprinting of transgenes in lymphocytes. Nucleic Acids Res. 2008;36:2320–2329. doi: 10.1093/nar/gkn085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt CM, Raychaudhuri S, Schaefer B, Chakraborty AK, Robey EA. Directed migration of positively selected thymocytes visualized in real time. PLoS Biol. 2005;3:e160. doi: 10.1371/journal.pbio.0030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygourakis K. Quantification and regulation of cell migration. Tissue Eng. 1996;2:1–16. doi: 10.1089/ten.1996.2.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.