Abstract
Summary: This review hopes to improve the selection of new tuberculosis (TB) vaccines by providing several perspectives on the immunization of humans, mice, guinea pigs, rabbits, and monkeys which have not usually been considered. (i) In human TB vaccine trials, the low rate of healing of Mycobacterium bovis BCG lesions (used as the control group) would distinguish individuals who might be helped by vaccination from the 95% who do not need it and would make these trials more conclusive. (ii) The rabbit immune response to Mycobacterium tuberculosis is much more effective in arresting tuberculosis than those of other laboratory animals, so pulmonary tubercle counting in rabbits should be included in all preclinical TB vaccine testing. (iii) Both delayed-type hypersensitivity (DTH) and cell-mediated immunity (CMI) are necessary to control the growth of M. tuberculosis. The testing of new TB vaccines in mice or in guinea pigs may not detect important antigens needed for human immunization. Mice respond poorly to tuberculin-like antigens that cause DTH. Guinea pigs respond poorly to antigens that cause CMI. Rabbits and humans respond well to both DTH and CMI antigens. Since monkeys are very susceptible to M. tuberculosis, they may not be as useful as rabbits for preclinical vaccine evaluation. (iv) Critical antigens (possibly ESAT-6 or CFP-10) might increase the immunity of the host to a greater extent than that produced by a natural M. tuberculosis infection and therefore would be useful in both prophylaxis and immunotherapy. Such critical antigens would increase the host's ability to neutralize key components of M. tuberculosis that enable it to survive in both laboratory animals and humans.
INTRODUCTION
New tuberculosis (TB) vaccines (better than the current Mycobacterium bovis BCG vaccines) are greatly needed to control this disease, which every year kills 2 to 3 million persons in the world today. Clinical trials of new vaccines are very expensive and less precise than the testing of these vaccines with laboratory animals, so it behooves us to obtain as much information as possible from laboratory animals before clinical trials are undertaken.
This is not a review of the current immunological literature that dissects the various components of the immune process, but it is an analysis of how delayed-type hypersensitivity (DTH) and cell-mediated immunity (CMI) in humans, mice, guinea pigs, rabbits, and monkeys could influence TB vaccine selection. In other words, this review focuses on the overall “woods” rather than on the individual “trees” that comprise the woods. The woods are the presence and the characteristics of the pulmonary tuberculous lesions themselves. The trees are the many individual factors (genes, transduction factors, cytokines, and microbicidins) that may affect the development of such lesions.
This review suggests (i) that in clinical trials the selection among new TB vaccines would be more precise if the rates of healing of the positive-control BCG group were taken into consideration; (ii) that in preclinical trials the selection of new TB vaccines would be more precise if tubercle counting in rabbits was always included, along with evaluations of mice and guinea pigs; and (iii) that the evaluations of new vaccines in rabbits would detect differences between two candidate TB vaccines better than would evaluations in mice and guinea pigs, because the immunity to Mycobacterium tuberculosis developed by rabbits is much stronger than that developed by the other two species; i.e., the difference between vaccinated and unvaccinated rabbits would therefore span a larger range.
The inclusion of tubercle counting in rabbits (a species that develops both good DTH and good CMI) would enable a more precise selection of new TB vaccines. In current preclinical TB vaccine evaluations, tubercle counting in rabbits has not been undertaken before the start of more-expensive clinical trials. However, the use of rabbits in preclinical TB vaccine testing could reduce the number of inconclusive clinical trials and save much time (as well as millions of dollars) in the development of better TB vaccines for worldwide use. Vaccine evaluation with rabbits seems to be even more pertinent than vaccine evaluation with monkeys, because monkeys also have a relatively weak immune response to M. tuberculosis and therefore would respond less well to vaccines.
With today's emphasis on molecular biology, many fundamental concepts of TB pathogenesis are often overlooked in the selection of new TB vaccines. This review calls many of these fundamental concepts to our attention.
WHY SOME CLINICAL TRIALS FAIL TO DETECT BENEFITS FROM BCG VACCINES
BCG vaccination usually increases host resistance to infection with virulent tubercle bacilli in almost every common laboratory animal. In fact, in laboratory animals, BCG is usually the standard to which new candidate vaccines are compared. BCG should also increase resistance in humans. Therefore, why do some clinical trials fail to show any benefit from BCG vaccination? Below are some possibilities.
High and Low Responders
The high-responding group includes individuals who convert their tuberculin skin tests but show no evidence of tuberculosis. This group, which comprises roughly 95% of healthy human beings, arrests the disease without vaccination (3, 97). These individuals do not need BCG, because they produce a good immune response without it.
The remaining 5% of individuals (the low-responding group) develop clinically active disease and may even die from it. These individuals evidently produce an insufficient immune response, so an effective TB vaccine could reduce the number of clinically active tuberculosis cases to 1%. In other words, the TB vaccine would protect about 80% of this group (6, 7, 74, 92). Complete protection of every individual may never be achieved.
Note that the 95%, 4%, and 1% of individuals approximate those found in populations in industrial countries (e.g., the United States and Europe). Developing countries with a high percentage of immunodeficient individuals (e.g., human immunodeficiency virus [HIV]) would have a different proportion in each group (27). The percentages found in industrial countries are used herein merely to designate each group in a simple manner.
High Prevalence of HIV
A high prevalence of infection with HIV exists in some developing countries, especially in sub-Saharan Africa. HIV infection lowers host acquired (adaptive) immunity to the tubercle bacillus (11, 27). Therefore, HIV-infected persons would respond less well to BCG vaccination than would persons who are not infected with HIV.
BCG vaccination would protect some M. tuberculosis-infected/HIV-infected individuals from developing clinically active disease when the HIV only partly decreased their immune response. In other words, HIV infection would transfer some individuals from the 95% group (who did not need the vaccine) to the intermediate group (who could be helped by the vaccine). However, BCG vaccination would have no benefit or could even be detrimental if HIV greatly lowered the immune response. In this case, HIV would transfer individuals from the 4% intermediate group (that would benefit from the vaccine) to the 1% immunodeficient group (that could not be helped by the vaccine). In the Karonga/Malawi BCG trial, 57% of cases of clinical tuberculosis were directly attributable to HIV infection (27).
High Prevalence of Intestinal Helminths and Poor Nutrition
In developing countries, tuberculosis and intestinal worm infections often occur in the same groups of people (106). Worm infections may cause some debilitation and lower host resistance to tuberculosis (106). Therefore, worm-infested populations would respond less well to BCG vaccination than would noninfested populations (47, 106). Poor nutrition may possibly have a similar effect (18, 62).
Environmental Mycobacteria
Environmental mycobacteria could have increased immunity in unvaccinated control groups (20, 27, 48), so the beneficial effects of BCG in the Malawi trial (27) and in other inconclusive trials (13, 20, 21, 48) would be hard to detect.
Absence of Booster PPD Skin Testing
Many individuals in every unvaccinated control group might show a booster reaction if tested again with tuberculin (purified protein derivative [PPD]) (23, 77, 101). If they did, they would already have substantial immunity from a healed natural infection, so BCG vaccination would provide relatively little additional benefit.
Variations in BCG Strains
Although the currently available BCG vaccines used in these trials were genetically different from the original BCG (13, 21, 48, 80, 88), recent studies with guinea pigs indicated that these differences may be small and not critical (56). However, when currently available BCG vaccines were evaluated with mice, some major differences were found (58).
Human Genetic Differences
BCG vaccination reduced clinical tuberculosis in North American Indians by about 80% (6, 7). This indigenous population had probably not been exposed to the tubercle bacillus for as many centuries as had the populations in Europe and Asia and therefore might have had more individuals in the low-responding group who could benefit from vaccination (98). In other words, fewer individuals were in the high-responding group, who did not need BCG immunization. Also, the BCG strains used in these studies may have more closely resembled those originally developed by Calmette and Guérin.
In contrast, the BCG trial in Chingleput, South India (102), could have been unsuccessful partly because most of the individuals who were not infected with HIV were in the high-responding group, who required no vaccination. In other words, the number of individuals in the low-responding group who could be helped by vaccination was small and hard to detect. Also, some of the factors listed above may have made the benefits of BCG vaccination unrecognizable.
Conclusion
I am not a statistician, but I believe that even the large number of individuals in BCG clinical trials does not compensate for every factor listed above. Clinical trials would be much more precise if more of these factors were identified and were accurately balanced between the control and vaccinated groups beforehand. In other words, the many factors listed above make it hard to detect those individuals for whom BCG vaccination actually prevented clinical disease (see Identifying the “∼4%” Group That Would Be Helped by BCG Vaccination below).
IDENTIFYING THE “∼4%” GROUP THAT WOULD BE HELPED BY BCG VACCINATION
Most clinical trials take relatively few of the above-described factors into consideration. If we had a way to eliminate from the trial the ∼95% of individuals who can arrest an early primary pulmonary TB lesion without clinically active disease as well as to eliminate from the trial the ∼1% of individuals who cannot be helped by the vaccine (because of some immunodeficiency), then the remaining ∼4% would undoubtedly show benefits from BCG vaccination comparable to those found for laboratory animals.
This ∼4% group was never specifically identified in the human populations that participated in recorded TB vaccine trials. However, with rabbits, Lurie et al. reported a way to do so (68), and this method could easily be used for human trials. Lurie found that his inbred resistant rabbits healed dermal BCG lesions faster than did his inbred susceptible rabbits. Therefore, Lurie chose the rabbits that healed their dermal BCG lesions the fastest as breeders for his resistant stock and chose the rabbits that healed their dermal BCG lesions the slowest as breeders for his two susceptible stocks.
Similarly, if standardized, the rate of healing of BCG skin lesions could be used on a sample of the human population for whom a clinical trial was planned. BCG lesions with intermediate rates of healing would identify the size of the ∼4% group that could benefit from the vaccine. If the ∼4% intermediate group was sufficiently large, the benefits of a good tuberculosis vaccine would be easily recognized, but if this ∼4% intermediate group was small, the candidate vaccines should probably be evaluated in a more favorable human population.
Also, during the main clinical trial, the rates of healing in the BCG-vaccinated group could be determined. (BCG is often used as a positive control for new vaccines.) Individuals in the ∼4% group (who could benefit from vaccination) would again show intermediate rates of BCG healing. Next, several years later, during the statistical analysis of the trial, the amount of clinically active TB developed in these ∼4% of vaccinees could be compared to the amount in the unvaccinated control group. I predict that these ∼4% of BCG vaccinees will always show a reduced amount of clinically active pulmonary tuberculosis.
Unfortunately, during the main clinical trial, there is no way of identifying this ∼4% group within the nonvaccinated control group (which would have greatly improved the statistics). Nevertheless, the identification of the ∼4% group in only the BCG-vaccinated group could reduce statistical variation enough to regain confidence in BCG vaccination for clinical use.
Note that (i) since millions of persons receive intradermal BCG, the delayed healing of their BCG lesions could identify individuals who are genetically most susceptible to M. tuberculosis; (ii) the rate of healing of intradermal BCG lesions in newborns should not be compared with rate of healing of intradermal BCG lesions in older individuals, because the immune system of newborns is still developing (see BCG in Newborn Infants below); and (iii) also, if an individual had previously received BCG (or had an inapparent TB infection), the next dermal BCG lesion would progress more rapidly and heal faster (15).
STOPPING THE DEVELOPMENT OF PRIMARY PULMONARY TUBERCLES IS A MAIN REASON FOR VACCINATION AGAINST TUBERCULOSIS FOR BOTH HUMANS AND LABORATORY ANIMALS
TB vaccines are usually given to prevent clinically apparent disease, i.e., to prevent early primary pulmonary tubercles from progressing to an X-ray-visible size. Vaccines have little or no effect on the activation of pulmonary alveolar macrophages (AM), because most AM are nonspecifically activated by ingesting a variety of inhaled particles (36, 41) and not by the expanded antigen-specific lymphocyte population produced by the vaccine.
In rabbits and in humans, most early pulmonary tubercles (caused by M. tuberculosis) are arrested by the host's immune response. Therefore, vaccine efficacy in rabbits can be measured by a reduction in the number of primary tubercles seen 5 weeks after the aerosol inhalation of virulent M. tuberculosis (see Tubercle Count Method below). However, early tubercles in mice and guinea pigs are not easily arrested. Therefore, we strongly advise that tubercle counting in rabbits be included in all preclinical TB vaccine evaluations.
Effective vaccination of mice and guinea pigs slows or inhibits bacillary growth, decreases bacillary titers in the lungs (see Comparisons of Tuberculosis in Humans, Rabbits, Mice, and Guinea Pigs below), and prolongs the life of the host. Vaccination would also lower the number of visible primary pulmonary tubercles if the mice and guinea pigs were sacrificed at the appropriate time. However, mice and guinea pigs develop relatively poor TB immunity, because they usually die of the disease. Therefore, the reduction in the number of visible primary tubercles should not be nearly as great as the reduction produced by the same vaccine in rabbits (and humans). Also, in mice and guinea pigs, many tiny primary tubercles that were not seen at the time of sacrifice would probably become visible at a later date.
TUBERCLE COUNT METHOD
Tubercle counting has been performed mainly for rabbits. The number of grossly visible primary pulmonary tubercles (Fig. 1) is counted about 5 weeks after an aerosol exposure to virulent M. tuberculosis (31, 33, 35, 64, 65, 67, 68, 71). The number of visible primary tubercles is decreased by (i) the number of M. tuberculosis bacilli that are immediately destroyed by pulmonary alveolar macrophages and (ii) the number of early tubercles that the immune response prevents from reaching a visible size.
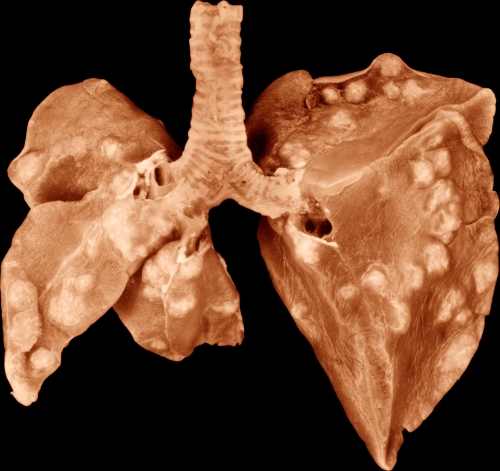
FIG. 1.
Formalin-fixed lungs of a commercial New Zealand White rabbit that inhaled about 33,000 virulent human-type tubercle bacilli (H37Rv) 5 weeks previously. Upon dissection, these lungs contained 131 grossly visible primary tubercles with no apparent grossly visible secondary tubercles. The “ratio” (i.e., the number of tubercle bacilli estimated to be inhaled divided by the number of grossly visible primary tubercles produced) was 250. In other words, in this rabbit, 250 viable H37Rv tubercle bacilli must be inhaled to produce each visible primary pulmonary tubercle. Effective BCG (and other effective vaccines for tuberculosis) should increase this ratio at least 5-fold (35, 68). Small areas of caseous necrosis are visible in many of the tubercles. On the left, this photograph shows the ventral surface of the right upper lobe, right middle lobe, and azygous lobe. On the right, the photograph shows the ventral surface of the left upper lobe and left lower lobe. The right lower lobe (RLL) had been removed for culture. This RLL contained 23 grossly visible tubercles and 1.35 × 105 culturable tubercle bacilli. Magnification, ×1.04. (Reproduced from reference 31 with permission of the publisher.)
Mice and guinea pigs develop one primary lesion for every 3 to 15 inhaled virulent M. tuberculosis bacilli (Table 1) (37, 55, 85) and usually die of the disease. In other words, they are much more susceptible than rabbits and humans. Prior BCG vaccination would reduce the amount of tuberculosis in mice and guinea pigs, because their primary lesions would be smaller, and the disease would progress more slowly (Fig. 2 and 3). However, in these hosts relatively few primary lesions would probably be fully arrested by the immune response. The challenge of mice and guinea pigs with M. tuberculosis of reduced virulence would make tubercle counting much more applicable to these hosts, but such studies have yet to be done. Counting of primary pulmonary tubercles in mice may require microscopy (see references 38 and 39).
TABLE 1.
Number of inhaled tubercle bacilli required to produce one primary pulmonary tubercle (the “ratio”) and the amount of multiplication during the logarithmic growth phase in unvaccinated rabbits, mice, and guinea pigsa
| Animal | Average no. of inhaled virulent bovine-type bacilli (Ravenel) required to produce 1 pulmonary tubercle | Average no. of inhaled virulent human-type bacilli (H37Rv) required to produce 1 pulmonary tubercle | Increase in no. of virulent bovine-type bacilli (Ravenel) before stationary phase | Increase in no. of virulent human-type bacilli (H37Rv) before stationary phase |
|---|---|---|---|---|
| Lurie's resistant rabbitsb (including commercial rabbits) | 3-15 | 300-3,000 | 1,000,000×e | 1,000×e |
| Mice (C57BL/6)c | 3-15 | 3-15 | 10,000× | 10,000×f |
| Guinea pigsd | 3-15 | 3-15 | No data | 1,000×g |
Adapted from reference 33. Note that this table shows the responses of the most frequently used laboratory animal species to the inhalation of virulent M. tuberculosis and virulent M. bovis. It also shows the importance of tissue-damaging DTH in limiting bacillary titers. Guinea pigs (with strong DTH) develop lower bacillary titers than do mice (with weak DTH). However, in spite of fewer viable bacilli, the progression of the disease seems to be more rapid in guinea pigs because of their relatively poor cell-mediated immunity. Monkeys are very susceptible to M. tuberculosis, and, as with mice and guinea pigs, only 3 to 15 inhaled virulent tubercle bacilli seem to be required to produce one visible primary pulmonary lesion (see Tuberculosis in Nonhuman Primates).
Lurie's resistant rabbits (e.g., strain III) (64, 65, 67, 69). Commercially available New Zealand White rabbits seem to be similar (24, 25, 33, 35, 37, 44, 71).
From reference 81. Bacillary multiplication in other mouse strains may be different from those listed here for C57BL/6 mice (see references 75 and 76). C57BL/6 is a relatively resistant strain of mouse.
From Fig. 4.
From Fig. 2.
From Fig. 3.
FIG. 2.
Number of viable virulent human-type (H37Rv) tubercle bacilli in the lungs of unvaccinated or BCG-vaccinated C57BL/6 mice at each interval following quantitative airborne infection. (C57BL/6 is a relatively resistant strain of mouse.) The vaccinated mice received 106 viable BCG tubercle bacilli subcutaneously 6 weeks before they were challenged by aerosol with H37Rv. Other strains of mice may respond somewhat differently (45). Note that the initial logarithmic growth phase was followed by a plateau and is similar to that of the guinea pigs represented in Fig. 3 and to that of the rabbits represented in Fig. 4. Note also that the H37Rv bacillary titers reached higher levels in nonvaccinated mice and guinea pigs than in rabbits. Vaccination of mice lowers mycobacterial titers, because an effective immune response occurs faster. However, vaccination does not arrest the disease in mice (or in guinea pigs). (Courtesy of Ian M. Orme, Colorado State University, Fort Collins, CO; reproduced with permission.)
FIG. 3.
Number of viable virulent human-type tubercle bacilli (H37Rv) in the lungs of BCG-vaccinated or control guinea pigs at each interval following quantitative airborne infection. Note that the in vivo bacillary growth curves for guinea pigs resemble those found for mice (Fig. 2) and for rabbits (Fig. 4). (Other experiments showed that the stationary phase in guinea pigs continues at least 18 weeks [2]). BCG-vaccinated guinea pigs are tuberculin positive before challenge. Therefore, the logarithmic growth stage ends sooner, and the number of viable tubercle bacilli is markedly reduced. In the vaccinated host, the disease is less severe, and the animals live longer. However, they usually die of the disease, because they do not develop effective CMI. The guinea pigs were immunized intradermally with live BCG 6 weeks before the aerosol challenge. Note also that BCG vaccination of guinea pigs lowers the bacillary titers 2 to 3 logs, whereas BCG in mice (Fig. 2) lowers these titers only 1 log (see also reference 49). BCG makes the guinea pigs strongly tuberculin positive but produces only a weak DTH in mice. This comparison further supports the principle that tissue-damaging DTH is an important immunological host defense against the intracellular multiplication of tubercle bacilli. However, Fig. 2 and 3 are not strictly comparable, because the mice represented in Fig. 2 inhaled more tubercle bacilli than the guinea pigs represented in Fig. 3. (Reproduced from reference 95 with permission of the American Thoracic Society. Copyright © American Thoracic Society.)
Three facts should be considered in evaluating TB vaccines by tubercle counting in any laboratory animal. (i) The more virulent the challenge strain of tubercle bacillus, the smaller will be the number of inhaled bacilli required to generate a visible primary tubercle (Table 1) and the smaller will be the difference in tubercle counts produced by effective and noneffective vaccines. (ii) Currently available rabbits are not inbred. Therefore, at least 18 rabbits in each group will often be required to get statistically significant results, i.e., 18 in the nonvaccinated group, 18 in the BCG-vaccinated group, and also 18 in each group receiving a new vaccine. This number was derived from data in reference 35 by the late Helen Abbey of our Department of Biostatistics. (iii) Rabbits, mice, guinea pigs, and monkeys often respond differently to each antigen in M. tuberculosis. Such species differences are unavoidable. This is the main reason why TB vaccines should be evaluated in several laboratory animal species before clinical trials are begun. Nevertheless, rabbits, like the majority of human beings, have high native and acquired resistance to infection by virulent M. tuberculosis and would therefore be more likely than other laboratory species to respond to TB antigens in similar ways. Unfortunately, except for the studies of Lurie et al. prior to 1964 (33, 64, 68, 69) and the few studies by our TB group here at the Johns Hopkins Center for Tuberculosis Research (33, 35, 44, 71), tubercle counting in rabbits has been almost completely neglected.
THE LARGEST DIFFERENCE BETWEEN TWO CANDIDATE VACCINES WILL BE FOUND IN HOSTS WITH THE STRONGEST IMMUNE RESPONSE
The differences between two TB vaccines may be harder to distinguish in mice and guinea pigs than in rabbits, because mice and guinea pigs do not stop the growth of M. tuberculosis as well as rabbits and humans do: the more effective the host's control of virulent tubercle bacilli, the more effective will be its immune response to vaccination. In other words, differences between two vaccines will be more easily recognized for animals that develop the strongest immune response, because the difference between the control and the vaccinated animals will span a larger range.
This concept was clearly illustrated by Lurie et al. (68), who showed that the number of visible primary pulmonary tubercles (produced by M. tuberculosis) in susceptible rabbits was not appreciably decreased by prior BCG vaccination, whereas the number of these primary tubercles in resistant rabbits was decreased to 20% of those found in the unvaccinated controls; i.e., the increased immunity produced by BCG in Lurie's resistant rabbits prevented 80% of the developing tubercles from reaching a visible size.
In other words, Lurie's susceptible rabbits developed rather poor immunity during infection with virulent M. tuberculosis and therefore developed relatively little increase in immunity from vaccination. His resistant rabbits developed rather good immunity during infection with virulent M. tuberculosis and therefore developed a substantial increase in immunity from vaccination. Commercially available New Zealand White rabbits resemble Lurie's resistant rabbits (24, 25, 35, 44, 71).
Similar to Lurie's susceptible rabbits, mice and guinea pigs develop relatively poor overall immunity during infection with virulent M. tuberculosis. Therefore, vaccination of mice and guinea pigs should have relatively little effect on stopping the eventual progression of primary pulmonary tubercles. In fact, the disease progresses in these susceptible hosts and would usually be lethal in time (83). However, mice and guinea pigs show a good immune response to some M. tuberculosis antigens and would be useful in selecting these antigens for TB vaccines (see below).
EFFECTIVE TB VACCINES MUST PRODUCE APPROPRIATE AMOUNTS OF DELAYED- TYPE HYPERSENSITIVITY AND CELL- MEDIATED IMMUNITY
Role of DTH and CMI in the Pathogenesis of Tuberculosis
TB is the classic disease with which to study the interplay between delayed-type hypersensitivity (DTH) and cell-mediated immunity (CMI) (30, 33, 40). DTH and CMI are similar immunological processes produced by Th1 lymphocytes. However, DTH and CMI inhibit the growth of M. tuberculosis by different mechanisms. In guinea pigs, rabbits, and humans, DTH kills nonactivated macrophages (that become overloaded with M. tuberculosis) by producing solid caseous necrosis (in which the bacilli do not grow) (30, 33), whereas CMI activates macrophages so that ingested M. tuberculosis cells are inhibited or even killed (30, 33, 70). Macrophages that are activated by CMI before they ingest tubercle bacilli are probably more effective than macrophages that are activated by CMI after they have ingested tubercle bacilli.
In guinea pigs, in rabbits, and, undoubtedly, in humans, tuberculin-like DTH stops the initial (intracellular) logarithmic growth of tubercle bacilli in early pulmonary TB lesions by causing solid caseous necrosis, in which the bacillus does not grow. This conclusion is derived from correlating bacillary numbers with the histopathology observed. In the tuberculous host, tuberculin sensitivity and caseous necrosis always develop at the same time (64; also see references 16 and 87). Before tuberculin sensitivity develops, the bacillus multiplies intracellularly without injuring the macrophage in which it resides (64).
Nonactivated macrophages continually enter tuberculous lesions, until the lesions are fully healed (32). Tubercle bacilli grow easily in these nonactivated macrophages. However, DTH kills these overloaded macrophages and stops further bacillary growth, often (as stated above) by causing solid caseous necrosis, in which bacilli do not grow.
Many macrophages that have been activated by CMI surround the caseous center of the lesion. Such activated macrophages ingest, inhibit, and even kill any free tubercle bacilli that they encounter (including the bacilli released from macrophages killed by DTH). Numerous CMI-activated macrophages are necessary to stop the progression of the lesion.
In brief, within every TB lesion, nonactivated and activated macrophages are always present. Therefore, (i) both local DTH and local CMI are needed to arrest the disease, and (ii) good TB vaccines will enhance both DTH and CMI in the proper proportion.
Antigens Producing DTH and/or CMI
The main difference between antigens eliciting DTH and antigens eliciting CMI is the concentration at which they produce these in vivo effects. In tuberculous lesions, tuberculin-like DTH antigens kill nonactivated macrophages within which tubercle bacilli have multiplied extensively at very low local concentrations, because the local concentration of the tuberculin-like products soon reaches tissue-damaging levels (30, 33). CMI antigens evidently activate macrophages to inhibit M. tuberculosis growth at higher concentrations (30, 33).
For skin testing of people, 1 tuberculin unit (1 TU) of PPD (first strength) or 5 TU (intermediate strength) is frequently used. One TU contains 0.00002 mg of PPD in the 0.1 ml used for the intradermal injection. If the second strength of PPD (250 TU, i.e., 0.005 mg) is injected intradermally in a person who is known to be strongly tuberculin positive, caseous necrosis will develop at the site of the tuberculin injection. In other words, the concentration of tuberculin is still very low when it can stop the intracellular multiplication of the bacillus by killing bacillus-laden macrophages.
In a host with a good immune response, tuberculin-like DTH antigens may be toxic when the host becomes hypersensitive to them, but CMI antigens are usually nontoxic. Nevertheless, CMI antigens in very large concentrations would probably produce necrosis, and tuberculin-like DTH antigens in very small concentrations do, in fact, activate macrophages without necrosis (5).
A list of DTH- and CMI-producing antigens has never been made. Many of those so far identified are reviewed in references 3, 4, 52, 61, 64, 79, and 89. In general, the proteins, peptides, and carbohydrates in tuberculin seem to produce DTH, and other proteins complexed with carbohydrates and lipids seem to produce CMI (reviewed in reference 33).
Note that to date, no laboratory has analyzed the known TB antigens for the amount of DTH and the amount of CMI that each antigen produces. For DTH, such an investigation would involve determining the minimal concentration of each antigen that elicits a positive (antigen-specific) skin test in tuberculous rabbits, guinea pigs, or even humans and, possibly, the minimal concentration of each antigen required in vitro to kill macrophages that contain live tubercle bacilli (see reference 61). For CMI, such an investigation would involve determining the concentration of each antigen that activates macrophages sufficiently to inhibit the intracellular growth of virulent tubercle bacilli. Unfortunately, the concentrations found in vitro may or may not match those found locally in vivo.
WHEN VACCINATED FOR TB, LABORATORY ANIMAL SPECIES DEVELOP DIFFERENT AMOUNTS OF DTH AND CMI
Immune Response to DTH Antigens
Mice develop little DTH to tuberculin-like antigens. Guinea pigs and rabbits develop considerable DTH. Humans are more sensitive to tuberculin than any laboratory animal species (Table 2) (37, 42). In other words, tuberculin-like DTH antigens produce caseous necrosis in humans at very low concentrations and in guinea pigs and rabbits at somewhat higher concentrations, but caseous necrosis usually does not occur in mice. For this reason, I propose that mice would be a poor species with which to recognize tuberculin-like DTH antigens in new vaccines, and guinea pigs and rabbits would be rather good species.
TABLE 2.
Characteristics of tuberculosis in humans and in laboratory animalsa
| Species | TB characteristicf |
|||
|---|---|---|---|---|
| Tuberculin-type allergy (DTH) | Caseous necrosis (due to DTH)b | CMI | Cavity formation | |
| Isolated human populationsc | +++++ | +++++ | ++ | +++ |
| Rhesus monkeysd | ++ | +++++ | ++ | +++ |
| Guinea pigs | +++ | +++++ | ++ | + |
| Modern humans | ||||
| Immunocompetent | +++++ | +++++ | ++++ | +++++ |
| Immunosuppressed | + | +++++ | + | + |
| Rabbitse | ||||
| Resistant | ++ | +++ | ++++ | ++++ |
| Susceptible | ++ | +++++ | ++ | 0 |
| Mice | + | +/− | +++ | 0 |
Reproduced from reference 37 and based on data from a table by Francis (50). References 29 and 100 reproduce the entire Francis table (which contains many other animal species, including elephants).
Tissue-damaging DTH is the cause of caseous necrosis. Tubercle bacilli are dormant in solid caseum, and many do not survive there. In arrested human and rabbit tuberculous lesions, solid caseum is often encapsulated by fibrous tissue.
Isolated human populations refer to those who had been exposed to M. tuberculosis only during the last several centuries, such as the Senegalese troops brought from Africa to Europe during the first World War (14, 28; see reference 98).
Cynomolgus monkeys are more resistant to tuberculosis than rhesus monkeys and may even arrest the disease (see the text).
The characteristics of tuberculosis in Lurie's inbred resistant and susceptible rabbits are reviewed in references 64 and 66.
The assignment of + to +++++ is an estimate for a given species as a whole. Good CMI and DTH are both needed to arrest the disease. Guinea pigs have good DTH but relatively poor CMI, and mice have good CMI but relatively poor DTH. Both species show progressive disease leading to their death. Most humans and rabbits (infected with virulent human-type tubercle bacilli) have adequate amounts of DTH and CMI to arrest the disease.
Immune Response to CMI Antigens
CMI antigens (see Effective TB Vaccines Must Produce Appropriate Amounts of Delayed-Type Hypersensitivity and Cell-Mediated Immunity above) that are as specific as tuberculin is for DTH remain to be identified. CMI antigens should be studied (i) for their ability to activate macrophages and (ii) for their ability to cause caseous necrosis. I propose that the immune response in mice (Fig. 2) is mainly a CMI response, because mice show little (or no) caseous necrosis and show only a weak reaction to tuberculin. Also, I propose that the immune response in guinea pigs (Fig. 3) is mainly a DTH response, because guinea pigs show considerable caseous necrosis and show a rather strong reaction to tuberculin. For this reason, I propose that mice would be a good species with which to recognize CMI antigens in new vaccines and that guinea pigs would be a rather poor species to do so.
Immune Response in both Mice and Guinea Pigs Together
The evaluation of new vaccines with both mice and in guinea pigs may compensate for the proposed deficiencies in both species. However, the inclusion of rabbits (a species for which DTH and CMI usually work together to arrest the disease) would make such evaluations more complete.
Since different antigens are recognized to different degrees by each laboratory animal species, vaccine evaluation with all common laboratory species—mice, guinea pigs, and rabbits (and even monkeys)—should provide the most information before expensive clinical trials are begun.
Note that after M. tuberculosis is inhaled, the strong DTH developed by humans probably stops the logarithmic growth of the bacillus sooner than does the DTH in rabbits or guinea pigs. In fact, in most tuberculin-positive humans, arrested TB primary lesions are so small that they cannot be identified by X ray during life (99).
COMPARISONS OF TUBERCULOSIS IN HUMANS, RABBITS, MICE, AND GUINEA PIGS
In rabbits, numerous inhaled M. tuberculosis cells are destroyed by pulmonary alveolar macrophages (Fig. 4) (30, 33, 37, 64, 69), but in mice and guinea pigs, relatively few inhaled M. tuberculosis cells are destroyed by alveolar macrophages (Table 1) (33, 37, 55). In humans, many tubercle bacilli seem to be destroyed soon after their inhalation by alveolar macrophages, because family members of tuberculous patients can inhale tubercle bacilli for many months and even years and still remain tuberculin negative (see reference 98).
FIG. 4.
Number of viable human- or bovine-type tubercle bacilli in the lungs of Lurie's natively resistant and susceptible inbred rabbits at each interval following quantitative airborne infection (1, 33, 64). Commercially available New Zealand White rabbits resemble Lurie's resistant strain of rabbits (24, 25, 33, 35, 37, 44, 71). This graph makes several points: (i) higher bacillary titers occur with the bovine type than with the human type, because the bovine type is much more virulent for rabbits; (ii) higher bacillary titers occur with Lurie's susceptible rabbits, because they have less native and acquired immunity; (iii) many human-type tubercle bacilli (but not many of the more-virulent bovine type) are destroyed soon after they are inhaled by the resident pulmonary alveolar macrophages (AM); (iv) the AM of the resistant rabbits destroy more human-type tubercle bacilli than the AM of the susceptible rabbits; (v) the multiplications of the highly virulent bovine-type and the less-virulent human-type bacilli in both susceptible and resistant rabbits are the same during the logarithmic growth phase (i.e., both types of bacilli grow equally well in nonactivated macrophages); and (vi) acquired (adaptive) immunity has less effect on reducing bacillary numbers when the infecting strain is more virulent. Human-type tubercle bacilli are more virulent for mice and guinea pigs than they are for rabbits. Therefore, TB vaccines would be less beneficial for mice and guinea pigs than would TB vaccines for rabbits. Also, TB vaccines in mice and guinea pigs would have less ability to detect differences in two or more proposed vaccines than would TB vaccines (tested by tubercle counting) in rabbits. This graph shows the increase in the number of viable bacilli relative to the initial number deposited in the pulmonary alveoli, which is the number zero in the graph. (The inhaled dose of the human type was roughly 100 times the inhaled dose of the bovine type because of differences in their virulences.) Additional rabbits would be needed to obtain the standard errors for each time point. However, these results are consistent with the tubercle count data on these same rabbit races (1, 64, 69). The number of human- and bovine-type tubercle bacilli in the lungs of the resistant rabbits failed to decrease during the period illustrated, because liquefaction and cavity formation occurred (with the extracellular multiplication of the bacilli) (64). Liquefaction usually did not occur in the susceptible rabbits (64), probably because their macrophages develop lower levels of hydrolytic enzymes (see reference 34). (Reproduced from reference 1 with permission of the American Thoracic Society. Copyright © American Thoracic Society.)
In rabbits, the innate and acquired immune responses are highly effective in preventing tiny pulmonary tubercles from reaching a visible size. Rabbits must inhale an average of 300 to 3,000 M. tuberculosis cells to produce one visible primary tubercle (Table 1) (33, 64, 69). Humans must usually inhale an average of 20 to 200 bacilli to do so (estimated by the late Richard L. Riley). However, in mice and guinea pigs, the majority of inhaled M. tuberculosis bacilli produce visible tubercles (Table 1) (33, 55, 78, 85).
Rabbits and most humans will eventually stop the progression of visible primary pulmonary tubercles produced by virulent M. tuberculosis (33, 64), but mice and guinea pigs usually cannot do so (75). Therefore, most primary tubercles in mice and guinea pigs progress, cause metastatic lesions, and eventually kill the host (83).
In rabbits, guinea pigs, and humans, the solid caseum inhibits and even kills some tubercle bacilli due to toxic fatty acids and low pH (33, 54, 63, 84). However, in mice, overloaded macrophages apparently undergo apoptosis, usually without forming necrosis. In mice, the vasculature in the tubercles usually remains patent (33, 37), whereas in rabbits, guinea pigs, and humans, thrombosis of the tubercle's central vasculature is a major cause of the caseous necrosis (see reference 26).
With time, cavitary tuberculosis usually occurs in rabbits infected with virulent M. bovis and occasionally occurs in rabbits infected with M. tuberculosis (24, 25, 33, 34, 64). Cavities frequently occur in tuberculous adult humans beings. However, cavities do not occur in mice and only rarely occur in guinea pigs (96). Many of these species differences are described in references 12, 37, and 49.
Note that since a typical caseum does not form in mice, extracellular tubercle bacilli are not inhibited but remain ready to divide. These bacilli are soon ingested by viable macrophages, and if these macrophages have not been activated, the bacilli will again start intracellular growth. Actively multiplying tubercle bacilli released from killed mouse macrophages into nonnecrotic tissues probably grow better when ingested by nearby nonactivated macrophages than do relatively dormant bacilli escaping from the solid caseous tissue found in other hosts. Therefore, the inability of mice to form typical caseous necrosis seems to be a factor in explaining why mice usually die of progressing pulmonary granulomas in spite of their ability to develop appreciable CMI. (References 104 and 105 discuss the role of CD8+ T cells in mouse tuberculosis.)
TUBERCULOSIS IN NONHUMAN PRIMATES
Monkeys are genetically more similar to humans than to other animal laboratory species, but their response to inhaled M. tuberculosis is more like that of “isolated” human populations (Table 2) (14, 37, 50, 53), such as the Senegalese troops who were brought from Africa to Europe during the first World War (14, 64). These troops developed the susceptible (hematogenously spread) childhood type of tuberculosis rather than the chronic cavitary (bronchial spread) type found in most adults today. In other words, such “isolated” human beings did not have the resistance to M. tuberculosis that most human beings developed after living with the disease for numerous centuries (see references 28 and 98).
Two strains of nonhuman primates are being used to evaluate TB vaccines: rhesus macaques (Macaca mulatta) (43a, 49, 53, 59, 60) and cynomolgus macaques (Macaca fascicularis) (17, 49, 59, 103). Quantitative airborne infection of young rhesus monkeys showed that a progressing primary tubercle was produced by the majority of inhaled viable bacillary units of virulent M. tuberculosis that reached the alveolar spaces (10, 86). Cynomolgus monkeys are natively more resistant than rhesus monkeys. In fact, some cynomolgus monkeys infected with a low M. tuberculosis dose become tuberculin positive with no other evidence of the disease (17, 103). However, both monkey strains seem to be more susceptible to M. tuberculosis than the majority of today's human population (Table 2). Also, in both monkey strains, the type of tuberculosis produced varies considerably.
Younger monkeys in both groups (2 to 5 kg in weight) tend to develop a more rapid childhood type of TB, and older monkeys (over 7 kg in weight) tend to develop a more chronic, slowly progressive type (51). In other words, the immune system of monkeys (like that of humans) takes several years to mature. Young monkeys were used for studies reported in references 53 and 103, and older, mature monkeys were used for studies reported in references 17 and 59. Liquefaction and cavity formation were occasionally observed for older monkeys after an intratracheal challenge with a rather high dose of M. tuberculosis (59) and after an aerosol challenge with a lower dose (9).
Quantitative airborne infection of rhesus monkeys with 12 to 49 units of M. tuberculosis was sufficient to produce multiple grossly visible pulmonary tubercles (9, 86). Therefore, rhesus monkeys are as susceptible as guinea pigs to this disease.
Quantitative airborne infection of cynomolgus monkeys remains to be reported. Scientists at the Tulane National Primate Research Center in Covington, LA, are exposing vaccinated cynomolgus monkeys (along with controls) to aerosols of M. tuberculosis. If they count the number of primary tubercles developed, they could calculate the “ratio” (31, 33, 64, 68), i.e., the number of inhaled M. tuberculosis cells required to produce one visible primary tubercle (Table 1). Ratios of 10 to 50 define a susceptible animal that would develop rather poor immunity from a vaccine. Ratios of 500 to 1,200 would define a resistant animal that (similarly to commercial rabbits) would develop good immunity from a vaccine. We truly need such information to decide whether two vaccines compared by using monkeys would produce as decisive a result as the same vaccines compared by using rabbits. Such “ratios” cannot be obtained if monkeys are challenged intratracheally (59, 103) or bronchoscopically (17, 60), because these procedures deposit numerous tubercle bacilli into one locale.
In nonhuman primates, tubercle counts would best be made 5 to 10 weeks after challenge with virulent M. tuberculosis by aerosol. At such times, the primary lesions could be easily distinguished from metastatic lesions, because the primary lesions are much larger. To compare two or more TB vaccines in nonhuman primates, further studies are needed on the effect of age and on the effect of very low inhaled doses of M. tuberculosis. However, all monkeys appear to be much more susceptible to M. tuberculosis than are human beings and therefore would not be an adequate substitute for rabbits.
Primate facilities that expose monkeys to M. tuberculosis by aerosol could easily be used to infect rabbits. The Tulane National Primate Research Center uses USAMRIID's head-only aerosol exposure apparatus (46), which works well for commercial New Zealand White rabbits (24, 33, 35, 44, 71). Comparisons of the efficacy of new TB vaccines in rabbits by tubercle counting should be more pertinent to the majority of modern-day human beings than comparisons of them in monkeys and should also be less costly.
In brief, human beings and rabbits prevent most primary pulmonary tubercles caused by M. tuberculosis from developing into clinically active disease, whereas monkeys (being much more susceptible) do not do so. The good overall immune response of human beings is best modeled by tubercle counting in rabbits, where the protective effects of a good vaccine are easily recognized by the reduction in the number of visible primary tubercles produced by an aerosol of M. tuberculosis. Good vaccines would show less benefit in monkeys, mice, and guinea pigs, because they are species that develop a less effective immune response to M. tuberculosis than that which rabbits and humans develop (see above).
BCG IN NEWBORN INFANTS
The vaccination of newborn infants is somewhat different from the vaccination of more mature individuals. In clinical trials, newborn infants have usually benefited from BCG immunization (8, 19, 20, 22, 48, 91). Since the cell-mediated immune system of newborns is relatively underdeveloped, live BCG would multiply more extensively in them than in older individuals. The larger number of BCG bacilli would persist until these newborns become more immunocompetent. At that time, these young vaccinees would be more effectively immunized than older vaccinees, because these youngsters had received a greater antigenic stimulus. On the other hand, because of their underdeveloped immune system, newborns would respond less well than older individuals to nonviable vaccines, unless those antigens persisted until the newborns were more immunocompetent.
TB VACCINES HAVE THEIR MAIN EFFECTS EARLY IN DISEASE
Prophylactic vaccination expands antigen-specific T-lymphocyte populations producing DTH and CMI and also expands antigen-specific B-cell populations producing antibodies. These expanded antigen-specific lymphocyte populations rapidly enter developing TB lesions (94). The logarithmic growth of inhaled virulent bacilli is stopped sooner, bacillary titers are reduced, and the host lives longer.
However, in both vaccinated and unvaccinated hosts, the virulent tubercle bacilli in the challenge infection will soon supply large quantities of antigens that will probably exceed the quantity supplied by the vaccine. These M. tuberculosis antigens may, in time, cause the acquired (adaptive) immunity developed in unvaccinated hosts to approximate that developed in vaccinated hosts. Therefore, the main effect of prophylactic TB vaccination would be the rapid immune response soon after M. tuberculosis enters the host and not after the infection has induced its own immunity, but see Prophylactic Immunization and Immunotherapy with Critical TB Antigens below.
PROPHYLACTIC IMMUNIZATION AND IMMUNOTHERAPY WITH CRITICAL TB ANTIGENS
Vaccines containing critical antigens may, however, increase host resistance above that produced during an active TB infection. Vaccines containing critical antigens would expand the corresponding Th1 lymphocyte population, and this Th1 population would be increased even further by an active TB infection, even though the infecting virulent strain contained only small amounts of such critical antigens.
Immunotherapy with critical antigens in patients who already have active tuberculosis could have a similar beneficial effect. Unfortunately, the use of critical antigens for both prophylactic immunization and immunotherapy is still in developmental stages (reviewed in references 3, 4, 43, 43a, 57, 89, and 90).
The effects of primary vaccination with live attenuated tubercle bacilli (often BCG) followed by a booster vaccination with critical antigens months or years later are currently being evaluated (3, 43, 73, 79, 82, 89). This two-step vaccination regimen is most promising, because it combines the multiple antigens of intact viable tubercle bacilli with the critical antigens that are found to have the greatest effect on host resistance.
Modified vaccinia virus Ankara expressing immunodominant secreted antigen 85A (MVA85A) is already in clinical trials as a booster for persons who have had a positive tuberculin skin test from BCG or a naturally acquired (arrested or latent) TB infection (43, 72, 73, 93). It is too soon to know whether individuals receiving an MVA85A booster vaccination will develop less clinical tuberculosis than BCG-vaccinated individuals who did not receive the booster.
Early-secreted antigenic target 6-kDa protein (ESAT-6), culture filtrate protein 10 (CFP-10), recombinant fusion protein Mtb72F, and others (see references 3, 4, and 46) might be promising critical antigens to boost the host's immune response (89). To date, however, ESAT-6 and CFP-10 have been used mainly with human peripheral blood mononuclear cells to (i) diagnose latent and active TB and (ii) assess the immune response to new TB vaccines (43, 72, 73, 93). (BCG does not contain these two antigens.)
With the genome of M. tuberculosis now known, many possible critical antigens should soon become available for testing (discussed in reference 82). Some of these M. tuberculosis antigens may be more effective (or critical) than others in controlling the growth of the tubercle bacillus. The best TB vaccine would enhance those critical antigens the most. The addition of pulmonary tubercle counting in rabbits to the current methods of antigen selection for TB vaccines should make such selections more precise.
ACTIVATION OF MACROPHAGES IS A MAJOR GENETIC FACTOR IN RESISTANCE TO TUBERCULOSIS
Pulmonary alveolar macrophages (AM) are nonspecifically activated by ingesting inhaled particles. The macrophages in tuberculous lesions are specifically activated by immune processes. In Lurie's inbred susceptible rabbits, both nonspecifically activated macrophages (i.e., pulmonary alveolar macrophages) and immunologically activated macrophages (in tuberculous lesions) cannot destroy virulent human-type tubercle bacilli as well as can the macrophages in his inbred resistant rabbits (Fig. 4) (64, 69). Therefore, his susceptible rabbits apparently have a defective macrophage-activating system. Whether such a defect occurs in other susceptible animals (or even in humans [98]) remains to be determined. In humans, defects in the immune system, such as that found in HIV/AIDS, are a major cause of the increased susceptibility to tuberculosis (11, 27).
DISCUSSION OF VARIOUS COMBINATIONS OF FACTORS THAT SHOULD BE CONSIDERED IN PRECLINICAL (AND CLINICAL) TESTING OF NEW TUBERCULOSIS VACCINES
TB Vaccination and Bacillary Virulence
Vaccination will have less effect on arresting the disease in hosts where M. tuberculosis is of high virulence. The amount of acquired (adaptive) host resistance is superimposed and determined by the amount of innate (genetic) host resistance (64). Therefore, the disease produced in mice, guinea pigs, and monkeys (which have rather low resistance to M. tuberculosis) would be helped less by vaccination than would the disease produced in rabbits and humans (which have high resistance to M. tuberculosis). The differences between two candidate vaccines would be more apparent in rabbits and humans, because they would develop considerable acquired (adaptive) resistance to all good TB vaccines.
TB Vaccination and Bacillary Titers
Good TB vaccines would lower the bacillary titers in the lungs of all common laboratory species. These vaccines would produce good DTH in guinea pigs, good CMI in mice, and both good DTH and good CMI in rabbits and humans. The good DTH response of guinea pigs seems to lower the pulmonary bacillary titers more effectively than the good CMI response of mice (compare Fig. 3 with Fig. 2). However, because of their poor CMI, guinea pigs seem to die sooner than do mice.
TB Vaccination and Tubercle Counts
Good TB vaccines lower primary pulmonary tubercle counts by producing both DTH and CMI. In immunized rabbits, most primary pulmonary tubercles that are not seen at 5 weeks after aerosol infection remain arrested. In mice and guinea pigs, many nonvisible pulmonary tubercles at 5 weeks may become visible at 10 or 20 weeks, but such studies remain to be performed. These species differences reflect the susceptibility of the host (Table 2). However, tubercle counting in all laboratory animals (including monkeys) could measure vaccine efficacy if the animal is necropsied at the right time.
Virulence, Bacillary Titers, and Tubercle Counts
In general, the greater the bacillary virulence, the greater will be the bacillary titer in the host (Fig. 4) and the fewer the number of inhaled bacilli will be required to generate one visible primary pulmonary tubercle (Table 1).
CONCLUSIONS
This review is an effort to improve the selection of new TB vaccines by providing certain perspectives on the immunization of humans, mice, guinea pigs, rabbits, and monkeys that have not usually been considered in TB vaccine selection. Briefly, these perspectives are as follows.
(i) In human trials, BCG vaccination has not been consistently beneficial. However, in laboratory animals, BCG has consistently increased host resistance to challenge with M. tuberculosis. We propose that the rate of healing of BCG lesions (used as a control for new vaccines in clinical trials) will identify the 95% of humans who arrest infection with M. tuberculosis without the need for vaccination. In the remaining 5% of individuals, the benefits of BCG vaccination should be easier to recognize and should be more consistent with those found in laboratory animals.
(ii) The arrest of early pulmonary tubercles by the immune process before they become clinically apparent is the very purpose of TB vaccination. Early tubercles in mice and guinea pigs are not as easily arrested, but most early pulmonary tubercles caused by M. tuberculosis in rabbits and humans are arrested.
(iii) Because of the expense, tubercle counting in rabbits has not been undertaken before starting much more expensive clinical trials. However, tubercle counting in rabbits could select the most effective new TB vaccines more precisely than any other procedure. Therefore, tubercle counting could save much time and could save millions of dollars in getting better TB vaccines into clinical use.
(iv) In mice and guinea pigs, differences between two TB vaccines are harder to distinguish, because the ability of these animals to stop the growth of M. tuberculosis is much less effective than the ability of rabbits and humans to do so. The more effective the immune response to virulent tubercle bacilli, the more effective will be the immune response to vaccination. In other words, differences between two vaccines may be more easily recognized by pulmonary tubercle counts in animals that develop a good immune response, because the difference between the control and the vaccinated animals spans a wider range.
(v) Both cell-mediated immunity (CMI) and delayed-type hypersensitivity (DTH) must be produced in a host to arrest the progress of tuberculosis. CMI and DTH are similar immunological processes involving Th1 lymphocytes. However, CMI and DTH inhibit the growth of M. tuberculosis by different mechanisms. CMI activates macrophages so that they inhibit the growth of the M. tuberculosis cells that they ingest. DTH kills nonactivated macrophages that become overloaded with M. tuberculosis by producing solid caseous necrosis in which the bacillus does not grow. (Nonactivated macrophages are present in every active tuberculous lesion and may ingest tubercle bacilli.)
(vi) DTH and CMI are produced by different M. tuberculosis antigens, and new vaccines must contain these antigens in the proper amounts. Mice (infected with M. tuberculosis) have weak tuberculin sensitivity (DTH) and apparently good CMI, and they usually die of the disease. Guinea pigs (infected with M. tuberculosis) have good tuberculin sensitivity (DTH) and apparently weak CMI, and they also usually die of the disease. However, most humans and rabbits (infected with M. tuberculosis) usually survive the disease. Therefore, we concluded that mice do not respond well to DTH-producing antigens and that guinea pigs apparently do not respond well to CMI-producing antigens. However, humans and rabbits (species that usually arrest the disease produced by M. tuberculosis) evidently respond well to both DTH- and CMI-producing antigens.
(vii) The antigens recognized by mice and those recognized by guinea pigs together may (or may not) be the same as the antigens recognized by rabbits. Also, the antigens recognized by rabbits may (or may not) be the same as the antigens recognized by humans. Such differences and similarities remain to be investigated. Therefore, we urge investigators to always include rabbits along with mice, guinea pigs, and, perhaps, monkeys in the preclinical testing of new TB vaccines in order to make preclinical studies more complete.
(viii) Vaccines containing critical antigens (possibly ESAT-6 or CFP-10) might increase the immunity of the host to a greater extent than antigens produced by a natural M. tuberculosis infection. Such critical antigens would increase the host's ability to neutralize key components of M. tuberculosis. However, only some critical antigens have so far been identified. When identified, these critical antigens could then be used for TB prophylaxis and/or TB immunotherapy.
Acknowledgments
I am indebted to Paul J. Converse for many helpful suggestions concerning this report and to William R. Bishai and Ying Zhang for reviewing it. They are members of our Johns Hopkins Center for Tuberculosis Research.
Biography
 Arthur M. Dannenberg, Jr., spent his 60-year research career unraveling the intricacies of tuberculosis in the rabbit model. He received his M.D. from Harvard Medical School in 1947 and Ph.D. in Microbiology from the University of Pennsylvania in 1952 under Professor Max B. Lurie, who was the foremost experimental pathologist of tuberculosis at that time. Dr. Lurie outlined the research career that Dr. Dannenberg pursued for the rest of his life. Dr. Dannenberg was a Postdoctoral Fellow in biochemistry at the University of Utah under Emil L. Smith (1952 to 1954), a Lt. Cdr. in infectious disease at U.S. Naval Medical Research Unit 1 at the University of California (1954 to 1956), an Assistant Professor in the experimental pathology of tuberculosis at the University of Pennsylvania (1956 to 1964), and an Associate Professor, and then a full Professor, at the Johns Hopkins Bloomberg School of Public Health, where he continued his studies (1964 to present).
Arthur M. Dannenberg, Jr., spent his 60-year research career unraveling the intricacies of tuberculosis in the rabbit model. He received his M.D. from Harvard Medical School in 1947 and Ph.D. in Microbiology from the University of Pennsylvania in 1952 under Professor Max B. Lurie, who was the foremost experimental pathologist of tuberculosis at that time. Dr. Lurie outlined the research career that Dr. Dannenberg pursued for the rest of his life. Dr. Dannenberg was a Postdoctoral Fellow in biochemistry at the University of Utah under Emil L. Smith (1952 to 1954), a Lt. Cdr. in infectious disease at U.S. Naval Medical Research Unit 1 at the University of California (1954 to 1956), an Assistant Professor in the experimental pathology of tuberculosis at the University of Pennsylvania (1956 to 1964), and an Associate Professor, and then a full Professor, at the Johns Hopkins Bloomberg School of Public Health, where he continued his studies (1964 to present).
REFERENCES
- 1.Allison, M. J., P. Zappasodi, and M. P. Lurie. 1962. Host-parasite relationships in natively resistant and susceptible rabbits on quantitative inhalation of tubercle bacilli. Their significance for the nature of genetic resistance. Am. Rev. Respir. Dis. 85:553-569. [DOI] [PubMed] [Google Scholar]
- 2.Alsaadi, A.-I., and D. W. Smith. 1973. The fate of virulent and attenuated mycobacteria in guinea pigs infected by the respiratory route. Am. Rev. Respir. Dis. 107:1041-1046. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, P. 2007. Vaccine strategies against latent tuberculosis infection. Trends Microbiol. 15:7-13. [DOI] [PubMed] [Google Scholar]
- 4.Andersen, P. 2007. Tuberculosis vaccines—an update. Nat. Rev. Microbiol. 5:484-487. [DOI] [PubMed] [Google Scholar]
- 5.Ando, M. 1973. Macrophage activation in tuberculin reactions of rabbits with primary BCG infection and reinfection. J. Reticuloendothel. Soc. 14:132-145. [PubMed] [Google Scholar]
- 6.Aronson, J. D. 1957. The status of BCG vaccination in the United States and Canada. Adv. Tuberc. Res. 8:131-153. [PubMed] [Google Scholar]
- 7.Aronson, J. D., C. F. Aronson, and H. C. Taylor. 1958. A twenty-year appraisal of BCG vaccination in the control of tuberculosis. Arch. Intern. Med. 101:881-883. [DOI] [PubMed] [Google Scholar]
- 8.Aronson, J. D., and A. M. Dannenberg. 1935. Effect of vaccination with BCG on tuberculosis in infancy and in childhood. Correlation of reactions to tuberculin tests, roentgenologic diagnosis and mortality. Am. J. Dis. Child. 50:1117-1130. [Google Scholar]
- 9.Barclay, W. R., R. L. Anacker, W. Brehmer, W. Leif, and E. Ribi. 1970. Aerosol-induced tuberculosis in subhuman primates and the course of the disease after intravenous BCG vaccination. Infect. Immun. 2:574-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barclay, W. R., W. M. Busey, D. W. Dalgard, R. C. Good, B. W. Janicki, J. E. Kasik, E. Ribi, C. E. Ulrich, and E. Wolinsky. 1973. Protection of monkeys against airborne tuberculosis by aerosol vaccination with bacillus Calmette-Guérin. Am. Rev. Respir. Dis. 107:351-358. [DOI] [PubMed] [Google Scholar]
- 11.Barnes, P. F., A. B. Bloch, P. T. Davidson, and D. E. Snider, Jr. 1991. Tuberculosis in patients with human immunodeficiency virus infection. N. Engl. J. Med. 324:1644-1650. [DOI] [PubMed] [Google Scholar]
- 12.Basaraba, R. J. 2008. Experimental tuberculosis: the role of comparative pathology in the discovery of improved tuberculosis treatment strategies. Tuberculosis 88(Suppl. 1):S37-S47. [DOI] [PubMed] [Google Scholar]
- 13.Bloom, B. R., and P. E. M. Fine. 1994. The BCG experience: implications for future vaccines against tuberculosis, p. 531-557. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. ASM Press, Washington, DC.
- 14.Borrel, A. 1920. Pneumonie et tuberculose chez les troupes noires. Ann. Inst. Pasteur (Paris) 34:105-148. [Google Scholar]
- 15.Bothamley, G. H., E. Cooper, D. Shingadia, and A. Mellanby. 2003. Tuberculin testing before BCG vaccination. BMJ 327:243-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canetti, G. 1955. The tubercle bacillus in the pulmonary lesion of man: histobacteriology and its bearing on the therapy of pulmonary tuberculosis. Springer, New York, NY.
- 17.Capuano, S. V. III, D. A. Croix, S. Pawar, A. Zinovik, A. Myers, P. L. Lin, S. Bissel, C. Fuhrman, E. Klein, and J. L. Flynn. 2003. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect. Immun. 71:5831-5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cegielski, J. P., and D. N. McMurray. 2004. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int. J. Tuber. Lung Dis. 8:286-298. [PubMed] [Google Scholar]
- 19.Citron, K. M. 1993. BCG vaccination against tuberculosis: international perspectives. Vaccinate the newborn in developing countries and those at risk in developed countries. BMJ 306:222-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 21.Comstock, G. W. 1994. Field trials of tuberculosis vaccines: how could we have done them better? Control. Clin. Trials 15:247-276. [DOI] [PubMed] [Google Scholar]
- 22.Comstock, G. W. 2000. Simple, practical ways to assess the protective efficacy of a new tuberculosis vaccine. Clin. Infect. Dis. 30(Suppl. 3):S250-S253. [DOI] [PubMed] [Google Scholar]
- 23.Comstock, G. W., and S. F. Woolpert. 1978. Tuberculin conversions: true or false? Am. Rev. Respir. Dis. 118:215-217. [DOI] [PubMed] [Google Scholar]
- 24.Converse, P. J., A. M. Dannenberg, Jr., J. E. Estep, K. Sugisaki, Y. Abe, B. H. Schofield, and M. L. Pitt. 1996. Cavitary tuberculosis produced in rabbits by aerosolized virulent tubercle bacilli. Infect. Immun. 64:4776-4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Converse, P. J., A. M. Dannenberg, Jr., T. Shigenaga, D. N. McMurray, S. W. Phalen, J. L. Stanford, G. A. Rook, T. Koru-Sengul, H. Abbey, J. E. Estep, and M. L. Pitt. 1998. Pulmonary bovine-type tuberculosis in rabbits: bacillary virulence, inhaled dose effects, tuberculin sensitivity, and Mycobacterium vaccae immunotherapy. Clin. Diagn. Lab. Immunol. 5:871-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Courtade, E. T., T. Tsuda, C. R. Thomas, and A. M. Dannenberg, Jr. 1975. Capillary density in developing and healing tuberculous lesions. Am. J. Pathol. 78:243-260. [PMC free article] [PubMed] [Google Scholar]
- 27.Crampin, A. C., J. R. Glynn, and P. E. M. Fine. 2009. What has Karonga taught us? Tuberculosis studied over three decades. Int. J. Tuber. Lung Dis. 13:153-164. [PMC free article] [PubMed] [Google Scholar]
- 28.Daniel, T. M. 1998. The early history of tuberculosis in central East Africa: insights from the clinical records of the first twenty years of Mengo Hospital and review of relevant literature. Int. J. Tuber. Lung Dis. 2:784-790. [PubMed] [Google Scholar]
- 29.Dannenberg, A. M., Jr. 1984. Pathogenesis of tuberculosis: native and acquired resistance in animals and humans, p. 344-354. In L. Leive and D. Schlessinger (ed.), Microbiology—1984. American Society for Microbiology, Washington, DC.
- 30.Dannenberg, A. M., Jr. 1993. Immunopathogenesis of pulmonary tuberculosis. Hosp. Pract. (Off. Ed.) 28:51-58. [DOI] [PubMed] [Google Scholar]
- 31.Dannenberg, A. M., Jr. 1998. Lurie's tubercle-count method to test TB vaccine efficacy in rabbits. Front. Biosci. 3:27-33. [DOI] [PubMed] [Google Scholar]
- 32.Dannenberg, A. M., Jr. 2003. Macrophage turnover, division and activation within developing, peak and “healed” tuberculous lesions produced in rabbits by BCG. Tuberculosis (Edinb.) 83:251-260. [DOI] [PubMed] [Google Scholar]
- 33.Dannenberg, A. M., Jr. 2006. Pathogenesis of human tuberculosis: insights from the rabbit model. ASM Press, Washington, DC.
- 34.Dannenberg, A. M., Jr. 2009. Liquefaction and cavity formation in pulmonary TB: a simple method in rabbit skin to test inhibitors. Tuberculosis (Edinb.) 89:243-247. [DOI] [PubMed] [Google Scholar]
- 35.Dannenberg, A. M., Jr., W. R. Bishai, N. Parrish, R. Ruiz, W. Johnson, B. C. Zook, J. W. Boles, and M. L. M. Pitt. 2000. Efficacies of BCG and vole bacillus (Mycobacterium microti) vaccines in preventing clinically apparent pulmonary tuberculosis in rabbits: a preliminary report. Vaccine 19:796-800. [DOI] [PubMed] [Google Scholar]
- 36.Dannenberg, A. M., Jr., M. S. Burstone, P. C. Walter, and J. W. Kinsley. 1963. A histochemical study of phagocytic and enzymatic functions of rabbit mononuclear and polymorphonuclear exudate cells and alveolar macrophages. I. Survey and quantitation of enzymes, and states of cellular activation. J. Cell Biol. 17:465-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dannenberg, A. M., Jr., and F. M. Collins. 2001. Progressive pulmonary tuberculosis is not due to increasing numbers of viable bacilli in rabbits, mice and guinea pigs, but is due to a continuous host response to mycobacterial products. Tuberculosis (Edinb.) 81:229-242. [DOI] [PubMed] [Google Scholar]
- 38.Dannenberg, A. M., Jr., and E. M. Scott. 1958. Melioidosis: pathogenesis and immunology in mice and hamsters. II. Studies with avirulent strains of Malleomyces pseudomallei. Am. J. Pathol. 34:1099-1121. [PMC free article] [PubMed] [Google Scholar]
- 39.Dannenberg, A. M., Jr., and E. M. Scott. 1960. Melioidosis: pathogenesis and immunology in mice and hamsters. III. The effect of vaccination with avirulent strains of Pseudomonas pseudomallei on the resistance to the establishment and the resistance to the progress of respiratory melioidosis caused by virulent strains: all-or-none aspects of this disease. J. Immunol. 84:233-246. [PubMed] [Google Scholar]
- 40.Dannenberg, A. M., Jr., and J. F. Tomashefski, Jr. 1998. Pathogenesis of pulmonary tuberculosis, p. 2447-2471. In A. P. Fishman (ed.), Fishman's pulmonary diseases and disorders, 3rd ed., vol. 2. McGraw-Hill, New York, NY. [Google Scholar]
- 41.Dannenberg, A. M., Jr., P. C. Walter, and F. A. Kapral. 1963. A histochemical study of phagocytic and enzymatic functions of rabbit mononuclear and polymorphonuclear exudate cells and alveolar macrophages. II. The effect of particle ingestion on enzyme activity; two phases of in vitro activation. J. Immunol. 90:448-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dharmadhikari, A. S., and E. A. Nardell. 2008. What animal models teach humans about tuberculosis. Am. J. Respir. Cell Mol. Biol. 39:503-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dockrell, H. M., and Y. Zhang. 2009. A courageous step down the road toward a new tuberculosis vaccine. Am. J. Respir. Clin. Care Med. 179:628-629. [DOI] [PubMed] [Google Scholar]
- 43a.Doherty, T. M., and P. Andersen. 2002. Tuberculosis vaccine development. Curr. Opin. Pulm. Med. 8:183-187. [DOI] [PubMed] [Google Scholar]
- 44.Dorman, S. E., C. L. Hatem, S. Tyagi, K. Aird, J. Lopez-Molina, M. L. Pitt, B. C. Zook, A. M. J. Dannenberg, W. R. Bishai, and Y. C. Manabe. 2004. Susceptibility to tuberculosis: clues from studies with inbred and outbred New Zealand White rabbits. Infect. Immun. 72:1700-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunn, P. L., and R. J. North. 1995. Virulence ranking of some Mycobacterium tuberculosis and Mycobacterium bovis strains according to their ability to multiply in the lungs, induce lung pathology, and cause mortality in mice. Infect. Immun. 63:3428-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dutta, N. K., S. Mehra, P. J. Didier, C. J. Roy, L. A. Doyle, X. Alvarez, M. Ratterree, N. A. Be, G. Lamichhane, S. K. Jain, M. R. Lacey, A. A. Lackner, and D. Kaushal. 2010. Genetic requirements for the survival of tubercle bacilli in primates. J. Infect. Dis. 201:1743-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elias, D., D. Wolday, H. Akuffo, B. Petros, U. Bronner, and S. Britton. 2001. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin. Exp. Immunol. 123:219-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fine, P. E. M. 2000. BCG vaccines and vaccination, p. 503-522. In L. R. Reichman, and E. S. Hershfield, (ed.), Tuberculosis. A comprehensive international approach. Marcel Dekker, New York, NY.
- 49.Flynn, J. A., A. M. Cooper, and W. R. Bishai. 2005. Animal models of tuberculosis, p. 547-560. In S. T. Cole, K. D. Eisenach, D. N. McMurray, and W. R. Jacobs, Jr. (ed.), Tuberculosis and the tubercle bacillus. ASM Press, Washington, DC.
- 50.Francis, J. 1958. Tuberculosis in animals and man: a study in comparative pathology. Cassell, London, United Kingdom.
- 51.Good, R. C. 1973. Tuberculosis and bacterial infection, p. 39-60. In G. H. Bourne (ed.), Nonhuman primates and medical research. Academic Press, New York, NY.
- 52.Gupta, U. D., V. M. Katoch, and D. N. McMurray. 2007. Current status of TB vaccines. Vaccine 25:3742-3751. [DOI] [PubMed] [Google Scholar]
- 53.Habel, K. 1947. Tuberculosis in a laboratory monkey colony: its spread and control. Am. Rev. Tuberc. 55:77-92. [DOI] [PubMed] [Google Scholar]
- 54.Hemsworth, G. R., and I. Kochan. 1978. Secretion of antimycobacterial fatty acids by normal and activated macrophages. Infect. Immun. 19:170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ho, R. S., J. S. Fok, G. E. Harding, and D. W. Smith. 1978. Host-parasite relationship in experimental airborne tuberculosis. VII. Fate of Mycobacterium tuberculosis in primary lung lesions and in primary lesion-free lung tissue infected as a result of bacillemia. J. Infect. Dis. 138:237-241. [DOI] [PubMed] [Google Scholar]
- 56.Horwitz, M. A., G. Harth, B. J. Dillon, and S. Maslesa-Galic. 2009. Commonly administered BCG strains including an evolutionary early strain and evolutionary late strains of disparate genealogy induce comparative immunity against tuberculosis. Vaccine 27:441-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horwitz, M. A., G. Harth, B. J. Dillon, and S. Maslesa-Galic. 2005. Enhancing the protective efficacy of Mycobacterium bovis BCG vaccination against tuberculosis by boosting with Mycobacterium tuberculosis major secretory protein. Infect. Immun. 73:4676-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lagranderie, M. R. R., A.-M. Balazuc, E. Deriaud, C. D. Leclerc, and M. Gheorghiu. 1996. Comparison of immune responses of mice immunized with five different Mycobacterium bovis BCG vaccine strains. Infect. Immun. 64:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langermans, J. A., P. Andersen, D. van Soolingen, R. A. Vervenne, P. A. Frost, T. van der Laan, L. A. van Pinxteren, J. van den Hombergh, S. Kroon, I. Peekel, S. Florquin, and A. W. Thomas. 2001. Divergent effect of bacillus Calmette-Guèrin (BCG) vaccination on Mycobacterium tuberculosis infection in highly related macaque species: implications for primate models in tuberculosis vaccine research. Proc. Natl. Acad. Sci. U. S. A. 98:11497-11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewinsohn, D. M., I. S. Tydeman, M. Frieder, J. E. Grotzke, R. A. Lines, S. Ahmed, K. D. Prongay, S. L. Primack, L. M. A. Colgin, A. D. Lewis, and D. A. Lewinsohn. 2006. High resolution radiographic and fine immunologic definition of TB disease progression in the rhesus macaque. Microbes Infect. 8:2587-2598. [DOI] [PubMed] [Google Scholar]
- 61.Lewinsohn, D. A., E. Winata, G. M. Swarbrick, K. E. Tanner, M. S. Cook, M. D. Null, M. E. Cansler, A. Sette, J. Sidney, and D. M. Lewinsohn. 2007. Immunodominant tuberculosis CD8 antigens preferentially restricted by HLA-B. PLoS Pathog. 3:1240-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu, P. T., S. Stenger, D. H. Tang, and R. L. Modlin. 2007. Vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 179:2060-2063. [DOI] [PubMed] [Google Scholar]
- 63.Long, E. R. 1958. The chemistry and chemotherapy of tuberculosis, 3rd ed. Lippincott Williams & Wilkins, Baltimore, MD.
- 64.Lurie, M. B. 1964. Resistance to tuberculosis: experimental studies in native and acquired defensive mechanisms. Harvard University Press, Cambridge, MA.
- 65.Lurie, M. B., S. Abramson, and A. G. Heppleston. 1952. On the response of genetically resistant and susceptible rabbits to the quantitative inhalation of human-type tubercle bacilli and the nature of resistance to tuberculosis. J. Exp. Med. 95:119-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lurie, M. B., and A. M. Dannenberg, Jr. 1965. Macrophage function in infectious disease with inbred rabbits. Bacteriol. Rev. 29:466-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lurie, M. B., A. G. Heppleston, S. Abramson, and I. B. Swartz. 1950. An evaluation of the method of quantitative airborne infection and its use in the study of the pathogenesis of tuberculosis. Am. Rev. Tuberc. 61:765-797. [PubMed] [Google Scholar]
- 68.Lurie, M. B., P. Zappasodi, E. Cardona-Lynch, and A. M. Dannenberg, Jr. 1952. The response to the intracutaneous inoculation of BCG as an index of native resistance to tuberculosis. J. Immunol. 68:369-387. [PubMed] [Google Scholar]
- 69.Lurie, M. B., P. Zappasodi, and C. Tickner. 1955. On the nature of genetic resistance to tuberculosis in the light of the host-parasite relationships in natively resistant and susceptible rabbits. Am. Rev. Tuberc. 72:297-329. [DOI] [PubMed] [Google Scholar]
- 70.Mackaness, G. B. 1968. The immunology of antituberculous immunity. Am. Rev. Respir. Dis. 97:337-344. [DOI] [PubMed] [Google Scholar]
- 71.Manabe, Y. C., A. M. Dannenberg, Jr., S. K. Tyagi, C. L. Hatem, M. Yoder, S. C. Woolwine, B. C. Zook, M. L. Pitt, and W. R. Bishai. 2003. Different strains of Mycobacterium tuberculosis cause various spectrums of disease in the rabbit model of tuberculosis. Infect. Immun. 71:6004-6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McShane, H., A. A. Pathan, C. R. Sander, S. M. Keating, S. C. Gilbert, K. Huygen, H. A. Fletcher, and A. V. Hill. 2004. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 10:1240-1244. [DOI] [PubMed] [Google Scholar]
- 73.McShane, H., A. A. Pathan, C. R. Sander, N. P. Goonetilleke, H. A. Fletcher, and A. V. S. Hill. 2005. Boosting BCG with MVA85A: the first candidate subunit vaccine for tuberculosis in clinical trials. Tuberculosis 85:47-52. [DOI] [PubMed] [Google Scholar]
- 74.Medical Research Council. 1972. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and in early adult life. Bull. World Health Organ. 46:371-385. [PMC free article] [PubMed] [Google Scholar]
- 75.Medina, E., and R. J. North. 1998. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology 93:270-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Medina, E., and R. J. North. 1999. Genetically susceptible mice remain proportionally more susceptible to tuberculosis after vaccination. Immunology 96:16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Menzies, D. 1999. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am. J. Respir. Crit. Care Med. 159:15-21. [DOI] [PubMed] [Google Scholar]
- 78.Middlebrook, G. 1961. Immunologic aspects of airborne infection: reactions to inhaled antigens. Bacteriol. Rev. 25:331-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mitsuyama, M., and D. N. McMurray. 2007. Tuberculosis: vaccine and drug development. Tuberculosis 87:510-513. [DOI] [PubMed] [Google Scholar]
- 80.Mostowy, S., A. G. Tsolaki, P. M. Small, and M. A. Behr. 2003. The in vitro evolution of BCG vaccines. Vaccine 21:4270-4274. [DOI] [PubMed] [Google Scholar]
- 81.North, R. J., L. Ryan, R. LaCource, T. Mogues, and M. E. Goodrich. 1999. Growth rate of mycobacteria in mice is an unreliable indicator of mycobacterial virulence. Infect. Immun. 67:5483-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Olsen, A. W., and P. Andersen. 2003. A novel TB vaccine; strategies to combat a complex pathogen. Immunol. Lett. 85:207-211. [DOI] [PubMed] [Google Scholar]
- 83.Orme, I. M., and A. A. Izo. 2005. Tuberculosis vaccine preclinical screening and development, p. 561-571. In S. T. Cole, K. D. Eisenach, D. N. McMurray, and W. R. Jacobs, Jr. (ed.), Tuberculosis and the tubercle bacillus. ASM Press, Washington, DC.
- 84.Poole, J., and H. Florey. 1970. Chronic inflammation and tuberculosis, p. 1183-1224. In H. Florey (ed.), General pathology, 4th ed. Saunders, Philadelphia, PA.
- 85.Ratcliffe, H. L. 1952. Tuberculosis induced by droplet nuclei infection: pulmonary tuberculosis of predetermined initial intensity in mammals. Am. J. Hyg. 55:36-48. [PubMed] [Google Scholar]
- 86.Ribi, E., R. L. Anacker, W. R. Barclay, W. Brehmer, S. C. Harris, W. R. Leif, and J. Simmons. 1971. Efficacy of mycobacterial cell walls against airborne tuberculosis in the rhesus monkey. J. Infect. Dis. 123:527-538. [DOI] [PubMed] [Google Scholar]
- 87.Rich, A. R. 1951. The pathogenesis of tuberculosis, 2nd ed. C. C. Thomas, Springfield, IL.
- 88.Ritz, N., W. A. Hanekom, R. Robins-Browne, W. J. Britton, and N. Curtis. 2008. Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol. Rev. 32:821-841. [DOI] [PubMed] [Google Scholar]
- 89.Rook, G. A. W., K. Dheda, and A. Zumla. 2005. Immune response to tuberculosis in developing countries: implications for new vaccines. Nat. Rev. Immunol. 5:661-667. [DOI] [PubMed] [Google Scholar]
- 90.Rook, G. A., G. Seah, and A. Ustianowski. 2001. M. tuberculosis: immunology and vaccination. Eur. Respir. J. 17:537-557. [DOI] [PubMed] [Google Scholar]
- 91.Rosenthal, S. R. 1955. Standardization and efficacy of BCG vaccination against tuberculosis. Twenty year study: a critical evaluation. JAMA 157:801-807. [DOI] [PubMed] [Google Scholar]
- 92.Rosenthal, S. R., E. Loewinsohn, M. L. Graham, D. Liveright, M. G. Thorne, and V. Johnson. 1961. BCG vaccination against tuberculosis in Chicago. A twenty-year study statistically analyzed. Pediatrics 28:622-641. [PubMed] [Google Scholar]
- 93.Sander, C. R., A. A. Pathan, N. E. R. Beveridge, I. Poulton, A. Minassian, N. Alder, J. Van Wijgerden, A. V. S. Hill, F. V. Gleeson, R. J. O. Davies, G. Pasvol, and H. McShane. 2009. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis-infected individuals. Am. J. Respir. Crit. Care Med. 179:724-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shigenaga, T., A. M. Dannenberg, Jr., D. B. Lowrie, W. Said, M. J. Urist, H. Abbey, B. H. Schofield, P. Mounts, and K. Sugisaki. 2001. Immune responses in tuberculosis: antibodies and CD4-CD8 lymphocytes with vascular adhesion molecules and cytokines (chemokines) cause a rapid antigen-specific cell infiltration at sites of bacillus Calmette-Guerin reinfection. Immunology 102:466-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smith, D. W., D. N. McMurray, E. H. Wiegeshaus, A. A. Grover, and G. E. Harding. 1970. Host-parasite relationships in experimental airborne tuberculosis. IV. Early events in the course of infection in vaccinated and nonvaccinated guinea pigs. Am. Rev. Respir. Dis. 102:937-949. [DOI] [PubMed] [Google Scholar]
- 96.Smith, D. W., and G. E. Harding. 1977. Animal model of human disease. Pulmonary tuberculosis. Animal model: experimental airborne tuberculosis in the guinea pig. Am. J. Pathol. 89:273-276. [PMC free article] [PubMed] [Google Scholar]
- 97.Smith, P. G., and A. R. Moss. 1994. Epidemiology of tuberculosis, p. 47-59. In B. R. Bloom (ed.) Tuberculosis: pathogenesis, protection, and control. ASM Press, Washington, DC.
- 98.Stead, W. W. 2001. Variation in vulnerability to tuberculosis in America today: random or legacies of different ancestral epidemics? Int. J. Tuber. Lung Dis. 5:807-814. [PubMed] [Google Scholar]
- 99.Sutherland, I., and I. Lindgren. 1979. The protective effect of BCG vaccination as indicated by autopsy studies. Tubercle 60:225-231. [DOI] [PubMed] [Google Scholar]
- 100.Thoen, C. O. 1994. Tuberculosis in wild and domestic mammals, p. 157-162. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. ASM Press, Washington, DC.
- 101.Thompson, N. J., J. L. Glassroth, D. E. Snider, Jr., and L. S. Farer. 1979. The booster phenomenon in serial tuberculin testing. Am. Rev. Respir. Dis. 119:587-597. [DOI] [PubMed] [Google Scholar]
- 102.Tuberculosis Research Centre (ICMR), Chennai. 1999. Fifteen year follow up of trial of BCG vaccines in south India for tuberculosis prevention. Indian J. Med. Res. 110:56-69. [PubMed] [Google Scholar]
- 103.Walsh, G. P., E. V. Tan, E. C. dela Cruz, R. M. Abalos, L. G. Villahermosa, L. J. Young, R. V. Cellona, J. B. Nazareno, and M. A. Horwitz. 1996. The Philippine cynomolgus monkey (Macaca fasicularis) provides a new nonhuman primate model of tuberculosis that resembles human disease. Nat. Med. 2:430-436. [DOI] [PubMed] [Google Scholar]
- 104.Woodworth, J. S., and S. M. Behar. 2006. Mycobacterium tuberculosis-specific CD8+ T cells and their role in immunity. Crit. Rev. Immunol. 26:317-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu Y., J. S. Woodworth, D. S. Shin, S. Morris, and S. M. Behar. 2008. Vaccine-elicited 10-kilodalton culture filtrate protein-specific CD8+ T cells are sufficient to mediate protection against Mycobacterium tuberculosis infection. Infect. Immun. 76:2249-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zevallos, K., G. Sandhu, K. Sacksteder, P. Yori, M. Kosek, W. Pan, C. Banda, B. Herrera, T. Valencia, R. H. Gilman, C. Vidal, G. Meza, K. C. Vergara, H. H. Garcia, and C. A. Evans. Human immunity against MTB antigens is augmented by treating intestinal helminths. J. Infect. Dis., in press.