Abstract
Summary: Melanized or dematiaceous fungi are associated with a wide variety of infectious syndromes. Many are soil organisms and are generally distributed worldwide, though certain species appear to have restricted geographic ranges. Though they are uncommon causes of disease, melanized fungi have been increasingly recognized as important pathogens, with most reports occurring in the past 20 years. The spectrum of diseases with which they are associated has also broadened and includes allergic disease, superficial and deep local infections, pneumonia, brain abscess, and disseminated infection. For some infections in immunocompetent individuals, such as allergic fungal sinusitis and brain abscess, they are among the most common etiologic fungi. Melanin is a likely virulence factor for these fungi. Diagnosis relies on careful microscopic and pathological examination, as well as clinical assessment of the patient, as these fungi are often considered contaminants. Therapy varies depending upon the clinical syndrome. Local infection may be cured with excision alone, while systemic disease is often refractory to therapy. Triazoles such as voriconazole, posaconazole, and itraconazole have the most consistent in vitro activity. Further studies are needed to better understand the pathogenesis and optimal treatment of these uncommon infections.
INTRODUCTION
Melanin is a ubiquitous compound found in many microbes and animals. Its functions are varied but are based on the unique molecular characteristics of its structure, which make it an extremely stable molecule, resistant to a variety of destructive physicochemical processes (83, 109, 324). In recent years its pathogenic role in fungi has become well described (123, 292, 375, 460, 546). This review will focus on fungi that are considered to be melanized as a primary feature, particularly with regard to their phenotypic appearance (macroscopic and microscopic morphologies) and appearance in tissue (histology).
The terms used to describe these fungi have evolved over the past several decades. As Sporothrix schenckii was one of the earliest melanized fungi described, “sporotrichoid” was often used to describe similar fungi, though currently it has been replaced by other, more useful terms. “Phaeoid,” “phaeo-sporotrichose,” and “dematiaceous” have also been mentioned in the literature (574). “Phaeo” comes from the Greek meaning “dark” and has been commonly used, particularly when describing infections due to these fungi as “phaeohyphomycosis,” i.e., infection caused by dark-walled fungi, as suggested by Ajello et al. (12, 630). It has been suggested that the term “dematiaceous” is not appropriate given its etymologic derivation from the Greek “deme,” meaning bundle, though it has become fairly entrenched in medical mycological literature and will likely persist in nomenclature (574). The term “melanized” has become more utilized recently, given its specific meaning. For the purposes of this review, however, the terms “dematiaceous,” “melanized,” “dark,” and “phaeoid” are used interchangeably to denote fungal elements containing melanin.
The presence of melanin alone is probably not a useful criterion for inclusion in this group of clinically important fungi, as melanin has been demonstrated in practically all “nondematiaceous” clinical fungi examined in the literature, including Histoplasma capsulatum, Paracoccidioides brasiliensis, Aspergillus spp., and even Candida albicans (293, 521, 548, 599, 757). One might contrast the fungi discussed here as heavily melanized, with brown-pigmented hyphae in tissue often discernible without the use of any staining procedure. At present, no quantitative measure of melanin is available to distinguish dematiaceous from other fungi. In addition, Sporothrix schenckii, with a yeast form in tissue, is well known and well described (177) as the agent of a unique clinical entity, sporotrichosis, and only issues regarding its mycology will be discussed here.
Melanized fungi are common in the environment (see Ecology below) and are often isolated in the microbiology lab, where they may be considered contaminants. Indeed, only 10% of dematiaceous lab isolates are likely to have clinical significance (72, 607). Clinical disease due to these fungi is uncommon, with one estimate from a large metropolitan area of one case/million persons/year (617). Despite their rarity in clinical practice, melanized fungi have become increasingly recognized as important pathogens, particularly in immunocompromised patients, though individuals with apparently “normal” immune systems have also been reported to have invasive, often fatal infections (627, 628).
The clinical syndromes caused by these fungi are differentiated based on histologic findings into eumycetoma, chromoblastomycosis, and phaeohyphomycosis. Eumycetoma is a deep tissue infection, usually of the lower extremities, characterized by the presence of mycotic granules (572). It is associated with a relatively small group of fungi. Chromoblastomycosis is caused primarily by a few species of fungi that produce characteristic sclerotic bodies in tissue and is usually seen in tropical areas (501, 572). Phaeohyphomycosis is a term generally reserved for the remainder of clinical syndromes caused by melanized fungi (623, 630). For the purposes of this review, these will be arbitrarily divided into allergic disease, superficial and deep local infections, pulmonary disease, central nervous system (CNS) infection, and disseminated disease. We do not aim to review every publication regarding melanized fungi, but rather we seek to provide a broad, yet in-depth overview of the field as it currently stands, recognizing that it will continue to evolve and expand with our increasing knowledge of and experience with these clinically important fungi.
ECOLOGY
Melanized or dematiaceous fungi as defined above are frequently considered ubiquitous saprobes inhabiting living and dead plant material and, for the most part, residing in the soil. We now know, however, that these generalized assumptions are incorrect for the group as a whole, as several etiologic agents occupy specific ecological niches or microenvironments, and the knowledge of their natural ecology contributes to our understanding of their opportunistic/pathogenic potential (175, 177, 606, 779). It has been suggested (175) that our use of the term “dematiaceous” be restricted to those ubiquitous, mostly plant-associated hyphomycetous fungi with brown hyphae (220, 221), such as Alternaria, Bipolaris, Curvularia, and Exserohilum in the order Pleosporales. The natural ecology of melanized fungi in several other orders is more restricted (187). For example, fungi in the order Calosphaeriales belong mostly to woody-plant- or wood-inhabiting genera such as Phaeoacremonium, Phialemonium, and Pleurostomophora, whereas some genera in the order Chaetothyriales, such as Exophiala, may have specific microenvironments and are characterized as “microextremophiles.” The ability of some species in this genus, such as E. xenobiotica, to grow in high concentrations of xenobiotics (606) such as xylene, toluene, or creosote-treated utility poles, as well as to cause human disease, is truly remarkable. Species in the genus Exophiala and related genera are frequently referred to as the “black yeast-like fungi” and are so named because of their ability to produce budding, yeast-like cells at some point in their life cycle as well as dark hyphae. The ecology of Pseudallescheria and Scedosporium was also recently investigated by examining the occurrence of these species in natural and human-dominated environments (393). These findings demonstrated increasing environmental recovery with increasing human habitation and a concomitant elevation in nitrogen concentrations. Another genus defined by its residence in a particular environmental niche is the halophilic genus Hortaea in the order Capnodiales. Hortaea werneckii, the agent of tinea nigra, is found in subtropical saltwater habitats and is manifested by its opportunistic adherence to the dead, salty keratin layers of the human hand (87). Thus, while several genera are considered “ubiquitous,” many prefer well-defined microenvironments which, for some genera, predispose them to causing disease where similar conditions exist in the host.
In addition, there are species that appear to be geographically restricted, such as Rhinocladiella mackenziei, which has been seen primarily in patients from the Middle East (726). While Scedosporium prolificans has been reported from many locations, most clinical cases originate from Australia and Spain, for unclear reasons (76, 336). This may be due to environmental features that preferentially support specific fungal species.
CLASSIFICATION, TAXONOMY, AND NOMENCLATURE
Classification of fungi is simply their assignment into defined categories. A classification system is composed of hierarchical groups which may be further subdivided to indicate degrees of relationships. The basic unit of classification is the species, although there is currently no universally acceptable definition for this unit. Taxonomy is the arrangement of these fungi into a classification. With multilocus sequencing providing classification insights unavailable to the phenotypic systematists (those who study the relationships and classification of organisms and the processes by which they have evolved), new phylogenetic classification schemes have emerged. Taylor et al. have provided an excellent treatment of the phylogenetic concepts underlying the definition of species in fungi (737). The abbreviated classification scheme to the ordinal level for ascomycetous melanized fungi covered in this review is based upon the most recent work of Hibbett et al. (340) and the Myconet “Outline of Ascomycotya” (463).
Kingdom: Fungi
- Phylum: Ascomycota
- Subphylum: Pezizomycotina
- Class: Dothideomycetes
- Orders: Capnodiales, Dothideales, Pleosporales, Botryosphaeriales
- Class: Eurotiomycetes
- Order: Chaetothyriales
- Class: Sordariomycetes
- Orders: Microascales, Sordariales, Calosphaeriales, Ophiostomatales
As seen above and in Table 1, clinically significant melanized fungi span several ascomycetous orders in the kingdom Fungi.
TABLE 1.
Salient features of selected clinically significant dematiaceous fungia
| Genus type | Order | Genus or species | Salient phenotypic and/or diagnostic featuresb |
|---|---|---|---|
| Anamorphic (asexual) hyphomycete (conidia | Capnodiales | Hortaea werneckii | Colonies olivaceous to black, mucoid to yeast-like, restricted; broad hyphae, wide annellated zones produce pale brown 1- to multicelled annelloconidia |
| borne free) | Cladosporium spp. | Colonies olivaceous to black, velvety; conidiophores simple or branched, with or without nodes or swellings; ramoconidia (“shield cells”) give rise to branching chains of fragile, dark, mostly 1- or 2-celled conidia with prominent attachment scares (hila) | |
| Dothideales | Aureobasidium pullulans | Colonies of A. pullulans var. pullulans mucoid, cream to pink initially and later brown to black, while those of A. pullulans var. melanigenum black at the outset; hyaline blastoconidia borne synchronously from hyaline hyphae; dark, thick-walled chlamydospores; DNA sequencing necessary for reliable differentiation of A. pullulans and H. dematioides | |
| Hormonema dematioides | Colonies similar to those seen in A. pullulans; hyaline blastoconidia produced asynchronously by percurrent proliferation from hyaline and dark hyphae | ||
| Pleosporales | Alternaria spp. | Colonies woolly, pale to olivaceous to black, with rapid growth; large, dark, euseptatec, muriform conidia in chains; A. infectoria conidia may be sparse and have long apical beaks serving as secondary conidiogenous cells | |
| Bipolaris spp. | Colonies woolly, gray to black, with rapid growth, bipolar germination, geniculate conidiophores, flattened hilum; B. spicifera has 3 distoseptad and 4 cells, while B. hawaiiensis has predominately 5 distosepta and 6 cells | ||
| Curvularia spp. | Colonies woolly, gray to black, with rapid growth; geniculate conidiophores; conidia euseptate and curved (pronounced to subtle) due to swollen middle cell which is darker in C. lunata; C. lunata var. aeria produces large stroma visible with the naked eye | ||
| Exserohilum spp. | Colonies woolly, gray to black, with rapid growth; geniculate conidiophores, truncate protruding hilum; E. rostratum has 7-9 distosepta; 8-10 cells; prominent dark basal and distal septa; E. longirostratum has longer conidia with central curvature; E. mcginnisii has subtle warty projections on conidia | ||
| Chaetothyriales | Exophiala spp. | Colonies mucoid initially, later more filamentous; conidiogenous cells predominately annellidic; phialides sometimes present; annellated black yeast synanamorph often present; many species very similar microscopically; nitrate positive; DNA sequencing of ITS region facilitates species identification; maximum temp varies; most frequently seen clinical species include E. xenobiotica, E. oligosperma, E. lecanii-corni, and E. phaeomuriformis | |
| Exophiala dermatitidis | Colonies black, mucoid; nitrate negative; growth at 40°C; black yeast E. exophialae synanamorph present; most common clinical Exophiala species; accurately identified by phenotypic features; obsolete name Wangiella dermatitidis | ||
| Cladophialophora spp. | Colonies black, velvety; growth rates and temperatures vary for individual species; microscopically similar to Cladosporium spp. but lack conidiophores, “shield cells,” and prominent hila; conidia are nonfragile (remain intact in chains); neurotropic species include C. bantiana and C. modesta; other human pathogenic species include C. arxii, C. boppii, C. carrionii, C. devriesii, C. emmonsii, C. mycetomatis, C. samoënsis, andC. saturnica | ||
| Fonsecaea spp. | Colonies olivaceous to black, velvety; conidia formed from swollen denticles giving rise to secondary and tertiary conidia in chains of up to four conidia; conidia also formed on sympodial conidiophores like in Rhinocladiella and occasionally from discrete phialides like in Phialophora; F. pedrosoi an agent of chromoblastomycosis, F. monophora an agent of both chromoblastomycosis and cerebral phaeohyphomycosis | ||
| Ochroconis gallopava | Colonies are brownish, velvety, with a maroon diffusing pigment; 2-celled, clavate conidia borne from denticles; growth at 45°C; no growth on media containing cycloheximide; neurotropic; obsolete names Dactylaria gallopava, D. constricta var. gallopava | ||
| Phialophora spp. | Colonies olivaceous to black, velvety; three species are clinically significant; P. verrucosa has dark, funnel-shaped collarettes; P. americana has deep, vase-shaped collarettes; the slow-growing P. europaea has very short collarettes | ||
| Rhinocladiella spp. | Colonies olivaceous to black, velvety; long, erect, brown, unbranched sympodial conidiophores; 1-celled pale ellipsoidal conidia borne on crowded denticles; an Exophiala yeast synanamorph may be present; R. mackenziei is a neurotropic species in the genus with relatively few conidia per fertile part of the geniculate conidiophore; conidia 1-celled, pale brown, ellipsoidal with a prominent truncate hilum; poor growth at 25°C, good growth at 40°C; obsolete name Ramichloridium mackenziei; other pathogenic species include R. aquaspersa and R. similis | ||
| Veronaea botryosa | Colonies gray to blackish-brown, woolly; long, brown conidiophores; pale brown, 2-celled conidia with a rounded apex and truncate base borne from closely spaced intercalary conidiogenous cells | ||
| Microascales | Scedosporium spp. | Colonies pale to yellowish-gray to darker gray, some with orange reverse, woolly; conidiogenous cells annellidic; some species produce a Pseudallescheria teleomorph and a Graphium synanamorph; similar human pathogenic species in the Pseudallescheria boydii species complex as defined by recent molecular studies include S. apiospermum, S. boydii, S. aurantiacum, and S. dehoogii; S. prolificans (obsolete name S. inflatum) possessing inflated annellidic conidiogenous cells, is unrelated to members of the P. boydii species complex | |
| Scopulariopsis spp. | Colonies gray to olivaceous-brown, woolly; conidiogenous cells annellidic; several very similar dark species are anamorphs of various Microascus spp. | ||
| Sordariales | Madurella mycetomatis | Colonies very slow growing and often heaped; dark brown to black; diffusible brown pigment; unlike M. grisea, M. mycetomatis grows at 40°C and fails to assimilate sucrose; precise identification facilitated by ITS sequencing | |
| Myceliophthora thermophila | Colonies light brown, powdery; ill-defined margin; conidia borne from ampulliform swellings are hyaline and smooth initially becoming rough and brown at maturity; growth at 48°C | ||
| Acrophialophora fusispora | Colonies centrally dark front and reverse; unbranched, erect, brown, echinulate conidiophores are anchored by a foot cell; chains of conidia with fine or coarse spirals produced from apex of brown conidiophores and inflated phialides on hyaline hyphae; growth at 40°C | ||
| Calosphaeriales | Phialemonium spp. | Colonies buff to gray to yellow; conidiogenous cells phialides and adelophialides (reduced phialides lacking a basal septum); P. obovatum has obovate conidia and a green diffusing pigment; sporodochia-producing isolates of P. curvatum have been reported | |
| Phaeoacremonium spp. | Colonies range from buff to pale yellow to pale or dark pink to various shades of brown; hyphae brown; conidiophores often have small warts (exudates); three distinct types of phialides may be present (types I, II, and III); polyphialides may also be present; 1-celled conidia aggregate at apices of phialides and are commonly reniform (kidney shaped) to allantoid (sausage shaped); human pathogenic species that grow at 40°C include P. parasiticum (obsolete name Phialophora parasitica), P. rubrigenum, P. alvesii, P. amstelodamense, P. krajedenii, P. tardicrescens, and P. venezuelense | ||
| Pleurostomophora spp. | Colonies of P. richardsiae (obsolete name Phialophora richardsiae) dark brown, velvety; phialides with prominent flaring collarettes bear globose, brown conidia while phialides with indistinct collarettes bear pale allantoid to cylindrical conidia; colonies of P. repens (obsolete name Phialophora repens) pale brown, phialides lack flaring collarettes, and conidia are pale, allantoid to cylindrical | ||
| Coniochaetales | Lecythophora spp. | Colonies moist, salmon to orange; conidiogenous cells primarily adelophialides; conidia aggregate at apices of conidiogenous cells; L. mutabilis distinguished from L. hoffmannii by dark chlamydoconidia | |
| Ophiostomatales | Sporothrix spp. | Colonies initially cream-colored, moist, with a finely wrinkled surface, becoming brownish-grayish with the production of dark sessile conidia; hyaline, budding cigar-shaped yeast cells present in host and at 35°C; S. schenckii is a species complex as determined by calmodulin sequencing; human pathogenic species include S. schenckii (sessile conidia triangular to oval); S. globosa (sessile conidia globose, no growth at 37°C); S. brasiliensis (sessile conidia subglobose, geographically restricted to Brazil); S.luriei (dark sessile conidia absent) | |
| Anamorphic (asexual) coelomycete (conidia borne within enclosed or semienclosed structures; organisms treated here have pycnidial conidiomata; frequently acquired by traumatic implantation) | Pleosporales | Phoma, Pleurophoma, Pleurophomopsis spp. | Colonies pale to light brownish-gray to darker gray, woolly; pycnidia brown to black; conidia small (4-6 μm), oblong, sometimes slightly curved, hyaline, often guttulate (containing small droplets); species are very similar and best differentiated by ITS sequencing |
| Coniothyrium, Paraconiothyrium, Microsphaeropsis spp. | Colonies pale gray to grayish-brown to brownish-black, some producing dark diffusible pigments into the agar, woolly; pycnidia brown to black; conidia mostly oblong of various sizes, pale brown to dark; species are very similar and best differentiated by ITS sequencing | ||
| Pyrenochaeta spp. | Colonies olivaceous to gray-black, restricted, velvety; pycnidia brown to black with setae surrounding the ostiole; conidia 1-celled, hyaline | ||
| Botryosphaeriales | Lasiodiplodia theobromae | Colonies grayish-black, woolly; pycnidia ostiolate, sometimes with setae; conidiogenous cells annellidic; large conidia 20-30 by 10-15μm, initially aseptate and hyaline; 1 septate, dark, longitudinally striate at maturity; obsolete name Botryodiplodia theobromae | |
| Neoscytalidium dimidiatum | Colonies woolly, black, with rapid growth, filling plate within a few days; an otherwise similar hyaline variant is also referred to as N. dimidiatum; 1- and 2-celled, dark or hyaline arthroconidia not separated by disjunctor cells; thin hyaline and wide (10-12 μm) dark or hyaline hyphae; multilocular pycnidial coelomycetous synanamorph requires several weeks to mature on plant-based media and produces versicolored conidia (middle cell darker); no longer referred to as Nattrassia mangiferae as this organism is an unrelated fruit pathogen now known as Neofusicoccum mangiferae | ||
| Macrophomina phaseolina | Colonies gray, woolly, with a dark diffusing pigment and small black dots representing immature/mature sclerotia; pycnidia and conidia usually not formed in culture; identification by sequencing | ||
| Sordariales | Phomopsis spp. | Colonies pale to light brown or gray, woolly; pycnidia brown to black, may be multilocular; conidia of two types, alpha conidia ellipsoidal while beta conidia long, filamentous, curved | |
| Teleomorphic (sexual) (produce ascomata, asci, and ascospores in culture) | Sordariales | Chaetomium spp. | Colonies olivaceous to grayish-brown, woolly; ascomata perithecial (opening at top) and covered with setae (hairs); large, reddish-brown elliptical ascospores; C. globosum, setae coiled, ascospores subglobose, growth at 35°C, no growth at 42°; C. atrobrunneum, neurotropic, setae mostly straight, ascospores narrowly fusoidal, growth at 42°C; C. perlucidum, neurotropic, very similar to C. atrobrunneum in colony morphology setae, and ascospore size; growth at 42°C |
| Achaetomium strumarium | Colonies pale to light brown with reddish-brown diffusing pigment after 2 weeks, woolly; ascomata perithecial with long, slightly curved setae; ascospores hyaline to dark, 13-17.5 by 8.5-11 μm, fusoidal; neurotropic species with growth at 40°C | ||
| Pleosporales | Leptosphaeria spp. | Colonies dark, velvety to slightly woolly, slow-growing; ascomata cleistothecial (no opening); ascospores hyaline, mostly with 4-6 septa; L. senegalensis and L. thompkinsii distinguished by ascospore features | |
| Microascales | Microascus spp. | Colonies initially white to gray to brownish; ascomata perithecial, developing as black dots in concentric rings on the agar, and may exude a cirrhus of red ascospores at maturity; species treated here have very similar, dark Scopulariopsis anamorphs; M. cinereus, short perithecial necks and orange-segment-shaped ascospores; M. cirrosus, longer perithecial necks with heart-shaped ascospores; M. trigonosporus, longer perithecial necks with triangular ascospores | |
| Pseudallescheria spp. | Colonies pale to yellowish gray to gray to brownish, woolly; ascomata cleistothecial; Scedosporium and Graphium anamorphs present; human pathogenic species as defined by recent molecular studies are Pseudallescheria boydii (anamorph Scedosporium boydii), Pseudallescheria apiosperma (anamorph Scedosporium apiospermum, heterothallic, does not form a teleomorph in culture, d-ribose negative), P. ellipsoidea |
Adapted from Table 14-1 from reference 724 with permission. This list is not all inclusive. Pictures of all organisms are available at doctorfungus.org or on the CD-ROM of the Atlas of Clinical Fungi, pilot version of 3rd ed. (174).
On potato flake agar at 25°C.
True septa continuous with outer wall.
Pseudosepta where only inner walls are involved.
Nomenclature refers to assigning formal scientific names. This process is regulated by the International Code of Botanical Nomenclature (ICBN) (http://www.bgbm.org/iapt/nomenclature/code/default.htm) to facilitate a stable naming system and to avoid and reject names which are in error or are ambiguous (789). Lack of adherence to these requisites often invalidates a taxon name and results in multiple names for the same organism. Other reasons for name changes include the placement of fungi into new genera as determined by phylogenetic studies, which frequently occurs within this heterogeneous group of fungi. When this occurs, the species epithet is retained, but it may require modification in keeping with the rules of Latin grammar. An example of recent changes for melanized fungi include the movement of Phialophora richardsiae to Pleurostomophora richardsiae and of Phialophora parasitica to Phaeoacremonium parasiticum. Discovery of a previously unrecognized teleomorph (sexual or meiotic state) may also precipitate a name change. A recent example is found in the discovery of the teleomorph for Scedosporium apiospermum, which was incorrectly thought to be Pseudallescheria boydii. We now know through the work of Gilgado et al. that the teleomorph for S. apiospermum is the heterothallic ascomycete Pseudallescheria apiosperma, as evidenced by the production of cleistothecia (round sexual structures containing asci and ascospores) and ascospores (the sexual reproductive propagules) between compatible mating strains of S. apiospermum (283). As the teleomorph name takes precedence over the anamorph (asexual, mitotic) name, the correct binomial would be the sexual state. Whether this name would be adopted by clinicians in everyday usage remains problematic.
Also confusing for clinicians and laboratorians alike is the naming convention that permits the use of more than one name for the same fungus. This is allowed when a particular form of the fungus is the one more commonly seen in the laboratory. Fungi recovered in culture commonly display only an anamorphic state. They may be either heterothallic isolates with no known teleomorph or homothallic strains failing to produce their sexual state in vitro. A few clinically significant homothallic melanized fungi do form both anamorphs and teleomorphs in culture. In this situation, as mentioned above, the teleomorph name takes precedence over the anamorph name, e.g., Pseudallescheria boydii rather than Scedosporium boydii and Microascus cinereus rather than Scopulariopsis cinerea. Additionally, in some genera, such as Pseudallescheria, two separate anamorphs which are distinctively different microscopically may be produced, and these are referred to as synanamorphs (another asexual form of the same fungus). Some homothallic strains, however, lack anamorphs and are known only by the name of the sexual state. Examples would include members of the genus Chaetomium. The advent of sequencing characterization has provided the tools necessary to reevaluate the evolutionary relationships of these black molds, and today multilocus molecular phylogenetic studies are clearly redefining previously described entities, uncovering new species and varieties, and correlating these with their natural habitats.
IDENTIFICATION OF ETIOLOGIC AGENTS
Over 150 species and 70 genera of dematiaceous fungi have been implicated in human and animal disease (Table 2). As the number of patients immunocompromised as a result of diseases and medical therapy increases, additional species are being reported as causes of human disease, expanding an already long list of potential pathogens. Identification of melanized etiologic agents known to cause human or animal disease has traditionally been based upon phenotypic features of the isolate observed in culture (175, 177, 220, 221). This practice continues to be the mainstay of fungal identification in most routine settings. More recently, molecular techniques employed for classification purposes and those provided by research facilities have provided additional tools for the characterization of these molds. Extensive sequencing for some genera has illustrated the concept of “species complexes,” or the inclusion of several separate species into what was formerly referred to as a single species. This has been clearly demonstrated in the genera Exophiala (825), Scedosporium (281-283), and Phaeoacremonium (525). The “splitting” of these species into separate taxa has of necessity changed our reporting practices. As an example, laboratories previously comfortable with discriminating only between Exophiala (Wangiella) dermatitidis and E. jeanselmei are now aware of several other clinically significant species that are not easily separated by phenotypic features alone (177, 184, 825) and that E. jeanselmei is in fact one of the less frequent agents of disease. Therefore, species other than E. dermatitidis are best reported as an Exophiala sp., not E. dermatitidis, unless sequencing has provided a species identification. These “new and improved” reporting techniques, however, must be communicated to clinicians in a manner consistent with their understanding of current organism terminology and the associated mycoses.
TABLE 2.
Melanized fungi in human diseasea
| Genus | Species |
|---|---|
| Achaetomiumd | A. strumarium |
| Acrophialophorab | A. fusispora |
| Alternariab | A. alternata, A. chlamydospora, A. dianthicola, A. infectoria, A. longipes, A. tenuissima |
| Anthopsisb | A. deltoidea |
| Arniumd | A. leporinum |
| Arthriniumb | A. phaeospermum |
| Ascotrichad | A. chartarum |
| Aureobasidiumb | A. pullulans |
| Bipolarisb | B. australiensis, B. hawaiiensis, B. papendorfii, B. spicifera |
| Botryomycesb | B. caespitosus |
| Chaetomiumd | C. atrobrunneum, C. funicola, C. globosum, C. murorum, C. perlucidum |
| Cladophialophorab | C. arxii, C. bantiana, C. boppii, C. carrionii, C. devriesii, C. emmonsii, C. modesta, C. mycetomatis, C. saturnica, C. samoënsis |
| Cladorrhinumb | C. bulbillosum |
| Cladosporiumb | C. cladosporioides, C. herbarum, C. oxysporum, C. sphaerospermum |
| Colletotrichumc | C. coccodes, C. crassipes, C. dematium, C. gloeosporioides, C. graminicola |
| Coniothyriumb | C. fuckelii |
| Corynesporab | C. cassiicola |
| Curvulariab | C. brachyspora, C. clavata, C. geniculata, C. inequalis, C. lunata, C. pallescens, C. senegalensis, C. verruculosa |
| Cyphellophorab | C. laciniata, C. pluriseptata |
| Dichotomophthorab | D. portulacae |
| Dichotomophthoropsisb | D. nymphaearum |
| Dissitimurusb | D. exedrus |
| Drechslerab | D. biseptata |
| Exophialab | E. asiatica, E. attenuata, E. bergeri, E. castellanii, E. dermatitidis, E. jeanselmei, E. lecanii-corni, E. moniliae, E. oligosperma, E. phaeomuriformis, E. pisciphila, E. salmonis, E. spinifera, E. xenobiotica |
| Exserohilumb | E. longirostratum, E. mcginnisii, E. rostratum |
| Fonsecaeab | F. monophora, F. pedrosoi |
| Hormonemab | H. dematioides |
| Hortaeaeb | H. werneckii |
| Lasiodiplodiac | L. theobromae |
| Lecythophorab | L. hoffmannii, L. mutabilis |
| Leptosphaeriad | L. senegalensis, L. thompkinsii |
| Macrophominac | M. phaseolina |
| Madurellab | M. grisea, M. mycetomatis |
| Microascusd | M. cinereus, M. cirrosus, M. trigonosporus |
| Monilliellab | M. suaveolens |
| Microsphaeropsisc | M. arundinis, M. olivacea |
| Myceliophthorab | M. thermophila |
| Mycocentrosporab | M. acerina |
| Mycoleptodiscusb | M. indicus |
| Neoscytalidiumc | N. dimidiatum |
| Neotestudinad | N. rosatii |
| Nigrosporab | N. sphaerica |
| Ochrocladosporiumb | O. elatum |
| Ochroconisb | O. gallopava, O. humicola, O. tshawytschae |
| Oidiodendronb | O. cerealis |
| Phaeoacremoniumb | P. alvesii, P. amstelodamense, P. griseorubrum, P. krajdenii, P. parasiticum, P. rubrigenum, P. sphinctrophorum, P. tardicrescens, P. venezuelense |
| Phaeosclerab | P. dematioides |
| Phaeotrichoconisb | P. crotalariae |
| Phialemoniumb | P. curvatum, P. obovatum |
| Phialophorab | P. americana, P. bubakii, P. europaea, P. reptans, P. verrucosa |
| Phomab | P. cruris-hominis, P. dennisii var. oculo-hominis, P. eupyrena, P. glomerata, P. herbarum, P. minutella, P. minutispora, P. sorghina |
| Piedraiad | P. hortae |
| Pleurophomac | P. cava |
| Pleurophomopsisc | P. lignicola |
| Pleurostomophorab | P. repens, P. richardsiae |
| Pseudochaetosphaeronemab | P. larense |
| Pseudomicrodochiumb | P. suttonii |
| Pyrenochaetab | P. mackinnonii, P. romeroi, P. unguis-hominis |
| Rhinocladiellab | R. aquaspersa, R. basitona, R. mackenziei, R. similis |
| Sarcinomycesb | S. phaeomuriformis |
| Scedosporiuma | S. prolificans |
| Scopulariopsisb | S. asperula, S. brumptii, S. fusca |
| Sphaeropsisc | S. subglobosa |
| Stenellab | S. araguata |
| Taeniolellab | T. stillbospora |
| Tetraploab | T. aristata |
| Thermomycesb | T. lanuginosus |
| Ulocladiumb | U. chartarum |
| Veronaeab | V. botryosa |
Some doubtful cases have been omitted; the list may not be all inclusive. Some genera that are outside the taxonomic orders discussed in the text but that contain melanized structures are included. Adapted from reference 493 with permission of the publisher.
Anamorphic hyphomycete.
Anamorphic coelomycete.
Teleomorphic ascomycete.
Phenotypic Identification
The level to which black molds can or should be identified in the routine laboratory may depend on several factors, such as the genus of the organism recovered, whether or not an epidemiologic investigation is warranted, and/or the level of identification required for appropriate patient management. The phenotypic identification of black molds is based primarily upon their macroscopic morphology (color, growth rate, and growth characteristics on standardized media), their microscopic morphology (hyphae, conidiogenous cells [specialized cells that produce the conidia], conidia [asexual reproductive propagules], etc.), and a limited number of physiologic features (primarily cycloheximide tolerance, nitrate assimilation, urea hydrolysis, and growth at various salt concentrations). Only genus-level identification may be possible or required for genera with several similar, closely related species, such as described above for Exophiala. In some other genera, certain species are clearly associated with a particular type of mycosis, and a combination of morphologic features, temperature, and physiology can provide a species-level identification. This is the case for the agent of cerebral phaeohyphomycosis, Cladophialophora bantiana.
Macroscopic morphology.
The medium (see “Isolation Procedures and Culture” in Diagnosis below) is an important consideration in the identification of melanized fungi. The use of a medium that promotes growth most consistently matching the original description of the organism is preferred, and this typically is a plant-based medium. The most commonly used is potato dextrose agar (PDA) or variations thereof. It provides colony colors that are close to those originally described, and it is usually adequate for conidiation. Other plant-based media include malt extract agar, V-8 juice agar, cereal agar, carnation leaf agar, cornmeal dextrose agar, and others. A more complete list of media and reagents may be found in the Manual of Clinical Microbiology, 9th ed. (441), and in the Atlas of Clinical Fungi, 2nd ed. (175). Phaeoid molds vary considerably in their colony colors. Although this characteristic is highly dependent upon environmental conditions, it is one that can be useful in the initial separation of genera/species. While most species are various shades of pale gray to dark gray to black, others may be brown or very pale or may turn darker only with the production of certain structures. Others may be some shade of purple or distinguished by diffusible pigments. Etiologic agents which are typically brown on PDA include Ochroconis gallopava, Pleurostomophora richardsiae, Pleurostomophora repens, some Phaeoacremonium species, Wallemia sebi, Myceliophthora thermophila, and Veronaea botryosa. The “pale list” includes fungi which seldom turn dark, such as Phialemonium species. Lecythophora mutabilis remains lightly colored until the production of dark chlamydospores. Ochroconis gallopava exudes a wine-red pigment into the agar (more pronounced on Sabouraud dextrose agar [SDA]), and several Phaeoacremonium species exhibit purple to lavender colonies.
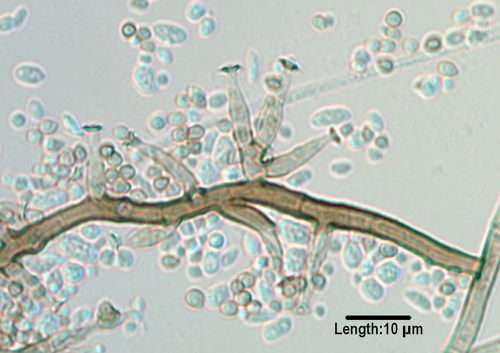
Microscopic morphology and pleomorphism.
Variable microscopic morphology in the same fungus, also referred to as pleomorphism or pleoanamorphism, is another feature useful in the phenotypic identification of black molds. Some fungi may display more than one form, such as yeast-like growth initially and more filamentous growth subsequently. This is common in the black yeasts such as Exophiala and related genera. Pleoanamorphism may also be exhibited by different types of anamorphic structures (synanamorphs), such as the Graphium state in Pseudallescheria or variably shaped conidia in Pleurostomophora richardsiae (Fig. 1). Identification of homothallic ascomycetes is typically based on the type of ascomata produced (primarily cleistothecia [round, closed structures containing asci and ascospores] or perithecia [round to pear-shaped structures with an opening or ostiole containing asci and ascospores], as in Pseudallescheria or Chaetomium/Achaetomium/Microascus, respectively) and differences in ascospore morphology. Ascospores may be of various sizes, shapes, colors, and ornamentations. The bulk of clinical black molds, however, are heterothallic ascomycetes. These mitosporic fungi are identified mostly by their methods of conidiogenesis and the morphology of their conidia. The majority of mitosporic isolates are hyphomycetes with their conidia borne free in the aerial mycelium. Also seen are coelomycetes, whose conidia are borne within asexual structures known as conidiomata. The methods of conidiogenesis (blastic [blown-out = blastoconidia, as seen in many genera] or thallic [formed from preexisting hyphae = arthroconidia, as in Neoscytalidium]), the types of conidiogenous cells (primarily annellidic [Scedosporium, Scopulariopsis, and Hortaea] or phialidic [many genera]), and the morphology of the conidia are taken in aggregate to form the basis for a morphologic identification. Annelloconidia are formed from percurrent, indeterminate conidiogenous cells that produce rings or annellations and become longer and narrower with the production of conidia, while phialoconidia are formed from conidiogenous cells with collarettes that may be quite distinct or subtle, and the conidiogenous cell remains the same size and shape with conidial production. It should be pointed out, however, that these morphologic features used to identify anamorphic species lacking teleomorphs are strictly phenotypic and do not define their phylogenetic placement within the order (157).
FIG. 1.
Conidiogenous cells of Pleurostomophora richardsiae, demonstrating prominent flaring collarettes as well as the two types of conidia (oval and globose) produced by this species. (Unless otherwise noted, in this and subsequent figures light microscopy photomicrographs of conidiogenous cells and/or conidia were taken from slide culture preparations grown on potato flakes agar for 7 days at 25°C.)
Physiologic features.
Physiologic characteristics may also assist in separation of various genera/species. However, only those that are available in routine laboratories are widely employed. The ability or inability of isolates to grow on media containing cycloheximide (referred to as cycloheximide tolerance), nitrate assimilation, urease activity, and salt tolerance, particularly for halophilic strains, are all useful adjuncts to the morphologic examination. Larger reference labs and research facilities also may use a battery of carbon assimilation profiles. Temperature tolerance is also useful in segregating potential pathogens. Those that fail to grow at 35°C are more likely to be recovered from superficial sites, while those capable of growth at this temperature have the potential for more invasive human disease. Several clinically significant dematiaceous molds are thermotolerant to thermophilic, with maximum growth temperatures to 45°C and beyond. A partial listing of these potentially neurotropic species includes E. dermatitidis, O. gallopava, C. bantiana, C. modesta, C. emmonsii, R. mackenziei, Acrophialophora fusispora, Fonsecaea monophora, and some aggressive Achaetomium and Chaetomium species.
Molecular Characterization
Molecular characterization of fungi is a mature discipline in the molecular systematics arena, with multilocus datasets, extensive taxon sampling, and rigorous analytical methods being the norm (340). Its use in the clinical laboratory, however, is mostly restricted to epidemiologic studies and to identification of unusual/uncommon or difficult-to-identify isolates. Molecular identification of most species relies on sequencing of ribosomal genes and comparison with published databases, notably those in GenBank; however, over 10% of these deposits may be erroneous (176). Private databases are also sometimes utilized for particular genera; however, these are difficult to access and may also contain incorrect deposits. Also, various methods and genes or gene regions such as the internal transcribed spacer regions ITS1 and ITS2, the D1/D2 domains, β-tubulin, actin, calmodulin, manganese superoxide dismutase, ATPase subunit 6, chitin synthase, mitochondrial small-subunit (SSU) rRNA, translation elongation factor 1α, and others are utilized, so that interlaboratory standardization of sequencing is lacking. Several International Society for Human and Animal Mycology (ISHAM) working groups are addressing standardization of fungal sequencing (58) as is the Clinical and Laboratory Standards Institute (CLSI) (146). Genera for which substantial sequencing data are available and for which species distinction appears to be satisfactory include those known as black yeasts, i.e., Exophiala and related genera (ITS) (825), Sporothrix species (calmodulin) (480), Phaeoacremonium species (β-tubulin and actin) (525), and Pseudallescheria/Scedosporium species (ribosomal DNA [rDNA] gene cluster, β-tubulin, calmodulin, and translation elongation factor 1α) (330). Molecular characterization should always be evaluated in light of phenotypic features, and sequence data for uncommon and/or potentially new species should be compared with those for ex-type strains.
ANAMORPHIC HYPHOMYCETE GENERA
Capnodiales
Hortaea
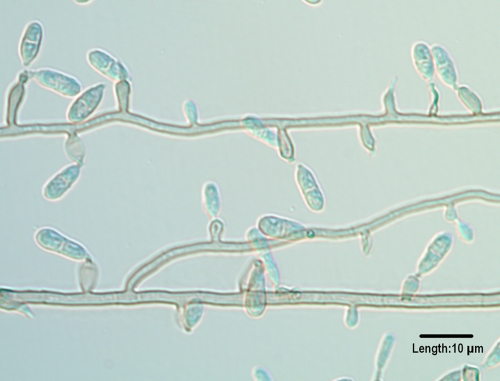
Hortaea werneckii is the etiologic agent of tinea nigra, an asymptomatic, superficial mycosis causing hyperchromic plaques without keratinolysis in the dead keratin layers of the skin (186, 685) and mostly restricted to the palms of the hands (tinea nigra palmaris) and soles of the feet (tinea nigra plantaris) (87). It is a halophilic organism whose natural habitat is in tropical and subtropical hypersaline environments (823), and it is thought be acquired through superficially abraded skin (186). Colonies are restricted, black, moist, and yeast-like initially, later becoming filamentous. Wide hyphae are densely septate, thick walled, and brown. Intercalary or lateral annellidic conidiogenous cells produce brown, two-celled ellipsoidal conidia with a darkened central septum. ITS sequencing facilitates molecular identification (823) and clearly distinguishes H. werneckii from other closely related halophilic and acidophilic (H. acidophila) nonpathogenic species (347).
Cladosporium.
The genus Cladosporium has recently undergone molecular and morphologic scrutiny (157), with many organisms being reassigned to other genera. One example is the transfer of Cladosporium elatum to Ochrocladosporium elatum. The genus is extremely ubiquitous, and although it is an agent of allergic disease in indoor settings, few species are documented to cause disease. The species complexes Cladosporium cladosporioides and C. oxysporum are the ones most commonly cited in cases of cutaneous and subcutaneous disease (313, 586, 641, 776) and occasionally deeper infections (396, 429); however, they are commonly contaminants, making the nature of reports doubtful. The inability of Cladosporium species to grow on media containing cycloheximide, their prominent “shield cells,” and conidia that are fragile (easily detached) and possess dark hila (attachment scars) are all features distinguishing Cladosporium from the unequivocally pathogenic Cladophialophora species.
Dothideales
Aureobasidium.
Recent molecular characterization of Aureobasidium pullulans and closely related organisms by multilocus sequence analysis (ITS, partial 28S rDNA, β-tubulin, translation elongation factor 1α, and elongase), expanding the work of de Hoog and Yulova and of Yulova et al. (173, 820), has shown that the genus Aureobasidium contains a single species and several varieties containing differing amounts of melanin and having various salt (820, 823) and temperature (824) tolerances. The mode of conidiogenesis is primarily synchronous rather than percurrent, as in Hormonema; however, features of conidiogenesis are difficult to ascertain with certainty. Sequencing is usually required for a definitive identification. Two varieties are human pathogens, A. pullulans var. pullulans, and A. pullulans var. melanigenum. In the former, colonies remain pink for approximately 1 week, tolerate 15% salt, and have a maximum temperature of 30°C, while in the latter, colonies are black at the outset, tolerate 10% salt, and have a maximum temperature of 35°C. Aureobasidium is an opportunistic pathogen of humans and animals recovered in cases of catheter-related septicemia (117, 360), disseminated infections (344, 663), chromoblastomycosis (616), and peritonitis (144, 367).
Hormonema.
As noted above, Hormonema species are phenotypically similar to Aureobasidium pullulans; however, conidiogenesis is primarily percurrent rather than synchronous. There are rare reports of cutaneous phaeohyphomycosis (149) and fungal peritonitis (690) due to this organism, both of which were reported prior to molecular characterization.
Pleosporales
Alternaria.
Alternaria is a large genus of plant pathogen species that are only occasionally implicated in opportunistic human disease. Cutaneous and subcutaneous phaeohyphomycosis in immunosuppressed individuals is the most common presentation (275, 577, 587, 798). Organ transplantation (280) and Cushing's syndrome appear to be major risk factors for cutaneous/subcutaneous disease, while bone marrow recipients are at risk for sinusitis (577). Ocular disease in individuals exposed to soil and garbage (577) is the next most common presentation, while onychomycosis is rarely reported. There are also occasional reports of allergic fungal sinusitis (67). While several species, such as A. chlamydospora (65, 703), A. longipes (275), and A. tenuissima (124, 642, 644), have been reported, most clinical isolates have been shown to be either A. alternata (176, 497, 710) or A. infectoria (99, 551, 648). ITS region sequences have demonstrated that A. longipes and A. tenuissima cannot be distinguished from A. alternata. Conidial production by Alternaria infectoria is sparse, and colonies may be pale.
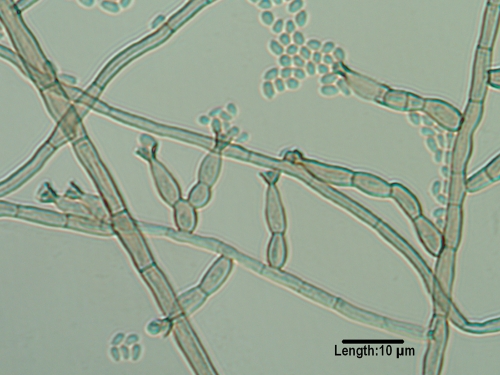
Bipolaris.
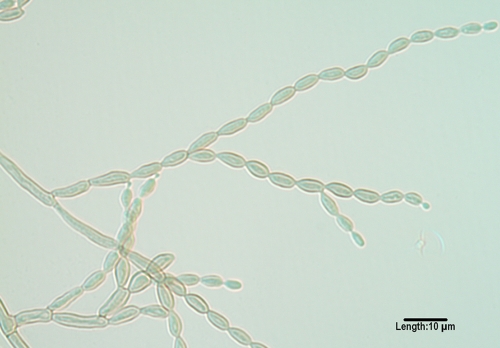
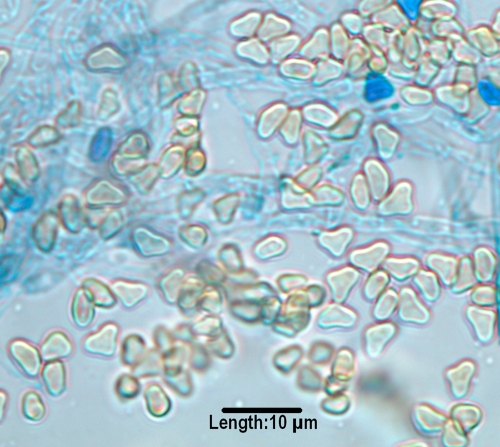
The most common mycosis attributed to Bipolaris spp. is allergic fungal sinusitis (125, 246, 417, 444, 508, 580). Other disease associations include subcutaneous lesions, keratitis, and peritoneal dialysis-associated peritonitis (508). Extension to the central nervous system via the nasal sinuses highlights the neurotropic potential of the genus, though this is very rare (260, 817). Clinically significant species inciting human disease include B. spicifera, B. hawaiiensis (Fig. 2), and B. australiensis. They are differentiated morphologically by conidial size and the number of distoseptations (pseudosepta where only inner walls are involved) (20). Conidia demonstrate bipolar germination, hence the genus name “Bipolaris.”
FIG. 2.
Conidia of Bipolaris hawaiiensis, demonstrating mostly five distosepta and six cells being borne from a geniculate conidiophore/conidiogeous cell.
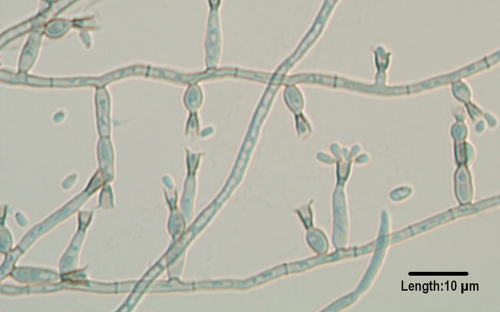
Curvularia.
Curvularia species are common in dead plant material and may cause a variety of human mycoses, including fungal keratitis, invasive sinusitis (215), onychomycosis, black grain eumycotic mycetoma (378), endocarditis (104), subcutaneous disease (813), and peritonitis (98, 241, 631) as well as systemic infections (175, 177). Additional reports involved fatal cerebral phaeohyphomycosis in an immunocompetent individual (121), endophthalmitis (579), and contaminated saline-filled breast implants (392). Clinical isolates include C. geniculata, C. lunata, C. pallescens, C. senegalensis, C. brachyspora, C. clavata, C. verruculosa, and C. inaequalis (Fig. 3) (598). C. lunata is the most common clinical species, and C. lunata var. aeria (Fig. 4) may produce large, upright stroma in culture that are visible with the naked eye.
FIG. 3.
Conidia of Curvularia inaequalis with mostly five septa and six cells borne from a geniculate conidiogenous cells.
FIG. 4.
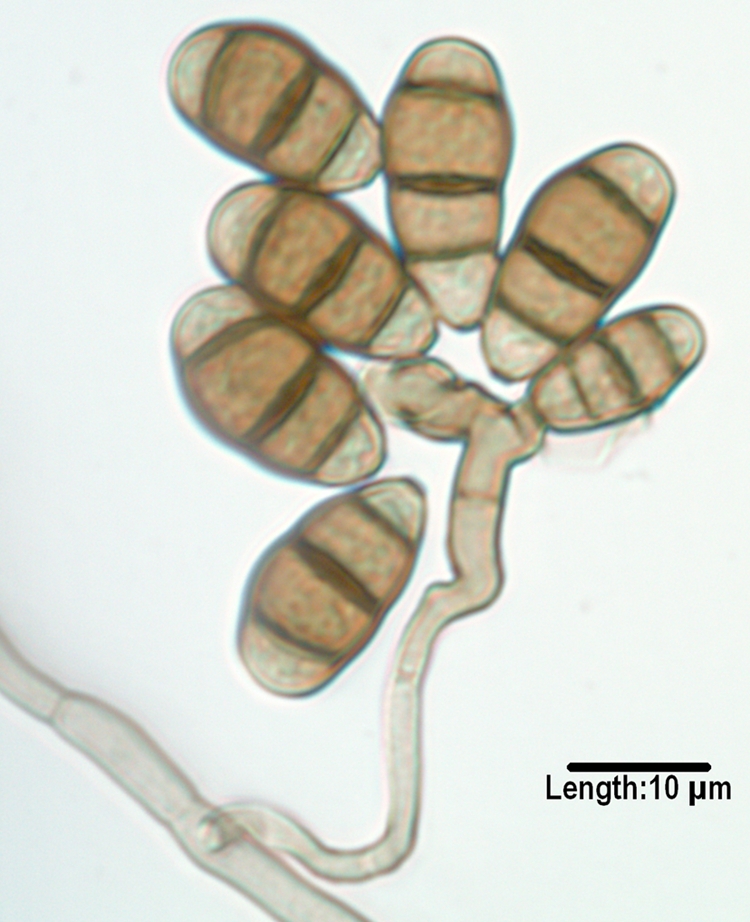
Conidia of Curvularia lunata var. aeria borne from a geniculate conidiogenous cell. Note that the middle cell is slightly enlarged, and septa are eusepta (true septa continuous with the outer wall).
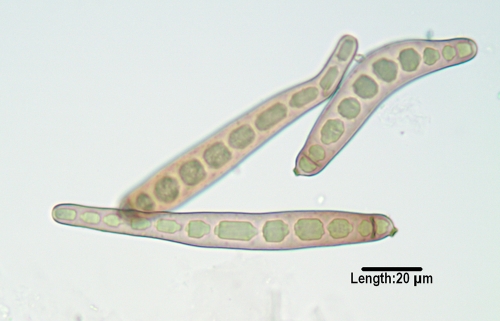
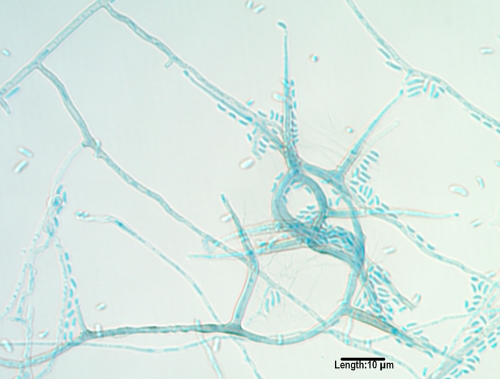
Exserohilum.
Three Exserohilum species recovered from humans are E. rostratum, E. longirostratum, and E. mcginnisii, although molecular studies suggest that they may be the same species (175, 177). The genus is characterized by its long, multidistoseptate conidia and a protruding hilum. E. rostratum exhibits darkened basal and distal septa, and E. longirostratum has conidia that are noticeably longer and centrally curved (Fig. 5), while E. mcginnisii has conidia with warty projections on their outer walls. Not all authorities agree that E. rostratum and E. longirostratum are separate species. Species are opportunistic and are etiologic agents of sinusitis (566), which may extend to the central nervous system (46), and keratitis (488), as well as cutaneous and subcutaneous mycoses (359, 508, 580). A fatal disseminated case was reported in a patient with aplastic anemia (37).
FIG. 5.
Multidistoseptate conidia of Exserohilum longirostratum, demonstrating a prominent basal septum (true septum) and a protruding hilum.
Chaetothyriales
Exophiala.
Species in the genus Exophiala are frequently referred to as “black yeasts” due to the ability of several species to form a budding yeast-like synanamorph as well as hyphal forms. Colonies are olivaceous-black with a black reverse and are initially moist or yeast-like, later becoming velvety at maturity. Asexual replication is by annellidic conidiogenous cells, and conidia are formed in clusters both from intercalary conidiogenous loci and at the tips of annellides. Some species may occasionally form conidia in chains (182) (catenate) on nutritionally deficient media or display phialides as well as annellides (183). Species are very similar microscopically, and unequivocal differentiation is facilitated by physiologic features such a temperature tolerance and nitrate assimilation and by molecular characterization. Some waterborne psychrophilic species such as E. pisciphila are pathogens of fish (436, 438), while others such as E. mesophila are found in dental unit water lines (604) and municipal drinking water (300). The most clinically important species are thermotolerant (719). In a recent study of U.S. clinical isolates, reidentification of strains by ITS sequencing showed the most common species to be E. dermatitidis (29%), E. xenobiotica (20%), and E. oligosperma (19%) (94, 825). While many clinical isolates are reported as E. jeanselmei, which has been regarded as a major agent of subcutaneous phaeohyphomycosis, this species made up only 8% of the isolates, and molecular studies clearly showed E. jeanselmei to be a heterogeneous complex of species (184, 780). Exophiala jeanselmei has been redefined clinically as an agent of traumatic cutaneous infection eventually leading to eumycetoma (27, 52, 683). Exophiala dermatitidis is distinguished phenotypically by its mostly mucoid colonies, ability to grow at 40°C, lack of nitrate assimilation (569), and yeast cells surrounded by capsules (819), which it shares with another aggressive species, E. spinifera (180, 214, 536, 699). The range of mycoses incited by E. dermatitidis include neurotropic infections in young, immunocompetent individuals (restricted to Asia) (138, 345, 492, 494), systemic lymphadenitis (13), cutaneous and subcutaneous infections in mostly immunocompromised individuals (346, 492), colonization of airways in cystic fibrosis patients (597), and mycoses related to continuous ambulatory peritoneal dialysis (CAPD) (783). It is also an opportunist in lungs of cystic fibrosis patients (320, 355) and may be recovered from the stool in patients with diarrhea (178). It has been recovered from Turkish steam baths (489) and associated with free-living amoebae in hospital water (128). E. phaeomuriformis, which is similar in morphology to E. dermatitidis, can grow at a maximum temperature of 38°C (490). Exophiala spinifera and the similar E. attenuata (780) have long, spine-like conidiophores. E. spinifera is an agent of serious disseminated mycoses in adolescents (180) and of cases of subcutaneous phaeohyphomycosis (327, 699). E. xenobiotica, which is capable of growing in the presence of high concentrations of xenobiotics such as xylene, toluene, or creosote-treated utility poles, was the agent of subcutaneous phaeohyphomycosis in a non-Hodgkin lymphoma patient (36). E. asiatica is a newly described species causing a fatal, disseminated cerebral phaeohyphomycosis in China (452).
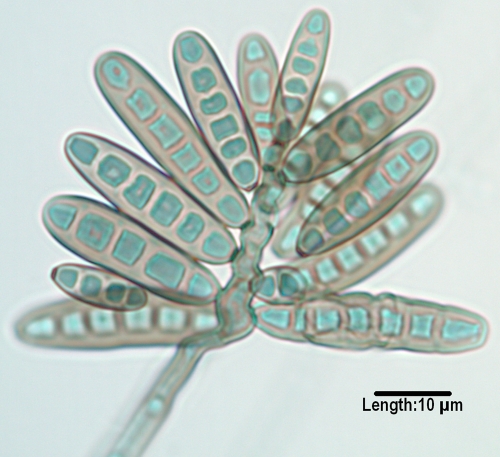
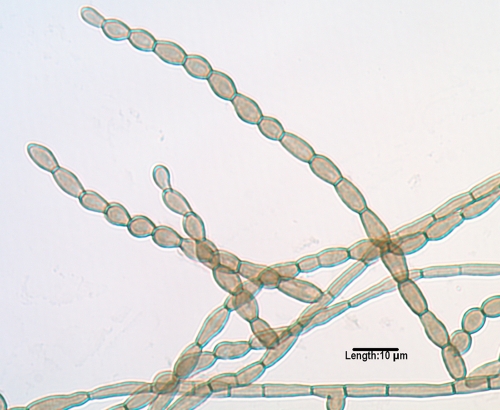
Cladophialophora.
Cladophialophora species, although morphologically similar to Cladosporium species, are differentiated by belonging to a different order, the Chaetothyriales rather than the Capnodiales; by lacking conidiophores, “shield cells,” or prominent hila (attachment points); by their ability to grow on media containing cycloheximide; and by having dry, nonfragile chains of conidia. The genus has recently been reevaluated by multilocus sequencing and currently contains seven species associated with humans (51). C. bantiana, (Fig. 6), previously characterized at the molecular level (279), is a neurotropic species with growth at 40°C and is the causative agent of numerous cases of cerebral phaeohyphomycosis (204, 272, 353, 395, 628, 733), many of which occur in immunocompetent individuals and most of which are fatal. The species has also been reported as an agent of eumycetoma (89), along with Madurella mycetomatis (51). Less common species occasionally incriminated in deep and superficial mycoses include C. modesta, C. arxii, C. devriesii, C. emmonsii (Fig. 7), C. boppii, and C. saturnica (47, 51, 295, 505, 516, 568, 748). C. carrionii and the recently described C. samoënsis are agents of chromoblastomycosis (51, 229, 446, 610, 826). C. yegresii is considered a closely related environmental sister species to C. carrionii (181, 782).
FIG. 6.
Long, nonfragile chains of conidia as seen in Cladophialophora bantiana.
FIG. 7.
Long, nonfragile chains of conidia produced by a less common species of Cladophialophora, C. emmonsii. Note that conidiophores and prominent hila (attachment scars) are absent.
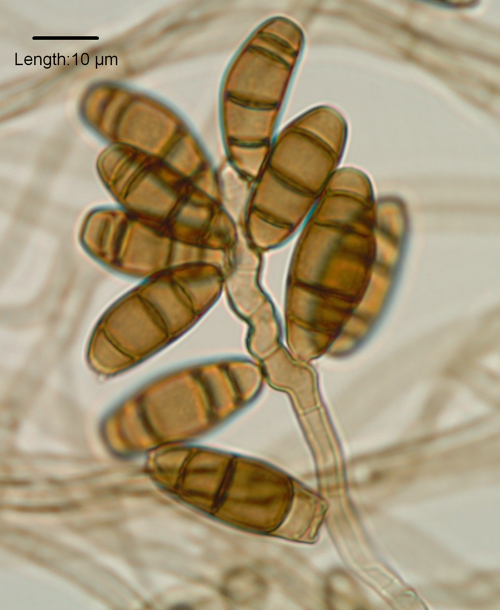
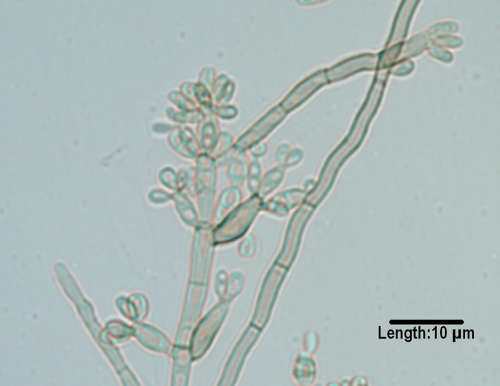
Fonsecaea.
The genus Fonsecaea is comprised of two species (174, 533). F. pedrosoi is known almost exclusively as an agent of chromoblastomycosis (45, 515, 610, 695), while the newly described F. monophora (Fig. 8) is known as an agent of chromoblastomycosis (808, 809, 810) and subcutaneous disease and, more recently, cerebral phaeohyphomycosis (721, 733). Prior reported cases of central nervous system and/or other deep tissue infections (520, 545, 701) should most likely be attributed to F. monophora. A murine model of disseminated infection with F. monophora was recently reported (113). Both species form conidia from swollen denticles which give rise to secondary and tertiary conidia in short chains of up to four conidia. Conidia may also be formed from sympodial conidiophores, as in Rhinocladiella, and in balls from discrete phialides with collarettes, as in Phialophora. Molecular characterization is required for unequivocal differentiation.
FIG. 8.
Conidial formation in Fonsecaea monophora. Conidia are formed from swollen denticles which give rise to secondary and tertiary conidia in chains of up to four conidia. The same type of conidiogenesis occurs in F. pedrosoi.
Ochroconis.
O. gallopava was initially observed to cause central nervous system disease in poultry (354). It has subsequently been shown to be an etiologic agent of neurotropic infections in immunocompromised humans (692) as well as pulmonary infections in immunocompetent hosts (348, 554). O. gallopava has colonies that are brownish rather than gray or olivaceous, produces a maroon diffusing pigment more pronounced on SDA than on PDA, grows at 40°C, fails to grow on media containing cycloheximide, and displays clavate, two-celled, hyaline conidia borne on long denticles (Fig. 9).
FIG. 9.
Two-celled, clavate (club-shaped) conidia of Ochroconis gallopava borne on long, thin denticles.
Phialophora.
Some human pathogens with phialidic conidiogenesis previously assigned to Phialophora (263) have been moved to other genera, namely, Phaeoacremonium (525) and Pleurostomophora (777), leaving only those species that are filamentous throughout their life cycle. Both P. verrucosa and P. americana produce their conidia from phialides with conspicuous darkened collarettes; these are funnel shaped and vase shaped in P. verrucosa (Fig. 10) and P. americana (Fig. 11), respectively. Sequencing has demonstrated a close relatedness, suggesting that the species may be synonymous (185, 811). P. verrucosa is primarily an agent of chromoblastomycosis (257, 770), although other reported infections include endocarditis, keratitis, and osteomyelitis (209, 760). A recently described species implicated in superficial infections, P. europaea, has very short collarettes (179).
FIG. 10.
Dark, funnel-shaped collarettes at the tips of the conidiogenous cells (phialides) in Phialophora verrucosa. Also note the oval-shaped conidia.
FIG. 11.
Deep, dark, vase-shaped collarettes in Phialophora americana.
Rhinocladiella.
Four species of Rhinocladiella are known agents of human disease. R. mackenziei (formerly Ramichloridium mackenziei and also thought to be synonymous with Ramichloridium obovoideum, which is now unrelated in the genus Pleurothecium as P. obovoideum) (43) is a frequently fatal neurotropic organism previously thought to be restricted to individuals residing in or immigrating from Middle Eastern countries (114, 394, 726). It has now been reported as the etiologic agent of a brain abscess in a man from India, an area where it is not endemic, who reported no travel outside the country (48). R. aquaspersa is an occasional agent of chromoblastomycosis (39, 589, 693). R. basitona was recovered from subcutaneous lesions in a man from Japan (43). R. similis (184) appears to be the agent reported under the name R. atrovirens in cases of mycetoma (535) and cerebral phaeohyphomycosis in an AIDS patient (193).
Veronaea.
Initial reports of infection due to Veronaea botryosa were clustered in China; however, a more global distribution is now recognized, with cases seen in Libya, Philippines, an island in the Indian Ocean, and the United States. Two cases are noteworthy as agents of subcutaneous disease in heart (725) and liver (251) transplant recipients. The genus has recently been reexamined at the molecular level by Arzanlou et al. (43).
Microascales
Scedosporium.
The genus Scedosporium and its associated teleomorph Pseudallescheria were extensively reviewed by Cortez et al. in 2008 (153); therefore, the information provided here will augment that previously published and/or highlight new taxonomy, distribution, and disease. Scedosporium prolificans, which is closely related but not a member of this complex, appears to occupy a more restricted geographic range, with infections occurring mainly in Australia, Spain, and the United States (747). Clinical discussion (see Clinical Syndromes and Their Management below) will be limited to S. prolificans, as related species have been extensively reviewed elsewhere (153) and may not reveal phaeoid hyphae in tissue, in contrast to the case for S. prolificans. Infection with this organism is of major concern in all settings due to its refractoriness to antifungal therapy and associated high mortality (18, 29, 76, 96, 119, 153, 312, 475, 538, 664, 802, 806). A recent review of 162 cases reported in the literature summarizes major risk factors as malignancy (46%), cystic fibrosis (12%), and solid organ transplantation (9%) and chief clinical presentations as disseminated infection (44%) and pulmonary mycoses (29%), followed by bone and joint infections (10%) (638). All disseminated infections were in individuals with underlying disease, primarily hematological malignancies; 70% of these had positive blood cultures, and mortality in this group was 88%. Molecular characterization by ITS, D1/D2, translation elongation factor 1α, and the chitin synthase genes for 20 cases of S. prolificans infection occurring in Germany between 1993 and 2007 suggests the possibility of two or three distinct genotypes (747). This finding may further our understanding of the epidemiology of this organism. Multiple genotypes were previously suggested by inter-simple-sequence-repeat (ISSR) fingerprinting (708). Increased numbers of infections with S. prolificans have also been reported from France (304) and Australia (190). Inflated annellides, a key microscopic feature in the identification of this organism, may be subtle in some isolates and easily overlooked. However, the colony color of S. prolificans is always darker than for other Scedosporium species.
Scopulariopsis.
The genus Scopulariopsis is unusual in containing both hyaline and dark species. Most pigmented species associated with disease are anamorphs of various Microascus species detailed in Teleomorphic Genera below. Scopulariopsis shares an annellidic method of conidiogenesis with Scedosporium species but can be differentiated from this genus by conidial formation in chains rather than in clumps.
Sordariales
Madurella.
An agent of dark grain mycetoma primarily in West Africa, M. mycetomatis has recently been proven to be a member of the Sordariales (172), unlike M. grisea, which resides in the Pleosporales. Isolates are very slow growing, produce a brown diffusible pigment, grow at 40°C, and frequently remain sterile in culture; however, lateral phialides and globose conidia are occasionally produced. Precise identification is facilitated by DNA sequencing. Molecular characterization of 38 different M. mycetomatis isolates from Sudan has shown them to have identical DNA patterns, suggesting that host susceptibility rather than differential virulence is the determining factor in clinical presentations (10).
Myceliophthora.
Myceliophthora thermophila is a thermophilic fungus common in high-temperature areas such as compost and exhibits growth at 50°C. Colonies are pale brown, and conidia are borne from ampulliform swellings. Reports suggest that its recovery from tissue, even with a heavy fungal burden, may be difficult (196). The organism is also uncommonly seen in the laboratory and may provide identification challenges. It has been fatal in a disseminated case (95) and in a patient with aortic involvement with medial necrosis (234). A severe case of osteomyelitis was also reported following extensive injury to a knee and distal femur following a barnyard pitchfork injury (196).
Acrophialophora.
Acrophialophora fusispora is an uncommonly seen agent occasionally microscopically misidentified as Scedosporium prolificans. The two species have similarly inflated conidiogenous cells, although they are phialidic versus annellidic and conidia are produced in chains rather than clusters in A. fusispora and S. prolificans, respectively. The organism grows at 40°C, colonies display a striking darkening centrally (both front and reverse), and it produces finely echinulate conidia demonstrating various degrees of spiral banding. It has been reported as an agent of cerebral phaeohyphomycosis in a leukemic child (24), as an agent of keratitis (691), and as an agent of keratouveitis in association with a retained intraocular lens (41).
Calosphaeriales
Phialemonium.
The genus Phialemonium was initially described to accommodate organisms closely resembling Acremonium spp. but containing pigmentation, although colonies often remain pale (264). Colonies are typically moist to slightly filamentous, and conidiogenous cells are a mixture of medium-length phialides and adelophialides (short phialides lacking a basal septum). The genus currently contains two species of clinical interest, P. obovatum and P. curvatum. P. obovatum produces a green, diffusible pigment; has obovate conidia (like an upside-down egg); and has been reported as an agent of fatal endocarditis in a neonate (273). P. curvatum isolates range from cream to yellowish to pale brownish and have allantoid (curved) conidia. Infections attributed to P. curvatum include cutaneous and subcutaneous disease, disseminated infection, endophthalmitis, peritonitis, arthritis, and fungemia (167, 264, 308, 793). Also reported are cases of hemodialysis-associated endovascular infections (608) and endocarditis (561), with some cases linked to intracavernous penile injections in men frequenting impotence clinics (717). Several recent cases have demonstrated sporodochial formation in P. curvatum, a feature not previously seen in this species (167, 608, 793). Rivero et al. have recently reviewed published Phialemonium cases (634).
Phaeoacremonium.
The genus Phaeoacremonium initially accommodated isolates with features similar to those seen in both Acremonium and Phialophora (159). It differs from the former by having pigmented hyphae and conidiophores and from the latter by having indistinct collarettes and warty conidiogenous cells. A recent morphologic and molecular characterization of the genus using β-tubulin sequences (525) has more clearly defined the genus and provided differential features for clinically significant species. Human pathogens include P. parasiticum (obsolete Phialophora parasitica) (Fig. 12) (335), P. alvesii (567), P. amstelodamense, P. griseorubrum, P. krajdenii (525), P. rubrigenum (491), P. tardicrescens, and P. venezuelense (309, 525). Infections caused by P. parasiticum include subcutaneous abscesses (245), thorn-induced arthritis (651), and disseminated infection (54). Colony colors may range from yellowish brown to orange-brown to brown to lavender.
FIG. 12.
Melanized hyphae, demonstrating warts (bottom), long robust phialides, and allantoid (curved) conidia of Phaeoacremonium parasiticum.
Pleurostomophora.
Clinically significant species in the mostly wood-inhabiting genus Pleurostomophora include P. richardsiae (obsolete Phialophora richardsiae) and P. repens (obsolete Phialophora repens), and individuals acquiring these mycoses are commonly immunocompromised (369, 601, 815). Species are anamorphs of the genus Pleurostoma. P. richardsiae is characterized microscopically by distinctive flaring collarettes (Fig. 1) and both globose and oval conidia. The colonies of both species tend to be brown rather than gray or olivaceous. Human infections include subcutaneous cases (311) and bone disease (761).
Coniochaetales
Lecythophora.
Two Lecythophora species, L. mutabilis and L. hoffmannii, are of clinical significance. Both produce orange, moist colonies initially, with central darkening in L. mutabilis as pigmented chlamydospores are produced. Organisms are agents of endophthalmitis (677), sinusitis (485), and prosthetic valve endocarditis (207). Recent large-subunit rDNA sequencing confirms the association of Lecythophora species with teleomorphs in the genus Coniochaeta (792) in the order Coniochaetales (361).
Ophiostomatales
Sporothrix.
Sporotrichosis occurs worldwide, with the primary agent of disease being Sporothrix schenckii. The disease is commonly acquired by implantation of the fungus from various types of woody/plant material. Lymphocutaneous lesions are the norm; however, pulmonary disease and disseminated infections may occur in patients with underlying diseases (177). As a dimorphic fungus, it exhibits cigar-shaped yeasts in tissue and at 35°C and filamentous growth in culture. Only the sessile conidia borne along the sides of the hyphae are melanized. In a recent study characterizing the genus by calmodulin sequencing (480) and critically reviewing morphologic/physiologic features, these sessile conidia were shown to vary according to species within the S. schenckii species complex (479). They are elongate to triangular in S. schenckii and globose to subglobose in S. brasiliensis and S. globosa.
ANAMORPHIC COELOMYCETE GENERA
Pleosporales
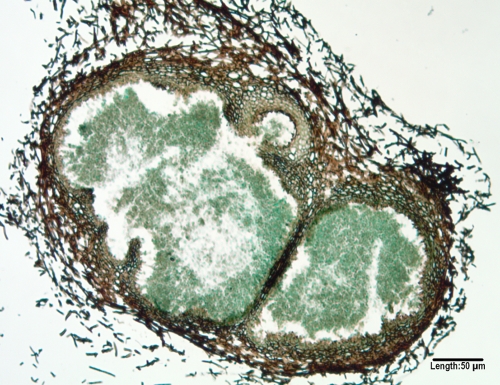
Phoma and Phoma-like pycnidial coelomycetes.
Several genera of morphologically similar pycnidial coelomycetes are occasionally recovered in cases of human subcutaneous disease (307, 585, 704), endophthalmitis (685), and deep tissue infection (411); however, their documentation and reporting as etiologic agents is limited by a lack of adequate identification (727). They include Phoma, Pleurophoma, Pleurophomopsis, and Pyrenochaeta species, with small, hyaline, typically one-celled conidia, and Coniothyrium (411, 704), Paraconiothyrium (773), and Microsphaeropsis species (307, 585, 685), with pale brown to dark, one-celled conidia (Fig. 13). The morphologic features of species within several sections in the genus Phoma have been detailed by Boerema et al. (84). Species in these similar genera are best differentiated by ITS sequencing.
FIG. 13.
A GMS-stained cross section of a multilocular pycnidium of a Microsphaeropsis species produced on carnation leaf agar after 5 weeks of incubation at 25°C.
Botryosphaeriales
Lasiodiplodia.
L. theobromae is a pycnidial coelomycetous organism incriminated in cases of subcutaneous disease (720), pneumonia in a liver transplant recipient (805), and ocular infections (615, 705). Conidia may take several weeks to mature and are distinctive, large (20 to 30 by 10 to 15 μm), and hyaline and single celled initially, becoming dark, striated, and two celled at maturity. The organism was formerly known as Botryodiplodia theobromae.
Macrophomina.
Macrophomina phaseolina has been recently reported as an agent of disseminated disease in a renal transplant recipient (735) and as an agent of a cutaneous infection in a child with acute myeloid leukemia (714). The species is difficult to identify without sequencing, as the isolate typically remains sterile in culture, producing only sclerotia (sterile hard masses of hyphal elements).
Neoscytalidium.
Neoscytalidium dimidiatum, previously known as Scytalidium dimidiatum (518, 519) is a rapidly growing, black, woolly, arthroconidia-producing mold. Microscopically similar hyaline variants lacking melanin, formerly referred to as Scytalidium hyalinum (639), should also be referred to as N. dimidiatum. The species may also produce a coelomycetous pycnidial synanmorph with extended incubation on appropriate media. The name Nattrassia mangiferae has now been placed in the new genus Neofusicoccum (158, 735). This organism is a plant pathogen, and the name should not be used for human isolates. N. dimidiatum primarily produces infections mimicking those caused by dermatophytes on skin and nails (218, 459), although there are occasional reports of ocular infections (26) and deep mycoses in immunocompromised individuals (73, 476, 694, 801).
Sordariales
Phomopsis.
There are only rare reports of Phomopsis species in human disease. Similarly to Phoma and related genera, they are rarely identified beyond the genus level. They are recognized by their black, pycnidial conidiomata (globose to subglobose structures lined with conidiogenous cells) that produce hyaline alpha (ellipsoidal) and beta (long, filamentous, curved) conidia. One report concerns a case of osteomyelitis of the finger in a diabetic patient (727).
TELEOMORPHIC GENERA
Sordariales
Chaetomium and Achaetomium.
Two ascomycetous genera known to produce their sexual state in culture are Achaetomium and Chaetomium. The fruiting body in both is a perithecium (a flask-shaped ascoma with an apical opening). Rarely are conidia produced. Species are differentiated mostly phenotypically by the size and shape of ascomata and the type of setae they possess, the size and shape of their brownish ascospores, and temperature tolerance. Most species fail to grow at 35°C and above and are common degraders of various organic compounds. The human pathogen C. globosum grows at 35°C but not 40°C, and reports of invasive disease due to this and other, unidentified species (34, 449, 742, 814) are inadequately documented and most likely due to neurotropic species. Chaetomium atrobrunneum (314) and C. perlucidum do grow at 40°C, are neurotropic (64), and should be considered in the differential diagnosis of CNS fungal disease. A key for identification of clinically significant species has been published by Barron et al. (64). The closely related Achaetomium strumarium is pale in culture and produces a reddish-purple diffusible pigment, ascospores similar to those of pathogenic Chaetomium spp., and occasional lateral, sessile conidia. It is also neurotropic and an agent of CNS phaeohyphomycosis with growth at 40°C (1, 40).
Pleosporales
Leptosphaeria.
Leptosphaeria senegalensis and the related L. tompkinsii are agents of black grain mycetoma mostly restricted to northern West Africa and India (177). In culture, colonies are slow growing and woolly, and black closed ascomata (cleistothecia) are immersed in the agar. Maturation of ascomata and ascospores is facilitated on plant-based media, and species are differentiated by ascospore features (216, 217).
Microascales
Microascus.
Several pigmented Scopulariopsis species go on to produce their Microascus perithecial teleomorphs in culture. Several of these species have been documented as agents of fatal disease, particularly in transplant recipients. M. cinereus caused a brain abscess in a bone marrow transplant recipient (53), suppurative cutaneous granulomata in a patient with chronic granulomatous disease (483), and endocarditis of a prosthetic valve (129). M. cirrosus was the etiologic agent of disseminated disease in a pediatric bone marrow recipient (424), and M. trigonosporus was reported in a fatal pneumonia in another bone marrow transplant recipient (517). Microascus species are differentiated primarily by the size/shape of the perithecia, the length of the perithecial necks (Fig. 14), and the size and shape of the reddish-brown ascospores, which are orange section shaped in M. cinereus, heart shaped in M. cirrosus (Fig. 15 ), and triangular in M. trigonosporus (Fig. 16).
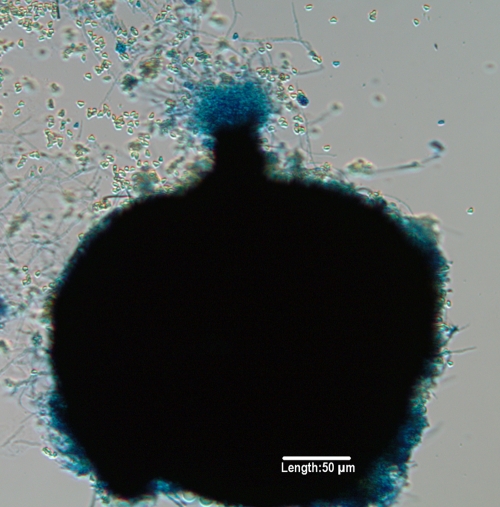
FIG. 14.
Perithecium of Microascus trigonosporus formed on potato flake agar after 3 weeks of incubation at 25°C. Note ascospores being released from the ostiole in the neck of the perithecium.
FIG. 15.
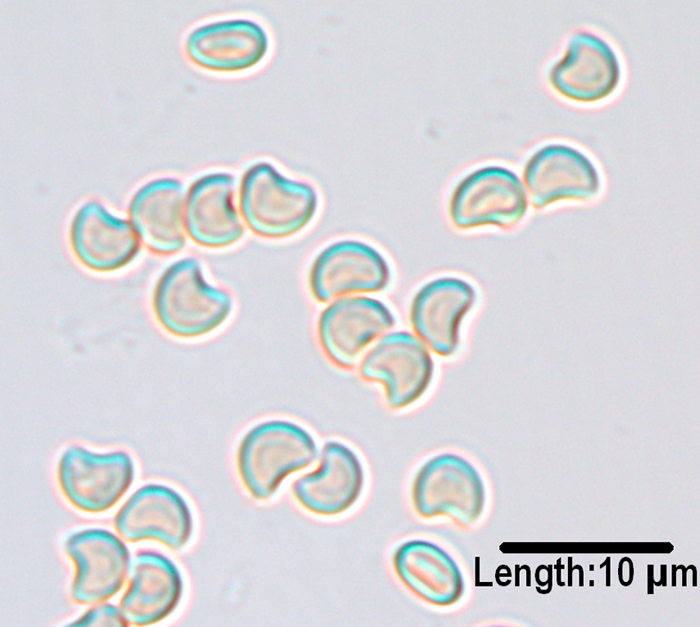
Heart-shaped ascospores of Microascus cirrosus produced on potato flake agar after 3 weeks of incubation at 25°C.
FIG. 16.
Triangular ascospores of Microascus trigonosporus produced on potato flake agar after 3 weeks of incubation at 25°C.
Pseudallescheria.
As discussed above for the anamorphic genus Scedosporium, Cortez et al. extensively reviewed Pseudallescheria/Scedosporium in 2008 (153), and so only subsequent taxonomic changes will be discussed here. The human pathogenic species as defined by recent molecular studies are as follows: Pseudallescheria boydii (anamorph Scedosporium boydii), Pseudallescheria apiosperma (anamorph Scedosporium apiospermum, heterothallic, not forming its teleomorph in culture, and d-ribose negative), and Pseudallescheria ellipsoidea (281-283). Other species of clinical interest in the P. boydii species complex include S. aurantiacum (190, 281) and S. dehoogii (282).
PATHOGENESIS
Surveys of outdoor air for fungal spores routinely show dematiaceous fungi (687). This suggests that all individuals are exposed, though few develop disease. Exposure is primarily from inhalation or minor trauma, which is frequently not even noticed by the patient. Relatively little is known regarding the pathogenic mechanisms by which melanized fungi cause disease, particularly in immunocompetent individuals.
Role of Melanin
One of the likely virulence factors is the presence of melanin in the cell wall, which is common to all dematiaceous fungi, though relatively few species have been studied (439, 522, 573, 666). Melanin in fungi is derived primarily from either dihydroxyphenylalanine (l-DOPA) or dihydroxynaphthalene (DHN) (437). Dematiaceous fungi contain only DHN melanin; l-DOPA melanin has not been described to our knowledge (122, 274, 439, 766). It is generally localized to the cell wall, though the exact mechanism of its production is poorly understood. In the species F. pedrosoi, melanin is produced in melanosomes associated with Fe2+ and Ca2+ and then transported to the cell wall (253). Melanin is extremely resistant to a variety of physicochemical agents, including free radical compounds, toxic metals, desiccation, and even ionizing radiation (165, 249, 331, 795). A species of Chaetomium was isolated from grass that had been frozen in a glacier for over 5,000 years (331).
Considerable work has been done to elucidate the virulence potentials of several fungi (dematiaceous and nondematiaceous) that contain melanin, notably Aspergillus fumigatus, Cryptococcus neoformans, E. dermatiditis, and S. prolificans (123, 134, 201, 203, 205, 284, 428, 549, 573, 653, 671, 730, 756). There are multiple proposed mechanisms by which melanin may act as a virulence factor (109, 324, 375). It may confer a protective advantage by scavenging free radicals and hypochlorite that are produced by phagocytic cells in their oxidative burst and that would normally kill most organisms (376, 671). In addition, melanin may bind to hydrolytic enzymes, thereby preventing their action on the plasma membrane, and to antifungal drugs, preventing their action (370, 375, 547, 768). There is also evidence that certain melanized fungi are less susceptible to phagocytosis and killing by neutrophils and macrophages (334, 584). These multiple functions may help explain the pathogenic potential of some dematiaceous fungi, even in immunocompetent hosts. Specifically, in E. dermatitidis, disruption of melanin production leads to markedly reduced virulence in animal models and restriction of hyphal growth (103, 201, 203, 428). However, hyphae of S. prolificans were found to be more susceptible to damage from neutrophils than A. fumigatus (284). Melanin has also been shown to reduce the susceptibility of M. mycetomatis to ketoconazole and itraconazole by binding these drugs (766). Though only a minority of dematiaceous fungi have been studied, it is likely that melanin plays a critical role in pathogenesis for clinically important species.
Other Putative Virulence Factors
It is interesting to note that most allergic disease and eosinophilia is caused by three genera, Alternaria, Bipolaris, and Curvularia (622). The virulence factors in these fungi that are responsible for eliciting allergic reactions are not well understood, though Alternaria was found to stimulate the degranulation of eosinophils, possibly due to an aspartic protease (372, 496). These organisms are very common in the environment, so exposure is practically universal, though the incidence of allergic disease is relatively low, suggesting that host factors may play a role. A study by Schubert et al. found that HLA-DQB1*03 was associated with allergic fungal sinusitis (676). Further studies are needed to better delineate the importance of virulence factors other than melanin.
DIAGNOSIS
The timely and accurate diagnosis of fungal infections by melanized fungi consists of a multifaceted approach. With the exponential increase in immunocompromised individuals, particularly those in tertiary care cancer centers (72), this becomes imperative to prevent potentially fatal outcomes. Standard conventional diagnostic procedures include direct microscopy, histopathological stains to document tissue invasion, radiographic and computerized tomography (CT) findings, and isolation procedures to recover the fungus and identify the etiologic agent. The clinical presentation and diagnostic findings segregate these infections into the major categories of eumycetoma, chromoblastomycosis, and phaeohyphomycosis. Phaeohyphomycosis maybe further delineated depending upon whether infections are superficial or deep, by their anatomic location, and by the host's response. The microscopic features seen in phaeohyphomycosis, however, are similar regardless of the anatomic site. The confusion surrounding the placement of members of the Sporothrix schenckii and Pseudallescheria boydii species complexes within the dark molds is related to their tissue presentation as yeast cells and hyaline hyphae, respectively. As the term phaeohyphomycosis is commonly used to describe fungi with dark hyphae in tissue, these organisms would be excluded; however, both produce melanized conidia in culture. As a thorough review of infections caused by Pseudallescheria/Scedosporium species has recently been published (153), this paper will concentrate on taxonomic changes and new species documented as etiologic agents subsequent to that review.
Initial Specimen Processing
The appropriate specimen collection, transport, and processing procedures are important considerations in the demonstration of melanized fungi in tissue and their recovery in culture. The most useful diagnostic specimens are those collected at the source of infection; however, specimens peripheral to the site of infection, such as blood cultures in hematogenously disseminated disease, may also be diagnostic in the absence of focal manifestations or when foci are not easily accessible. Appropriate specimens for the recovery of fungi are detailed elsewhere in several reference works (723). Specimens commonly obtained for recovery of melanized fungi include tissue biopsy specimens, aspirates, and body fluids. Surgically obtained specimens should always be cultured as well as processed for histopathology, and the inoculum should be finely sliced or minced rather than ground (as in the case for recovery of H. capsulatum). Gross examination may occasionally reveal evidence of melanized fungi as well (Fig. 17). Small volumes of sterile body fluids may also be concentrated by syringe filtration (0.2 μm). Several blood culture systems are available, and the maximum amount of blood recommended should always be used. Swab cultures from superficial sites are usually not representative of the disease process, frequently contain indigenous contaminating mycobiota, and should generally be avoided. Grains or granules should also be washed several times in antimicrobial-containing saline to avoid bacterial overgrowth (504). Also compromising etiologic agent recovery is a delay in specimen transport. Optimally, most specimens should be processed within 2 h of collection, and cerebrospinal fluid should never be refrigerated (723).
FIG. 17.
Bipolaris spicifera colonies in stomach mucosa of patient with disseminated disease (autopsy). (Reproduced from reference 624 [original Fig. 15-6A] with kind permission of Springer Science and Business Media.)
Guidelines regarding the handling of potentially infectious fungi in the laboratory setting are available. It is suggested that cultures of certain well-known pathogenic fungi, such as Coccidioides immitis/posadasii and H. capsulatum, be worked with in a biosafety level (BSL) 3 facility, which requires a separate negative-pressure room, though clinical samples may be handled under BSL 2 conditions (130). Recently, certain agents of phaeohyphomycosis, in particular C. bantiana, have been included in the list of fungi that should be kept under BSL 2 containment (130), and in Europe this mold is considered a hazard category 3 agent (one that can cause severe human disease) (231). This seems reasonable given its propensity, albeit rare, for causing life-threatening infection in healthy individuals.
Direct Microscopy
Due to the ubiquitous nature of melanized fungi, examination of direct specimens is critical, as the finding of fungal elements within tissue is required to document a black mold as the etiologic agent when recovered in culture. It should also be noted that in individuals receiving antifungal therapy, hyphae seen in tissue may be the only evidence of disease, as growth in culture may be severely suppressed or absent. Conversely, recovery in culture without visualization in tissue should be interpreted with caution. Isolation of the same organism multiple times or from multiple sites also supports its role in disease when microscopic evidence is lacking. Commonly used methods for the direct examination of specimens include the Gram stain, several different concentrations of KOH preparations (with or without the incorporation of mycological stains), and the fluorescent calcofluor white stain (652). The Gram stain and KOH preparations are rapid, easily performed tests that should not be overlooked when making an initial assessment of fungal disease with appropriate clinical specimens. Each is described in detail in various microbiology texts (441, 591). Calcofluor and related fluorochromes that bind to cellulose and chitin in fungal cell walls provide another rapid stain for demonstrating fungi by utilizing fluorescence (326). A fluorescence microscope with broadband excitation filters in the range of 300 to 412 nm (322) and eye barriers are required (441). Diagnostic structures seen by direct microscopy also vary according to the clinical presentation. In cases of eumycetoma incited by dark fungi, the demonstration and appearance of pigmented grains or granules (bundles of hyphae often embedded in a cement-like matrix) from pus, exudates, bandage gauze, and biopsied tissue are highly significant and narrow the potential etiologic agents to a limited number of black molds known to cause mycetoma. Members of the Pseudallescheria boydii species complex and Phaeoacremonium species, however, produce pale grains in tissue (159, 503). Fungi responsible for grains or granules expressed from draining sinus tracts are best visualized in permanent histopathological preparations, as are sclerotic bodies seen in chromoblastomycosis. Fungal elements seen in phaeohyphomycosis are frequently detected by direct microscopy; however, tissue invasion is best documented by permanent histopathological stains. The Gram stain may also be useful in some settings with fungi often demonstrating variable staining. Note that the hyphae are often Gram negative while the conidia are Gram positive; however, either structure may be Gram variable.
Histopathology and Special Stains
Several histopathological stains are useful for the demonstration of melanized fungi (670). The most frequently used hematoxylin-and-eosin (H&E) stain demonstrates pigmentation in hyphae that are strongly melanized (Fig. 18). In fungi that are only lightly pigmented, hyphae may be misidentified as hyaline rather than dark. The melanin Fontana-Masson stain (Fig. 19) is useful in these situations to visualize the phaeoid nature of hyphae in tissue, though other molds may occasionally stain strongly as well (414). An additional stain useful for dark hyphae is the periodic acid-Schiff (PAS) stain (Fig. 20). It is frequently preferred over H&E due to the more vivid colors of hyphae, which stain a bright pink-purple against a green background; however, it may overshadow the melanin when present. For practically all fungal histopathology, a Gomori methenamine silver (GMS) stain is ordered. Its utility is in the dramatic visualization of hyphae as dark elements against a green background; however, it fails to discriminate between pigmented and nonpigmented fungal elements (Fig. 21). Note that in Fig. 19 and 21 many of the fungal elements are either short stubby hyphae, pseudohyphae, or moniform (bead-like) hyphae. This is not an uncommon tissue presentation with several dematiaceous genera and is quite different from that seen in aspergillosis, fusariosis, or zygomycosis. Chromoblastomycosis (Fig. 22) presents in tissue as brown, compact muriform hyphal elements with horizontal and vertical cross walls variously referred to as sclerotic bodies, Medlar bodies, or “copper pennies” (610).
FIG. 18.
H&E stain of melanized, moniliform hyphal elements of Cladophialophora bantiana from a brain abscess.
FIG. 19.
Bipolaris spicifera in lung tissue (Fontana-Masson stain; magnification ×100). (Reproduced from reference 624 [original Fig. 15-13B] with kind permission of Springer Science and Business Media.)
FIG. 20.
PAS stain of Ochrocladosporium elatum, formerly Cladosporium elatum, from sinus tissue.
FIG. 21.
GMS stain of Rhinocladiella mackenziei from a brain abscess. Note the many moniform hyphal elements often seen with melanized fungi.
FIG. 22.
GMS stain of sclerotic bodies produced by Fonsecaea pedrosoi.
Isolation Procedures and Culture
These ubiquitous fungi can be contaminants in cultures, making the determination of clinical significance problematic. A high degree of clinical suspicion as well as correlation with appropriate clinical findings is required when interpreting culture results. The recovery of dematiaceous fungi from clinical specimens requires the appropriate media and incubation conditions. Various references list suggested schemes for primary isolation media (722, 723). A common approach is to include nonselective as well as selective media, such as those containing cycloheximide, media enriched for fastidious organisms such as brain heart infusion agar (BHI) and inhibitory mold agar (IMA), and also media containing antimicrobial agents to suppress bacteria in specimens collected from nonsterile sites. A nonselective medium frequently employed is SDA. While growth is adequate, the color of the colonies is often cream to pale orange or light brown, making their recognition as phaeoid genera difficult. When these same isolates are transferred to plant-based media, they assume their more typical olivaceous to dark brown to black color. The additional time required for these subcultures can have potentially devastating consequences in the profoundly compromised individual, and therefore the use of a plant-based medium initially, such as PDA, is highly recommended. Cultures are commonly incubated at 30°C; however, room temperature at or near 25°C may also be used. Cultures should be examined every day for the first 3 days and twice a week thereafter. Most phaeoid molds are recovered within a week, and incubation of negative cultures beyond 3 weeks is seldom necessary (430). Substantially longer incubation, however, may be required for development of diagnostic structures in some isolates, particularly for coelomycetes or homothallic ascomycetes. All filamentous organisms should be manipulated and examined under a certified biological safety cabinet.
Radiology
There are few radiologic features that distinguish melanized fungi from other molds as causes of infection. The “dot-in-circle” sign has been noted to be specific for the finding of eumycetoma in magnetic resonance imaging (MRI) studies (141). This is felt to be due to the low signal intensity produced by the fungal grains in tissue.
Antigen Testing and Serology
There are no widely available serologic or antigen tests available to specifically detect melanized fungi in blood or tissue. However, serum antigen testing for 1,3-β-d-glucan (primarily for Candida spp.) and galactomannan (primarily for Aspergillus spp.) may cross-react with melanized fungi, though usually with low levels (164, 419). However, in immunocompromised patients with cultures positive for dematiaceous fungi, a positive serum galactomannan test may indicate concomitant infection with Aspergillus; careful clinical correlation is advised (71). Further studies are needed to better understand the nature and likelihood of cross-reactivity. Serum enzyme-linked immunosorbent assays (ELISAs) for F. pedrosoi and C. carrionii have been developed to aid in the diagnosis of chromoblastomycosis, though relatively low sensitivity and specificity have limited their usefulness, and only small numbers of patients have been studied (552, 775, 778).
Molecular Diagnostics
In an effort to improve the rapidity with which invasive fungal infections are detected as well as the sensitivity and specificity of diagnostic tests, recent assays have focused on nonculture methods, in particular nucleic acid-based methods, such as PCR assays. Despite advances in the direct diagnosis of other, more common genera such as Candida, Aspergillus and Fusarium, the direct diagnosis of infections incited by melanized species remains a challenge. However, studies have begun to examine the potential of identifying species within this diverse group of fungi using PCR of highly conserved regions of ribosomal DNA (2). A panfungal PCR assay described by Lau et al. targeting the ITS1 region was able to detect several species of dark molds from fresh, formalin-fixed, or paraffin-embedded tissue specimens, including S. prolificans, Exophiala spp., Exserohilum rostratum, and Microsphaeropsis arundinis (445). In a real-time PCR assay targeting part of the 28S large-subunit rRNA gene, Vollmer et al. were able to amplify Aureobasidium pullulans in clinical specimens from intensive-care patients with either artificial respiration or infective endocarditis (785). While these methods and those to be refined in the foreseeable future will provide a more rapid diagnosis for some agents of phaeohyphomycosis, the diversity of black molds increasing in immunocompromised individuals makes their identification from direct materials a daunting task. Currently, a greater utility of molecular methods is in the identification, taxonomy, and phylogenetic placement of these melanized fungi.
IN VITRO ANTIFUNGAL SUSCEPTIBILITY
In vitro antifungal susceptibility testing has advanced considerably in the past several years, especially when one considers that a standardized method for testing yeasts was not available until 1997 (534), the first standardized method for filamentous fungi was not available until 2002, and both were updated in 2008 (147, 148). Due to the relatively recent development of antifungal susceptibility testing, the available in vitro data for dematiaceous fungi are relatively sparse, and often rely on small numbers of isolates per species. An important issue is that much of the older literature is often inconsistent with regard to methodology, making reliable observations difficult. In addition, as defined interpretive breakpoints are not available for any of the molds, guidelines for interpreting in vitro data frequently rely on close approximations to breakpoints for Candida species, as well as achievable concentrations of the drug using standard dosing regimens. A MIC of ≤1 μg/ml is often used as an indicator of potential susceptibility for most drugs used to treat black molds, excluding flucytosine (5-FC) (<50 μg/ml), recognizing that there are significant differences in pharmacological properties between the various agents as well as differences in drug concentrations tested. Lower MICs typically suggest better activity. The in vitro activities of several antifungal agents against a variety of dematiaceous fungi are presented in Table 3. The data are from a compilation of the current literature (49, 50, 161-163, 224-228, 250, 266, 294, 315, 382-384, 391, 532, 553, 625, 781, 782); however, clinical correlates are not available. Antifungal susceptibility testing of etiologic agents, when warranted, may assist in appropriate patient management.
TABLE 3.
In vitro activities of antifungal agents against selected melanized fungia
| Species | Activityb |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AmB | Itra | Vori | Posa | Isavu | Ravu | Keto | Terb | 5-FC | Casp | Mica | Anid | |
| Alternaria spp. | + | ++ | + | ++ | + | + | − | + | + | |||
| Aureobasidium pullulans | + | ++ | ++ | |||||||||
| Bipolaris spp. | + | ++ | ++ | ++ | + | + | + | + | + | |||
| Chaetomium spp. | + | ++ | ++ | ++ | + | − | ||||||
| Cladosporium spp. | + | ++ | ++ | ++ | + | + | ||||||
| Cladophialophora bantiana | ++ | ++ | ++ | ++ | + | + | + | + | ++ | + | ||
| Curvularia spp. | + | ++ | ++ | ++ | + | + | + | + | ||||
| Exophiala spp. | + | ++ | ++ | ++ | + | + | + | + | + | + | ||
| Exophiala dermatitidis | ++ | ++ | ++ | ++ | + | + | + | + | + | + | ||
| Exserohilum spp. | ++ | ++ | ++ | + | + | |||||||
| Fonsecaea pedrosoi | ++ | ++ | ++ | + | ++ | ++ | + | + | + | + | ||
| Lasiodiplodia theobromae | + | |||||||||||
| Madurella mycetomatis | + | ++ | ++ | ++ | + | − | + | |||||
| Ochroconis gallopava | + | ++ | + | |||||||||
| Phialemonium spp. | + | + | + | |||||||||
| Phialophora spp. | + | ++ | ++ | + | + | + | + | + | ||||
| Rhinocladiella spp. | + | ++ | ++ | + | + | |||||||
| Rhinocladiella mackenziei | + | + | + | + | + | + | − | − | ||||
| Scedosporium/Pseudallescheria spp. | − | + | ++ | + | ||||||||
| Scedosporium prolificans | − | − | − | − | − | − | − | − | ||||
| Scopulariopsis brumptii | − | − | − | − | ||||||||
| Veronaea botryosa | + | + | ||||||||||
| Wallemia sebi | + | + | + | + | + | − | + | |||||
Adapted from reference 625 with permission of Expert Reviews Ltd.
Abbreviations: AmB, amphotericin B; 5-FC, flucytosine; Keto, ketoconazole; Itra, itraconazole; Vori, voriconazole; Posa, posaconazole; Ravu, ravuconazole; Casp, caspofungin; Mica, micafungin; Anid, anidulafungin; Terb, terbinafine; Isavu, isavuconazole. ++, good activity suggested based on consistently low MICs and testing against at least five isolates of a particular genus or species; +, potential/marginal activity suggested based on inconsistent MICs or very few isolates of a particular genus or species; −, no significant activity suggested based on consistently high MICs. The results do not represent formally defined CLSI breakpoints.
Polyenes
Amphotericin B.
Amphotericin B generally has good in vitro activity against most clinically important dematiaceous fungi. However, some species have been consistently resistant (MICs of ≥2 μg/ml) in vitro, including S. prolificans and S. brumptii (507), while other species have occasionally been found to be resistant, including Curvularia spp., Exophiala spp., and R. mackenziei (507, 726). Significant toxicity often limits use of the standard formulation, primarily due to renal insufficiency, electrolyte disturbances, and infusion-related side effects. However, nephrotoxicity has been significantly reduced by the development of lipid-associated formulations (339). Use of these preparations allows for much higher doses than possible with standard amphotericin B, which may improve their efficacy against these fungi. In addition, lipid amphotericin B preparations may achieve higher concentrations in brain as well (407).
Natamycin.
Natamycin is a polyene antifungal used exclusively as topical therapy in eye infections, particularly keratitis. It has broad spectrum of activity against most relevant molds (Aspergillus and Fusarium) and is available in concentrations of up to 5%, which is generally well tolerated (478, 650). Data for susceptibility against common dematiaceous fungi are very limited, though one study did show activity against Curvularia (800).
Azoles
The triazole agents itraconazole, voriconazole, and posaconazole demonstrate the most consistent in vitro activity against dematiaceous fungi, except against S. prolificans and S. brumptii, which are resistant to all azoles (120, 227, 507, 509). Only voriconazole is available as an intravenous (i.v.) formulation. All of these agents have significant drug interactions that must be considered during therapy (310). In addition, therapeutic drug monitoring is becoming increasingly utilized as data correlating serum levels with clinical response and toxicity accumulate (706).
Other azoles have a limited role in the therapy of these infections. Ketoconazole was the first oral azole and has a relatively broad spectrum. However, a number of side effects have significantly limited its current use with the availability of newer agents that are much better tolerated. Sparse in vitro data are available for dematiaceous fungi, but good activity is noted for the most common fungi causing chromoblastomycosis and mycetoma (35, 767). Fluconazole has negligible activity against dematiaceous molds (132, 259) and essentially no role in therapy given the variety of other options available, though anecdotal success has been reported (198).
Itraconazole.
Though itraconazole was the first oral azole with significant activity against dematiaceous fungi and has had the most clinical use in therapy, concerns over adverse effects and the lack of an intravenous formulation have reduced its use in recent years. For itraconazole, the capsule form requires an acidic environment for absorption, while the suspension with cyclodextrin does not, being more consistently absorbed. Itraconazole demonstrates good activity against the vast majority of dematiaceous fungi tested (226-228, 506, 507). MICs generally are ≤0.125 μg/ml for this group of fungi.
Voriconazole.
Voriconazole has become the treatment of choice for invasive aspergillosis, supplanting amphotericin B for this indication (385, 745). It has also become a commonly used agent for treating many other invasive mold infections, especially those caused by dematiaceous fungi. The i.v. form is particularly useful in critically ill patients. It is generally well tolerated, though visual side effects are common but rarely limit therapy (745). In addition, like itraconazole, it has a broad spectrum of activity that includes most dematiaceous fungi (227, 228, 507). However, MICs may be slightly higher for voriconazole, though the clinical significance of this is unclear.
Posaconazole.
Posaconazole is the most recently released azole and has the broadest spectrum of any oral agent (338, 401, 707). It is currently available only orally, though an i.v. formulation is being developed. It is generally very well tolerated. Oral absorption is significantly improved if it is administered with food, particularly food with a high fat content. The published in vitro data are relatively limited for dematiaceous fungi, but good activity is demonstrated against most species tested, including Bipolaris spp., C. bantiana, and R. mackenziei (14, 52, 224, 592). Posaconazole may be useful in cases of CNS disease, even that due to refractory molds (600).
Investigational azoles.
Isavuconazole is a broad-spectrum azole with both oral and i.v. forms that has not been approved for use at this writing. Limited in vitro data exist for dematiaceous fungi (294, 532). Ravuconazole is another investigational azole with activity against a wide variety of molds (259).
Flucytosine
Flucytosine (5-FC) is unique in its mechanism of action, inhibiting DNA and RNA synthesis (252, 774). In the United States it is available only in oral form. The development of resistance during monotherapy has resulted in its use in combination therapy for systemic mycoses, most notably cryptococcal meningitis (774). In vitro studies with dematiaceous fungi are limited, though activity against C. bantiana, Exophiala spp., and Fonsecaea (F.) pedrosoi, the major etiologic agent of chromoblastomycosis, has been shown (111, 202).
Allylamines
Allylamines, like the azoles, also inhibit ergosterol synthesis, but they act on squalene epoxidase, an enzyme two synthetic steps before the target of azoles. Their clinical role has been limited to treatment of dermatophyte infections, though there has been recent interest in potentially expanding their clinical spectrum (333, 626). Terbinafine is the only oral allylamine available for systemic use. However, its extensive binding to serum proteins and distribution into skin and adipose tissue have diminished enthusiasm for its use in treating serious systemic fungal infections (356, 381, 468, 655). In vitro studies against dematiaceous fungi are emerging, and broad-spectrum activity has been seen, including against Alternaria, Curvularia, and Bipolaris and agents of chromoblastomycosis (35, 382, 506). The in vitro testing range for this agent is typically between 0.004 and 2 μg/ml.
Echinocandins
The echinocandins are the latest group of antifungal agents to be developed and have a unique mechanism of action, inhibiting 1,3-β-d-glucan synthesis and thereby disrupting the fungal cell wall (118). Caspofungin, micafungin, and anidulafungin are available only in an intravenous formulation and are generally well tolerated and, notably, have very few significant drug interactions (145). They are generally considered therapeutically equivalent, based on studies conducted on invasive Candida infections. In vitro studies with dematiaceous fungi are limited, with variable activity noted against many dematiaceous fungi, including Curvularia, Bipolaris, and F. pedrosoi (224, 225). S. prolificans appears to be resistant (225, 509). Micafungin may have lower MICs for C. bantiana than other echinocandins (225). In general, MICs for dematiaceous fungi are higher than those for Aspergillus spp.
Other Agents
A variety of drugs have been explored for activity against these fungi, given their refractory nature. Miltefosine, a drug originally developed as an anticancer drug and found to be effective in leishmaniasis, has antifungal activity against a variety of dematiaceous fungi, including S. prolificans (797). Nikkomycin, a chitin synthase inhibitor with activity against Coccidioides immitis, was found to have relatively poor activity against dematiaceous fungi (454).
Antifungal Combinations
Uses of antifungal combinations are being increasingly studied as strategies for treatment of refractory fungal infections, though not extensively for dematiaceous fungi. However, for C. bantiana and S. prolificans, the most common fungi causing CNS and disseminated diseases, respectively, novel approaches are needed to improve therapy, and a variety of combinations have been studied in vitro and in vivo (see Animal Models of Infection below). In a murine model of C. bantiana, combination therapy was found to be superior to monotherapy for all agents tested (481).
Given that no single antifungal agent has significant activity against S. prolificans, numerous combinations have been studied to improve efficacy. The combination of itraconazole and terbinafine has been studied against S. prolificans, which is otherwise generally resistant to all agents. In vitro, synergistic activity against most isolates of this species was found, and no antagonism was noted (511). Voriconazole and terbinafine also display similar synergy in vitro (510). The mechanism is presumably potent inhibition of ergosterol synthesis at two different steps of the pathway by these agents. However, this should be interpreted with caution, as terbinafine is not recommended for systemic infections. The combination of voriconazole with micafungin and amphotericin B was found to be synergistic in vitro against S. prolificans, though double combinations also lowered individual MICs (637). Other reports suggest synergy against S. prolificans with voriconazole and caspofungin or with micafungin and amphotericin B (160, 716, 821). Importantly, antagonism was not observed with any of these combinations. Gil-Lamaignere et al. showed a synergistic effect of voriconazole or posaconazole with neutrophils in vitro against hyphae of S. prolificans (285). Older literature also suggests synergy with ketoconazole and 5-FC for a variety of dematiaceous fungi (152). This may be applicable to other azoles as well.
ANIMAL MODELS OF INFECTION
There are relatively few animal studies with dematiaceous fungi. One of the earliest studies was a murine model of infection with E. dermatitidis and F. pedrosoi (603). Amphotericin B and 5-FC were active alone or in combination, though ketoconazole was not. In another study, 5-FC had the broadest activity against C. bantiana, O. gallopava, and E. dermatitidis in mice, followed by amphotericin B and fluconazole (despite resistance in vitro) (202). Terbinafine was ineffective in vivo, despite good in vitro activity (202).
More recent studies have focused on therapy with posaconazole in refractory mycoses due to R. mackenziei, C. bantiana, and E. dermatiditis. Al-Abdely et al. found posaconazole to be more effective than amphotericin B or itraconazole in murine models of central nervous system infection with R. mackenziei and C. bantiana (14, 15). Posaconazole was also found to be effective in a model of disseminated E. dermatiditis infection (301). In another murine model of C. bantiana infection, posaconazole and flucytosine improved survival alone, though the combination of posaconazole, flucytosine, and micafungin yielded the greatest benefit (481). In a recent murine model of F. monophora, posaconazole was associated with significantly better survival than amphotericin B or itraconazole (113). Posaconazole was associated with improved survival compared with amphotericin B or caspofungin in a murine model of Exophiala infection (633).
S. prolificans was studied in a murine model, and the combination of micafungin with either voriconazole or amphotericin B was associated with improved survival, though the triple combination of all three agents was not more effective (637). Posaconazole with granulocyte-macrophage colony-stimulating factor (GM-CSF) against S. prolificans did not improve survival in one study (698), though liposomal amphotericin B with G-CSF did improve survival in a murine model (560).
CLINICAL SYNDROMES AND THEIR MANAGEMENT
A wide variety of clinical syndromes have been associated with melanized fungi, reflecting their diverse nature (Table 4). The number of published articles relating to these fungi has risen steadily in recent years. They may be considered opportunists or true pathogens, and many of the various clinical presentations can occur in both healthy and immunocompromised individuals. In reviewing the literature, it is seen that case reports often lack crucial details of medical history, diagnostic studies, therapy, and especially clinical follow-up. This limits their usefulness in determining the efficacy of the therapy. However, since randomized trials are not practical given the rarity of these infections, we are left to manage with the available data.
TABLE 4.
Clinical syndromes, associated dematiaceous fungi, and suggested therapya
| Clinical syndrome | Commonly associated fungal genera or species | Therapyb |
|---|---|---|
| Eumycetoma | Madurella, Pyrenochaetae, Leptosphaeria | Azole ± Terb |
| Chromoblastomycosis | Fonsecaea(F. pedrosoi), Phialophora, Rhinocladiella | Azole ± Terb |
| Phaeohyphomycosis | ||
| Allergic fungal sinusitis | Bipolaris, Curvularia | Surgery + steroids ± Itra |
| Allergic bronchopulmonary mycosis | Bipolaris, Curvularia | Steroids ± Itra |
| Onychomycosis | Alternaria, Scopulariopsis | Itra or Terb ± topical agents |
| Tinea nigra | Hortaea werneckii, Stenella araguata | Topical agents |
| Subcutaneous nodules | Alternaria, Exophiala, Phialophora | Surgery ± azole |
| Keratitis | Curvularia, Bipolaris, Exserohilum | Topical natamycin ± topical azole |
| Bone and joint infection | Scedosporium prolificans, Alternaria | Vori ± Terb |
| Peritonitis | Curvularia, Exophiala, Alternaria | Catheter removal ± AmB or azole |
| Pneumonia | Ochroconis, Exophiala, Chaetomium | Vori (L-AmB if severe) |
| Brain abscess | Cladophialophora bantiana, Rhinocladiella mackenziei, Ochroconis | Azole + L-AmB or echinocandin ± 5-FC (see text) |
| Disseminated disease | Scedosporium prolificans, Bipolaris, Exophiala | Vori + Terb ± echinocandin, Vori ± echinocandin or L-AmB (see text) |
Adapted from reference 625 with permission of Expert Reviews Ltd.
Abbreviations: Vori, voriconazole; Itra, itraconazole; Terb, terbinafine; L-AmB, lipid amphotericin B; 5-FC, flucytosine; azole, voriconazole, posaconazole, or itraconazole; +, with; ±, with or without.
Eumycetoma
Mycetoma is one of the oldest infections described in recorded writings, being mentioned as “pada valmikam” (anthill foot) in the ancient Vedic hymns of India (455). Eumycetoma is due to fungi and accounts for one-third to one-half of all cases of mycetoma (473). The first report in the modern medical literature was in 1846 by Godfrey (289). It is a chronic subcutaneous infection caused by a small group of fungi and characterized by the presence of grains, or sclerotia, in tissue (502). These grains are usually white or black, depending on the fungal species involved, and are composed of fungal cells surrounded by a dense extracellular matrix containing a melanin compound, which gives it a dark color and likely has a role in protecting the organism from host defenses (9). Eumycetoma is common in many tropical and subtropical areas of the world. The species involved are often associated with a particular geographic region. M. mycetomatis is one of the most common species, particularly in Africa and India (9). Many other species have been implicated, including Pyrenochaeta romeroi (South America), Leptosphaeria senegalensis (Africa), E. jeanselmei, Curvularia spp., and P. verrucosa (9).
In contrast to chromoblastomycosis and subcutaneous phaeohyphomycosis, which may be cured with surgical techniques alone, eumycetoma almost always requires prolonged systemic antifungal therapy in addition to surgery due to the extensive and deep tissue involvement. The most experience has been with ketoconazole and itraconazole, though itraconazole appears to have more consistent clinical activity (9, 27, 30, 116, 127). Recently, reports of success using voriconazole and posaconazole have been published (431, 447, 462, 537). Therapy generally is continued for at least 3 months, though courses of 6 to 24 months or longer are often required. In refractory cases, combination therapy has also been used, adding either flucytosine or terbinafine to an active triazole (350, 447). Surgery can help to reduce disease burden and occasionally cure small, localized lesions that do not involve bone (30). Amphotericin B is largely ineffective and impractical given the duration of therapy that is often required (632).
Chromoblastomycosis
Chromoblastomycosis is a slowly progressive, chronic subcutaneous mycosis that is seen predominantly in tropical areas (610). Pedroso was one of the first to report of this disease in 1920 (581). The term chromoblastomycosis was introduced by Terra et al. in 1922 (739). Minor trauma typically precedes the lesions, though many patients do not recall this. Nodular lesions can progress over years to form large, verrucous plaques. Histopathology is characterized by the presence of muriform sclerotic bodies (Medlar bodies or “copper pennies”) in tissue, which defines this condition (232, 610). By far the most common species is F. pedrosoi, followed by Phialophora verrucosa and, less commonly, Cladophialophora carrioni and Rhinocladiella aquaspersa (610). They can also cause other clinical syndromes, often leading to confusion in the literature as some authors refer to any disease caused by these fungi as “_______ chromoblastomycosis” (66). Other fungi have been implicated, though some reports do not clearly describe the pathognomonic features and so are questionable (594). It is not understood how and under what conditions sclerotic bodies are formed in tissue. Melanin is thought to play an important role, though other compounds such as peptidases, glycosphingolipids, and sialidase may be involved in pathogenesis as well (666).
Therapy is difficult and various modalities have been used, usually over a period of several months and even years. Besides antifungal therapy, surgery, cryotherapy, thermotherapy, and even laser therapy have been tried (90). In a large series, cryotherapy, itraconazole, or the combination resulted in the largest number of cures (88). In developing countries, where systemic antifungals are not easily available or are too expensive, the use of cryotherapy alone in a systematic manner over several months has led to good cure rates as well (126). Such physical therapies are most effective on small, localized lesions. The exact mechanism of this effect is unclear.
Antifungal therapy is essential for moderate to severe or widespread disease. As a single agent, itraconazole appears to be the most effective, and it is the agent with which there is the most clinical experience (88, 426, 610, 611, 620). A variety of other treatments have also been successful, including ketoconazole, flucytosine, local heat therapy, and amphotericin B (44, 377, 515). However, the overall cure rate was only 57% in one large series of 100 cases from Brazil, despite use of multiple modalities (515). Recently, terbinafine has been found to have in vitro and clinical activity (91, 684). In refractory cases, the combination of itraconazole and terbinafine has been found to be useful, and some experts recommend this as first-line therapy for moderate to severe disease (90, 317, 610).
Phaeohyphomycosis
The remainder of clinical syndromes can be grouped under the term phaeohyphomycosis. For the purposes of this review, they will be arbitrarily divided into allergic disease, superficial infection, deep local infection, pulmonary infection, central nervous system infection, and disseminated infection.
Allergic disease.
Allergic responses to dematiaceous fungi may actually represent the most common clinical manifestation of these fungi. Though asthma has many associated environmental factors, several studies have linked it with exposure to molds and to dematiaceous fungi, Alternaria spp. and Cladosporium spp. in particular (108, 278, 456, 555). The effect has also correlated with seasonal fluctuations in outdoor mold counts (555). In addition, Alternaria has been associated with severe asthma exacerbation in some individuals (541). A frequent finding is the presence of elevated Alternaria-specific IgE (456). However, most melanized fungi do not elicit such a response, and it remains unclear why only a few genera are associated with allergic disease.
(i) Allergic fungal sinusitis.
Allergic fungal sinusitis is a relatively common condition, with estimates of 6 to 9% of all cases of chronic sinusitis requiring surgery (673). Patients with this condition usually present with chronic sinus symptoms that are not responsive to antibiotics. Previously, Aspergillus was thought to be the most common fungus responsible for allergic sinusitis, but it is now appreciated that disease due to dematiaceous fungi actually comprises the majority of cases (239, 674). Geographic variation has also been reported, with an increased incidence in the southern United States (240).
The most common species isolated are Alternaria, Bipolaris, and Curvularia, though other rare fungi (Epicoccum and Nodulisporium) have also been reported (155, 544, 673). However, fungi are frequently isolated from normal individuals as well (668). Criteria have been suggested for this disease, and these include (i) nasal polyps; (ii) the presence of allergic mucin, containing Charcot-Leyden crystals and eosinophils; (iii) hyphal elements in the mucosa without evidence of tissue invasion, (iv) positive skin test to fungal allergens; and (v) on computed tomography (CT) scans, characteristic areas of central hyperattenuation within the sinus cavity. Not all are considered by experts to be necessary for diagnosis (357, 675). Diagnosis generally depends on demonstration of allergic mucin, with or without actual culture of the organism. Therapy consists of surgery to remove the mucin, which is often tenacious, and systemic steroids, though patients have been cured by surgical therapy alone (498, 631, 732). Antifungal therapy, usually in the form of itraconazole, may play a role in reducing the requirement for steroids, but this is not routinely recommended and small, randomized studies showed no benefit when it is used as primary therapy in addition to surgery (425, 654). However, in refractory cases, itraconazole may improve outcomes (135, 680). Other azoles (voriconazole) have only rarely been used for this disease (223).
In rare cases, patients may present with often chronic symptoms of mass effect due to the inflammation extending from sinuses into adjacent structures, including the orbits (102, 125, 150, 379). These are almost always immunocompetent patients. In addition to surgery and steroids, systemic antifungal therapy is often given.
(ii) ABPM.
Allergic bronchopulmonary mycosis (ABPM) is similar in presentation to allergic bronchopulmonary aspergillosis (ABPA), which is typically seen in patients with asthma or cystic fibrosis (5, 658). There is a suggestion that allergic fungal sinusitis and allergic bronchopulmonary mycosis may actually be a continuum of disease and should be referred to as sinobronchial allergic mycosis (SAM) (771). Criteria for the diagnosis of ABPA in patients with asthma include (i) asthma, (ii) positive skin test for fungal allergens, (iii) elevated IgE levels, (iv) Aspergillus-specific IgE, and (v) proximal bronchiectasis (5). Similar criteria for ABPM are not established but may include elevated IgE levels, positive skin tests, and response to systemic steroids.
In reviewing cases of ABPM due to dematiaceous fungi, essentially all cases are found to be due to Bipolaris or Curvularia (323, 432, 526, 622, 631, 658, 753). Asthma was common in these cases, but bronchiectasis was often not present, perhaps reflecting somewhat different pathogenic mechanisms. All cases had either eosinophilia or elevated IgE levels. Therapy was primarily systemic steroids, with a slow taper over 2 to 3 months or longer, if necessary. Itraconazole has been used as a steroid-sparing agent in ABPA, but its efficacy is not clear and routine use of itraconazole is not generally recommended (5).
Superficial infections.
these cases of superficial infections involve only keratinized tissues, such as the fingernails and toenails and the stratum corneum. Consequences of these infections are generally cosmetic. Relatively few fungi are responsible for the majority of infections.
(i) Onychomycosis.
Dematiaceous fungi are rare causes of onychomycosis. Clinical features may include a history of trauma, involvement of only one or two toenails, and lack of response to standard systemic therapy (316). Alternaria, Scopulariopsis, and Neoscytalidium have been reported, with the last genus being highly resistant to therapy (68, 316, 643, 752). In one study, Neoscytalidium infection was associated with plucking of green tea leaves (68). Itraconazole and terbinafine are the most commonly used systemic agents and may be combined with topical therapy for refractory cases (316, 752). No published data are available for the newer azole agents.
(ii) Tinea nigra.
Tinea nigra is an uncommon infection confined to the stratum corneum. The characteristic appearance is that of a pigmented macule, usually on the palms or soles, and may be bilateral (440, 682). It is usually asymptomatic. The most common reported cause is Hortaea werneckii (previously Phaeoannellomyces werneckii), with some cases due to Stenella araguata (87, 588, 682). Most cases are associated with exposure to sandy beaches in tropical regions, where H. werneckii is found in areas of high salinity (588). However, individual cases due to Scopulariopsis brevicaulis, Phoma eupyrena, and Chaetomium globosum with findings consistent with tinea nigra have also been reported (57, 154, 287). Diagnosis is made by skin scraping, and biopsy is not needed. Although systemic antifungals have been given with success (318), topical therapy with azoles or keratolytics is very effective (87, 588).
Deep local infections.
Deep local infections are a heterogenous group of infectious syndromes that are typically caused by local trauma. Virtually any of the melanized fungi discussed in this review may cause these infections. While they are rarely life-threatening, even in immunocompromised patients, considerable morbidity can result due to difficulties in treatment and complications.
(i) Subcutaneous lesions.
Subcutaneous lesions are the most common case reports of infection due to melanized fungi in the literature. Alternaria spp. are by far the most common etiologic agent, with a recent review cataloguing over 156 cases up to 2007 (195, 191, 275, 373, 542, 577, 710, 751). Exophiala spp. and Phialophora spp. are the next most common fungi, followed by Cladosporium spp., Exserohilum spp., Veronaea botryosa, and many others with scattered case reports (7, 12, 19, 23, 32, 36, 56, 61, 65, 81, 140, 143, 194, 209, 230, 241, 251, 258, 306, 313, 342, 362, 365, 369, 387, 413, 418, 421, 457, 469, 482, 486, 487, 495, 539, 559, 556, 568, 571, 576, 590, 609, 613, 614, 646, 659, 694, 699, 720, 728, 749, 764, 776, 807, 818). Minor trauma is the usual inciting factor, though it is frequently unrecognized by the patient. Occasionally wood splinters or other vegetable matter is found upon skin biopsy or excision of the lesion (499, 524, 559, 660).
Many patients are immunocompetent, and they often are from a rural background, i.e., farmers with frequent, minor trauma from plant material or gardeners (133, 192, 206, 233, 277, 359, 576, 751, 815). Organ transplantation is also a common risk factor (28, 38, 112, 142, 235, 236, 262, 271, 286, 296, 465, 484, 514, 556, 587, 645, 679, 704, 725). Apparently nosocomial cases have also been reported, with skin irritations from dressings or i.v. sites as possible risk factors (237). Lesions typically occur on exposed areas of the body and often appear as isolated cystic or papular lesions. Presentation is usually indolent, with weeks to months of gradual enlarging mass, though pain is often absent. Severely immunocompromised patients are at increased risk of subsequent dissemination, though this may rarely occur in apparently immunocompetent patients as well. Occasionally, infection may extend to involve joints or bone, requiring more extensive surgery or prolonged antifungal therapy (198).
Multiple therapeutic options are available, usually depending on the immune status of the patient and the extent of lesions. Oral systemic therapy with an azole antifungal agent in conjunction with surgery is frequently employed and has been used successfully, particularly in immunocompromised patients. This is to prevent possible disseminated infection, though this is actually very rare in all but the most immunosuppressed patients. Terbinafine has also been used successfully, particularly in patients failing azole therapy (7). Surgical excision alone has been successful in a number of cases, even in organ transplant patients (12, 194, 235, 387, 418, 558, 587, 646, 686, 720, 764). The Mohs surgical technique, which was developed for removing melanoma, may be a useful surgical approach, as it spares tissue and completely removes the pathological lesion in staged surgeries (86). Patients for whom prolonged antifungal therapy is problematic may also benefit from this technique, such as transplant patients, who often are on immunosuppressive medications that interact with oral triazole antifungals. For multiple lesions where resection may be difficult, antifungal therapy alone has also been successful, even in immunocompromised patients (251, 514). A variety of alternative therapies have been successfully employed as well, including cryotherapy, thermotherapy (local heat application), and supersaturated potassium iodide (SSKI) (286, 313, 751). These are particularly relevant in developing countries where systemic antifungal therapy is difficult to obtain or too expensive for patients. Recurrences may occur several months to over a year after therapy is complete, so careful clinical follow-up is important (38, 142).
(ii) Keratitis.
Fungal keratitis is an important ophthalmologic problem, particularly in tropical areas of the world (298). In one large series, 40% of all infectious keratitis was caused by fungi, almost exclusively molds (298). The most common fungi are Fusarium and Aspergillus, followed by dematiaceous fungi (up to 8 to 17% of cases) (298, 715). Many species can cause disease, with Bipolaris and Curvularia most common, though Lasiodiplodia theobromae may cause more severe disease (55, 75, 213, 298, 743, 744). Approximately half the cases are associated with trauma; prior eye surgery, diabetes, and contact lens use have also been noted as important risk factors (298, 743). Diagnosis rests on potassium hydroxide (KOH) smear and culture, with many dematiaceous fungi associated with pigmented plaques (270).
Many cases of keratitis due to dematiaceous fungi have come from India (77, 136, 210, 269, 582). In a large experience of keratitis due to dematiaceous fungi, 88 cases were examined (269). The most common dematiaceous genus causing keratitis was Curvularia, followed by Bipolaris, Exserohilum, and Lasiodiplodia. Almost half the cases were associated with trauma. Most patients received topical agents only (5% natamycin with or without an azole), though more severe cases also received oral ketoconazole. Overall response was 72% in those available for follow-up. Surgery was needed in 13 patients, with an additional 6 requiring enucleation due to poor response. Itraconazole topically has also been used with success (582).
In a study from the United States of 43 cases of Curvularia keratitis, almost all were associated with trauma (800). Plants were the most common source, though several cases of metal injury were seen as well. Topical natamycin was used almost exclusively, with only a few severe cases requiring adjunctive therapy, usually with an azole. Of the oral agents, itraconazole had the best in vitro activity, though the majority of isolates were resistant to flucytosine. Surgery, including penetrating keratoplasty, was required in 19% of patients. At the end of therapy, only 78% had a visual acuity of 20/40 or better. Other case series from the United States have noted a rise in contact lens use as a risk factor in recent years (374, 388). In the southern United States, cases are more frequently seen during warm, humid months (799).
Topical polyenes, such as amphotericin B and natamycin, are commonly used, but oral and topical itraconazole have been found to be useful as well (298, 744). Use of voriconazole has become more common, with topical preparations well described (211), but published cases involving dematiaceous fungi are infrequent (564). A series of Alternaria keratitis cases that were refractory to natamycin responded to topical azoles (758). A recent analysis of clinical trials involving fungal keratitis suggested that none of the available agents was highly effective (247). Many patients are left with residual visual deficits at the end of therapy. Clearly, further advances in therapy are needed for this debilitating disease.
(iii) Bone and joint infections.
There are relatively few case reports of isolated osteoarticular infections due to dematiaceous fungi, perhaps reflecting the significant trauma often required for implantation into these deeper tissues, though some cases did not have noticeable trauma. S. prolificans is the most common cause, with all except two cases occurring in young children (166, 299, 325, 406, 453, 474, 716, 718). In addition to surgery, various antifungal therapies have been employed, with three cases using the combination of voriconazole and terbinafine with success (166, 299, 406). Unusual therapies have also been tried, including irrigation with polyhexamethylbiguanide and oral therapy with miltefosine (406, 716). Whether these actually improved the likelihood of a clinical response is unknown.
Alternaria alternata has been reported to cause palatal ulcers with associated osteomyelitis in patients with chronic sinusitis (198, 265, 297). Although immune tolerance has been suggested in these cases, no convincing evidence is available. Recurrences were common despite prolonged antifungal therapy.
Other organisms associated with single case reports include P. obovatum, E. oligosperma, C. arxii, M. mycetomatis, P. richardsiae, F. pedrosoi, and P. parasiticum (94, 390, 398, 472, 512, 689, 812). Itraconazole was the most common single agent used with success (398, 512, 689). Antifungal therapy for all these cases is usually prolonged, i.e., >6 months and up to 2 years.
(iv) Peritonitis.
Peritonitis occurs essentially only in patients receiving peritoneal dialysis (6, 8, 69, 97, 106, 115, 117, 144, 197, 261, 302, 305, 367, 405, 416, 461, 466, 563, 598, 605, 618, 619, 656, 690, 762, 765, 783, 784). The presentation is usually subacute, with many patients being without significant symptoms. The genera isolated included Curvularia (eight cases); Exophiala (five); Alternaria (four); Bipolaris (four); Aureobasidium (three); and Lecythophora, Hormonema, and Phialemonium (one each). Eosinophils in peritoneal fluid were not uncommon and were associated with a variety of species. Catheter removal was considered critical, though one case with amphotericin B lock therapy in the catheter with systemic fluconazole resulted in cure with catheter retention (106). Outcomes were generally good, with only three deaths, two associated with persistent infection and the other with a retained catheter (405, 618, 690).
(v) Miscellaneous infections.
Various anecdotal cases of unusual infectious syndromes have been reported. A case of epididymitis due to E. jeanselmei was reported in a 54-year-old male who had received multiple needle aspirations for a symptomatic hydrocele (248). Surgical excision alone resulted in cure. In another case, a 5-year-old asymptomatic girl was noted to have “black grains” in her urine 3 weeks after treatment for a urinary tract infection. Hyphae and conidia identified as a Curvularia sp. were observed in a wet mount but did not grow in culture (635). She received no therapy, and the condition spontaneously resolved. E. dermatitidis was isolated from a case of otitis externa in a 19-year-old immunocompetent female, along with Pseudomonas (404). She responded to antibiotics and topical antimycotics. E. jeanselmei was isolated from esophageal brushings of a patient with Barrett's esophagus, and biopsy revealed hyphal elements as well (669). The patient was treated with ketoconazole, though little clinical improvement was noted after 5 months. A case of acute, invasive sinusitis due to E. rostratum was reported in an 18-year-old female with aplastic anemia and persistent neutropenia (442). She underwent surgery and prolonged antifungal therapy with liposomal amphotericin B and voriconazole-itraconazole, although she died of her underlying disease with persistent evidence of infection at autopsy. In contrast to the case for allergic sinusitis, such presentations are rare in immunocompromised patients.
Pulmonary infection.
Pulmonary infection is usually seen in immunocompromised patients or those with underlying lung disease, and it may be due to a wide variety of species, including S. prolificans, C. bantiana, Chaetomium spp., Ochroconis gallopava, Exophiala spp., Alternaria, Cladophialophora boppii, F. pedrosoi, L. theobromae, Aureobasidium pullulans, Curvularia spp., Sarcinosporin inkin, and P. verrucosa (1, 17, 63, 92, 101, 107, 199, 219, 267, 303, 348, 351, 352, 402, 427, 429, 443, 464, 477, 520, 528, 554, 583, 701, 734, 736, 805, 814). Clinical manifestations include pneumonia, asymptomatic solitary pulmonary nodules, and endobronchial lesions which may cause hemoptysis. Therapy has consisted of systemic antifungal agents, usually amphotericin B or itraconazole initially, followed by itraconazole for a more prolonged period. Mortality rates are high in immunocompromised patients (>40%). Experience with voriconazole is accumulating and appears promising (17, 199, 219, 348). Posaconazole was effective in a case of Alternaria pneumonia in a patient with leukemia that was refractory to amphotericin B and voriconazole (528). Occasional cases of solitary pulmonary nodules in immunocompetent patients may be cured with surgical resection alone (92, 303).
Central nervous system infection.
Central nervous system infection is a rare but frequently fatal manifestation of phaeohyphomycosis, often in immunocompetent individuals. In a review of 101 cases of central nervous system infection due to dematiaceous fungi (628), the most common presentation was found to be brain abscess (11, 24, 21, 34, 42, 53, 60, 79, 82, 100, 110, 114, 137, 138, 156, 168, 169, 193, 212, 222, 254, 255, 290, 314, 319, 329, 337, 343, 371, 394, 396, 408, 410, 412, 415, 422, 458, 500, 505, 513, 523, 527, 529-531, 562, 570, 578, 602, 629, 649, 661, 662, 665, 667, 678, 681, 692, 702, 711, 726, 738, 746, 763, 786, 787, 790, 791, 803, 816). What is truly unique about this disease is that over half the cases were in patients with no risk factor or immunodeficiency. In addition, no specific exposures were associated with onset of infection, though many cases seem to occur in rural areas. Typical symptoms included headache, neurologic deficits, and seizures, though rarely all three. The most common species was C. bantiana, accounting for half the cases. Other species included Rhinocladiella mackenziei, Ochroconis gallopava, Bipolaris spicifera, Exophiala dermatitidis, and Chaetomium strumarium. Mortality was >70%. Since that review in 2004, over 50 cases have been reported, with C. bantiana remaining the most frequently seen isolate (131, 4, 17, 16, 22, 31, 40, 70, 78, 93, 121, 139, 170, 189, 243, 244, 268, 272, 276, 328, 332, 341, 363, 380, 399, 420, 433, 448, 450, 467, 476, 545, 575, 636, 657, 696, 700, 721, 731, 733, 754, 759). A new species, Fonsecaea monophora, has been reported since that time and appears to have a predilection for causing CNS disease, in contrast to its related species, F. pedrosoi (420, 721, 733). Encephalitis with diffuse brain involvement is a rare presentation, with essentially 100% mortality (1, 80).
The pathogenesis may be hematogenous spread from an initial, presumably subclinical pulmonary focus, though this remains speculation. Animal models of C. bantiana reliably replicate CNS infection with intravenous or intranasal inoculation, though these are generally immunosuppressed mice (15, 200). However, it remains unclear why these fungi preferentially cause CNS disease in immunocompetent individuals.
Meningitis has also been described, usually in immunocompromised patients (3, 17, 59, 74, 260, 366, 423, 444, 470, 729). However, cases with iatrogenic complications related to contaminated steroid preparations injected epidurally have been reported (131). These can be difficult to treat, and mortality is high (>60%).
Many therapeutic strategies have been used in the treatment of brain abscess, though it is unclear if any result in significantly improved outcomes. The retrospective analysis of 101 reported cases mentioned above suggested that the combination of amphotericin B, flucytosine, and itraconazole may be associated with improved survival, though it was not frequently used. Subsequent reports have documented various regimens, some using voriconazole or posaconazole with clinical success, though failures have also been reported. Voriconazole was unsuccessful in treating three out of four cases of C. bantiana brain abscess, though two of these patients were immunocompromised, and one received concomitant phenytoin, which may have reduced levels of voriconazole (243, 450, 467, 754). Despite these reports, voriconazole may have a role in therapy of phaeohyphomycotic brain abscess, as it has been successfully used in cases of Aspergillus and S. apiospermum brain abscess (188, 540). Posaconazole has been reported to be effective in a case of R. mackenziei brain abscess, which represents the first reported survival of infection due to this species (16). For cases due to C. bantiana, addition of flucytosine to azole therapy may be useful given its in vitro activity against this species specifically and in vivo and clinical data (481, 628). Based on the experience described above and animal studies, a combination of agents is likely to be more effective than monotherapy, though the optimal combination remains unclear and should be based on the individual case.
What does appear to be consistent is that complete excision of brain abscess whenever feasible is associated with better outcomes than aspiration or partial excision. In a series of 10 cases due to C. bantiana at one institution, all surviving patients were able to have complete resection of the brain lesion (268). In another case, repeated surgical excisions alone resulted in cure (189). However, outcomes remain poor, with an overall mortality of >70%.
Disseminated infection.
Disseminated infection is the most uncommon manifestation of infection caused by melanized fungi. In a review of 72 cases (627), most patients were immunocompromised, though occasional patients without known immunodeficiency or risk factors developed disseminated disease as well (3, 24, 73, 76, 95, 105, 119, 171, 208, 238, 242, 246, 288, 291, 308, 321, 345, 386, 389, 397, 400, 402, 403, 409, 423, 424, 435, 471, 485, 516, 538, 543, 550, 557, 578, 593, 595, 596, 612, 621, 627, 640, 647, 672, 688, 712, 713, 740, 755, 769, 772, 794, 804, 806, 822). In contrast to most invasive mold infections, blood cultures were positive in over half the cases. The most common isolate was S. prolificans, accounting for over a third of cases. Since that review, S. prolificans remains the most frequent cause of disseminated disease, almost exclusively in immunocompromised patients (33, 62, 96, 151, 304, 358, 364, 368, 434, 697, 709, 747, 750, 796). E. dermatitidis, in contrast, is commonly seen in immunocompetent patients, particularly from Asia (13, 349, 565). A variety of other molds were reported in disseminated disease, including E. oligosperma, Chaetomium perlucidum, O. gallopava, Lecythophora mutabilis, P. parasiticum, B. spicifera, Exserohilum sp., E. spinifera, and Curvularia lunata (25, 64, 85, 207, 256, 335, 417, 451, 536, 741, 788). Interestingly, peripheral eosinophilia has been observed in 9% of cases, and these were generally due to Bipolaris and Curvularia. These same species are often associated with allergic disease.
The mortality rate was >70%, despite aggressive antifungal therapy. There were no antifungal regimens associated with improved survival for disseminated infection. Scedosporium prolificans is generally resistant to all available antifungal agents, and infection with S. prolificans was associated with nearly 100% mortality in the absence of recovery from neutropenia, indicating the importance of the host response in this infection. However, recent case reports have suggested that the combination of itraconazole or voriconazole with terbinafine may be synergistic against this species and improve outcomes, though clinical experience is limited (358, 750, 796). Some case reports utilized colony-stimulating factors and/or leukocyte infusions to augment antifungal therapy (96, 368, 796).
Other combinations or therapies have not been shown to be consistently effective, though clinical experience is limited, and will likely be confined to anecdotal reports, given the rarity of this infection. Recent successful case reports have used itraconazole, voriconazole, and posaconazole for a variety of different species (207, 368, 417, 536, 565). Amphotericin B alone is not generally effective (25, 64, 368).
CONCLUSIONS
Melanized fungi remain uncommon causes of infection in humans but have become increasingly recognized in a wide variety of clinical syndromes. Many species across a broad range of genera are associated with disease, which leads to daunting challenges in diagnostic testing. However, relatively few are responsible for the majority of clinical cases. Alternaria is a frequent cause of subcutaneous lesions, Bipolaris and Curvularia are often associated with allergic disease, and C. bantiana and S. prolificans are the most common causes of brain abscess and disseminated disease, respectively. Taxonomy is constantly evolving as molecular methods shed new light on relationships between species. Melanin appears to be an important virulence factor for these fungi, though much additional work is needed to better understand the pathogenic mechanisms underlying these infections, particularly in immunocompetent patients. Life-threatening infections are rare but may be seen even in individuals with no apparent risk factors, especially in cases of brain abscess. As these are typically soil organisms and common laboratory contaminants, sometimes they are disregarded as nonpathogenic. However, the clinical setting in which they are isolated should always be considered when evaluating their potential as etiologic agents and before making decisions regarding therapy. Diagnosis depends on a high degree of clinical suspicion and careful mycological and pathological examination of clinical specimens. Molecular diagnostic techniques are progressing but are not standardized or reliable for the diverse species encountered.
Therapy for many infectious syndromes has evolved with the advent of several new antifungal agents in recent years. The oral triazoles voriconazole, posaconazole, and itraconazole demonstrate the most consistent in vitro activity against this group of fungi and are widely used, though voriconazole is usually the drug of choice in most clinical settings. High doses of amphotericin B lipid formulations may have a role in the treatment of refractory cases or for severe infections in unstable patients, though it is usually not effective as a single agent. Once the patient is stable, “consolidation” therapy with a broad-spectrum oral azole is often employed until a complete response is achieved. Terbinafine has broad activity against melanized fungi, and interest in its use beyond dermatophyte infections is increasing. It appears to provide synergistic activity with azole antifungals, and this may be a useful strategy against refractory subcutaneous infections such as chromoblastomycosis and mycetoma that often do not respond to conventional monotherapy. In addition, the use of terbinafine with voriconazole for disseminated S. prolificans infection has been successful with what is otherwise an almost universally fatal infection. It should be pointed out that in these disseminated cases, recovery of immune function, especially phagocytic cells, is critically important as well. Flucytosine has limited activity against dematiaceous fungi, though it may have a role in therapy of chromoblastomycosis and of brain abscess due to C. bantiana, in particular. Echinocandins do not appear to be useful as single agents but may be considered in combination therapy of difficult cases. Combination therapy is a potentially useful therapeutic strategy for refractory infections, particularly brain abscess and disseminated disease. However, it is not clear which antifungal drug combinations are most effective. Therapy is evolving for many of the clinical syndromes described, and randomized clinical trials to address this issue are impractical given the sporadic nature of cases. Detailed case reporting of both successful and unsuccessful clinical experiences will be important in attempting to define optimal therapy for infections caused by dematiaceous fungi.
Biography
 Sanjay Revankar, M.D., is an Associate Professor at Wayne State University, Department of Medicine, Division of Infectious Diseases, Detroit, MI. He completed his residency in internal medicine at the University of Michigan and fellowships in infectious diseases and mycology at the University of Texas Health Science Center, San Antonio, and the Fungus Testing Laboratory, respectively. His research interests include basic and clinical mycology, especially unusual mold infections.
Sanjay Revankar, M.D., is an Associate Professor at Wayne State University, Department of Medicine, Division of Infectious Diseases, Detroit, MI. He completed his residency in internal medicine at the University of Michigan and fellowships in infectious diseases and mycology at the University of Texas Health Science Center, San Antonio, and the Fungus Testing Laboratory, respectively. His research interests include basic and clinical mycology, especially unusual mold infections.
 Deanna A. Sutton, Ph.D., MT,SM(ASCP), RM,SM(NRCM), is an Associate Professor in the Department of Pathology at the University of Texas Health Science Center in San Antonio, TX, and the Administrative Director of the Fungus Testing Laboratory (FTL). As a member of the FTL, her research interests encompass fungal taxonomy, antifungal susceptibility trends, and identification of rare molds encountered in human and veterinary medicine. She has taught numerous medical mycology courses and has been an invited workshop presenter both nationally and internationally. She currently serves as an Associate Editor of Medical Mycology; is on the editorial board of several peer-reviewed journals, including the Journal of Clinical Microbiology and Antimicrobial Agents and Chemotherapy; and has written extensively on invasive fungal infections caused by uncommon and emerging molds in humans and animals.
Deanna A. Sutton, Ph.D., MT,SM(ASCP), RM,SM(NRCM), is an Associate Professor in the Department of Pathology at the University of Texas Health Science Center in San Antonio, TX, and the Administrative Director of the Fungus Testing Laboratory (FTL). As a member of the FTL, her research interests encompass fungal taxonomy, antifungal susceptibility trends, and identification of rare molds encountered in human and veterinary medicine. She has taught numerous medical mycology courses and has been an invited workshop presenter both nationally and internationally. She currently serves as an Associate Editor of Medical Mycology; is on the editorial board of several peer-reviewed journals, including the Journal of Clinical Microbiology and Antimicrobial Agents and Chemotherapy; and has written extensively on invasive fungal infections caused by uncommon and emerging molds in humans and animals.
REFERENCES
- 1.Abbott, S. P., L. Sigler, R. McAleer, D. A. McGough, M. G. Rinaldi, and G. Mizell. 1995. Fatal cerebral mycoses caused by the ascomycete Chaetomium strumarium. J. Clin. Microbiol. 33:2692-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abliz, P., K. Fukushima, K. Takizawa, and K. Nishimura. 2004. Identification of pathogenic dematiaceous fungi and related taxa based on large subunit ribosomal DNA D1/D2 domain sequence analysis. FEMS Immunol. Med. Microbiol. 40:41-49. [DOI] [PubMed] [Google Scholar]
- 3.Adam, R. D., M. L. Paquin, E. A. Petersen, M. A. Saubolle, M. G. Rinaldi, J. G. Corcoran, J. N. Galgiani, and R. E. Sobonya. 1986. Phaeohyphomycosis caused by the fungal genera Bipolaris and Exserohilum. A report of 9 cases and review of the literature. Medicine 65:203-217. [DOI] [PubMed] [Google Scholar]
- 4.Adeyemi, O. A., O. Lie, R. Bernstein, N. Gottardi-Littell, K. Muro, D. Patil, and G. A. Noskin. 2007. Woman with multiple brain abscesses. Clin. Infect. Dis. 45:1351-1352:1397-1399. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal, R. 2009. Allergic bronchopulmonary aspergillosis. Chest 135:805-826. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal, S., N. L. Goodman, and H. H. Malluche. 1993. Peritonitis due to Exophiala jeanselmei in a patient undergoing continuous ambulatory peritoneal dialysis. Am. J. Kidney Dis. 21:673-675. [DOI] [PubMed] [Google Scholar]
- 7.Agger, W. A., D. Andes, and J. W. Burgess. 2004. Exophiala jeanselmei infection in a heart transplant recipient successfully treated with oral terbinafine. Clin. Infect. Dis. 38:e112-115. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad, S., R. J. Johnson, S. Hillier, W. R. Shelton, and M. G. Rinaldi. 1985. Fungal peritonitis caused by Lecythophora mutabilis. J. Clin. Microbiol. 22:182-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed, A. O., W. van Leeuwen, A. Fahal, W. van de Sande, H. Verbrugh, and A. van Belkum. 2004. Mycetoma caused by Madurella mycetomatis: a neglected infectious burden. Lancet Infect. Dis. 4:566-574. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed, A. O., W. van Vianen, M. T. ten Kate, W. W. van de Sande, A. van Belkum, A. H. Fahal, H. A. Verbrugh, and I. A. Bakker-Woudenberg. 2003. A murine model of Madurella mycetomatis eumycetoma. FEMS Immunol. Med. Microbiol. 37:29-36. [DOI] [PubMed] [Google Scholar]
- 11.Ajanee, N., M. Alam, K. Holmberg, and J. Khan. 1996. Brain abscess caused by Wangiella dermatitidis: case report. Clin. Infect. Dis. 23:197-198. [DOI] [PubMed] [Google Scholar]
- 12.Ajello, L., L. K. Georg, R. T. Steigbigel, and C. J. Wang. 1974. A case of phaeohyphomycosis caused by a new species of Phialophora. Mycologia 66:490-498. [PubMed] [Google Scholar]
- 13.Alabaz, D., F. Kibar, S. Arikan, B. Sancak, U. Celik, N. Aksaray, and M. Turgut. 2009. Systemic phaeohyphomycosis due to Exophiala (Wangiella) in an immunocompetent child. Med. Mycol. 47:653-657. [DOI] [PubMed] [Google Scholar]
- 14.Al-Abdely, H. M., L. Najvar, R. Bocanegra, A. Fothergill, D. Loebenberg, M. G. Rinaldi, and J. R. Graybill. 2000. SCH 56592, amphotericin B, or itraconazole therapy of experimental murine cerebral phaeohyphomycosis due to Ramichloridium obovoideum (“Ramichloridium mackenziei”). Antimicrob. Agents Chemother. 44:1159-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Abdely, H. M., L. K. Najvar, R. Bocanegra, and J. R. Graybill. 2005. Antifungal therapy of experimental cerebral phaeohyphomycosis due to Cladophialophora bantiana. Antimicrob. Agents Chemother. 49:1701-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Abdely, H. M., A. M. Alkhunaizi, J. A. Al Tawfiq, M. Hassounah, M. G. Rinaldi, and D. A. Sutton. 2005. Successful therapy of cerebral phaeohyphomycosis due to Ramichloridium mackenziei with the new triazole posaconazole. Med. Mycol. 43:91-95. [DOI] [PubMed] [Google Scholar]
- 17.Al-Aidaroos, A., I. Bin-Hussain, H. El Solh, A. Kofide, S. Thawadi, A. Belgaumi, and A. Al Ahmari. 2007. Invasive Chaetomium infection in two immunocompromised pediatric patients. Pediatr. Infect. Dis. J. 26:456-458. [DOI] [PubMed] [Google Scholar]
- 18.Al-Astruey-Izquierdo, A., M. Cuenca-Estrella, A. Monzon, and J. L. Rodriguez-Tudela. 2007. Prevalence and susceptibility testing of new species of Pseudallescheria and Scedosporium in a collection of clinical mold isolates. Antimicrob. Agents Chemother. 51:748-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Attar, A., C. G. Williams, and R. J. Redett. 2006. Rare lower extremity invasive fungal infection in an immunosuppressed patient: Exserohilum longirostratum. Plast. Reconstr. Surg. 117:e44-47. [DOI] [PubMed] [Google Scholar]
- 20.Alcorn, J. L. 1983. Generic concepts in Drechslera, Bipolaris and Exserohilum. Mycotaxon 17:1-86. [Google Scholar]
- 21.Aldape, K. D., H. S. Fox, J. P. Roberts, N. L. Ascher, J. R. Lake, and H. A. Rowley. 1991. Cladosporium trichoides cerebral phaeohyphomycosis in a liver transplant recipient. Report of a case. Am. J. Clin. Pathol. 95:499-502. [DOI] [PubMed] [Google Scholar]
- 22.Alhabib, K. F., and E. A. Bryce. 2003. Xylohypha bantiana multiple brain abscesses in a patient with systemic lupus erythematosus. Can. J. Infect. Dis. 14:119-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allred, B. J. 1990. Subcutaneous phaeohyphomycosis due to Exophiala jeanselmei in an immunosuppressed patient: case report. New Zealand Med. J. 103:321-322. [PubMed] [Google Scholar]
- 24.Al-Mohsen, I. Z., D. A. Sutton, L. Sigler, E. Almodovar, N. Mahgoub, H. Frayha, S. Al-Hajjar, M. G. Rinaldi, and T. J. Walsh. 2000. Acrophialophora fusispora brain abscess in a child with acute lymphoblastic leukemia: review of cases and taxonomy. J. Clin. Microbiol. 38:4569-4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Obaid, I., S. Ahmad, Z. U. Khan, B. Dinesh, and H. M. Hejab. 2006. Catheter-associated fungemia due to Exophiala oligosperma in a leukemic child and review of fungemia cases caused by Exophiala species. Eur. J. Clin. Microbiol. Infect. Dis. 25:729-732. [DOI] [PubMed] [Google Scholar]
- 26.Al-Rajhi, A. A., A. H. Awad, S. S. al-Hedaithy, R. K. Forster, and K. C. Caldwell. 1993. Scytalidium dimidiatum fungal endophthalmitis. Br. J. Ophthalmol. 77:388-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Tawfiq, J. A., and S. S. Amr. 2009. Madura leg due to Exophiala jeanselmei successfully treated with surgery and itraconazole therapy. Med. Mycol. 47:648-652. [DOI] [PubMed] [Google Scholar]
- 28.Altomare, G. F., G. L. Capella, V. Boneschi, and M. A. Viviani. 2000. Effectiveness of terbinafine in cutaneous alternariosis. Br. J. Dermatol. 142:840-841. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez, M., P. B. Lopez, C. Rayon, G. J. Garcia, M. C. Roson Porto, M. Gonzalez, J. V. Martinez-Suarez, and J. L. Rodriguez-Tudela. 1995. Nosocomial outbreak caused by Scedosporium prolificans (inflatum): four fatal cases in leukemic patients. J. Clin. Microbiol. 33:3290-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ameen, M., and R. Arenas. 2009. Developments in the management of mycetomas. Clin. Exp. Dermatol. 34:1-7. [DOI] [PubMed] [Google Scholar]
- 31.Amr, S. S., and J. A. Al-Tawfiq. 2007. Aspiration cytology of brain abscess from a fatal case of cerebral phaeohyphomycosis due to Ramichloridium mackenziei. Diagn. Cytopathol. 35:695-699. [DOI] [PubMed] [Google Scholar]
- 32.Anandan, V., V. Nayak, S. Sundaram, and P. Srikanth. 2008. An association of Alternaria alternata and Scopulariopsis brevicaulis in cutaneous phaeohyphomycosis. Indian J. Dermatol. Venereol. Leprol. 74:244-247. [DOI] [PubMed] [Google Scholar]
- 33.Ananda-Rajah, M. R., A. Grigg, and M. A. Slavin. 2008. Breakthrough disseminated Scedosporium prolificans infection in a patient with relapsed leukaemia on prolonged voriconazole followed by posaconazole prophylaxis. Mycopathologia 166:83-86. [DOI] [PubMed] [Google Scholar]
- 34.Anandi, V., T. J. John, A. Walter, J. C. Shastry, M. K. Lalitha, A. A. Padhye, L. Ajello, and F. W. Chandler. 1989. Cerebral phaeohyphomycosis caused by Chaetomium globosum in a renal transplant recipient. J. Clin. Microbiol. 27:2226-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrade, T. S., L. G. Castro, R. S. Nunes, V. M. Gimenes, and A. E. Cury. 2004. Susceptibility of sequential Fonsecaea pedrosoi isolates from chromoblastomycosis patients to antifungal agents. Mycoses 47:216-221. [DOI] [PubMed] [Google Scholar]
- 36.Aoyama, Y., M. Nomura, S. Yamanaka, Y. Ogawa, and Y. Kitajima. 2009. Subcutaneous phaeohyphomycosis caused by Exophiala xenobiotica in a non-Hodgkin lymphoma patient. Med. Mycol. 47:95-99. [DOI] [PubMed] [Google Scholar]
- 37.Aquino, V. M., J. M. Norvell, K. Krisher, and M. M. Mustafa. 1995. Fatal disseminated infection due to Exserohilum rostratum in a patient with aplastic anemia: case report and review. Clin. Infect. Dis. 20:176-178. [DOI] [PubMed] [Google Scholar]
- 38.Ara, M., C. Aspiroz, P. Zaballos, V. Alcalde, R. Alvarez, A. Rezusta, and J. A. Gimenez. 2006. Relapse of cutaneous Alternaria infectoria in a renal transplant recipient after 2 years. Acta Derm. Venereol. 86:154-155. [DOI] [PubMed] [Google Scholar]
- 39.Arango, M., C. Jaramillo, A. Cortes, and A. Restrepo. 1998. Auricular chromoblastomycosis caused by Rhinocladiella aquaspersa. Med. Mycol. 36:43-45. [PubMed] [Google Scholar]
- 40.Aribandi, M., C. Bazan III, and M. G. Rinaldi. 2005. Magnetic resonance imaging findings in fatal primary cerebral infection due to Chaetomium strumarium. Australas. Radiol. 49:166-169. [DOI] [PubMed] [Google Scholar]
- 41.Arthur, S., L. L. Steed, D. J. Apple, Q. Peng, G. Howard, and M. Escobar-Gomez. 2001. Scedosporium prolificans keratouveitis in association with a contact lens retained intraocularly over a long term. J. Clin. Microbiol. 39:4579-4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arunkumar, M. J., V. Rajshekhar, M. J. Chandy, P. P. Thomas, and C. K. Jacob. 2000. Management and outcome of brain abscess in renal transplant recipients. Postgrad. Med. J. 76:207-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arzanlou, M., J. Z. Groenewald, W. Gams, U. Braun, H. D. Shin, and P. W. Crous. 2007. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Stud. Mycol. 58:57-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Attapattu, M. C. 1997. Chromoblastomycosis—a clinical and mycological study of 71 cases from Sri Lanka. Mycopathologia 137:145-151. [DOI] [PubMed] [Google Scholar]
- 45.Attili, D. S., G. S. De Hoog, and A. A. Pizzirani-Kleiner. 1998. rDNA-RFLP and ITS1 sequencing of species of the genus Fonsecaea, agents of chromoblastomycosis. Med. Mycol. 36:219-225. [PubMed] [Google Scholar]
- 46.Aviv, J. E., W. Lawson, E. J. Bottone, V. P. Sachdev, P. M. Som, and H. F. Biller. 1990. Multiple intracranial mucoceles associated with phaeohyphomycosis of the paranasal sinuses. Arch. Otolaryngol. Head Neck Surg. 116:1210-1213. [DOI] [PubMed] [Google Scholar]
- 47.Badali, H., V. O. Carvalho, V. Vicente, D. Attili-Angelis, I. B. Kwiatkowski, A. H. Gerrits Van Den Ende, and G. S. De Hoog. 2009. Cladophialophora saturnica sp. nov., a new opportunistic species of Chaetothyriales revealed using molecular data. Med. Mycol. 47:51-62. [DOI] [PubMed] [Google Scholar]
- 48.Badali, H., J. Chander, S. Bansal, A. Aher, S. S. Borkar, J. F. Meis, and G. S. De Hoog. 2010. First autochthonous case of Rhinocladiella mackenziei cerebral abscess outside the Middle East. J. Clin. Microbiol. 48:646-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Badali, H., G. S. De Hoog, I. Curfs-Breuker, B. Andersen, and J. F. Meis. 2009. In vitro activities of eight antifungal drugs against 70 clinical and environmental isolates of Alternaria species. J. Antimicrob. Chemother. 63:1295-1297. [DOI] [PubMed] [Google Scholar]
- 50.Badali, H., G. S. de Hoog, I. Curfs-Breuker, and J. F. Meis. 2010. In vitro activities of antifungal drugs against Rhinocladiella mackenziei, an agent of fatal brain infection. J. Antimicrob. Chemother. 65:175-177. [DOI] [PubMed] [Google Scholar]
- 51.Badali, H., C. Gueidan, M. J. Najafzadeh, A. Bonifaz, A. H. van den Ende, and G. S. de Hoog. 2008. Biodiversity of the genus Cladophialophora. Stud. Mycol. 61:175-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Badali, H., M. J. Najafzadeh, M. Van Esbroeck, E. van den Enden, B. Tarazooie, J. F. Meis, and G. S. de Hoog. 2010. The clinical spectrum of Exophiala jeanselmei, with a case report and in vitro antifungal susceptibility of the species. Med. Mycol. 48:318-327. [DOI] [PubMed] [Google Scholar]
- 53.Baddley, J. W., S. A. Moser, D. A. Sutton, and P. G. Pappas. 2000. Microascus cinereus (anamorph Scopulariopsis) brain abscess in a bone marrow transplant recipient. J. Clin. Microbiol. 38:395-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baddley, J. W., L. Mostert, R. C. Summerbell, and S. A. Moser. 2006. Phaeoacremonium parasiticum infections confirmed by beta-tubulin sequence analysis of case isolates. J. Clin. Microbiol. 44:2207-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Badenoch, P. R., C. L. Halliday, D. H. Ellis, K. J. Billing, and R. A. Mills. 2006. Ulocladium atrum keratitis. J. Clin. Microbiol. 44:1190-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baker, J. G., I. F. Salkin, P. Forgacs, J. H. Haines, and M. E. Kemna. 1987. First report of subcutaneous phaeohyphomycosis of the foot caused by Phoma minutella. J. Clin., Microbiol. 25:2395-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bakerspigel, A. 1970. The isolation of Phoma hibernica from a lesion on a leg. Sabouraudia 7:261-264. [PubMed] [Google Scholar]
- 58.Balajee, S. A., A. M. Borman, M. E. Brandt, J. Cano, M. Cuenca-Estrella, E. Dannaoui, J. Guarro, G. Haase, C. C. Kibbler, W. Meyer, K. O'Donnell, C. A. Petti, J. L. Rodriguez-Tudela, D. Sutton, A. Velegraki, and B. L. Wickes. 2009. Sequence-based identification of Aspergillus, Fusarium, and Mucorales species in the clinical mycology laboratory: where are we and where should we go from here? J. Clin. Microbiol. 47:877-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banerjee, T. K., A. K. Patwari, R. Dutta, V. K. Anand, and A. Chabra. 2002. Cladosporium bantianum meningitis in a neonate. Indian J. Pediatr. 69:721-723. [DOI] [PubMed] [Google Scholar]
- 60.Banerjee, U., A. K. Mohapatra, C. Sarkar, and R. Chaudhery. 1989. Cladosporiosis (cerebral phaeohyphomycosis) of brain—a case report. Mycopathologia 105:163-166. [DOI] [PubMed] [Google Scholar]
- 61.Baradkar, V. P., M. Mathur, and S. Kumar. 2009. Phaeohyphomycosis of subcutaneous tissue caused by Phaeoacremonium parasiticum. Indian J. Med. Microbiol. 27:66-69. [PubMed] [Google Scholar]
- 62.Barbaric, D., and P. J. Shaw. 2001. Scedosporium infection in immunocompromised patients: successful use of liposomal amphotericin B and itraconazole. Med. Pediatr. Oncol. 37:122-125. [DOI] [PubMed] [Google Scholar]
- 63.Barenfanger, J., F. Ramirez, R. P. Tewari, and L. Eagleton. 1989. Pulmonary phaeohyphomycosis in a patient with hemoptysis. Chest 95:1158-1160. [DOI] [PubMed] [Google Scholar]
- 64.Barron, M. A., D. A. Sutton, R. Veve, J. Guarro, M. Rinaldi, E. Thompson, P. J. Cagnoni, K. Moultney, and N. E. Madinger. 2003. Invasive mycotic infections caused by Chaetomium perlucidum, a new agent of cerebral phaeohyphomycosis. J. Clin. Microbiol. 41:5302-5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bartolome, B., R. Valks, J. Fraga, V. Buendia, J. Fernandez-Herrera, and A. Garcia-Diez. 1999. Cutaneous alternariosis due to Alternaria chlamydospora after bone marrow transplantation. Acta Dermatol. Venereol. 79:244. [DOI] [PubMed] [Google Scholar]
- 66.Barton, K., D. Miller, and S. C. Pflugfelder. 1997. Corneal chromoblastomycosis. Cornea 16:235-239. [PubMed] [Google Scholar]
- 67.Bartynski, J. M., T. V. McCaffrey, and E. Frigas. 1990. Allergic fungal sinusitis secondary to dermatiaceous fungi—Curvularia lunata and Alternaria. Otolaryngol. Head Neck Surg. 103:32-39. [DOI] [PubMed] [Google Scholar]
- 68.Barua, P., S. Barua, B. Borkakoty, and J. Mahanta. 2007. Onychomycosis by Scytalidium dimidiatum in green tea leaf pluckers: report of two cases. Mycopathologia 164:193-195. [DOI] [PubMed] [Google Scholar]
- 69.Bava, A. J., A. Fayad, C. Cespedes, and M. Sandoval. 2003. Fungal peritonitis caused by Bipolaris spicifera. Med. Mycol. 41:529-531. [DOI] [PubMed] [Google Scholar]
- 70.Beeram, V., S. Challa, and P. Vannemreddy. 2008. Cerebral mycetoma with cranial osteomyelitis. J. Neurosurg. Pediatr. 1:493-495. [DOI] [PubMed] [Google Scholar]
- 71.Ben-Ami, R., P. R. Lasala, R. E. Lewis, and D. P. Kontoyiannis. 2010. Lack of galactomannan reactivity in dematiaceous molds recovered from cancer patients with phaeohyphomycosis. Diagn. Microbiol. Infect. Dis. 66:200-203. [DOI] [PubMed] [Google Scholar]
- 72.Ben-Ami, R., R. E. Lewis, I. I. Raad, and D. P. Kontoyiannis. 2009. Phaeohyphomycosis in a tertiary care cancer center. Clin. Infect. Dis. 48:1033-1041. [DOI] [PubMed] [Google Scholar]
- 73.Benne, C. A., C. Neeleman, M. Bruin, G. S. De Hoog, and A. Fleer. 1993. Disseminating infection with Scytalidium dimidiatum in a granulocytopenic child. Eur. J. Clin. Microbiol. Infect. Dis. 12:118-121. [DOI] [PubMed] [Google Scholar]
- 74.Bennett, J. E., H. Bonner, A. E. Jennings, and R. I. Lopez. 1973. Chronic meningitis caused by Cladosporium trichoides. Am. J. Clin. Pathol. 59:398-407. [DOI] [PubMed] [Google Scholar]
- 75.Ben-Simon, G. J., I. S. Barequet, and A. Grinbaum. 2002. More than tears in your eyes (Exophiala jeanselmei keratitis). Cornea 21:230-231. [DOI] [PubMed] [Google Scholar]
- 76.Berenguer, J., J. L. Rodriguez-Tudela, C. Richard, M. Alvarez, M. A. Sanz, L. Gaztelurrutia, J. Ayats, and J. V. Martinez-Suarez. 1997. Deep infections caused by Scedosporium prolificans. A report on 16 cases in Spain and a review of the literature. Scedosporium prolificans Spanish Study Group. Medicine 76:256-265. [DOI] [PubMed] [Google Scholar]
- 77.Bharathi, M. J., R. Ramakrishnan, S. Vasu, R. Meenakshi, and R. Palaniappan. 2003. Epidemiological characteristics and laboratory diagnosis of fungal keratitis. A three-year study. Indian J. Ophthalmol. 51:315-321. [PubMed] [Google Scholar]
- 78.Bhat, S. V., D. L. Paterson, M. G. Rinaldi, and P. J. Veldkamp. 2007. Scedosporium prolificans brain abscess in a patient with chronic granulomatous disease: successful combination therapy with voriconazole and terbinafine. Scand. J. Infect. Dis. 39:87-90. [DOI] [PubMed] [Google Scholar]
- 79.Bhatia, R., P. Tandon, and N. K. Misra. 1986. Inflammatory lesions of the basal ganglia and thalamus: review of twenty-one cases. Neurosurgery 19:983-988. [DOI] [PubMed] [Google Scholar]
- 80.Biggs, P. J., R. L. Allen, J. M. Powers, and H. P. Holley, Jr. 1986. Phaeohyphomycosis complicating compound skull fracture. Surg. Neurol. 25:393-396. [DOI] [PubMed] [Google Scholar]
- 81.Bilu, D., S. Movahedi-Lankarani, R. A. Kazin, C. Shields, and M. Moresi. 2004. Cutaneous Bipolaris infection in a neutropenic patient with acute lymphoblastic leukemia. J. Cutan. Med. Surg. 8:446-449. [DOI] [PubMed] [Google Scholar]
- 82.Binford, C. H., R. K. Thompson, M. E. Gorham, and C. W. Emmons. 1952. Mycotic brain abscess due to Cladosporium trichoides, a new species. Am. J. Clin. Pathol. 22:535-542. [DOI] [PubMed] [Google Scholar]
- 83.Bloomfield, B. J., and M. Alexander. 1967. Melanins and resistance of fungi to lysis. J. Bacteriol. 93:1276-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boerema, G. H., J. de Gruyter, M. E. Noordeloos, and M. E. C. Hamers. 2004. Phoma identification manual. Differentiation of specific and infra-specific taxa in culture. CABI Publishing, Cambridge, MA.
- 85.Boggild, A. K., S. M. Poutanen, S. Mohan, and M. A. Ostrowski. 2006. Disseminated phaeohyphomycosis due to Ochroconis gallopavum in the setting of advanced HIV infection. Med. Mycol. 44:777-782. [DOI] [PubMed] [Google Scholar]
- 86.Bogle, M. A., M. S. Rabkin, and A. K. Joseph. 2004. Mohs micrographic surgery for the eradication of phaeohyphomycosis of the hand. Dermatol. Surg. 30:231-233. [DOI] [PubMed] [Google Scholar]
- 87.Bonifaz, A., H. Badali, G. S. de Hoog, M. Cruz, J. Araiza, M. A. Cruz, L. Fierro, and R. M. Ponce. 2008. Tinea nigra by Hortaea werneckii, a report of 22 cases from Mexico. Stud. Mycol. 61:77-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonifaz, A., E. Carrasco-Gerard, and A. Saul. 2001. Chromoblastomycosis: clinical and mycologic experience of 51 cases. Mycoses 44:1-7. [DOI] [PubMed] [Google Scholar]
- 89.Bonifaz, A., S. De Hoog, M. R. McGinnis, A. Saul, O. Rodriguez-Cortes, J. Araiza, M. Cruz, and P. Mercadillo. 2009. Eumycetoma caused by Cladophialophora bantiana successfully treated with itraconazole. Med. Mycol. 47:111-114. [DOI] [PubMed] [Google Scholar]
- 90.Bonifaz, A., V. Paredes-Solis, and A. Saul. 2004. Treating chromoblastomycosis with systemic antifungals. Expert. Opin. Pharmacother. 5:247-254. [DOI] [PubMed] [Google Scholar]
- 91.Bonifaz, A., A. Saul, V. Paredes-Solis, J. Araiza, and L. Fierro-Arias. 2005. Treatment of chromoblastomycosis with terbinafine: experience with four cases. J. Dermatolog. Treat. 16:47-51. [DOI] [PubMed] [Google Scholar]
- 92.Borges, M. C., Jr., S. Warren, W. White, and E. V. Pellettiere. 1991. Pulmonary phaeohyphomycosis due to Xylohypha bantiana. Arch. Pathol. Lab. Med. 115:627-629. [PubMed] [Google Scholar]
- 93.Borkar, S. A., M. S. Sharma, G. Rajpal, M. Jain, I. Xess, and B. S. Sharma. 2008. Brain abscess caused by Cladophialophora bantiana in an immunocompetent host: need for a novel cost-effective antifungal agent. Indian J. Med. Microbiol. 26:271-274. [DOI] [PubMed] [Google Scholar]
- 94.Bossler, A. D., S. S. Richter, A. J. Chavez, S. A. Vogelgesang, D. A. Sutton, A. M. Grooters, M. G. Rinaldi, G. S. de Hoog, and M. A. Pfaller. 2003. Exophiala oligosperma causing olecranon bursitis. J. Clin. Microbiol. 41:4779-4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bourbeau, P., D. A. McGough, H. Fraser, N. Shah, and M. G. Rinaldi. 1992. Fatal disseminated infection caused by Myceliophthora thermophila, a new agent of mycosis: case history and laboratory characteristics. J. Clin. Microbiol. 30:3019-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bouza, E., P. Munoz, L. Vega, M. Rodriguez-Creixems, J. Berenguer, and A. Escudero. 1996. Clinical resolution of Scedosporium prolificans fungemia associated with reversal of neutropenia following administration of granulocyte colony-stimulating factor. Clin. Infect. Dis. 23:192-193. [DOI] [PubMed] [Google Scholar]
- 97.Brackett, R. W., A. N. Shenouda, S. S. Hawkins, and W. B. Brock. 1988. Curvularia infection complicating peritoneal dialysis. South. Med. J. 81:943-944. [DOI] [PubMed] [Google Scholar]
- 98.Brandt, M. E., and D. W. Warnock. 2003. Epidemiology, clinical manifestations, and therapy of infections caused by dematiaceous fungi. J. Chemother. 15(Suppl. 2):36-47. [DOI] [PubMed] [Google Scholar]
- 99.Brasch, J., J. O. Busch, and G. S. de Hoog. 2008. Cutaneous phaeohyphomycosis caused by Alternaria infectoria. Acta Dermatol. Venereol. 88:160-161. [DOI] [PubMed] [Google Scholar]
- 100.Brown, J. W., III, J. Nadell, C. V. Sanders, and L. Sardenga. 1976. Brain abscess caused by Cladosporium trichoides (bantianum): a case with paranasal sinus involvement. South. Med. J. 69:1519-1521. [DOI] [PubMed] [Google Scholar]
- 101.Brubaker, L. H., J. C. Steele, Jr., and J. P. Rissing. 1988. Cure of Curvularia pneumonia by amphotericin B in a patient with megakaryocytic leukemia. Arch. Pathol. Lab. Med. 112:1178-1179. [PubMed] [Google Scholar]
- 102.Brummund, W., V. P. Kurup, G. J. Harris, J. A. Duncavage, and J. A. Arkins. 1986. Allergic sino-orbital mycosis. A clinical and immunologic study. JAMA 256:3249-3253. [PubMed] [Google Scholar]
- 103.Brush, L., and N. P. Money. 1999. Invasive hyphal growth in Wangiella dermatitidis is induced by stab inoculation and shows dependence upon melanin biosynthesis. Fungal Genet. Biol. 28:190-200. [DOI] [PubMed] [Google Scholar]
- 104.Bryan, C. S., C. W. Smith, D. E. Berg, and R. B. Karp. 1993. Curvularia lunata endocarditis treated with terbinafine: case report. Clin. Infect. Dis. 16:30-32. [DOI] [PubMed] [Google Scholar]
- 105.Bryan, M. G., D. M. Elston, C. Hivnor, and B. A. Honl. 2000. Phaeohyphomycosis in a premature infant. Cutis 65:137-140. [PubMed] [Google Scholar]
- 106.Buchanan, W. E., M. J. Quinn, and J. A. Hasbargen. 1994. Peritoneal catheter colonization with Alternaria: successful treatment with catheter preservation. Perit. Dial. Int. 14:91-92. [PubMed] [Google Scholar]
- 107.Burns, K. E., N. P. Ohori, and A. T. Iacono. 2000. Dactylaria gallopava infection presenting as a pulmonary nodule in a single-lung transplant recipient. J. Heart Lung Transplant. 19:900-902. [DOI] [PubMed] [Google Scholar]
- 108.Bush, R. K., and J. J. Prochnau. 2004. Alternaria-induced asthma. J. Allergy Clin. Immunol. 113:227-234. [DOI] [PubMed] [Google Scholar]
- 109.Butler, M. J., and A. W. Day. 1998. Fungal melanins: a review. Can. J. Microbiol. 44:1115-1136. [Google Scholar]
- 110.Buxi, T. B., K. Prakash, R. Vohra, and D. Bhatia. 1996. Imaging in phaeohyphomycosis of the brain: case report. Neuroradiology 38:139-141. [DOI] [PubMed] [Google Scholar]
- 111.Caligiorne, R. B., M. A. Resende, P. H. Melillo, C. P. Peluso, F. H. Carmo, and V. Azevedo. 1999. In vitro susceptibility of chromoblastomycosis and phaeohyphomycosis agents to antifungal drugs. Med. Mycol. 37:405-409. [DOI] [PubMed] [Google Scholar]
- 112.Calista, D., M. Leardini, and F. Arcangeli. 2003. Subcutaneous Exophiala jeanselmei infection in a heart transplant patient. Eur. J. Dermatol. 13:489. [PubMed] [Google Scholar]
- 113.Calvo, E., F. J. Pastor, M. M. Rodriguez, E. Mayayo, V. Salas, and J. Guarro. 2010. Murine model of a disseminated infection by the novel fungus Fonsecaea monophora and successful treatment with posaconazole. Antimicrob. Agents Chemother. 54:919-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Campbell, C. K., and S. S. A. Al-Hedaithy. 1993. Phaeohyphomycosis of the brain caused by Ramichloridium mackenziei sp. nov. in Middle Eastern countries. J. Med. Vet. Mycol. 31:325-332. [Google Scholar]
- 115.Canon, H. L., S. C. Buckingham, R. J. Wyatt, and D. P. Jones. 2001. Fungal peritonitis caused by Curvularia species in a child undergoing peritoneal dialysis. Pediatr. Nephrol. 16:35-37. [DOI] [PubMed] [Google Scholar]
- 116.Capoor, M. R., G. Khanna, D. Nair, A. Hasan, Rajni, M. Deb, and P. Aggarwal. 2007. Eumycetoma pedis due to Exophiala jeanselmei. Indian J. Med. Microbiol. 25:155-157. [DOI] [PubMed] [Google Scholar]
- 117.Caporale, N. E., L. Calegari, D. Perez, and E. Gezuele. 1996. Peritoneal catheter colonization and peritonitis with Aureobasidium pullulans. Perit. Dial. Int. 16:97-98. [PubMed] [Google Scholar]
- 118.Cappelletty, D., and K. Eiselstein-McKitrick. 2007. The echinocandins. Pharmacotherapy 27:369-388. [DOI] [PubMed] [Google Scholar]
- 119.Carreter de Granda, M. E., C. Richard, E. Conde, A. Iriondo, F. Marco de Lucas, R. Salesa, and A. Zubizarreta. 2001. Endocarditis caused by Scedosporium prolificans after autologous peripheral blood stem cell transplantation. Eur. J. Clin. Microbiol. Infect. Dis. 20:215-217. [DOI] [PubMed] [Google Scholar]
- 120.Carrillo, A. J., and J. Guarro. 2001. In vitro activities of four novel triazoles against Scedosporium spp. Antimicrob. Agents Chemother. 45:2151-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Carter, E., and C. Boudreaux. 2004. Fatal cerebral phaeohyphomycosis due to Curvularia lunata in an immunocompetent patient. J. Clin. Microbiol. 42:5419-5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carzaniga, R., D. Fiocco, P. Bowyer, and R. J. O'Connell. 2002. Localization of melanin in conidia of Alternaria alternata using phage display antibodies. Mol. Plant Microbe Interact. 15:216-224. [DOI] [PubMed] [Google Scholar]
- 123.Casadevall, A., A. L. Rosas, and J. D. Nosanchuk. 2000. Melanin and virulence in Cryptococcus neoformans. Curr. Opin. Microbiol. 3:354-358. [DOI] [PubMed] [Google Scholar]
- 124.Castanet, J., J. P. Lacour, M. Toussaint-Gary, C. Perrin, S. Rodot, and J. P. Ortonne. 1995. Alternaria tenuissima plurifocal cutaneous infection. Ann. Dermatol. Venereol. 122:115-118. [PubMed] [Google Scholar]
- 125.Castelnuovo, P., F. De Bernardi, C. Cavanna, F. Pagella, P. Bossolesi, P. Marone, and C. Farina. 2004. Invasive fungal sinusitis due to Bipolaris hawaiiensis. Mycoses 47:76-81. [DOI] [PubMed] [Google Scholar]
- 126.Castro, L. G., E. R. Pimentel, and C. S. Lacaz. 2003. Treatment of chromomycosis by cryosurgery with liquid nitrogen: 15 years' experience. Int. J. Dermatol. 42:408-412. [DOI] [PubMed] [Google Scholar]
- 127.Castro, L. G., and J. Piquero-Casals. 2008. Clinical and mycologic findings and therapeutic outcome of 27 mycetoma patients from Sao Paulo, Brazil. Int. J. Dermatol. 47:160-163. [DOI] [PubMed] [Google Scholar]
- 128.Cateau, E., T. Mergey, C. Kauffmann-Lacroix, and M. H. Rodier. 2009. Relationships between free living amoebae and Exophiala dermatitidis: a preliminary study. Med. Mycol. 47:115-118. [DOI] [PubMed] [Google Scholar]
- 129.Celard, M., E. Dannaoui, M. A. Piens, E. Gueho, G. Kirkorian, T. Greenland, F. Vandenesch, and S. Picot. 1999. Early Microascus cinereus endocarditis of a prosthetic valve implanted after Staphylococcus aureus endocarditis of the native valve. Clin. Infect. Dis. 29:691-692. [DOI] [PubMed] [Google Scholar]
- 130.Centers for Disease Control and Prevention. 2009. Biosafety in microbiological and biomedical laboratories, 5th ed., p. 170-177. Centers for Disease Control and Prevention, Atlanta, GA.
- 131.Centers for Disease Control and Prevention. 2002. Exophiala infection from contaminated injectable steroids prepared by a compounding pharmacy—United States, July-November 2002. MMWR Morb. Mortal. Wkly. Rep. 51:1109-1112. [PubMed] [Google Scholar]
- 132.Cermeno-Vivas, J. R., J. M. Torres-Rodriguez, J. C. Fung-Tomc, E. Huczko, B. Minassian, and D. P. Bonner. 2001. In vitro susceptibility of dematiaceous fungi to ten antifungal drugs using an agar diffusion test. Rev. Iberoam. Micol. 18:113-117. [PubMed] [Google Scholar]
- 133.Chabasse, D., C. de Bievre, E. Legrand, J. P. Saint-Andre, L. de Gentile, B. Cimon, and J. P. Bouchara. 1995. Subcutaneous abscess caused by Pleurophomopsis lignicola Petr: first case. J. Med. Vet. Mycol. 33:415-417. [PubMed] [Google Scholar]
- 134.Chai, L. Y., M. G. Netea, J. Sugui, A. G. Vonk, W. W. van de Sande, A. Warris, K. J. Kwon-Chung, and B. J. Kullberg. 2009. Aspergillus fumigatus conidial melanin modulates host cytokine response. Immunobiology [Epub ahead of print.] doi: 10.1016/j.imbio.2009.10.002. [DOI] [PMC free article] [PubMed]
- 135.Chan, K. O., K. A. Genoway, and A. R. Javer. 2008. Effectiveness of itraconazole in the management of refractory allergic fungal rhinosinusitis. J. Otolaryngol. Head Neck Surg. 37:870-874. [PubMed] [Google Scholar]
- 136.Chander, J., N. Singla, N. Agnihotri, S. K. Arya, and A. Deep. 2008. Keratomycosis in and around Chandigarh: a five-year study from a north Indian tertiary care hospital. Indian J. Pathol. Microbiol. 51:304-306. [DOI] [PubMed] [Google Scholar]
- 137.Chandramukhi, A., M. G. Ramadevi, and S. K. Shankar. 1983. Cerebral cladosporiosis—a neuropathological and microbiological study. Clin. Neurol. Neurosurg. 85:245-253. [DOI] [PubMed] [Google Scholar]
- 138.Chang, C. L., D. S. Kim, D. J. Park, H. J. Kim, C. H. Lee, and J. H. Shin. 2000. Acute cerebral phaeohyphomycosis due to Wangiella dermatitidis accompanied by cerebrospinal fluid eosinophilia. J. Clin. Microbiol. 38:1965-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chang, X., R. Li, J. Yu, X. Bao, and J. Qin. 2009. Phaeohyphomycosis of the central nervous system caused by Exophiala dermatitidis in a 3-year-old immunocompetent host. J. Child Neurol. 24:342-345. [DOI] [PubMed] [Google Scholar]
- 140.Chen, Y. T., H. C. Lin, C. C. Huang, and Y. H. Lo. 2006. Cutaneous phaeohyphomycosis caused by an itraconazole and amphotericin B resistant strain of Veronaea botryosa. Int. J. Dermatol. 45:429-432. [DOI] [PubMed] [Google Scholar]
- 141.Cherian, R. S., M. Betty, M. T. Manipadam, V. M. Cherian, P. M. Poonnoose, A. T. Oommen, and R. A. Cherian. 2009. The “dot-in-circle” sign—a characteristic MRI finding in mycetoma foot: a report of three cases. Br. J. Radiol. 82:662-665. [DOI] [PubMed] [Google Scholar]
- 142.Chua, J. D., S. M. Gordon, J. Banbury, G. S. Hall, and G. W. Procop. 2001. Relapsing Exophiala jeanselmei phaeohyphomycosis in a lung-transplant patient. Transplant. Infect. Dis. 3:235-238. [DOI] [PubMed] [Google Scholar]
- 143.Chuan, M. T., and M. C. Wu. 1995. Subcutaneous phaeohyphomycosis caused by Exophiala jeanselmei: successful treatment with itraconazole. Int. J. Dermatol. 34:563-566. [DOI] [PubMed] [Google Scholar]
- 144.Clark, E. C., S. M. Silver, G. E. Hollick, and M. G. Rinaldi. 1995. Continuous ambulatory peritoneal dialysis complicated by Aureobasidium pullulans peritonitis. Am. J. Nephrol. 15:353-355. [DOI] [PubMed] [Google Scholar]
- 145.Cleary, J. D. 2009. Echinocandins: pharmacokinetic and therapeutic issues. Curr. Med. Res. Opin. 25:1741-1750. [PubMed] [Google Scholar]
- 146.Clinical Laboratory Standards Institute. 2008. Interpretive criteria for identification of bacteria and fungi by sequencing; approved guideline. CLSI MM18-A. Clinical Laboratory Standards Institute, Wayne, PA.
- 147.Clinical Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Revised M38-A2. Clinical Laboratory Standards Institute, Wayne, PA.
- 148.Clinical Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A3, 3rd ed. Clinical Laboratory Standards Institute, Wayne, PA.
- 149.Coldiron, B. M., E. L. Wiley, and M. G. Rinaldi. 1990. Cutaneous phaeohyphomycosis caused by a rare fungal pathogen, Hormonema dematioides: successful treatment with ketoconazole. J. Am. Acad. Dermatol. 23:363-367. [DOI] [PubMed] [Google Scholar]
- 150.Colton, R., A. Zeharia, B. Karmazyn, N. Buller, Y. Levy, R. Inbal, S. Zmira, and E. Yaniv. 2002. Exserohilum sinusitis presenting as proptosis in a healthy adolescent male. J. Adolesc. Health 30:73-75. [DOI] [PubMed] [Google Scholar]
- 151.Cooley, L., D. Spelman, K. Thursky, and M. Slavin. 2007. Infection with Scedosporium apiospermum and S. prolificans, Australia. Emerg. Infect. Dis. 13:1170-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Corrado, M. L., M. Kramer, M. Cummings, and R. H. Eng. 1982. Susceptibility of dematiaceous fungi to amphotericin B, miconazole, ketoconazole, flucytosine and rifampin alone and in combination. Sabouraudia 20:109-113. [DOI] [PubMed] [Google Scholar]
- 153.Cortez, K. J., E. Roilides, F. Quiroz-Telles, J. Meletiadis, C. Antachopoulos, T. Knudsen, W. Buchanan, J. Milanovich, D. A. Sutton, A. Fothergill, M. G. Rinaldi, Y. R. Shea, T. Zaoutis, S. Kottilil, and T. J. Walsh. 2008. Infections caused by Scedosporium spp. Clin. Microbiol. Rev. 21:157-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Costa, A. R., E. Porto, C. d. Lacaz, N. T. de Melo, M. d. Calux, and N. Y. Valente. 1988. Cutaneous and ungual phaeohyphomycosis caused by species of Chaetomium Kunze (1817) ex Fresenius, 1829. J. Med. Vet. Mycol. 26:261-268. [PubMed] [Google Scholar]
- 155.Cox, G. M., W. A. Schell, R. L. Scher, and J. R. Perfect. 1994. First report of involvement of Nodulisporium species in human disease. J. Clin. Microbiol. 32:2301-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Crichlow, D. K., F. T. Enrile, and M. Y. Memon. 1973. Cerebellar abscess due to Cladosporium trichoides (bantianum): case report. Am. J. Clin. Pathol. 60:416-421. [DOI] [PubMed] [Google Scholar]
- 157.Crous, P. W., U. Braun, K. Schubert, and J. Z. Groenewald. 2007. Delimiting Cladosporium from morphologically similar genera. Stud. Mycol. 58:33-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Crous, P. W., B. Slippers, M. J. Wingfield, J. Rheeder, W. F. Marasas, A. J. Philips, A. Alves, T. Burgess, P. Barber, and J. Z. Groenewald. 2006. Phylogenetic lineages in the Botryosphaeriaceae. Stud. Mycol. 55:235-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Crous, P. W., W. Gams, M. J. Wingfield, and P. S. van Wyk. 1996. Phaeoacremonium gen. nov. associated with wilt and decline disease of woody hosts and human infections. Mycologia 88:786-796. [Google Scholar]
- 160.Cuenca-Estrella, M., A. Alastruey-Izquierdo, L. Alcazar-Fuoli, L. Bernal-Martinez, A. Gomez-Lopez, M. J. Buitrago, E. Mellado, and J. L. Rodriguez-Tudela. 2008. In vitro activities of 35 double combinations of antifungal agents against Scedosporium apiospermum and Scedosporium prolificans. Antimicrob. Agents Chemother. 52:1136-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cuenca-Estrella, M., A. Gomez-Lopez, E. Mellado, M. J. Buitrago, A. Monzon, and J. L. Rodriguez-Tudela. 2006. Head-to-head comparison of the activities of currently available antifungal agents against 3,378 Spanish clinical isolates of yeasts and filamentous fungi. Antimicrob. Agents Chemother. 50:917-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cuenca-Estrella, M., A. Gomez-Lopez, E. Mellado, G. Garcia-Effron, A. Monzon, and J. L. Rodriguez-Tudela. 2005. In vitro activity of ravuconazole against 923 clinical isolates of nondermatophyte filamentous fungi. Antimicrob. Agents Chemother. 49:5136-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cuenca-Estrella, M., B. Ruiz-Diez, J. V. Martinez-Suarez, A. Monzon, and J. L. Rodriguez-Tudela. 1999. Comparative in-vitro activity of voriconazole (UK-109,496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J. Antimicrob. Chemother. 43:149-151. [DOI] [PubMed] [Google Scholar]
- 164.Cuetara, M. S., A. Alhambra, M. D. Moragues, E. Gonzalez-Elorza, J. Ponton, and A. del Palacio. 2009. Detection of (1,3)-beta-d-glucan as an adjunct to diagnosis in a mixed population with uncommon proven invasive fungal diseases or with an unusual clinical presentation. Clin. Vaccine Immunol. 16:423-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dadachova, E., and A. Casadevall. 2008. Ionizing radiation: how fungi cope, adapt, and exploit with the help of melanin. Curr. Opin. Microbiol. 11:525-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dalton, P. A., W. J. Munckhof, and D. W. Walters. 2006. Scedosporium prolificans: an uncommon cause of septic arthritis. Aust. N. Z. J. Surg. 76:661-663. [DOI] [PubMed] [Google Scholar]
- 167.Dan, M., O. Yossepowitch, D. Hendel, O. Shwartz, and D. A. Sutton. 2006. Phialemonium curvatum arthritis of the knee following intra-articular injection of a corticosteroid. Med. Mycol. 44:571-574. [DOI] [PubMed] [Google Scholar]
- 168.Dar, L., S. Singh, U. Banerjee, A. Verma, and R. Bhatia. 1993. Brain abscess due to Xylohypha bantiana. Indian J. Med. Microbiol. 11:148-150. [Google Scholar]
- 169.Dastur, H. M., A. P. Chaukar, and M. D. Rebello. 1966. Cerebral chromoblastomycosis due to Cladosporium trichoides (bantianum). I. A review and case report. Neurol. India 14:1-5. [PubMed] [Google Scholar]
- 170.Deb, S., A. K. Khan, B. Debasish, and B. Subroto. 2005. Intracranial necrotizing granuloma caused by Cladophialophora bantiana. Neurol. India 53:335-336. [DOI] [PubMed] [Google Scholar]
- 171.De Battle, J., M. Motje, R. Balanza, R. Guardia, and R. Ortiz. 2000. Disseminated infection caused by Scedosporium prolificans in a patient with multilineal leukemia. J. Clin. Microbiol. 38:1694-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.De Hoog, G. S., D. Adelmann, A. O. Ahmed, and A. van Belkum. 2004. Phylogeny and typification of Madurella mycetomatis, with a comparison of other agents of eumycetoma. Mycoses 47:121-130. [DOI] [PubMed] [Google Scholar]
- 173.De Hoog, G. S., and N. A. Yurlova. 1994. Conidiogenesis, nutritional physiology and taxonomy of Aureobasidium and Hormonema. Antonie Van Leeuwenhoek 65:41-54. [DOI] [PubMed] [Google Scholar]
- 174.De Hoog, G. S., D. Attili-Angelis, V. A. Vicente, A. H. Van Den Ende, and F. Queiroz-Telles. 2004. Molecular ecology and pathogenic potential of Fonsecaea species. Med. Mycol. 42:405-416. [DOI] [PubMed] [Google Scholar]
- 175.De Hoog, G. S., J. Guarro, J. Gene, and M. J. Figueras. 2000. Atlas of clinical fungi. Centraalbureau voor Schimmelcultures, Utrecht, Netherlands.
- 176.De Hoog, G. S., and R. Horre. 2002. Molecular taxonomy of the Alternaria and Ulocladium species from humans and their identification in the routine laboratory. Mycoses 45:259-276. [DOI] [PubMed] [Google Scholar]
- 177.De Hoog, G. S., J. Guarro, J. Gené, and M. J. Fígueras. 2009. Atlas of clinical fungi, pilot version of 3rd ed., CD-ROM. Centraalbureau voor Schimmelcultures, Baarn, Netherlands.
- 178.De Hoog, G. S., T. Matos, M. Sudhadham, K. F. Luijsterburg, and G. Haase. 2005. Intestinal prevalence of the neurotropic black yeast Exophiala (Wangiella) dermatitidis in healthy and impaired individuals. Mycoses 48:142-145. [DOI] [PubMed] [Google Scholar]
- 179.De Hoog, G. S., P. Mayser, G. Haase, R. Horre, and A. M. Horrevorts. 2000. A new species, Phialophora europaea, causing superficial infections in humans. Mycoses 43:409-416. [DOI] [PubMed] [Google Scholar]
- 180.De Hoog, G. S., N. Poonwan, and A. H. G. Gerrits van den Ende. 1999. Taxonomy of Exophiala spinifera and its relationship to E. jeanselmei. Stud. Mycol. 43:133-142. [Google Scholar]
- 181.De Hoog, G. S., A. S. Nishikaku, G. Fernandez-Zeppenfeldt, C. Padin-Gonzalez, E. Burger, H. Badali, N. Richard-Yegres, and A. H. van den Ende. 2007. Molecular analysis and pathogenicity of the Cladophialophora carrionii complex, with the description of a novel species. Stud. Mycol. 58:219-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.De Hoog, G. S., K. Takeo, E. Gottlich, K. Nishimura, and M. Miyaji. 1995. A human isolate of Exophiala (Wangiella) dermatitidis forming a catenate synanamorph that links the genera Exophiala and Cladophialophora. J. Med. Vet. Mycol. 33:355-358. [PubMed] [Google Scholar]
- 183.De Hoog, G. S., K. Takeo, S. Yoshida, E. Gottlich, K. Nishimura, and M. Miyaji. 1994. Pleoanamorphic life cycle of Exophiala (Wangiella) dermatitidis. Antonie Van Leeuwenhoek 65:143-153. [DOI] [PubMed] [Google Scholar]
- 184.De Hoog, G. S., V. Vicente, R. B. Caligiorne, S. Kantarcioglu, K. Tintelnot, A. H. Gerrits van den Ende, and G. Haase. 2003. Species diversity and polymorphism in the Exophiala spinifera clade containing opportunistic black yeast-like fungi. J. Clin. Microbiol. 41:4767-4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.De Hoog, G. S., X. O. Weenink, and A. H. G. Gerrits van den Ende. 1999. Taxonomy of the Phialophora verrucosa complex with the description of four new species. Stud. Mycol. 43:107-142. [Google Scholar]
- 186.De Hoog, G. S., and A. H. G. Gerrits van den Ende. 1992. Nutritional pattern and ecophysiology of Hortae werneckii, agent of human tinea nigra. Antonie Van Leeuwenhoek 62:321-329. [DOI] [PubMed] [Google Scholar]
- 187.De Hoog, G. S., and R. G. Vitale. 2007. Bipolaris, Exophiala, Scedosporium, Sporothrix, and other dematiaceous fungi, p. 1898-1917. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 188.De Lastours, V., A. Lefort, M. Zappa, V. Dufour, N. Belmatoug, and B. Fantin. 2003. Two cases of cerebral aspergillosis successfully treated with voriconazole. Eur. J. Clin. Microbiol. Infect. Dis. 22:297-299. [DOI] [PubMed] [Google Scholar]
- 189.Delfino, D., S. De Hoog, L. Polonelli, M. Benecchi, F. Fanti, S. Galatioto, G. Manti, and V. Cusumano. 2006. Survival of a neglected case of brain abscess caused by Cladophialophora bantiana. Med. Mycol. 44:651-654. [DOI] [PubMed] [Google Scholar]
- 190.Delhaes, L., A. Harun, S. C. Chen, Q. Nguyen, M. Slavin, C. H. Heath, K. Maszewska, C. Halliday, V. Robert, T. C. Sorrell, T. A. Auscedo Study Group, and W. Meyer. 2008. Molecular typing of Australian Scedosporium isolates showing genetic variability and numerous S. aurantiacum. Emerg. Infect. Dis. 14:282-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Del Palacio, A., C. Gomez-Hernando, F. Revenga, E. Carabias, A. Gonzalez, M. S. Cuetara, and E. M. Johnson. 1996. Cutaneous Alternaria alternata infection successfully treated with itraconazole. Clin. Exp. Dermatol. 21:241-243. [DOI] [PubMed] [Google Scholar]
- 192.Del Palacio, H. A., J. M. Conde-Zurita, P. S. Reyes, and N. A. Rodriguez. 1983. A case of Alternaria alternata (Fr.) Keissler infection of the knee. Clin. Exp. Dermatol. 8:641-646. [DOI] [PubMed] [Google Scholar]
- 193.Del Palacio-Hernanz, A., M. K. Moore, C. K. Campbell, A. del Palacio-Perez-Medel, and R. del Castillo-Cantero. 1989. Infection of the central nervous system by Rhinocladiella atrovirens in a patient with acquired immunodeficiency syndrome. J. Med. Vet. Mycol. 27:127-130. [PubMed] [Google Scholar]
- 194.De Monbrison, F., M. A. Piens, B. Ample, S. Euvrard, P. Cochat, and S. Picot. 2004. Two cases of subcutaneous phaeohyphomycosis due to Exophiala jeanselmei, in cardiac transplant and renal transplant patients. Br. J. Dermatol. 150:597-598. [DOI] [PubMed] [Google Scholar]
- 195.De Moragas, J. M., G. Prats, and G. Verger. 1981. Cutaneous alternariosis treated with miconazole. Arch. Dermatol. 117:292-294. [DOI] [PubMed] [Google Scholar]
- 196.Destino, L., D. A. Sutton, A. L. Helon, P. L. Havens, J. G. Thometz, R. E. Willoughby, Jr., and M. J. Chusid. 2006. Severe osteomyelitis caused by Myceliophthora thermophila after a pitchfork injury. Ann. Clin. Microbiol. Antimicrob. 5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.DeVault, G. A., Jr., S. T. Brown III, J. W. King, M. Fowler, and A. Oberle. 1985. Tenckhoff catheter obstruction resulting from invasion by Curvularia lunata in the absence of peritonitis. Am. J. Kidney Dis. 6:124-127. [DOI] [PubMed] [Google Scholar]
- 198.Diaz, M., R. Puente, and M. A. Trevino. 1990. Response of long-running Alternaria alternata infection to fluconazole. Lancet 336:513. [DOI] [PubMed] [Google Scholar]
- 199.Diemert, D., D. Kunimoto, C. Sand, and R. Rennie. 2001. Sputum isolation of Wangiella dermatitidis in patients with cystic fibrosis. Scand. J. Infect. Dis. 33:777-779. [DOI] [PubMed] [Google Scholar]
- 200.Dixon, D. M., W. G. Merz, H. L. Elliott, and S. Macleay. 1987. Experimental central nervous system phaeohyphomycosis following intranasal inoculation of Xylohypha bantiana in cortisone-treated mice. Mycopathologia 100:145-153. [DOI] [PubMed] [Google Scholar]
- 201.Dixon, D. M., J. Migliozzi, C. R. Cooper, Jr., O. Solis, B. Breslin, and P. J. Szaniszlo. 1992. Melanized and non-melanized multicellular form mutants of Wangiella dermatitidis in mice: mortality and histopathology studies. Mycoses 35:17-21. [DOI] [PubMed] [Google Scholar]
- 202.Dixon, D. M., and A. Polak. 1987. In vitro and in vivo drug studies with three agents of central nervous system phaeohyphomycosis. Chemotherapy 33:129-140. [DOI] [PubMed] [Google Scholar]
- 203.Dixon, D. M., A. Polak, and P. J. Szaniszlo. 1987. Pathogenicity and virulence of wild-type and melanin-deficient Wangiella dermatitidis. J. Med. Vet. Mycol. 25:97-106. [DOI] [PubMed] [Google Scholar]
- 204.Dixon, D. M., T. J. Walsh, W. G. Merz, and M. R. McGinnis. 1989. Infections due to Xylohypha bantiana (Cladosporium trichoides). Rev. Infect. Dis. 11:515-525. [DOI] [PubMed] [Google Scholar]
- 205.Doering, T. L., J. D. Nosanchuk, W. K. Roberts, and A. Casadevall. 1999. Melanin as a potential cryptococcal defence against microbicidal proteins. Med. Mycol. 37:175-181. [PubMed] [Google Scholar]
- 206.Dooley, D. P., M. L. Beckius, B. S. Jeffery, C. K. McAllister, W. H. Radentz, A. R. Feldman, M. G. Rinaldi, S. R. Bailey, and J. H. Keeling. 1989. Phaeohyphomycotic cutaneous disease caused by Pleurophoma in a cardiac transplant patient. J. Infect. Dis. 159:503-507. [DOI] [PubMed] [Google Scholar]
- 207.Drees, M., B. L. Wickes, M. Gupta, and S. Hadley. 2007. Lecythophora mutabilis prosthetic valve endocarditis in a diabetic patient. Med. Mycol. 45:463-467. [DOI] [PubMed] [Google Scholar]
- 208.Drouhet, E., and B. Dupont. 1983. Laboratory and clinical assessment of ketoconazole in deep-seated mycoses. Am. J. Med. 74:30-47. [DOI] [PubMed] [Google Scholar]
- 209.Duggan, J. M., M. D. Wolf, and C. A. Kauffman. 1995. Phialophora verrucosa infection in an AIDS patient. Mycoses 38:215-218. [DOI] [PubMed] [Google Scholar]
- 210.Dunlop, A. A., E. D. Wright, S. A. Howlader, I. Nazrul, R. Husain, K. McClellan, and F. A. Billson. 1994. Suppurative corneal ulceration in Bangladesh. A study of 142 cases examining the microbiological diagnosis, clinical and epidemiological features of bacterial and fungal keratitis. Aust. N. Z. J. Ophthalmol. 22:105-110. [DOI] [PubMed] [Google Scholar]
- 211.Dupuis, A., N. Tournier, G. Le Moal, and N. Venisse. 2009. Preparation and stability of voriconazole eye drop solution. Antimicrob. Agents Chemother. 53:798-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Duque, O. 1961. Meningo-encephalitis and brain abscess caused by Cladosporium and Fonsecaea. Am. J. Clin. Pathol. 36:505-517. [DOI] [PubMed] [Google Scholar]
- 213.Durkin, S. R., T. Henderson, R. Raju, and D. Ellis. 2008. Successful treatment of phaeohyphomycotic keratitis caused by Bipolaris australiensis. Clin. Exp. Ophthalmol. 36:697-699. [DOI] [PubMed] [Google Scholar]
- 214.Dutriaux, C., I. Saint-Cyr, N. Desbois, D. Cales-Quist, A. Diedhou, and A. M. Boisseau-Garsaud. 2005. Subcutaneous phaeohyphomycosis due to Exophiala spinifera in a renal transplant recipient. Ann. Dermatol. Venereol. 132:259-262. [DOI] [PubMed] [Google Scholar]
- 215.Ebright, J. R., P. H. Chandrasekar, S. Marks, M. R. Fairfax, A. Aneziokoro, and M. R. McGinnis. 1999. Invasive sinusitis and cerebritis due to Curvularia clavata in an immunocompetent adult. Clin. Infect. Dis. 28:687-689. [DOI] [PubMed] [Google Scholar]
- 216.El-Ani, A. S. 1966. A new species of Leptosphaeria, an etiologic agent of mycetoma. Mycologia 58:406-411. [PubMed] [Google Scholar]
- 217.El-Ani, A. S., and M. A. Gordon. 1965. The ascospore sheath and taxonomy of Leptosphaeria senegalensis. Mycologia 57:275-278. [PubMed] [Google Scholar]
- 218.Elewski, B. E. 1996. Onychomycosis caused by Scytalidium dimidiatum. J. Am. Acad. Dermatol. 35:336-338. [DOI] [PubMed] [Google Scholar]
- 219.Elinav, H., U. Izhar, S. Benenson, D. Admon, C. Hidalgo-Grass, I. Polacheck, and M. Korem. 2009. Invasive Scytalidium dimidiatum infection in an immunocompetent adult. J. Clin. Microbiol. 47:1259-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ellis, M. B. 1971. Dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew, United Kingdom.
- 221.Ellis, M. B. 1976. More dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew, United Kingdom.
- 222.Emmens, R. K., D. Richardson, W. Thomas, S. Hunter, R. A. Hennigar, J. R. Wingard, and F. S. Nolte. 1996. Necrotizing cerebritis in an allogeneic bone marrow transplant recipient due to Cladophialophora bantiana. J. Clin. Microbiol. 34:1330-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Erwin, G. E., and J. E. Fitzgerald. 2007. Case report: allergic bronchopulmonary aspergillosis and allergic fungal sinusitis successfully treated with voriconazole. J. Asthma 44:891-895. [DOI] [PubMed] [Google Scholar]
- 224.Espinel-Ingroff, A. 1998. Comparison of In vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Espinel-Ingroff, A. 2003. In vitro antifungal activities of anidulafungin and micafungin, licensed agents and the investigational triazole posaconazole as determined by NCCLS methods for 12,052 fungal isolates: review of the literature. Rev. Iberoam. Micol. 20:121-136. [PubMed] [Google Scholar]
- 226.Espinel-Ingroff, A. 2001. In vitro fungicidal activities of voriconazole, itraconazole, and amphotericin B against opportunistic moniliaceous and dematiaceous fungi. J. Clin. Microbiol. 39:954-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Espinel-Ingroff, A., K. Boyle, and D. J. Sheehan. 2001. In vitro antifungal activities of voriconazole and reference agents as determined by NCCLS methods: review of the literature. Mycopathologia 150:101-115. [DOI] [PubMed] [Google Scholar]
- 228.Espinel-Ingroff, A., E. Johnson, H. Hockey, and P. Troke. 2008. Activities of voriconazole, itraconazole and amphotericin B in vitro against 590 moulds from 323 patients in the voriconazole phase III clinical studies. J. Antimicrob. Chemother. 61:616-620. [DOI] [PubMed] [Google Scholar]
- 229.Esterre, P., A. Andriantsimahavandy, E. R. Ramarcel, and J. L. Pecarrere. 1996. Forty years of chromoblastomycosis in Madagascar: a review. Am. J. Trop. Med. Hyg. 55:45-47. [DOI] [PubMed] [Google Scholar]
- 230.Estes, S. A., W. G. Merz, and L. G. Maxwell. 1977. Primary cutaneous phaeohyphomycosis caused by Drechslera spicifera. Arch. Dermatol. 113:813-815. [PubMed] [Google Scholar]
- 231.European Economic Community. 2000. Directive 2000/54/EC, p. 19. In Official journal of the European Communities. Office for Official Publications of the European Communities, Luxembourg.
- 232.Fader, R. C., and M. R. McGinnis. 1988. Infections caused by dematiaceous fungi: chromoblastomycosis and phaeohyphomycosis. Infect. Dis. Clin. N. Am. 2:925-938. [PubMed] [Google Scholar]
- 233.Fan, Y. M., W. M. Huang, S. F. Li, G. F. Wu, W. Li, and R. Y. Chen. 2009. Cutaneous phaeohyphomycosis of foot caused by Curvularia clavata. Mycoses 52:544-546. [DOI] [PubMed] [Google Scholar]
- 234.Farina, C., A. Gamba, R. Tambini, H. Beguin, and J. L. Trouillet. 1998. Fatal aortic Myceliophthora thermophila infection in a patient affected by cystic medial necrosis. Med. Mycol. 36:113-118. [PubMed] [Google Scholar]
- 235.Farina, C., E. Gotti, D. Mouniee, P. Boiron, and A. Goglio. 2007. Phaeoacremonium parasiticum subcutaneous infection in a kidney-transplanted patient successfully treated by surgery. Transpl. Infect. Dis. 9:253-255. [DOI] [PubMed] [Google Scholar]
- 236.Farina, C., E. Gotti, A. Parma, L. Naldi, and A. Goglio. 2007. Pheohyphomycotic soft tissue disease caused by Alternaria alternata in a kidney transplant patient: a case report and literature review. Transplant. Proc. 39:1655-1659. [DOI] [PubMed] [Google Scholar]
- 237.Feldman, D. L., E. Fitzpatrick, O. Schaening, and L. I. Lutwick. 1995. Nosocomial phaeomycotic cyst of the hand. Ann. Plastic Surg. 35:113-115. [DOI] [PubMed] [Google Scholar]
- 238.Feltkamp, M. C., M. J. Kersten, J. van der Lelie, J. D. Burggraaf, G. S. de Hoog, and E. J. Kuijper. 1997. Fatal Scedosporium prolificans infection in a leukemic patient. Eur. J. Clin. Microbiol. Infect. Dis. 16:460-464. [DOI] [PubMed] [Google Scholar]
- 239.Ferguson, B. J. 2000. Definitions of fungal rhinosinusitis. Otolaryngol. Clin. N. Am. 33:227-235. [DOI] [PubMed] [Google Scholar]
- 240.Ferguson, B. J., L. Barnes, J. M. Bernstein, D. Brown, C. E. Clark III, P. R. Cook, W. S. DeWitt, S. M. Graham, B. Gordon, A. R. Javer, J. H. Krouse, F. A. Kuhn, H. L. Levine, S. C. Manning, B. F. Marple, A. H. Morgan, J. D. Osguthorpe, D. Skedros, B. M. Rains III, H. H. Ramadan, J. E. Terrell, and A. J. Yonkers. 2000. Geographic variation in allergic fungal rhinosinusitis. Otolaryngol. Clin. N. Am. 33:441-449. [DOI] [PubMed] [Google Scholar]
- 241.Fernandez, M., D. E. Noyola, S. N. Rossmann, and M. S. Edwards. 1999. Cutaneous phaeohyphomycosis caused by Curvularia lunata and a review of Curvularia infections in pediatrics. Pediatr. Infect. Dis. J. 18:727-731. [DOI] [PubMed] [Google Scholar]
- 242.Ferraro, F. A., and M. A. Morgan. 1989. A case of disseminated Phialophora parasitica infection. Arch. Pathol. Lab. Med. 113:1379-1381. [PubMed] [Google Scholar]
- 243.Fica, A., M. C. Diaz, M. Luppi, R. Olivares, L. Saez, M. Baboor, and P. Vasquez. 2003. Unsuccessful treatment with voriconazole of a brain abscess due to Cladophialophora bantiana. Scand. J. Infect. Dis. 35:892-893. [DOI] [PubMed] [Google Scholar]
- 244.Filizzola, M. J., F. Martinez, and S. J. Rauf. 2003. Phaeohyphomycosis of the central nervous system in immunocompetent hosts: report of a case and review of the literature. Int. J. Infect. Dis. 7:282-286. [DOI] [PubMed] [Google Scholar]
- 245.Fincher, R. M., J. F. Fisher, A. A. Padhye, L. Ajello, and J. C. Steele, Jr. 1988. Subcutaneous phaeohyphomycotic abscess caused by Phialophora parasitica in a renal allograft recipient. J. Med. Vet. Mycol. 26:311-314. [PubMed] [Google Scholar]
- 246.Flanagan, K. L., and A. D. Bryceson. 1997. Disseminated infection due to Bipolaris australiensis in a young immunocompetent man: case report and review. Clin. Infect. Dis. 25:311-313. [DOI] [PubMed] [Google Scholar]
- 247.Florcruz, N. V., and I. Peczon, Jr. 2008. Medical interventions for fungal keratitis. Cochrane Database Syst. Rev. 23:CD004241. [DOI] [PubMed] [Google Scholar]
- 248.Flynn, B. J., P. P. Bourbeau, P. J. Cera, L. M. Scicchitano, R. L. Jordan, and W. T. Yap. 1999. Phaeohyphomycosis of the epididymis caused by Exophiala jeanselmei. J. Urol. 162:492-493. [PubMed] [Google Scholar]
- 249.Fogarty, R. V., and J. M. Tobin. 1996. Fungal melanins and their interactions with metals. Enzyme Microb. Technol. 19:311-317. [DOI] [PubMed] [Google Scholar]
- 250.Fothergill, A. W., M. G. Rinaldi, and D. A. Sutton. 2009. Antifungal susceptibility testing of Exophiala spp.: a head-to-head comparison of amphotericin B, itraconazole, posaconazole and voriconazole. Med. Mycol. 47:41-43. [DOI] [PubMed] [Google Scholar]
- 251.Foulet, F., C. Duvoux, C. de Bievre, C. Hezode, and S. Bretagne. 1999. Cutaneous phaeohyphomycosis caused by Veronaea bothryosa in a liver transplant recipient successfully treated with itraconazole. Clin. Infect. Dis. 29:689-690. [DOI] [PubMed] [Google Scholar]
- 252.Francis, P., and T. J. Walsh. 1992. Evolving role of flucytosine in immunocompromised patients: new insights into safety, pharmacokinetics, and antifungal therapy. Clin. Infect. Dis. 15:1003-1018. [DOI] [PubMed] [Google Scholar]
- 253.Franzen, A. J., M. M. Cunha, K. Miranda, J. Hentschel, H. Plattner, M. B. da Silva, C. G. Salgado, W. de Souza, and S. Rozental. 2008. Ultrastructural characterization of melanosomes of the human pathogenic fungus Fonsecaea pedrosoi. J. Struct. Biol. 162:75-84. [DOI] [PubMed] [Google Scholar]
- 254.Freitas, A., D. B. Pedral-Sampaio, N. L. Espinheira, E. Daltro, J. Sampaio, P. Athanasio, C. Lacaz, and R. Badaro. 1997. Cladophialophora bantiana (previously Cladosporium trichoides): first report of a case in Brazil. Braz. J. Infect. Dis. 1:313-316. [PubMed] [Google Scholar]
- 255.Friedman, A. D., J. M. Campos, L. B. Rorke, D. A. Bruce, and A. M. Arbeter. 1981. Fatal recurrent Curvularia brain abscess. J. Pediatr. 99:413-415. [DOI] [PubMed] [Google Scholar]
- 256.Fukushima, N., K. Mannen, S. Okamoto, T. Shinogi, K. Nishimoto, and E. Sueoka. 2005. Disseminated Ochroconis gallopavum infection in a chronic lymphocytic leukemia: a case report and review of the literature on hematological malignancies. Intern. Med. 44:879-882. [DOI] [PubMed] [Google Scholar]
- 257.Fukushiro, R. 1983. Chromomycosis in Japan. Int. J. Dermatol. 22:221-229. [DOI] [PubMed] [Google Scholar]
- 258.Fukushiro, R., S. Udagawa, Y. Kawashima, and Y. Kawamura. 1986. Subcutaneous abscesses caused by Ochroconis gallopavum. J. Med. Vet. Mycol. 24:175-182. [PubMed] [Google Scholar]
- 259.Fung-Tomc, J. C., E. Huczko, B. Minassian, and D. P. Bonner. 1998. In vitro activity of a new oral triazole, BMS-207147 (ER-30346). Antimicrob. Agents Chemother. 42:313-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Fuste, F. J., L. Ajello, R. Threlkeld, and J. E. Henry, Jr. 1973. Drechslera hawaiiensis: causative agent of a fatal fungal meningo-encephalitis. Sabouraudia 11:59-63. [DOI] [PubMed] [Google Scholar]
- 261.Gadallah, M. F., R. White, M. A. el Shahawy, F. Abreo, A. Oberle, and J. Work. 1995. Peritoneal dialysis complicated by Bipolaris hawaiiensis peritonitis: successful therapy with catheter removal and oral itraconazol without the use of amphotericin-B. Am. J. Nephrol. 15:348-352. [DOI] [PubMed] [Google Scholar]
- 262.Gallelli, B., M. Viviani, M. Nebuloni, A. V. Marzano, C. Pozzi, P. Messa, and G. B. Fogazzi. 2006. Skin infection due to Alternaria species in kidney allograft recipients: report of a new case and review of the literature. J. Nephrol. 19:668-672. [PubMed] [Google Scholar]
- 263.Gams, W. 2000. Phialophora and some similar morphologically little-differentiated anamorphs of divergent ascomycetes. Stud. Mycol. 45:187-199. [Google Scholar]
- 264.Gams, W., and M. R. McGinnis. 1983. Phialemonium, a new anamorph genus intermediate between Phialophora and Acremonium. Mycologia 75:977-987. [Google Scholar]
- 265.Garau, J., R. D. Diamond, L. B. Lagrotteria, and S. A. Kabins. 1977. Alternaria osteomyelitis. Ann. Intern. Med. 86:747-748. [DOI] [PubMed] [Google Scholar]
- 266.Garcia-Effron, G., A. Gomez-Lopez, E. Mellado, A. Monzon, J. L. Rodriguez-Tudela, and M. Cuenca-Estrella. 2004. In vitro activity of terbinafine against medically important non-dermatophyte species of filamentous fungi. J. Antimicrob. Chemother. 53:1086-1089. [DOI] [PubMed] [Google Scholar]
- 267.Garcia-Ruiz, J. C., E. Amutio, I. Hernandez, C. Alvarez, F. Floristan, I. Zuazua, A. Alvarez-Blanco, and J. Ponton. 1998. Clinical resolution of Scedosporium prolificans pneumonia associated with treatment with liposomal amphotericin B in a patient with acute leukemia. Rev. Iberoam. Micol. 15:158-159. [PubMed] [Google Scholar]
- 268.Garg, N., I. B. Devi, G. V. Vajramani, S. Nagarathna, S. Sampath, B. A. Chandramouli, A. Chandramuki, and S. K. Shankar. 2007. Central nervous system cladosporiosis: an account of ten culture-proven cases. Neurol. India 55:282-288. [DOI] [PubMed] [Google Scholar]
- 269.Garg, P., U. Gopinathan, K. Choudhary, and G. N. Rao. 2000. Keratomycosis: clinical and microbiologic experience with dematiaceous fungi. Ophthalmology 107:574-580. [DOI] [PubMed] [Google Scholar]
- 270.Garg, P., G. K. Vemuganti, S. Chatarjee, U. Gopinathan, and G. N. Rao. 2004. Pigmented plaque presentation of dematiaceous fungal keratitis: a clinicopathologic correlation. Cornea 23:571-576. [DOI] [PubMed] [Google Scholar]
- 271.Garrison, A. P., G. W. Procop, V. Vincek, J. Moon, M. I. Morris, S. Doblecki-Lewis, T. J. Cleary, D. Brust, and I. Rosa-Cunha. 2008. A case of subcutaneous Mycoleptodiscus indicus infection in a liver transplant recipient successfully treated with antifungal therapy. Transpl. Infect. Dis. 10:218-220. [DOI] [PubMed] [Google Scholar]
- 272.Garzoni, C., L. Markham, P. Bijlenga, and J. Garbino. 2008. Cladophialophora bantiana: a rare cause of fungal brain abscess. Clinical aspects and new therapeutic options. Med. Mycol. 46:481-486. [DOI] [PubMed] [Google Scholar]
- 273.Gavin, P. J., D. A. Sutton, and B. Z. Katz. 2002. Fatal endocarditis in a neonate caused by the dematiaceous fungus Phialemonium obovatum: case report and review of the literature. J. Clin. Microbiol. 40:2207-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Geis, P. A., M. H. Wheeler, and P. J. Szaniszlo. 1984. Pentaketide metabolites of melanin synthesis in the dematiaceous fungus Wangiella dermatitidis. Arch. Microbiol. 137:324-328. [DOI] [PubMed] [Google Scholar]
- 275.Gene, J., A. Azon-Masoliver, J. Guarro, F. Ballester, I. Pujol, M. Llovera, and C. Ferrer. 1995. Cutaneous phaeohyphomycosis caused by Alternaria longipes in an immunosuppressed patient. J. Clin. Microbiol. 33:2774-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.George, I. A., M. S. Mathews, R. Karthik, L. John, A. Sundar, O. C. Abraham, and V. Joseph. 2008. Fatal cerebral abscess caused by Cladophialophora bantiana. J. Assoc. Physicians India 56:470-472. [PubMed] [Google Scholar]
- 277.Gerdsen, R., M. Uerlich, G. S. De Hoog, T. Bieber, and R. Horre. 2001. Sporotrichoid phaeohyphomycosis due to Alternaria infectoria. Br. J. Dermatol. 145:484-486. [DOI] [PubMed] [Google Scholar]
- 278.Gergen, P. J., P. C. Turkeltaub, and M. G. Kovar. 1987. The prevalence of allergic skin test reactivity to eight common aeroallergens in the U.S. population: results from the second National Health and Nutrition Examination Survey. J. Allergy Clin. Immunol. 80:669-679. [DOI] [PubMed] [Google Scholar]
- 279.Gerrits van den Ende, A. H. G., and G. S. de Hoog. 1999. Variability and molecular diagnostics of the neurotropic species Cladophialophora bantiana. Stud. Mycol. 43:151-162. [Google Scholar]
- 280.Gilaberte, M., R. Bartralot, J. M. Torres, F. S. Reus, V. Rodriguez, A. Alomar, and R. M. Pujol. 2005. Cutaneous alternariosis in transplant recipients: clinicopathologic review of 9 cases. J. Am. Acad. Dermatol. 52:653-659. [DOI] [PubMed] [Google Scholar]
- 281.Gilgado, F., J. Cano, J. Gene, and J. Guarro. 2005. Molecular phylogeny of the Pseudallescheria boydii species complex: proposal of two new species. J. Clin. Microbiol. 43:4930-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Gilgado, F., J. Cano, J. Gene, D. A. Sutton, and J. Guarro. 2008. Molecular and phenotypic data supporting distinct species statuses for Scedosporium apiospermum and Pseudallescheria boydii and the proposed new species Scedosporium dehoogii. J. Clin. Microbiol. 46:766-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Gilgado, F., J. Gene, J. Cano, and J. Guarro. 2010. Heterothallism in Scedosporium apiospermum and description of its teleomorph Pseudallescheria apiosperma sp. nov. Med. Mycol. 48:122-128. [DOI] [PubMed] [Google Scholar]
- 284.Gil-Lamaignere, C., A. Maloukou, J. L. Rodriguez-Tudela, and E. Roilides. 2001. Human phagocytic cell responses to Scedosporium prolificans. Med. Mycol. 39:169-175. [DOI] [PubMed] [Google Scholar]
- 285.Gil-Lamaignere, C., E. Roilides, J. Mosquera, A. Maloukou, and T. J. Walsh. 2002. Antifungal triazoles and polymorphonuclear leukocytes synergize to cause increased hyphal damage to Scedosporium prolificans and Scedosporium apiospermum. Antimicrob. Agents Chemother. 46:2234-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Gilmour, T. K., E. Rytina, P. B. O'Connell, and J. C. Sterling. 2001. Cutaneous alternariosis in a cardiac transplant recipient. Australas. J. Dermatol. 42:46-49. [DOI] [PubMed] [Google Scholar]
- 287.Ginarte, M., M. Pereiro, Jr., V. Fernandez-Redondo, and J. Toribio. 1996. Plantar infection by Scopulariopsis brevicaulis. Dermatology 193:149-151. [DOI] [PubMed] [Google Scholar]
- 288.Girardi, L. S., R. Malowitz, G. T. Tortora, and E. D. Spitzer. 1993. Aureobasidium pullulans septicemia. Clin. Infect. Dis. 16:338-339. [DOI] [PubMed] [Google Scholar]
- 289.Godfrey, J. 1846. Diseases of the foot not hitherto described. Lancet i:593-594. [Google Scholar]
- 290.Goel, A., A. Satoskar, A. P. Desai, and S. K. Pandya. 1992. Brain abscess caused by Cladosporium trichoides. Br. J. Neurosurg. 6:591-593. [DOI] [PubMed] [Google Scholar]
- 291.Gold, W. L., H. Vellend, I. E. Salit, I. Campbell, R. Summerbell, M. Rinaldi, and A. E. Simor. 1994. Successful treatment of systemic and local infections due to Exophiala species. Clin. Infect. Dis. 19:339-341. [DOI] [PubMed] [Google Scholar]
- 292.Gomez, B. L., and J. D. Nosanchuk. 2003. Melanin and fungi. Curr. Opin. Infect. Dis. 16:91-96. [DOI] [PubMed] [Google Scholar]
- 293.Gomez, B. L., J. D. Nosanchuk, S. Diez, S. Youngchim, P. Aisen, L. E. Cano, A. Restrepo, A. Casadevall, and A. J. Hamilton. 2001. Detection of melanin-like pigments in the dimorphic fungal pathogen Paracoccidioides brasiliensis in vitro and during infection. Infect. Immun. 69:5760-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Gonzalez, G. M. 2009. In vitro activities of isavuconazole against opportunistic filamentous and dimorphic fungi. Med. Mycol. 47:71-76. [DOI] [PubMed] [Google Scholar]
- 295.Gonzalez, M. S., B. Alfonso, D. Seckinger, A. A. Padhye, and L. Ajello. 1984. Subcutaneous phaeohyphomycosis caused by Cladosporium devriesii, sp. nov. Sabouraudia 22:427-432. [PubMed] [Google Scholar]
- 296.Gonzalez-Lopez, M. A., R. Salesa, M. C. Gonzalez-Vela, H. Fernandez-Llaca, J. F. Val-Bernal, and J. Cano. 2007. Subcutaneous phaeohyphomycosis caused by Exophiala oligosperma in a renal transplant recipient. Br. J. Dermatol. 156:762-764. [DOI] [PubMed] [Google Scholar]
- 297.Goodpasture, H. C., T. Carlson, B. Ellis, and G. Randall. 1983. Alternaria osteomyelitis. Evidence of specific immunologic tolerance. Arch. Pathol. Lab. Med. 107:528-530. [PubMed] [Google Scholar]
- 298.Gopinathan, U., P. Garg, M. Fernandes, S. Sharma, S. Athmanathan, and G. N. Rao. 2002. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center in South India. Cornea 21:555-559. [DOI] [PubMed] [Google Scholar]
- 299.Gosbell, I. B., V. Toumasatos, J. Yong, R. S. Kuo, D. H. Ellis, and R. C. Perrie. 2003. Cure of orthopaedic infection with Scedosporium prolificans, using voriconazole plus terbinafine, without the need for radical surgery. Mycoses 46:233-236. [DOI] [PubMed] [Google Scholar]
- 300.Gottlich, E., W. van der Lubbe, B. Lange, S. Fiedler, I. Melchert, M. Reifenrath, H. C. Flemming, and S. de Hoog. 2002. Fungal flora in groundwater-derived public drinking water. Int. J. Hyg. Environ. Health 205:269-279. [DOI] [PubMed] [Google Scholar]
- 301.Graybill, J. R., L. K. Najvar, E. Johnson, R. Bocanegra, and D. Loebenberg. 2004. Posaconazole therapy of disseminated phaeohyphomycosis in a murine model. Antimicrob. Agents Chemother. 48:2288-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Greig, J., M. Harkness, P. Taylor, C. Hashmi, S. Liang, and J. Kwan. 2003. Peritonitis due to the dermatiaceous mold Exophiala dermatitidis complicating continuous ambulatory peritoneal dialysis. Clin. Microbiol. Infect. 9:713-715. [DOI] [PubMed] [Google Scholar]
- 303.Greig, J. R., M. A. Khan, N. S. Hopkinson, B. G. Marshall, P. O. Wilson, and S. U. Rahman. 2001. Pulmonary infection with Scedosporium prolificans in an immunocompetent individual. J. Infect. 43:15-17. [DOI] [PubMed] [Google Scholar]
- 304.Grenouillet, F., F. Botterel, J. Crouzet, F. Larosa, Y. Hicheri, J. M. Forel, P. Helias, S. Ranque, and L. Delhaes. 2009. Scedosporium prolificans: an emerging pathogen in France? Med. Mycol. 47:343-350. [DOI] [PubMed] [Google Scholar]
- 305.Guarner, J., C. Del Rio, P. Williams, and J. E. McGowan, Jr. 1989. Fungal peritonitis caused by Curvularia lunata in a patient undergoing peritoneal dialysis. Am. J. Med. Sci. 298:320-323. [DOI] [PubMed] [Google Scholar]
- 306.Guarro, J., H. C. Gugnani, N. Sood, R. Batra, E. Mayayo, J. Gene, and S. Kakkar. 2008. Subcutaneous phaeohyphomycosis caused by Wallemia sebi in an immunocompetent host. J. Clin. Microbiol. 46:1129-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Guarro, J., E. Mayayo, J. Tapiol, C. Aguilar, and J. Cano. 1999. Microsphaeropsis olivacea as an etiological agent of human skin infection. Med. Mycol. 37:133-137. [PubMed] [Google Scholar]
- 308.Guarro, J., M. Nucci, T. Akiti, J. Gene, J. Cano, M. D. Barreiro, and C. Aguilar. 1999. Phialemonium fungemia: two documented nosocomial cases. J. Clin. Microbiol. 37:2493-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Guarro, J., A. M. Silvestre, Jr., G. Verkley, J. Cano, O. F. Gompertz, J. Gene, M. M. Ogawa, J. Tomimori-Yamashita, S. P. Teixeira, and F. A. de Almeida. 2006. Limitations of DNA sequencing for diagnosis of a mixed infection by two fungi, Phaeoacremonium venezuelense and a Plectophomella sp., in a transplant recipient. J. Clin. Microbiol. 44:4279-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Gubbins, P. O., and S. Heldenbrand. 2010. Clinically relevant drug interactions of current antifungal agents. Mycoses 53:95-113. [DOI] [PubMed] [Google Scholar]
- 311.Gueho, E., A. Bonnefoy, J. Luboinski, J. C. Petit, and G. S. De Hoog. 1989. Subcutaneous granuloma caused by Phialophora richardsiae: case report and review of the literature. Mycoses 32:219-223. [DOI] [PubMed] [Google Scholar]
- 312.Guerrero, A., P. Torres, M. T. Duran, B. Ruiz-Diez, M. Rosales, and J. L. Rodriguez-Tudela. 2001. Airborne outbreak of nosocomial Scedosporium prolificans infection. Lancet 357:1267-1268. [DOI] [PubMed] [Google Scholar]
- 313.Gugnani, H. C., V. Ramesh, N. Sood, J. Guarro, H. Moin Ul, A. Paliwal-Joshi, and B. Singh. 2006. Cutaneous phaeohyphomycosis caused by Cladosporium oxysporum and its treatment with potassium iodide. Med. Mycol. 44:285-288. [DOI] [PubMed] [Google Scholar]
- 314.Guppy, K. H., C. Thomas, K. Thomas, and D. Anderson. 1998. Cerebral fungal infections in the immunocompromised host: a literature review and a new pathogen—Chaetomium atrobrunneum: case report. Neurosurgery 43:1463-1469. [DOI] [PubMed] [Google Scholar]
- 315.Gupta, A. K., and Y. Kohli. 2003. In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and nondermatophytes, and in vitro evaluation of combination antifungal activity. Br. J. Dermatol. 149:296-305. [DOI] [PubMed] [Google Scholar]
- 316.Gupta, A. K., J. E. Ryder, R. Baran, and R. C. Summerbell. 2003. Non-dermatophyte onychomycosis. Dermatol. Clin. 21:257-268. [DOI] [PubMed] [Google Scholar]
- 317.Gupta, A. K., P. R. Taborda, and A. D. Sanzovo. 2002. Alternate week and combination itraconazole and terbinafine therapy for chromoblastomycosis caused by Fonsecaea pedrosoi in Brazil. Med. Mycol. 40:529-534. [DOI] [PubMed] [Google Scholar]
- 318.Gupta, G., A. D. Burden, G. S. Shankland, M. E. Fallowfield, and M. D. Richardson. 1997. Tinea nigra secondary to Exophiala werneckii responding to itraconazole. Br. J. Dermatol. 137:483-484. [DOI] [PubMed] [Google Scholar]
- 319.Gupta, S. K., K. S. Manjunath-Prasad, B. S. Sharma, V. K. Khosla, V. K. Kak, M. Minz, and V. K. Sakhuja. 1997. Brain abscess in renal transplant recipients: report of three cases. Surg. Neurol. 48:284-287. [DOI] [PubMed] [Google Scholar]
- 320.Haase, G., H. Skopnik, T. Groten, G. Kusenbach, and H. G. Posselt. 1991. Long-term fungal cultures from sputum of patients with cystic fibrosis. Mycoses 34:373-376. [DOI] [PubMed] [Google Scholar]
- 321.Halaby, T., H. Boots, A. Vermeulen, A. Van Der Ven, H. Beguin, H. Van Hooff, and J. Jacobs. 2001. Phaeohyphomycosis caused by Alternaria infectoria in a renal transplant patient. J. Clin. Microbiol. 39:1952-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Haldane, D. J., and E. Robart. 1990. A comparison of calcofluor white, potassium hydroxide, and culture for the laboratory diagnosis of superficial fungal infection. Diagn. Microbiol. Infect. Dis. 13:337-339. [DOI] [PubMed] [Google Scholar]
- 323.Halwig, J. M., D. A. Brueske, P. A. Greenberger, R. B. Dreisin, and H. M. Sommers. 1985. Allergic bronchopulmonary curvulariosis. Am. Rev. Resp. Dis. 132:186-188. [DOI] [PubMed] [Google Scholar]
- 324.Hamilton, A. J., and B. L. Gomez. 2002. Melanins in fungal pathogens. J. Med. Microbiol. 51:189-191. [DOI] [PubMed] [Google Scholar]
- 325.Harkness, B., D. Andresen, A. Kesson, and D. Isaacs. 2009. Infections following lawnmower and farm machinery-related injuries in children. J. Paediatr. Child Health 45:525-528. [DOI] [PubMed] [Google Scholar]
- 326.Harrington, B. J., and G. J. Hageage. 2003. Calcofluor white: a review of its uses and applications in clinical mycology and parasitology. Lab. Med. 34:361-367. [Google Scholar]
- 327.Harris, J. E., D. A. Sutton, A. Rubin, B. Wickes, G. S. De Hoog, and C. Kovarik. 2009. Exophiala spinifera as a cause of cutaneous phaeohyphomycosis: case study and review of the literature. Med. Mycol. 47:87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Harrison, D. K., S. Moser, and C. A. Palmer. 2008. Central nervous system infections in transplant recipients by Cladophialophora bantiana. South. Med. J. 101:292-296. [DOI] [PubMed] [Google Scholar]
- 329.Hart, A. P., D. A. Sutton, P. J. McFeeley, and M. Kornfeld. 2001. Cerebral phaeohyphomycosis caused by a dematiaceous Scopulariopsis species. Clin. Neuropathol. 20:224-228. [PubMed] [Google Scholar]
- 330.Harun, A., H. Perdomo, F. Gilgado, S. C. Chen, J. Cano, J. Guarro, and W. Meyer. 2009. Genotyping of Scedosporium species: a review of molecular approaches. Med. Mycol. 47:406-414. [DOI] [PubMed] [Google Scholar]
- 331.Haselwandter, K., and M. R. Ebner. 1994. Microorganisms surviving for 5300 years. FEMS Microbiol. Lett. 116:189-193. [DOI] [PubMed] [Google Scholar]
- 332.Hauck, E. F., M. McGinnis, and H. J. Nauta. 2008. Cerebral phaeohyphomycosis mimics high-grade astrocytoma. J. Clin. Neurosci. 15:1061-1066. [DOI] [PubMed] [Google Scholar]
- 333.Hay, R. J. 1999. Therapeutic potential of terbinafine in subcutaneous and systemic mycoses. Br. J. Dermatol. 141:36-40. [DOI] [PubMed] [Google Scholar]
- 334.Hayakawa, M., E. E. Ghosn, M. da Gloria Teixeria de Sousa, K. S. Ferreira, and S. R. Almeida. 2006. Phagocytosis, production of nitric oxide and pro-inflammatory cytokines by macrophages in the presence of dematiaceous fungi that cause chromoblastomycosis. Scand. J. Immunol. 64:382-387. [DOI] [PubMed] [Google Scholar]
- 335.Heath, C. H., J. L. Lendrum, B. L. Wetherall, S. L. Wesselingh, and D. L. Gordon. 1997. Phaeoacremonium parasiticum infective endocarditis following liver transplantation. Clin. Infect. Dis. 25:1251-1252. [DOI] [PubMed] [Google Scholar]
- 336.Heath, C. H., M. A. Slavin, T. C. Sorrell, R. Handke, A. Harun, M. Phillips, Q. Nguyen, L. Delhaes, D. Ellis, W. Meyer, and S. C. Chen. 2009. Population-based surveillance for scedosporiosis in Australia: epidemiology, disease manifestations and emergence of Scedosporium aurantiacum infection. Clin. Microbiol. Infect. 15:689-693. [DOI] [PubMed] [Google Scholar]
- 337.Heney, C., E. Song, A. Kellen, F. Raal, S. D. Miller, and V. Davis. 1989. Cerebral phaeohyphomycosis caused by Xylohypha bantiana. Eur. J. Clin. Microbiol. Infect. Dis. 8:984-988. [DOI] [PubMed] [Google Scholar]
- 338.Herbrecht, R. 2004. Posaconazole: a potent, extended-spectrum triazole anti-fungal for the treatment of serious fungal infections. Int. J. Clin. Pract. 58:612-624. [DOI] [PubMed] [Google Scholar]
- 339.Herbrecht, R., S. Natarajan-Ame, Y. Nivoix, and V. Letscher-Bru. 2003. The lipid formulations of amphotericin B. Expert Opin. Pharmacother. 4:1277-1287. [DOI] [PubMed] [Google Scholar]
- 340.Hibbett, D. S., M. Binder, J. F. Bischoff, M. Blackwell, P. F. Cannon, O. E. Eriksson, S. Huhndorf, T. James, P. M. Kirk, R. Lucking, H. Thorsten Lumbsch, F. Lutzoni, P. B. Matheny, D. J. McLaughlin, M. J. Powell, S. Redhead, C. L. Schoch, J. W. Spatafora, J. A. Stalpers, R. Vilgalys, M. C. Aime, A. Aptroot, R. Bauer, D. Begerow, G. L. Benny, L. A. Castlebury, P. W. Crous, Y. C. Dai, W. Gams, D. M. Geiser, G. W. Griffith, C. Gueidan, D. L. Hawksworth, G. Hestmark, K. Hosaka, R. A. Humber, K. D. Hyde, J. E. Ironside, U. Koljalg, C. P. Kurtzman, K. H. Larsson, R. Lichtwardt, J. Longcore, J. Miadlikowska, A. Miller, J. M. Moncalvo, S. Mozley-Standridge, F. Oberwinkler, E. Parmasto, V. Reeb, J. D. Rogers, C. Roux, L. Ryvarden, J. P. Sampaio, A. Schussler, J. Sugiyama, R. G. Thorn, L. Tibell, W. A. Untereiner, C. Walker, Z. Wang, A. Weir, M. Weiss, M. M. White, K. Winka, Y. J. Yao, and N. Zhang. 2007. A higher-level phylogenetic classification of the fungi. Mycol. Res. 111:509-547. [DOI] [PubMed] [Google Scholar]
- 341.Hipolito, E., E. Faria, A. F. Alves, G. S. de Hoog, J. Anjos, T. Goncalves, P. V. Morais, and H. Estevao. 2009. Alternaria infectoria brain abscess in a child with chronic granulomatous disease. Eur. J. Clin. Microbiol. Infect. Dis. 28:377-380. [DOI] [PubMed] [Google Scholar]
- 342.Hironaga, M., K. Nakano, I. Yokoyama, and J. Kitajima. 1989. Phialophora repens, an emerging agent of subcutaneous phaeohyphomycosis in humans. J. Clin. Microbiol. 27:394-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Hironaga, M., and S. Watanabe. 1980. Cerebral phaeohyphomycosis caused by Cladosporium bantianum: a case in a female who had cutaneous alternariosis in her childhood. Sabouraudia 18:229-235. [DOI] [PubMed] [Google Scholar]
- 344.Hirsch, B. E., B. F. Farber, J. F. Shapiro, and S. Kennelly. 1996. Successful treatment of Aureobasidium pullulans prosthetic hip infection. Infect. Dis. Clin. Pract. 5:205-207. [Google Scholar]
- 345.Hiruma, M., A. Kawada, H. Ohata, Y. Ohnishi, H. Takahashi, M. Yamazaki, A. Ishibashi, K. Hatsuse, M. Kakihara, and M. Yoshida. 1993. Systemic phaeohyphomycosis caused by Exophiala dermatitidis. Mycoses 36:1-7. [DOI] [PubMed] [Google Scholar]
- 346.Hohl, P. E., H. P. Holley, Jr., E. Prevost, L. Ajello, and A. A. Padhye. 1983. Infections due to Wangiella dermatitidis in humans: report of the first documented case from the United States and a review of the literature. Rev. Infect. Dis. 5:854-864. [DOI] [PubMed] [Google Scholar]
- 347.Holker, U., J. Bend, R. Pracht, L. Tetsch, T. Muller, M. Hofer, and G. S. de Hoog. 2004. Hortaea acidophila, a new acid-tolerant black yeast from lignite. Antonie Van Leeuwenhoek 86:287-294. [DOI] [PubMed] [Google Scholar]
- 348.Hollingsworth, J. W., S. Shofer, and A. Zaas. 2007. Successful treatment of Ochroconis gallopavum infection in an immunocompetent host. Infection 35:367-369. [DOI] [PubMed] [Google Scholar]
- 349.Hong, K. H., J. W. Kim, S. J. Jang, E. Yu, and E. C. Kim. 2009. Liver cirrhosis caused by Exophiala dermatitidis. J. Med. Microbiol. 58:674-677. [DOI] [PubMed] [Google Scholar]
- 350.Hood, S. V., C. B. Moore, J. S. Cheesbrough, A. Mene, and D. W. Denning. 1997. Atypical eumycetoma caused by Phialophora parasitica successfully treated with itraconazole and flucytosine. Br. J. Dermatol. 136:953-956. [PubMed] [Google Scholar]
- 351.Hoppin, E. C., E. L. McCoy, and M. G. Rinaldi. 1983. Opportunistic mycotic infection caused by Chaetomium in a patient with acute leukemia. Cancer 52:555-556. [DOI] [PubMed] [Google Scholar]
- 352.Hopwood, V., E. G. Evans, J. Matthews, and D. W. Denning. 1995. Scedosporium prolificans, a multi-resistant fungus, from a U.K. AIDS patient. J. Infect. 30:153-155. [DOI] [PubMed] [Google Scholar]
- 353.Horré, R., and G. S. De Hoog. 1999. Primary cerebral infections by melanized fungi: a review. Stud. Mycol. 43:176-193. [Google Scholar]
- 354.Horré, R., G. S. de Hoog, C. Kluczny, G. Marklein, and K. P. Schaal. 1999. rDNA diversity of Ochroconis and Scolecobasidium species isolated from humans and animals. Stud. Mycol. 43:194-205. [Google Scholar]
- 355.Horré, R., K. P. Schaal, B. Siekmeier, B. Sterzik, G. S. de Hoog, and N. Schnitzler. 2004. Isolation of fungi, especially Exophiala dermatitidis, in patients suffering from cystic fibrosis. Respiration 71:360-366. [DOI] [PubMed] [Google Scholar]
- 356.Hosseini-Yeganeh, M., and A. J. McLachlan. 2002. Physiologically based pharmacokinetic model for terbinafine in rats and humans. Antimicrob. Agents Chemother. 46:2219-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Houser, S. M., and J. P. Corey. 2000. Allergic fungal rhinosinusitis: pathophysiology, epidemiology, and diagnosis. Otolaryngol. Clin. N. Am. 33:399-409. [DOI] [PubMed] [Google Scholar]
- 358.Howden, B. P., M. A. Slavin, A. P. Schwarer, and A. M. Mijch. 2003. Successful control of disseminated Scedosporium prolificans infection with a combination of voriconazole and terbinafine. Eur. J. Clin. Microbiol. Infect. Dis. 22:111-113. [DOI] [PubMed] [Google Scholar]
- 359.Hsu, M. M., and J. Y. Lee. 1993. Cutaneous and subcutaneous phaeohyphomycosis caused by Exserohilum rostratum. J. Am. Acad. Dermatol. 28:340-344. [DOI] [PubMed] [Google Scholar]
- 360.Huang, Y. T., S. J. Liaw, C. H. Liao, J. L. Yang, D. M. Lai, Y. C. Lee, and P. R. Hsueh. 2008. Catheter-related septicemia due to Aureobasidium pullulans. Int. J. Infect. Dis. 12:e137-139. [DOI] [PubMed] [Google Scholar]
- 361.Huhndorf, S., A. Miller, and F. Fernádez. 2004. Molecular systematics of the Sordariales: the order and the family Lasiosphaeriaceaae redefined. Mycologia 2:368-387. [PubMed] [Google Scholar]
- 362.Hurley, M. A., and B. Saffran. 1997. Subcutaneous phaeohyphomycosis: overview and case report. J. Foot Ankle Surg. 36:230-235. [DOI] [PubMed] [Google Scholar]
- 363.Husain, S., B. D. Alexander, P. Munoz, R. K. Avery, S. Houston, T. Pruett, R. Jacobs, E. A. Dominguez, J. G. Tollemar, K. Baumgarten, C. M. Yu, M. M. Wagener, P. Linden, S. Kusne, and N. Singh. 2003. Opportunistic mycelial fungal infections in organ transplant recipients: emerging importance of non-Aspergillus mycelial fungi. Clin. Infect. Dis. 37:221-229. [DOI] [PubMed] [Google Scholar]
- 364.Husain, S., P. Munoz, G. Forrest, B. D. Alexander, J. Somani, K. Brennan, M. M. Wagener, and N. Singh. 2005. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin. Infect. Dis. 40:89-99. [DOI] [PubMed] [Google Scholar]
- 365.Hussey, S. M., R. Gander, P. Southern, and M. P. Hoang. 2005. Subcutaneous phaeohyphomycosis caused by Cladophialophora bantiana. Arch. Pathol. Lab. Med. 129:794-797. [DOI] [PubMed] [Google Scholar]
- 366.Huttova, M., K. Kralinsky, J. Horn, I. Marinova, K. Iligova, J. Fric, S. Spanik, J. Filka, J. Uher, J. Kurak, and V. Krcmery, Jr. 1998. Prospective study of nosocomial fungal meningitis in children—report of 10 cases. Scand. J. Infect. Dis. 30:485-487. [DOI] [PubMed] [Google Scholar]
- 367.Ibanez, P. R., J. Chacon, A. Fidalgo, J. Martin, V. Paraiso, and J. L. Munoz-Bellido. 1997. Peritonitis by Aureobasidium pullulans in continuous ambulatory peritoneal dialysis. Nephrol. Dial. Transplant. 12:1544-1545. [DOI] [PubMed] [Google Scholar]
- 368.Idigoras, P., E. Perez-Trallero, L. Pineiro, J. Larruskain, M. C. Lopez-Lopategui, N. Rodriguez, and J. M. Gonzalez. 2001. Disseminated infection and colonization by Scedosporium prolificans: a review of 18 cases, 1990-1999. Clin. Infect. Dis. 32:e158-e165. [DOI] [PubMed] [Google Scholar]
- 369.Ikai, K., H. Tomono, and S. Watanabe. 1988. Phaeohyphomycosis caused by Phialophora richardsiae. J. Am. Acad. Dermatol. 19:478-481. [DOI] [PubMed] [Google Scholar]
- 370.Ikeda, R., T. Sugita, E. S. Jacobson, and T. Shinoda. 2003. Effects of melanin upon susceptibility of Cryptococcus to antifungals. Microbiol. Immunol. 47:271-277. [DOI] [PubMed] [Google Scholar]
- 371.Imwidthaya, P. 1994. Systemic fungal infections in Thailand. J. Med. Vet. Mycol. 32:395-399. [DOI] [PubMed] [Google Scholar]
- 372.Inoue, Y., Y. Matsuwaki, S. H. Shin, J. U. Ponikau, and H. Kita. 2005. Nonpathogenic, environmental fungi induce activation and degranulation of human eosinophils. J. Immunol. 175:5439-5447. [DOI] [PubMed] [Google Scholar]
- 373.Iwatsu, T. 1988. Cutaneous alternariosis. Arch. Dermatol. 124:1822-1825. [PubMed] [Google Scholar]
- 374.Iyer, S. A., S. S. Tuli, and R. C. Wagoner. 2006. Fungal keratitis: emerging trends and treatment outcomes. Eye Contact Lens 32:267-271. [DOI] [PubMed] [Google Scholar]
- 375.Jacobson, E. S. 2000. Pathogenic roles for fungal melanins. Clin. Microbiol. Rev. 13:708-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Jacobson, E. S., and G. M. Compton. 1996. Discordant regulation of phenoloxidase and capsular polysaccharide in Cryptococcus neoformans. J. Med. Vet. Mycol. 34:289-291. [DOI] [PubMed] [Google Scholar]
- 377.Jacyk, W. K. 1979. Chromomycosis due to Cladosporium carrionii treated with 5-fluorocytosine. A case report from northern Nigeria. Cutis 23:649-650. [PubMed] [Google Scholar]
- 378.Janaki, C., G. Sentamilselvi, V. R. Janaki, S. Devesh, and K. Ajithados. 1999. Case report. Eumycetoma due to Curvularia lunata. Mycoses 42:345-346. [DOI] [PubMed] [Google Scholar]
- 379.Jay, W. M., R. W. Bradsher, B. LeMay, N. Snyderman, and E. J. Angtuaco. 1988. Ocular involvement in mycotic sinusitis caused by Bipolaris. Am. J. Ophthalmol. 105:366-370. [DOI] [PubMed] [Google Scholar]
- 380.Jayakeerthi, S. R., M. Dias, S. Nagarathna, B. Anandh, A. Mahadevan, and A. Chandramuki. 2004. Brain abscess due to Cladophialophora bantiana. Indian J. Med. Microbiol. 22:193-195. [PubMed] [Google Scholar]
- 381.Jensen, J. C. 1989. Clinical pharmacokinetics of terbinafine (Lamisil). Clin. Exp. Dermatol. 14:110-113. [DOI] [PubMed] [Google Scholar]
- 382.Jessup, C. J., N. S. Ryder, and M. A. Ghannoum. 2000. An evaluation of the in vitro activity of terbinafine. Med. Mycol. 38:155-159. [DOI] [PubMed] [Google Scholar]
- 383.Johnson, E. M., A. Szekely, and D. W. Warnock. 1998. In-vitro activity of voriconazole, itraconazole and amphotericin B against filamentous fungi. J. Antimicrob. Chemother. 42:741-745. [DOI] [PubMed] [Google Scholar]
- 384.Johnson, E. M., A. Szekely, and D. W. Warnock. 1999. In vitro activity of Syn-2869, a novel triazole agent, against emerging and less common mold pathogens. Antimicrob. Agents Chemother. 43:1260-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Johnson, L. B., and C. A. Kauffman. 2003. Voriconazole: a new triazole antifungal agent. Clin. Infect. Dis. 36:630-637. [DOI] [PubMed] [Google Scholar]
- 386.Juma, A. 1993. Phialophora richardsiae endocarditis of aortic and mitral valves in a diabetic man with a porcine mitral valve. J. Infect. 27:173-175. [DOI] [PubMed] [Google Scholar]
- 387.Jumaa, P. A., C. Lightowler, L. R. Baker, and S. S. Das. 1995. Cutaneous infection caused by Phialophora richardsiae treated successfully by surgical excision in an immunocompromised patient. J. Infect. 30:261-262. [DOI] [PubMed] [Google Scholar]
- 388.Jurkunas, U., I. Behlau, and K. Colby. 2009. Fungal keratitis: changing pathogens and risk factors. Cornea 28:638-643. [DOI] [PubMed] [Google Scholar]
- 389.Kaczmarski, E. B., J. A. Liu Yin, J. A. Tooth, E. M. Love, and I. W. Delamore. 1986. Systemic infection with Aureobasidium pullulans in a leukaemic patient. J. Infect. 13:289-291. [DOI] [PubMed] [Google Scholar]
- 390.Kaell, A. T., and I. Weitzman. 1983. Acute monoarticular arthritis due to Phialophora parasitica. Am. J. Med. 74:519-522. [DOI] [PubMed] [Google Scholar]
- 391.Kahn, J. N., M. J. Hsu, F. Racine, R. Giacobbe, and M. Motyl. 2006. Caspofungin susceptibility in Aspergillus and non-Aspergillus molds: inhibition of glucan synthase and reduction of beta-d-1,3 glucan levels in culture. Antimicrob. Agents Chemother. 50:2214-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Kainer, M. A., H. Keshavarz, B. J. Jensen, M. J. Arduino, M. E. Brandt, A. A. Padhye, W. R. Jarvis, and L. K. Archibald. 2005. Saline-filled breast implant contamination with Curvularia species among women who underwent cosmetic breast augmentation. J. Infect. Dis. 192:170-177. [DOI] [PubMed] [Google Scholar]
- 393.Kaltseis, J., J. Rainer, and G. S. De Hoog. 2009. Ecology of Pseudallescheria and Scedosporium species in human-dominated and natural environments and their distribution in clinical samples. Med. Mycol. 47:398-405. [DOI] [PubMed] [Google Scholar]
- 394.Kanj, S. S., S. S. Amr, and G. D. Roberts. 2001. Ramichloridium mackenziei brain abscess: report of two cases and review of the literature. Med. Mycol. 39:97-102. [DOI] [PubMed] [Google Scholar]
- 395.Kantarcioglu, A. S., and G. S. de Hoog. 2004. Infections of the central nervous system by melanized fungi: a review of cases presented between 1999 and 2004. Mycoses 47:4-13. [DOI] [PubMed] [Google Scholar]
- 396.Kantarcioglu, A. S., A. Yucel, and G. S. De Hoog. 2002. Case report. Isolation of Cladosporium cladosporioides from cerebrospinal fluid. Mycoses 45:500-503. [DOI] [PubMed] [Google Scholar]
- 397.Karim, M., H. Sheikh, M. Alam, and Y. Sheikh. 1993. Disseminated Bipolaris infection in an asthmatic patient: case report. Clin. Infect. Dis. 17:248-253. [DOI] [PubMed] [Google Scholar]
- 398.Karuppal, R., C. M. Kumaran, A. Marthya, C. V. Manoj Kumar, M. P. Narayanan, R. V. Raman, and S. Thomas. 2009. Tibial osteomyelitis due to Fonsecaea pedrosoi in an immunocompetent patient: case report. J. Foot Ankle Surg. 48:569-572. [DOI] [PubMed] [Google Scholar]
- 399.Kashgari, T. Q., H. Al-Miniawi, and M. K. Moawad Hanna. 2000. Cerebral phaeohyphomycosis caused by Ramichloridium mackenziei in the Eastern Province of Saudi Arabia. Ann. Saudi Med. 20:457-460. [DOI] [PubMed] [Google Scholar]
- 400.Kaufman, S. M. 1971. Curvularia endocarditis following cardiac surgery. Am. J. Clin. Pathol. 56:466-470. [DOI] [PubMed] [Google Scholar]
- 401.Keating, G. M. 2005. Posaconazole. Drugs 65:1553-1567. [DOI] [PubMed] [Google Scholar]
- 402.Kenney, R. T., K. J. Kwon-Chung, A. T. Waytes, D. A. Melnick, H. I. Pass, M. J. Merino, and J. I. Gallin. 1992. Successful treatment of systemic Exophiala dermatitidis infection in a patient with chronic granulomatous disease. Clin. Infect. Dis. 14:235-242. [DOI] [PubMed] [Google Scholar]
- 403.Kent, D., T. Wong, R. Osgood, K. Kosinski, G. Coste, and D. Bor. 1998. Fungemia due to Hormonema dematioides following intense avian exposure. Clin. Infect. Dis. 26:759-760. [DOI] [PubMed] [Google Scholar]
- 404.Kerkmann, M. L., K. Piontek, H. Mitze, and G. Haase. 1999. Isolation of Exophiala (Wangiella) dermatitidis in a case of otitis externa. Clin. Infect. Dis. 29:939-940. [DOI] [PubMed] [Google Scholar]
- 405.Kerr, C. M., J. R. Perfect, P. C. Craven, J. H. Jorgensen, D. J. Drutz, J. D. Shelburne, H. A. Gallis, and R. A. Gutman. 1983. Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis. Ann. Intern. Med. 99:334-336. [DOI] [PubMed] [Google Scholar]
- 406.Kesson, A. M., M. C. Bellemore, T. J. O'Mara, D. H. Ellis, and T. C. Sorrell. 2009. Scedosporium prolificans osteomyelitis in an immunocompetent child treated with a novel agent, hexadecylphospocholine (miltefosine), in combination with terbinafine and voriconazole: a case report. Clin. Infect. Dis. 48:1257-1261. [DOI] [PubMed] [Google Scholar]
- 407.Kethireddy, S., and D. Andes. 2007. CNS pharmacokinetics of antifungal agents. Expert Opin. Drug Metab. Toxicol. 3:573-581. [DOI] [PubMed] [Google Scholar]
- 408.Keyser, A., F. X. Schmid, H. J. Linde, J. Merk, and D. E. Birnbaum. 2002. Disseminated Cladophialophora bantiana infection in a heart transplant recipient. J. Heart Lung Transplant. 21:503-505. [DOI] [PubMed] [Google Scholar]
- 409.Khan, J. A., S. T. Hussain, S. Hasan, P. McEvoy, and A. Sarwari. 2000. Disseminated Bipolaris infection in an immunocompetent host: an atypical presentation. J. Pak. Med. Assoc. 50:68-71. [PubMed] [Google Scholar]
- 410.Khan, Z. U., S. J. Lamdhade, M. Johny, J. Al Khalidi, A. Thussu, H. N. Yossef, I. Al Obaid, and A. A. Nasser. 2002. Additional case of Ramichloridium mackenziei cerebral phaeohyphomycosis from the Middle East. Med. Mycol. 40:429-433. [DOI] [PubMed] [Google Scholar]
- 411.Kiehn, T. E., B. Polsky, E. Punithalingam, F. F. Edwards, A. E. Brown, and D. Armstrong. 1987. Liver infection caused by Coniothyrium fuckelii in a patient with acute myelogenous leukemia. J. Clin. Microbiol. 25:2410-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Kim, R. C., C. J. Hodge, Jr., H. V. Lamberson, Jr., and L. B. Weiner. 1981. Traumatic intracerebral implantation of Cladosporium trichoides. Neurology 31:1145-1148. [DOI] [PubMed] [Google Scholar]
- 413.Kimura, M., A. Goto, T. Furuta, T. Satou, S. Hashimoto, and K. Nishimura. 2003. Multifocal subcutaneous phaeohyphomycosis caused by Phialophora verrucosa. Arch. Pathol. Lab. Med. 127:91-93. [DOI] [PubMed] [Google Scholar]
- 414.Kimura, M., and M. R. McGinnis. 1998. Fontana-Masson-stained tissue from culture-proven mycoses. Arch. Pathol. Lab. Med. 122:1107-1111. [PubMed] [Google Scholar]
- 415.King, A. B., and T. S. Collette. 1952. Brain abscess due to Cladosporium trichoides. Bull. Johns Hopkins Hosp. 91:298-305. [PubMed] [Google Scholar]
- 416.King, D., L. Pasarell, D. M. Dixon, M. R. McGinnis, and W. G. Merz. 1993. A phaeohyphomycotic cyst and peritonitis caused by Phialemonium species and a reevaluation of its taxonomy. J. Clin. Microbiol. 31:1804-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Kobayashi, H., A. Sano, N. Aragane, M. Fukuoka, M. Tanaka, F. Kawaura, Y. Fukuno, E. Matsuishi, and S. Hayashi. 2008. Disseminated infection by Bipolaris spicifera in an immunocompetent subject. Med. Mycol. 46:361-365. [DOI] [PubMed] [Google Scholar]
- 418.Kondo, Y., M. Hiruma, A. Matsushita, S. Matsuba, K. Nishimura, and K. Takamori. 2007. Cutaneous phaeohyphomycosis caused by Veronaea botryosa observed as sclerotic cells in tissue. Int. J. Dermatol. 46:625-627. [DOI] [PubMed] [Google Scholar]
- 419.Koo, S., J. M. Bryar, J. H. Page, L. R. Baden, and F. M. Marty. 2009. Diagnostic performance of the (1,3)-beta-d-glucan assay for invasive fungal disease. Clin. Infect. Dis. 49:1650-1659. [DOI] [PubMed] [Google Scholar]
- 420.Koo, S., M. Klompas, F. M. Marty, J. M. Bryar, J. H. Page, and L. R. Baden. 2010. Fonsecaea monophora cerebral phaeohyphomycosis: case report of successful surgical excision and voriconazole treatment and review. Med. Mycol. 48:769-774. [DOI] [PubMed] [Google Scholar]
- 421.Kotylo, P. K., K. S. Israel, J. S. Cohen, and M. S. Bartlett. 1989. Subcutaneous phaeohyphomycosis of the finger caused by Exophiala spinifera. Am. J. Clin. Pathol. 91:624-627. [DOI] [PubMed] [Google Scholar]
- 422.Kralovic, S. M., and J. C. Rhodes. 1995. Phaeohyphomycosis caused by Dactylaria (human dactylariosis): report of a case with review of the literature. J. Infect. 31:107-113. [DOI] [PubMed] [Google Scholar]
- 423.Krcmery, V., Jr., S. Spanik, A. Danisovicova, Z. Jesenska, and M. Blahova. 1994. Aureobasidium mansoni meningitis in a leukemia patient successfully treated with amphotericin B. Chemotherapy 40:70-71. [DOI] [PubMed] [Google Scholar]
- 424.Krisher, K. K., N. B. Holdridge, M. M. Mustafa, M. G. Rinaldi, and D. A. McGough. 1995. Disseminated Microascus cirrosus infection in pediatric bone marrow transplant recipient. J. Clin. Microbiol. 33:735-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Kuhn, F. A., and A. R. Javer. 2000. Allergic fungal rhinosinusitis: perioperative management, prevention of recurrence, and role of steroids and antifungal agents. Otolaryngol. Clin. N. Am. 33:419-433. [DOI] [PubMed] [Google Scholar]
- 426.Kumarasinghe, S. P., and M. P. Kumarasinghe. 2000. Itraconazole pulse therapy in chromoblastomycosis. Eur. J. Dermatol. 10:220-222. [PubMed] [Google Scholar]
- 427.Kusenbach, G., H. Skopnik, G. Haase, F. Friedrichs, and H. Dohmen. 1992. Exophiala dermatitidis pneumonia in cystic fibrosis. Eur. J. Ped. 151:344-346. [DOI] [PubMed] [Google Scholar]
- 428.Kwon-Chung, K. J., I. Polacheck, and T. J. Popkin. 1982. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J. Bacteriol. 150:1414-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Kwon-Chung, K. J., I. S. Schwartz, and B. J. Rybak. 1975. A pulmonary fungus ball produced by Cladosporium cladosporioides. Am. J. Clin. Pathol. 64:564-568. [DOI] [PubMed] [Google Scholar]
- 430.Labarca, J. A., E. A. Wagar, A. E. Grasmick, H. M. Kokkinos, and D. A. Bruckner. 1998. Critical evaluation of 4-week incubation for fungal cultures: is the fourth week useful? J. Clin. Microbiol. 36:3683-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Lacroix, C., E. de Kerviler, P. Morel, F. Derouin, and M. Feuilhade de Chavin. 2005. Madurella mycetomatis mycetoma treated successfully with oral voriconazole. Br. J. Dermatol. 152:1067-1068. [DOI] [PubMed] [Google Scholar]
- 432.Lake, F. R., J. H. Froudist, R. McAleer, R. L. Gillon, A. E. Tribe, and P. J. Thompson. 1991. Allergic bronchopulmonary fungal disease caused by Bipolaris and Curvularia. Aust. N. Z. J. Med. 21:871-874. [DOI] [PubMed] [Google Scholar]
- 433.Lakshmi, V., C. Padmasri, P. Umabala, C. Sundaram, and M. Panigrahi. 2008. Cerebral phaeohyphomycosis due to Cladophialophora bantiana. Indian J. Med. Microbiol. 26:392-395. [DOI] [PubMed] [Google Scholar]
- 434.Lamaris, G. A., G. Chamilos, R. E. Lewis, A. Safdar, I. I. Raad, and D. P. Kontoyiannis. 2006. Scedosporium infection in a tertiary care cancer center: a review of 25 cases from 1989-2006. Clin. Infect. Dis. 43:1580-1584. [DOI] [PubMed] [Google Scholar]
- 435.Lampert, R. P., J. H. Hutto, W. H. Donnelly, and S. T. Shulman. 1977. Pulmonary and cerebral mycetoma caused by Curvularia pallescens. J. Pediatr. 91:603-605. [DOI] [PubMed] [Google Scholar]
- 436.Langdon, J. S., and W. L. McDonald. 1987. Cranial Exophiala pisciphila infection in Salmo salar in Australia. Bull. Eur. Assoc. Fish Pathol. 7:35-37. [Google Scholar]
- 437.Langfelder, K., M. Streibel, B. Jahn, G. Haase, and A. A. Brakhage. 2003. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 38:143-158. [DOI] [PubMed] [Google Scholar]
- 438.Langvad, F., O. Pedersen, and K. Engjom. 1985. A fungal disease caused by Exophiala sp. nova in farmed Atlantic salmon in Western Norway, p. 323-328. In A. E. Ellis (ed.), Fish and shellfish pathology. Academic Press, London, United Kingdom.
- 439.Lanisnik Rizner, T., and M. H. Wheeler. 2003. Melanin biosynthesis in the fungus Curvularia lunata (teleomorph: Cochliobolus lunatus). Can. J. Microbiol. 49:110-119. [DOI] [PubMed] [Google Scholar]
- 440.Larangeira de Almeida, H., Jr., R. N. Dallazem, L. S. Dossantos, and S. A. Hallal. 2007. Bilateral tinea nigra in a temperate climate. Dermatol. Online J. 13:25. [PubMed] [Google Scholar]
- 441.LaRocco, M. T. 2007. Reagents, stains, and media: mycology, p. 1737-1744. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 442.Lasala, P. R., M. B. Smith, M. R. McGinnis, K. Sackey, J. A. Patel, and S. Qiu. 2005. Invasive Exserohilum sinusitis in a patient with aplastic anemia. Pediatr. Infect. Dis. J. 24:939-941. [DOI] [PubMed] [Google Scholar]
- 443.Lastoria, C., A. Cascina, F. Bini, A. Di Matteo, C. Cavanna, C. Farina, E. Carretto, and F. Meloni. 2009. Pulmonary Cladophialophora boppii infection in a lung transplant recipient: case report and literature review. J. Heart Lung Transplant. 28:635-637. [DOI] [PubMed] [Google Scholar]
- 444.Latham, R. H. 2000. Bipolaris spicifera meningitis complicating a neurosurgical procedure. Scand. J. Infect. Dis. 32:102-103. [DOI] [PubMed] [Google Scholar]
- 445.Lau, A., S. Chen, T. Sorrell, D. Carter, R. Malik, P. Martin, and C. Halliday. 2007. Development and clinical application of a panfungal PCR assay to detect and identify fungal DNA in tissue specimens. J. Clin. Microbiol. 45:380-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Lavelle, P. 1980. Chromoblastomycosis in Mexico. PAHO Sci. Publ. 396:235-247. [Google Scholar]
- 447.Lee, D. K., and A. K. Schwartz. 2007. Primary mycetoma osteomyelitis of the calcaneus with active subcutaneous nodules. J. Foot Ankle Surg. 46:302-306. [DOI] [PubMed] [Google Scholar]
- 448.Lee, Y. M., P. A. Tambyah, K. H. Lee, K. C. Tan, and S. G. Lim. 2003. Successful treatment of Xylohypha bantiana brain abscess mimicking invasive cerebral aspergillosis in a liver transplant recipient. J. Infect. 47:348-351. [DOI] [PubMed] [Google Scholar]
- 449.Lesire, V., E. Hazouard, P. F. Dequin, M. Delain, M. Therizol-Ferly, and A. Legras. 1999. Possible role of Chaetomium globosum in infection after autologous bone marrow transplantation. Intensive Care Med. 25:124-125. [DOI] [PubMed] [Google Scholar]
- 450.Levin, T. P., D. E. Baty, T. Fekete, A. L. Truant, and B. Suh. 2004. Cladophialophora bantiana brain abscess in a solid-organ transplant recipient: case report and review of the literature. J. Clin. Microbiol. 42:4374-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Levy, I., J. Stein, S. Ashkenazi, Z. Samra, G. Livni, and I. Yaniv. 2003. Ecthyma gangrenosum caused by disseminated Exserohilum in a child with leukemia: a case report and review of the literature. Pediatr. Dermatol. 20:495-497. [DOI] [PubMed] [Google Scholar]
- 452.Li, D. M., R. Y. Li, G. S. De Hoog, Y. X. Wang, and D. L. Wang. 2009. Exophiala asiatica, a new species from a fatal case in China. Med. Mycol. 47:101-109. [DOI] [PubMed] [Google Scholar]
- 453.Li, J. Y., T. Y. Yong, D. I. Grove, and P. T. Coates. 2008. Successful control of Scedosporium prolificans septic arthritis and probable osteomyelitis without radical surgery in a long-term renal transplant recipient. Transpl. Infect. Dis. 10:63-65. [DOI] [PubMed] [Google Scholar]
- 454.Li, R. K., and M. G. Rinaldi. 1999. In vitro antifungal activity of nikkomycin Z in combination with fluconazole or itraconazole. Antimicrob. Agents Chemother. 43:1401-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Lichon, V., and A. Khachemoune. 2006. Mycetoma: a review. Am. J. Clin. Dermatol. 7:315-321. [DOI] [PubMed] [Google Scholar]
- 456.Lin, R. Y., and K. D. Williams. 2003. Hypersensitivity to molds in New York City in adults who have asthma. Allergy Asthma Proc. 24:13-18. [PubMed] [Google Scholar]
- 457.Lin, S. C., P. L. Sun, Y. M. Ju, and Y. J. Chan. 2009. Cutaneous phaeohyphomycosis caused by Exserohilum rostratum in a patient with cutaneous T-cell lymphoma. Int. J. Dermatol. 48:295-298. [DOI] [PubMed] [Google Scholar]
- 458.Lirng, J. F., R. D. Tien, A. K. Osumi, J. F. Madden, R. P. McLendon, and D. Sexton. 1995. Cerebral phaeohyphomycosis complicated with brain abscess: a case report. Chin. Med. J. 55:491-495. [PubMed] [Google Scholar]
- 459.Little, M. G., and M. L. Hammond. 1995. Scytalidium dimidiatum in Australia. Australas. J. Dermatol. 36:204-205. [DOI] [PubMed] [Google Scholar]
- 460.Liu, G. Y., and V. Nizet. 2009. Color me bad: microbial pigments as virulence factors. Trends Microbiol. 17:406-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Lopes, J. O., S. H. Alves, J. P. Benevenga, F. B. Brauner, M. S. Castro, and E. Melchiors. 1994. Curvularia lunata peritonitis complicating peritoneal dialysis. Mycopathologia 127:65-67. [DOI] [PubMed] [Google Scholar]
- 462.Loulergue, P., A. Hot, E. Dannaoui, A. Dallot, S. Poiree, B. Dupont, and O. Lortholary. 2006. Successful treatment of black-grain mycetoma with voriconazole. Am. J. Trop. Med. Hyg. 75:1106-1107. [PubMed] [Google Scholar]
- 463.Lumbsch, H. T., and S. M. Huhndorf. 2007. Outline of Ascomycota. Myconet 13:1-58. [Google Scholar]
- 464.Lundstrom, T. S., M. R. Fairfax, M. C. Dugan, J. A. Vazquez, P. H. Chandrasekar, E. Abella, and C. Kasten-Sportes. 1997. Phialophora verrucosa infection in a BMT patient. Bone Marrow Transplant. 20:789-791. [DOI] [PubMed] [Google Scholar]
- 465.Luque, P., F. A. Garcia-Gil, J. Larraga, B. Jimenez, E. Tome-Zelaya, M. T. Serrano, and M. E. Barrao. 2006. Treatment of cutaneous infection by Alternaria alternata with voriconazole in a liver transplant patient. Transplant. Proc. 38:2514-2515. [DOI] [PubMed] [Google Scholar]
- 466.Lye, W. C. 1993. Peritonitis due to Wangiella dermatitidis in a patient on CAPD. Perit. Dial. Int. 13:319-320. [PubMed] [Google Scholar]
- 467.Lyons, M. K., J. E. Blair, and K. O. Leslie. 2005. Successful treatment with voriconazole of fungal cerebral abscess due to Cladophialophora bantiana. Clin. Neurol. Neurosurg. 107:532-534. [DOI] [PubMed] [Google Scholar]
- 468.Machard, B., P. Misslin, and M. Lemaire. 1989. Influence of plasma protein binding on the brain uptake of an antifungal agent, terbinafine, in rats. J. Pharm. Pharmacol. 41:700-704. [DOI] [PubMed] [Google Scholar]
- 469.Madan, V., D. Bisset, P. Harris, S. Howard, and M. H. Beck. 2006. Phaeohyphomycosis caused by Exophiala salmonis. Br. J. Dermatol. 155:1082-1084. [DOI] [PubMed] [Google Scholar]
- 470.Madrigal, V., J. Alonso, E. Bureo, F. J. Figols, and R. Salesa. 1995. Fatal meningoencephalitis caused by Scedosporium inflatum (Scedosporium prolificans) in a child with lymphoblastic leukemia. Eur. J. Clin. Microbiol. Infect. Dis. 14:601-603. [DOI] [PubMed] [Google Scholar]
- 471.Maertens, J., K. Lagrou, H. Deweerdt, I. Surmont, G. E. Verhoef, J. Verhaegen, and M. A. Boogaerts. 2000. Disseminated infection by Scedosporium prolificans: an emerging fatality among haematology patients. Case report and review. Ann. Hematol. 79:340-344. [DOI] [PubMed] [Google Scholar]
- 472.Magnon, K. C., M. Jalbert, and A. A. Padhye. 1993. Osteolytic phaeohyphomycosis caused by Phialemonium obovatum. Arch. Pathol. Lab. Med. 117:841-843. [PubMed] [Google Scholar]
- 473.Maiti, P. K., A. Ray, and S. Bandyopadhyay. 2002. Epidemiological aspects of mycetoma from a retrospective study of 264 cases in West Bengal. Trop. Med. Int. Health 7:788-792. [DOI] [PubMed] [Google Scholar]
- 474.Malekzadeh, M., G. D. Overturf, S. B. Auerbach, L. Wong, and M. Hirsch. 1990. Chronic, recurrent osteomyelitis caused by Scedosporium inflatum. Pediatr. Infect. Dis. J. 9:357-359. [DOI] [PubMed] [Google Scholar]
- 475.Malloch, D., and I. F. Salkin. 1984. A new species of Scedosporium associated with osteomyelitis in humans. Mycotaxon 21:247-255. [Google Scholar]
- 476.Mani, R. S., Y. T. Chickabasaviah, S. Nagarathna, A. Chandramuki, M. R. Shivprakash, J. Vijayan, D. K. Prashantha, P. S. Vasudevan, A. Natarajan, and J. Kovoor. 2008. Cerebral phaeohyphomycosis caused by Scytalidium dimidiatum: a case report from India. Med. Mycol. 46:705-711. [DOI] [PubMed] [Google Scholar]
- 477.Manian, F. A., and M. J. Brischetto. 1993. Pulmonary infection due to Exophiala jeanselmei: successful treatment with ketoconazole. Clin. Infect. Dis. 16:445-446. [DOI] [PubMed] [Google Scholar]
- 478.Manzouri, B., G. C. Vafidis, and R. K. Wyse. 2001. Pharmacotherapy of fungal eye infections. Expert Opin. Pharmacother. 2:1849-1857. [DOI] [PubMed] [Google Scholar]
- 479.Marimon, R., J. Cano, J. Gene, D. A. Sutton, M. Kawasaki, and J. Guarro. 2007. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J. Clin. Microbiol. 45:3198-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Marimon, R., J. Gene, J. Cano, L. Trilles, M. Dos Santos Lazera, and J. Guarro. 2006. Molecular phylogeny of Sporothrix schenckii. J. Clin. Microbiol. 44:3251-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Marine, M., F. J. Pastor, and J. Guarro. 2009. Combined antifungal therapy in a murine model of disseminated infection by Cladophialophora bantiana. Med. Mycol. 47:45-49. [DOI] [PubMed] [Google Scholar]
- 482.Markham, W. D., R. D. Key, A. A. Padhye, and L. Ajello. 1990. Phaeohyphomycotic cyst caused by Tetraploa aristata. J. Med. Vet. Mycol. 28:147-150. [PubMed] [Google Scholar]
- 483.Marques, A. R., K. J. Kwon-Chung, S. M. Holland, M. L. Turner, and J. I. Gallin. 1995. Suppurative cutaneous granulomata caused by Microascus cinereus in a patient with chronic granulomatous disease. Clin. Infect. Dis. 20:110-114. [DOI] [PubMed] [Google Scholar]
- 484.Marques, S. A., R. M. Camargo, R. C. Summerbell, G. S. De Hoog, P. Ishioka, L. M. Chambo-Cordaro, and M. E. Marques. 2006. Subcutaneous phaeohyphomycosis caused by Phaeoacremonium parasiticum in a renal transplant patient. Med. Mycol. 44:671-676. [DOI] [PubMed] [Google Scholar]
- 485.Marriott, D. J., K. H. Wong, E. Aznar, J. L. Harkness, D. A. Cooper, and D. Muir. 1997. Scytalidium dimidiatum and Lecythophora hoffmannii: unusual causes of fungal infections in a patient with AIDS. J. Clin. Microbiol. 35:2949-2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Martinez-Gonzalez, M. C., M. M. Verea, D. Velasco, F. Sacristan, J. Del Pozo, J. Garcia-Silva, and E. Fonseca. 2008. Three cases of cutaneous phaeohyphomycosis by Exophiala jeanselmei. Eur. J. Dermatol. 18:313-316. [DOI] [PubMed] [Google Scholar]
- 487.Maslen, M. M., T. Collis, and R. Stuart. 1996. Lasiodiplodia theobromae isolated from a subcutaneous abscess in a Cambodian immigrant to Australia. J. Med. Vet. Mycol. 34:279-283. [DOI] [PubMed] [Google Scholar]
- 488.Mathews, M. S., and S. V. Maharajan. 1999. Exserohilum rostratum causing keratitis in India. Med. Mycol. 37:131-132. [PubMed] [Google Scholar]
- 489.Matos, T., G. S. de Hoog, A. G. de Boer, I. de Crom, and G. Haase. 2002. High prevalence of the neurotrope Exophiala dermatitidis and related oligotrophic black yeasts in sauna facilities. Mycoses 45:373-377. [DOI] [PubMed] [Google Scholar]
- 490.Matos, T., G. Haase, A. H. Gerrits van den Ende, and G. S. de Hoog. 2003. Molecular diversity of oligotrophic and neurotropic members of the black yeast genus Exophiala, with accent on E. dermatitidis. Antonie Van Leeuwenhoek 83:293-303. [DOI] [PubMed] [Google Scholar]
- 491.Matsui, T., K. Nishimoto, S. Udagawa, H. Ishihara, and T. Ono. 1999. Subcutaneous phaeohyphomycosis caused by Phaeoacremonium rubrigenum in an immunosuppressed patient. Nippon Ishinkin. Gakkai Zasshi. 40:99-102. [DOI] [PubMed] [Google Scholar]
- 492.Matsumoto, T., A. A. Padhye, L. Ajello, and P. G. Standard. 1984. Critical review of human isolates of Wangiella dermatitidis. Mycologia 76:232-249. [Google Scholar]
- 493.Matsumoto, T., L. Ajello, T. Matsuda, P. J. Szaniszlo, and T. J. Walsh. 1994. Developments in hyalohyphomycosis and phaeohyphomycosis. J. Med. Vet. Mycol. 32(Suppl. 1):329-349. [DOI] [PubMed] [Google Scholar]
- 494.Matsumoto, T., K. Nishimoto, K. Kimura, A. A. Padhye, L. Ajello, and M. R. McGinnis. 1984. Phaeohyphomycosis caused by Exophiala moniliae. Sabouraudia 22:17-26. [PubMed] [Google Scholar]
- 495.Matsushita, A., L. Jilong, M. Hiruma, M. Kobayashi, T. Matsumoto, H. Ogawa, and A. A. Padhye. 2003. Subcutaneous phaeohyphomycosis caused by Veronaea botryosa in the People's Republic of China. J. Clin. Microbiol. 41:2219-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Matsuwaki, Y., K. Wada, T. A. White, L. M. Benson, M. C. Charlesworth, J. L. Checkel, Y. Inoue, K. Hotta, J. U. Ponikau, C. B. Lawrence, and H. Kita. 2009. Recognition of fungal protease activities induces cellular activation and eosinophil-derived neurotoxin release in human eosinophils. J. Immunol. 183:6708-6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Mayser, P., M. Nilles, and G. S. De Hoog. 2002. Case report. Cutaneous phaeohyphomycosis due to Alternaria alternata. Mycoses 45:338-340. [DOI] [PubMed] [Google Scholar]
- 498.McAleer, R., D. B. Kroenert, J. L. Elder, and J. H. Froudist. 1981. Allergic bronchopulmonary disease caused by Curvularia lunata and Drechslera hawaiiensis. Thorax 36:338-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.McCown, H. F., and E. E. Sahn. 1997. Subcutaneous phaeohyphomycosis and nocardiosis in a kidney transplant patient. J. Am. Acad. Dermatol. 36:863-866. [DOI] [PubMed] [Google Scholar]
- 500.McGill, H. C., and J. W. Brueck. 1956. Brain abscess due to Hormodendrum species. Arch. Pathol. 62:303-311. [PubMed] [Google Scholar]
- 501.McGinnis, M. R. 1983. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J. Am. Acad. Dermatol. 8:1-16. [DOI] [PubMed] [Google Scholar]
- 502.McGinnis, M. R. 1996. Mycetoma. Dermatol. Clin. 14:97-104. [DOI] [PubMed] [Google Scholar]
- 503.McGinnis, M. R., A. A. Padhye, and L. Ajello. 1982. Pseudallescheria boydii Negroni et Fischer, 1943, and its later synonym Petriellidium Mallock, 1970. Mycotaxon 14:94-102. [Google Scholar]
- 504.McGinnis, M. R., and A. A. Padhye. 2003. Fungi causing eumycotic mycetoma, p. 1848-1856. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 505.McGinnis, M. R., S. M. Lemon, D. H. Walker, G. S. De Hoog, and G. Haase. 1999. Fatal cerebritis caused by a new species of Cladophialophora. Stud. Mycol. 43:166-171. [Google Scholar]
- 506.McGinnis, M. R., and L. Pasarell. 1998. In vitro evaluation of terbinafine and itraconazole against dematiaceous fungi. Med. Mycol. 36:243-246. [PubMed] [Google Scholar]
- 507.McGinnis, M. R., and L. Pasarell. 1998. In vitro testing of susceptibilities of filamentous ascomycetes to voriconazole, itraconazole, and amphotericin B, with consideration of phylogenetic implications. J. Clin. Microbiol. 36:2353-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.McGinnis, M. R., M. G. Rinaldi, and R. E. Winn. 1986. Emerging agents of phaeohyphomycosis: pathogenic species of Bipolaris and Exserohilum. J. Clin. Microbiol. 24:250-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Meletiadis, J., J. F. Meis, J. W. Mouton, J. L. Rodriquez-Tudela, J. P. Donnelly, and P. E. Verweij. 2002. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob. Agents Chemother. 46:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Meletiadis, J., J. W. Mouton, J. F. Meis, and P. E. Verweij. 2003. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob. Agents Chemother. 47:106-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Meletiadis, J., J. W. Mouton, J. L. Rodriguez-Tudela, J. F. Meis, and P. E. Verweij. 2000. In vitro interaction of terbinafine with itraconazole against clinical isolates of Scedosporium prolificans. Antimicrob. Agents Chemother. 44:470-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Menon, S., and J. C. Edwards. 1994. Mycotic arthritis of the knee due to Madurella grisea. Br. J. Rheumatol. 33:292-295. [DOI] [PubMed] [Google Scholar]
- 513.Middleton, F. G., P. F. Jurgenson, J. P. Utz, S. Shadomy, and H. J. Shadomy. 1976. Brain abscess caused by Cladosporium trichoides. Arch. Intern. Med. 136:444-448. [PubMed] [Google Scholar]
- 514.Miele, P. S., C. S. Levy, M. A. Smith, E. M. Dugan, R. H. Cooke, J. A. Light, and D. R. Lucey. 2002. Primary cutaneous fungal infections in solid organ transplantation: a case series. Am. J. Transplant. 2:678-683. [DOI] [PubMed] [Google Scholar]
- 515.Minotto, R., C. D. Bernardi, L. F. Mallmann, M. I. Edelweiss, and M. L. Scroferneker. 2001. Chromoblastomycosis: a review of 100 cases in the state of Rio Grande do Sul, Brazil. J. Am. Acad. Dermatol. 44:585-592. [DOI] [PubMed] [Google Scholar]
- 516.Mitchell, D. M., M. Fitz-Henley, and J. Horner-Bryce. 1990. A case of disseminated phaeohyphomycosis caused by Cladosporium devriesii. West Indian Med. J. 39:118-123. [PubMed] [Google Scholar]
- 517.Mohammedi, I., M. A. Piens, C. Audigier-Valette, J. C. Gantier, L. Argaud, O. Martin, and D. Robert. 2004. Fatal Microascus trigonosporus (anamorph Scopulariopsis) pneumonia in a bone marrow transplant recipient. Eur. J. Clin. Microbiol. Infect. Dis. 23:215-217. [DOI] [PubMed] [Google Scholar]
- 518.Moore, M. K. 1986. Hendersonula toruloidea and Scytalidium hyalinum infections in London, England. J. Med. Vet. Mycol. 24:219-230. [PubMed] [Google Scholar]
- 519.Moore, M. K. 1992. The infection of human skin and nail by Scytalidium species. Curr. Top. Med. Mycol. 4:1-42. [DOI] [PubMed] [Google Scholar]
- 520.Morris, A., W. A. Schell, D. McDonagh, S. Chaffee, and J. R. Perfect. 1995. Pneumonia due to Fonsecaea pedrosoi and cerebral abscesses due to Emericella nidulans in a bone marrow transplant recipient. Clin. Infect. Dis. 21:1346-1348. [DOI] [PubMed] [Google Scholar]
- 521.Morris-Jones, R., B. L. Gomez, S. Diez, M. Uran, S. D. Morris-Jones, A. Casadevall, J. D. Nosanchuk, and A. J. Hamilton. 2005. Synthesis of melanin pigment by Candida albicans in vitro and during infection. Infect. Immun. 73:6147-6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Morris-Jones, R., S. Youngchim, J. M. Hextall, B. L. Gomez, S. D. Morris-Jones, R. J. Hay, A. Casadevall, J. D. Nosanchuk, and A. J. Hamilton. 2004. Scytalidium dimidiatum causing recalcitrant subcutaneous lesions produces melanin. J. Clin. Microbiol. 42:3789-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Morton, S. J., K. Midthun, and W. G. Merz. 1986. Granulomatous encephalitis caused by Bipolaris hawaiiensis. Arch. Pathol. Lab. Med. 110:1183-1185. [PubMed] [Google Scholar]
- 524.Moskowitz, L. B., T. J. Cleary, M. R. McGinnis, and C. B. Thomson. 1983. Phialophora richardsiae in a lesion appearing as a giant cell tumor of the tendon sheath. Arch. Pathol. Lab. Med. 107:374-376. [PubMed] [Google Scholar]
- 525.Mostert, L., J. Z. Groenewald, R. C. Summerbell, V. Robert, D. A. Sutton, A. A. Padhye, and P. W. Crous. 2005. Species of Phaeoacremonium associated with infections in humans and environmental reservoirs in infected woody plants. J. Clin. Microbiol. 43:1752-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Mroueh, S., and A. Spock. 1992. Allergic bronchopulmonary disease caused by Curvularia in a child. Ped. Pulm. 12:123-126. [DOI] [PubMed] [Google Scholar]
- 527.Mukherji, S. K., and M. Castillo. 1995. Cerebral phaeohyphomycosis caused by Xylohypha bantiana: MR findings. Am. J. Roentgenol. 164:1304-1305. [DOI] [PubMed] [Google Scholar]
- 528.Mullane, K., A. A. Toor, C. Kalnicky, T. Rodriguez, J. Klein, and P. Stiff. 2007. Posaconazole salvage therapy allows successful allogeneic hematopoietic stem cell transplantation in patients with refractory invasive mold infections. Transpl. Infect. Dis. 9:89-96. [DOI] [PubMed] [Google Scholar]
- 529.Musella, R. A., and G. H. Collins. 1971. Cerebral chromoblastomycosis. Case report. J. Neurosurg. 35:219-222. [DOI] [PubMed] [Google Scholar]
- 530.Nadkarni, T. D., A. Goel, A. Shenoy, and A. P. Karapurkar. 1993. Cladosporium bantianum (trichoides) infection of the brain. J. Postgrad. Med. 39:43-44. [PubMed] [Google Scholar]
- 531.Naim, U. R., E. S. Mahgoub, and A. H. Chagla. 1988. Fatal brain abscesses caused by Ramichloridium obovoideum: report of three cases. Acta Neurochirurg. 93:92-95. [DOI] [PubMed] [Google Scholar]
- 532.Najafzadeh, M. J., H. Badali, M. T. Illnait-Zaragozi, G. S. De Hoog, and J. F. Meis. 2010. In vitro activities of eight antifungal drugs against 55 clinical isolates of Fonsecaea spp. Antimicrob. Agents Chemother. 54:1636-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Najafzadeh, M. J., C. Gueidan, H. Badali, A. H. Van Den Ende, L. Xi, and G. S. De Hoog. 2009. Genetic diversity and species delimitation in the opportunistic genus Fonsecaea. Med. Mycol. 47:17-25. [DOI] [PubMed] [Google Scholar]
- 534.National Committe for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committe for Clinical Laboratory Standards, Wayne, PA.
- 535.Ndiaye, B., M. Develoux, M. T. Dieng, A. Kane, O. Ndir, G. Raphenon, and M. Huerre. 2000. Current report of mycetoma in Senegal: report of 109 cases. J. Mycol. Méd. 10:140-144. [Google Scholar]
- 536.Negroni, R., S. H. Helou, N. Petri, A. M. Robles, A. Arechavala, and M. H. Bianchi. 2004. Case study: posaconazole treatment of disseminated phaeohyphomycosis due to Exophiala spinifera. Clin. Infect. Dis. 38:e15-20. [DOI] [PubMed] [Google Scholar]
- 537.Negroni, R., A. Tobon, B. Bustamante, M. A. Shikanai-Yasuda, H. Patino, and A. Restrepo. 2005. Posaconazole treatment of refractory eumycetoma and chromoblastomycosis. Rev. Inst. Med. Trop. Sao Paulo 47:339-346. [DOI] [PubMed] [Google Scholar]
- 538.Nenoff, P., U. Gutz, K. Tintelnot, A. Bosse-Henck, M. Mierzwa, J. Hofmann, L. C. Horn, and U. F. Haustein. 1996. Disseminated mycosis due to Scedosporium prolificans in an AIDS patient with Burkitt lymphoma. Mycoses 39:461-465. [DOI] [PubMed] [Google Scholar]
- 539.Neoh, C. Y., S. H. Tan, and P. Perera. 2007. Cutaneous phaeohyphomycosis due to Cladophialophora bantiana in an immunocompetent patient. Clin. Exp. Dermatol. 32:539-540. [DOI] [PubMed] [Google Scholar]
- 540.Nesky, M. A., E. C. McDougal, and J. E. Peacock, Jr. 2000. Pseudallescheria boydii brain abscess successfully treated with voriconazole and surgical drainage: case report and literature review of central nervous system pseudallescheriasis. Clin. Infect. Dis. 31:673-677. [DOI] [PubMed] [Google Scholar]
- 541.Neukirch, C., C. Henry, B. Leynaert, R. Liard, J. Bousquet, and F. Neukirch. 1999. Is sensitization to Alternaria alternata a risk factor for severe asthma? A population-based study. J. Allergy Clin. Immunol. 103:709-711. [DOI] [PubMed] [Google Scholar]
- 542.Neumeister, B., W. Hartmann, M. Oethinger, B. Heymer, and R. Marre. 1994. A fatal infection with Alternaria alternata and Aspergillus terreus in a child with agranulocytosis of unknown origin. Mycoses 37:181-185. [DOI] [PubMed] [Google Scholar]
- 543.Nielsen, K., H. Lang, A. C. Shum, K. Woodruff, and J. D. Cherry. 1993. Disseminated Scedosporium prolificans infection in an immunocompromised adolescent. Pediatr. Infect. Dis. J. 12:882-884. [DOI] [PubMed] [Google Scholar]
- 544.Noble, J. A., S. A. Crow, D. G. Ahearn, and F. A. Kuhn. 1997. Allergic fungal sinusitis in the southeastern U. S. A.: involvement of a new agent Epicoccum nigrum Ehrenb. ex Schlecht. 1824. J. Med. Vet. Mycol. 35:405-409. [PubMed] [Google Scholar]
- 545.Nobrega, J. P., S. Rosemberg, A. M. Adami, E. M. Heins-Vaccari, S. Lacaz Cda, and T. de Brito. 2003. Fonsecaea pedrosoi cerebral phaeohyphomycosis (“chromoblastomycosis”): first human culture-proven case reported in Brazil. Rev. Inst. Med. Trop. Sao Paulo. 45:217-220. [DOI] [PubMed] [Google Scholar]
- 546.Nosanchuk, J. D., and A. Casadevall. 2003. The contribution of melanin to microbial pathogenesis. Cell. Microbiol. 5:203-223. [DOI] [PubMed] [Google Scholar]
- 547.Nosanchuk, J. D., and A. Casadevall. 2006. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob. Agents Chemother. 50:3519-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Nosanchuk, J. D., B. L. Gomez, S. Youngchim, S. Diez, P. Aisen, R. M. Zancope-Oliveira, A. Restrepo, A. Casadevall, and A. J. Hamilton. 2002. Histoplasma capsulatum synthesizes melanin-like pigments in vitro and during mammalian infection. Infect. Immun. 70:5124-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Nosanchuk, J. D., A. L. Rosas, S. C. Lee, and A. Casadevall. 2000. Melanisation of Cryptococcus neoformans in human brain tissue. Lancet 355:2049-2050. [DOI] [PubMed] [Google Scholar]
- 550.Nucci, M., T. Akiti, G. Barreiros, F. Silveira, S. G. Revankar, D. A. Sutton, and T. F. Patterson. 2001. Nosocomial fungemia due to Exophiala jeanselmei var. jeanselmei and a Rhinocladiella species: newly described causes of bloodstream infection. J. Clin. Microbiol. 39:514-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Nulens, E., E. De Laere, H. Vandevelde, L. B. Hilbrands, A. J. Rijs, W. J. Melchers, and P. E. Verweij. 2006. Alternaria infectoria phaeohyphomycosis in a renal transplant patient. Med. Mycol. 44:379-382. [DOI] [PubMed] [Google Scholar]
- 552.Oberto-Perdigon, L., H. Romero, M. Perez-Blanco, and R. Apitz-Castro. 2005. An ELISA test for the study of the therapeutic evolution of chromoblastomycosis by Cladophialophora carrionii in the endemic area of Falcon State, Venezuela. Rev. Iberoam. Micol. 22:39-43. [DOI] [PubMed] [Google Scholar]
- 553.Odabasi, Z., V. L. Paetznick, J. R. Rodriguez, E. Chen, and L. Ostrosky-Zeichner. 2004. In vitro activity of anidulafungin against selected clinically important mold isolates. Antimicrob. Agents Chemother. 48:1912-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Odell, J. A., S. Alvarez, D. G. Cvitkovich, D. A. Cortese, and B. L. McComb. 2000. Multiple lung abscesses due to Ochroconis gallopavum, a dematiaceous fungus, in a nonimmunocompromised wood pulp worker. Chest 118:1503-1505. [DOI] [PubMed] [Google Scholar]
- 555.O'Driscoll, B. R., L. C. Hopkinson, and D. W. Denning. 2005. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulm. Med. 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Ogawa, M. M., N. Z. Galante, P. Godoy, O. Fischman-Gompertz, F. Martelli, A. L. Colombo, J. Tomimori, and J. O. Medina-Pestana. 2009. Treatment of subcutaneous phaeohyphomycosis and prospective follow-up of 17 kidney transplant recipients. J. Am. Acad. Dermatol. 61:977-985. [DOI] [PubMed] [Google Scholar]
- 557.Ogden, P. E., D. L. Hurley, and P. T. Cain. 1992. Fatal fungal endarteritis caused by Bipolaris spicifera following replacement of the aortic valve. Clin. Infect. Dis. 14:596-598. [DOI] [PubMed] [Google Scholar]
- 558.Ohira, S., K. Isoda, H. Hamanaka, K. Takahashi, K. Nishimoto, and H. Mizutani. 2002. Case report. Phaeohyphomycosis caused by Phialophora verrucosa developed in a patient with non-HIV acquired immunodeficiency syndrome. Mycoses 45:50-54. [PubMed] [Google Scholar]
- 559.O'Quinn, R. P., J. L. Hoffmann, and A. S. Boyd. 2001. Colletotrichum species as emerging opportunistic fungal pathogens: a report of 3 cases of phaeohyphomycosis and review. J. Am. Acad. Dermatol. 45:56-61. [DOI] [PubMed] [Google Scholar]
- 560.Ortoneda, M., J. Capilla, I. Pujol, F. J. Pastor, E. Mayayo, J. Fernandez-Ballart, and J. Guarro. 2002. Liposomal amphotericin B and granulocyte colony-stimulating factor therapy in a murine model of invasive infection by Scedosporium prolificans. J. Antimicrob. Chemother. 49:525-529. [DOI] [PubMed] [Google Scholar]
- 561.Osherov, A., E. Schwammenthal, R. Kuperstein, J. Strahilevitz, and M. S. Feinberg. 2006. Phialemonium curvatum prosthetic valve endocarditis with an unusual echocardiographic presentation. Echocardiography 23:503-505. [DOI] [PubMed] [Google Scholar]
- 562.Osiyemi, O. O., L. M. Dowdy, S. M. Mallon, and T. Cleary. 2001. Cerebral phaeohyphomycosis due to a novel species: report of a case and review of the literature. Transplantation 71:1343-1346. [DOI] [PubMed] [Google Scholar]
- 563.O'Sullivan, F. X., B. R. Stuewe, J. M. Lynch, J. W. Brandsberg, T. B. Wiegmann, R. V. Patak, W. G. Barnes, and G. R. Hodges. 1981. Peritonitis due to Drechslera spicifera complicating continuous ambulatory peritoneal dialysis. Ann. Intern. Med. 94:213-214. [DOI] [PubMed] [Google Scholar]
- 564.Ozbek, Z., S. Kang, J. Sivalingam, C. J. Rapuano, E. J. Cohen, and K. M. Hammersmith. 2006. Voriconazole in the management of Alternaria keratitis. Cornea 25:242-244. [DOI] [PubMed] [Google Scholar]
- 565.Oztas, E., B. Odemis, M. Kekilli, M. Kurt, B. M. Dinc, E. Parlak, A. Kalkanci, and N. Sasmaz. 2009. Systemic phaeohyphomycosis resembling primary sclerosing cholangitis caused by Exophiala dermatitidis. J. Med. Microbiol. 58:1243-1246. [DOI] [PubMed] [Google Scholar]
- 566.Padhye, A. A., L. Ajello, M. A. Wieden, and K. K. Steinbronn. 1986. Phaeohyphomycosis of the nasal sinuses caused by a new species of Exserohilum. J. Clin. Microbiol. 24:245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Padhye, A. A., M. S. Davis, D. Baer, A. Reddick, K. K. Sinha, and J. Ott. 1998. Phaeohyphomycosis caused by Phaeoacremonium inflatipes. J. Clin. Microbiol. 36:2763-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Padhye, A. A., W. B. Helwig, N. G. Warren, L. Ajello, F. W. Chandler, and M. R. McGinnis. 1988. Subcutaneous phaeohyphomycosis caused by Xylohypha emmonsii. J. Clin. Microbiol. 26:709-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Padhye, A. A., M. R. McGinnis, and L. Ajello. 1978. Thermotolerance of Wangiella dermatitidis. J. Clin. Microbiol. 8:424-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Palaoglu, S., A. Sav, T. Basak, Y. Yalcinlar, and B. W. Scheithauer. 1993. Cerebral phaeohyphomycosis. Neurosurgery 33:894-897. [DOI] [PubMed] [Google Scholar]
- 571.Palencarova, E., Z. Jesenska, L. Plank, S. Straka, T. Baska, A. Hajtman, and J. Pec. 1995. Phaeohyphomycosis caused by Alternaria species and Phaeosclera dematioides Sigler, Tsuneda and Carmichael. Clin. Exp. Dermatol. 20:419-422. [DOI] [PubMed] [Google Scholar]
- 572.Pang, K. R., J. J. Wu, D. B. Huang, and S. K. Tyring. 2004. Subcutaneous fungal infections. Dermatol. Ther. 17:523-531. [DOI] [PubMed] [Google Scholar]
- 573.Paolo, W. F., Jr., E. Dadachova, P. Mandal, A. Casadevall, P. J. Szaniszlo, and J. D. Nosanchuk. 2006. Effects of disrupting the polyketide synthase gene WdPKS1 in Wangiella [Exophiala] dermatitidis on melanin production and resistance to killing by antifungal compounds, enzymatic degradation, and extremes in temperature. BMC Microbiol. 6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Pappagianis, D., and L. Ajello. 1994. Dematiaceous—a mycologic misnomer? J. Med. Vet. Mycol. 32:319-321. [DOI] [PubMed] [Google Scholar]
- 575.Pardo, F., E. Ferrer, P. A. Romero, and M. L. Perez del Molino. 2006. Cerebral phaeiohyphomycosis due to Cladophialophora bantiana. Enferm. Infecc. Microbiol. Clin. 24:593-594. [DOI] [PubMed] [Google Scholar]
- 576.Parra, I. H., R. Galimberti, G. Galimberti, B. Guanella, and A. Kowalczuk. 2008. Lymphocutaneous nocardiosis and cutaneous pheohyphomycosis in a liver transplant recipient. Int. J. Dermatol. 47:571-574. [DOI] [PubMed] [Google Scholar]
- 577.Pastor, F. J., and J. Guarro. 2008. Alternaria infections: laboratory diagnosis and relevant clinical features. Clin. Microbiol. Infect. 14:734-746. [DOI] [PubMed] [Google Scholar]
- 578.Patel, R., C. A. Gustaferro, R. A. Krom, R. H. Wiesner, G. D. Roberts, and C. V. Paya. 1994. Phaeohyphomycosis due to Scopulariopsis brumptii in a liver transplant recipient. Clin. Infect. Dis. 19:198-200. [DOI] [PubMed] [Google Scholar]
- 579.Pathengay, A., G. Y. Shah, T. Das, and S. Sharma. 2006. Curvularia lunata endophthalmitis presenting with a posterior capsular plaque. Indian J. Ophthalmol. 54:65-66. [DOI] [PubMed] [Google Scholar]
- 580.Pauzner, R., A. Goldschmied-Reouven, I. Hay, Z. Vered, Z. Ziskind, N. Hassin, and Z. Farfel. 1997. Phaeohyphomycosis following cardiac surgery: case report and review of serious infection due to Bipolaris and Exserohilum species. Clin. Infect. Dis. 25:921-923. [DOI] [PubMed] [Google Scholar]
- 581.Pedroso, A. G. 1920. 4 casos de dermatite verrucosa produzida pela Phialophora verrucosa. Ann. Paulistas Med. Cirurgia 11:53-61. [Google Scholar]
- 582.Peerapur, B. V., S. D. Rao, S. Patil, and B. G. Mantur. 2004. Keratomycosis due to Exserohilum rostratum—a case report. Indian J. Med. Microbiol. 22:126-127. [PubMed] [Google Scholar]
- 583.Pellon Daben, R., E. Marco de Lucas, L. Martin Cuesta, T. Piedra Velasco, J. Arnaiz Garcia, R. Landeras, M. Lopez Duarte, and A. Bermudez. 2008. Imaging findings of pulmonary infection caused by Scedosporium prolificans in a deep immunocompromised patient. Emerg. Radiol. 15:47-49. [DOI] [PubMed] [Google Scholar]
- 584.Peltroche-Llacsahuanga, H., N. Schnitzler, S. Jentsch, A. Platz, S. De Hoog, K. G. Schweizer, and G. Haase. 2003. Analyses of phagocytosis, evoked oxidative burst, and killing of black yeasts by human neutrophils: a tool for estimating their pathogenicity? Med. Mycol. 41:7-14. [DOI] [PubMed] [Google Scholar]
- 585.Pendle, S., K. Weeks, M. Priest, A. Gill, B. Hudson, G. Kotsiou, and R. Pritchard. 2004. Phaeohyphomycotic soft tissue infections caused by the coelomycetous fungus Microsphaeropsis arundinis. J. Clin. Microbiol. 42:5315-5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Pereiro, M., Jr., J. Jo-Chu, and J. Toribio. 1998. Phaeohyphomycotic cyst due to Cladosporium cladosporioides. Dermatology 197:90-92. [PubMed] [Google Scholar]
- 587.Pereiro, M., Jr., M. M. Pereiro Ferreiros, G. S. De Hoog, and J. Toribio. 2004. Cutaneous infection caused by Alternaria in patients receiving tacrolimus. Med. Mycol. 42:277-282. [DOI] [PubMed] [Google Scholar]
- 588.Perez, C., M. T. Colella, C. Olaizola, C. Hartung de Capriles, S. Magaldi, and S. Mata-Essayag. 2005. Tinea nigra: report of twelve cases in Venezuela. Mycopathologia 160:235-238. [DOI] [PubMed] [Google Scholar]
- 589.Perez-Blanco, M., G. Fernandez-Zeppenfeldt, R. Hernandez, F. Yegres, and D. Borelli. 1998. Chromomycosis by Rhinocladiella aquaspera: the first case in Venezuela. Rev. Iberoam. Micol. 15:51-54. [PubMed] [Google Scholar]
- 590.Petrini, B., F. Farnebo, M. A. Hedblad, and P. Appelgren. 2006. Concomitant late soft tissue infections by Cladophialophora bantiana and Mycobacterium abscessus following tsunami injuries. Med. Mycol. 44:189-192. [DOI] [PubMed] [Google Scholar]
- 591.Pfaller, M. A., and M. R. McGinnis. 2009. The laboratory and clinical mycology, p. 55-77. In E. J. Anaissie, M. R. McGinnis, M. A. Pfaller (ed.), Clinical mycology, 2nd ed. Churchill Livingstone, Philadelphia, PA.
- 592.Pfaller, M. A., S. A. Messer, R. J. Hollis, and R. N. Jones. 2002. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob. Agents Chemother. 46:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Pickles, R. W., D. E. Pacey, D. B. Muir, and W. H. Merrell. 1996. Experience with infection by Scedosporium prolificans including apparent cure with fluconazole therapy. J. Infect. 33:193-197. [DOI] [PubMed] [Google Scholar]
- 594.Piepenbring, M., O. A. Caceres Mendez, A. A. Espino Espinoza, R. Kirschner, and H. Schofer. 2007. Chromoblastomycosis caused by Chaetomium funicola: a case report from Western Panama. Br. J. Dermatol. 157:1025-1029. [DOI] [PubMed] [Google Scholar]
- 595.Pierach, C. A., G. Gulmen, G. J. Dhar, and J. C. Kiser. 1973. Phialophora mutabilis endocarditis. Ann. Intern. Med. 79:900-901. (Letter.) [DOI] [PubMed] [Google Scholar]
- 596.Pierce, N. F., J. C. Millan, B. S. Bender, and J. L. Curtis. 1986. Disseminated Curvularia infection. Arch. Pathol. Lab. Med. 110:871. [PubMed] [Google Scholar]
- 597.Pihet, M., J. Carrere, B. Cimon, D. Chabasse, L. Delhaes, F. Symoens, and J. P. Bouchara. 2009. Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis—a review. Med. Mycol. 47:387-397. [DOI] [PubMed] [Google Scholar]
- 598.Pimentel, J. D., K. Mahadevan, A. Woodgyer, L. Sigler, C. Gibas, O. C. Harris, M. Lupino, and E. Athan. 2005. Peritonitis due to Curvularia inaequalis in an elderly patient undergoing peritoneal dialysis and a review of six cases of peritonitis associated with other Curvularia spp. J. Clin. Microbiol. 43:4288-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Pirt, S. J., and B. I. Rowley. 1969. Melanin production in Aspergillus nidulans. Biochem. J. 114:9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Pitisuttithum, P., R. Negroni, J. R. Graybill, B. Bustamante, P. Pappas, S. Chapman, R. S. Hare, and C. J. Hardalo. 2005. Activity of posaconazole in the treatment of central nervous system fungal infections. J. Antimicrob. Chemother. 56:745-755. [DOI] [PubMed] [Google Scholar]
- 601.Pitrak, D. L., E. W. Koneman, R. C. Estupinan, and J. Jackson. 1988. Phialophora richardsiae infection in humans. Rev. Infect. Dis. 10:1195-1203. [DOI] [PubMed] [Google Scholar]
- 602.Podnos, Y. D., P. Anastasio, L. De La Maza, and R. B. Kim. 1999. Cerebral phaeohyphomycosis caused by Ramichloridium obovoideum (Ramichloridium mackenziei): case report. Neurosurgery 45:372-375. [DOI] [PubMed] [Google Scholar]
- 603.Polak, A. 1984. Antimycotic therapy of experimental infections caused by dematiaceous fungi. Sabouraudia 22:279-289. [DOI] [PubMed] [Google Scholar]
- 604.Porteous, N. B., A. M. Grooters, S. W. Redding, E. H. Thompson, M. G. Rinaldi, G. S. De Hoog, and D. A. Sutton. 2003. Identification of Exophiala mesophila isolated from treated dental unit waterlines. J. Clin. Microbiol. 41:3885-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Prasad, K. N., N. Prasad, A. Gupta, R. K. Sharma, A. K. Verma, and A. Ayyagari. 2004. Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis: a single centre Indian experience. J. Infect. 48:96-101. [DOI] [PubMed] [Google Scholar]
- 606.Prenafeta-Boldu, F. X., R. Summerbell, and G. Sybren de Hoog. 2006. Fungi growing on aromatic hydrocarbons: biotechnology's unexpected encounter with biohazard? FEMS Microbiol. Rev. 30:109-130. [DOI] [PubMed] [Google Scholar]
- 607.Pritchard, R. C., and D. B. Muir. 1987. Black fungi: a survey of dematiaceous hyphomycetes from clinical specimens identified over a five-year period in a reference laboratory. Pathology 19:281-284. [DOI] [PubMed] [Google Scholar]
- 608.Proia, L. A., M. K. Hayden, P. L. Kammeyer, J. Ortiz, D. A. Sutton, T. Clark, H. J. Schroers, and R. C. Summerbell. 2004. Phialemonium: an emerging mold pathogen that caused 4 cases of hemodialysis-associated endovascular infection. Clin. Infect. Dis. 39:373-379. [DOI] [PubMed] [Google Scholar]
- 609.Qiu-Xia, C., L. Chang-Xing, H. Wen-Ming, S. Jiang-Qiang, L. Wen, and L. Shun-Fang. 2008. Subcutaneous phaeohyphomycosis caused by Cladosporium sphaerospermum. Mycoses 51:79-80. [DOI] [PubMed] [Google Scholar]
- 610.Queiroz-Telles, F., P. Esterre, M. Perez-Blanco, R. G. Vitale, C. G. Salgado, and A. Bonifaz. 2009. Chromoblastomycosis: an overview of clinical manifestations, diagnosis and treatment. Med. Mycol. 47:3-15. [DOI] [PubMed] [Google Scholar]
- 611.Queiroz-Telles, F., K. S. Purim, J. N. Fillus, G. F. Bordignon, R. P. Lameira, J. Van Cutsem, and G. Cauwenbergh. 1992. Itraconazole in the treatment of chromoblastomycosis due to Fonsecaea pedrosoi. Int. J. Dermatol. 31:805-812. [DOI] [PubMed] [Google Scholar]
- 612.Rabodonirina, M., S. Paulus, F. Thevenet, R. Loire, E. Gueho, O. Bastien, J. F. Mornex, M. Celard, and M. A. Piens. 1994. Disseminated Scedosporium prolificans (S. inflatum) infection after single-lung transplantation. Clin. Infect. Dis. 19:138-142. [DOI] [PubMed] [Google Scholar]
- 613.Rajendran, C., B. K. Khaitan, R. Mittal, M. Ramam, M. Bhardwaj, and K. K. Datta. 2003. Phaeohyphomycosis caused by Exophiala spinifera in India. Med. Mycol. 41:437-441. [DOI] [PubMed] [Google Scholar]
- 614.Rallis, E., and E. Frangoulis. 2006. Successful treatment of subcutaneous phaeohyphomycosis owing to Exophiala jeanselmei with oral terbinafine. Int. J. Dermatol. 45:1369-1370. [DOI] [PubMed] [Google Scholar]
- 615.Rebell, G., and R. K. Forster. 1976. Lasiodiplodia theobromae as a cause of keratomycoses. Sabouraudia 14:155-170. [DOI] [PubMed] [Google Scholar]
- 616.Redondo-Bellon, P., M. Idoate, M. Rubio, and J. Ignacio Herrero. 1997. Chromoblastomycosis produced by Aureobasidium pullulans in an immunosuppressed patient. Arch. Dermatol. 133:663-664. [PubMed] [Google Scholar]
- 617.Rees, J. R., R. W. Pinner, R. A. Hajjeh, M. E. Brandt, and A. L. Reingold. 1998. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992-1993: results of population-based laboratory active surveillance. Clin. Infect. Dis. 27:1138-1147. [PubMed] [Google Scholar]
- 618.Reiss-Levy, E., and P. Clingan. 1981. Peritonitis caused by Alternaria alternata. Med. J. Aust. 2:44. [PubMed] [Google Scholar]
- 619.Remon, C., I. J. de la Calle, F. Vallejo Carrion, S. Perez-Ramos, and E. Fernandez Ruiz. 1996. Exophiala jeanselmei peritonitis in a patient on CAPD. Perit. Dial. Int. 16:536-538. [PubMed] [Google Scholar]
- 620.Restrepo, A., A. Gonzalez, I. Gomez, M. Arango, and C. de Bedout. 1988. Treatment of chromoblastomycosis with itraconazole. Ann. N. Y. Acad. Sci. 544:504-516. [DOI] [PubMed] [Google Scholar]
- 621.Restrepo, A., M. R. McGinnis, D. Malloch, A. Porras, N. Giraldo, A. Villegas, and J. Herrera. 1984. Fungal endocarditis caused by Arnium leporinum following cardiac surgery. Sabouraudia 22:225-234. [PubMed] [Google Scholar]
- 622.Revankar, S. G. 2004. Dematiaceous fungi. Semin. Resp. Crit. Care Med. 25:183-189. [DOI] [PubMed] [Google Scholar]
- 623.Revankar, S. G. 2006. Phaeohyphomycosis. Infect. Dis. Clin. North Am. 20:609-620. [DOI] [PubMed] [Google Scholar]
- 624.Revankar, S. G. 2007. Phaeohyphomycosis, p. 243-252. In C. A. Kauffman (ed.), Atlas of fungal infections, 2nd ed. Current Medicine, Philadelphia, PA.
- 625.Revankar, S. G. 2005. Therapy of infections caused by dematiaceous fungi. Expert Rev. Anti Infect. Ther. 3:601-612. [DOI] [PubMed] [Google Scholar]
- 626.Revankar, S. G., M. D. Nailor, and J. D. Sobel. 2008. Use of terbinafine in rare and refractory mycoses. Future Microbiol. 3:9-17. [DOI] [PubMed] [Google Scholar]
- 627.Revankar, S. G., J. E. Patterson, D. A. Sutton, R. Pullen, and M. G. Rinaldi. 2002. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin. Infect. Dis. 34:467-476. [DOI] [PubMed] [Google Scholar]
- 628.Revankar, S. G., D. A. Sutton, and M. G. Rinaldi. 2004. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin. Infect. Dis. 38:206-216. [DOI] [PubMed] [Google Scholar]
- 629.Riley, O., and S. H. Mann. 1960. Brain abscess caused by Cladosporioum trichoides. Am. J. Clin. Pathol. 33:525-531. [Google Scholar]
- 630.Rinaldi, M. 1996. G. Phaeohyphomycosis. Dermatol. Clin. 14:147-153. [DOI] [PubMed] [Google Scholar]
- 631.Rinaldi, M. G., P. Phillips, J. G. Schwartz, R. E. Winn, G. R. Holt, F. W. Shagets, J. Elrod, G. Nishioka, and T. B. Aufdemorte. 1987. Human Curvularia infections. Report of five cases and review of the literature. Diagn. Microbiol. Infect. Dis. 6:27-39. [DOI] [PubMed] [Google Scholar]
- 632.Rios-Fabra, A., A. R. Moreno, and R. E. Isturiz. 1994. Fungal infection in Latin American countries. Infect. Dis. Clin. North Am. 8:129-154. [PubMed] [Google Scholar]
- 633.Rivard, R. G., S. McCall, M. E. Griffith, J. S. Hawley, R. A. Ressner, H. Borra, J. E. Moon, M. L. Beckius, C. K. Murray, and D. R. Hospenthal. 2007. Efficacy of caspofungin and posaconazole in a murine model of disseminated Exophiala infection. Med. Mycol. 45:685-689. [DOI] [PubMed] [Google Scholar]
- 634.Rivero, M., A. Hidalgo, A. Alastruey-Izquierdo, M. Cia, L. Torroba, and J. L. Rodriguez-Tudela. 2009. Infections due to Phialemonium species: case report and review. Med. Mycol. 47:766-774. [DOI] [PubMed] [Google Scholar]
- 635.Robson, A. M., and R. D. Craver. 1994. Curvularia urinary tract infection: a case report. Pediatr. Nephrol. 8:83-84. [DOI] [PubMed] [Google Scholar]
- 636.Roche, M., R. M. Redmond, S. O'Neill, and E. Smyth. 2005. A case of multiple cerebral abscesses due to infection with Cladophialophora bantiana. J. Infect. 51:e285-288. [DOI] [PubMed] [Google Scholar]
- 637.Rodriguez, M. M., E. Calvo, C. Serena, M. Marine, F. J. Pastor, and J. Guarro. 2009. Effects of double and triple combinations of antifungal drugs in a murine model of disseminated infection by Scedosporium prolificans. Antimicrob. Agents Chemother. 53:2153-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Rodriguez-Tudela, J. L., J. Berenguer, J. Guarro, A. S. Kantarcioglu, R. Horre, G. S. de Hoog, and M. Cuenca-Estrella. 2009. Epidemiology and outcome of Scedosporium prolificans infection, a review of 162 cases. Med. Mycol. 47:359-370. [DOI] [PubMed] [Google Scholar]
- 639.Roeijmans, H. J., G. S. De Hoog, C. S. Tan, and M. J. Figge. 1997. Molecular taxonomy and GC/MS of metabolites of Scytalidium hyalinum and Nattrassia mangiferae (Hendersonula toruloidea). J. Med. Vet. Mycol. 35:181-188. [PubMed] [Google Scholar]
- 640.Rohwedder, J. J., J. L. Simmons, H. Colfer, and B. Gatmaitan. 1979. Disseminated Curvularia lunata infection in a football player. Arch. Intern. Med. 139:940-941. [PubMed] [Google Scholar]
- 641.Romano, C., R. Bilenchi, C. Alessandrini, and C. Miracco. 1999. Case report. Cutaneous phaeohyphomycosis caused by Cladosporium oxysporum. Mycoses 42:111-115. [DOI] [PubMed] [Google Scholar]
- 642.Romano, C., M. Fimiani, M. Pellegrino, L. Valenti, L. Casini, C. Miracco, and E. Faggi. 1996. Cutaneous phaeohyphomycosis due to Alternaria tenuissima. Mycoses 39:211-215. [DOI] [PubMed] [Google Scholar]
- 643.Romano, C., E. Paccagnini, and E. M. Difonzo. 2001. Onychomycosis caused by Alternaria spp. in Tuscany, Italy from 1985 to 1999. Mycoses 44:73-76. [DOI] [PubMed] [Google Scholar]
- 644.Romano, C., L. Valenti, C. Miracco, C. Alessandrini, E. Paccagnini, E. Faggi, and E. M. Difonzo. 1997. Two cases of cutaneous phaeohyphomycosis by Alternaria alternata and Alternaria tenuissima. Mycopathologia 137:65-74. [DOI] [PubMed] [Google Scholar]
- 645.Romano, C., L. Vanzi, D. Massi, and E. M. Difonzo. 2005. Subcutaneous alternariosis. Mycoses 48:408-412. [DOI] [PubMed] [Google Scholar]
- 646.Ronan, S. G., I. Uzoaru, V. Nadimpalli, J. Guitart, and J. R. Manaligod. 1993. Primary cutaneous phaeohyphomycosis: report of seven cases. J. Cutan. Pathol. 20:223-228. [DOI] [PubMed] [Google Scholar]
- 647.Roncoroni, A. J., and J. Smayevsky. 1988. Arthritis and endocarditis from Exophiala jeanselmei infection. Ann. Intern. Med. 108:773. [DOI] [PubMed] [Google Scholar]
- 648.Roosje, P. J., G. S. de Hoog, J. P. Koeman, and T. Willemse. 1993. Phaeohyphomycosis in a cat caused by Alternaria infectoria E. G. Simmons. Mycoses 36:451-454. [DOI] [PubMed] [Google Scholar]
- 649.Rossmann, S. N., P. L. Cernoch, and J. R. Davis. 1996. Dematiaceous fungi are an increasing cause of human disease. Clin. Infect. Dis. 22:73-80. [DOI] [PubMed] [Google Scholar]
- 650.Rotowa, N. A., H. J. Shadomy, and S. Shadomy. 1990. In vitro activities of polyene and imidazole antifungal agents against unusual opportunistic fungal pathogens. Mycoses 33:203-211. [DOI] [PubMed] [Google Scholar]
- 651.Rowland, M. D., and W. E. Farrar. 1987. Thorn-induced Phialophora parasitica arthritis treated successfully with synovectomy and ketaconazole. Am. J. Med. Sci. 30:393-395. [Google Scholar]
- 652.Ruchel, R., M. Schaffrinski, K. R. Seshan, and G. T. Cole. 2000. Vital staining of fungal elements in deep-seated mycotic lesions during experimental murine mycoses using the parenterally applied optical brightener Blankophor. Med. Mycol. 38:231-237. [DOI] [PubMed] [Google Scholar]
- 653.Ruiz-Diez, B., and J. V. Martinez-Suarez. 2003. Isolation, characterization, and antifungal susceptibility of melanin-deficient mutants of Scedosporium prolificans. Curr. Microbiol. 46:228-232. [DOI] [PubMed] [Google Scholar]
- 654.Rupa, V., M. Jacob, M. S. Mathews, A. Job, M. Kurien, and S. M. Chandi. 2002. Clinicopathological and mycological spectrum of allergic fungal sinusitis in South India. Mycoses 45:364-367. [DOI] [PubMed] [Google Scholar]
- 655.Ryder, N. S., and I. Frank. 1992. Interaction of terbinafine with human serum and serum proteins. J. Med. Vet. Mycol. 30:451-460. [DOI] [PubMed] [Google Scholar]
- 656.Ryoo, N. H., J. S. Ha, D. S. Jeon, J. R. Kim, and E. A. Hwang. 2009. Alternaria peritonitis after contact with a cat. Perit. Dial. Int. 29:235-236. [PubMed] [Google Scholar]
- 657.Saberi, H., A. Kashfi, S. Hamidi, S. A. Tabatabai, and P. Mansouri. 2003. Cerebral phaeohyphomycosis masquerading as a parafalcian mass: case report. Surg. Neurol. 60:354-359. [DOI] [PubMed] [Google Scholar]
- 658.Saenz, R. E., W. D. Brown, and C. V. Sanders. 2001. Allergic bronchopulmonary disease caused by Bipolaris hawaiiensis presenting as a necrotizing pneumonia: case report and review of literature. Am. J. Med. Sci. 321:209-212. [DOI] [PubMed] [Google Scholar]
- 659.Safdar, A. 2003. Curvularia—favorable response to oral itraconazole therapy in two patients with locally invasive phaeohyphomycosis. Clin. Microbiol. Infect. 9:1219-1223. [DOI] [PubMed] [Google Scholar]
- 660.Saint-Jean, M., G. St-Germain, C. Laferriere, and B. Tapiero. 2007. Hospital-acquired phaeohyphomycosis due to Exserohilum rostratum in a child with leukemia. Can. J. Infect. Dis. Med. Microbiol. 18:200-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Salama, A. D., T. Rogers, G. M. Lord, R. I. Lechler, and P. D. Mason. 1997. Multiple Cladosporium brain abscesses in a renal transplant patient: aggressive management improves outcome. Transplantation 63:160-162. [DOI] [PubMed] [Google Scholar]
- 662.Salem, F. A., D. W. Kannangara, and R. Nachum. 1983. Cerebral chromomycosis. Arch. Neurol. 40:173-174. [DOI] [PubMed] [Google Scholar]
- 663.Salkin, I. F., J. A. Martinez, and M. E. Kemna. 1986. Opportunistic infection of the spleen caused by Aureobasidium pullulans. J. Clin. Microbiol. 23:828-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Salkin, I. F., M. R. McGinnis, M. J. Dykstra, and M. G. Rinaldi. 1988. Scedosporium inflatum, an emerging pathogen. J. Clin. Microbiol. 26:498-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Sandhyamani, S., R. Bhatia, L. N. Mohapatra, and S. Roy. 1981. Cerebral cladosporiosis. Surg. Neurol. 15:431-434. [DOI] [PubMed] [Google Scholar]
- 666.Santos, A. L., V. F. Palmeira, S. Rozental, L. F. Kneipp, L. Nimrichter, D. S. Alviano, M. L. Rodrigues, and C. S. Alviano. 2007. Biology and pathogenesis of Fonsecaea pedrosoi, the major etiologic agent of chromoblastomycosis. FEMS Microbiol. Rev. 31:570-591. [DOI] [PubMed] [Google Scholar]
- 667.Santosh, V., N. Khanna, S. K. Shankar, L. Pal, S. Das, A. Chandramukhi, and V. R. Kolluri. 1995. Primary mycotic abscess of the brain caused by Fonsecaea pedrosoi. Case report. J. Neurosurg. 82:128-130. [DOI] [PubMed] [Google Scholar]
- 668.Sasama, J., D. A. Sherris, S. H. Shin, G. M. Kephart, E. B. Kern, and J. U. Ponikau. 2005. New paradigm for the roles of fungi and eosinophils in chronic rhinosinusitis. Curr. Opin. Otolaryngol. Head Neck Surg. 13:2-8. [DOI] [PubMed] [Google Scholar]
- 669.Sautter, R. E., M. D. Bliss, D. Morrow, and R. E. Lee. 1984. Isolation of Exophiala jeanselmei associated with esophageal pathology—three cases, laboratory and clinical features. Mycopathologia 87:105-109. [DOI] [PubMed] [Google Scholar]
- 670.Schnadig, V. J., and G. L. Woods. 2009. Histology of fungal infections, p. 79-108. In E. J. Anaissie, M. R. McGinnis, and M. A. Pfaller (ed.), Clinical mycology, 2nd ed. Churchill Livingstone, Philadelphia, PA.
- 671.Schnitzler, N., H. Peltroche-Llacsahuanga, N. Bestier, J. Zundorf, R. Lutticken, and G. Haase. 1999. Effect of melanin and carotenoids of Exophiala (Wangiella) dermatitidis on phagocytosis, oxidative burst, and killing by human neutrophils. Infect. Immun. 67:94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Schonheyder, H. C., H. E. Jensen, W. Gams, O. Nyvad, P. Van Nga, B. Aalbaek, and J. Stenderup. 1996. Late bioprosthetic valve endocarditis caused by Phialemonium aff. curvatum and Streptococcus sanguis: a case report. J. Med. Vet. Mycol. 34:209-214. [PubMed] [Google Scholar]
- 673.Schubert, M. S. 2004. Allergic fungal sinusitis. Otolaryngol. Clin. N. Am. 37:301-326. [DOI] [PubMed] [Google Scholar]
- 674.Schubert, M. S. 2004. Allergic fungal sinusitis: pathogenesis and management strategies. Drugs 64:363-374. [DOI] [PubMed] [Google Scholar]
- 675.Schubert, M. S. 2009. Allergic fungal sinusitis: pathophysiology, diagnosis and management. Med. Mycol. 47(Suppl. 1):S324-S330. [DOI] [PubMed] [Google Scholar]
- 676.Schubert, M. S., P. S. Hutcheson, R. J. Graff, L. Santiago, and R. G. Slavin. 2004. HLA-DQB1 *03 in allergic fungal sinusitis and other chronic hypertrophic rhinosinusitis disorders. J. Allergy Clin. Immunol. 114:1376-1383. [DOI] [PubMed] [Google Scholar]
- 677.Scott, I. U., V. Cruz-Villegas, H. W. Flynn, Jr., and D. Miller. 2004. Delayed-onset, bleb-associated endophthalmitis caused by Lecythophora mutabilis. Am. J. Ophthalmol. 137:583-585. [DOI] [PubMed] [Google Scholar]
- 678.Seaworth, B. J., K. J. Kwon-Chung, J. D. Hamilton, and J. R. Perfect. 1983. Brain abscess caused by a variety of Cladosporium trichoides. Am. J. Clin. Pathol. 79:747-752. [DOI] [PubMed] [Google Scholar]
- 679.Segner, S., F. Jouret, J. F. Durant, L. Marot, and N. Kanaan. 2009. Cutaneous infection by Alternaria infectoria in a renal transplant patient. Transpl. Infect. Dis. 11:330-332. [DOI] [PubMed] [Google Scholar]
- 680.Seiberling, K., and P. J. Wormald. 2009. The role of itraconazole in recalcitrant fungal sinusitis. Am. J. Rhinol. Allergy 23:303-306. [DOI] [PubMed] [Google Scholar]
- 681.Sekhon, A. S., J. Galbraith, B. W. Mielke, A. K. Garg, and G. Sheehan. 1992. Cerebral phaeohyphomycosis caused by Xylohypha bantiana, with a review of the literature. Eur. J. Epidemiol. 8:387-390. [DOI] [PubMed] [Google Scholar]
- 682.Severo, L. C., M. C. Bassanesi, and A. T. Londero. 1994. Tinea nigra: report of four cases observed in Rio Grande do Sul (Brazil) and a review of Brazilian literature. Mycopathologia 126:157-162. [DOI] [PubMed] [Google Scholar]
- 683.Severo, L. C., F. M. Oliveira, G. Vettorato, and A. T. Londero. 1999. Mycetoma caused by Exophiala jeanselmei. Report of a case successfully treated with itraconazole and review of the literature. Rev. Iberoam. Micol. 16:57-59. [PubMed] [Google Scholar]
- 684.Sevigny, G. M., and F. A. Ramos-Caro. 2000. Treatment of chromoblastomycosis due to Fonsecaea pedrosoi with low-dose terbinafine. Cutis 66:45-46. [PubMed] [Google Scholar]
- 685.Shah, C. V., D. B. Jones, and E. R. Holz. 2001. Microsphaeropsis olivacea keratitis and consecutive endophthalmitis. Am. J. Ophthalmol. 131:142-143. [DOI] [PubMed] [Google Scholar]
- 686.Sheikh, S. S., and S. S. Amr. 2007. Mycotic cysts: report of 21 cases including eight pheomycotic cysts from Saudi Arabia. Int. J. Dermatol. 46:388-392. [DOI] [PubMed] [Google Scholar]
- 687.Shelton, B. G., K. H. Kirkland, W. D. Flanders, and G. K. Morris. 2002. Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl. Environ. Microbiol. 68:1743-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Shigemori, M., K. Kawakami, T. Kitahara, O. Ijichi, M. Mizota, N. Ikarimoto, and K. Miyata. 1996. Hepatosplenic abscess caused by Curvularia boedijn in a patient with acute monocytic leukemia. Pediatr. Infect. Dis. J. 15:1128-1129. [DOI] [PubMed] [Google Scholar]
- 689.Shigemura, T., K. Agematsu, T. Yamazaki, K. Eriko, G. Yasuda, K. Nishimura, and K. Koike. 2009. Femoral osteomyelitis due to Cladophialophora arxii in a patient with chronic granulomatous disease. Infection 37:469-473. [DOI] [PubMed] [Google Scholar]
- 690.Shin, J. H., S. K. Lee, S. P. Suh, D. W. Ryang, N. H. Kim, M. G. Rinaldi, and D. A. Sutton. 1998. Fatal Hormonema dematioides peritonitis in a patient on continuous ambulatory peritoneal dialysis: criteria for organism identification and review of other known fungal etiologic agents. J. Clin. Microbiol. 36:2157-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Shukla, P. K., Z. A. Khan, B. Lal, P. K. Agrawal, and O. P. Srivastava. 1983. Clinical and experimental keratitis caused by the Colletotrichum state of Glomerella cingulata and Acrophialophora fusispora. Sabouraudia 21:137-147. [PubMed] [Google Scholar]
- 692.Sides, E. H., III, J. D. Benson, and A. A. Padhye. 1991. Phaeohyphomycotic brain abscess due to Ochroconis gallopavum in a patient with malignant lymphoma of a large cell type. J. Med. Vet. Mycol. 29:317-322. [PubMed] [Google Scholar]
- 693.Sidrim, J. J. C., R. H. O. Menezes, G. C. Paixao, M. F. G. Rocha, R. S. N. Brilhante, A. M. A. Oliveria, and M. J. N. Diogenes. 1999. Rhinocladiella aquaspersa: limite imprecise entre chromoblastomycose et phaeohyphomycose? J. Mycol. Méd. 9:114-118. [Google Scholar]
- 694.Sigler, L., R. C. Summerbell, L. Poole, M. Wieden, D. A. Sutton, M. G. Rinaldi, M. Aguirre, G. W. Estes, and J. N. Galgiani. 1997. Invasive Nattrassia mangiferae infections: case report, literature review, and therapeutic and taxonomic appraisal. J. Clin. Microbiol. 35:433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Silva, J. P., W. de Souza, and S. Rozental. 1998. Chromoblastomycosis: a retrospective study of 325 cases on Amazonic Region (Brazil). Mycopathologia 143:171-175. [DOI] [PubMed] [Google Scholar]
- 696.Silveira, E. R., M. A. Resende, V. S. Mariano, W. A. Coura, L. D. Alkmim, L. B. Vianna, C. E. Starling, G. G. Cruz, L. H. Benicio, A. M. Paula, J. A. Gomes, G. D. Santos, M. A. Macedo, R. E. Salum, M. Gontijo, A. L. Rabello, and R. B. Caligiorne. 2003. Brain abscess caused by Cladophialophora (Xylohypha) bantiana in a renal transplant patient. Transpl. Infect. Dis. 5:104-107. [DOI] [PubMed] [Google Scholar]
- 697.Simarro, E., F. Marin, A. Morales, E. Sanz, J. Perez, and J. Ruiz. 2001. Fungemia due to Scedosporium prolificans: a description of two cases with fatal outcome. Clin. Microbiol. Infect. 7:645-647. [DOI] [PubMed] [Google Scholar]
- 698.Simitsopoulou, M., C. Gil-Lamaignere, N. Avramidis, A. Maloukou, S. Lekkas, E. Havlova, L. Kourounaki, D. Loebenberg, and E. Roilides. 2004. Antifungal activities of posaconazole and granulocyte-macrophage colony-stimulating factor ex vivo and in mice with disseminated infection due to Scedosporium prolificans. Antimicrob. Agents Chemother. 48:3801-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Singal, A., D. Pandhi, S. N. Bhattacharya, S. Das, S. Aggarwal, and K. Mishra. 2008. Pheohyphomycosis caused by Exophiala spinifera: a rare occurrence. Int. J. Dermatol. 47:44-47. [DOI] [PubMed] [Google Scholar]
- 700.Singh, H., S. Irwin, S. Falowski, M. Rosen, L. Kenyon, D. Jungkind, and J. Evans. 2008. Curvularia fungi presenting as a large cranial base meningioma: case report. Neurosurgery 63:e177. [DOI] [PubMed] [Google Scholar]
- 701.Singh, N., R. Agarwal, D. Gupta, M. R. Shivaprakash, and A. Chakrabarti. 2006. An unusual case of mediastinal mass due to Fonsecaea pedrosoi. Eur. Respir. J. 28:662-664. [DOI] [PubMed] [Google Scholar]
- 702.Singh, N., F. Y. Chang, T. Gayowski, and I. R. Marino. 1997. Infections due to dematiaceous fungi in organ transplant recipients: case report and review. Clin. Infect. Dis. 24:369-374. [DOI] [PubMed] [Google Scholar]
- 703.Singh, S. M., J. Naidu, and M. Pouranik. 1990. Ungual and cutaneous phaeohyphomycosis caused by Alternaria alternata and Alternaria chlamydospora. J. Med. Vet. Mycol. 28:275-278. [PubMed] [Google Scholar]
- 704.Siu, K., and A. K. Izumi. 2004. Phaeohyphomycosis caused by Coniothyrium. Cutis 73:127-130. [PubMed] [Google Scholar]
- 705.Slomovic, A. R., R. K. Forster, and H. Gelender. 1985. Lasiodiplodia theobromae panophthalmitis. Can. J. Ophthalmol. 20:225-228. [PubMed] [Google Scholar]
- 706.Smith, J., and D. Andes. 2008. Therapeutic drug monitoring of antifungals: pharmacokinetic and pharmacodynamic considerations. Ther. Drug Monit. 30:167-172. [DOI] [PubMed] [Google Scholar]
- 707.Smith, W. J., R. H. Drew, and J. R. Perfect. 2009. Posaconazole's impact on prophylaxis and treatment of invasive fungal infections: an update. Expert Rev. Anti Infect. Ther. 7:165-181. [DOI] [PubMed] [Google Scholar]
- 708.Sole, M., J. Cano, J. L. Rodriguez-Tudela, J. Ponton, D. A. Sutton, R. Perrie, J. Gene, V. Rodriguez, and J. Guarro. 2003. Molecular typing of clinical and environmental isolates of Scedosporium prolificans by inter-simple-sequence-repeat polymerase chain reaction. Med. Mycol. 41:293-300. [DOI] [PubMed] [Google Scholar]
- 709.Song, M. J., J. H. Lee, and N. Y. Lee. 2009. Fatal Scedosporium prolificans infection in a paediatric patient with acute lymphoblastic leukaemia. Mycoses [Epub ahead of print.] doi: 10.1111/j.1439-0507.2009.01765.x. [DOI] [PubMed]
- 710.Sood, N., H. C. Gugnani, J. Guarro, A. Paliwal-Joshi, and V. K. Vijayan. 2007. Subcutaneous phaeohyphomycosis caused by Alternaria alternata in an immunocompetent patient. Int. J. Dermatol. 46:412-413. [DOI] [PubMed] [Google Scholar]
- 711.Sood, P., V. Dogra, A. Thakur, B. Mishra, A. Mandal, and S. Sinha. 2000. Brain abscess due to Xylohypha bantiana. Scand. J. Infect. Dis. 32:708-709. [DOI] [PubMed] [Google Scholar]
- 712.Sparrow, S. A., L. A. Hallam, B. E. Wild, and D. L. Baker. 1992. Scedosporium inflatum: first case report of disseminated infection and review of the literature. Pediatr. Hematol. Oncol. 9:293-295. [DOI] [PubMed] [Google Scholar]
- 713.Spielberger, R. T., B. R. Tegtmeier, M. R. O'Donnell, and J. I. Ito. 1995. Fatal Scedosporium prolificans (S. inflatum) fungemia following allogeneic bone marrow transplantation: report of a case in the United States. Clin. Infect. Dis. 21:1067. [DOI] [PubMed] [Google Scholar]
- 714.Srinivasan, A., B. L. Wickes, A. M. Romanelli, L. Debelenko, J. E. Rubnitz, D. A. Sutton, E. H. Thompson, A. W. Fothergill, M. G. Rinaldi, R. T. Hayden, and J. L. Shenep. 2009. Cutaneous infection caused by Macrophomina phaseolina in a child with acute myeloid leukemia. J. Clin. Microbiol. 47:1969-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 715.Srinivasan, M. 2004. Fungal keratitis. Curr. Opin. Ophthalmol. 15:321-327. [DOI] [PubMed] [Google Scholar]
- 716.Steinbach, W. J., W. A. Schell, J. L. Miller, and J. R. Perfect. 2003. Scedosporium prolificans osteomyelitis in an immunocompetent child treated with voriconazole and caspofungin, as well as locally applied polyhexamethylene biguanide. J. Clin. Microbiol. 41:3981-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Strahilevitz, J., G. Rahav, H. J. Schroers, R. C. Summerbell, Z. Amitai, A. Goldschmied-Reouven, E. Rubinstein, Y. Schwammenthal, M. S. Feinberg, Y. Siegman-Igra, E. Bash, I. Polacheck, A. Zelazny, S. J. Howard, P. Cibotaro, O. Shovman, and N. Keller. 2005. An outbreak of Phialemonium infective endocarditis linked to intracavernous penile injections for the treatment of impotence. Clin. Infect. Dis. 40:781-786. [DOI] [PubMed] [Google Scholar]
- 718.Studahl, M., T. Backteman, F. Stalhammar, E. Chryssanthou, and B. Petrini. 2003. Bone and joint infection after traumatic implantation of Scedosporium prolificans treated with voriconazole and surgery. Acta Paediatr. 92:980-982. [DOI] [PubMed] [Google Scholar]
- 719.Sudduth, E. J., A. J. Crumbley III, and W. E. Farrar. 1992. Phaeohyphomycosis due to Exophiala species: clinical spectrum of disease in humans. Clin. Infect. Dis. 15:639-644. [DOI] [PubMed] [Google Scholar]
- 720.Summerbell, R. C., S. Krajden, R. Levine, and M. Fuksa. 2004. Subcutaneous phaeohyphomycosis caused by Lasiodiplodia theobromae and successfully treated surgically. Med. Mycol. 42:543-547. [DOI] [PubMed] [Google Scholar]
- 721.Surash, S., A. Tyagi, G. S. De Hoog, J. S. Zeng, R. C. Barton, and R. P. Hobson. 2005. Cerebral phaeohyphomycosis caused by Fonsecaea monophora. Med. Mycol. 43:465-472. [DOI] [PubMed] [Google Scholar]
- 722.Sutton, D. A. 2008. Basic mycology, p. 15-35. In D. R. Hospenthal and M. G. Rinaldi (ed.), Diagnosis and treatment of human mycoses. Humana Press, Towata, NJ.
- 723.Sutton, D. A. 2007. Specimen collection, transport, and processing: mycology, p. 1728-1735. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 724.Sutton, D. A., M. G. Rinaldi, S. E. Sanche. 2009. Dematiaceous fungi, p. 329-354. In E. J. Anaissie, M. R. McGinnis, and M. A. Pfaller (ed.). Clinical mycology, 2nd ed. Elsevier, Philadelphia, PA.
- 725.Sutton, D. A., M. G. Rinaldi, and M. Kielhofner. 2004. First U.S. report of subcutaneous phaeohyphomycosis caused by Veronaea botryosa in a heart transplant recipient and review of the literature. J. Clin. Microbiol. 42:2843-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726.Sutton, D. A., M. Slifkin, R. Yakulis, and M. G. Rinaldi. 1998. U.S. case report of cerebral phaeohyphomycosis caused by Ramichloridium obovoideum (R. mackenziei): criteria for identification, therapy, and review of other known dematiaceous neurotropic taxa. J. Clin. Microbiol. 36:708-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Sutton, D. A., W. D. Timm, G. Morgan-Jones, and M. G. Rinaldi. 1999. Human phaeohyphomycotic osteomyelitis caused by the coelomycete Phomopsis Saccardo 1905: criteria for identification, case history, and therapy. J. Clin. Microbiol. 37:807-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Suzuki, Y., S. Udagawa, H. Wakita, N. Yamada, H. Ichikawa, F. Furukawa, and M. Takigawa. 1998. Subcutaneous phaeohyphomycosis caused by Geniculosporium species; a new fungal pathogen. Br. J. Dermatol. 138:346-350. [DOI] [PubMed] [Google Scholar]
- 729.Symmers, W. S. C. 1960. A case of cerebral chromoblastomycosis (Cladosporiosis) occurring in Britain as a complication of polyarteritis treated with cortisone. Brain 83:37-51. [DOI] [PubMed] [Google Scholar]
- 730.Szaniszlo, P. J. 2002. Molecular genetic studies of the model dematiaceous pathogen Wangiella dermatitidis. Int. J. Med. Microbiol. 292:381-390. [DOI] [PubMed] [Google Scholar]
- 731.Taj-Aldeen, S. J., M. Almaslamani, A. Alkhal, I. A. Bozom, A. M. Romanelli, B. L. Wickes, A. W. Fothergill, and D. A. Sutton. 2010. Cerebral phaeohyphomycosis due to Rhinocladiella mackenziei (formerly Ramichloridium mackenziei): a taxonomic update and review of the literature. Med. Mycol. 48:546-556. [DOI] [PubMed] [Google Scholar]
- 732.Taj-Aldeen, S. J., A. A. Hilal, and W. A. Schell. 2004. Allergic fungal rhinosinusitis: a report of 8 cases. Am. J. Otolaryngol. 25:213-218. [DOI] [PubMed] [Google Scholar]
- 733.Takei, H., J. C. Goodman, and S. Z. Powell. 2007. Cerebral phaeohyphomycosis caused by Cladophialophora bantiana and Fonsecaea monophora: report of three cases. Clin. Neuropathol. 26:21-27. [DOI] [PubMed] [Google Scholar]
- 734.Tamm, M., M. Malouf, and A. Glanville. 2001. Pulmonary Scedosporium infection following lung transplantation. Transplant. Infect. Dis. 3:189-194. [DOI] [PubMed] [Google Scholar]
- 735.Tan, D. H., L. Sigler, C. F. Gibas, and I. W. Fong. 2008. Disseminated fungal infection in a renal transplant recipient involving Macrophomina phaseolina and Scytalidium dimidiatum: case report and review of taxonomic changes among medically important members of the Botryosphaeriaceae. Med. Mycol. 46:285-292. [DOI] [PubMed] [Google Scholar]
- 736.Tan, H. P., H. E. Wahlstrom, J. U. Zamora, and T. Hassanein. 1997. Aureobasidium pneumonia in a post liver transplant recipient: a case report. Hepatol. Gastroenterol. 44:1215-1218. [PubMed] [Google Scholar]
- 737.Taylor, J. W., D. J. Jacobson, S. Kroken, T. Kasuga, D. M. Geiser, D. S. Hibbett, and M. C. Fisher. 2000. Phylogenetic species recognition and species concepts in fungi. Fungal Genet. Biol. 31:21-32. [DOI] [PubMed] [Google Scholar]
- 738.Tekkok, I. H., M. J. Higgins, and E. C. Ventureyra. 1996. Posttraumatic gas-containing brain abscess caused by Clostridium perfringens with unique simultaneous fungal suppuration by Myceliophthora thermophila: case report. Neurosurgery 39:1247-1251. [DOI] [PubMed] [Google Scholar]
- 739.Terra, F., T. M. Fonseca, and O. E. Area Leao. 1922. Novo typo de dermatite verrucosa mycose por Achroteca com associacao de leishmaniose. Brazil Med. 36:363-368. [Google Scholar]
- 740.Terreni, A. A., A. F. DiSalvo, A. S. Baker, Jr., W. B. Crymes, P. R. Morris, and H. Dowda, Jr. 1990. Disseminated Dactylaria gallopava infection in a diabetic patient with chronic lymphocytic leukemia of the T-cell type. Am. J. Clin. Pathol. 94:104-107. [DOI] [PubMed] [Google Scholar]
- 741.Tessari, G., A. Forni, R. Ferretto, M. Solbiati, G. Faggian, A. Mazzucco, and A. Barba. 2003. Lethal systemic dissemination from a cutaneous infection due to Curvularia lunata in a heart transplant recipient. J. Eur. Acad. Dermatol. Venereol. 17:440-442. [DOI] [PubMed] [Google Scholar]
- 742.Thomas, C., D. Mileusnic, R. B. Carey, M. Kampert, and D. Anderson. 1999. Fatal Chaetomium cerebritis in a bone marrow transplant patient. Hum. Pathol. 30:874-879. [DOI] [PubMed] [Google Scholar]
- 743.Thomas, P. A. 2003. Current perspectives on ophthalmic mycoses. Clin. Microbiol. Rev. 16:730-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Thomas, P. A. 2003. Fungal infections of the cornea. Eye 17:852-862. [DOI] [PubMed] [Google Scholar]
- 745.Thompson, G. R., and J. S. Lewis. 2010. Pharmacology and clinical use of voriconazole. Expert Opin. Drug Metab. Toxicol. 6:83-94. [DOI] [PubMed] [Google Scholar]
- 746.Tintelnot, K., G. S. De Hoog, E. Thomas, W. I. Steudel, K. Huebner, and H. P. Seeliger. 1991. Cerebral phaeohyphomycosis caused by an Exophiala species. Mycoses 34:239-244. [DOI] [PubMed] [Google Scholar]
- 747.Tintelnot, K., G. Just-Nubling, R. Horre, B. Graf, I. Sobottka, M. Seibold, A. Haas, U. Kaben, and G. S. De Hoog. 2009. A review of German Scedosporium prolificans cases from 1993 to 2007. Med. Mycol. 47:351-358. [DOI] [PubMed] [Google Scholar]
- 748.Tintelnot, K., P. von Hunnius, G. S. de Hoog, A. Polak-Wyss, E. Gueho, and F. Masclaux. 1995. Systemic mycosis caused by a new Cladophialophora species. J. Med. Vet. Mycol. 33:349-354. [PubMed] [Google Scholar]
- 749.Tokuhisa, Y., Y. Hagiya, M. Hiruma, and K. Nishimura. 2010. Phaeohyphomycosis of the face caused by Exophiala oligosperma. Mycoses [Epub ahead of pring.] doi: 10.1111/j.1439-0507.2009.01845.x. [DOI] [PubMed]
- 750.Tong, S. Y., A. Y. Peleg, J. Yoong, R. Handke, J. Szer, and M. Slavin. 2007. Breakthrough Scedosporium prolificans infection while receiving voriconazole prophylaxis in an allogeneic stem cell transplant recipient. Transpl. Infect. Dis. 9:241-243. [DOI] [PubMed] [Google Scholar]
- 751.Torres-Rodriguez, J. M., M. P. Gonzalez, J. M. Corominas, and R. M. Pujol. 2005. Successful thermotherapy for a subcutaneous infection due to Alternaria alternata in a renal transplant recipient. Arch. Dermatol. 141:1171-1173. [DOI] [PubMed] [Google Scholar]
- 752.Tosti, A., B. M. Piraccini, S. Lorenzi, and M. Iorizzo. 2003. Treatment of non-dermatophyte mold and Candida onychomycosis. Dermatol. Clin. 21:491-497. [DOI] [PubMed] [Google Scholar]
- 753.Travis, W. D., K. J. Kwon-Chung, D. E. Kleiner, A. Geber, W. Lawson, H. I. Pass, and D. Henderson. 1991. Unusual aspects of allergic bronchopulmonary fungal disease: report of two cases due to Curvularia organisms associated with allergic fungal sinusitis. Hum. Pathol. 22:1240-1248. [DOI] [PubMed] [Google Scholar]
- 754.Trinh, J. V., W. J. Steinbach, W. A. Schell, J. Kurtzberg, S. S. Giles, and J. R. Perfect. 2003. Cerebral phaeohyphomycosis in an immunodeficient child treated medically with combination antifungal therapy. Med. Mycol. 41:339-345. [DOI] [PubMed] [Google Scholar]
- 755.Tsai, C. Y., Y. C. Lu, L. Wang, T. L. Hsu, and J. Sung. 1966. Systemic chromoblastomycosis due to Hormodendrum dermatitidis (Kano) Conant. Am. J. Clin. Pathol. 46:103-114. [DOI] [PubMed] [Google Scholar]
- 756.Tsai, H. F., Y. C. Chang, R. G. Washburn, M. H. Wheeler, and K. J. Kwon-Chung. 1998. The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J. Bacteriol. 180:3031-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 757.Tsai, H. F., M. H. Wheeler, Y. C. Chang, and K. J. Kwon-Chung. 1999. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 181:6469-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758.Tu, E. Y. 2009. Alternaria keratitis: clinical presentation and resolution with topical fluconazole or intrastromal voriconazole and topical caspofungin. Cornea 28:116-119. [DOI] [PubMed] [Google Scholar]
- 759.Tunuguntla, A., M. M. Saad, J. Abdalla, and J. W. Myers. 2005. Multiple brain abscesses caused by Cladophialophora bantianum: a challenging case. Tenn. Med. 98:227-. 228:235. [PubMed] [Google Scholar]
- 760.Turiansky, G. W., P. M. Benson, L. C. Sperling, P. Sau, I. F. Salkin, M. R. McGinnis, and W. D. James. 1995. Phialophora verrucosa: a new cause of mycetoma. J. Am. Acad. Dermatol. 32:311-315. [DOI] [PubMed] [Google Scholar]
- 761.Uberti-Foppa, C., L. Fumagalli, N. Gianotti, A. M. Viviani, R. Vaiani, and E. Gieho. 1995. First case of osteomyelitis due to Phialophora richardsiae in a patient with HIV infection. AIDS 9:975-976. [PubMed] [Google Scholar]
- 762.Ujhelyi, M. R., R. H. Raasch, C. M. van der Horst, and W. D. Mattern. 1990. Treatment of peritonitis due to Curvularia and Trichosporon with amphotericin B. Rev. Infect. Dis. 12:621-627. [DOI] [PubMed] [Google Scholar]
- 763.Umabala, P., V. Lakshmi, A. R. Murthy, V. S. Prasad, C. Sundaram, and H. Beguin. 2001. Isolation of a Nodulisporium species from a case of cerebral phaeohyphomycosis. J. Clin. Microbiol. 39:4213-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764.Umemoto, N., T. Demitsu, M. Kakurai, K. Sasaki, R. Azuma, E. Iida, K. Yoneda, M. Kawasaki, and T. Mochizuki. 2009. Two cases of cutaneous phaeohyphomycosis due to Exophiala jeanselmei: diagnostic significance of direct microscopical examination of the purulent discharge. Clin. Exp. Dermatol. 34:e351-353. [DOI] [PubMed] [Google Scholar]
- 765.Vachharajani, T. J., F. Zaman, S. Latif, R. Penn, and K. D. Abreo. 2005. Curvularia geniculata fungal peritonitis: a case report with review of literature. Int. Urol. Nephrol. 37:781-784. [DOI] [PubMed] [Google Scholar]
- 766.van de Sande, W. W., J. de Kat, J. Coppens, A. O. Ahmed, A. Fahal, H. Verbrugh, and A. van Belkum. 2007. Melanin biosynthesis in Madurella mycetomatis and its effect on susceptibility to itraconazole and ketoconazole. Microbes Infect. 9:1114-1123. [DOI] [PubMed] [Google Scholar]
- 767.van de Sande, W. W., A. Luijendijk, A. O. Ahmed, I. A. Bakker-Woudenberg, and A. van Belkum. 2005. Testing of the in vitro susceptibilities of Madurella mycetomatis to six antifungal agents by using the Sensititre system in comparison with a viability-based 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay and a modified NCCLS method. Antimicrob. Agents Chemother. 49:1364-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 768.van Duin, D., A. Casadevall, and J. D. Nosanchuk. 2002. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibilities to amphotericin B and caspofungin. Antimicrob. Agents Chemother. 46:3394-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 769.Vartian, C. V., D. M. Shlaes, A. A. Padhye, and L. Ajello. 1985. Wangiella dermatitidis endocarditis in an intravenous drug user. Am. J. Med. 78:703-707. [DOI] [PubMed] [Google Scholar]
- 770.Velazquez, L. F., A. Restrepo, and G. Calle. 1976. Cromomicosis: experiencia de doce azos. Acta Med. Colomb. 1:165-171. [Google Scholar]
- 771.Venarske, D. L., and R. D. deShazo. 2002. Sinobronchial allergic mycosis: the SAM syndrome. Chest 121:1670-1676. [DOI] [PubMed] [Google Scholar]
- 772.Ventin, M., C. Ramirez, and J. Garau. 1987. Exophiala dermatitidis de Hoog from a valvular aortal prothesis. Mycopathologia 99:45-46. [DOI] [PubMed] [Google Scholar]
- 773.Verkley, G. J. M., M. da Silva, D. T. Wicklow, and P. W. Crous. 2004. Paraconiothyrium, a new genus to accommodate the mycoparasite Coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species. Stud. Mycol. 50:323-335. [Google Scholar]
- 774.Vermes, A., H. J. Guchelaar, and J. Dankert. 2000. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J. Antimicrob. Chemother. 46:171-179. [DOI] [PubMed] [Google Scholar]
- 775.Vidal, M. S., L. G. de Castro, S. C. Cavalecate, and S. Lacaz Cda. 2003. Immunoprecipitation techniques and Elisa in the detection of anti-Fonsecaea pedrosoi antibodies in chromoblastomycosis. Rev. Inst. Med. Trop. Sao Paulo 45:315-318. [DOI] [PubMed] [Google Scholar]
- 776.Vieira, M. R., A. Milheiro, and F. A. Pacheco. 2001. Phaeohyphomycosis due to Cladosporium cladosporioides. Med. Mycol. 39:135-137. [DOI] [PubMed] [Google Scholar]
- 777.Vijaykrishna, D., L. Mostert, R. Jeewon, W. Gams, K. D. Hyde, and P. W. Crous. 2005. Pleurostomophora, an anamorph of Pleurostoma (Calosphaeriales), a new anamorph genus morphologically similar to Phialophora. Stud. Mycol. 50:387-395. [Google Scholar]
- 778.Villalba, E., and J. F. Yegres. 1988. Detection of circulating antibodies in patients affected by chromoblastomycosis by Cladosporium carrionii using double immunodiffusion. Mycopathologia 102:17-19. [DOI] [PubMed] [Google Scholar]
- 779.Vincente, V. A., D. Attili-Angelis, M. R. Pie, F. Queiroz-Telles, L. M. Cruz, M. J. Najafzadeh, G. S. de Hoog, J. Zhao, and A. Pizzirani-Kleiner. 2008. Environmental isolation of black yeast-like fungi involved in human infection. Stud. Mycol. 61:137-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 780.Vitale, R. G., and G. S. De Hoog. 2002. Molecular diversity, new species and antifungal susceptibilities in the Exophiala spinifera clade. Med. Mycol. 40:545-556. [DOI] [PubMed] [Google Scholar]
- 781.Vitale, R. G., G. S. De Hoog, and P. E. Verweij. 2003. In vitro activity of amphotericin B, itraconazole, terbinafine and 5-fluocytosine against Exophiala spinifera and evaluation of post-antifungal effects. Med. Mycol. 41:301-307. [DOI] [PubMed] [Google Scholar]
- 782.Vitale, R. G., M. Perez-Blanco, and G. S. De Hoog. 2009. In vitro activity of antifungal drugs against Cladophialophora species associated with human chromoblastomycosis. Med. Mycol. 47:35-40. [DOI] [PubMed] [Google Scholar]
- 783.Vlassopoulos, D., G. Kouppari, D. Arvanitis, K. Papaefstathiou, A. Dounavis, A. Velegraki, and V. Hadjiconstantinou. 2001. Wangiella dermatitidis peritonitis in a CAPD patient. Perit. Dial. Int. 21:96-97. [PubMed] [Google Scholar]
- 784.Vogelgesang, S. A., J. W. Lockard, M. J. Quinn, and J. A. Hasbargen. 1990. Alternaria peritonitis in a patient undergoing continuous ambulatory peritoneal dialysis. Perit. Dial. Int. 10:313. [PubMed] [Google Scholar]
- 785.Vollmer, T., M. Stormer, K. Kleesiek, and J. Dreier. 2008. Evaluation of novel broad-range real-time PCR assay for rapid detection of human pathogenic fungi in various clinical specimens. J. Clin. Microbiol. 46:1919-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786.Vukmir, R. B., S. Kusne, P. Linden, W. Pasculle, A. W. Fothergill, J. Sheaffer, J. Nieto, R. Segal, H. Merhav, and A. J. Martinez. 1994. Successful therapy for cerebral phaeohyphomycosis due to Dactylaria gallopava in a liver transplant recipient. Clin. Infect. Dis. 19:714-719. [DOI] [PubMed] [Google Scholar]
- 787.Walz, R., M. Bianchin, M. L. Chaves, M. R. Cerski, L. C. Severo, and A. T. Londero. 1997. Cerebral phaeohyphomycosis caused by Cladophialophora bantiana in a Brazilian drug abuser. J. Med. Vet. Mycol. 35:427-431. [PubMed] [Google Scholar]
- 788.Wang, T. K., W. Chiu, S. Chim, T. M. Chan, S. S. Wong, and P. L. Ho. 2003. Disseminated Ochroconis gallopavum infection in a renal transplant recipient: the first reported case and a review of the literature. Clin. Nephrol. 60:415-423. [DOI] [PubMed] [Google Scholar]
- 789.Warnock, D. W. 2007. Taxonomy and classification of fungi, p. 1721-1727. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 790.Watson, K. C. 1962. Cerebral chromoblastomycosis. J. Pathol. Bacteriol. 84:233-237. [DOI] [PubMed] [Google Scholar]
- 791.Watson, K. C., and G. M. Lines. 1957. Brain abscess due to the fungus Hormodendrum. S. African Med. J. 31:1081-1082. [PubMed] [Google Scholar]
- 792.Weber, E., C. Görke, and D. Begerow. 2002. The Lecythophora-Coniochaeta complelx: II. Molecular studies based on sequences of the large subunit of ribosomal DNA. Nova Hedwigia 74:187-200. [Google Scholar]
- 793.Weinberger, M., I. Mahrshak, N. Keller, A. Goldscmied-Reuven, N. Amariglio, M. Kramer, A. Tobar, Z. Samra, S. D. Pitlik, M. G. Rinaldi, E. Thompson, and D. Sutton. 2006. Isolated endogenous endophthalmitis due to a sporodochial-forming Phialemonium curvatum acquired through intracavernous autoinjections. Med. Mycol. 44:253-259. [DOI] [PubMed] [Google Scholar]
- 794.Westerman, D. A., B. R. Speed, and H. M. Prince. 1999. Fatal disseminated infection by Scedosporium prolificans during induction therapy for acute leukemia: a case report and literature review. Pathology 31:393-394. [DOI] [PubMed] [Google Scholar]
- 795.Wheeler, M. H., and A. A. Bell. 1988. Melanins and their importance in pathogenic fungi. Curr. Top. Med. Mycol. 2:338-387. [DOI] [PubMed] [Google Scholar]
- 796.Whyte, M., H. Irving, P. O'Regan, M. Nissen, D. Siebert, and R. Labrom. 2005. Disseminated Scedosporium prolificans infection and survival of a child with acute lymphoblastic leukemia. Pediatr. Infect. Dis. J. 24:375-377. [DOI] [PubMed] [Google Scholar]
- 797.Widmer, F., L. C. Wright, D. Obando, R. Handke, R. Ganendren, D. H. Ellis, and T. C. Sorrell. 2006. Hexadecylphosphocholine (miltefosine) has broad-spectrum fungicidal activity and is efficacious in a mouse model of cryptococcosis. Antimicrob. Agents Chemother. 50:414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 798.Wiest, P. M., K. Wiese, M. R. Jacobs, A. B. Morrissey, T. I. Abelson, W. Witt, and M. M. Lederman. 1987. Alternaria infection in a patient with acquired immunodeficiency syndrome: case report and review of invasive alternaria infections. Rev. Infect. Dis. 9:799-803. [DOI] [PubMed] [Google Scholar]
- 799.Wilhelmus, K. R. 2005. Climatology of dematiaceous fungal keratitis. Am. J. Ophthalmol. 140:1156-1157. [DOI] [PubMed] [Google Scholar]
- 800.Wilhelmus, K. R., and D. B. Jones. 2001. Curvularia keratitis. Trans. Am. Ophthalmol. Soc. 99:111-130. [PMC free article] [PubMed] [Google Scholar]
- 801.Willinger, B., G. Kopetzky, F. Harm, P. Apfalter, A. Makristathis, A. Berer, A. Bankier, and S. Winkler. 2004. Disseminated infection with Nattrassia mangiferae in an immunosuppressed patient. J. Clin. Microbiol. 42:478-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 802.Wilson, C. M., E. J. O'Rourke, M. R. McGinnis, and I. F. Salkin. 1990. Scedosporium inflatum: clinical spectrum of a newly recognized pathogen. J. Infect. Dis. 161:102-107. [DOI] [PubMed] [Google Scholar]
- 803.Wilson, E. 1982. Cerebral abscess caused by Cladosporium bantianum. Case report. Pathology 14:91-96. [DOI] [PubMed] [Google Scholar]
- 804.Wise, K. A., B. R. Speed, D. H. Ellis, and J. H. Andrew. 1993. Two fatal infections in immunocompromised patients caused by Scedosporium inflatum. Pathology 25:187-189. [DOI] [PubMed] [Google Scholar]
- 805.Woo, P. C., S. K. Lau, A. H. Ngan, H. Tse, E. T. Tung, and K. Y. Yuen. 2008. Lasiodiplodia theobromae pneumonia in a liver transplant recipient. J. Clin. Microbiol. 46:380-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 806.Wood, G. M., J. G. McCormack, D. B. Muir, D. H. Ellis, M. F. Ridley, R. Pritchard, and M. Harrison. 1992. Clinical features of human infection with Scedosporium inflatum. Clin. Infect. Dis. 14:1027-1033. [DOI] [PubMed] [Google Scholar]
- 807.Woollons, A., C. R. Darley, S. Pandian, P. Arnstein, J. Blackee, and J. Paul. 1996. Phaeohyphomycosis caused by Exophiala dermatitidis following intra-articular steroid injection. Br. J. Dermatol. 135:475-477. [PubMed] [Google Scholar]
- 808.Xi, L., C. Lu, J. Sun, X. Li, H. Liu, J. Zhang, Z. Xie, and G. S. De Hoog. 2009. Chromoblastomycosis caused by a meristematic mutant of Fonsecaea monophora. Med. Mycol. 47:77-80. [DOI] [PubMed] [Google Scholar]
- 809.Xi, L., J. Sun, C. Lu, H. Liu, Z. Xie, K. Fukushima, K. Takizawa, M. J. Najafzadeh, and G. S. De Hoog. 2009. Molecular diversity of Fonsecaea (Chaetothyriales) causing chromoblastomycosis in southern China. Med. Mycol. 47:27-33. [DOI] [PubMed] [Google Scholar]
- 810.Xie, Z., J. Zhang, L. Xi, X. Li, L. Wang, C. Lu, and J. Sun. 2010. A chronic chromoblastomycosis model by Fonsecaea monophora in Wistar rat. Med. Mycol. 48:201-206. [DOI] [PubMed] [Google Scholar]
- 811.Yamagishi, Y., K. Kawasaki, and H. Ishizaki. 1997. Mitochondrial DNA analysis of Phialophora verrucosa. Mycoses 40:329-334. [DOI] [PubMed] [Google Scholar]
- 812.Yangco, B. G., D. TeStrake, and J. Okafor. 1984. Phialophora richardsiae isolated from infected human bone: morphological, physiological and antifungal susceptibility studies. Mycopathologia 86:103-111. [DOI] [PubMed] [Google Scholar]
- 813.Yau, Y. C., J. de Nanassy, R. C. Summerbell, A. G. Matlow, and S. E. Richardson. 1994. Fungal sternal wound infection due to Curvularia lunata in a neonate with congenital heart disease: case report and review. Clin. Infect. Dis. 19:735-740. [DOI] [PubMed] [Google Scholar]
- 814.Yeghen, T., L. Fenelon, C. K. Campbell, D. W. Warnock, A. V. Hoffbrand, H. G. Prentice, and C. C. Kibbler. 1996. Chaetomium pneumonia in patient with acute myeloid leukaemia. J. Clin. Pathol. 49:184-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 815.Yehia, M., M. Thomas, H. Pilmore, W. Van Der Merwe, and I. Dittmer. 2004. Subcutaneous black fungus (phaeohyphomycosis) infection in renal transplant recipients: three cases. Transplantation 77:140-142. [DOI] [PubMed] [Google Scholar]
- 816.Yoshimori, R. N., R. A. Moore, H. H. Itabashi, and D. G. Fujikawa. 1982. Phaeohyphomycosis of brain: granulomatous encephalitis caused by Drechslera spicifera. Am. J. Clin. Pathol. 77:363-370. [DOI] [PubMed] [Google Scholar]
- 817.Young, C. N., J. G. Swart, D. Ackermann, and K. Davidge-Pitts. 1978. Nasal obstruction and bone erosion caused by Drechslera hawaiiensis. J. Laryngol. Otol. 92:137-143. [DOI] [PubMed] [Google Scholar]
- 818.Yu, J., S. Yang, Y. Zhao, and R. Li. 2006. A case of subcutaneous phaeohyphomycosis caused by Chaetomium globosum and the sequences analysis of C. globosum. Med. Mycol. 44:541-545. [DOI] [PubMed] [Google Scholar]
- 819.Yurlova, N. A., and G. S. de Hoog. 2002. Exopolysaccharides and capsules in human pathogenic Exophiala species. Mycoses 45:443-448. [DOI] [PubMed] [Google Scholar]
- 820.Yurlova, N. A., G. S. de Hoog, and A. H. G. Gerrits van den Ende. 1999. Taxonomy of Aureobasidium and allied genera. Stud. Mycol. 43:63-69. [Google Scholar]
- 821.Yustes, C., and J. Guarro. 2005. In vitro synergistic interaction between amphotericin B and micafungin against Scedosporium spp. Antimicrob. Agents Chemother. 49:3498-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 822.Zaharopoulos, P., V. J. Schnadig, K. D. Davie, R. E. Boudreau, and V. W. Weedn. 1988. Multiseptate bodies in systemic phaeohyphomycosis diagnosed by fine needle aspiration cytology. Acta Cytol. 32:885-891. [PubMed] [Google Scholar]
- 823.Zalar, P., G. S. de Hoog, and N. Gunde-Cimerman. 1999. Ecology of halotolerant dothideaceous black yeasts. Stud. Mycol. 43:38-48. [Google Scholar]
- 824.Zalar, P., C. Gostincar, G. S. de Hoog, V. Ursic, M. Sudhadham, and N. Gunde-Cimerman. 2008. Redefinition of Aureobasidium pullulans and its varieties. Stud. Mycol. 61:21-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 825.Zeng, J. S., D. A. Sutton, A. W. Fothergill, M. G. Rinaldi, M. J. Harrak, and G. S. de Hoog. 2007. Spectrum of clinically relevant Exophiala species in the United States. J. Clin. Microbiol. 45:3713-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 826.Zeppenfeldt, G., N. Richard-Yegres, F. Yegres, and R. Hernández. 1994. Cladosporium carrionii: hongo dimorfo en cactáceas de la zona endémica para la cromomicosis en Venezuela. Rev. Iberoam. Micol. 11:61-63. [Google Scholar]