Abstract
Summary: There are many neglected nonenteric protozoa able to cause serious morbidity and mortality in humans, particularly in the developing world. Diseases caused by certain protozoa are often more severe in the presence of HIV. While information regarding neglected tropical diseases caused by trypanosomatids and Plasmodium is abundant, these protozoa are often not a first consideration in Western countries where they are not endemic. As such, diagnostics may not be available in these regions. Due to global travel and immigration, this has become an increasing problem. Inversely, in certain parts of the world (particularly sub-Saharan Africa), the HIV problem is so severe that diseases like microsporidiosis and toxoplasmosis are common. In Western countries, due to the availability of highly active antiretroviral therapy (HAART), these diseases are infrequently encountered. While free-living amoebae are rarely encountered in a clinical setting, when infections do occur, they are often fatal. Rapid diagnosis and treatment are essential to the survival of patients infected with these organisms. This paper reviews information on the diagnosis and treatment of nonenteric protozoal diseases in immunocompromised people, with a focus on patients infected with HIV. The nonenteric microsporidia, some trypanosomatids, Toxoplasma spp., Neospora spp., some free-living amoebae, Plasmodium spp., and Babesia spp. are discussed.
INTRODUCTION
A previous report by Stark et al. (533) reviewed the clinical significance of various enteric protozoa in immunocompromised (IC) patients. While enteric protozoan infections are important in IC groups, there is also a large number of nonenteric protozoa capable of causing significant morbidity and mortality, particularly in IC patients. The clinical significance of tissue protozoa in HIV-infected patients has been reviewed previously by others (308), although due to recent developments, an update is warranted. This paper reviews the scientific literature obtainable mostly from the last decade pertaining to protozoan infections in IC patients. The role of certain tissue protozoan infections in human pregnancy is also discussed.
Several nonenteric protozoa are capable of infecting multiple cell/tissue types. Furthermore, virtually every human cell/tissue type is capable of hosting a number of parasitic and potentially parasitic protozoa. This is particularly true for IC patients, whose reduced immunity allows these infections to progress to full capacity without challenge.
The majority of protozoa discussed here have completely adapted to a parasitic life and are unable to survive in the absence of their preferred host(s). The nonenteric microsporidia, the trypanosomatids (Leishmania spp., Trypanosoma spp., and most lower trypanosomatids), Toxoplasma gondii, Neospora caninum, Plasmodium spp., and Babesia spp. are all among the true parasites that will be discussed herein. While many of these protozoa are responsible for zoonoses, some have a limited host range that does not usually include humans under normal circumstances. However, in cases of severe immunosuppression, parasites that rarely infect humans may do so to cause life-threatening disease. This is true for the microsporidia and some lower trypanosomatids whose host range is usually restricted to a few lower vertebrates and invertebrates.
The coccidian parasite T. gondii is capable of infecting virtually all warm-blooded organisms (180, 274) but can complete the sexual component of its life cycle only in the intestine of cats (178, 274). Human contact with Toxoplasma is common (2, 13, 622), with one-third of the human population believed to be infected (599). Despite this, Toxoplasma infections are typically benign (599), with clinically apparent toxoplasmosis occurring almost exclusively in severely IC patients (405, 599).
The role of the coccidian N. caninum in IC patients is vague, although recent research suggests a role for N. caninum as an opportunistic organism in IC groups. Neospora is closely related to Toxoplasma, although Neospora is best known as a pathogen of veterinary significance. While no human infections with N. caninum have been confirmed, the high incidence of N. caninum antibodies in patients with HIV compared to non-HIV-infected groups suggests a potential role for Neospora as an opportunistic organism in IC patients (361).
Members of the genus Babesia have a broad host range, which includes various rodents and ruminants (56, 75, 307). Several Babesia species are capable of infecting humans, although Babesia microti and Babesia divergens are most often associated with human infection (106). While some Babesia spp. have the ability to cause clinical disease in immunocompetent (ICT) humans, there are a number of IC groups that are predisposed to a more severe form of babesiosis.
Several parasitic protozoa have evolved to cause a potentially fatal disease in ICT humans. Several species of Leishmania, Trypanosoma, and Plasmodium are among this group of potentially lethal protozoa. In IC patients infected with these protozoa, disease progression is often more rapid and severe than that in ICT patients. Immunocompromised patients may also present with unusual clinical signs. The reduction in the immune capacity observed for HIV-infected patients also means that the complete eradication of these protozoa is unlikely and the risk of disease relapse is high. The human-infecting species of Leishmania and Trypanosoma are also zoonoses (93, 123, 202, 418, 445), and the existence of animal reservoirs makes the control of these organisms particularly difficult.
Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium knowlesi are the only five malaria parasites known to infect humans. Plasmodium knowlesi is thought to be the only zoonotic malaria parasite, with the ability to cause disease in some nonhuman primates (350, 415). Plasmodium falciparum causes the most severe form of malaria, killing approximately 2.7 million people each year (546). Plasmodium falciparum is probably an obligate human parasite without any animal reservoirs, as it lacks the ability to infect gorillas and chimpanzees (412).
According to the World Health Organization (WHO), malaria and HIV are among the two most important global health problems of our time (603). Malaria is a huge economic burden in tropical and subtropical countries. The disease is believed to cost Africa alone approximately $12 billion U.S. annually (603). While the topic is of great significance, the importance of Plasmodium-HIV coinfections will not be discussed here in any further detail. This topic is thoroughly covered in the scientific literature, and there is a wealth of current resources available (46, 63, 109, 276, 308, 439, 557, 566, 570, 591, 597). The WHO Regional Office for South-East Asia website (http://www.searo.who.int/index.htm) also covers this topic in great detail. One document available from the WHO website is particularly relevant (603).
Some species of protozoa are not parasitic under normal circumstances but will invade the tissues of other organisms if an opportunity is presented. These organisms have no specific adaptations that enable them to parasitize mammalian tissues and normally survive freely in the environment. However, human and animal infections do occur on rare occasions. Infections with these protozoa are almost always fatal (456, 568, 594). These organisms are free-living amoebae, and the most clinically important of these include Acanthamoeba spp., Naegleria spp., Balamuthia mandrillaris, and Sappinia pedata.
The clinical importance of these pathogenic tissue protozoa in IC patients is reviewed. Immunocompromised groups discussed in this paper include transplant patients and pregnant women, although particular attention is paid to those infected with HIV.
NONENTERIC PROTOZOA IN PREGNANCY
The importance of tissue protozoa in pregnancy is a well-discussed topic in the scientific literature. This topic is also relevant to the focus of this paper because in the strictest sense, pregnant women are also IC. This controlled state of immunosuppression enables the pregnant mother to accommodate fetal antigens that would otherwise be considered foreign (618). As such, this section has been included in the manuscript as an introduction to the topic. More detailed information pertaining to the importance of various tissue protozoa (including Plasmodium spp.) in pregnancy was reported previously (43, 63, 70, 72, 73, 161, 192, 195, 203, 342, 432, 482, 499, 564, 565, 600, 616, 624). For information regarding malaria in pregnancy, the WHO website is useful (see http://www.who.int/features/2003/04b/en/).
During pregnancy, the maternal immune system is shifted to favor a T helper 2 (TH2) immune response, while the T helper 1 (TH1) response is downregulated (436, 618). This downregulation of the inflammatory TH1-type immune response allows the mother to tolerate fetal antigens. However, the TH1 response is essential for gamma interferon (IFN-γ) production and cytotoxic-T-cell activation, which are paramount to the eradication of intracellular pathogens such as Leishmania, Trypanosoma cruzi, and T. gondii (236, 421, 435, 436, 556). The production of IFN-γ is of particular importance to the control of Toxoplasma infections (289, 429, 435). Generally, a healthy mother will keep these infections in check for the duration of the pregnancy. However, the immature immune system of the fetus means that it is vulnerable to infections that may be able to cross the uterine-placental barrier.
In a normal pregnancy, the fetal and maternal circulations are partitioned by a layer of trophoblast cells, which prevent the direct mixing of the two (236). Large blood components such as lymphocytes and erythrocytes do not cross this partition. However, oxygen, nutrients, certain proteins, and endocrine molecules are allowed passage by means of active or passive transport (34). This means that intracellular parasites with limited host cell specificity, such as Leishmania (macrophages, neutrophils, and dendritic cells), Babesia (erythrocytes), and the extracellular parasite Trypanosoma brucei, are unlikely to pose a direct threat to fetal tissues for the duration of the pregnancy. However, at the time of birth a small amount of blood is exchanged between mother and neonate when the placenta separates from the uterine wall (236). This could allow the transfer of T. brucei, Babesia, or Leishmania infections from mother to neonate. Even so, congenital infections with Babesia (515), Leishmania (52), and T. brucei (480) are uncommon compared to congenital infections with Toxoplasma and T. cruzi.
For intracellular protozoa with a broad range of potential host cells, congenital transmission is reported more frequently. Toxoplasma gondii and T. cruzi are able to infect virtually all cell types and by these means have the ability to cross the placental-uterine barrier.
Chagas' Disease and Pregnancy
The transplacental transmission of Chagas' disease occurs with an efficiency of around 5% to 6% for infections acquired in certain regions of Bolivia, Chile, and Paraguay (71, 465, 553). In other areas where the disease is endemic, an efficiency of 1 to 2% or lower has been reported (465). Recently, due to an increase in migration, congenital Chagas' disease has become an increasing public health concern in countries where the disease is not endemic (70, 223, 399, 615). Approximately 26% of all new T. cruzi infections are thought to be the result of congenital transmission (42).
Congenital Chagas' disease is thought to occur exclusively in pregnant woman who have lesions on their placenta (71). These lesions cause damage to the trophoblast partition, allowing trypanosomes to enter the fetal circulation (71). Most congenital infections are asymptomatic or cause nonspecific illness in neonates (42). However, a portion of these infections can result in abortion, low birth weight, hepatosplenomegaly, meningoencephalitis, respiratory insufficiency, anemia, and premature birth (42, 71, 553). Congenital infections with T. cruzi have a mortality rate of around 14% (71). Speculatively, as with Toxoplasma infections, the outcome for the fetus may be dependent on the time during gestation at which trypanosomes begin to parasitize fetal tissues.
Toxoplasmosis and Pregnancy
Toxoplasma gondii is the most significant protozoan pathogen in human pregnancy. Unlike T. cruzi, T. gondii has a worldwide distribution and a broad host range, which includes most warm-blooded animals. Approximately one-third of the entire human population is thought to be infected with Toxoplasma. Furthermore, the efficiency of congenital Toxoplasma transmission in humans was reported to be as high as 19.8% in one study (270). However, Innes et al. (290) suggested that the vertical transmission of Toxoplasma is not so efficient. Regardless, the importance of Toxoplasma to human pregnancy is significant.
In primary Toxoplasma infections, the control of the parasite is dependent on a strong TH1 immune response characterized by the activation of cytotoxic T cells and the production of IFN-γ (436). This TH1 response suppresses the replication and spread of Toxoplasma tachyzoites, which eventually leads to a benign infection. However, the TH1-type response is downregulated during pregnancy. If the mother was infected with Toxoplasma prior to her pregnancy, the risk to the fetus is low. This is due to the development of immunological memory in the mother prior to pregnancy. In this case, the maternal immune system is able to suppress infections before they spread to the trophoblast cells of the placental-uterine partition (435). However, if the mother acquires a primary infection before 20 weeks of gestation, severe fetopathies can result. These can include any combination of the following: chorioretinitis, hydrocephalus, intracranial calcification, and convulsions (538). The overproduction of IFN-γ due to a primary Toxoplasma infection early in a pregnancy may also lead to abortion (435). If infection occurs after 20 weeks of gestation, mild fetopathies (if any) may be observed (538). The fetus can produce antigen-specific responses from around 20 to 22 weeks of gestation (300). This reduction in the severity of Toxoplasma-related fetopathies after 20 weeks coincides with the appearance of the fetal immune system after approximately 20 weeks of gestation.
Other Protozoa in Pregnancy
Given the close relationship between Toxoplasma and Neospora and the high prevalence and efficiency of congenital Neospora transmission in cattle (607), Neospora may also present a risk in human pregnancies. Furthermore, data from experimental infections carried out with nonhuman primates suggest that transplacental transmission is also possible in humans (30). However, the role of N. caninum in human pregnancy is yet to be elucidated.
To our knowledge, cases of congenital transmission of all other tissue protozoa discussed in this paper (the microsporidia, nonhuman Trypanosoma, lower trypanosomatids, and free-living amoebae) have never been reported.
TISSUE-ASSOCIATED PROTOZOA
Nonenteric Microsporidia
Organism and disease.
While the microsporidia are often described as protozoa (25, 363, 577, 578), recent reports indicated that they are actually more closely related to fungi (199, 256, 351, 376). However, in accordance with convention, the microsporidia are described here as protozoa.
The term microsporidia is used as general nomenclature for approximately 1,200 species of obligate intracellular parasites belonging to the phylum Microsporidia. These ancient organisms are ubiquitous in nature but are usually recognized as parasites of invertebrates (531). However, due to the emergence of the global HIV/AIDS pandemic, these organisms are now recognized as opportunistic infectious agents worldwide. The microsporidia are now recognized as significant human pathogens, with infections occurring mainly, but not exclusively, in severely IC patients.
Encephalitozoon spp. are the second most common cause of microsporidial infection in humans after the enteric microsporidian Enterocytozoon bieneusi. Three Encephalitozoon species are known to cause disease in humans: Encephalitozoon cuniculi, Encephalitozoon hellem, and Encephalitozoon intestinalis, previously known as Septata intestinalis. Encephalitozoon species are the most common cause of disseminated microsporidiosis (554), and all Encephalitozoon species have the propensity to disseminate in IC or immunosuppressed patients (166, 217, 540). Encephalitozoon intestinalis is often associated with enteric disease (115, 197) but can infect the kidneys (55), nasal mucosa (167), skin (317), eyes (304), and gallbladder (252). Encephalitozoon intestinalis may also be detected in saliva, urine, and bronchoalveolar lavage fluid (167). Encephalitozoon hellem can cause pulmonary disease (498), keratitis/keratoconjunctivitis (304), kidney disease, and nasal polyps (252). Encephalitozoon cuniculi can infect the intestines, liver, peritoneum, kidneys, and eyes (252, 304). One case study also described an undefined species of Encephalitozoon as the cause of sexually transmissible urethritis in an HIV-infected patient (45).
Trachipleistophora species are less frequently encountered than Encephalitozoon species. Trachipleistophora hominis is known to infect the myocardium and skeletal muscle of HIV-infected patients (132) as well as the conjunctiva, kidneys, and nasal sinuses (252). Trachipleistophora anthropophthera is associated with disseminated disease (576).
Nosema ocularum, Anncaliia (formerly Brachiola) algerae, Microsporidium ceylonensis, Microsporidium africanum, and Vittaforma corneae are causes of keratitis in ICT individuals following eye trauma (168, 586). Anncaliia algerae was also reported to cause myositis in an HIV-negative woman receiving a range of immunosuppressive drugs for the treatment of rheumatoid arthritis (82). Anncaliia (formerly Brachiola) vesicularum and Pleistophora species are associated with skeletal muscle infections in HIV-infected patients (81, 252, 598). In IC patients, Anncaliia (formerly Brachiola) connori infects the smooth muscle, cardiac muscle, kidneys, liver, lungs, and brain (252, 598). A member of the genus Nosema was recently reported to cause keratitis in an ICT patient following bathing in a contaminated water source (133).
Transplant patients and patients suffering from leukocyte malignancies are also predisposed to infections with microsporidia. Pulmonary infection in a leukemia patient by an undefined species of microsporidia has been described (314). An undefined Encephalitozoon was described as the cause of disease in the graft of an HIV-negative renal transplant patient, resulting in renal dysfunction (348). Occasionally, the microsporidia may infect immunologically healthy individuals. One such case involved an unusual E. hellem infection where spores were being shed in the stool of an HIV-negative, immunologically healthy traveler (398). Table 1 summarizes the disease spectra associated with various species of tissue microsporidia.
TABLE 1.
Disease spectra observed for humans infected with various tissue-associated microsporidial species
| Species of Microsporidia associated with human disease | Disease spectrum and/or tissue(s) involved |
|---|---|
| Encephalitozoon intestinalis | Disseminated disease, enteric disease; kidneys, nasal mucosa, skin, eyes, and gallbladder; has also been detected in saliva, urine, and bronchoalveolar lavage fluid |
| Encephalitozoon hellem | Disseminated disease, pulmonary disease, keratitis, keratoconjunctivitis, kidney disease, nasal polyps |
| Encephalitozoon cuniculi | Disseminated disease, CNS, intestine, liver, peritoneum, kidney, and eyes |
| Undefined Encephalitozoon species | Sexually transmissible urethritis |
| Trachipleistophora hominis | Myocardium, skeletal muscle, conjunctiva, kidney, and nasal sinuses |
| Trachipleistophora anthropophthera | Disseminated disease |
| Nosema ocularum, Microsporidium ceylonensis, Microsporidiaafricanum, Vittaforma corneae | Keratitis in immunocompetent hosts following eye trauma |
| Anncaliia (formerly Brachiola) algerae | Keratitis in immunocompetent hosts following eye trauma; myositis in an immunosuppressed patient |
| Anncaliia (formerly Brachiola) vesicularum | Skeletal muscle infections |
| Pleistophora species | Skeletal muscle infections |
| Anncaliia (formerly Brachiola) connori | Disseminated disease infecting the smooth muscle, cardiac muscle, kidney, liver, lungs, and brain |
The transmission of microsporidiosis is poorly understood, although infection must occur through the direct contact of spores with potential host cells. In cases of ocular infection, transmission results from the direct contact of spores with the eye (304). The inhalation or ingestion of spores from the environment is the probable mode of transmission for some Encephalitozoon species (163).
Diagnosis.
The diagnosis of microsporidiosis usually relies on microscopy. The use of PCR can be difficult due to the species-specific nature of most PCR assays and the broad range of microsporidia capable of infecting tissues. A PCR-restriction fragment length polymorphism (RFLP) assay is available, which can differentiate between five species of microsporidia (528) and may be useful if applied to DNA extracted from infected tissues or fluids. A PCR method for the differentiation of a number of Encephalitozoon species and E. bieneusi has also been described, although this method requires downstream processing such as sequencing, RFLP, and/or Southern blot hybridization (212). Real-time PCR assays have been developed for the detection of E. intestinalis DNA in stool specimens, which may be adapted to tissue specimens (390, 611). There are several other PCR assays available that may be useful for identifying and/or differentiating various microsporidia (55, 59, 148, 166, 200, 211, 359).
In cases of ocular infection, microscopic examination of corneal scrapings is usually employed for diagnosis (207). Various stains may be applied to fixed corneal scrapings, although a potassium hydroxide-calcofluor white stain was found to be most efficient in one study (303). A PCR assay has also been developed for the detection of V. corneae DNA in corneal scrapings (468). Another PCR assay for the detection of V. corneae DNA in trichrome-stained smears of corneal scrapings has also been developed (98).
Calcofluor white and a modified trichrome blue stain (165) are useful for the detection of microsporidia in clinical specimens such as bronchoalveolar lavage fluid or urine. Calcofluor white shows greater sensitivity than trichrome blue, although differentiation between calcofluor-stained yeasts and microsporidia may be difficult (165). While it is less sensitive, the trichrome blue stain enables better differentiation between yeast cells and microsporidia (165).
Indirect fluorescent antibody techniques (IFATs) are also available for some Encephalitozoon species (529), as are a broad range of non-species-specific stains that can be applied to tissue biopsy specimens and smears (529, 595). If differentiation between species is necessary and PCR or species-specific IFAT is unavailable, transmission electron microscopy (TEM) (Fig. 1) performed on fixed tissue sections may be useful (55). A direct agglutination test (DAT) is also available, which can detect anti-E. cuniculi antibodies (302).
FIG. 1.
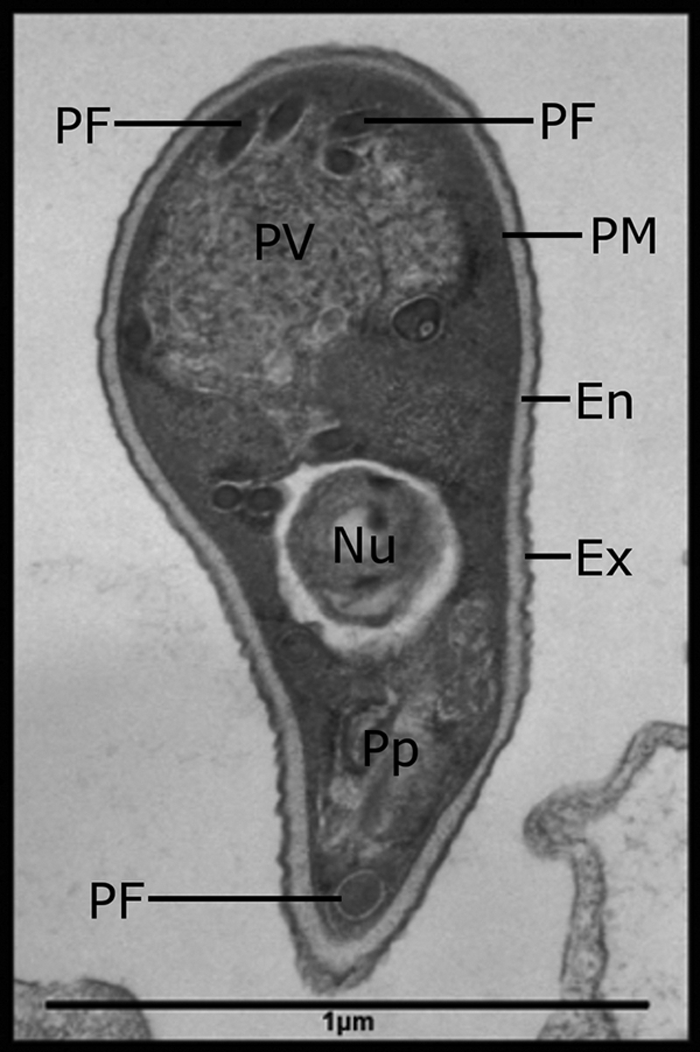
Transmission electron micrograph of an Encephalitozoon cuniculi spore. The main characteristics are labeled, including the polar filament (PF), posterior vacuole (PV), plasma membrane (PM), endospore (EN), exospore (EX), polarplast (Pp), and nucleus (Nu).
Treatment.
Albendazole is the drug of choice for the treatment of disseminated microsporidian infections (39). Albendazole demonstrates good antimicrosporidial activity against Encephalitozoon species, particularly E. hellem (162, 166, 209), in vivo and in vitro. The drug itraconazole may also be useful when combined with albendazole therapy for infections caused by Trachipleistophora or Anncaliia (39). Fumagillin is useful for the treatment of ocular microsporidiosis and is applied to the site of infection in the form of eye drops (39). Oral clindamycin therapy may be effective for the treatment of disseminated E. intestinalis infection (316, 317). Immune restoration following highly active antiretroviral therapy (HAART) is also necessary for the resolution or remission of microsporidial disease occurring as a result of HIV infection (224, 393).
Given the close relationship between the microsporidia and fungi, it is not surprising that certain antifungal compounds (particularly some benzimidazoles) exhibit good antimicrosporidial activity (164, 233, 489, 491, 532, 617). As such, the future discovery of novel antifungal compounds may also have implications for future antimicrosporidial therapy.
Leishmania
Organism and disease.
Leishmania spp. are obligate intracellular parasites that cause three major syndromes in humans: visceral leishmaniasis (VL), cutaneous leishmaniasis (CL), and mucocutaneous leishmaniasis (MCL) (264). Leishmaniasis is transmitted between hosts via an insect vector, which includes species of sandfly from the genera Phlebotomus and Lutzomyia (609). Infection occurs when an infected female fly takes a blood meal from an uninfected host, injecting flagellated promastigotes into the bite wound (609). Promastigotes are then phagocytosed by macrophages, neutrophils, or dendritic cells (58, 475). Once inside the host cell phagolysosome, promastigotes transform into nonflagellated amastigotes, where they multiply by binary fission, eventually destroying the host cell (460, 609). While the process of promastigote entry into host cells is generally described as occurring by means of phagocytosis, this process is now thought to be more complex, involving a series of host-parasite cell interactions (324, 408, 475). Infections with visceral Leishmania species can also be transmitted via blood transfusion and organ transplantation (379).
Various terrestrial mammals are potential reservoirs of Leishmania infection, including rodents and canids (264, 609). Most cases of New World leishmaniasis are the result of zoonotic transmission from either domestic canids or native wildlife (282). In these regions, the recent emergence of zoonotic leishmaniasis is an important public health concern, mostly attributable to poverty, urbanization, and human migration (12, 282).
Approximately 12 million people are thought to be infected with Leishmania worldwide (150). Leishmania infects 500,000 people annually, resulting in approximately 50,000 associated deaths (374). Approximately 19,000 to 24,000 new cases of VL are reported each year in sub-Saharan Africa alone, with most cases occurring in the East African countries of Sudan, Eritrea, Ethiopia, Kenya, and Somalia (283). In these countries, most cases of VL are caused by Leishmania donovani, although Leishmania infantum is occasionally identified as the causative agent (283). The highest incidence of VL in sub-Saharan Africa occurs in Sudan, particularly on the Ethiopian border. Old World CL is also endemic to parts of sub-Saharan Africa, where the causative agent is usually Leishmania major (283). New World CL and MCL are endemic to parts of Central and South America, where the causal species is usually Leishmania braziliensis (218). A list of the clinical syndromes and/or diseases associated with various trypanosomatids (including Leishmania) is summarized in Table 2.
TABLE 2.
Disease spectra observed for humans infected with various trypanosomatids
| Clinical syndrome or disease spectrum | Species | Distribution | Vector genus | Description |
|---|---|---|---|---|
| Visceral leishmaniasis | Leishmania donovani | Old World; parts of central Asia and East Africa | Phlebotomus | These Leishmania species may cause other syndromes (such as CL) in IC patients [see “Leishmania and HIV. (i) Atypical clinical features”] |
| Leishmania infantuma | Old World; Mediterranean basin, Central Asia, Middle East, China | Phlebotomus | ||
| Leishmania chagasia | New World; parts of Central and South America | Lutzomyia | ||
| Leishmania tropica | Old World; Mediterranean basin, Middle East, Southwest Asia | Phlebotomus | ||
| Cutaneous leishmaniasis | Leishmania tropica | Old World; Mediterranean basin, Middle East, Southwest Asia | Phlebotomus | These Leishmania species may cause other syndromes (such as VL) in IC patients [see “Leishmania and HIV. (i) Atypical clinical features”] |
| Leishmania major | Old World; Middle East, Southwest Asia, sub-Saharan Africa | |||
| Leishmania aethiopica | Old World; Ethiopia, Kenya | |||
| Leishmania killicki | North Africa | |||
| Leishmania mexicana | New World; parts of Central America, South America, and the Southern regions of the United States | Lutzomyia | ||
| Leishmania amazonensis | ||||
| Leishmania venezuelensis | ||||
| Leishmania braziliensis | ||||
| Leishmania panamensis | ||||
| Leishmania guyanensis | ||||
| Leishmania peruviana | ||||
| Leishmania lainsoni | ||||
| Leishmania colombiensis | ||||
| Leishmania pifanoi | ||||
| Leishmania garnhami | ||||
| Mucocutaneous leishmaniasis | Leishmania panamensis | Parts of Central and South America | Lutzomyia | These Leishmania species may cause other syndromes (such as VL) in IC patients [see “Leishmania and HIV. (i) Atypical clinical features”] |
| Leishmania braziliensis | ||||
| Leishmania braziliensis | ||||
| Leishmania guyanensis | ||||
| Chagas' disease | Trypanosoma cruzi | Parts of Central and South America and South/Southwestern United States | Triatoma | In ICT patients, Chagas' disease is often associated with cardiopathies; in IC patients, Chagas' disease can also include CNS manifestations [see “Chagas' disease. (iv) Chagas' disease and HIV”] |
| African sleeping sickness (“nagana” in animals) | Trypanosoma brucei rhodesiense | Sub-Saharan Africa; Central and West Africa | Glossina | There is no evidence to indicate that the clinical course of sleeping sickness is worsened in IC patients |
| Trypanosoma brucei gambiense | ||||
| Sleeping sickness-like syndrome (“nagana” in animals) | Trypanosoma congolense | Sub-Saharan Africa | Glossina | The disease-causing potential of T. congolense is not certain, as the patient in this single report was also infected with a T. brucei species |
| Fever, chills, and sensory impairments (“surra” in animals) | Trypanosoma brucei evansi | Southeast Asia, Africa, and South America | Any hematophagous fly species and, in South America, vampire bats | T. b. evansi relies on mechanical transmission between hosts; therefore, any hematophagous insect or animal is a potential vector; in this single case report, the patient also experienced behavioral changes; the infection was partly attributed to a dysfunctional human trypanolytic factor |
| Transient fever (“nagana” in animals) | Trypanosoma brucei brucei | Sub-Saharan Africa | Glossina | In this case, T. b. brucei caused transient, self-limiting infection characterized by fever and severe dyspnea; only a single reported case; human infections with T. b. brucei are controversial |
| Fever, anemia, anorexia, and sometimes edema | Trypanosoma(Herpetosoma)lewisi | Malaysia, Africa, and India | Several species of flea | Trypanosoma lewisi infection is usually restricted to rodents; children seem to be more susceptible to T. lewisi infection than adults |
| Cutaneous leishmaniasis-like disease | Unnamed, highly divergent member of the genus Leishmania | Martinique | Vector unknown or nonexistent | Only 2 reported cases; this organism caused a small cutaneous, ulcerative lesion on the eyebrow of an ICT patient; for an HIV-infected patient, diffuse, cutaneous, nonulcerative lesions were reported |
| Visceral leishmaniasis-like syndrome | Undefined lower trypanosomatid | Spain | Vector unknown or nonexistent | Only 1 case reported; patient suffered from symptoms similar to those of VL, including pancytopenia and hepatosplenomegaly; the organism was similar to yet morphologically different from Leishmania; this species was blunt ended and had a denser kinetoplast |
| Leptomonas pulexsimulantis-like organism | Brazil | No vector; a monoxenous trypanosomatid usually infecting fleas | Only 1 case reported; patient suffered from symptoms similar to those of VL, including splenomegaly and fever |
It is under debate as to whether L. chagasi and L. infantum are different species. Some believe that L. infantum was originally brought to the New World during the Spanish and Portuguese occupation of South America and that these species are identical.
(i) Visceral leishmaniasis.
Visceral leishmaniasis, resulting from the replication of amastigotes within mononuclear phagocytes of the liver, spleen, and bone marrow, usually results in fever, severe cachexia, hepatosplenomegaly, and pancytopenia (170, 264, 408). The symptoms of VL usually develop after an incubation period of weeks to years (264), and the disease is invariably fatal if left untreated.
(ii) Cutaneous leishmaniasis.
CL is characterized by lesions on the surface of the skin resulting from the replication of Leishmania amastigotes within mononuclear phagocytes of the skin (Fig. 2 and 3). The morphology of skin lesions can vary greatly. Lesions can exist as ulcers, nodules, plaques, or papules. Ulcerative lesions eventually heal to leave atrophic scars. Patients can have multiple lesions at various localities. Typically, cutaneous lesions appear as well-defined ulcers with raised borders (248). Other unusual lesion morphologies (vegetative, verrucous, crusted, and lupoid) were described by Guimarães et al. (248). Cutaneous leishmaniasis can also present as disseminated skin nodules or papules (Fig. 3) (248). Ulcerative lesions can be painful, and secondary bacterial infections can occur. Cutaneous leishmaniasis can remain subclinical or may become clinically apparent after an incubation period of weeks to months (27, 247, 264, 473).
FIG. 2.
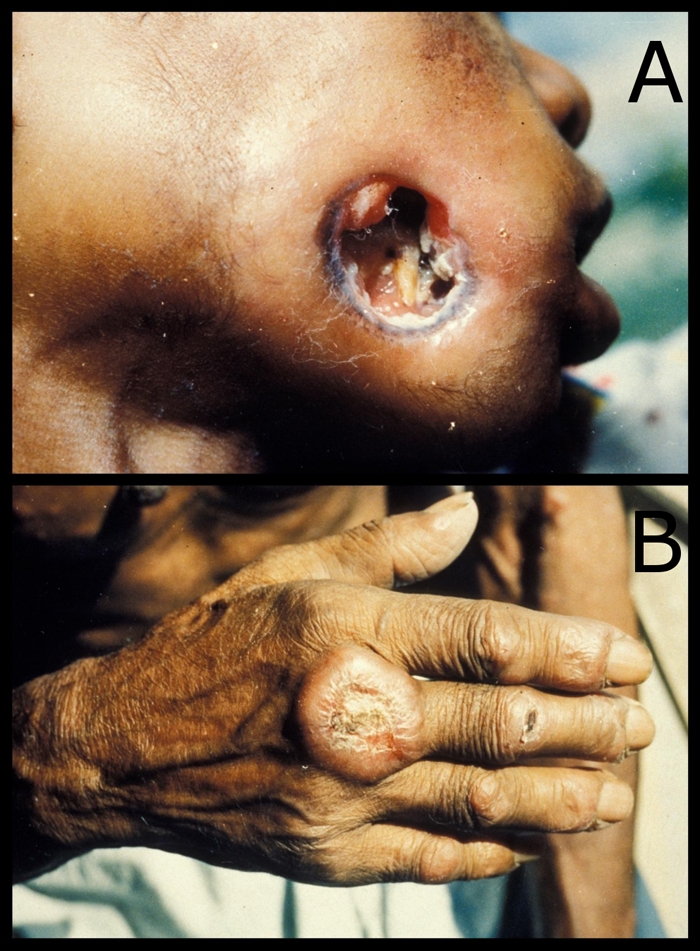
Clinical presentation of cutaneous leishmaniasis. (A) Ulcerative lesion of the face resulting in complete perforation of the cheek. (B) Partially healed cutaneous lesion of the hand.
FIG. 3.
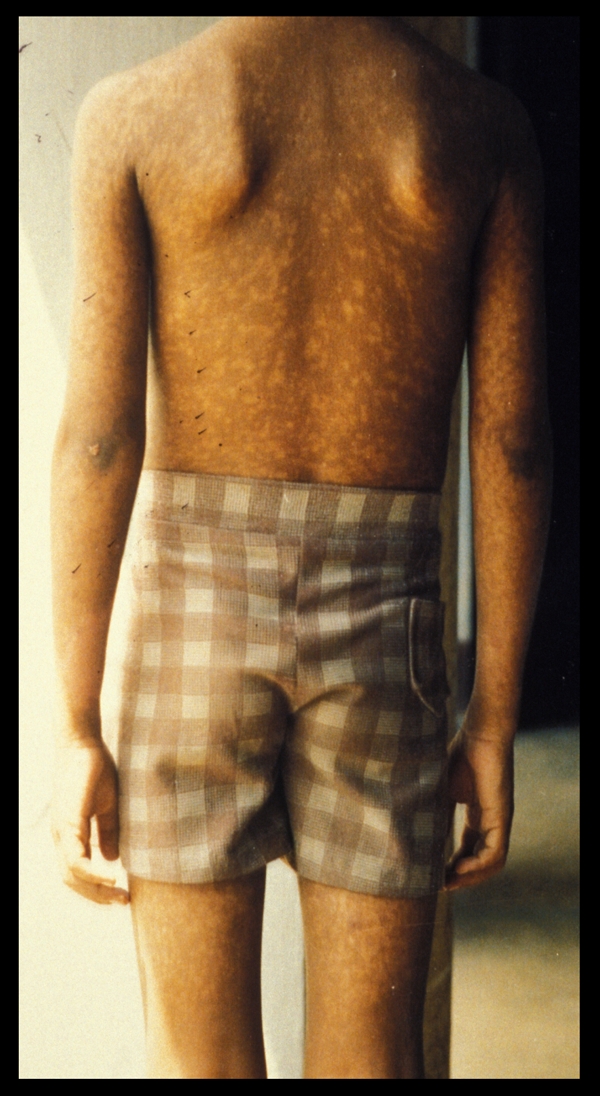
Diffuse cutaneous leishmaniasis presenting as widespread, nonulcerative plaques.
Usually, lesions undergo complete resolution in ICT individuals. In contrast, lesions in HIV-infected individuals are more severe, larger, and more diffuse and will increase in size without treatment (135, 247). Some cutaneous Leishmania species also have the ability to induce T-cell anergy and apoptosis (442).
(iii) Mucocutaneous leishmaniasis.
Mucocutaneous leishmaniasis is restricted to the New World and usually follows a cutaneous infection. The species most often associated with MCL is Leishmania braziliensis, although Leishmania panamensis and Leishmania guyanensis may occasionally cause MCL (264, 609). In MCL, Leishmania parasites travel from the skin through the lymphatics and/or blood vessels to the mucous membranes of the mouth, nose, throat, and soft palate (219). This results in the destruction of the naso-oropharyngeal membranes, and perforation of the nasal septum can occur (14, 173, 264, 609). Mucocutaneous leishmaniasis can occur concurrently with a cutaneous infection or months to years after cutaneous lesions heal (219). The risk of developing MCL following CL is believed to be less than 5% in ICT individuals (122, 219). While MCL can result in severe disfigurement, the mortality rate is low (219).
Leishmania and HIV.
Since the 1980s, Leishmania-HIV coinfections (especially infections with L. infantum) have become an important public health concern, particularly in European countries surrounding the Mediterranean basin. A study from 1998 reported that 10% of AIDS patients in southern Spain were also infected with Leishmania (440). Later studies estimated that 25% to 70% of adult patients with VL in the southern regions of Spain, France, and Italy were also infected with HIV (83). In fact, approximately 90% of all Leishmania-HIV coinfections identified by the WHO up to the year 2001 came from Spain, Italy, France, and Portugal (10). Much of the current literature regarding Leishmania-HIV coinfection in southern Europe comes from Spain, where L. infantum is endemic and VL is the most common syndrome encountered. In Spain, prior to 1985, most patients with VL were ICT children, but after 1998, approximately 80% of patients with VL were IC adults (362). Visceral leishmaniasis is also an important disease in HIV-infected persons in East Africa, South America, and Asia (10, 159).
In HIV-infected patients, the associated reduction in immune function allows latent infections of Leishmania to become clinically apparent (605). In one study, the prevalence of clinically apparent VL in HIV-infected patients was 2,320 times greater than that in ICT patients (362). Furthermore, the clinical course of VL is far more severe in HIV-infected patients than in ICT individuals (18).
Intravenous drug use is probably the largest risk factor for obtaining a Leishmania-HIV coinfection in southern Europe (83, 440, 449), with some studies reporting 80% to 90% of all coinfected patients being intravenous drug users (11, 362). Furthermore, a more recent report from the WHO stated that 64% of Leishmania-HIV coinfections reported in southern Europe between 2001 and 2006 were of intravenous drug users (604). It is probable that the sharing of needles facilitates the concurrent transmission of Leishmania and HIV in many of these cases (362).
(i) Atypical clinical features.
Leishmania-HIV-coinfected patients will often present with atypical clinical features. For example, several studies reported cutaneous or mucocutaneous infections caused by the visceral species L. infantum (83, 120, 449). A case of post-kala-azar dermal leishmaniasis was also reported for an HIV-infected patient coinfected with L. infantum (534). Leishmania donovani can cause cutaneous disease in the presence of HIV (189), while Leishmania mexicana can cause visceral disease (463). While MCL usually follows a cutaneous infection, MCL has been reported as the first clinical manifestation of AIDS even before CL or other more typical AIDS-defining illnesses (135). In a kidney transplant patient, concurrent cutaneous, visceral, and ocular leishmaniases as a result of L. braziliensis infection were also described (239). Interestingly, one report noted that 20% to 40% of HIV-infected patients with VL do not exhibit splenomegaly, which is a typical clinical feature of VL in ICT individuals (395).
In other cases of Leishmania-HIV coinfection, involvement of the lungs (83, 362, 584), gastrointestinal system (83, 362), pancreas (104), and eye (239) has also been reported.
(ii) Leishmania and HIV progression.
Leishmania-HIV coinfections present a unique problem to clinicians, as Leishmania and HIV are thought to complement each other's disease progression. Some studies suggested that Leishmania may directly increase the rate of replication of HIV in human macrophages (158, 610, 623). Visceral Leishmania species also enhance the progression of HIV infections via the production of certain antigens that induce apoptosis of CD4+ cells (450). Therefore, CD4+ cell counts are an unreliable indicator of the effectiveness of antiretroviral therapy in these patients (17). Human immunodeficiency virus infection is thought to increase the chance of developing VL by 100 to 1,000 times in areas of endemicity (420). The presence of HIV in VL also improves the chances of relapse and reduces the likelihood of a therapeutic response to treatment (420). Cure of clinically apparent leishmaniasis may be achieved temporarily for HIV-infected patients, although it is unlikely that Leishmania parasites will be completely eradicated (155, 534).
Diagnosis.
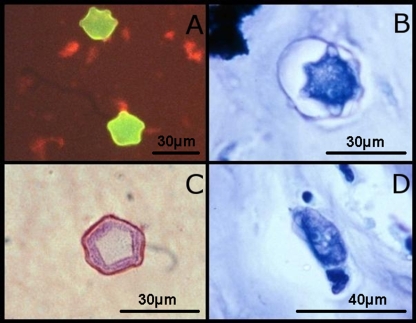
Conventional (23, 126, 137, 145, 205, 237) and real-time (91, 154, 396, 569) PCR assays, immunoassays (292, 484, 527), culture systems (17, 53, 527, 535), and microscopy (6, 130, 190) are available for the diagnosis of leishmaniasis. Light microscopy has been described as the method of choice for the diagnosis of leishmaniasis (408, 487). Any of the Romanowsky-based stains, such as a Wright stain (319) or Giemsa stain (130, 466), can be used for the direct microscopic identification of Leishmania parasites in tissue sections, smears, or tissue aspirates (Fig. 4B and D) (10). The inoculation of tissue aspirate material onto Novy-MacNeal-Nicolle agar for the cultivation of live Leishmania parasites may also be useful for diagnosis but may be impractical for some diagnostic laboratories (487). In Ethiopia, where L. donovani VL is endemic and resources are strained, the use of microscopy can also be impractical. In this case, an L. donovani-specific DAT is preferred (17). The DAT (and modifications of this test) demonstrate sensitivities of between 89% and 95% (10). The quantitative buffy coat (QBC) tube technique is a rapid and simple technique that is also useful for the diagnosis of VL (356).
FIG. 4.

Microscopic images from clinical samples. (A) Trypanosoma brucei trypomastigotes in a blood smear (Leishman stain). (B) Oval-shaped Leishmania infantum amastigotes from a bone marrow aspirate (Leishman stain). (C) Toxoplasma gondii tachyzoites from CSF (Giemsa stain). (D) Typical round Leishmania amastigotes from a bone marrow aspirate (Leishman stain).
Several other serological tests are available, including a commercial IFAT and the rK39 dipstick test (487). The rK39 dipstick test is rapid and inexpensive and displays sensitivities of >90% for the detection of L. donovani-associated kala-azar in India. However, sensitivities as low as 20% have been reported for HIV-infected patients with VL in Europe (10). In cases of severe immunodeficiency, enzyme-linked immunosorbent assays (ELISAs) and other serological tests can be of limited use, as antibodies to Leishmania may be absent despite an active infection (18, 158). Furthermore, antibodies to some antigenic epitopes of Leishmania can cross-react with those of Trypanosoma (and other protozoa), which can make the use of some serological tests problematic (10, 79, 381, 581). However, a recently developed latex agglutination test for the detection of Leishmania antigen in patient urine samples is available, which demonstrates good sensitivity and specificity (10).
PCR performed on DNA extracted from fluids, tissue scrapings, or punch biopsy specimens is a sensitive and specific alternative to serology and antigen detection (438, 527). PCR applied to peripheral blood specimens is useful for the diagnosis of VL in HIV-infected individuals (10). Conventional and real-time PCR assays for the diagnosis of CL have demonstrated greater sensitivity than microscopy or parasite culture (130, 391, 516). PCR performed on DNA extracted from paraffin-embedded tissues is also useful for the diagnosis of CL (490).
To ensure an accurate diagnosis, it is recommended that multiple diagnostic techniques are applied (where possible) when Leishmania infection is suspected in HIV-infected patients (143, 170, 441).
Treatment.
The antimonial compounds (usually sodium stibogluconate and meglumine antimoniate), miltefosine, and amphotericin B are the mainstay of antileishmanial therapy (10, 17, 158, 239, 464, 488).
In developing countries, the pentavalent antimonial compounds are usually the first line of treatment for leishmaniasis due to their availability and low cost (17, 158). However, these compounds are quite toxic (17, 125, 149, 158, 362). Furthermore, due to their widespread use for over 70 years, resistance has meant that antimonials are virtually useless in parts of India and Nepal (24, 374). In regions of India where antimonial resistance was apparent, pentamidine was once used as an alternative. However, due to severe toxic side effects, the use of pentamidine was abandoned (10, 158). Generally, the pentavalent antimonials are not used in developed countries, where amphotericin B in various forms is favored due to reduced toxic side effects. The comparatively low toxicity of amphotericin B enables treatment with higher dosages and, subsequently, shorter courses of therapy (609).
Liposomal amphotericin B is the treatment of choice for VL (488), although it is expensive and may not be feasible in developing countries (158). In India, oral miltefosine therapy has gained widespread use for the treatment of VL due to its effectiveness and comparatively mild side effects (10). However, as with the antimonials, resistance to miltefosine is likely to become a future problem in India (408). Other drugs that have demonstrated anti-Leishmania activity include paromomycin (488), sitamaquine (464), and aminosidine (158), although these drugs have not gained widespread use. Interestingly, the HIV protease inhibitors indinavir and saquinavir have direct leishmaniacidal activity in vitro (497).
In Ethiopia, sodium stibogluconate is the first-line treatment for Leishmania-HIV-coinfected patients despite severe side effects, including a 33% chance that the patient will die of drug-induced pancreatitis (17). Unfortunately, less-toxic drugs such as amphotericin B and miltefosine are not as accessible in Ethiopia (17). Furthermore, Ethiopian patients with a Leishmania-HIV coinfection are less responsive to miltefosine than to antimonials (10).
In a recent study, the efficacy of various regimens for the treatment of American CL and MCL was reviewed (240). The findings of that study indicated that the conventional antimonial drugs (sodium stibogluconate or meglumine antimoniate injected intramuscularly) are efficacious for the treatment of New World CL and MCL (240). Oral allopurinol and oral pentoxifylline were also found to be good adjuvants to antimonial therapy (240). Furthermore, the efficacy of the drug miltefosine was found to be species dependent, being more effective against L. panamensis infections than against L. mexicana and L. braziliensis infections (240).
As with other protozoan-HIV coinfections, HAART resulting in immune reconstitution has greatly reduced the incidence of Leishmania-HIV coinfections in symptomatic patients (10, 147, 151, 488).
Trypanosoma
Members of the genus Trypanosoma are the causative agents of human African trypanosomiasis (African sleeping sickness) and American trypanosomiasis (Chagas' disease), two blood-borne diseases, each with an insect vector (32). Usually, two species of Trypanosoma are associated with human trypanosomiasis. These are Trypanosoma cruzi and Trypanosoma brucei gambiense/Trypanosoma brucei rhodesiense. A broad range of terrestrial mammals, including cattle, dogs, cats, and wild animals, behave as potential reservoir hosts for these species (160, 202, 287, 315).
Both species of Trypanosoma infecting humans are transmitted by blood-feeding insects, although they differ fundamentally in their modes of transmission. Trypanosoma brucei is transmitted in the salivary secretions of tsetse flies from the genus Glossina, while T. cruzi is transmitted in the feces of triatomine bugs (1, 127). Trypanosoma brucei infection occurs when the tsetse fly takes a blood meal (105, 196). Trypomastigotes from the fly's saliva enter the bite wound and multiply extracellularly in the blood, lymph, or spinal fluid. Humans become infected with T. cruzi when a triatomine bug takes a blood meal and defecates on the host's skin. Triatomine salival secretions cause the host to itch and unknowingly rub the triatomine feces (containing trypomastigotes) into the bite wound. Microabrasions caused by host scratching also allow the entry of trypomastigotes into the host (32). Trypanosoma cruzi trypomastigotes travel to various host tissues through the blood and lymph. The trypomastigotes eventually enter host cells, where they transform into the amastigote stage and multiply by binary fission until the host cell is destroyed. Trypanosoma cruzi may also be transmitted congenitally, via blood transfusion, or via organ transplantation (160, 425). Congenital transmission of T. brucei has also been reported although less frequently (105, 313, 413, 427, 480).
Trypanosoma cruzi and T. brucei also differ in distribution, with T. cruzi being endemic in the Southern/Southwestern regions of the United States and parts of Central and South America (494), while T. brucei is confined to parts of Central and Southern Africa (32). African sleeping sickness and Chagas' disease are both potentially fatal in the absence of treatment (32, 103) regardless of the presence of HIV.
Interestingly, human infections with several species of non-human-infecting trypanosomes have also been reported. The non-human-infecting trypanosomes Trypanosoma brucei brucei, Trypanosoma brucei evansi, Trypanosoma congolense, and Trypanosoma (Herpetosoma) lewisi are usually associated with animal disease but are now recognized as the potential agents of a transient, self-limiting illness in ICT humans (138, 299, 305, 495, 521, 560). As most human infections with the non-human-infecting trypanosomes were not associated with immune deficiencies, the role of these organisms in IC patients is yet to be defined. However, given their ability to cause illness in ICT humans, it is likely that these organisms will be recognized in the future as opportunistic agents in IC patients. As such, several case reports of human infection with these trypanosomes are also discussed.
Chagas' disease.
Approximately 8 to 9 million cases of Chagas' disease are thought to exist worldwide, with approximately 50,000 cases reported annually (282). Trypanosoma cruzi infections in Latin America and the Caribbean are between 5 and 10 times more common than malaria in these regions (282). The Pan-American Health Organization (PAHO) estimates that 7.7 million people in 21 countries where the disease is endemic (including the United States) are infected with T. cruzi (494). In recent years, Chagas' disease has become a public health concern in countries where the disease is not endemic due to immigration from areas where the disease is endemic (293, 294, 502, 601, 615). As such, the screening of solid-organ and blood donors for Chagas' disease has become increasingly important (92, 193, 220, 379). In Canada and some European countries, blood and organ donors are required to fill out a questionnaire in relation to Chagas' disease, and individuals known to be infected are prohibited from donating organs (379, 502). In the United States, screening of blood donors with a recommended enzyme immunoassay has been implemented but is not yet mandatory (502).
The clinical course of Chagas' disease is divided into three stages: the acute, intermediate, and chronic stages (160, 394, 454). At the initial site of infection, inflammation and swelling usually occur. The acute stage begins when symptoms appear 6 to 10 days after the initial infection and continue for up to 2 months (32, 394). Most infections occur during childhood (32).
(i) Acute stage.
The acute phase of Chagas' disease often remains unnoticed or misdiagnosed, as the symptoms experienced, including anorexia, fever, malaise, nausea, vomiting, and diarrhea, are not particular to Chagas' disease (160). On rare occasions, death does occur in the acute phase of infection, usually due to myocarditis or meningoencephalitis (32, 457).
(ii) Intermediate stage.
The acute phase of Chagas' disease is usually followed by a period of asymptomatic carriage of parasites, which usually remain with the patient for the rest of their life. Approximately 50% of all T. cruzi-infected patients are in this intermediate phase of Chagas' disease (454). These patients are generally asymptomatic and unaware of their disease. However, the presence of a T. cruzi infection can be demonstrated by serology, PCR, or parasite culture (454).
(iii) Chronic stage.
Around 30% of all Chagas' disease patients will develop chronic disease, characterized by severe myopathies and organ and tissue damage after an incubation period of 10 to 30 years (32, 87). During this stage, cardiomegaly, megacolon, and megaesophagus may be observed in patients (543). The damage inflicted on the heart is often permanent and severe to the point where late-stage Chagas' disease patients may require heart transplantation (84, 139). Heart failure and sudden death will usually occur in the absence of treatment (32, 543). An abnormal electrocardiogram (ECG) may be apparent for years prior to the appearance of symptoms such as cardiomegaly. Therefore, an ECG can be used for the early detection of chronic Chagas' disease in patients with a known T. cruzi infection (222). Echocardiography and chest radiography are also employed to detect heart abnormalities associated with chronic Chagas' disease (222).
The management of Chagasic heart disease is usually dependent on individual patient circumstances (44, 221, 437, 481). For the treatment of bradyarrhythmias, a pacemaker can be fitted (222). For the treatment of certain ventricular arrhythmias, a cardioverter defibrillator may be implanted (222). Patients with refractory heart failure are usually assessed as candidates for heart transplantation (222). In transplant patients, there is a risk of reactivation of the disease in response to immunosuppressive antirejection therapy (44). There is also a risk of reobtaining a donor-derived infection with T. cruzi (222, 330). Chemotherapeutic compounds used for other heart conditions, such as the β-blockers or the drug digoxin, may be prescribed for the treatment of Chagas' disease-associated cardiomyopathies in some cases (44).
The exact pathological mechanisms associated with chronic Chagas' disease are not completely understood (44, 249). Direct tissue damage due to the cycle of host cell infection and destruction is thought to account, to some degree, for the damage inflicted during chronic disease. However, it is now thought that much of the damage associated with chronic Chagas' disease is due to an autoimmune reaction. In most cases, the T. cruzi parasite load is disproportionately low compared to the damage inflicted during chronic Chagas' disease (44). As such, the host's own immune system is thought to play a major role in the pathogenesis of chronic disease (44, 249). Several antigens expressed by T. cruzi are reported to mimic human proteins, including the cardiac myosin heavy chain and the cardiac muscarinic acetylcholine receptor (44, 131, 353). This results in the development of a cross-reactive autoimmune response (234). As such, the pathogenesis of chronic Chagas' disease is thought to be more a case of postinfectious autoimmunity rather than damage inflicted directly by T. cruzi parasites (44, 249).
(iv) Chagas' disease and HIV.
In areas of endemicity, reactivated Chagas' disease may be the first presentation of HIV infection (20), and the lethality of T. cruzi-HIV coinfection is high (369). In regions where the disease is endemic, reactivation of latent Chagas' disease not only occurs in patients infected with HIV (118, 201, 476, 496) but also has been reported for those with leukemia (9) or lymphoma (208) and transplant recipients (8, 40, 216).
Typically, severe immunosuppression alters the clinical course of Chagas' disease to include central nervous system (CNS) manifestations, which are not usually seen for ICT patients (20). Human immunodeficiency virus-infected patients with Chagas' disease may experience acute meningoencephalitis, fever, headaches, seizures, and vomiting along with myocarditis and other pathologies observed for ICT patients (567). Human immunodeficiency virus-infected patients with Chagas' disease may also present with necrotic brain lesions (349). Unusual pathologies have also been reported. In one case, parasitosis of the cervix uteri was observed for an HIV-infected patient, diagnosed by a cervical biopsy (113). Another unusual case was reported for a kidney transplant patient who developed Chagasic skin lesions (216).
(v) Diagnosis.
In HIV-infected individuals, the diagnosis of Chagasic brain pathologies begins with a computed tomography (CT) scan or magnetic resonance imaging (MRI) (20, 174, 369, 567). Meningoencephalitis and myocarditis as seen for T. cruzi-HIV coinfection may be easily confused with those observed for Toxoplasma-HIV coinfections (20, 522). Trypanosoma cruzi necrotic brain lesions are also indistinguishable from those of Toxoplasma. Differentiation between these pathogens by PCR or microscopy is essential, as incorrect treatment may result in disease progression and patient death.
A definitive diagnosis can be made by the direct identification of trypomastigotes in stained blood smears or in the buffy coat (20, 543). The QBC tube technique may be a useful diagnostic tool during the acute phase of Chagas' disease (443, 444) but is less useful for diagnosis during the chronic phase of the disease (15). In the acute phase of Chagas' disease, live trypanosomes may be observable in an unstained blood smear viewed at a ×100 magnification (49). The use of the Romanowsky-based stains (usually a Giemsa, Wright, or Leishman's stain) is suitable for the detection of trypomastigotes in thin blood smears (49, 487). For examination of amastigotes of T. cruzi in tissue biopsy specimens, a hematoxylin-and-eosin stain is often employed. The following websites contain excellent images of T. cruzi parasites in stained blood smears and heart tissue biopsy specimens: http://www.dpd.cdc.gov/dpdx/HTML/ImageLibrary/TrypanosomiasisAmerican_il.htm and http://apps.who.int/tdr/publications/tdr-image-library?disease=Chagas+disease&location=&idNumber=&descText=&photographer=&creditSource=&x=19&y=10.
Due to a reduction in CD4+ counts, trypomastigotes may be observed more frequently in the blood of HIV-infected patients than in the blood of ICT individuals (496). In one study, the mean number of T. cruzi genome equivalents in patients with chronic Chagas' disease was 25.83 ± 26.32 per ml of blood (62). However, in an HIV-infected patient with cerebral Chagas' disease, 280 T. cruzi genome equivalents per ml of blood were detected (74). Both of those studies utilized a quantitative PCR assay (62, 74). Generally, blood microscopy is useful only for detecting trypanosomes in the acute phase of Chagas' disease (160).
In chronic Chagas' disease, serological and molecular techniques should be used preferentially to blood microscopy due to their higher sensitivity. PCR performed on DNA extracted from peripheral blood is useful for the early diagnosis of reactivated Chagas' disease (373) and for monitoring treatment efficacy (74, 171). PCR may also be performed on tissue biopsy specimens for the molecular characterization of strains/isolates (74) or to demonstrate the chronic persistence of trypanosomes in tissues (40). A sensitive real-time PCR assay has also been developed for the detection of T. cruzi DNA in blood specimens (446). The diagnosis of congenital Chagas' disease can be made by the microscopic observation of T. cruzi parasites in umbilical cord blood specimens, although PCR is a more sensitive alternative (42). Immunological techniques such as ELISA or IFAT can also be useful for the diagnosis of Chagas' disease (99, 100, 160, 238, 372).
A combination of diagnostic tools, including direct detection methods (molecular tools and/or microscopy) and serology, is recommended for an accurate diagnosis of Chagas' disease (160). In the case of the CNS involvement observed frequently for HIV-infected patients, trypanosomes will often be observable microscopically in the cerebrospinal fluid (CSF) (118).
(vi) Treatment.
The drugs of choice for the treatment of Chagas' disease are benznidazole and nifurtimox (20, 567). Both drugs are proven to reduce the severity of acute Chagas' disease but are less effective against chronic Chagas' disease (543). These drugs both require extended courses and may cause severe side effects (41, 543). Over 80% of patients are cured of symptoms (20), although trypanosomes will usually persist asymptomatically (543). The drug allopurinol has also demonstrated antitrypanosomal activity (550) and has comparatively few side effects (543). With the completion of the T. cruzi and T. brucei genome projects, a push toward a more focused identification of new drug targets using bioinformatic approaches has become apparent in recent years (346, 400).
African sleeping sickness.
There are four known subspecies of Trypanosoma brucei, two of which cause human disease. In Western and Central Africa, T. brucei gambiense causes a chronic form of disease, while in Eastern and Southern Africa, T. brucei rhodesiense causes an acute form (32, 184, 269). Infection with either subspecies is usually fatal if left untreated (196).
Millions of people from 36 countries in sub-Saharan Africa are at risk of obtaining a T. brucei infection. According to the WHO, prevalences of 50% occur in some African communities, including villages in the Democratic Republic of Congo, Angola, and southern Sudan. In these communities, sleeping sickness was considered the first or second cause of mortality even before HIV/AIDS (606). Approximately 50,000 to 70,000 people in sub-Saharan Africa are believed to be infected with T. brucei, with approximately 17,000 new cases reported annually (283).
Two stages of African sleeping sickness exist: the early hemolymphatic stage and the successive CNS stage (68, 183). Shortly after the initial infection, a localized inflammatory nodule forms near the bite wound, which may ulcerate (32). This nodule is referred to as a trypanosomal chancre. Trypanosomal chancre does not always occur and its appearance is often dependent on the strain (530) and/or subspecies (32). Trypanosomal chancre occurs in approximately 19% of T. b. rhodesiense infections and rarely in T. b. gambiense infections (32, 68). Three to four weeks later, the chancre heals while T. brucei trypomastigotes spread to the bloodstream and lymphatics (61, 184).
(i) The hemolymphatic stage.
During the hemolymphatic stage, patients will usually suffer from headache, arthralgia, weakness, fever, and malaise (315). Patients infected with the T. b. rhodesiense subspecies will suffer a more acute form of disease, which can involve anemia, thrombocytopenia, disseminated intravascular coagulation, and heart pathologies (100). Pancarditis and pericardial effusion can occur, eventually leading to heart failure. Pulmonary edema may result in death. Hepatosplenomegaly may also occur (68).
The clinical course of a T. b. gambiense-type infection is far more subtle and, as a result, may remain unrecognized or misdiagnosed. In T. b. gambiense infections, after several weeks patients will exhibit swollen lymph nodes, often on the posterior of the neck (referred to as Winterbottom's sign) (32).
(ii) The CNS stage.
The second stage of African sleeping sickness involves invasion of the CNS by trypomastigotes (196). This second stage occurs within a few weeks in T. b. rhodesiense infections but over a matter of months to years in T. b. gambiense infections. The clinical manifestations of the CNS stage are quite diverse. Patients will experience the sleeping disorders typical of African sleeping sickness, such as a reversal of sleep-wake cycles and a strong urge to sleep (315). Patients can also experience any range or combination of psychological, motor, and sensory disorders (32, 196, 315). Headache, weight loss, and endocrine disorders such as impotence are also typical (32). If the second stage of African sleeping sickness is left untreated, coma and eventual death will ensue.
(iii) African sleeping sickness and HIV.
To our knowledge, there is little association between the occurrence of severe African sleeping sickness and HIV (384). Dedet and Pratlong (141) also described the absence of any significant association between sleeping sickness and HIV. While T. cruzi is an intracellular parasite, T. brucei is extracellular and exists only in the lymph, the bloodstream, and, eventually, the CSF. It was suggested that being extracellular, the control of T. brucei infection is based on a T-cell-independent immunoglobulin response. Therefore, HIV infection does not place sleeping sickness patients at a significantly increased risk of developing more-severe forms of the disease. As such, the treatment of sleeping sickness patients in the presence of HIV infection is often successful (384). There is little in the literature describing the clinical course of sleeping sickness in HIV-infected patients, although based on current information, the clinical course is similar to that observed for ICT individuals.
(iv) Variable surface glycoprotein.
The extracellular life of T. brucei means that it is in frequent contact with the humoral immune response. Despite this, T. brucei is a successful extracellular parasite capable of inducing chronic infections that can last for several years (100). This is due to its ability to alter the expression of certain immunogenic glycoproteins on its surface. The existence of these variable surface glycoproteins (VSGs) allows T. brucei to remain extracellular and still evade the host's humoral immune response.
VSG is the predominant surface antigen in African trypanosomes and covers the entire plasma membrane (585). Trypanosoma brucei has approximately 1,250 to 2,000 genes and pseudogenes that control the expression of VSGs (68, 545). During a T. brucei infection, specific antibodies to a given VSG are raised by the host. However, T. brucei is able to switch on a different VSG gene to express a new VSG that is not recognized by the host immune system. When antibodies are raised to this new VSG, T. brucei switches its surface coat once again. This strategy enables T. brucei to evade the host immune response. This phenomenon also explains the fluctuation of trypanosome numbers in the blood of the host often observed throughout the course of a T. brucei infection (100).
The production of excreted, soluble VSG is another immune evasion strategy employed by T. brucei (585). Soluble VSGs divert the host's antibody response by binding circulating anti-VSG antibodies, leaving them unavailable for binding to VSG that is associated with the trypanosome surface. Certain VSGs can also induce the production of autoantibodies by molecular mimicry (585), a phenomenon which is reminiscent of T. cruzi infection.
(v) Diagnosis.
If neurological symptoms are apparent, an MRI scan of the brain is often used to identify brain abnormalities (315). However, a definitive diagnosis must be made through the use of serology, PCR, or microscopy. The demonstration of trypanosomes in Giemsa- or Wright-Giemsa-stained smears of blood or CSF is useful for a definitive diagnosis (Fig. 4A) (315, 352). This may be more difficult in T. b. gambiense infections than in T. b. rhodesiense infections due to the low number of parasites circulating in the blood and/or CSF (352). In T. b. gambiense infections, parasite loads can vary between 100 and 10,000 trypanosomes/ml; hence, trypomastigotes may be readily observed in blood smears or not at all (100). The following link contains excellent images of T. brucei in stained blood smears: http://www.dpd.cdc.gov/dpdx/HTML/ImageLibrary/TrypanosomiasisAfrican_il.htm.
The concentration and separation of whole-blood constituents by high-speed centrifugation (the microhematocrit centrifugation technique) can allow the direct visualization of live, motile trypanosomes under low-power (magnification of ×100 to ×200) microscopy, and it is more sensitive than stained smears (100). The QBC tube technique can also be used for the diagnosis of T. brucei infections. In one study, the QBC tube technique demonstrated a sensitivity of 95% at a blood trypanosome concentration of 450 trypanosomes per ml (16). In that same study, the microhematocrit centrifugation technique displayed a sensitivity of 95% at a blood trypanosome concentration of 7,500 trypanosomes per ml (16).
A number of immunological assays are available for the detection of anti-T. brucei antibodies in human sera (194, 352, 561). The card agglutination test for trypanosomiasis (CATT) is the most important serological tool available in areas where T. b. gambiense infection is endemic (352). This 5-min test is used extensively in Africa for the screening of populations at risk of obtaining T. b. gambiense sleeping sickness (68, 101, 352, 524).
PCR of blood, CSF, or lymph aspirate demonstrates good sensitivity (296, 352). Molecular techniques are useful for both the detection and strain typing of T. brucei. Restriction fragment length polymorphism (RFLP) and PCR for T. brucei have enabled extensive research into the molecular epidemiology of T. brucei sleeping sickness (232, 269, 271). Some molecular techniques can differentiate Trypanosoma brucei at the subspecies level and may be useful for determining the most appropriate treatment option (271). A number of sensitive molecular assays are also available for the detection of African trypanosomes. These include a recently developed oligochromatography technique (397), a real-time PCR (36), a loop-mediated isothermic amplification (LAMP) assay (337), and a conventional PCR assay (341).
Determining the protein concentration in CSF can be used to estimate the progression of African sleeping sickness. In sleeping sickness patients, CSF protein concentrations can range from between 100 and 2,000 mg/liter (100). Generally, a CSF protein concentration of >750 mg/liter reflects damage to the blood-brain barrier (100). However, due to various impracticalities such as the need for labile reagents and a lack of standardization, this technique is no longer recommended for field laboratories (100).
Interestingly, experimental T. b. rhodesiense infections in vervet monkeys suggested that disease progression and efficacy of treatment can be monitored by measuring the concentrations of serum IgM and interleukin-10 (IL-10) (403). However, this approach is likely to have limitations in the field similar to those of the CSF protein concentration method. Instead, the CSF white blood cell (WBC) count is preferred. Generally, a WBC count of 10 to 20 cells/μl of CSF is indicative of late-stage sleeping sickness and requires timely treatment (100).
(vi) Treatment.
The drugs pentamidine, suramin, melarsoprol, eflornithine (dl-α-difluoromethylornithine [DFMO]), and benznidazole are the mainstay of treatment regimens for African sleeping sickness (32). Melarsoprol is widely used for treating late-stage sleeping sickness despite the occurrence of drug-induced, potentially fatal encephalopathies observed for 2 to 12% of patients (51, 61, 225, 233). However, if used under the right regimen, parasitological cure may be achieved with melarsoprol (51). Pentamidine and suramin are used for the treatment of early-stage sleeping sickness (61). Pentamidine is considered the drug of choice for early-stage T. b. gambiense infections (68). Eflornithine is used for the treatment of late-stage T. b. gambiense infections but has many adverse side effects and demonstrates limited activity against T. b. rhodesiense infections (76, 269). Recently, combination nifurtimox-eflornithine therapy has been proposed as a new treatment option for late-stage T. b. gambiense infection because it is as efficacious as eflornithine monotherapy and reduces the likelihood of inducing drug resistance (233, 455).
As with T. cruzi, the T. brucei genome project has enabled the development of bioinformatic approaches for the selection of new drug targets and the development of new drugs (67, 346, 400). Another recent approach to drug development involves the screening of natural compounds, derived mostly from plants, for the presence of antitrypanosomal activity (225).
Non-human-infecting Trypanosoma species.
Human infections with non-human-infecting Trypanosoma species are rarely reported. However, T. b. brucei, T. b. evansi, T. congolense, and T. lewisi infections have been identified in several cases of human disease (138, 285, 299, 305, 311, 495, 521, 560, 573). These trypanosomes infect a range of domestic, feral, and wild animals (124, 261, 262, 371) and probably come into frequent contact with humans.
It is unknown whether immunosuppression is a predisposing factor for infections with the non-human-infecting trypanosomes. One human infection was reported prior to the emergence of HIV (299). In some more-recent cases, patients were HIV negative (285, 305). In other cases, the existence of an immune deficiency was not considered or was simply not discussed (495, 521, 560). While the role of these trypanosomes in IC patients is unknown, infections with these organisms are likely to be reported for IC patients in the future.
(i) Human trypanolytic factor.
Due to the coevolution of humans alongside the endemic trypanosomes of Africa, humans and some primates have evolved a non-immune-mediated mechanism designed to specifically eliminate African trypanosomes (365, 423, 424). The human serum components involved in the specific lysis of trypanosomes are referred to as human trypanolytic factor (HTLF) (424). The key components of HTLF are the apolipoprotein L-I and the haptoglobin-related protein (572). These components are found in the serum of all humans (572).
Originally, only two African trypanosomes were thought to be resistant to HTLF: T. brucei gambiense and T. brucei rhodesiense (424). However, recent reports suggest that certain subtypes of non-human-infecting trypanosomes may also be resistant to HTLF (285, 365, 416). It is plausible that this resistance to HTLF has enabled some human infections with non-human-infecting trypanosomes to occur.
A similar inbuilt antitrypanosomal mechanism does not exist for T. cruzi. This is probably due to the comparatively recent arrival of humans in the Americas approximately 9,000 years ago (365).
(ii) Trypanosoma congolense.
Trypanosoma congolense is a pathogen of livestock animals in sub-Saharan Africa, causing weight loss, anemia, and immunosuppression (124). Like T. brucei, T. congolense has a tsetse fly vector, and so its range is restricted to that of the tsetse fly (124).
A single case of human infection with T. congolense was reported for a 50-year-old woman from Cote d'Ivoire who was also infected with a species of T. brucei (560). The poor condition of the patient suggested that she was in the later stage of sleeping sickness, although no trypanosomes were identified in the patient's CSF. The woman had a weakly positive CATT result and a negative latex agglutination test. Trypanosomes were identified microscopically but were morphologically different from T. brucei and similar to T. congolense (comparatively short, with poor motility and lacking a free flagellum).
Species-specific PCR for T. brucei spp. and T. congolense indicated that the woman was infected with both of these species. The patient was treated with pentamidine and fully recovered (560). Given that the patient in this case was also infected with a T. brucei sp., it is impossible to determine whether T. congolense contributed significantly to the patient's disease. However, the possible role of T. congolense as a human pathogen should not be dismissed.
Recently, Xong et al. (614) found that certain strains of T. congolense were completely resistant to HTLF. This finding suggests that more cases of human T. congolense infection are likely to be reported in the future.
(iii) Trypanosoma brucei evansi.
Trypanosoma brucei evansi was only recently identified as a subspecies of T. brucei (344). Trypanosoma brucei evansi is endemic to parts of Southeast Asia, Africa, and South America and causes a potentially fatal disease called “surra” in livestock and companion animals, including horses, dogs, cattle, goats, pigs, and camels (69, 471). Trypanosoma brucei evansi exists only as the bloodstream form and lacks the ability to enter the procyclic form in the gut of the tsetse fly (345). As such, T. b. evansi relies entirely on mechanical transmission between hosts by means of various species of hematophagous fly and, in South America, by vampire bats (261).
To our knowledge, only a single case of human T. b. evansi infection has been reported (305). The patient was a cattle farmer from India who experienced intermittent fever with chills and sweating. The patient later developed signs of sensory deficit and became violent. Giemsa-stained smears enabled the detection of trypanosomes in peripheral blood, although none were observed in the CSF. The patient was treated with a regimen of intravenous suramin as recommended for T. b. rhodesiense sleeping sickness, and his symptoms resolved. Three individual PCR assays were employed to identify the causative agent. Surprisingly, the etiological agent was identified as T. b. evansi (305).
Interestingly, genetic characterization of this strain of T. b. evansi indicated that it was not dissimilar from a number of other T. b. evansi reference strains (559). It was therefore hypothesized that the case from India may have been the result of an immunodeficiency in the patient or a deficiency in the patient's HTLF (559). A follow-up study performed by Vanhollebeke et al. (573) confirmed this hypothesis by identifying mutations in both alleles of the patient's apolipoprotein L-I gene, which rendered this patient's major HTLF protein dysfunctional.
While this case appears to be extremely rare and unusual, a recent report by Lai et al. (345) indicated that certain strains of T. b. evansi display natural resistance to HTLF. Therefore, as with T. congolense, more infections with T. b. evansi will probably be encountered in the future.
(iv) Trypanosoma brucei brucei.
In sub-Saharan Africa, T. b. brucei is known as a cause of a potentially fatal animal sleeping sickness, or “nagana,” in livestock (47, 138). Trypanosoma brucei brucei is morphologically identical to T. b. rhodesiense and T. b. gambiense. Trypanosoma brucei brucei also infects the same range of vertebrate hosts and relies on the tsetse fly for its transmission. The major difference between these species is that T. b. brucei is thought to be sensitive to HTLF and therefore lacks the ability to infect humans (47).
Human infections with T. b. brucei are somewhat controversial due to the similarity between T. b. brucei and the human-infecting T. brucei subspecies (231, 269). Cases of human infection with particular strains of T. b. brucei have been identified in the past, although these trypanosomes are now referred to as “T. b. gambiense group 2” (138, 231, 558). However, some authors question whether human-infective T. brucei isolates are truly different from non-human-infective isolates (269).
Regardless, a recent report identified an atypical human Trypanosoma infection, which was eventually attributed to a subtype of T. b. brucei (138). In 2003, a 10-month-old child from Ghana was admitted to a local hospital with fever, severe dyspnea, and paleness. The patient's blood was examined for malaria using Giemsa-stained smears. No malarial parasites were identified, although numerous trypanosomes were visible. The necessary drugs were not available for treatment at the time of diagnosis, and the patient did not receive treatment. In an examination 2 years later, no trypanosomes were observed in the patient's Giemsa-stained smears. The child appeared healthy and had a negative CATT result. DNA was extracted from the archived Giemsa-stained slides for species identification by various taxon-specific PCRs. The species was identified as either T. b. brucei or T. b. gambiense group 2. Based on various unusual aspects of this case, including geographic location and the patient's ability to spontaneously clear the infection, T. b. gambiense group 2 infection was considered unlikely. As such, the infection was attributed to T. b. brucei (138).
While infections with T. b. brucei are controversial, it is possible that certain human-infective subtypes exist that are yet to be identified. In any case, it seems likely that infections with human-infecting T. b. brucei subtypes would go mistaken for T. b. gambiense or T. b. rhodesiense. This error would have little impact on the management of patients.
(v) Trypanosoma (Herpetosoma) lewisi.
Trypanosoma lewisi is a member of the subgenus Herpetosoma, which includes several species of trypanosomes that are usually obligate parasites of rodents (298, 365). Trypanosoma lewisi infection is transmitted by various species of flea and has a worldwide distribution (298, 365). Members of the subgenus Herpetosoma are morphologically indistinguishable from each other and are often described collectively as T. lewisi-like organisms (298). In rodents, T. lewisi-like infections are thought to be nonpathogenic and self-limiting due to the organism's limited capacity to alter its surface coat (298, 365). In humans, however, T. lewisi-like organisms have been reported to cause a transient disease characterized by fever, anemia, anorexia (285, 299, 495), and, in some cases, generalized edema (285).
To our knowledge, only six cases of T. lewisi-like infection in humans have been reported (285, 299, 311, 495, 521). Poor living conditions associated with an infestation of feral rodents are a common characteristic in most cases. Not surprisingly, all reports originate from developing countries, including Malaysia (299), India (311, 521), Thailand (495), and Africa (285). In those reports, the diagnosis of T. lewisi infection was based on the morphology of trypanosomes in peripheral blood films, although more-recent reports also utilized molecular techniques (285, 495). While some early reports of T. lewisi-like infection were not supported with molecular data (as molecular techniques were not yet available) (299, 521), it should be remembered that human-infecting trypanosomes are not endemic to southeast Asia. As such, these early reports from Malaysia and India are probably accurate in their diagnoses (299, 521).
Generally, T. lewisi-like organisms induce a transient infection that is spontaneously cleared by the patient in the absence of specific treatment. In most cases, CNS involvement was not reported. However, in one severe case, trypomastigotes were present in peripheral blood smears and CSF (285). The patient was treated with melarsoprol, and subsequent blood and CSF films were negative for trypanosomes (285).
Unlike some opportunistic infections discussed in this paper, human T. lewisi infection is not restricted to patients who are IC. In one case, the patient was confirmed to be HIV negative (285). Another case was reported prior to the emergence of HIV (299). In some cases, immunodeficiency was either not considered or not discussed (495). However, all patients who did not receive treatment spontaneously cleared their infection in a matter of days, which is suggestive of a fully functional immune system. Based on these points, it is unlikely that immunosuppression was a predisposing factor for T. lewisi-like infection in these cases. However, given that the majority of infections occurred in infants, it is possible that infants are more susceptible to T. lewisi-like infection, perhaps due to an undeveloped immune system (285, 299, 311, 365, 495).
The mechanisms that allow T. lewisi-like organisms to infect humans are not understood. It has been speculated that certain subtypes of T. lewisi may be resistant to trypanolysis, like some African trypanosomes (365). However, phylogenetic studies based on the small-subunit (SSU) ribosomal DNA (rDNA) indicated that T. lewisi and other Herpetosoma strains cluster together in a group separate from that of the African trypanosomes (371). Given that HTLF has specific activity against African trypanosomes only (and not T. cruzi), it seems logical that infections with T. lewisi-like organisms occur (in part) due to the lack of any inherent, protective mechanism against Herpetosoma in humans rather than the resistance of T. lewisi to HTLF. Unfortunately, no studies exist which explore the effect of normal human serum on T. lewisi-like organisms.
Regardless, it is unlikely that immunodeficiency has played a role in the T. lewisi-like infections reported. However, given the apparent susceptibility of infants to T. lewisi infection, it is plausible that IC patients may be at a greater risk of developing T. lewisi-associated disease than ICT individuals.
Lower Trypanosomatids
The “lower trypanosomatids” are a group of trypanosomatid parasites that usually infect invertebrate hosts. These include Blastocrithidia, Crithidia, and Leptomonas species, all of which are considered nonpathogenic to ICT humans. These organisms are known to have only one invertebrate host in their life cycle and are often referred to as the “monoxenous trypanosomatids.”
To our knowledge, only four cases of monoxenous trypanosomatid infection have been reported for humans, and only three of these were HIV-infected patients. However, it should be noted that two of these cases (the infections from Martinique described below) involved an organism that was later identified as a highly divergent member of the genus Leishmania (407). As such, this organism may not be considered a true lower trypanosomatid.
HIV-infected patient from Martinique.
The earliest case, an HIV-infected patient from Martinique who presented with a disease which resembled diffuse cutaneous leishmaniasis, was reported by Dedet et al. (142). The patient was treated with meglumine antimoniate for suspected Leishmania infection and zidovudine for HIV. Skin lesions improved following treatment, although the patient eventually died. Parasites were isolated in culture from skin biopsy material for further study. The patient was negative for Leishmania antibodies, and isoenzyme analysis revealed that the parasite was probably not of the genus Leishmania. The morphology of these organisms in transmission electron micrographs suggested that they were a species of monoxenous trypanosomatid.
ICT patient from Martinique.
A second case report, also from Martinique, identified the same organism as that described above for the previous Martinique case as being the cause of a localized cutaneous lesion in an ICT patient (54). The patient was treated with pentamidine, and the lesion disappeared within 2 months.
In later studies, efforts were made to better characterize this trypanosomatid. Through the use of various molecular techniques and phylogenetic analysis, the organism was identified as a highly divergent member of the genus Leishmania (407). DNA sequences from this unnamed species clustered with Leishmania enriettii and were basal to all other euleishmania species, although the support for this was low (407). Regardless, these analyses clearly indicated that this organism was a member of the genus Leishmania.
Given its relationship to Leishmania, it was postulated that this organism, like other Leishmania species, would also have a digenetic life cycle (407). It is therefore likely that this organism has a number of vertebrate hosts and a hematophagous insect vector.
Unidentified insect trypanosomatid.
The third case of a lower trypanosomatid-HIV coinfection was reported for an intravenous drug abuser from Madrid, Spain (297). Based on clinical findings, including hepatosplenomegaly and pancytopenia, VL was suspected. An immunofluorescent antibody test for the detection of Leishmania antibodies was inconclusive. Bone marrow aspirates were negative for amastigotes, although promastigotes were isolated in culture. The patient was treated for 21 days with meglumine antimonite and recovered. After 3 years no relapses had occurred.
The isolated organism was morphologically different from Leishmania spp., as it was blunt ended and had a denser kinetoplast. The organism was not infective for BALB/c mice or hamsters. Furthermore, an infection could not be established in the gut of the sandfly Phlebotomus perniciosus. As such, the organism was suspected to be a lower trypanosomatid.
Isoenzyme analysis of the cultured organism demonstrated that it was not similar to other Leishmania reference strains. DNA cross-hybridization was performed by using DNA from the organism with a range of other lower trypanosomatid species as well as T. cruzi. No homology was observed between this organism and all other species tested. As such, this organism was assumed to be a newly discovered non-human-infecting trypanosomatid.
Given that the patient was an intravenous drug user, it was postulated that the infection could have been obtained by washing syringes in water that had been contaminated with insect feces (297). However, other authors suggested that this infection was more likely to have occurred by the accidental inhalation of parasitized insects (140).
Leptomonas-like organism.
The most recent report of a lower trypanosomatid-HIV coinfection in a Brazilian male was described by Pacheco et al. (417). The patient presented to a local hospital complaining of weakness and fever. Physical examination revealed an enlargement of the cervical lymph nodes and splenomegaly. The patient was from an area where CL is endemic, and a visceralizing L. braziliensis infection was suspected. The patient was positive for Leishmania antibodies by IFAT. Promastigote forms similar to those of Leishmania were inoculated into a culture from the patient's bone marrow aspirate and cultivated for further study.
Isoenzyme analysis, kinetoplast DNA (kDNA) restriction analysis, and Southern blot hybridization revealed that this organism was not a member of the genus Leishmania or Trypanosoma or of the subgenus Sauroleishmania. However, kDNA restriction analysis demonstrated that this organism was genetically similar to Leptomonas pulexsimulantis, a flagellate found in fleas. The patient was treated for 20 days with N-methyl-glucantime antimoniate and completely recovered. No relapse was reported for 2 years after treatment.
Additional remarks.
The prospect of parasitological cure for patients infected with lower trypanosomatids appears to be quite good, even in the presence of HIV. In all of these cases, patients presented with symptoms similar to those observed for the common Leishmania syndromes and responded well to antimonial treatment or pentamidine in one case (54). Therefore, in cases where infection with lower trypanosomatids is misdiagnosed as leishmaniasis and treated as such, therapy is likely to be successful. Infection with these organisms is rare in humans, and consequently, these organisms will be rarely exposed to common anti-Leishmania drugs. This makes the development of drug resistance in these organisms highly unlikely. However, the unusual nature of these infections also means that they are likely to go undiagnosed.
Toxoplasma gondii
Organism and disease.
Toxoplasma gondii is an obligate intracellular parasite with a worldwide distribution and broad host range, which includes most mammals and birds (429). Despite intense debates regarding the phylogeny of this organism (181, 268, 388), T. gondii still remains the only species within its genus. The sexual cycle of Toxoplasma (the production of oocysts) occurs only in the intestine of cats (178, 274, 599), and human infections usually occur by two routes: either via the ingestion of oocysts shed by a feline definitive host or by the ingestion of meat containing tissue cysts from an intermediate host (178). Toxoplasma infections are also transmissible via blood transfusion (204), organ transplantation (156), and the congenital route (599). The congenital transmission of Toxoplasma may represent the most clinically important route of human infection, although its importance is currently under debate (270, 290).
Toxoplasma tissue cysts occur in both intermediate and definitive hosts and are resistant to the host immune system. These resistant structures harbor the slow-growing bradyzoite form of Toxoplasma (599). The rupture of tissue cysts results in the release of bradyzoites, which differentiate into the rapidly multiplying tachyzoite form (178). Tissue cysts can persist in the host indefinitely, presumably due to a cycle of tissue cyst rupture and reinfection of new host cells (599), followed by suppression by the host immune system and the subsequent formation of new tissue cysts. In IC patients, the suppression of this cycle is inadequate. In these patients, tachyzoites are allowed to spread rapidly via the host's circulation to cause widespread tissue damage.
Approximately one-third of the human population is believed to be infected with T. gondii (429). In ICT patients, Toxoplasma infection is usually subclinical (405, 599). However, in IC patients, Toxoplasma causes a range of pathologies. Encephalitis is the most common clinical manifestation of toxoplasmosis in HIV-infected patients (116) and is usually attributable to the reactivation of a latent infection (21, 429, 537). Generally, CD4+ T-lymphocyte counts of less than 100 cells/μl of blood permit the reactivation of toxoplasmosis (328). Prior to the AIDS pandemic, Toxoplasma encephalitis was rarely reported but is now considered an important AIDS-defining illness (255).
In humans, close contact with cats and the consumption of rare or undercooked meat increase the risk of acquiring a primary Toxoplasma infection (134, 178, 179, 274, 402, 429). As such, the risk of obtaining a primary Toxoplasma infection is no higher for IC patients than for ICT individuals (328, 472). Interestingly, one study identified intravenous drug use as a major risk factor for Toxoplasma infection (328). As such, the sharing of needles between intravenous drug users may also facilitate the transmission of Toxoplasma.
In IC patients, Toxoplasma can cause a range of ocular complications (4, 19, 448), pulmonary disease (433, 526), myocarditis (3, 244, 347), cystitis (277), polymyositis (254), pancreatitis (119), and hepatitis (383). Other complications in HIV-infected patients include gastrointestinal disease, panhypopituitarism, diabetes insipidus, orchitis, myositis, and a syndrome characterized by inappropriate antidiuretic hormone secretion (599). Disseminated toxoplasmosis can also occur in IC individuals (22, 422, 562) and may lead to septic shock (364). Bone marrow involvement has also been reported (65). A case of concurrent cerebral toxoplasmosis and Chagas' disease was also reported for an HIV-infected patient (619). A recent case study also reported the unusual localization of a “toxoplasmic abscess” on the spinal cord of an HIV-infected patient (447).
While generally benign, Toxoplasma infections in ICT patients can cause polymyositis (254), hepatitis (175), meningoencephalitis (451), and ocular disease (112, 254, 278, 485). Toxoplasma is recognized as the most common cause of retinitis in ICT individuals and the second most common cause of retinitis in AIDS patients after cytomegalovirus (339). Transplant patients are also at an increased risk of clinically apparent toxoplasmosis (156, 177, 206, 453).
In sub-Saharan Africa, toxoplasmosis is a significant albeit neglected disease. Due to poor access to HAART, toxoplasmosis remains a common AIDS-defining illness in this region (283). Unfortunately, few studies have been carried out to assess the impact of toxoplasmosis in sub-Saharan Africa. However, a number of serological studies have been performed (419). In a study from Botswana, 6.5% of 46 HIV-infected patients tested positive for anti-T. gondii antibodies (602). A study from the town of Nazaret in Ethiopia showed that 39/65 (60%) people tested were positive for Toxoplasma by serology (402). This was probably due to the custom of eating raw or undercooked mutton (more than 50% of people in this study admitted to doing this) in these areas and also due to the habit of keeping cats for the control of rats and mice (402). In a study from Burkina Faso, 25.3% of pregnant women were found to be seropositive for Toxoplasma (525).
In a recent report, Africa was among the regions associated with a high Toxoplasma seroprevalence. Other regions also associated with a high seroprevalence of Toxoplasma include the Middle East, parts of Southeast Asia, Latin America, and parts of Eastern/Central Europe (419). Western European countries and the United States were associated with a lower Toxoplasma seroprevalence (419).
Diagnosis.
The diagnosis of Toxoplasma encephalitis usually involves a CT scan or MRI of the brain. However, Toxoplasma-associated brain abnormalities may be indistinguishable from AIDS-related cerebral lymphoma (64, 392) or cerebral Chagas' disease (121, 429). Therefore, microscopy, molecular techniques, and/or parasite cultivation should be employed for a definitive diagnosis (428, 583). The direct demonstration of Toxoplasma tachyzoites in cerebral tissues is the method of choice for a definitive diagnosis of cerebral toxoplasmosis (429). The microscopic observation of free Toxoplasma tachyzoites in stained smears of blood or CSF is indicative of an active infection and is a useful diagnostic tool (Fig. 4C). The cultivation of Toxoplasma tachyzoites from CSF can also be useful but may be impractical due to the strict culture requirements of Toxoplasma.
The use of certain antibody detection assays is limited, as these assays cannot differentiate between active and benign infections (429). However, ELISAs that detect antibodies against certain excreted/secreted antigens of Toxoplasma may be useful. Human immunodeficiency virus-infected patients tend to display higher antibody titers to certain excreted/secreted antigens than patients with a benign infection. As such, these ELISAs may be used as a marker for active Toxoplasma infection in IC patients (429). A latex agglutination test (519) and several other ELISAs are also available for the detection of anti-Toxoplasma antibodies (2, 338, 519, 613).
PCR performed on CSF can be useful in cases where cerebral toxoplasmosis is suspected but tachyzoites are not visible (541, 621). Several PCR assays are available. One PCR assay exhibited 100% sensitivity and a specificity of 94.4% when applied to DNA extracted from CSF (583). A nested PCR assay demonstrated 33% sensitivity and 100% specificity (108). A multiplex real-time PCR assay that is optimized for the diagnosis of cerebral toxoplasmosis is also available (406). This assay simultaneously amplifies two Toxoplasma genes and displays a specificity of 100% and a sensitivity of 68.8% (406). Other real-time PCR assays are also available (116, 433). Quantitative real-time PCR assays have demonstrated excellent sensitivity (429) and may also be used to monitor a patient's response to anti-Toxoplasma therapy. In contrast to antibody techniques, a positive PCR result obtained from peripheral blood or CSF is indicative of an active infection (429). Moreover, HIV-infected patients generally demonstrate a high parasite load in the circulation compared to ICT individuals. In these patients, PCR applied to peripheral blood specimens could eliminate the requirement for invasive CNS biopsy techniques (429). A number of PCR assays have also been optimized for use on amniotic fluid for the diagnosis of congenital toxoplasmosis (188, 404, 409).
As mentioned above in the section on pregnancy, primary Toxoplasma infections acquired prior to the commencement of a pregnancy pose little threat to the fetus. Similarly, primary Toxoplasma infections obtained after 20 weeks of gestation will result in minimal fetopathies, if any. However, a primary Toxoplasma infection obtained prior to 20 weeks of gestation can have serious effects on the fetus. A useful T. gondii IgG avidity test is available, which can be used to estimate the time that a Toxoplasma infection was acquired relative to a pregnancy (301). This can help to predict the level of potential risk that the infection will pose to the fetus. However, it should also be mentioned that the results of serological tests are often difficult to interpret for pregnant women and neonates (514). For these patients, an interpretation of serological test results requires experience and expertise (514). In the presence of HIV infection, this IgG avidity test may be less useful. In one study, a pregnant mother infected with HIV who acquired a Toxoplasma infection while pregnant displayed an antibody profile similar to that of ICT patients with a latent infection (343).
Treatment.
The drugs pyrimethamine and sulfadiazine are the mainstays of anti-Toxoplasma therapy (39, 429). In cases where patients do not respond to traditional pyrimethamine and sulfadiazine therapy or where patients do not tolerate sulfadiazine, pyrimethamine and clindamycin therapy is an effective alternative (39). The use of the drug leucovorin (folinic acid) in combination with pyrimethamine-sulfadiazine or pyrimethamine-clindamycin therapy is recommended to prevent various hematological side effects associated with the use of pyrimethamine (39, 429). The drug atovaquone (309, 552) and combination azithromycin-pyrimethamine therapy may also be useful for the treatment of cerebral toxoplasmosis in HIV-infected patients (608). In cases of ocular toxoplasmosis, the intravitreal injection of clindamycin can be effective (612). With regard to pregnancy, pyrimethamine and sulfadiazine should be used only after the 20th week of gestation (250). Before the 20th week, spiramycin can be used (250).
Interestingly, there is evidence to suggest that certain antiretroviral drugs have a direct inhibitory effect on Toxoplasma (157). As is the case with other protozoa, the availability of HAART has led to a major reduction in AIDS-associated toxoplasmosis (5, 382, 429). In particular, cases of Toxoplasma encephalitis have been greatly reduced to the extent that they are now very uncommon in regions with access to HAART (382, 429, 537).
Neospora caninum
The organism.
Neospora caninum is an apicomplexan parasite closely related to T. gondii. Neospora has a canine definitive host and is best known for its veterinary significance. In cattle, N. caninum is known as a cause of widespread abortion, resulting in great economic losses (470), and the presence of dogs on cattle farms is a major risk factor for bovine neosporosis (230). The life stages of Neospora are morphologically similar to those of Toxoplasma. The similarity between T. gondii and N. caninum is such that some authors question the validity of the genus Neospora (280).
Speculatively, close contact with dogs and the consumption of undercooked meat (particularly beef) are probably the most likely risk factors for obtaining an N. caninum infection. However, no clinically apparent human infections with N. caninum have been reported. Regardless, a reduction in immune capacity as a result of HIV infection could allow infections with N. caninum to become clinically apparent. The possibility of such an occurrence is supported by the ability of lower trypanosomatids and the microsporidia, which do not usually infect ICT humans, to do so in the presence of HIV infection.
Neospora antibodies in human sera.
Several studies investigated the prevalence of N. caninum antibodies in human sera. One study found that 76 women with a history of spontaneous abortion were negative for N. caninum antibodies (434). In contrast, another study found 69/1,029 human serum samples obtained from blood donors to be positive for N. caninum antibodies (555). In that study, only 27.5% of sera positive for anti-Neospora antibodies were also positive for anti-Toxoplasma antibodies, which suggests that antigenic cross-reactivity between these organisms did not take place (555). In a later study, anti-N. caninum antibodies were detected in patients with HIV and in patients with neurological complaints (361). In that study, an immunoblot analysis was used to differentiate patients with circulating antibodies to Neospora, Toxoplasma, or both organisms (361). In contrast, an English study found no human exposure to N. caninum antigens at all, despite a large study population, which consisted of 3,232 serum samples from the general population and 518 serum samples from a high-risk group (farm workers) (387). However, a recent study from Egypt found a Neospora seroprevalence of 7.92% in human serum samples (288). In a study from France, no sera from ICT women were positive for anti-N. caninum antibodies, although 4/400 serum samples from HIV-infected patients tested positive for anti-N. caninum antibodies (478). However, given the low antibody titers observed by this study, the possibility of low-level cross-reactivity could not be ruled out (478).
Additional remarks.
In all studies that reported the detection of anti-N. caninum antibodies in human sera, no evidence of clinical disease attributable to N. caninum was provided (288, 478, 555). As such, clinically apparent neosporosis is unlikely to occur in ICT humans. However, the results of one of these reports suggest a potential role for N. caninum as an opportunistic organism in IC individuals (361). In that study, 23/61 patients infected with HIV tested positive for anti-N. caninum antibodies (361). In that same study, 9/50 patients with neurological complaints also tested positive for anti-N. caninum antibodies (361). The prevalences obtained for the HIV group and the neurological-complaints group were significantly higher than the prevalences obtained for the newborn infant group and healthy subjects. This suggests that N. caninum may have the potential to infect humans in the presence of HIV and that Neospora infections may be linked to neurological complaints (361).
While N. caninum has not been linked directly to human disease, the possibility of clinically apparent N. caninum infections should not dismissed. In cases where T. gondii infections are suspected, N. caninum infection may be ruled out through the use of PCR performed on DNA extracted from tissue biopsy specimens or CSF. A number of sensitive PCR assays exist for N. caninum, which may be useful (31, 483). The tachyzoite stage of N. caninum is morphologically similar to that of Toxoplasma. It is quite possible that N. caninum could have been mistaken for Toxoplasma in CSF smears from IC patients if microscopy alone was used. As such, it is recommended that tissues that are thought to be infected with T. gondii should also be tested with a Neospora-specific PCR.
BLOOD PROTOZOA
Babesia spp.
Organism and disease.
Babesia spp. are hemoprotozoan parasites of the order Piroplasmida transmitted by ticks of the genus Ixodes (286). Members of the genus Babesia share some similarities with the genus Plasmodium, including an arthropod vector and the ability to invade erythrocytes. While several species of Babesia can infect humans, Babesia microti and Babesia divergens are the species most often associated with human infection (187, 229, 281). Small mammals (235, 307, 492, 539, 574) and ruminants (275, 354, 501, 542, 574) serve as reservoirs for Babesia spp., and humans usually become infected after an infected Ixodes tick takes a blood meal. However, transmission via blood transfusion has also been widely reported (50, 86, 246, 272, 366, 379).
Human babesiosis is becoming increasingly common, particularly in IC patients (286). While the prognosis of Babesia infection is often good for otherwise healthy ICT individuals (389), Babesia infections are more likely to be severe or even life threatening for IC patients (326, 331, 574). Infections of IC patients are also less responsive to treatment (333, 592). In contrast, Babesia infections may be self-limiting or subclinical in ICT patients (574). The risk of developing severe babesiosis also increases with age, with individuals over the age of 50 years being predisposed to severe symptoms (263, 334, 389). Other groups predisposed to a severe, potentially lethal form of babesiosis include those with HIV (198, 214, 592), those receiving immunosuppressive therapy (including transplant patients), those with leukocyte malignancies, and those without a spleen (226, 331, 333, 366, 389, 574). The predisposition of splenectomized humans to a more severe form of disease is a feature that is also common to malaria parasites. Immunocompromised individuals are also more likely to suffer a relapse of babesiosis after apparent clinical cure (198).
In ICT patients, the symptoms of babesiosis include fever, sweats, chills, fatigue, and a mild-to-moderate hemolytic anemia (574). Patients who are elderly, IC, or asplenic will experience similar symptoms but with increased severity. An isolated case of splenic rupture due to a B. microti infection has also been reported (340). In ICT patients, Babesia infections can persist for many months, even if treatment has been initiated (335, 574). While patients suffering from malaria and babesiosis may have similar symptoms, cerebral involvement, as observed for Plasmodium falciparum infection, is not known to occur in human Babesia infections (582).
A case of babesiosis in a pregnant mother has also been reported, although congenital infection did not occur (467). To our knowledge, only one possible case of congenital Babesia infection has occurred (515). As such, congenital Babesia infection is an extremely rare event, if it occurs at all.
Nomenclature of human-infecting Babesia species.
Generally, B. microti is associated with human babesiosis in the United States, while B. divergens is associated with European babesiosis (187). However, the nomenclature of human-infecting Babesia parasites has recently become more complex due to the emergence of new isolates in Europe, the United States, Africa, South America, and Asia. The classification of human-infecting Babesia species was initially dependent on morphology and host specificity, although later studies based on molecular techniques indicated that these criteria are inadequate (114, 459).
Human-infecting Babesia parasites which could not be classified as either B. microti or B. divergens were often designated a short acronym to identify them. Examples include WA1, EU1, CA1, MO1, KO, and KY (35, 114, 322, 574). However, based on molecular and immunological evidence, some of these acronyms are now known to represent the same species (114, 574). Furthermore, several studies have demonstrated that these organisms represent previously undescribed species of Babesia (265, 279, 547, 574). As such, some of these organisms have recently been allocated species names (114, 265).
(i) Babesia duncani (WA1).
The first report of a human infection with the WA1 isolate of Babesia was described in 1995 by Quick et al. (459) in Washington State. The patient was a 41-year-old ICT male with an intact spleen. The organism was morphologically indistinguishable from B. microti. However, molecular and serological studies indicated that this organism was distinct from B. microti, B. divergens, and several other Babesia species (459, 547). Furthermore, the disease caused by WA1 in laboratory rodents was far more severe than that which is typically observed for B. microti (459). In 1995, Persing et al. (431) identified four WA1-like infections (one of which was fatal) in splenectomized patients from Northern California. These isolates described by Persing et al. (431) were identified by the acronyms CA1, CA2, CA3, and CA4 (114). Ribosomal DNA sequences from these four isolates were virtually identical (431). Later reports also described infections with WA1-type organisms, which were acquired via blood transfusion in patients from Washington State (267) (isolates WA2 and WA3) and California (327) (isolates CA5 and CA6). Herwaldt et al. (267) PCR amplified sections of the SSU rDNAs from WA2 and WA3, which were found to be identical to each other and to WA1. Kjemtrup et al. (327) later demonstrated that that the SSU rDNAs from CA5 and CA6 were 99.9% similar to that of the WA1 isolate. Using phylogenetic analysis of the SSU rDNAs of various Babesia isolates, Kjemtrup et al. (327) also demonstrated that the human isolates CA1, CA3, and CA4 were extremely similar to Babesia isolates from mule deer and the bighorn sheep but distinct from the other WA1-type parasites. In 2006, Conrad et al. (114) confirmed the same phylogenetic relationships by constructing a strict consensus tree based on ITS2 sequences. In that study, Conrad et al. (114) also proposed the species name Babesia duncani for the WA1-type isolates (WA1, WA2, WA3, CA5, and CA6). To our knowledge, the organisms CA1, CA2, CA3, and CA4 have not been given a species name.
(ii) Babesia divergens-like organisms.
In 1996, Herwaldt et al. (263) described a case of fatal babesiosis in a 73-year-old splenectomized man from Missouri. Molecular and immunological characterization of this organism (MO1) revealed that it was similar to B. divergens (associated with human babesiosis in Europe) and dissimilar to B. microti and WA1 (263). In a later report, a similar organism (KY) was identified as a cause of acute babesiosis in a patient from Kentucky (35). In 2004, Herwaldt et al. (266) reported another Babesia divergens-like infection in Washington State in an 82-year-old man without a spleen. The SSU rDNA of this organism was 99.5% similar to that of B. divergens (266). Interestingly, Holman (279) found that the SSU rDNA and internal transcribed spacer (ITS) sequences from the KY isolate and a Babesia isolate found in cotton-tailed rabbits on Nantucket Island, MA, were 100% identical. However, the ITS1 and ITS2 regions were several bases longer in the KY/rabbit isolates than the ITS regions of B. divergens (279). Moreover, studies based on morphology and infectivity in cows demonstrated that these isolates (the KY/rabbit isolates) were distinct from B. divergens (279). The KY/rabbit isolate, MO1, and the Washington state Babesia divergens-like isolates are now collectively referred to as B. divergens-like organisms (574).
(iii) Babesia venatorum (EU1).
In 2003, Herwaldt et al. (265) identified two non-B. divergens infections in two asplenic men from Austria and Italy. This isolate became known as “EU1.” PCR and sequence analysis revealed that this organism was not B. divergens but was similar to Babesia odocoilei (a species known to infect white-tailed deer of the United States) (265). Herwaldt et al. proposed the species name B. venatorum for the EU1 isolate (265). In a later study, Haselbarth et al. (253) identified a Babesia infection in a 63-year-old splenecto- mized German patient with nodular lymphocyte-predominant Hodgkin's lymphoma. Sequence analysis of the SSU rDNA of this isolate revealed that it was 99% similar to the EU1 isolates (253). Recently, EU1 Babesia parasites have been identified in roe deer from France (56) and in Ixodes ticks from Poland (107). To our knowledge, only three human cases of Babesia venatorum-like infection have been reported (253, 265).
(iv) Other Babesia isolates.
Cases of human babesiosis are not as common outside Europe and the United States. However, several cases of human babesiosis have been confirmed in Asia, Central/South America, and Africa. In these cases, species names have not been allocated.
Rios et al. (474) microscopically identified an unknown species of Babesia in the blood of a subject from Columbia. Rios et al. also detected anti-Babesia antibodies in the sera of several Colombian subjects, although no Babesia parasites were observed (474).
Kim et al. (322) identified the first case of human Babesia infection in South Korea. Sequencing of the SSU rDNA of this isolate (KO1) determined that it was similar to a Babesia parasite isolated from sheep in China (322). In Taiwan, Shih et al. (518) discovered an asymptomatic infection with a species of Babesia in a 51-year-old woman. This new isolate (TW1) was antigenically similar to B. microti but not identical (518).
A case of transfusion-associated babesiosis has also been reported in Japan (385). The etiological agent from this case (385) was later isolated from the blood donor (493). The rDNA sequence of this organism was 99.2% similar to that of B. microti, although serum from the donor was only weakly reactive to B. microti isolates from the United States (493). This Japanese isolate became known as the “Kobe strain” (493). Interestingly, a mouse trapped near the residence of the blood donor was infected with a Babesia isolate with an rDNA sequence identical to that of the Kobe strain (596). Kobe strain-like Babesia isolates have also been detected in field rodents in central Taiwan and Southeastern Mainland China (492).
Cases of human babesiosis have also been reported in South Africa (77), Mexico (326), India (375), and Egypt (191).
Diagnosis.
A definitive diagnosis of Babesia infection is usually made by the observation of Babesia parasites in Giemsa-stained thin blood smears (49, 273, 574). Babesia parasites appear as piroplasm or “tear-drop” forms, ring forms, amoeboid forms, and tetrad or “Maltese cross” forms (49, 114). Low-grade infections may be overlooked, where less than 1% of erythrocytes may contain Babesia parasites (273, 332). In symptomatic patients, Babesia is more readily detectable by microscopy (273). The following website provides excellent images of Babesia parasites in Giemsa-stained blood smears: http://www.dpd.cdc.gov/dpdx/HTML/ImageLibrary/Babesiosis_il.htm.
Babesia parasites can resemble P. falciparum in Giemsa-stained smears, which can be problematic for diagnosis (257). In these cases, a patient's travel history can be useful. However, Babesia and Plasmodium can be distinguished by the presence or absence of pigment deposits (hemozoin) in parasitized erythrocytes. Pigment deposits occur only in Plasmodium infections (257).
Other diagnostic techniques are also available. In the past, the inoculation of live laboratory rodents with patient blood specimens was a useful diagnostic tool. One study described rodent inoculation as being as sensitive and specific as PCR (336). However, rodent inoculation may be impractical for some laboratories. Furthermore, the technique can be problematic due to various susceptibilities of different species of rodent to various species of Babesia. Diagnosis by live-animal inoculation is also quite slow (273). Due to its limitations, the inoculation of laboratory rodents is not widely used. The QBC tube technique may also be useful for the microscopic diagnosis of Babesia infections (102).
PCR is useful for diagnosis of Babesia infection (336). Vannier and Krause (574) recommended that PCR be used for diagnosis when Babesia infection is suspected and Giemsa-stained blood smears appear negative or ambiguous (574). Molecular techniques are also useful when the identification of the causal species is desired. The sequencing of PCR products has been used widely for phylogenetic analyses of human-infecting Babesia species (263, 266, 273).
Serological techniques such as ELISA (360) or IFAT (574) may also be useful, although IFAT is more frequently used (273). When using IFAT, titers greater than 1:1,024 usually occur for B. microti infections in the early, acute phase of infection and may be indicative of a recent and/or active infection (574). Serology is less useful for B. divergens infections, as the onset of B. divergens babesiosis is more rapid, and disease symptoms become apparent before seroconversion takes place (574).
Treatment.
Originally, the standard treatment for Babesia infection was clindamycin and quinine therapy (286, 582). However, combination therapy with atovaquone and azithromycin is preferred, as it is just as effective and is associated with fewer adverse reactions (574, 582). In cases of severe babesiosis, aggressive therapy combining large blood transfusions followed by prophylaxis (often an infusion of intravenous clindamycin) is an effective treatment (582).
In a recent animal study, imidocarb diproprionate demonstrated good activity against Babesia caballi in horses (510). Imidocarb is not currently licensed for human use, although it has been used under special license to successfully treat some human infections (582). This drug may be useful as an alternative treatment for human babesiosis in the future.
FREE-LIVING AMOEBAE
Acanthamoeba spp.
Organism and disease.
Acanthamoeba spp. are ubiquitous, free-living amoebae that infect humans opportunistically. These organisms are the etiological agent of fatal granulomatous amoebic encephalitis (GAE), usually associated with IC individuals (511, 593, 594). Acanthamoeba exists in a range of environments, including soil, air, freshwater, salt water, and sewage (320). While humans are in frequent contact with environmental sources of Acanthamoeba species, this organism infrequently causes disease (320). Acanthamoeba can be acquired by washing of the face in pond water, getting sand or dust in the eye, inhalation of contaminated material, or traumatic injection or entry through preexisting wounds or lesions (377, 579). The clinical course of Acanthamoeba infection is slow and subtle compared to that of infections with other free-living amoebae (28, 504).
The disease spectrum of Acanthamoeba infection is very broad in IC patients. In ICT individuals, Acanthamoeba is most often known as a cause of keratitis in contact lens wearers (Fig. 5) (26, 78, 284, 320). Occasionally, ICT individuals will suffer from GAE, although the disease is more common in IC individuals (377, 594). Patients with GAE will often present with seizures, headaches, confusion, and locomotor difficulties (172, 367). GAE is almost always fatal, regardless of immune status, with an approximate survival rate of less than 7% (594). Patients with Acanthamoeba-HIV coinfection usually do not survive, particularly patients with disseminated disease (90, 323, 377, 411, 477, 486, 509). In IC patients, Acanthamoeba infections also occur in the skin (386) and bones (512). Rhinosinusitis (401, 477), keratitis (251, 318, 358), otitis (185, 513), vasculitis (260, 486), and endophthalmitis (259) have also been reported for Acanthamoeba-HIV-coinfected patients. Skin lesions are the most common presenting manifestation of Acanthamoeba in AIDS (377) and may be present in the absence of CNS involvement (551).
FIG. 5.
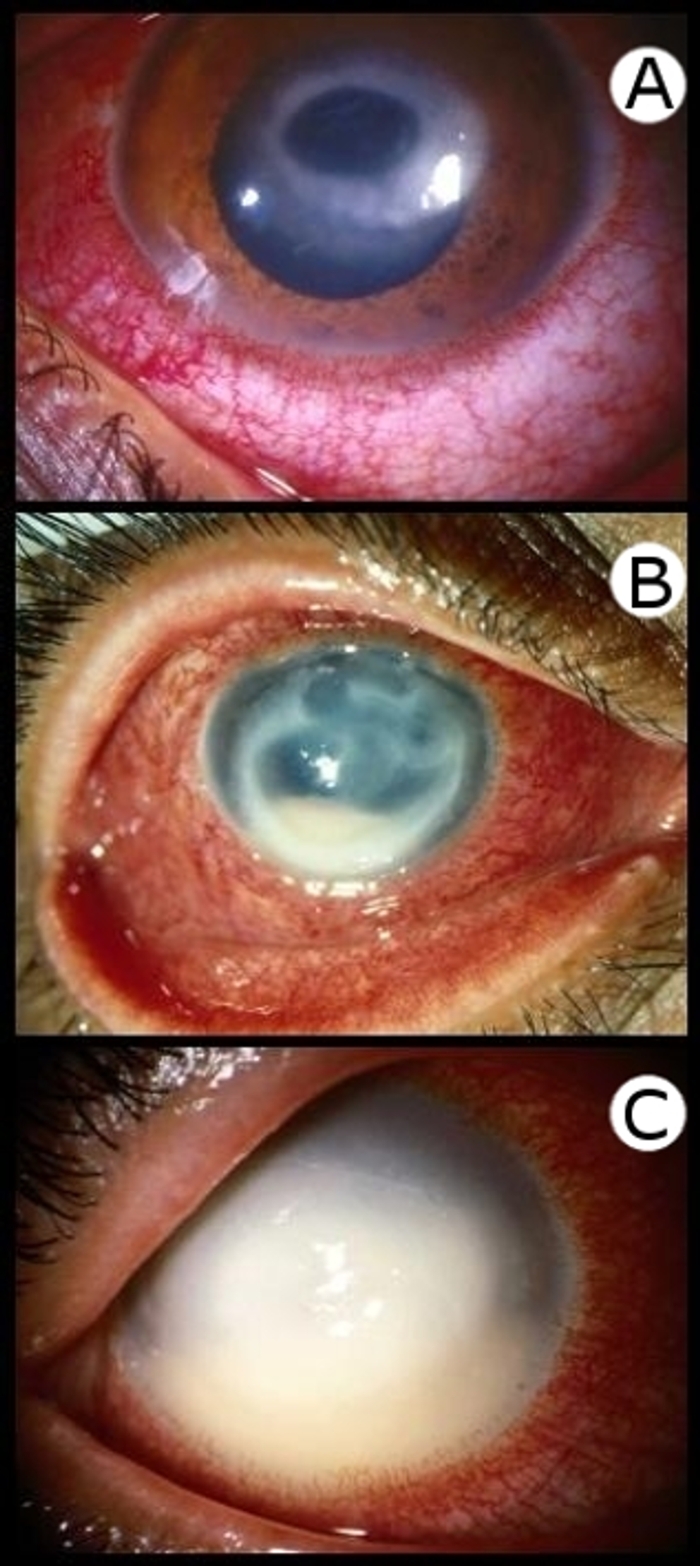
Clinical presentation of Acanthamoeba keratitis. (Courtesy of Paul Badenoch.) (A) Keratitis demonstrating ring-stage infiltrate. (B) Keratitis with multiple ring infiltrates and hypopyon. (C) Keratitis with near-total suppuration.
Acanthamoeba has been reported as a cause of disease in other IC groups. In lung transplant recipients, infections involving the skin, lungs, and brain as well as sinusitis and widely disseminated disease have been reported (176, 411, 571, 580). Acanthamoeba has also been reported to cause disease in kidney and liver transplant recipients (215, 536). An unusual case of Acanthamoeba infection was reported for a patient who was severely IC (CD4+ cell count of 182 cells/mm3) due to an infection with Mycobacterium tuberculosis (594). In that study, pulmonary and CNS involvements were apparent, with concurrent dermal lesions (594).
Diagnosis.
The diagnosis of Acanthamoeba infection usually requires microscopy of stained tissue sections or wet mounts (377). Giemsa stain, Gram stain, or the fluorescent stain calcofluor white can be used (Fig. 6). The following website provides excellent microscopic images of free-living amoebae: http://www.dpd.cdc.gov/dpdx/HTML/ImageLibrary/FreeLivingAmebic_il.htm.
FIG. 6.
Fixed, stained sections from corneal scrapings showing cysts and a trophozoite of Acanthamoeba. (Courtesy of Paul Badenoch.) (A) Acanthamoeba cysts stained with calcofluor white. (B) Acanthamoeba cyst stained with a Giemsa stain. (C) Acanthamoeba cyst stained with a Gram stain. (D) Acanthamoeba trophozoite stained with a Giemsa stain.
If CNS involvement is suspected, an MRI is often employed to determine the presence of brain lesions (367, 377). The cultivation of Acanthamoeba from clinical specimens can also be diagnostic. The cultivation of trophozoites from CSF, brain tissue, corneal scrapings, or material from cutaneous/sinus lesions can be achieved by inoculation onto neomycin-nalidixic acid (NNA) agar plates containing a layer of Escherichia coli or Enterobacter aerogenes cells. Inoculation of biopsy material onto monolayers of cultured mammalian cells can also be performed (377). Immunofluorescent antibody test (377) and PCR (367, 594) are also available for the diagnosis of Acanthamoeba infection. In a study performed on a single patient suffering from GAE, PCR was found to be the only technique to return a positive result for four CSF samples, dermal specimens, a bronchoalveolar lavage specimen, lung biopsy specimens, and brain biopsy specimens, while culture, microscopy, and immunofluorescence repeatedly returned negative results (594). Several monoclonal antibodies to surface antigens of Acanthamoeba cysts have also been developed, which could be useful for the development of new diagnostic tests in the future (563).
Treatment.
A broad range of drugs have been used to treat Acanthamoeba infections but with various successes. Drugs that show some anti-Acanthamoeba activity include propamidine isethionate, polyhexamethylene biguanide, itraconazole, clotrimazole, pentamidine isethionate, amphotericin B, flucytosine, chlorhexidine gluconate, hydroxystilbamidine, paromomycin, cotrimoxazole, rifampin, polymyxin, sulfadiazine, trimethoprim-sulfamethoxazole, azithromycin, dibromopropamidine, hexamidine, and ketoconazole (215, 377, 401, 411, 579). In one study, propamidine proved to be the most effective anti-Acanthamoeba agent against 19 corneal isolates (357). The drug polyhexamethylene biguanide was also effective (357). The drugs pentamidine, hexamidine, chlorhexidine, and chloroxylenol demonstrated intermediate activity, while neomycin, amphotericin B, and povidone-iodine had poor activity (357).
In some cases, surgical intervention may be necessary for the treatment of Acanthamoeba infections. In severe cases of sinusitis and rhinosinusitis, sinus debridement surgery may be necessary (401, 580). Surgical intervention may also be necessary for Acanthamoeba keratitis where penetrating keratoplasty or complete evisceration of the eye may be required (579).
Despite the broad range of drugs trialed, the most effective treatment for acanthamoebiasis remains undefined, and many drug treatments have shown various efficacies (215, 320, 323, 377, 571, 594). To date, there is no proven regimen for the treatment of Acanthamoeba infection (233). As such, once an Acanthamoeba infection is confirmed, therapy should be applied rapidly and aggressively.
Balamuthia mandrillaris
Organism and disease.
Balamuthia mandrillaris is a ubiquitous free-living amoeba that infects humans opportunistically. In humans, Balamuthia is known as a cause of lethal granulomatous encephalitis. Balamuthia is believed to exist in all environments and has been isolated from environmental soil samples (186, 505) and recreational bathing water (241). Humans become infected either through the inhalation of contaminated material or through cuts or abrasions in the skin. It is also thought that Balamuthia can enter the brain through the nose via the olfactory nerves (321). Balamuthia infection in animals has also been reported (85, 325). In fact, B. mandrillaris was first isolated in 1993 from a mandrill baboon that had died from meningoencephalitis (590). Approximately 150 cases of human Balamuthia infection were reported worldwide between the years 1990 and 2008 (94), with an additional 10 cases reported in California in 2009 (508). The clinical course of Balamuthia infection is generally subacute, often extending over a matter of weeks to months and usually concluding with the death of the patient (28, 310, 452, 469, 544).
The disease spectrum of B. mandrillaris infection can include skin lesions, rhinitis (588), and disseminated disease (523). However, the most common clinical manifestation of Balamuthia infection is GAE, which presents with a clinical picture similar to that of Acanthamoeba GAE. Encephalitis resulting from Balamuthia infection is often referred to as Balamuthia amoebic encephalitis (BAE). Numerous cases of BAE have been reported for ICT and IC persons alike (129, 152, 153, 245, 291, 430, 456). Balamuthia amoebic encephalitis is almost invariably fatal (430, 456, 568) regardless of immune status, with most cases being diagnosed postmortem. However, survivals have been reported (144, 306).
Compared to infections with Naegleria fowleri (discussed below), the clinical course of Balamuthia infection is slow (144, 306). In some of these cases, CNS symptoms are experienced for weeks to months prior to diagnosis and treatment (144, 430). In one patient, CNS involvement did not become apparent until 18 months after the disease presented in the form of a facial lesion (469). In this case, a more timely diagnosis could have improved the outcome (469). While Acanthamoeba infection is usually associated with IC individuals (377, 594), Balamuthia is known to infect ICT individuals but with a noticeable preference for young children and the elderly (28, 144, 245, 306, 355, 456, 520, 544). Also in contrast to Acanthamoeba, Balamuthia is not known as a cause of keratitis. Otherwise, the disease spectra associated with these amoebae are similar (588).
Patients infected with Balamuthia will often experience fever, headache, nausea, vomiting, stiff neck, and focal neurological signs that accompany brain lesions (380, 430). Other neurological signs can include changes in personality and mental status, seizures, and sleepiness (380). Hydrocephalus may also be experienced (28, 182). Cutaneous lesions have been reported as a presenting feature of Balamuthia infection in some cases (153, 456, 469). Susceptibility to Balamuthia infection is increased in the presence of cancers, diabetes, drug abuse, alcoholism, organ transplantation, and HIV (380, 426, 430, 587). Several cases of Balamuthia infection have been reported for AIDS patients (380, 620). While most Balamuthia-HIV coinfections manifest as BAE, disseminated disease due to Balamuthia-HIV coinfection has also been reported (523). In one AIDS patient, encephalitis without a granulomatous reaction was observed, along with involvement of the kidneys, adrenal glands, thyroid gland, and liver (523).
Diagnosis.
Neurological abnormalities will usually prompt a CT scan or MRI of the brain (291). Brain lesions caused by Balamuthia can appear similar to those caused by other protozoa, which may lead to a misdiagnosis and incorrect treatment (523). For a definitive diagnosis, microscopy, PCR, and/or IFAT must be employed. Most Balamuthia cases are diagnosed by IFAT (380, 430, 544), which is considered the “gold standard” for the detection of Balamuthia (523). Diagnosis by staining and microscopy of fixed tissue sections is also employed but usually after the patient's death (144). For staining of fixed tissue sections, a hematoxylin-and-eosin stain is often used (144, 380, 452). Other alternative stains such as a Gomori's methenamine silver stain or a periodic acid-Schiff stain are also useful (380). Even so, Balamuthia may still be inconspicuous in stained tissue sections (430), and laboratories without access to IFAT may underreport the incidence of Balamuthia infection (430). As such, Balamuthia infection should not be excluded solely on the basis of a negative microscopy result. If IFAT is not available, PCR or electron microscopy may be employed to confirm the presence of a Balamuthia infection (380, 452, 544). DNA extracted from deparaffinized brain tissue biopsy specimens can be tested with PCR using Balamuthia-specific primers (544). A multiplex PCR assay that can differentiate between infections with Acanthamoeba, Balamuthia, and N. fowleri in a single test has also been developed (462).
Treatment.
Treatment regimens for Balamuthia infections are poorly defined. Balamuthia infections are usually fatal, and in studies which reported “favorable” outcomes (the long-term survival of patients), neurological damage was sometimes permanent and treatment regimens had to be continued for years (144). However, some drugs do demonstrate anti-Balamuthia activity. In one in vitro study, miltefosine demonstrated anti-Balamuthia activity, while the drug voriconazole had virtually no effect (506). In another in vitro study, pentamidine and propamidine were shown to be amoebicidal, while amphotericin B was only amoebastatic (507). In the few cases in which patients survived, the treatment regimens employed were as follows: (i) pentamidine (300 mg intravenous once daily), sulfadiazine (1.5 g four times daily), fluconazole (400 mg daily), and clarithromycin (500 mg three times daily) (306) and (ii) fluconazole (400 mg daily), sulfadiazine (1.5 g every 6 h), and clarithromycin (500 mg three times daily) (144).
Usually, the prognosis for BAE patients is very poor, and survival is rare. Further research is required to facilitate the discovery of more effective anti-Balamuthia compounds.
Naegleria fowleri
Organism and disease.
Naegleria fowleri is a free-living amoeboflagellate, first identified as a human pathogen in South Australia by Malcolm Fowler in 1965 (117, 210). Naegleria fowleri is recognized as the cause of primary amoebic meningoencephalitis (PAM), an often fatal disease. Naegleria infections are obtained exclusively through contact with contaminated bodies of water (29, 33, 48, 60, 89), presumably when the water enters the nasal cavity (117). The protozoa then invade the central nervous system via the olfactory nerve (60). The species Naegleria italica and Naegleria australiensis are also considered potentially pathogenic members of the genus Naegleria (479).
Naegleria species are ubiquitous in wet environments and have recently been identified in domestic water sources (378), cooling waters from power plants (37), and well water (48). Several novel Naegleria spp. have recently been isolated from freshwater sediments in Arctic and sub-Antarctic regions (146). However, these species grew only between room temperature and 30°C (146) and therefore probably lack the ability to parasitize mammalian tissues. Human and animal infections with Naegleria are usually associated with temperatures greater than 30°C (95, 117, 136).
Despite the ubiquity of Naegleria, infections are comparatively rare, and the estimated risk of becoming infected with Naegleria is low (80). However, in one Australian study, an “outbreak” of N. fowleri infection was described where 19 cases of probable PAM occurred between 1947 and 1972 (117). These infections were thought to be associated with a contaminated drinking water pipeline from which N. fowleri was eventually isolated in 1972. Some patients recalled having water from this source splashed onto their face and in their nose. Furthermore, some of these pipes were in direct contact with sunlight, and temperatures within these pipes were reported to reach 35°C to 45°C in summer. These conditions would have promoted the growth of the vegetative form of N. fowleri (117).
Similarly to B. mandrillaris, N. fowleri infections are associated with healthy ICT persons, often children (111, 128, 258, 410, 517), rather than IC patients (60). In contrast to other free-living amoebae, N. fowleri infections are restricted to the CNS (60). To our knowledge, only one case of PAM has been reported for an HIV-infected patient, and parasitic involvement was restricted to the CNS (110). Furthermore, one study described a case where an organ donor had died of PAM, but the single recipient of the kidneys, pancreas, a lung, and liver failed to contract PAM (38, 379).
In the single case of Naegleria-HIV coinfection, the patient suffered from a range of neurological symptoms, including headache, somnolence, dysarthria, left hemiparesis, and motor incoordination. The patient died 8 days after admission to the hospital (110).
The progression of PAM is rapid, with death often occurring within a week after the onset of symptoms (29, 89). Survival from PAM is rare, with only 7 reports of survival out of approximately 300 cases reported prior to 2002 (295). Naegleria fowleri rapidly destroys the tissues of the CNS, leading to various neurological disturbances, including taste and olfactory impairments, nausea, vomiting, anorexia, headache, dizziness, confusion, and, finally, coma and death (29, 89).
Diagnosis.
Primary amoebic meningoencephalitis is usually diagnosed from wet mounts of CSF, where active amoebae may be observed (110, 312, 575). Giemsa- or trichrome-stained CSF smears may aid in the early diagnosis of PAM by enabling the differentiation of amoebae from host cells based on the nuclear structure (589). Fixed hematoxylin-and-eosin stains may also be useful (503).
Since the onset of PAM is extremely rapid (usually less than a week), there is insufficient time for the patients' immune systems to develop a detectable immune response. Therefore, serology is not useful for the diagnosis of PAM (589). The diagnosis of PAM should include PCR (500, 575). A PCR assay with the ability to detect Naegleria in formalin-fixed brain sections with a high level of sensitivity was developed (500). A real-time PCR assay that can distinguish between multiple members of the genus Naegleria by melting-curve analysis has also been developed (479). This assay could easily be adapted to DNA extracted from the CSF and may be useful in identifying the causal species of a Naegleria infection. A recently developed real-time PCR assay is also available for the detection of N. fowleri (370, 458). Naegleria may also be cultured from fresh CSF onto nonnutrient agar or various other medium formulations for further characterization (97, 312).
Treatment.
As with the other free-living amoebae, no proven treatment regimens exist for PAM. However, amphotericin B is probably the drug of choice for the treatment of N. fowleri infection due to its successful use in a few cases of PAM (66, 575). Amphotericin B has also demonstrated good anti-Naegleria activity in vitro (242, 243, 548, 549). However, amphotericin B therapy is not always successful (503). The drugs fluconazole and rifampin have also been used in combination with amphotericin B to successfully treat PAM (575). The drug ketoconazole has also shown anti-Naegleria activity in in vitro studies (414, 549). Drugs reported to be ineffective against Naegleria include trimethoprim (96), penicillin, sulfadiazine, chloramphenicol, oxytetracycline hydrochloride, streptomycin, methotrexate, emetine, quinine, and metronidazole (88).
While survival of patients with N. fowleri infection is rare, it has been hypothesized that the prognosis for PAM patients could be improved by a combination of therapies, including multiroute amphotericin B therapy (including the intrathecal route), a range of antimycotic and antibacterial drugs, intrathecal anti-Naegleria immunoglobulin, and the anti-inflammatory drug dexamethasone (7). Unfortunately, the rapid course of PAM means that the survival of patients relies on timely and aggressive therapy. Given the lack of any proven treatments for PAM, the prognosis for those diagnosed with the disease is poor. Clearly, more research is required to identify more anti-Naegleria compounds and to develop treatment regimens for PAM.
Sappinia pedata
To our knowledge, only a single case of human Sappinia pedata infection has been reported (228, 461, 588). This organism is a free-living amoeba that has been isolated from environmental sources such as soil and tree bark (461). Given the existence of this single isolated case, Sappinia is mentioned only briefly.
The patient in question was a 38-year-old ICT male who presented with neurological signs, including headache, blurred vision, photophobia, and vomiting, for 2 to 3 days. Magnetic resonance imaging scans revealed a mass in the posterior left temporal lobe, which was later excised. The mass was found to contain trophozoites of an amoeboid organism which was thought to be of the species Sappinia diploidea (228). The patient was treated with a range of antiamoebic drugs, including azithromycin, pentamidine, itraconazole, and flucytosine (227). The outcome was favorable, and the patient survived (227, 228). In a later study, three real-time PCR assays were used to ascertain that the etiological agent was more likely to be S. pedata than S. diploidea (461).
CONCLUSIONS
Protozoan pathogens with the ability to cause disease in human tissues are a major global health concern. In most cases, the prognosis for patients with protozoal disease of solid tissues and/or blood is greatly worsened in the presence of HIV. Due to the debilitation of the immune system seen in HIV infection, organisms such as Toxoplasma, which usually cause benign infections, can cause potentially fatal disease. Similarly, the microsporidia and some lower trypanosomatids, which are usually noninfectious to ICT humans, can cause serious disease in HIV-infected patients.
While patients who adhere to antiretroviral therapy may experience a partial and/or temporary reconstitution of the immune system and resolution of some disease symptoms of protozoal origin, HIV infection is also complicated by immune reconstitution disorders (IRDs). Any pathogen capable of causing an opportunistic infection has the potential to cause IRD after the commencement of antiretroviral therapy (213). However, some pathogens have a lower tendency to cause IRDs than others. Toxoplasma-associated IRDs are an uncommon event (429), although granulomatous inflammation is often triggered in response to antiretroviral therapy in the presence of Leishmania infections (10, 213). Immune reconstitution disorders are characterized by inflammation at the site of a given infection and may be confused as an immunodeficiency-related disease rather than IRD. If a patient has been treated recently for a particular pathogen, IRD may occur in response to dead pathogens still present in the tissues (213). It is important that clinicians be aware of this paradoxical disease syndrome in order to prevent a misdiagnosis of IRD with opportunistic disease. An awareness of IRD is also necessary so that IRD is not mistaken for a failure of antiretroviral therapy.
In IC patients, many tissue-infecting protozoa demonstrate the ability to cause disease states that are atypical. Moreover, different species of parasitic protozoa can cause similar disease states in the presence of HIV. One example of this is the ability of Toxoplasma and T. cruzi to cause morphologically similar necrotic brain lesions. Atypical disease presentations and the similarity between certain disease states caused by unrelated protozoa in IC patients can make a correct diagnosis challenging. These similarities can lead to misdiagnosis and the inappropriate treatment of patients. It is important that clinicians and diagnostic laboratories are made aware of the pathogenic potential of various protozoa in the presence of HIV and are also aware of the various techniques available for the diagnosis of different protozoal infections. Information relating to the diagnostic techniques available for various tissue protozoa is summarized in Table 3.
TABLE 3.
Diagnostic techniques available for various blood and tissue protozoan infections
| Organism(s) | Techniques | Description |
|---|---|---|
| Microsporidia | Light microscopy, TEM, PCR, real-time PCR, IFAT, DAT | Light microscopy is cheap and applicable to any clinical specimen; however, differentiation of species may be difficult by light microscopy; transmission electron microscopy may be useful for differentiation of species, although it can be expensive; several PCR and real-time PCR assays have been developed for detection of various species, although PCR requires a special apparatus; real-time PCR can be more rapid than conventional PCR, as it does not require agarose gel electrophoresis; generally, real-time PCR is more sensitive than conventional PCR; sequencing of PCR products may be necessary for species differentiation; indirect fluorescent antibody tests and DAT are available for some species only |
| Leishmania | Conventional and real-time PCR, light microscopy, culture systems, serological techniques, DAT, rK39 dipstick test, latex agglutination test, QBC tube technique | Staining of blood films followed by light microscopy is definitive but is less sensitive than molecular techniques; real-time PCR can also be used to monitor patients' responses to therapy but requires a special apparatus; sequencing of PCR products can also allow differentiation of species; culture systems are useful but may be impractical; in Ethiopia DAT is preferred for diagnosis of L. donovani infection, as it is cheap, rapid, and sensitive; the rK39 dipstick test is cheap, rapid, and sensitive for diagnosis in ICT patients; however, the rK39 test demonstrated low sensitivities of ∼20% in European VL patients infected with HIV; the latex agglutination test is sensitive and noninvasive (applied to urine specimens); enzyme-linked immunosorbent assay and other serological techniques may be of limited use in IC patients, as circulating antibodies may not be detected during an active infection; the QBC tube technique is useful for the diagnosis of VL |
| Trypanosoma cruzi | Light microscopy, real-time PCR, PCR, ELISA, IFAT, QBC tube technique | Light microscopy is definitive; trypanosomes are more readily observed in peripheral blood specimens in the acute stage and in IC patients; the blood stains are useful for blood stages, and hematoxylin and eosin stains are used for diagnosis of the intracellular stages, as seen in solid tissues; live, motile trypanosomes may be observed microscopically in the buffy coat or CSF (if CNS involvement is apparent); PCR and real-time PCR are sensitive but require a special apparatus; PCR can also be useful for monitoring treatment efficacy; the QBC tube technique is useful for diagnosis during the acute phase of Chagas' disease but is less useful during the chronic phase of the disease |
| Trypanosoma brucei rhodesiense, Trypanosoma brucei gambiense | Light microscopy, PCR, real-time PCR, ELISA, CATT, QBC tube technique | The blood stains are useful for light microscopic diagnosis when applied to blood or CSF; the microhematocrit centrifugation technique also enables the visualization of live motile trypanosomes microscopically; several PCRs and real-time PCRs are also available; PCR is sensitive and can allow differentiation of T. brucei subtypes; enzyme-linked immunosorbent assay is also useful; the CATT is available for T. b. gambiense sleeping sickness and is the most widely used technique in areas of endemicity, as it is cheap, rapid, and sensitive; a CSF white blood cell count is useful to predict the stage of sleeping sickness; the QBC tube technique has demonstrated greater sensitivity for detection of African trypanosomes than the microhematocrit centrifugation technique |
| Non-human-infecting Trypanosoma | Light microscopy, various molecular techniques | No specific diagnostics are available, as human infections are rare; infections are typically diagnosed by a combination of light microscopy and various molecular techniques |
| Lower trypanosomatids | TEM, light microscopy, various molecular techniques | As with the non-human-infecting Trypanosoma spp., no specific diagnostics are available, as human infections are rare; infections were typically diagnosed by a combination of light microscopy and various molecular techniques; transmission electron microscopy was used in 1 case |
| Toxoplasma gondii | Light microscopy, PCR, real-time PCR, ELISA, IgG avidity test for pregnancy | Observation of live free tachyzoites in stained smears of blood or CSF is diagnostic of an active infection; enzyme-linked immunosorbent assay can be useful but fails to differentiate between active and benign infections; PCR performed on peripheral blood is also diagnostic for an active Toxoplasma infection; real-time PCR assays are available, which are extremely sensitive and specific. The IgG avidity test is useful in pregnancy to predict the risk that a Toxoplasma infection poses to the fetus; however, the results of serological tests can be complex and difficult to interpret for pregnant woman and neonates |
| Neospora caninum | Serology, PCR | Tachyzoites of Neospora are virtually indistinguishable from those of Toxoplasma; as such, light microscopy cannot differentiate the two; few diagnostics are available for human infections, as human neosporosis has never been reported; however, various serological techniques and PCR assays have been developed, which show good sensitivity and specificity |
| Babesia | Light microscopy, PCR, laboratory rodent inoculation, ELISA, IFAT, QBC tube technique | Microscopic analysis of stained blood smears is useful for diagnosis in the early stages of infection or in IC patients; live-rodent inoculation is sensitive and was once used for diagnosis but has been abandoned due to practicality issues; PCR can be applied to blood specimens and is sensitive and extremely useful for the characterization of new isolates; enzyme-linked immunosorbent assay and IFAT may be useful for diagnosis of B. microti infections but are less applicable to B. divergens infections; for definitive confirmation of Babesia infection at the species level, amplification of the SSU rDNA genes by Babesia-specific PCR followed by sequencing of the PCR product can be performed; the QBC tube technique can also be used for the microscopic diagnosis of Babesia infection |
| Acanthamoeba (GAE) | Light microscopy, cultivation, IFAT, PCR | Acanthamoeba spp. can be identified in Gram or Giemsa stains of CSF; calcofluor white can also be used for microscopic detection of Acanthamoeba; cultivation of trophozoites from CSF or tissue lesions by inoculation of the specimen onto neomycin-nalidixic acid agar plates seeded with a layer of Escherichia coli or Enterobacter aerogenes cells is a useful diagnostic tool; cultivation of Acanthamoeba can also provide the material for downstream molecular characterization; indirect fluorescent antibody techniques and PCR are also available; PCR is probably the most sensitive technique available for diagnosis of Acanthamoeba infection |
| Balamuthia (BAE) | Light microscopy, PCR, IFAT, electron microscopy | Several stains are useful for light microscopic diagnosis, although the hematoxylin and eosin stain is usually applied to fixed tissue sections; however, Balamuthia trophozoites may be difficult to see; the use of an immunofluorescent antibody technique is a sensitive and specific alternative to light microscopy and is the gold standard for diagnosis of Balamuthia infection; PCR is also useful for diagnosis, as it is sensitive; some PCR assays can distinguish between Balamuthia and other free-living amoebae; electron microscopy is useful, although it is expensive |
| Naegleria fowleri (PAM) | Light microscopy, PCR, real-time PCR, cultivation | As the onset of PAM is rapid, live, motile trophozoites can be readily observed in wet mounts of patient CSF; light microscopy of fixed, stained tissue smears is also useful. PCR and real-time PCR are available; cultivation of Naegleria from CSF enables downstream molecular analysis of isolates |
| Sappinia pedata | Light microscopy, PCR | Only 1 reported case, which was diagnosed by light microscopy; recently, 3 real-time PCR assays were also developed for Sappinia spp. |
Prior to the AIDS pandemic, infections with the microsporidia were rarely observed (169), and only four cases of infections with lower trypanosomatids have been reported to date, three of these in HIV-infected persons. Given the recent identification of the lower trypanosomatids as opportunistic infectious agents in IC individuals, it is not unreasonable to sug- gest that other opportunistic infectious agents may present themselves in the future. Given the findings of recent serological studies (361, 478), it is possible that N. caninum may present itself as an opportunistic infections agent in IC patients in the future. It is recommended that in cases where Toxoplasma infection is suspected, PCR for the detection of N. caninum DNA should also be used to assess the possible role of N. caninum as an opportunistic pathogen in IC patients. Given their ability to infect ICT humans, it is also possible that certain nonhuman Trypanosoma species may also become recognized as opportunistic organisms in IC patients.
An awareness of the potential for organisms that rarely infect humans to occasionally cause serious disease is paramount to the survival of patients infected with these organisms. This is true for the free-living amoebae, particularly N. fowleri, due to the rapid onset of disease following the appearance of symptoms. In such cases, rapid and aggressive therapy could mean the difference between life and death. Knowledge of the various drugs available for the treatment of numerous protozoal diseases is, of course, paramount to the survival of patients, and clinicians should be aware of the most effective (and current) treatments available. A current list of treatment regimens available for various protozoal infections is summarized in Table 4.
TABLE 4.
Suggested antimicrobial therapy for infections with various blood- and tissue-associated protozoaa
| Organism | Suggested antimicrobial therapyc |
|---|---|
| Microsporidia | Effective HAART leading to immune restoration can result in clinical cure of microsporidiosis; restoration of CD4+ cell counts of >100 cells/μl should lead to clinical improvement; for treatment of disseminated infections with Encephalitozoon hellem, Encephalitozoon cuniculi, or Encephalitozoon intestinalis, albendazole (400 mg orally twice daily for 3 weeks) can be used; there is no established treatment for Pleistophora or Anncaliia species infections, although albendazole therapy as described above is recommended; for disseminated infections with Trachipleistophora, albendazole (400 mg oral twice daily for 3 weeks) plus itraconazole (400 mg oral daily for 3 weeks) are recommended; clindamycin has also demonstrated some anti-E. intestinalis activity (300 mg orally every 6 h)b; treatment for ocular infections with E. hellem, E. cuniculi, Vittaforma corneae, or Nosema ocularum includes fumigillin (Fumidil B) at 3 mg/ml in saline (final concn of 70 μg/ml fumigillin) in eye drop form plus albendazole (400 mg orally twice daily for 3 weeks) |
| New World CL and MCL (Mexico, Central America, South America) | Always begin treatment of MCL with antimonial therapy; recommended primary therapy of sodium stibogluconate or meglumine antimoniate (20 mg/kg of body wt/day i.v. for 28 days); in Brazil, antimony and pentoxifylline (400 mg orally 3 times a day for 30 days) are superior to antimonial treatment alone; alternatives are amphotericin B (1 mg/kg i.v. every other day for 20 doses), liposomal amphotericin B (3 mg/kg/day i.v. for 6 days [3 weeks for MCL]), or miltefosine (2.5 mg/kg/day orally for 28 days); oral miltefosine is effective against L. panamensis, marginal against L. mexicana, and ineffective against L. braziliensis |
| Old World CL and MCL (Europe, Asia, and Africa) | Stibogluconate or meglumine antimoniate (20 mg/kg/day i.v. for 10 days) |
| Visceral leishmaniasis | Recommended primary therapy of liposomal amphotericin B (3 mg/kg/day i.v. for 5 days followed by 3 mg/kg on days 14 and 21); alternatives are liposomal amphotericin B (10 mg/kg/day i.v. for 2 days), stibogluconate or meglumine antimoniate (20 mg/kg/day i.v. in a single dose for 28 days), miltefosine (1.5-2.5 mg/kg/day orally for 28 days), or standard amphotericin B (1 mg/kg i.v. every other day for 20 days) |
| Trypanosoma cruzi | For adults, nifurtimox (8-10 mg/kg/day orally [after meals] divided into 4 doses for 120 days); for children 11-16 yr of age, nifurtimox (12.5-15 mg/kg/day orally [after meals] divided into 4 doses for 90 days); for children <11 yr of age, nifurtimox (15-20 mg/kg/day orally [after meals] divided into 4 doses each day for 90 days); an alternative is benznidazole (5-7 mg/kg/day orally divided into 2 doses each day for 30-90 days) |
| Trypanosoma brucei gambiense sleeping sickness (lymphatic stage) | Recommended therapy is pentamidine (4 mg/kg/day i.m. for 10 days); an alternative is suramin (100-mg i.v. test dose followed by 1 g i.v. on days 1, 3, 7, 14, and 21) |
| Trypanosoma brucei gambiense sleeping sickness (late CNS stage) | Recommended therapy is melarsoprol (2.2 mg/kg/day i.v. for 10 days); an alternative is eflornithine (100 mg/kg i.v. every 6 h for 14 days) |
| Trypanosoma brucei rhodesiense sleeping sickness (lymphatic stage) | Suramin (100-mg i.v. test dose followed by 1 g i.v. on days 1, 3, 7, 14, and 21) |
| Trypanosoma brucei rhodesiense sleeping sickness (late CNS stage) | Melarsoprol (2-3.6 mg/kg/day i.v. for 3 days, repeat after 7 days, and repeat for a third time 7 days after the second course) |
| Toxoplasma gondii | Treatment is often complex, depending on immune status and the presence or absence of pregnancy; no recommended treatment for immunologically healthy individuals unless there is evidence of severe symptoms or organ damage; treatment of congenital toxoplasmosis is also very complex; for acute infection in pregnancy at <18 wks of gestation, spiramycin (1 g every 8 h orally until delivery) can be used if amniotic fluid is PCR negative; for acute infection in pregnancy at >18 wks of gestation and if amniotic fluid is PCR positive, pyrimethamine (50 mg every 12 h orally for 2 days and then 50 mg/day orally) plus sulfadiazine (75 mg/kg orally for 1 dose and then 50 mg/kg every 12 h orally) plus leucovorin (10-20 mg/day orally) can be used; in cases of chorioretinitis, meningitis, or lowered resistance due to cytotoxic drugs or steroids in non-AIDS patients, pyrimethamine (200 mg/day orally for 1 dose and then 50-75 mg every 24 h) plus sulfadiazine (1-1.5 mg orally 4 times daily) plus leucovorin (5-20 mg orally 3 times per week) can be used (continue for 2 weeks after symptoms subside), and also add prednisone (1 mg/kg/day i.v. in 2 divided doses to reduce CSF protein or vision-threatening inflammation) |
| AIDS-related cerebral toxoplasmosis | For prevention of cerebral toxoplasmosis in AIDS patients (prophylaxis), trimethoprim-sulfamethoxazole DSe (1 tablet orally every 24 h) or trimethoprim-sulfamethoxazole SSf (1 tablet orally every 24 h); alternatives are dapsone (50 mg orally every 24 h) plus pyrimethamine (50 mg orally per week) plus leucovorin (10-25 mg orally every 24 h) or atovaquone (1,500 mg orally every 24 h); recommended therapy for cerebral toxoplasmosis is pyrimethamine (200 mg orally for 1 dose) followed by pyrimethamine (75 mg/day orally) plus sulfadiazine (1-1.5 g orally every 6 h) plus oral leucovorin (10-20 mg daily) continued for 4-6 weeks after resolution of symptoms or trimethoprim-sulfamethoxazole (10-50 mg/kg/day orally or i.v. divided into 12 hourly doses) for 30 days; alternatives for use in patients with sulfa intolerance are pyrimethamine (200 mg/kg orally for 1 dose) followed by pyrimethamine (75 mg/kg/day orally) plus leucovorin (10-20 mg orally daily) plus one of either (i) clindamycin (600 mg orally or i.v. every 6 h), (ii) clarithromycin (1 g orally twice daily), (iii) azithromycin (1.2-1.5 g orally every 24 h), or (iv) atovaquone (750 mg orally every 6 h), with treatment for 4-6 weeks after resolution of symptoms; for suppression after resolution of cerebral toxoplasmosis, sulfadiazine (500-1,000 mg orally 4 times daily) plus pyrimethamine (25-50 mg orally every 24 h) plus leucovorin (10-25 mg orally every 24 h) (discontinue if CD4+ cell count is >200 for at least 3 mo), clindamycin (300-450 mg orally every 6-8 h) plus pyrimethamine (25-50 mg orally every 24 h) plus leucovorin (10-25 mg orally every 24 h), or atovaquone (750 mg orally every 6-12 h) is recommended |
| Babesia spp. | Recommended therapy is atovaquone (750 mg orally twice a day for 7-10 days) plus azithromycin (500 mg orally for 1 dose and then 250 mg every 24 h for 7 days); an alternative is clindamycin (600 mg orally 3 times a day) plus quinine (650 mg orally 3 times a day for 7-10 days); adults can receive i.v. clindamycin (1.2 g twice daily); immunocompromised patients should be treated for more than 6 wk |
| Free-living amoebae | For these organisms no proven treatment regimens exist; these treatment options are based on successful regimens described in case reports |
| Acanthamoeba spp. | Treatment is a 3-mo course of oral cotrimoxazole plus oral rifampin, a lipid formulation of i.v. amphotericin B plus oral voriconazole, high-dose i.v. amphotericin B plus oral 5-fluorocytosine, or oral trimethoprim-sulfamethoxazole plus oral rifampin plus oral ketoconazole; for treatment of cutaneous lesions, topical chlorhexidine and 2% ketoconazole cream and oral itraconazole are used; for treatment of Acanthamoeba keratitis, propamidine (0.1%) and neomycin-gramicidin-polymyxin eye drops, PHMB (0.02%) eye drops, or chlorhexidine (0.02%) eye drops are used |
| Balamuthia mandrillaris | Pentamidine (300 mg i.v. once a day), sulfadiazine (1.5 g orally 4 times daily), fluconazole (400 mg orally daily), and clarithromycin (500 mg orally 3 times daily) or fluconazole (400 mg oral daily), sulfadiazine (1.5g orally every 6 h), clarithromycin (500 mg orally 3 times daily) |
| Naegleria fowleri | Intrathecal amphotericin B (1.5 mg/kg/day in 2 divided doses for 3 days); this is followed by intrathecal amphotericin B (1 mg/kg/day for 6 days), followed by intrathecal amphotericin B (1.5 mg/day for 2 days) and then intrathecal amphotericin B (1 mg/day every other day for 8 days) |
| Sappinia pedata | Azithromycin, pentamidine, itraconazole, and flucytosine therapyd |
Information regarding neglected tropical diseases caused by the trypanosomes, Leishmania, and Plasmodium is abundant in the literature. However, the extent of the HIV problem in some areas of the world (particularly sub-Saharan Africa) allows diseases like toxoplasmosis and microsporidiosis to remain a common health problem in these locations. In Western countries, due to the availability of HAART, these diseases are less frequently encountered. Due to global travel and immigration, nonendemic tropical diseases are increasingly encountered (57, 329, 534). A recent example of this is the diagnosis of cutaneous leishmaniasis in Sydney, Australia (329, 534). As some tissue protozoa are nonendemic in Western countries or are very rarely encountered, many of these protozoa are not a first consideration in the differential diagnosis; hence, diagnostics are often not always available. This is presently being remedied at St. Vincent's Hospital in Sydney.
Acknowledgments
We thank Paul Badenoch for graciously donating the photographs used to produce Fig. 5 and 6.
Biography
 J. L. N. Barratt is a Ph.D. student at the University of Technology, Sydney (UTS), working in collaboration with St. Vincent's Hospital, under the supervision of Professor John Ellis and Dr. Damien Stark. He completed his honors degree at the UTS under the supervision of Professor John Ellis on the protozoan parasite Neospora caninum. His current research focuses on human enteric protozoa, with a particular focus on the trichomonad parasite Dientamoeba fragilis.
J. L. N. Barratt is a Ph.D. student at the University of Technology, Sydney (UTS), working in collaboration with St. Vincent's Hospital, under the supervision of Professor John Ellis and Dr. Damien Stark. He completed his honors degree at the UTS under the supervision of Professor John Ellis on the protozoan parasite Neospora caninum. His current research focuses on human enteric protozoa, with a particular focus on the trichomonad parasite Dientamoeba fragilis.
 J. Harkness graduated from Monash University medical school in Melbourne, Australia, in 1967. Training as a junior medical officer was at the Alfred Hospital in Melbourne, followed by training in pathology at the Alfred Hospital, Hammersmith Hospital, London, United Kingdom, and the Mayo Clinic, Rochester, NY. He was appointed Director of Microbiology at St. Vincent's Hospital, Sydney, Australia, in 1975. He is an Associate Professor in the Medical Faculty, University of New South Wales, and Adjunct Professor at the University of Technology in Sydney. He is actively involved in teaching medical undergraduates and postgraduates. He is interested in infections in the immunocompromised host, antimicrobial therapy, and parasitology. Research interests in the laboratory in particular are in parasitology and mycology.
J. Harkness graduated from Monash University medical school in Melbourne, Australia, in 1967. Training as a junior medical officer was at the Alfred Hospital in Melbourne, followed by training in pathology at the Alfred Hospital, Hammersmith Hospital, London, United Kingdom, and the Mayo Clinic, Rochester, NY. He was appointed Director of Microbiology at St. Vincent's Hospital, Sydney, Australia, in 1975. He is an Associate Professor in the Medical Faculty, University of New South Wales, and Adjunct Professor at the University of Technology in Sydney. He is actively involved in teaching medical undergraduates and postgraduates. He is interested in infections in the immunocompromised host, antimicrobial therapy, and parasitology. Research interests in the laboratory in particular are in parasitology and mycology.
 D. Marriott graduated from the University of New South Wales (UNSW) Medical School in 1978 and was awarded F.R.A.C.P. in 1985 and F.R.C.P.A. in 1986. She is an Associate Professor, School of Medicine, UNSW, and Adjunct Professor at the University of Technology, Sydney. She has a long-standing interest in infections in immunocompromised patients and was a founding member and past president of the Australasian Society for HIV Medicine. She is active in clinical research, particularly mycology and parasitology, and is an enthusiastic teacher and clinician.
D. Marriott graduated from the University of New South Wales (UNSW) Medical School in 1978 and was awarded F.R.A.C.P. in 1985 and F.R.C.P.A. in 1986. She is an Associate Professor, School of Medicine, UNSW, and Adjunct Professor at the University of Technology, Sydney. She has a long-standing interest in infections in immunocompromised patients and was a founding member and past president of the Australasian Society for HIV Medicine. She is active in clinical research, particularly mycology and parasitology, and is an enthusiastic teacher and clinician.
 J. T. Ellis completed a Ph.D. on leishmaniasis with Professor J. M. Crampton at the Liverpool School of Tropical Medicine in 1986 and subsequently did postdoctoral research on Eimeria vaccines at Houghton Poultry Research Station (with Dr. M. Elaine Rose and colleagues) and parasite phylogeny (with Professor A. M. Johnson at the Flinders University of South Australia). He was appointed to the academic staff of the University Technology Sydney in 1992 as a Lecturer in Microbiology. Over the last 19 years he has continued to study parasitic protozoa of both veterinary and medical importance. He is currently Professor of Molecular Biology, and his present research interests include the development of vaccines and diagnostics for protozoal diseases of economic importance. He was awarded the degree of D.Sc. by Liverpool University in 2006 for his pioneering research on the biology of cyst-forming coccidia. He is a Fellow of the Royal Society for Tropical Medicine and Hygiene and a member of the Australian, British, and American Societies for Parasitology.
J. T. Ellis completed a Ph.D. on leishmaniasis with Professor J. M. Crampton at the Liverpool School of Tropical Medicine in 1986 and subsequently did postdoctoral research on Eimeria vaccines at Houghton Poultry Research Station (with Dr. M. Elaine Rose and colleagues) and parasite phylogeny (with Professor A. M. Johnson at the Flinders University of South Australia). He was appointed to the academic staff of the University Technology Sydney in 1992 as a Lecturer in Microbiology. Over the last 19 years he has continued to study parasitic protozoa of both veterinary and medical importance. He is currently Professor of Molecular Biology, and his present research interests include the development of vaccines and diagnostics for protozoal diseases of economic importance. He was awarded the degree of D.Sc. by Liverpool University in 2006 for his pioneering research on the biology of cyst-forming coccidia. He is a Fellow of the Royal Society for Tropical Medicine and Hygiene and a member of the Australian, British, and American Societies for Parasitology.
 D. Stark is a Senior Hospital Scientist in the Microbiology Department at St. Vincent's Hospital, Sydney, and is also an Associate, Department of Medical and Molecular Biosciences, Institute for the Biotechnology of Infectious Disease, University of Technology, Sydney (UTS). His research interests include clinical, diagnostic, and molecular parasitology, with a special interest in all aspects of D. fragilis research.
D. Stark is a Senior Hospital Scientist in the Microbiology Department at St. Vincent's Hospital, Sydney, and is also an Associate, Department of Medical and Molecular Biosciences, Institute for the Biotechnology of Infectious Disease, University of Technology, Sydney (UTS). His research interests include clinical, diagnostic, and molecular parasitology, with a special interest in all aspects of D. fragilis research.
REFERENCES
- 1.Abad-Franch, F., A. Paucar, C. Carpio, C. A. Cuba, H. M. Aguilar, and M. A. Miles. 2001. Biogeography of Triatominae (Hemiptera: Reduviidae) in Ecuador: implications for the design of control strategies. Mem. Inst. Oswaldo Cruz 96:611-620. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Madi, M. A., N. Al-Molawi, and J. M. Behnke. 2008. Seroprevalence and epidemiological correlates of Toxoplasma gondii infections among patients referred for hospital-based serological testing in Doha, Qatar. Parasit. Vectors 1:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht, H., H. J. Stellbrink, S. Fenske, H. Schafer, and H. Greten. 1994. Successful treatment of Toxoplasma gondii myocarditis in an AIDS patient. Eur. J. Clin. Microbiol. Infect. Dis. 13:500-504. [DOI] [PubMed] [Google Scholar]
- 4.Aleixo, A. L., E. I. Benchimol, E. de Souza Neves, C. S. Silva, L. C. Coura, and M. R. Amendoeira. 2009. Frequency of lesions suggestive of ocular toxoplasmosis among a rural population in the State of Rio de Janeiro. Rev. Soc. Bras. Med. Trop. 42:165-169. (In Portugese.) [DOI] [PubMed] [Google Scholar]
- 5.Alfonzo, M., D. Blanc, C. Troadec, M. Huerre, M. Eliaszewicz, G. Gonzalez, Y. Koyanagi, and D. Scott-Algara. 2002. Temporary restoration of immune response against Toxoplasma gondii in HIV-infected individuals after HAART, as studied in the hu-PBMC-SCID mouse model. Clin. Exp. Immunol. 129:411-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aliaga, L., F. Cobo, J. D. Mediavilla, J. Bravo, A. Osuna, J. M. Amador, J. Martin-Sanchez, E. Cordero, and J. M. Navarro. 2003. Localized mucosal leishmaniasis due to Leishmania (Leishmania) infantum: clinical and microbiologic findings in 31 patients. Medicine (Baltimore) 82:147-158. [DOI] [PubMed] [Google Scholar]
- 7.Alisky, J. M. 2008. Survival of Naegleria fowleri primary amebic meningocephalitis (PAM) could be improved with an intensive multi-route chemo- and biotherapeutic regimen. Med. Hypotheses 71:969-971. [DOI] [PubMed] [Google Scholar]
- 8.Altclas, J., A. Sinagra, M. Dictar, C. Luna, M. T. Veron, A. M. De Rissio, M. M. Garcia, C. Salgueira, and A. Riarte. 2005. Chagas disease in bone marrow transplantation: an approach to preemptive therapy. Bone Marrow Transplant. 36:123-129. [DOI] [PubMed] [Google Scholar]
- 9.Altclas, J., A. Sinagra, G. Jaimovich, C. Salgueira, C. Luna, A. Requejo, V. Milovic, A. De Rissio, L. Feldman, and A. Riarte. 1999. Reactivation of chronic Chagas' disease following allogeneic bone marrow transplantation and successful pre-emptive therapy with benznidazole. Transpl. Infect. Dis. 1:135-137. [DOI] [PubMed] [Google Scholar]
- 10.Alvar, J., P. Aparicio, A. Aseffa, M. Den Boer, C. Canavate, J. P. Dedet, L. Gradoni, R. Ter Horst, R. Lopez-Velez, and J. Moreno. 2008. The relationship between leishmaniasis and AIDS: the second 10 years. Clin. Microbiol. Rev. 21:334-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvar, J., B. Gutierrez-Solar, I. Pachon, E. Calbacho, M. Ramirez, R. Valles, J. L. Guillen, C. Canavate, and C. Amela. 1996. AIDS and Leishmania infantum. New approaches for a new epidemiological problem. Clin. Dermatol. 14:541-546. [DOI] [PubMed] [Google Scholar]
- 12.Alvar, J., S. Yactayo, and C. Bern. 2006. Leishmaniasis and poverty. Trends Parasitol. 22:552-557. [DOI] [PubMed] [Google Scholar]
- 13.Alvarado-Esquivel, C., A. Torres-Castorena, O. Liesenfeld, C. R. Garcia-Lopez, S. Estrada-Martinez, A. Sifuentes-Alvarez, J. F. Marsal-Hernandez, R. Esquivel-Cruz, F. Sandoval-Herrera, J. A. Castaneda, and J. P. Dubey. 2009. Seroepidemiology of Toxoplasma gondii infection in pregnant women in rural Durango, Mexico. J. Parasitol. 95:271-274. [DOI] [PubMed] [Google Scholar]
- 14.Amato, V. S., A. R. Padilha, A. C. Nicodemo, M. I. Duarte, M. Valentini, D. E. Uip, M. Boulos, and V. A. Neto. 2000. Use of itraconazole in the treatment of mucocutaneous leishmaniasis: a pilot study. Int. J. Infect. Dis. 4:153-157. [DOI] [PubMed] [Google Scholar]
- 15.Amato Neto, V., M. H. Lopes, C. R. De Marchi, and F. Maia de Silva. 1998. An attempt to detect Trypanosoma cruzi in the peripheral blood of patients with Chagas' disease, in chronic phase, using quantitative buffy coat (QBC). Rev. Soc. Bras. Med. Trop. 31:231-233. (In Portugese.) [DOI] [PubMed] [Google Scholar]
- 16.Ancelle, T., A. Paugam, F. Bourlioux, A. Merad, and J. P. Vigier. 1997. Detection of trypanosomes in blood by the quantitative buffy coat (QBC) technique: experimental evaluation. Med. Trop. (Mars.) 57:245-248. (In French.) [PubMed] [Google Scholar]
- 17.Anema, A., and K. Ritmeijer. 2005. Treating HIV/AIDS and leishmaniasis coinfection in Ethiopia. CMAJ 172:1434-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angarano, G., P. Maggi, M. A. Rollo, A. M. B. Larocca, M. Quarto, A. Scalone, and L. Gradoni. 1998. Diffuse necrotic hepatic lesions due to visceral leishmaniasis in AIDS. J. Infect. 36:167-169. [DOI] [PubMed] [Google Scholar]
- 19.Antoniazzi, E., R. Guagliano, V. Meroni, S. Pezzotta, and P. E. Bianchi. 2008. Ocular impairment of toxoplasmosis. Parassitologia 50:35-36. [PubMed] [Google Scholar]
- 20.Antunes, A. C., F. M. Cecchini, F. B. Bolli, P. P. Oliveira, R. G. Reboucas, T. L. Monte, and D. Fricke. 2002. Cerebral trypanosomiasis and AIDS. Arq. Neuropsiquiatr. 60:730-733. [PubMed] [Google Scholar]
- 21.Antunes, F. 2004. Central nervous system AIDS-related diseases. Acta Neurochir. 146:1071-1074. [DOI] [PubMed] [Google Scholar]
- 22.Arnold, S. J., M. C. Kinney, M. S. McCormick, S. Dummer, and M. A. Scott. 1997. Disseminated toxoplasmosis. Unusual presentations in the immunocompromised host. Arch. Pathol. Lab. Med. 121:869-873. [PubMed] [Google Scholar]
- 23.Arora, S. K., S. Gupta, S. Bhardwaj, N. Sachdeva, and N. L. Sharma. 2008. An epitope-specific PCR test for diagnosis of Leishmania donovani infections. Trans. R. Soc. Trop. Med. Hyg. 102:41-45. [DOI] [PubMed] [Google Scholar]
- 24.Ashutosh, S. Sundar, and N. Goyal. 2007. Molecular mechanisms of antimony resistance in Leishmania. J. Med. Microbiol. 56:143-153. [DOI] [PubMed] [Google Scholar]
- 25.Asmuth, D. M., P. C. DeGirolami, M. Federman, C. R. Ezratty, D. K. Pleskow, G. Desai, and C. A. Wanke. 1994. Clinical features of microsporidiosis in patients with AIDS. Clin. Infect. Dis. 18:819-825. [DOI] [PubMed] [Google Scholar]
- 26.Badenoch, P. R. 1991. The pathogenesis of Acanthamoeba keratitis. Aust. N. Z. J. Ophthalmol. 19:9-20. [PubMed] [Google Scholar]
- 27.Bailey, M. S., and D. N. Lockwood. 2007. Cutaneous leishmaniasis. Clin. Dermatol. 25:203-211. [DOI] [PubMed] [Google Scholar]
- 28.Bakardjiev, A., P. H. Azimi, N. Ashouri, D. P. Ascher, D. Janner, F. L. Schuster, G. S. Visvesvara, and C. Glaser. 2003. Amebic encephalitis caused by Balamuthia mandrillaris: report of four cases. Pediatr. Infect. Dis. J. 22:447-453. [DOI] [PubMed] [Google Scholar]
- 29.Barnett, N. D., A. M. Kaplan, R. J. Hopkin, M. A. Saubolle, and M. F. Rudinsky. 1996. Primary amoebic meningoencephalitis with Naegleria fowleri: clinical review. Pediatr. Neurol. 15:230-234. [DOI] [PubMed] [Google Scholar]
- 30.Barr, B. C., P. A. Conrad, K. W. Sverlow, A. F. Tarantal, and A. G. Hendrickx. 1994. Experimental fetal and transplacental Neospora infection in the nonhuman primate. Lab. Invest. 71:236-242. [PubMed] [Google Scholar]
- 31.Barratt, J., S. Al Qassab, M. P. Reichel, and J. T. Ellis. 2008. The development and evaluation of a nested PCR assay for detection of Neospora caninum and Hammondia heydorni in feral mouse tissues. Mol. Cell. Probes 22:228-233. [DOI] [PubMed] [Google Scholar]
- 32.Barrett, M. P., R. J. Burchmore, A. Stich, J. O. Lazzari, A. C. Frasch, J. J. Cazzulo, and S. Krishna. 2003. The trypanosomiases. Lancet 362:1469-1480. [DOI] [PubMed] [Google Scholar]
- 33.Barwick, R. S., D. A. Levy, G. F. Craun, M. J. Beach, and R. L. Calderon. 2000. Surveillance for waterborne-disease outbreaks—United States, 1997-1998. MMWR CDC Surveill. Summ. 49:1-21. [PubMed] [Google Scholar]
- 34.Bauer, M. K., J. E. Harding, N. S. Bassett, B. H. Breier, M. H. Oliver, B. H. Gallaher, P. C. Evans, S. M. Woodall, and P. D. Gluckman. 1998. Fetal growth and placental function. Mol. Cell. Endocrinol. 140:115-120. [DOI] [PubMed] [Google Scholar]
- 35.Beattie, J. F., M. L. Michelson, and P. J. Holman. 2002. Acute babesiosis caused by Babesia divergens in a resident of Kentucky. N. Engl. J. Med. 347:697-698. [DOI] [PubMed] [Google Scholar]
- 36.Becker, S., J. R. Franco, P. P. Simarro, A. Stich, P. M. Abel, and D. Steverding. 2004. Real-time PCR for detection of Trypanosoma brucei in human blood samples. Diagn. Microbiol. Infect. Dis. 50:193-199. [DOI] [PubMed] [Google Scholar]
- 37.Behets, J., P. Declerck, Y. Delaedt, L. Verelst, and F. Ollevier. 2007. Survey for the presence of specific free-living amoebae in cooling waters from Belgian power plants. Parasitol. Res. 100:1249-1256. [DOI] [PubMed] [Google Scholar]
- 38.Bennett, W. M., J. F. Nespral, M. W. Rosson, and K. M. McEvoy. 2008. Use of organs for transplantation from a donor with primary meningoencephalitis due to Naegleria fowleri. Am. J. Transplant. 8:1334-1335. [DOI] [PubMed] [Google Scholar]
- 39.Benson, C. A., J. E. Kaplan, H. Masur, A. Pau, and K. K. Holmes. 2005. Treating opportunistic infections among HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association/Infectious Diseases Society of America. Clin. Infect. Dis. 40:S131-S235. [PubMed] [Google Scholar]
- 40.Benvenuti, L. A., A. Roggerio, N. V. Sambiase, A. Fiorelli, and M. de Lourdes Higuchi. 2005. Polymerase chain reaction in endomyocardial biopsies for monitoring reactivation of Chagas' disease in heart transplantation: a case report and review of the literature. Cardiovasc. Pathol. 14:265-268. [DOI] [PubMed] [Google Scholar]
- 41.Bern, C., S. P. Montgomery, B. L. Herwaldt, A. Rassi, Jr., J. A. Marin-Neto, R. O. Dantas, J. H. Maguire, H. Acquatella, C. Morillo, L. V. Kirchhoff, R. H. Gilman, P. A. Reyes, R. Salvatella, and A. C. Moore. 2007. Evaluation and treatment of Chagas disease in the United States: a systematic review. JAMA 298:2171-2181. [DOI] [PubMed] [Google Scholar]
- 42.Bern, C., M. Verastegui, R. H. Gilman, C. Lafuente, G. Galdos-Cardenas, M. Calderon, J. Pacori, M. Del Carmen Abastoflor, H. Aparicio, M. F. Brady, L. Ferrufino, N. Angulo, S. Marcus, C. Sterling, and J. H. Maguire. 2009. Congenital Trypanosoma cruzi transmission in Santa Cruz, Bolivia. Clin. Infect. Dis. 49:1667-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bialek, R., and J. Knobloch. 1999. Parasitic infections in pregnancy and congenital protozoan infections. Part I. Protozoan infections. Z. Geburtshilfe Neonatol. 203:55-62. (In German.) [PubMed] [Google Scholar]
- 44.Biolo, A., A. L. Ribeiro, and N. Clausell. 2010. Chagas cardiomyopathy—where do we stand after a hundred years? Prog. Cardiovasc. Dis. 52:300-316. [DOI] [PubMed] [Google Scholar]
- 45.Birthistle, K., P. Moore, and P. Hay. 1996. Microsporidia: a new sexually transmissable cause of urethritis. Genitourin. Med. 72:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birx, D., M. de Souza, and J. N. Nkengasong. 2009. Laboratory challenges in the scaling up of HIV, TB, and malaria programs: the interaction of health and laboratory systems, clinical research, and service delivery. Am. J. Clin. Pathol. 131:849-851. [DOI] [PubMed] [Google Scholar]
- 47.Bishop, J. R., M. Shimamura, and S. L. Hajduk. 2001. Insight into the mechanism of trypanosome lytic factor-1 killing of Trypanosoma brucei brucei. Mol. Biochem. Parasitol. 118:33-40. [DOI] [PubMed] [Google Scholar]
- 48.Blair, B., P. Sarkar, K. R. Bright, F. Marciano-Cabral, and C. P. Gerba. 2008. Naegleria fowleri in well water. Emerg. Infect. Dis. 14:1499-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blevins, S. M., R. A. Greenfield, and M. S. Bronze. 2008. Blood smear analysis in babesiosis, ehrlichiosis, relapsing fever, malaria, and Chagas disease. Cleve. Clin. J. Med. 75:521-530. [DOI] [PubMed] [Google Scholar]
- 50.Blue, D., V. Graves, L. McCarthy, J. Cruz, S. Gregurek, and D. Smith. 2009. Fatal transfusion-transmitted Babesia microti in the Midwest. Transfusion 49:8. [DOI] [PubMed] [Google Scholar]
- 51.Blum, J., and C. Burri. 2002. Treatment of late stage sleeping sickness caused by T. b. gambiense: a new approach to the use of an old drug. Swiss Med. Wkly. 132:51-56. [DOI] [PubMed] [Google Scholar]
- 52.Boehme, C. C., U. Hain, A. Novosel, S. Eichenlaub, E. Fleischmann, and T. Loscher. 2006. Congenital visceral leishmaniasis. Emerg. Infect. Dis. 12:359-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boggild, A. K., C. Miranda-Verastegui, D. Espinosa, J. Arevalo, V. Adaui, G. Tulliano, A. Llanos-Cuentas, and D. E. Low. 2007. Evaluation of a microculture method for isolation of Leishmania parasites from cutaneous lesions of patients in Peru. J. Clin. Microbiol. 45:3680-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boisseau-Garsaud, A. M., D. Cales-Quist, N. Desbois, J. Jouannelle, A. Jouannelle, F. Pratlong, and J. P. Dedet. 2000. A new case of cutaneous infection by a presumed monoxenous trypanosomatid in the island of Martinique (French West Indies). Trans. R. Soc. Trop. Med. Hyg. 94:51-52. [DOI] [PubMed] [Google Scholar]
- 55.Boldorini, R., G. Monga, A. Tosoni, E. S. Didier, M. Nebuloni, G. Costanzi, G. Mazzucco, and J. M. Orenstein. 1998. Renal Encephalitozoon (Septata) intestinalis infection in a patient with AIDS. Post-mortem identification by means of transmission electron microscopy and PCR. Virchows Arch. 432:535-539. [DOI] [PubMed] [Google Scholar]
- 56.Bonnet, S., M. Jouglin, M. L'Hostis, and A. Chauvin. 2007. Babesia sp. EU1 from roe deer and transmission within Ixodes ricinus. Emerg. Infect. Dis. 13:1208-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boonstra, E. 2002. Trypanosomiasis—a real risk for tourists visiting national parks in Tanzania. Tidsskr. Nor. Laegeforen. 122:35-37. (In Norwegian.) [PubMed] [Google Scholar]
- 58.Bosetto, M. C., and S. Giorgio. 2007. Leishmania amazonensis: multiple receptor-ligand interactions are involved in amastigote infection of human dendritic cells. Exp. Parasitol. 116:306-310. [DOI] [PubMed] [Google Scholar]
- 59.Botero, J. H., M. N. Montoya, A. L. Vanegas, A. Diaz, L. Navarro-i-Martinez, F. J. Bornay, F. Izquierdo, C. del Aguila, and P. Agudelo Sdel. 2004. Frequency of intestinal microsporidian infections in HIV-positive patients, as diagnosis by quick hot Gram chromotrope staining and PCR. Biomedica 24:375-384. (In Spanish.) [PubMed] [Google Scholar]
- 60.Bottone, E. J. 1993. Free-living amebas of the genera Acanthamoeba and Naegleria: an overview and basic microbiologic correlates. Mt. Sinai J. Med. 60:260-270. [PubMed] [Google Scholar]
- 61.Bouteille, B., O. Oukem, S. Bisser, and M. Dumas. 2003. Treatment perspectives for human African trypanosomiasis. Fundam. Clin. Pharmacol. 17:171-181. [DOI] [PubMed] [Google Scholar]
- 62.Braga, M. S., L. Lauria-Perez, E. R. Arganaraz, R. J. Nascimento, and A. R. Teixeira. 2000. Persistent infections in chronic Chagas' disease patients treated with anti-Trypanosoma cruzi nitroderivatives. Rev. Inst. Med. Trop. Sao Paulo 42:157-161. [DOI] [PubMed] [Google Scholar]
- 63.Briand, V., C. Badaut, and M. Cot. 2009. Placental malaria, maternal HIV infection and infant morbidity. Ann. Trop. Paediatr. 29:71-83. [DOI] [PubMed] [Google Scholar]
- 64.Brightbill, T. C., M. J. Post, G. T. Hensley, and A. Ruiz. 1996. MR of Toxoplasma encephalitis: signal characteristics on T2-weighted images and pathologic correlation. J. Comput. Assist. Tomogr. 20:417-422. [DOI] [PubMed] [Google Scholar]
- 65.Brouland, J.-P., J. Audouin, P. Hofman, A. Le Tourneau, D. Basset, B. Rio, R. Zittoun, and J. Diebold. 1996. Bone marrow involvement by disseminated toxoplasmosis in acquired immunodeficiency syndrome: the value of bone marrow trephine biopsy and immunohistochemistry for the diagnosis. Hum. Pathol. 27:302-306. [DOI] [PubMed] [Google Scholar]
- 66.Brown, R. L. 1991. Successful treatment of primary amebic meningoencephalitis. Arch. Intern. Med. 151:1201-1202. [PubMed] [Google Scholar]
- 67.Brun, R., and O. Balmer. 2006. New developments in human African trypanosomiasis. Curr. Opin. Infect. Dis. 19:415-420. [DOI] [PubMed] [Google Scholar]
- 68.Brun, R., J. Blum, F. Chappuis, and C. Burri. 2010. Human African trypanosomiasis. Lancet 375:148-159. [DOI] [PubMed] [Google Scholar]
- 69.Brun, R., H. Hecker, and Z. R. Lun. 1998. Trypanosoma evansi and T. equiperdum: distribution, biology, treatment and phylogenetic relationship (a review). Vet. Parasitol. 79:95-107. [DOI] [PubMed] [Google Scholar]
- 70.Brutus, L., J. A. Santalla, N. A. Salas, D. Schneider, and J. P. Chippaux. 2009. Screening for congenital infection by Trypanosoma cruzi in France. Bull. Soc. Pathol. Exot. 102:300-309. (In French.) [PubMed] [Google Scholar]
- 71.Brutus, L., D. Schneider, J. Postigo, M. Romero, J. Santalla, and J. P. Chippaux. 2008. Congenital Chagas disease: diagnostic and clinical aspects in an area without vectorial transmission, Bermejo, Bolivia. Acta Trop. 106:195-199. [DOI] [PubMed] [Google Scholar]
- 72.Buekens, P., O. Almendares, Y. Carlier, E. Dumonteil, M. Eberhard, R. Gamboa-Leon, M. James, N. Padilla, D. Wesson, and X. Xiong. 2008. Mother-to-child transmission of Chagas' disease in North America: why don't we do more? Matern. Child Health J. 12:283-286. [DOI] [PubMed] [Google Scholar]
- 73.Buffolano, W. 2008. Congenital toxoplasmosis: the state of the art. Parassitologia 50:37-43. [PubMed] [Google Scholar]
- 74.Burgos, J. M., S. B. Begher, J. M. Freitas, M. Bisio, T. Duffy, J. Altcheh, R. Teijeiro, H. Lopez Alcoba, F. Deccarlini, H. Freilij, M. J. Levin, J. Levalle, A. M. Macedo, and A. G. Schijman. 2005. Molecular diagnosis and typing of Trypanosoma cruzi populations and lineages in cerebral Chagas disease in a patient with AIDS. Am. J. Trop. Med. Hyg. 73:1016-1018. [PubMed] [Google Scholar]
- 75.Burkot, T. R., B. S. Schneider, N. J. Pieniazek, C. M. Happ, J. S. Rutherford, S. B. Slemenda, E. Hoffmeister, G. O. Maupin, and N. S. Zeidner. 2000. Babesia microti and Borrelia bissettii transmission by Ixodes spinipalpis ticks among prairie voles, Microtus ochrogaster, in Colorado. Parasitology 121(Pt. 6):595-599. [PubMed] [Google Scholar]
- 76.Burri, C., and R. Brun. 2003. Eflornithine for the treatment of human African trypanosomiasis. Parasitol. Res. 90(Suppl. 1):S49-S52. [DOI] [PubMed] [Google Scholar]
- 77.Bush, J. B., M. Isaacson, A. S. Mohamed, F. T. Potgieter, and D. T. de Waal. 1990. Human babesiosis—a preliminary report of 2 suspected cases in South Africa. S. Afr. Med. J. 7:699. [PubMed] [Google Scholar]
- 78.Butler, T. K., J. J. Males, L. P. Robinson, A. W. Wechsler, G. L. Sutton, J. Cheng, P. Taylor, and K. McClellan. 2005. Six-year review of Acanthamoeba keratitis in New South Wales, Australia: 1997-2002. Clin. Exp. Ophthalmol. 33:41-46. [DOI] [PubMed] [Google Scholar]
- 79.Caballero, Z. C., O. E. Sousa, W. P. Marques, A. Saez-Alquezar, and E. S. Umezawa. 2007. Evaluation of serological tests to identify Trypanosoma cruzi infection in humans and determine cross-reactivity with Trypanosoma rangeli and Leishmania spp. Clin. Vaccine Immunol. 14:1045-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cabanes, P. A., F. Wallet, E. Pringuez, and P. Pernin. 2001. Assessing the risk of primary amoebic meningoencephalitis from swimming in the presence of environmental Naegleria fowleri. Appl. Environ. Microbiol. 67:2927-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cali, A., P. M. Takvorian, S. Lewin, M. Rendel, C. S. Sian, M. Wittner, H. B. Tanowitz, E. Keohane, and L. M. Weiss. 1998. Brachiola vesicularum, n. g., n. sp., a new microsporidium associated with AIDS and myositis. J. Eukaryot. Microbiol. 45:240-251. [DOI] [PubMed] [Google Scholar]
- 82.Cali, A., L. M. Weiss, and P. M. Takvorian. 2004. An analysis of the microsporidian genus Brachiola, with comparisons of human and insect isolates of Brachiola algerae. J. Eukaryot. Microbiol. 51:678-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Calza, L., A. D'Antuono, G. Marinacci, R. Manfredi, V. Colangeli, B. Passarini, R. Orioli, O. Varoli, and F. Chiodo. 2004. Disseminated cutaneous leishmaniasis after visceral disease in a patient with AIDS. J. Am. Acad. Dermatol. 50:461-465. [DOI] [PubMed] [Google Scholar]
- 84.Campos, S. V., T. M. Strabelli, V. Amato Neto, C. P. Silva, F. Bacal, E. A. Bocchi, and N. A. Stolf. 2008. Risk factors for Chagas' disease reactivation after heart transplantation. J. Heart Lung Transplant. 27:597-602. [DOI] [PubMed] [Google Scholar]
- 85.Canfield, P. J., L. Vogelnest, M. I. Cunningham, and G. S. Visvesvara. 1997. Amoebic meningoencephalitis caused by Balamuthia mandrillaris in an orangutan. Aust. Vet. J. 75:97-100. [DOI] [PubMed] [Google Scholar]
- 86.Cangelosi, J. J., B. Sarvat, J. C. Sarria, B. L. Herwaldt, and A. J. Indrikovs. 2008. Transmission of Babesia microti by blood transfusion in Texas. Vox Sang. 95:331-334. [DOI] [PubMed] [Google Scholar]
- 87.Carod-Artal, F. J. 2007. Stroke: a neglected complication of American trypanosomiasis (Chagas' disease). Trans. R. Soc. Trop. Med. Hyg. 101:1075-1080. [DOI] [PubMed] [Google Scholar]
- 88.Carter, R. F. 1969. Sensitivity to amphotericin B of a Naegleria sp. isolated from a case of primary amoebic meningoencephalitis. J. Clin. Pathol. 22:470-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caruzo, G., and J. Cardozo. 2008. Primary amoebic meningoencephalitis: a new case from Venezuela. Trop. Doct. 38:256-257. [DOI] [PubMed] [Google Scholar]
- 90.Casper, T., D. Basset, C. Leclercq, J. Fabre, N. Peyron-Raison, and J. Reynes. 1999. Disseminated acanthamoeba infection in a patient with AIDS: response to 5-fluorocytosine therapy. Clin. Infect. Dis. 29:944-945. [DOI] [PubMed] [Google Scholar]
- 91.Castilho, T. M., L. M. Camargo, D. McMahon-Pratt, J. J. Shaw, and L. M. Floeter-Winter. 2008. A real-time polymerase chain reaction assay for the identification and quantification of American Leishmania species on the basis of glucose-6-phosphate dehydrogenase. Am. J. Trop. Med. Hyg. 78:122-132. [PubMed] [Google Scholar]
- 92.Castro, E. 2009. Chagas' disease: lessons from routine donation testing. Transfus. Med. 19:16-23. [DOI] [PubMed] [Google Scholar]
- 93.Cattand, P. 2001. The epidemiology of human African trypanosomiasis: a complex multifactorial history. Med. Trop. (Mars.) 61:313-322. (In French.) [PubMed] [Google Scholar]
- 94.CDC. 2008. Balamuthia amebic encephalitis—California, 1999-2007. MMWR Morb. Mortal. Wkly. Rep. 57:768-771. [PubMed] [Google Scholar]
- 95.CDC. 2003. Primary amebic meningoencephalitis—Georgia, 2002. MMWR Morb. Mortal. Wkly. Rep. 52:962-964. [PubMed] [Google Scholar]
- 96.Cerva, L. 1980. Naegleria fowleri: trimethoprim sensitivity. Science 209:1541. [DOI] [PubMed] [Google Scholar]
- 97.Cervantes-Sandoval, I., J. J. de Serrano-Luna, J. L. Tapia-Malagon, J. Pacheco-Yepez, A. Silva-Olivares, S. Galindo-Gomez, V. Tsutsumi, and M. Shibayama. 2007. Characterization of Naegleria fowleri strains isolated from human cases of primary amoebic meningoencephalitis in Mexico. Rev. Invest. Clin. 59:342-347. [PubMed] [Google Scholar]
- 98.Chan, K. S., and T. H. Koh. 2008. Extraction of microsporidial DNA from modified trichrome-stained clinical slides and subsequent species identification using PCR sequencing. Parasitology 135:701-703. [DOI] [PubMed] [Google Scholar]
- 99.Chandler, F. W., and J. C. Watts. 1988. Immunofluorescence as an adjunct to the histopathologic diagnosis of Chagas' disease. J. Clin. Microbiol. 26:567-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chappuis, F., L. Loutan, P. Simarro, V. Lejon, and P. Buscher. 2005. Options for field diagnosis of human African trypanosomiasis. Clin. Microbiol. Rev. 18:133-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chappuis, F., E. Stivanello, K. Adams, S. Kidane, A. Pittet, and P. A. Bovier. 2004. Card agglutination test for trypanosomiasis (CATT) end-dilution titer and cerebrospinal fluid cell count as predictors of human African trypanosomiasis (Trypanosoma brucei gambiense) among serologically suspected individuals in southern Sudan. Am. J. Trop. Med. Hyg. 71:313-317. [PubMed] [Google Scholar]
- 102.Chatel, G., M. Gulletta, A. Matteelli, A. Marangoni, L. Signorini, O. Oladeji, and S. Caligaris. 1999. Diagnosis of tick-borne relapsing fever by the quantitative buffy coat fluorescence method. Am. J. Trop. Med. Hyg. 60:738-739. [DOI] [PubMed] [Google Scholar]
- 103.Checchi, F., J. A. Filipe, M. P. Barrett, and D. Chandramohan. 2008. The natural progression of gambiense sleeping sickness: what is the evidence? PLoS Negl. Trop. Dis. 2:e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chehter, E. Z., M. A. Longo, A. A. Laudanna, and M. I. Duarte. 2001. Pancreatic involvement in co-infection visceral leishmaniasis and HIV: histological and ultrastructural aspects. Rev. Inst. Med. Trop. Sao Paulo 43:75-78. [DOI] [PubMed] [Google Scholar]
- 105.Chimelli, L., and F. Scaravilli. 1997. Trypanosomiasis. Brain Pathol. 7:599-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cichocka, A., and B. Skotarczak. 2001. Babesosis—difficulty of diagnosis. Wiad. Parazytol. 47:527-533. (In Polish.) [PubMed] [Google Scholar]
- 107.Cieniuch, S., J. Stanczak, and A. Ruczaj. 2009. The first detection of Babesia EU1 and Babesia canis canis in Ixodes ricinus ticks (Acari, Ixodidae) collected in urban and rural areas in northern Poland. Pol. J. Microbiol. 58:231-236. [PubMed] [Google Scholar]
- 108.Cingolani, A., A. De Luca, A. Ammassari, R. Murri, A. Linzalone, R. Grillo, and A. Antinori. 1996. PCR detection of Toxoplasma gondii DNA in CSF for the differential diagnosis of AIDS-related focal brain lesions. J. Med. Microbiol. 45:472-476. [DOI] [PubMed] [Google Scholar]
- 109.Clark, T., and L. Fitzgerald. 2007. Malaria, tuberculosis, and HIV/AIDS. J. Midwifery Womens Health 52:e33-e35. [DOI] [PubMed] [Google Scholar]
- 110.Clavel, A., L. Franco, S. Letona, J. Cuesta, A. Barbera, M. Varea, J. Quilez, F. Javier Castillo, and R. Gomez-Lus. 1996. Primary amebic meningoencephalitis in a patient with AIDS: unusual protozoological findings. Clin. Infect. Dis. 23:1314-1315. [DOI] [PubMed] [Google Scholar]
- 111.Cogo, P. E., M. Scagli, S. Gatti, F. Rossetti, R. Alaggio, A. M. Laverda, L. Zhou, L. Xiao, and G. S. Visvesvara. 2004. Fatal Naegleria fowleri meningoencephalitis, Italy. Emerg. Infect. Dis. 10:1835-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Commodaro, A. G., R. N. Belfort, L. V. Rizzo, C. Muccioli, C. Silveira, M. N. Burnier, Jr., and R. Belfort, Jr. 2009. Ocular toxoplasmosis: an update and review of the literature. Mem. Inst. Oswaldo Cruz 104:345-350. [DOI] [PubMed] [Google Scholar]
- 113.Concetti, H., M. Retegui, G. Perez, and H. Perez. 2000. Chagas' disease of the cervix uteri in a patient with acquired immunodeficiency syndrome. Hum. Pathol. 31:120-122. [DOI] [PubMed] [Google Scholar]
- 114.Conrad, P. A., A. M. Kjemtrup, R. A. Carreno, J. Thomford, K. Wainwright, M. Eberhard, R. Quick, S. R. Telford III, and B. L. Herwaldt. 2006. Description of Babesia duncani n. sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int. J. Parasitol. 36:779-789. [DOI] [PubMed] [Google Scholar]
- 115.Conteas, C. N., E. S. Didier, and O. G. Berlin. 1997. Workup of gastrointestinal microsporidiosis. Dig. Dis. 15:330-345. [DOI] [PubMed] [Google Scholar]
- 116.Contini, C. 2008. Clinical and diagnostic management of toxoplasmosis in the immunocompromised patient. Parassitologia 50:45-50. [PubMed] [Google Scholar]
- 117.Cooter, R. 2002. The history of the discovery of primary amoebic meningoencephalitis. Aust. Fam. Physician 31:399-400. [PubMed] [Google Scholar]
- 118.Cordova, E., A. Boschi, J. Ambrosioni, C. Cudos, and M. Corti. 2008. Reactivation of Chagas disease with central nervous system involvement in HIV-infected patients in Argentina, 1992-2007. Int. J. Infect. Dis. 12:587-592. [DOI] [PubMed] [Google Scholar]
- 119.Coronado, O., A. Mastroianni, P. Scarani, R. Manfredi, and F. Chiodo. 1995. Toxoplasmic pancreatitis in a patient with AIDS. Med. Mal. Infect. 25:1014-1016. (In French.) [Google Scholar]
- 120.Cortes, P., N. Cardenosa, J. Romani, M. Gallego, C. Munoz, J. L. Barrio, C. Riera, and M. Portus. 1997. Oral leishmaniasis in an HIV-positive patient caused by two different zymodemes of Leishmania infantum. Trans. R. Soc. Trop. Med. Hyg. 91:438-439. [DOI] [PubMed] [Google Scholar]
- 121.Corti, M. 2000. AIDS and Chagas' disease. AIDS Patient Care STDs 14:581-588. [DOI] [PubMed] [Google Scholar]
- 122.Costa, J. W., Jr., D. A. Milner, Jr., and J. H. Maguire. 2003. Mucocutaneous leishmaniasis in a US citizen. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 96:573-577. [DOI] [PubMed] [Google Scholar]
- 123.Coura, J. R., A. C. Junqueira, O. Fernandes, S. A. Valente, and M. A. Miles. 2002. Emerging Chagas disease in Amazonian Brazil. Trends Parasitol. 18:171-176. [DOI] [PubMed] [Google Scholar]
- 124.Coustou, V., F. Guegan, N. Plazolles, and T. Baltz. 2010. Complete in vitro life cycle of Trypanosoma congolense: development of genetic tools. PLoS Negl. Trop. Dis. 4:e618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Croft, S. L., S. Sundar, and A. H. Fairlamb. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:111-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cruz, I., C. Canavate, J. M. Rubio, M. A. Morales, C. Chicharro, F. Laguna, M. Jimenez-Mejias, G. Sirera, S. Videla, and J. Alvar. 2002. A nested polymerase chain reaction (Ln-PCR) for diagnosing and monitoring Leishmania infantum infection in patients co-infected with human immunodeficiency virus. Trans. R. Soc. Trop. Med. Hyg. 96:S185-S189. [DOI] [PubMed] [Google Scholar]
- 127.Cruz-Lopez, L., E. A. Malo, J. C. Rojas, and E. D. Morgan. 2001. Chemical ecology of triatomine bugs: vectors of Chagas disease. Med. Vet. Entomol. 15:351-357. [DOI] [PubMed] [Google Scholar]
- 128.Cubero-Menendez, O., and D. Cubero-Rego. 2004. Primary amoebic meningoencephalitis: a case report. Rev. Neurol. (Paris) 38:336-338. (In French.) [PubMed] [Google Scholar]
- 129.Cuevas, P. M., P. G. Smoje, M. L. Jofre, D. W. Ledermann, H. I. Noemi, C. F. Berwart, L. J. Latorre, and B. S. Gonzalez. 2006. Granulomatous amoebic meningoencephalitis by Balamuthia mandrillaris: case report and literature review. Rev. Chilena Infectol. 23:237-242. (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 130.Culha, G., S. Uzun, K. Ozcan, H. R. Memisoglu, and K. P. Chang. 2006. Comparison of conventional and polymerase chain reaction diagnostic techniques for leishmaniasis in the endemic region of Adana, Turkey. Int. J. Dermatol. 45:569-572. [DOI] [PubMed] [Google Scholar]
- 131.Cunha-Neto, E., A. M. Bilate, K. V. Hyland, S. G. Fonseca, J. Kalil, and D. M. Engman. 2006. Induction of cardiac autoimmunity in Chagas heart disease: a case for molecular mimicry. Autoimmunity 39:41-54. [DOI] [PubMed] [Google Scholar]
- 132.Curry, A., N. J. Beeching, J. D. Gilbert, G. Scott, P. L. Rowland, and B. J. Currie. 2005. Trachipleistophora hominis infection in the myocardium and skeletal muscle of a patient with AIDS. J. Infect. 51:e139-e144. [DOI] [PubMed] [Google Scholar]
- 133.Curry, A., H. S. Mudhar, S. Dewan, E. U. Canning, and B. E. Wagner. 2007. A case of bilateral microsporidial keratitis from Bangladesh—infection by an insect parasite from the genus Nosema. J. Med. Microbiol. 56:1250-1252. [DOI] [PubMed] [Google Scholar]
- 134.Dabritz, H. A., and P. A. Conrad. 2010. Cats and toxoplasma: implications for public health. Zoonoses Public Health 57:34-52. [DOI] [PubMed] [Google Scholar]
- 135.Da-Cruz, A. M., D. V. Filgueiras, Z. Coutinho, W. Mayrink, G. Grimaldi, Jr., P. M. De Luca, S. C. Mendonca, and S. G. Coutinho. 1999. Atypical mucocutaneous leishmaniasis caused by Leishmania braziliensis in an acquired immunodeficiency syndrome patient: T-cell responses and remission of lesions associated with antigen immunotherapy. Mem. Inst. Oswaldo Cruz 94:537-542. [DOI] [PubMed] [Google Scholar]
- 136.Daft, B. M., G. S. Visvesvara, D. H. Read, H. Kinde, F. A. Uzal, and M. D. Manzer. 2005. Seasonal meningoencephalitis in Holstein cattle caused by Naegleria fowleri. J. Vet. Diagn. Invest. 17:605-609. [DOI] [PubMed] [Google Scholar]
- 137.Deborggraeve, S., M. Boelaert, S. Rijal, S. De Doncker, J. C. Dujardin, P. Herdewijn, and P. Buscher. 2008. Diagnostic accuracy of a new Leishmania PCR for clinical visceral leishmaniasis in Nepal and its role in diagnosis of disease. Trop. Med. Int. Health 13:1378-1383. [DOI] [PubMed] [Google Scholar]
- 138.Deborggraeve, S., M. Koffi, V. Jamonneau, F. A. Bonsu, R. Queyson, P. P. Simarro, P. Herdewijn, and P. Buscher. 2008. Molecular analysis of archived blood slides reveals an atypical human Trypanosoma infection. Diagn. Microbiol. Infect. Dis. 61:428-433. [DOI] [PubMed] [Google Scholar]
- 139.de Carvalho, V. B., E. F. Sousa, J. H. Vila, J. P. da Silva, M. R. Caiado, S. R. Araujo, R. Macruz, and E. J. Zerbini. 1996. Heart transplantation in Chagas' disease. 10 years after the initial experience. Circulation 94:1815-1817. [DOI] [PubMed] [Google Scholar]
- 140.Dedet, J. P. 1996. Parasitism of HIV-infected patients by insect flagellates. Lancet 347:1409. [DOI] [PubMed] [Google Scholar]
- 141.Dedet, J. P., and F. Pratlong. 2000. Leishmania, Trypanosoma and monoxenous trypanosomatids as emerging opportunistic agents. J. Eukaryot. Microbiol. 47:37-39. [DOI] [PubMed] [Google Scholar]
- 142.Dedet, J. P., B. Roche, F. Pratlong, D. Cales-Quist, J. Jouannelle, J. C. Benichou, and M. Huerre. 1995. Diffuse cutaneous infection caused by a presumed monoxenous trypanosomatid in a patient infected with HIV. Trans. R. Soc. Trop. Med. Hyg. 89:644-646. [DOI] [PubMed] [Google Scholar]
- 143.De Doncker, S., V. Hutse, S. Abdellati, S. Rijal, B. M. Singh Karki, S. Decuypere, D. Jacquet, D. Le Ray, M. Boelaert, S. Koirala, and J. C. Dujardin. 2005. A new PCR-ELISA for diagnosis of visceral leishmaniasis in blood of HIV-negative subjects. Trans. R. Soc. Trop. Med. Hyg. 99:25-31. [DOI] [PubMed] [Google Scholar]
- 144.Deetz, T. R., M. H. Sawyer, G. Billman, F. L. Schuster, and G. S. Visvesvara. 2003. Successful treatment of Balamuthia amoebic encephalitis: presentation of 2 cases. Clin. Infect. Dis. 37:1304-1312. [DOI] [PubMed] [Google Scholar]
- 145.de Fatima Arruda Pereira, E., V. Thomaz-Soccol, H. C. Lima, A. Thomaz-Soccol, E. A. de Castro, F. Mulinari-Brenner, F. Queiroz-Telles, and E. Luz. 2008. Molecular diagnosis of leishmaniosis in the Parana state of southern Brazil. Exp. Dermatol. 17:1024-1030. [DOI] [PubMed] [Google Scholar]
- 146.De Jonckheere, J. F. 2006. Isolation and molecular identification of free-living amoebae of the genus Naegleria from Arctic and sub-Antarctic regions. Eur. J. Protistol. 42:115-123. [DOI] [PubMed] [Google Scholar]
- 147.de La Rosa, R., J. A. Pineda, J. Delgado, J. Macias, F. Morillas, J. A. Mira, A. Sanchez-Quijano, M. Leal, and E. Lissen. 2002. Incidence of and risk factors for symptomatic visceral leishmaniasis among human immunodeficiency virus type 1-infected patients from Spain in the era of highly active antiretroviral therapy. J. Clin. Microbiol. 40:762-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Delbac, F., and C. Vivares. 1997. A PCR technique for detecting and differentiating known microsporidia in AIDS patients. J. Eukaryot. Microbiol. 44:75S. [DOI] [PubMed] [Google Scholar]
- 149.Delgado, J., J. Macias, J. A. Pineda, J. E. Corzo, M. P. Gonzalez-Moreno, R. de la Rosa, A. Sanchez-Quijano, M. Leal, and E. Lissen. 1999. High frequency of serious side effects from meglumine antimoniate given without an upper limit dose for the treatment of visceral leishmaniasis in human immunodeficiency virus type-1-infected patients. Am. J. Trop. Med. Hyg. 61:766-769. [DOI] [PubMed] [Google Scholar]
- 150.Delgado, O., S. Silva, V. Coraspe, M. A. Rivas, A. J. Rodriguez-Morales, P. Navarro, and C. Franco-Paredes. 2008. Cutaneous leishmaniasis imported from Colombia to Northcentral Venezuela: implications for travel advice. Travel Med. Infect. Dis. 6:376-379. [DOI] [PubMed] [Google Scholar]
- 151.del Giudice, P., M. Mary-Krause, C. Pradier, S. Grabar, P. Dellamonica, P. Marty, J. A. Gastaut, D. Costagliola, and E. Rosenthal. 2002. Impact of highly active antiretroviral therapy on the incidence of visceral leishmaniasis in a French cohort of patients infected with human immunodeficiency virus. J. Infect. Dis. 186:1366-1370. [DOI] [PubMed] [Google Scholar]
- 152.Denney, C. F., V. J. Iragui, L. D. Uber-Zak, N. C. Karpinski, E. J. Ziegler, G. S. Visvesvara, and S. L. Reed. 1997. Amebic meningoencephalitis caused by Balamuthia mandrillaris: case report and review. Clin. Infect. Dis. 25:1354-1358. [DOI] [PubMed] [Google Scholar]
- 153.Deol, I., L. Robledo, A. Meza, G. S. Visvesvara, and R. J. Andrews. 2000. Encephalitis due to a free-living amoeba (Balamuthia mandrillaris): case report with literature review. Surg. Neurol. 53:611-616. [DOI] [PubMed] [Google Scholar]
- 154.de Paiva Cavalcanti, M., M. E. Felinto de Brito, W. V. de Souza, Y. de Miranda Gomes, and F. G. Abath. 2009. The development of a real-time PCR assay for the quantification of Leishmania infantum DNA in canine blood. Vet. J. 182:356-358. [DOI] [PubMed] [Google Scholar]
- 155.Dereure, J., H. D. Thanh, T. Lavabre-Bertrand, G. Cartron, F. Bastides, D. Richard-Lenoble, and J. P. Dedet. 2003. Visceral leishmaniasis. Persistence of parasites in lymph nodes after clinical cure. J. Infect. 47:77-81. [DOI] [PubMed] [Google Scholar]
- 156.Derouin, F., and H. Pelloux. 2008. Prevention of toxoplasmosis in transplant patients. Clin. Microbiol. Infect. 14:1089-1101. [DOI] [PubMed] [Google Scholar]
- 157.Derouin, F., and M. Santillana-Hayat. 2000. Anti-Toxoplasma activities of antiretroviral drugs and interactions with pyrimethamine and sulfadiazine in vitro. Antimicrob. Agents Chemother. 44:2575-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Desjeux, P. 1999. Global control and Leishmania HIV co-infection. Clin. Dermatol. 17:317-325. [DOI] [PubMed] [Google Scholar]
- 159.Desjeux, P., and J. Alvar. 2003. Leishmania/HIV co-infections: epidemiology in Europe. Ann. Trop. Med. Parasitol. 97(Suppl. 1):3-15. [DOI] [PubMed] [Google Scholar]
- 160.Diaz, J. H. 2008. Recognizing and reducing the risks of Chagas disease (American trypanosomiasis) in travelers. J. Travel Med. 15:184-195. [DOI] [PubMed] [Google Scholar]
- 161.Di Carlo, P., A. Romano, M. G. Schimmenti, A. Mazzola, and L. Titone. 2008. Materno-fetal Toxoplasma gondii infection: critical review of available diagnostic methods. Infez. Med. 16:28-32. (In Italian.) [PubMed] [Google Scholar]
- 162.Didier, E. S. 1997. Effects of albendazole, fumagillin, and TNP-470 on microsporidial replication in vitro. Antimicrob. Agents Chemother. 41:1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Didier, E. S. 2005. Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop. 94:61-76. [DOI] [PubMed] [Google Scholar]
- 164.Didier, E. S., J. A. Maddry, P. J. Brindley, M. E. Stovall, and P. J. Didier. 2005. Therapeutic strategies for human microsporidia infections. Expert Rev. Anti Infect. Ther. 3:419-434. [DOI] [PubMed] [Google Scholar]
- 165.Didier, E. S., J. M. Orenstein, A. Aldras, D. Bertucci, L. B. Rogers, and F. A. Janney. 1995. Comparison of three staining methods for detecting microsporidia in fluids. J. Clin. Microbiol. 33:3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Didier, E. S., L. B. Rogers, A. D. Brush, S. Wong, V. Traina-Dorge, and D. Bertucci. 1996. Diagnosis of disseminated microsporidian Encephalitozoon hellem infection by PCR-Southern analysis and successful treatment with albendazole and fumagillin. J. Clin. Microbiol. 34:947-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Didier, E. S., L. B. Rogers, J. M. Orenstein, M. D. Baker, C. R. Vossbrinck, T. Van Gool, R. Hartskeerl, R. Soave, and L. M. Beaudet. 1996. Characterization of Encephalitozoon (Septata) intestinalis isolates cultured from nasal mucosa and bronchoalveolar lavage fluids of two AIDS patients. J. Eukaryot. Microbiol. 43:34-43. [DOI] [PubMed] [Google Scholar]
- 168.Didier, E. S., J. A. Shadduck, P. J. Didier, N. Millichamp, and C. R. Vossbrinck. 1991. Studies on ocular microsporidia. J. Protozool. 38:635-638. [PubMed] [Google Scholar]
- 169.Didier, E. S., M. E. Stovall, L. C. Green, P. J. Brindley, K. Sestak, and P. J. Didier. 2004. Epidemiology of microsporidiosis: sources and modes of transmission. Vet. Parasitol. 126:145-166. [DOI] [PubMed] [Google Scholar]
- 170.Diehl, A. R., R. P. Dos Santos, R. Zimmerman, L. P. Luz, T. Weiss, P. Jacobson, and L. Z. Goldani. 2004. Microscopy and polymerase chain reaction detection of Leishmania chagasi in the pleural and ascitic fluid of a patient with AIDS: case report and review of diagnosis and therapy of visceral leishmaniasis. Can. J. Infect. Dis. Med. Microbiol. 15:231-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Diez, M., L. Favaloro, A. Bertolotti, J. M. Burgos, C. Vigliano, M. P. Lastra, M. J. Levin, A. Arnedo, C. Nagel, A. G. Schijman, and R. R. Favaloro. 2007. Usefulness of PCR strategies for early diagnosis of Chagas' disease reactivation and treatment follow-up in heart transplantation. Am. J. Transplant. 7:1633-1640. [DOI] [PubMed] [Google Scholar]
- 172.Di Gregorio, C., F. Rivasi, N. Mongiardo, B. De Rienzo, S. Wallace, and G. S. Visvesvara. 1992. Acanthamoeba meningoencephalitis in a patient with acquired immunodeficiency syndrome. Arch. Pathol. Lab. Med. 116:1363-1365. [PubMed] [Google Scholar]
- 173.Di Lella, F., V. Vincenti, D. Zennaro, A. Afeltra, A. Baldi, D. Giordano, E. Pasanisi, A. Bacciu, S. Bacciu, and G. Di Lella. 2006. Mucocutaneous leishmaniasis: report of a case with massive involvement of nasal, pharyngeal and laryngeal mucosa. Int. J. Oral Maxillofac. Surg. 35:870-872. [DOI] [PubMed] [Google Scholar]
- 174.Di Lorenzo, G. A., M. A. Pagano, A. L. Taratuto, M. L. Garau, F. J. Meli, and M. D. Pomsztein. 1996. Chagasic granulomatous encephalitis in immunosuppressed patients. Computed tomography and magnetic resonance imaging findings. J. Neuroimaging 6:94-97. [DOI] [PubMed] [Google Scholar]
- 175.Dogan, N., S. Kabukcuoglu, and E. Vardareli. 2007. Toxoplasmic hepatitis in an immunocompetent patient. Turkiye Parazitol. Derg. 31:260-263. (In Turkish.) [PubMed] [Google Scholar]
- 176.Duarte, A. G., F. Sattar, B. Granwehr, J. F. Aronson, Z. Wang, and S. Lick. 2006. Disseminated acanthamoebiasis after lung transplantation. J. Heart Lung Transplant. 25:237-240. [DOI] [PubMed] [Google Scholar]
- 177.Duband, S., J. Cornillon, E. Tavernier, J. M. Dumollard, D. Guyotat, and M. Peoc'h. 2008. Toxoplasmosis with hemophagocytic syndrome after bone marrow transplantation: diagnosis at autopsy. Transpl. Infect. Dis. 10:372-374. [DOI] [PubMed] [Google Scholar]
- 178.Dubey, J. P. 2009. History of the discovery of the life cycle of Toxoplasma gondii. Int. J. Parasitol. 39:877-882. [DOI] [PubMed] [Google Scholar]
- 179.Dubey, J. P. 2008. The history of Toxoplasma gondii—the first 100 years. J. Eukaryot. Microbiol. 55:467-475. [DOI] [PubMed] [Google Scholar]
- 180.Dubey, J. P. 2010. Toxoplasma gondii infections in chickens (Gallus domesticus): prevalence, clinical disease, diagnosis and public health significance. Zoonoses Public Health 57:60-73. [DOI] [PubMed] [Google Scholar]
- 181.Dubey, J. P., B. C. Barr, J. R. Barta, I. Bjerkas, C. Bjorkman, B. L. Blagburn, D. D. Bowman, D. Buxton, J. T. Ellis, B. Gottstein, A. Hemphill, D. E. Hill, D. K. Howe, M. C. Jenkins, Y. Kobayashi, B. Koudela, A. E. Marsh, J. G. Mattsson, M. M. McAllister, D. Modry, Y. Omata, L. D. Sibley, C. A. Speer, A. J. Trees, A. Uggla, S. J. Upton, D. J. Williams, and D. S. Lindsay. 2002. Redescription of Neospora caninum and its differentiation from related coccidia. Int. J. Parasitol. 32:929-946. [DOI] [PubMed] [Google Scholar]
- 182.Duke, B. J., R. W. Tyson, R. DeBiasi, J. E. Freeman, and K. R. Winston. 1997. Balamuthia mandrillaris meningoencephalitis presenting with acute hydrocephalus. Pediatr. Neurosurg. 26:107-111. [DOI] [PubMed] [Google Scholar]
- 183.Dumas, M. 2000. Sleeping sickness, a reemerging sickness. Bull. Acad. Natl. Med. 184:1867-1882; discussion 1882-1885. (In French.) [PubMed] [Google Scholar]
- 184.Dumas, M., and B. Bouteille. 1996. Human African trypanosomiasis. C. R. Seances Soc. Biol. Fil. 190:395-408. (In French.) [PubMed] [Google Scholar]
- 185.Dunand, V. A., S. M. Hammer, R. Rossi, M. Poulin, M. A. Albrecht, J. P. Doweiko, P. C. DeGirolami, E. Coakley, E. Piessens, and C. A. Wanke. 1997. Parasitic sinusitis and otitis in patients infected with human immunodeficiency virus: report of five cases and review. Clin. Infect. Dis. 25:267-272. [DOI] [PubMed] [Google Scholar]
- 186.Dunnebacke, T. H., F. L. Schuster, S. Yagi, and G. C. Booton. 2004. Balamuthia mandrillaris from soil samples. Microbiology 150:2837-2842. [DOI] [PubMed] [Google Scholar]
- 187.Dvorakova, H. M., and M. Dvorackova. 2007. Babesiosis, a little known zoonosis. Epidemiol. Mikrobiol. Imunol. 56:176-180. (In Czech.) [PubMed] [Google Scholar]
- 188.Eida, O. M., M. M. Eida, and A. B. Ahmed. 2009. Evaluation of polymerase chain reaction on amniotic fluid for diagnosis of congenital toxoplasmosis. J. Egypt. Soc. Parasitol. 39:541-550. [PubMed] [Google Scholar]
- 189.Elamin, E. M., S. Guerbouj, A. M. Musa, I. Guizani, E. A. Khalil, M. M. Mukhtar, A. M. Elkadaro, H. S. Mohamed, M. E. Ibrahim, M. M. Abdel Hamid, M. El Azhari, and A. M. El Hassan. 2005. Uncommon clinical presentations of cutaneous leishmaniasis in Sudan. Trans. R. Soc. Trop. Med. Hyg. 99:803-808. [DOI] [PubMed] [Google Scholar]
- 190.Elamin, E. M., I. Guizani, S. Guerbouj, M. Gramiccia, A. M. El Hassan, T. Di Muccio, M. A. Taha, and M. M. Mukhtar. 2008. Identification of Leishmania donovani as a cause of cutaneous leishmaniasis in Sudan. Trans. R. Soc. Trop. Med. Hyg. 102:54-57. [DOI] [PubMed] [Google Scholar]
- 191.El-Bahnasawy, M. M., and T. A. Morsy. 2008. Egyptian human babesiosis and general review. J. Egypt. Soc. Parasitol. 38:265-272. [PubMed] [Google Scholar]
- 192.Elbez-Rubinstein, A., D. Ajzenberg, M. L. Darde, R. Cohen, A. Dumetre, H. Yera, E. Gondon, J. C. Janaud, and P. Thulliez. 2009. Congenital toxoplasmosis and reinfection during pregnancy: case report, strain characterization, experimental model of reinfection, and review. J. Infect. Dis. 199:280-285. [DOI] [PubMed] [Google Scholar]
- 193.El Ghouzzi, M. H., E. Boiret, F. Wind, C. Brochard, S. Fittere, L. Paris, D. Mazier, N. Sansonetti, and P. Bierling. 2010. Testing blood donors for Chagas disease in the Paris area, France: first results after 18 months of screening. Transfusion 50:575-583. [DOI] [PubMed] [Google Scholar]
- 194.Elrayah, I. E., M. A. Rhaman, L. T. Karamalla, K. M. Khalil, and P. Buscher. 2007. Evaluation of serodiagnostic tests for T. b. gambiense human African trypanosomiasis in southern Sudan. East. Mediterr. Health J. 13:1098-1107. [DOI] [PubMed] [Google Scholar]
- 195.Elsheikha, H. M. 2008. Congenital toxoplasmosis: priorities for further health promotion action. Public Health 122:335-353. [DOI] [PubMed] [Google Scholar]
- 196.Enanga, B., R. J. Burchmore, M. L. Stewart, and M. P. Barrett. 2002. Sleeping sickness and the brain. Cell. Mol. Life Sci. 59:845-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Endeshaw, T., A. Kebede, J. J. Verweij, A. Zewide, K. Tsige, Y. Abraham, D. Wolday, T. Woldemichael, T. Messele, A. M. Polderman, and B. Petros. 2006. Intestinal microsporidiosis in diarrheal patients infected with human immunodeficiency virus-1 in Addis Ababa, Ethiopia. Jpn. J. Infect. Dis. 59:306-310. [PubMed] [Google Scholar]
- 198.Falagas, M. E., and M. S. Klempner. 1996. Babesiosis in patients with AIDS: a chronic infection presenting as fever of unknown origin. Clin. Infect. Dis. 22:809-812. [DOI] [PubMed] [Google Scholar]
- 199.Fast, N. M., J. M. Logsdon, Jr., and W. F. Doolittle. 1999. Phylogenetic analysis of the TATA box binding protein (TBP) gene from Nosema locustae: evidence for a microsporidia-fungi relationship and spliceosomal intron loss. Mol. Biol. Evol. 16:1415-1419. [DOI] [PubMed] [Google Scholar]
- 200.Fedorko, D. P., N. A. Nelson, E. S. Didier, D. Bertucci, R. M. Delgado, and A. M. Hruszkewycz. 2001. Speciation of human microsporidia by polymerase chain reaction single-strand conformation polymorphism. Am. J. Trop. Med. Hyg. 65:397-401. [DOI] [PubMed] [Google Scholar]
- 201.Ferreira, M. S., A. Nishioka Sde, M. T. Silvestre, A. S. Borges, F. R. Nunes-Araujo, and A. Rocha. 1997. Reactivation of Chagas' disease in patients with AIDS: report of three new cases and review of the literature. Clin. Infect. Dis. 25:1397-1400. [DOI] [PubMed] [Google Scholar]
- 202.Fevre, E. M., K. Picozzi, J. Jannin, S. C. Welburn, and I. Maudlin. 2006. Human African trypanosomiasis: epidemiology and control. Adv. Parasitol. 61:167-221. [DOI] [PubMed] [Google Scholar]
- 203.Figueiro-Filho, E. A., G. Duarte, P. El-Beitune, S. M. Quintana, and T. L. Maia. 2004. Visceral leishmaniasis (kala-azar) and pregnancy. Infect. Dis. Obstet. Gynecol. 12:31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Figueroa Damian, R. 1998. Risk of transmission of infectious diseases by transfusion. Ginecol. Obstet. Mex. 66:277-283. (In Spanish.) [PubMed] [Google Scholar]
- 205.Fisa, R., C. Riera, P. Lopez-Chejade, I. Molina, M. Gallego, V. Falco, E. Ribera, and M. Portus. 2008. Leishmania infantum DNA detection in urine from patients with visceral leishmaniasis and after treatment control. Am. J. Trop. Med. Hyg. 78:741-744. [PubMed] [Google Scholar]
- 206.Fishman, J. A. 2007. Infection in renal transplant recipients. Semin. Nephrol. 27:445-461. [DOI] [PubMed] [Google Scholar]
- 207.Font, R. L., A. N. Samaha, M. J. Keener, P. Chevez-Barrios, and J. D. Goosey. 2000. Corneal microsporidiosis. Report of case, including electron microscopic observations. Ophthalmology 107:1769-1775. [DOI] [PubMed] [Google Scholar]
- 208.Fontes Rezende, R. E., M. A. Lescano, L. N. Zambelli Ramalho, J. F. de Castro Figueiredo, R. Oliveira Dantas, U. Garzella Meneghelli, and J. L. Pimenta Modena. 2006. Reactivation of Chagas' disease in a patient with non-Hodgkin's lymphoma: gastric, oesophageal and laryngeal involvement. Trans. R. Soc. Trop. Med. Hyg. 100:74-78. [DOI] [PubMed] [Google Scholar]
- 209.Fournier, S., O. Liguory, C. Sarfati, F. David-Ouaknine, F. Derouin, J. M. Decazes, and J. M. Molina. 2000. Disseminated infection due to Encephalitozoon cuniculi in a patient with AIDS: case report and review. HIV Med. 1:155-161. [DOI] [PubMed] [Google Scholar]
- 210.Fowler, M., and R. F. Carter. 1965. Acute pyogenic meningitis probably due to Acanthamoeba sp.: a preliminary report. Br. Med. J. ii:740-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Franzen, C., and A. Muller. 1999. Molecular techniques for detection, species differentiation, and phylogenetic analysis of microsporidia. Clin. Microbiol. Rev. 12:243-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Franzen, C., A. Muller, P. Hartmann, P. Hegener, M. Schrappe, V. Diehl, G. Fatkenheuer, and B. Salzberger. 1998. Polymerase chain reaction for diagnosis and species differentiation of microsporidia. Folia Parasitol. (Praha) 45:140-148. [PubMed] [Google Scholar]
- 213.French, M. A. 2009. HIV/AIDS: immune reconstitution inflamatory syndrome. A reappraisal. Clin. Infect. Dis. 48:101-107. [DOI] [PubMed] [Google Scholar]
- 214.Froberg, M. K., D. Dannen, and J. S. Bakken. 2004. Babesiosis and HIV. Lancet 363:704. [DOI] [PubMed] [Google Scholar]
- 215.Fung, K. T., A. P. Dhillon, J. E. McLaughlin, S. B. Lucas, B. Davidson, K. Rolles, D. Patch, and A. K. Burroughs. 2008. Cure of Acanthamoeba cerebral abscess in a liver transplant patient. Liver Transpl. 14:308-312. [DOI] [PubMed] [Google Scholar]
- 216.Gallerano, V., J. Consigli, S. Pereyra, S. Gomez Zanni, C. Danielo, R. H. Gallerano, and A. Guidi. 2007. Chagas' disease reactivation with skin symptoms in a patient with kidney transplant. Int. J. Dermatol. 46:607-610. [DOI] [PubMed] [Google Scholar]
- 217.Gamboa-Dominguez, A., J. De Anda, J. Donis, F. Ruiz-Maza, G. S. Visvesvara, and H. Diliz. 2003. Disseminated Encephalitozoon cuniculi infection in a Mexican kidney transplant recipient. Transplantation 75:1898-1900. [DOI] [PubMed] [Google Scholar]
- 218.Garcia, A. L., R. Parrado, S. De Doncker, H. Bermudez, and J. C. Dujardin. 2007. American tegumentary leishmaniasis: direct species identification of Leishmania in non-invasive clinical samples. Trans. R. Soc. Trop. Med. Hyg. 101:368-371. [DOI] [PubMed] [Google Scholar]
- 219.Garcia, L. S. (ed.). 2001. Diagnostic medical parasitology, 4th ed. ASM Press, Washington, DC.
- 220.Garraud, O., G. Andreu, M. H. Elghouzzi, S. Laperche, and J. J. Lefrere. 2007. Measures to prevent transfusion-associated protozoal infections in non-endemic countries. Travel Med. Infect. Dis. 5:110-112. [DOI] [PubMed] [Google Scholar]
- 221.Gascon, J., P. Albajar, E. Canas, M. Flores, J. Gomez i Prat, R. N. Herrera, C. A. Lafuente, H. L. Luciardi, A. Moncayo, L. Molina, J. Munoz, S. Puente, G. Sanz, B. Trevino, X. Sergio-Salles, et al. 2008. Diagnosis, management and treatment of chronic Chagas' heart disease in areas where Trypanosoma cruzi infection is not endemic. Enferm. Infecc. Microbiol. Clin. 26:99-106. (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 222.Gascon, J., P. Albajar, E. Canas, M. Flores, J. Gomez i Prat, R. N. Herrera, C. A. Lafuente, H. L. Luciardi, A. Moncayo, L. Molina, J. Munoz, S. Puente, G. Sanz, B. Trevino, and X. Sergio-Salles. 2007. Diagnosis, management and treatment of chronic Chagas' heart disease in areas where Trypanosoma cruzi infection is not endemic. Rev. Esp. Cardiol. 60:285-293. (In Spanish.) [PubMed] [Google Scholar]
- 223.Gascon, J., C. Bern, and M. J. Pinazo. 2010. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 115:22-27. [DOI] [PubMed] [Google Scholar]
- 224.Gazzard, B. 2005. AIDS and the gastrointestinal tract. Medicine 33:24-26. [Google Scholar]
- 225.Gehrig, S., and T. Efferth. 2008. Development of drug resistance in Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense. Treatment of human African trypanosomiasis with natural products (review). Int. J. Mol. Med. 22:411-419. [PubMed] [Google Scholar]
- 226.Gelfand, J. A., and M. V. Callahan. 2003. Babesiosis: an update on epidemiology and treatment. Curr. Infect. Dis. Rep. 5:53-58. [DOI] [PubMed] [Google Scholar]
- 227.Gelman, B. B., V. Popov, G. Chaljub, R. Nader, S. J. Rauf, H. W. Nauta, and G. S. Visvesvara. 2003. Neuropathological and ultrastructural features of amebic encephalitis caused by Sappinia diploidea. J. Neuropathol. Exp. Neurol. 62:990-998. [DOI] [PubMed] [Google Scholar]
- 228.Gelman, B. B., S. J. Rauf, R. Nader, V. Popov, J. Borkowski, G. Chaljub, H. W. Nauta, and G. S. Visvesvara. 2001. Amoebic encephalitis due to Sappinia diploidea. JAMA 285:2450-2451. [DOI] [PubMed] [Google Scholar]
- 229.Genchi, C. 2007. Human babesiosis, an emerging zoonosis. Parassitologia 49(Suppl. 1):29-31. [PubMed] [Google Scholar]
- 230.Ghalmi, F., B. China, R. Kaidi, and B. Losson. 2009. First epidemiological study on exposure to Neospora caninum in different canine populations in the Algiers District (Algeria). Parasitol. Int. 58:444-450. [DOI] [PubMed] [Google Scholar]
- 231.Gibson, W. 2007. Resolution of the species problem in African trypanosomes. Int. J. Parasitol. 37:829-838. [DOI] [PubMed] [Google Scholar]
- 232.Gibson, W. 2009. Species-specific probes for the identification of the African tsetse-transmitted trypanosomes. Parasitology 136:1501-1507. [DOI] [PubMed] [Google Scholar]
- 233.Gilbert, D. N., R. C. Moellering, G. M. Eliopoulas, H. F. Chambers, and M. S. Saag (ed.). 2009. The Sanford guide to antimicrobial therapy. Antimicrobial Therapy, Inc., Sperryville, VA.
- 234.Girones, N., H. Cuervo, and M. Fresno. 2005. Trypanosoma cruzi-induced molecular mimicry and Chagas' disease. Curr. Top. Microbiol. Immunol. 296:89-123. [DOI] [PubMed] [Google Scholar]
- 235.Goethert, H. K., C. Lubelcyzk, E. LaCombe, M. Holman, P. Rand, R. P. Smith, Jr., and S. R. Telford III. 2003. Enzootic Babesia microti in Maine. J. Parasitol. 89:1069-1071. [DOI] [PubMed] [Google Scholar]
- 236.Goldsby, R. A., T. J. Kindt, B. A. Osborne, and J. Kuby (ed.). 2003. Immunology, 5th ed. W. H. Freeman and Company, New York, NY.
- 237.Gomes, A. H., I. M. Armelin, S. Z. Menon, and V. L. Pereira-Chioccola. 2008. Leishmania (V.) braziliensis: detection by PCR in biopsies from patients with cutaneous leishmaniasis. Exp. Parasitol. 119:319-324. [DOI] [PubMed] [Google Scholar]
- 238.Gomes, Y. M. 1997. PCR and sero-diagnosis of chronic Chagas' disease. Biotechnological advances. Appl. Biochem. Biotechnol. 66:107-119. [DOI] [PubMed] [Google Scholar]
- 239.Gontijo, C. M., R. S. Pacheco, F. Orefice, E. Lasmar, E. S. Silva, and M. N. Melo. 2002. Concurrent cutaneous, visceral and ocular leishmaniasis caused by Leishmania (Viannia) braziliensis in a kidney transplant patient. Mem. Inst. Oswaldo Cruz 97:751-753. [DOI] [PubMed] [Google Scholar]
- 240.Gonzalez, U., M. Pinart, M. Rengifo-Pardo, A. Macaya, J. Alvar, and J. A. Tweed. 2009. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database Syst. Rev. 2009:CD004834. [DOI] [PubMed] [Google Scholar]
- 241.Gornik, K. 2005. Pathogenic properties of free-living amoebae isolated from natural and man-made bathing sites in the province of Western Pomerania. Ann. Acad. Med. Stetin. 51:127-133. (In Polish.) [PubMed] [Google Scholar]
- 242.Goswick, S. M., and G. M. Brenner. 2003. Activities of azithromycin and amphotericin B against Naegleria fowleri in vitro and in a mouse model of primary amebic meningoencephalitis. Antimicrob. Agents Chemother. 47:524-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Goswick, S. M., and G. M. Brenner. 2003. Activities of therapeutic agents against Naegleria fowleri in vitro and in a mouse model of primary amebic meningoencephalitis. J. Parasitol. 89:837-842. [DOI] [PubMed] [Google Scholar]
- 244.Grange, F., E. L. Kinney, J. J. Monsuez, M. Rybojad, F. Derouin, M. A. Khuong, and M. Janier. 1990. Successful therapy for Toxoplasma gondii myocarditis in acquired immunodeficiency syndrome. Am. Heart J. 120:443-444. [DOI] [PubMed] [Google Scholar]
- 245.Griesemer, D. A., L. L. Barton, C. M. Reese, P. C. Johnson, J. A. Gabrielsen, D. Talwar, and G. S. Visvesvara. 1994. Amebic meningoencephalitis caused by Balamuthia mandrillaris. Pediatr. Neurol. 10:249-254. [DOI] [PubMed] [Google Scholar]
- 246.Gubernot, D. M., C. T. Lucey, K. C. Lee, G. B. Conley, L. G. Holness, and R. P. Wise. 2009. Babesia infection through blood transfusions: reports received by the US Food and Drug Administration, 1997-2007. Clin. Infect. Dis. 48:25-30. [DOI] [PubMed] [Google Scholar]
- 247.Guiguemde, R. T., O. S. Sawadogo, C. Bories, K. L. Traore, D. Nezien, L. Nikiema, F. Pratlong, P. Marty, R. Houin, and M. Deniau. 2003. Leishmania major and HIV co-infection in Burkina Faso. Trans. R. Soc. Trop. Med. Hyg. 97:168-169. [DOI] [PubMed] [Google Scholar]
- 248.Guimarães, L. H., P. R. Machado, E. L. Lago, D. J. Morgan, A. Schriefer, O. Bacellar, and E. M. Carvalho. 2009. Atypical manifestations of tegumentary leishmaniasis in a transmission area of Leishmania braziliensis in the state of Bahia, Brazil. Trans. R. Soc. Trop. Med. Hyg. 103:712-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Gutierrez, F. R., P. M. Guedes, R. T. Gazzinelli, and J. S. Silva. 2009. The role of parasite persistence in pathogenesis of Chagas heart disease. Parasite Immunol. 31:673-685. [DOI] [PubMed] [Google Scholar]
- 250.Haberkorn, A. 1996. Chemotherapy of human and animal coccidioses: state and perspectives. Parasitol. Res. 82:193-199. [DOI] [PubMed] [Google Scholar]
- 251.Hansen, B., and G. Kronborg. 2003. Acanthamoeba keratitis in a non-contact lens wearer with human immunodeficiency virus. Scand. J. Infect. Dis. 35:207-209. [DOI] [PubMed] [Google Scholar]
- 252.Hart, C. A., N. J. Beeching, B. I. Duerden, A. Curry, N. French, S. Kariuki, S. M. Graham, M. A. Gordon, P. G. Hoggard, S. Kewn, and D. J. Back. 2000. Infections in AIDS. J. Med. Microbiol. 49:947-967. [DOI] [PubMed] [Google Scholar]
- 253.Haselbarth, K., A. M. Tenter, V. Brade, G. Krieger, and K. P. Hunfeld. 2007. First case of human babesiosis in Germany—clinical presentation and molecular characterisation of the pathogen. Int. J. Med. Microbiol. 297:197-204. [DOI] [PubMed] [Google Scholar]
- 254.Hassene, A., A. Vital, A. Anghel, S. Guez, and C. Series. 2008. Acute acquired toxoplasmosis presenting as polymyositis and chorioretinitis in immunocompetent patient. Joint Bone Spine 75:603-605. [DOI] [PubMed] [Google Scholar]
- 255.Haverkos, H. W. 1987. Assessment of therapy for Toxoplasma encephalitis. The TE Study Group. Am. J. Med. 82:907-914. [DOI] [PubMed] [Google Scholar]
- 256.Hawksworth, D. L. 2006. Pandora's mycological box: molecular sequences vs. morphology in understanding fungal relationships and biodiversity. Rev. Iberoam. Micol. 23:127-133. (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 257.Healy, G. R., and T. K. Ruebush II. 1980. Morphology of Babesia microti in human blood smears. Am. J. Clin. Pathol. 73:107-109. [DOI] [PubMed] [Google Scholar]
- 258.Hebbar, S., I. Bairy, N. Bhaskaranand, S. Upadhyaya, M. S. Sarma, and A. K. Shetty. 2005. Fatal case of Naegleria fowleri meningo-encephalitis in an infant: case report. Ann. Trop. Paediatr. 25:223-226. [DOI] [PubMed] [Google Scholar]
- 259.Heffler, K. F., T. J. Eckhardt, A. C. Reboli, and D. Stieritz. 1996. Acanthamoeba endophthalmitis in acquired immunodeficiency syndrome. Am. J. Ophthalmol. 122:584-586. [DOI] [PubMed] [Google Scholar]
- 260.Helton, J., M. Loveless, and C. R. White, Jr. 1993. Cutaneous acanthamoeba infection associated with leukocytoclastic vasculitis in an AIDS patient. Am. J. Dermatopathol. 15:146-149. [DOI] [PubMed] [Google Scholar]
- 261.Herrera, H. M., A. M. Davila, A. Norek, U. G. Abreu, S. S. Souza, P. S. D'Andrea, and A. M. Jansen. 2004. Enzootiology of Trypanosoma evansi in Pantanal, Brazil. Vet. Parasitol. 125:263-275. [DOI] [PubMed] [Google Scholar]
- 262.Herrera, H. M., A. Norek, T. P. Freitas, V. Rademaker, O. Fernandes, and A. M. Jansen. 2005. Domestic and wild mammals infection by Trypanosoma evansi in a pristine area of the Brazilian Pantanal region. Parasitol. Res. 96:121-126. [DOI] [PubMed] [Google Scholar]
- 263.Herwaldt, B., D. H. Persing, E. A. Precigout, W. L. Goff, D. A. Mathiesen, P. W. Taylor, M. L. Eberhard, and A. F. Gorenflot. 1996. A fatal case of babesiosis in Missouri: identification of another piroplasm that infects humans. Ann. Intern. Med. 124:643-650. [DOI] [PubMed] [Google Scholar]
- 264.Herwaldt, B. L. 1999. Leishmaniasis. Lancet 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 265.Herwaldt, B. L., S. Caccio, F. Gherlinzoni, H. Aspock, S. B. Slemenda, P. Piccaluga, G. Martinelli, R. Edelhofer, U. Hollenstein, G. Poletti, S. Pampiglione, K. Loschenberger, S. Tura, and N. J. Pieniazek. 2003. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg. Infect. Dis. 9:942-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Herwaldt, B. L., G. de Bruyn, N. J. Pieniazek, M. Homer, K. H. Lofy, S. B. Slemenda, T. R. Fritsche, D. H. Persing, and A. P. Limaye. 2004. Babesia divergens-like infection, Washington State. Emerg. Infect. Dis. 10:622-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Herwaldt, B. L., A. M. Kjemtrup, P. A. Conrad, R. C. Barnes, M. Wilson, M. G. McCarthy, M. H. Sayers, and M. L. Eberhard. 1997. Transfusion-transmitted babesiosis in Washington State: first reported case caused by a WA1-type parasite. J. Infect. Dis. 175:1259-1262. [DOI] [PubMed] [Google Scholar]
- 268.Heydorn, A. O., and H. Mehlhorn. 2001. Further remarks on Hammondia hammondi and the taxonomic importance of obligate heteroxeny. Parasitol. Res. 87:573-577. [DOI] [PubMed] [Google Scholar]
- 269.Hide, G. 1999. History of sleeping sickness in East Africa. Clin. Microbiol. Rev. 12:112-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Hide, G., E. K. Morley, J. M. Hughes, O. Gerwash, M. S. Elmahaishi, K. H. Elmahaishi, D. Thomasson, E. A. Wright, R. H. Williams, R. G. Murphy, and J. E. Smith. 2009. Evidence for high levels of vertical transmission in Toxoplasma gondii. Parasitology 136:1877-1885. [DOI] [PubMed] [Google Scholar]
- 271.Hide, G., and A. Tait. 2009. Molecular epidemiology of African sleeping sickness. Parasitology 136:1491-1500. [DOI] [PubMed] [Google Scholar]
- 272.Hildebrandt, A., K. P. Hunfeld, M. Baier, A. Krumbholz, S. Sachse, T. Lorenzen, M. Kiehntopf, H. J. Fricke, and E. Straube. 2007. First confirmed autochthonous case of human Babesia microti infection in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 26:595-601. [DOI] [PubMed] [Google Scholar]
- 273.Hildebrandt, A., A. M. Tenter, E. Straube, and K.-P. Hunfeld. 2008. Human babesiosis in Germany: just overlooked or truly new? Int. J. Med. Microbiol. 298:336-346. [Google Scholar]
- 274.Hill, D. E., S. Chirukandoth, and J. P. Dubey. 2005. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim. Health Res. Rev. 6:41-61. [DOI] [PubMed] [Google Scholar]
- 275.Hilpertshauser, H., P. Deplazes, M. Schnyder, L. Gern, and A. Mathis. 2006. Babesia spp. identified by PCR in ticks collected from domestic and wild ruminants in southern Switzerland. Appl. Environ. Microbiol. 72:6503-6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hobbs, C. V., T. Voza, A. Coppi, B. Kirmse, K. Marsh, W. Borkowsky, and P. Sinnis. 2009. HIV protease inhibitors inhibit the development of preerythrocytic-stage Plasmodium parasites. J. Infect. Dis. 199:134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Hofman, P., H. Quintens, J. F. Michiels, B. Taillan, and A. Thyss. 1993. Toxoplasma cystitis associated with acquired immunodeficiency syndrome. Urology 42:589-592. [DOI] [PubMed] [Google Scholar]
- 278.Holland, G. N. 2009. Ocular toxoplasmosis: the influence of patient age. Mem. Inst. Oswaldo Cruz 104:351-357. [DOI] [PubMed] [Google Scholar]
- 279.Holman, P. J. 2006. Phylogenetic and biologic evidence that Babesia divergens is not endemic in the United States. Ann. N. Y. Acad. Sci. 1081:518-525. [DOI] [PubMed] [Google Scholar]
- 280.Holmdahl, O. J., J. G. Mattsson, A. Uggla, and K. E. Johansson. 1994. The phylogeny of Neospora caninum and Toxoplasma gondii based on ribosomal RNA sequences. FEMS Microbiol. Lett. 119:187-192. [DOI] [PubMed] [Google Scholar]
- 281.Homer, M. J., I. Aguilar-Delfin, S. R. Telford III, P. J. Krause, and D. H. Persing. 2000. Babesiosis. Clin. Microbiol. Rev. 13:451-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Hotez, P. J., M. E. Bottazzi, C. Franco-Paredes, S. K. Ault, and M. R. Periago. 2008. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl. Trop. Dis. 2:e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Hotez, P. J., and A. Kamath. 2009. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 3:e412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Houang, E., D. Lam, D. Fan, and D. Seal. 2001. Microbial keratitis in Hong Kong: relationship to climate, environment, and contact-lens disinfection. Trans. R. Soc. Trop. Med. Hyg. 95:361-367. [DOI] [PubMed] [Google Scholar]
- 285.Howie, S., M. Guy, L. Fleming, W. Bailey, H. Noyes, J. A. Faye, J. Pepin, B. Greenwood, H. Whittle, D. Molyneux, and T. Corrah. 2006. A Gambian infant with fever and an unexpected blood film. PLoS Med. 3:e355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Hunfeld, K. P., A. Hildebrandt, and J. S. Gray. 2008. Babesiosis: recent insights into an ancient disease. Int. J. Parasitol. 38:1219-1237. [DOI] [PubMed] [Google Scholar]
- 287.Hutchinson, O. C., E. M. Fevre, M. Carrington, and S. C. Welburn. 2003. Lessons learned from the emergence of a new Trypanosoma brucei rhodesiense sleeping sickness focus in Uganda. Lancet Infect. Dis. 3:42-45. [DOI] [PubMed] [Google Scholar]
- 288.Ibrahim, H. M., P. Huang, T. A. Salem, R. M. Talaat, M. I. Nasr, X. Xuan, and Y. Nishikawa. 2009. Prevalence of Neospora caninum and Toxoplasma gondii antibodies in northern Egypt. Am. J. Trop. Med. Hyg. 80:263-267. [PubMed] [Google Scholar]
- 289.Innes, E. A. 1997. Toxoplasmosis: comparative species susceptibility and host immune response. Comp. Immunol. Microbiol. Infect. Dis. 20:131-138. [DOI] [PubMed] [Google Scholar]
- 290.Innes, E. A., P. M. Bartley, D. Buxton, and F. Katzer. 2009. Ovine toxoplasmosis. Parasitology 136:1887-1894. [DOI] [PubMed] [Google Scholar]
- 291.Intalapaporn, P., C. Suankratay, S. Shuangshoti, K. Phantumchinda, S. Keelawat, and H. Wilde. 2004. Balamuthia mandrillaris meningoencephalitis: the first case in southeast Asia. Am. J. Trop. Med. Hyg. 70:666-669. [PubMed] [Google Scholar]
- 292.Islam, M. Z., M. Itoh, H. Takagi, A. U. Islam, A. R. Ekram, A. Rahman, A. Takesue, Y. Hashiguchi, and E. Kimura. 2008. Enzyme-linked immunosorbent assay to detect urinary antibody against recombinant rKRP42 antigen made from Leishmania donovani for the diagnosis of visceral leishmaniasis. Am. J. Trop. Med. Hyg. 79:599-604. [PubMed] [Google Scholar]
- 293.Jackson, Y. 2009. International migration: global issue, local impact. The example of two parasites. Rev. Med. Suisse 5:1022-1025. (In French.) [PubMed] [Google Scholar]
- 294.Jackson, Y., A. Angheben, B. Carrilero Fernandez, J. M. Jansa i Lopez del Vallado, J. G. Jannin, and P. Albajar-Vinas. 2009. Management of Chagas disease in Europe. Experiences and challenges in Spain, Switzerland and Italy. Bull. Soc. Pathol. Exot. 102:326-329. (In French.) [PubMed] [Google Scholar]
- 295.Jain, R., S. Prabhakar, M. Modi, R. Bhatia, and R. Sehgal. 2002. Naegleria meningitis: a rare survival. Neurol. India 50:470-472. [PubMed] [Google Scholar]
- 296.Jamonneau, V., P. Solano, and G. Cuny. 2001. Use of molecular biology in the diagnosis of human African trypanosomiasis. Med. Trop. (Mars.) 61:347-354. (In French.) [PubMed] [Google Scholar]
- 297.Jimenez, M. I., R. Lopez-Velez, R. Molina, C. Canavate, and J. Alvar. 1996. HIV co-infection with a currently non-pathogenic flagellate. Lancet 347:264-265. [DOI] [PubMed] [Google Scholar]
- 298.Jittapalapong, S., T. Inpankaew, N. Sarataphan, V. Herbreteau, J. P. Hugot, S. Morand, and R. W. Stich. 2008. Molecular detection of divergent trypanosomes among rodents of Thailand. Infect. Genet. Evol. 8:445-449. [DOI] [PubMed] [Google Scholar]
- 299.Johnson, P. D. 1933. A case of infection by Trypanosoma lewisi in a child. Trans. R. Soc. Trop. Med. Hyg. XXVI:467-468. [Google Scholar]
- 300.Jones, C. A., J. A. Holloway, and J. O. Warner. 2002. Phenotype of fetal monocytes and B lymphocytes during the third trimester of pregnancy. J. Reprod. Immunol. 56:45-60. [DOI] [PubMed] [Google Scholar]
- 301.Jones, J. L., A. Krueger, J. Schulkin, and P. M. Schantz. 2010. Toxoplasmosis prevention and testing in pregnancy, survey of obstetrician-gynaecologists. Zoonoses Public Health 57:27-33. [DOI] [PubMed] [Google Scholar]
- 302.Jordan, C. N., A. M. Zajac, K. S. Snowden, and D. S. Lindsay. 2006. Direct agglutination test for Encephalitozoon cuniculi. Vet. Parasitol. 135:235-240. [DOI] [PubMed] [Google Scholar]
- 303.Joseph, J., S. Murthy, P. Garg, and S. Sharma. 2006. Use of different stains for microscopic evaluation of corneal scrapings for diagnosis of microsporidial keratitis. J. Clin. Microbiol. 44:583-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Joseph, J., G. K. Vemuganti, and S. Sharma. 2005. Microsporidia: emerging ocular pathogens. Indian J. Med. Microbiol. 23:80-91. [DOI] [PubMed] [Google Scholar]
- 305.Joshi, P. P., V. R. Shegokar, R. M. Powar, S. Herder, R. Katti, H. R. Salkar, V. S. Dani, A. Bhargava, J. Jannin, and P. Truc. 2005. Human trypanosomiasis caused by Trypanosoma evansi in India: the first case report. Am. J. Trop. Med. Hyg. 73:491-495. [PubMed] [Google Scholar]
- 306.Jung, S., R. L. Schelper, G. S. Visvesvara, and H. T. Chang. 2004. Balamuthia mandrillaris meningoencephalitis in an immunocompetent patient: an unusual clinical course and a favorable outcome. Arch. Pathol. Lab. Med. 128:466-468. [DOI] [PubMed] [Google Scholar]
- 307.Karbowiak, G. 2004. Zoonotic reservoir of Babesia microti in Poland. Pol. J. Microbiol. 53(Suppl.):61-65. [PubMed] [Google Scholar]
- 308.Karp, C. L., and P. G. Auwaerter. 2007. Coinfection with HIV and tropical infectious diseases. I. Protozoal pathogens. Clin. Infect. Dis. 45:1208-1213. [DOI] [PubMed] [Google Scholar]
- 309.Katlama, C., B. Mouthon, D. Gourdon, D. Lapierre, and F. Rousseau. 1996. Atovaquone as long-term suppressive therapy for toxoplasmic encephalitis in patients with AIDS and multiple drug intolerance. Atovaquone Expanded Access Group. AIDS 10:1107-1112. [PubMed] [Google Scholar]
- 310.Katz, J. D., A. H. Ropper, L. Adelman, M. Worthington, and P. Wade. 2000. A case of Balamuthia mandrillaris meningoencephalitis. Arch. Neurol. 57:1210-1212. [DOI] [PubMed] [Google Scholar]
- 311.Kaur, R., V. K. Gupta, A. C. Dhariwal, D. C. Jain, and L. Shiv. 2007. A rare case of trypanosomiasis in a two month old infant in Mumbai, India. J. Commun. Dis. 39:71-74. [PubMed] [Google Scholar]
- 312.Kaushal, V., D. K. Chhina, S. Ram, G. Singh, R. K. Kaushal, and R. Kumar. 2008. Primary amoebic meningoencephalitis due to Naegleria fowleri. J. Assoc. Physicians India 56:459-462. [PubMed] [Google Scholar]
- 313.Kazyumba, L., M. Wery, and J. F. Ruppol. 1978. Congenital transmission of Trypanosoma gambiense. Ann. Soc. Belg. Med. Trop. 58:65-66. [PubMed] [Google Scholar]
- 314.Kelkar, R., P. S. Sastry, S. S. Kulkarni, T. K. Saikia, P. M. Parikh, and S. H. Advani. 1997. Pulmonary microsporidial infection in a patient with CML undergoing allogeneic marrow transplant. Bone Marrow Transplant. 19:179-182. [DOI] [PubMed] [Google Scholar]
- 315.Kennedy, P. G. 2004. Human African trypanosomiasis of the CNS: current issues and challenges. J. Clin. Invest. 113:496-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Kester, K. E., G. W. Turiansky, and P. L. McEvoy. 1998. Nodular cutaneous microsporidiosis in a patient with AIDS and successful treatment with long-term oral clindamycin therapy. Ann. Intern. Med. 128:911-914. [DOI] [PubMed] [Google Scholar]
- 317.Kester, K. E., G. S. Visvesara, and P. McEvoy. 2000. Organism responsible for nodular cutaneous microsporidiosis in a patient with AIDS. Ann. Intern. Med. 133:925. [DOI] [PubMed] [Google Scholar]
- 318.Kettesy, B., T. Komar, A. Berta, and L. Modis. 2008. Acanthamoeba keratitis. Orv. Hetil. 149:2037-2045. (In Hungarian.) [DOI] [PubMed] [Google Scholar]
- 319.Khachonsaksumet, V., and M. Riganti. 2002. Rapid Wright's stain for the detection of imported Leishmania tropica. Southeast Asian J. Trop. Med. Public Health 33:509-511. [PubMed] [Google Scholar]
- 320.Khan, N. A. 2003. Pathogenesis of Acanthamoeba infections. Microb. Pathog. 34:277-285. [DOI] [PubMed] [Google Scholar]
- 321.Kiderlen, A. F., and U. Laube. 2004. Balamuthia mandrillaris, an opportunistic agent of granulomatous amebic encephalitis, infects the brain via the olfactory nerve pathway. Parasitol. Res. 94:49-52. [DOI] [PubMed] [Google Scholar]
- 322.Kim, J. Y., S. H. Cho, H. N. Joo, M. Tsuji, S. R. Cho, I. J. Park, G. T. Chung, J. W. Ju, H. I. Cheun, H. W. Lee, Y. H. Lee, and T. S. Kim. 2007. First case of human babesiosis in Korea: detection and characterization of a novel type of Babesia sp. (KO1) similar to ovine babesia. J. Clin. Microbiol. 45:2084-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kim, S. Y., M. J. Syms, M. R. Holtel, and K. K. Nauschuetz. 2000. Acanthamoeba sinusitis with subsequent dissemination in an AIDS patient. Ear Nose Throat J. 79:168, 171-174. [PubMed] [Google Scholar]
- 324.Kima, P. E. 2007. The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int. J. Parasitol. 37:1087-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Kinde, H., D. H. Read, B. M. Daft, M. Manzer, R. W. Nordhausen, D. J. Kelly, P. A. Fuerst, G. Booton, and G. S. Visvesvara. 2007. Infections caused by pathogenic free-living amebas (Balamuthia mandrillaris and Acanthamoeba sp.) in horses. J. Vet. Diagn. Invest. 19:317-322. [DOI] [PubMed] [Google Scholar]
- 326.Kjemtrup, A. M., and P. A. Conrad. 2000. Human babesiosis: an emerging tick-borne disease. Int. J. Parasitol. 30:1323-1337. [DOI] [PubMed] [Google Scholar]
- 327.Kjemtrup, A. M., B. Lee, C. L. Fritz, C. Evans, M. Chervenak, and P. A. Conrad. 2002. Investigation of transfusion transmission of a WA1-type babesial parasite to a premature infant in California. Transfusion 42:1482-1487. [DOI] [PubMed] [Google Scholar]
- 328.Kodym, P., S. Hrda, L. Machala, H. Rozsypal, M. Stankova, and M. Maly. 2006. Prevalence and incidence of Toxoplasma infection in HIV-positive patients in the Czech Republic. J. Eukaryot. Microbiol. 53(Suppl. 1):S160-S161. [DOI] [PubMed] [Google Scholar]
- 329.Konecny, P., and D. J. Stark. 2007. An Australian case of New World cutaneous leishmaniasis. Med. J. Aust. 186:315-317. [DOI] [PubMed] [Google Scholar]
- 330.Kotton, C. N. 2009. Donor-derived infections in transplant patients. Clin. Microbiol. Newsl. 31:63-67. [Google Scholar]
- 331.Krause, P. J. 2002. Babesiosis. Med. Clin. North Am. 86:361-373. [DOI] [PubMed] [Google Scholar]
- 332.Krause, P. J. 2003. Babesiosis diagnosis and treatment. Vector Borne Zoonotic Dis. 3:45-51. [DOI] [PubMed] [Google Scholar]
- 333.Krause, P. J., B. E. Gewurz, D. Hill, F. M. Marty, E. Vannier, I. M. Foppa, R. R. Furman, E. Neuhaus, G. Skowron, S. Gupta, C. McCalla, E. L. Pesanti, M. Young, D. Heiman, G. Hsue, J. A. Gelfand, G. P. Wormser, J. Dickason, F. J. Bia, B. Hartman, S. R. Telford III, D. Christianson, K. Dardick, M. Coleman, J. E. Girotto, and A. Spielman. 2008. Persistent and relapsing babesiosis in immunocompromised patients. Clin. Infect. Dis. 46:370-376. [DOI] [PubMed] [Google Scholar]
- 334.Krause, P. J., K. McKay, J. Gadbaw, D. Christianson, L. Closter, T. Lepore, S. R. Telford III, V. Sikand, R. Ryan, D. Persing, J. D. Radolf, and A. Spielman. 2003. Increasing health burden of human babesiosis in endemic sites. Am. J. Trop. Med. Hyg. 68:431-436. [PubMed] [Google Scholar]
- 335.Krause, P. J., A. Spielman, S. R. Telford III, V. K. Sikand, K. McKay, D. Christianson, R. J. Pollack, P. Brassard, J. Magera, R. Ryan, and D. H. Persing. 1998. Persistent parasitemia after acute babesiosis. N. Engl. J. Med. 339:160-165. [DOI] [PubMed] [Google Scholar]
- 336.Krause, P. J., S. Telford III, A. Spielman, R. Ryan, J. Magera, T. V. Rajan, D. Christianson, T. V. Alberghini, L. Bow, and D. Persing. 1996. Comparison of PCR with blood smear and inoculation of small animals for diagnosis of Babesia microti parasitemia. J. Clin. Microbiol. 34:2791-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Kuboki, N., N. Inoue, T. Sakurai, F. Di Cello, D. J. Grab, H. Suzuki, C. Sugimoto, and I. Igarashi. 2003. Loop-mediated isothermal amplification for detection of African trypanosomes. J. Clin. Microbiol. 41:5517-5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Kuk, S., and M. Ozden. 2007. A four-year investigation of the seropositivity of Toxoplasma gondii in our hospital. Turkiye Parazitol. Derg. 31:1-3. (In Turkish.) [PubMed] [Google Scholar]
- 339.Kuo, I., and N. A. Rao. 1999. Ocular disease in AIDS. Springer Semin. Immunopathol. 21:161-177. [DOI] [PubMed] [Google Scholar]
- 340.Kuwayama, D. P., and R. J. Briones. 2008. Spontaneous splenic rupture caused by Babesia microti infection. Clin. Infect. Dis. 46:e92-e95. [DOI] [PubMed] [Google Scholar]
- 341.Kyambadde, J. W., J. C. Enyaru, E. Matovu, M. Odiit, and J. F. Carasco. 2000. Detection of trypanosomes in suspected sleeping sickness patients in Uganda using the polymerase chain reaction. Bull. World Health Organ. 78:119-124. [PMC free article] [PubMed] [Google Scholar]
- 342.Lagerberg, R. E. 2008. Malaria in pregnancy: a literature review. J. Midwifery Womens Health 53:209-215. [DOI] [PubMed] [Google Scholar]
- 343.Lago, E. G., G. S. Conrado, C. S. Piccoli, R. L. Carvalho, and A. L. Bender. 2009. Toxoplasma gondii antibody profile in HIV-infected pregnant women and the risk of congenital toxoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 28:345-351. [DOI] [PubMed] [Google Scholar]
- 344.Lai, D. H., H. Hashimi, Z. R. Lun, F. J. Ayala, and J. Lukes. 2008. Adaptations of Trypanosoma brucei to gradual loss of kinetoplast DNA: Trypanosoma equiperdum and Trypanosoma evansi are petite mutants of T. brucei. Proc. Natl. Acad. Sci. U. S. A. 105:1999-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Lai, D. H., Q. P. Wang, Z. Li, A. G. Luckins, S. A. Reid, and Z. R. Lun. 2010. Investigations into human serum sensitivity expressed by stocks of Trypanosoma brucei evansi. Int. J. Parasitol. 40:705-710. [DOI] [PubMed] [Google Scholar]
- 346.Landfear, S. M. 2008. Drugs and transporters in kinetoplastid protozoa. Adv. Exp. Med. Biol. 625:22-32. [DOI] [PubMed] [Google Scholar]
- 347.Lanjewar, D. N., S. V. Agale, A. R. Chitale, and S. R. Joshi. 2006. Sudden death due to cardiac toxoplasmosis. J. Assoc. Physicians India 54:244-245. [PubMed] [Google Scholar]
- 348.Latib, M. A., M. D. Pascoe, M. S. Duffield, and D. Kahn. 2001. Microsporidiosis in the graft of a renal transplant recipient. Transpl. Int. 14:274-277. [DOI] [PubMed] [Google Scholar]
- 349.Lazo, J., A. C. Meneses, A. Rocha, M. S. Ferreira, J. O. Marquez, E. Chapadeiro, and E. R. Lopes. 1998. Chagasic meningoencephalitis in the immunodeficient. Arq. Neuropsiquiatr. 56:93-97. [DOI] [PubMed] [Google Scholar]
- 350.Lee, K. S., J. Cox-Singh, and B. Singh. 2009. Morphological features and differential counts of Plasmodium knowlesi parasites in naturally acquired human infections. Malar. J. 8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Lee, S. C., N. Corradi, E. J. Byrnes III, S. Torres-Martinez, F. S. Dietrich, P. J. Keeling, and J. Heitman. 2008. Microsporidia evolved from ancestral sexual fungi. Curr. Biol. 18:1675-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Lejon, V., M. Boelaert, J. Jannin, A. Moore, and P. Buscher. 2003. The challenge of Trypanosoma brucei gambiense sleeping sickness diagnosis outside Africa. Lancet Infect. Dis. 3:804-808. [DOI] [PubMed] [Google Scholar]
- 353.Leon, J. S., M. D. Daniels, K. M. Toriello, K. Wang, and D. M. Engman. 2004. A cardiac myosin-specific autoimmune response is induced by immunization with Trypanosoma cruzi proteins. Infect. Immun. 72:3410-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.L'Hostis, M., A. Chauvin, A. Valentin, E. Precigout, and A. Gorenflot. 1997. Survey of Babesia divergens antibody kinetics in cattle in western France. Vet. Res. 28:481-488. [PubMed] [Google Scholar]
- 355.Li, Q., X. H. Yang, and J. Qian. 2005. September 2004: a 6-year-old girl with headache and stiff neck. Brain Pathol. 15:93-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Liarte, D. B., I. L. Mendonca, F. C. Luz, E. A. Abreu, G. W. Mello, T. J. Farias, A. F. Ferreira, M. A. Millington, and C. H. Costa. 2001. QBC for the diagnosis of human and canine american visceral leishmaniasis: preliminary data. Rev. Soc. Bras. Med. Trop. 34:577-581. (In Portugese.) [DOI] [PubMed] [Google Scholar]
- 357.Lim, L., D. J. Coster, and P. R. Badenoch. 2000. Antimicrobial susceptibility of 19 Australian corneal isolates of Acanthamoeba. Clin. Exp. Ophthalmol. 28:119-124. [DOI] [PubMed] [Google Scholar]
- 358.Lindsay, R. G., G. Watters, R. Johnson, S. E. Ormonde, and G. R. Snibson. 2007. Acanthamoeba keratitis and contact lens wear. Clin. Exp. Optom. 90:351-360. [DOI] [PubMed] [Google Scholar]
- 359.Lippert, U., J. Schottelius, and C. Manegold. 2003. Disseminated microspiridiosis (Encephalitozoon intestinalis) in a patient with HIV infection. Dtsch. Med. Wochenschr. 128:1769-1772. (In German.) [DOI] [PubMed] [Google Scholar]
- 360.Loa, C. C., M. E. Adelson, E. Mordechai, I. Raphaelli, and R. C. Tilton. 2004. Serological diagnosis of human babesiosis by IgG enzyme-linked immunosorbent assay. Curr. Microbiol. 49:385-389. [DOI] [PubMed] [Google Scholar]
- 361.Lobato, J., D. A. Silva, T. W. Mineo, J. D. Amaral, G. R. Segundo, J. M. Costa-Cruz, M. S. Ferreira, A. S. Borges, and J. R. Mineo. 2006. Detection of immunoglobulin G antibodies to Neospora caninum in humans: high seropositivity rates in patients who are infected by human immunodeficiency virus or have neurological disorders. Clin. Vaccine Immunol. 13:84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Lopez-Velez, R., J. A. Perez-Molina, A. Guerrero, F. Baquero, J. Villarrubia, L. Escribano, C. Bellas, F. Perez-Corral, and J. Alvar. 1998. Clinicoepidemiologic characteristics, prognostic factors, and survival analysis of patients coinfected with human immunodeficiency virus and Leishmania in an area of Madrid, Spain. Am. J. Trop. Med. Hyg. 58:436-443. [DOI] [PubMed] [Google Scholar]
- 363.Lopez-Velez, R., M. C. Turrientes, C. Garron, P. Montilla, R. Navajas, S. Fenoy, and C. del Aguila. 1999. Microsporidiosis in travelers with diarrhea from the tropics. J. Travel Med. 6:223-227. [DOI] [PubMed] [Google Scholar]
- 364.Lucet, J. C., M. P. Bailly, J. P. Bedos, M. Wolff, B. Gachot, and F. Vachon. 1993. Septic shock due to toxoplasmosis in patients infected with the human immunodeficiency virus. Chest 104:1054-1058. [DOI] [PubMed] [Google Scholar]
- 365.Lun, Z. R., S. A. Reid, D. H. Lai, and F. J. Li. 2009. Atypical human trypanosomiasis: a neglected disease or just an unlucky accident? Trends Parasitol. 25:107-108. [DOI] [PubMed] [Google Scholar]
- 366.Lux, J. Z., D. Weiss, J. V. Linden, D. Kessler, B. L. Herwaldt, S. J. Wong, J. Keithly, P. Della-Latta, and B. E. Scully. 2003. Transfusion-associated babesiosis after heart transplant. Emerg. Infect. Dis. 9:116-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.MacLean, R. C., N. Hafez, S. Tripathi, C. G. Childress, N. R. Ghatak, and F. Marciano-Cabral. 2007. Identification of Acanthamoeba sp. in paraffin-embedded CNS tissue from an HIV+ individual by PCR. Diagn. Microbiol. Infect. Dis. 57:289-294. [DOI] [PubMed] [Google Scholar]
- 368.Reference deleted.
- 369.Madalosso, G., A. C. Pellini, M. J. Vasconcelos, A. F. Ribeiro, L. Weissmann, G. S. Oliveira Filho, A. C. Penalva de Oliveira, and J. E. Vidal. 2004. Chagasic meningoencephalitis: case report of a recently included AIDS-defining illness in Brazil. Rev. Inst. Med. Trop. Sao Paulo 46:199-202. [DOI] [PubMed] [Google Scholar]
- 370.Madarova, L., K. Trnkova, F. Sona, K. Cyril, and O. Margita. 2010. A real-time PCR diagnostic method for detection of Naegleria fowleri. Exp. Parasitol. 126:37-41. [DOI] [PubMed] [Google Scholar]
- 371.Maia da Silva, F., A. Marcili, P. A. Ortiz, S. Epiphanio, M. Campaner, J. L. Catao-Dias, J. J. Shaw, E. P. Camargo, and M. M. Teixeira. 2010. Phylogenetic, morphological and behavioural analyses support host switching of Trypanosoma (Herpetosoma) lewisi from domestic rats to primates. Infect. Genet. Evol. 10:522-529. [DOI] [PubMed] [Google Scholar]
- 372.Malan, A. K., E. Avelar, S. E. Litwin, H. R. Hill, and C. M. Litwin. 2006. Serological diagnosis of Trypanosoma cruzi: evaluation of three enzyme immunoassays and an indirect immunofluorescent assay. J. Med. Microbiol. 55:171-178. [DOI] [PubMed] [Google Scholar]
- 373.Maldonado, C., S. Albano, L. Vettorazzi, O. Salomone, J. C. Zlocowski, C. Abiega, M. Amuchastegui, R. Cordoba, and T. Alvarellos. 2004. Using polymerase chain reaction in early diagnosis of re-activated Trypanosoma cruzi infection after heart transplantation. J. Heart Lung Transplant. 23:1345-1348. [DOI] [PubMed] [Google Scholar]
- 374.Maltezou, H. C. 2008. Visceral leishmaniasis: advances in treatment. Recent Pat. Antiinfect Drug Discov. 3:192-198. [DOI] [PubMed] [Google Scholar]
- 375.Marathe, A., J. Tripathi, V. Handa, and V. Date. 2005. Human babesiosis—a case report. Indian J. Med. Microbiol. 23:267-269. [PubMed] [Google Scholar]
- 376.Marcet-Houben, M., G. Marceddu, and T. Gabaldon. 2009. Phylogenomics of the oxidative phosphorylation in fungi reveals extensive gene duplication followed by functional divergence. BMC Evol. Biol. 9:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Marciano-Cabral, F., and G. Cabral. 2003. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16:273-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Marciano-Cabral, F., R. MacLean, A. Mensah, and L. LaPat-Polasko. 2003. Identification of Naegleria fowleri in domestic water sources by nested PCR. Appl. Environ. Microbiol. 69:5864-5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Martin-Davila, P., J. Fortun, R. Lopez-Velez, F. Norman, M. Montes de Oca, P. Zamarron, M. I. Gonzalez, A. Moreno, T. Pumarola, G. Garrido, A. Candela, and S. Moreno. 2008. Transmission of tropical and geographically restricted infections during solid-organ transplantation. Clin. Microbiol. Rev. 21:60-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Martinez, A. J., F. L. Schuster, and G. S. Visvesvara. 2001. Balamuthia mandrillaris: its pathogenic potential. J. Eukaryot. Microbiol. 2001(Suppl.):6S-9S. [DOI] [PubMed] [Google Scholar]
- 381.Martinkovic, F., and A. Marinculic. 2006. Antibodies against Leishmania cross-react with Crithidia luciliae: indirect immunofluorescence and dot-ELISA study in dogs. Parasitol. Res. 98:378-380. [DOI] [PubMed] [Google Scholar]
- 382.Maschke, M., O. Kastrup, S. Esser, B. Ross, U. Hengge, and A. Hufnagel. 2000. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART). J. Neurol. Neurosurg. Psychiatry 69:376-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Mastroianni, A., O. Coronado, P. Scarani, R. Manfredi, and F. Chiodo. 1996. Liver toxoplasmosis and acquired immunodeficiency syndrome. Recenti Prog. Med. 87:353-355. (In Italian.) [PubMed] [Google Scholar]
- 384.Matete, G. O., and O. A. Kajejo. 2005. Human African trypanosomiasis and human immunodeficiency virus co-infection in Western Kenya. East Afr. Med. J. 82:20-23. [DOI] [PubMed] [Google Scholar]
- 385.Matsui, T., R. Inoue, K. Kajimoto, A. Tamekane, A. Okamura, Y. Katayama, M. Shimoyama, K. Chihara, A. Saito-Ito, and M. Tsuji. 2000. First documentation of transfusion-associated babesiosis in Japan. Rinsho Ketsueki 41:628-634. (In Japanese.) [PubMed] [Google Scholar]
- 386.May, L. P., G. S. Sidhu, and M. R. Buchness. 1992. Diagnosis of Acanthamoeba infection by cutaneous manifestations in a man seropositive to HIV. J. Am. Acad. Dermatol. 26:352-355. [DOI] [PubMed] [Google Scholar]
- 387.McCann, C. M., A. J. Vyse, R. L. Salmon, D. Thomas, D. J. Williams, J. W. McGarry, R. Pebody, and A. J. Trees. 2008. Lack of serologic evidence of Neospora caninum in humans, England. Emerg. Infect. Dis. 14:978-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Mehlhorn, H., and A. O. Heydorn. 2000. Neospora caninum: is it really different from Hammondia heydorni or is it a strain of Toxoplasma gondii? An opinion. Parasitol. Res. 86:169-178. [DOI] [PubMed] [Google Scholar]
- 389.Meister, J. 1999. Human babesiosis: a case study. Clin. Excell. Nurse Pract. 3:214-216. [PubMed] [Google Scholar]
- 390.Menotti, J., B. Cassinat, C. Sarfati, O. Liguory, F. Derouin, and J. M. Molina. 2003. Development of a real-time PCR assay for quantitative detection of Encephalitozoon intestinalis DNA. J. Clin. Microbiol. 41:1410-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Mihoubi, I., F. de Monbrison, N. Romeuf, T. Moulahem, and S. Picot. 2006. Outsourced real-time PCR diagnosis of cutaneous leishmaniasis in the outbreak region of Constatine, Algeria. Med. Trop. (Mars.) 66:39-44. (In French.) [PubMed] [Google Scholar]
- 392.Miller, R. F., M. A. Hall-Craggs, D. C. Costa, N. S. Brink, F. Scaravilli, S. B. Lucas, I. D. Wilkinson, P. J. Ell, B. E. Kendall, and M. J. Harrison. 1998. Magnetic resonance imaging, thallium-201 SPET scanning, and laboratory analyses for discrimination of cerebral lymphoma and toxoplasmosis in AIDS. Sex. Transm. Infect. 74:258-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Mofenson, L. M., M. T. Brady, S. P. Danner, K. L. Dominguez, R. Hazra, E. Handelsman, P. Havens, S. Nesheim, J. S. Read, L. Serchuck, and R. Van Dyke. 2009. Guidelines for the prevention and treatment of opportunistic infections among HIV-exposed and HIV-infected children: recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR Recomm. Rep. 58:1-166. [PMC free article] [PubMed] [Google Scholar]
- 394.Moncayo, A., and M. I. Ortiz Yanine. 2006. An update on Chagas disease (human American trypanosomiasis). Ann. Trop. Med. Parasitol. 100:663-677. [DOI] [PubMed] [Google Scholar]
- 395.Mondain-Miton, V., M. Toussaint-Gari, P. Hofman, P. Marty, M. Carles, F. De Salvador, F. Miton, Y. Le Fichoux, and P. Dellamonica. 1995. Atypical leishmaniasis in a patient infected with human immunodeficiency virus. Clin. Infect. Dis. 21:663-665. [DOI] [PubMed] [Google Scholar]
- 396.Mortarino, M., A. Franceschi, F. Mancianti, C. Bazzocchi, C. Genchi, and C. Bandi. 2004. Quantitative PCR in the diagnosis of Leishmania. Parassitologia 46:163-167. [PubMed] [Google Scholar]
- 397.Mugasa, C. M., T. Laurent, G. J. Schoone, P. A. Kager, G. W. Lubega, and H. D. Schallig. 2009. Nucleic acid sequence-based amplification with oligochromatography for detection of Trypanosoma brucei in clinical samples. J. Clin. Microbiol. 47:630-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Muller, A., R. Bialek, A. Kamper, G. Fatkenheuer, B. Salzberger, and C. Franzen. 2001. Detection of microsporidia in travelers with diarrhea. J. Clin. Microbiol. 39:1630-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Munoz, J., M. Portus, M. Corachan, V. Fumado, and J. Gascon. 2007. Congenital Trypanosoma cruzi infection in a non-endemic area. Trans. R. Soc. Trop. Med. Hyg. 101:1161-1162. [DOI] [PubMed] [Google Scholar]
- 400.Myler, P. J. 2008. Searching the Tritryp genomes for drug targets. Adv. Exp. Med. Biol. 625:133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Nachega, J. B., P. Rombaux, B. Weynand, G. Thomas, and F. Zech. 2005. Successful treatment of Acanthamoeba rhinosinusitis in a patient with AIDS. AIDS Patient Care STDs 19:621-625. [DOI] [PubMed] [Google Scholar]
- 402.Negash, T., G. Tilahun, and G. Medhin. 2008. Seroprevalence of Toxoplasma gondii in Nazaret town, Ethiopia. East Afr. J. Public Health 5:211-214. [PubMed] [Google Scholar]
- 403.Ngotho, M., J. M. Kagira, H. E. Jensen, S. M. Karanja, I. O. Farah, and J. Hau. 2009. Immunospecific immunoglobulins and IL-10 as markers for Trypanosoma brucei rhodesiense late stage disease in experimentally infected vervet monkeys. Trop. Med. Int. Health 14:736-747. [DOI] [PubMed] [Google Scholar]
- 404.Nishikawa, A., H. Yamada, T. Yamamoto, Y. Mizue, Y. Akashi, T. Hayashi, T. Nihei, M. Nishiwaki, and J. Nishihira. 2009. A case of congenital toxoplasmosis whose mother demonstrated serum low IgG avidity and positive tests for multiplex-nested PCR in the amniotic fluid. J. Obstet. Gynaecol. Res. 35:372-378. [DOI] [PubMed] [Google Scholar]
- 405.Nissapatorn, V., C. Lee, K. F. Quek, C. L. Leong, R. Mahmud, and K. A. Abdullah. 2004. Toxoplasmosis in HIV/AIDS patients: a current situation. Jpn. J. Infect. Dis. 57:160-165. [PubMed] [Google Scholar]
- 406.Nogui, F. L., S. Mattas, G. Turcato, Jr., and D. S. Lewi. 2009. Neurotoxoplasmosis diagnosis for HIV-1 patients by real-time PCR of cerebrospinal fluid. Braz. J. Infect. Dis. 13:18-23. [DOI] [PubMed] [Google Scholar]
- 407.Noyes, H., F. Pratlong, M. Chance, J. Ellis, G. Lanotte, and J. P. Dedet. 2002. A previously unclassified trypanosomatid responsible for human cutaneous lesions in Martinique (French West Indies) is the most divergent member of the genus Leishmania ss. Parasitology 124:17-24. [DOI] [PubMed] [Google Scholar]
- 408.Ogden, G. B., and P. C. Melby. 2009. Encyclopedia of microbiology, 3rd ed., p. 663-673. Elsevier Inc., Philadelphia, PA.
- 409.Okay, T. S., L. Yamamoto, L. C. Oliveira, E. R. Manuli, H. F. Andrade, Jr., and G. M. Del Negro. 2009. Significant performance variation among PCR systems in diagnosing congenital toxoplasmosis in Sao Paulo, Brazil: analysis of 467 amniotic fluid samples. Clinics (Sao Paulo) 64:171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Okuda, D. T., H. J. Hanna, S. W. Coons, and J. B. Bodensteiner. 2004. Naegleria fowleri hemorrhagic meningoencephalitis: report of two fatalities in children. J. Child Neurol. 19:231-233. [PubMed] [Google Scholar]
- 411.Oliva, S., M. Jantz, R. Tiernan, D. L. Cook, and M. A. Judson. 1999. Successful treatment of widely disseminated acanthamoebiasis. South. Med. J. 92:55-57. [DOI] [PubMed] [Google Scholar]
- 412.Ollomo, B., S. Karch, P. Bureau, N. Elissa, A. J. Georges, and P. Millet. 1997. Lack of malaria parasite transmission between apes and humans in Gabon. Am. J. Trop. Med. Hyg. 56:440-445. [DOI] [PubMed] [Google Scholar]
- 413.Olowe, S. A. 1975. A case of congenital trypanosomiasis in Lagos. Trans. R. Soc. Trop. Med. Hyg. 69:57-59. [DOI] [PubMed] [Google Scholar]
- 414.Ondarza, R. N., A. Iturbe, and E. Hernandez. 2006. In vitro antiproliferative effects of neuroleptics, antimycotics and antibiotics on the human pathogens Acanthamoeba polyphaga and Naegleria fowleri. Arch. Med. Res. 37:723-729. [DOI] [PubMed] [Google Scholar]
- 415.Ong, C. W., S. Y. Lee, W. H. Koh, E. E. Ooi, and P. A. Tambyah. 2009. Monkey malaria in humans: a diagnostic dilemma with conflicting laboratory data. Am. J. Trop. Med. Hyg. 80:927-928. [PubMed] [Google Scholar]
- 416.Otto, M. A., A. S. da Silva, L. T. Gressler, M. H. Farret, K. C. Tavares, R. A. Zanette, L. C. Miletti, and S. G. Monteiro. 2010. Susceptibility of Trypanosoma evansi to human blood and plasma in infected mice. Vet. Parasitol. 168:1-4. [DOI] [PubMed] [Google Scholar]
- 417.Pacheco, R. S., M. C. Marzochi, M. Q. Pires, C. M. Brito, F. Madeira Mde, and E. G. Barbosa-Santos. 1998. Parasite genotypically related to a monoxenous trypanosomatid of dog's flea causing opportunistic infection in an HIV positive patient. Mem. Inst. Oswaldo Cruz 93:531-537. [DOI] [PubMed] [Google Scholar]
- 418.Padilla, A. M., J. D. Marco, P. Diosque, M. A. Segura, M. C. Mora, M. M. Fernandez, E. L. Malchiodi, and M. A. Basombrio. 2002. Canine infection and the possible role of dogs in the transmission of American tegumentary leishmaniosis in Salta, Argentina. Vet. Parasitol. 110:1-10. [DOI] [PubMed] [Google Scholar]
- 419.Pappas, G., N. Roussos, and M. E. Falagas. 2009. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol. 39:1385-1394. [DOI] [PubMed] [Google Scholar]
- 420.Paredes, R., J. Munoz, I. Diaz, P. Domingo, M. Gurgui, and B. Clotet. 2003. Leishmaniasis in HIV infection. J. Postgrad. Med. 49:39-49. [DOI] [PubMed] [Google Scholar]
- 421.Parodi, C., A. M. Padilla, and M. A. Basombrio. 2009. Protective immunity against Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz 104(Suppl. 1):288-294. [DOI] [PubMed] [Google Scholar]
- 422.Patel, R. 1999. Disseminated toxoplasmosis after liver transplantation. Clin. Infect. Dis. 29:705-706. [DOI] [PubMed] [Google Scholar]
- 423.Pays, E., and B. Vanhollebeke. 2008. Mutual self-defence: the trypanolytic factor story. Microbes Infect. 10:985-989. [DOI] [PubMed] [Google Scholar]
- 424.Pays, E., B. Vanhollebeke, L. Vanhamme, F. Paturiaux-Hanocq, D. P. Nolan, and D. Perez-Morga. 2006. The trypanolytic factor of human serum. Nat. Rev. Microbiol. 4:477-486. [DOI] [PubMed] [Google Scholar]
- 425.Pays, J. F. 1998. American human trypanosomiasis 90 years after its discovery by Carlos Chagas. I. Epidemiology and control. Med. Trop. (Mars.) 58:391-402. (In French.) [PubMed] [Google Scholar]
- 426.Peleg, A. Y., S. Husain, E. J. Kwak, F. P. Silveira, M. Ndirangu, J. Tran, K. A. Shutt, R. Shapiro, N. Thai, K. Abu-Elmagd, K. R. McCurry, A. Marcos, and D. L. Paterson. 2007. Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal CD-52 antibody. Clin. Infect. Dis. 44:204-212. [DOI] [PubMed] [Google Scholar]
- 427.Pepin, J., C. Guern, F. Milord, L. Ethier, M. Bokelo, and P. J. Schechter. 1989. The use of difluoromethylornithine in congenital trypanosomiasis due to Trypanosoma brucei-gambiense. Med. Trop. (Mars.) 49:83-85. (In French.) [PubMed] [Google Scholar]
- 428.Peregudova, A. B., V. I. Shakhgil'dian, D. B. Goncharov, T. N. Ermak, I. M. Tishkevich, O. Shipulina, N. V. Gorlova, and B. M. Gruzdev. 2007. Cerebral toxoplasmosis in HIV-infected patients. Ter. Arkh. 79:36-39. (In Russian.) [PubMed] [Google Scholar]
- 429.Pereira-Chioccola, V. L., J. E. Vidal, and C. Su. 2009. Toxoplasma gondii infection and cerebral toxoplasmosis in HIV-infected patients. Future Microbiol. 4:1363-1379. [DOI] [PubMed] [Google Scholar]
- 430.Perez, M. T., and L. M. Bush. 2007. Fatal amebic encephalitis caused by Balamuthia mandrillaris in an immunocompetent host: a clinicopathological review of pathogenic free-living amebae in human hosts. Ann. Diagn. Pathol. 11:440-447. [DOI] [PubMed] [Google Scholar]
- 431.Persing, D. H., B. L. Herwaldt, C. Glaser, R. S. Lane, J. W. Thomford, D. Mathiesen, P. J. Krause, D. F. Phillip, and P. A. Conrad. 1995. Infection with a Babesia-like organism in northern California. N. Engl. J. Med. 332:298-303. [DOI] [PubMed] [Google Scholar]
- 432.Petersen, E. 2007. Toxoplasmosis. Semin. Fetal Neonatal Med. 12:214-223. [DOI] [PubMed] [Google Scholar]
- 433.Petersen, E., B. Edvinsson, B. Lundgren, T. Benfield, and B. Evengard. 2006. Diagnosis of pulmonary infection with Toxoplasma gondii in immunocompromised HIV-positive patients by real-time PCR. Eur. J. Clin. Microbiol. Infect. Dis. 25:401-404. [DOI] [PubMed] [Google Scholar]
- 434.Petersen, E., M. Lebech, L. Jensen, P. Lind, M. Rask, P. Bagger, C. Bjorkman, and A. Uggla. 1999. Neospora caninum infection and repeated abortions in humans. Emerg. Infect. Dis. 5:278-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Pfaff, A. W., A. Abou-Bacar, V. Letscher-Bru, O. Villard, A. Senegas, M. Mousli, and E. Candolfi. 2007. Cellular and molecular physiopathology of congenital toxoplasmosis: the dual role of IFN-gamma. Parasitology 134:1895-1902. [DOI] [PubMed] [Google Scholar]
- 436.Pfaff, A. W., and E. Candolfi. 2003. Immune responses to protozoan parasites and its relevance to diagnosis in immunocompromised patients. Eur. J. Protistol. 39:428-434. [Google Scholar]
- 437.Phan, B. A., M. A. Laflamme, A. Stempien-Otero, A. P. Limaye, F. S. Buckner, and W. C. Levy. 2006. Confirmation of Chagas' cardiomyopathy following heart transplantation. Heart Vessels 21:325-327. [DOI] [PubMed] [Google Scholar]
- 438.Piarroux, R., F. Gambarelli, H. Dumon, M. Fontes, S. Dunan, C. Mary, B. Toga, and M. Quilici. 1994. Comparison of PCR with direct examination of bone marrow aspiration, myeloculture, and serology for diagnosis of visceral leishmaniasis in immunocompromised patients. J. Clin. Microbiol. 32:746-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Pierce, S. K., and L. H. Miller. 2009. World Malaria Day 2009: what malaria knows about the immune system that immunologists still do not. J. Immunol. 182:5171-5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Pineda, J. A., J. A. Gallardo, J. Macias, J. Delgado, C. Regordan, F. Morillas, F. Relimpio, J. Martin-Sanchez, A. Sanchez-Quijano, M. Leal, and E. Lissen. 1998. Prevalence of and factors associated with visceral leishmaniasis in human immunodeficiency virus type 1-infected patients in southern Spain. J. Clin. Microbiol. 36:2419-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Pinero, J., E. Martinez, R. Pacheco, Z. Aragon, F. De Armas, A. Del Castillo, and B. Valladares. 1999. PCR-ELISA for diagnosis of mucocutaneous leishmaniasis. Acta Trop. 73:21-29. [DOI] [PubMed] [Google Scholar]
- 442.Pinheiro, R. O., E. F. Pinto, A. B. Benedito, U. G. Lopes, and B. Rossi-Bergmann. 2004. The T-cell anergy induced by Leishmania amazonensis antigens is related with defective antigen presentation and apoptosis. An. Acad. Bras. Cienc. 76:519-527. [DOI] [PubMed] [Google Scholar]
- 443.Pinto, A. Y., S. A. Valente, and V. da Costa Valente. 2004. Emerging acute Chagas disease in Amazonian Brazil: case reports with serious cardiac involvement. Braz. J. Infect. Dis. 8:454-460. [DOI] [PubMed] [Google Scholar]
- 444.Pinto, A. Y., S. A. Valente, V. da Costa Valente, A. G. Ferreira, Jr., and J. R. Coura. 2008. Acute phase of Chagas disease in the Brazilian Amazon region: study of 233 cases from Para, Amapa and Maranhao observed between 1988 and 2005. Rev. Soc. Bras. Med. Trop. 41:602-614. (In Portugese.) [DOI] [PubMed] [Google Scholar]
- 445.Pinto, C. M., S. Ocana-Mayorga, M. S. Lascano, and M. J. Grijalva. 2006. Infection by trypanosomes in marsupials and rodents associated with human dwellings in Ecuador. J. Parasitol. 92:1251-1255. [DOI] [PubMed] [Google Scholar]
- 446.Piron, M., R. Fisa, N. Casamitjana, P. Lopez-Chejade, L. Puig, M. Verges, J. Gascon, J. Gomez i Prat, M. Portus, and S. Sauleda. 2007. Development of a real-time PCR assay for Trypanosoma cruzi detection in blood samples. Acta Trop. 103:195-200. [DOI] [PubMed] [Google Scholar]
- 447.Pittner, Y., J. F. Dufour, G. David, A. Boibieux, and D. Peyramond. 2009. Spinal cord toxoplasmosis in HIV infection. Med. Mal. Infect. 39:401-405. (In French.) [DOI] [PubMed] [Google Scholar]
- 448.Pleyer, U., N. Torun, and O. Liesenfeld. 2007. Ocular toxoplasmosis. Ophthalmologe 104:603-615. (In German.) [DOI] [PubMed] [Google Scholar]
- 449.Postigo, C., R. Llamas, C. Zarco, R. Rubio, F. Pulido, J. R. Costa, and L. Iglesias. 1997. Cutaneous lesions in patients with visceral leishmaniasis and HIV infection. J. Infect. 35:265-268. [DOI] [PubMed] [Google Scholar]
- 450.Potestio, M., P. D'Agostino, G. C. Romano, S. Milano, V. Ferlazzo, A. Aquino, G. Di Bella, R. Caruso, G. Gambino, G. Vitale, S. Mansueto, and E. Cillari. 2004. CD4+ CCR5+ and CD4+ CCR3+ lymphocyte subset and monocyte apoptosis in patients with acute visceral leishmaniasis. Immunology 113:260-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Pradhan, S., R. Yadav, and V. N. Mishra. 2007. Toxoplasma meningoencephalitis in HIV-seronegative patients: clinical patterns, imaging features and treatment outcome. Trans. R. Soc. Trop. Med. Hyg. 101:25-33. [DOI] [PubMed] [Google Scholar]
- 452.Prasad, K., R. Bhatia, M. V. Srivastava, V. Pardasani, A. Garg, and A. Rishi. 2008. Fatal subacute necrotising brainstem encephalitis in a young man due to a rare parasitic (Balamuthia) infection. Pract. Neurol. 8:112-117. [DOI] [PubMed] [Google Scholar]
- 453.Prasil, P. 2009. Current options for the diagnosis and therapy of toxoplasmosis in HIV-negative patients. Klin. Mikrobiol. Infekc. Lek. 15:83-90. (In Czech.) [PubMed] [Google Scholar]
- 454.Prata, A. 2001. Clinical and epidemiological aspects of Chagas disease. Lancet Infect. Dis. 1:92-100. [DOI] [PubMed] [Google Scholar]
- 455.Priotto, G., S. Kasparian, W. Mutombo, D. Ngouama, S. Ghorashian, U. Arnold, S. Ghabri, E. Baudin, V. Buard, S. Kazadi-Kyanza, M. Ilunga, W. Mutangala, G. Pohlig, C. Schmid, U. Karunakara, E. Torreele, and V. Kande. 2009. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Lancet 374:56-64. [DOI] [PubMed] [Google Scholar]
- 456.Pritzker, A. S., B. K. Kim, D. Agrawal, P. M. Southern, Jr., and A. G. Pandya. 2004. Fatal granulomatous amebic encephalitis caused by Balamuthia mandrillaris presenting as a skin lesion. J. Am. Acad. Dermatol. 50:S38-S41. [DOI] [PubMed] [Google Scholar]
- 457.Punukollu, G., R. M. Gowda, I. A. Khan, V. S. Navarro, and B. C. Vasavada. 2007. Clinical aspects of the Chagas' heart disease. Int. J. Cardiol. 115:279-283. [DOI] [PubMed] [Google Scholar]
- 458.Puzon, G. J., J. A. Lancaster, J. T. Wylie, and I. J. Plumb. 2009. Rapid detection of Naegleria fowleri in water distribution pipeline biofilms and drinking water samples. Environ. Sci. Technol. 43:6691-6696. [DOI] [PubMed] [Google Scholar]
- 459.Quick, R. E., B. L. Herwaldt, J. W. Thomford, M. E. Garnett, M. L. Eberhard, M. Wilson, D. H. Spach, J. W. Dickerson, S. R. Telford III, K. R. Steingart, R. Pollock, D. H. Persing, J. M. Kobayashi, D. D. Juranek, and P. A. Conrad. 1993. Babesiosis in Washington State: a new species of Babesia? Ann. Intern. Med. 119:284-290. [DOI] [PubMed] [Google Scholar]
- 460.Quintana, E., Y. Torres, C. Alvarez, A. Rojas, M. E. Forero, and M. Camacho. 2010. Changes in macrophage membrane properties during early Leishmania amazonensis infection differ from those observed during established infection and are partially explained by phagocytosis. Exp. Parasitol. 124:258-264. [DOI] [PubMed] [Google Scholar]
- 461.Qvarnstrom, Y., A. J. da Silva, F. L. Schuster, B. B. Gelman, and G. S. Visvesvara. 2009. Molecular confirmation of Sappinia pedata as a causative agent of amoebic encephalitis. J. Infect. Dis. 199:1139-1142. [DOI] [PubMed] [Google Scholar]
- 462.Qvarnstrom, Y., G. S. Visvesvara, R. Sriram, and A. J. da Silva. 2006. Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J. Clin. Microbiol. 44:3589-3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Ramos-Santos, C., O. Hernandez-Montes, G. Sanchez-Tejeda, and A. Monroy-Ostria. 2000. Visceral leishmaniosis caused by Leishmania (L.) mexicana in a Mexican patient with human immunodeficiency virus infection. Mem. Inst. Oswaldo Cruz 95:733-737. [DOI] [PubMed] [Google Scholar]
- 464.Rashid, J. R., P. M. Nyakundi, G. Kirigi, D. Kinoti, and M. K. Wasunna. 2002. Compassionate use of sitamaquine in an HIV-positive patient with visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 96:533-534. [DOI] [PubMed] [Google Scholar]
- 465.Rassi, A., Jr., A. Rassi, and J. A. Marin-Neto. 2010. Chagas disease. Lancet 375:1388-1402. [DOI] [PubMed] [Google Scholar]
- 466.Rastogi, V., and P. S. Nirwan. 2007. Cutaneous leishmaniasis: an emerging infection in a non-endemic area and a brief update. Indian J. Med. Microbiol. 25:272-275. [DOI] [PubMed] [Google Scholar]
- 467.Raucher, H. S., H. Jaffin, and J. L. Glass. 1984. Babesiosis in pregnancy. Obstet. Gynecol. 63:7S-9S. [PubMed] [Google Scholar]
- 468.Reddy, A. K., P. K. Balne, K. Gaje, and P. Garg. 2009. Polymerase chain reaction for the diagnosis and species identification of microsporidia in patients with keratitis. Clin. Microbiol. Infect. [Epub ahead of print.] doi: 10.1111/j.1469-0691.2009.03152.x. [DOI] [PubMed]
- 469.Reed, R. P., C. M. Cooke-Yarborough, A. L. Jaquiery, K. Grimwood, A. S. Kemp, J. C. Su, and J. R. Forsyth. 1997. Fatal granulomatous amoebic encephalitis caused by Balamuthia mandrillaris. Med. J. Aust. 167:82-84. [DOI] [PubMed] [Google Scholar]
- 470.Reichel, M. P., and J. T. Ellis. 2009. Neospora caninum—how close are we to development of an efficacious vaccine that prevents abortion in cattle? Int. J. Parasitol. 39:1173-1187. [DOI] [PubMed] [Google Scholar]
- 471.Reid, S. A. 2002. Trypanosoma evansi control and containment in Australasia. Trends Parasitol. 18:219-224. [DOI] [PubMed] [Google Scholar]
- 472.Reiter-Owona, I., R. Bialek, J. K. Rockstroh, and H. M. Seitz. 1998. The probability of acquiring primary toxoplasma infection in HIV-infected patients: results of an 8-year retrospective study. Infection 26:20-25. [DOI] [PubMed] [Google Scholar]
- 473.Reithinger, R., J. C. Dujardin, H. Louzir, C. Pirmez, B. Alexander, and S. Brooker. 2007. Cutaneous leishmaniasis. Lancet Infect. Dis. 7:581-596. [DOI] [PubMed] [Google Scholar]
- 474.Rios, L., G. Alvarez, and S. Blair. 2003. Serological and parasitological study and report of the first case of human babesiosis in Colombia. Rev. Soc. Bras. Med. Trop. 36:493-498. (In Portugese.) [DOI] [PubMed] [Google Scholar]
- 475.Rittig, M. G., and C. Bogdan. 2000. Leishmania-host-cell interaction: complexities and alternative views. Parasitol. Today 16:292-297. [DOI] [PubMed] [Google Scholar]
- 476.Rivera, J., L. D. Hillis, and B. D. Levine. 2004. Reactivation of cardiac Chagas' disease in acquired immune deficiency syndrome. Am. J. Cardiol. 94:1102-1103. [DOI] [PubMed] [Google Scholar]
- 477.Rivera, M. A., and T. A. Padhya. 2002. Acanthamoeba: a rare primary cause of rhinosinusitis. Laryngoscope 112:1201-1203. [DOI] [PubMed] [Google Scholar]
- 478.Robert-Gangneux, F., and F. Klein. 2009. Serologic screening for Neospora caninum, France. Emerg. Infect. Dis. 15:987-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Robinson, B. S., P. T. Monis, and P. J. Dobson. 2006. Rapid, sensitive, and discriminating identification of Naegleria spp. by real-time PCR and melting-curve analysis. Appl. Environ. Microbiol. 72:5857-5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Rocha, G., A. Martins, G. Gama, F. Brandao, and J. Atouguia. 2004. Possible cases of sexual and congenital transmission of sleeping sickness. Lancet 363:247. [DOI] [PubMed] [Google Scholar]
- 481.Rocha, M. O., M. M. Teixeira, and A. L. Ribeiro. 2007. An update on the management of Chagas cardiomyopathy. Expert Rev. Anti Infect. Ther. 5:727-743. [DOI] [PubMed] [Google Scholar]
- 482.Rogerson, S. J. 2010. Malaria in pregnancy and the newborn. Adv. Exp. Med. Biol. 659:139-152. [DOI] [PubMed] [Google Scholar]
- 483.Romano, A., A. Trisciuoglio, D. Grande, and E. Ferroglio. 2009. Comparison of two PCR protocols for the detection of Neospora caninum DNA in rodents. Vet. Parasitol. 159:159-161. [DOI] [PubMed] [Google Scholar]
- 484.Romero, H. D., L. de Almeida Silva, M. L. Silva-Vergara, V. Rodrigues, R. T. Costa, S. F. Guimaraes, W. Alecrim, H. Moraes-Souza, and A. Prata. 2009. Comparative study of serologic tests for the diagnosis of asymptomatic visceral leishmaniasis in an endemic area. Am. J. Trop. Med. Hyg. 81:27-33. [PubMed] [Google Scholar]
- 485.Ronday, M. J., J. S. Stilma, R. F. Barbe, W. J. McElroy, L. Luyendijk, A. H. Kolk, M. Bakker, A. Kijlstra, and A. Rothova. 1996. Aetiology of uveitis in Sierra Leone, West Africa. Br. J. Ophthalmol. 80:956-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Rosenberg, A. S., and M. B. Morgan. 2001. Disseminated acanthamoebiasis presenting as lobular panniculitis with necrotizing vasculitis in a patient with AIDS. J. Cutan. Pathol. 28:307-313. [DOI] [PubMed] [Google Scholar]
- 487.Rosenblatt, J. E. 2009. Laboratory diagnosis of infections due to blood and tissue parasites. Clin. Infect. Dis. 49:1103-1108. [DOI] [PubMed] [Google Scholar]
- 488.Rosenthal, E., and P. Marty. 2003. Recent understanding in the treatment of visceral leishmaniasis. J. Postgrad. Med. 49:61-68. [DOI] [PubMed] [Google Scholar]
- 489.Rossi, P., C. Urbani, G. Donelli, and E. Pozio. 1999. Resolution of microsporidial sinusitis and keratoconjunctivitis by itraconazole treatment. Am. J. Ophthalmol. 127:210-212. [DOI] [PubMed] [Google Scholar]
- 490.Safaei, A., M. H. Motazedian, and M. Vasei. 2002. Polymerase chain reaction for diagnosis of cutaneous leishmaniasis in histologically positive, suspicious and negative skin biopsies. Dermatology 205:18-24. [DOI] [PubMed] [Google Scholar]
- 491.Sagoo, M. S., J. S. Mehta, S. Hau, L. D. Irion, A. Curry, R. E. Bonshek, and S. J. Tuft. 2007. Microsporidium stromal keratitis: in vivo confocal findings. Cornea 26:870-873. [DOI] [PubMed] [Google Scholar]
- 492.Saito-Ito, A., N. Takada, F. Ishiguro, H. Fujita, Y. Yano, X. H. Ma, and E. R. Chen. 2008. Detection of Kobe-type Babesia microti associated with Japanese human babesiosis in field rodents in central Taiwan and southeastern mainland China. Parasitology 135:691-699. [DOI] [PubMed] [Google Scholar]
- 493.Saito-Ito, A., M. Tsuji, Q. Wei, S. He, T. Matsui, M. Kohsaki, S. Arai, T. Kamiyama, K. Hioki, and C. Ishihara. 2000. Transfusion-acquired, autochthonous human babesiosis in Japan: isolation of Babesia microti-like parasites with hu-RBC-SCID mice. J. Clin. Microbiol. 38:4511-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Sanchez-Sancho, F., N. E. Campillo, and J. A. Paez. 2010. Chagas disease: progress and new perspectives. Curr. Med. Chem. 17:423-452. [DOI] [PubMed] [Google Scholar]
- 495.Sarataphan, N., M. Vongpakorn, B. Nuansrichay, N. Autarkool, T. Keowkarnkah, P. Rodtian, R. W. Stich, and S. Jittapalapong. 2007. Diagnosis of a Trypanosoma lewisi-like (Herpetosoma) infection in a sick infant from Thailand. J. Med. Microbiol. 56:1118-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Sartori, A. M., M. H. Lopes, L. A. Benvenuti, B. Caramelli, A. di Pietro, E. V. Nunes, L. P. Ramirez, and M. A. Shikanai-Yasuda. 1998. Reactivation of Chagas' disease in a human immunodeficiency virus-infected patient leading to severe heart disease with a late positive direct microscopic examination of the blood. Am. J. Trop. Med. Hyg. 59:784-786. [DOI] [PubMed] [Google Scholar]
- 497.Savoia, D., T. Allice, and P.-A. Tovo. 2005. Antileishmanial activity of HIV protease inhibitors. Int. J. Antimicrob. Agents 26:92-94. [DOI] [PubMed] [Google Scholar]
- 498.Scaglia, M., L. Sacchi, G. P. Croppo, A. da Silva, S. Gatti, S. Corona, A. Orani, A. M. Bernuzzi, N. J. Pieniazek, S. B. Slemenda, S. Wallace, and G. S. Visvesvara. 1997. Pulmonary microsporidiosis due to Encephalitozoon hellem in a patient with AIDS. J. Infect. 34:119-126. [DOI] [PubMed] [Google Scholar]
- 499.Schantz-Dunn, J., and N. M. Nour. 2009. Malaria and pregnancy: a global health perspective. Rev. Obstet. Gynecol. 2:186-192. [PMC free article] [PubMed] [Google Scholar]
- 500.Schild, M., C. Gianinazzi, B. Gottstein, and N. Muller. 2007. PCR-based diagnosis of Naegleria sp. infection in formalin-fixed and paraffin-embedded brain sections. J. Clin. Microbiol. 45:564-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Schmid, N., P. Deplazes, S. Hoby, M. P. Ryser-Degiorgis, R. Edelhofer, and A. Mathis. 2008. Babesia divergens-like organisms from free-ranging chamois (Rupicapra r. rupicapra) and roe deer (Capreolus c. capreolus) are distinct from B. divergens of cattle origin—an epidemiological and molecular genetic investigation. Vet. Parasitol. 154:14-20. [DOI] [PubMed] [Google Scholar]
- 502.Schmunis, G. A., and Z. E. Yadon. 2010. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 115:14-21. [DOI] [PubMed] [Google Scholar]
- 503.Schoeman, C. J., A. E. van der Vyver, and G. S. Visvesvara. 1993. Primary amoebic meningo-encephalitis in southern Africa. J. Infect. 26:211-214. [DOI] [PubMed] [Google Scholar]
- 504.Schuster, F. L. 2002. Cultivation of pathogenic and opportunistic free-living amebas. Clin. Microbiol. Rev. 15:342-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Schuster, F. L., T. H. Dunnebacke, G. C. Booton, S. Yagi, C. K. Kohlmeier, C. Glaser, D. Vugia, A. Bakardjiev, P. Azimi, M. Maddux-Gonzalez, A. J. Martinez, and G. S. Visvesvara. 2003. Environmental isolation of Balamuthia mandrillaris associated with a case of amebic encephalitis. J. Clin. Microbiol. 41:3175-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Schuster, F. L., B. J. Guglielmo, and G. S. Visvesvara. 2006. In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J. Eukaryot. Microbiol. 53:121-126. [DOI] [PubMed] [Google Scholar]
- 507.Schuster, F. L., and G. S. Visvesvara. 1996. Axenic growth and drug sensitivity studies of Balamuthia mandrillaris, an agent of amebic meningoencephalitis in humans and other animals. J. Clin. Microbiol. 34:385-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Schuster, F. L., S. Yagi, S. Gavali, D. Michelson, R. Raghavan, I. Blomquist, C. Glastonbury, A. W. Bollen, D. Scharnhorst, S. L. Reed, S. Kuriyama, G. S. Visvesvara, and C. A. Glaser. 2009. Under the radar: Balamuthia amebic encephalitis. Clin. Infect. Dis. 48:879-887. [DOI] [PubMed] [Google Scholar]
- 509.Schwarzwald, H., P. Shah, J. Hicks, M. Levy, M. L. Wagner, and M. W. Kline. 2003. Disseminated Acanthamoeba infection in a human immunodeficiency virus-infected infant. Pediatr. Infect. Dis. J. 22:197-199. [PubMed] [Google Scholar]
- 510.Schwint, O. N., M. W. Ueti, G. H. Palmer, L. S. Kappmeyer, M. T. Hines, R. T. Cordes, D. P. Knowles, and G. A. Scoles. 2009. Imidocarb dipropionate clears persistent Babesia caballi infection with elimination of transmission potential. Antimicrob. Agents Chemother. 53:4327-4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Seijo Martinez, M., G. Gonzalez-Mediero, P. Santiago, A. Rodriguez De Lope, J. Diz, C. Conde, and G. S. Visvesvara. 2000. Granulomatous amebic encephalitis in a patient with AIDS: isolation of Acanthamoeba sp. group II from brain tissue and successful treatment with sulfadiazine and fluconazole. J. Clin. Microbiol. 38:3892-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Selby, D. M., R. S. Chandra, T. A. Rakusan, B. Loechelt, B. M. Markle, and G. S. Visvesvara. 1998. Amebic osteomyelitis in a child with acquired immunodeficiency syndrome: a case report. Pediatr. Pathol. Lab. Med. 18:89-95. [PubMed] [Google Scholar]
- 513.Sell, J. J., F. W. Rupp, and W. W. Orrison, Jr. 1997. Granulomatous amebic encephalitis caused by acanthamoeba. Neuroradiology 39:434-436. [DOI] [PubMed] [Google Scholar]
- 514.Sensini, A. 2006. Toxoplasma gondii infection in pregnancy: opportunities and pitfalls of serological diagnosis. Clin. Microbiol. Infect. 12:504-512. [DOI] [PubMed] [Google Scholar]
- 515.Sethi, S., D. Alcid, H. Kesarwala, and R. W. Tolan, Jr. 2009. Probable congenital babesiosis in infant, New Jersey, U.S.A. Emerg. Infect. Dis. 15:788-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Shahbazi, F., S. Shahabi, B. Kazemi, M. Mohebali, A. R. Abadi, and Z. Zare. 2008. Evaluation of PCR assay in diagnosis and identification of cutaneous leishmaniasis: a comparison with the parasitological methods. Parasitol. Res. 103:1159-1162. [DOI] [PubMed] [Google Scholar]
- 517.Shenoy, S., G. Wilson, H. V. Prashanth, K. Vidyalakshmi, B. Dhanashree, and R. Bharath. 2002. Primary meningoencephalitis by Naegleria fowleri: first reported case from Mangalore, South India. J. Clin. Microbiol. 40:309-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Shih, C. M., L. P. Liu, W. C. Chung, S. J. Ong, and C. C. Wang. 1997. Human babesiosis in Taiwan: asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J. Clin. Microbiol. 35:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Shin, D. W., D. Y. Cha, Q. J. Hua, G. H. Cha, and Y. H. Lee. 2009. Seroprevalence of Toxoplasma gondii infection and characteristics of seropositive patients in general hospitals in Daejeon, Korea. Korean J. Parasitol. 47:125-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Shirabe, T., Y. Monobe, and G. S. Visvesvara. 2002. An autopsy case of amebic meningoencephalitis. The first Japanese case caused by Balamuthia mandrillaris. Neuropathology 22:213-217. [DOI] [PubMed] [Google Scholar]
- 521.Shrivastava, K. K., and G. P. Shrivastava. 1974. Two cases of Trypanosoma (Herpetosoma) species infection of man in India. Trans. R. Soc. Trop. Med. Hyg. 68:143-144. [DOI] [PubMed] [Google Scholar]
- 522.Silva, N., L. O'Bryan, E. Medeiros, H. Holand, J. Suleiman, J. S. de Mendonca, N. Patronas, S. G. Reed, H. G. Klein, H. Masur, and R. Badaro. 1999. Trypanosoma cruzi meningoencephalitis in HIV-infected patients. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20:342-349. [DOI] [PubMed] [Google Scholar]
- 523.Silva-Vergara, M. L., E. R. Da Cunha Colombo, E. De Figueiredo Vissotto, A. C. Silva, J. E. Chica, R. M. Etchebehere, and S. J. Adad. 2007. Disseminated Balamuthia mandrillaris amoeba infection in an AIDS patient from Brazil. Am. J. Trop. Med. Hyg. 77:1096-1098. [PubMed] [Google Scholar]
- 524.Simarro, P. P., J. R. Franco, P. Ndongo, E. Nguema, F. J. Louis, and J. Jannin. 2006. The elimination of Trypanosoma brucei gambiense sleeping sickness in the focus of Luba, Bioko Island, Equatorial Guinea. Trop. Med. Int. Health 11:636-646. [DOI] [PubMed] [Google Scholar]
- 525.Simpore, J., A. Savadogo, D. Ilboudo, M. C. Nadambega, M. Esposito, J. Yara, S. Pignatelli, V. Pietra, and S. Musumeci. 2006. Toxoplasma gondii, HCV, and HBV seroprevalence and co-infection among HIV-positive and -negative pregnant women in Burkina Faso. J. Med. Virol. 78:730-733. [DOI] [PubMed] [Google Scholar]
- 526.Sing, A., L. Leitritz, A. Roggenkamp, H. J. Kolb, A. Szabados, V. Fingerle, I. B. Autenrieth, and J. Heesemann. 1999. Pulmonary toxoplasmosis in bone marrow transplant recipients: report of two cases and review. Clin. Infect. Dis. 29:429-433. [DOI] [PubMed] [Google Scholar]
- 527.Singh, S., and R. Sivakumar. 2003. Recent advances in the diagnosis of leishmaniasis. J. Postgrad. Med. 49:55-60. [DOI] [PubMed] [Google Scholar]
- 528.Sironi, M., C. Bandi, S. Novati, and M. Scaglia. 1997. A PCR-RFLP method for the detection and species identification of human microsporidia. Parassitologia 39:437-439. [PubMed] [Google Scholar]
- 529.Slodkowicz-Kowalska, A. 2004. Laboratory diagnostics of human microsporidiosis. Wiad. Parazytol. 50:679-689. (In Polish.) [PubMed] [Google Scholar]
- 530.Smith, D. H., and J. W. Bailey. 1997. Human African trypanosomiasis in south-eastern Uganda: clinical diversity and isoenzyme profiles. Ann. Trop. Med. Parasitol. 91:851-856. [DOI] [PubMed] [Google Scholar]
- 531.Smith, J. E. 2009. The ecology and evolution of microsporidian parasites. Parasitology 136:1901-1914. [DOI] [PubMed] [Google Scholar]
- 532.Sridhar, M. S., and S. Sharma. 2003. Microsporidial keratoconjunctivitis in a HIV-seronegative patient treated with debridement and oral itraconazole. Am. J. Ophthalmol. 136:745-746. [DOI] [PubMed] [Google Scholar]
- 533.Stark, D., J. L. N. Barratt, S. van Hal, D. Marriott, J. Harkness, and J. Ellis. 2009. Clinical significance of enteric protozoa in the immunosuppressed human population. Clin. Microbiol. Rev. 22:634-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Stark, D., S. Pett, D. Marriott, and J. Harkness. 2006. Post-kala-azar dermal leishmaniasis due to Leishmania infantum in a human immunodeficiency virus type 1-infected patient. J. Clin. Microbiol. 44:1178-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Stefanidou, M. P., M. Antoniou, A. V. Koutsopoulos, Y. T. Neofytou, K. Krasagakis, S. Kruger-Krasagakis, Y. Tselentis, and A. D. Tosca. 2008. A rare case of leishmaniasis recidiva cutis evolving for 31 years caused by Leishmania tropica. Int. J. Dermatol. 47:588-589. [DOI] [PubMed] [Google Scholar]
- 536.Steinberg, J. P., R. L. Galindo, E. S. Kraus, and K. G. Ghanem. 2002. Disseminated acanthamebiasis in a renal transplant recipient with osteomyelitis and cutaneous lesions: case report and literature review. Clin. Infect. Dis. 35:e43-e49. [DOI] [PubMed] [Google Scholar]
- 537.Stenzel, W., H. Pels, P. Staib, P. Impekoven, N. Bektas, and M. Deckert. 2004. Concomitant manifestation of primary CNS lymphoma and Toxoplasma encephalitis in a patient with AIDS. J. Neurol. 251:764-766. [DOI] [PubMed] [Google Scholar]
- 538.Stray-Pedersen, B. 1993. Toxoplasmosis in pregnancy. Baillieres Clin. Obstet. Gynaecol. 7:107-137. [DOI] [PubMed] [Google Scholar]
- 539.Sun, Y., G. Liu, L. Yang, R. Xu, and W. Cao. 2008. Babesia microti-like rodent parasites isolated from Ixodes persulcatus (Acari: Ixodidae) in Heilongjiang Province, China. Vet. Parasitol. 156:333-339. [DOI] [PubMed] [Google Scholar]
- 540.Svedhem, V., M. Lebbad, B. Hedkvist, C. Del Aguila, P. Hedman, R. Larsson, R. Navajas, and A. Aust-Kettis. 2002. Disseminated infection with Encephalitozoon intestinalis in AIDS patients: report of 2 cases. Scand. J. Infect. Dis. 34:703-705. [DOI] [PubMed] [Google Scholar]
- 541.Tachikawa, N., M. Goto, Y. Hoshino, H. Gatanaga, A. Yasuoka, T. Wakabayashi, H. Katano, S. Kimura, S. Oka, and A. Iwamoto. 1999. Detection of Toxoplasma gondii, Epstein-Barr virus, and JC virus DNAs in the cerebrospinal fluid in acquired immunodeficiency syndrome patients with focal central nervous system complications. Intern. Med. 38:556-562. [DOI] [PubMed] [Google Scholar]
- 542.Tampieri, M. P., R. Galuppi, C. Bonoli, G. Cancrini, A. Moretti, and M. Pietrobelli. 2008. Wild ungulates as Babesia hosts in northern and central Italy. Vector Borne Zoonotic Dis. 8:667-674. [DOI] [PubMed] [Google Scholar]
- 543.Tanowitz, H. B., L. V. Kirchhoff, D. Simon, S. A. Morris, L. M. Weiss, and M. Wittner. 1992. Chagas' disease. Clin. Microbiol. Rev. 5:400-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Tavares, M., J. M. Correia da Costa, S. S. Carpenter, L. A. Santos, C. Afonso, A. Aguiar, J. Pereira, A. I. Cardoso, F. L. Schuster, S. Yagi, R. Sriram, and G. S. Visvesvara. 2006. Diagnosis of first case of Balamuthia amoebic encephalitis in Portugal by immunofluorescence and PCR. J. Clin. Microbiol. 44:2660-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Taylor, J. E., and G. Rudenko. 2006. Switching trypanosome coats: what's in the wardrobe? Trends Genet. 22:614-620. [DOI] [PubMed] [Google Scholar]
- 546.Taylor-Robinson, D., K. Jones, and P. Garner. 2007. Malaria: uncomplicated, caused by Plasmodium falciparum. Clin. Evid. 2007:pii0919. [PMC free article] [PubMed] [Google Scholar]
- 547.Thomford, J. W., P. A. Conrad, S. R. Telford III, D. Mathiesen, B. H. Bowman, A. Spielman, M. L. Eberhard, B. L. Herwaldt, R. E. Quick, and D. H. Persing. 1994. Cultivation and phylogenetic characterization of a newly recognized human pathogenic protozoan. J. Infect. Dis. 169:1050-1056. [DOI] [PubMed] [Google Scholar]
- 548.Tiewcharoen, S., V. Junnu, and P. Chinabut. 2002. In vitro effect of antifungal drugs on pathogenic Naegleria spp. Southeast Asian J. Trop. Med. Public Health 33:38-41. [PubMed] [Google Scholar]
- 549.Tiewcharoen, S., V. Junnu, and S. Suvoutho. 2003. Effect of antifungal drugs on pathogenic Naegleria spp isolated from natural water sources. J. Med. Assoc. Thai. 86:876-882. [PubMed] [Google Scholar]
- 550.Tomimori-Yamashita, J., P. D. Deps, D. R. Almeida, M. M. Enokihara, M. T. De Seixas, and E. Freymuller. 1997. Cutaneous manifestation of Chagas' disease after heart transplantation: successful treatment with allopurinol. Br. J. Dermatol. 137:626-630. [DOI] [PubMed] [Google Scholar]
- 551.Torno, M. S., Jr., R. Babapour, A. Gurevitch, and M. D. Witt. 2000. Cutaneous acanthamoebiasis in AIDS. J. Am. Acad. Dermatol. 42:351-354. [DOI] [PubMed] [Google Scholar]
- 552.Torres, R. A., W. Weinberg, J. Stansell, G. Leoung, J. Kovacs, M. Rogers, and J. Scott. 1997. Atovaquone for salvage treatment and suppression of toxoplasmic encephalitis in patients with AIDS. Atovaquone/Toxoplasmic Encephalitis Study Group. Clin. Infect. Dis. 24:422-429. [DOI] [PubMed] [Google Scholar]
- 553.Torrico, F., C. Alonso-Vega, E. Suarez, P. Rodriguez, M. C. Torrico, M. Dramaix, C. Truyens, and Y. Carlier. 2004. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am. J. Trop. Med. Hyg. 70:201-209. [PubMed] [Google Scholar]
- 554.Tosoni, A., M. Nebuloni, A. Ferri, S. Bonetto, S. Antinori, M. Scaglia, L. Xiao, H. Moura, G. S. Visvesvara, L. Vago, and G. Costanzi. 2002. Disseminated microsporidiosis caused by Encephalitozoon cuniculi III (dog type) in an Italian AIDS patient: a retrospective study. Mod. Pathol. 15:577-583. [DOI] [PubMed] [Google Scholar]
- 555.Tranas, J., R. A. Heinzen, L. M. Weiss, and M. M. McAllister. 1999. Serological evidence of human infection with the protozoan Neospora caninum. Clin. Diagn. Lab. Immunol. 6:765-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Tripathi, P., V. Singh, and S. Naik. 2007. Immune response to Leishmania: paradox rather than paradigm. FEMS Immunol. Med. Microbiol. 51:229-242. [DOI] [PubMed] [Google Scholar]
- 557.Troye-Blomberg, M., and K. Berzins. 2008. Immune interactions in malaria co-infections with other endemic infectious diseases: implications for the development of improved disease interventions. Microbes Infect. 10:948-952. [DOI] [PubMed] [Google Scholar]
- 558.Truc, P., P. Formenty, P. B. Diallo, C. Komoin-Oka, and F. Lauginie. 1997. Confirmation of two distinct classes of zymodemes of Trypanosoma brucei infecting man and wild mammals in Cote d'Ivoire: suspected difference in pathogenicity. Ann. Trop. Med. Parasitol. 91:951-956. [DOI] [PubMed] [Google Scholar]
- 559.Truc, P., W. Gibson, and S. Herder. 2007. Genetic characterization of Trypanosoma evansi isolated from a patient in India. Infect. Genet. Evol. 7:305-307. [DOI] [PubMed] [Google Scholar]
- 560.Truc, P., V. Jamonneau, P. N′Guessan, L. N′Dri, P. B. Diallo, and G. Cuny. 1998. Trypanosoma brucei ssp. and T. congolense: mixed human infection in Cote d'Ivoire. Trans. R. Soc. Trop. Med. Hyg. 92:537-538. [DOI] [PubMed] [Google Scholar]
- 561.Truc, P., V. Lejon, E. Magnus, V. Jamonneau, A. Nangouma, D. Verloo, L. Penchenier, and P. Buscher. 2002. Evaluation of the micro-CATT, CATT/Trypanosoma brucei gambiense, and LATEX/T b gambiense methods for serodiagnosis and surveillance of human African trypanosomiasis in West and Central Africa. Bull. World Health Organ. 80:882-886. [PMC free article] [PubMed] [Google Scholar]
- 562.Tschirhart, D., and E. C. Klatt. 1988. Disseminated toxoplasmosis in the acquired immunodeficiency syndrome. Arch. Pathol. Lab. Med. 112:1237-1241. [PubMed] [Google Scholar]
- 563.Turner, M. L., E. J. Cockerell, H. M. Brereton, P. R. Badenoch, M. Tea, D. J. Coster, and K. A. Williams. 2005. Antigens of selected Acanthamoeba species detected with monoclonal antibodies. Int. J. Parasitol. 35:981-990. [DOI] [PubMed] [Google Scholar]
- 564.Uneke, C. J. 2008. Diagnosis of Plasmodium falciparum malaria in pregnancy in sub-Saharan Africa: the challenges and public health implications. Parasitol. Res. 102:333-342. [DOI] [PubMed] [Google Scholar]
- 565.Uneke, C. J. 2008. Impact of placental Plasmodium falciparum malaria on pregnancy and perinatal outcome in sub-Saharan Africa. Part III: placental malaria, maternal health, and public health. Yale J. Biol. Med. 81:1-7. [PMC free article] [PubMed] [Google Scholar]
- 566.Uneke, C. J., and A. Ogbonna. 2009. Malaria and HIV co-infection in pregnancy in sub-Saharan Africa: impact of treatment using antimalarial and antiretroviral agents. Trans. R. Soc. Trop. Med. Hyg. 103:761-767. [DOI] [PubMed] [Google Scholar]
- 567.Vaidian, A. K., L. M. Weiss, and H. B. Tanowitz. 2004. Chagas' disease and AIDS. Kinetoplastid Biol. Dis. 3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Valverde, J., J. E. Arrese, and G. E. Pierard. 2006. Granulomatous cutaneous centrofacial and meningocerebral amebiasis. Am. J. Clin. Dermatol. 7:267-269. [DOI] [PubMed] [Google Scholar]
- 569.van der Meide, W., J. Guerra, G. Schoone, M. Farenhorst, L. Coelho, W. Faber, I. Peekel, and H. Schallig. 2008. Comparison between quantitative nucleic acid sequence-based amplification, real-time reverse transcriptase PCR, and real-time PCR for quantification of Leishmania parasites. J. Clin. Microbiol. 46:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Van Geertruyden, J. P., J. Menten, R. Colebunders, E. Korenromp, and U. D'Alessandro. 2008. The impact of HIV-1 on the malaria parasite biomass in adults in sub-Saharan Africa contributes to the emergence of antimalarial drug resistance. Malar. J. 7:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Van Hamme, C., M. Dumont, M. Delos, and J. M. Lachapelle. 2001. Cutaneous acanthamoebiasis in a lung transplant patient. Ann. Dermatol. Venereol. 128:1237-1240. (In French.) [PubMed] [Google Scholar]
- 572.Vanhollebeke, B., and E. Pays. The trypanolytic factor of human serum: many ways to enter the parasite, a single way to kill. Mol. Microbiol. 76:806-814. [DOI] [PubMed]
- 573.Vanhollebeke, B., P. Truc, P. Poelvoorde, A. Pays, P. P. Joshi, R. Katti, J. G. Jannin, and E. Pays. 2006. Human Trypanosoma evansi infection linked to a lack of apolipoprotein L-I. N. Engl. J. Med. 355:2752-2756. [DOI] [PubMed] [Google Scholar]
- 574.Vannier, E., and P. J. Krause. 2009. Update on babesiosis. Interdiscip. Perspect. Infect. Dis. 2009:984568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Vargas-Zepeda, J., A. V. Gomez-Alcala, J. A. Vasquez-Morales, L. Licea-Amaya, J. F. De Jonckheere, and F. Lares-Villa. 2005. Successful treatment of Naegleria fowleri meningoencephalitis by using intravenous amphotericin B, fluconazole and rifampicin. Arch. Med. Res. 36:83-86. [DOI] [PubMed] [Google Scholar]
- 576.Vavra, J., A. T. Yachnis, J. A. Shadduck, and J. M. Orenstein. 1998. Microsporidia of the genus Trachipleistophora—causative agents of human microsporidiosis: description of Trachipleistophora anthropophthera n. sp. (Protozoa: Microsporidia). J. Eukaryot. Microbiol. 45:273-283. [DOI] [PubMed] [Google Scholar]
- 577.Velasquez, J. N., S. Carnevale, E. A. Guarnera, J. H. Labbe, A. Chertcoff, M. G. Cabrera, and M. I. Rodriguez. 1996. Detection of the microsporidian parasite Enterocytozoon bieneusi in specimens from patients with AIDS by PCR. J. Clin. Microbiol. 34:3230-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Velasquez, J. N., C. Di Risio, S. Carnevale, M. Corti, J. Benetucci, J. P. Bossini, M. Cabrera, A. Chertcoff, J. H. Labbe, M. Rodriguez, H. Bessaso, and E. A. Guarnera. 1997. Morphological study of Enterocytozoon bineousi in patients with AIDS and chronic diarrhea. Acta Gastroenterol. Latinoam. 27:241-245. (In Spanish.) [PubMed] [Google Scholar]
- 579.Vemuganti, G. K., G. Pasricha, S. Sharma, and P. Garg. 2005. Granulomatous inflammation in Acanthamoeba keratitis: an immunohistochemical study of five cases and review of literature. Indian J. Med. Microbiol. 23:231-238. [PubMed] [Google Scholar]
- 580.Vernon, S. E., B. C. Acar, S. M. Pham, and D. Fertel. 2005. Acanthamoeba infection in lung transplantation: report of a case and review of the literature. Transpl. Infect. Dis. 7:154-157. [DOI] [PubMed] [Google Scholar]
- 581.Vexenat Ade, C., J. M. Santana, and A. R. Teixeira. 1996. Cross-reactivity of antibodies in human infections by the kinetoplastid protozoa Trypanosoma cruzi, Leishmania chagasi and Leishmania (viannia) braziliensis. Rev. Inst. Med. Trop. Sao Paulo 38:177-185. [DOI] [PubMed] [Google Scholar]
- 582.Vial, H. J., and A. Gorenflot. 2006. Chemotherapy against babesiosis. Vet. Parasitol. 138:147-160. [DOI] [PubMed] [Google Scholar]
- 583.Vidal, J. E., F. A. Colombo, A. C. de Oliveira, R. Focaccia, and V. L. Pereira-Chioccola. 2004. PCR assay using cerebrospinal fluid for diagnosis of cerebral toxoplasmosis in Brazilian AIDS patients. J. Clin. Microbiol. 42:4765-4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Vijayan, V. K. 2009. Parasitic lung infections. Curr. Opin. Pulm. Med. 15:274-282. [DOI] [PubMed] [Google Scholar]
- 585.Vincendeau, P., and B. Bouteille. 2006. Immunology and immunopathology of African trypanosomiasis. An. Acad. Bras. Cienc. 78:645-665. [DOI] [PubMed] [Google Scholar]
- 586.Visvesvara, G. S., M. Belloso, H. Moura, A. J. Da Silva, I. N. Moura, G. J. Leitch, D. A. Schwartz, P. Chevez-Barrios, S. Wallace, N. J. Pieniazek, and J. D. Goosey. 1999. Isolation of Nosema algerae from the cornea of an immunocompetent patient. J. Eukaryot. Microbiol. 46:10S. [PubMed] [Google Scholar]
- 587.Visvesvara, G. S., A. J. Martinez, F. L. Schuster, G. J. Leitch, S. V. Wallace, T. K. Sawyer, and M. Anderson. 1990. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J. Clin. Microbiol. 28:2750-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Visvesvara, G. S., H. Moura, and F. L. Schuster. 2007. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 50:1-26. [DOI] [PubMed] [Google Scholar]
- 589.Visvesvara, G. S., and F. L. Schuster. 2008. Opportunistic free-living amebae, part II. Clin. Microbiol. Newsl. 30:159-166. [Google Scholar]
- 590.Visvesvara, G. S., F. L. Schuster, and A. J. Martinez. 1993. Balamuthia mandrillaris, n. g., n. sp., agent of amebic meningoencephalitis in humans and other animals. J. Eukaryot. Microbiol. 40:504-514. [DOI] [PubMed] [Google Scholar]
- 591.Vitoria, M., R. Granich, C. F. Gilks, C. Gunneberg, M. Hosseini, W. Were, M. Raviglione, and K. M. De Cock. 2009. The global fight against HIV/AIDS, tuberculosis, and malaria: current status and future perspectives. Am. J. Clin. Pathol. 131:844-848. [DOI] [PubMed] [Google Scholar]
- 592.Vyas, J. M., S. R. Telford, and G. K. Robbins. 2007. Treatment of refractory Babesia microti infection with atovaquone-proguanil in an HIV-infected patient: case report. Clin. Infect. Dis. 45:1588-1590. [DOI] [PubMed] [Google Scholar]
- 593.Walia, R., J. G. Montoya, G. S. Visvesvera, G. C. Booton, and R. L. Doyle. 2007. A case of successful treatment of cutaneous Acanthamoeba infection in a lung transplant recipient. Transpl. Infect. Dis. 9:51-54. [DOI] [PubMed] [Google Scholar]
- 594.Walochnik, J., A. Aichelburg, O. Assadian, A. Steuer, G. Visvesvara, N. Vetter, and H. Aspock. 2008. Granulomatous amoebic encephalitis caused by Acanthamoeba amoebae of genotype T2 in a human immunodeficiency virus-negative patient. J. Clin. Microbiol. 46:338-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Weber, R., R. T. Bryan, D. A. Schwartz, and R. L. Owen. 1994. Human microsporidial infections. Clin. Microbiol. Rev. 7:426-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Wei, Q., M. Tsuji, A. Zamoto, M. Kohsaki, T. Matsui, T. Shiota, S. R. Telford III, and C. Ishihara. 2001. Human babesiosis in Japan: isolation of Babesia microti-like parasites from an asymptomatic transfusion donor and from a rodent from an area where babesiosis is endemic. J. Clin. Microbiol. 39:2178-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Weiss, G. E., P. D. Crompton, S. Li, L. A. Walsh, S. Moir, B. Traore, K. Kayentao, A. Ongoiba, O. K. Doumbo, and S. K. Pierce. 2009. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J. Immunol. 183:2176-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Weiss, L. M. 2001. Microsporidia: emerging pathogenic protists. Acta Trop. 78:89-102. [DOI] [PubMed] [Google Scholar]
- 599.Weiss, L. M., and J. P. Dubey. 2009. Toxoplasmosis: a history of clinical observations. Int. J. Parasitol. 39:895-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Wellems, T. E., K. Hayton, and R. M. Fairhurst. 2009. The impact of malaria parasitism: from corpuscles to communities. J. Clin. Invest. 119:2496-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Wendel, S. Transfusion transmitted Chagas disease: is it really under control? Acta Trop. 115:28-34. [DOI] [PubMed]
- 602.Wester, C. W., H. Bussmann, S. Moyo, A. Avalos, T. Gaolathe, N. Ndwapi, M. Essex, R. R. MacGregor, and R. G. Marlink. 2006. Serological evidence of HIV-associated infection among HIV-1-infected adults in Botswana. Clin. Infect. Dis. 43:1612-1615. [DOI] [PubMed] [Google Scholar]
- 603.WHO. 2004. Malaria and HIV interactions and their implications for public health policy. Report of a technical consultation, Geneva, Switzerland, 23-25 June. World Health Organization, Geneva, Switzerland. http://www.who.int/hiv/pub/prev_care/malariahiv.pdf.
- 604.WHO. 2007. Report of the Fifth Consultative Meeting on Leishmania/HIV Coinfection. World Health Organization, Geneva, Switzerland. http://www.who.int/leishmaniasis/resources/Leishmaniasis_hiv_coinfections5.pdf.
- 605.WHO. 1995. Report on the Consultative Meeting on Leishmanial/HIV Coinfection. Addis Ababa, Ethiopia, 20-22 March 2007. World Health Organization, Geneva, Switzerland. http://www.who.int/leishmaniasis/resources/Leishmaniasis_hiv_coinfection5.pdf.
- 606.WHO. 2006. World Health Organization online fact sheet: African trypanosomiasis (sleeping sickness). World Health Organization, Geneva, Switzerland. http://www.who.int/mediacentre/factsheets/fs259/en/.
- 607.Williams, D. J., C. S. Hartley, C. Bjorkman, and A. J. Trees. 2009. Endogenous and exogenous transplacental transmission of Neospora caninum—how the route of transmission impacts on epidemiology and control of disease. Parasitology 136:1895-1900. [DOI] [PubMed] [Google Scholar]
- 608.Wiselka, M. J., R. Read, and R. G. Finch. 1996. Response to oral and intravenous azithromycin in a patient with Toxoplasma encephalitis and AIDS. J. Infect. 33:227-229. [DOI] [PubMed] [Google Scholar]
- 609.Wittner, M., and H. B. Tanowitz. 2000. Leishmaniasis in infants and children. Semin. Pediatr. Infect. Dis. 11:196-201. [Google Scholar]
- 610.Wolday, D., N. Berhe, H. Akuffo, and S. Britton. 1999. Leishmania-HIV interaction: immunopathogenic mechanisms. Parasitol. Today 15:182-187. [DOI] [PubMed] [Google Scholar]
- 611.Wolk, D. M., S. K. Schneider, N. L. Wengenack, L. M. Sloan, and J. E. Rosenblatt. 2002. Real-time PCR method for detection of Encephalitozoon intestinalis from stool specimens. J. Clin. Microbiol. 40:3922-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Wong, R., R. Dell'Omo, M. Marino, B. Hussein, N. Okhravi, and C. E. Pavesio. 2009. Toxoplasma gondii: an atypical presentation of Toxoplasma as optic disc swelling and hemispherical retinal vein occlusion treated with intravitreal clindamycin. Int. Ophthalmol. 29:195-198. [DOI] [PubMed] [Google Scholar]
- 613.Wu, K., X. G. Chen, H. Li, H. Yan, P. L. Yang, Z. R. Lun, and X. Q. Zhu. 2009. Diagnosis of human toxoplasmosis by using the recombinant truncated surface antigen 1 of Toxoplasma gondii. Diagn. Microbiol. Infect. Dis. 64:261-266. [DOI] [PubMed] [Google Scholar]
- 614.Xong, H. V., P. De Baetselier, E. Pays, and S. Magez. 2002. Selective pressure can influence the resistance of Trypanosoma congolense to normal human serum. Exp. Parasitol. 102:61-65. [DOI] [PubMed] [Google Scholar]
- 615.Yadon, Z. E., and G. A. Schmunis. 2009. Congenital Chagas disease: estimating the potential risk in the United States. Am. J. Trop. Med. Hyg. 81:927-933. [DOI] [PubMed] [Google Scholar]
- 616.Yartey, J. 2009. Cochrane review on ITNs for preventing malaria in pregnancy. Int. J. Epidemiol. 38:35-36. [DOI] [PubMed] [Google Scholar]
- 617.Yee, R. W., F. O. Tio, J. A. Martinez, K. S. Held, J. A. Shadduck, and E. S. Didier. 1991. Resolution of microsporidial epithelial keratopathy in a patient with AIDS. Ophthalmology 98:196-201. [DOI] [PubMed] [Google Scholar]
- 618.Yip, L., J. McCluskey, and R. Sinclair. 2006. Immunological aspects of pregnancy. Clin. Dermatol. 24:84-87. [DOI] [PubMed] [Google Scholar]
- 619.Yoo, T. W., A. Mlikotic, M. E. Cornford, and C. K. Beck. 2004. Concurrent cerebral American trypanosomiasis and toxoplasmosis in a patient with AIDS. Clin. Infect. Dis. 39:e30-e34. [DOI] [PubMed] [Google Scholar]
- 620.Zagardo, M. T., R. J. Castellani, G. H. Zoarski, and S. C. Bauserman. 1997. Granulomatous amebic encephalitis caused by leptomyxid amebae in an HIV-infected patient. Am. J. Neuroradiol. 18:903-908. [PMC free article] [PubMed] [Google Scholar]
- 621.Zambrano, Y., A. Chiarello, A. Soca, I. Villalobos, M. Marrero, M. Soler, J. Laferte, and M. Alvarez. 2006. Use of polymerase chain reaction for the diagnosis of central nervous system infections. Invest. Clin. 47:337-347. (In Spanish.) [PubMed] [Google Scholar]
- 622.Zarkovic, A., C. McMurray, N. Deva, S. Ghosh, D. Whitley, and S. Guest. 2007. Seropositivity rates for Bartonella henselae, Toxocara canis and Toxoplasma gondii in New Zealand blood donors. Clin. Exp. Ophthalmol. 35:131-134. [DOI] [PubMed] [Google Scholar]
- 623.Zhao, C., B. Papadopoulou, and M. J. Tremblay. 2004. Leishmania infantum enhances human immunodeficiency virus type-1 replication in primary human macrophages through a complex cytokine network. Clin. Immunol. 113:81-88. [DOI] [PubMed] [Google Scholar]
- 624.Zoller, T., T. J. Naucke, J. May, B. Hoffmeister, H. Flick, C. J. Williams, C. Frank, F. Bergmann, N. Suttorp, and F. P. Mockenhaupt. 2009. Malaria transmission in non-endemic areas: case report, review of the literature and implications for public health management. Malar. J. 8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]