Abstract
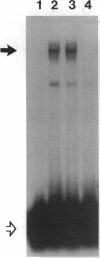
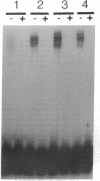
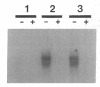
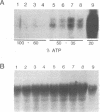
Renal ischemia results in both a profound fall in cellular ATP and a rapid induction of the 70 kD heat-shock protein family, HSP-70. The present studies examined the relationship between cellular ATP and induction of the stress response in renal cortex. Cellular ATP, continuously monitored by in vivo 31P-NMR spectroscopy, was reduced and maintained at specific, stable levels in renal cortex by partial aortic occlusion for 45 min. Activation of heat-shock transcription factor (HSF) was detected by gel retardation assay and transcription was confirmed by Northern analysis. Activation of HSF was not present, and HSP-70 mRNA induction did not occur when ATP levels were maintained above 60% preocclusion (control) levels. Reduction in cortical ATP levels to 35-50% preocclusion values resulted in HSF activation and low-level expression of inducible HSP-70 mRNA. Cellular ATP of 20-25% control values resulted in a greater level of HSF activation and subsequent HSP-70 mRNA elaboration. HSF was activated at the end of 15 min of total occlusion. The studies indicate that a 50% reduction in cellular ATP in the renal cortex must occur before the stress response is detectable, that reduction of ATP below 25% control levels produces a more vigorous response, and that reperfusion is not required for initiation of a heat-shock response in the kidney. Cellular ATP, or the metabolic consequences associated with ATP depletion, may be a threshold factor for initiation of a stress response in the kidney.
Full text
PDF

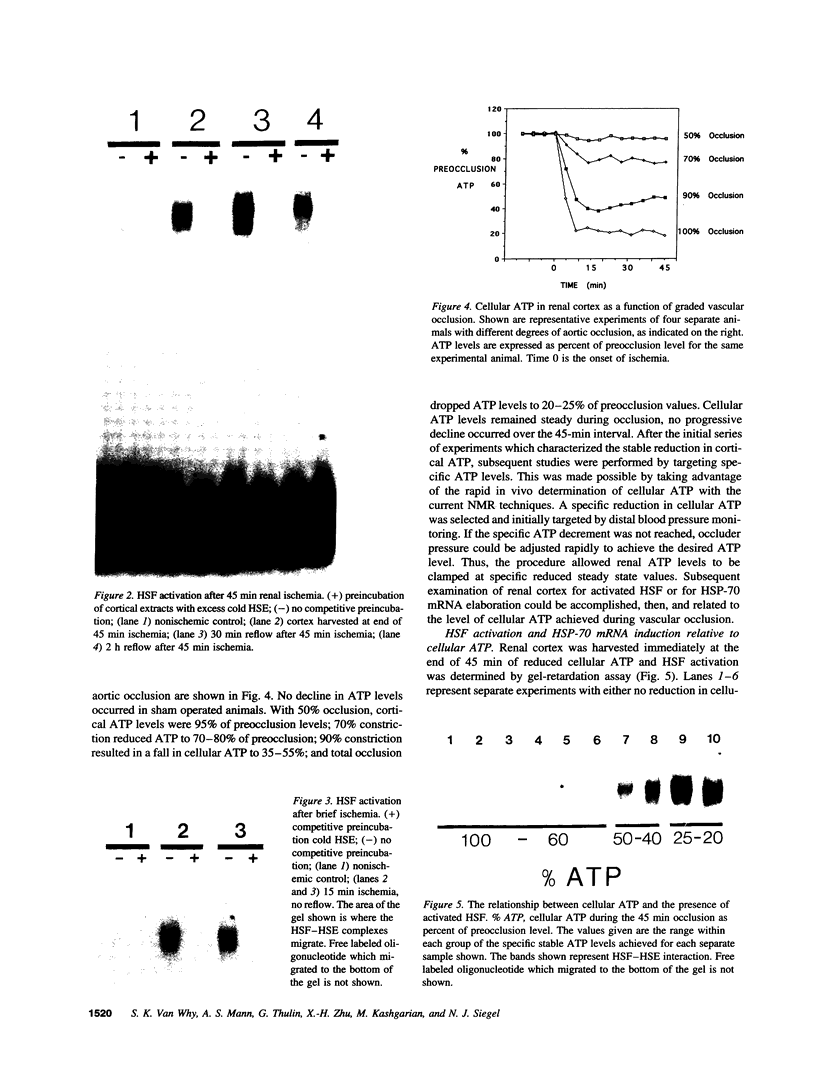
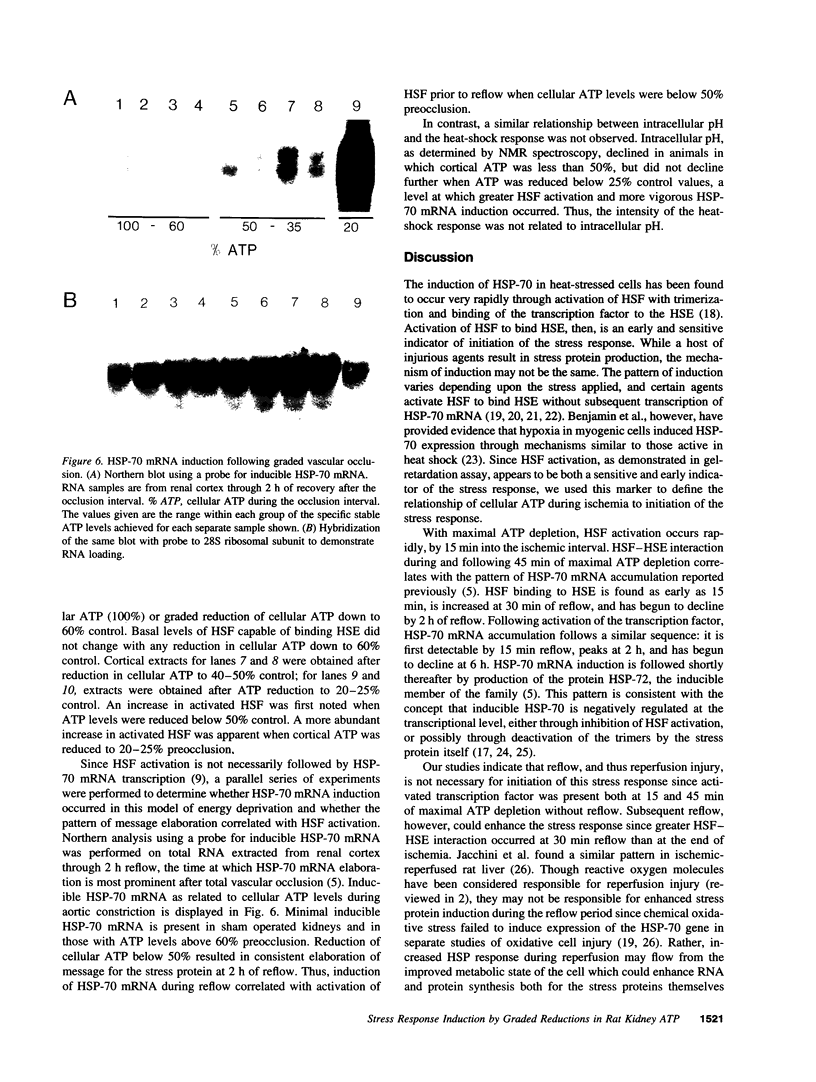


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abravaya K., Myers M. P., Murphy S. P., Morimoto R. I. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev. 1992 Jul;6(7):1153–1164. doi: 10.1101/gad.6.7.1153. [DOI] [PubMed] [Google Scholar]
- Abravaya K., Phillips B., Morimoto R. I. Attenuation of the heat shock response in HeLa cells is mediated by the release of bound heat shock transcription factor and is modulated by changes in growth and in heat shock temperatures. Genes Dev. 1991 Nov;5(11):2117–2127. doi: 10.1101/gad.5.11.2117. [DOI] [PubMed] [Google Scholar]
- Abravaya K., Phillips B., Morimoto R. I. Heat shock-induced interactions of heat shock transcription factor and the human hsp70 promoter examined by in vivo footprinting. Mol Cell Biol. 1991 Jan;11(1):586–592. doi: 10.1128/mcb.11.1.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler R., Welch W. J., Voellmy R. Heat shock gene regulation by nascent polypeptides and denatured proteins: hsp70 as a potential autoregulatory factor. J Cell Biol. 1992 Jun;117(6):1151–1159. doi: 10.1083/jcb.117.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann R. P., Lovett M., Welch W. J. Examining the function and regulation of hsp 70 in cells subjected to metabolic stress. J Cell Biol. 1992 Jun;117(6):1137–1150. doi: 10.1083/jcb.117.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin I. J., Horie S., Greenberg M. L., Alpern R. J., Williams R. S. Induction of stress proteins in cultured myogenic cells. Molecular signals for the activation of heat shock transcription factor during ischemia. J Clin Invest. 1992 May;89(5):1685–1689. doi: 10.1172/JCI115768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin I. J., Kröger B., Williams R. S. Activation of the heat shock transcription factor by hypoxia in mammalian cells. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6263–6267. doi: 10.1073/pnas.87.16.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. J., Udelsman R., Feulner G. J., Norton D. D., Holbrook N. J. Stress-induced heat shock protein 70 expression in adrenal cortex: an adrenocorticotropic hormone-sensitive, age-dependent response. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9873–9877. doi: 10.1073/pnas.88.21.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkan S. C., Emami A., Schwartz J. H. Heat stress protein-associated cytoprotection of inner medullary collecting duct cells from rat kidney. Am J Physiol. 1993 Sep;265(3 Pt 2):F333–F341. doi: 10.1152/ajprenal.1993.265.3.F333. [DOI] [PubMed] [Google Scholar]
- Boydstun I. I., Thulin G., Zhu X. H., Avison M. J., Gaudio K. M., Siegel N. J. Disassociation of postischemic recovery of renal adenosine triphosphate and cellular integrity. Pediatr Res. 1993 Jun;33(6):595–597. doi: 10.1203/00006450-199306000-00013. [DOI] [PubMed] [Google Scholar]
- Bruce J. L., Price B. D., Coleman C. N., Calderwood S. K. Oxidative injury rapidly activates the heat shock transcription factor but fails to increase levels of heat shock proteins. Cancer Res. 1993 Jan 1;53(1):12–15. [PubMed] [Google Scholar]
- Emami A., Schwartz J. H., Borkan S. C. Transient ischemia or heat stress induces a cytoprotectant protein in rat kidney. Am J Physiol. 1991 Apr;260(4 Pt 2):F479–F485. doi: 10.1152/ajprenal.1991.260.4.F479. [DOI] [PubMed] [Google Scholar]
- Goldenberg C. J., Luo Y., Fenna M., Baler R., Weinmann R., Voellmy R. Purified human factor activates heat shock promoter in a HeLa cell-free transcription system. J Biol Chem. 1988 Dec 25;263(36):19734–19739. [PubMed] [Google Scholar]
- Hensold J. O., Hunt C. R., Calderwood S. K., Housman D. E., Kingston R. E. DNA binding of heat shock factor to the heat shock element is insufficient for transcriptional activation in murine erythroleukemia cells. Mol Cell Biol. 1990 Apr;10(4):1600–1608. doi: 10.1128/mcb.10.4.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurivich D. A., Sistonen L., Kroes R. A., Morimoto R. I. Effect of sodium salicylate on the human heat shock response. Science. 1992 Mar 6;255(5049):1243–1245. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- Karn H., Ovsenek N., Heikkila J. J. Examination of the DNA sequence-specific binding properties of heat shock transcription factor in Xenopus laevis embryos. Biochem Cell Biol. 1992 Oct-Nov;70(10-11):1006–1013. doi: 10.1139/o92-144. [DOI] [PubMed] [Google Scholar]
- Lis J., Wu C. Protein traffic on the heat shock promoter: parking, stalling, and trucking along. Cell. 1993 Jul 16;74(1):1–4. doi: 10.1016/0092-8674(93)90286-y. [DOI] [PubMed] [Google Scholar]
- Liu R. Y., Kim D., Yang S. H., Li G. C. Dual control of heat shock response: involvement of a constitutive heat shock element-binding factor. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3078–3082. doi: 10.1073/pnas.90.7.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto R. I. Cells in stress: transcriptional activation of heat shock genes. Science. 1993 Mar 5;259(5100):1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Mosser D. D., Theodorakis N. G., Morimoto R. I. Coordinate changes in heat shock element-binding activity and HSP70 gene transcription rates in human cells. Mol Cell Biol. 1988 Nov;8(11):4736–4744. doi: 10.1128/mcb.8.11.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissim I., Hardy M., Pleasure J., Nissim I., States B. A mechanism of glycine and alanine cytoprotective action: stimulation of stress-induced HSP70 mRNA. Kidney Int. 1992 Sep;42(3):775–782. doi: 10.1038/ki.1992.347. [DOI] [PubMed] [Google Scholar]
- Palleros D. R., Welch W. J., Fink A. L. Interaction of hsp70 with unfolded proteins: effects of temperature and nucleotides on the kinetics of binding. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5719–5723. doi: 10.1073/pnas.88.13.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel N. J., Devarajan P., Van Why S. Renal cell injury: metabolic and structural alterations. Pediatr Res. 1994 Aug;36(2):129–136. doi: 10.1203/00006450-199408000-00001. [DOI] [PubMed] [Google Scholar]
- Skowyra D., Georgopoulos C., Zylicz M. The E. coli dnaK gene product, the hsp70 homolog, can reactivate heat-inactivated RNA polymerase in an ATP hydrolysis-dependent manner. Cell. 1990 Sep 7;62(5):939–944. doi: 10.1016/0092-8674(90)90268-j. [DOI] [PubMed] [Google Scholar]
- Sorger P. K. Heat shock factor and the heat shock response. Cell. 1991 May 3;65(3):363–366. doi: 10.1016/0092-8674(91)90452-5. [DOI] [PubMed] [Google Scholar]
- Tacchini L., Schiaffonati L., Pappalardo C., Gatti S., Bernelli-Zazzera A. Expression of HSP 70, immediate-early response and heme oxygenase genes in ischemic-reperfused rat liver. Lab Invest. 1993 Apr;68(4):465–471. [PubMed] [Google Scholar]
- Van Why S. K., Hildebrandt F., Ardito T., Mann A. S., Siegel N. J., Kashgarian M. Induction and intracellular localization of HSP-72 after renal ischemia. Am J Physiol. 1992 Nov;263(5 Pt 2):F769–F775. doi: 10.1152/ajprenal.1992.263.5.F769. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M. The cell biology of ischemic renal injury. Kidney Int. 1991 Mar;39(3):476–500. doi: 10.1038/ki.1991.58. [DOI] [PubMed] [Google Scholar]