Abstract
Although vaginal immunization has been explored as a strategy to induce mucosal immunity in the female reproductive tract, this site displays unique immunological features that probably evolved to inhibit anti-paternal T cell responses after insemination to allow successful pregnancy. We previously demonstrated that estradiol, which induces an estrus-like state, prevented CD8+ T cell priming during intravaginal immunization of mice. We now show that estradiol prevented antigen loading of vaginal antigen presenting cells (APC) after intravaginal immunization. Histological examination confirmed that estradiol prevented penetration of peptide antigen into the vaginal wall. Removal of the estradiol-induced mucus barrier by mucinase partially restored antigen loading of vaginal APC and CD8+ T cell proliferation in vivo. The estradiol-induced mucus barrier may thus prevent exposure to antigens delivered intravaginally, supplementing additional estradiol-dependent mechanism(s) that inhibit CD8+ T cell priming after insemination or vaginal vaccination.
Keywords: CD8+ T cell, vagina, estrogen, antigen presentation, reproductive immunology
INTRODUCTION
Vaginal immunization can induce mucosal immunity in the female reproductive tract (FRT) but there are several unusual features of vaginal immune responses that may have evolved to protect against deleterious immune reactions to paternal antigens and thus ensure successful development of the semi-allogeneic embryo and fetus. Insemination introduces immunogenic components, including male cells and cellular debris, that may induce allogeneic cellular and/or humoral immune responses against the implanting embryo or fetus [1, 2]. However, infertility caused by female immune responses against sperm antigens is rare [3], and CD8+ T cell responses to male antigens post-coitus are difficult to detect [2]. In some experimental models, even allogeneic insemination of TCR transgenic females by male mice that express the TCR transgenic ligand are unable to induce T cell activation, or tolerance, or damage to the developing allogeneic fetuses overexpressing this TCR ligand [4] (Seavey and Mosmann, unpublished data). The presence of immunogenic components in the semen combined with the lack of response to male antigen suggests that the reproductive tract is hyporesponsive to antigen deposited during sexual intercourse.
The menstrual cycle influences vaginal immunization in rats, mice and humans. Human antibody responses to vaginal immunization were higher during the follicular than the luteal phase of the human menstrual cycle [5]. In mice, vaginal immunization during estrus led to reduced T cell responses as measured by antigen-specific proliferation of lymph node cells and footpad swelling, both normally CD4-dominated responses [6]. Immune inhibition during estrus may occur locally, as estrus enhanced mouse antibody responses induced by oral immunization [7].
The menstrual hormone, estradiol (E2) influences structural features of the female reproductive tract (FRT), such as endometrial thickening and increased mucus production [8, 9]. E2 also clearly regulates immune responses in the uterus and vagina, which may help to explain the inhibition of immune responsiveness at estrogen-dominated phases of the menstrual cycle. E2 inhibits the ability of rat vaginal cells to present antigen and induce T cell proliferation [10, 11]. This effect is mediated at least in part by the effect of TGFβ on rat vaginal [11] or uterine [12] antigen-presenting (APC). E2 also potentiated the suppressive activity of human regulatory T cells [13], providing another potential explanation for reduction of T cell responses during estrus. In mice, we showed that E2 profoundly inhibited priming of CD4+ and CD8+ T cell immune responses by the vaginal, but not subcutaneous route [2].
As most previous studies focused on human and rat T cell proliferative responses likely to be mediated mainly by CD4+ T cells, we sought to determine whether the inhibition of mouse CD8+ T cell responses in our model was due to a mechanism similar to the well-established effect of TGFβ on rat APC, or to increased suppression. Using a mouse model for reproductive immunology, ovarectomized mice treated with exogenous estradiol, we found that the vaginal APC from E2-treated mice were fully capable of presenting antigen to CD8+ T cells if pulsed in vivo, but that penetration of antigen, even peptide antigen, into the vaginal wall was blocked in E2-treated mice, as measured either functionally by antigen presentation, or physically using fluorescent-labeled peptide. This effect was partially abrogated by mucinase treatment, implicating the E2-enhanced mucus barrier as one of multiple mechanisms that reduce exposure and responsiveness of the maternal immune system to vaginal antigens during estrus. This mechanism may help to limit the inhibition of anti-paternal CD8+ T cell responses to the brief estrogen-dominated fertile period in each cycle, without interfering with potential anti-pathogen responses at other times and locations. The mucus barrier may also influence the effectiveness of vaginal vaccines administered during difference phases of the menstrual cycle.
METHODS AND MATERIALS
Mice
Six to eight week old mice, C57Bl/6 (B6) (H2b), BALB/c (H2d), and B6 mice transgenic for the membrane bound form of OVA (B6.mOVA) were purchased from the Jackson Laboratory (Bar Harbor, ME), and ovariectomized (OVX) female C57Bl/6 mice from Taconic Labs (Hudson, NY). The anti-Ld 2C and anti-ovalbumin (OVA) OT-I TCR transgenic mice on a C57Bl/6 background were generous gifts from Jonathan Schneck and Nick Crispe, respectively.
Cells, reagents and peptides
All ex vivo work was performed using RPMI (Cellgro, Mediatech, Inc) containing Penicillin-Streptomycin (Cellgro, Mediatech, Inc), L-Glutamine (Cellgro, Mediatech, Inc), 10% FBS (Perbio, HyClone), and 2-β-mercaptoethanol (Sigma-Aldrich). Peptides were obtained from Invitrogen (Carlsbad, CA). The peptide pSYGL (SIYRYYGL) binds to H2Kb and is an alternate specificity of H2Ld-restricted 2C TCR transgenic T cells [14]. The peptide pOVA (SIINFEKL) binds to H2Kb and stimulates OT-I TCR transgenic CD8+ T cells. Naïve CD8+ OT-I T cells (CD8α+, CD90.2+, CD44−, CD62L+) were isolated using a BD FACSAria cell sorter.
Digestion of vagina and ex vivo pulsing of vaginal cells
For Reverse Elispot experiments involving mouse vaginal cells we perfused the vagina with saline, cut it into fragments, then digested with 0.1% collagenase IV (Sigma-Aldrich), 0.01% DNase (Roche), and 200U/ml of hyaluronidase (Sigma-Aldrich) in cell culture medium for 45 minutes at 37°C, 5% CO2. After digestion, cells were passed over a cell strainer to remove tissue debris then counted and either used immediately in Reverse Elispot assays or pulsed with peptide depending on the experiment. For ex vivo pulsing vaginal cells were pulsed with 10μM of pSIINFEKL peptide in 96-well plates in cell culture media for 1.5hrs at 37°C. After pulsing cells were washed 3X then prepared for Reverse Elispot assays.
Antibodies and flow cytometry
Anti-mouse IAb (AF6-120.1), Kb (AF6-88.5), IgG1 (A85-1), CD90.2 (53-2.1) Db (KH95), CD11b (M1/70) were purchased from BD Pharmingen (San Diego, CA). Anti-CD44 (IM7), CD45.1 (A20), CD62L (MEL-14), Vα2 (B20.1), and Vβ5 (MR9-4) were purchased from eBioscience (San Diego, CA), and anti-mouse CD8α (5H10) from Caltag (Burlingame, CA). 7-amino-actinomycin D (7-AAD) was obtained from Calbiochem (La Jolla, CA). The anti-mouse 2C transgenic TCR clonotypic antibody producing hybridoma, clone 1B2, was a generous gift from Jonathan Schneck. All samples were analyzed on a BD LSR II flow cytometer and data processed using FlowJo (Treestar Software, Ashland, Oregon). The 25-D1.16 monoclonal antibody (recognizing the pSIINFEKL/H2Kb complex [15]) was obtained from hybridoma supernatants.
Hormone replacement
Throughout this report we utilize an artificial mouse model system to better help us elucidate the subtle immunoregulation of the female reproductive tract; ovarectomized female mice supplemented with estradiol to induce an estrus-like state as also used by several other investigators [16, 17]. Ovariectomized mice used for intravaginal immunization experiments were injected with either PBS or water soluble cyclodextrin-encapsulated 17β-estradiol (Sigma-Aldrich) at 1μg/mouse/day i.p. in 200μl of PBS. Mice were treated with hormones starting 3 days before immunization and continuing for the duration of the experiment (i.e., mice were treated daily with hormone). To induce estrus in normal mice, B6 female mice were injected with 5 I.U. of pregnant mare serum gonadotropin (PMSG) (Sigma-Aldrich) in 200ul of PBS i.p. 24-36 hours before mating. To induce ovulation, mice treated with PMSG were given 5 I.U. of human gonadotropin (hCG) (Sigma-Aldrich) in 200ul of PBS i.p. at the time of mating (i.e., pairing). Using PMSG and hCG hormones to induce ovulation has been described previously (9). To reduce the possibility of vaginal tissue damage the estrous status was confirmed by observing enlargement of the vaginal labia rather than by taking vaginal smears.
Adoptive transfer of TCR transgenic cells
For the transfer experiment (Fig. 5B) we adoptively transferred whole splenocytes from transgenic mice labeled with 5uM of 5(6)-Carboxyfluorescein diacetate N-succinimidyl ester (CFSE) (Sigma-Aldrich). Five million anti-OVA T cells (OT-1) as whole spleen were transferred into female hosts i.p. in sterile HBSS. OT-I cells express a transgenic TCR that is restricted to the pSIINFEKL peptide in the context of H2Kb.
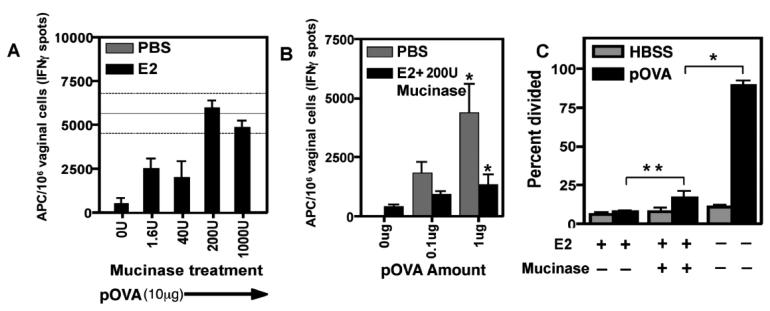
FIGURE 5. Mucinase treatment partially restores both loading of peptide antigen on vaginal APC and anti-paternal CD8+ T cell responses after IVAG immunization.
A, Estradiol-treated mice were immunized intravaginally with 10μg of pOVA peptide and 0U, 1.6U, 40U, 200U, or 1000U of mucinase hyaluronidase. PBS-treated mice were immunized with pOVA but not mucinase. The mean for PBS-treated mice is shown as a solid horizontal line and the confidence interval, for this group, as flanking dashed lines. After 12 hours, APC in vaginal tissues were enumerated by Reverse Elispot using in vitro effector OT-I cells. Antigen loading onto vaginal APC were quantitated by the ability of the extracted APC to stimulate effector T cells to secrete IFNγ. The graph shows mean±SEM; N=3 mice per group. B, Estradiol-treated mice were immunized intravaginally with 0μg, 0.1μg, or 1μg of pOVA peptide with 200U of mucinase. PBS-treated mice were immunized in the absence of mucinase. Reverse Elispot enumerated APC in the vaginal tissue. The graph shows mean±SEM; N=3 per group except 0μg (N=2); * indicates p<0.05. C, CFSE-labeled OT-I cells were adoptively transferred into OVX B6 females, which were treated with PBS or E2 then immunized intravaginally with 10μg of pOVA with or without 200U of mucinase. Three days post-immunization, OT-I proliferation in para-aortic lymph nodes was analyzed by flow cytometry. The graph shows percent divided of gated CD8α+ CD45.1+ Vα2+ Vβ5+ cells as mean±SEM; N=3 mice per group; * indicates p<0.05. Figures A, B, and C were each representative of three independent experiments.
Reverse Elispot assay
This assay has previous been described [2]. Briefly, numbers of APC were measured by titrating different concentrations of APC populations with constant, numbers of TCR transgenic T cells in Elispot assays for IL-2 or IFNγ. T cells only secrete cytokines upon APC stimulation, and so the number of spots counted in represented the number of APC; not the number of T cells. Sorted naïve CD8+ T cells were used to detect APC that could prime naïve T cells – measured by IL-2 secretion, and 5 day in vitro-activated effector CD8+ T cells were used to detect APC that could restimulate effector T cells – measured by IFNγ secretion. Filtration plates with 96 wells (MAIP N4550; Millipore, Bedford, MA) were coated with 2μg/ml of purified anti-mouse IFNγ (AN18), anti-IL-4 (11B11), or anti-IL-2 (JES6-1A12) in 1X PBS. Constant numbers (30,000/well) of CD8+ T cells were added to each well and APCs were titrated across the plate. Cytokines were detected by the addition of 50μl of biotinylated anti-mouse IFNγ (XMG1.2), anti-mouse IL-2 (JES6-5H4), or anti-mouse IL-4 (BVD6-24G2) at 2μg/ml in PBSTB (PBS/0.1% Polyoxyethylene (20) Sorbitan Monolaurate/1% Bovine serum albumin) (Sigma-Aldrich) to each well. Both coating and detection antibodies were purchased from eBioscience. After overnight (16hr) incubation at 37°C, 5% CO2 plates were washed in PBST (1X PBS/0.1% Polyoxyethylene (20) Sorbitan Monolaurate), 1μg/ml of alkaline-phosphatase conjugated streptavidin (Jackson Labs, Bar Harbor, ME) in PBSTB was incubated on the plate for 30 minutes at 25°C, then washed off using PBST. Plates were developed using the alkaline phosphatase substrate kit III (vector laboratories, Burlingame, CA). All assays contained RPMI medium plus 10% FCS. Spots were counted using the Immunospot C.T.L. scanner and counting software (CTL, Cleveland, OH).
Isolation of male reproductive organ cells
Paired reproductive organs (seminal vesicles, vas deferens, epididymis, and testicles) from 6-10 month old B6 or B6.mOVA transgenic males were removed and glass-homogenized to release cells. Cells and debris were pelleted by centrifugation at 10,000xg using a Sorvall RC 26 Plus centrifuge (Sorvall, Asheville, NC). We used 0.02% of combined two testicles suspended in saline and pelleted at a total volume of 10μl in HBSS per female mouse (6-8 weeks old) immunized intravaginally. Age matched males were used for the testicular immunization study and mass differences between different mouse testicles were negligible.
Immunizations and mucinase treatments
Fertility hormone-treated mice were immunized intravaginally (IVAG) using a micropipette to instill 10μl of HBSS solution into the upper cervicovaginal region. Mice were anesthetized for the entire procedure using 2,2,2-Tribromoethanol at 240mg/kg of total body weight. Mucinase treatment was performed using Hyaluronidase VI purified from bovine testis (Sigma-Aldrich) in PBS at 0, 1.6, 40, 200, and 1000 units per mouse, where 1 unit=129ng of enzyme. The mucinase enzyme remained in the vaginal tract for the duration of the experiment.
Histology and fluorescent microscopy
Vaginal tissues were removed, fixed in buffered formaldehyde solution (Mallinckrodt, Phillipsburg, NJ) overnight at 4°C in the dark. The mid-vaginal region was excised from between the cervix and bladder duct and embedded in paraffin wax using a Microm EC 350-1/2 wax embedder [18]. Four-micron sections were cut using a Microm HM 355S microtome and stored at 4°C in the dark until stained. For the staining of tissue sections using the nuclear dye DAPI the tissue sections were first deparafinized as described elsewhere [18]. We used 0.1μM of DAPI nuclear stain (Molecular Probes, Carlsbad, CA) in cell culture media and stained the deparafinized sections for 5 minutes at 25°C in the dark; sections were then washed several times with 1X PBS to remove excess DAPI dye. Slides were cover-slipped using anti-fading aqueous mounting medium (Biomeda, Foster City, CA) and stored at 4°C in the dark until analyzed.
Image collection and analysis
Images were collected on a Nikon Eclipse E600 fluorescent microscope (Tokyo, Japan) using SPOT software (v3.4, 2002). All images in a single channel were exposed for the same length of time: FITC (15 seconds), DAPI (0.5 seconds). Percent stained for the pOVA-FITC peptide was measured by gating regions on tissue images and measuring the total area positive for FITC using the Image Pro Plus software (v3.0, Media Cybernetics, Baltimore, MD). Digital images were overlaid using Adobe Photoshop (v9.0, 2005).
Software and statistical analysis
Significance was determined using the Mann-Whitney, non-parametric test or the unpaired student's t test. Significance was assumed at a p-value < 0.05. Statistical software used was GraphPad Prism (v4.0a, 2003). All studies were repeated at least once.
RESULTS
Functional antigen-presenting vaginal cells are absent in IVAG-immunized, estradiol-treated mice
We have previously shown that estradiol inhibits local CD8+ T cell immune responses to intravaginal immunization [2], but the mechanism of this effect was not identified. Possible mechanisms of FRT-specific inhibition include inhibiting T cell activation in localized lymphoid tissue, inhibiting costimulatory or migratory properties of APC, altering surface expression of MHC molecules, or limiting access of potential APC to luminal antigen.
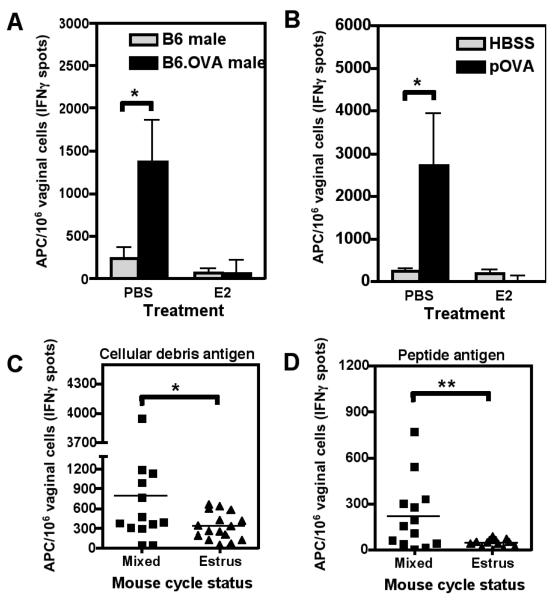
To determine if vaginal cells could process and cross-present antigen from male cells and debris likely to be introduced during coitus, homogenates of male reproductive organs from B6 or B6.mOVA transgenic mice were instilled into the vaginal tract of estradiol-treated or untreated ovariectomized B6 mice. These homogenates were used as a source of antigen expressed by normal mouse tissues, but were not surrogates for the complex array of mediators in semen. After 12 hours, cells were extracted from the vaginal tissue and tested for the ability to re-stimulate effector OT-I TCR transgenic CD8+ T cells to secrete IFNγ in the Reverse Elispot assay. This assay measures the frequency of APC able to stimulate antigen specific, naïve T cells (measured by IL-2 secretion), or antigen specific, effector T cells (measured by IFNγ secretion) (see Methods). The B6.mOVA but not B6 extracts induced substantial numbers of vaginal APC that restimulated OVA-specific OT-I effector T cells (Fig. 1A, *p<0.05) in control mice, but these cells could not be detected in estradiol-treated mice (Fig. 1A). A similar reduction in the numbers of functional vaginal APC by estradiol was observed after immunization with the OVA peptide (SIINFEKL) recognized by OT-I cells (Fig. 1B, *p<0.05).
FIGURE 1. Estradiol prevents the generation of peptide pulsed vaginal APC after intravaginal immunization.
A, PBS or estradiol (E2) treated ovariectomized (OVX) mice were immunized intravaginally (IVAG) with B6 or B6.mOVA male reproductive organ homogenates. After 12 hours, vaginal cells were extracted and analyzed for APC activity in Reverse Elispot assays that measure APC stimulation via the activation of antigen specific effector (measured by IFNγ secretion) or naïve (measured by IL-2 secretion) T cells. The graph shows mean±SEM of the frequency of vaginal APC inducing IFNγ synthesis by effector OT-I T cells. * indicates p<0.05; N=3 mice per group, graph representative of experiment repeated in duplicate. B, PBS or E2 treated OVX mice were immunized IVAG with HBSS alone or pSIINFEKL peptide (pOVA). Twelve hours post-insemination vagina were removed and processed for Reverse Elispots. The graph shows mean±SEM of the frequency of vaginal APC inducing IFNγ secretion by OT-I cells. * indicates p<0.05; N=3 mice per group, graph representative of experiment repeated in triplicate. C and D, Normal B6 female mice were treated with PMSG to induce a high proportion of estrus, and mice in estrus were identified by labial swelling. The ‘mixed’ group contained normal mice from all estrous states. Mice were immunized IVAG with B6.mOVA male reproductive organ homogenates (C) or pSIYRYYGL peptide (D). After 12 hours, vaginal cells were extracted and APC enumerated as in parts A and B, using OT-I (C) or 2C (D) effector T cells. * indicates p<0.05, ** indicates p<0.01 by the unpaired student's t test; N≥13 mice per group.
We used non-ovariectomized mice to confirm results observed in the ovariectomized model (Fig. 1C+D). Normal, cycling, age-matched, B6 female mice, were treated with PMSG and hCG to induce estrus in a high proportion of mice. Normal or estrous mice were immunized IVAG with either B6.mOVA male extracts (Fig. 1C, tested on OT-I effector T cells) or peptide (pSIYRYYGL) (Fig. 1D, tested on 2C effector T cells). Antigen-presenting vaginal cells were present in substantial numbers in some but not all of the normal cycling mice, whereas mice selected to be in estrus gave significantly lower APC numbers from the vagina after IVAG immunization. This confirms that in normal mice, the frequency of functional APC was reduced in the estrous (estradiol-dominated) phase, as also seen in the estradiol-treated ovariectomized mice.
To determine whether estradiol acted by reducing costimulatory function on the APC, we used the anti-OVA T cell hybridoma B3Z stably transfected with LacZ under the NFAT promoter [19] to measure vaginal APC without the need for costimulation. This assay also detected OVA-presenting APC in vaginal cells from PBS- but not estradiol-treated mice (data not shown). Thus estradiol may prevent the uptake, processing or presentation of antigen; reduce the number of potential APC in the vaginal tissue; or convert the APC to a suppressive or non-stimulatory state.
Vaginal APC from estradiol treated mice can stimulate CD8+ T cell responses ex vivo
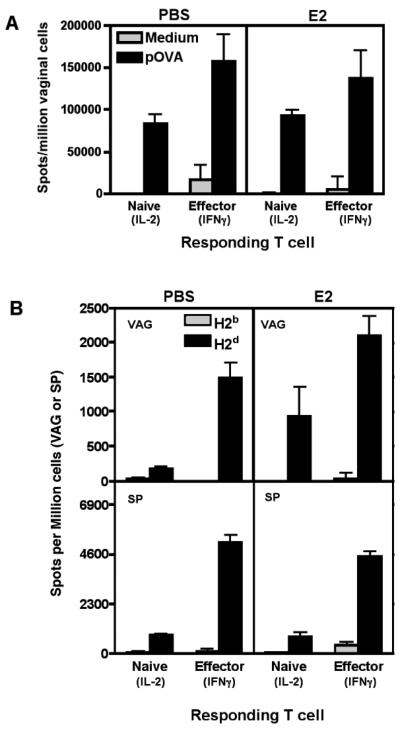
To distinguish between defects in antigen uptake and processing versus other antigen-presenting functions, we pulsed vaginal cells from estradiol- or PBS-treated mice with peptide ex vivo and tested for APC function. In contrast to the results of in vivo peptide administration (Fig. 1), ex vivo-pulsed vaginal cells from both groups of mice presented peptide to either naïve or effector CD8+ T cells (Fig. 2A, p>0.05). To test for the possibility that low in vivo MHC antigen levels were increased during the in vitro antigen pulsing incubation, alloreactive CD8+ T cells (TCR transgenic 2C T cells recognizing H2Ld) were used to detect vaginal APC by the Reverse Elispot assay without the need for exogenous peptide pulsing. Estradiol-treated mice had similar or even higher numbers of vaginal APC than PBS-treated mice, confirming that cells capable of presenting antigen are present in estradiol-treated vaginal tissue (Fig. 2B). The spleen was used as a tissue specific control (Fig. 2B, bottom panel). Estradiol also did not alter MHC class I expression levels on vaginal APC as measured by flow cytometry (data not shown). Thus the reduction of vaginal APC numbers by estradiol may be due to lack of antigen loading, rather than inhibition of APC function or removal from the FRT of cells capable of presenting antigen.
FIGURE 2. Vaginal APC from estradiol-treated mice are functional and can stimulate CD8+ T cell responses ex vivo.
A, Vaginal cells were extracted from PBS or E2 treated mice and pulsed with 10μg/ml of pSIINFEKL (pOVA) peptide or medium alone at 37°C for 1.5 hours. Pulsed cells were analyzed in Reverse Elispot assays containing either sorted naïve (IL-2) or in vitro-activated effector (IFNγ) OT-I cells. Graph shows mean±SEM of the frequency of vaginal APC inducing IFNγ or IL-2 secretion. Graph representative of three experiments. B, Cells were extracted from vaginal tissues (VAG) or spleens (SP) of PBS or E2 treated mice, and analyzed in Reverse Elispot assays using naïve anti-H2d 2C cells (IL-2) or in vitro activated effector anti-H2d 2C cells (IFNγ). Graphs show mean±SEM of the frequency of APC. N=3 mice per group. Graph representative of three independent experiments. Non-significant groups had a p-value greater than 0.05.
The frequency of peptide pulsed cells is reduced in the vagina of estradiol treated mice
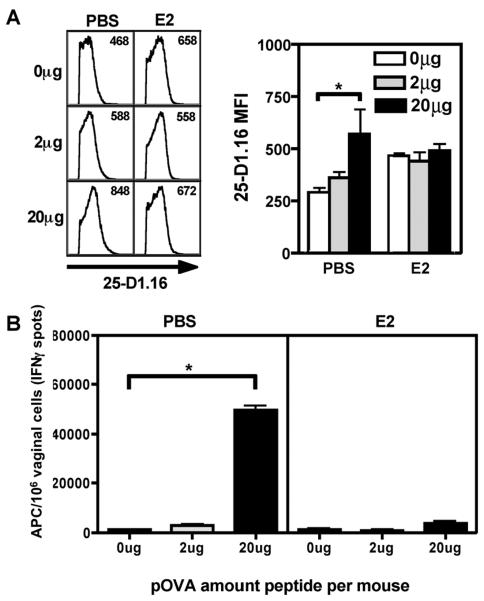
We used the anti-pSIINFEKL/H2Kb antibody (25-D1.16) to track peptide-loaded APCs more directly. Mice were immunized intravaginally with increasing concentrations of pSIINFEKL peptide and after 12 hours vaginal APC from PBS- or estradiol-treated mice were analyzed by flow cytometry. We used anti-CD11b antibody to detect both macrophages and DCs, and anti-H-2b to detect the target MHC antigen for the 25-D1.16 peptide/MHC-specific antibody. In vivo peptide pulsing significantly increased the MFI of 25-D1.16+ cells in the saline group (Fig. 3A, *p<0.05), but not the estradiol-treated group. The higher background in the estradiol-treated group may be due to increased epithelial cell growth and keratinization, which leads to higher auto-fluorescence [20, 21]. The decreased peptide binding in the estradiol-treated group directly correlated with decreased numbers of APC stimulating effector CD8+ T cells ex vivo as enumerated by Reverse Elispot assays (Fig. 3B) further supporting the hypothesis that estradiol reduces vaginal CD8+ T cell priming by limiting APC access to antigen.
FIGURE 3. Estradiol reduced the frequency of in vivo peptide-pulsed vaginal cells.
A, PBS or E2 treated mice were immunized IVAG with pSIINFEKL (pOVA) peptide at 0μg, 2μg, or 20μg per mouse. After 12 hours vaginal cells were extracted and analyzed by flow cytometry using the anti-pSIINFEKL/H2Kb antibody 25-D1.16. Left panel: representative histograms showing 25-D1.16 positive cells gated on live CD11b+ H2Db+ vaginal cells; mean fluorescence intensity (MFI) is shown in the upper right hand corner of each histogram. Right panel: Mean±SEM of MFI from 25-D1.16. * indicates p<0.05; N=3 mice per group. B, The cell populations described in A were analyzed in Reverse Elispot assays using in vitro activated effector OT-I cells. * indicates p<0.05; N=3 mice per group. Graph representative of two independent experiments.
Estradiol modifies the vaginal tissue environment, limiting luminal antigen penetration and subsequent APC loading in vivo
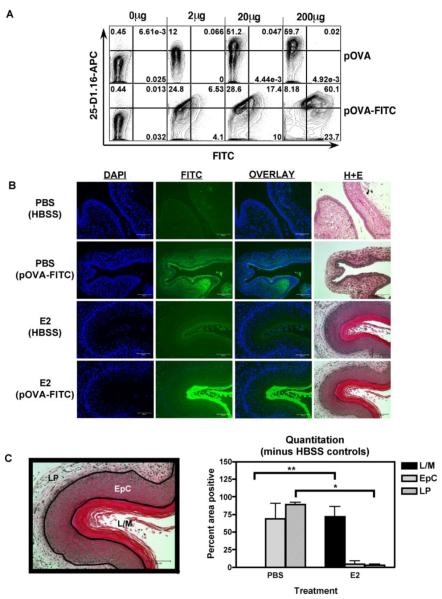
Since our data suggested that estradiol inhibited antigen penetration or loading, we next measured the physical penetration of a peptide antigen into the vaginal tissue using fluorescein-labeled pSIINFEKL OVA peptide. Antigenically, the fluorescein-labeled pOVA peptide behaved similarly to unlabeled peptide, as splenocytes pulsed with the pOVA-FITC peptide were recognized by the 25-D1.16 antibody (Fig. 4A), and pOVA-FITC-pulsed splenocytes were recognized by peptide-specific effector CD8+ cells in Reverse Elispot assays (data not shown).
FIGURE 4. Estradiol inhibited penetration of pOVA-FITC into the vaginal wall.
A, B6 female splenocytes were pulsed with FITC-labeled or unlabeled pSIINFEKL peptide at 0μg, 2μg, 20μg or 200μg/ml in cell culture medium for 1.5hrs, stained with 25-D1.16 antibody, then analyzed by flow cytometry. B, PBS or E2 treated mice were immunized IVAG with pSIINFEKL-FITC peptide at 20μg per mouse or HBSS alone. Twelve hours after immunization vaginal tissues were fixed, embedded, and sectioned. Sequential sections were stained with the nuclear dye DAPI or with haematoxylin and eosin. Exposure times were 0.5 seconds for DAPI and 15 seconds for FITC. Representative image of an experiment repeated twice. C, The percent of the area staining positive for pSIINFEKL-FITC binding (mean±SEM) is shown in the graph, and an example of boundary assignments is shown on the left. N=3 mice per group; * and ** indicates p<0.05; Lumen/mucus (L/M); Epithelial layer (EpC); Lamina propria (LP).
PBS- or estradiol- treated mice were immunized intravaginally with peptide or PBS, and vaginal sections were prepared 12 hours later. Substantial peptide fluorescence was observed in vaginal wall tissue in PBS-treated mice (Fig. 4B). The strong fluorescent peptide signal observed in the lamina propria of PBS-treated mice could be due to inter-cellular channels that lead to the lamina propria [22]. In contrast, in estradiol-treated mice, intense pOVA-FITC peptide staining was visible in the thick mucus barrier, whereas little staining was evident in the vaginal tissue (visualized by nuclear DAPI staining and the sequential H+E sections, Fig. 4B). We quantified the proportion of pOVA-FITC staining of mucus, epithelial layers and lamina propria regions using image analysis software. pOVA-FITC peptide was distributed through most of the epithelial layer and lamina propria in the saline treated mice (Fig. 4C, *p<0.05), but was present only in the thick mucus layer of estradiol-treated mice (Fig. 4C, **p<0.05).
The estradiol-induced mucus barrier decreases antigen penetration and subsequent CD8+ T cell responses
Because of the striking lack of penetration of peptide antigen beyond the mucus barrier, we tested whether the mucus layer was responsible for the estradiol-induced reduction of in vivo peptide pulsing and anti-paternal CD8+ T cell responses. The mucinase, hyaluronidase, was used to remove the mucus barrier in estradiol-treated and control mice immunized intravaginally with pOVA. Mucinase treatment restored the number of in vivo-pulsed APC detectable ex vivo by the Reverse Elispot assay at the highest peptide concentration (Fig. 5A), however, estradiol still partially inhibited peptide pulsing at lower antigen doses (Fig. 5B). To determine if the estradiol-mediated inhibition of in vivo CD8+ T cell proliferation could also be restored by mucus removal, we adoptively transferred CFSE labeled OT-I cells into OVX female B6 mice. After treatment with E2 or PBS for three days, the mice were immunized IVAG with pOVA peptide with or without 200U of mucinase enzyme. Three days post-immunization the proliferation of OT-I cells in the spleen and para-aortic lymph nodes was analyzed by flow cytometry. Mucinase significantly, but only partially, restored the anti-peptide responses in estradiol-treated mice (Fig. 5C, **p<0.05). Thus mucinase enhanced APC presentation of vaginally-administered antigen peptide, and so estradiol-induced mucus appeared to be one mechanism preventing antigen penetration and loading onto APC in the vaginal tissue. The partial restoration suggests that estradiol probably also inhibited peptide loading of APC by additional mechanisms such as the substantial thickening of the epithelial layer in estradiol-treated mice (Figure 4B).
DISCUSSION
In addition to previously-described mechanisms, our results show that estrogen-induced vaginal mucus inhibits antigen penetration and hence immune reactivity against antigens delivered to the vaginal lumen by vaccination, and the same mechanism potentially contributes to the prevention of maternal responses to paternal antigens introduced during insemination.
Using this model, we can extrapolate that estradiol-induced mucus may contribute to the failed initiation of anti-paternal responses during insemination. Due to the extensive degradation of paternally-derived cells after mating, it is likely that maternal exposure to paternal antigens includes live cells, cellular debris and degradation products including peptides. Although mucus is known to block penetration of large particles, such as bacteria, into the vaginal wall, it is more surprising that, given its high water content, mucus also prevents penetration of antigens as small as peptides. Thus vaginal mucus may cause substantial variability in responses to intravaginal vaccination at different stages of the hormonal cycle, and may provides effective barrier for the spectrum of antigens likely to be introduced during insemination.
Our results showing that APC removed from the hormone-controlled environment of the vagina can stimulate mouse CD8+ and CD4+ (not shown) T cells ex vivo is in contrast to the inhibition of T cell proliferation stimulated by rat vaginal cells presenting antigen ex vivo [10, 11]. In addition to the potential differences between species, this discrepancy may also be influenced by the short-term cytokine secretion for antigen presentation used in our studies, compared to the longer-term proliferation assay used in previous work. In addition, proliferation in response to protein antigens may be mainly mediated by CD4+ T cells, whereas most of our experiments focused on CD8+ T cells.
Our demonstration of the role for mucus in inhibiting presentation of even a peptide antigen adds to several other mechanisms that help to reduce infections by pathogens such as HIV transferred during intercourse [23]. Mucus forms a barrier that impedes the penetration of microorganisms, macromolecules, and toxins from entering host tissues. The major components of mucus are mucins, a family of large, heavily glycosylated proteins. The vaginal mucus barrier is maximally developed during the ovulatory and luteal phases, and is initiated by the rising estrogen levels during proliferative and follicular phases. Mucus also contains immunomodulatory properties. Mid-cycle cervical mucus adversely affected the bioactive properties of IL-4 and the immunoglobulins IgA and IgG by decreasing their recovery from aqueous media after contact [24]. Cervical mucus contains antagonists of two key inflammatory cytokines, IL-8 and IL-1, both involved in the recruitment of phagocytic and inflammatory cell types [25]. Thus mucus may regulate immune parameters in the female reproductive tract by both physical and biochemical mechanisms.
Additional functions of estradiol may account for the partial inhibition of antigen presentation and CD8+ T cell activation that remains even after mucinase treatment. For example, estradiol induces the proliferation of vaginal epithelial cells [20] resulting in thickening of the epithelial layer which may reduce antigen penetration to the underlying APC. Estradiol also inhibits APC maturation [26] and APC function [10, 11]; expands T-regulatory cell populations [27]; upregulates the suppressive ligand, PD-1, on several APCs; and reduces expression of both chemokine receptors and T cell migratory activity [28]. In view of all these mechanisms, it is not surprising that there is residual estradiol-mediated inhibition of CD8+ T cell immunization in mucinase-treated animals.
Our studies using vaginal immunization and analysis of vaginal APC are relevant to models of IVAG vaccination, and may also provide information on immune responses against vaginal pathogens at different stages of the hormonal cycle. However, these results are only partially relevant to the regulation of maternal immune responses against paternal antigens delivered during insemination, because in mice the majority of the ejaculate is delivered to the uterine lumen, although a percentage does contact the vaginal wall via the coital plug [29]. Also, semen contains several potent immunomodulators that further modify the maternal response.
Mice normally mate only during estrus, so exposure to paternal antigens normally occurs only during estrogen-induced inhibition of immune priming. Unlike estrus cycling mammals (e.g., rodents), humans can be sexually active throughout the menstrual cycle, although there is a strong preference for mating when fertile [30]. As human semen deposition is therefore not as strongly restricted to the high-estrogen phase of the cycle, additional mechanisms probably prevent anti-paternal responses. Both human and mouse semen contain large quantities of the immunosuppressive cytokine TGFβ, and human semen also contains PGE2 which also suppresses T cell responses [31]. If anti-paternal T cell responses are generated, several mechanisms may prevent anti-fetal attack later during pregnancy [32-34], including restricted antigen presentation [35]. Although unrestricted mating in humans may reduce the importance of antigen blockade by estradiol-induced mucus as a mechanism for lack of responsiveness to paternal antigens, inhibition of antigen penetration by mucus may still contribute to the reduced efficacy of human vaccination during the proliferative phase [5].
Although the levels of estradiol used in our studies result in higher circulating hormone levels than occur in natural estrous cycles, higher levels of systemically administered exogenous estradiol may be required to achieve the levels of hormone in the FRT that are provided by local production. The exogenous levels in our study are similar to those used in other studies of the effect of reproductive hormones on the immune system [16, 17]. Furthermore, the experiments in Figure 1C, D also show a reduction in vaginal wall APC numbers in mice selected for being in the estrous phase after hormone induction, thus reinforcing our hypothesis that estradiol can reduce antigen presentation during normal estrous cycles.
We propose that access to antigen deposited in the vaginal tract is partially restricted by an estradiol-induced mucus barrier that lines the vaginal lumen during the estrous phase in mice. This physical barrier may contribute to immunological ignorance to paternal antigens, complementing the immunoregulatory components in the seminal fluid [1] and the female reproductive tract [36] that further enhance maternal tolerance or ignorance to paternal antigens during insemination [5, 6]. These data may have implications for the design of intravaginal immunization strategies attempting to induce efficient cell mediated and humoral immunity in the reproductive tract, such as vaccines for sexually transmitted pathogens.
ACKNOWLEDGMENTS
We thank James Kobie, Stephen Dewhurst, Andrea Sant and Jim Miller for reviewing this manuscript; Nathan Laniewski and James Kobie for cell sorting help; Deb Fowell for the use of her Fluorescent microscope; Edith Lord for the B3Z clone; Nick Crispe for the 25-D1.16 hybridoma and OT-I mice, and Jonathan Schneck for the 2C TCR transgenic mice.
Abbreviations used in this paper
- FRT
female reproductive tract
- OVX
ovariectomized
- IVAG
intravaginal
- DC
dendritic cell
- E2
17β-estradiol
Footnotes
DISCLOSURES
The authors have no financial conflict of interest.
REFERENCES
- 1.Robertson SA, Sharkey DJ. The role of semen in induction of maternal immune tolerance to pregnancy. Semin Immunol. 2001;13(4):243–54. doi: 10.1006/smim.2000.0320. [DOI] [PubMed] [Google Scholar]
- 2.Seavey MM, Mosmann TR. Paternal antigen-bearing cells transferred during insemination do not stimulate anti-paternal CD8+ T cells: role of estradiol in locally inhibiting CD8+ T cell responses. J Immunol. 2006;177(11):7567–78. doi: 10.4049/jimmunol.177.11.7567. [DOI] [PubMed] [Google Scholar]
- 3.Robertson SA. Control of the immunological environment of the uterus. Rev Reprod. 2000;5(3):164–74. doi: 10.1530/ror.0.0050164. [DOI] [PubMed] [Google Scholar]
- 4.Rogers AM, Boime I, Connolly J, Cook JR, Russell JH. Maternal-fetal tolerance is maintained despite transgene-driven trophoblast expression of MHC class I, and defects in Fas and its ligand. Eur J Immunol. 1998;28(11):3479–87. doi: 10.1002/(SICI)1521-4141(199811)28:11<3479::AID-IMMU3479>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 5.Kozlowski PA, Williams SB, Lynch RM, Flanigan TP, Patterson RR, Cu-Uvin S, et al. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J Immunol. 2002;169(1):566–74. doi: 10.4049/jimmunol.169.1.566. [DOI] [PubMed] [Google Scholar]
- 6.Black CA, Rohan LC, Cost M, Watkins SC, Draviam R, Alber S, et al. Vaginal mucosa serves as an inductive site for tolerance. J Immunol. 2000;165(9):5077–83. doi: 10.4049/jimmunol.165.9.5077. [DOI] [PubMed] [Google Scholar]
- 7.Gockel CM, Bao S, Holland MK, Beagley KW. Influence of the murine oestrous cycle on the induction of mucosal immunity. Am J Reprod Immunol. 2003;50(5):369–79. doi: 10.1034/j.1600-0897.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- 8.Haas GG, Jr., Nicosia SV, Wolf DP. Influence of estrogens on vascular transudation and mucus production in the rabbit endocervix. Fertil Steril. 1987;48(6):1036–42. doi: 10.1016/s0015-0282(16)59605-8. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, et al. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J Biol Chem. 2006;281(36):26683–92. doi: 10.1074/jbc.M601522200. [DOI] [PubMed] [Google Scholar]
- 10.Wira CR, Rossoll RM. Antigen-presenting cells in the female reproductive tract: influence of sex hormones on antigen presentation in the vagina. Immunology. 1995;84(4):505–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Wira CR, Roche MA, Rossoll RM. Antigen presentation by vaginal cells: role of TGFbeta as a mediator of estradiol inhibition of antigen presentation. Endocrinology. 2002;143(8):2872–9. doi: 10.1210/endo.143.8.8938. [DOI] [PubMed] [Google Scholar]
- 12.Wira CR, Rossoll RM. Oestradiol regulation of antigen presentation by uterine stromal cells: role of transforming growth factor-beta production by epithelial cells in mediating antigen-presenting cell function. Immunology. 2003;109(3):398–406. doi: 10.1046/j.1365-2567.2003.01670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prieto GA, Rosenstein Y. Oestradiol potentiates the suppressive function of human CD4 CD25 regulatory T cells by promoting their proliferation. Immunology. 2006;118(1):58–65. doi: 10.1111/j.1365-2567.2006.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Eisen HN, Kranz DM. A model T-cell receptor system for studying memory T-cell development. Microbes Infect. 2003;5(3):233–40. doi: 10.1016/s1286-4579(03)00016-9. [DOI] [PubMed] [Google Scholar]
- 15.Mareeva T, Lebedeva T, Anikeeva N, Manser T, Sykulev Y. Antibody specific for the peptide.major histocompatibility complex. Is it T cell receptor-like? J Biol Chem. 2004;279(43):44243–9. doi: 10.1074/jbc.M407021200. [DOI] [PubMed] [Google Scholar]
- 16.Charles R, Wira MAC-G, Grant Katherine S. Endocrine Regulation of the Mucosal Immune System in the Female Reproductive Tract. In: Mestecky MELaF., editor. Mucosal Immunology. Third Edition ed. Elsevier Academic Press; Burlington, MA 01803, USA: 2005. pp. 1661–75. [Google Scholar]
- 17.Bhavanam S, Snider DP, Kaushic C. Intranasal and subcutaneous immunization under the effect of estradiol leads to better protection against genital HSV-2 challenge compared to progesterone. Vaccine. 2008 doi: 10.1016/j.vaccine.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 18.Wira CR, Rossoll RM, Kaushic C. Antigen-presenting cells in the female reproductive tract: influence of estradiol on antigen presentation by vaginal cells. Endocrinology. 2000;141(8):2877–85. doi: 10.1210/endo.141.8.7594. [DOI] [PubMed] [Google Scholar]
- 19.Sanderson S, Shastri N. LacZ inducible, antigen/MHC-specific T cell hybrids. Int Immunol. 1994;6(3):369–76. doi: 10.1093/intimm/6.3.369. [DOI] [PubMed] [Google Scholar]
- 20.Inada K, Hayashi S, Iguchi T, Sato T. Establishment of a primary culture model of mouse uterine and vaginal stroma for studying in vitro estrogen effects. Exp Biol Med (Maywood) 2006;231(3):303–10. doi: 10.1177/153537020623100310. [DOI] [PubMed] [Google Scholar]
- 21.Hisano N. Effects on hamster vaginal development of a single dose of testosterone or estradiol given neonatally. Acta Anat (Basel) 1977;97(4):361–70. doi: 10.1159/000144755. [DOI] [PubMed] [Google Scholar]
- 22.King BF. Ultrastructure of the nonhuman primate vaginal mucosa: epithelial changes during the menstrual cycle and pregnancy. J Ultrastruct Res. 1983;82(1):1–18. doi: 10.1016/s0022-5320(83)90092-8. [DOI] [PubMed] [Google Scholar]
- 23.Lederman MM, Offord RE, Hartley O. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat Rev Immunol. 2006;6(5):371–82. doi: 10.1038/nri1848. [DOI] [PubMed] [Google Scholar]
- 24.Ginsburg KA, Wolf NA, Fidel PL. Potential effects of midcycle cervical mucus on mediators of immune reactivity. Fertil Steril. 1997;67(1):46–50. doi: 10.1016/s0015-0282(97)81854-7. [DOI] [PubMed] [Google Scholar]
- 25.Kataranovski M, Radojcic L, Prokic V, Vojvodic D. Presence of interleukin-8 and the IL-1 receptor antagonist in the cervical mucus of fertile and infertile women. Vojnosanit Pregl. 2004;61(4):359–64. doi: 10.2298/vsp0404359k. [DOI] [PubMed] [Google Scholar]
- 26.Nalbandian G, Paharkova-Vatchkova V, Mao A, Nale S, Kovats S. The selective estrogen receptor modulators, tamoxifen and raloxifene, impair dendritic cell differentiation and activation. J Immunol. 2005;175(4):2666–75. doi: 10.4049/jimmunol.175.4.2666. [DOI] [PubMed] [Google Scholar]
- 27.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, et al. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. 2004;173(4):2227–30. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 28.Lang TJ. Estrogen as an immunomodulator. Clin Immunol. 2004;113(3):224–30. doi: 10.1016/j.clim.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Carballada R, Esponda P. Structure of the vaginal plugs generated by normal rats and by rats with partially removed seminal vesicles. J Exp Zool. 1993;265(1):61–8. doi: 10.1002/jez.1402650109. [DOI] [PubMed] [Google Scholar]
- 30.Wilcox AJ, Baird DD, Dunson DB, McConnaughey DR, Kesner JS, Weinberg CR. On the frequency of intercourse around ovulation: evidence for biological influences. Hum Reprod. 2004;19(7):1539–43. doi: 10.1093/humrep/deh305. [DOI] [PubMed] [Google Scholar]
- 31.Choudhry MA, Hockberger PE, Sayeed MM. PGE2 suppresses mitogen-induced Ca2+ mobilization in T cells. Am J Physiol. 1999;277(6 Pt 2):R1741–8. doi: 10.1152/ajpregu.1999.277.6.R1741. [DOI] [PubMed] [Google Scholar]
- 32.Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270(5236):630–3. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 33.Jiang SP, Vacchio MS. Multiple mechanisms of peripheral T cell tolerance to the fetal "allograft". J Immunol. 1998;160(7):3086–90. [PubMed] [Google Scholar]
- 34.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 35.Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117(5):1399–411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206(1):306–35. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]