Abstract
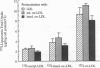
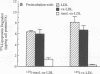
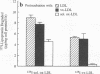
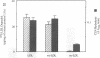
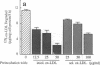
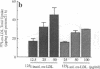
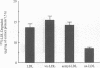
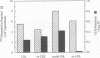
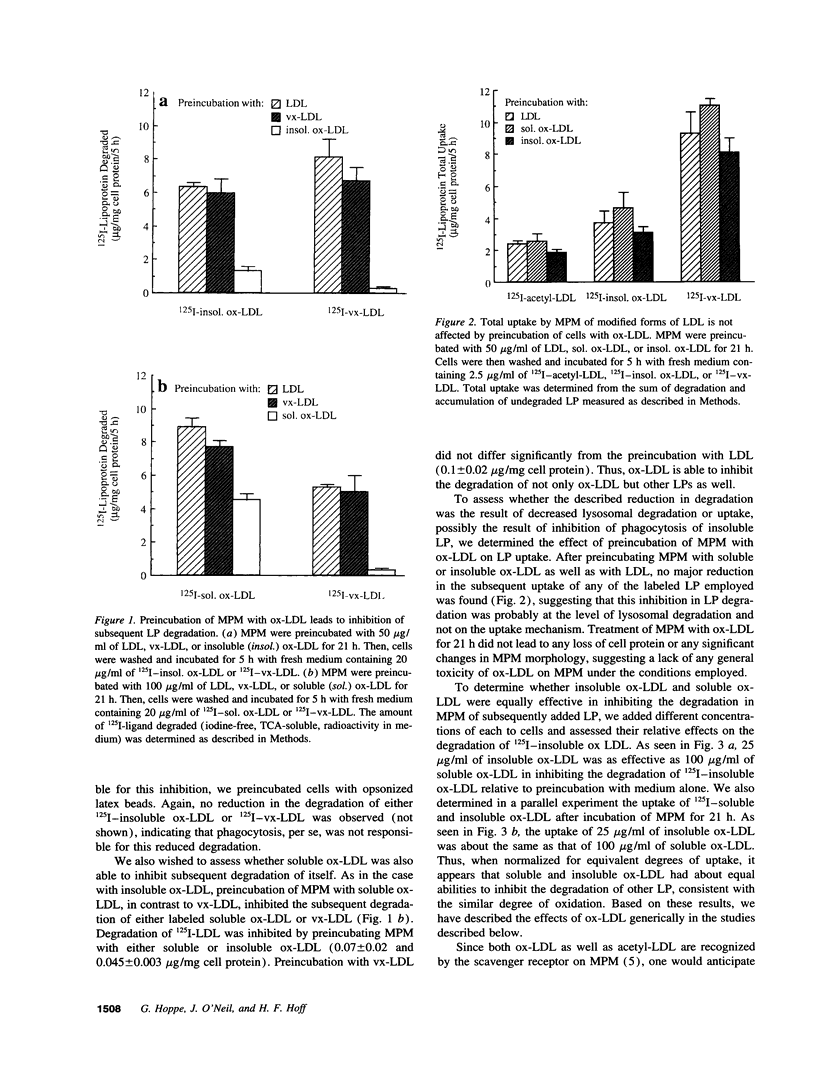
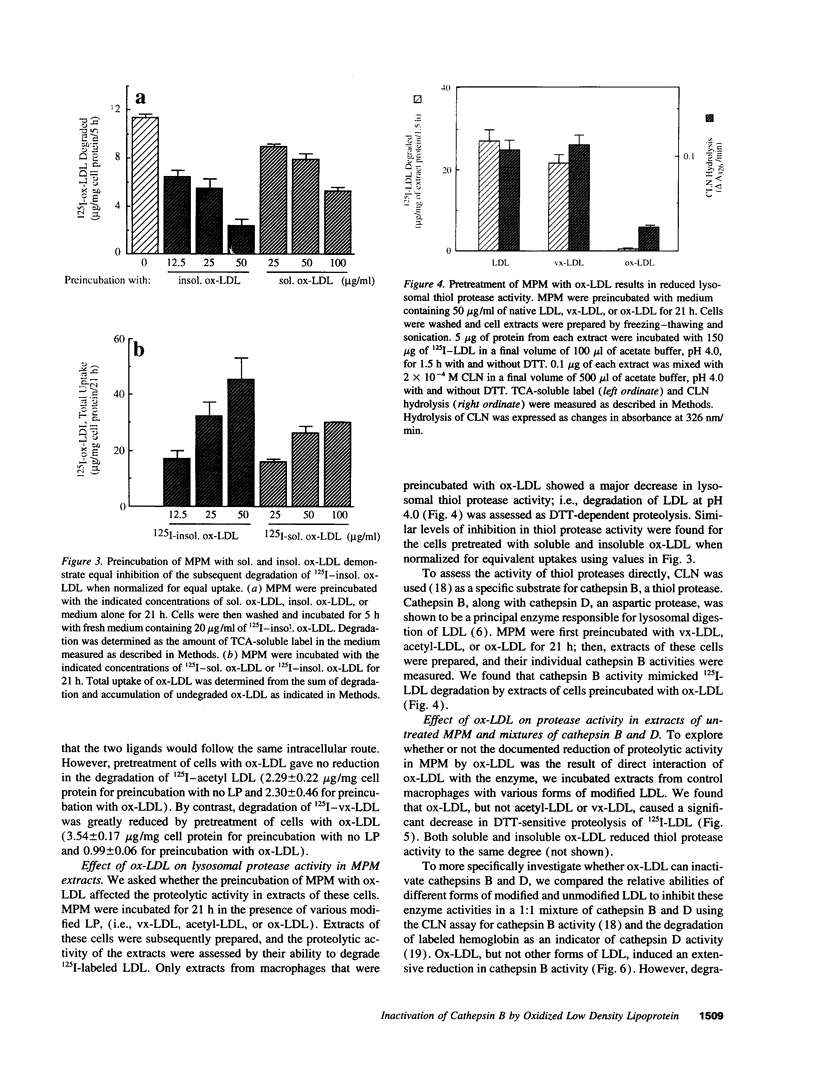
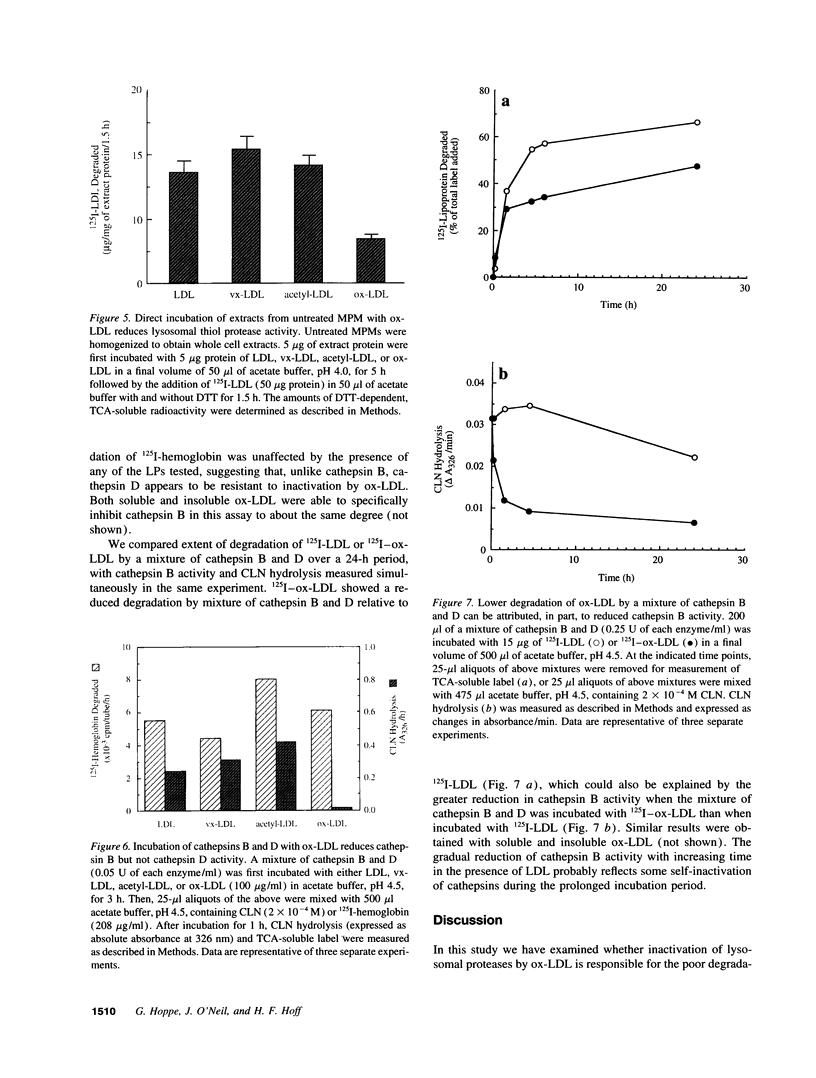
Deficient processing of apo B in oxidized LDL (ox-LDL) by macrophage lysosomal proteases has been documented and attributed to modifications in apo B. We have investigated whether direct inactivation of lysosomal proteases by ox-LDL could also be responsible for this deficient degradation. When mouse peritoneal macrophages (MPM) were preincubated for 21 h at 37 degrees C with ox-LDL, LDL, or vortex-aggregated LDL, only ox-LDL inhibited the subsequent degradation of 125I-labeled forms of the above lipoproteins. Uptake of labeled lipoproteins was not appreciably affected by preincubation with ox-LDL, suggesting that the inhibition was at the level of lysosomal degradation. Thiol protease activity of cell extracts at pH 4.0, was reduced in MPM preincubated with ox-LDL relative to cells preincubated with LDL or medium alone. Extracts from untreated MPM, or mixtures of cathepsin B and D, showed a reduced ability to degrade 125I-LDL at pH 4.5 and reduced cathepsin B activity, after incubation with ox-LDL relative to incubation with LDL. Thus, the reduced degradation of lipoproteins in MPM pretreated with ox-LDL could be due to direct inactivation of the lysosomal protease, cathepsin B.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Kita T., Yokode M., Narumiya S., Kawai C. Multiple receptors for modified low density lipoproteins in mouse peritoneal macrophages: different uptake mechanisms for acetylated and oxidized low density lipoproteins. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1375–1382. doi: 10.1016/0006-291x(89)92262-6. [DOI] [PubMed] [Google Scholar]
- Bajkowski A. S., Frankfater A. Specific spectrophotometric assays for cathepsin B1. Anal Biochem. 1975 Sep;68(1):119–127. doi: 10.1016/0003-2697(75)90685-5. [DOI] [PubMed] [Google Scholar]
- Ball R. Y., Bindman J. P., Carpenter K. L., Mitchinson M. J. Oxidized low density lipoprotein induces ceroid accumulation by murine peritoneal macrophages in vitro. Atherosclerosis. 1986 May;60(2):173–181. doi: 10.1016/0021-9150(86)90009-2. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Schaur R. J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff H. F., O'Neil J., Chisolm G. M., 3rd, Cole T. B., Quehenberger O., Esterbauer H., Jürgens G. Modification of low density lipoprotein with 4-hydroxynonenal induces uptake by macrophages. Arteriosclerosis. 1989 Jul-Aug;9(4):538–549. doi: 10.1161/01.atv.9.4.538. [DOI] [PubMed] [Google Scholar]
- Hoff H. F., Whitaker T. E., O'Neil J. Oxidation of low density lipoprotein leads to particle aggregation and altered macrophage recognition. J Biol Chem. 1992 Jan 5;267(1):602–609. [PubMed] [Google Scholar]
- Hoff H. F., Zyromski N., Armstrong D., O'Neil J. Aggregation as well as chemical modification of LDL during oxidation is responsible for poor processing in macrophages. J Lipid Res. 1993 Nov;34(11):1919–1929. [PubMed] [Google Scholar]
- Jessup W., Mander E. L., Dean R. T. The intracellular storage and turnover of apolipoprotein B of oxidized LDL in macrophages. Biochim Biophys Acta. 1992 Jun 22;1126(2):167–177. doi: 10.1016/0005-2760(92)90287-6. [DOI] [PubMed] [Google Scholar]
- Khoo J. C., Miller E., McLoughlin P., Steinberg D. Enhanced macrophage uptake of low density lipoprotein after self-aggregation. Arteriosclerosis. 1988 Jul-Aug;8(4):348–358. doi: 10.1161/01.atv.8.4.348. [DOI] [PubMed] [Google Scholar]
- Lougheed M., Zhang H. F., Steinbrecher U. P. Oxidized low density lipoprotein is resistant to cathepsins and accumulates within macrophages. J Biol Chem. 1991 Aug 5;266(22):14519–14525. [PubMed] [Google Scholar]
- Roma P., Bernini F., Fogliatto R., Bertulli S. M., Negri S., Fumagalli R., Catapano A. L. Defective catabolism of oxidized LDL by J774 murine macrophages. J Lipid Res. 1992 Jun;33(6):819–829. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sparrow C. P., Parthasarathy S., Steinberg D. A macrophage receptor that recognizes oxidized low density lipoprotein but not acetylated low density lipoprotein. J Biol Chem. 1989 Feb 15;264(5):2599–2604. [PubMed] [Google Scholar]
- Steinbrecher U. P., Zhang H. F., Lougheed M. Role of oxidatively modified LDL in atherosclerosis. Free Radic Biol Med. 1990;9(2):155–168. doi: 10.1016/0891-5849(90)90119-4. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tang J. Cathepsin D from porcine and bovine spleen. Methods Enzymol. 1981;80(Pt 100):565–581. doi: 10.1016/s0076-6879(81)80045-6. [DOI] [PubMed] [Google Scholar]
- Uchida K., Stadtman E. R. Covalent attachment of 4-hydroxynonenal to glyceraldehyde-3-phosphate dehydrogenase. A possible involvement of intra- and intermolecular cross-linking reaction. J Biol Chem. 1993 Mar 25;268(9):6388–6393. [PubMed] [Google Scholar]
- Uchida K., Stadtman E. R. Selective cleavage of thioether linkage in proteins modified with 4-hydroxynonenal. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5611–5615. doi: 10.1073/pnas.89.12.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witztum J. L., Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991 Dec;88(6):1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]