Abstract
The recently reported modest success of the RV144 Thai trial vaccine regimen in preventing HIV-1 acquisition has focused interest on the potential contribution to that protection of vaccine-elicited CD4+ T cell responses. We evaluated the induction of virus-specific CD4+ T cell responses in rhesus monkeys using a series of diverse vaccine vectors. We assessed both the magnitudes and functional profiles of the antigen-specific CD4+ T cells by measuring cytokine production, memory differentiation, and the expression of mucosal homing molecules. We found that DNA prime/recombinant MVA boost immunizations induced particularly high frequency virus-specific CD4+ T cell responses with polyfunctional repertoires, and these responses were partially preserved following SHIV-89.6P challenge. The majority of the vaccine-elicited CD4+ T cells were CD28+ memory T cells that expressed low levels of β7. Neither the magnitudes nor the functional profiles of the virus-specific CD4+ T cells generated by vaccination were associated with a preservation of CD4+ T cells or control of viral replication following SHIV-89.6P challenge. Interestingly, monkeys primed with recombinant Ad5 immunogens showed a dramatic expansion of both the magnitude and polyfunctionality of the vaccine-elicited CD4+ T cell responses following envelope protein boost. These results demonstrate that vaccine strategies that include recombinant MVA or recombinant Ad5 vectors can elicit robust CD4+ T cell responses.
Keywords: HIV-vaccine, CD4+ T cell responses, Rhesus monkey, SHIV-89.6P
INTRODUCTION
The findings in the recently reported HIV-1 vaccine trial in Thailand (RV144) have refocused interest on vaccine-elicited CD4+ T cell and antibody responses. In this human vaccine trial, a recombinant canarypox priming immunization followed by an envelope protein boosting immunization generated modest protection against the acquisition of an HIV-1 infection (Rerks-Ngarm et al., 2009). Since this vaccine regimen elicited CD4+ T cell and antibody responses but not CD8+ T cell responses, attention is turning to the evaluation of vaccine strategies that induce robust and durable responses of these types.
Virus-specific CD4+ T cells play a central role in the immune containment of HIV-1. They contribute to HIV-1 clearance both by providing help for B cell responses and by maintaining effective cytotoxic T lymphocytes (CTLs) (Kalams and Walker, 1998). Functional CD4+ T cells are also required at the time of immune priming for the normal development of memory CD8+ T cells (Janssen et al., 2003; Shedlock and Shen, 2003). It is therefore important to explore vaccine strategies for eliciting antigen-specific CD4+ T cell responses.
While the evaluation of HIV-1 vaccine candidates has, for the most part, relied on determining the magnitude of vaccine-elicited T lymphocyte IFN-γ responses, emerging data are indicating that different vaccine modalities can induce cellular immune responses that are qualitatively very different (Casimiro et al., 2003; Cox et al., 2008; Hansen et al., 2009; Hovav et al., 2007; Sun et al., 2008). For example, some vaccine modalities bias T cells to central memory and others to effector memory responses. Moreover, different vaccine platforms induce T lymphocyte responses with different functional profiles. It will be important to evaluate diverse characteristics of vaccine-induced CD4+ T cell responses.
The present study was initiated to explore quantitative and qualitative differences in the virus-specific CD4+ T cell responses generated in rhesus monkeys using different vaccine modalities. We had the opportunity to carry out an evaluation of these immune responses elicited by different vaccine vectors expressing the same HIV and SIV gene inserts.
RESULTS
Study design
The immunization and challenge schedule for each experimental group of monkeys is summarized in Table 1 (Letvin et al., 2004; Letvin et al., 2006; Santra et al., 2004; Santra et al., 2009; Santra et al., 2007; Seaman et al., 2005). In the homologous prime-boost experimental groups, 6 monkeys received 1012 particles of rAd5 as a priming immunization at weeks 0 and 8, followed by a homologous rAd5 boost at week 26. Six monkeys received 109 pfu of recombinant MVA at weeks 0 and 8, followed by homologous boosting immunizations at weeks 26 and 43. Another 4 monkeys received 5 mg plasmid DNA at weeks 0, 4, and 8, followed by a homologous DNA plasmid boosting immunization at week 42. In the heterologous DNA-rAd5 experimental group, 17 monkeys received 4 mg inoculations of plasmid DNA at weeks 0, 4, and 8 followed by a heterologous rAd5 boosting immunization at week 26. Twelve monkeys in the DNA-rMVA experimental group received 5 mg inoculations of plasmid DNA at weeks 0, 4, and 8 followed by a heterologous recombinant MVA boosting immunization at week 42. Six monkeys in the rAd5-protein group received 1011 particles of rAd5 as a priming immunization at weeks 0 and 3, followed by an HIV-1 envelope protein boost (SF162 gp140 delta V2) at week 39. At 12 weeks (DNA-rAd5), 18 weeks (DNA-rMVA, DNA-DNA), 21 weeks (rMVA-rMVA) or 22 weeks (rAd5-rAd5) after the final immunizations, five groups of experimental and 14 control monkeys were challenged intravenously with 50 50% monkey infectious doses of pathogenic SHIV-89.6P from the same virus stock.
Table 1.
Immunization and challenge schedule
| Group | Priming immunization | Boosting immunization | SHIV-89.6P challenge | Reference |
|---|---|---|---|---|
| rAd5-rAd5 (n = 6) | rAd5, 1012 particles; weeks 0 and 8 | rAd5, 1012 particles; week 26 | week 48 | Santra et al., 2009 |
| rMVA-rMVA (n = 6) | rMVA, 109 pfu; weeks 0 and 8 | rMVA, 109 pfu; weeks 26 and 43 | week 64 | Santra et al., 2007 |
| DNA-DNA (n = 4) | DNA, 5 mg; weeks 0, 4 and 8 IL-2/Ig plasmid, 5 mg; weeks 0 and 4 |
DNA, 5 mg; week 42 | week 60 | Santra et al., 2004 |
| DNA-rAd5 (n = 17) | DNA, 4 mg; weeks 0, 4 and 8 | rAd5, 1012 particles; week 26 | week 38 |
Letvin et al., 2004
Letvin et al., 2006 |
| DNA-rMVA (n = 12) | DNA, 5 mg; weeks 0, 4 and 8 IL-2/Ig plasmid, 5 mg; weeks 0 and 4 |
rMVA, 109 pfu; week 42 | week 60 | Santra et al., 2004 |
| rAd5-protein (n = 6) | rAd5, 1011 particles; weeks 0 and 3 | HIV-1 SF162 gp140, 100 μg adjuvanted with MF59; week 39 | – | – |
Virus-specific CD4+ T cell responses following vaccination and following SHIV-89.6P challenge
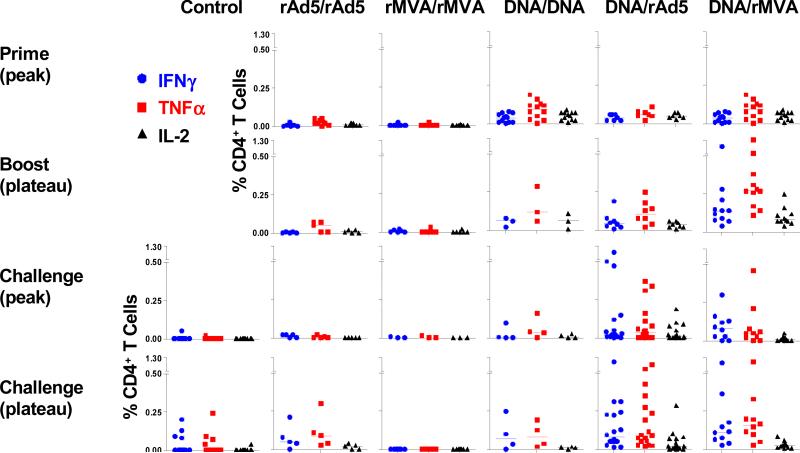
We first sought to characterize the virus-specific CD4+ T cell responses elicited by various well-established vaccine vectors. PBMCs from monkeys receiving homologous or heterologous vaccination regimens were isolated at two weeks following priming immunization (prime, peak), 12 weeks following boosting immunization (boost, plateau), two weeks following challenge (challenge, peak), and 12 weeks following challenge (challenge, plateau). PBMCs were exposed to pools of overlapping peptides spanning the SIV Gag or HIV-1 Env protein, and the fractions of CD4+ T cells producing IFN-γ, TNF-α, or IL-2 were determined by intracellular cytokine staining (Fig. 1).
Figure 1. Antigen-specific CD4+ T cell responses following vaccination and following SHIV-89.6P challenge.
Peripheral blood lymphocytes from monkeys receiving homologous (rAd5/rAd5, rMVA/rMVA, DNA/DNA) or heterologous vaccination regimens (DNA/rAd5, DNA/rMVA) were exposed to pools of overlapping peptides spanning the SIV Gag or HIV-1 Env protein and the fractions of CD4+ T cells producing IFN-γ, TNF-α, or IL-2 are shown in the upper two panels. All experimental and 14 control monkeys were then challenged with SHIV-89.6P, and the antigen-specific CD4+ T cell responses that developed in these monkeys following challenge are shown in the lower two panels.
Very few virus-specific CD4+ T cells were detected in monkeys that received a rAd5 or rMVA priming immunization. In contrast, monkeys primed with plasmid DNA developed substantial virus-specific CD4+ T cell responses that could be measured by IFN-γ, TNF-α or IL-2 production. Homologous rAd5, rMVA, or DNA boosting elicited no further increase in the magnitude of the virus-specific CD4+ T cell responses. However, the monkeys that received DNA immunizations developed a dramatic expansion of their virus-specific CD4+ T cell responses following a heterologous rMVA boosting. A substantial expansion of virus-specific CD4+ T cell responses was also seen after heterologous rAd5 boosting. Of the vaccine regimens that we evaluated, DNA prime, heterologous rMVA boost immunization elicited the highest frequency virus-specific CD4+ T cell responses.
We then sought to evaluate whether these different vaccination regimens affected the magnitude of the CD4+ T cell responses that developed in these monkeys following a SHIV challenge (Fig. 1, lower two panels). The virus-specific CD4+ T cell responses of these groups of vaccinated monkeys fell two weeks after challenge, consistent with the well described loss of naïve CD4+ T cells during the first two weeks after SHIV-89.6P infection (Nishimura et al., 2005). However, these virus-specific CD4+ T cell responses returned to baseline levels 12 weeks following SHIV-89.6P challenge. As expected, the control monkeys only generated low frequency virus-specific CD4+ T cell responses following challenge. Twelve weeks following SHIV-89.6P challenge, highly significant differences were found between the magnitudes of the CD4+ T cell responses in the control monkeys and the groups of monkeys receiving DNA prime/recombinant viral vector boost vaccinations (Kruskal-Wallis tests, P < 0.01).
Functional profiles of virus-specific CD4+ T cells following vaccination and following SHIV-89.6P challenge
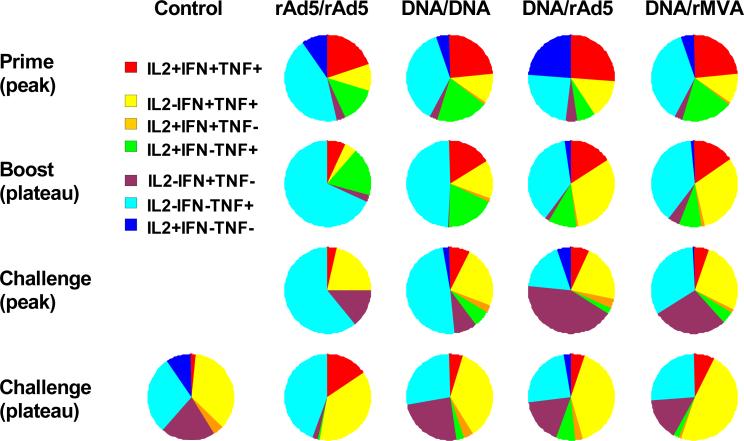
We previously showed that heterologous prime-boost vaccine regimens induced high frequency virus-specific CD8+ T cell responses with polyfunctional repertoires. Moreover, the functional profiles of the vaccine-induced virus-specific CD8+ T cells were associated with control of viral replication following SHIV-89.6P challenge (Sun et al., 2008). We wanted to determine whether these different vaccine strategies induced qualitatively different virus-specific CD4+ T cell responses. Total virus-specific cytokine-producing CD4+ T cells were divided into 7 distinct populations based on their production of IFN-γ, TNF-α, and IL-2, either individually or in any combination. The functional profiles of the vaccine-induced CD4+ T cells are shown by expressing each type of cytokine response as a proportion of the total response. The mean values for the animals in each experimentally vaccinated group are shown in a series of pie charts (Fig. 2).
Figure 2. Cytokine profiles of antigen-specific CD4+ T cells following vaccination and following SHIV-89.6P challenge.
PBL isolated from the different cohorts of rhesus monkeys at the indicated times following vaccination and following virus challenge were exposed to pools of overlapping peptides spanning the Gag or Env proteins and cytokine production was measured by intracellular cytokine staining. Antigen-specific cytokine-producing CD4+ T cells were divided into seven distinct populations based on their production of IFN-γ, TNF-α, and IL-2 either individually or in any combination. Cytokine profiles were determined by expressing each cytokine response as a proportion of the total antigen-specific cytokine-producing CD4+ T cell response and data are presented as the mean values from each experimental group in a pie chart.
Monkeys primed with rAd5 or DNA developed polyfunctional virus-specific CD4+ T cell responses that were predominantly cytokine triple positive or cytokine double positive (IFN-γ+TNF-α+IL-2- and IFN-γ-TNF-α+IL-2+). Homologous or heterologous boosting did not further expand the representation of polyfunctional virus-specific CD4+ T cell responses. In addition, the cytokine profiles of the virus-specific CD4+ T cells of these groups of vaccinated monkeys were indistinguishable twelve weeks following SHIV-89.6P challenge, with most cells being IL-2-IFN-γ+TNF-α+ (Fig. 2, lower two panels). Moreover, no significant differences were found between the control and vaccinated groups in the representation of polyfunctional virus-specific CD4+ T cells observed following SHIV-89.6P challenge.
Expression of memory- and mucosal homing-associated molecules on virus-specific CD4+ T cells following vaccination and following challenge
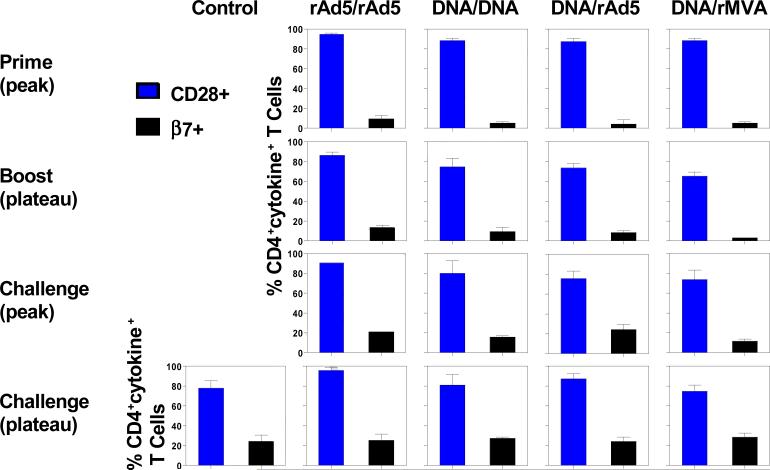
Having failed to detect a qualitative difference in the cytokine profiles of the virus-specific CD4+ T cell elicited by these various vaccine regimens, we sought to determine whether these different vaccine regimens induced qualitatively different virus-specific CD4+ T cell responses as measured by β7 and CD28 expression (Fig. 3). β7 integrins are expressed on mucosal lymphocytes and mediate lymphocyte trafficking to and retention in mucosal tissues (Gorfu, Rivera-Nieves, and Ley, 2009). We therefore evaluated the expression of β7 on virus-specific CD4+ T cells following vaccination and following challenge in these cohorts of monkeys. Few virus-specific CD4+ T cells expressed β7 after rAd5 or DNA priming. Homologous or heterologous boost immunization did not expand the proportion of β7+ virus-specific CD4+ T cells. Therefore, virus-specific CD4+ T cells elicited by vaccination expressed low levels of this mucosal homing molecule. However, approximately 20-30% of the virus-specific CD4+ T cells began to express β7 after SHIV-89.6P challenge, and no significant differences were detected between the control and vaccinated animals. A detailed phenotypic analysis was also done to assess the memory differentiation of these virus-specific CD4+ T cells. Virus-specific CD4+ T cells were divided into central and effector memory cells based on their expression of CD28 (Pitcher et al., 2002). Interestingly, virus-specific CD4+ T cells were predominantly CD28+ memory cells, and no significant differences were detected in the relative representation of these CD28+CD4+ T cells between the different groups of animals following vaccination and following challenge.
Figure 3. Expression of memory- and mucosal homing-associated molecules on antigen-specific CD4+ T cells following vaccination and following challenge.
PBL isolated from the different cohorts of rhesus monkeys at the indicated times following vaccination or challenge were exposed to pools of overlapping peptides spanning the Gag or Env proteins, and the fractions of CD4+ T cells producing IFN-γ, TNF-α, or IL-2 were determined by intracellular cytokine staining. The sum total production of all three cytokines was determined by flow cytometric analysis. Expression of CD28 (memory-associated molecule) and β7 (mucosal homing-associated molecule) on these antigen-specific cytokine-producing CD4+ T cells is presented as mean ± SEM.
Magnitude and quality of vaccine-induced virus-specific CD4+ T cell responses were not associated with preservation of CD4+ T cells and control of viral replication following SHIV-89.6P challenge
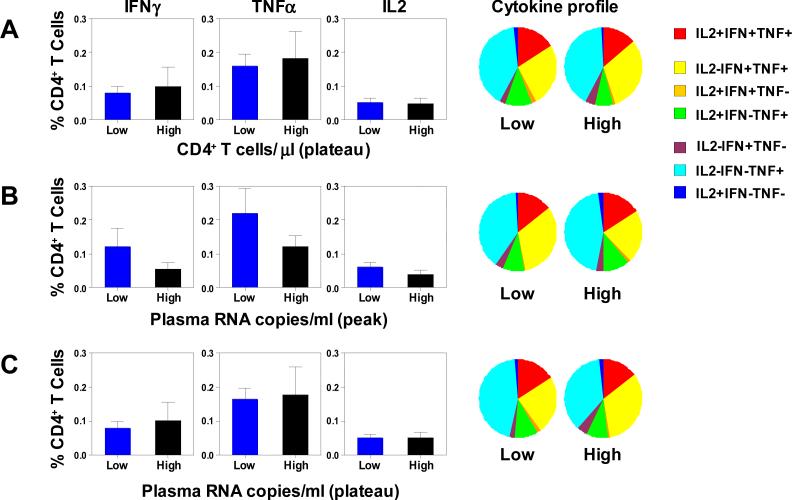
We have previously shown that both the magnitude and functional profile of the virus-specific CD8+ T cells generated by vaccination were associated with control of viral replication following SHIV-89.6P challenge (Sun et al., 2008). In the current study, we evaluated whether an association also exists between the magnitude and quality of vaccine-elicited virus-specific CD4+ T cell responses and disease progression following viral infection. To evaluate this possibility, we divided all 33 animals that received experimental vaccines into halves, low and high, on the basis of the magnitudes of their CD4+ T cell counts, as well as the magnitudes of their peak and their plateau plasma viral RNA levels. Both the quantity and quality of virus-specific CD4+ T cell responses after the boost immunizations were then compared in these cohorts (Fig. 4). The vaccine-elicited virus-specific CD4+ T cells were of comparable magnitudes and had comparable functional profiles in animals that demonstrated low or high set-point CD4+ T cell counts post-challenge. Furthermore, the vaccine-elicited virus-specific CD4+ T cells were also comparable in the monkeys with low or high peak and set point viral RNA levels. Thus, neither the magnitudes nor the functional profiles of the virus-specific CD4+ T cells generated by vaccination were associated with a preservation of CD4+ T cells or control of viral replication following SHIV-89.6P challenge.
Figure 4. Magnitude and functionality of the vaccine-elicited antigen-specific CD4+ T cell responses were not associated with preserved CD4+ T cells or reduced plasma viral RNA levels following SHIV-89.6P challenge.
The 33 animals that received different vaccine regimens were divided into halves, low and high, based on the magnitudes of their plateau CD4+ T cell counts (A), peak plasma viral RNA levels (B) or plateau plasma viral RNA levels (C). PBL isolated following the boost immunizations were exposed to pools of overlapping peptides spanning the Gag or Env proteins, and the fractions of CD4+ T cells producing IFN-γ, TNF-α, or IL-2 were determined by intracellular cytokine staining. In the left panels, the magnitudes of the vaccine-elicited antigen-specific CD4+ T cell responses are presented as means ± SEM in bar charts. In the right panels, functional profiles of the vaccine-elicited antigen-specific CD4+ T cells are shown as the mean values from each group of monkeys in pie charts.
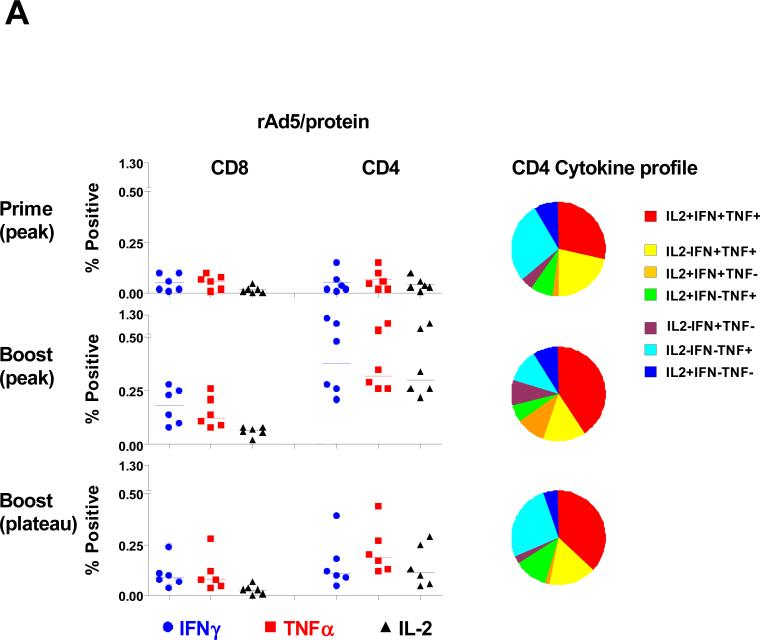
rAd5 prime, envelope protein boost induced high frequency envelope-specific CD4+ T cell responses with polyfunctional repertoires
In the recently reported RV144 HIV-1 vaccine trial in Thailand, a recombinant canarypox priming immunization followed by an envelope protein boosting immunization generated modest protection against acquiring an HIV-1 infection (Rerks-Ngarm et al., 2009). We were therefore interested to evaluate both the magnitude and functional profile of the vaccine-elicited envelope-specific CD4+ T cell responses in 6 rhesus monkeys that received a recombinant vector priming immunization followed by an HIV-1 envelope boosting immunization.
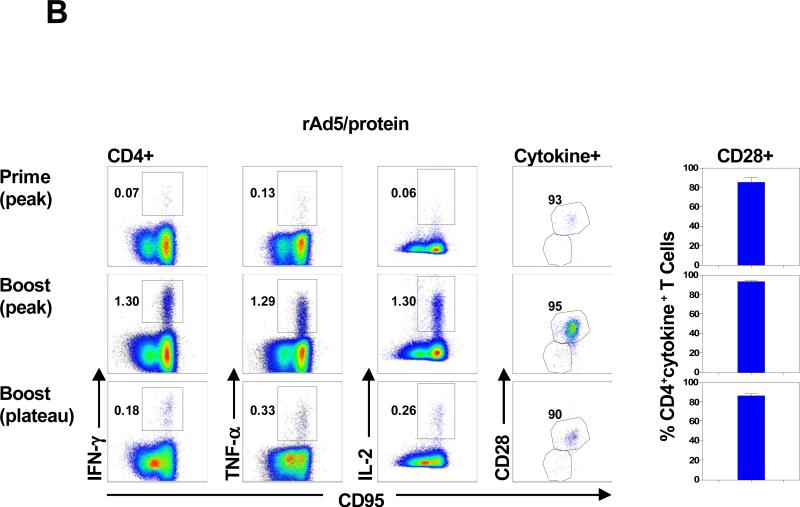
Monkeys primed with rAd5 developed envelope-specific T cell responses that included both CD4+ and CD8+ T cells (Fig. 5A). However, a dramatic CD4+ T cell-biased expansion of envelope-specific T cell responses was seen two weeks after the envelope protein boost. We then analyzed the functional profile of these envelope-specific CD4+ T cells. Monkeys primed with rAd5 developed polyfunctional envelope-specific CD4+ T cell responses that were predominantly IFN-γ+TNF-α+IL-2+ or IFN-γ+TNF-α+IL-2-. Envelope protein boosting induced a further expansion of the polyfunctional envelope-specific CD4+ T cell responses, with 75% of the responses made up of either cytokine double positive or triple positive CD4+ T cells. Further, the cytokine profiles of these cells were unchanged 12 weeks following the boost immunization. A detailed phenotypic analysis was also done to assess the memory differentiation of these envelope-specific CD4+ T cells. Consistent with previous results, the envelope-specific CD4+ T cells elicited by the protein boosting immunization were predominantly CD28+ memory cells (Fig. 5B). These results indicate that rAd5 prime/envelope protein boost immunization induced high frequency envelope-specific CD28+ memory CD4+ T cell responses with polyfunctional repertoires.
Figure 5. HIV-1 envelope-specific CD4+ T cell responses following rAd5 prime and envelope protein boost.
(A) PBL from 6 animals that received a rAd5 priming immunization followed by an HIV-1 envelope boosting immunization were exposed to pools of overlapping peptides spanning the HIV-1 envelope protein and the fractions of CD4+ or CD8+ T cell producing IFN-γ, TNF-α, or IL-2 are shown in the left panel. Functional profiles of the vaccine-elicited envelope-specific CD4+ T cells are shown as the mean values in pie charts in the right panel. (B) Expression of IFN-γ, TNF-α, or IL-2 by CD4 + T cells from a representative rhesus monkey following rAd5 prime and envelope protein boost immunization is shown in the left panels. Expression of CD28 on envelope-specific cytokine-producing CD4+ T cells from a representative rhesus monkey is shown in the middle panel. Expression of CD28 on these envelope-specific cytokine-producing CD4+ T cells is presented as mean ± SEM in the right panel.
DISCUSSION
In the wake of the recently reported modest success of the RV144 Thai vaccine trial, interest has turned to the potential contribution of vaccine-elicited CD4+ T cell and antibody responses to protection against HIV-1 acquisition. In the current study, we found that different vectors generated virus-specific CD4+ T cell responses of different magnitudes and with different functional profiles. Of the vaccine regimens that we evaluated, DNA prime/rMVA boost immunization elicited the highest frequency virus-specific CD4+ T cell responses, and these cells had polyfunctional repertoires. A substantial expansion of the virus-specific CD4+ T cell responses was also seen in plasmid DNA primed monkeys after rAd5 boosting.
Immediately after a SHIV-89.6P challenge, the virus-specific CD4+ T cell populations in these vaccinated monkeys decreased, likely as a consequence of the well described loss of CD4+ T cells during the first weeks following a pathogenic CXCR4-tropic SHIV infection (Nishimura et al., 2005). Although these virus-specific CD4+ T cell responses decreased following SHIV-89.6P challenge, highly significant differences were observed between the magnitudes of these responses in the control monkeys and groups of monkeys receiving heterologous prime-boost vaccinations. These results demonstrate that immunization by DNA prime, heterologous vector boost induced high frequency HIV-1- and SIV-specific CD4+ T cell responses that are partially preserved following an intravenous infection with a pathogenic CXCR4-tropic SHIV.
We also evaluated the expression of memory- and mucosal homing-associated molecules on SIV-specific CD4+ T cells following vaccination and following SHIV-89.6P challenge. We found that after a priming immunization very few of the virus-specific CD4+ T cells expressed β7, the mucosal homing-associated molecule. Moreover, a homologous or heterologous boosting immunization did not expand the proportion of β7+ virus-specific CD4+ T cells. Therefore, virus-specific CD4+ T cells elicited by vaccination expressed low levels of this mucosal homing molecule. A detailed memory phenotypic analysis showed that majority of the virus-specific CD4+ T cells elicited by vaccination were CD28+ central memory T cells, and no significant differences were detected in the relative representation of these CD28+CD4+ T cells in the various groups of animals following vaccination and following challenge. Therefore, these results suggest that these T cell vaccine strategies generated CD28+ memory CD4+ T cell populations that home to the lymphoid tissue.
We have previously shown that both the magnitude and functional profile of the virus-specific CD8+ T cells generated by vaccination were associated with control of viral replication following SHIV-89.6P challenge (Sun et al., 2008). In the current study, we found that neither the magnitudes nor the functional profiles of the virus-specific CD4+ T cells generated by vaccination were associated with a preservation of CD4+ T cells or control of viral replication following SHIV-89.6P challenge. It is now clear that CD8+ T cell responses are associated with the containment of HIV/SIV replication in acute infection and they are also important for the maintenance of viral control during chronic infection (Borrow et al., 1997; Schmitz et al., 1999). CD4+ T cells provide help for B cell responses and maintain effective cytotoxic T lymphocytes (CTL) (Kalams and Walker, 1998). Functional CD4+ T cells are also required at the time of immune priming for the development of long-term memory CD8+ T cells (Janssen et al., 2003; Shedlock and Shen, 2003). Therefore, although we did not document a direct association between the magnitudes of the virus-specific CD4+ T cell population generated by vaccination and the control of viral replication post-challenge, we previously reported that a survival advantage was associated with the magnitude of the virus-specific CD4+ T cell responses generated by vaccination and also associated with the preservation of the virus-specific CD4+ T cell responses following SIVmac251 infection (Sun et al., 2006). The difference between these findings in the setting of a SHIV-89.6P and an SIVmac251 infection may reflect differences in the pathogenic consequences of a CXCR4-tropic and a CCR5-tropic lentivirus.
We used archived PBMCs from 51 rhesus monkeys from previously reported studies that received different homologous or heterologous prime-boost immunizations. There are therefore caveats that should be acknowledged when interpreting these findings. The differences in the immunization and challenge schedules for each experimental group of monkeys in these studies might have influenced the functional T cell data. In addition, the administration of the IL-2/Ig plasmid during DNA vaccine priming in the DNA/DNA and DNA/rMVA groups may have led to augmented immune responses that were capable of controlling viremia and preventing disease progression following a SHIV-89.6P challenge. Nevertheless, the findings in the present study demonstrate that vaccine strategies that include recombinant MVA or recombinant Ad5 vectors can elicit robust CD4+ T cell responses.
In the current study we found that rhesus monkeys primed with rAd5 showed a dramatic expansion of virus-specific CD4+ T cell responses following an envelope protein boost. In addition, boosting with MF59-adjuvanted protein elicited a further expansion of the polyfunctional Env-specific CD4+ T cell responses, with 3/4 of the responses made up of either cytokine double positive or triple positive CD4+ T cells. Further, these polyfunctional Env-specific CD4+ T cell responses were well preserved 12 weeks following the protein boost immunization. These results indicate that a protein boost immunization induced durable, high frequency envelope-specific CD4+ T cell responses with polyfunctional repertoires.
MATERIALS AND METHODS
Selection of rhesus monkeys
Heparinized blood samples were obtained from Mamu-A*01- rhesus monkeys (Macaca mulatta). All animals were maintained in accordance with the NIH Guide to the Care and Use of Laboratory Animals (National Research Council, 1996) and with the approval of the Institutional Animal Care and Use Committee of Harvard Medical School and the National Institutes of Health.
Immunization and challenge of rhesus monkeys
Archived PBL from 51 rhesus monkeys from previously reported studies were assigned to six experimental groups that received different homologous or heterologous prime-boost immunizations (Letvin et al., 2004; Letvin et al., 2006; Santra et al., 2004; Santra et al., 2009; Santra et al., 2007; Seaman et al., 2005). Plasmid DNA, rAd5 and rMVA vaccine vectors were constructed as previously described and all vaccine regimens were administered by intramuscular injection using a needle-free Biojector system. In the rAd5-protein group, 6 monkeys received 100μg of SF162 (delta V2) gp140 protein administered with MF59 adjuvant as a boosting immunization (Burke et al., 2009). The immunization and challenge schedules for each experimental group of monkeys are summarized in Table 1. Five groups of experimentally vaccinated and 14 Mamu-A*01- control monkeys were challenged intravenously with 50 50% monkey infectious doses of pathogenic SHIV-89.6P from the same virus stock.
CD4+ T lymphocyte counts and plasma viral RNA levels
Peripheral blood CD4+ T lymphocyte counts were calculated by multiplying the total lymphocyte count by the percentage of CD3+CD4+ T cells determined by mAb staining and flow cytometric analysis. Plasma viral RNA levels were measured by an ultra-sensitive branched DNA amplification assay with a detection limit of 125 copies per ml (Siemens Diagnostics, Berkeley, CA).
Antibodies
The antibodies used in this study were directly coupled to fluorescein isothiocyanate (FITC), phycoerythrin (PE), phycoerythrin-Texas red (ECD), peridinium chlorophyll protein-Cy5.5 (PerCP-Cy5.5), phycoerythrin-Cy7 (PE-Cy7), AmCyan, Pacific Blue®, allophycocyanin (APC), Alexa Fluor® 700 and Quantum-Dot 605. All reagents were validated and titrated using rhesus monkey PBL. The following mAbs were used: anti-TNF-α-FITC (MAb11; BD Biosciences, San Jose, CA), anti-β7-PE (FIB504; BD Biosciences), anti-CD95-ECD (DX2; BD Biosciences), anti-CD28-PerCP-Cy5.5 (L293; BD Biosciences), anti-IFN-γ-PE-Cy7 (B27; BD Biosciences), anti-CD3-Pacific Blue® (SP34-2; BD Biosciences), anti-IL-2-APC (MQ1-17H12; BD Biosciences), anti-CD8α-Alexa Fluor® 700 (RPA-T8; BD Biosciences), and anti-CD4-Qantum-Dot 605 (unconjugated anti-CD4 antibody was obtained from BD Bioscience, Qantum-Dot 605 was obtained from Invitrogen, Carlsbad, CA). A violet fluorescent reactive dye (ViViD; Invitrogen) was also used as a viability marker to exclude dead cells in the analysis.
PBL stimulation and intracellular cytokine staining
Purified PBL were isolated from EDTA-anticoagulated blood and frozen in the vapor phase of liquid nitrogen. Cells were later thawed and allowed to rest for 6 h at 37°C in a 5% CO2 environment. The viability of these cells was >90%. PBL were then incubated at 37°C in a 5% CO2 environment for 6 h in the presence of RPMI/10% fetal calf serum alone (unstimulated), a pool of 15-mer Gag or Env peptides (2 μg/ml of each peptide; AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, Germantown, MD), or staphylococcal enterotoxin B (SEB) (5 μg/ml, Sigma-Aldrich, St. Louis, MO) as a positive control. All cultures contained Monensin (GolgiStop; BD Biosciences) as well as 1 μg/ml anti-CD49d (BD Biosciences). Anti-CD28 and anti-CD49d mAbs are usually used to co-stimulate T cell activation in intracellular cytokine staining assays. Since we included anti-CD28 antibody in our staining panel of mAbs, we excluded the anti-CD28 mAb in the stimulation phase of the assay to avoid the down regulation of this molecule. The cultured cells were stained with mAbs specific for cell surface molecules including CD3, CD4, CD8, CD28, CD95 and β7. After fixing with Cytofix/Cytoperm solution (BD Biosciences), cells were permeabilized and stained with antibodies specific for IFN-γ, TNF-α, and IL-2. Labeled cells were fixed in 1% formaldehyde-PBS.
Flow cytometric analysis
Samples were collected on a BD LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star, Ashland, OR). Approximately 500,000 to 1,000,000 events were collected per sample. Doublets were excluded by forward scatter (FSC)-area versus FSC-height. Dead cells were excluded by their staining with amine reactive dye. CD4+ and CD8+ T cells were determined by their expression of CD3, CD4 or CD8. Functional analyses were done by plotting the expression of each cytokine molecule against another, and a boolean combination of single functional gates was generated using FlowJo software. The frequency of cells producing IFN-γ, TNF-α, and IL-2, either individually or in any combination, was determined from the FlowJo, formatted in PESTLE, and analyzed using SPICE software (both PESTLE and SPICE software were provided by M. Roederer, Bethesda, NIH). All values used for analysis are background subtracted. Responses were considered positive when the percentage of total cytokine-producing cells was at least twice that of the background.
Statistical analyses
Statistical analyses and graphical presentations were computed with GraphPad Prism. The Kruskal-Wallis test for multiple groups (or the Mann-Whitney test for two groups) was used to compare the cellular immune responses of the different groups of experimental animals.
ACKNOWLEDGMENTS
We are grateful to Mario Roederer for helpful conversations and Michelle Lifton for technical assistance. The protein and adjuvant were provided by Dr. Susan Barnett (Novartis). This work was supported in part by funds from the intramural research program of the Vaccine Research Center, NIAID, NIH, the Harvard Medical School CFAR grant AI060354, and the NIH grant N01-AI30033.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, Meyers H, Nelson JA, Gairin JE, Hahn BH, Oldstone MB, Shaw GM. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3(2):205–11. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- Burke B, Gomez-Roman VR, Lian Y, Sun Y, Kan E, Ulmer J, Srivastava IK, Barnett SW. Neutralizing antibody responses to subtype B and C adjuvanted HIV envelope protein vaccination in rabbits. Virology. 2009;387(1):147–56. doi: 10.1016/j.virol.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, Tang A, Chen M, Huang L, Harris V, Freed DC, Wilson KA, Dubey S, Zhu DM, Nawrocki D, Mach H, Troutman R, Isopi L, Williams D, Hurni W, Xu Z, Smith JG, Wang S, Liu X, Guan L, Long R, Trigona W, Heidecker GJ, Perry HC, Persaud N, Toner TJ, Su Q, Liang X, Youil R, Chastain M, Bett AJ, Volkin DB, Emini EA, Shiver JW. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003;77(11):6305–13. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KS, Clair JH, Prokop MT, Sykes KJ, Dubey SA, Shiver JW, Robertson MN, Casimiro DR. DNA gag/adenovirus type 5 (Ad5) gag and Ad5 gag/Ad5 gag vaccines induce distinct T-cell response profiles. J Virol. 2008;82(16):8161–71. doi: 10.1128/JVI.00620-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorfu G, Rivera-Nieves J, Ley K. Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med. 2009;9(7):836–50. doi: 10.2174/156652409789105525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr., Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15(3):293–9. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovav AH, Panas MW, Osuna C, Cayabyab MJ, Autissier P, Letvin NL. The impact of a boosting immunogen on the differentiation of secondary memory CD8+ T cells. J Virol. 2007 doi: 10.1128/JVI.01519-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421(6925):852–6. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- Kalams SA, Walker BD. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188(12):2199–204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin NL, Huang Y, Chakrabarti BK, Xu L, Seaman MS, Beaudry K, Korioth-Schmitz B, Yu F, Rohne D, Martin KL, Miura A, Kong WP, Yang ZY, Gelman RS, Golubeva OG, Montefiori DC, Mascola JR, Nabel GJ. Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys. J Virol. 2004;78(14):7490–7. doi: 10.1128/JVI.78.14.7490-7497.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L, Yang ZY, Chakrabarti B, Rao SS, Schmitz JE, Montefiori DC, Barker BR, Bookstein FL, Nabel GJ. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312(5779):1530–3. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. National Academic Press; Washington, D.C.: 1996. [Google Scholar]
- Nishimura Y, Brown CR, Mattapallil JJ, Igarashi T, Buckler-White A, Lafont BA, Hirsch VM, Roederer M, Martin MA. Resting naive CD4+ T cells are massively infected and eliminated by X4-tropic simian-human immunodeficiency viruses in macaques. Proc Natl Acad Sci U S A. 2005;102(22):8000–5. doi: 10.1073/pnas.0503233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168(1):29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Santra S, Barouch DH, Korioth-Schmitz B, Lord CI, Krivulka GR, Yu F, Beddall MH, Gorgone DA, Lifton MA, Miura A, Philippon V, Manson K, Markham PD, Parrish J, Kuroda MJ, Schmitz JE, Gelman RS, Shiver JW, Montefiori DC, Panicali D, Letvin NL. Recombinant poxvirus boosting of DNA-primed rhesus monkeys augments peak but not memory T lymphocyte responses. Proc Natl Acad Sci U S A. 2004;101(30):11088–93. doi: 10.1073/pnas.0401954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra S, Sun Y, Korioth-Schmitz B, Fitzgerald J, Charbonneau C, Santos G, Seaman MS, Ratcliffe SJ, Montefiori DC, Nabel GJ, Ertl HC, Letvin NL. Heterologous prime/boost immunizations of rhesus monkeys using chimpanzee adenovirus vectors. Vaccine. 2009;27(42):5837–45. doi: 10.1016/j.vaccine.2009.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra S, Sun Y, Parvani JG, Philippon V, Wyand MS, Manson K, Gomez-Yafal A, Mazzara G, Panicali D, Markham PD, Montefiori DC, Letvin NL. Heterologous prime/boost immunization of rhesus monkeys by using diverse poxvirus vectors. J Virol. 2007;81(16):8563–70. doi: 10.1128/JVI.00744-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283(5403):857–60. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Seaman MS, Xu L, Beaudry K, Martin KL, Beddall MH, Miura A, Sambor A, Chakrabarti BK, Huang Y, Bailer R, Koup RA, Mascola JR, Nabel GJ, Letvin NL. Multiclade human immunodeficiency virus type 1 envelope immunogens elicit broad cellular and humoral immunity in rhesus monkeys. J Virol. 2005;79(5):2956–63. doi: 10.1128/JVI.79.5.2956-2963.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300(5617):337–9. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- Sun Y, Santra S, Schmitz JE, Roederer M, Letvin NL. Magnitude and quality of vaccine-elicited T-cell responses in the control of immunodeficiency virus replication in rhesus monkeys. J Virol. 2008;82(17):8812–9. doi: 10.1128/JVI.00204-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Schmitz JE, Buzby AP, Barker BR, Rao SS, Xu L, Yang ZY, Mascola JR, Nabel GJ, Letvin NL. Virus-specific cellular immune correlates of survival in vaccinated monkeys after simian immunodeficiency virus challenge. J Virol. 2006;80(22):10950–6. doi: 10.1128/JVI.01458-06. [DOI] [PMC free article] [PubMed] [Google Scholar]