Abstract
The goal of the present study was to determine if older adults benefited from attention to a specific sensory modality in a voluntary attention task and evidenced changes in voluntary or involuntary attention when compared to younger adults. Suppressing and enhancing effects of voluntary attention were assessed using two cued forced-choice tasks, one that asked participants to localize and one that asked them to categorize visual and auditory targets. Involuntary attention was assessed using the same tasks, but with no attentional cues. The effects of attention were evaluated using traditional comparisons of means and Cox proportional hazards models. All analyses showed that older adults benefited behaviorally from selective attention in both visual and auditory conditions, including robust suppressive effects of attention. Of note, the performance of the older adults was commensurate with that of younger adults in almost all analyses, suggesting that older adults can successfully engage crossmodal attention processes. Thus, age-related increases in distractibility across sensory modalities are likely due to mechanisms other than deficits in attentional processing.
Keywords: Aging, Crossmodal, Elderly, Human, Proportional hazards, Selective attention
Introduction
A 20-year-old man sits in a subway car reading a newspaper, seemingly oblivious to the conversations going on around him. Although his hearing is good, he could not tell you what those conversations were about if you asked him; he is simply ignoring them. Could a healthy 75-year-old person do the same?
Attention can be thought of as the ability to restrict processing to only the most important or salient stimuli in the environment, and different kinds of attention have been identified based on what stimulus characteristics are used to restrict processing [for review see (Kastner and Ungerleider 2000; Corbetta and Shulman 2002)]. Cross-modal attention is the ability to restrict processing based on sensory modality (Spence and Driver 1997; Spence et al. 2001), for example, the ability to focus selectively on what you are seeing and ignore stimuli from other senses. The ability to ignore has both voluntary and involuntary components. To continue with the example above, the man is able to voluntarily suppress processing of the sounds around him in order to focus on his reading. However, a particularly loud or deviant sound would involuntarily capture his attention regardless of his voluntary focus. A person could be more distractible because he is less effective at suppressing potential distractors with endogenous (voluntary) attention, or because changes in exogenous (involuntary) attention, stimulus processing, or baseline cognitive state make him more vulnerable to distraction.
Most research on aging and attention has focused on attention within one modality, such as visual attention during a visual search task or visual spatial attention (Lavie and Cox 1997; Maylor and Lavie 1998; Madden et al. 2002, 2004; Madden and Langley 2003) [for review see (Plude et al. 1994; Groth and Allen 2000)]. Little research has addressed the effects of aging on crossmodal selective attention. Poliakoff et al. (2006) have one of the only studies directly assessing crossmodal selective attention and aging. Their study examined reaction times and error rates for young, young-old, and old-old participants. Subjects completed blocks where they were cued to perform a visual task with either no distractors or tactile distractors, and vice versa. Posture was also modulated across task blocks. The researchers observed that relative to the no-distractor condition, older adults were more slowed and committed more errors than younger adults during a tactile task with visual distractors. Importantly, this indicates that older adults may be more distractible than younger adults, suggesting that crossmodal attention is impaired in older adults under certain task conditions.
As mentioned above, an increase in distractibility when people are instructed to attend could occur if older adults did not engage voluntary selective attention, exhibited changes in stimulus processing or exogenous capture, or had a change in baseline state. The present study aimed to address the question of whether or not older adults can successfully instantiate crossmodal selective attention and to examine potential age-related effects on exogenous attentional pull. The ability to invoke voluntary attention is typically tested using informative cues. Prior to presentation of the target stimulus, the participant is given a cue that provides them with task-relevant information. If the subject is speeded when an informative cue is provided, it is interpreted that the person has been able to restrict their processing appropriately in response to the cue. A classic example of this task is a cued spatial attention task where the subject's task is to identify the spatial location of the target (Posner et al. 1980). A cue is presented that either contains information about the likely location of the target or contains no spatial information. If reaction time is speeded on the informative trials, it is inferred that the subject was able to restrict processing using voluntary attention. In contrast to tests of voluntary attention, involuntary attention is typically not tested with cues, as the measure of interest is automatic rather than consciously controlled processes. Spence, Driver, and colleagues (Spence and Driver 1997; Spence et al. 2001) investigated both exogenous and endogenous crossmodal attention. They established that younger adults are able to use informative cues to direct their attention to a sensory modality, and their thorough exploration of potential caveats in the design of crossmodal paradigms provided the basis for the experiments reported here.
The study reported here was interested in investigating both voluntary and involuntary crossmodal attention. Rather than using distraction as a measure of attentional control, this task aimed to test whether or not crossmodal selective attention could be invoked as compared to divided attention. Healthy older and younger adults completed cued and uncued versions of a task where they were required to identify the spatial location of a visual or auditory target, closely following Spence and Driver's (1997) design. In addition, participants performed cued and uncued versions of a non-spatial task in which they were required to categorize visual or auditory targets. It was hypothesized that younger adults would better engage voluntary attention, therefore exhibiting greater effects of endogenous attention than older adults, but older adults would be more influenced by involuntary attentional capture than younger subjects.
Methods
Subjects
A total of 26 younger adults and 26 older adults were tested. Data were excluded for participants that did not complete all paradigms or had errors in data acquisition, did not follow instructions, or were greater than 3 standard deviations from the group mean in 3 of the 4 tasks. This resulted in slightly different numbers of data points for each task. The number of subjects included in each task is reflected in Tables 2 and 4. Major subject characteristics are listed in Table 1.
Table 2.
Reaction times and attentional effects to cued tasks
| N | Valid | Neutral | Invalid | Benefit | Cost | |
|---|---|---|---|---|---|---|
| Spatial | ||||||
| Visual target | ||||||
| Younger | 25 | 416.9 (14.0) | 433.7 (15.4) | 437.9 (16.1) | 16.8 (4.2) | 4.2 (5.7) |
| Older | 23 | 486.5 (15.6) | 504.6 (16.4) | 497.3 (16.0) | 18.0 (4.7) | −7.3 (5.0) |
| Auditory target | ||||||
| Younger | 25 | 425.8 (17.4) | 433.4 (19.1) | 451.6 (22.9) | 7.6 (5.0) | 18.2 (7.0) |
| Older | 23 | 504.5 (21.8) | 519.0 (22.6) | 530.4 (22.5) | 14.5 (8.5) | 25.9 (8.2) |
| Non-spatial | ||||||
| Visual target | ||||||
| Younger | 25 | 658.6 (20.1) | 666.4 (20.6) | 733.3 (30.5) | 7.8 (4.7) | 67.0 (15.5) |
| Older | 24 | 691 (16.8) | 700 (19.0) | 762 (27.6) | 8.6 (5.5) | 62.7 (13.2) |
| Auditory target | ||||||
| Younger | 25 | 810 (33.7) | 834 (34.4) | 902 (38.8) | 24 (11.2) | 67.8 (16.9) |
| Older | 24 | 863 (27.5) | 895 (31.5) | 947 (35.6) | 32.1 (11.9) | 52.0 (11.9) |
Mean reaction times are reported in milliseconds with standard error of the mean (SEM) in parentheses for the spatial and non-spatial cued tasks for each age group. Benefit and cost are also represented Fig. 1, but are reported here numerically for convenience
Table 4.
Reaction times and modality shift effect for uncued tasks
| N | Same | Switch | MSE | |
|---|---|---|---|---|
| Spatial | ||||
| Visual | ||||
| Younger | 23 | 394 (12.5) | 422 (14.1) | 28.2 (4.5) |
| Older | 24 | 457.0 (17.5) | 495.9 (20.8) | 38.9 (4.9) |
| Auditory | ||||
| Younger | 23 | 417.5 (14.8) | 435.1 (13.8) | 17.6 (3.3) |
| Older | 24 | 498.1 (22.5) | 522.4 (22.7) | 24.3 (4.8) |
| Non-spatial | ||||
| Visual | ||||
| Younger | 23 | 625.0 (22.0) | 648.1 (24.0) | 23.2 (6.4) |
| Older | 24 | 655.9 (19.7) | 682.7 (20.7) | 31.4 (26.9) |
| Auditory | ||||
| Younger | 23 | 788.8 (30.3) | 829.6 (32.0) | 40.9 (12.0) |
| Older | 24 | 884.7 (36.0) | 915.1 (39.2) | 32.2 (10.0) |
Mean reaction times are reported in milliseconds with standard error of the mean in parentheses for older and younger adults for both spatial and non-spatial tasks
Table 1.
Major subject characteristics
| Age (years) | Education (years) | Right handed (n) | MME | Male (n) | |
|---|---|---|---|---|---|
| Younger | 28.3 (5.9) | 16.1 (2.6) | 23 | 28.8 (1.5) | 13 |
| Older | 67.9 (3.5) | 14.8 (2.6) | 22 | 28.5 (1.4) | 11 |
Means are listed with standard deviations in parentheses
MME mini-mental state exam
As this was a study of healthy aging, participants were excluded for evidence of dementia, considered to be a score of greater than 2.5 standard deviations from the mean for their age and education on the mini-mental state examination (MMSE) (Bravo and Herbert 1997), as well as self-reported diagnoses or medications consistent with psychiatric disorders, neurological problems, head injuries, stroke, or diabetes. Potential participants were further screened for evidence of alcoholism with the alcohol use disorders identification test (Bohn et al. 1995). Participants who reported a diagnosis of depression were allowed to participate if they had been receiving treatment for 3 months or longer and were currently non-symptomatic as assessed using the Center for Epidemiological Studies Depression Scale (CES-D) (Haringsma et al. 2004). In addition, participants were required to have functional color vision as evidenced by a score of less than 7 on the Concise Edition of Ishihara's Test for Colour-blindness (Kanehara and Co., Tokyo, Japan), corrected visual acuity of 20/40 or better in both eyes measured with a modified Snell visual acuity exam, and no more than moderate hearing loss, defined as 50 dB measured with a digital audiometer (Digital Recordings, Halifax, Nova Scotia), audioscope (Welch Allyn, Skaneateles Falls, NY), or audiologists at the Wake Forest University Department of Speech and Hearing.
Design
Participants completed spatial and non-spatial tasks testing endogenous and exogenous attention, for a total of four tasks (spatial–endogenous, spatial–exogenous, non-spatial–endogenous, non-spatial–exogenous). Subjects completed the experiments over the course of two visits, spatial tasks on one day and non-spatial on another. The order of spatial versus non-spatial tasks was randomized across subjects. Within the spatial and non-spatial tasks, the order of presentation of endogenous and exogenous tasks was randomized. All experiments were completed in a sound and light attenuated booth (Whisper Room, Morristown, PA, USA). All stimuli were presented and reaction time and accuracy data collected using E-Prime stimulus presentation software (Psychology Software Tools, Pittsburgh, PA, USA). Reported reaction times are from correct responses only. Each participant's reaction time data were cleaned for outliers by removing responses that were more than three standard deviations from that participant's mean on the task.
Stimuli for spatial tasks
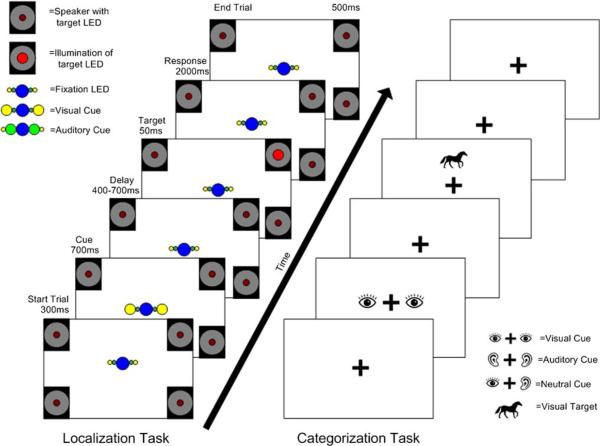
Spatial tasks were based on paradigms developed by Spence, Driver, and colleagues (Spence and Driver 1997; Spence et al. 2001) to test crossmodal attention in younger adults. Participants faced an array of four speakers and four red light emitting diodes (LEDs) arranged at the corners of an imaginary rectangle where speakers and LEDs were located 52° right and left of fixation and 18° above and below midline (Fig. 1). Participants were instructed to press the right button if they saw or heard a target in one of the two locations on their right and the left button if they saw or heard a target from the left locations. Auditory targets were 150-ms white noise stimuli (five 20-ms white noise bursts separated by 10-ms gaps) from one speaker, and visual targets were a 50-ms illumination of one of the four red LEDs. A 5-cm-long row of five LEDs was located at the center of the display. A blue central LED served as fixation. The fixation LED remained illuminated throughout the task, and subjects were instructed to remain focused on the fixation. The fixation LED was flanked by two green LEDs whose illumination served as the cue to attend to the auditory sense. The green LEDs were in turn flanked by two yellow LEDs whose illumination directed participants to attend to their sense of vision. Illumination of both yellow and green LEDs served as a divided attention cue. Participants were seated 45 cm from fixation with fixation at eye level.
Fig. 1.
Diagrammic overview of paradigm structure. The left panel shows a schematic representation of the spatial task, where circles represent light emitting diodes (LEDs) and squares indicate speaker position. Illumination of LEDs is represented by larger circles. This example is a validly cued visual trial, where the visual cue is followed by illumiation of the top right LED. The right panel is an example of the non-spatial task, illustrated with one of the visual farm animal targets. Both examples show visual targets, but auditory targets were presented with equal frequency
Stimuli for non-spatial tasks
The non-spatial task was designed to closely replicate the crossmodal attention demands of the spatial task, but with a design that did not use spatial location as a target. During non-spatial tasks, participants were seated with their chin in a comfortably adjusted chin rest 52.1 cm from a 17-in. LG 915FT + monitor run by a Dell PC computer and were instructed to maintain fixation on a black fixation cross located at the center of the screen at all times (Fig. 1). Visual targets were black silhouettes of animals presented for 250 ms against a white screen above or below fixation. Auditory targets were 250-ms clips of animal sounds (edited for length using Goldwave software, http://www. goldwave.com) presented through speakers flanking the monitor with volume adjusted to be clearly audible. During the task, participants indicated with a button press whether the target they saw or heard was a farm animal or a zoo animal. Button assignment was randomized, so that half the participants pressed the left button to indicate they saw or heard a zoo animal and the other half pressed the right button. An attentional cue was presented prior to the presentation of the target. The attentional cue consisted of two black and white pictures, one located to the left of fixation and one to the right, and an auditory cue. The visual attention cue was two eyes and the word “see” being spoken, the auditory cue was two ears and the word “hear”, and one ear and one eye with the word “both” spoken directed subjects to divide their attention between the visual and auditory modalities.
Endogenous design
The endogenous tasks were a cued spatial task and a cued non-spatial task. Tasks using informative cues were chosen as a measure of endogenous attention based on previous research that has shown the effects of attentional cues in spatial [e.g., (Posner et al. 1980)] and crossmodal (Spence and Driver 1997; Spence et al. 2001) attention. The timeline for the two cued tasks was identical except for the duration of the targets (Fig. 1). Each trial began with a delay whose length varied randomly between 500 and 700 ms. The delay was followed by presentation of an attentional cue for 750 ms. After another delay that randomly varied between 400 and 700 ms, the target was presented, and participants had 2,000 ms in which to make a response. Participants completed a practice block of 24 trials, which they could repeat if necessary, and then 7 blocks of 60 trials each. In each block, 40 trials were validly cued, 10 were invalidly cued, and 10 were preceded by a divided attention cue, for a total 420 trials of 280 valid trials, 70 invalid trials, and 70 neutral trials. Subjects were informed during the instructions that the cues would not always be correct, but were correct about 80% of the time (valid and neutral trials), and that they should pay attention to the cued sense.
Exogenous design
The exogenous paradigms were identical to the endogenous tasks except they contained no cues. Each trial began with a delay period that randomly varied in duration between 500 and 700 ms. The target was presented after the delay, and again participants had 2,000 ms in which to make a response. Participants completed a practice block of 24 trials, and then 4 blocks with 80 trials each. Trials could either be “same” trials or “switch” trials. In same trials, the sensory modality of the target matched the sensory modality of the preceding target, e.g., a visual target preceded by a visual target. In switch trials, the sensory modality of the target was different from the sensory modality of the preceding trial, e.g., a visual target preceded by an auditory target. Overall, there were a total of 79 same and 79 switch trials in each modality.
The measure of interest in the exogenous tasks was the modality shift effect (MSE) (Cohen and Rist 1992; Spence and Driver 1997). The modality shift effect captures the slowing that occurs when a person switches processing from one sensory modality to the other. The MSE is believed to be completely exogenous and is calculated by determining the difference between reaction time on same and switch trials. Because the only difference between the targets in the same and switch trials is the sensory modality of the trial preceding them, the MSE is thought to index the exogenous pull of the preceding sensory modality. Therefore, the MSE calculated on visual trials would reflect the exogenous influence of the auditory modality on the visual, and vice versa. The fact that the MSE is involuntary does not mean that it is necessarily helpful or unhelpful to the task at hand, but simply that the relationship between modalities that it reflects is an automatic rather than conscious or voluntary effect.
Analysis methods
Two different analysis methods were used to investigate potential age-related differences in attentional effects. Mean reaction times were compared, as these are most commonly used in the literature and provide fundamental and valuable information about the central tendencies of the data. However, traditional comparisons of the mean are parametric and rely on the assumption of a normal distribution, an assumption that does not always hold for reaction time data. In addition, simple comparisons of the mean are not always preferred in aging research, where it may be necessary to correct for concerns of general cognitive slowing (Verhaeghen and Cerella 2002). Therefore, in addition to traditional comparisons of the mean, Cox proportional hazards modeling was performed as a means to capture relationships between conditions using a semiparametric design with the potential to be more sensitive to age-related differences in the data. The Cox proportional hazards analysis is described below.
Measures of central tendency
For the endogenous tasks, the primary measures of interest were the attentional effects (benefit and cost). The benefit is determined by calculating the difference between average response times to neutrally and validly cued trials (neutral–valid), and cost is the difference between neutrally and invalidly cued trials (invalid–neutral). To assess the contribution of each of these aspects of attention, repeated measures analyses of variance (ANOVAs) were performed using SPSS (SPSS Inc., Chicago, IL, USA). Previous research has observed speeding on validly cued trials (benefit) and slowing on invalidly cued trials (cost) relative to divided attention trials, as discussed in detail by Spence and Driver (Spence and Driver 1997; Spence et al. 2001). The specific goal of this analysis was to determine if there were differential costs or benefits associated with age. Due to the fact that attentional effects have been observed to differ between auditory and visual modalities (Spence and Driver 1997; Spence et al. 2001), separate ANOVAs were used to assess auditory and visual responses. A secondary analysis was performed on the reaction times for each trial type to further explore the outcomes of the cost-benefit analysis. Response times were analyzed using ANOVAs of cue condition (valid, invalid, and neutral) × age.
For the exogenous tasks, repeated measures ANOVAs were used to test the effects of condition (same, switch) × age on visual and auditory reaction times. The effects of age on the MSE were examined using a two-sample t test.
Proportional hazards analyses
Proportional hazards analyses are most commonly used to evaluate survival data (or any time-to-event outcomes) in epidemiological and medical studies, but also can be useful in comparing conditions in psychophysical experiments, as they do not assume a particular distribution but still allow reaction time data to be modeled by explanatory variables similar to multiple regression (Kleinbaum 1996; Wenger and Gibson 2004). These properties can be observed by looking at Eq. 1, the formula for the Cox proportional hazards model (Kleinbaum 1996):
| (1) |
In this formula, Xi represents the explanatory variables in the model, h0(t) is the baseline hazard function, and the exponential base e is raised to the linear sum of the p explanatory variables. The Cox proportional hazards model can be said to be semiparametric, as no parametric assumption is made about the shape of the baseline hazard function, but comparison of two conditions is constrained by the assumption that the baseline hazard function is the same in both conditions. The output of primary interest in a Cox proportional hazards analysis is the hazard ratio (HR), which is calculated as the hazard function (Eq. 1) for one condition divided by the hazard function for another condition [Eq. 2 (Kleinbaum 1996)]:
| (2) |
Because the baseline hazard function is the same in both conditions, this term cancels out and the HR then reflects the relationship between the explanatory variables in each condition. The HR has a straightforward interpretation as an increase or decrease in the occurrence of an event under the experimental condition compared to the control condition. For instance, in these experiments, reaction times to correctly cued targets are compared to reaction times to neutrally cued targets. The numerator of Eq. 2 would represent the valid condition and the denominator the neutral condition. If subjects respond faster when they are correctly cued, the ratio will be larger than 1, reflecting the proportion of reaction time gain from the validly cued condition.
As mentioned above, the Cox proportional hazards model used in this analysis compares two conditions, assuming their hazard functions share the same shape over time and are therefore parallel (i.e., “proportional” hazards). If the hazard functions of the two group distributions being compared are not parallel, this fundamental assumption of the model has been violated and corrective procedures must be undertaken. In analyses of our data, log-log plots and time-dependent interactions added in the Cox models indicated that this assumption did not hold for older and younger adults (i.e., they had different baseline hazard functions). In the log-log plots, this was evident in crossing of the distribution curves (they were not parallel). Therefore, stratified Cox models were used to account for the non-parallel baseline hazards (Kleinbaum 1996; Therneau and Grambsch 2000). S-plus v7.0 was used to perform all Cox regression analysis (Insightful Corporation, Seattle, WA). Repeated measures due to multiple reaction times per person were accounted for by using the “cluster” command in S-plus (Therneau and Grambsch 2000).
Results
Over all four task types, accuracy was quite high for both groups (young = 98.3%, older 97.9%). Each condition had accuracy above 96% for both younger and older participants, and there were no significant differences in accuracy between groups on any task.
Endogenous spatial task
The cost, benefit, and reaction times for each condition and each age group are reported in Table 2 and depicted graphically in Fig. 2. The ANOVA examining attentional effects (cost vs. benefit) × age to visual targets showed a main effect of attention effects [F(1, 46) = 12.6, P < 0.01], reflecting that benefit was larger than cost to visual targets. However, no main effect of age [F(1, 46) = 1.3, P = 0.26], or interaction between attentional effects and Age [F(1, 46) = 1.4, P = 0.24], was observed. It should be noted that cost was very small for younger adults (4.2 ms) and was actually slightly positive in older adults (−7.3 ms). That is, invalid trials were actually slightly faster than neutral. However, this is not meaningful as one sample t tests showed that visual cost was not significantly different from zero for either younger (t = −0.067, P = 0.95) or older (t = −0.65, P = 0.52) adults. Analysis of cost and benefit to auditory targets showed no significant results in main effects of Age [F(1, 46) = 0.00, P = 0.99], attentional effects [F(1, 46) = 0.23, P = 0.64], or interaction between them [F(1, 46) = 0.76, P = 0.39].
Fig. 2.
Mean results of endogenous attention tasks. Attentional effects (cost and benefit) are shown in milliseconds. Error bars represent standard error of the mean
The cost-benefit outcomes were further explored by analyzing reaction times to evaluate the actual reaction time differences for each cue condition (valid, neutral, invalid) in each of the groups and sensory modalities. The ANOVA for reaction times to visual targets (cue condition × age) revealed a main effect of age [F(1, 46) = 9.5, P < 0.01] reflecting the anticipated outcome that younger adults were significantly faster than older adults in all three cue conditions. There was also a main effect of cue condition [F(2, 92) = 12.5, P < 0.01] with validly cued trials being consistently faster than neutral or invalid trials. The interaction between condition and age was not significant [F(2, 92) = 1.4, P = 0.26], indicating that differences between cue conditions did not vary by age group.
Results for the auditory modality in the spatial task were similar. There was a main effect of age in the ANOVA for reaction times to auditory targets [F(1, 46) = 7.7, P < 0.01] due to the fact that older participants were slower than younger participants in all three cue conditions. A main effect of cue condition was also observed [F(2, 92) = 10.8, P < 0.01], but the interaction between cue condition and age was not significant [F(2, 92) = 0.2, P = 0.78].
Cost and benefit were also analyzed using the Cox proportional hazards method (Table 3). Both older and younger adults showed benefits of attention to visual targets when valid and neutrally cued trials were compared. Younger adults showed a hazard ratio (HR) of 1.12, indicating that they were speeded approximately 12% by the presence of a valid cue. Older adults had a HR of 1.11, or approximately 11% speeding, and there was no significant between-group difference (z = 0.27, P = 0.61). Attentional cost as assessed with Cox proportional hazards was negligible in both groups. Younger adults slowed approximately 1% (HR = 0.99) and older adults did not show slowing (HR = 1.01). There was a trend toward a difference between groups in attentional cost to visual targets in the spatial task, but the groups were not significantly different (z = 1.78, P = 0.08).
Table 3.
Proportional hazards results for all conditions
| Spatial |
Non-spatial |
|||||
|---|---|---|---|---|---|---|
| Younger | Older | P value | Younger | Older | P value | |
| Visual target | ||||||
| Benefit | 1.12 | 1.11 | 0.61 | 1.06 | 1.06 | 0.99 |
| Cost | 0.99 | 1.01 | 0.08 | 0.75 | 0.72 | 0.38 |
| Auditory target | ||||||
| Benefit | 1.06 | 1.06 | 0.92 | 1.10 | 1.13 | 0.33 |
| Cost | 0.91 | 0.91 | 0.79 | 0.90 | 0.95 | 0.30 |
Columns show the hazard ratio (HR) for younger and older adults, along with the P value from Cox proportional hazards regression for the interaction term showing that cost and benefit do not vary with respect to age group. Benefit represents the ratio of valid to neutral trials. Values above 1 indicate speeding to the validly cued trials. Cost represents the ratio of invalid to neutral trials. Values below 1 show slowing on invalidly cued trials
In response to auditory targets, both younger and older adults showed a benefit of approximately 6% (young HR = 1.06, old HR = 1.06) when validly and neutrally cued trials were compared. No age-related differences in auditory benefit were noted in the spatial task using Cox proportional hazards (z = 0.09, P = 0.92). A HR of 0.91, or slowing of around 9%, was observed in both younger and older adults when attentional cost was computed. Again, no significant between-group difference was noted (z = 0.27, P = 0.79). These results support the findings from the traditional comparison of means, which also noted larger cost than benefit in the auditory condition of the spatial task and no differences in cost and benefit between older and younger adults.
Endogenous non-spatial task
An ANOVA examining attention effects × age for visual targets in the non-spatial task revealed a main effect of attention, [F(1, 47) = 26.5, P < 0.01] reflecting the fact that cost was much larger than benefit to visual targets for both older and younger adults (see Table 2). No other significant effects were observed [main effect of age: F(1, 47) = 0.03, P = 0.87; interaction of attention effect × age: F(1, 47) = 0.05, P = 0.82]. Similar outcomes were observed for the auditory targets with a main effect of attention [F(1, 47) = 4.8, P < 0.05], reflecting that cost was larger than benefit for both older and younger adults. Again, no main effect of age [F(1, 47) = 0.1, P = 0.74] or an interaction between age and attentional effect [F(1, 47) = 0.7, P = 0.42] was observed.
The analysis of visual reaction times in the non-spatial task reflected a main effect of cue condition [F(2, 94) = 41.7, P < 0.01], but no main effect of age [F(1, 47) = 1.1, P = 0.31] or interaction between age and cue condition [F(1, 94) = 0.03, P = 0.97] was detected. For auditory reaction times a main effect of cue condition [F(2, 94) = 38.6, P < 0.01], but no effect of age [F(1, 47) = 1.3, P = 0.26] or an interaction between cue condition and age [F(2, 94) = 0.3, P = 0.74].
Proportional hazards analysis showed significant attentional effects in both age groups but no between-group differences. Both younger and older adults displayed an attentional benefit to visual targets in the non-spatial task of approximately 6% (HR = 1.06). No significant difference between age groups was noted (z = 0.01, P [ 0.99). Both age groups showed very similar attentional cost, with a HR of 0.75 for younger adults and 0.72 for older adults, indicating slowing close to 30% in the presence of an invalid cue relative to a neutral cue. No significant difference in visual attentional cost was noted using Cox proportional hazards in the non-spatial task (z = −0.87, P = 0.38). These findings reiterate the results of the comparison of means showing small but significant benefits, robust costs, and no difference between older and younger participants for either cost or benefit.
In response to auditory targets, younger adults showed benefit of approximately 10% (HR = 1.10). Similarly, a benefit of approximately 13% was noted for older adults, (HR = 1.13). No significant effects of age group were noted for auditory benefit in the spatial task using Cox proportional hazards analysis (z = 0.98, P = 0.33). Auditory cost was more disparate between the age groups, although the difference between the groups was still non-significant (z = 1.03, P = 0.30). Younger adults had a HR of 0.89 between invalid and neutral trials, indicating slowing of approximately 11%, and older adults showed slowing of approximately 5% (HR = 0.95). These results differ slightly from traditional comparison of means where cost was substantially larger than benefit. However, the main finding of no significant differences in attentional effects in younger and older adults was supported by both analyses.
Exogenous spatial task
Reaction times and MSEs for both uncued tasks are reported in Table 4. The ANOVA of condition (same/switch) × age for visual targets demonstrated main effects of both condition [F(1, 45) = 105.5, P < 0.01] and age [F(1, 45) = 8.4, P < 0.01] but no interaction between the two [F(1, 45) = 2.67, P = 0.11]. The MSE for visual targets did not show a difference between younger and older adults (t = −1.6, P = 0.12). The outcomes for auditory targets were the same as those for visual. A main effect of both condition [F(1, 45) = 51.4, P < 0.01] and age [F(1, 45) = 9.8, P < 0.01] was observed but no interaction [F(1, 45) = 1.31, P = 0.26], and comparison of the auditory MSE showed no effect of age group [t = −1.1, P = 0.25]. Using Cox proportional hazards analysis, younger adults demonstrated a HR of 1.27 (approximately a 27% difference) between same and switch trials for visual targets, which was not significantly different from the HR of 1.28 or approximately 28% difference observed in older adults (z = 0.36, P = 0.72). In response to auditory targets, younger adults exhibited a HR of 1.13 or approximately 13% difference and older adults a HR of 1.15, which again did not differ significantly with age (z = 0.53, P = 0.60). Both traditional ANOVA and Cox proportional hazards revealed no significant age-related difference in the exogenous influence of the visual or auditory modalities.
Exogenous non-spatial task
A main effect of condition (same/switch) was found for both visual [F(1, 45) = 29.7, P < 0.01] and auditory [F(1, 45) = 19.8, P < 0.01] targets in the non-spatial task. No main effects of age [visual: F(1, 45) = 1.18, P = 0.29] or interaction of condition and age [F(1, 45) = 0.15, P = 0.70] were observed for the visual modality. No Condition × Age interaction was observed in the auditory modality [F(1, 45) = 0.43, P = 0.52], but the main effect of age showed a trend toward a significant effect [F(1, 45) = 3.51, P = 0.07]. As in the spatial task, no significant age difference was demonstrated for visual (t = −0.97, P = 0.34) or auditory (t = 0.54, P = 0.59) MSEs. However, proportional hazards analysis did suggest a trend for an age difference in the visual MSE, where younger adults showed a HR of 1.15 or an approximately 15% effect of switching modalities, while older adults showed a HR of 1.19, a difference that trended toward significance (z = −1.83, P = 0.07). The auditory MSE was not significantly different between age groups in the proportional hazard analysis (z = −0.53, P = 0.60). In this comparison, a HR of 1.13 or approximately 13% effect was observed in younger adults, and a HR of 1.11 or approximately 11% effect was seen in older adults. Both traditional comparisons of the mean and Cox proportional hazards failed to find a difference between younger and older adults in visual or auditory exogenous influence in the non-spatial task.
Discussion
Previous work showing that older adults are more influenced by crossmodal distractors than younger adults (Alain and Woods 1999; Poliakoff et al. 2006) led to the hypothesis that older adults would fail to instantiate voluntary crossmodal selective attention and their involuntary attention would be more easily captured than younger adults. However, the results presented here do not support these hypotheses. The findings of the reported experiments are remarkably consistent across different tasks, different sensory modalities, different kinds of attention, and different analyses: attentional effects were not different from those observed in younger adults. Older adults were able to use informative cues to direct their attention to a specific sensory modality and were not differentially influenced by exogenous attentional capture. Importantly, this suggests that the ability to engage cross-modal selective attention is preserved with aging, in spite of the fact that older adults are differentially affected by crossmodal distractors.
Endogenous tasks
Two tasks were used to test crossmodal attention, a spatial task and a non-spatial task. Results from both paradigms are similar to the results Spence and Driver observed in the spatial task most akin to the one performed here (Spence and Driver 1997). In the cued spatial task, benefit was larger than cost for both age groups in response to visual targets, but cost was larger than benefit to auditory targets. The interpretation of these findings is that the effects of visual attention, evident in visual benefit and auditory cost, are greater than auditory attention in the spatial task. Our findings suggest this is true for both older and younger adults.
One interesting difference between the tasks is that in the non-spatial task, benefit is larger for the auditory than the visual modality. Findings from our spatial task as well as others (Spence and Driver 1997) show larger benefit for the visual modality. This difference in results could be due to the relative dominance of the visual modality in spatial localization, the more complex nature of the categorization task, or the use of crossmodal cues in the non-spatial task. This spatial task was meant to closely parallel previous studies, where visual cues are used to cue both auditory and visual attention (Spence and Driver 1997; Spence et al. 2001). The authors cite previous literature showing that the modality shift effect primarily affects target rather than non-target stimuli. However, it seems possible that switching from a valid visual cue to an auditory target could have the effect of minimizing speeding associated with benefit. Using a cue with an auditory component might therefore result in larger auditory benefit. Future studies will be needed to investigate these potential differences further and replicate these results using this or other non-spatial tasks.
Another effect of potential interest is that of response repetition. In these tasks, response type (left/right, farm/zoo) choices were randomized. Therefore, the paradigms contained response type switches and modality switches, but the crossmodal switching effect should not be biased by the response type effect. Future studies may investigate the possibility that older adults could be more speeded when a response is repeated, such as two farm animal stimuli or two right-sided stimuli, than younger adults or conversely more slowed if the response switched.
Exogenous tasks
To our knowledge, this is the first investigation of the MSE in healthy aging adults, but event-related potential (ERP) and behavioral studies of attentional capture by distracting information have shown that older adults process more extraneous information than their younger counterparts (Alain and Woods 1999; Andres et al. 2006; Healey et al. 2008). Given this information, we hypothesized that the MSE would be larger for older adults. Findings from our studies of exogenous attention were less conclusive than those from the cued tasks, in that there was a trend for significance in the Cox proportional hazards analysis for the auditory MSE in the non-spatial task. Nevertheless, the fact remains that these results do not convincingly support our hypothesis that older adults are more influenced by exogenous attentional pull than younger adults. This suggests that older adults are not more influenced by exogenous attentional pull than younger adults, in spite of the fact that previous studies indicate they process more distracting information. In fact, the observations here that older adults are able to engage endogenous crossmodal attention and are not influenced more by exogenous pull than their younger counterparts suggest that older adults should not be more distractible. However, this assertion rests on the assumption that distraction is a measure of attentional function.
Does paying attention mean not distracted?
A recent paper from our laboratory (Hugenschmidt et al. 2009) presents data that may elucidate this apparent conflict in findings. In that experiment, multisensory integration was used as an index of background distractor processing during crossmodal attention. It was observed that older adults showed greater multisensory integration than younger adults during divided attention. When selective attention was invoked, multisensory integration was virtually abolished in younger adults. Older adults reduced the amount of integration observed proportionally to younger adults. However, even though they showed equivalent suppression, older adults still integrated more under selective attention conditions because they integrated more during the divided attention baseline condition.
These findings make three important points. First, as in the experiments reported here, older adults were able to successfully engage crossmodal selective attention as measured through suppression of multisensory integration. Second, in spite of this, older adults processed more background information during selective attention, consistent with previous research indicating that the ability to inhibit responses to distracting stimuli in both the visual and auditory modalities decreases with age (Folk and Lincourt 1996; Alain and Woods 1999; Groth and Allen 2000; Gaeta et al. 2001; Tales et al. 2002; McCarley et al. 2004; Fabiani et al. 2006; Rowe et al. 2006; Yang and Hasher 2007). Finally, these observations indicate that distraction may not be a reliable measure of attentional function. That is, there are times when the amount of distraction can be dissociated from the ability to successfully invoke attentional networks and their attendant enhancement and suppression. As an anecdotal example of this, imagine you are eating in a crowded restaurant. You may have to attend closely to follow the conversation at your table. In spite of the fact that you are attending very well, you may still be more distracted than you would be in a quieter environment. Your level of distraction would not indicate that you are unable to modulate sensory perception with attention, but rather that the level of background noise is still distracting even after modulation.
This idea is further supported by a survey of attentional literature. As mentioned in the introduction, very little research has directly examined the effects of aging on crossmodal attention. However, there are cued studies of other forms of attention that indicate that older adults successfully engage and benefit from attention (Bahramali et al. 1999; Groth and Allen 2000; Verhaeghen and Cerella 2002; Madden et al. 2004; Ballesteros et al. 2008). In these studies, selective attention (crossmodal or unimodal) is defined as a relative measure, commonly as increases or decreases in reaction time or accuracy when compared to a referent condition (e.g., divided attention). This means that if significant differences are noted between selective and divided attention, participants are able to instantiate selective attention. In the present study, older and younger adults were both able to engage selective attention relative to divided attention. However, these findings do not necessarily mean that resistance to distractibility is equivalent across different age groups. Older adults could be more distractible, but still show a relative improvement due to selective attention, as was observed in the multisensory integration paper mentioned above (Hugenschmidt et al. 2009).
In fact, increased distractibility in spite of intact attentional function might be expected if older adults had a higher sensory load in the baseline referent condition. In this situation, older adults would work harder at all levels of processing to attain the same behavioral results as younger adults. There is some evidence from ERP literature to support the idea that baseline sensory functioning may be altered in healthy aging. A study by Alain and Woods (1999) compared event-related potentials in older, middle-aged and younger adults who were asked to perform a simple visual task, while a train of standard and deviant tones were played in the background. The researchers observed that middle-aged and older adults showed larger sensory-evoked potentials to the standard background auditory stimuli than younger adults. In addition, they observed that the mismatch negativity (MMN) was smaller for older adults than younger adults. The mismatch negativity (MMN) is a waveform computed as the difference between a standard and a deviant stimulus, typically in a train of auditory tones. The smaller MMN in older adults indicates that they process standard and deviant tones more equally than younger adults. Together, these findings support the idea that older adults may be more distracted because of a higher sensory load at baseline. They shower larger cortical responses to unattended auditory stimuli, which makes discrimination of deviants from background standard stimuli more difficult.
To return to the example given in the introduction, could the hypothetical 75-year old ignore the sounds on the subway while reading his paper as well as the 20-year old? The results from this study would suggest yes; the older man is not more susceptible to the exogenous pull of stimuli and is able to effectively engage crossmodal attention. However, these data may only tell half of the story; the older man may in fact be more susceptible to distraction even though he is attending as well as the younger man if his sensory processes are changing.
Implications for multisensory integration
The finding of intact crossmodal attentional function in older adults has direct bearing on multisensory processes. Previous studies have observed that older adults show increased multisensory integration relative to younger adults (Strupp et al. 1999; Laurienti et al. 2006). In younger adults, multisensory integration is modulated by stimulus characteristics, such as intensity, timing, and location [for review see (Stein and Meredith 1993; Stein and Stanford 2008)] but also by top down cognitive processes, such as semantic congruence (Laurienti et al. 2004) or attention (Alsius et al. 2005; Talsma and Woldorff 2005; Talsma et al. 2007; van Atteveldt et al. 2007; Mozolic et al. 2008a). Given that it is anecdotally believed that attention falters with age, and that previous research suggests this may be the case [e.g., (Maylor and Lavie 1998; Poliakoff et al. 2006)], it might be hypothesized that increased integration in older adults results from attentional deficits. That is, multisensory integration is no longer adequately constrained by crossmodal attention. The results of this behavioral experiment suggest that increased integration in older adults is not due to a failure of attention.
At present, it is thought that a dorsal attentional network including prefrontal and parietal cortices acts to increase neural activity in relevant cortices and decrease activity in unrelated or competing regions (Desimone and Duncan 1995; Kastner and Ungerleider 2001; Corbetta and Shulman 2002). In crossmodal attention, this means slightly increasing activity in the attended modality and more heavily suppressing neural activity in competing or ignored modalities (Roland 1982; Kawashima et al. 1995; Ghatan et al. 1998; Fox et al. 2005; Johnson and Zatorre 2005; Mozolic et al. 2008b). If attention were dysfunctional in older adults, it might be hypothesized that increased distractibility and multisensory integration in older adults occurs because the dorsal attentional network does not adequately suppress neural activity in competing modalities. However, the results of the present experiment suggest that older adults can enhance and suppress commensurately with older adults, and that changes in stimulus processing may be due to fundamental changes in sensory processing or alterations in bottom-up processes.
Limitations and future directions
The results of this study combined with previous findings suggest that crossmodal attention is intact in healthy aging, but that baseline sensory load may be altered in older adults. However, no tests of baseline functioning were directly undertaken here, and participants were not tested in any tasks with distractors, which would allow direct comparison of attentional functioning and distractor processing in the same subjects. It is also important to keep in mind that specific task characteristics can heavily influence outcomes in psychophysical paradigms, e.g., (Maylor and Lavie 1998; Madden and Langley 2003). For instance, attentional research suggests that the relative timing of the cue and target as well as specific characteristics of the task such as the cue, target, and fixation can be influential in the ability to detect age-related differences in attention. One study that specifically manipulated the delay between cue and target presentation showed that aging-related attentional differences may be more apparent at short cue-target delay times (Castel et al. 2003). Cue-target delay time was not specifically manipulated in this study, but this may be an important avenue for future studies investigating the effects of aging on crossmodal attention. In addition, psychophysical techniques are limited in their ability to assess baseline sensory function in that they do not directly measure neural activity. Ideally, future studies will use functional neuroimaging techniques (including cerebral perfusion, EEG, MEG) to directly assess the impact of distractors and baseline sensory functioning on attentional performance in aging adults. Finally, research on a healthy aging population is necessary to characterize behavior in the absence of disease, but it must be acknowledged that the results presented here are based on highly successful agers and may not apply broadly to the aging population. Future studies using a more inclusive aging population may shed light on this issue.
Acknowledgments
The authors would like to thank Ms. Debra Hege for her invaluable assistance. Research support was provided by NIH#NS042658, the Roena Kulynych Memory and Cognition Research Center, and the Wake Forest University GCRC #RR07122.
Footnotes
Conflict of interest statement The authors report no actual or potential conflicts of interest relating to this research.
References
- Alain C, Woods DL. Age-related changes in processing auditory stimuli during visual attention: evidence for deficits in inhibitory control and sensory memory. Psychol Aging. 1999;14:507–519. doi: 10.1037//0882-7974.14.3.507. [DOI] [PubMed] [Google Scholar]
- Alsius A, Navarra J, Campbell R, Soto-Faraco S. Audiovisual integration of speech falters under high attention demands. Curr Biol. 2005;15:839–843. doi: 10.1016/j.cub.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Andres P, Parmentier FB, Escera C. The effect of age on involuntary capture of attention by irrelevant sounds: a test of the frontal hypothesis of aging. Neuropsychologia. 2006;44:2564–2568. doi: 10.1016/j.neuropsychologia.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Bahramali H, Gordon E, Lagopoulos J, Lim CL, Li W, Leslie J, Wright J. The effects of age on late components of the ERP and reaction time. Exp Aging Res. 1999;25:69–80. doi: 10.1080/036107399244147. [DOI] [PubMed] [Google Scholar]
- Ballesteros S, Reales JM, Mayas J, Heller MA. Selective attention modulates visual and haptic repetition priming: effects in aging and Alzheimer's disease. Exp Brain Res. 2008;189:473–483. doi: 10.1007/s00221-008-1441-6. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Babor TF, Kranzler HR. The alcohol use disorders identification test (audit): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423. doi: 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- Bravo G, Herbert R. Age- and education-specific reference values for the Mini-Mental and Modified Mini-Mental State Examinations derived from a non-demented elderly population. Int J Geriatr Psychiatry. 1997;12:1008–1018. doi: 10.1002/(sici)1099-1166(199710)12:10<1008::aid-gps676>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Castel AD, Chasteen AL, Scialfa CT, Pratt J. Adult age differences in the time course of inhibition of return. J Gerontol B Psychol Sci Soc Sci. 2003;58:P256–P259. doi: 10.1093/geronb/58.5.p256. [DOI] [PubMed] [Google Scholar]
- Cohen R, Rist F. The modality shift effect. Further explorations at the crossroads. Ann N Y Acad Sci. 1992;658:163–181. doi: 10.1111/j.1749-6632.1992.tb22844.x. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Low KA, Wee E, Sable JJ, Gratton G. Reduced suppression or labile memory? Mechanisms of inefficient filtering of irrelevant information in older adults. J Cogn Neurosci. 2006;18:637–650. doi: 10.1162/jocn.2006.18.4.637. [DOI] [PubMed] [Google Scholar]
- Folk CL, Lincourt AE. The effects of age on guided conjunction search. Exp Aging Res. 1996;22:99–118. doi: 10.1080/03610739608254000. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaeta H, Friedman D, Ritter W, Cheng J. An event-related potential evaluation of involuntary attentional shifts in young and older adults. Psychol Aging. 2001;16:55–68. doi: 10.1037/0882-7974.16.1.55. [DOI] [PubMed] [Google Scholar]
- Ghatan PH, Hsieh JC, Petersson KM, Stone-Elander S, Ingvar M. Coexistence of attention-based facilitation and inhibition in the human cortex. Neuroimage. 1998;7:23–29. doi: 10.1006/nimg.1997.0307. [DOI] [PubMed] [Google Scholar]
- Groth KE, Allen PA. Visual attention and aging. Front Biosci. 2000;5:D284–D297. doi: 10.2741/groth. [DOI] [PubMed] [Google Scholar]
- Haringsma R, Engels GI, Beekman AT, Spinhoven P. The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry. 2004;19:558–563. doi: 10.1002/gps.1130. [DOI] [PubMed] [Google Scholar]
- Healey MK, Campbell KL, Hasher L. Cognitive aging and increased distractibility: costs and potential benefits. Prog Brain Res. 2008;169:353–363. doi: 10.1016/S0079-6123(07)00022-2. [DOI] [PubMed] [Google Scholar]
- Hugenschmidt CE, Mozolic JL, Laurienti PJ. Suppression of multisensory integration by modality-specific attention in aging. Neuroreport. 2009;20:349–353. doi: 10.1097/WNR.0b013e328323ab07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, Zatorre RJ. Attention to simultaneous unrelated auditory and visual events: behavioral and neural correlates. Cereb Cortex. 2005;15:1609–1620. doi: 10.1093/cercor/bhi039. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. The neural basis of biased competition in human visual cortex. Neuropsychologia. 2001;39:1263–1276. doi: 10.1016/s0028-3932(01)00116-6. [DOI] [PubMed] [Google Scholar]
- Kawashima R, O'Sullivan BT, Roland PE. Positron-emission tomography studies of cross-modality inhibition in selective attentional tasks: closing the “mind's eye”. Proc Natl Acad Sci USA. 1995;92:5969–5972. doi: 10.1073/pnas.92.13.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinbaum DG. Survival analysis: a self-learning text. Springer; New York: 1996. [Google Scholar]
- Laurienti PJ, Kraft RA, Maldjian JA, Burdette JH, Wallace MT. Semantic congruence is a critical factor in multisensory behavioral performance. Exp Brain Res. 2004;158:405–414. doi: 10.1007/s00221-004-1913-2. [DOI] [PubMed] [Google Scholar]
- Laurienti PJ, Burdette JH, Maldjian JA, Wallace MT. Enhanced multisensory integration in older adults. Neurobiol Aging. 2006;27:1155–1163. doi: 10.1016/j.neurobiolaging.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Lavie N, Cox S. The efficiency of visual selective attention: efficient visual search leads to inefficient distractor rejection. Psychol Sci. 1997;8:395–398. [Google Scholar]
- Madden DJ, Langley LK. Age-related changes in selective attention and perceptual load during visual search. Psychol Aging. 2003;18:54–67. doi: 10.1037/0882-7974.18.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Langley LK, Hawk TC, Coleman RE. Aging and attentional guidance during visual search: functional neuroanatomy by positron emission tomography. Psychol Aging. 2002;17:24–43. doi: 10.1037//0882-7974.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Cabeza R, Huettel SA. Age-related preservation of top-down attentional guidance during visual search. Psychol Aging. 2004;19:304–309. doi: 10.1037/0882-7974.19.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylor EA, Lavie N. The influence of perceptual load on age differences in selective attention. Psychol Aging. 1998;13:563–573. doi: 10.1037//0882-7974.13.4.563. [DOI] [PubMed] [Google Scholar]
- McCarley JS, Mounts JR, Kramer AF. Age-related differences in localized attentional interference. Psychol Aging. 2004;19:203–210. doi: 10.1037/0882-7974.19.1.203. [DOI] [PubMed] [Google Scholar]
- Mozolic JL, Hugenschmidt CE, Peiffer AM, Laurienti PJ. Modality-specific selective attention attenuates multisensory integration. Exp Brain Res. 2008a;184:39–52. doi: 10.1007/s00221-007-1080-3. [DOI] [PubMed] [Google Scholar]
- Mozolic JL, Joyner D, Hugenschmidt CE, Peiffer AM, Kraft RA, Maldjian JA, Laurienti PJ. Cross-modal deactivations during modality-specific selective attention. BMC Neurol. 2008b;8:35. doi: 10.1186/1471-2377-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plude DJ, Enns JT, Brodeur D. The development of selective attention: a life-span overview. Acta Psychol (Amst) 1994;86:227–272. doi: 10.1016/0001-6918(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Poliakoff E, Ashworth S, Lowe C, Spence C. Vision and touch in ageing: crossmodal selective attention and visuotactile spatial interactions. Neuropsychologia. 2006;44:507–517. doi: 10.1016/j.neuropsychologia.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol. 1980;109:160–174. [PubMed] [Google Scholar]
- Roland PE. Cortical regulation of selective attention in man. A regional cerebral blood flow study. J Neurophysiol. 1982;48:1059–1078. doi: 10.1152/jn.1982.48.5.1059. [DOI] [PubMed] [Google Scholar]
- Rowe G, Valderrama S, Hasher L, Lenartowicz A. Attentional disregulation: a benefit for implicit memory. Psychol Aging. 2006;21:826–830. doi: 10.1037/0882-7974.21.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence C, Driver J. On measuring selective attention to an expected sensory modality. Percept Psychophys. 1997;59:389–403. doi: 10.3758/bf03211906. [DOI] [PubMed] [Google Scholar]
- Spence C, Nicholls ME, Driver J. The cost of expecting events in the wrong sensory modality. Percept Psychophys. 2001;63:330–336. doi: 10.3758/bf03194473. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The merging of the senses. MIT Press; Cambridge, MA: 1993. [Google Scholar]
- Stein BE, Stanford TR. Multisensory integration: current issues from the perspective of the single neuron. Nat Rev Neurosci. 2008;9:255–266. doi: 10.1038/nrn2331. [DOI] [PubMed] [Google Scholar]
- Strupp M, Arbusow V, Borges Pereira C, Dieterich M, Brandt T. Subjective straight-ahead during neck muscle vibration: effects of ageing. Neuroreport. 1999;10:3191–3194. doi: 10.1097/00001756-199910190-00012. [DOI] [PubMed] [Google Scholar]
- Tales A, Troscianko T, Wilcock GK, Newton P, Butler SR. Age-related changes in the preattentional detection of visual change. Neuroreport. 2002;13:969–972. doi: 10.1097/00001756-200205240-00014. [DOI] [PubMed] [Google Scholar]
- Talsma D, Woldorff MG. Selective attention and multisensory integration: multiple phases of effects on the evoked brain activity. J Cogn Neurosci. 2005;17:1098–1114. doi: 10.1162/0898929054475172. [DOI] [PubMed] [Google Scholar]
- Talsma D, Doty TJ, Woldorff MG. Selective attention and audiovisual integration: is attending to both modalities a prerequisite for early integration? Cereb Cortex. 2007;17:679–690. doi: 10.1093/cercor/bhk016. [DOI] [PubMed] [Google Scholar]
- Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. Springer; New York: 2000. [Google Scholar]
- van Atteveldt NM, Formisano E, Goebel R, Blomert L. Top-down task effects overrule automatic multisensory responses to letter-sound pairs in auditory association cortex. Neuroimage. 2007;36:1345–1360. doi: 10.1016/j.neuroimage.2007.03.065. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J. Aging, executive control, and attention: a review of meta-analyses. Neurosci Biobehav Rev. 2002;26:849–857. doi: 10.1016/s0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- Wenger MJ, Gibson BS. Using hazard functions to assess changes in processing capacity in an attentional cuing paradigm. J Exp Psychol Hum Percept Perform. 2004;30:708–719. doi: 10.1037/0096-1523.30.4.708. [DOI] [PubMed] [Google Scholar]
- Yang L, Hasher L. The enhanced effects of pictorial distraction in older adults. J Gerontol B Psychol Sci Soc Sci. 2007;62:P230–P233. doi: 10.1093/geronb/62.4.p230. [DOI] [PubMed] [Google Scholar]