Abstract
Summary: The entry of anti-infectives into the central nervous system (CNS) depends on the compartment studied, molecular size, electric charge, lipophilicity, plasma protein binding, affinity to active transport systems at the blood-brain/blood-cerebrospinal fluid (CSF) barrier, and host factors such as meningeal inflammation and CSF flow. Since concentrations in microdialysates and abscesses are not frequently available for humans, this review focuses on drug CSF concentrations. The ideal compound to treat CNS infections is of small molecular size, is moderately lipophilic, has a low level of plasma protein binding, has a volume of distribution of around 1 liter/kg, and is not a strong ligand of an efflux pump at the blood-brain or blood-CSF barrier. When several equally active compounds are available, a drug which comes close to these physicochemical and pharmacokinetic properties should be preferred. Several anti-infectives (e.g., isoniazid, pyrazinamide, linezolid, metronidazole, fluconazole, and some fluoroquinolones) reach a CSF-to-serum ratio of the areas under the curves close to 1.0 and, therefore, are extremely valuable for the treatment of CNS infections. In many cases, however, pharmacokinetics have to be balanced against in vitro activity. Direct injection of drugs, which do not readily penetrate into the CNS, into the ventricular or lumbar CSF is indicated when other effective therapeutic options are unavailable.
INTRODUCTION
Central nervous system (CNS) infections caused by pathogens with a reduced sensitivity to drugs are a therapeutic challenge. This is particularly true for infections caused by penicillin-resistant pneumococci, methicillin-resistant staphylococci, multiresistant Gram-negative aerobic bacilli, or several other organisms (including Aspergillus spp., Scedosporium apiospermum, and Nocardia asteroides) that affect primarily the CNS in immunocompromised patients. This review aims to increase the awareness of the peculiarities of the pharmacokinetics of anti-infectives within the CNS.
The intracranial-intraspinal space consists of several compartments. Even in individual regions of one compartment, e.g., cerebrospinal fluid (CSF), strong differences in drug concentrations can occur between the ventricular, cisternal, and lumbar parts of the compartment (227). This most probably is also true for the extracellular space of the CNS (43). Since the majority of studies of humans reported drug concentrations in ventricular and lumbar CSF and since drug concentrations in tissue homogenates (i.e., not only in brain tissue) are generally difficult to interpret, this review will focus on CSF.
Pharmacokinetic data concerning the entry of many drugs into the intracranial compartments are incomplete. For this reason, the review will provide clues based on the drugs' physicochemical properties, which help to assess which compounds are most promising for the treatment of CNS infections.
PHYSIOLOGY OF THE BLOOD-BRAIN/ BLOOD-CSF BARRIER
The first experiments demonstrating the existence of the blood-brain/blood-CSF barrier were performed by Paul Ehrlich at the end of the 19th century: he injected anilin dyes into the blood of experimental animals and noticed that all organs with the exception of the brain were stained (60). At the beginning of the 20th century, Ehrlich's student Edwin Goldmann injected trypan blue intravenously (i.v.) or subcutaneously. Trypan blue stained the choroid plexus and the dura mater but did not substantially enter the CSF. Conversely, after the direct injection of trypan blue into the CSF, the brain and spinal cord were stained, indicating the absence of a tight diffusional barrier between CSF and brain tissue (80, 81). Previously, data on the function of the blood-brain/blood-CSF barrier was reported: the intrathecal injection of 30 mg sodium ferrocyanid caused convulsions, whereas the intravenous injection of doses 2 orders of magnitude higher did not cause CNS symptoms (126).
Later, experiments performed with larger hydrophilic compounds, e.g., inulin (201) and horseradish peroxidase (27), showed that the morphological correlate of the blood-brain barrier are the cells of the cerebrovascular endothelium linked by tight junctions (203). Between the dura mater and arachnoidea, several flat cell layers are linked by tight junctions and tight gap junctions and are covered by an incomplete basement membrane (159). In small brain regions, the capillary endothelia do not possess tight junctions (median eminence of the hypothalamus, area postrema at the floor of the fourth ventricle, and subfornical organ at the roof of the third ventricle [187, 259]). Via these regions, large hydrophilic molecules, which cannot penetrate tight junctions and cell membranes, enter the interstitial space of the brain and the CSF. The surface of brain capillaries without tight junctions is approximately 1/5,000 of the total capillary surface of the brain (38). The morphological correlate of the blood-CSF barrier is the cylindric epithelium of the choroid plexus linked by tight junctions (43, 66, 144).
In summary, the central nervous compartments are bordered by a diffusional barrier consisting of at least one lipid membrane with the exception of a few leaky regions allowing even large hydrophilic molecules, such as immunoglobulins, entry into the extracellular space of the brain and the CSF. The blood-brain/blood-CSF barrier was therefore compared with a second cell membrane surrounding the whole CNS (187). To view the blood-brain/blood-CSF barrier as a simple lipid membrane surrounding the CNS, however, is too simplistic. In order to ensure the proper function of the CNS, a variety of small molecules able to penetrate lipid membranes to a certain extent, e.g., catecholamines and neuropeptides, have to be inactivated. Several compounds with a low molecular weight are cleaved enzymatically at the blood-brain/blood-CSF barrier. These enzymatic activities gave rise to the concept of the enzymatic blood-brain/blood-CSF barrier (for a review, see references 79 and 152).
Other compounds, in particular substrates for brain metabolism, enter the brain more readily than by diffusion along a concentration gradient. Important examples of facilitated diffusion through the blood-brain barrier are glucose, amino acids, and the prodrug l-3,4-dihydroxyphenylalanine (l-DOPA). Penicillin G is a ligand of a high-affinity transport system facilitating its entry into the intracranial compartments, which is of minor clinical importance because of its low capacity (233). Conversely, several active drug efflux transporters contribute strongly to the function of the blood-brain/blood-CSF barrier (129, 149, 218, 219, 233).
PHYSIOLOGY OF THE EXCHANGE OF DRUGS BETWEEN BLOOD AND THE DIFFERENT INTRACRANIAL COMPARTMENTS
The intracranial space and vertebral canal cannot be looked at as a single physiological compartment: it is divided into the CSF space and the extracellular and intracellular spaces of the brain and spinal cord (Fig. 1) (42, 43). Within the individual parts of the same compartment, concentrations often differ (43). After i.v. injection, most drugs achieve higher concentrations in the lumbar than in the ventricular CSF. The injection of drugs into the lumbar CSF frequently does not produce therapeutic concentrations in cisternal or ventricular CSF (104, 227). Elimination half-lives appear to be longer after injection into the lumbar than into the ventricular CSF: the CSF elimination half-life of a hydrophilic radioactive compound (111indium-diethylene triamine pentaacetic acid [111In-DTPA]) injected into the lumbar CSF space was estimated to be 12.4 to 131.1 h (median, 31.7 h) (167). Conversely, the elimination half-life of the large hydrophilic drug vancomycin after intraventricular injection ranged from 3.0 to 20.5 h (median, 5.2 h) (191, 192, 204).
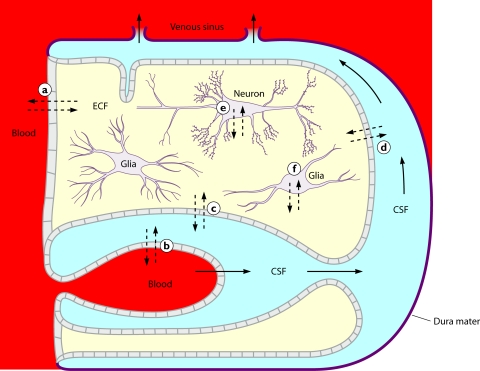
FIG. 1.
Intracranial fluid compartments. Continuous arrows represent the direction of the CSF flow. Interrupted arrows indicate where a diffusion of water or solutes can occur between brain capillaries, CSF, and nervous tissue: (a) across the blood-brain barrier; (b) across the epithelium of the choroid plexus; (c) across the ependyma; (d) across the pia-glial membranes at the surface of the brain and spinal cord; (e and f) across the cell membranes of neurons and glial cells. The thick line represents the dura mater and arachnoidea surrounding the system. (Reproduced from reference 42 with permission of Churchill Livingstone.)
No diffusional barrier exists between the interstitial space of the nervous tissue and the CSF. Even large molecules can enter the interstitial space of the nervous tissue from the CSF space by diffusion (202). The exchange between the CSF space and the interstitial space of the brain was studied for a patient receiving amphotericin B intraventricularly: the CSF space was estimated to be 139 ml, whereas the volume of distribution in the nervous tissue was estimated to be 677 ml, and the transfer constant between both spaces was 0.78/h, corresponding to a half-life of 0.9 h for the exchange between both compartments (14).
Approximately two-thirds of the CSF is produced by the choroid plexus as an ultrafiltrate with low concentrations of most blood-derived proteins and drugs. One-third of the CSF originates from the extracellular space of the brain and spinal cord; i.e., there is a continuous flow within the extracellular space of the brain and spinal cord toward the CSF space impeding the establishment of equal drug concentrations in the CSF space and extracellular fluid of the nervous tissue by diffusion. Humans and animals differ considerably with respect to their relationships between the CSF production rate and volume of the CSF space (the ratio of both parameters strongly increases in small animals, illustrated by CSF turnover rates of approximately 0.4%/min for humans and 0.7 to 1.9%/min for rats and mice) (42, 43). For this reason, quantitative data derived from animals are difficult to relate to conditions in humans.
At present, for many compounds, it is unknown exactly how, after systemic administration, the drug concentration in the interstitial space of the nervous tissue is related to the CSF concentration. Both concentrations should be of the same order of magnitude. The drug concentrations in brain homogenates do not reflect the concentrations in the extracellular space but rather reflect an average concentration of several compartments depending on how homogenates were prepared, how the drug of interest was extracted, and by what method of quantitation the concentration was measured.
Microdialysis has been used to quantify concentrations of anti-infectives in the extracellular space of the brain of humans (150). It relies on repeated sampling and can be used to study pharmacokinetics in the extracellular space of brain tissue. In the brain, it is an invasive procedure requiring the surgical placement of a catheter. Drug concentrations measured by microdialysis are influenced by properties of the probe and perfusion solution, by the postsurgery interval in relation to surgical trauma, and tissue integrity properties (45). For these reasons, drug concentrations within the cerebral extracellular space (CES) are usually estimated after the perfusion of the microdialysis probe with drug solutions of different concentrations and the calculation of the loss or gain of the drug in these solutions by the no-net-flow method (150). Because of the limited access to brain tissue in humans, the methodological problems of extracting brain homogenates, and the obstacles of measuring exact drug concentrations by microdialysis in humans (45, 150), the CSF concentration in our view, at present, still represents the closest approximation of drug concentrations in the extracellular space of the central nervous compartments available for the majority of drugs in humans. A recent study with rats comparing the unbound brain concentrations by a brain homogenate method, by brain microdialysis, and by determinations of CSF concentrations came to the conclusion that CSF concentrations may be used as a surrogate method to predict brain interstitial fluid concentrations of drugs (128).
According to the concept of the “sink action” of the CSF, the drug concentration in the interstitial space of the brain and myelon should be somewhat higher than that in the neighboring CSF (43); this concept suggests that the CSF has a function similar to that of the lymphatic system in other tissues. The extracellular fluid of the nervous tissue drains via the CSF space into the venous blood by means of the arachnoid granulations and along cranial and spinal nerve roots. The arachnoid granulations are one-way valves that transport the CSF into the venous blood without filtration; i.e., large molecules and even bacteria or erythrocytes and leukocytes can pass these valves (43, 124).
In order to describe the entry of drugs into the central nervous compartments and their removal from these compartments, intercompartmental clearances (CLin and CLout) can be defined (68, 215). CLout consists of two components: (i) diffusion back into blood through the blood-brain/blood-CSF barrier (CLrev) and (ii) bulk flow of the interstitial fluid of the brain and CSF into the venous blood through the arachnoid granulations and cranial and spinal nerve roots (CLb) (215). Both components cannot be distinguished in humans without invasive methods. When CSF production and absorption are not severely affected, the average volume of CSF produced every hour (CLb) is approximately 20 to 30 ml (39, 61, 180, 255). The CSF production rate is influenced by age, cerebral perfusion pressure, and several drugs, including compounds frequently used, e.g., diuretics (43). CSF production shows a circadian variation, with a minimum production of 12 ± 7 ml/h at approximately 18 h and a nightly peak production at approximately 2 h of 42 ± 2 ml/h (180). For the above-mentioned patient receiving amphotericin B intraventricularly, the average CSF production was estimated to be 32.4 ml/h (14).
For compounds that are not actively transported through the blood-CSF and blood-brain barrier, CLin and CLrev are determined by diffusion and are approximately equal:
 |
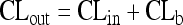 |
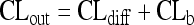 |
This implies that in the absence of active transport, the CLout is always greater than the CLin by approximately 20 to 30 ml/h. It also implies that for the majority of anti-infectives, steady-state CSF concentrations are substantially below the corresponding plasma concentrations: in the absence of active transport, for a drug that diffuses poorly through lipid membranes, the CLout is much larger than the CLin. For a drug that readily penetrates lipid membranes and is not removed actively from CSF and brain, the CLout and CLin are similar, because the CLin and CLrev are large compared to the CLb. For the exact determination of the CLin, repeated measurements of CSF drug concentrations early after an intravenous drug injection are necessary (215). The CLout can be measured most accurately by dividing the dose by the area under the drug concentration-time curve in CSF (AUCCSF) after a drug injection into the CSF: CLout = dose injected into the CSF/AUCCSF.
Such data from humans are available for some drugs only. The ratio of the inward and outward clearances for a drug is determined by the ratio of the area of its concentration-time curve in CSF and that in serum after an intravenous injection (166): CLin/CLout = AUCCSF/AUCS.
In the absence of active transport, a rough estimate of CLin (=CLdiff) can be obtained by the equations (CLdiff + 25 ml/h)/CLdiff = 1 + 25 ml/h/CLdiff = AUCS/AUCCSF and CLdiff = 25 ml/h/(AUCS/AUCCSF −1).
For humans, the ratio of the AUCCSF/AUCS or the CSF-to-serum drug concentration in steady state is the most accurate parameter to characterize drug penetration into the CSF.
In addition to host conditions (age, degree of meningeal inflammation and affection of CSF flow), drug concentrations within the CSF depend on the physicochemical properties of the drug (see below). Physiologically, the CSF-to-serum albumin ratio reflecting the permeability of the blood-CSF barrier and the CSF flow is high in newborns, is lowest around the age of 4 years, and then increases with age, probably because of a reduced turnover of CSF in healthy aging (22, 143). At equal plasma concentrations and in the absence of meningeal inflammation, CSF drug concentrations are often higher in infants and older persons than in children and young adults.
Depending on the pathogen responsible and the severity of disease, central nervous system (CNS) infections cause an increase of the permeability of the blood-CSF/blood-brain barrier and/or a decrease of the CSF flow (216), frequently leading to an increase of drug concentrations in the central nervous compartments during inflammation.
METHODS TO STUDY DRUG CONCENTRATIONS IN THE CNS IN HUMANS
The most simple way to study the entry of drugs into the CNS is to measure drug concentrations in the lumbar CSF collected by a single lumbar puncture during a continuous drug infusion. When the drug is administered to the central compartment and equilibrium has been reached, the CSF-to-serum steady-state concentration ratio can be determined by a single measurement of the drug concentration in serum and CSF. This type of study is ideal to characterize the relationship between entry and exit rates, or the CLin and CLout. A continuous infusion is, however, difficult to justify for severely ill patients, unless data regarding the drug studied show that a continuous infusion has an equal or superior pharmacodynamic effect (e.g., in the case of time-dependent antibiotics [see below]) compared to a bolus infusion.
After a bolus injection or a short-duration drug infusion into the central compartment, pharmacokinetic data characterizing the time course of the entry and exit of the drug from the CSF can be generated. For this purpose, CSF and serum concentrations at different intervals between the infusion and the lumbar puncture have to be studied with a relative large cohort of patients in order to extrapolate concentration-versus-time curves in CSF. Measurement of CSF concentrations at a single time point after a drug injection or short-duration infusion can lead to wrong conclusions depending on the interval between drug infusion and CSF withdrawal: because of the lag of the concentration-time curve in CSF compared to the respective curve in serum, CSF-to-serum concentration ratios at individual time points strongly depend on the interval between drug administration to the systemic circulation and CSF and serum sampling (179) (Fig. 2 A and B). Even when a median time of 8 weeks had elapsed between the commencement of the most recent treatment and sample collection from HIV patients, the CSF-to-serum concentration ratios (median [range]) of drugs determined from single paired CSF and serum (plasma) samples showed a very high variation (abacavir, 0.039 [0.0 to 2.36]; indinavir, 0.111 [0.0 to 0.47]; lamivudine, 0.229 [0.0 to 4.90]; nevirapine, 0.626 [0.41 to 0.77]; ritonavir, 0.0 [0.0 to 0.52]; stavudine, 0.204 [0.0 to 0.204]; zidovudine, 0.02 [0.0 to 6.74]) (12), which is partly explained by different intervals between dosing and CSF sampling. For this reason, in Table S1 in the supplemental material, absolute CSF concentrations only and not concentration ratios are reported, unless the original study clearly documented the attainment of steady state.
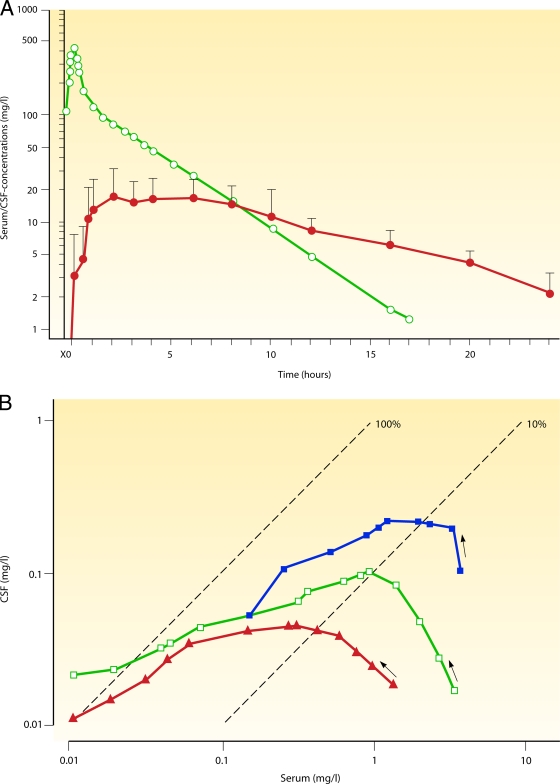
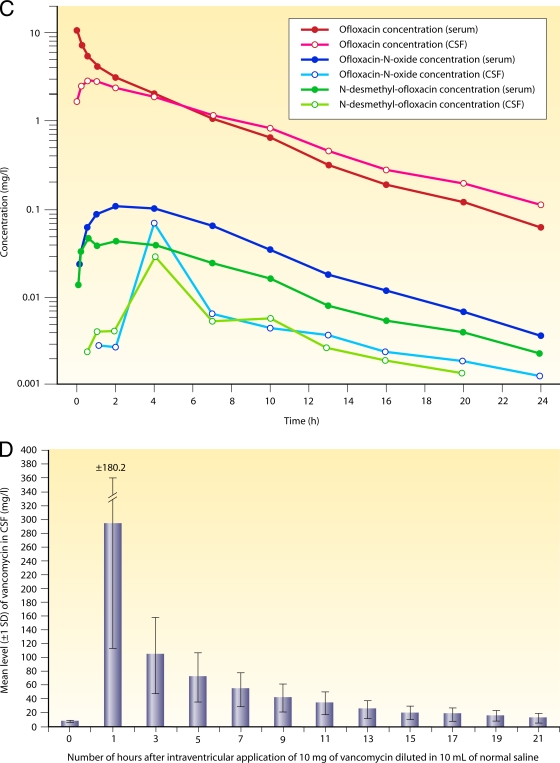
FIG. 2.
Drug exchange between blood and CSF. (A) Intravenous injection of a small hydrophilic compound moderately entering the CNS. Shown are semilogarithmic concentration-versus-time curves of fosfomycin in serum (open circles) and ventricular CSF (filled circles) after the intravenous application of 10 g. The AUCCSF-to-AUCS ratio was 0.138. The concentrations in CSF lagged behind those in serum, and the elimination half-life in CSF was considerably longer than that in serum, suggesting that the drug was eliminated mainly by bulk flow. (Reproduced from reference 115 with permission from Urban & Vogel, Munich, Germany.) (B) Double-logarithmic hysteresis loops illustrating the CSF-to-serum concentration ratios of the relatively hydrophilic fluoroquinolone ciprofloxacin over 24 h after intravenous infusion of 200 mg. The concentration ratio increased with the interval between infusion and CSF and serum sampling in three patients (open squares, closed squares, and closed triangles). Please note that this ratio can attain almost any value depending on the interval between infusion and measurement. (Reproduced from reference 173 with permission of Oxford University Press.) (C) Intravenous injection of a moderately lipophilic fluoroquinolone with a relatively low molecular mass readily crossing the blood-CSF barrier. Shown are concentration-versus-time curves in serum and ventricular CSF of ofloxacin and its metabolites after the intravenous application of 400 mg. Please note that after the initial distribution phase, serum (red filled circles) and CSF (red open circles) ofloxacin concentrations almost ran in parallel, indicating that CSF bulk flow was negligible compared to diffusion across the blood-CSF barrier. The overall penetration of ofloxacin into the CSF was greater than that of its more hydrophilic metabolites ofloxacin-N-oxide (serum, blue filled circles; CSF, blue open circles) and N-desmethyl-ofloxacin (serum, green filled circles; CSF, green open circles) (AUCCSF0-24 h/AUCS0-24 h of 0.62 ± 0.09 versus 0.14 ± 0.10 and 0.37 ± 0.35; P < 0.05). (Reproduced from reference 169.) (D) Intraventricular injection of a large hydrophilic compound with low entry into the CNS when administered systemically (vancomycin). Hour 0 denotes the time point immediately before the intraventricular (re)injection of 10 mg vancomycin. Columns represent means ± standard deviations (SD) of data from three patients. Please note that therapeutic CSF levels are encountered over 24 h. (Reproduced from reference 191 with permission of the University of Chicago Press.)
For patients with external ventriculostomy, CSF and serum drug concentrations can be determined repeatedly. These data can be used for a detailed calculation of pharmacokinetic parameters of the drug exchange between CSF and serum (Fig. 2) and for the estimation of steady-state CSF concentrations (171).
The limitations of microdialysis in patients are discussed above. In the future, an increasing number of labeled anti-infectives (e.g., see reference 117) for the noninvasive characterization of drug entry into the CNS by magnetic resonance imaging (MRI) or positron emission tomography (PET) will be available for use for humans.
Surgery for diagnostic and therapeutic purposes allows single measurements of drug concentrations in brain tissue and abscesses. When interpreting tissue concentrations, it has to be considered that brain tissue does not represent a single compartment. Data on drug concentrations in brain abscesses are of great value for the successful treatment of this disease.
Suitable publications for this review were identified by a PUBMED search using the following algorithm: name of the antibiotic and (cerebrospinal or brain) and (concentration or concentrations) and human.
Moreover, we contacted the manufacturers for information on the penetration of the respective drugs into the cerebrospinal fluid. Concerning the penetration of some drugs (e.g., broad-spectrum cephalosporins) into the CNS, a plethora of information has been published, and we were unable to cite all relevant literature. For compounds where several publications were identified by this method, the amount of information was reduced by omitting studies of single cases unless they contained detailed pharmacokinetic data. We apologize for any bias or omission of publications of equal importance which may have occurred.
Pharmacokinetic data were extracted from the full text of publications written in English or German. For non-English and non-German publications, only the English abstract was used to extract information. When this search did not identify suitable publications, other search strategies were employed.
When the data presented in the original publication allowed further analysis, pharmacokinetic parameters were assessed by noncompartmental methods. The elimination rate constants in serum (KS) and in CSF (KCSF) were estimated by log-linear regression, and the terminal half-lives in serum and CSF were calculated as ln2/K. The areas under the concentration-versus-time curve in serum/plasma (AUCS) and CSF (AUCCSF) were estimated by the linear trapezoidal rule. Extrapolation from the last concentration measured to infinity was performed by dividing the last concentration measured in serum and CSF by the KS and KCSF, respectively. For the assessment of CSF penetration, the AUCCSF was divided by the AUCS. Clearance (CL) after intravenous infusion or clearance divided by bioavailability (CL/F) after oral administration was estimated as the dose divided by the AUCS. The apparent volume of distribution (Vd) was estimated by dose/(AUCS·KS).
PENETRATION OF ANTI-INFECTIVES INTO THE CSF AND BRAIN TISSUE IN THE ABSENCE OF MENINGEAL INFLAMMATION
Drug concentrations measured in the absence of meningeal inflammation represent the minimum concentrations that can be encountered early in the course of a CNS infection or during its resolution. For this reason, it is highly desirable to reach effective drug concentrations in the CNS compartments not only with inflamed but also with uninflamed meninges.
The entry of drugs, including antibiotics, into the cerebrospinal fluid and extracellular space of the brain is governed by the following factors.
Molecular Size
The diffusion coefficient in liquids is inversely proportional to the hydrodynamic radius of the compound, and the hydrodynamic radius is approximately proportional to the square root of the molecular mass; i.e., the entry of a compound into the CSF depends on the square root of the molecular mass (67). Although the penetration of large hydrophilic compounds into the CSF is low, there is no absolute cutoff. Molecules as large as IgM are present in normal CSF at approximately 1/1,000 of their serum concentration.
Lipophilicity
Since the central nervous compartments are surrounded by at least one cell layer, i.e., two lipid membranes per cell, linked predominantly by tight junctions, the whole CNS can be viewed to be surrounded by lipid layers. The lipophilicity of a compound increases its ability to penetrate these membranes. The lipophilicity of a drug can be characterized by the octanol-water partition coefficient or more easily by chromatographic retention parameters determined by high-performance liquid chromatography in a reversed-phase system and is usually expressed as log P (123, 184, 198, 225, 245). Log P values of anti-infectives are given in Table S3 in the supplemental material. For cephalosporins, a significant relationship was found between lipophilicity and diffusion across the blood-brain barrier in rats. Cephalosporins exhibiting a moderate lipophilicity diffused well into CSF (198). Since very lipophilic compounds tend to be highly protein bound and to bind to lipid membranes, the ideal octanol-water partition coefficient at pH 7.4 for diffusion from plasma into CSF is around 1 to 10, corresponding to a log P of 0 to 1 (178).
For compounds that can be present in either an ionized or a nonionized form, penetration into the central nervous compartments is pH dependent: they penetrate more readily through lipid membranes in their unionized form than in their ionized form. Since the pH of the blood of healthy individuals is higher than that of the CSF (pH 7.4 versus 7.3; during bacterial meningitis the difference increases—CSF pH values down to below 7.0 can be encountered), for weak acids such as penicillins and cephalosporins, the fraction of unionized drug in CSF is higher than that in plasma. This implies that in severe meningitis, β-lactam antibiotics diffuse more readily from the central nervous compartments to the blood than vice versa (240).
Plasma Protein Binding
Binding to plasma proteins also strongly influences the entry of drugs into the central nervous compartments. It is generally believed that in the presence of an intact barrier, only the plasma fraction unbound can freely penetrate, because binding proteins (in particular albumin and globulins) pass the blood-brain/blood-CSF barrier only to a small degree (183). In a comparative study of humans, the CSF penetration of ceftriaxone (plasma protein binding, 90 to 95%) as estimated by AUCCSF/AUCS ratio was 1 order of magnitude lower than that of cefotaxime (plasma protein binding below 40%) (175, 178).
Based on molecular size, lipophilicity, and plasma protein binding, the penetration of a drug into CSF can be estimated. We have published a preliminary nomogram for this purpose (178).
Active Transport
The CSF concentrations and CSF-to-serum AUC ratios of antibiotics depend not only on their physicochemical properties but also on their differential affinity to transport systems (Fig. 3). Anti-infectives are ligands of several active transport systems responsible for the removal of toxic compounds from the CNS (233). The influence of these systems on the drug concentrations in the intracranial compartments is variable.
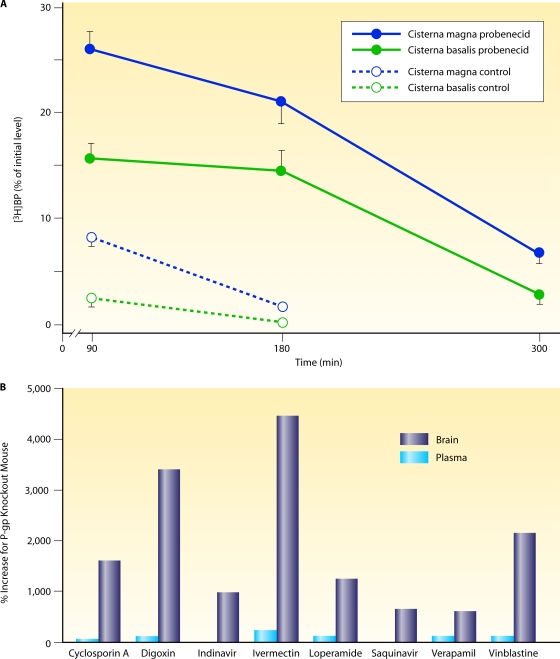
FIG. 3.
Effects of inhibitors of drug efflux pumps on the penetration of antibiotics into the central nervous system. (A) Concentration of radioactively labeled benzylpenicillin (BP) in the cisterna magna (CM) and the cisterna basalis (CB) after its application into the CM in control and probenecid-pretreated dogs. Concentrations of benzylpenicillin (means ± standard errors of the means [SEM]; n = 4) in the CM and CB over time (minutes), expressed as the percentage of the concentration in the CM at 5 min after drug application. Inhibition of the efflux pump by probenecid increases the CSF concentrations by a factor of 3 to 15. (Reproduced from reference 253 with permission of Elsevier B.V.) (B) Increased drug concentrations in P-glycoprotein knockout mice (149). The percent increases (concentration in knockout mice divided by the concentration in wild-type mice) in brain (dark blue bars) and plasma (light blue bars) for different drugs investigated by Schinkel and coworkers are shown. Contrarily to brain levels, plasma concentrations were not changed substantially, indicating a key role for P-gp at the blood-brain/blood-CSF barrier. (Reproduced from reference 149 with permission of the American Society for Pharmacology and Experimental Therapeutics.)
The removal of the neurotoxic antiparasitic drug ivermectin (used in humans for the treatment of river blindness and filariasis) from the CNS by P-glycoprotein (P-gp) (Mdr3), an abundant transporter protein at the blood-brain barrier, is a well-known example of the strong impact of an active transport system on the drug concentration within the CNS. It was discovered when P-gp-deficient mice were treated for mites and displayed an increased sensitivity to the neurotoxic effects of ivermectin (100-fold) compared to wild-type control mice (218, 219). In a patient with an intact P-gp system treated with ivermectin at 0.2 mg/kg of body weight/day subcutaneously, the drug and its metabolites were below the limit of detection in CSF (244). P-gp has an extremely broad spectrum of ligands favoring lipophilic compounds. The pump handles substrates from approximately 300 to 4,000 Da in mass (Fig. 3B) (149). The molecular mechanism of P-gp was recently unraveled by X-ray structure analysis (6). The exposure of the blood-brain barrier to some anti-infectives may upregulate P-gp: in vitro, the treatment of a human brain microvessel endothelial cell line with rifampin or the HIV proteinase inhibitor atazanavir or ritonavir caused increases in the P-gp expression levels by 1.8-, 6-, and 2-fold, respectively (271). This efflux pump system particularly affects the concentrations of several HIV proteinase inhibitors and macrolides within the CNS.
Organic anion transporter 3 (Oat3) and peptide transporter 2 (PEPT2) are located mainly in the choroid plexus (116). Several penicillins and cephalosporins are ligands of Oat3 (161), an active outward transport system for weak organic acids which decreases the CSF concentrations of these compounds, particularly under conditions with an intact blood-CSF/blood-brain barrier, and which is inhibited by probenecid. Several clinical observations suggested that cephalothin, a strong ligand of Oat3, is unsuitable for the treatment of bacterial meningitis because of its rapid removal from the CSF (233). In experimental animals, in the absence of meningeal inflammation, probenecid increased the CSF concentrations of penicillin G after intracisternal injection by a factor of 3 to 15 (Fig. 3A) and the CSF-to-serum concentration ratio during continuous i.v. infusion by 2 to 3 times (40, 234, 253). This effect tended to be weaker during meningitis (40). The cephalosporins cefadroxil and cephalexin are ligands of PEPT2. The influence of the transport system of weak organic acids on the pharmacokinetics of most β-lactam antibiotics in the CNS is moderate (e.g., see references 37 and 232). Ceftriaxone, cefotaxime, other larger cephalosporins, and carbapenems have minimal affinity to Oat3 and PEPT2, and ceftriaxone, cefotaxime, and meropenem belong to the standard therapy of community- and hospital-acquired bacterial meningitis.
Detailed reviews on the affinities of anti-infectives and other drugs for the different transport systems located at the blood-CSF/blood-brain barrier have been reported recently (129, 149, 233). At present, based on physicochemical properties, it is difficult to predict whether a drug is a strong ligand of P-gp or not (6).
Metabolism within the CNS
The choroid plexus and other regions of the CNS are able to metabolize drugs such as phenobarbital and dexamethasone (82). Isolated choroid plexus can metabolize cephalothin to desacetyl-cephalothin (182). To our knowledge, the metabolism of cefotaxime and many other antibiotics within the CNS has not been studied systematically.
PENETRATION OF ANTI-INFECTIVES INTO THE CSF AND BRAIN TISSUE IN THE PRESENCE OF MENINGEAL INFLAMMATION
In bacterial meningitis, the blood-CSF/blood-brain barrier is becoming leaky by the opening of intercellular tight junctions of the vessel walls particularly within venules (197). Moreover, the CSF outflow resistance increases, leading to a moderate reduction of the CSF production and absorption rates (216), and the activity of P-gp can be inhibited by proinflammatory cytokines (254). These three mechanisms (increased drug entry into the CSF and delayed removal by a reduction of the CSF bulk flow and by the inhibition of the activity of efflux pumps) synergistically lead to a rise of the CSF concentrations, particularly of drugs that do not enter the CSF readily in the absence of meningeal inflammation. Under the conditions of a severe disruption of the blood-brain/blood-CSF barrier, the physicochemical properties of drugs, which govern drug entry into the CNS in the absence of meningeal inflammation, become less important.
In critically ill patients, hydrophilic antibiotics (e.g., β-lactams, aminoglycosides, and glycopeptides) often have an increased volume of distribution (lowering serum concentrations and AUCS) and a reduced drug clearance (increasing serum concentrations and AUCS) (207). Which of these mechanisms inversely influencing CSF penetration has a greater impact in an individual is difficult to assess. Lipophilic antibiotics (e.g., fluoroquinolones, macrolides, tigecycline, and lincosamides) have lesser alterations in the volume of distribution but can also develop reduced drug clearances, causing an increase of the AUC in plasma (207). In experimental animals sepsis increased cerebral albumin extravasation as an indicator of the impaired integrity of the blood-brain/blood-CSF barrier (69, 92). Therefore, in the majority of cases, sepsis should lead to a moderate increase in the concentrations of anti-infectives in the CSF and other intracranial compartments.
The CSF of neonates contains high concentrations of proteins transferred from blood across the epithelial cells of the immature choroid plexus, and permeability to small lipid-insoluble molecules is greater in the developing than in the mature brain (214). For this reason, at equal serum levels, higher CSF concentrations of hydrophilic anti-infectives can be expected in the neonatal CSF than in the CSF of older children and adults.
GENERAL PROPERTIES OF CLASSES OF ANTI-INFECTIVES CONCERNING PENETRATION INTO THE CNS
β-Lactam Antibiotics
β-Lactams are compounds with a molecular mass of around 400 Da and plasma protein binding ranging from 0 to 95%. They are weak acids with pKa values of between 2.75 and 4. Their affinity for drug efflux pumps varies from strong (e.g., cephalothin) to almost absent (e.g., ceftriaxone) (233). Based on the AUCCSF/AUCS ratio, the penetration of all β-lactam antibiotics into the CSF in the absence of meningeal inflammation is generally below 0.15, i.e., relatively poor (Table 1 and see Table S1 in the supplemental material). This class of antibiotics is characterized by a high level of activity against susceptible pathogens and a relatively low toxicity. In the presence of moderately susceptible pathogens, the daily dose of several β-lactam antibiotics can be increased without a high rate of serious side effects: daily cefotaxime doses of up to 24 g for the treatment of meningitis caused by pneumococci with a reduced sensitivity to broad-spectrum cephalosporins were well tolerated (251), and the daily i.v. dose of ampicillin can be increased to 15 g and beyond for adults with normal renal function.
TABLE 1.
CSF penetration and clinical use of different classes of antibioticsa
| Compound (reference[s] for CSF penetration) | AUCCSF/AUCSb |
Relationship of CSF concn to MIC with usual doses | Compound(s) with broad clinical experience for CNS infections | Description | |
|---|---|---|---|---|---|
| Uninflamed or mildly inflamed meninges | Strong meningeal inflammation | ||||
| Penicillins Penicillin (46, 107, 108, 194, 246) | 0.02 | 0.2 | CSF concn with uninflamed meninges close to the MICs for moderately susceptible bacteria | Penicillin G, ampicillin, amoxicillin | Low toxicity; daily dose can be increased up to 15-20 g (ampicillin) |
| Nafcillin (164) | |||||
| Cloxacillin (46, 217) | 0.0087 | ||||
| Amoxicillin (18, 35) | 0.058 | ||||
| Ampicillin (35, 46, 72) | |||||
| Mezlocillin (94) | |||||
| Piperacillin (51, 168) | 0.034 | 0.32 | |||
| β-Lactamase inhibitors | 0.07 | 0.1 | CSF concn with inflamed and uninflamed meninges below the concn used in vitro for susceptibility testing (1-4 mg/liter) | Sulbactam | Little experience with in vivo activity in meningitis in humans; high-dose sulbactam (up to 8 g/day) was used successfully to treat Acinetobacter meningitis |
| Clavulanate (18) | 0.037 | 0.084 | |||
| Sulbactam (72) | |||||
| Tazobactam (168) | 0.106 | ||||
| Cephalosporins | 0.007-0.1 | 0.15 | CSF concn with uninflamed meninges close to the MICs of moderately susceptible bacteria; because of binding to plasma proteins, AUC ratio for ceftriaxone is approx 1 order of magnitude lower than that of cefotaxime | Cefazolin, cefotaxime, ceftriaxone, ceftazidime, cefepime, cefpirome | Low toxicity; daily dose can be increased up to 12-24 g (cefotaxime) |
| Cefazolin (111) | |||||
| Cephaloridine (46) | |||||
| Cefuroxime (112, 229) | |||||
| Cefotaxime (96, 175, 194, 195, 230) | 0.12 | 0.04, 0.17 | |||
| Ceftriaxone (47, 118, 141, 162, 175, 195, 236) | 0.007 | ||||
| Ceftazidime (24, 70, 83, 156, 160, 172, 265) | 0.057 | ||||
| Cefixime (165) | |||||
| Cefepime (213) | 0.103 | ||||
| Cefpirome (73, 181, 262) | 0.145, 0.31 | ||||
| Carbapenems | 0.2 | 0.3 | CSF concn with uninflamed meninges close to the MICs for moderately susceptible bacteria | Meropenem | Meropenem meningitis dose of 6 g/day; high proconvulsive activity of imipenem |
| Imipenem (15, 155, 263) | 0.14 | ||||
| Meropenem (34, 41, 142, 170) | 0.047, 0.21, 0.25 | 0.39 | |||
| Aminoglycosides | 0.2 | Not available | CSF concn with uninflamed meninges close to the MICs for moderately susceptible bacteria | Gentamicin, amikacin | High toxicity precludes strong increase of the daily dose; consider intrathecal application |
| Gentamicin (28, 46) | |||||
| Netilmicin (29, 55, 177) | 0.24 | ||||
| Amikacin (26, 76) | |||||
| Fluoroquinolones | 0.3-0.7 | 0.7-0.9 | CSF concn above the MIC for susceptible bacteria with uninflamed and inflamed meninges | Ciprofloxacin, (lev)ofloxacin, moxifloxacin | Effective compounds with favorable CNS pharmacokinetics; suitable therapy for susceptible bacteria (Gram-negative aerobic bacilli, L.monocytogenes) |
| Ciprofloxacin (173, 261) | 0.24, 0.43 | 0.92 | |||
| Ofloxacin (169) | 0.62 | ||||
| Levofloxacin (189, 223) | 0.71 | ||||
| Moxifloxacin (4, 5, 105) | 0.46 | 0.79 (0.71-0.94) | |||
| Chloramphenicol (46, 74, 270) | 0.6-0.7 | 0.6-0.7 | CSF concn above the MIC for susceptible bacteria with uninflamed and inflamed meninges | Chloramphenicol | Bacteriostatic, risk of aplastic anemia; reserve antibiotic |
| Macrolides (98) | Despite adequate CSF concn, bacteriostatic against S. pneumoniae | Erythromycin, clarithromycin | Case reports suggest effectiveness in CNS infections caused by Mycoplasma, Chlamydia, and Legionella spp. | ||
| Clarithromycin (137) | Not available | 0.18 | |||
| Tetracyclines | Ratios of individual CSF and serum samples suggest AUC ratio ∼0.2 | Ratios of individual CSF and serum samples suggest AUC ratio ∼0.2 | CSF concn close to the MIC for susceptible bacteria | Doxycycline | Documented effectiveness for neuroborreliosis, -brucellosis, and -syphilis |
| Doxycycline (56, 107, 108, 269) | |||||
| Fosfomycin (75, 115, 193) | 0.18 (0.09-0.27) | Not available | CSF concn above the MIC for susceptible pathogens with both inflamed and uninflamed meninges | Fosfomycin | Reserve antibiotic for S. aureus and P. aeruginosa CNS infections |
| Linezolid (20, 252) | 0.9 (0.8-1) | Not available | CSF concn above the MIC for susceptible pathogens with both inflamed and uninflamed meninges | Linezolid | Reserve antibiotic for S. aureus and Enterococcus sp. CNS infections |
| Metronidazole (93, 101, 258) | Not available | 0.87 | CSF concn above the MIC for susceptible pathogens with both inflamed and uninflamed meninges | Metronidazole | Standard therapy for CNS infections by anaerobic bacteria |
| Rifamycins | CSF concn above the MIC for susceptible pathogens with both inflamed and uninflamed meninges | Rifampin | Standard therapy of tuberculous meningitis; favorable clinical experience with S. aureus and S. pneumoniae CNS infections | ||
| Rifampin (52, 62, 89, 106, 150, 163, 174) | 0.22 | Not available | |||
| Trimethoprim and sulfamethoxazole (57, 125, 257) | With high doses, CSF concn above the MIC for susceptible pathogens with both inflamed and | Cotrimoxazole | Reserve antibiotic for L. monocytogenes and T. gondii CNS | ||
| Trimethoprim | 0.18 | 0.42-0.51 | uninflamed meninges | infections | |
| Sulfamethoxazole | 0.12 | 0.24-0.30 | |||
| Glycopeptides | With uninflamed meninges, CSF concn below or close to the MICs forsusceptible bacteria | Vancomycin | Standard therapy for CNS infections by methicillin-resistant S. aureus and multiresistant S. pneumoniae; high CSF-to-serum ratios were determined during continuous infusion of a high vancomycin dose (60 mg/kg/day) (2); other reports suggested lower CSF penetration | ||
| Vancomycin (2, 31, 65, 192, 205) | 0.18, 0.14 | 0.30 (0.29-0.48) | |||
| Antituberculosis drugs | Not available | Although AUC ratios are not available for all compounds, isoniazide, pyrazinamide, and ethionamide readily enter the CSF; streptomycin behaves like other aminoglycosides | Isoniazide, pyrazinamide, ethambutol, streptomycin | Limited data suggest moderate CSF penetration of ethambutol | |
| Isoniazid (53, 54, 62, 106, 228) | 0.86 (0.78-1.17) | ||||
| Pyrazinamide (54, 106) | |||||
| Ethambutol (25, 85) | |||||
| Streptomycin (62, 106) | |||||
| Ethionamide (62) | |||||
| Antiherpesvirus nucleoside analogues | Not available | CSF concn above the IC of susceptible viruses with both inflamed and uninflamed meninges | Acyclovir, ganciclovir | High-dose intravenous acyclovir and ganciclovir are the treatment of choice for CNS infections by herpesviruses | |
| Acyclovir (134) | 0.31 | ||||
| Valacyclovir (135) | 0.19 | ||||
| Ganciclovir | |||||
| Foscarnet (90, 199, 231) | 0.27, 0.43 | 0.23, 0.66 | CSF concn above the IC of susceptible viruses with both inflamed and uninflamed meninges | Foscarnet | Reserve compound for cytomegalovirus CNS infections |
| Antiretroviral drugs | Not available | CSF penetration depends strongly on the compound studied; based on animal exptl and clinical data, zidovudine, abacavir, stavudine, amprenavir-ritonavir, fosamprenavir-ritonavir, indinavir-ritonavir, and lopinavir-ritonavir are considered to reach an effective CNS concn (121, 241, 264) | Zidovudine, abacavir, delavirdine, nevirapine, ritonavir-lamivudine, delavirdine, nevirapine, boostered amprenavir, indinavir, or lopinavir | Effective concn in the CNS probably prevents dementia and development of resistant mutants | |
| Abacavir (12, 146, 249) | 0.35 | ||||
| Lamivudine (12, 71, 249) | |||||
| Stavudine (12, 71, 88, 249) | |||||
| Zidovudine (12, 71, 209, 249) | 0.75 | ||||
| Efavirenz (12, 238) | |||||
| Nevirapine (12, 249) | |||||
| Amprenavir (212) | |||||
| Atazanavir (250) | |||||
| Darunavir (267) | |||||
| Indinavir (12, 86, 122, 140) | 0.06-0.15 | ||||
| Lopinavir (30, 268) | 0.29 | ||||
| Ritonavir (12, 114) | |||||
| Saquinavir (12, 114) | |||||
| Raltegravir (266) | |||||
| Enfuvirtide (248) | |||||
| Maraviroc (248) | |||||
| Antifungal drugs | Not available | Fluconazole, flucytosine, and voriconazole readily enter the CSF in the absence and presence of meningeal inflammation and reach therapeutic levels; with both conventional and liposomal amphotericin B, CSF concentrations are low | Fluconazole, flucytosine, voriconazole, amphotericin B | Standard therapy (amphotericin B, flucytosine, and fluconazole for Cryptococcus neoformans and Candida albicans; voriconazole for Aspergillus spp.) for CNS mycoses; consider intrathecal therapy with amphotericin B | |
| Flucytosine (23) | 0.74 | ||||
| Fluconazole (16, 138, 148, 242) | 0.86 (0.74-0.89) | ||||
| Voriconazole (48, 63, 133) | 0.46 | ||||
| Itraconazole (97) | |||||
| Posaconazole (210) | |||||
| Amphotericin B (110, 127) | Low | ||||
| Micafungin (185) | |||||
| Antiparasitic drugs | Not available | Albendazole penetrates the central nervous compartments more readily than praziquantel; high-dose sulfadiazine and pyrimethamine result in effective CSF concn in humans | Albendazole, sulfadiazine, pyrimethamine | Albendazole is the therapy of choice in neurocysticercosis; the combination of pyrimethamine and sulfadiazine is standard therapy for cerebral toxoplasmosis | |
| Albendazole (103, 157, 237) | 0.38, 0.43 | ||||
| Praziquantel (103, 186) | 0.24 | ||||
| Sulfadiazine (3, 19) | 0.27-0.33 | ||||
| Dapsone (77, 206) | |||||
| Pyrimethamine (147, 260) | |||||
| Intraventricular therapy | ≫1 | ≫1 | High CSF concn with uninflamed and inflamed meninges; with large and/or hydrophilic compounds, which do not readily cross the blood-CSF barrier, the relatively low CSF turnover rate in humans allows once-daily dosing | Option when i.v. administration will not lead to CSF concn above the MIC for the causative organism | |
| Colistin (100, 139, 272) | |||||
| Gentamicin (113, 200, 256) | |||||
| Netilmicin (55) | |||||
| Vancomycin (191, 192, 204) | |||||
| Daptomycin (64) | |||||
| Amphotericin B (14) | |||||
For detailed pharmacokinetic data, see Tables S1.1 to S1.4 and S2 in the supplemental material. The statements on CSF concentrations are based only on dosing regimens usually employed for CNS infections (243) (Table S1).
Boldface type indicates estimates of CSF penetration of the antibiotic class based on available data.
β-Lactam antibiotics can cause epileptic seizures after intrathecal but also after intravenous administration. Epileptic seizures have been observed for up to 33% of children treated with imipenem i.v. for bacterial meningitis (263). After intraventricular injection in rats, cefazolin, imipenem, and aztreonam were most potent in inducing epileptic seizures (49). For this reason, meropenem instead of imipenem is recommended for the therapy of CNS infections.
For the new carbapenems doripenem and ertapenem, no data concerning their CNS penetrations in humans are available.
Since the systemic dose of several β-lactam antibiotics can be increased to ensure higher CSF concentrations and because of the proconvulsive activity of this antibiotic class, intraventricular injection of β-lactam antibiotics is not indicated in spite of their poor percent CSF penetration.
The AUCCSF/AUCS ratio of the β-lactamase inhibitor tazobactam in patients with uninflamed meninges is approximately 0.1 (168). For four patients with uninflamed meninges receiving 200 mg clavulanic acid, the AUCCSF/AUCS was 0.037 ± 0.015, and the elimination half-life in CSF was 1.89 ± 0.70 h, compared to 1.49 ± 0.50 h in serum (F. Sörgel, unpublished observation). In view of the practice of using high constant concentrations of β-lactamase inhibitors for in vitro susceptibility testing of β-lactams (e.g., tazobactam at 4 mg/ml) and of the low absolute CSF concentrations after the administration of 0.2 to 1 g of these compounds, larger amounts of β-lactamase inhibitors than the standard doses may be necessary to treat CNS infections (87, 168).
In animal meningitis models using β-lactamase-producing pathogens, high-dose β-lactamase inhibitors effectively protected the coadministered penicillin (109, 154). Six patients with Acinetobacter baumannii meningitis were cured with systemic sulbactam (4 g/day) plus ampicillin, and two did not respond (99). For one adult treated with 1 g sulbactam every 3 h (i.e., 8 g/day) plus ampicillin at 16 g/day, A. baumannii meningitis was cured, and treatment was tolerated without severe side effects (33).
Aminoglycosides
Aminoglycosides are hydrophilic compounds with a molecular mass of around 400 Da and low plasma protein binding. Their CSF penetration is considered to be particularly poor; however, based on the AUCCSF/AUCS ratio, their penetration into the CSF in the absence of meningeal inflammation is not lower than those of β-lactam antibiotics and other hydrophilic antibiotics with a similar molecular mass (177). The therapeutic index of aminoglycosides is low: because of nephro- and ototoxicity, the systemic dose of aminoglycosides can be increased in narrow limits only. This impedes the attainment of effective CSF concentrations, particularly when inflammation is mild to moderate. Therefore, most of the experience with intrathecal therapy has been gathered with aminoglycosides (Table 2 and see Table S2 in the supplemental material). Traditionally, an i.v. aminoglycoside has been used together with ampicillin to treat Listeria monocytogenes CNS infections. Recent data, however, suggest that the addition of aminoglycosides to treatment for listeriosis does not improve the patients' outcome (153). The indications for i.v. use of aminoglycosides for meningitis (e.g., see reference 243) have decreased over the last decades with the introduction of less-toxic antibiotics into clinical practice.
TABLE 2.
Intraventricular application of antibiotics to reach effective concentrations within the CNSa
| Antibiotic | Dose for adults | Severe reported side effect(s) |
|---|---|---|
| Gentamicin | 5 mg every 24 h | Hearing loss (temporary), epileptic seizures, aseptic meningitis, eosinophilic CSF pleocytosis |
| Tobramycin | 5 mg every 24 h | Similar to those of gentamicin |
| Amikacin | 30 mg every 24 h | Similar to those of gentamicin |
| Streptomycin | Up to 1 mg/kg every (24-)48 h | Hearing loss (temporary), epileptic seizures, radiculitis, transverse myelitis, arachnoiditis, paraplegia |
| Vancomycin | 5-20 mg every 24 h | Hearing loss (temporary) |
| Colistin (polymyxin E) methanesulfonate (12,500 IU = 1mg) | 10 (1.6-20) mg every 24 h | Meningeal inflammation; with high doses, epileptic seizures, loss of appetite, agitation, eosinophilia, edema, pain, albuminuria |
| Daptomycin | 5-10 mg every 72 h | Fever |
| Amphotericin B | 0.1-0.5 mg every 24 h | Tinnitus, fever, shivering, Parkinson syndrome |
Fluoroquinolones
Most fluoroquinolones are moderately lipophilic drugs with a molecular mass of around 300 Da and low binding to plasma proteins (approximately 20 to 40%) (21). Around the physiological pH, most agents are uncharged, which favors their CNS penetration. Based on the AUCCSF/AUCS ratio, their penetration into the CSF in the absence of meningeal inflammation is much higher than that of β-lactam antibiotics (see Table S1.1 in the supplemental material). Fluoroquinolones are of great value for the treatment of CNS infections by Gram-negative aerobic bacilli (ciprofloxacin) and by Mycobacterium tuberculosis (moxifloxacin) (4, 5). The activity of most fluoroquinolones is too low to treat Streptococcus pneumoniae meningitis (176). Because of their relatively high CNS toxicity, a strong increase of their systemic dose as with β-lactam antibiotics is not feasible. Since several fluoroquinolones enter the CNS readily, intrathecal injections of fluoroquinolones are unnecessary.
Chloramphenicol
Chloramphenicol, a small inhibitor of bacterial protein synthesis, is active against a variety of bacteria and readily enters the CSF (74, 270). It has been used extensively in the last decades for the treatment of bacterial meningitis. In industrialized countries, chloramphenicol is restricted mostly to topical uses because of the risk of induction of aplastic anemia. However, it remains a valuable reserve antibiotic for patients with allergy to β-lactam antibiotics or with CNS infections caused by multiresistant pathogens.
Macrolides
Macrolide antibiotics are basic highly lipophilic compounds. Macrolides are inhibitors of P-gp (59). The macrolide ivermectin, used to treat different human parasitoses, including river blindness, is efficiently removed from the CNS by P-gp (218, 219). P-gp restricts topical erythromycin absorption across the cornea, and the ocular bioavailability of P-gp substrates can be significantly enhanced by P-gp inhibitors (50).
Macrolides penetrate well into tissue, but because of their relatively high molecular mass (around 750 Da) and probably also because of their affinity for P-gp, they do not reach sufficient CSF concentrations in the absence of meningeal inflammation (190). Due to a lack of bactericidal activity in experimental S. pneumoniae meningitis (221), their role in the treatment of CNS infections is limited to Mycoplasma sp. and Legionella pneumophila encephalitis.
Tetracyclines and Tigecycline
Among tetracyclines, experience for the treatment of CNS infections is greatest with doxycycline. The lipophilic drug doxycycline is readily absorbed after oral application (>80%). Its protein binding is >80%, and its elimination half-life is long, ranging from 12 to 25 h (1). In small studies, doxycycline was not inferior to ceftriaxone for the treatment of neuroborreliosis (e.g., see reference 107). It is part of standard treatment protocols for neurobrucellosis and has been used successfully to treat neurosyphilis. Although systematic pharmacokinetic studies are lacking, based on the CSF-to-serum concentration ratios of individual samples, the CSF penetration is approximately 0.2 in both the absence and the presence of meningeal inflammation (108, 269). Since daily doses of 200 mg produce CSF concentrations close to the MICs for susceptible bacteria, the daily dose has been increased to up to 400 mg (269). The new glycylcycline tigecycline reaches CSF concentrations of approximately 10% of those found in serum with uninflamed meninges (208).
Fosfomycin
Fosfomycin is a bactericidal antibiotic that is active against many Gram-positive and distinct Gram-negative bacteria. Fosfomycin is a very small hydrophilic compound with a molecular mass of only 138 Da, which is neither metabolized nor protein bound (115). Because of its low molecular mass and despite its hydrophilicity, it enters the CNS in the presence and absence of meningeal inflammation more readily than β-lactam antibiotics (115, 193). In the United States, uncomplicated urinary tract infection represents the only indication for oral fosfomycin tromethamine. Based on early difficulties in determining the in vitro activity of fosfomycin, there was initial skepticism about its efficacy and application range. However, correctly executed experiments documenting its efficacy led to a broad use of intravenous fosfomycin in Europe and many other regions of the world, including the treatment of CNS infections by multiresistant bacteria (196).
Oxazolidinones
Linezolid, the first oxazolidinone, which is active against Gram-positive bacteria, has amphiphilic properties, possesses a Vd of approximately 1 liter/kg, and readily enters the CSF with an AUCCSF/AUCS ratio close to 1 (20, 252). Although primarily bacteriostatic, linezolid has been employed successfully for CNS infections caused by multiresistant organisms (211).
Metronidazole and Clindamycin
Metronidazole is a small lipophilic compound that is active against most anaerobic bacteria penetrating well into most tissues, including abscess contents. In animal studies, metronidazole readily penetrated the blood-CSF/blood-brain barrier, and data regarding the entry into human CSF and brain abscess confirmed this finding (93, 101, 258). Metronidazole is part of the standard therapy of bacterial brain abscess. Clindamycin possesses a molecular mass of 425 Da and is highly bound to plasma proteins. Although its penetration into the CNS is considered poor, it reached therapeutic CSF concentrations after a single dose of 1,200 mg in 10 AIDS patients, 8 without meningeal inflammation (78). This finding and data from animal experiments support the use of high-dose clindamycin in CNS infections with susceptible organisms.
Rifamycins
Rifamycins are lipophilic compounds with a molecular mass above 800 Da. Because of the relatively high molecular mass and a protein binding rate of approximately 80%, the penetration of rifampin into the CSF is moderate. As a consequence of their lipophilicity, the CSF concentrations of rifamycins are almost independent of the state of the blood-CSF barrier (62, 163, 174). When rifampin was used with ceftriaxone for the treatment of experimental meningitis caused by penicillin-resistant S. pneumoniae in rabbits, bacteriologic cure occurred promptly, with or without dexamethasone therapy (188). Although clinical data on the efficacy of rifampin in patients with S. pneumoniae meningitis are scarce, some authors recommended that in areas with high rates of occurrence of penicillin-resistant pneumococcal strains, the combination of ceftriaxone plus rifampin instead of vancomycin should be preferred, or rifampin, vancomycin, and ceftriaxone should be administered together when adjunctive dexamethasone is used (188, 243).
At high doses, rifabutin reached bactericidal CSF concentrations in a rabbit model of S. pneumoniae meningitis (220). Because of frequent side effects at high doses (e.g., arthralgia, uveitis, and stomatitis), rifabutin has rarely been used to treat CNS infections (136).
Sulfonamides and Trimethoprim
Sulfonamides and trimethoprim are small lipophilic antibiotics. The penetration of sulfamethoxazole, sulfadiazine, and trimethoprim into the CSF in both the absence and the presence of meningeal inflammation is higher than the penetration of β-lactam antibiotics and aminoglycosides (3, 125, 257). At high doses, these agents have qualified for the treatment of CNS infections with susceptible bacteria (e.g., Listeria monocytogenes, Nocardia asteroides, and Stenotrophomonas maltophilia) but also with fungi and parasites (e.g., paracoccidioidomycosis and toxoplasmosis). Bone marrow toxicity can limit increases of the daily dose or the duration of treatment.
Glycopeptides
Glycopeptides are hydrophilic antibiotics with a high molecular mass (above 1,400 Da). Their plasma protein binding rate ranges from <50% (vancomycin) to approximately 90% (teicoplanin) (17). Although not as toxic as aminoglycosides on kidneys and the inner ear, toxicity is still high enough to limit an increase of the dose. For patients with inflamed meninges, bactericidal CSF concentrations of vancomycin against susceptible pathogens are reached during high intravenous doses (2). Adjunctive dexamethasone therapy as recommended for community-acquired bacterial meningitis in patients reduced CSF vancomycin concentrations substantially, resulting in a delay in CSF sterilization in a rabbit model of experimental meningitis (188). Based on these data, those authors concluded that when dexamethasone is used as an adjunctive therapy for bacterial meningitis in areas with high rates of occurrence of penicillin-resistant pneumococci, the combination of rifampin and ceftriaxone should be preferred instead of vancomycin and ceftriaxone (188) (see below). A recent study of humans with dexamethasone-treated meningitis, however, found adequate vancomycin CSF concentrations in steady state during a continuous infusion of a high dose of 60 mg/kg/day. It was concluded that appropriate concentrations of vancomycin in CSF may be obtained even with a concomitant use of steroids provided that the vancomycin dosage is high enough (205).
With uninflamed meninges and after i.v. administration of a single dose of 1,000 mg, CSF vancomycin concentrations were below 0.5 mg/liter (166). Similarly, in adults after severe head trauma receiving four 500-mg doses of vancomycin/day, drug concentrations in the cerebral interstitial space measured by microdialysis 5 to 6 h after dosing were ≤1.2 mg/liter, or approximately 8% of the corresponding serum concentrations, whereas mean subcutaneous tissue levels were 43% of the serum concentrations (31).
Because of its strong binding to plasma proteins, teicoplanin penetration into the CSF is lower than that of vancomycin (235). For this reason, it has been administered to treat CNS infections in rare cases only (102). Successful intrathecal administration of teicoplanin has been reported (130).
Daptomycin and Fusidic Acid
Daptomycin is the first compound of a new class of antibiotics, the cyclic lipopeptides. In an animal model of meningitis, the mean CSF penetration was 6%, and the compound was rapidly bactericidal (36). Daptomycin was successfully used to treat meningitis caused by methicillin-resistant staphylococci in a 41-year-old woman (119). CSF concentrations in humans after intravenous application, however, have not yet been reported. Successful treatment of ventriculitis with intrathecal administration of 10 mg followed by 5 mg daptomycin every third day was reported, and high peak and trough CSF levels were measured (64) (Table 2 and see Table S2 in the supplemental material). The penetration of fusidic acid into the CSF is low, and its use for CNS infections is uncommon (46, 89, 151).
Polypeptides
Polypeptides consist of large hydrophilic compounds with a high systemic toxicity. For the treatment of CNS infections, the majority of data have been gathered with colistin. Colistin is used for the treatment of carbapenem-resistant Acinetobacter sp. CNS infections in children and adults (32, 100). In the absence and presence of ventriculitis, CSF penetration of colistin is poor. The CSF-to-serum concentration ratios were 0.051 to 0.057 (139) and 0.16 (estimated from Fig. 1 in reference 100). Because the relatively high toxicity after i.v. administration does not allow an increase of the systemic daily dose, colistin is frequently administered intrathecally (Table 2). To our knowledge, pharmacokinetics after intraventricular administration have not been characterized.
Antituberculosis Drugs
Pyrazinamide and isoniazid are moderately lipophilic small molecules and therefore ideal agents for the treatment of CNS infections. For this reason, treatment of tuberculous meningitis must always include isoniazid and pyrazinamide unless the pathogen is resistant to these drugs (62). The lipophilic reserve antituberculosis agent ethionamide also readily enters the CSF (62). The CSF penetrations of ethambutol and of the larger compounds streptomycin (hydrophilic) and rifampin (lipophilic; plasma protein binding rate of approximately 80%) are lower, and these compounds do not always reach CSF concentrations that are active against M. tuberculosis, in particular when meningeal inflammation is less pronounced or resolves during reconvalescence (106). When resistant strains of M. tuberculosis cause meningitis, moxifloxacin reaches high CSF concentrations in the presence and absence of meningeal inflammation and has been used successfully for this indication (4, 5). The entry of isoniazid, pyrazinamide, rifampin, and streptomycin into CSF during tuberculous meningitis was not inhibited by concomitant corticosteroid treatment (20 mg dexamethasone/day i.v. or 60 mg prednisolone/day orally [p.o.]) (106).
Drugs for the Treatment of Herpesviruses
The entry of acyclovir into CSF was moderate after administration of both oral acyclovir and valacyclovir (see Table S1.2 in the supplemental material) (134, 135). In the absence of meningeal inflammation, the CSF penetration of ganciclovir estimated by the AUCCSF/AUCS ratio in a nonhuman primate model was 0.155 ± 0.071 (224), suggesting a CSF penetration similar to that of acyclovir. We found no information concerning the penetration of cidofovir and famciclovir into human CSF.
Antiretroviral Drugs
In the absence of complicating opportunistic infections, the blood-CSF/blood-brain barrier of patients with HIV encephalopathy as assessed by the CSF-to-serum albumin ratio is intact or slightly impaired (8). For this reason, for clinical purposes, with these drugs no differentiation is necessary between penetration in the presence of uninflamed meninges and that in the presence of inflamed meninges.
The penetration of drugs for the treatment of HIV infections into the CNS strongly depends on the compound studied. Valid data for humans do not exist for all drugs. Protease inhibitors are, to various degrees, ligands of the P-gp transport pump located at the blood-brain/blood-CSF barrier, making them more or less susceptible to active efflux from the central nervous compartments (149, 266).
Poor penetration of antiretroviral drugs into the CNS allows continued HIV replication in the CNS. This may promote the selection of drug-resistant HIV mutants. In a study comparing drug resistance profiles in blood and CSF, different resistance profiles concerning at least one drug were observed for 45% of HIV patients (12). Enfuvirtide, the first compound of the new class of HIV fusion inhibitors (molecular mass above 4,000 Da), penetrates poorly into the CNS. Recently, the selection of enfuvirtide-resistant HIV within the CNS during salvage therapy with the suppression of HIV RNA in plasma below the quantification limit was reported (248). The use of antiretroviral compounds with a poor penetration into the CNS is probably associated with an increased risk of cognitive decline (121). Based on the best available evidence, considering chemical characteristics, CSF pharmacology, and effectiveness in the CNS in humans and animal models, a CNS penetration-effectiveness (CPE) index has been established to assess the ability of a drug to inhibit retroviral replication within the CNS (121). Antiretrovirals with sufficient CNS penetration to inhibit HIV replication in the brain are listed in Table 1, and available pharmacokinetic data for humans are listed in Table S1.2 in the supplemental material.
Antifungal Agents
The classical fungicidal agent amphotericin B, active against most fungi of clinical importance, is a large lipophilic molecule. Its size impedes the entry of the drug into the CNS. In animal experiments and scarce reports for humans, the CSF concentrations of unencapsulated amphotericin B were below 1% of the corresponding serum or plasma levels (110, 127). Only one study of five neonates who possessed a leaky blood-CSF barrier claimed, without presenting the actual concentrations measured, that amphotericin CSF concentrations were 40 to 90% of the corresponding serum levels. Consequently, a systemic application of standard doses results in low, frequently subtherapeutic levels in the CNS compartments. An increase of the daily dose is limited by the toxicity of amphotericin B. Encapsulation into liposomes is able to decrease toxicity, thereby allowing an increase of the daily dose. Since the entry of the drug into CSF and brain is governed by the free fraction of drug in serum, the amphotericin B CSF concentrations after the intravenous application of a colloidal dispersion and of several liposomal preparations were also below 1% of the respective serum concentrations (110). In spite of these low CSF concentrations, there is a large body of clinical experience of the successful use of different amphotericin B preparations for CNS infections in humans (110). In a rabbit model of Candida albicans meningoencephalitis, 5 mg/kg/day of liposomal amphotericin B and 1 mg/kg/day of amphotericin B deoxycholate were approximately equally effective and resulted in similar CSF concentrations (84). For humans with AIDS-associated cryptococcal meningitis, a 3-week course of 4 mg/kg liposomal amphotericin B had equal clinical efficacy but resulted in earlier CSF sterilization and fewer side effects than 0.7 mg/kg amphotericin B, suggesting some advantages of liposomal amphotericin B for the treatment of CNS infections (120). However, when high amphotericin B CSF concentrations are necessary, intrathecal administration should be considered (for dosing, see Table 2; for CSF concentrations and pharmacokinetics, see Table S1.3 and S2 in the supplemental material).
5-Flucytosine does not bind to plasma proteins and possesses excellent penetration into the CSF: 1 to 2 h after dosing, CSF concentrations amounted to 74.4% ± 5.6% of the corresponding serum levels (23).
The ability of the echinocandins caspofungin, micafungin, and anidulafungin to penetrate the blood-CSF/blood-brain barrier is poor as a consequence of their high molecular mass (above 1,000 Da) (222). Micafungin CSF concentrations were 0.007 and 0.017 mg/liter in a patient with cerebral aspergillosis treated with 300 mg daily and equaled the MIC of micafungin for Aspergillus (0.007 to 0.015 mg/liter) (185). Micafungin concentrations determined for several subcompartments of the CNS after intravenous administration in experimental rabbits with Candida meningoencephalitis were highest in the meninges and the choroid plexus, but micafungin was not reliably detected in CSF. Based on pharmacokinetic-pharmacodynamic data and Monte Carlo simulations, the appropriate dose to induce a near-maximum effect in a majority of infants with Candida meningoencephalitis was estimated to lie between 9 and 15 mg/kg (95).
Intraventricular caspofungin (1 to 2 mg/day), together with systemic voriconazole, was used successfully to treat a 2-year-old child with Scedosporium apiospermum brain abscesses and ventriculitis without reporting CSF levels (158). For the other echinocandins, no experience with intraventricular treatment is available. Despite the low penetration into intracranial compartments, several case reports concerning the successful systemic combination therapy of echinocandins with other antifungals for cerebral mycoses have been published. The contribution of the echinocandins to treatment success, however, was not always evident.
The less rapidly fungicidal azoles differ considerably with respect to their penetration into the central nervous compartments: in animal models, CSF and brain tissue concentrations of itraconazole and posaconazole were low, whereas the smaller azoles voriconazole and, in particular, fluconazole readily penetrated the blood-CSF/blood-brain barrier (222). Itraconazole exhibits significant affinity for P-gp, whereas the other azoles are only weak substrates of this efflux pump (110). Human studies documented the excellent entry of fluconazole into the central nervous compartments (242), whereas the voriconazole CSF-to-serum concentration ratios as a measure of CSF penetration were 0.46 (median) (133), and itraconazole CSF concentrations were low (110) (see Table S1.3 in the supplemental material). Because of its favorable pharmacokinetic parameters, voriconazole was more effective than amphotericin B in patients with invasive aspergillosis, including those with CNS aspergillosis (91).
Antiparasitic Drugs
Pyrimethamine (249 Da) (protein binding of approximately 90%; plasma half-life of approximately 4 days) is a critical part of therapy for persons with cerebral toxoplasmosis. Levels in CSF reach approximately 10 to 25% of the corresponding serum levels, and the absolute CSF concentrations are above those effective in vitro (147).
Atovaquone is active in mouse models of cerebral toxoplasmosis and is used in humans for this indication, when other drugs fail or cannot be administered due to severe side effects. To our knowledge, no data concerning its entry into human CSF or brain tissue have been published.
Albendazole (small and lipophilic) is metabolized outside the CNS in the liver to albendazole sulfoxide (ASOX), the active metabolite, which readily penetrates the blood-brain/blood-CSF barrier (237). Praziquantel has a slightly higher molecular mass, and it enters the CNS at a lower percentage. This may explain the better therapeutic results with albendazole than with praziquantel in neurocysticercosis reported by several studies (e.g., see reference 103).
PENETRATION OF ANTI-INFECTIVES INTO THE CENTRAL NERVOUS COMPARTMENTS
A summary of data gathered on the penetration of antibacterial, antifungal, and antiviral agents used for CNS infections in humans is presented in Table 1. Pharmacokinetic parameters determined by individual studies are presented in Table S1 and S2 in the supplemental material. In the tables in the supplemental material, means ± standard deviations or medians (minima to maxima) of pharmacokinetic parameters are presented (depending on the data reported). Since the CSF flow in relation to the volume of the CSF space is species dependent (0.38% per minute in humans and approximately 1% per minute in rats) (43), animal experiments are useful for establishing relationships between physicochemical properties of anti-infectives and penetration into the CNS but are not suitable to estimate pharmacokinetic parameters of the drug exchange between blood and CNS or drug concentrations in CSF in humans. Animal data are therefore not included in Tables S1 and S2. Physicochemical properties of anti-infectives useful to estimate entry into the CNS are listed in Table S3.
We do not report MICs or minimal bactericidal concentrations (MBCs) (bacteria and fungi) or concentrations that inhibit viral replication by 50% (IC50), because these values strongly depend on the susceptibility of the individual clinical isolate (e.g., see reference 122). In Table 1 we comment on the relationship between CSF levels achieved and the MIC (bacteria and fungi) and IC50 (viruses) of susceptible pathogens. The physician treating CNS infections is encouraged to exactly determine the susceptibility of the infectious agent by quantitative methods before making decisions on the probable in vivo efficacy of a therapy based on data presented in Table 1 or Tables S1.1 to S1.4 in the supplemental material.
RELATIONSHIP BETWEEN DRUG CONCENTRATION WITHIN THE INTRACRANIAL COMPARTMENTS AND ANTIMICROBIAL ACTIVITY
The relationship between drug concentration and effect has been studied most thoroughly with meningitis models. The most important determinant predicting efficacy in meningitis is the relationship between the actual concentration in CSF of the antibacterial studied and its MBC for the infecting organism (131). Many antibacterials at equal concentrations are less effective in sterilizing the CSF in vivo than killing bacteria in vitro. The main causes of this phenomenon are the relatively low bacterial growth rate in CSF and the acidic CSF during bacterial meningitis impeding the activity of several antibiotics, including aminoglycosides and macrolides (e.g., see reference 221), whereas drug binding to CSF proteins appears to be of minor importance because of the relatively low CSF protein concentration. In experimental meningitis models, β-lactam antibiotics are most active in CSF at concentrations ≥10× the MBC (239). The bactericidal rates of fluoroquinolones positively correlate with concentrations in CSF relative to the respective MBCs (176). Only when this ratio exceeded 10 did these antibiotics exhibit rapid bactericidal activities in CSF.
Carefully designed experiments showed that of the parameters time above MBC, Cmax/MBC, and AUCCSF/MBC, in the case of β-lactams, only time above the MBC was independently correlated with outcome (131). With several β-lactam antibiotics, the maximum bactericidal activity was achieved only when drug CSF concentrations exceeded the MBC of the causative organism for the entire dosing interval. Delayed regrowth of bacteria after exposure to and subsequent removal of an antibacterial (postantibiotic effect) appears to be minimal or absent in the CSF in vivo with β-lactams (131).
Generally, the pharmacodynamic properties of antibiotics in CSF are similar to those in other body sites; β-lactam agents and vancomycin are time dependent, whereas the quinolones and aminoglycosides are concentration dependent. Once-daily dosing of aminoglycosides was as effective as multiple-daily-dosing regimens in experimental meningitis models, probably because of drug-induced prolonged persistent effects. Fluoroquinolones produced less prolonged persistent effects in meningitis than in other infections and were slightly less effective for meningitis when administered once daily (11). In contrast to infections at other sites, quinolone concentrations need to continuously exceed the MBC for maximal effectiveness; i.e., in CNS infections, fluoroquinolones show some features of time-dependent antibiotics (132).
In experimental fungal infections of the brain, with the probable exception of cryptococcal meningitis, outcome correlates closer with brain parenchymal antifungal penetration than with antifungal CSF concentrations (110). This appears to be particularly true for cerebral aspergillosis (222).
To our knowledge, detailed pharmacokinetic-pharmacodynamic studies of experimental fungal CNS infections are lacking. In a neutropenic mouse disseminated candidiasis model, the peak serum level in relation to the MIC (peak serum level/MIC ratio) was the parameter best predictive of the outcome of amphotericin B treatment. The total amount of drug necessary to achieve various microbiological outcomes over the treatment period was 4.8- to 7.6-fold lower when the dosing schedule called for large single doses than when the same amount of total drug was administered in 2 to 6 doses; i.e., amphotericin behaved as a dose-dependent anti-infective agent (10). Conversely, when triazoles were used in the same experimental model, the AUC/MIC ratio correlated best with outcome, suggesting that a long time above the MIC was more important for the in vivo efficacy of the triazoles than of amphotericin B (9).
For individual HIV strains, drug concentrations that inhibit viral replication can be estimated in vitro (IC50). Blood and the intracranial compartments appear to represent separate compartments of HIV replication, in which different patterns of HIV resistance have been identified (264). To control HIV infection in the CNS, the drug concentrations in the extracellular space of the brain and spinal cord are probably most important. It is still a matter of debate how in vitro resistance analysis can be converted into information that can be implemented into clinical practice and whether effective CNS concentrations are necessary to prevent HIV-induced dementia (264). For phenotypic assays, bioinformatics analyses linking resistance values and clinical response data have been performed (247). By combining data on drug levels in plasma and CSF with phenotypic resistance data, it may be possible to estimate whether the patient's treatment protocol is sufficient to overcome his viral resistance pattern (12). Based on the best available evidence, considering chemical characteristics, CSF pharmacology, and effectiveness in the CNS in humans and animal models, a CNS penetration-effectiveness (CPE) index has been established to assess the ability of a drug to inhibit retroviral replication within the CNS (121). The CPE score correlated with low CSF HIV DNA levels and with improvements of several neuropsychological parameters in HIV-infected patients (121, 241).
METHODS TO INCREASE DRUG CONCENTRATIONS IN CENTRAL NERVOUS SYSTEM COMPARTMENTS
The easiest way to increase drug concentrations within the CNS is to increase the systemic dose. Several anti-infectives possess low toxicity. Therefore, the daily dose can be increased without severe side effects: the standard dose of meropenem for extracerebral infections is three 1-g doses/day, whereas for meningitis in adults, the standard dose is three 2-g doses/day (34, 41, 170). A well-established example of this strategy is the increase of the cefotaxime dose from 6 g up to 24 g/day when meningitis is caused by S. pneumoniae with reduced sensitivity to broad-spectrum cephalosporins (251). Acinetobacter baumannii meningitis has been treated successfully with 2 g ampicillin plus 1 g sulbactam every 3 h without severe adverse drug effects (33). The daily dose of fluconazole can be increased up to 1,600 mg with tolerable side effects (44, 58).
Drug entry into brain tissue and CSF can be increased by the artificial temporary disruption of the diffusional barriers between blood, CSF, and brain tissue, e.g., by the intra-arterial injection of hyperosmotic compounds or alkyl glycerols (149). This strategy used in experimental tumor therapy is dangerous for CNS infections, because it can aggravate brain edema. For the treatment of CNS infections, the intravenous administration of prodrugs with better penetration into the CNS than the active compound, of drugs included in surface-modified liposomes, or of adjuvant drugs inhibiting efflux pumps at the blood-CSF and/or blood-brain barrier appears to be more promising, (e.g., see references 7, 149, 215, 233, and 264). With the exception of the use of probenecid to increase the CSF concentrations of penicillins, these approaches have not gained access to clinical practice.
An effective but invasive method to establish high drug concentrations within the CNS is intraventricular injection in addition to intravenous administration. This method is most useful with antibiotics with a low penetration into the CSF and/or a high systemic toxicity, which precludes increasing the daily systemic dose (e.g., aminoglycosides, vancomycin, amphotericin B, and polymyxins). It should be noted that the administration of anti-infectives into the ventricular or lumbar CSF is almost always an off-label use and therefore requires a clear indication. For a large hydrophilic compound such as vancomycin, the apparent volume of distribution after intraventricular injection was 0.25 liters (204). Vancomycin and other molecules with a large molecular mass and hydrophilicity have a long elimination half-life in CSF, as they do not readily cross the blood-CSF barrier after intracisternal injection by diffusion. The long half-lives in CSF ensure therapeutic CSF concentrations with once-daily dosing (see Table S2 in the supplemental) (e.g., see references 191 and 204). The high interpatient variations of CSF elimination half-lives is caused by the heterogeneity of these critically ill patients (e.g., CSF flow out of an external ventriculostomy, obstruction of ventricles or the subarachnoid space by blood, or drugs affecting the CSF secretion).
In infants with Gram-negative meningitis and ventriculitis, the routine use of intraventricular aminoglycosides in addition to intravenous antibiotics resulted in a 3-fold-increased mortality compared to standard treatment with intravenous antibiotics alone (145, 226). For this reason, intraventricular antibiotic therapy should not be used when effective systemic therapy is available. When exactly intrathecal administration of anti-infectives should be used to treat CNS infections is a matter of debate (e.g., see references 13 and 226). In the presence of multiresistant organisms, sometimes no other treatment options are available. Current treatment protocols are listed in Table 2. For other drugs, the clinician in charge of the patient must consult the literature on the safety of the compound or the group of antibiotics which he considers to administer intrathecally. If no data at all are available, one must at least ensure that the pH and osmolarity of the solution are compatible with intrathecal administration. Medications administered intrathecally should be preservative free. Injections must be performed slowly and with a small volume in order to avoid a rise of the intracranial pressure in patients with increased CSF outflow resistance. To minimize regions within the CNS with subtherapeutic antibiotic levels, we recommend concomitant systemic therapy whenever possible.
Because of their relatively low systemic toxicity and high potential to induce epileptic seizures after intracerebroventricular application (49), β-lactam antibiotics are not good candidates for intraventricular use despite several casuistic reports.
CONCLUSIONS
Knowledge of the pharmacokinetics of anti-infectives at the blood-brain/blood-CSF barrier may help to improve the treatment of CNS infections. Frequently, the physician in charge of a patient has to consider data concerning pharmacokinetics and in vitro/in vivo activity. The ideal compound to treat CNS infections is small, is moderately lipophilic (lipid-water partition coefficient at pH 7.4 of around 1 to 10), has a low level of plasma protein binding, has a volume of distribution of around 1 liter/kg, and is not a strong ligand of P-gp or another efflux pump located at the blood-brain or blood-CSF barrier. When several approximately equally active compounds are available, a drug that comes close to these physicochemical and pharmacokinetic properties should be preferred. Some anti-infectives (e.g., isoniazid, pyrazinamide, linezolid, metronidazole, fluconazole, and some fluoroquinolones) achieve an AUCCSF/AUCS ratio close to 1.0 and, therefore, are extremely valuable drugs for the treatment of CNS infections with susceptible pathogens. In most cases, however, pharmacokinetics have to be balanced against in vitro activity. Some highly active compounds whose permeability of the blood-CSF/blood-brain barrier is low can be administered directly into the ventricular or lumbar CSF. It should be noted that only injection into the ventricles ensures distribution in the whole CSF space (227), that concomitant intravenous therapy is advisable, and that a clear indication for the direct injection of an anti-infective into the CSF must be present.
Supplementary Material
Acknowledgments
We thank Martin Munz and Sven Hüttner, Institute for Biomedical & Pharmaceutical Research, Heroldsberg (IBMP), for their help in compiling the physicochemical properties of anti-infectives. We also thank Martina Kinzig, IBMP, and Hilmar Prange and the colleagues of the Neurological Intensive Care Unit of the University of Göttingen for their continuous support in generating original data discussed in this review.
We gratefully acknowledge financial support from the Else Kröner Fresenius Stiftung to R.N. and H.E. and from the European Commission (CAREPNEUMO) to R.N.
This work is dedicated to Hilmar Prange on the occasion of his 65th birthday.
Biography
 Roland Nau, M.D., M.Sc., studied Medicine, Sociology, and Philosophy and then specialized in Neurology. He was trained in basic sciences under the supervision of Otto Creutzfeldt and Michael Conlon, Max Planck Institutes for Biophysical Chemistry and Experimental Medicine, Göttingen, Germany, and Martin Täuber, University of California, San Francisco. For over 20 years, together with Hilmar Prange, he has been studying the entry of anti-infectives and other drugs into the cerebrospinal fluid (CSF) in patients and experimental animals. Research interests are focused on infections of the central nervous system. He published approximately 180 papers in Medline-listed journals and is member of the Editorial Board of Chemotherapy. From 2005 to 2007 he headed the Neurochemical Laboratory, Department of Neurology, University of Göttingen, specializing in CSF analysis. Since 2008 he has served as Chief of the Department of Geriatrics, Protestant Hospital Göttingen-Weende, and is Associate Professor of the School of Medicine, University of Göttingen.
Roland Nau, M.D., M.Sc., studied Medicine, Sociology, and Philosophy and then specialized in Neurology. He was trained in basic sciences under the supervision of Otto Creutzfeldt and Michael Conlon, Max Planck Institutes for Biophysical Chemistry and Experimental Medicine, Göttingen, Germany, and Martin Täuber, University of California, San Francisco. For over 20 years, together with Hilmar Prange, he has been studying the entry of anti-infectives and other drugs into the cerebrospinal fluid (CSF) in patients and experimental animals. Research interests are focused on infections of the central nervous system. He published approximately 180 papers in Medline-listed journals and is member of the Editorial Board of Chemotherapy. From 2005 to 2007 he headed the Neurochemical Laboratory, Department of Neurology, University of Göttingen, specializing in CSF analysis. Since 2008 he has served as Chief of the Department of Geriatrics, Protestant Hospital Göttingen-Weende, and is Associate Professor of the School of Medicine, University of Göttingen.
 Fritz Sörgel, Ph.D., studied Pharmacy and Medicine. He is Associate Professor of the School of Medicine, University of Duisburg-Essen, Germany, and Founder and Director of the Institute for Biomedical and Pharmaceutical Research, Nürnberg, Germany. He published about 170 papers in Medline-listed journals and is Editor-in-Chief of Chemotherapy and Editorial Board Member of Recent Patents on Anti-Infective Drug Discovery and Journal of Bioequivalence & Bioavailability. He specialized in pharmacokinetics and bioanalytics of therapeutic and illicit drugs, focusing on anti-infectives, biosimilars, antineoplastic agents, and medical history on Paul Ehrlich. He organized two World Conferences to honor Ehrlich's 150th birthday (2004) and the Nobel Prize awarded to Ehrlich (2008). From 2006 to 2009 he served as President of the International Society of Anti-Infective Pharmacology (ISAP). He held various other functions, including Board Member of the Paul Ehrlich Society, Germany. He was given the Federal Order of Merit of Germany in 2007.
Fritz Sörgel, Ph.D., studied Pharmacy and Medicine. He is Associate Professor of the School of Medicine, University of Duisburg-Essen, Germany, and Founder and Director of the Institute for Biomedical and Pharmaceutical Research, Nürnberg, Germany. He published about 170 papers in Medline-listed journals and is Editor-in-Chief of Chemotherapy and Editorial Board Member of Recent Patents on Anti-Infective Drug Discovery and Journal of Bioequivalence & Bioavailability. He specialized in pharmacokinetics and bioanalytics of therapeutic and illicit drugs, focusing on anti-infectives, biosimilars, antineoplastic agents, and medical history on Paul Ehrlich. He organized two World Conferences to honor Ehrlich's 150th birthday (2004) and the Nobel Prize awarded to Ehrlich (2008). From 2006 to 2009 he served as President of the International Society of Anti-Infective Pharmacology (ISAP). He held various other functions, including Board Member of the Paul Ehrlich Society, Germany. He was given the Federal Order of Merit of Germany in 2007.
 Helmut Eiffert, M.D., Ph.D., studied Medicine and Chemistry and then specialized in Infectious Diseases. He received his degrees from Georg August University Göttingen (Germany). He conducted research at the Institute of Organic Chemistry concerning the molecular structure and function of new antibiotics from natural sources. He then joined Norbert Hilschmann, Department of Immunochemistry, Max Planck Institute of Experimental Medicine, Göttingen, Germany, as a research fellow and worked in the field of the human immune system of secretions. He spent residencies at the Departments of Internal Medicine, Neuropediatrics, Clinical Chemistry, and Medical Microbiology. He is now Associate Professor, Attending Physician, Infectiologist, and Vice-Director of the Department of Medical Microbiology, University Hospital, Göttingen. His main research interests are pathogenesis and therapy of infectious diseases of the nervous system.
Helmut Eiffert, M.D., Ph.D., studied Medicine and Chemistry and then specialized in Infectious Diseases. He received his degrees from Georg August University Göttingen (Germany). He conducted research at the Institute of Organic Chemistry concerning the molecular structure and function of new antibiotics from natural sources. He then joined Norbert Hilschmann, Department of Immunochemistry, Max Planck Institute of Experimental Medicine, Göttingen, Germany, as a research fellow and worked in the field of the human immune system of secretions. He spent residencies at the Departments of Internal Medicine, Neuropediatrics, Clinical Chemistry, and Medical Microbiology. He is now Associate Professor, Attending Physician, Infectiologist, and Vice-Director of the Department of Medical Microbiology, University Hospital, Göttingen. His main research interests are pathogenesis and therapy of infectious diseases of the nervous system.
Footnotes
Supplemental material for this article may be found at http://cmr.asm.org/.
REFERENCES
- 1.Agwuh, K. N., and A. MacGowan. 2006. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 58:256-265. [DOI] [PubMed] [Google Scholar]
- 2.Albanese, J., M. Leone, B. Bruguerolle, M. L. Ayem, B. Lacarelle, and C. Martin. 2000. Cerebrospinal fluid penetration and pharmacokinetics of vancomycin administered by continuous infusion to mechanically ventilated patients in an intensive care unit. Antimicrob. Agents Chemother. 44:1356-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert, F., G. B. Bishop-Freudling, and H. Vergin. 1984. Diffusion of tetroxoprim and sulfadiazine in the cerebrospinal fluid of neurosurgery patients. Fortschr. Med. 102:1064-1066. (In German.) [PubMed] [Google Scholar]
- 4.Alffenaar, J. W., P. M. de Vries, G. J. Luijckx, D. van Soolingen, T. S. van der Werf, and R. van Altena. 2008. Plasma and cerebrospinal fluid pharmacokinetics of moxifloxacin in a patient with tuberculous meningitis. Antimicrob. Agents Chemother. 52:2293-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alffenaar, J. W., R. van Altena, H. J. Bokkerink, G. J. Luijckx, D. van Soolingen, R. E. Aarnoutse, and T. S. van der Werf. 2009. Pharmacokinetics of moxifloxacin in cerebrospinal fluid and plasma in patients with tuberculous meningitis. Clin. Infect. Dis. 49:1080-1082. [DOI] [PubMed] [Google Scholar]
- 6.Aller, S. G., J. Yu, A. Ward, Y. Weng, S. Chittaboina, R. Zhuo, P. M. Harrell, Y. T. Trinh, Q. Zhang, I. L. Urbatsch, and G. Chang. 2009. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 323:1718-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson, B. D., M. E. Morgan, and D. Singhal. 1995. Enhanced oral bioavailability of DDI after administration of 6-Cl-ddP, an adenosine deaminase-activated prodrug, to chronically catheterized rats. Pharm. Res. 12:1126-1133. [DOI] [PubMed] [Google Scholar]
- 8.Andersson, L. M., L. Hagberg, D. Fuchs, B. Svennerholm, and M. Gisslen. 2001. Increased blood-brain barrier permeability in neuro-asymptomatic HIV-1-infected individuals—correlation with cerebrospinal fluid HIV-1 RNA and neopterin levels. J. Neurovirol. 7:542-547. [DOI] [PubMed] [Google Scholar]
- 9.Andes, D., K. Marchillo, T. Stamstad, and R. Conklin. 2003. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:3165-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andes, D., T. Stamsted, and R. Conklin. 2001. Pharmacodynamics of amphotericin B in a neutropenic-mouse disseminated-candidiasis model. Antimicrob. Agents Chemother. 45:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andes, D. R., and W. A. Craig. 1999. Pharmacokinetics and pharmacodynamics of antibiotics in meningitis. Infect. Dis. Clin. North Am. 13:595-618. [DOI] [PubMed] [Google Scholar]
- 12.Antinori, A., C. F. Perno, M. L. Giancola, F. Forbici, G. Ippolito, R. M. Hoetelmans, and S. C. Piscitelli. 2005. Efficacy of cerebrospinal fluid (CSF)-penetrating antiretroviral drugs against HIV in the neurological compartment: different patterns of phenotypic resistance in CSF and plasma. Clin. Infect. Dis. 41:1787-1793. [DOI] [PubMed] [Google Scholar]
- 13.Arnell, K., P. Enblad, T. Wester, and J. Sjolin. 2007. Treatment of cerebrospinal fluid shunt infections in children using systemic and intraventricular antibiotic therapy in combination with externalization of the ventricular catheter: efficacy in 34 consecutively treated infections. J. Neurosurg. 107:213-219. [DOI] [PubMed] [Google Scholar]
- 14.Atkinson, A. J., Jr., and D. D. Bindschadler. 1969. Pharmacokinetics of intrathecally administered amphotericin B. Am. Rev. Respir. Dis. 99:917-924. [DOI] [PubMed] [Google Scholar]
- 15.Aubert, G., G. Jacquemond, B. Pozzetto, R. Duthel, D. Boylot, J. Brunon, and G. Dorche. 1991. Pharmacokinetic evidence of imipenem efficacy in the treatment of Klebsiella pneumoniae nosocomial meningitis. J. Antimicrob. Chemother. 28:316-317. [DOI] [PubMed] [Google Scholar]
- 16.Bafeltowska, J. J., and E. Buszman. 2005. Pharmacokinetics of fluconazole in the cerebrospinal fluid of children with hydrocephalus. Chemotherapy 51:370-376. [DOI] [PubMed] [Google Scholar]
- 17.Bailey, E. M., M. J. Rybak, and G. W. Kaatz. 1991. Comparative effect of protein binding on the killing activities of teicoplanin and vancomycin. Antimicrob. Agents Chemother. 35:1089-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakken, J. S., J. N. Bruun, P. Gaustad, and T. C. Tasker. 1986. Penetration of amoxicillin and potassium clavulanate into the cerebrospinal fluid of patients with inflamed meninges. Antimicrob. Agents Chemother. 30:481-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barraviera, B., P. C. Pereira, R. P. Mendes, J. M. Machado, C. R. Lima, and D. A. Meira. 1989. Evaluation of acetylator phenotype, renal function and serum sulfadiazine levels in patients with paracoccidioidomycosis treated with cotrimazine (a combination of sulfadiazine and trimethoprim). Mycopathologia 108:107-112. [DOI] [PubMed] [Google Scholar]
- 20.Beer, R., K. W. Engelhardt, B. Pfausler, G. Broessner, R. Helbok, P. Lackner, C. Brenneis, S. T. Kaehler, A. Georgopoulos, and E. Schmutzhard. 2007. Pharmacokinetics of intravenous linezolid in cerebrospinal fluid and plasma in neurointensive care patients with staphylococcal ventriculitis associated with external ventricular drains. Antimicrob. Agents Chemother. 51:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergogne-Berezin, E. 2002. Clinical role of protein binding of quinolones. Clin. Pharmacokinet. 41:741-750. [DOI] [PubMed] [Google Scholar]
- 22.Blennow, K., P. Fredman, A. Wallin, C. G. Gottfries, I. Karlsson, G. Langstrom, I. Skoog, L. Svennerholm, and C. Wikkelso. 1993. Protein analysis in cerebrospinal fluid. II. Reference values derived from healthy individuals 18-88 years of age. Eur. Neurol. 33:129-133. [DOI] [PubMed] [Google Scholar]
- 23.Block, E. R., and J. E. Bennett. 1972. Pharmacological studies with 5-fluorocytosine. Antimicrob. Agents Chemother. 1:476-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blumer, J. L., S. C. Aronoff, C. M. Myers, C. A. O'Brien, J. D. Klinger, and M. D. Reed. 1985. Pharmacokinetics and cerebrospinal fluid penetration of ceftazidime in children with meningitis. Dev. Pharmacol. Ther. 8:219-231. [DOI] [PubMed] [Google Scholar]
- 25.Bobrowitz, I. D. 1972. Ethambutol in tuberculous meningitis. Chest 61:629-632. [DOI] [PubMed] [Google Scholar]
- 26.Briedis, D. J., and H. G. Robson. 1978. Cerebrospinal fluid penetration of amikacin. Antimicrob. Agents Chemother. 13:1042-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brightman, M. W., and T. S. Reese. 1969. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 40:648-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruckner, O., M. Alexander, and F. Martens. 1980. Gentamicin concentrations in cerebrospinal fluid of patients with inflamed and uninflamed meninges. Infection 8:86-89. (In German.) [DOI] [PubMed] [Google Scholar]
- 29.Bruckner, O., M. Trautmann, D. Kolodziejczyk, M. Alexander, and H. Collmann. 1983. Netilmicin in human CSF after parenteral administration in patients with slightly and severely impaired blood CSF barrier. J. Antimicrob. Chemother. 11:565-571. [DOI] [PubMed] [Google Scholar]
- 30.Capparelli, E. V., D. Holland, C. Okamoto, B. Gragg, J. Durelle, J. Marquie-Beck, G. van den Brande, R. Ellis, and S. Letendre. 2005. Lopinavir concentrations in cerebrospinal fluid exceed the 50% inhibitory concentration for HIV. AIDS 19:949-952. [DOI] [PubMed] [Google Scholar]
- 31.Caricato, A., M. Pennisi, A. Mancino, G. Vigna, C. Sandroni, A. Arcangeli, and M. Antonelli. 2006. Levels of vancomycin in the cerebral interstitial fluid after severe head injury. Intensive Care Med. 32:325-328. [DOI] [PubMed] [Google Scholar]
- 32.Cascio, A., A. Conti, L. Sinardi, C. Iaria, F. F. Angileri, G. Stassi, T. David, A. Versaci, M. Iaria, and A. David. 3 November 2009, posting date. Post-neurosurgical multidrug-resistant Acinetobacter baumannii meningitis successfully treated with intrathecal colistin. A new case and a systematic review of the literature. Int. J. Infect. Dis. [Epub ahead of print.] doi: 10.1016/j.ijid.2009.06.032. [DOI] [PubMed]
- 33.Cawley, M. J., C. Suh, S. Lee, and B. H. Ackerman. 2002. Nontraditional dosing of ampicillin-sulbactam for multidrug-resistant Acinetobacter baumannii meningitis. Pharmacotherapy 22:527-532. [DOI] [PubMed] [Google Scholar]
- 34.Chou, Y. W., Y. H. Yang, J. H. Chen, C. C. Kuo, and S. H. Chen. 2007. Quantification of meropenem in plasma and cerebrospinal fluid by micellar electrokinetic capillary chromatography and application in bacterial meningitis patients. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 856:294-301. [DOI] [PubMed] [Google Scholar]
- 35.Clumeck, N., J. P. Thys, R. Vanhoof, M. P. Vanderlinden, J. P. Butzler, and E. Yourassowsky. 1978. Amoxicillin entry into human cerebrospinal fluid: comparison with ampicillin. Antimicrob. Agents Chemother. 14:531-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cottagnoud, P., M. Pfister, F. Acosta, M. Cottagnoud, L. Flatz, F. Kuhn, H. P. Muller, and A. Stucki. 2004. Daptomycin is highly efficacious against penicillin-resistant and penicillin- and quinolone-resistant pneumococci in experimental meningitis. Antimicrob. Agents Chemother. 48:3928-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Craft, J. C., W. E. Feldman, and J. D. Nelson. 1979. Clinicopharmacological evaluation of amoxicillin and probenecid against bacterial meningitis. Antimicrob. Agents Chemother. 16:346-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crone, C. 1971. The blood-brain barrier—facts and questions, p. 52-62. In B. K. Siesjö and S. C. Sörensen (ed.), Ion homeostasis of the brain. Munksgaard, Kopenhagen, Denmark.
- 39.Cserr, H. F., D. N. Cooper, P. K. Suri, and C. S. Patlak. 1981. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am. J. Physiol. 240:F319-F328. [DOI] [PubMed] [Google Scholar]
- 40.Dacey, R. G., and M. A. Sande. 1974. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob. Agents Chemother. 6:437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dagan, R., L. Velghe, J. L. Rodda, and K. P. Klugman. 1994. Penetration of meropenem into the cerebrospinal fluid of patients with inflamed meninges. J. Antimicrob. Chemother. 34:175-179. [DOI] [PubMed] [Google Scholar]
- 42.Davson, H. 1967. Physiology of the cerebrospinal fluid. Churchill, London, United Kingdom.
- 43.Davson, H., K. Welch, and M. B. Segal. 1987. Physiology and pathophysiology of the cerebrospinal fluid. Churchill Livingstone, London, United Kingdom.
- 44.Debruyne, D. 1997. Clinical pharmacokinetics of fluconazole in superficial and systemic mycoses. Clin. Pharmacokinet. 33:52-77. [DOI] [PubMed] [Google Scholar]
- 45.de Lange, E. C., and M. Danhof. 2002. Considerations in the use of cerebrospinal fluid pharmacokinetics to predict brain target concentrations in the clinical setting: implications of the barriers between blood and brain. Clin. Pharmacokinet. 41:691-703. [DOI] [PubMed] [Google Scholar]
- 46.de Louvois, J., P. Gortvai, and R. Hurley. 1977. Antibiotic treatment of abscesses of the central nervous system. Br. Med. J. 2:985-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Rio, M., G. H. McCracken, Jr., J. D. Nelson, D. Chrane, and S. Shelton. 1982. Pharmacokinetics and cerebrospinal fluid bactericidal activity of ceftriaxone in the treatment of pediatric patients with bacterial meningitis. Antimicrob. Agents Chemother. 22:622-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denes, E., N. Pichon, M. Debette-Gratien, B. Bouteille, and J. M. Gaulier. 2004. Pharmacokinetics of voriconazole in the cerebrospinal fluid of an immunocompromised patient with a brain abscess due to Aspergillus fumigatus. Clin. Infect. Dis. 39:603-604. [DOI] [PubMed] [Google Scholar]
- 49.De Sarro, A., D. Ammendola, M. Zappala, S. Grasso, and G. B. De Sarro. 1995. Relationship between structure and convulsant properties of some beta-lactam antibiotics following intracerebroventricular microinjection in rats. Antimicrob. Agents Chemother. 39:232-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dey, S., S. Gunda, and A. K. Mitra. 2004. Pharmacokinetics of erythromycin in rabbit corneas after single-dose infusion: role of P-glycoprotein as a barrier to in vivo ocular drug absorption. J. Pharmacol. Exp. Ther. 311:246-255. [DOI] [PubMed] [Google Scholar]
- 51.Dickinson, G. M., D. G. Droller, R. L. Greenman, and T. A. Hoffman. 1981. Clinical evaluation of piperacillin with observations on penetrability into cerebrospinal fluid. Antimicrob. Agents Chemother. 20:481-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D'Oliveira, J. J. 1972. Cerebrospinal fluid concentrations of rifampin in meningeal tuberculosis. Am. Rev. Respir. Dis. 106:432-437. [DOI] [PubMed] [Google Scholar]
- 53.Donald, P. R., W. L. Gent, H. I. Seifart, J. H. Lamprecht, and D. P. Parkin. 1992. Cerebrospinal fluid isoniazid concentrations in children with tuberculous meningitis: the influence of dosage and acetylation status. Pediatrics 89:247-250. [PubMed] [Google Scholar]
- 54.Donald, P. R., and H. Seifart. 1988. Cerebrospinal fluid pyrazinamide concentrations in children with tuberculous meningitis. Pediatr. Infect. Dis. J. 7:469-471. [DOI] [PubMed] [Google Scholar]
- 55.Donauer, E., G. Drumm, J. Moringlane, C. Ostertag, and R. Kivelitz. 1987. Intrathecal administration of netilmicin in gentamicin-resistant ventriculitis. Acta Neurochir. (Wien) 86:83-88. [DOI] [PubMed] [Google Scholar]
- 56.Dotevall, L., and L. Hagberg. 1989. Penetration of doxycycline into cerebrospinal fluid in patients treated for suspected Lyme neuroborreliosis. Antimicrob. Agents Chemother. 33:1078-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dudley, M. N., R. E. Levitz, R. Quintiliani, J. M. Hickingbotham, and C. H. Nightingale. 1984. Pharmacokinetics of trimethoprim and sulfamethoxazole in serum and cerebrospinal fluid of adult patients with normal meninges. Antimicrob. Agents Chemother. 26:811-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duswald, K. H., A. Penk, and L. Pittrow. 1997. High-dose therapy with fluconazole > or = 800 mg day−1. Mycoses 40:267-277. [DOI] [PubMed] [Google Scholar]
- 59.Eberl, S., B. Renner, A. Neubert, M. Reisig, I. Bachmakov, J. Konig, F. Dorje, T. E. Murdter, A. Ackermann, H. Dormann, K. G. Gassmann, E. G. Hahn, S. Zierhut, K. Brune, and M. F. Fromm. 2007. Role of p-glycoprotein inhibition for drug interactions: evidence from in vitro and pharmacoepidemiological studies. Clin. Pharmacokinet. 46:1039-1049. [DOI] [PubMed] [Google Scholar]
- 60.Ehrlich, P. 1885. Über das Sauerstoffbedürfnis des Organismus. Eine farbenanalytische Studie. Hirschwald-Verlag, Berlin, Germany.
- 61.Ekstedt, J. 1977. CSF hydrodynamic studies in man. 1. Method of constant pressure CSF infusion. J. Neurol. Neurosurg. Psychiatry 40:105-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ellard, G. A., M. J. Humphries, and B. W. Allen. 1993. Cerebrospinal fluid drug concentrations and the treatment of tuberculous meningitis. Am. Rev. Respir. Dis. 148:650-655. [DOI] [PubMed] [Google Scholar]
- 63.Elter, T., M. Sieniawski, A. Gossmann, C. Wickenhauser, U. Schroder, H. Seifert, J. Kuchta, J. Burhenne, K. D. Riedel, G. Fatkenheuer, and O. A. Cornely. 2006. Voriconazole brain tissue levels in rhinocerebral aspergillosis in a successfully treated young woman. Int. J. Antimicrob. Agents 28:262-265. [DOI] [PubMed] [Google Scholar]
- 64.Elvy, J., D. Porter, and E. Brown. 2008. Treatment of external ventricular drain-associated ventriculitis caused by Enterococcus faecalis with intraventricular daptomycin. J. Antimicrob. Chemother. 61:461-462. [DOI] [PubMed] [Google Scholar]
- 65.Fan-Havard, P., M. C. Nahata, M. H. Bartkowski, W. J. Barson, and E. J. Kosnik. 1990. Pharmacokinetics and cerebrospinal fluid (CSF) concentrations of vancomycin in pediatric patients undergoing CSF shunt placement. Chemotherapy 36:103-108. [DOI] [PubMed] [Google Scholar]
- 66.Farquhar, M. G., and G. E. Palade. 1963. Junctional complexes in various epithelia. J. Cell Biol. 17:375-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Felgenhauer, K. 1980. Protein filtration and secretion at human body fluid barriers. Pflugers Arch. 384:9-17. [DOI] [PubMed] [Google Scholar]
- 68.Fenstermacher, J. D., R. G. Blasberg, and C. S. Patlak. 1981. Methods for quantifying the transport of drugs across brain barrier systems. Pharmacol. Ther. 14:217-248. [DOI] [PubMed] [Google Scholar]
- 69.Flierl, M. A., P. F. Stahel, D. Rittirsch, M. Huber-Lang, A. D. Niederbichler, L. M. Hoesel, B. M. Touban, S. J. Morgan, W. R. Smith, P. A. Ward, and K. Ipaktchi. 2009. Inhibition of complement C5a prevents breakdown of the blood-brain barrier and pituitary dysfunction in experimental sepsis. Crit. Care 13:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fong, I. W., and K. B. Tomkins. 1984. Penetration of ceftazidime into the cerebrospinal fluid of patients with and without evidence of meningeal inflammation. Antimicrob. Agents Chemother. 26:115-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Foudraine, N. A., R. M. Hoetelmans, J. M. Lange, F. de Wolf, B. H. van Benthem, J. J. Maas, I. P. Keet, and P. Portegies. 1998. Cerebrospinal-fluid HIV-1 RNA and drug concentrations after treatment with lamivudine plus zidovudine or stavudine. Lancet 351:1547-1551. [DOI] [PubMed] [Google Scholar]
- 72.Foulds, G., T. J. McBride, A. K. Knirsch, W. J. Rodriguez, and W. N. Khan. 1987. Penetration of sulbactam and ampicillin into cerebrospinal fluid of infants and young children with meningitis. Antimicrob. Agents Chemother. 31:1703-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Friedland, I. R., E. Sultan, K. H. Lehr, and B. Lenfant. 1998. Concentrations of cefpirome in cerebrospinal fluid of children with bacterial meningitis after a single intravenous dose. Antimicrob. Agents Chemother. 42:199-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Friedman, C. A., F. C. Lovejoy, and A. L. Smith. 1979. Chloramphenicol disposition in infants and children. J. Pediatr. 95:1071-1077. [DOI] [PubMed] [Google Scholar]
- 75.Friedrich, H., E. Engel, and J. Potel. 1987. Fosfomycin levels in the cerebrospinal fluid of patients with and without meningitis. Immun. Infekt. 15:98-102. (In German.) [PubMed] [Google Scholar]
- 76.Gaillard, J. L., C. Silly, A. Le Masne, B. Mahut, F. Lacaille, G. Cheron, V. Abadie, P. Hubert, V. Matha, C. Coustere, et al. 1995. Cerebrospinal fluid penetration of amikacin in children with community-acquired bacterial meningitis. Antimicrob. Agents Chemother. 39:253-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gatti, G., J. Hossein, M. Malena, M. Cruciani, and M. Bassetti. 1997. Penetration of dapsone into cerebrospinal fluid of patients with AIDS. J. Antimicrob. Chemother. 40:113-115. [DOI] [PubMed] [Google Scholar]
- 78.Gatti, G., M. Malena, R. Casazza, M. Borin, M. Bassetti, and M. Cruciani. 1998. Penetration of clindamycin and its metabolite N-demethylclindamycin into cerebrospinal fluid following intravenous infusion of clindamycin phosphate in patients with AIDS. Antimicrob. Agents Chemother. 42:3014-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghersi-Egea, J. F., B. Leininger-Muller, R. Cecchelli, and J. D. Fenstermacher. 1995. Blood-brain interfaces: relevance to cerebral drug metabolism. Toxicol. Lett. 82-83:645-653. [DOI] [PubMed] [Google Scholar]
- 80.Goldmann, E. E. 1909. Die äußere und innere Sekretion des gesunden und kranken Organismus im Lichte der “vitalen Färbung.” Beitr. Klin. Chirurg. 64:192-265. [Google Scholar]
- 81.Goldmann, E. E. 1913. Vitalfärbung am Zentralnervensystem. Abh. Preußischen Akad. Wiss. Phys. Math. Kl. 1:1-60. [Google Scholar]
- 82.Gradinaru, D., A. L. Minn, Y. Artur, A. Minn, and J. M. Heydel. 2009. Drug metabolizing enzyme expression in rat choroid plexus: effects of in vivo xenobiotics treatment. Arch. Toxicol. 83:581-586. [DOI] [PubMed] [Google Scholar]
- 83.Green, H. T., M. A. O'Donoghue, M. D. Shaw, and C. Dowling. 1989. Penetration of ceftazidime into intracranial abscess. J. Antimicrob. Chemother. 24:431-436. [DOI] [PubMed] [Google Scholar]
- 84.Groll, A. H., N. Giri, V. Petraitis, R. Petraitiene, M. Candelario, J. S. Bacher, S. C. Piscitelli, and T. J. Walsh. 2000. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J. Infect. Dis. 182:274-282. [DOI] [PubMed] [Google Scholar]
- 85.Gundert-Remy, U., M. Klett, and E. Weber. 1973. Concentration of ethambutol in cerebrospinal fluid in man as a function of the non-protein-bound drug fraction in serum. Eur. J. Clin. Pharmacol. 6:133-136. [DOI] [PubMed] [Google Scholar]
- 86.Haas, D. W., J. Stone, L. A. Clough, B. Johnson, P. Spearman, V. L. Harris, J. Nicotera, R. H. Johnson, S. Raffanti, L. Zhong, P. Bergqwist, S. Chamberlin, V. Hoagland, and W. D. Ju. 2000. Steady-state pharmacokinetics of indinavir in cerebrospinal fluid and plasma among adults with human immunodeficiency virus type 1 infection. Clin. Pharmacol. Ther. 68:367-374. [DOI] [PubMed] [Google Scholar]
- 87.Hanninen, P., and T. Rossi. 1986. Penetration of sulbactam into cerebrospinal fluid of patients with viral meningitis or without meningitis. Rev. Infect. Dis. 8(Suppl. 5):S609-S611. [DOI] [PubMed] [Google Scholar]
- 88.Haworth, S. J., B. Christofalo, R. D. Anderson, and L. M. Dunkle. 1998. A single-dose study to assess the penetration of stavudine into human cerebrospinal fluid in adults. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17:235-238. [DOI] [PubMed] [Google Scholar]
- 89.Hedberg, A., H. G. Hardemark, B. Olsson-Liljequist, and J. Sjolin. 2004. Penetration of fusidic acid and rifampicin into cerebrospinal fluid in low-grade inflammatory meningitis caused by Staphylococcus epidermidis. Clin. Microbiol. Infect. 10:765-768. [DOI] [PubMed] [Google Scholar]
- 90.Hengge, U. R., N. H. Brockmeyer, R. Malessa, U. Ravens, and M. Goos. 1993. Foscarnet penetrates the blood-brain barrier: rationale for therapy of cytomegalovirus encephalitis. Antimicrob. Agents Chemother. 37:1010-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 92.Hofer, S., C. Bopp, C. Hoerner, K. Plaschke, R. M. Faden, E. Martin, H. J. Bardenheuer, and M. A. Weigand. 2008. Injury of the blood brain barrier and up-regulation of icam-1 in polymicrobial sepsis. J. Surg. Res. 146:276-281. [DOI] [PubMed] [Google Scholar]
- 93.Hoffmann, H. G., D. Forster, and B. Muirhead. 1984. Metronidazole concentration of the cerebrospinal fluid from slightly inflamed meninges. Arzneimittelforschung 34:830-831. (In German.) [PubMed] [Google Scholar]
- 94.Hoffmann, H. G., D. Forster, and I. Schoen. 1980. Concentrations of mezlocillin in cerebrospinal fluid in vira meningitis. Infection 8:162-164. (In German.) [DOI] [PubMed] [Google Scholar]
- 95.Hope, W. W., D. Mickiene, V. Petraitis, R. Petraitiene, A. M. Kelaher, J. E. Hughes, M. P. Cotton, J. Bacher, J. J. Keirns, D. Buell, G. Heresi, D. K. Benjamin, Jr., A. H. Groll, G. L. Drusano, and T. J. Walsh. 2008. The pharmacokinetics and pharmacodynamics of micafungin in experimental hematogenous Candida meningoencephalitis: implications for echinocandin therapy in neonates. J. Infect. Dis. 197:163-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Humbert, G., A. Leroy, S. R. Nair, and C. E. Cherubin. 1984. Concentrations of cefotaxime and the desacetyl metabolite in serum and CSF of patients with meningitis. J. Antimicrob. Chemother. 13:487-494. [DOI] [PubMed] [Google Scholar]
- 97.Imai, T., T. Yamamoto, S. Tanaka, M. Kashiwagi, S. Chiba, H. Matsumoto, and T. Uede. 1999. Successful treatment of cerebral aspergillosis with a high oral dose of itraconazole after excisional surgery. Intern. Med. 38:829-832. [DOI] [PubMed] [Google Scholar]
- 98.Jaruratanasirikul, S., R. Hortiwakul, T. Tantisarasart, N. Phuenpathom, and S. Tussanasunthornwong. 1996. Distribution of azithromycin into brain tissue, cerebrospinal fluid, and aqueous humor of the eye. Antimicrob. Agents Chemother. 40:825-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jimenez-Mejias, M. E., J. Pachon, B. Becerril, J. Palomino-Nicas, A. Rodriguez-Cobacho, and M. Revuelta. 1997. Treatment of multidrug-resistant Acinetobacter baumannii meningitis with ampicillin/sulbactam. Clin. Infect. Dis. 24:932-935. [DOI] [PubMed] [Google Scholar]
- 100.Jimenez-Mejias, M. E., C. Pichardo-Guerrero, F. J. Marquez-Rivas, D. Martin-Lozano, T. Prados, and J. Pachon. 2002. Cerebrospinal fluid penetration and pharmacokinetic/pharmacodynamic parameters of intravenously administered colistin in a case of multidrug-resistant Acinetobacter baumannii meningitis. Eur. J. Clin. Microbiol. Infect. Dis. 21:212-214. [DOI] [PubMed] [Google Scholar]
- 101.Jokipii, A. M., V. V. Myllyla, E. Hokkanen, and L. Jokipii. 1977. Penetration of the blood brain barrier by metronidazole and tinidazole. J. Antimicrob. Chemother. 3:239-245. [DOI] [PubMed] [Google Scholar]
- 102.Jourdan, C., J. Convert, A. Peloux, O. Boussaid, J. Grando, and S. Tigaud. 1996. Adequate intrathecal diffusion of teicoplanin after failure of vancomycin, administered in continuous infusion in three cases of shunt associated meningitis. Pathol. Biol. (Paris) 44:389-392. (In French.) [PubMed] [Google Scholar]
- 103.Jung, H., M. Hurtado, M. Sanchez, M. T. Medina, and J. Sotelo. 1990. Plasma and CSF levels of albendazole and praziquantel in patients with neurocysticercosis. Clin. Neuropharmacol. 13:559-564. [DOI] [PubMed] [Google Scholar]
- 104.Kaiser, A. B., and Z. A. McGee. 1975. Aminoglycoside therapy of Gram-negative bacillary meningitis. N. Engl. J. Med. 293:1215-1220. [DOI] [PubMed] [Google Scholar]
- 105.Kanellakopoulou, K., A. Pagoulatou, K. Stroumpoulis, M. Vafiadou, H. Kranidioti, H. Giamarellou, and E. J. Giamarellos-Bourboulis. 2008. Pharmacokinetics of moxifloxacin in non-inflamed cerebrospinal fluid of humans: implication for a bactericidal effect. J. Antimicrob. Chemother. 61:1328-1331. [DOI] [PubMed] [Google Scholar]
- 106.Kaojarern, S., K. Supmonchai, P. Phuapradit, C. Mokkhavesa, and S. Krittiyanunt. 1991. Effect of steroids on cerebrospinal fluid penetration of antituberculous drugs in tuberculous meningitis. Clin. Pharmacol. Ther. 49:6-12. [DOI] [PubMed] [Google Scholar]
- 107.Karlsson, M., S. Hammers-Berggren, L. Lindquist, G. Stiernstedt, and B. Svenungsson. 1994. Comparison of intravenous penicillin G and oral doxycycline for treatment of Lyme neuroborreliosis. Neurology 44:1203-1207. [DOI] [PubMed] [Google Scholar]
- 108.Karlsson, M., S. Hammers, I. Nilsson-Ehle, A. S. Malmborg, and B. Wretlind. 1996. Concentrations of doxycycline and penicillin G in sera and cerebrospinal fluid of patients treated for neuroborreliosis. Antimicrob. Agents Chemother. 40:1104-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kern, W., S. L. Kennedy, M. Sachdeva, E. R. Sande, D. Gunderson, and M. G. Tauber. 1990. Evaluation of piperacillin-tazobactam in experimental meningitis caused by a beta-lactamase-producing strain of K1-positive Escherichia coli. Antimicrob. Agents Chemother. 34:697-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kethireddy, S., and D. Andes. 2007. CNS pharmacokinetics of antifungal agents. Expert Opin. Drug Metab. Toxicol. 3:573-581. [DOI] [PubMed] [Google Scholar]
- 111.Klekner, A., A. Ga'spa'r, S. Kardos, J. Szabo, and G. Cse'csei. 2003. Cefazolin prophylaxis in neurosurgery monitored by capillary electrophoresis. J. Neurosurg. Anesthesiol. 15:249-254. [DOI] [PubMed] [Google Scholar]
- 112.Kossmann, T., V. Hans, R. Stocker, H. G. Imhof, B. Joos, O. Trentz, and M. C. Morganti-Kossmann. 1996. Penetration of cefuroxime into the cerebrospinal fluid of patients with traumatic brain injury. J. Antimicrob. Chemother. 37:161-167. [DOI] [PubMed] [Google Scholar]
- 113.Kourtopoulos, H., and S. E. Holm. 1976. Intraventricular treatment of Serratia marcescens meningitis with gentamicin. Pharmacokinetic studies of gentamicin concentration in one case. Scand. J. Infect. Dis. 8:57-60. [PubMed] [Google Scholar]
- 114.Kravcik, S., K. Gallicano, V. Roth, S. Cassol, N. Hawley-Foss, A. Badley, and D. W. Cameron. 1999. Cerebrospinal fluid HIV RNA and drug levels with combination ritonavir and saquinavir. J. Acquir. Immune Defic. Syndr. 21:371-375. [PubMed] [Google Scholar]
- 115.Kuhnen, E., G. Pfeifer, and C. Frenkel. 1987. Penetration of fosfomycin into cerebrospinal fluid across non-inflamed and inflamed meninges. Infection 15:422-424. [DOI] [PubMed] [Google Scholar]
- 116.Kuroda, M., H. Kusuhara, H. Endou, and Y. Sugiyama. 2005. Rapid elimination of cefaclor from the cerebrospinal fluid is mediated by a benzylpenicillin-sensitive mechanism distinct from organic anion transporter 3. J. Pharmacol. Exp. Ther. 314:855-861. [DOI] [PubMed] [Google Scholar]
- 117.Langer, O., M. Mitterhauser, M. Brunner, M. Zeitlinger, W. Wadsak, B. X. Mayer, K. Kletter, and M. Muller. 2003. Synthesis of fluorine-18-labeled ciprofloxacin for PET studies in humans. Nucl. Med. Biol. 30:285-291. [DOI] [PubMed] [Google Scholar]
- 118.Latif, R., and A. S. Dajani. 1983. Ceftriaxone diffusion into cerebrospinal fluid of children with meningitis. Antimicrob. Agents Chemother. 23:46-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee, D. H., B. Palermo, and M. Chowdhury. 2008. Successful treatment of methicillin-resistant Staphylococcus aureus meningitis with daptomycin. Clin. Infect. Dis. 47:588-590. [DOI] [PubMed] [Google Scholar]
- 120.Leenders, A. C., P. Reiss, P. Portegies, K. Clezy, W. C. Hop, J. Hoy, J. C. Borleffs, T. Allworth, R. H. Kauffmann, P. Jones, F. P. Kroon, H. A. Verbrugh, and S. de Marie. 1997. Liposomal amphotericin B (AmBisome) compared with amphotericin B both followed by oral fluconazole in the treatment of AIDS-associated cryptococcal meningitis. AIDS 11:1463-1471. [DOI] [PubMed] [Google Scholar]
- 121.Letendre, S., J. Marquie-Beck, E. Capparelli, B. Best, D. Clifford, A. C. Collier, B. B. Gelman, J. C. McArthur, J. A. McCutchan, S. Morgello, D. Simpson, I. Grant, and R. J. Ellis. 2008. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch. Neurol. 65:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Letendre, S. L., E. V. Capparelli, R. J. Ellis, J. A. McCutchan, and the HIV Neurobehavioral Research Center Group. 2000. Indinavir population pharmacokinetics in plasma and cerebrospinal fluid. Antimicrob. Agents Chemother. 44:2173-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Levin, V. A. 1980. Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J. Med. Chem. 23:682-684. [DOI] [PubMed] [Google Scholar]
- 124.Levine, J. E., J. T. Povlishock, and D. P. Becker. 1982. The morphological correlates of primate cerebrospinal fluid absorption. Brain Res. 241:31-41. [DOI] [PubMed] [Google Scholar]
- 125.Levitz, R. E., M. N. Dudley, R. Quintiliani, L. D. Mullany, and C. H. Nightingale. 1984. Cerebrospinal fluid penetration of trimethoprim-sulphamethoxazole in two patients with Gram-negative bacillary meningitis. J. Antimicrob. Chemother. 13:400-401. [DOI] [PubMed] [Google Scholar]
- 126.Lewandowsky, M. 1900. Zur Lehre der Cerebrospinalflüssigkeit. Z. Klin. Med. 40:480-494. [Google Scholar]
- 127.Liu, H., H. Davoudi, and T. Last. 1995. Determination of amphotericin B in cerebrospinal fluid by solid-phase extraction and liquid chromatography. J. Pharm. Biomed. Anal. 13:1395-1400. [DOI] [PubMed] [Google Scholar]
- 128.Liu, X., K. Van Natta, H. Yeo, O. Vilenski, P. E. Weller, P. D. Worboys, and M. Monshouwer. 2009. Unbound drug concentration in brain homogenate and cerebral spinal fluid at steady state as a surrogate for unbound concentration in brain interstitial fluid. Drug Metab. Dispos. 37:787-793. [DOI] [PubMed] [Google Scholar]
- 129.Loscher, W., and H. Potschka. 2005. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2:86-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Losonsky, G. A., A. Wolf, R. S. Schwalbe, J. Nataro, C. B. Gibson, and E. W. Lewis. 1994. Successful treatment of meningitis due to multiply resistant Enterococcus faecium with a combination of intrathecal teicoplanin and intravenous antimicrobial agents. Clin. Infect. Dis. 19:163-165. [DOI] [PubMed] [Google Scholar]
- 131.Lutsar, I., and I. R. Friedland. 2000. Pharmacokinetics and pharmacodynamics of cephalosporins in cerebrospinal fluid. Clin. Pharmacokinet. 39:335-343. [DOI] [PubMed] [Google Scholar]
- 132.Lutsar, I., G. H. McCracken, Jr., and I. R. Friedland. 1998. Antibiotic pharmacodynamics in cerebrospinal fluid. Clin. Infect. Dis. 27:1117-1127. [DOI] [PubMed] [Google Scholar]
- 133.Lutsar, I., S. Roffey, and P. Troke. 2003. Voriconazole concentrations in the cerebrospinal fluid and brain tissue of guinea pigs and immunocompromised patients. Clin. Infect. Dis. 37:728-732. [DOI] [PubMed] [Google Scholar]
- 134.Lycke, J., O. Andersen, B. Svennerholm, L. Appelgren, and C. Dahlof. 1989. Acyclovir concentrations in serum and cerebrospinal fluid at steady state. J. Antimicrob. Chemother. 24:947-954. [DOI] [PubMed] [Google Scholar]
- 135.Lycke, J., C. Malmestrom, and L. Stahle. 2003. Acyclovir levels in serum and cerebrospinal fluid after oral administration of valacyclovir. Antimicrob. Agents Chemother. 47:2438-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Malessa, R., H. C. Diener, T. Olbricht, B. Bohmer, and N. H. Brockmeyer. 1994. Successful treatment of meningoencephalitis caused by Mycobacterium avium intracellulare in AIDS. Clin. Invest. 72:850-852. [DOI] [PubMed] [Google Scholar]
- 137.Maniu, C. V., W. C. Hellinger, S. Y. Chu, R. Palmer, and S. Alvarez-Elcoro. 2001. Failure of treatment for chronic Mycobacterium abscessus meningitis despite adequate clarithromycin levels in cerebrospinal fluid. Clin. Infect. Dis. 33:745-748. [DOI] [PubMed] [Google Scholar]
- 138.Manosuthi, W., P. Chetchotisakd, T. Nolen, D. Wallace, S. Sungkanuparph, T. Anekthananon, K. Supparatpinyo, P. Pappas, R. Larsen, S. Filler, and D. Andes. 2010. Monitoring and impact of fluconazole serum and cerebrospinal fluid concentration in HIV-associated cryptococcal meningitis-infected patients. HIV Med. 11:276-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Markantonis, S. L., N. Markou, M. Fousteri, N. Sakellaridis, S. Karatzas, I. Alamanos, E. Dimopoulou, and G. Baltopoulos. 2009. Penetration of colistin into cerebrospinal fluid. Antimicrob. Agents Chemother. 53:4907-4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Martin, C., A. Sonnerborg, J. O. Svensson, and L. Stahle. 1999. Indinavir-based treatment of HIV-1 infected patients: efficacy in the central nervous system. AIDS 13:1227-1232. [DOI] [PubMed] [Google Scholar]
- 141.Martin, E., J. R. Koup, U. Paravicini, and K. Stoeckel. 1984. Pharmacokinetics of ceftriaxone in neonates and infants with meningitis. J. Pediatr. 105:475-481. [DOI] [PubMed] [Google Scholar]
- 142.Matsuda, T., K. Ikawa, K. Ikeda, N. Morikawa, R. Tsumura, M. Shibukawa, K. Iida, and K. Kurisu. 2009. LC method for the determination of meropenem in cerebrospinal fluid: application totherapeutic drug monitoring. Chromatographia 69:1031-1034. [Google Scholar]
- 143.May, C., J. A. Kaye, J. R. Atack, M. B. Schapiro, R. P. Friedland, and S. I. Rapoport. 1990. Cerebrospinal fluid production is reduced in healthy aging. Neurology 40:500-503. [DOI] [PubMed] [Google Scholar]
- 144.McComb, J. G. 1983. Recent research into the nature of cerebrospinal fluid formation and absorption. J. Neurosurg. 59:369-383. [DOI] [PubMed] [Google Scholar]
- 145.McCracken, G. H., Jr., S. G. Mize, and N. Threlkeld. 1980. Intraventricular gentamicin therapy in Gram-negative bacillary meningitis of infancy. Report of the Second Neonatal Meningitis Cooperative Study Group. Lancet i:787-791. [PubMed] [Google Scholar]
- 146.McDowell, J. A., G. E. Chittick, J. R. Ravitch, R. E. Polk, T. M. Kerkering, and D. S. Stein. 1999. Pharmacokinetics of [(14)C]abacavir, a human immunodeficiency virus type 1 (HIV-1) reverse transcriptase inhibitor, administered in a single oral dose to HIV-1-infected adults: a mass balance study. Antimicrob. Agents Chemother. 43:2855-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McLeod, R., D. Mack, R. Foss, K. Boyer, S. Withers, S. Levin, J. Hubbell, and the Toxoplasmosis Study Group. 1992. Levels of pyrimethamine in sera and cerebrospinal and ventricular fluids from infants treated for congenital toxoplasmosis. Antimicrob. Agents Chemother. 36:1040-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Menichetti, F., M. Fiorio, A. Tosti, G. Gatti, M. Bruna Pasticci, F. Miletich, M. Marroni, D. Bassetti, and S. Pauluzzi. 1996. High-dose fluconazole therapy for cryptococcal meningitis in patients with AIDS. Clin. Infect. Dis. 22:838-840. [DOI] [PubMed] [Google Scholar]
- 149.Miller, D. S., B. Bauer, and A. M. Hartz. 2008. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol. Rev. 60:196-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mindermann, T., W. Zimmerli, and O. Gratzl. 1998. Rifampin concentrations in various compartments of the human brain: a novel method for determining drug levels in the cerebral extracellular space. Antimicrob. Agents Chemother. 42:2626-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mindermann, T., W. Zimmerli, Z. Rajacic, and O. Gratzl. 1993. Penetration of fusidic acid into human brain tissue and cerebrospinal fluid. Acta Neurochir. (Wien) 121:12-14. [DOI] [PubMed] [Google Scholar]
- 152.Minn, A., J. F. Ghersi-Egea, R. Perrin, B. Leininger, and G. Siest. 1991. Drug metabolizing enzymes in the brain and cerebral microvessels. Brain Res. Brain Res. Rev. 16:65-82. [DOI] [PubMed] [Google Scholar]
- 153.Mitja, O., C. Pigrau, I. Ruiz, X. Vidal, B. Almirante, A. M. Planes, I. Molina, D. Rodriguez, and A. Pahissa. 2009. Predictors of mortality and impact of aminoglycosides on outcome in listeriosis in a retrospective cohort study. J. Antimicrob. Chemother. 64:416-423. [DOI] [PubMed] [Google Scholar]
- 154.Mizen, L., G. Woodnutt, I. Kernutt, and E. J. Catherall. 1989. Simulation of human serum pharmacokinetics of ticarcillin-clavulanic acid and ceftazidime in rabbits, and efficacy against experimental Klebsiella pneumoniae meningitis. Antimicrob. Agents Chemother. 33:693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Modai, J., D. Vittecoq, J. M. Decazes, and A. Meulemans. 1985. Penetration of imipenem and cilastatin into cerebrospinal fluid of patients with bacterial meningitis. J. Antimicrob. Chemother. 16:751-755. [DOI] [PubMed] [Google Scholar]
- 156.Modai, J., D. Vittecoq, J. M. Decazes, M. Wolff, and A. Meulemans. 1983. Penetration of ceftazidime into cerebrospinal fluid of patients with bacterial meningitis. Antimicrob. Agents Chemother. 24:126-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moskopp, D., and E. Lotterer. 1993. Concentrations of albendazole in serum, cerebrospinal fluid and hydatidous brain cyst. Neurosurg. Rev. 16:35-37. [DOI] [PubMed] [Google Scholar]
- 158.Mursch, K., S. Trnovec, H. Ratz, D. Hammer, R. Horre, A. Klinghammer, S. de Hoog, and J. Behnke-Mursch. 2006. Successful treatment of multiple Pseudallescheria boydii brain abscesses and ventriculitis/ependymitis in a 2-year-old child after a near-drowning episode. Childs Nerv. Syst. 22:189-192. [DOI] [PubMed] [Google Scholar]
- 159.Nabeshima, S., T. S. Reese, D. M. Landis, and M. W. Brightman. 1975. Junctions in the meninges and marginal glia. J. Comp. Neurol. 164:127-169. [DOI] [PubMed] [Google Scholar]
- 160.Nadstawek, J., H. P. Weil, C. Frenkel, E. Kuhnen, W. Mauersberger, and U. Hornchen. 1989. Serum and cerebrospinal fluid kinetics of ceftazidime. Immun. Infekt. 17:212-216. (In German.) [PubMed] [Google Scholar]
- 161.Nagata, Y., H. Kusuhara, H. Endou, and Y. Sugiyama. 2002. Expression and functional characterization of rat organic anion transporter 3 (rOat3) in the choroid plexus. Mol. Pharmacol. 61:982-988. [DOI] [PubMed] [Google Scholar]
- 162.Nahata, M. C., D. E. Durrell, and W. J. Barson. 1986. Ceftriaxone kinetics and cerebrospinal fluid penetration in infants and children with meningitis. Chemotherapy 32:89-94. [DOI] [PubMed] [Google Scholar]
- 163.Nahata, M. C., P. Fan-Havard, W. J. Barson, H. M. Bartkowski, and E. J. Kosnik. 1990. Pharmacokinetics, cerebrospinal fluid concentration, and safety of intravenous rifampin in pediatric patients undergoing shunt placements. Eur. J. Clin. Pharmacol. 38:515-517. [DOI] [PubMed] [Google Scholar]
- 164.Nahata, M. C., P. Fan-Havard, E. J. Kosnik, M. H. Bartkowski, and W. J. Barson. 1990. Pharmacokinetics and cerebrospinal fluid concentration of nafcillin in pediatric patients undergoing cerebrospinal fluid shunt placement. Chemotherapy 36:98-102. [DOI] [PubMed] [Google Scholar]
- 165.Nahata, M. C., V. M. Kohlbrenner, and W. J. Barson. 1993. Pharmacokinetics and cerebrospinal fluid concentrations of cefixime in infants and young children. Chemotherapy 39:1-5. [DOI] [PubMed] [Google Scholar]
- 166.Nau, R. 1992. Pharmakokinetische Untersuchungen zum Übertritt verschiedener Antibiotika und Osmotherapeutika in den ventrikulären Liquor cerebrospinalis des Menschen. Fachbereich Medizin, Universität Göttingen, Göttingen, Germany.
- 167.Nau, R., D. Emrich, and H. W. Prange. 1993. Inverse correlation between disappearance of intrathecally injected 111In-DTPA from CSF with CSF protein content and CSF-to-serum albumin ratio. J. Neurol. Sci. 115:102-104. [DOI] [PubMed] [Google Scholar]
- 168.Nau, R., M. Kinzig-Schippers, F. Sorgel, S. Schinschke, R. Rossing, C. Muller, H. Kolenda, and H. W. Prange. 1997. Kinetics of piperacillin and tazobactam in ventricular cerebrospinal fluid of hydrocephalic patients. Antimicrob. Agents Chemother. 41:987-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nau, R., M. Kinzig, T. Dreyhaupt, H. Kolenda, F. Sorgel, and H. W. Prange. 1994. Kinetics of ofloxacin and its metabolites in cerebrospinal fluid after a single intravenous infusion of 400 milligrams of ofloxacin. Antimicrob. Agents Chemother. 38:1849-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nau, R., C. Lassek, M. Kinzig-Schippers, A. Thiel, H. W. Prange, and F. Sorgel. 1998. Disposition and elimination of meropenem in cerebrospinal fluid of hydrocephalic patients with external ventriculostomy. Antimicrob. Agents Chemother. 42:2012-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nau, R., and H. W. Prange. 1996. Estimation of steady state antibiotic concentration in cerebrospinal fluid from single-dose kinetics. Eur. J. Clin. Pharmacol. 49:407-409. [DOI] [PubMed] [Google Scholar]
- 172.Nau, R., H. W. Prange, M. Kinzig, A. Frank, A. Dressel, P. Scholz, H. Kolenda, and F. Sorgel. 1996. Cerebrospinal fluid ceftazidime kinetics in patients with external ventriculostomies. Antimicrob. Agents Chemother. 40:763-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nau, R., H. W. Prange, J. Martell, S. Sharifi, H. Kolenda, and J. Bircher. 1990. Penetration of ciprofloxacin into the cerebrospinal fluid of patients with uninflamed meninges. J. Antimicrob. Chemother. 25:965-973. [DOI] [PubMed] [Google Scholar]
- 174.Nau, R., H. W. Prange, S. Menck, H. Kolenda, K. Visser, and J. K. Seydel. 1992. Penetration of rifampicin into the cerebrospinal fluid of adults with uninflamed meninges. J. Antimicrob. Chemother. 29:719-724. [DOI] [PubMed] [Google Scholar]
- 175.Nau, R., H. W. Prange, P. Muth, G. Mahr, S. Menck, H. Kolenda, and F. Sorgel. 1993. Passage of cefotaxime and ceftriaxone into cerebrospinal fluid of patients with uninflamed meninges. Antimicrob. Agents Chemother. 37:1518-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nau, R., T. Schmidt, K. Kaye, J. L. Froula, and M. G. Tauber. 1995. Quinolone antibiotics in therapy of experimental pneumococcal meningitis in rabbits. Antimicrob. Agents Chemother. 39:593-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nau, R., P. Scholz, S. Sharifi, S. Rohde, H. Kolenda, and H. W. Prange. 1993. Netilmicin cerebrospinal fluid concentrations after an intravenous infusion of 400 mg in patients without meningeal inflammation. J. Antimicrob. Chemother. 32:893-896. [DOI] [PubMed] [Google Scholar]
- 178.Nau, R., F. Sorgel, and H. W. Prange. 1994. Lipophilicity at pH 7.4 and molecular size govern the entry of the free serum fraction of drugs into the cerebrospinal fluid in humans with uninflamed meninges. J. Neurol. Sci. 122:61-65. [DOI] [PubMed] [Google Scholar]
- 179.Nau, R., G. Zysk, A. Thiel, and H. W. Prange. 1993. Pharmacokinetic quantification of the exchange of drugs between blood and cerebrospinal fluid in man. Eur. J. Clin. Pharmacol. 45:469-475. [DOI] [PubMed] [Google Scholar]
- 180.Nilsson, C., F. Stahlberg, C. Thomsen, O. Henriksen, M. Herning, and C. Owman. 1992. Circadian variation in human cerebrospinal fluid production measured by magnetic resonance imaging. Am. J. Physiol. 262:R20-R24. [DOI] [PubMed] [Google Scholar]
- 181.Nix, D. E., J. H. Wilton, N. Velasquez, J. L. Budny, H. B. Lassman, P. Mitchell, K. Divan, and J. J. Schentag. 1992. Cerebrospinal fluid penetration of cefpirome in patients with non-inflamed meninges. J. Antimicrob. Chemother. 29(Suppl. A):51-57. [DOI] [PubMed] [Google Scholar]
- 182.Nolan, C. M., and W. C. Ulmer, Jr. 1980. A study of cephalothin and desacetylcephalothin in cerebrospinal fluid in therapy for experimental pneumococcal meningitis. J. Infect. Dis. 141:326-330. [DOI] [PubMed] [Google Scholar]
- 183.Norrby, S. R. 1985. Role of cephalosporins in the treatment of bacterial meningitis in adults. Overview with special emphasis on ceftazidime. Am. J. Med. 79:56-61. [DOI] [PubMed] [Google Scholar]
- 184.Ohno, K., K. D. Pettigrew, and S. I. Rapoport. 1978. Lower limits of cerebrovascular permeability to nonelectrolytes in the conscious rat. Am. J. Physiol. 235:H299-H307. [DOI] [PubMed] [Google Scholar]
- 185.Okugawa, S., Y. Ota, K. Tatsuno, K. Tsukada, S. Kishino, and K. Koike. 2007. A case of invasive central nervous system aspergillosis treated with micafungin with monitoring of micafungin concentrations in the cerebrospinal fluid. Scand. J. Infect. Dis. 39:344-346. [DOI] [PubMed] [Google Scholar]
- 186.Overbosch, D., J. C. van de Nes, E. Groll, H. W. Diekmann, A. M. Polderman, and H. Mattie. 1987. Penetration of praziquantel into cerebrospinal fluid and cysticerci in human cysticercosis. Eur. J. Clin. Pharmacol. 33:287-292. [DOI] [PubMed] [Google Scholar]
- 187.Pardridge, W. M., R. Sakiyama, and G. Fierer. 1983. Transport of propranolol and lidocaine through the rat blood-brain barrier. Primary role of globulin-bound drug. J. Clin. Invest. 71:900-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Paris, M. M., S. M. Hickey, M. I. Uscher, S. Shelton, K. D. Olsen, and G. H. McCracken, Jr. 1994. Effect of dexamethasone on therapy of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob. Agents Chemother. 38:1320-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pea, F., E. Ferrari, F. Pavan, D. Roman-Pognuz, F. Bandello, and M. Furlanut. 2005. Levofloxacin disposition over time in aqueous humor of patients undergoing cataract surgery. Antimicrob. Agents Chemother. 49:2554-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Periti, P., T. Mazzei, E. Mini, and A. Novelli. 1989. Clinical pharmacokinetic properties of the macrolide antibiotics. Effects of age and various pathophysiological states (part II). Clin. Pharmacokinet. 16:261-282. [DOI] [PubMed] [Google Scholar]
- 191.Pfausler, B., H. P. Haring, A. Kampfl, J. Wissel, M. Schober, and E. Schmutzhard. 1997. Cerebrospinal fluid (CSF) pharmacokinetics of intraventricular vancomycin in patients with staphylococcal ventriculitis associated with external CSF drainage. Clin. Infect. Dis. 25:733-735. [DOI] [PubMed] [Google Scholar]
- 192.Pfausler, B., H. Spiss, R. Beer, A. Kampl, K. Engelhardt, M. Schober, and E. Schmutzhard. 2003. Treatment of staphylococcal ventriculitis associated with external cerebrospinal fluid drains: a prospective randomized trial of intravenous compared with intraventricular vancomycin therapy. J. Neurosurg. 98:1040-1044. [DOI] [PubMed] [Google Scholar]
- 193.Pfausler, B., H. Spiss, P. Dittrich, M. Zeitlinger, E. Schmutzhard, and C. Joukhadar. 2004. Concentrations of fosfomycin in the cerebrospinal fluid of neurointensive care patients with ventriculostomy-associated ventriculitis. J. Antimicrob. Chemother. 53:848-852. [DOI] [PubMed] [Google Scholar]
- 194.Pfister, H. W., V. Preac-Mursic, B. Wilske, and K. M. Einhaupl. 1989. Cefotaxime vs penicillin G for acute neurologic manifestations in Lyme borreliosis. A prospective randomized study. Arch. Neurol. 46:1190-1194. [DOI] [PubMed] [Google Scholar]
- 195.Pfister, H. W., V. Preac-Mursic, B. Wilske, E. Schielke, F. Sorgel, and K. M. Einhaupl. 1991. Randomized comparison of ceftriaxone and cefotaxime in Lyme neuroborreliosis. J. Infect. Dis. 163:311-318. [DOI] [PubMed] [Google Scholar]
- 196.Popovic, M., D. Steinort, S. Pillai, and C. Joukhadar. 2010. Fosfomycin: an old, new friend? Eur. J. Clin. Microbiol. Infect. Dis. 29:127-142. [DOI] [PubMed] [Google Scholar]
- 197.Quagliarello, V. J., A. Ma, H. Stukenbrok, and G. E. Palade. 1991. Ultrastructural localization of albumin transport across the cerebral microvasculature during experimental meningitis in the rat. J. Exp. Med. 174:657-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Radouane, A., F. Pehourcq, G. Tramu, E. E. Creppy, and B. Bannwarth. 1996. Influence of lipophilicity on the diffusion of cephalosporins into the cerebrospinal fluid. Fundam. Clin. Pharmacol. 10:309-313. [DOI] [PubMed] [Google Scholar]
- 199.Raffi, F., A. M. Taburet, B. Ghaleh, A. Huart, and E. Singlas. 1993. Penetration of foscarnet into cerebrospinal fluid of AIDS patients. Antimicrob. Agents Chemother. 37:1777-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rahal, J. J., Jr., P. J. Hyams, M. S. Simberkoff, and E. Rubinstein. 1974. Combined intrathecal and intramuscular gentamicin for Gram-negative meningitis. Pharmacologic study of 21 patients. N. Engl. J. Med. 290:1394-1398. [DOI] [PubMed] [Google Scholar]
- 201.Rall, D. P., W. W. Oppelt, and C. S. Patlak. 1962. Extracellular space of brain as determined by diffusion of inulin from the ventricular system. Life Sci. 2:43-48. [Google Scholar]
- 202.Reese, T. S., N. Feder, and M. W. Brightman. 1971. Electron microscopic study of the blood-brain and blood-cerebrospinal fluid barriers with microperoxidase. J. Neuropathol. Exp. Neurol. 30:137-138. [PubMed] [Google Scholar]
- 203.Reese, T. S., and M. J. Karnovsky. 1967. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 34:207-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Reesor, C., A. W. Chow, A. Kureishi, and P. J. Jewesson. 1988. Kinetics of intraventricular vancomycin in infections of cerebrospinal fluid shunts. J. Infect. Dis. 158:1142-1143. [DOI] [PubMed] [Google Scholar]
- 205.Ricard, J. D., M. Wolff, J. C. Lacherade, B. Mourvillier, N. Hidri, G. Barnaud, G. Chevrel, L. Bouadma, and D. Dreyfuss. 2007. Levels of vancomycin in cerebrospinal fluid of adult patients receiving adjunctive corticosteroids to treat pneumococcal meningitis: a prospective multicenter observational study. Clin. Infect. Dis. 44:250-255. [DOI] [PubMed] [Google Scholar]
- 206.Rich, J. D., and M. Mirochnick. 1996. Dapsone penetrates cerebrospinal fluid during Pneumocystis carinii pneumonia prophylaxis. Diagn. Microbiol. Infect. Dis. 24:77-79. [DOI] [PubMed] [Google Scholar]
- 207.Roberts, J. A., and J. Lipman. 2009. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 37:840-851, 859. [DOI] [PubMed] [Google Scholar]
- 208.Rodvold, K. A., M. H. Gotfried, M. Cwik, J. M. Korth-Bradley, G. Dukart, and E. J. Ellis-Grosse. 2006. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J. Antimicrob. Chemother. 58:1221-1229. [DOI] [PubMed] [Google Scholar]
- 209.Rolinski, B., J. R. Bogner, I. Sadri, U. Wintergerst, and F. D. Goebel. 1997. Absorption and elimination kinetics of zidovudine in the cerebrospinal fluid in HIV-1-infected patients. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 15:192-197. [DOI] [PubMed] [Google Scholar]
- 210.Ruping, M. J., N. Albermann, F. Ebinger, I. Burckhardt, C. Beisel, C. Muller, J. J. Vehreschild, M. Kochanek, G. Fatkenheuer, C. Bangard, A. J. Ullmann, W. Herr, K. Kolbe, M. Hallek, and O. A. Cornely. 2008. Posaconazole concentrations in the central nervous system. J. Antimicrob. Chemother. 62:1468-1470. [DOI] [PubMed] [Google Scholar]
- 211.Rupprecht, T. A., and H. W. Pfister. 2005. Clinical experience with linezolid for the treatment of central nervous system infections. Eur. J. Neurol. 12:536-542. [DOI] [PubMed] [Google Scholar]
- 212.Sadler, B. M., G. E. Chittick, R. E. Polk, D. Slain, T. M. Kerkering, S. D. Studenberg, Y. Lou, K. H. Moore, J. L. Woolley, and D. S. Stein. 2001. Metabolic disposition and pharmacokinetics of [14C]-amprenavir, a human immunodeficiency virus type 1 (HIV-1) protease inhibitor, administered as a single oral dose to healthy male subjects. J. Clin. Pharmacol. 41:386-396. [DOI] [PubMed] [Google Scholar]
- 213.Saez-Llorens, X., E. Castano, R. Garcia, C. Baez, M. Perez, F. Tejeira, and G. H. McCracken, Jr. 1995. Prospective randomized comparison of cefepime and cefotaxime for treatment of bacterial meningitis in infants and children. Antimicrob. Agents Chemother. 39:937-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Saunders, N. R., M. D. Habgood, and K. M. Dziegielewska. 1999. Barrier mechanisms in the brain. II. Immature brain. Clin. Exp. Pharmacol. Physiol. 26:85-91. [DOI] [PubMed] [Google Scholar]
- 215.Sawchuk, R. J., and M. A. Hedaya. 1990. Modeling the enhanced uptake of zidovudine (AZT) into cerebrospinal fluid. 1. Effect of probenecid. Pharm. Res. 7:332-338. [DOI] [PubMed] [Google Scholar]
- 216.Scheld, W. M., R. G. Dacey, H. R. Winn, J. E. Welsh, J. A. Jane, and M. A. Sande. 1980. Cerebrospinal fluid outflow resistance in rabbits with experimental meningitis. Alterations with penicillin and methylprednisolone. J. Clin. Invest. 66:243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Schievink, H. I., H. Mattie, R. T. Thomeer, and E. Van Strijen. 1993. The passage of cloxacillin into cerebrospinal fluid in the absence of meningitis. Br. J. Clin. Pharmacol. 36:57-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Schinkel, A. H. 1999. P-glycoprotein, a gatekeeper in the blood-brain barrier. Adv. Drug Deliv. Rev. 36:179-194. [DOI] [PubMed] [Google Scholar]
- 219.Schinkel, A. H., J. J. Smit, O. van Tellingen, J. H. Beijnen, E. Wagenaar, L. van Deemter, C. A. Mol, M. A. van der Valk, E. C. Robanus-Maandag, H. P. te Riele, et al. 1994. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 77:491-502. [DOI] [PubMed] [Google Scholar]
- 220.Schmidt, H., G. Zysk, R. R. Reinert, W. Bruck, A. Stringaris, V. Chen, K. Stuertz, F. Fischer, R. Bartels, K. J. Schaper, S. Weinig, and R. Nau. 1997. Rifabutin for experimental pneumococcal meningitis. Chemotherapy 43:264-271. [DOI] [PubMed] [Google Scholar]
- 221.Schmidt, T., J. Froula, and M. G. Tauber. 1993. Clarithromycin lacks bactericidal activity in cerebrospinal fluid in experimental pneumococcal meningitis. J. Antimicrob. Chemother. 32:627-632. [DOI] [PubMed] [Google Scholar]
- 222.Schwartz, S., and E. Thiel. 2009. Cerebral aspergillosis: tissue penetration is the key. Med. Mycol. 47(Suppl. 1):S387-S393. [DOI] [PubMed] [Google Scholar]
- 223.Scotton, P. G., F. Pea, M. Giobbia, M. Baraldo, A. Vaglia, and M. Furlanut. 2001. Cerebrospinal fluid penetration of levofloxacin in patients with spontaneous acute bacterial meningitis. Clin. Infect. Dis. 33:e109-e111. [DOI] [PubMed] [Google Scholar]
- 224.Serabe, B. M., D. J. Murry, R. Dauser, J. Nuchtern, J. Durfee, L. McGuffey, S. Berg, and S. M. Blaney. 1999. Plasma and CSF pharmacokinetics of ganciclovir in nonhuman primates. Cancer Chemother. Pharmacol. 43:415-418. [DOI] [PubMed] [Google Scholar]
- 225.Shah, M. V., K. L. Audus, and R. T. Borchardt. 1989. The application of bovine brain microvessel endothelial-cell monolayers grown onto polycarbonate membranes in vitro to estimate the potential permeability of solutes through the blood-brain barrier. Pharm. Res. 6:624-627. [DOI] [PubMed] [Google Scholar]
- 226.Shah, S., A. Ohlsson, and V. Shah. 2004. Intraventricular antibiotics for bacterial meningitis in neonates. Cochrane Database Syst. Rev. 2004:CD004496. [DOI] [PubMed] [Google Scholar]
- 227.Shapiro, W. R., D. F. Young, and B. M. Mehta. 1975. Methotrexate: distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. N. Engl. J. Med. 293:161-166. [DOI] [PubMed] [Google Scholar]
- 228.Shin, S. G., J. K. Roh, N. S. Lee, J. G. Shin, I. J. Jang, C. W. Park, and H. J. Myung. 1990. Kinetics of isoniazid transfer into cerebrospinal fluid in patients with tuberculous meningitis. J. Korean Med. Sci. 5:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sirinavin, S., S. Chiemchanya, P. Visudhipan, and S. Lolekha. 1984. Cefuroxime treatment of bacterial meningitis in infants and children. Antimicrob. Agents Chemother. 25:273-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sjolin, J., N. Eriksson, P. Arneborn, and O. Cars. 1991. Penetration of cefotaxime and desacetylcefotaxime into brain abscesses in humans. Antimicrob. Agents Chemother. 35:2606-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sjovall, J., S. Bergdahl, G. Movin, S. Ogenstad, and M. Saarimaki. 1989. Pharmacokinetics of foscarnet and distribution to cerebrospinal fluid after intravenous infusion in patients with human immunodeficiency virus infection. Antimicrob. Agents Chemother. 33:1023-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Spector, R. 1986. Ceftriaxone pharmacokinetics in the central nervous system. J. Pharmacol. Exp. Ther. 236:380-383. [PubMed] [Google Scholar]
- 233.Spector, R. 2010. Nature and consequences of mammalian brain and CSF efflux transporters: four decades of progress. J. Neurochem. 112:13-23. [DOI] [PubMed] [Google Scholar]
- 234.Spector, R., and A. V. Lorenzo. 1974. Inhibition of penicillin transport from the cerebrospinal fluid after intracisternal inoculation of bacteria. J. Clin. Invest. 54:316-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Stahl, J. P., J. Croize, M. Wolff, J. J. Garaud, P. Leclercq, F. Vachon, and M. Micoud. 1987. Poor penetration of teicoplanin into cerebrospinal fluid in patients with bacterial meningitis. J. Antimicrob. Chemother. 20:141-142. [DOI] [PubMed] [Google Scholar]
- 236.Steele, R. W., L. B. Eyre, R. W. Bradsher, R. E. Weinfeld, I. H. Patel, and J. Spicehandler. 1983. Pharmacokinetics of ceftriaxone in pediatric patients with meningitis. Antimicrob. Agents Chemother. 23:191-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Takayanagui, O. M., P. S. Bonato, S. A. Dreossi, and V. L. Lanchote. 2002. Enantioselective distribution of albendazole metabolites in cerebrospinal fluid of patients with neurocysticercosis. Br. J. Clin. Pharmacol. 54:125-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tashima, K. T., A. M. Caliendo, M. Ahmad, J. M. Gormley, W. D. Fiske, J. M. Brennan, and T. P. Flanigan. 1999. Cerebrospinal fluid human immunodeficiency virus type 1 (HIV-1) suppression and efavirenz drug concentrations in HIV-1-infected patients receiving combination therapy. J. Infect. Dis. 180:862-864. [DOI] [PubMed] [Google Scholar]
- 239.Tauber, M. G., C. A. Doroshow, C. J. Hackbarth, M. G. Rusnak, T. A. Drake, and M. A. Sande. 1984. Antibacterial activity of beta-lactam antibiotics in experimental meningitis due to Streptococcus pneumoniae. J. Infect. Dis. 149:568-574. [DOI] [PubMed] [Google Scholar]
- 240.Thea, D., and M. Barza. 1989. Use of antibacterial agents in infections of the central nervous system. Infect. Dis. Clin. North Am. 3:553-570. [PubMed] [Google Scholar]
- 241.Tozzi, V., P. Balestra, M. F. Salvatori, C. Vlassi, G. Liuzzi, M. L. Giancola, M. Giulianelli, P. Narciso, and A. Antinori. 2009. Changes in cognition during antiretroviral therapy: comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders. J. Acquir. Immune Defic. Syndr. 52:56-63. [DOI] [PubMed] [Google Scholar]
- 242.Tucker, R. M., P. L. Williams, E. G. Arathoon, B. E. Levine, A. I. Hartstein, L. H. Hanson, and D. A. Stevens. 1988. Pharmacokinetics of fluconazole in cerebrospinal fluid and serum in human coccidioidal meningitis. Antimicrob. Agents Chemother. 32:369-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Tunkel, A. R., B. J. Hartman, S. L. Kaplan, B. A. Kaufman, K. L. Roos, W. M. Scheld, and R. J. Whitley. 2004. Practice guidelines for the management of bacterial meningitis. Clin. Infect. Dis. 39:1267-1284. [DOI] [PubMed] [Google Scholar]
- 244.Turner, S. A., J. D. Maclean, L. Fleckenstein, and C. Greenaway. 2005. Parenteral administration of ivermectin in a patient with disseminated strongyloidiasis. Am. J. Trop. Med. Hyg. 73:911-914. [PubMed] [Google Scholar]
- 245.van Bree, J. B., A. G. de Boer, M. Danhof, L. A. Ginsel, and D. D. Breimer. 1988. Characterization of an “in vitro” blood-brain barrier: effects of molecular size and lipophilicity on cerebrovascular endothelial transport rates of drugs. J. Pharmacol. Exp. Ther. 247:1233-1239. [PubMed] [Google Scholar]
- 246.van der Valk, P. G., E. J. Kraai, P. C. van Voorst Vader, H. Haaxma-Reiche, and J. A. Snijder. 1988. Penicillin concentrations in cerebrospinal fluid (CSF) during repository treatment regimen for syphilis. Genitourin. Med. 64:223-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Van Laethem, K., and A. M. Vandamme. 2006. Interpreting resistance data for HIV-1 therapy management—know the limitations. AIDS Rev. 8:37-43. [PubMed] [Google Scholar]
- 248.van Lelyveld, S. F., M. Nijhuis, F. Baatz, I. Wilting, W. M. van den Bergh, M. Kurowski, D. de Jong, A. I. Hoepelman, and A. M. Wensing. 2010. Therapy failure following selection of enfuvirtide-resistant HIV-1 in cerebrospinal fluid. Clin. Infect. Dis. 50:387-390. [DOI] [PubMed] [Google Scholar]
- 249.van Praag, R. M., E. C. van Weert, R. P. van Heeswijk, X. J. Zhou, J. P. Sommadossi, S. Jurriaans, J. M. Lange, R. M. Hoetelmans, and J. M. Prins. 2002. Stable concentrations of zidovudine, stavudine, lamivudine, abacavir, and nevirapine in serum and cerebrospinal fluid during 2 years of therapy. Antimicrob. Agents Chemother. 46:896-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Vernazza, P., S. Daneel, V. Schiffer, L. Decosterd, W. Fierz, T. Klimkait, M. Hoffmann, and B. Hirschel. 2007. The role of compartment penetration in PI-monotherapy: the Atazanavir-Ritonavir Monomaintenance (ATARITMO) Trial. AIDS 21:1309-1315. [DOI] [PubMed] [Google Scholar]
- 251.Viladrich, P. F., C. Cabellos, R. Pallares, F. Tubau, J. Martinez-Lacasa, J. Linares, and F. Gudiol. 1996. High doses of cefotaxime in treatment of adult meningitis due to Streptococcus pneumoniae with decreased susceptibilities to broad-spectrum cephalosporins. Antimicrob. Agents Chemother. 40:218-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Villani, P., M. B. Regazzi, F. Marubbi, P. Viale, L. Pagani, F. Cristini, B. Cadeo, G. Carosi, and R. Bergomi. 2002. Cerebrospinal fluid linezolid concentrations in postneurosurgical central nervous system infections. Antimicrob. Agents Chemother. 46:936-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Vladic, A., N. Strikic, D. Jurcic, M. Zmajevic, M. Klarica, and M. Bulat. 2000. Homeostatic role of the active transport in elimination of [3H]benzylpenicillin out of the cerebrospinal fluid system. Life Sci. 67:2375-2385. [DOI] [PubMed] [Google Scholar]
- 254.von Wedel-Parlow, M., P. Wolte, and H. J. Galla. 2009. Regulation of major efflux transporters under inflammatory conditions at the blood-brain barrier in vitro. J. Neurochem. 111:111-118. [DOI] [PubMed] [Google Scholar]
- 255.Wagshul, M. E., J. J. Chen, M. R. Egnor, E. J. McCormack, and P. E. Roche. 2006. Amplitude and phase of cerebrospinal fluid pulsations: experimental studies and review of the literature. J. Neurosurg. 104:810-819. [DOI] [PubMed] [Google Scholar]
- 256.Wald, S. L., and R. L. McLaurin. 1980. Cerebrospinal fluid antibiotic levels during treatment of shunt infections. J. Neurosurg. 52:41-46. [DOI] [PubMed] [Google Scholar]
- 257.Wang, E. E., and C. G. Prober. 1983. Ventricular cerebrospinal fluid concentrations of trimethoprim-sulphamethoxazole. J. Antimicrob. Chemother. 11:385-389. [DOI] [PubMed] [Google Scholar]
- 258.Warner, J. F., R. L. Perkins, and L. Cordero. 1979. Metronidazole therapy of anaerobic bacteremia, meningitis, and brain abscess. Arch. Intern. Med. 139:167-169. [PubMed] [Google Scholar]
- 259.Weindl, A. 1973. Neuroendocrine aspects of circumventricular organs, p. 3-32. In F. Ganong and L. Martini (ed.), Frontiers in neuroendicrionology. Oxford University Press, New York, NY.
- 260.Weiss, L. M., C. Harris, M. Berger, H. B. Tanowitz, and M. Wittner. 1988. Pyrimethamine concentrations in serum and cerebrospinal fluid during treatment of acute Toxoplasma encephalitis in patients with AIDS. J. Infect. Dis. 157:580-583. [DOI] [PubMed] [Google Scholar]
- 261.Wolff, M., L. Boutron, E. Singlas, B. Clair, J. M. Decazes, and B. Regnier. 1987. Penetration of ciprofloxacin into cerebrospinal fluid of patients with bacterial meningitis. Antimicrob. Agents Chemother. 31:899-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wolff, M., P. Chavanet, A. Kazmierczak, A. Pechinot, C. Dematons, H. Portier, and B. Lenfant. 1992. Diffusion of cefpirome into the cerebrospinal fluid of patients with purulent meningitis. J. Antimicrob. Chemother. 29(Suppl. A):59-62. [DOI] [PubMed] [Google Scholar]
- 263.Wong, V. K., H. T. Wright, Jr., L. A. Ross, W. H. Mason, C. B. Inderlied, and K. S. Kim. 1991. Imipenem/cilastatin treatment of bacterial meningitis in children. Pediatr. Infect. Dis. J. 10:122-125. [DOI] [PubMed] [Google Scholar]
- 264.Wynn, H. E., R. C. Brundage, and C. V. Fletcher. 2002. Clinical implications of CNS penetration of antiretroviral drugs. CNS Drugs 16:595-609. [DOI] [PubMed] [Google Scholar]
- 265.Yeh, H. H., Y. H. Yang, Y. W. Chou, J. Y. Ko, C. A. Chou, and S. H. Chen. 2005. Determination of ceftazidime in plasma and cerebrospinal fluid by micellar electrokinetic chromatography with direct sample injection. Electrophoresis 26:927-934. [DOI] [PubMed] [Google Scholar]
- 266.Yilmaz, A., M. Gisslen, S. Spudich, E. Lee, A. Jayewardene, F. Aweeka, and R. W. Price. 2009. Raltegravir cerebrospinal fluid concentrations in HIV-1 infection. PLoS One 4:e6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Yilmaz, A., A. Izadkhashti, R. W. Price, P. W. Mallon, M. De Meulder, P. Timmerman, and M. Gisslen. 2009. Darunavir concentrations in cerebrospinal fluid and blood in HIV-1-infected individuals. AIDS Res. Hum. Retroviruses 25:457-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yilmaz, A., L. Stahle, L. Hagberg, B. Svennerholm, D. Fuchs, and M. Gisslen. 2004. Cerebrospinal fluid and plasma HIV-1 RNA levels and lopinavir concentrations following lopinavir/ritonavir regimen. Scand. J. Infect. Dis. 36:823-828. [DOI] [PubMed] [Google Scholar]
- 269.Yim, C. W., N. M. Flynn, and F. T. Fitzgerald. 1985. Penetration of oral doxycycline into the cerebrospinal fluid of patients with latent or neurosyphilis. Antimicrob. Agents Chemother. 28:347-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yogev, R., W. M. Kolling, and T. Williams. 1981. Pharmacokinetic comparison of intravenous and oral chloramphenicol in patients with Haemophilus influenzae meningitis. Pediatrics 67:656-660. [PubMed] [Google Scholar]
- 271.Zastre, J. A., G. N. Chan, P. T. Ronaldson, M. Ramaswamy, P. O. Couraud, I. A. Romero, B. Weksler, M. Bendayan, and R. Bendayan. 2009. Up-regulation of P-glycoprotein by HIV protease inhibitors in a human brain microvessel endothelial cell line. J. Neurosci. Res. 87:1023-1036. [DOI] [PubMed] [Google Scholar]
- 272.Ziai, W. C., and J. J. Lewin III. 2009. Improving the role of intraventricular antimicrobial agents in the management of meningitis. Curr. Opin. Neurol. 22:277-282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.