Abstract
Acetohexamide is a drug used to treat type II diabetes and is tightly bound to the protein human serum albumin (HSA) in the circulation. It has been proposed that the binding of some drugs with HSA can be affected by the non-enzymatic glycation of this protein. This study used high-performance affinity chromatography to examine the changes in acetohexamide-HSA binding that take place as the glycation of HSA is increased. It was found in frontal analysis experiments that the binding of acetohexamide to glycated HSA could be described by a two-site model involving both strong and weak affinity interactions. The average association equilibrium constant (Ka) for the high affinity interactions was in the range of 1.2–2.0 × 105 M−1 and increased in moving from normal to HSA with glycation levels that might be found in advanced diabetes. It was found through competition studies that acetohexamide was binding at both Sudlow sites I and II on the glycated HSA. The Ka for acetohexamide at Sudlow site I increased by 40% in going from normal HSA to minimally glycated HSA but then decreased back to near-normal values in going to more highly glycated HSA. At Sudlow site II, the Ka for acetohexamide first decreased by about 40% and then increased in going from normal HSA to minimally glycated HSA and more highly glycated HSA. This information demonstrates the importance of conducting both frontal analysis and site-specific binding studies in examining the effects of glycation on the interactions of a drug with HSA.
Keywords: Acetohexamide, Human serum albumin, Glycation, Drug-protein binding, High-performance affinity chromatography
1. Introduction
Human serum albumin (HSA) is the most abundant protein in human plasma. It is a globular, non-glycosylated protein that contains a single chain of 585 amino acids and has a serum concentration of ~40 g/L [1–8]. This protein has two major binding sites for drugs (i.e., Sudlow sites I and II) [9,10]. Sudlow site I, also known as the warfarin-azapropazone site, binds to bulky heterocyclic compounds such as coumarin compounds, sulfonamides, and salicylate [3,6,8,11]. Sudlow site II, or the indole-benzodiazepine site, binds aromatic carboxylic acids and profens such as ibuprofen and ketoprofen [3,6,8,11]. HSA binds many endogenous and exogenous compounds at these and other sites, acting as the major transport protein for such compounds in the circulation [8]. The binding of drugs to HSA can greatly affect their pharmacologic properties and influence the activity of a drug by altering its free fraction in blood [8,12].
The interactions of drugs with HSA can be affected by competition for binding sites with co-administered drugs or endogenous compounds, but they may also be affected by modifications such as glycation [8,13]. Glycation occur when free amine groups on a protein become non-enzymatically linked to reducing sugars such as glucose to form a Schiff base, which can then later rearrange to form a more stable Amadori product. This process is especially prevalent in diabetes, in which blood sugar levels are elevated. The normal level of HSA glycation is around 6–13%, while a diabetic individual may have 20–30% of HSA glycated in the circulation [1,2,8]. Some of the primary glycation sites on HSA have been shown to be at or near Sudlow sites I and II, which has created interest in the possible role these modifications may have on drug binding at these regions [2,14,15].
Acetohexamide (Figure 1) is a sulfonylurea drug that is used in the treatment of type II, or non-insulin dependent, diabetes [16,17]. Like many other sulfonylurea compounds, acetohexamide binds tightly to HSA. It has been demonstrated that this binding occurs at both Sudlow sites I and II, with equilibrium constants in the range of 4.2 × 104 to 1.3 × 105 M−1 at 37°C for normal HSA [18]. One past study conducted at pH 7.4 and 37ºC using equilibrium gel filtration found a 44% decrease in the binding of acetohexamide to highly glycated HSA versus normal HSA, as determined when combining similar concentrations for the drug and protein [19]. Fluorescence quenching experiments using moderately glycated HSA at pH 7.4 and 20ºC, plus up to a ten-fold excess of drug, suggested there was a slight decrease in the affinity of acetohexamide for glycated HSA versus normal HSA [20]. As these past results indicate, there is still a need for binding studies under temperature and glycation conditions that are more typical of those expected in blood during diabetes and for data that more clearly shows how glycation affects the interactions of acetohexamide with HSA.
Figure 1.
Structure of acetohexamide.
This study will use high-performance affinity chromatography (HPAC) to examine the binding of acetohexamide to HSA with various levels of glycation. The use of normal HSA columns in HPAC has been shown in numerous studies to give good correlation with the drug binding behavior noted for normal HSA in solution [21]. The benefits of using HPAC for this research when compared to traditional methods such as equilibrium dialysis or ultrafiltration include the good precision and reproducibility of HPAC, its small sample requirements, and its ease of automation [21,22]. HPAC has recently been used to characterize the binding of acetohexamide with normal HSA [18] and to examine the effects of glycation on the binding of HSA with warfarin and L-tryptophan (i.e., site-selective probes for Sudlow sites I and II) [23]. This current report will build on these previous studies by first using HPAC to determine how the overall association equilibrium constants and binding capacities of acetohexamide on HSA are affected during increasing levels of glycation, as examined by using frontal analysis. Zonal elution experiments will then be used to provide site-specific information on how the binding of acetohexamide at Sudlow sites I and II is changed as the glycation of HSA is increased. The information gained from this research should provide a better description of how acetohexamide binds to HSA during diabetes. Such information, in turn, should aid in the future development of improved patient treatment regimes based on acetohexamide and related drugs.
2. Experimental
2.1 Reagents
The acetohexamide, warfarin (≥ 97%), L-tryptophan (98%), D-(+)-glucose (99.5%), sodium azide (>95%), HSA (essentially fatty acid free, ≥ 96%), and commercial glycated HSA (Lot 058K6087) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The Nucleosil Si-300 (7 micron particle diameter, 300 Å pore size) was purchased from Macherey-Nagel (Düren, Germany). Reagents used in the bicinchoninic acid (BCA) protein assay were from Pierce (Rockford, IL, USA). The enzymatic assay kit for fructosamine was from Diazyme Laboratories (San Diego, CA, USA). Sterile 17 × 100 mm culture tubes were purchased from Fisher Scientific (Pittsburg, PA, USA). Slide-A-Lyzer 7K (7 kDa, MW cutoff) dialysis cassettes with various sample volumes (0.5–3, 3–12 or 12–30 ml) were obtained from Thermo Scientific (Rockford, IL, USA). The Econo-Pac 10 DG columns (30 × 10 ml) were from Bio-Rad (Hercules, CA, USA). All solutions were made using water from a Nanopure system (Barnstead, Dubuque, IA, USA) and filtered through 0.20 μm GNWP nylon membranes from Millipore (Billerica, MA, USA).
2.2 Apparatus
The HPLC system was from Jasco (Tokyo, Japan) and consisted of a DG-2080-53 three-solvent degasser, two PU-2080 isocratic pumps, a AS-2055 autosampler, a CO-2060 column oven, and a UV-2075 UV/Vis detector, along with a Rheodyne Advantage PF six-port valve (Cotati, CA, USA). The HPLC system hardware was controlled by EZChrom Elite software v3.2.1 (Scientific Software, Pleasanton, CA, USA) via Jasco LC Net hardware. Labview 5.1 (National Instruments, Austin, TX, USA) was used to analyze the frontal analysis curves. PeakFit 4.12 (Jandel Scientific Software, San Rafael, CA, USA) was used to determine the central moments of peaks obtained from zonal elution experiments. Linear regression was performed using Excel 2003 (Microsoft Corporation, Redmond, WA, USA) and non-linear regression was carried out using DataFit (Oakdale Engineering, PA, USA).
2.3 Methods
2.3.1 Preparation of glycated HSA and HSA columns
Diol silica was made using Nucleosil Si-300 silica, and glycated HSA was immobilized to the diol silica by the Schiff base method [23–26]. A control support was also made in this manner but with no protein being added during the immobilization step. A BCA assay was performed in triplicate on each support to determine the protein content, using glycated HSA as the standard and the control support as the blank. Although both the Schiff base immobilization method and glycation involve free amine residues on a protein, previous studies have determined that the conditions typically present during these processes lead to the involvement of different residues on HSA, with the Schiff base immobilization method predominantly using the N-terminus or a few lysines that are not present at Sudlow sites I or II (see Refs. [27–29] for more details). Because of this difference, the prior glycation of HSA should not have affected the ability of this protein to be immobilized by the Schiff base method or the relative activities noted at Sudlow sites I and II for this immobilized protein. This result has been confirmed in recent studies looking at the binding of warfarin and L-tryptophan to glycated HSA columns that were identical to those used in this current study [23].
This report used three batches of glycated HSA, with each batch having known and different levels of glycation. The first HSA sample was a commercial in vitro preparation (gHSA1), as prepared under proprietary conditions by incubating a fixed concentration of D-glucose with HSA at 37 ºC for periods of time that were no longer than a week. The second and third samples of glycated HSA (gHSA2 and gHSA3) were prepared in a similar fashion at 37 ºC and over four weeks using moderate or high levels of D-glucose (i.e., 15 or 30 mM), as described previously [23]. The resulting protein solutions were stored at −80 °C. A portion of each solution was lyophilized and the resulting protein sample was stored at −80 °C until use. A commercial fructosamine assay designed for work with serum samples was modified to determine the glycation levels in each HSA sample, as described in Ref. [23].
2.3.2. Chromatographic methods
After each type of glycated HSA had been immobilized, the resulting supports were downward slurry-packed into separate 2.0 cm × 2.1 mm I.D. columns at 3500 psi (24 MPa) using pH 7.4, 0.067 M potassium phosphate buffer as the packing solution. The corresponding control supports were packed into separate columns under the same conditions. These columns were stored at 4 °C in the packing solution and used over a period of one year and fewer than 500 sample applications. Similar columns containing normal HSA have been found in previous work to have good stability for drug-protein binding studies under such conditions [30].
The acetohexamide, R-warfarin, and L-tryptophan solutions were made in pH 7.4, 0.067 M potassium phosphate buffer. This buffer was also used as the application and elution buffer in the chromatographic studies. All solutions were filtered through a 0.2 μM nylon filter and degassed for 10–15 min prior to use. A flow rate of 0.5 ml/min was used for both the frontal analysis and zonal elution studies. This flow rate has been shown in previous studies to give reproducible retention factors and binding capacities for drug binding studies conducted on similar columns containing normal HSA [31,32]. All of the chromatographic studies were performed at 37 °C.
In the frontal analysis experiments, the column was first equilibrated with pH 7.4, 0.067 M potassium phosphate buffer and then switched to the desired sample solution using an automated six-port valve. Once the analyte had saturated the column and produced a breakthrough curve, the valve was switched and pH 7.4, 0.067 M phosphate buffer was used to elute the remaining analyte from the column. The analyte solutions contained acetohexamide at concentrations that ranged from 1–1000 μM. Although the pKa of sulfonylurea drugs like acetohexamide range from 5.2–6.2, even the solutions containing the highest concentration of acetohexamide gave less than a 0.05 change in pH for the pH 7.4 buffer, and the pH of this solution was found to vary by less than 0.05 units over the course of this study. The elution of acetohexamide was monitored at 248 nm for the 1–7.5 μM solutions and at 315 nm for the 10–1000 μM solutions (note: the detection wavelength was changed to keep the absorbance values within a linear range of response as the concentration was increased). All experiments were performed in triplicate and the resulting breakthrough curves were analyzed using the equal areas method [21]. Non-specific binding was corrected by subtracting the results for the control column from the results for each glycated HSA column.
The zonal elution competition studies were performed by using R-warfarin as a probe for Sudlow site I and L-tryptophan as a probe for Sudlow site II [9,10,23]. Recent studies have shown that these probe compounds are specific for these sites even when using HSA that has been glycated at levels like those used in this current study [23]. Concentrations of acetohexamide ranging from 1–20 μM were placed in the mobile phase for the competition experiments. The injected samples consisted of 20 μL of 5 μM R-warfarin or L-tryptophan, conditions which have been shown to represent linear elution conditions for similar HSA columns [33,34]. The elution of R-warfarin or L-tryptophan was monitored at 308 or 280 nm, respectively. Injections consisting of 20 μL of 20 μM sodium nitrate (i.e., a non-retained solute) were made under the same conditions to determine the column void time, with the elution of sodium nitrate being monitored at 205 nm. The resulting peaks were fit to an exponentially-modified Gaussian curve to find their central moments for retention time or void time measurements.
3. Results and Discussion
3.1 Preparation of Glycated HSA
Previous studies have compared the interactions of acetohexamide to HSA versus glycated HSA by examining either changes in the binding capacities of these proteins or the differences in their apparent binding constants [19,20]. However, these past studies did not compare the binding of acetohexamide with HSA having various levels of glycation and did not consider how the binding of this drug to specific sites on HSA may change with glycation. These studies also conducted binding studies at lower temperatures than are present in the body [20] or used higher glucose concentrations for HSA glycation than are found in the body during diabetes [19]. These limitations were overcome in this present study by conducting all interaction studies at 37ºC and by using HSA that had undergone various known levels of glycation under conditions similar to those found in serum during diabetes.
There were three preparations of in vitro glycated HSA that were utilized in this study. The first preparation (referred to as gHSA1, and obtained from a commercial source) had a relatively low level of glycation of 1.31 (± 0.05) mol hexose/mol HSA. This preparation was used to represent minimally glycated HSA, as might be found during pre-diabetes or early state diabetes. The second sample, gHSA2, had a glycation level of 2.34 (± 0.13) mol hexose/mol HSA and was made using glucose conditions typical of those seen in many patients with diabetes [35]. The third sample, gHSA3, contained 3.35 (± 0.14) mol hexose/mol HSA and was used to represent a case of uncontrolled, advanced diabetes. After these various preparations had been immobilized, their supports were found to have protein contents of 29 (± 4), 47 (± 8), and 40 (± 3) mg protein/g silica, respectively (i.e., ~440–710 nmol HSA/g silica). These protein levels were comparable to those reported for normal HSA supports made by the same immobilization method [26].
3.2. Frontal analysis studies
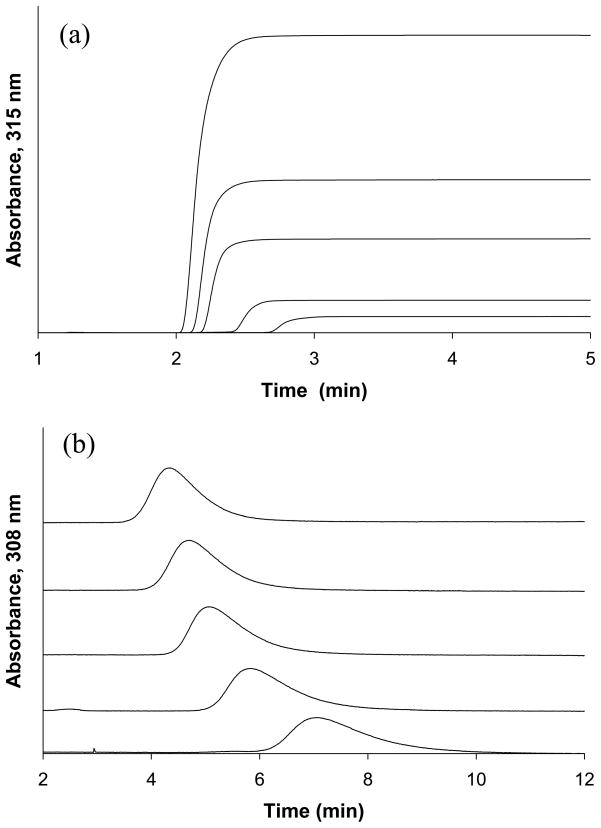
Breakthrough curves were collected for acetohexamide on each glycated HSA column. An example of such an experiment is given in Figure 2(a), as acquired for the gHSA2 column. This data was first analyzed by using a double-reciprocal plot of 1/mLapp versus 1/[Acetohexamide], as prepared according to Eqn. (1) (see Table 1 for a list of all equations used in this report). An example of this type of plot is provided in Figure 3. Deviations from a linear response in such a plot were noted for all of the glycated HSA columns, indicating that more than one type of binding site for acetohexamide was present (e.g., see behavior predicted for a two-site model by Eqns. (2)–(3) in Table 1). The same type of behavior has been reported in previous studies examining the binding of acetohexamide to columns containing normal HSA [18]. One advantage of using frontal analysis for this type of analysis was that it allowed the association equilibrium constants and binding capacities of the column to be measured independently from one another [21,22], thus making it possible to obtain values for the association equilibrium constants that were not affected by moderate column-to-column variations in the amount of immobilized HSA that was present.
Figure 2.
Examples of (a) breakthrough curves for acetohexamide and (b) zonal elution competition studies for injections of R-warfarin in the presence of acetohexamide. These results were obtained on the gHSA2 column. The concentrations of acetohexamide in (a) were as follows (from left-to-right): 1000, 500, 300, 100 and 50 μM. In (b) using 20 μL of 5 μM R-warfarin was injected in the presence of the following concentrations of acetohexamide in the mobile phase (from top-to-bottom): 20, 15, 10, 5 and 1 μM.
Table 1.
| Frontal analysis | Predicted responsea | ||
|---|---|---|---|
| Two-site binding of A with L |
|
||
| |||
| |||
| Linear response to a two-site model at low concentrations of A |
|
||
| Zonal Elution | |||
| Direct competition of A with agent I in the mobile phase for a single set of binding sites on L |
|
||
Abbreviations and terms: Ka, association equilibrium constant; mL, moles of binding sites; mLapp, apparent moles of analyte required to reach the central position of the breakthrough curve at a given concentration of applied analyte; α1, fraction of all binding regions that make up the high affinity binding sites (i.e., α1 = mL1,tot/mLtot); β2, ratio of the association equilibrium constant for any lower affinity site (e.g., Ka2) versus the highest affinity site, where β2 = Ka2/Ka1 and 0 < Ka2 < Ka1; tR, measured retention time; tM, column void time; k, retention factor, where k = (tR − tM)/tM; KaI and KaA, association equilibrium constants for the competing agent and probe, respectively, at their site of competition; VM, column void volume.
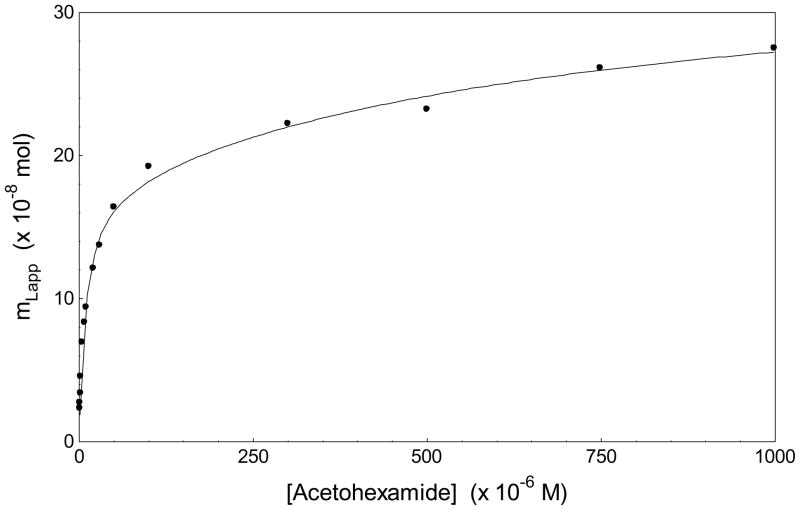
Figure 3.
A double-reciprocal plot for the binding of acetohexamide to a column containing a preparation of gHSA1. The best-fit line obtained when fitting the linear region of this plot (i.e., the data at 1–10 μM acetohexamide) to Eqn. (4) was as follows: y = 360 (± 10) x + [7.4 (± 0.5) × 107], r = 0.998, n = 7. The error bars represent a range of ± 1 SD.
The linear range in the double-reciprocal plots was used with the behavior predicted by Eqn. (4) at low analyte concentrations to provide an initial estimate of the association equilibrium constant for the highest affinity sites of acetohexamide in each column. This linear range occurred at acetohexamide concentrations below 10 μM and gave correlation coefficients of 0.998–0.999 (n = 6–7) over this range. The estimated association equilibrium constants obtained by this approach for the high affinity binding regions of acetohexamide were as follows: gHSA1, 2.0 (± 0.2) ×105 M−1; gHSA2, 2.0 (± 0.2) × 105 M−1; and gHSA3, 1.9 (± 0.1) × 105 M−1. Use of the same approach with acetohexamide on a normal HSA column has given a previous estimate of 2.0 (± 0.2) ×105 M−1 for these high affinity interactions [18]. Thus, at first glance, the interactions of acetohexamide did not appear to have any significant change at these high affinity sites as the extent of glycation for HSA was varied.
A more detailed analysis was conducted by fitting the frontal analysis data to a two-site model by using Eqn. (3). This type of fit has been shown in the past to give the best description for the binding of acetohexamide to columns containing normal HSA, involving both a group of low affinity interactions and a set of high affinity sites [18]. This same type of model was found to give a good fit for the results obtained on each glycated HSA column, with correlation coefficients of 0.998–0.999 (n = 15). Only random variations in the data about the best-fit lines were observed in these plots, along with a small sum of the square of the residuals (range, 2.0 × 10−18 to 4.9 × 10−18). In comparison, the use of a single-site model with the same data gave non-random variations of the residuals about the best-fit line, correlation coefficients of 0.979–0.990, and a larger sum of the square of the residuals (3.5 × 10−17 to 8.4 × 10−17).
Table 2 summarizes the association equilibrium constants and amount of each class of binding sites that were noted for the glycated HSA columns when using the two-site model. The association equilibrium constant (Ka) values for the high affinity interactions gave good general agreement with the values estimated from the linear regions of plots made according to Eqn. (4), but a gradual increase in these values with the level of glycation was also observed. This increase occurred in going from normal HSA (i.e., using a value obtained from Ref. [18]) or gHSA1, with values of 1.2–1.3 × 105 M−1, to the gHSA2 and gHSA3 samples, which both gave a Ka value of 2.0 × 105 M−1 for their high affinity sites (i.e., a 1.5- to 1.7-fold increase). This increase (e.g., as measured between normal HSA and gHSA1 versus gHSA3) was significant at the 95% confidence level. These results agreed with data obtained using acetohexamide and soluble gHSA3 or normal HSA in ultrafiltration studies (data not shown). The amount of these high affinity regions were all in the general range of 1.5–2.4 × 10−8 mol, which represented specific activities of 0.8–1.3 for acetohexamide.
Table 2.
Binding parameters obtained for acetohexamide with glycated HSA by frontal analysis and using a two-site modela
| High affinity sites | Low affinity sites | |||||
|---|---|---|---|---|---|---|
| Type of column | Ka1 (× 105 M−1) | mL1 (× 10−8 mol) | Specific activity (mol/mol HSA) | Ka2 (× 103 M−1) | mL2 (× 10−8 mol) | Specific activity (mol/mol HSA) |
| HSAb | 1.3 (± 0.2) | 2.4 (± 0.1) | 1.3 (± 0.1) | 0.35 (± 0.03) | 9.3 (± 5.5) | 5.2 (± 3.1) |
| gHSA1 | 1.2 (± 0.2) | 1.7 (± 0.1) | 1.3 (± 0.2) | 1.4 (± 0.8) | 1.7 (± 0.4) | 1.3 (± 0.3) |
| gHSA2 | 2.0 (± 0.6) | 1.8 (± 0.3) | 0.80 (± 0.20) | 11 (± 3) | 2.4 (± 0.3) | 1.1 (± 0.2) |
| gHSA3 | 2.0 (± 0.3) | 1.5 (± 0.1) | 0.80 (± 0.09) | 4.1 (± 0.7) | 3.0 (± 0.1) | 1.6 (± 0.5) |
The values in parenthesis represent ± 1 S.D. These values were determined by using error propagation and the standard deviations of the slopes and intercepts that were obtained from best-fit lines generated according to Eqn. (3).
The results for normal HSA were obtained from Ref. [18] and were acquired using immobilization and chromatographic conditions identical to those used in this current study for glycated HSA.
The average association equilibrium constants estimated for the lower affinity sites increased from 0.35 × 103 M−1 to 1.4–11 × 103 M−1 in going from normal HSA to the glycated HSA samples. However, the estimated specific activities of these lower affinity sites decreased from 5.2 to 1.1–1.6 for the same protein preparations. This combined effect resulted in an increase in the product Ka2 n2 (i.e., Ka2 multiplied by the specific activity for the low affinity sites) from approximately 1.8 × 103 M−1 for normal HSA and gHSA1 to 6.6–12.1 × 103 for gHSA2 and gHSA3, a change that was significant at the 95% confidence level. Such a result suggests that a small amount of glycation (e.g., as in gHSA1) did not have any appreciable effect on these lower affinity interactions, while a greater degree of glycation (e.g., as in gHSA2 and gHSA3) did raise the extent of lower affinity interactions for acetohexamide with HSA.
3.3 Competition studies at Sudlow site I
Zonal elution competition studies were next performed to examine binding of acetohexamide at Sudlow site I as the level of glycation for HSA was varied. These experiments were carried out using R-warfarin as a site-selective probe. An example of such a study is given in Figure 2(b). When a plot of 1/k vs. [Acetohexamide] was made for injections of R-warfarin, a linear response was obtained for all glycated HSA columns that were tested. These plots gave correlation coefficients of 0.970–0.992 (n = 6) and only random deviations in the data about the best-fit lines. In addition, the best-fit intercepts agreed within ± 2 S.D. with the actual intercepts measured in the absence of any acetohexamide in the mobile phase. These results indicated that there was direct competition between R-warfarin and acetohexamide on the glycated HSA columns, indicating that acetohexamide was binding at Sudlow site I. The same type of behavior has been noted for acetohexamide on columns containing normal HSA [18].
The association equilibrium constant for acetohexamide at Sudlow site I was found by taking the ratio of the slope to the intercept for plots of 1/k versus [Acetohexamide] when injecting R-warfarin as a probe, as indicated by Eqn. (5) (Note: the use of this approach and equation to measure an association equilibrium constant at a given binding site provides a value that is independent of the amount of immobilized protein and of column-to-column variations in this protein content [21,22]). The results that were obtained from these plots are summarized in Table 3. In going from normal HSA to gHSA1, there was about a 40% increase in binding affinity for acetohexamide at Sudlow site I. This increase was statistically significant at the 95% confidence level. This was followed by a similar, statistically significant decrease for gHSA2 and gHSA3 to values that were comparable to those for normal HSA (i.e., values only 3–10% below Ka for normal HSA and within of ± 1 S.D. of this value). This trend is believed to be linked to differences in the glycation patterns of these samples as a result of differences in their preparation conditions and the resulting modifications and/or local alterations in the structure of HSA that occurred at or near Sudlow site I as a result of these modifications in the early, intermediate and advanced stages of glycation.
Table 3.
Association equilibrium constants (Ka) obtained at Sudlow sites I and II for acetohexamide with glycated HSAa
| Type of column | Ka, Sudlow site I (× 104 M−1) | Ka, Sudlow Site II (× 104 M−1) |
|---|---|---|
| HSAb | 4.2 (± 0.4) | 13 (± 1) |
| gHSA1 | 5.9 (± 0.5) | 7.9 (± 0.7) |
| gHSA2 | 3.8 (± 0.3) | 11 (± 1) |
| gHSA3 | 4.1 (± 0.6) | 12 (± 1) |
The values in parenthesis represent ± 1 S.D. These values were determined by using error propagation and the standard deviations of the slopes and intercepts that were obtained from best-fit lines generated according to Eqn. (5).
The results for normal HSA were obtained from Ref. [18] and were acquired using immobilization and chromatographic conditions identical to those used in this current study for glycated HSA.
It was possible from plots prepared according to Eqn. (5) to also determine how the specific activity of Sudlow site I changed for acetohexamide as the level of glycation for HSA was increased. This was done by using the intercept of such a plot along with the previously-measured Ka values for R-warfarin with each type of glycated HSA used in this study [23] and the known protein content of the columns. No significant change in specific activity was seen for acetohexamide at Sudlow site in going from normal HSA to the gHSA1 or gHSA2 columns. However, up to a 30% decrease in specific activity may have been present when comparing gHSA3 to normal HSA or gHSA1 (i.e., changes significant at the 90% and 95% confidence levels, respectively).
Previous binding studies did not find any significant changes in the affinity or specific activity for R-warfarin at Sudlow site I in going from normal HSA to glycated HSA when using columns identical to those employed in this current report [23]. The different behavior seen here for acetohexamide versus the earlier results for R-warfarin indicates that the effects of glycation-related modifications at or near Sudlow site I can differ from one drug or solute. Work with additional pharmaceutical agents, including other sulfonylurea compounds, is in progress to further determine the extent to which these interactions can vary between structurally-related drugs that bind to this region of HSA. Such differences have already been noted in competition experiments that have been conducted with tolbutamide at Sudlow site I, which gave a 1.2- to 1.3-fold increase in Ka for this drug in going from the normal HSA column to all of the glycated HSA columns that were used in this current report [36].
3.4 Competition studies at Sudlow site II
Competition studies based on zonal elution experiments were also carried out for acetohexamide at Sudlow site II for the various samples of glycated HSA that were examined in this report. These experiments were conducted by using L-tryptophan as a site-selective probe. Plots of 1/k vs. [Acetohexamide] that were generated during the injection of L-tryptophan gave a linear response for each glycated HSA column. These plots had correlation coefficients in the range of 0.991–0.996 (n = 5 or 6), along with only random deviations in the data about the best-fit lines. The best-fit intercepts also agreed within ± 2 S.D. with the actual intercepts noted in the absence of any acetohexamide in the mobile phase. From these results it was determined that there was direct competition between L-tryptophan and acetohexamide on the glycated HSA columns, as has also been noted on columns containing normal HSA [18]. These data indicated that acetohexamide was binding at Sudlow site II on the various samples of glycated HSA that were tested.
The association equilibrium constants that were calculated for acetohexamide at Sudlow site II are given in Table 3. The pattern noted here was just the opposite of the trend seen in the previous section for acetohexamide at Sudlow site I. For instance, in going from normal HSA to gHSA1, there was a 40% decrease in binding affinity for acetohexamide (significant at the 95% confidence level), followed by an increase back to values for gHSA2 and gHSA3 that were only 8–15% below those for normal HSA. This trend is again believed to be linked to changes in the types of glycated-related modifications that occur at or near Sudlow site II as a result of different stages of glycation. It is interesting to note that the different changes seen for acetohexamide at Sudlow sites I and II produced a net average binding strength at these high affinity regions that was comparable to that for the other HSA samples. This explains why only smaller differences were noted in the frontal analysis data when comparing these samples.
Data from the competition studies were used to compare the specific activities for acetohexamide at Sudlow site II on each of the glycated HSA columns. This was carried out using the same approach as described in the previous section but now used the competition data and the known protein content of each column along with previously-measured values of Ka for L-tryptophan on the glycated HSA samples that were used in this study [23]. In this case, no significant change at either the 90% or 95% confidence level was seen in the specific activity of Sudlow site II for acetohexamide when comparing any of the glycated HSA columns or versus the specific activity expected for normal HSA.
Prior frontal analysis experiments have indicated that there is almost a five- to six-fold change in affinity for L-tryptophan at Sudlow site II in going from normal HSA to any of the glycated HSA samples used in this current report [23]. As was noted in the last section for Sudlow site I, this difference from the results seen here for acetohexamide indicate that the effects of glycation-related modifications can vary between solutes that bind to the same region of HSA. For example, competition experiments using tolbutamide have found that there is a 1.1-to 1.4-fold increase in Ka for this drug at Sudlow site II in going from normal HSA to any of the glycated HSA samples that were tested in this current report [36].
4. Conclusions
These studies used high-performance affinity chromatography as a tool to see how the binding of acetohexamide with HSA changes as the level of glycation for this protein is increased. As has been found for normal HSA [18], frontal analysis indicated that the binding of acetohexamide to glycated HSA followed a two site model that included a group of high affinity sites and a set of lower affinity interactions. The Ka values for the high affinity interactions were in the general range of 1.2–2.0 × 105 M−1 and appeared to increase in going from normal or minimally glycated HSA to more highly glycated HSA. An increase in the extent of lower affinity interactions were also noted in going from normal or minimally glycated HSA to the more highly glycated samples of HSA.
Competition studies indicated that the association equilibrium constant of acetohexamide at Sudlow site I initially increased in going from normal HSA to minimally glycated HSA. The value of Ka then decreased in going to more highly glycated HSA. Different behavior was seen at Sudlow site II, in which the Ka for acetohexamide first decreased and then increased in going from normal HSA to minimally glycated HSA and more highly glycated HSA. Such information demonstrates the importance of conducting both frontal analysis and site-specific binding studies in examining the effects of glycation on the interactions of a drug with HSA. The results noted in this current study may have clinical relevance in that sulfonylurea drugs such as acetohexamide are often used to help patients with poor glycemic control in diabetes. However, obtaining normal blood sugar levels in diabetic patients can be elusive and difficult to routinely accomplish in the long term [35].
The results on this study suggest that there may be difference in the binding of drugs such as acetohexamide to HSA as the levels of glucose in blood are normalized in these patients (i.e., which would tend to gradually lower glycation levels) or as the diabetes progresses (which would tend to increase glycation). Binding experiments similar to those described in this report are now being conducted with in vivo glycated HSA to further investigate the effects of changes in glycation.
The differences in binding seen in this report in the competition studies for the various preparations of HSA are thought to be linked to changes in the preparation conditions and in the glycated-related modifications that occur at or near Sudlow sites I and II in the presence of various stages of glycation. The belief that these variations are related to glycation patterns rather than immobilization effects is supported by the fact that the same columns have been found with other solutes to have either no changes in binding affinity (e.g., as noted for warfarin at Sudlow site I) [23] or a more consistent change in binding strength as the level of glycation is increased (e.g., as seen for L-tryptophan at Sudlow site II [23] and for tolbutamide at both Sudlow sites I or II [36]). Future work with a new method for non-covalently entrapping HSA will be used to further investigate these trends [37]. Structural studies using tools from quantitative proteomics are also being carried out to determine which residues in HSA may lead to these changes in binding properties upon glycation (e.g., see Ref. [28]). The data that can be obtained through such research should result in a better understanding of how glycation can change the interactions of drugs such as acetohexamide to HSA during diabetes. This information, in turn, should aid in the later development of better patient treatments based on acetohexamide and related drugs.
Figure 4.
A plot of mLapp versus [Acetohexamide] for the gHSA1 column, as analyzed according to Eqn. (3) and using a two-site model. The best-fit values that were obtained for the association equilibrium constants and binding capacities are summarized in Table 3.
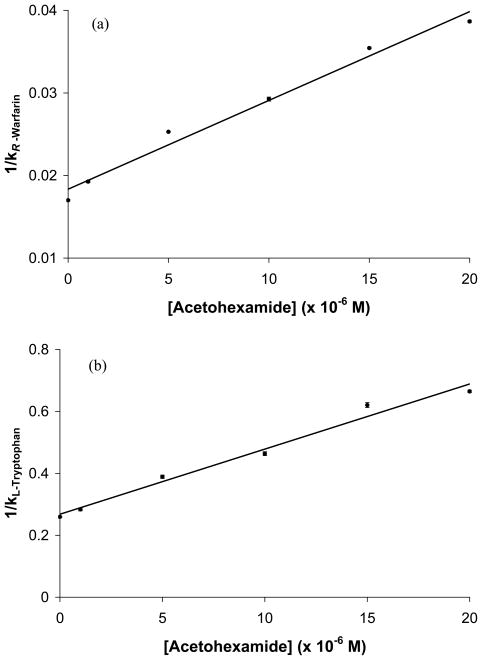
Figure 5.
Change in the retention factor for (a) R-warfarin or (b) L-tryptophan as the mobile phase concentration of acetohexamide was varied on a gHSA1 column, as analyzed according to Eqn. (5). The best-fit line in (a) was y = 1100 (± 100) x + 0.018 (± 0.001), r = 0.991, n = 6. The best-fit line in (b) was y = [2.1 (± 0.1) × 104] x + 0.27 (± 0.02), r = 0.991, n = 6. The error bars represent a range of ± 1 SD.
Acknowledgments
This research was supported by National Institutes of Health under grant R01 DK069629 and was conducted in facilities that were renovated under NIH grant RR015468-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mendez DL, Jensen RA, McElroy LA, Pena JM, Esquerra RM. Arch Biochem Biophys. 2005;444:92. doi: 10.1016/j.abb.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Nakajou K, Watanabe H, Kragh-Hansen U, Maruyama T, Otagiri M. Biochim Biophys Acta. 2003;1623:88. doi: 10.1016/j.bbagen.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Ascenzi P, Bocedi A, Notari S, Fanali G, Fesce R, Fasano M. Mini-Rev Med Chem. 2006;6:483. doi: 10.2174/138955706776361448. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Zied OK, Al-Shihi OIK. J Am Chem Soc. 2008;130:10793. doi: 10.1021/ja8031289. [DOI] [PubMed] [Google Scholar]
- 5.Petitpas I, Bhattacharya AA, Twine S, East M, Curry S. J Biol Chem. 2001;276:22804. doi: 10.1074/jbc.M100575200. [DOI] [PubMed] [Google Scholar]
- 6.Fasano M, Curry S, Terreno E, Galliano M, Fanali G, Narciso P, Notari S, Ascenzi P. IUBMB Life. 2005;57:787. doi: 10.1080/15216540500404093. [DOI] [PubMed] [Google Scholar]
- 7.Dockal M, Carter DC, Ruker F. J Biol Chem. 1999;274:29303. doi: 10.1074/jbc.274.41.29303. [DOI] [PubMed] [Google Scholar]
- 8.Colmenarejo G. Med Res Rev. 2003;23:275. doi: 10.1002/med.10039. [DOI] [PubMed] [Google Scholar]
- 9.Sudlow G, Birkett DJ, Wade DN. Mol Pharmacol. 1975;11:824. [PubMed] [Google Scholar]
- 10.Sudlow G, Birkett DJ, Wade DN. Mol Pharmacol. 1976;12:1052. [PubMed] [Google Scholar]
- 11.Dockal M, Chang M, Carter DC, Ruker F. Protein Sci. 2000;9:1455. doi: 10.1110/ps.9.8.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertucci C, Andrisano V, Gotti R, Cavrini V. J Chromatogr B. 2002;768:147. doi: 10.1016/s0378-4347(01)00494-7. [DOI] [PubMed] [Google Scholar]
- 13.Ascoli GA, Domenici E, Bertucci C. Chirality. 2006;18:667. doi: 10.1002/chir.20301. [DOI] [PubMed] [Google Scholar]
- 14.Iberg N, Fluckiger R. J Biol Chem. 1986;261:13542. [PubMed] [Google Scholar]
- 15.Garlick RL, Mazer JS. J Biol Chem. 1983;258:6142. [PubMed] [Google Scholar]
- 16.Imamura Y, Kojima Y, Ichibagase H. Chem Pharm Bull (Tokyo) 1985;33:1281. doi: 10.1248/cpb.33.1281. [DOI] [PubMed] [Google Scholar]
- 17.Skillman TG, Feldman JM. Am J Med. 1981;70:361. doi: 10.1016/0002-9343(81)90773-7. [DOI] [PubMed] [Google Scholar]
- 18.Joseph KS, Hage DS. J Chromatogr B. 2010;878:1590. doi: 10.1016/j.jchromb.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuchiya S, Sakurai T, Sekiguchi SI. Biochem Pharmacol. 1984;33:2967. doi: 10.1016/0006-2952(84)90595-1. [DOI] [PubMed] [Google Scholar]
- 20.Koyama H, Sugioka N, Uno A, Mori S, Nakajima K. Biopharm Drug Dispos. 1997;18:791. doi: 10.1002/(sici)1099-081x(199712)18:9<791::aid-bdd66>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Hage DS. J Chromatogr B. 2002;768:3. doi: 10.1016/s0378-4347(01)00482-0. [DOI] [PubMed] [Google Scholar]
- 22.Schiel JE, Joseph KS, Hage DS. In: Adv Chromatogr. Grinsberg N, Grushka E, editors. Taylor & Francis; New York: 2010. [Google Scholar]
- 23.Joseph KS, Hage DS. J Pharm Biomed Anal. 2010;53:811. doi: 10.1016/j.jpba.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loun B, Hage DS. J Chromatogr. 1992;579:225. [PubMed] [Google Scholar]
- 25.Ruhn PF, Garver S, Hage DS. J Chromatogr A. 1994;669:9. doi: 10.1016/0021-9673(94)80332-3. [DOI] [PubMed] [Google Scholar]
- 26.Joseph KS, Moser AC, Basiaga S, Schiel JE, Hage DS. J Chromatogr A. 2009;1216:3492. doi: 10.1016/j.chroma.2008.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wa C, Cerny RL, Clarke WA, Hage DS. Clin Chim Acta. 2007;385:48. doi: 10.1016/j.cca.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnaby O, Wa C, Cerny RL, Clarke W, Hage DS. Clin Chim Acta. 2010;411:1102. doi: 10.1016/j.cca.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wa C, Cerny RL, Hage DS. Anal Chem. 2006;78:7967. doi: 10.1021/ac0609935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Hage DS. J Chromatogr A. 1997;766:15. doi: 10.1016/s0021-9673(96)01040-0. [DOI] [PubMed] [Google Scholar]
- 31.Loun B, Hage DS. Anal Chem. 1994;66:3814. doi: 10.1021/ac00093a043. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Hage DS. J Chromatogr. 1993;645:241. doi: 10.1016/0021-9673(93)83383-4. [DOI] [PubMed] [Google Scholar]
- 33.Conrad ML, Moser AC, Hage DS. J Sep Sci. 2009;32:1145. doi: 10.1002/jssc.200800567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moser AC, Kingsbury C, Hage DS. J Pharm Biomed Anal. 2006;41:1101. doi: 10.1016/j.jpba.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Powers AC. In: Harrison's Principles of Internal Medicine. Kasper DL, Fauci AS, Longo DL, Braunwald E, Hauser SL, Jameson JL, editors. Chap 323 McGraw-Hill; 2005. [Google Scholar]
- 36.Joseph KS. PhD Dissertation. University of Nebraska; Lincoln, NE: 2010. [Google Scholar]
- 37.Jackson AJ, Xuan H, Hage DS. Anal Biochem. 2010;404:106. doi: 10.1016/j.ab.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]