Abstract
Summary: The complement system comprises several fluid-phase and membrane-associated proteins. Under physiological conditions, activation of the fluid-phase components of complement is maintained under tight control and complement activation occurs primarily on surfaces recognized as “nonself” in an attempt to minimize damage to bystander host cells. Membrane complement components act to limit complement activation on host cells or to facilitate uptake of antigens or microbes “tagged” with complement fragments. While this review focuses on the role of complement in infectious diseases, work over the past couple of decades has defined several important functions of complement distinct from that of combating infections. Activation of complement in the fluid phase can occur through the classical, lectin, or alternative pathway. Deficiencies of components of the classical pathway lead to the development of autoimmune disorders and predispose individuals to recurrent respiratory infections and infections caused by encapsulated organisms, including Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae. While no individual with complete mannan-binding lectin (MBL) deficiency has been identified, low MBL levels have been linked to predisposition to, or severity of, several diseases. It appears that MBL may play an important role in children, who have a relatively immature adaptive immune response. C3 is the point at which all complement pathways converge, and complete deficiency of C3 invariably leads to severe infections, including those caused by meningococci and pneumococci. Deficiencies of the alternative and terminal complement pathways result in an almost exclusive predisposition to invasive meningococcal disease. The spleen plays an important role in antigen processing and the production of antibodies. Splenic macrophages are critical in clearing opsonized encapsulated bacteria (such as pneumococci, meningococci, and Escherichia coli) and intraerythrocytic parasites such as those causing malaria and babesiosis, which explains the fulminant nature of these infections in persons with anatomic or functional asplenia. Paramount to the management of patients with complement deficiencies and asplenia is educating patients about their predisposition to infection and the importance of preventive immunizations and seeking prompt medical attention.
INTRODUCTION
Over the past 3 decades, with the advent of molecular biology techniques, the development of knockout and transgenic animals, and the solving of solution and crystal structures of several complement components, invaluable insights into the intricate functioning of complement have been gained. It is very clear that the functions of complement extend well beyond its originally described function, i.e., that of combating infections. This review seeks to provide an understanding of the biology of the complement system and how complement deficiencies predispose individuals to infectious diseases, as an update of the previous comprehensive review on the role of complement in infections in this journal by Figueroa and Densen (128). In addition, the infectious complications of splenectomy will be discussed.
THE COMPLEMENT SYSTEM
Activation of the Complement System
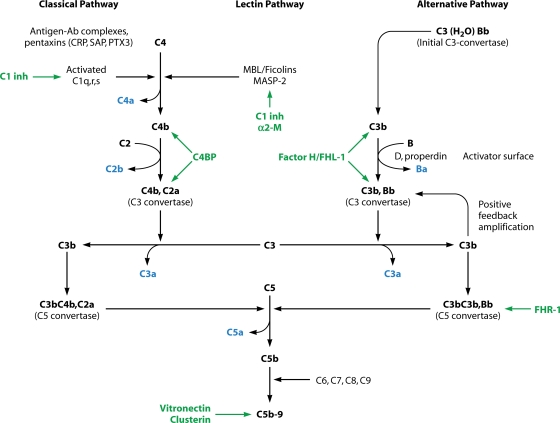
Complement activation in the fluid phase occurs through three pathways, which are called the classical, lectin, and alternative pathways. A simplified schematic overview of these three pathways is shown in Fig. 1. Key features of the individual soluble proteins that comprise the complement system are provided in Table 1.
FIG. 1.
Schematic representing the activation of the complement cascade. The fragments released into solution are indicated in blue. The key fluid-phase regulators are indicated in green. Ab, antibody; CRP, C-reactive protein; SAP, serum amyloid P component; PTX3, pentraxin 3; C1 inh, C1 inhibitor; α2-M, α2-macroglobulin; C4BP, C4b-binding protein; FHL-1, factor H-like protein-1; FHR-1, factor H-related molecule-1.
TABLE 1.
Characteristics of proteins that activate the complement cascade
| Category and component | Approx serum concn (μg/ml) | Mol mass (kDa) | Structurea | No. of genetic loci | Chromosomal assignment |
|---|---|---|---|---|---|
| Complement activator proteins | |||||
| Classical pathway proteins | |||||
| C1q | 70 | 459 | 18 polypeptide chains; 6A, 6B, 6C; A-B and C-C linked by disulfides | 3 (A, B, C) | 1p34-1p36.3 |
| C1r | 34 | 173 | Comprises a CUB (C1r/C1s, uEGF, bone morphogenetic protein) module, an epidermal growth factor (EGF)-like module, a second CUB module, two complement control protein (CCP) modules, and a C-terminal chymotrypsin-like serine protease domain; dimer (A and B chains linked by disulfide bond) | 1 | 12p13 |
| C1s | 31 | 80 | Dimer (A and B chains linked by disulfide bond); modular structure described for C1r | 1 | 12p13 |
| C4 | 600 | 206 | β-α-γ; 1 β-α and 2 α-γ disulfide bonds | 2 (C4A, C4B) | 6p21.3 |
| C2 | 11-35 | 100 | 1 chain | 1 | 6p21.3 |
| Alternative pathway proteins | |||||
| Factor B | 200 | 90 | 1 chain | 1 | 6p21.3 |
| Factor D (adipsin) | 1-2 | 25 | 1 chain | 1 | 19p13.3 |
| Lectin pathway proteins | |||||
| Mannan-binding lectin (MBL) | 1-5 | ∼25 (subunit monomer) | Subunit, trimers of identical polypeptides; subunits organized into larger oligomers (n ∼ 2 for variant [B, C, and D] alleles and n = 4-6 for wild-type [A] allele) | 1 | 10q11.2-q21 |
| Ficolin-1 (M-ficolin; ficolin/P35-related protein) | 0.04-0.1 (monocytes and PMNs main source) | ∼32 (subunit monomer) | Subunit, trimers of identical polypeptides; subunits organized into larger oligomers | 1 | 9q34 |
| Ficolin-2 (L-ficolin; hucolin; EBP-37; ficolin/P35) | 3-4 | 34 (subunit monomer) | As with ficolin-1 | 1 | 9q34 |
| Ficolin-3 (H-ficolin; Hakata antigen; thermolabile β-2 macroglycoprotein; thermolabile substance) | 18 | 35 (subunit monomer) | As with ficolin-1 | 1 | Chr 1 |
| MASP-1 | 6 | 97 | Active form consists of heavy and light chains linked by disulfide bond | 1 | 3q27-28 |
| MASP-2 | 0.02-0.8 | 83 | Active form consists of A and B chains linked by disulfide bond | 1 | 1p36.3-p36.2 |
| MASP-3 | 2-12.9 | 105 | Activation splits 105-kDa disulfide-linked dimer into A (58 kDa) and B (42 kDa); B chain is serine protease domain | 1 | 3q27-28 |
| MAp19 | 0.5 | 19 | Alternatively spliced version of MASP-2, contains first 2 domains and 4 additional C-terminal amino acids; head-to-tail homodimer | 1 | 1p36.3-p36.2 |
| C3 | 1,000-1,500 | 190 | β-α, linked by disulfide bond; crystal structure shows organization into 13 domains (8 macroglobulin domains, CUB, thioester [TED], anaphylatoxin, linker, and C345c domain) | 1 | 19p13.3-p13.2 |
| Terminal complement components | |||||
| C5 | 75 | 190 | β-α, linked by disulfide bond | 1 | 9q34.1 |
| C6 | 45 | 100 | 1 chain | 1 | 5p13 |
| C7 | 90 | 95 | 1 chain | 1 | 5p13 |
| C8 | 55-80 | 151 | α-γ dimer linked by disulfide, noncovalently associated with β | 3 | (α,β)1p32; (γ) 9q34.3 |
| C9 | 60 | 71 | 1 chain | 1 | 5p13 |
| Complement-regulatory proteins | |||||
| Positive regulator | |||||
| Properdin | 5-10 | 55 | Cyclic polymers in head-to-tail orientation; dimers-trimers-tetramers in 26:54:20 ratio; each monomer comprises 6 thrombospondin-like repeats (TSRs) and has 14 sites of C-mannosylation | 1 | Xp11.4-p11.23 |
| Negative regulators | |||||
| C1 inhibitor | 150 | 104 | 1 chain; highly glycosylated | 1 | 11q11-q13.1 |
| C4b-binding protein (C4BP) | 150-300 | ∼550 | 7 disulfide-linked α-chains (8 SCRs) linked to β-chain (3 SCRs) via disulfide (major isoform, α7/β1); minor isoforms α7/β0 and α6/β1 | 2 | (α,β) 1q32 |
| Factor H | 500 | 155 | 1 chain (20 SCRs) | 1 | 1q32 |
| Factor H-like protein-1 (FHL-1) | 25 | 43 | 1 chain (7 SCRs; identical to 7 N-terminal SCRs of factor H, plus 4 unique C-terminal amino acids) | 1 | 1q32 |
| Factor H-related molecule-1 (FHR-1) | 70-100 | 37 | 1 chain (5 SCRs; the 3 C-terminal SCRs bear 100, 100, and 97% homology with the three C-terminal SCRs of fH, respectively) | 1 | 1q32 |
| Vitronectin (S-protein) | 500 | 75 (65-kDa proteolytic | 1 chain | 1 | 17q11 |
| fragment also seen) | |||||
| Clusterin (SP-40,40; apolipoprotein J) | 100-300 | 60 (predicted); 80 (observed) | Heterodimer linked by 5-disulfide bond motif | 1 | 8p21-p12 |
| Factor J | ∼5 | ∼24 | Highly glycosylated cationic protein (pI > 9.6) | ? | ? |
SCRs, short consensus repeats.
The classical pathway.
The classical pathway is usually initiated by binding of antibodies to their target antigens. Binding of an antibody to its target exposes a binding site for C1q in the trimolecular C1 complex. C1q then binds to appropriately spaced Fc regions of immunoglobulin molecules. Therefore, it is important for IgG molecules to achieve a critical density on a surface in order to engage C1q. However, because IgM is a pentameric molecule and each target-bound IgM can bind to a C1q molecule, IgM is, on a molar basis, a more potent activator of the classical pathway than IgG. Human IgG subclasses also differ in their ability to activate complement; in general, IgG1, IgG2, and IgG3 activate complement in the order IgG3 > IgG1 > IgG2, while IgG4 does not activate complement.
The classical pathway is also activated when members of the pentraxin family (which includes C-reactive protein [CRP], serum amyloid P component [SAP], and pentraxin 3 [PTX3]) bind to surfaces and engage C1q (315, 377, 495). A novel mechanism of classical pathway activation is initiated by the binding of certain pneumococcal polysaccharides to the specific intracellular adhesion molecule (ICAM)-grabbing nonintegrin R1 (SIGN-R1). SIGN-R1 is one of five receptors that was discovered during efforts to identify the murine homolog of a human C-type lectin called dendritic cell-specific ICAM-3-grabbing nonintegrin (DC-SIGN) (218).
The C1 complex is a multimolecular protease that is formed by the association of one molecule of C1q, the recognition protein of the complex, and the Ca2+-dependent catalytic subunit, the tetramer C1s-C1r-C1r-C1s, which comprises two copies of each of the modular proteases C1r and C1s (13, 230). Binding of C1q generates a conformational signal that results in autoactivation of C1r, which in turn activates C1s. Both C1r and C1s are activated through cleavage of a single Arg-Ile bond.
Activated C1s cleaves the 77-amino-acid C4a fragment from the N terminus of the α chain of C4 to form the metastable C4b molecule. This results in exposure of the internal thioester bond of C4b (246), which can react readily with nucleophilic groups (i.e., electron-donating groups), such as with —OH to form an ester linkage or with —NH2 to form an amide linkage (96). Alternatively, the carbonyl group can react with water and become hydrolyzed. There are two isoforms of C4 present in normal human serum, which are called C4A and C4B (16). A His residue in the α chain at position 1106 imparts to C4B the ability to form ester linkages. The presence of an Asp residue at position 1106 results in C4A functionality and preferential amide bond formation with target —NH2 groups (56). Differences in the binding properties of C4A (amide) and C4B (ester) may have important functional consequences. C4B is believed to possess greater hemolytic activity than C4A. On the other hand C4A binds to complement receptor 1 (CR1) more efficiently and may play an important role in clearing immune complexes from the bloodstream; persons who are deficient in C4A or who possess lower copy numbers of the C4A gene have a higher incidence of autoimmune diseases (68, 491). In the next step in classical pathway activation, C2 binds to C4b deposited on a surface. C2 is also cleaved by activated C1s into the C2a fragment, which remains attached noncovalently to C4b, and C2b, which is released into solution. C4b,2a forms the C3 convertase (C3-cleaving enzyme) of the classical pathway. In this manner a single C1 complex can cleave several substrate molecules and serve to amplify complement activation.
The lectin pathway.
As with the C1 complex of the classical pathway, the lectin pathway also comprises recognition molecules (mannan-binding lectin [MBL] and the ficolins) and catalytic proteins (MBL-associated serine protease 1 [MASP-1], MASP-2, MASP-3, and MBL-associated protease 19 [MAp-19]). MBL consists of large oligomers assembled from identical polypeptide chains (100) and bears structural similarity to C1q.
MBL preferentially recognizes glucans, lipophosphoglycans, and glycoinositol phospholipids that contain mannose, glucose, fucose, or N-acetylglucosamine (GlcNAc) as their terminal hexose (Hex) (476). Such carbohydrate micropatterns are found in limited amounts in glycoproteins of higher animals, and these are not arranged in a repetitive pattern in the membrane that would facilitate binding to MBL (145). Furthermore, mammalian carbohydrates often terminate in sialic acid residues, which shield the relevant neutral sugars, and thus are not recognized by MBL (145).
MASPs are homologs of C1r and C1s of the classical pathway. MASP-2 bound to MBL cleaves C4. Recently, MASP-1 was shown to serve the important role of cleaving pro-factor D to its active form, factor D. Consistent with this observation, serum from a MASP-1/3-deficient mouse was unable to activate the alternative pathway (433). MASP-3 is found complexed to ficolin-3 and may inhibit the ability of ficolin-3 to activate complement (409).
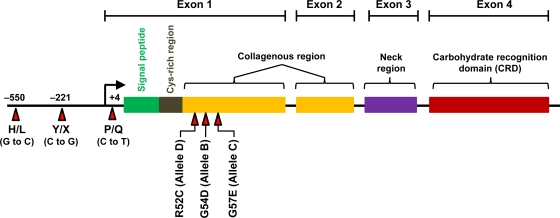
Humans and New World monkeys have a single MBL gene (mbl2), while rodents have two homologous forms of MBL, which are designated MBL-A and MBL-C and are the products of mbl1 and mbl2 genes, respectively (176, 245, 301). Exon 1 harbors three missense single-nucleotide polymorphisms (SNPs) that result in amino acid changes in the first part of the collagenous region. These SNPs result in Gly54Asp, Gly57Glu, and Arg52Cys changes and are termed the “B,” “C,” and “D” alleles, respectively (the wild-type MBL molecule is termed “A”). The B, C, and D alleles collectively are referred to as the “O” alleles, and each of these three SNPs interferes with formation of high-order MBL oligomers. In addition to the SNPs in exon 1, there are several other polymorphisms located in the MBL promoter region, some of which influence MBL expression levels. Three relevant polymorphisms are G/C at position −550 (termed H/L), C/G at position −221 (termed Y/X), and C/T at position +4 of the 5′ untranslated portion of mbl2 (termed P/Q) (270, 271). A schematic of the mbl2 gene and its associated polymorphisms is provided in Fig. 2. The promoter alleles are found in linkage disequilibrium with the exon 1 SNPs, which results in a limited number of haplotypes (HYPA, LYPA, LYQA, and LXPA with the normal A allele in exon 1 and HYPD, LYPB, and LYQC on chromosomes with variant [B, C, or D] alleles in exon 1). When the A, or wild-type, alleles are in cis with promoter −550/−221 haplotypes HY, LY, and LX, the MBL concentrations are high, intermediate, and low, respectively (441).
FIG. 2.
Schematic representation of the mbl2 gene and its genetic polymorphisms that determine MBL expression levels. Polymorphisms are indicated by the red arrows.
The alternative pathway.
The alternative pathway is phylogenetically the oldest arm of the complement system. This pathway does not require initiation by antibodies and thus serves to protect the host from invading pathogens prior to the development of specific immune responses. The alternative pathway is capable of autoactivation because of a process termed “tickover” of C3. Spontaneous “tickover” of C3 results in generation of a conformationally altered C3 molecule called C3(H2O) that is capable of binding factor B. Once factor B associates with C3(H2O), factor B itself undergoes a conformational change, which renders it susceptible to cleavage by the serine protease factor D, generating Ba and Bb. The Bb fragment remains associated with C3(H2O) and through its own serine protease domain can cleave the C3a fragment from the N terminus of the α chain of C3 to yield C3b. Cleavage of C3 results in a conformational change in the molecule and exposure of its internal thioester bond. The calculated half-life of the thioester of this metastable C3b molecule is ∼60 μs (282, 405). Within this short period, the nascent C3b must find a suitable electron donor in the form of an —OH or —NH2 group on a biological surface to form a covalent ester or amide bond, respectively; failure to do so will result in reaction of C3b with a water molecule, and inactive C3b will remain in solution. The labile nature of activated C3b ensures that C3b binding occurs only proximate to the site of complement activation, thereby preventing indiscriminate tissue damage. Surface-bound C3b can then bind to factor B and generate more C3 convertases and thus set into motion the positive feedback amplification loop that is a feature unique to the alternative pathway. Properdin, the only known positive regulator of the complement system, serves to stabilize the alternative pathway C3 convertase and extends its half-life 5- to 10-fold to ∼7 min (122). Recent data suggest that properdin can bind directly to certain surfaces such as zymosan, rabbit erythrocytes (RBCs), and apoptotic cells and to bacteria such as Neisseria gonorrhoeae and “rough” Escherichia coli (which lack O-antigen repeating units on their lipopolysaccharide [LPS]) and initiate alternative pathway activation (420). However, commercially available purified properdin preparations, as used in that study, contain aggregates of properdin that result from freeze-thawing of the protein (335), which could result in spuriously high avidity. The purified physiological (or native) forms of properdin (dimers, trimers, and tetramers) do not bind to neisseriae (1). Purified native properdin can bind to zymosan and late apoptotic or necrotic cells (127, 490), but it remains to be determined whether binding occurs in the context of serum that contains molecules that might interfere with direct binding of properdin to surfaces (299).
The terminal complement pathway (membrane attack complex).
All pathways of complement converge at the level of C3. C4b,C2a, and C3b,Bb are C3 convertases of the classical and alternative pathways, respectively. Efficient cleavage of C3 results in the incorporation of an additional C3b molecule in the C3 convertase complexes, which results in genesis of C5 convertases by changing the Km for C5 by over 1,000-fold from far above physiological C5 concentrations to well below them (337). Cleavage of C5 by C5 convertases results in release of the C5a fragment, a potent anaphylatoxin and chemoattractant.
The membrane attack complex is formed by the sequential fusion of C6, C7, C8, and C9 to C5b. Fusion is accompanied by hydrophilic-amphiphilic transition of the complex and results in the generation of an integral membrane attack complex. C7 plays a critical role in the hydrophilic-amphiphilic transition because it confers on the intermediate complex C5b-7 the ability to bind directly to target cell membranes (355, 359). The membrane-bound C5b-7 complex facilitates the incorporation of C8 and C9 into the membrane attack complex, resulting in formation of a transmembrane pore.
Regulation of Complement Activation
To facilitate the selective activation of complement on invading microbes or at sites of tissue injury, but not on normal cells, the complement cascade is kept under strict control by several fluid-phase and membrane-bound proteins (Table 2). The main fluid-phase inhibitors of the classical pathway of complement are C1 inhibitor and C4b-binding protein (C4BP), the alternative pathway is inhibited by factor H, and vitronectin and clusterin regulate the terminal pathway.
TABLE 2.
Complement receptors and membrane-bound complement inhibitors
| Category and protein | Characteristic(s) |
|---|---|
| Complement ligands with complement-inhibitory function | |
| CR1 | Cofactor for factor I cleavage of C3b to iC3b and further to C3d and of C4b to C4d; binds to MBL and C1q; clearance of opsonized pathogens and C3b/C4b associated with immune complexes (“immune adherence”) |
| CD46 | Cofactor for factor I cleavage of C3b and C4b; serves as receptor for pathogens such as measles virus and (?) N. gonorrhoeae |
| CD55 | Accelerates decay of C3 convertase assembled on cells |
| CD59 | Inhibits assembly of membrane attack complex (C9 polymerization) |
| Complement ligands | |
| CR2 | Binds primarily to C3d and C3dg; part of the CR2/CD19/CD81 complex that mediates B cell responses to antigens linked to C3 fragments |
| CR3 | Ligand for iC3b; phagocytosis |
| CR4 | Binds C3d/C3dg; function not known |
| CRIg | Ligand for the β-chain of C3b/iC3b; role for pathogen clearance in mouse model |
| C1qR | Ligand for C1q; phagocytosis |
| SIGN-R1 | Complement receptor identified as one of the murine homologs of DC-SIGN; binds selected pneumococcal polysaccharides and C1q and can activate the classical pathway in an antibody-independent manner |
| Receptors for anaphylatoxins | |
| C3aR | Binds C3a/C3a des-Arg; vasodilatation |
| C5aR | Binds C5a/C5a des-Arg; chemotaxis; possible modulatory role in airway inflammation |
In addition to inactivating complement system proteases (C1r, C1s, and MASP-2), C1 inhibitor inhibits contact system proteases (factor XII and plasma kallikrein), an intrinsic coagulation protease (factor XI), and the fibrinolytic proteases (plasmin and tissue plasminogen activator). Thus, C1 inhibitor also serves to limit the generation of bradykinin. Excessive bradykinin production results in increased vascular permeability, the hallmark of hereditary angioneurotic edema (HAE), which is caused by decreased C1 inhibitor levels (HAE is commonly referred to as C1 esterase inhibitor deficiency). C1 inhibitor deficiency is inherited as an autosomal dominant condition with incomplete penetrance. HAE is characterized by episodes of local subcutaneous edema and submucosal edema involving the upper respiratory and gastrointestinal tracts. Patients with HAE type I have absent or low levels of protein. Patients with HAE type II have a single-base substitution that leads to production of a dysfunctional protein. Type I HAE and type II HAE are clinically indistinguishable. A third form of HAE (type III), seen exclusively in women, is the result of a missense mutation of the coagulation factor XII gene (39) and is precipitated or worsened by high estrogen levels; C1 inhibitor levels and function are normal in these women.
A central aspect of the immune system is the ability to distinguish self from nonself. C3 undergoes low-grade spontaneous activation in the fluid phase and binds to —OH groups on cell surfaces. Restriction of alternative pathway activation on cell surfaces is mediated by membrane-bound complement regulators such as CD46, CR1, and CD55 (discussed below). Although factor H has always been considered a fluid-phase inhibitor of the alternative pathway, it also plays a key role in regulating complement on tissue surfaces. Critical to the ability of factor H to act specifically at cell surfaces is its ability to bind to select polyanions (292). Heparin has often been used as a model polyanion to study factor H-polyanion interactions. The presence of clusters of sialic acid or other polyanions such as sialylated glycolipids (295) and chondroitin sulfate C on a cell surface enhances the interaction between factor H and C3b (121, 336), which simultaneously competitively decreases the interaction of factor B with C3b, thus preventing C3 convertase formation.
The major cell surface-associated molecules that regulate complement are complement receptor 1 (CR1) (immune adherence receptor; CD35), CD46 (membrane cofactor protein [MCP]), CD55, and CD59. Some membrane-associated complement inhibitors, such as CR1 and CD46, also serve as complement receptors. Others, such as CR2, CR3, and CR4, serve as receptors for complement components but do not possess any complement-inhibitory activity. A newly discovered complement receptor (complement receptor of the Ig superfamily [CRIg]) binds to C3b and iC3b (the latter is formed by further processing of C3b by factor I in concert with either factor H, CR1, or MCP) and inhibits the C3 convertase of the alternative pathway (220, 483). Their features have been summarized in Table 2.
Recently, C7 was found associated with vimentin expressed on the surface of endothelial cells. This cell-associated C7 serves as a “trap” for terminal complement components and forms membrane-associated terminal complement complex (SC5b-9) (42). Unlike soluble SC5b-9, membrane-associated SC5b-9 does not stimulate endothelial cells to secrete interleukin-8 (IL-8), express adhesion molecules, or increase endothelial cell layer permeability and in fact can block the proinflammatory effects of soluble SC5b-9 (42).
Complement Receptors
CR1 binds to C3b, C4b, C1q, and MBL. Human erythrocyte CR1 mediates binding of complement-opsonized immune complexes or microorganisms to the cell, and this forms the basis for the phenomenon of immune adherence (316). These bound complexes or organisms are carried to the spleen or liver, where they are removed; in the process the CR1 molecule is also lost from the RBC surface.
CR1 carries the Knops blood group antigens, which include the McCoy (McC), Swain-Langley (Sl), and York (Yk) antigens (308). Two epitopes of the Knops collection of antigens, called McC(b+) and Sla−, are expressed in only about 1% of Caucasians but in about 40 to 70% of African and African-American populations (307). In severe Plasmodium falciparum malaria, infected erythrocytes rosette with uninfected erythrocytes, which obstructs the microvasculature and contributes to the pathogenesis of cerebral malaria (378). RBCs of persons with the Sla− CR1 polymorphism exhibit reduced adhesion to the domain of the malarial antigen that binds normal erythrocytes. Thus, a high frequency of the CR1 Sla− polymorphism may confer an advantage to populations in regions where malaria is endemic (440); this finding has not been confirmed in other studies (498).
The CR1 copy number on erythrocytes constitutes another polymorphism. The CR1 copy number per RBC is under the control of high (H)- and low (L)-copy-number alleles, whose frequencies are 0.73 and 0.27, respectively. Erythrocytes with low expression of CR1 (<100 molecules per cell) show greatly reduced rosetting with P. falciparum-infected RBCs. Thus, low RBC CR1 expression levels could be protective against cerebral malaria. Indeed, 80% of the natives of Papua New Guinea, where malaria is endemic, have low CR1 levels. In contrast, this polymorphism does not correlate with CR1 levels in Africans, and consistently, CR1 H/L polymorphisms were not associated with severe malaria in Gambian patients (25).
Paradoxically, two studies performed in Thailand showed that patients with severe malaria have a higher frequency of the L/L genotype and express lower RBC CR1 levels than patients with uncomplicated malaria, suggesting that high CR1 expression protects against severe disease (438). Circulating immune complexes could contribute to the pathogenesis of cerebral malaria, and high CR1 expression could facilitate clearance of these complexes and protect against severe disease.
CR1 serves as a complement-regulatory protein and has been discussed above. CR2 is the receptor for C3d/C3dg (these fragments are formed by the cleavage of iC3b by factor I and CR1) and can also bind to iC3b. CR2 also serves as the receptor for the Epstein-Barr virus (EBV) glycoprotein 350/220. CR2 also plays a key role in coupling the innate recognition of microbial antigens to B cell activation. Activation of complement results in deposition of C3 fragments on foreign antigens or microbes; the interaction of C3-tagged antigens with CR2 (CD21) results in formation of the CD21/CD19/CD81 complex and B cell activation, as discussed below.
CR3 (also known as Mac-1, CD11b/CD18, or αMβ2-integrin) is an important phagocytic receptor for iC3b-coated microbes; it plays a key role in extravasation of leukocytes from the circulation to sites of injury or infection and in the homing of lymphocytes to tissues. CR3 is used by certain intracellular pathogens, such as Mycobacterium tuberculosis, Cryptococcus neoformans, and Francisella tularensis, to adhere to and gain entry into cells.
The receptors for C3a and C5a are both transmembrane G protein-coupled receptors. No cases of deficiency of these receptors in humans have been described, but studies in animal models have suggested that they may modulate the severity of sepsis and airway hyperresponsiveness.
Functions of the Complement System
Innate immunity: role in combating infections.
The complement system is adapted to selectively recognize foreign surfaces and target them for elimination. The cascade is delicately balanced to facilitate activation on pathogens and at the same time minimize nonspecific damage to bystander host cells. Activation of complement results in C3b deposition and formation of C5 convertases, which leads to C5b-9 insertion into membranes. Gram-negative bacteria can be killed by appropriately inserted C5b-9 complexes. However, Gram-positive bacteria and fungi possess thick cell walls and are intrinsically resistant to C5b-9-mediated lysis. Despite their resistance to complement-mediated lysis, Gram-positive bacteria and fungi posses numerous strategies to limit complement activation (discussed below) in order to limit anaphylatoxin generation, polymorphonuclear leukocyte (PMN) influx, and subsequent opsonophagocytosis. Complement activation can also facilitate neutralization of viruses. Specific antibody and the early components of the classical pathway are often sufficient for neutralization (72). The alternative pathway in conjunction with IgG can also lyse cells infected with various DNA and RNA viruses.
Link between innate immunity and adaptive immunity.
Complement plays a critical role in adaptive immune responses. The covalent binding of C3b to an antigen is important to mark the antigen for uptake by phagocytic cells or retention by follicular dendritic cells (FDCs) for recognition by cognate B cells. CD21 (which binds iC3b, C3dg, and C3d) and CD35 (which binds C3b and C4b) on B cells play a key role in enhancing B cell immunity. Studies in mice have elucidated the mechanism of B cell activation. In mice, CD21 and CD35 are coexpressed and represent splice products of a single locus (the Cr2 locus) (242, 303) mainly on B cells and FDCs. On B cells, CD21 forms a receptor complex with CD19 and the tetraspanin protein, CD81. CD19 is a transmembrane protein that serves as a signaling/adaptor molecule. The CD19/CD21/CD81 complex functions to enhance B cell antigen receptor (BCR) signaling, in part by prolonging the association of the BCR with lipid rafts (67). Hen egg lysozyme bearing two or three tandem copies of C3d is 1,000- and 10,000-fold more immunogenic than hen egg lysozyme alone, respectively (92).
A second mechanism by which complement enhances B cell immunity is by localization of antigen to FDCs within lymphoid follicles. High expression of CD21 and CD35 on FDCs facilitates efficient trapping of immune complexes bound to C3 fragments within the lymphoid compartment. An intact classical pathway and CD21 and CD35 are all necessary for the uptake of immune complexes by FDCs (21), although the mechanism for uptake is not clear.
Studies in C3 knockout mice have shown reduced T helper cell-dependent IgG responses (233). Separate from an antigen-presenting cell (APC) defect, the lack of C3 prevents C3a and C5a generation; receptors for these two anaphylatoxins may be important in the pulmonary response to viruses such as influenza virus. CD46 also plays an important role in the regulation of T cells. Cross-linking of CD46 (which is also the receptor for the vaccine strain of measles virus and certain adenoviruses) with an anti-CD46 antibody or with C3b (a ligand for CD46) inhibits monocyte IL-12 production, which may contribute to the immunosuppression seen with measles virus infection (219). Coengagement of CD3 and CD46 in the presence of IL-2 induces a T-regulatory 1 (Tr1)-specific cytokine phenotype in CD4+ T cells. These IL-10-producing T cells proliferate, suppress the activation of bystander T cells, and acquire a memory phenotype (227). These findings indicate a critical role for complement in the differentiation of regulatory T cells.
Miscellaneous functions of the complement system.
Removal of senescent cells occurs by a process of apoptosis or programmed cell death. Under normal circumstances, apoptotic cells are cleared by macrophages and dendritic cells. Complement activation on, and clearance of, apoptotic cells is initiated by binding of C1q (234). Lack of clearance of apoptotic cells may be responsible for the formation of autoantibodies against complexes of proteins, nucleic acids, and phospholipids (99, 178, 434). The complement system is important for solubilization and clearance of immune complexes. Activation of the classical pathway inhibits the formation of insoluble immune complexes in plasma (185), and alternative pathway activation solubilizes immune complexes that have formed or are deposited in tissues. C3b and C4b associated with immune complexes can bind to CR1 on RBCs (390). These CR1-bound immune complexes are removed as RBCs traverse the spleen and the RBCs are returned to the circulation. Correspondingly, disease flares in systemic lupus erythematosus (SLE) are associated with decreases in erythrocyte CR1 expression (31, 76, 223). The discussion above represents only a restricted view of the rather complex and sometimes apparently paradoxical role of complement in autoimmunity. For a more detailed discussion of complement in the pathogenesis of SLE, the reader is referred to reviews by Walport and Pickering (348, 473).
In addition to their role in innate immunity against invading pathogens, complement proteins can also modulate diverse developmental processes, such as cell survival, growth, and differentiation in various tissues (286, 364) and in tissue or organ regeneration (89, 228, 283, 287). A critical role for the classical pathway in synaptic remodeling in mice was recently identified (427).
COMPLEMENT DEFICIENCIES
Acquired Deficiencies of Complement
Deficiencies of complement proteins may be acquired or inherited. Acquired complement deficiencies are relatively common and may occur as a result of decreased synthesis, increased protein loss, or increased consumption. The liver is the most important organ for the synthesis of several complement proteins, and therefore, low complement levels are often seen in persons with advanced liver disease. Patients with alcoholic cirrhosis and low levels of C3, C4, and CH50 were reported to have an increased risk of infections, including pneumonia caused by Streptococcus pneumoniae and septicemia with Staphylococcus aureus and E. coli (197). Complement deficiencies may result from increased protein loss associated with nephritic syndrome or protein-losing enteropathies. Increased consumption of complement often accompanies immune complex disease, vasculitis, or development of autoantibodies against complement proteins. The association of acquired complement deficiency and meningococcal disease is discussed below.
Autoantibodies that are directed against and stabilize the alternative pathway C3 convertase are called C3 nephritic factor (C3 NeF). Enhanced stability of the C3 convertase results in complement consumption and a persistent acquired subtotal C3 deficiency. Individuals with this disorder have a high risk of meningococcal disease (408), consistent with the observation that their serum shows decreased bactericidal activity against meningococci (407). Uncontrolled complement activation results in complement-containing deposits within the glomerular basement membrane (type II membranoproliferative glomerulonephritis [MPGN II]). Another interesting clinical entity that is associated with C3 Nef is partial lipodystrophy, which is characterized by the gradual onset of bilateral and symmetric loss of subcutaneous fat from the face, neck, upper extremities, thorax, and abdomen, in a “cephalocaudal” sequence, and spares the lower extremities (298). Complement activation may target the adipose tissue because fat cells are a major reservoir of factor D (also called adipsin), the activating enzyme of the alternative pathway.
Hypocomplementemic urticarial vasculitis syndrome (HUVS) (also called SLE-related syndrome or chronic hypocomplementemic cutaneous vasculitis) is a disorder associated with anti-C1q antibodies (281) that leads to classical pathway activation and chronic decreases in C1, C2, C4, and C3 levels (84). Patients with this disorder have clinical features that resemble SLE but lack other serological markers (ANA or anti-dsDNA) for SLE. The typical patient is a young female with a chronic rash, angioedema, and arthralgia. Although the rash has been termed urticaria, it has atypical features, including the absence of pruritus and its persistent nature. Histologically, the skin lesions show perivasculitis or a leukocytoclastic vasculitis. Normal levels of C1 inhibitor distinguish this syndrome from hereditary angioedema.
Inherited Deficiencies of Complement Activation
The previous review article on the same subject (128) has provided an excellent and comprehensive list of all complement-deficient patients and their clinical manifestations. We will not attempt to recatalog all cases of complement deficiency in this review, in part because the disease associations have not changed. Pertinent new studies on the subject will be cited in context of the prior data presented by Figueroa and Densen (128).
Classical pathway deficiencies.
Evidence for the importance of the classical pathway in prevention of autoimmunity is provided by the observation that homozygous hereditary deficiency of each of the early proteins of the classical pathway of complement activation is very strongly associated with the development of SLE (346). Deficiencies of C1 components, C2, and C4 are inherited in an autosomal recessive manner. Such deficiencies are the strongest genetic factors in susceptibility to the development of SLE that have been characterized in humans. There is a hierarchy of prevalence and disease severity according to the position of the protein in the classical pathway. The most prevalent and most severe disease is associated with deficiency of the proteins of the C1 complex and with total C4 deficiency; almost every human with C1q deficiency (474), over 75% of all individuals with total C4 deficiency, and 57% of individuals with C1r/s deficiency have SLE (54, 492), often with severe clinical manifestations. In contrast, C2 deficiency is associated with a much lower prevalence of disease, estimated at ∼10%. The female preponderance usually seen with SLE is not seen in complement-deficient patients. Symptoms usually occur at a younger age, and there may be a higher prevalence of anti-Ro antibodies (294, 362). The prevalence of complete deficiency of C4 or subunit proteins of the C1 complex in humans is extremely rare; fewer than 100 cases have been reported so far (14, 274, 492, 496). Of the approximately 30 cases of complete C4 deficiency reported in the literature, which include the cases reported previously (128), recurrent respiratory infections, pneumonias, and meningitis are common (284, 294, 384).
The complexity of genetic control of levels of circulating complement proteins is best exemplified by C4. In a haploid genome there are generally two C4 genes in tandem, which encode C4A and C4B. However, deletions or duplications of C4 can occur (55, 57, 396). The frequencies of C4 gene dosages of 2, 3, 4, 5, and 6 in the Caucasian population are 2%, 25.3%, 52%, 17.3%, and 3.3%, respectively. C4A is important for solubilization of immune complexes and their clearance through CR1 on erythrocytes. C4A deficiency predisposes individuals to lupus. Partial C4 deficiency of C4A or C4B is the most common inherited immune deficiency in humans, with a combined frequency of ∼30% in the normal Caucasian population (34). Complete deficiency of either C4A or C4B is relatively common and occurs in about 6% of the population (34). There is an inverse correlation between C4A gene copy number and susceptibility to SLE; zero copies (odds ratio [OR] = 5.267) and one copy (OR = 1.613) of C4A are risk factors for SLE, whereas the presence of ≥3 copies of C4A appears to be protective (OR = 0.574) (491).
Infections reported in persons with complete deficiencies of the components of the classical pathway (C1q/r/s, C4, and C2) include those with encapsulated bacteria such as S. pneumoniae, Haemophilus influenzae, and Neisseria meningitidis. It is not surprising that a similar spectrum of infections is seen in persons with hypo- or dysgammaglobulinemias, because classical pathway components form the “effector arm” of antibodies against these bacteria.
The role of C4 isoform (C4A and C4B) deficiency in determining predisposition to infections has been debated. Because the capsules of most bacteria that cause meningitis, such as N. meningitidis, S. pneumoniae, and H. influenzae, all possess —OH groups but not —NH2 groups, it was hypothesized that a deficiency of C4B (which preferentially forms ester linkages) would result in reduced complement activation on these bacterial pathogens and an increased incidence of invasive infections. Rowe et al. (379) found homozygous C4B deficiency in 5 of 46 children with bacterial meningitis (10.9%) and in only 7 of 223 controls (3.1%). There was no correlation between heterozygous C4B deficiency and either heterozygous or homozygous C4A deficiency and bacterial meningitis. Subsequently, Bishof and colleagues (33) found a significant association between C4B deficiency and bacteremia with N. meningitidis, S. pneumoniae, and H. influenzae only in white children (14% of cases versus 2% of controls). African-American children with meningitis, however, did not have an increased incidence of C4B deficiency. A larger study with 257 children (60) did not show an association between C4B deficiency and the development of either bacteremia or meningitis (2.3% of patients versus 3.7% of controls had C4B deficiency). Similarly, there was no increase in the frequency of C4B deficiency in patients with meningococcemia and a terminal complement deficiency (120). The last two reports suggested that C4B deficiency alone does not predispose individuals to infection with encapsulated bacteria. Consistent with this observation, no association between homozygous C4 isoform deficiency and patients with pneumococcal bacteremia or recurrent pneumonia (n = 80) was noted (108). Smaller case studies describe recurrent infections in C4 isoform-deficient persons. Gilliam et al. (159) reported an association between juvenile idiopathic arthritis (JIA) and C4 isoform deficiency. Three individuals with homozygous C4 isoform deficiency were reported to have frequent and severe recurrences (>10/year) of intraoral herpes simplex lesions (400). It was speculated that the combination of low C4 levels and impaired T cell recognition of viral peptides may have contributed to the recurrent nature of the disease. Another study found that low levels of IgG1 and IgG3 antibodies that mediated antibody-dependent cellular cytotoxicity were a predisposing factor for recurrent genital herpes. C4 isotype deficiencies were significantly more frequent in patients without neuralgias (401). Thus, while the antibodies produced by a T helper type 1 (Th1) response protected against recurrences, complement activation may have contributed to inflammation and neuropathic pain in these patients. A role for C4 in protection against certain fungal infections was suggested by the observation that C4B and C4A deficiencies were both associated with increased susceptibility to paracoccidioidomycosis in a study of 69 Brazilian patients and 225 healthy matched controls (91).
Relative to C1 and C4 deficiencies, homozygous C2 deficiency is common (1 in 10,000 individuals of Caucasian origin), while heterozygous C2 deficiency occurs in 1% of the population. Individuals with C2 deficiency may remain healthy and not suffer infectious complications. About 10 to 30% of C2-deficient individuals develop autoimmune disorders, including SLE, cutaneous lupus, and discoid lupus erythematosus (50, 454). One homozygous and 19 possible heterozygous C2-deficient individuals were identified in a cohort of patients with rheumatologic disorders, including 137 with SLE, 274 with juvenile rheumatoid arthritis, and 134 with rheumatoid arthritis. In comparison, only 6 of 509 normal individuals had heterozygous C2 deficiency, providing strong evidence of an association between C2 deficiency and these autoimmune disorders (162).
Among the homozygous C2-deficient patients described previously (128), N. meningitidis, S. pneumoniae bacteremia, H. influenzae meningitis, and recurrent respiratory infections were common. A case of S. aureus bacteremia was also documented in that series. Septic arthritis caused by serogroup Y N. meningitidis in a 12-year-old girl with C2 deficiency (199) and two cases of disseminated gonococcal infection (DGI), one in a 22-year-old man (290) and one in a 72-year-old woman (382), have subsequently been described.
The clinical manifestations of 40 individuals with homozygous C2 deficiency from 33 Swedish families over a 25-year period were studied by Jonsson et al. (216). Severe infection was the most common clinical manifestation; 23 patients had a history of invasive infections, mostly septicemia or meningitis caused by S. pneumoniae, and of these, 12 patients had recurrent infections. Other bacteria causing invasive infections included S. aureus (n = 3), H. influenzae type b (Hib) (n = 2), Streptococcus agalactiae, N. meningitidis (n = 3), Kingella kingae, Stenotrophomonas maltophilia, and enterococcal species (1 each). Nineteen patients had at least one episode of pneumonia, and recurrent pneumonia was seen in 10 patients. Repeated infections occurred mainly during infancy and childhood. SLE was found in 10 patients; 7 patients had undifferentiated connective tissue disease or vasculitis. Of note, cardiovascular disease occurred at a high rate, with a total of 10 acute myocardial infarctions and 5 cerebrovascular episodes in six patients. Causes of death among the C2-deficient patients were infection (n = 5), acute myocardial infarction (n = 3), and cancer (n = 1).
A follow-up study on the Swedish cohort by Jonsson et al. (215) found that homozygosity of G2M(n) (GM allotypes are genetic variants of the Ig constant heavy G chain) was associated with less severe infections in C2-deficient persons. Why C2-deficient patients homozygous for G2M(n) have a lower incidence of serious infections is not clear. Two observations could provide an explanation for this finding: first, higher IgG2 antibody responses to polysaccharides are seen in G2M(n) homozygous individuals (232, 388), and second, anticapsular IgG antibody can enhance complement activation through the alternative pathway (399). Evidence for the latter is provided by studies of two C2-deficient sisters, one of whom had meningitis with serogroup W-135 N. meningitidis. Immunization of the two sisters with the meningococcal polysaccharide vaccine resulted in production of IgG that could mediate bacterial killing by recruitment of the alternative pathway even in the absence of C2 (399).
The role of Ig in determining the susceptibility of 38 homozygous C2-deficient persons to infections was examined by Alper et al. (4). Increased susceptibility to bacterial infections was associated with significantly lower mean levels of IgG4 and IgA. Of the 13 homozygous C2-deficient individuals with infections, 85% had IgG4 deficiency, compared with 64% of 25 individuals without infections. In another report, the clinical courses of three C2-deficient patients (two were siblings) were recorded (256). All three children suffered infectious complications early in life. One child suffered pneumococcal meningitis, S. pneumoniae septic arthritis, and recurrent otitis media. IgA and IgG2 levels were slightly low. His sister had H. influenzae meningitis and also had recurrent otitis media. The third boy had episodes of meningococcal meningitis, bronchitis, and epiglottitis. In all instances, levels of either IgA and/or an IgG subclass was slightly decreased. An intact classical pathway appears to be important in eliciting normal IgG4 responses. A female child with C2 deficiency who presented at the age of 3 months with recurrent pneumococcal septicemia was reported (15). Although IgG subclass levels were normal, specific IgG responses to immunization against S. pneumoniae and H. influenzae were significantly impaired. In another report, of five patients with homozygous type I C2 deficiency (caused by a 28-bp deletion in the C2 gene) in two families, three suffered from recurrent infections (387). These patients had additional risk factors; two patients (one from each family) had IgG2 deficiency or IgA deficiency. All three patients had lower alternative pathway activity. The mean IgG4 levels in 24 patients with defects of the classical pathway (C1q, C1r, C2, or C4) were about 10-fold lower than normal values (30). In conclusion, C2 deficiency is associated with many abnormalities in serum Ig, some of which could contribute to increased susceptibility to infection.
Another variable that may have contributed, at least in part, to the development of severe infections in the C2-deficient Swedish cohort (215) was MBL concentration; MBL genotypes that predicted low MBL levels were associated with increased severity of infection (OR, 1.3). Interestingly, no patient with a combined C2 and MBL deficiency genotype had autoimmune manifestations. The structural gene for C2 lies between the genes encoding factor B and HLA-B (55). Lower factor B levels and lower alternative pathway activity have been cited as a possible contributing factor to the increased susceptibility of C2-deficient persons to infections (215, 319, 398, 455). Collectively, the data suggest that the etiology of increased risk of infections in C2-deficient persons is rather complicated and may involve defects in more than one aspect of the immune system. These recent findings underscore the redundancy in host defenses against invading pathogens. Ongoing analysis of risk factors for diseases on a genome-wide scale in large populations will undoubtedly shed further light on our understanding of the predisposing factors for infections.
Alternative pathway deficiencies.
Activation of the alternative pathway is mediated by C3, factor B, factor D, and properdin. Excessive alternative pathway activation in the fluid phase is kept in check by factors H and I. No person with an inherited complete deficiency of factor B has been reported. Partial deficiency of factor D was reported in twins who suffered from respiratory infections with H. influenzae and Proteus and Pseudomonas spp. The diagnosis was made in adulthood, but there was a history of recurrent infections since childhood (about 7 to 8 years of age). The first case of complete deficiency of factor D (the mode of inheritance is autosomal recessive) was of an adult with a history of N. meningitidis meningitis and two episodes of disseminated gonococcal infection (DGI) (188). Factor D deficiency was also identified in a 23-year-old woman with serogroup B meningococcal sepsis (28). Three additional cases of complete factor D deficiency were identified in this family, which had a high degree of consanguinity. A fifth family member, who suffered meningitis at the age of 20 years and died at the age of 71 from an episode of pneumococcal pneumonia, also was identified as having factor D deficiency by DNA analysis. A second case of meningitis occurred in another factor D-deficient member of this family during the course of the study, but the details of the infecting pathogen are not known. MBL and Fc receptors were also analyzed in an attempt to understand why only certain family members suffered infections, but no definite patterns were recognized. More recently, two cases of serogroup B meningococcal septic shock that occurred at the ages of 9 and 13 months in factor D-deficient siblings of consanguineous parents were reported (422). It is worth noting that immunization of all factor D-deficient patients in both of these studies resulted in normal antibody responses. This is in contrast to the case for C3-deficient patients, who have significantly impaired humoral immune responses (discussed below). A 6-day-old neonate with S. pneumoniae sepsis and probable factor D deficiency was described, but there were no genetic studies performed on any of the family members (477).
Properdin deficiency has been described in several families and is the only X-linked complement deficiency. Three types of properdin deficiency have been characterized. In type I deficiency, a nonsense mutation leads to a premature stop codon (479). Type II deficiency results in low (<10% of normal) levels of properdin in serum. Two point mutations were identified, one in intron 3 and one in exon 4, which may affect folding, secretion, and/or turnover of the protein (479). Type III deficiency is characterized by a point mutation that results in impaired properdin binding to C3b (141). The features of meningococcal disease in properdin-deficient individuals are discussed below.
Schejbel et al. (389) studied a large Pakistani family with properdin deficiency. The index case had a history of recurrent infections. A novel frameshift mutation in the properdin gene was identified. Screening of 24 available relatives revealed four affected males, four female carriers, and a male heterozygous carrier who was subsequently diagnosed with Klinefelter syndrome. There was a strong association between properdin deficiency and recurrent otitis media and recurrent pneumonia. This study was the first to associate properdin deficiency with infections other than meningococcal disease.
Total deficiency of the regulatory components of the alternative pathway, factors H and I (both inherited in an autosomal recessive manner), leads to uninhibited activation of the alternative pathway, consumption of C3, and the inability of serum to support bactericidal activity or opsonophagocytosis. Details of individual patients with factor H and factor I have been cataloged by Figueroa and Densen (128) and more recently by Reis et al. (365). Factor I-deficient patients appear to develop a greater number of recurrences and also display a broader range of infections. Recurrent otitis media, bronchitis, sinusitis, tonsillitis, and cutaneous abscesses have been described. As seen with C3 deficiency, invasive infections with S. pneumoniae, H. influenzae, and N. meningitidis (groups B, C, and W-135) have been reported (128, 365), and recurrences are common. In addition, cases of Streptococcus pyogenes and hemorrhagic measles and a case with 11 recurrences of aseptic meningitis (38) have been documented. Because of uninhibited C3 activation and C3b production, constant stimulation of CR1 on macrophages was thought to be responsible for lower CR1 expression and defective CR1-mediated opsonophagocytosis in factor I-deficient patients (357). Functional defects of CR3 were also described, although the mechanism for this observation remains undefined. Factor I deficiency also leads to autoimmune disorders, including immune complex glomerulonephritis and vasculitis.
Complete deficiency of factor H is also associated with an increased incidence of infections, including recurrent otitis media and bronchitis (250), H. influenzae otitis media (445), and invasive disease with N. meningitidis (groups B and X) (133, 320). About half of the reported patients with factor H deficiency do not have infectious complications. Renal disease (discussed below) is the major pathology associated with complete factor H deficiency. Despite the fact that deficiencies of factor H and factor I both result in depletion of functional C3, for reasons not entirely clear, infections appear to be more frequent and more severe in factor I-deficient patients.
Persons with factor H deficiency have a predilection for developing renal disease (347). Excessive complement activation because of complete deficiency of factor H, or because of mutations or autoantibodies that that result in loss of complement-inhibiting functions of factor H, leads to uninhibited complement activation and the development of type II membranoproliferative glomerulonephritis (MPGN II) (347). Interestingly, factor I deficiency, which also leads to complement activation and C3 consumption, does not lead to MPGN II. In the absence of factor I, there will not be any processing of C3b to iC3b or C3d. Using mice that were deficient in either factor H alone, factor I alone, or both factors H and I, Rose et al. (370) demonstrated that C3b breakdown products were essential for the genesis of MPGN II associated with factor H deficiency. The clinical manifestations and pathophysiology of renal disease in factor H deficiency have been reviewed by Pickering and Cook (347).
Lectin pathway deficiencies.
MBL levels vary widely among individuals, with serum levels ranging from 5 ng/ml to over 10 μg/ml. The genetic control of MBL levels is described above. It is interesting that no individual with complete MBL deficiency has been reported; none of about 10,000 individuals tested had a plasma MBL level of less than 3 ng/ml (209). The MBL level in each individual remains fairly stable throughout life. At birth, concentrations are two-thirds of adult levels, which are reached in a month, and there is a minor decline in old age (203). MBL is considered an acute-phase reactant, although the increase is slow (∼1 week after the inciting event) and modest (up to a 3-fold increase) (442).
Studies that address the correlation of MBL levels with infections must be interpreted with caution. First, the cutoff value that defines MBL deficiency varies from study to study, and values ranging from 50 ng/ml (69) to 1 μg/ml (317) have been used. Second, the finding that MBL levels were genetically determined led to several investigators using allotyping to correlate MBL levels with disease. While such studies have yielded useful insights, it must be noted that the MBL levels in individuals with the identical genotype for all MBL variants may differ by as much as 10-fold (424). The problems surrounding the interpretation of the role of MBL in clinical studies have been aptly summed up by Jensenius et al. (209): “Importantly, even among (healthy) individuals with identical allotypes, the concentration of MBL in plasma may vary considerably. Very little variation is seen in samples withdrawn over 1 year, but elevated MBL concentrations are observed during infections or after major operations with increases of up to 3-fold. Possibly this relatively small (compared with 1000-fold steady-state interindividual differences) acute-phase response has prompted investigators to base their investigations solely on allotypes analyses—and, sadly to say, in most cases they are only encompassing the structural allotypes. … there is no substitute for measuring the actual level of MBL in plasma. The reliance on structural allotypes will, everything else being equal, be expected to result in finding a lower than the true significance of the MBL association with whatever is being investigated.” While several studies have attempted to link MBL deficiency to a variety of noninfectious and infectious disorders, this review will focus on only the latter.
(i) MBL deficiency and infections.
Just prior to the discovery of MBL, Soothill and Harvey (416) found that the sera from 11 of 43 children with recurrent infections did not support opsonization of Saccharomyces cerevisiae (baker's yeast). These children commonly had infections of the skin and respiratory tract, with rare involvement of the bone, and S. aureus was the bacterium most frequently isolated. Other pathogens isolated included S. pyogenes, H. influenzae, Pseudomonas aeruginosa, and E. coli K1. About half of the patients had generalized rash and diarrhea. A similar defect in opsonization was seen in 4 of 72 healthy adults and 1 of 11 children with unrelated diseases, which was significantly lower than the incidence in the study population. The defect was not attributed to Ig deficiencies or lack of hemolytic activity but was corrected by normal serum. The same group reported defective yeast opsonization in four children with protracted diarrhea; plasma infusions corrected the opsonization defect in all four and resulted in symptomatic improvement in three children (51). They further analyzed 100 children with protracted diarrhea and found that 23% of children with failure to thrive had the defect versus only 4% of children with diarrhea without failure to thrive; the latter rate was similar to the rate of yeast opsonization defect in the general population. Subsequently, Super et al. (431) confirmed that the sera of about 5 to 7% of the general population failed to opsonize S. cerevisiae. These individuals had low MBL levels, and the opsonization defect was corrected by purified MBL in a dose-dependent fashion in an assay that measured the deposition of complement on a mannan-coated surface. Sumiya et al. (429) analyzed the mbl2 gene in three children with recurrent infections and low serum MBL levels and identified the codon 54 defect in exon 1. These early studies spurred a burst of investigations that attempted to define the association between MBL deficiency and susceptibility to infections.
The range of infections in four persons with MBL exon 1 genotype defects and one person with a combined MBL and IgA deficiency, whose ages ranged from 15 to 56 years, included recurrent skin abscesses, chronic cryptosporidial diarrhea, meningococcal meningitis with recurrent herpes simplex, and fatal lobar pneumonia due to Klebsiella (430). Kakkanaiah et al. (217) compared MBL concentrations in 47 HIV-negative adults who had recurrent infections with those in 50 healthy adult controls. Although the mean serum MBL concentrations in the patient group did not differ significantly from those in the control group, the proportion of individuals with less than 5 ng of serum MBL per ml was significantly larger in the patient group than in the control group (21% versus 4%). A second study group consisted of 73 pediatric and 56 adult patients with recurrent infections. Pediatric patients had significantly lower mean concentrations of serum MBL than their healthy controls. Again, there was no significant difference between the MBL concentrations in adult patients and adult controls, but the proportion of individuals with serum MBL concentrations of less than 5 ng/ml was significantly higher in both pediatric (22%) and adult (38%) patients than in their respective controls (4%). These results provide evidence that low concentrations of serum MBL are associated with recurrent infections not only in children but also in adults.
Consistent with the wide array of infections reported in persons with MBL deficiency, several in vitro studies have demonstrated that MBL binds to a diverse spectrum of pathogens and that bound MBL can promote C4 deposition on the pathogen surface (116, 181, 226, 231, 318, 465, 481). Such studies have prompted investigators to link MBL deficiency with infections, and this is discussed next.
(a) Meningococcal disease. Because of the importance of complement in host defenses against meningococcal infections, the interactions of MBL with N. meningitidis have been studied quite extensively. Case reports and studies of individual families have suggested an association between low MBL levels and meningococcal disease (23, 241, 279). A study of a Danish family suggested that the combination of MBL and properdin deficiencies may heighten the predisposition to meningococcal disease. Four members of this family suffered meningococcal disease, and one case was fatal. Two of six males with undetectable properdin levels had meningitis, and both these patients had MBL variant alleles that predicted low MBL levels. High MBL concentrations were seen in three of the remaining four properdin-deficient males, and none had meningitis (22).
Population-based studies substantiate a role for MBL in defenses against meningococcal disease. The frequency of variants of the MBL gene in children in the United Kingdom with meningococcal disease versus controls was ascertained in two independent studies. One study was hospital based (194 patients and 272 control patients with noninfectious disorders), and the other was community based (72 patients and 110 control healthy individuals). The proportion of homozygosity for MBL variant alleles was higher in patients with meningococcal disease than in controls in both the hospital study (7.7% versus 1.5%) and the community study (8.3% versus 2.7%) (187). In a previous study of Norwegian patients with meningococcal disease (76 with serogroup B disease and 25 with serogroup C disease), the proportions of individuals with low MBL levels (defined in that study as <100 ng/ml) were similar in cases and healthy blood donor controls (10.1% versus 12.5%) (151). A key difference in the two studies was that the mean age in the United Kingdom hospital cohort was 3.5 years, compared to 16 years in the Norwegian group. More recently, mutations in exon 1 of mbl2 that determine low MBL levels (codons 54, 52, and 57) were examined in a pediatric cohort (ages 2 to 215 months) with invasive meningococcal disease and compared to those in healthy age-matched volunteers with no history of meningococcal disease (117). The overall frequency of an MBL exon 1 variant genotype was significantly higher in patients than in controls (31.8% versus 8.2%). When stratified according to age, 39.3% of patients with disease onset at less than 24 months of age had an MBL structural variant genotype. This was further increased and most pronounced in children with disease onset at less than 12 months of age (57.1%). Clinical severity and outcome did not differ between patients with wild-type and mutant alleles (117). Collectively, these data support the notion that MBL plays an important role in protection against disease in early childhood prior to maturation of the adaptive immune system (457).
(b) Pneumococcal disease. A case-control study in Oxfordshire, England, found a 2.5-fold increase in the risk of invasive pneumococcal disease in study participants homozygous for mutations in mbl2 codon 52, 54, or 57 (381). Twelve percent of 229 patients with invasive pneumococcal disease were homozygous for these mutations, compared to only 5% of 353 controls; the results were replicated in 787 additional subjects, 109 of whom were MBL allele homozygotes. Neither heterozygosity for these codon variants nor the promoter polymorphism at position −221 was associated with susceptibility. Invasive infections with S. pneumoniae serotype 14 were overrepresented among the homozygotes. The type 14 pneumococcal polysaccharide has a linear backbone composed of Glc → GlcNAc → Gal, with Gal residues linked as monosaccharide side chains (478). Impaired recognition of GlcNAc may have predisposed patients homozygous for MBL mutations to type 14 pneumococcal disease.
However, another study of patients with invasive pneumococcal infections in Denmark did not find a statistically significant increased risk for MBL polymorphisms (240). The different conclusions may relate to the very low prevalence of homozygous MBL mutations in the latter study, a reflection of the smaller number of participants. Similarly, a small study of 63 Belgian patients with invasive pneumococcal disease found an increased, albeit statistically insignificant, association with MBL structural (codon 54, 52, and 57) polymorphisms. The −221 and −550 promoter allele distribution and the prevalence of the combined MBL structural and promoter −221 variant alleles were not significantly different between the patient group and the control group. The authors of this study stated that combining their data with the study by Kronberg et al. (240) yielded a significant risk of invasive pneumococcal infections in persons with MBL structural variants. The limited role of MBL in depositing C3 on pneumococci is supported by epidemiologic observations that show no association with MBL SNPs that result in low MBL levels and community-acquired pneumococcal pneumonia (113).
Eisen et al. (106) analyzed the association between mbl2 genotypes that result in low MBL levels (defined in this study as less <0.5 μg/ml) and the outcome of sepsis using data pooled from five studies with adults and one study with children and concluded that the risk of death was increased among MBL-deficient patients with S. pneumoniae infection (OR, 5.62) after adjustment for bacteremia, comorbidities, and age. Taking the data together, it could be concluded that while MBL deficiency may not be a risk factor for developing pneumococcal infection, persons with homozygous variant MBL alleles may have an increased risk of death from invasive pneumococcal disease.
(c) Tuberculosis. The high frequency of variant MBL alleles that result in low MBL levels in populations with a high prevalence of tuberculosis has raised speculation that low MBL levels may protect these individuals against disease. A summary of the studies that have attempted to correlate MBL genotype and/or MBL levels with tuberculosis is given in Table 3. In summary, there appears to be evidence that a critical “intermediate” level of MBL may be protective against intracellular pathogens such as Mycobacterium tuberculosis. One possible explanation is that high levels of MBL binding would lead to C3b/iC3b deposition and promote entry of bacteria into phagocytes through complement receptors such as CR1 and CR3. MBL binds to lipoarabinomannan (LAM) on the mycobacterial surface (149), which is also a mycobacterial ligand for mannose receptors on monocytes and macrophages. The absence of MBL would allow unimpeded access of LAM to mannose receptors and nonopsonic uptake of bacteria. Low levels of MBL that result in limited complement activation but that are sufficient to bind to LAM and block engagement of mannose receptors may be most beneficial for the host. This, however, is likely to be an oversimplified hypothesis, because MBL itself can bind to CR1 and could enhance bacterial uptake. Further, MBL bound to bacteria could affect ligation of macrophage receptors, which can modulate the release of cytokines such as IL-12 that play a critical role in host responses to infection (432).
TABLE 3.
MBL polymorphisms and infections with Mycobacterium tuberculosis
| Study description | Reference |
|---|---|
| MBL mutations compared in 277 patients with pulmonary tuberculosis (TB) and 288 household contacts; HYA/HYA subjects | |
| were protected against tuberculosis (OR, 0.09; P < 1 × 10−6); LYB/LYD subjects were susceptible to disease (OR, 49; | |
| P < 1 × 10−6) | 52 |
| Relationships between the susceptibility to TB exon 1 mutations and MBL levels in Turkish children; 27 children with | |
| pulmonary TB and 17 with extrapulmonary TB compared to 99 age-matched healthy controls; AB genotype (low MBL | |
| level) significantly lower in patients (9.1%) than in controls (27.3%) (P < 0.011); Median MBL levels significantly lower in | |
| the control group than in cases | 73 |
| Study of MBL levels and MBL2 gene polymorphisms in HIV-1-infected patients without TB (HIV+ TB−) (n = 151) and with | |
| TB (HIV+ TB+) (n = 109), HIV− TB+ patients (n = 148), and healthy controls (n = 146); MBL levels significantly higher | |
| among HIV− TB+ and HIV+ TB+ patients than controls and HIV+ TB− patients (P < 0.05); increased frequency of O/O | |
| genotype and YY genotype among HIV− TB+ patients than controls; HIV+ TB+ patients had significantly increased | |
| frequency of YA/YA diplotype (high MBL levels) compared to controls (P = 0.03); HIV+ TB+ patients had significantly | |
| decreased frequency of medium MBL expression diplotypes (XA/XA and YA/YO) compared to HIV+ TB− patients and | |
| healthy controls; YA/YA diplotype (high MBL) may predispose HIV+ patients to TB, while O/O genotype (low MBL) may | |
| predispose to TB in HIV− individuals; medium MBL expression diplotypes may protect against TB in HIV− patients | 2 |
| Six MBL SNPs (A/B, A/C, A/D, H/L, Y/X, and P/Q) studied in 152 Chinese males with pulmonary TB vs 293 healthy | |
| controls; none of the variants individually contributed to risk of TB, although there was a slightly increased risk with XB | |
| (low MBL) haplotype | 258 |
| MBL-2 structural and promoter polymorphisms in HIV infection and TB in a white Spanish population; 615 HIV+ with and | |
| without TB, 127 HIV− TB+ patients, 142 TB household contacts, and 344 controls; frequency of low producer or | |
| nonproducer mbl-2 genotypes lower in HIV patients than controls; HIV− TB+ patients had lower frequencies of low | |
| producer or nonproducer alleles and genotypes than HIV+ TB− patients and controls; positive correlation between | |
| incidence of TB and frequency of nonproducer mbl-2 alleles in Western Europe; MBL deficiency associated with lower risk | |
| of TB in HIV patients | 148 |
| MBL B allele frequency lower in controls than TB cases among African-Americans (P < 0.01) but no differences found | |
| between cases and controls of white and Hispanic ethnicity | 112 |
| Study of MBL alleles in 109 TB+ (and HIV-uninfected) patients living in Denmark and 250 white control subjects; low- | |
| expressing MBL genotype more frequent in controls than in white patients; similar trend in patients of other ethnic origin; | |
| heterozygosity for MBL variant alleles (low serum MBL) associated with protection against TB | 413 |
| No significant difference in frequency of MBL variant alleles in Turkish population with or without TB | 334 |
| Study of role of MBL in HIV and TB in Tanzania; HIV+ TB+ patients (n = 150) vs HIV− TB+ patients (n = 94) vs HIV− | |
| TB− controls (n = 113); HIV− TB+ patients had significantly higher MBL levels than controls, HIV+ TB+, and HIV+ | |
| TB− patients; low MBL associated with HIV risk; high MBL associated with TB risk | 154 |
| MBL alleles and TB in South African population; MBL B allele found in 22 of 79 (28%) of TB-negative controls compared | |
| with 12 of 91 (13%) of patients with pulmonary TB (P < 0.017) and 5 of 64 (8%) of patients with TB meningitis | |
| (P < 0.002); significantly lower serum MBL in TB-negative controls than in successfully treated | |
| TB patients (P < 0.004) | 190 |
| Correlation between B and C alleles (prevalence in sub-Saharan Africans, 29%) and TB infection; areas with high prevalence | |
| of variant alleles (low MBL) have higher incidence of TB; high incidence of TB may select for low-MBL-expressing alleles | 305 |
| No correlation between variant MBL alleles and TB in West African population | 25 |
(d) Malaria. A few studies have evaluated the association between MBL polymorphisms and malaria. The higher frequency of low-MBL-determining genotypes in regions where malaria is endemic raises the possibility that this disease could be a selection factor for variant MBL genes. In a matched case-control study with 870 Ghanaian children, the influence of six polymorphisms of the mbl2 gene on P. falciparum infection and severe malaria was examined. Heterozygosity for the C allele was found in 35% of healthy controls but in 42% of asymptomatically infected children (P = 0.01) and in 46% of patients with severe malaria (P = 0.007), suggesting that MBL could have a protective role in young children with immature immune systems (194). In a study of Gabonese children, increased MBL plasma levels and corresponding mbl2 genotypes were associated with lower concentrations of several cytokines and chemokines in plasma specimens from malaria patients (36). Another study of MBL levels and polymorphisms in Gabonese children participating in a prospective study of severe and mild malaria due to infection with P. falciparum showed that a higher proportion of patients with severe malaria than of subjects with mild malaria had lower levels of MBL (0.35 versus 0.19; P = 0.02). MBL B and C alleles (low MBL levels) were present at higher frequencies in persons with severe malaria (0.45 versus 0.31; P = 0.04) (261). These results suggest that compromised innate immune responses in the form of low MBL levels may be a risk factor for severe malaria in young children who lack mature acquired immune responses.
(e) Viral hepatitis. The studies examining the MBL genotypes or levels with hepatitis B and C disease progression or complications have been summarized in a recent review by Brown et al. (49). Disease progression is likely to be multifactorial and influenced by several factors, including ethnicity, alcohol consumption, concomitant infections, and HLA alleles. Several studies address only the mbl2 genotype (and in some cases only selected genotypes) without a measurement of serum MBL levels. Despite the differences in cohort characteristics and MBL genotypes studied, there appears to be a correlation between low-MBL-producing genotypes and hepatitis B disease progression. The role of MBL in hepatitis C virus (HCV) infection is less clear, but it appears that high MBL levels may correlate positively with pathology and response to treatment.
(f) HIV infection. In vitro studies have shown the ability of MBL to bind to HIV (180, 385) and prevent “trans infection” of T cells (418). Such data raise the possibility that MBL levels could play a role in the susceptibility to or clinical course of HIV infection. Clinical studies that have attempted to correlate MBL levels (either by mbl2 genotype or by measurement of serum MBL concentrations) with HIV acquisition or disease progression are listed in Table 4. Almost all studies have shown that low MBL levels are correlated with a higher rate of HIV infection. The ability of MBL to neutralize the virus or block its interaction with DC-SIGN may explain in part the protective role that high MBL levels play in HIV acquisition. On the other hand, data linking MBL levels with disease progression are conflicting. The rate of disease progression is often determined by coinfection with other pathogens, and the interactions of MBL with these coinfecting pathogens may confound interpretation of the data. As an example, high MBL levels may predispose individuals to tuberculosis. In situations where MBL does not neutralize HIV, MBL-coated viruses may be taken up intracellularly more rapidly, thus facilitating virus propagation. With the advent of better antiretroviral therapy and longer life expectancy of HIV-infected individuals in resource-sufficient settings, additional studies that examine the role of MBL in disease progression may be warranted.
TABLE 4.
Summary of studies examining the effect of MBL on HIV transmission or disease progression
| Study type and description | Reference |
|---|---|
| HIV transmission | |
| XA/XA haplotype in Argentinean children associated with ∼8-fold risk of acquiring HIV-1 (P = 0.054; OR, 8.11) | 278 |
| H promoter allele (−550) more frequent in prenatally HIV-exposed but uninfected Italian children than in infected | |
| children (48% vs 31%; P = 0.214) | 37 |
| Eight (8%) of the HIV-infected men were homozygous for the variant MBL alleles, compared with one (0.8%) of the | |
| healthy controls (P = 0.005) and 0% of high-risk controls (P = 0.05) | 150 |
| Frequency of MBL deficiency was significantly increased in HIV-infected patients compared with controls | |
| (12.1% and 3.5%, respectively) | 154 |
| Promoter (−550 [H/L] and −221 [X/Y]) alleles examined in a Brazilian population; CD4+ counts lower and viral load | |
| higher among seropositive patients with haplotypes LY, LX, and HX (intermediate to low MBL levels) than among | |
| those with HY (high MBL) haplotype (P < 0.05) | 463 |
| MBL concentrations significantly lower in asymptomatic HIV patients (P < 0.05) than in seronegative controls; very low | |
| (≤25 ng/ml) MBL serum concentrations were detected in 5/19 (26.3%) and 7/75 (9.3%) asymptomatic HIV-seropositive | |
| and HIV-seronegative individuals, respectively (P = 0.06) | 361 |
| Homozygosity for the MBL variant alleles was significantly higher in the HIV-1-infected group | 339 |
| No association between MBL alleles B, C, and D and susceptibility to HIV-1 infection (P = 1.0) in | |
| 278 HIV+ Columbians and controls | 273 |
| The mutant MBL G57E allele (either homozygous or compound heterozygous) is associated with susceptibility to HIV-1 | |
| infection in the Gabonese population (P = 0.019) | 305 |
| Study of 145 HIV-1-infected subjects and 99 healthy controls showed the presence of alleles MBL*A, MBL*B, and | |
| MBL*D, whose frequencies were 69%, 22%, and 09% among patients and 71%, 13%, and 16% among healthy controls | |
| respectively; the presence of the variant MBL*B was associated with higher plasma viral load levels, suggesting the | |
| importance of the MBL gene polymorphism in the clinical evolution of HIV-1-infected patients | 462 |
| HIV disease progression | |
| B variant allele present in 52% of children with rapidly progressing disease compared to 18.5% in | |
| slow progressors (P = 0.011) | 8 |
| Study examined effects of mbl2 alleles on HIV-1 disease progression and CNS impairment in children; children <2 years | |
| with MBL2-O/O (low MBL) experienced more rapid disease progression (O/O vs A/A, relative hazard [RH] = 1.54 and | |
| P = 0.02; O/O vs A/O, RH = 2.28 and P = 0.029) and rapid progression to CNS impairment (O/O vs A/A, RH = 2.78 | |
| and P = 0.027; O/O vs A/O, RH = 1.69 and P = 0.035) | 406 |
| Heterozygosity for coding mutations (O allele) delays disease progression; homozygosity for the −221 promoter | |
| polymorphism (X allele) accelerates rate of disease progression | 59 |
| Effect of MBL-2 polymorphisms on susceptibility and progression of HIV-1 infection in children; analysis of MBL-2 | |
| genotypes with respect to clinical classification (CDC clinical classification A, B, or C) yielded minimal differences; | |
| immunological categories 2 and 3 (<25% CD4+ T cells) were more likely to have MBL-2 variant alleles (P = 0.01); | |
| only 1/10 long-term nonprogressors had an MBL-2 mutation (A/D) with a corresponding protein level of 611 ng/ml | 103 |
| Homozygous promoter H/H (−550; high MBL) more frequent in Italian children with rapid progression (23%) than in | |
| slow progression (5%; P = 0.0194) | 37 |
| Median survival time shorter after AIDS diagnosis for men who carried variant alleles (both homozygous and | |
| heterozygous) than for men homozygous for the normal MBL allele (11 vs 18 mo; P = 0.007) | 150 |
| XA/XA haplotype in Argentinean children associated with ∼3-fold risk of progression to pediatric AIDS (P = 0.026; | |
| OR, 2.81); independent positive correlation between the rate of AIDS progression and MBL plasma concn | |
| (P = 0.008; OR, 1.28). | 278 |
| 2.5-yr follow-up of 80 HIV+ Danish patients; no difference in death rates in persons with high (>650 ng/ml), intermediate | |
| (101-650 ng/ml), and low (<101 ng/ml) MBL levels; no association between MBL level and decline in CD4+ T | |
| cells or length of time between HIV+ diagnosis and development of AIDS | 322 |
| HIV-1-infected men with the variant alleles progressed more slowly to AIDS (RH, 0.62) and death (RH, 0.73); CD4+ | |
| T-cell count determined at time of AIDS diagnosis was lower among persons with the mutation (97/mm3 vs 204/mm3; | |
| P = 0.03) | 263 |
(g) Fungal infections. Because mannan is an important component of fungal cell walls, the possible role of MBL in fungal infections has been investigated. Belgian women suffering from recurrent vulvovaginal candidiasis were more likely to possess the variant mbl2 codon 54 (allele B) than control women (20 versus 6.6%; P = 0.01) (97). Those authors also reported that the presence of the B allele was associated with significantly fewer recurrences among women who were placed on chronic fluconazole maintenance therapy. Carriage of MBL allele B was also more frequent in Brazilian women with recurrent vulvovaginal candidiasis (25.0%) than in women with acute vulvovaginal candidiasis (17.9%) or controls (10.6%) (160). MBL concentrations in cervicovaginal lavage fluid specimens from Chinese (257) and Latvian (17, 18) women with recurrent vulvovaginal candidiasis were lower than those in a control population. Interestingly, women with acute vulvovaginal candidiasis had higher local MBL levels than control women (257), possibly a reflection of the fact that MBL is an acute-phase reactant. In contrast, a study on Italian women did not show any increase in the rates of vulvovaginal candidiasis and MBL exon 1 mutations (297).
MBL may also have a protective role in deep-seated Candida infections. A study of patients with secondary peritonitis revealed that persons with MBL variant alleles had yeast isolated from the abdomen more frequently that persons with the wild-type MBL allele (39% versus 16%, respectively) (466). Patients with positive intra-abdominal yeast cultures had significantly lower MBL levels than did patients with negative cultures (median of 0.16 μg/ml versus 0.65 μg/ml).
A recent study compared MBL levels between 65 adult patients with proven or probable invasive aspergillosis and 78 immunocompromised control subjects with a febrile illness. Patients with invasive aspergillosis had lower mean MBL levels (281 ng/ml) than patients without aspergillosis (835 ng/ml). MBL deficiency, defined in that study as a level of <500 ng/ml, was seen more commonly in persons with invasive aspergillosis than in the control population (62% versus 32%) (243). A small study has cited an association between low MBL levels and chronic necrotizing pulmonary aspergillosis (CNPA) (also known as “semi-invasive aspergillosis”). This form of aspergillosis is usually seen in persons with preexisting damage to the lung parenchyma and/or mild immunocompromise, as may occur with alcoholism, diabetes, malnutrition, or low-dose steroid use. Seven of 10 white patients with CNPA (70%) had MBL haplotypes associated with low MBL levels, compared with 25.6% of the white control subjects (75). Another form of pulmonary aspergillosis is called allergic bronchopulmonary aspergillosis (ABPA). Studies of Indians with ABPA have shown that the 1011A allele in intron 1 was associated with higher MBL levels and higher levels of circulating eosinophils and IgE, the latter two being associated with disease severity (222, 267).
(h) CF. Cystic fibrosis (CF) is a hereditary disorder caused by a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene and affects the exocrine glands of the lungs, liver, pancreas, and intestines. Pulmonary infections are a major cause of morbidity in CF. Younger children become infected with bacteria such as H. influenzae and S. aureus, but infections with mucoid strains of P. aeruginosa and Burkholderia cepacia predominate in older children. The effect of MBL variant alleles on the course of CF has been addressed in several studies. A study of 149 CF patients showed that lung function was significantly impaired in carriers of MBL variant alleles compared to normal homozygotes (153). There was no statistical difference among A/A children (with normal MBL levels) and A/O or O/O children (with variant alleles) with respect to the frequency of colonization or the age of acquisition of P. aeruginosa infection. However, among those children colonized with P. aeruginosa, carriers of variant MBL alleles had significantly impaired lung function. B. cepacia infection was also more frequently seen in carriers of variant alleles. Why children with lower MBL levels are more predisposed to lung damage following colonization with P. aeruginosa remains unclear. The predicted survival of children with variant MBL alleles was 8 years less than that of MBL-sufficient children. The correlation between severity of lung disease in CF patients and low MBL alleles was confirmed in a subsequent study (493). Again, MBL sufficiency did not offer protection against colonization with P. aeruginosa. Davies et al. (83) reported MBL binding to B. cepacia but not to P. aeruginosa, which could provide an explanation for increased rates of colonization with the former pathogen in MBL-deficient persons with CF. Other studies, however, did not report an association between MBL levels and disease severity in CF (53, 328).
In a large study of 1,019 Canadian CF patients, Dorfman et al. (98) found that low MBL levels were associated with acquisition of P. aeruginosa at an earlier age. The effect of MBL was amplified in patients with genotypes that resulted in high production of transforming growth factor-β1 (TGF-β1). High-TGF-β1-producing genotypes have been shown to promote pulmonary fibrosis in CF patients (102). Thus, gene-gene interactions likely modulate the clinical course of CF lung disease, whereby high TGF-β1 production enhances the modulatory effect of MBL levels on the age of first bacterial infection and the rate of decline of pulmonary function.
(ii) Ficolin deficiency and infections.
The ficolin family of proteins, ficolin-1, -2, and -3 (Table 1), comprises the other members of the recognition proteins of the lectin pathway. Although inherited deficiencies of ficolins are rare, polymorphisms that influence the concentration and function of ficolin-2 (L-ficolin) have been described (198). A frameshift mutation (FCN3 + 1637delC, rs28357092) in exon 5 of FCN3, the gene encoding ficolin-3 (H-ficolin), has been observed in the heterozygous state in healthy persons with a frequency of 0.01 (311). In a recent study, Munthe-Fog et al. surveyed 1,282 patients with various immunodeficiencies (excluding HIV infection) and found that 23 were heterozygous and 1 was homozygous for the FCN3 mutation (310). Heterozygous individuals had about half the ficolin-3 levels seen in control normal individuals. The homozygous individual was a 32-year-old man who had unrelated parents of Macedonian and Albanian origin. He suffered multiple lower respiratory tract infections since early childhood and also had recurrent warts in his fingers. At the age of 20 he underwent a splenectomy for thrombocytopenia. At 26 years of age he had cerebral abscesses caused by nonhemolytic streptococci. Following this, he had multiple episodes of bacterial pneumonia, and sputum cultures yielded H. influenzae and P. aeruginosa. The patient had no measurable ficolin-3 levels and, as expected, no ability to activate C4 through ficolin-3 (310).
C3 deficiency.
C3 occupies a central position in the complement cascade and subserves several critical functions, which include solubilization of immune complexes, enhancing bacterial killing either through membrane attack complex formation or opsonophagocytosis, and potentiation of the humoral immune response. About 20 families with total C3 deficiency (inherited in an autosomal recessive manner) have been described (128, 365). Not surprisingly, C3-deficient individuals develop autoimmune disorders, infections, or both. Infections tend to be recurrent and severe. Invasive infections (meningitis, bacteremia, pneumonia, and otitis media) with S. pneumoniae, N. meningitidis, and H. influenzae have been reported (41, 43, 119, 132, 172, 184, 340, 386, 451).
Because of its role in bridging innate immunity and adaptive immunity, persons with C3 deficiency show impaired responses to immunization. Immunization of a 2-year-old child with total C3 deficiency and a history of recurrent pyogenic infections did not induce a long-term antibody response (158). Maturation of dendritic cells was impaired, with decreased CD1a expression and IL-12p70 secretion ability. These cells were unable to induce autologous B cell proliferation and Ig secretion in the presence of CD40 ligand and C3. The ability of CD4+ T cells to develop into T regulatory 1 cells after CD3 and CD46 activation in the presence of IL-2 was also significantly impaired. Thus, various aspects of the adaptive immune responses are impaired in C3 deficiency.
C3 deficiency can also result from excessive activation and consumption of complement. Such a situation arises with deficiencies of factor H or factor I or in the presence of C3 nephritic factor, as discussed above. C3 levels under conditions of excessive C3 consumption are less than 10% of normal levels. However, the presence of even small amounts of C3 reduces the severity and frequency of infections.
Terminal complement pathway deficiencies.
Invasive meningococcal disease and disseminated gonococcal infection (DGI) are the only conditions known to be associated with deficiencies of the terminal components of complement (C5 through C9). All late complement deficiencies are inherited in an autosomal recessive manner; heterozygous individuals usually express about half of the normal levels of the protein. In the cases reported by Figueroa and Densen (128), about 50% of all persons deficient in a terminal complement component had invasive meningococcal disease. While polymorphisms of terminal complement components have been described, their clinical significance is not clear.
Nonsense mutations were responsible for C5 deficiency in two African-American families (475). Homozygosity for double mutation in exon 40 of the C5 gene caused missense and frameshift mutations that resulted in production of a truncated and highly unstable C5 molecule in a Spanish family (87). One member of this family suffered from meningococcal sepsis and several episodes of pneumonia, and a second person had bacterial meningitis twice and frequent episodes of tonsillitis, pneumonia, and herpes. C5 deficiency secondary to a point mutation in exon 10 was identified in a 5-year-old Turkish boy with two episodes of bacterial meningitis (N. meningitidis was documented in one episode) (344).
An important distinction between C5 deficiency and deficiency of the other components of the terminal complement pathway is that C5-deficient patients lack the ability to mount an efficient chemotactic response because of the absence of C5a generation. Despite this additional defect, the bacterial etiology of systemic infections does not differ in these individuals (>95% Neisseria spp.) (128). While much attention has been focused on the high incidence of meningococcal disease in terminal-complement-deficient persons, there may also be increased susceptibility to disseminated gonococcal infection. The case report of a C5-deficient male with nine episodes of DGI (412) is illustrative.
C6 deficiency is usually the result of point mutations, and some of these have been identified in Japanese, mixed-race South African, African-American, Dutch, and Irish populations (193, 324, 338, 497). Subtotal C6 deficiency is the result of an abnormal 5′ splice donor site of intron 15 that results in an in-frame stop codon 17 codons downstream from the intron boundary. The result is a truncated polypeptide that is 13.5% smaller than normal C6. These individuals have low (1 to 2% of the normal mean) C6 levels, but even these low levels appear to be sufficient to form membrane attack complex (332) and support bactericidal activity against a group B meningococcal strain (10 to 35% of the killing compared with normal serum) (487). There have been no reported instances of meningococcal disease in any of the 10 individuals identified with subtotal C6 deficiency, suggesting that even a small amount of lytic activity in serum may be sufficient to alleviate the high risk of meningococcal disease seen with total deficiency of terminal complement components.
Subtotal C6 deficiency in combination with subtotal C7 deficiency has been reported in two families (126). The C7 molecule in the patients with C6/C7 subtotal deficiency show an abnormal IEF pattern caused by an R499S mutation, and these patients have plasma C7 levels that are ∼5% of normal (488, 489). The same mutation was found in a Russian patient who had two episodes of meningococcal disease as a child. This patient also had chronic otitis media that was only partially responsive to medical treatment; S. aureus and Bacteroides faecalis were cultured from middle ear pus (353). Complete deficiencies of C7 have been reported in several cases, and the molecular basis includes mutations at a 3′ splice acceptor site in intron 1 (125), a mutation at a 5′ splice donor site of intron 16, several point mutations (124, 489), and deletion of part of the gene (326).
C8 is an oligomeric protein that is composed of nonidentical α, β, and γ subunits. To date, no deficiency of the γ subunit of C8 has been identified. The most common cause of C8 deficiency is a C1309T mutation in the β subunit that leads to a premature stop codon (221). All cases of C8 deficiency involving C8β have been described in Caucasians. Mutations in C8α subunit are found almost exclusively in African or Hispanic populations (374). These mutations result in expression of a dysfunctional C8α-γ molecule that was present at low concentrations (∼1% of normal levels). Interestingly, very low levels of functional C8β were also observed (3% of normal levels) (437). The reason for the low β subunit levels is not clear. Possibilities include a requirement for C8α-γ for secretion or protection of C8β against proteolysis.
The highest prevalence of homozygous C9 deficiency (1:1,000) is seen in the Japanese population. These data were obtained from large-scale screening of blood donors or hospitalized patients (144, 183, 201). Most deficiencies result from point mutations that result in premature stop codons (484). C9 deficiency is very rare in other populations. Serum from C9-deficient individuals can support complement-dependent hemolysis of antibody-sensitized erythrocytes (254), although the reaction is 100 times slower than with C9-sufficient serum. Similarly, a serum-sensitive strain of E. coli could also be killed over 3 h, a rate ∼35-fold lower than with normal serum (254). Killing of a serum-sensitive strain of N. gonorrhoeae and a strain of N. meningitidis called Y6212 occurred in C9-depleted serum over 120 min; C9-depleted serum reconstituted with C9 killed bacteria within 30 min (179). Interestingly, the risks of meningococcal disease among C7- and C9-deficient persons in the Japanese population were approximately 10,000- and 1,400-fold higher than that in complement-sufficient controls (313). These data strongly support the notion that the predisposition to meningococcal disease is strongly linked to the efficiency of complement-dependent bactericidal activity. Thus, the ability of C9-deficient serum to kill Neisseria, albeit at a lower rate, is associated with an ∼10-fold-decreased risk of meningococcal infections in C9-deficient persons compared to individuals with deficiencies of C5 through C8, whose sera lack any hemolytic or bactericidal activity (254, 313).
Membrane complement protein deficiencies and infections: leukocyte adhesion deficiency (CD11b/CD18 or CR3 deficiency).
Leukocyte adhesion deficiency-1 (LAD-1) is caused by mutations in the β subunit of integrins that result in a failure to express CR3, LFA-1, CR4 (CD11c/CD18; p150,95), and αDβ2 on leukocytes (229), which adversely affects a variety of leukocyte functions, including adhesion, chemotaxis, iC3b-mediated opsonophagocytosis, and neutrophil respiratory burst. LAD-1 is an autosomal recessive condition that can result from one of several different described mutations. Reduced quantities of specific mRNA, altered size of mRNA because of alternative splicing (229), or apparently normal mRNA have all been described. Point mutations in CD18 can affect its ability to form heterodimers with the α chain.
This disorder is characterized by prolonged and recurrent infections, often with Staphylococcus and Pseudomonas, that begin in infancy. A history of delayed separation and infection of the umbilical cord (omphalitis) may be present. Infections of the soft tissues, mucosal surfaces, and intestines, often with extensive necrosis, can occur. Mortality is high, and about half of affected individuals die before 2 years of age. Acute gingivitis is almost universally seen in individuals who reach childhood. Because of defective adhesion of leukocytes to endothelium that results in defective margination, there is a persistent leukocytosis (2 to 20 times the normal) despite the absence of any active infection. Disease severity is inversely proportional to the amount of CD18 expression. Some mutations result in a failure to produce mRNA that results in severe deficiency (<0.3% expression), and these individuals often die early in infancy. Individuals who express small amounts of CR3 (2 to 10% of normal) have mild to moderate symptoms and sometimes survive to adulthood (9, 10, 200, 421).
MICROBIAL INTERACTIONS WITH COMPLEMENT
The success of a pathogen lies in its ability to evade host immune defenses. It should be emphasized that although most studies of complement-microbe interactions have been carried out with serum, complement components are present even at mucosal surfaces and in almost every body fluid studied. About 50 years ago, Roantree and Rantz showed that Gram-negative bacteria isolated from the bloodstream were almost always resistant to complement-dependent bactericidal activity, underscoring the importance of complement evasion in bacterial pathogenesis (368).
While Gram-negative bacteria can potentially be killed by complement alone by insertion of the membrane attack complex, Gram-positive bacteria and fungi are intrinsically resistant to the lytic activity of complement because they possess thick cell walls. However, complement deposition on gram-positive bacteria and fungi results in deposition of C3 fragments that act as opsonins. Activation of C5 results in generation of C5a, a potent chemotaxin that recruits polymorphonuclear leukocytes to facilitate phagocytosis. Limiting C3 deposition would therefore confer an advantage to microbes by dampening inflammation. On the other hand, certain intracellular microbes, such as M. tuberculosis, can use C3 fragments deposited on their surface to gain entry into their preferred milieu within cells.
Over the past 3 decades, numerous microbial complement evasion strategies have been elucidated. There are examples of microbes that block complement activation at every step (236, 369, 486). The presence of more than one complement evasion mechanism concomitantly has been described for several pathogens; in fact, the presence of multiple complement-dampening strategies appears to be the rule rather than the exception. A detailed review of microbial complement evasion mechanisms is beyond the scope of this review, and only a few examples are provided here.
Every microbe activates the complement system; however, the degree of complement activation varies among organisms. Effective insertion of C5b-9 into the bacterial membrane requires complement activation and C5 convertase formation close to the bacterial membrane. Complement activation is regulated by the K antigens (capsular polysaccharides) and O antigens (“smooth” LPS) on Klebsiella pneumoniae. In Klebsiella serotypes where the K antigens mask the LPS (e.g., O1:K1, O1:K10, and O1:K16 [449]), there is minimal complement activation because K antigens are poor complement activators. K. pneumoniae serotypes where the K antigens and LPS are both exposed at the bacterial surface (K2, K7, K19, K21, K22, and K66) activate complement (smooth LPS activates the alternative pathway (3), but C3b is located relatively distant from the outer membrane and therefore there is minimal C5b-9 inserted into the bacterial membrane (293). The chemical composition of the O antigenic repeats in Salmonella determines the rate of alternative pathway activation and initial C3b binding (170) as well as amplification of initially deposited C3b by the alternative pathway positive feedback loop (210). A critical density of long LPS molecules in Salmonella enterica serovar Montevideo was required to sterically hinder access of C5b-9 to the outer membrane (171). The presence of O-antigenic repeats could also interfere with assembly and insertion of the terminal complement pathway. Incorporation of C8 and C9 into the stably bound C5b-7 complex caused release of the entire complex from the bacterial surface of a smooth-LPS-bearing strain of S. enterica serovar Minnesota (212) but not from the surface of the serum-sensitive “deep rough” mutant strain with an LPS that comprised only lipid A and 2-keto-3-deoxyoctulosonic acid (KDO) (211).
Capsular polysaccharides play a key role in pathogenesis of a wide array of bacteria and fungi. Capsules are antiphagocytic and often limit complement activation. Capsules have diverse chemical structures, but in most instances they are polyanionic. Despite their ubiquitous presence, surprisingly little is known about the mechanism(s) by which they regulate complement. Sialic acid-containing capsules have received considerable attention, probably because the role of sialic acids in regulating the alternative pathway on erythrocytes has been well studied. The capsule of type III group B streptococci regulates the alternative pathway; removal of the terminal sialic acid residues by neuraminidase treatment converts the bacterium from a nonactivator to an activator of the alternative pathway (104, 105). Desialylation of N. meningitidis serogroup B, whose capsule is composed of homopolymers of α(2,8)-lined polysialic acid, also enhances alternative pathway activation (207). Of 109 group C strains recovered from persons with invasive disease in Spain, three isolates that were resistant to killing by anticapsule antibodies elicited by the group C conjugate vaccine (459) were found to be “hyperproducers” of capsule.
Sialic acid is used as a virulence factor by several microorganisms. The ability of Sindbis virus to activate the alternative pathway of complement is inversely related to its host-determined envelope sialic acid content (189). Trypomastigotes of Trypanosoma cruzi express a cell surface-associated trans-sialidase that enables the parasite to rapidly sialylate its surface when supplied with α(2,3)-linked sialic acid in the form of glycoconjugates in serum or on cell surfaces. Desialylation of the trypomastigotes results in an increased ratio of C3b to the inactive molecule, iC3b, and results in increased lysis of the organism (450).
The LPSs of several gram-negative bacteria, including H. influenzae, nontypeable H. influenzae, N. meningitidis, N. gonorrhoeae, Moraxella catarrhalis, Campylobacter jejuni, and Yersinia pestis, lack O-antigen repeats and are therefore sometimes referred to as lipooligosaccharide (LOS). H. influenzae, N. meningitidis. C. jejuni, and Y. pestis express capsules, while N. gonorrhoeae and M. catarrhalis do not. Capsular polysaccharide expression appears to be important for high-level serum resistance in H. influenzae and N. meningitidis and for their ability to cause invasive disease and meningitis. In contrast, nontypeable H. influenzae and unencapsulated N. meningitidis are relatively serum sensitive and very rarely cause bacteremia.
Bacteria that elaborate LOS have evolved sophisticated mechanisms to escape complement. It is interesting that several LOS glycan structures mimic host carbohydrates; for example, the lacto-N-neotetraose substitution resembles glycosphingolipids, and the Gal → Gal → Glc epitope mimics the PK-like epitope on erythrocytes (275-277). Incorporation of sialic acid onto LOS is a mechanism shared by several strains of N. meningitidis, N. gonorrhoeae, nontypeable H. influenzae, and C. jejuni (306, 456). Sialoglycans on erythrocytes regulate the alternative pathway by virtue of their ability to enhance the interactions of factor H with C3b while simultaneously decreasing factor B-C3b interactions (224); it is presumed that LOS sialic acid regulates the alternative pathway by a similar mechanism. A feature of sialylation of lacto-N-neotetraose expressing LOS that is unique to N. gonorrhoeae is the enhancement of factor H binding to the bacterial surface (268).
Effective complement activation on bacteria such as H. influenzae (including nontypeable strains), N. meningitidis, and N. gonorrhoeae requires “priming” by the antibody-dependent classical pathway (202, 251). In the absence of specific antibody, the alternative and MBL pathways cannot support bactericidal activity of serum, which may contribute to the higher incidence of disease with H. influenzae and N. meningitidis during the first 2 years of life. Colonization is an immunizing process (165); the development of a repertoire of protective antibodies with age as a result of colonization may be associated with decreased disease rates in older children and adults. Although the MBL pathway alone does not mediate bactericidal activity in vitro, it may play an important role prior to the development of antibodies by functioning as an opsonin. As discussed above, MBL deficiencies are associated with a wide range of infections.
Over the past decade, binding of complement inhibitors such as C1 inhibitor, factor H, factor H-like protein-1 (FHL-1) (an alternatively spliced variant of factor H that also has complement inhibiting function), C4b-binding protein (C4BP), and vitronectin to several bacteria, fungi, viruses, and parasites has been described (236). Binding of complement inhibitors is associated with complement inhibition on the bacterial surface.
Certain members of the pox- and herpesvirus families have proven to be particularly adept at expressing molecules that mimic the function of host complement inhibitors. Cells infected with vaccinia virus secrete a virus-encoded protein called the vaccine virus complement control protein (VCP) that resembles part of the human C4BP molecule (235) and can inhibit the classical and alternative pathways. Similarly, Kaposi's sarcoma-associated herpesvirus (KSHV) expresses a complement-downregulatory protein that is organized into domains characteristic of complement inhibitors such as factor H and C4BP (309, 419). Epstein-Barr virus (EBV) (302) and glycoproteins C-1 and C-2 (gC-1 and gC-2) of herpes simplex virus type 1 (HSV-1) (260, 289) also inhibit complement activation.
Several bacteria secrete proteases that degrade complement components or molecules that interfere with complement activation. These include P. aeruginosa, Serratia liquefaciens, Serratia marcescens, Y. pestis, and Porphyromonas gingivalis. S. aureus exemplifies a bacterium that possesses redundant strategies to inactivate complement. This bacterium expresses or secretes numerous proteins that can block complement activation at almost every step of the cascade in an effort to reduce chemotaxin production by the host (214, 369).
While development of an antibody response in most instances is protective, it can sometimes decrease killing by otherwise bactericidal serum and enhance the susceptibility to infection. Such antibodies are sometimes called “blocking antibodies.” In 1894, Pfeiffer and Isaeff (345) noted that animals given an excess of immune serum were more susceptible to infection caused by organisms that had been used to produce the immune serum (472). Antibodies that develop at a later stage of Brucella abortus infection were shown to block the bactericidal effects of antibodies that developed early following infection (499). Some patients with chronic urinary tract infections with E. coli (436) or P. aeruginosa (173) developed antibodies that blocked killing of the infecting strain by normal human serum.
Blocking antibodies may play an important role in neisserial infections. Sera from patients convalescing from meningococcal infection are sometimes less effective at killing meningococci than sera collected during the acute phase of infection. These sera can block killing by otherwise bactericidal normal human sera (443, 444). IgA purified from human serum following infection with group B, C, and Y meningococci blocked complement-mediated bacteriolysis by the same sera (168). In a separate study, sera from 24 of 28 military recruits with meningococcal disease lacked bactericidal activity; removal of IgA from these 24 sera uniformly enhanced the bactericidal activity of IgM present in the same sera (169). IgA1 directed against N. meningitidis serogroup C polysaccharide blocked the bactericidal activity of IgG; blocking was not because of competitive inhibition of IgG binding and did not require the Fc region of IgA1 for function (206).
Blocking antibody plays an important role in the pathogenesis of N. gonorrhoeae. In a longitudinal study of 243 female commercial sex workers who experienced frequent gonococcal infection, those with antibody to reduction modifiable protein (Rmp; protein III) were at increased risk of infection (adjusted odds ratio, 3.4) (354). Immunopurification studies confirmed the specificity of the gonococcal target for blocking antibodies as Rmp (366). Rmp is not the only gonococcal target for blocking antibodies; IgA directed against the LOS of N. gonorrhoeae could also block killing by otherwise bactericidal IgG (12).
MENINGOCOCCAL INFECTIONS
Because of the high prevalence and recurrent nature of meningococcal disease in complement-deficient persons, a discussion of its pathogenesis and interactions with the complement system is merited. Disseminated gonococcal infection (DGI) has also been reported in persons with complement deficiencies; probably the most spectacular case was that of a person with nine episodes of DGI (412). However, given the high frequency of transmission of gonorrhea among sexual contacts (252) and the strong association between certain auxotypes and phenotypes (including the ability to resist complement) of N. gonorrhoeae and dissemination (397), it is difficult to ascertain whether complement deficiency increases the risk for gonococcal dissemination. Figueroa and Densen (128) have made several seminal observations regarding the characteristics of meningococcal infection in complement-deficient patients based upon comprehensive cataloging of cases reported prior to 1991. The results of their findings are summarized below, in addition to newer data obtained since their review.
Epidemiology of Meningococcal Disease
Excluding epidemics, there are about 500,000 cases of meningococcal disease reported annually, with a mortality of about 10% (446, 447). About half of all cases occur in the “meningitis belt” of sub-Saharan Africa (244). While the rate of meningococcal disease in industrialized countries is typically <5/100,000 individuals, the incidence of disease during epidemics in sub-Saharan Africa can approach 1% (167, 372, 458). Meningococci have been classified into 13 serogroups based upon the chemical composition of their capsular polysaccharides (74, 143, 208). Most invasive isolates belong to serogroups A, B, C, W-135, and Y (341, 372). Serogroup A meningococci are responsible for large, cyclic epidemics in Africa and Asia, while 30 to 90% of endemic cases in the industrialized world are caused by serogroup B. Serogroup B can also cause persistent, epidemic disease, with incidences of 10/100,000 to 20/100,000, as observed, for example, in Norway, Cuba, Brazil, and New Zealand. These epidemics have led to the development and deployment of strain-specific outer membrane vesicle vaccines (196). Serogroup C causes smaller-scale outbreaks worldwide. Over the past 2 decades the incidence of serogroup Y disease in the United States has climbed, and it now accounts for a third of cases (288, 371). Serogroup W-135 strains were responsible for a large outbreak among pilgrims to the Hajj in Saudi Arabia in 2000 and 2001 and for an epidemic in Burkina Faso in 2002 (85). More recently, epidemics of serogroup X disease have been reported in Africa (95, 146).
Acquired Complement Deficiencies and Meningococcal Disease
While it is well established that inherited deficiencies of complement constitute a strong risk factor for meningococcal disease, the risk of meningococcal disease in persons with acquired complement defects is less clear. The complement profiles of two patients with severe hepatic failure that was complicated with meningococcemia were studied by Ellison et al. (110), who documented normal C1q levels but low or undetectable concentrations of C3 through C6, C8, C9, factor B, and factor I. Of 20 patients presenting with their first episode of invasive meningococcal disease, 6 had a CH50 level of less than 2 standard deviations below the normal mean (111). Of these patients, two had inherited C6 deficiency and one had inherited C8 deficiency. The remaining three had defects in multiple complement components associated with SLE or multiple myeloma. Garty et al. (155) found that 3 of 30 patients with invasive meningococcal disease who were treated at a hospital in Israel between 1970 and 1989 had complement deficiencies. One patient had a congenital C7 deficiency, and two had SLE and MPGN. The latter two patients had low C3, C4, and CH50 levels prior to the onset of infection. The incidence of meningococcal infection in the Jewish population of central Israel was estimated to be 1/100,000, and that of SLE with MPGN was estimated to be ∼250/100,000. The concomitant occurrence of these two rare entities supported an association between acquired complement deficiencies and meningococcal disease. Reports by Feliciano et al. (123) and Mitchell et al. (300) further supported an association between SLE and meningococcal disease. Hypocomplementemia as a result of C3 NeF has also been associated with meningococcal disease (408). A case of meningococcal disease in a patient with transient complement deficiency caused by poststreptococcal glomerulonephritis has been reported (82). Three episodes of DGI within an 18-month period in a patient with low complement levels secondary to type I MPGN (225) and gonococcal endocarditis in an SLE patient (448) have been reported. Collectively, the evidence suggests that individuals with acquired complement defects secondary to complement consumption states are at a higher risk for developing invasive neisserial disease.
Frequency of Hereditary Complement Deficiencies among Patients with Meningococcal Disease
As discussed above, the incidence of hereditary deficiencies of complement components varies widely depending on the complement protein and the population studied. While hereditary deficiency of C2 is relatively common (∼0.01%), complete deficiencies of other proteins such as C4 are very rare. C9 deficiencies are relatively common in the Japanese population and are found in ∼0.1% of blood donors. This contrasts with the low frequency (0.005%) of C7 deficiency in the same population. In contrast, C7 deficiency was relatively common in the Israeli Moroccan Jewish population; of a total of 365 healthy blood donors, 1 (0.27%) was homozygous for the mutant allele and 6 (1.6%) were heterozygous for the mutant allele (175). A relatively high frequency of C6 deficiency has been detected in the western Cape population of South Africa (330, 358). The association of complement deficiencies with invasive neisserial infections prompted several studies to estimate the frequency of complement deficiencies among patients with their first episode of systemic neisserial infection (24, 80, 94, 109, 111, 114, 134, 192, 247, 313, 321, 351, 363, 393). Figueroa and Densen (128) plotted the incidence of complement deficiency detected in patients with meningococcal disease versus the incidence of meningococcal disease in the general population and showed that the rate of complement deficiencies detected dropped in areas where the incidence of disease was high. For example, 0 of 47 persons with meningococcal disease in Denmark, where meningococcal disease was epidemic (∼35 cases per 1,000,000 population), had complement deficiencies (363). In contrast, complement deficiency was diagnosed in 8 of 16 persons (50%) with meningococcal disease in Japan, where disease was sporadic (with an incidence of 1 in 1,000,000) (313). The introduction of a hypervirulent clone into a nonimmune population would result in disease and spread of the strain efficiently among normal as well as complement-deficient individuals, and because the number of the former greatly exceeds the number of the latter, the relative proportion of complement-deficient persons affected will be small. In nonepidemic situations or when the population at large has developed immunity against the prevalent strain, the complement-deficient individuals are likely to be at a higher risk and would represent a greater proportion of cases.
Meningococcal Colonization and Invasion: the First Step in Virulence
Nasopharyngeal colonization is the first step in meningococcal pathogenesis. Humans are the only known reservoirs for N. meningitidis infection. Type IV pili are crucial for attachment of meningococci to epithelial cells. Encapsulated meningococci possess a highly negatively charged capsule which can interfere with the engagement of several bacterial adhesins with their cellular receptors. Type IV pili are polymeric filamentous proteins that protrude beyond the capsule and are instrumental in mediating initial attachment of bacteria to cells. Pili undergo extensive antigenic variation that regulates adhesion of bacteria to cells. Some pilus variants form bundles of pili that bind bacteria together and allow them to grow as microcolonies on the cell surface. Following the initial attachment, more intimate attachment occurs through other bacterial molecules, including opacity protein (Opa), neisserial adhesin A (NadA), and lipooligosaccharide (LOS). Mechanisms of adherence and invasion have been discussed by Virji (467) and Nassif et al. (314).
Complement Evasion Strategies of N. meningitidis
Capsular polysaccharide is the most important N. meningitidis virulence factor. With a few exceptions (136, 191, 468), almost every reported case of invasive disease is caused by encapsulated organisms. Capsular polysaccharide expression increases the resistance of bacteria to complement-dependent killing. The exact mechanism by which the various capsular polysaccharides confer serum resistance is not clear. While group B, C, W-135, and Y capsules possess sialic acid, others, such as those of groups A and X, do not. Jarvis and Vedros showed that desialylation of a group B meningococcus [group B capsule is a homopolymer of α(2,8)-linked polysialic acid] with neuraminidase increased alternative pathway activation (207). Group C capsule [a homopolymer of α(2,9)-linked polysialic acid] also regulated the alternative pathway. Group C strains that “hyperproduce” capsule because of an insertion element between the capsule synthesis (sia or syn) and capsule transport (ctr) operons inhibit the alternative complement pathway more effectively than strains that produce “normal” levels of capsule (459).
A majority of invasive meningococcal isolates express the sialylated lacto-N-neotetraose LOS species (213, 383). Neisserial LOS molecules mimic host carbohydrates (275, 276), which could contribute to the ability to evade host immunity. In contrast, carrier strains isolated from the nasopharynx tend to express LOS species that cannot be sialylated (213). These observations support a role for LOS sialic acid in pathogenesis of invasive disease. However, the role for LOS sialylation in mediating serum resistance in meningococci is not as clearly defined as for N. gonorrhoeae (115, 138).
Another membrane component that plays a role in enhancing meningococcal serum resistance is called factor H-binding protein (fHbp). This protein (also called lipidated protein 2086 [LP2086] and genome-derived neisserial antigen [GNA] 1870) was identified as a vaccine candidate by two groups using independent approaches: a “reverse vaccinology” approach (349) or fractionation of meningococcal outer membrane proteins to identify fractions that elicited bactericidal antibodies (L. Bernfield, L. Fletcher, A. Howell, J. Farley, R. Zagursky, M. Knauf, and G. Zlotnick, presented at the Proceedings of the 13th International Pathogenic Neisseria Conference, Oslo, Norway, 2002). fHbp is a principal component of two promising investigational recombinant protein vaccines (161). While all meningococcal strains isolated to date express fHbp, expression levels vary widely across strains (285). fHbp binds to factor H and enhances its ability to resist complement-dependent killing (269, 394). The role of blocking antibodies directed against capsule has been discussed above as part of the discussion of bacterial complement evasion strategies.
Roles of Natural Antibody and Opsonophagocytosis in Protective Immunity against Meningococcal Disease
Considering the high rates of carriage, the incidence of invasive meningococcal disease, even during epidemics, is relatively low. Cross-sectional population studies have shown that in a nonepidemic setting, about 10% of healthy humans carry meningococci in their nasopharynges (58, 61, 71, 425). In closed and semiclosed populations, such as in army barracks and in dormitories, carriage rates approached 100% during an epidemic. A study of the rates of carriage among children and young adults revealed that the lowest rates (<5%) were among young children aged 10 years or less and the highest carriage rates (20 to 30%) in young adults between 20 and 25 years of age (71). Similar carriage rates of ∼17% were reported among 15- to 19-year-olds in a large study carried out in the United Kingdom (272). About 50% of carriage isolates are unencapsulated, while almost every strain recovered from the bloodstream or the cerebrospinal fluid (CSF) is encapsulated. Unencapsulated strains adhere more efficiently to nasopharyngeal epithelial cells (426) and may therefore have an advantage over encapsulated strains in their ability to establish colonization.
The seminal studies of Goldschneider et al. published over 40 years ago showed that protection against invasive meningococcal disease correlated well with a serum bactericidal titer of ≥1:4 (i.e., the proportion of patient serum in the reaction mixture that results in >50% killing is 1:4 or less) using human complement as the complement source (164). The percentage of sera that lacked bactericidal activity reached a nadir in samples from children between 6 months and 2 years of age, which coincided with the age of maximum susceptibility to disease. In the next set of experiments, baseline serum samples were collected from army recruits at Fort Dix at their time of enrollment and from recruits who contracted meningococcal disease. Serum bactericidal assays were performed against the group C strain that caused an epidemic in the camp using the baseline sera from 54 patients and 444 controls; only 3 of 54 (5.6%) baseline sera from cases showed a bactericidal titer of 1:4 or greater, while 82.2% of control sera could kill the epidemic strain. The baseline sera from patients also did not kill heterologous strains; bactericidal titers of 1:4 or greater against a group A, B, or C strain were seen with only 17.4%, 13.0%, and 8.7% of baseline patient sera versus 72.2%, 77.8%, and 67.0% of control sera, respectively. This study clearly showed that while most patients who contracted disease did not have protective bactericidal titers against the homologous strain or against a heterologous group A, B, or C strain tested, as many as 35% of young adult males also lacked equivalent protective titers against at least one of the heterologous strains tested. Yet fewer than 1% of these adults contracted meningococcal disease during the epidemic conditions that prevailed at Fort Dix in the winter of 1967 to 1968. To better understand the dynamics of colonization and invasion, sera from 492 recruits were obtained at baseline and nasopharyngeal swabs were taken at 2-week intervals over an 8-week period. Of the 492 recruits, 54 (11%) had baseline bactericidal titers of less than 1:4 against the epidemic case strain. Of these 54 potentially susceptible individuals, 44 were colonized with meningococci over the 8-week period; 24 of the 44 isolates were group C (the epidemic was also caused by a group C strain). However, 11 recruits had bactericidal titers against their colonizing group C strain, which suggested that this group C strain was distinct from the epidemic strain. Of the remaining 13 recruits, 5 suffered invasive meningococcal disease, to yield an attack rate of 38.5%. It may therefore be concluded that not all susceptible individuals develop invasive infection despite being colonized with an invasive isolate.
Studies performed more recently in the United Kingdom evaluated the correlation between bactericidal titers and disease incidence (determined using laboratory surveillance data) in various age groups for serogroup C (452) and serogroup B (453). For serogroup C, the inverse relationship between bactericidal activity and disease incidence was roughly observed, but with a lower percentage of adults achieving putatively protective titers (∼30%) than in the study by Goldschneider et al. (164) (∼70%). For serogroup B, the highest incidence of disease was seen in infants 6 to 11 months of age, as (presumably) maternally derived antibodies decreased. However, despite the decreasing incidence of disease through childhood, there was no apparent increase in the proportion of children with putatively protective bactericidal titers. These data contrast with the data of Goldschneider et al. (165), who showed that the proportion of individuals with protective (≥1:4) bactericidal titers rose throughout childhood. Trotter et al. (452) reported a small secondary peak in disease incidence among teenagers, but the proportion of individuals with bactericidal titers of ≥1:4 also rose among this age group. Why the 2- to 12-year age group did not show a higher incidence of disease despite having a lower proportion of subjects with “protective” antibody levels is not clear. The simplest explanation is probably because of lower rates of carriage and exposure, but this alone cannot explain the high disease rates in infants.
The role of opsonophagocytosis in conferring protection against meningococcal disease (in the absence of serum bactericidal activity) has received little attention. The role of complement-dependent phagocytosis and complement-dependent bactericidal activity was studied with 62 strains of N. meningitidis by Ross and colleagues (375). Strains that belonged to serogroups B and 29E, but not serogroup A, C, W-135, and Y strains, were killed by neutrophils following incubation with C8-depleted pooled human serum. While similar amounts of C3 were deposited on group B and group Y strains, only the former were resistant to direct complement-dependent killing. Further, there was no correlation between opsonophagocytic killing and complement-dependent bactericidal activity among the group B and Y strains. In contrast to the C8-depleted pooled human serum, serum from an immunized C8-deficient patient could mediate opsonophagocytic killing, which suggested that opsonophagocytosis assumes a critical role in protection against meningococcal disease in persons deficient in components of the terminal complement pathway (C5 through C9). In particular, the efficacy of a meningococcal vaccine in such individuals would depend on opsonophagocytic activity.
The Fcγ receptor family in humans is broadly divided into FcγRI, FcγRII, and FcγRIII; FcγRII is further subdivided into FcγRIIA (CD32), FcγRIIB, and FcγRIIC, and FcγRIII comprises FcγRIIIA and FcγRIIIB (CD16). The reader is referred to reference 323 for an in-depth review of these receptors. A variety of human FcγR alleles with altered functionality exist; two Fcγ alleles in N. meningitidis whose roles have been investigated are the H/R131 forms of FcγRIIA and the NA1/NA2 forms of FcγRIIIB. In vitro studies demonstrated that IgG1-mediated phagocytosis of S. aureus, H. influenzae type b, and group B meningococci was less with PMNs homozygous for the NA1 allele of FcγRIIIB than with those homozygous for the NA2 allele (47). The difference was most apparent when complement was absent in the reaction mixture and with concomitant blockade of FcγRIIA. The H/R alleles at amino acid 131 of FcγRIIA determine the affinity for human IgG2, which is believed to be the dominant subclass response to encapsulated pathogens (403). H/H homozygosity results in high-affinity binding, while R/R homozygosity results in low-affinity binding; heterozygotes show intermediate affinity.
In a study of 15 persons with C6 or C8 deficiency from four families either with (n = 8) or without (n = 7) a history of meningococcal disease, the combined FcγRIIA (R/R 131)/FcγRIIIB (NA2/NA2) phenotype was seen in 6 individuals with a history of disease and in only 1 without a history of meningococcal disease (OR, 13.9). The authors also examined 15 properdin-deficient individuals from 5 families, 7 of whom experienced one episode each of meningococcal disease and 8 who never had meningococcal disease. There was no association between FcγRIIA phenotype and disease susceptibility in the properdin-deficient population. In a retrospective study of 25 children who survived fulminant meningococcal septic shock, 11 were homozygous for the R/R131 allele of FcγRIIA, which binds IgG2 with lower affinity than the H/H allele (46). The incidence of the R/R allele in meningococcal sepsis survivors (44%) was significantly higher than the prevalence of this allele in the healthy Caucasian population (23%). Correspondingly, PMNs with the R/R131 allotype phagocytosed N. meningitidis opsonized with polyclonal IgG2 antibodies less effectively than PMNs from H/H131 individuals.
FcγRIIA allotypes in 29 Russian terminal-complement-deficient patients who suffered recurrent episodes of meningococcal disease were evaluated. The risk of contracting meningococcal disease was 3.3 times higher in R/R131 homozygous individuals above the age of 10 than in the corresponding age-matched H/H homozygous individuals. H/H homozygosity was not protective in children under the age of 10 years. A possible explanation for the age-related protection of the H/H allele is that the older population may have had higher antibody levels than the younger population, and the protective effects of Fc receptors would be more evident in the presence of antibody. In the same study, meningococcal disease had a more severe course in 14 of 31 disease episodes in patients with R/R and R/H allotypes; in contrast, only 1 of 18 severe episodes was seen in patients with the H/H131 allotype (the remaining 17 were classified as having moderate disease). Thus, H/H131 individuals appear to have a higher acquired antibody-mediated phagocytosis-dependent resistance to meningococcal disease above the age of 10 years. H/H homozygotes may also have a relatively milder disease course.
Evidence for opsonophagocytic protection provides the rationale for immunization of terminal-complement-deficient patients against meningococcal disease. Antibody titers against group A and C polysaccharide were similar in 8 late-complement-deficient persons, 11 of their family members, and 7 unrelated normal individuals (11). The decline in antibody responses was more rapid in complement-deficient persons. However, the elicited anticapsular antibodies promoted opsonophagocytic killing. Enhancement in opsonophagocytic activity was similarly reported in three C7-deficient siblings following administration of the quadrivalent polysaccharide vaccine (391). Eighteen patients with late complement defects who were given the quadrivalent polysaccharide vaccine were followed for 2 years. Two episodes of meningococcal disease occurred in two patients at 9 and 12 months following administration of the vaccine (350). One of the patients was presumed to have group C disease based on the observation of a significant increase in anti-group C polysaccharide antibody titers. This patient had relatively low levels of anti-group C antibody 4 months preceding the infection, which could have accounted for the susceptibility to invasive disease. The serogroup that caused disease in the second patient was not known, and thus it is not clear why this individual contracted disease. Fijen et al. reported the response to immunization with the tetravalent capsular polysaccharide vaccine in 53 complement-deficient individuals (7, C3; 19, properdin; 27, terminal component) (131). All patients generated functional antibody responses to immunization that were comparable to that seen in normal individuals. The majority of patients were followed for 6 years postimmunization; six episodes of meningococcal disease occurred. Four of these were caused by group B strains that were not covered by the vaccine. The remaining two were caused by group Y. These latter infections both occurred in C8-deficient individuals, at 3.5 years and 5 years postimmunization. Antibody titers following administration of the polysaccharide (unconjugated) vaccine declines rapidly after 2 to 3 years, and therefore the occurrence of disease after this period does not necessarily constitute vaccine failure. Revaccination of two C3-deficient and 17 late-complement-deficient patients resulted in robust increases in antibody titers (101).
Platonov and colleagues studied the efficacy of the quadrivalent polysaccharide vaccine in Russian patients with late complement component deficiency who experienced between one and five meningococcal infections (352). Of 45 such patients, 31 were immunized with a quadrivalent meningococcal polysaccharide vaccine and were followed for 3 to 8 years. As reported in prior studies, there was a good IgG response at 1 month after vaccination, which remained elevated for about 3 years. Revaccination of these terminal-complement-deficient patients 3 years after the first dose restored the total Ig concentrations to those observed 1 year after the first dose. Six new episodes of meningococcal infection in four patients developed in the vaccine group. Among the 14 unimmunized patients, six episodes in six patients developed during the same study period. A significant survival benefit was detected, substantiating the need to vaccinate complement-deficient persons against meningococcal disease. Studies to determine the efficacy of the newer conjugate vaccines in complement-deficient patients will need to be carried out to document their efficacy in this population, although there is no reason to suspect that they will be less efficacious than the polysaccharide vaccine.
Correlates of the Severity of Meningococcal Disease
The use of quantitative PCR has enabled accurate quantification of bacterial loads in the bloodstream of patients with meningococcemia. A study of DNA levels in blood by real-time PCR in 1,045 patients throughout England and Wales showed that higher bacterial loads correlated with poorer outcomes (81). The median bacterial load in 95 patients who died was 5.29 log10 copies/ml, compared to a median bacterial load of 3.79 log10 copies/ml in the 950 survivors. Higher bacterial loads were associated with prolonged hospitalization; digit, limb, or soft tissue loss; and the need for hemodialysis. This study corroborated results from a previous study which also showed a correlation between higher bacterial load and severe disease (median of 8.4 × 106 DNA copies/ml) compared to milder disease (1.1 × 106 DNA copies/ml) (174). Bacterial load did not correlate with the duration of symptoms prior to admission.
Disease severity correlates with levels of circulation proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α), IL-1, and IL-6 (464, 470); chemokines such as monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), and IL-8 (304); endotoxin (44); and complement activation (44). It is not clear whether endotoxin released from bacteria results in complement activation or whether complement activation on the bacterial surface results in endotoxin release. The latter scenario may serve to explain why persons with terminal complement deficiencies have a more favorable outcome, and this is discussed below. The importance of lipid A structure in inciting inflammation was demonstrated in a recent study by Fransen et al. (140), who screened 464 meningococcal isolates for their ability to stimulate cytokines in vitro. About 9% of these strains elicited a weak cytokine response. Analysis of the LPSs of these strains revealed a penta-acylated lipid A because of mutations in the lpxL1 gene. Patients infected with lpxL1 mutant strains presented significantly less frequently with rash and had higher platelet counts, consistent with reduced cytokine induction and less activation of tissue factor-mediated coagulopathy. These data pointed to an important role for N. meningitidis LPS in determining the clinical course of meningococcal disease.
Characteristics of Meningococcal Disease in Terminal Complement Component Deficiency States
The features of meningococcal disease in terminal-complement-deficient persons were characterized previously by Figueroa and Densen and have been confirmed in subsequent studies. Terminal complement deficiency is associated with a 7,000- to 10,000-fold-higher risk of developing meningococcal disease (128). The risk of disease in C9-deficient individuals is lower at ∼1,400-fold, as discussed above, probably because C9-deficient serum can support hemolytic activity, albeit very weakly (128). About 40 to 50% of terminal-complement-deficient persons suffer recurrent infections. In some instances, relapses, defined as infection with the same serogroup recurring within 1 month of the prior infection, have been documented. A plot of the logarithm of the number of infections sustained by each terminal-complement-deficient patient against the number of episodes of infection yielded a straight line (128). The data supported the hypothesis that prior meningococcal disease does not protect these individuals from subsequent episodes of meningococcal disease. The risk of each episode of meningococcal disease was independent of prior episodes and was calculated to be 39.1% (128). It is not clear why prior infections do not protect this population from recurrent episodes, especially in light of data that demonstrate adequate antibody responses following natural infection (331, 358). These antibodies are capable of supporting bactericidal activity in combination with normal human complement. Immunization of these individuals with the polysaccharide vaccine also elicits an adequate antibody response that confers protection against disease (352). Infection is likely to result in strain- and serogroup-specific immunity, while vaccination with the quadrivalent vaccine would elicit protective antibodies against four of the five major serogroups.
While the median age of meningococcal infection in complement-sufficient persons is ∼3 years, the median age of the first infection in terminal-complement-deficient persons is 17 years (128). A possible explanation for this seemingly counterintuitive observation is that normal individuals develop durable natural immunity with age, while complement-deficient individuals always remain at risk for disease. Further, exposure to meningococcal strains may also increase during the teenage years.
An increased risk of meningococcal disease caused by serogroup W-135 and Y strains and other rare serogroups has been reported for complement-deficient patients (128, 132, 135, 329, 374). A study of South African patients showed a significant association between rare serogroups and complement deficiencies. There was a strong association between electrophoretic type (ET), cluster F strains, and complement deficiency (329). In contrast, serotyping, serosubtyping, and multilocus enzyme electrophoretic typing of meningococci showed no difference between isolates from 44 complement-deficient persons and from 50 complement-sufficient persons in the Netherlands with meningococcal disease (130).
The archives of the Netherlands Reference Laboratory for Bacterial Meningitis have data on all blood and CSF meningococcal isolates and have provided invaluable insights into the epidemiological aspects of meningococcal disease. A total of 7,732 cases of meningococcal disease were reported between 1959 and 1992. Serogroups A, B, and C represented 8%, 71%, and 18% of the cases, respectively; the remaining 3% were caused by “uncommon serogroups” (W-135, X, Y, Z, 29E, and nongroupable isolates). Fijen et al. (134) selected four cases each caused by group A, B, and C strains from every year of the study period and three cases caused by uncommon serogroups for every 10-year interval. Because of a doubling of the incidence of group B disease between 1989 and 1992, 55 additional group B cases were included in the analysis. Of the 460 selected cases, 176 were available for further study and constituted the first study group. The second group comprised 91 of the 225 patients with disease caused by uncommon serogroups (non-A, -B, or -C). In the first study group, 6 of 176 patients (3%) were diagnosed with a complement deficiency. One of 45 cases caused by group A (2%), 3 of 46 cases caused by group C (7%), and 2 of 6 cases caused by uncommon strains (33%) were diagnosed with complement deficiency. None of the selected group B cases were associated with complement deficiency. In the second group of 91 patients who were infected with uncommon serogroups, 30 (33%) had a complement deficiency. Of all patients under 5 years of age, only 2% had a complement deficiency, versus 19% of those above 5 years of age. Of the 32 patients with uncommon serogroups and complement deficiency, only 1 was under 5 years of age, highlighting the observation that complement-deficient persons are older than normal persons at the time of infection. Properdin deficiency was detected in 13, C3 deficiency syndromes were detected in 6 (3 with C3 NeF, 1 with factor H deficiency, and 2 with congenital C3 deficiency), and late complement deficiency was seen in 17. Among relatives of complement-deficient individuals, 27% had meningococcal disease (18% of the relatives of properdin-deficient individuals and 38% of the relatives of late-complement-component-deficient individuals became infected), versus only 2% of relatives of normal individuals. None of the properdin- or C3-deficient patients had recurrent disease, while 57% of the late-complement-deficient patients had two or more recurrences. In this study, persons with properdin or late complement component deficiencies had a slightly higher incidence of septic shock than the general population. The severity of recurrent episodes was similar among the late-component-deficient individuals.
The prior studies have categorized serogroups W-135, Y, and X as “uncommon” serogroups (and sometimes less virulent), and a relatively high incidence of complement deficiencies has been detected among persons infected with these strains. However, the epidemiology of meningococcal disease has changed over the past 2 decades. Serogroup Y disease, which was relatively uncommon (and still remains uncommon in Europe), is responsible for a third of cases in the United States. An epidemic of W-135 disease that began during the Hajj in 2000 (253) has spread to several countries. An epidemic caused by serogroup X has been reported in Niger. It would be highly unlikely that a large fraction of cases caused by these strains are in complement-deficient persons. A more likely explanation is that these W-135, X, and Y strains are more virulent than previously isolated strains from these serogroups. Considerable genetic exchange occurs among meningococci, and modern molecular typing methods have divided meningococci into clonal complexes. Certain clonal complexes, such as ST-11 (ET-37), ST-32 (ET-5), and ST-41/44 complexes, are examples of “hyperinvasive” lineages (494). Although the majority of strains within a particular lineage belong to a single serogroup, each lineage comprises strains that belong to more than one serogroup. Whether the strains that cause disease in complement-deficient persons belong to less virulent lineages is not known. It is interesting that meningococcal strains that cause invasive disease in complement-deficient persons are all serum resistant (374). Similar strains that cause disease in normal individuals are responsible for infections in complement-deficient persons (130). With a few exceptions (88), most strains that cause disseminated gonococcal infection (DGI) in complement-deficient individuals are also resistant to killing by serum from normal humans (374). The current knowledge of the mechanisms of neisserial serum resistance suggests that regulation of complement deposition occurs at or prior to the level of C3 deposition (395, 469). Less C3 binding would enable the organism to evade opsonophagocytosis, which is the major mechanism of immune defense in complement-deficient individuals. However, strains that cause disease in C3-deficient persons are also serum resistant. It therefore seems reasonable to conclude that virulence requires attributes in addition to serum resistance, and this could explain why certain lineages that possess these properties are “hypervirulent.”
Finally, it has been observed that meningococcal disease in late-complement-component-deficient persons is milder and is associated with lower mortality than that in their complement-sufficient counterparts (128, 374). Disease severity and mortality in meningococcemia are related to the level of complement activation and circulating endotoxin levels (44). In vitro studies have shown that an intact complement system is required for LPS release from rough E. coli strains (327, 439). Lehner et al. (248) followed endotoxin levels in a C6-deficient girl with meningococcemia. Endotoxin levels were low upon admission but rose dramatically following infusion of fresh frozen plasma, concomitant with correction of the C6 deficiency. The patient's serum obtained at the time of admission did not cause LPS release from E. coli, but serum from the patient following plasma transfusion did release LPS. Taken together, these data suggest that at least one reason for better clinical outcomes in late-complement-component-deficient persons with meningococcemia may be related to lower circulating endotoxin levels.
Meningococcal Disease in Properdin Deficiency
The frequency of properdin deficiency may be underestimated because the standard screening assays for complement deficiencies, such as the CH50, C3, and C4 levels, are usually normal. The alternative pathway hemolytic activity (AP50) is low, but values often lie within the normal range. Immunochemical assays can detect type I and II properdin deficiencies, while functional tests are necessary to diagnose type III deficiency. These tests are offered only by specialty laboratories. Cases often come to attention because of a family history of meningococcal disease.
Properdin deficiency is associated with an increased risk of meningococcal disease. About 50% of properdin-deficient individuals suffer from invasive meningococcal infection. In the cases listed by Figueroa and Densen, with the exception of recurrent S. pneumoniae bacteremia and otitis media in a child with a combined C2 and properdin deficiency, properdin deficiency was associated almost exclusively with N. meningitidis infections (128). Several subsequent studies have continued to report an association between properdin deficiency and meningococcal infections (78, 94, 142, 157, 389, 392, 417), including a case of bone and joint infection (376). Three index cases and six additional family members with properdin deficiency were detected among 101 survivors of meningococcal infections and 59 survivors of severe pneumococcal and H. influenzae infections (392). Two of the patients had meningococcal infection at 15 and 16 years of age, respectively, and one had H. influenzae meningitis at 1.5 years of age. The patients belonged to three nonrelated families of Tunisian Jews from different parts of Tunisia. In contrast to the previously described fulminant course of meningococcal infection with high fatality in some properdin-deficient patients (374), these two patients had relatively indolent disease.
Why only some but not all properdin-deficient persons contract meningococcal disease is not clear. Four members of a family with 10% of normal properdin levels were identified; two of these four individuals had meningococcal disease (142). Only two of nine properdin-deficient males in a Swiss family had meningitis caused by Neisseria meningitidis serogroup B without any sequelae (417). Interestingly, these two properdin-deficient patients with meningitis differed from the other properdin-deficient individuals in that they lacked the G2M(n) allotype; the presence of this allotype is associated with higher IgG2 antibody responses to T-independent antigens such as polysaccharides (232, 333, 388). The authors concluded that an additional factor(s) could determine the susceptibility of properdin-deficient persons to infection.
Concomitant dysfunction of the coagulation system could result in a more fulminant disease course. Fijen et al. (129) described a case of a 7-year-old properdin-deficient child with serogroup Y disease and extensive gangrene of the limbs and a pulmonary embolism who underwent multiple amputations. This child had concomitant protein C deficiency, which contributed to the clotting diathesis. A study of complement deficiencies in 29 children (mean age of 4.5 years) who survived meningococcal disease revealed one case of properdin deficiency. This patient also had heterozygous protein C deficiency and was the only group Y meningococcal case in that series (94).
High mortality has been cited as a reason for the lack of recurrences of meningococcal disease among properdin-deficient persons (93, 128, 374). Another reason for the lack of recurrences in this population may be because specific antibodies can support bactericidal activity in properdin-deficient serum (414, 415). In some instances, antimeningococcal IgG could enhance alternative pathway-mediated complement deposition in a properdin-dependent manner (414).
Factor H Levels and Predisposition to Meningococcal Disease
An SNP within a nuclear factor κB (NF-κB)-responsive element in the factor H gene (C-496T) regulates serum factor H levels; individuals with the C/C genotype have higher factor H levels (177). Genetic susceptibility to meningococcal disease was investigated in two independent studies, a case-control and family-based transmission-disequilibrium test (TDT), using two separate cohorts of United Kingdom Caucasian patients. Disease susceptibility was both genetically associated with the C/C homozygous genotype (OR, 2.0) and linked to the C allele. The association was most significant in serogroup C-infected patients (OR, 2.9) (177). Individuals possessing the factor H C-496T C/C genotype were more likely to have increased serum factor H levels and reduced bactericidal activity against meningococci and were at an increased risk of contracting meningococcal disease.
INFECTIONS IN SPLENECTOMIZED INDIVIDUALS
Causes of Hyposplenism
Splenic dysfunction can be the result of either anatomic or functional hyposplenism. Asplenia and splenic hypoplasia refer to the complete or partial lack of splenic tissue, respectively. The commonest cause of the absence of splenic tissue is surgical splenectomy. Individuals with sickle cell disease undergo autosplenectomy because of vaso-occlusion that leads to splenic infarctions and loss of splenic tissue. Congenital asplenia associated with congenital abnormalities of the cardiovascular system is called Ivemark syndrome (205). Isolated congenital hyposplenia and asplenia are very rare conditions.
Risk of Infection following Splenectomy
A retrospective review of the literature spanning the period 1966 to 1996 concluded that the risks of infection among children and adults were 3.3 and 3.2%, respectively (32). The mortality among children was slightly higher (1.7%) than that among adults (1.3%). The presence of hematological disorders was associated with higher infection rates; patients with thalassemia and sickle cell anemia had the highest rates of infection (8.2% and 7.3%) and mortality (5.1% and 4.8%), respectively. In a study of 538 splenectomized persons who were followed over 1,731 person-years, 38 developed bacteremia, and of these, 45% occurred during the first month after surgery (107). However, most of these early postsplenectomy bacteremias were caused by Enterobacteriaceae and occurred in patients with gastrointestinal malignancies, while only one was caused by S. pneumoniae. Beyond the early postoperative period, there was an 8-fold increase in the risk of bacteremia.
Estimates of the annual incidence of serious bacterial infections following splenectomy derived from cohort studies are 0.42% (77) and 0.23% (107) per year, with a lifetime risk of overwhelming infection of about 5% (262). The incidence of severe infection and its outcome were evaluated in 1,648 patients who underwent splenectomy in Scotland between 1988 and 1999 by Kyaw et al. (242a). The overall rate for a first severe infection was 7.0 per 100 person-years. The overall rates for a second infection and a third infection per 100 person-years were 44.9 and 109.3, respectively. Among the repeated episodes of severe infection, over half occurred within 6 months of the first severe infection. The susceptibility to severe infection was greatest in older age groups (5.5 per 100 person-years in those aged >50 years) and in patients splenectomized for hematologic malignancy (9.2 per 100 person-years). Between 50% and 80% of all severe infections or deaths occurred within 1 to 3 years following splenectomy. Whereas some series indicate that the risk of overwhelming postsplenectomy infection declines with the time elapsed following splenectomy (262), other series do not show a significant reduction (471). In conclusion, the risk of infections is higher in persons who undergo splenectomy for hematologic conditions (especially malignancies) and in those who have additional causes for immunosuppression, such as corticosteroid or cytotoxic medications. Overwhelming infection following splenectomy carries a mortality of 38 to 69% (471).
Pneumococcal Sepsis and Infections with Other Encapsulated Bacteria
S. pneumoniae is overwhelmingly the most common cause of postsplenectomy sepsis and accounts for 80 to 90% of cases (5, 471). Because it is a Gram-positive organism and possesses a cell wall, S. pneumoniae is resistant to lysis by insertion of a membrane attack complex. Phagocytosis mediated by leukocyte complement and Fc receptors is a major mechanism of pneumococcal killing. The importance of the antibody-dependent classical pathway in host defenses against pneumococci is evident from the increased risk of invasive pneumococcal disease in persons with a range of disorders that result in low levels of or dysfunctional immunoglobulins. The spleen is an important site for filtering complement- and antibody-coated bacteria from the bloodstream.
Splenic dysfunction is also associated with an increased incidence of invasive disease caused by other encapsulated bacteria such as N. meningitidis, E. coli, and H. influenzae type b. Antibodies play an important role in clearing these pathogens, and loss of splenic function would explain the predisposition of asplenic individuals to these infections. The widespread use of the conjugate vaccine over the past 2 decades has virtually eliminated invasive H. influenzae type b disease where it is used.
Malaria
Persons with splenectomy suffer a higher risk of malaria and babesiosis, both of which are protozoal infections that affect erythrocytes. A prospective study over a 1-year period of 33 Malawians who had undergone splenectomy secondary to trauma showed that splenectomized patients were twice as likely as controls to have P. falciparum parasitemia (19). Parasitemia was more likely to be associated with febrile symptoms in splenectomized individuals. Parasite densities reached significantly higher levels (90, 204, 356) and mature developmental parasite forms were seen more frequently in the peripheral blood of asplenic individuals (blood smears in acute P. falciparum infection usually show only early trophozoites or gametocytes). The course of P. falciparum or Plasmodium vivax malaria was not reported to be more severe in four splenectomized patients in Thailand with either P. falciparum or P. vivax infection; three of the four cases were categorized as having partial immunity against malaria (259). Acute P. falciparum malaria developed in two immigrants from Africa who underwent splenectomy for splenomegaly thought to be secondary to a lymphoproliferative disorder; malaria developed despite the two immigrants not leaving the area where malaria was not endemic following splenectomy (27).
The spleen plays an important role in removing RBCs that have reduced deformability. However, it is difficult to distinguish live from dead parasites based on light microscopy. The “persistence” of parasitemia following treatment for malaria in asplenic patients may be because of lack of “pitting” (removal of the parasite with a part of the RBC membrane by the spleen). Parasite clearance after artesunate treatment was markedly prolonged, although the parasites were presumed dead because they could not be cultured ex vivo. These observations confirm the central role of the spleen in the clearance of parasitized RBCs after antimalarial therapy. The failure of an asplenic patient to clear parasitemia based solely on a peripheral smear should not be presumed to indicate antimalarial drug resistance.
Babesiosis
Babesia microti is responsible for most cases of human babesiosis in the United States. Most cases have been reported in the coastal areas of southern New England from eastern Connecticut to Cape Cod and the chain of islands off its coast (Nantucket, Martha's Vineyard, Block Island, eastern Long Island, Shelter Island, and Fire Island). Because babesiosis is not a reportable disease, it is difficult to estimate its incidence. A few cases of Babesia duncani and related species have been reported in northern California and in Washington state. Other Babesia species related to Babesia divergens can also cause disease in splenectomized persons (186). Babesiosis is rare in Europe, and most reported cases have occurred in splenectomized persons and have been caused by B. divergens (373).
B. microti infections can vary in severity. About 25% of adults and 50% of children have a self-limiting flu-like illness. Fever, fatigue, malaise, chills, sweats, myalgias, and arthralgias are common manifestations. Shortness of breath, headache, anorexia, and nausea are less frequent manifestations. Fever is the most common finding on the physical exam. Hepatosplenomegaly and jaundice are infrequently seen. Severe disease, including pulmonary and renal complications, disseminated intravascular coagulation, cardiac failure, or a persistent relapsing course, occurs more commonly in immunocompromised persons (237). Laboratory findings show evidence of intravascular hemolysis, including low hemoglobin and hematocrit, low serum haptoglobin concentration, and hemoglobinuria. The reticulocyte count is elevated, the white count is normal or low, and thrombocytopenia is common. Parasitemia ranges from 1 to 10% but can reach as high as 80% in asplenic patients (45, 66). Liver enzymes (alkaline phosphatase, lactate dehydrogenase, and aspartate and alanine aminotransferases) and bilirubin are elevated.
B. microti infections can be fatal in 5 to 10% of cases. A univariate analysis of 139 cases of babesiosis in New York state showed that a history of cardiac abnormality, a history of splenectomy, the presence of a cardiac murmur, alkaline phosphatase levels of greater than 125 U/liter, white blood cell counts of greater than 5 × 109/liter, and parasitemia of 4% or higher were significantly associated with severe disease (480). Splenectomized persons suffer more severe disease, including complications such as noncardiogenic pulmonary edema, altered mental status, renal failure, and hemophagocytic syndrome (66, 296, 380, 410). The clinical course in such patients can often be prolonged (402). A severe case of babesiosis in an HIV-positive individual eventually responded to RBC exchange transfusion (265). A combination of azithromycin and atovaquone for 7 to 10 days is the recommended initial regimen for treatment of B. microti infections (238). Immunocompromised persons may require longer courses of treatment, perhaps for 2 weeks after clearance of parasitemia. A combination of quinine and clindamycin is also effective but may be associated with a higher incidence of side effects (238). Clindamycin and quinine are recommended for B. divergens infections, which tend to progress rapidly because they tend to infect splenectomized persons. Exchange transfusions to reduce the parasite burden should be considered for severely ill persons with parasite loads of >10%.
Capnocytophaga Infections
Capnocytophaga canimorsus, formerly called Centers for Disease Control group dysgenic fermenter (DF) 2, was first discovered in 1976 (35). This is a Gram-negative rod that is found in the oral flora of dogs and cats and can be transmitted to humans by bite (54% of cases), scratch (8.5%), or mere exposure to animals (27%). Although it is rare, C. canimorsus can cause serious infections, including fulminant sepsis, meningitis, and peripheral gangrene (249, 342, 428). Lion et al. (255) reviewed over 100 cases of C. canimorsus septicemia and found decreased host defenses due to splenectomy in 33%, alcohol abuse in 24%, and other causes of immunosuppression in 5%. There was no obvious predisposing factor in the remaining 40% of cases. Of 28 reported cases of C. canimorsus meningitis, splenectomy and alcohol abuse were reported for 18% and 25%, respectively. The clinical features and cerebrospinal fluid findings in Capnocytophaga meningitis were similar to those seen with other causes of bacterial meningitis (249). The mortality rate in patients with meningitis alone (5%) was lower than the mortality seen in cases of septicemia (30%) (249). Recently, a fatal case of Capnocytophaga cynodegmi sepsis and meningitis in a woman with diabetes and traumatic splenectomy was described. Prior to this report, C. cynodegmi was associated only with local infections following dog bites (48). C. canimorsus is susceptible to penicillin, ampicillin-sulbactam, clindamycin, imipenem, and extended-spectrum cephalosporins. Ampicillin-sulbactum should be given prophylactically to asplenic individuals who sustain dog bite injuries.
PREVENTION OF INFECTIONS IN PERSONS WITH DEFECTS OF COMPLEMENT ACTIVATION AND SPLENECTOMY
Immunization
Vaccines that were originally developed against N. meningitidis, H, influenzae, and S. pneumoniae comprised purified bacterial polysaccharides. Unfortunately, these vaccines were not immunogenic in children under the age of 2 years, who suffer the highest burden of disease. Unconjugated polysaccharide vaccines do not elicit a memory response and have no effect upon nasopharyngeal carriage. Only short-lived protection is seen even in adults. The introduction of conjugate vaccines and their widespread implementation has had a significant impact on the morbidity caused by these pathogens.
The widespread use of the conjugate vaccine against H. influenzae type b (Hib) since the late 1980s and early 1990s in most industrialized countries has reduced the incidence of carriage and has virtually eliminated disease in these nations. Hib was one of the most common causes of bacterial pneumonia and meningitis in children between 4 and 18 months of age. Hib infections continue to occur in resource-poor nations where children are not immunized or in instances where the primary childhood immunization series was not completed. Six major serotypes of H. influenzae (a to f) have been described, and cases caused by serotypes not covered by the vaccine are occasionally seen. Three different Hib conjugate vaccines are currently in use: polyribosyl-ribitol phosphate (PRP)-OMP (outer membrane protein of N. meningitidis), HbOC (CRM197), and PRP-T (tetanus). A fourth conjugate, PRP-D (diphtheria) was withdrawn because of its inferior immunogenicity and poorer protection compared to the other conjugate vaccine preparations (482). Because humans are the only reservoir for Hib, reduction in carriage rates can result in “herd immunity” by reducing the exposure of unvaccinated persons to the pathogen.
Of the 91 pneumococcal serotypes identified, about 11 account for 70% of cases of invasive pediatric infections worldwide (182). Currently, a 23-valent pneumococcal polysaccharide vaccine is available for use in adults. A heptavalent pneumococcal conjugate (PCV7) vaccine was approved for use in the United States in 2000 but recently has been succeeded by a 13-valent conjugate polysaccharide vaccine (Prevnar 13; Wyeth Pharmaceuticals Inc.). Prevnar 13 is approved for use in children between the ages of 6 weeks and 71 months for (i) routine vaccination of all children aged 2 to 59 months, (ii) vaccination of children aged 60 to 71 months with underlying medical conditions that increase their risk for pneumococcal disease or its complications, and (iii) vaccination of children who previously received one or more doses of PCV7 (62). A single dose of PCV13 may be administered to children 6 through 18 years of age with certain medical conditions (which include C1, C2, C3, and C4 deficiencies and splenectomy), regardless of whether they have previously received PCV7 or the pneumococcal polysaccharide vaccine.
A tetravalent unconjugated meningococcal polysaccharide vaccine was licensed for use in the United States in 1981. Currently, a tetravalent meningococcal conjugate (group A, C, W-135, and Y polysaccharides conjugated to diphtheria toxoid [Menactra; Sanofi Pasteur]) vaccine is used for the routine immunization of teenagers in the United States. In early 2010, the FDA approved a second quadrivalent meningococcal conjugate vaccine (group A, C, W-135, and Y polysaccharides conjugated to diphtheria CRM197 [Menveo; Novartis]) for use in persons between 11 and 55 years of age (63). Three monovalent serogroup C conjugate vaccines (using tetanus toxoid or CRM197 as the conjugate) are being used in several European countries, Canada, and Australia. Monovalent group A conjugates are being developed for use in countries in the African meningitis belt. The Meningitis Vaccine Project has developed a group A conjugate vaccine (MenAfriVac; Serum Institute of India), and phase 2 and 3 trials in India and Africa are under way (280). The development of vaccines against serogroup B meningococci using group B capsule has been problematic because of the structural similarity of group B polysaccharide with host tissue molecules such as neural cell adhesion molecule (N-CAM) (137). Outer membrane vesicle (OMV) vaccines derived from group B strains have been used with success to control epidemics caused by that specific strain in countries such as Norway (195) and Cuba (404). Over 60 million doses of the OMV vaccine developed at the Finlay Institute in Cuba have been administered in 16 countries in Latin America with a good safety profile. Most recently, a tailor-made vaccine against a group B strain responsible for an epidemic in New Zealand has been reported to elicit a protective bactericidal antibody titer (defined as a ≥4-fold increase in serum bactericidal titer or at least 1:8 if baseline bactericidal titers were <1:4) in ∼70% of infants given four doses of the vaccine (485), with a reported effectiveness of around 85% in children aged between 6 months and 2 years (147). The porin A (PorA) component in such vaccines appears to be the immunodominant component and may contribute to the relatively narrow strain coverage of the vaccine. OMV vaccines containing up to six PorA molecules were developed to broaden vaccine coverage (86). Efforts to develop a broadly protective vaccine against group B meningococcal strains using recently characterized meningococcal membrane proteins are under way (435).
The conjugate Hib vaccine elicits a protective antibody response in children when administered prior to (6) or after (239) splenectomy. The immunogenicity of the Hib conjugate vaccine was assessed in 57 patients with thalassemia, 32 of whom had undergone splenectomy (70). All patients achieved protective antibody levels (>1 μg/ml). Antibody titers declined to undetectable levels in four patients and decreased to concentrations of less than 1 μg/ml in another two patients at 2 to 3 years after vaccination. Studies to determine the duration of protection and timing of booster doses are required.
There are limited data on the efficacy of the pneumococcal conjugate vaccine in splenectomized patients, but the evidence suggests an adequate response in naïve individuals as well as in prior recipients of the 23-valent polysaccharide (unconjugated) vaccine (411, 423). The heptavalent conjugate vaccine (PCV7) was able to elicit high levels of IgG against all its seven constituent polysaccharides in two patients with recurrent pneumococcal bacteremia who had received the polysaccharide vaccine but failed to mount adequate antibody responses (312). The clinical efficacy of the newer 13-valent conjugate vaccine in this setting is not known and will need evaluation.
The United Kingdom recommendations are that splenectomized adults should receive two doses of the meningococcal serogroup C conjugate vaccine (in combination with Hib vaccine) 2 months apart, or one additional dose if fully immunized before splenectomy. The high prevalence of group C disease in the United Kingdom led to immunization with the monovalent group C conjugate vaccine. Asplenic children under the age of 1 year should be vaccinated according to the United Kingdom guidelines (two doses in infancy and a booster dose at 12 months of age) (http://www.dh.gov.uk/en/Publichealth/Healthprotection/Immunization/Greenbook/DH_4097254). The immune responses of 130 asplenic persons to this vaccine were measured. These individuals had a significantly lower geometric mean titer of serum bactericidal antibody titers than 48 age-matched controls. However, 80% of asplenic individuals achieved serum bactericidal titers of ≥1:8 with baby rabbit complement, which is considered protective. No differences between the two groups in the serogroup C-specific IgG geometric mean concentration were observed. Poorer responses were observed if the indication for splenectomy was medical or if the conjugate vaccine was administered less than 10 years after splenectomy. Individuals who did not achieve a bactericidal titer of ≥1:16 were offered a second dose of the vaccine; 61% of these individuals achieved a protective titer. The authors concluded that either the level of functional antibody in asplenic persons should be determined and nonresponders should receive a second dose or two doses of meningococcal vaccine should be routinely offered to this population. Similar studies are required to assess the efficacy of the quadrivalent meningococcal conjugate vaccine.
Of 111 splenectomized patients in the United Kingdom cohort, only 82% and 68% had received the Hib and group C meningococcal conjugate vaccines, respectively, indicating poor compliance with the United Kingdom immunization recommendations. Similarly, vaccine coverage in a cohort of splenectomized patients in the Netherlands was low; coverage against S. pneumoniae was only 64%, and only 32% were vaccinated against Hib and 27% against N. meningitidis (291). Education of patients and providers about the risk of infection associated with splenectomy and immunization to prevent at least some of these infections are of the utmost importance.
Prophylactic Antibiotics
As recommended for normal individuals, all complement-deficient persons should also be immunized with the conjugate pneumococcal, Hib, and meningococcal vaccines. However, the meningococcal vaccine does not protect against group B disease. Despite some reports suggesting a higher risk of disease caused by uncommon meningococcal serogroups in complement-deficient persons, group B disease still accounts for a significant proportion of infections in this population. An attempt to prevent meningococcal disease in complement deficient persons in an area where group B meningococcal disease was endemic was made by Potter et al. (358). Forty South African patients with homozygous C6 deficiency were identified in an area where group B meningococcal meningitis was endemic. In a prospective study over 2 to 4 years, patients with recurrent infections who received monthly doses of benzathine penicillin were protected from subsequent neisserial infection compared with those who did not receive penicillin. It is worth noting that C6-deficient patients with one or no meningococcal infections who did not receive antibiotic prophylaxis had significantly fewer infections than patients with recurrent disease.
With the inclusion of the pneumococcal, Hib, and meningococcal vaccines as part of routine childhood immunizations, the role for antibiotic prophylaxis in splenectomized individuals and in children with sickle cell disease needs to be reconsidered. Further, the global spread of penicillin-resistant S. pneumoniae and the potential for selection of resistant organisms have diminished the enthusiasm for antibiotic prophylaxis. Educating individuals with complement deficiencies or splenectomies about their increased risk for serious, life-threatening infections and the importance of seeking prompt medical attention for febrile illnesses is essential (360). A study in the Netherlands revealed that 43% of splenectomized patients had not received up-to-date information about the infection risks associated with their condition and that 50% were not aware of the need to contact a physician immediately in case of high fever (291).
MBL Replacement Therapy
MBL replacement therapy has been attempted in a child with recurrent infections (139) and in a 20-year-old woman with cystic fibrosis (152). It is not clear from the case reports whether the replacement therapy had any benefit. Phase I and II studies have established the safety of MBL replacement therapy using MBL purified from human plasma (Statens Serum Institut, Copenhagen, Denmark) (139, 460). This product did not elicit an antibody response to MBL. A dose of at least 6 mg twice a week results in plasma MBL concentrations of above 1 μg/ml, which is considered a reasonable target therapeutic level to treat infections (460, 461). There is a wide variation among individuals in the half-life of MBL (20), and therapeutic monitoring of drug levels may be required to optimize dosage and dosing intervals. However, the initiation of larger clinical trials with plasma-derived MBL did not receive support from the European Union on the grounds that therapeutic trials based on human plasma were ethically unacceptable. In addition, the potential risk of transmission of yet-undiscovered pathogens associated with plasma products and the possibility of production capacity being limited by the availability of human plasma led to the production of recombinant human MBL (rMBL) in human embryonic kidney (HEK) cell lines (209). Infusion of rMBL restored MBL function, was not associated with severe adverse effects, and had pharmacokinetics similar to those previously reported with plasma-derived MBL (343). Phase II trials with several patient groups are being considered, including patients with leukemia undergoing chemotherapy.
Replacement Therapy with Human Immunoglobulins
Primary antibody deficiencies were treated with intramuscular IgG (IMIG) preparations in the 1950s. Controlled trials comparing IMIG preparations with either no treatment or placebo were considered unethical because preliminary studies strongly suggested a beneficial effect of IgG replacement. The first controlled trials that compared IMIG with intravenous immunoglobulin (IVIG) were conducted in the 1970s and showed that IVIG was at least as effective (7) or superior (79, 325). IVIG therapy is the cornerstone of treatment for all IgG-deficient persons with recurrent infections. There are, however, no data that support the use of IVIG in preventing infections in splenectomized or complement-deficient persons.
Recommendations
Conjugate vaccines against S. pneumoniae, Hib, and N. meningitidis are now part of routine immunizations in children and adolescents in most industrialized nations. In the United States the Hib and pneumococcal conjugate vaccines are administered at 2, 4, and 6 months, with a booster dose at 12 to 15 months of age. The meningococcal quadrivalent conjugate vaccine is recommended at 11 to 12 years of age; teenagers between the ages of 13 and 18 and college freshmen who have not previously received the vaccine should also be vaccinated (29). While there are no large studies that examine the efficacy of these vaccines in splenectomized or complement-deficient persons, the Advisory Committee on Immunization Practices (ACIP) recommends the use of these vaccines; persons 18 years of age or younger should receive the conjugate pneumococcal vaccine (PCV13), and adults should receive the polysaccharide vaccine (62). A single revaccination may be given after 5 years. Children above the age of 2 years and with asplenia or C3 or terminal complement deficiencies should receive the meningococcal conjugate vaccine (64). Individuals with active autoimmune disorders that result in complement consumption and functional C3 deficiency should also be considered candidates for the meningococcal and pneumococcal vaccines. The ACIP recommends that immunizations be administered at least 2 weeks prior to elective splenectomy. If vaccination cannot be carried out prior to splenectomy, it should be done as soon as the patient has clinically stabilized after the surgery. Persons who have received the meningococcal polysaccharide vaccine previously may be revaccinated with the conjugate vaccine after 2 to 3 years. The ACIP recommends revaccination of high-risk individuals (those with persistent complement deficiencies, asplenia, or persistent and prolonged exposure [microbiology technicians or travelers to or residents of regions where the illness is hyperendemic or epidemic]) with the conjugate meningococcal vaccine (Menactra) after 5 years if they received their first vaccine dose at age ≥7 years or after 3 years if the first vaccine dose was administered between ages 2 and 6 years (65). Repeated doses of group A and C polysaccharides may induce immunologic hyporesponsiveness and should be avoided (40, 166, 264, 266). Hyporesponsiveness to group C polysaccharide can be overcome with use of the group C conjugate vaccine (163, 367).
Long-term oral penicillin prophylaxis was recommended for infants with sickle cell disease and functional asplenia based on a study that demonstrated an 84% reduction in the incidence of pneumococcal sepsis over a 15-month follow-up period (156). A study by Falletta et al. concluded that penicillin prophylaxis could safely be discontinued at 5 years of age in children who had not experienced severe pneumococcal infection or a splenectomy and were receiving comprehensive medical care (118). Whether prophylaxis would be beneficial for immunized children is not known. The emergence of antibiotic resistance is always a concern. Opinions regarding the use of antibiotics in splenectomized persons are widely divergent. A reasonable approach may to reserve antibiotics only for those splenectomized individuals who experience pneumococcal infections despite appropriate immunization.
CONCLUSION
Over the past 2 decades considerable insights have been gained into of the role of complement in the pathophysiology of various infectious and noninfectious disease states. Animal models have defined the role of complement in disease pathogenesis in vivo. Crystal and solution structures of various complement molecules have elucidated structure-function relationships and could lead to development of novel therapeutic agents that modulate complement function in diseases where complement plays a role in pathogenesis. Better vaccines have almost eradicated diseases such as Hib disease, and the future holds promise with the prospect of vaccines against diseases such as group B meningococcal infections. Education of patients and providers about the risks of infections associated with complement deficiency and splenectomy, the ability to reduce the incidence of several of these infections with appropriate immunizations, and the importance of seeking prompt medical attention when indicated could greatly reduce the morbidity and improve the quality of life of this population.
Acknowledgments
We are indebted to J. E. Figueroa and P. Densen for their previous outstanding contribution on the same subject in this journal. We are grateful to Caroline Trotter (University of Bristol, United Kingdom) for helpful discussions.
This work was supported by National Institutes of Health grants AI32725 and AI084048 (P.A.R) and AI054544 (S.R.).
We regret that because of the length of this article not all original papers could be cited, and any omissions are unintentional.
Biography
 Sanjay Ram earned his medical degree at the Seth G.S. Medical College and King Edward VII Memorial Hospital in Mumbai, India. He did a residency in internal medicine at the State University of New York at Buffalo, followed by an infectious diseases fellowship at the Boston University School of Medicine between 1994 and 1998. During his fellowship, Dr. Ram studied complement interactions with Neisseria gonorrhoeae at the Maxwell Finland Laboratory for Infectious Diseases under the mentorship of Dr. Peter Rice. He served as a faculty member at Boston University until 2006 and is now an Associate Professor at the University of Massachusetts at Worcester. Dr. Ram continues to study how the pathogenic neisseriae (N. gonorrhoeae and N. meningitidis) interact with and evade the complement system, in addition to serving as an infectious diseases consultant at the University of Massachusetts.
Sanjay Ram earned his medical degree at the Seth G.S. Medical College and King Edward VII Memorial Hospital in Mumbai, India. He did a residency in internal medicine at the State University of New York at Buffalo, followed by an infectious diseases fellowship at the Boston University School of Medicine between 1994 and 1998. During his fellowship, Dr. Ram studied complement interactions with Neisseria gonorrhoeae at the Maxwell Finland Laboratory for Infectious Diseases under the mentorship of Dr. Peter Rice. He served as a faculty member at Boston University until 2006 and is now an Associate Professor at the University of Massachusetts at Worcester. Dr. Ram continues to study how the pathogenic neisseriae (N. gonorrhoeae and N. meningitidis) interact with and evade the complement system, in addition to serving as an infectious diseases consultant at the University of Massachusetts.
 Lisa Lewis is currently an Assistant Professor in the Department of Medicine, Division of Infectious Diseases and Immunology, at the University of Massachusetts Medical School in Worcester, MA. She received her Ph.D. in microbiology and immunology from the State University of New York at Buffalo in 1996 and conducted her postdoctoral training at the University of Oklahoma Health Sciences Center (OUHSC) in Oklahoma City. She was later appointed Assistant Professor of Research and Director of the Laboratory for DNA Sequencing and Microbial Genomics at OUHSC. For the past 20 years her research has focused on the pathogenesis of Neisseria meningitidis and Neisseria gonorrhoeae. Currently, her research is focused on the interaction of Neisseria species with complement and on mechanisms that pathogenic neisseriae use to evade complement-mediated killing.
Lisa Lewis is currently an Assistant Professor in the Department of Medicine, Division of Infectious Diseases and Immunology, at the University of Massachusetts Medical School in Worcester, MA. She received her Ph.D. in microbiology and immunology from the State University of New York at Buffalo in 1996 and conducted her postdoctoral training at the University of Oklahoma Health Sciences Center (OUHSC) in Oklahoma City. She was later appointed Assistant Professor of Research and Director of the Laboratory for DNA Sequencing and Microbial Genomics at OUHSC. For the past 20 years her research has focused on the pathogenesis of Neisseria meningitidis and Neisseria gonorrhoeae. Currently, her research is focused on the interaction of Neisseria species with complement and on mechanisms that pathogenic neisseriae use to evade complement-mediated killing.
 Peter A. Rice earned his medical degree (M.D.) at the University of Pennsylvania School of Medicine. After clinical and research training in internal medicine and infectious diseases, Dr. Rice joined the medical faculty at Boston University School of Medicine, attaining the rank of Professor and Chief of the Section of Infectious Diseases. Recently he joined the Division of Infectious Diseases and Immunology at the University of Massachusetts Medical School. Dr. Rice has a longstanding interest in and is internationally recognized for his work on the immunologic interactions of Neisseria gonorrhoeae and the complement system. He has made many contributions to the understanding at the molecular level of how Neisseria species disarm human complement and thereby escape immune surveillance to go on and produce active infection, work that is particularly relevant in the design of effective antibody-based vaccines. In addition, Dr. Rice has developed rapid diagnostic testing for gonorrhea and is testing immunopharmacologic approaches for treating multi-antimicrobial-resistant N. gonorrhoeae infection.
Peter A. Rice earned his medical degree (M.D.) at the University of Pennsylvania School of Medicine. After clinical and research training in internal medicine and infectious diseases, Dr. Rice joined the medical faculty at Boston University School of Medicine, attaining the rank of Professor and Chief of the Section of Infectious Diseases. Recently he joined the Division of Infectious Diseases and Immunology at the University of Massachusetts Medical School. Dr. Rice has a longstanding interest in and is internationally recognized for his work on the immunologic interactions of Neisseria gonorrhoeae and the complement system. He has made many contributions to the understanding at the molecular level of how Neisseria species disarm human complement and thereby escape immune surveillance to go on and produce active infection, work that is particularly relevant in the design of effective antibody-based vaccines. In addition, Dr. Rice has developed rapid diagnostic testing for gonorrhea and is testing immunopharmacologic approaches for treating multi-antimicrobial-resistant N. gonorrhoeae infection.
REFERENCES
- 1.Agarwal, S., V. P. Ferreira, C. Cortes, M. K. Pangburn, P. A. Rice, and S. Ram. 2010. An evaluation of the role of properdin in alternative pathway activation on Neisseria meningitidis and Neisseria gonorrhoeae. J. Immunol. 185:507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alagarasu, K., P. Selvaraj, S. Swaminathan, S. Raghavan, G. Narendran, and P. R. Narayanan. 2007. Mannose binding lectin gene variants and susceptibility to tuberculosis in HIV-1 infected patients of South India. Tuberculosis (Edinburgh) 87:535-543. [DOI] [PubMed] [Google Scholar]
- 3.Alberti, S., D. Alvarez, S. Merino, M. T. Casado, F. Vivanco, J. M. Tomas, and V. J. Benedi. 1996. Analysis of complement C3 deposition and degradation on Klebsiella pneumoniae. Infect. Immun. 64:4726-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alper, C. A., J. Xu, K. Cosmopoulos, B. Dolinski, R. Stein, G. Uko, C. E. Larsen, D. P. Dubey, P. Densen, L. Truedsson, G. Sturfelt, and A. G. Sjoholm. 2003. Immunoglobulin deficiencies and susceptibility to infection among homozygotes and heterozygotes for C2 deficiency. J. Clin. Immunol. 23:297-305. [DOI] [PubMed] [Google Scholar]
- 5.Altamura, M., L. Caradonna, L. Amati, N. M. Pellegrino, G. Urgesi, and S. Miniello. 2001. Splenectomy and sepsis: the role of the spleen in the immune-mediated bacterial clearance. Immunopharmacol. Immunotoxicol. 23:153-161. [DOI] [PubMed] [Google Scholar]
- 6.Ambrosino, D. M., M. Y. Lee, D. Chen, and R. C. Shamberger. 1992. Response to Haemophilus influenzae type B conjugate vaccine in children undergoing splenectomy. J. Pediatr. Surg. 27:1045-1048. [DOI] [PubMed] [Google Scholar]
- 7.Ammann, A. J., R. F. Ashman, R. H. Buckley, W. R. Hardie, H. J. Krantmann, J. Nelson, H. Ochs, E. R. Stiehm, T. Tiller, D. W. Wara, and R. Wedgwood. 1982. Use of intravenous gamma-globulin in antibody immunodeficiency: results of a multicenter controlled trial. Clin. Immunol. Immunopathol. 22:60-67. [DOI] [PubMed] [Google Scholar]
- 8.Amoroso, A., M. Berrino, M. Boniotto, S. Crovella, E. Palomba, G. Scarlatti, C. Serra, P. A. Tovo, and S. Vatta. 1999. Polymorphism at codon 54 of mannose-binding protein gene influences AIDS progression but not HIV infection in exposed children. AIDS 13:863-864. [DOI] [PubMed] [Google Scholar]
- 9.Anderson, D. C., F. C. Schmalsteig, M. J. Finegold, B. J. Hughes, R. Rothlein, L. J. Miller, S. Kohl, M. F. Tosi, R. L. Jacobs, T. C. Waldrop, et al. 1985. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J. Infect. Dis. 152:668-689. [DOI] [PubMed] [Google Scholar]
- 10.Anderson, D. C., and T. A. Springer. 1987. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu. Rev. Med. 38:175-194. [DOI] [PubMed] [Google Scholar]
- 11.Andreoni, J., H. Kayhty, and P. Densen. 1993. Vaccination and the role of capsular polysaccharide antibody in prevention of recurrent meningococcal disease in late complement component-deficient individuals. J. Infect. Dis. 168:227-231. [DOI] [PubMed] [Google Scholar]
- 12.Apicella, M. A., M. A. J. Westerink, S. A. Morse, H. Schneider, P. A. Rice, and J. M. Griffiss. 1986. Bactericidal antibody response of normal human serum to the lipooligosaccharide of Neisseria gonorrhoeae. J. Infect. Dis. 153:520-526. [DOI] [PubMed] [Google Scholar]
- 13.Arlaud, G. J., C. Gaboriaud, N. M. Thielens, and V. Rossi. 2002. Structural biology of C1. Biochem. Soc Trans. 30:1001-1006. [DOI] [PubMed] [Google Scholar]
- 14.Atkinson, J. P., and P. M. Schneider. 2004. Genetic susceptibility and class III complement genes, p. 153-201. In R. G. Lahita (ed.), Systemic lupus erythematosus, 4th ed. Elsevier-Academic Press, San Diego, CA.
- 15.Attwood, J. T., Y. Williams, and C. Feighery. 2001. Impaired IgG responses in a child with homozygous C2 deficiency and recurrent pneumococcal septicaemia. Acta Paediatr. 90:99-101. [DOI] [PubMed] [Google Scholar]
- 16.Awdeh, Z. L., and C. A. Alper. 1980. Inherited structural polymorphism of the fourth component of human complement. Proc. Natl. Acad. Sci. U. S. A. 77:3576-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babula, O., G. Lazdane, J. Kroica, W. J. Ledger, and S. S. Witkin. 2003. Relation between recurrent vulvovaginal candidiasis, vaginal concentrations of mannose-binding lectin, and a mannose-binding lectin gene polymorphism in Latvian women. Clin. Infect. Dis. 37:733-737. [DOI] [PubMed] [Google Scholar]
- 18.Babula, O., G. Lazdane, J. Kroica, I. M. Linhares, W. J. Ledger, and S. S. Witkin. 2005. Frequency of interleukin-4 (IL-4) −589 gene polymorphism and vaginal concentrations of IL-4, nitric oxide, and mannose-binding lectin in women with recurrent vulvovaginal candidiasis. Clin. Infect. Dis. 40:1258-1262. [DOI] [PubMed] [Google Scholar]
- 19.Bach, O., M. Baier, A. Pullwitt, N. Fosiko, G. Chagaluka, M. Kalima, W. Pfister, E. Straube, and M. Molyneux. 2005. Falciparum malaria after splenectomy: a prospective controlled study of 33 previously splenectomized Malawian adults. Trans. R. Soc. Trop. Med. Hyg. 99:861-867. [DOI] [PubMed] [Google Scholar]
- 20.Bang, P., I. Laursen, K. Thornberg, J. Schierbeck, B. Nielsen, H. Valdimarsson, C. Koch, and M. Christiansen. 2008. The pharmacokinetic profile of plasma-derived mannan-binding lectin in healthy adult volunteers and patients with Staphylococcus aureus septicaemia. Scand. J. Infect. Dis. 40:44-48. [DOI] [PubMed] [Google Scholar]
- 21.Barrington, R. A., O. Pozdnyakova, M. R. Zafari, C. D. Benjamin, and M. C. Carroll. 2002. B lymphocyte memory: role of stromal cell complement and FcgammaRIIB receptors. J. Exp. Med. 196:1189-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bathum, L., H. Hansen, B. Teisner, C. Koch, P. Garred, K. Rasmussen, and P. Wang. 2006. Association between combined properdin and mannose-binding lectin deficiency and infection with Neisseria meningitidis. Mol. Immunol. 43:473-479. [DOI] [PubMed] [Google Scholar]
- 23.Bax, W. A., O. J. Cluysenaer, A. K. Bartelink, P. C. Aerts, R. A. Ezekowitz, and H. van Dijk. 1999. Association of familial deficiency of mannose-binding lectin and meningococcal disease. Lancet 354:1094-1095. [DOI] [PubMed] [Google Scholar]
- 24.Beatty, D. W., C. R. Ryder, and H. D. Heese. 1986. Complement abnormalities during an epidemic of group B meningococcal infection in children. Clin. Exp. Immunol. 64:465-470. [PMC free article] [PubMed] [Google Scholar]
- 25.Bellamy, R., C. Ruwende, K. P. McAdam, M. Thursz, M. Sumiya, J. Summerfield, S. C. Gilbert, T. Corrah, D. Kwiatkowski, H. C. Whittle, and A. V. Hill. 1998. Mannose binding protein deficiency is not associated with malaria, hepatitis B carriage nor tuberculosis in Africans. QJM 91:13-18. [DOI] [PubMed] [Google Scholar]
- 26.Reference deleted.
- 27.Bidegain, F., A. Berry, M. Alvarez, O. Verhille, F. Huguet, P. Brousset, J. Pris, B. Marchou, and J. F. Magnaval. 2005. Acute Plasmodium falciparum malaria following splenectomy for suspected lymphoma in 2 patients. Clin. Infect. Dis. 40:e97-100. [DOI] [PubMed] [Google Scholar]
- 28.Biesma, D. H., A. J. Hannema, H. van Velzen-Blad, L. Mulder, R. van Zwieten, I. Kluijt, and D. Roos. 2001. A family with complement factor D deficiency. J. Clin. Invest. 108:233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilukha, O. O., and N. Rosenstein. 2005. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 54:1-21. [PubMed] [Google Scholar]
- 30.Bird, P., and P. J. Lachmann. 1988. The regulation of IgG subclass production in man: low serum IgG4 in inherited deficiencies of the classical pathway of C3 activation. Eur. J. Immunol. 18:1217-1222. [DOI] [PubMed] [Google Scholar]
- 31.Birmingham, D. J., K. F. Gavit, S. M. McCarty, C. Y. Yu, B. H. Rovin, H. N. Nagaraja, and L. A. Hebert. 2006. Consumption of erythrocyte CR1 (CD35) is associated with protection against systemic lupus erythematosus renal flare. Clin. Exp. Immunol. 143:274-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bisharat, N., H. Omari, I. Lavi, and R. Raz. 2001. Risk of infection and death among post-splenectomy patients. J. Infect. 43:182-186. [DOI] [PubMed] [Google Scholar]
- 33.Bishof, N. A., T. R. Welch, and L. S. Beischel. 1990. C4B deficiency: a risk factor for bacteremia with encapsulated organisms. J. Infect. Dis. 162:248-250. [DOI] [PubMed] [Google Scholar]
- 34.Blanchong, C. A., E. K. Chung, K. L. Rupert, Y. Yang, Z. Yang, B. Zhou, J. M. Moulds, and C. Y. Yu. 2001. Genetic, structural and functional diversities of human complement components C4A and C4B and their mouse homologues, Slp and C4. Int. Immunopharmacol. 1:365-392. [DOI] [PubMed] [Google Scholar]
- 35.Bobo, R. A., and E. J. Newton. 1976. A previously undescribed gram-negative bacillus causing septicemia and meningitis. Am. J. Clin. Pathol. 65:564-569. [DOI] [PubMed] [Google Scholar]
- 36.Boldt, A. B., A. Luty, M. P. Grobusch, K. Dietz, A. Dzeing, M. Kombila, P. G. Kremsner, and J. F. Kun. 2006. Association of a new mannose-binding lectin variant with severe malaria in Gabonese children. Genes Immun. 7:393-400. [DOI] [PubMed] [Google Scholar]
- 37.Boniotto, M., S. Crovella, D. Pirulli, G. Scarlatti, A. Spano, L. Vatta, S. Zezlina, P. A. Tovo, E. Palomba, and A. Amoroso. 2000. Polymorphisms in the MBL2 promoter correlated with risk of HIV-1 vertical transmission and AIDS progression. Genes Immun. 1:346-348. [DOI] [PubMed] [Google Scholar]
- 38.Bonnin, A. J., H. J. Zeitz, and A. Gewurz. 1993. Complement factor I deficiency with recurrent aseptic meningitis. Arch. Intern. Med. 153:1380-1383. [PubMed] [Google Scholar]
- 39.Bork, K., S. E. Barnstedt, P. Koch, and H. Traupe. 2000. Hereditary angioedema with normal C1-inhibitor activity in women. Lancet 356:213-217. [DOI] [PubMed] [Google Scholar]
- 40.Borrow, R., H. Joseph, N. Andrews, M. Acuna, E. Longworth, S. Martin, N. Peake, R. Rahim, P. Richmond, E. Kaczmarski, and E. Miller. 2000. Reduced antibody response to revaccination with meningococcal serogroup A polysaccharide vaccine in adults. Vaccine 19:1129-1132. [DOI] [PubMed] [Google Scholar]
- 41.Borzy, M. S., and D. Houghton. 1985. Mixed-pattern immune deposit glomerulonephritis in a child with inherited deficiency of the third component of complement. Am. J. Kidney Dis. 5:54-59. [DOI] [PubMed] [Google Scholar]
- 42.Bossi, F., L. Rizzi, R. Bulla, A. Debeus, C. Tripodo, P. Picotti, E. Betto, P. Macor, C. Pucillo, R. Wurzner, and F. Tedesco. 2009. C7 is expressed on endothelial cells as a trap for the assembling terminal complement complex and may exert anti-inflammatory function. Blood 113:3640-3648. [DOI] [PubMed] [Google Scholar]
- 43.Botto, M., K. Y. Fong, A. K. So, A. Rudge, and M. J. Walport. 1990. Molecular basis of hereditary C3 deficiency. J. Clin. Invest. 86:1158-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandtzaeg, P., T. E. Mollnes, and P. Kierulf. 1989. Complement activation and endotoxin levels in systemic meningococcal disease. J. Infect. Dis. 160:58-65. [DOI] [PubMed] [Google Scholar]
- 45.Brasseur, P., and A. Gorenflot. 1992. Human babesiosis in Europe. Mem. Inst. Oswaldo Cruz 87(Suppl. 3):131-132. [DOI] [PubMed] [Google Scholar]
- 46.Bredius, R. G., B. H. Derkx, C. A. Fijen, T. P. de Wit, M. de Haas, R. S. Weening, J. G. van de Winkel, and T. A. Out. 1994. Fc gamma receptor IIa (CD32) polymorphism in fulminant meningococcal septic shock in children. J. Infect. Dis. 170:848-853. [DOI] [PubMed] [Google Scholar]
- 47.Bredius, R. G., C. A. Fijen, M. De Haas, E. J. Kuijper, R. S. Weening, J. G. Van de Winkel, and T. A. Out. 1994. Role of neutrophil Fc gamma RIIa (CD32) and Fc gamma RIIIb (CD16) polymorphic forms in phagocytosis of human IgG1- and IgG3-opsonized bacteria and erythrocytes. Immunology 83:624-630. [PMC free article] [PubMed] [Google Scholar]
- 48.Brenner, D. J., D. G. Hollis, G. R. Fanning, and R. E. Weaver. 1989. Capnocytophaga canimorsus sp. nov. (formerly CDC group DF-2), a cause of septicemia following dog bite, and C. cynodegmi sp. nov., a cause of localized wound infection following dog bite. J. Clin. Microbiol. 27:231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown, K. S., S. D. Ryder, W. L. Irving, R. B. Sim, and T. P. Hickling. 2007. Mannan binding lectin and viral hepatitis. Immunol. Lett. 108:34-44. [DOI] [PubMed] [Google Scholar]
- 50.Bussone, G., and L. Mouthon. 2009. Autoimmune manifestations in primary immune deficiencies. Autoimmun. Rev. 8:332-336. [DOI] [PubMed] [Google Scholar]
- 51.Candy, D. C., V. F. Larcher, J. H. Tripp, J. T. Harries, B. A. Harvey, and J. F. Soothill. 1980. Yeast opsonisation in children with chronic diarrhoeal states. Arch. Dis. Child. 55:189-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Capparelli, R., M. Iannaccone, D. Palumbo, C. Medaglia, E. Moscariello, A. Russo, and D. Iannelli. 2009. Role played by human mannose-binding lectin polymorphisms in pulmonary tuberculosis. J. Infect. Dis. 199:666-672. [DOI] [PubMed] [Google Scholar]
- 53.Carlsson, M., A. G. Sjoholm, L. Eriksson, S. Thiel, J. C. Jensenius, M. Segelmark, and L. Truedsson. 2005. Deficiency of the mannan-binding lectin pathway of complement and poor outcome in cystic fibrosis: bacterial colonization may be decisive for a relationship. Clin. Exp. Immunol. 139:306-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carneiro-Sampaio, M., B. L. Liphaus, A. A. Jesus, C. A. Silva, J. B. Oliveira, and M. H. Kiss. 2008. Understanding systemic lupus erythematosus physiopathology in the light of primary immunodeficiencies. J. Clin. Immunol. 28(Suppl. 1):S34-S41. [DOI] [PubMed] [Google Scholar]
- 55.Carroll, M. C., R. D. Campbell, D. R. Bentley, and R. R. Porter. 1984. A molecular map of the human major histocompatibility complex class III region linking complement genes C4, C2 and factor B. Nature 307:237-241. [DOI] [PubMed] [Google Scholar]
- 56.Carroll, M. C., D. M. Fathallah, L. Bergamaschini, E. M. Alicot, and D. E. Isenman. 1990. Substitution of a single amino acid (aspartic acid for histidine) converts the functional activity of human complement C4B to C4A. Proc. Natl. Acad. Sci. U. S. A. 87:6868-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carroll, M. C., A. Palsdottir, K. T. Belt, and R. R. Porter. 1985. Deletion of complement C4 and steroid 21-hydroxylase genes in the HLA class III region. EMBO J. 4:2547-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cartwright, K. A., J. M. Stuart, D. M. Jones, and N. D. Noah. 1987. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol. Infect. 99:591-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Catano, G., B. K. Agan, H. Kulkarni, V. Telles, V. C. Marconi, M. J. Dolan, and S. K. Ahuja. 2008. Independent effects of genetic variations in mannose-binding lectin influence the course of HIV disease: the advantage of heterozygosity for coding mutations. J. Infect. Dis. 198:72-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cates, K. L., P. Densen, J. C. Lockman, and R. P. Levine. 1992. C4B deficiency is not associated with meningitis or bacteremia with encapsulated bacteria. J. Infect. Dis. 165:942-944. [DOI] [PubMed] [Google Scholar]
- 61.Caugant, D. A., E. A. Hoiby, P. Magnus, O. Scheel, T. Hoel, G. Bjune, E. Wedege, J. Eng., and L. O. Froholm. 1994. Asymptomatic carriage of Neisseria meningitidis in a randomly sampled population. J. Clin. Microbiol. 32:323-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.CDC. 2010. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children. Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb. Mortal. Wkly. Rep. 59:258-261. [PubMed] [Google Scholar]
- 63.CDC. 2010. Licensure of a meningococcal conjugate vaccine (Menveo) and guidance for use. Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb. Mortal. Wkly. Rep. 59:273. [PubMed] [Google Scholar]
- 64.CDC. 2008. Report from the Advisory Committee on Immunization Practices (ACIP): decision not to recommend routine vaccination of all children aged 2-10 years with quadrivalent meningococcal conjugate vaccine (MCV4). MMWR Morb. Mortal. Wkly. Rep. 57:462-465. [PubMed] [Google Scholar]
- 65.CDC. 2009. Updated recommendation from the Advisory Committee on Immunization Practices (ACIP) for revaccination of persons at prolonged increased risk for meningococcal disease. MMWR Morb. Mortal. Wkly. Rep. 58:1042-1043. [PubMed] [Google Scholar]
- 66.Centeno-Lima, S., V. do Rosario, R. Parreira, A. J. Maia, A. M. Freudenthal, A. M. Nijhof, and F. Jongejan. 2003. A fatal case of human babesiosis in Portugal: molecular and phylogenetic analysis. Trop. Med. Int. Health 8:760-764. [DOI] [PubMed] [Google Scholar]
- 67.Cherukuri, A., P. C. Cheng, H. W. Sohn, and S. K. Pierce. 2001. The CD19/CD21 complex functions to prolong B cell antigen receptor signaling from lipid rafts. Immunity 14:169-179. [DOI] [PubMed] [Google Scholar]
- 68.Christiansen, F. T., R. L. Dawkins, G. Uko, J. McCluskey, P. H. Kay, and P. J. Zilko. 1983. Complement allotyping in SLE: association with C4A null. Aust. N. Z. J. Med. 13:483-488. [DOI] [PubMed] [Google Scholar]
- 69.Christiansen, O. B., D. C. Kilpatrick, V. Souter, K. Varming, S. Thiel, and J. C. Jensenius. 1999. Mannan-binding lectin deficiency is associated with unexplained recurrent miscarriage. Scand. J. Immunol. 49:193-196. [DOI] [PubMed] [Google Scholar]
- 70.Cimaz, R., C. Mensi, E. D'Angelo, E. Fantola, V. Milone, L. R. Biasio, V. Carnelli, and A. R. Zanetti. 2001. Safety and immunogenicity of a conjugate vaccine against Haemophilus influenzae type b in splenectomized and nonsplenectomized patients with Cooley anemia. J. Infect. Dis. 183:1819-1821. [DOI] [PubMed] [Google Scholar]
- 71.Claus, H., M. C. Maiden, D. J. Wilson, N. D. McCarthy, K. A. Jolley, R. Urwin, F. Hessler, M. Frosch, and U. Vogel. 2005. Genetic analysis of meningococci carried by children and young adults. J. Infect. Dis. 191:1263-1271. [DOI] [PubMed] [Google Scholar]
- 72.Cooper, N. R., and G. R. Nemerow. 1984. The role of antibody and complement in the control of viral infections. J. Invest. Dermatol. 83:121s-127s. [DOI] [PubMed] [Google Scholar]
- 73.Cosar, H., F. Ozkinay, H. Onay, N. Bayram, A. R. Bakiler, M. Anil, D. Can, and C. Ozkinay. 2008. Low levels of mannose-binding lectin confers protection against tuberculosis in Turkish children. Eur. J. Clin. Microbiol. Infect. Dis. 27:1165-1169. [DOI] [PubMed] [Google Scholar]
- 74.Craven, D. E., C. E. Frasch, L. F. Mocca, F. B. Rose, and R. Gonzalez. 1979. Rapid serogroup identification of Neisseria meningitidis by using antiserum agar: prevalence of serotypes in a disease-free military population. J. Clin. Microbiol. 10:302-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Crosdale, D. J., K. V. Poulton, W. E. Ollier, W. Thomson, and D. W. Denning. 2001. Mannose-binding lectin gene polymorphisms as a susceptibility factor for chronic necrotizing pulmonary aspergillosis. J. Infect. Dis. 184:653-656. [DOI] [PubMed] [Google Scholar]
- 76.Csipo, I., E. Kiss, P. Soltesz, P. Antal-Szalmas, G. Szegedi, J. H. Cohen, R. P. Taylor, and M. Kavai. 1999. Effect of plasmapheresis on ligand binding capacity and expression of erythrocyte complement receptor type 1 (CR1) of patients with systemic lupus erythematosus (SLE). Clin. Exp. Immunol. 118:458-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cullingford, G. L., D. N. Watkins, A. D. Watts, and D. F. Mallon. 1991. Severe late postsplenectomy infection. Br. J. Surg. 78:716-721. [DOI] [PubMed] [Google Scholar]
- 78.Cunliffe, N. A., N. Snowden, E. M. Dunbar, and M. R. Haeney. 1995. Recurrent meningococcal septicaemia and properdin deficiency. J. Infect. 31:67-68. [DOI] [PubMed] [Google Scholar]
- 79.Cunningham-Rundles, C., F. P. Siegal, E. M. Smithwick, A. Lion-Boule, S. Cunningham-Rundles, J. O'Malley, S. Barandun, and R. A. Good. 1984. Efficacy of intravenous immunoglobulin in primary humoral immunodeficiency disease. Ann. Intern. Med. 101:435-439. [DOI] [PubMed] [Google Scholar]
- 80.D'Amelio, R., A. Agostoni, R. Biselli, M. Brai, G. Caruso, M. Cicardi, A. Corvetta, L. Fontana, G. Misiano, R. Perricone, et al. 1992. Complement deficiency and antibody profile in survivors of meningococcal meningitis due to common serogroups in Italy. Scand. J. Immunol. 35:589-595. [DOI] [PubMed] [Google Scholar]
- 81.Darton, T., M. Guiver, S. Naylor, D. L. Jack, E. B. Kaczmarski, R. Borrow, and R. C. Read. 2009. Severity of meningococcal disease associated with genomic bacterial load. Clin. Infect. Dis. 48:587-594. [DOI] [PubMed] [Google Scholar]
- 82.Daskas, N., K. Farmer, R. Coward, and M. Erlewyn-Lajeunesse. 2007. Meningococcal disease associated with an acute post-streptococcal complement deficiency. Pediatr. Nephrol. 22:747-749. [DOI] [PubMed] [Google Scholar]
- 83.Davies, J., O. Neth, E. Alton, N. Klein, and M. Turner. 2000. Differential binding of mannose-binding lectin to respiratory pathogens in cystic fibrosis. Lancet 355:1885-1886. [DOI] [PubMed] [Google Scholar]
- 84.Davis, M. D., and J. D. Brewer. 2004. Urticarial vasculitis and hypocomplementemic urticarial vasculitis syndrome. Immunol. Allergy Clin. North Am. 24:183-213. [DOI] [PubMed] [Google Scholar]
- 85.Decosas, J., and J. B. Koama. 2002. Chronicle of an outbreak foretold: meningococcal meningitis W135 in Burkina Faso. Lancet Infect. Dis. 2:763-765. [DOI] [PubMed] [Google Scholar]
- 86.de Kleijn, E. D., R. de Groot, J. Labadie, A. B. Lafeber, G. van den Dobbelsteen, L. van Alphen, H. van Dijken, B. Kuipers, G. W. van Omme, M. Wala, R. Juttmann, and H. C. Rumke. 2000. Immunogenicity and safety of a hexavalent meningococcal outer-membrane-vesicle vaccine in children of 2-3 and 7-8 years of age. Vaccine 18:1456-1466. [DOI] [PubMed] [Google Scholar]
- 87.Delgado-Cervino, E., G. Fontan, and M. Lopez-Trascasa. 2005. C5 complement deficiency in a Spanish family. Molecular characterization of the double mutation responsible for the defect. Mol. Immunol. 42:105-111. [DOI] [PubMed] [Google Scholar]
- 88.Del Rio, C., D. S. Stephens, J. S. Knapp, R. J. Rice, and W. O. Schalla. 1989. Comparison of isolates of Neisseria gonorrhoeae causing meningitis and report of gonococcal meningitis in a patient with C8 deficiency. J. Clin. Microbiol. 27:1045-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Del Rio-Tsonis, K., P. A. Tsonis, I. K. Zarkadis, A. G. Tsagas, and J. D. Lambris. 1998. Expression of the third component of complement, C3, in regenerating limb blastema cells of urodeles. J. Immunol. 161:6819-6824. [PubMed] [Google Scholar]
- 90.Demar, M., E. Legrand, D. Hommel, P. Esterre, and B. Carme. 2004. Plasmodium falciparum malaria in splenectomized patients: two case reports in French Guiana and a literature review. Am. J. Trop. Med. Hyg. 71:290-293. [PubMed] [Google Scholar]
- 91.de Messias, I. J., A. Reis, M. Brenden, F. Queiroz-Telles, and G. Mauff. 1991. Association of major histocompatibility complex class III complement components C2, BF, and C4 with Brazilian paracoccidioidomycosis. Complement Inflamm. 8:288-293. [DOI] [PubMed] [Google Scholar]
- 92.Dempsey, P. W., M. E. Allison, S. Akkaraju, C. C. Goodnow, and D. T. Fearon. 1996. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science 271:348-350. [DOI] [PubMed] [Google Scholar]
- 93.Densen, P., J. M. Weiler, J. M. Griffiss, and L. G. Hoffmann. 1987. Familial properdin deficiency and fatal meningococcemia. Correction of the bactericidal defect by vaccination. N. Engl. J. Med. 316:922-926. [DOI] [PubMed] [Google Scholar]
- 94.Derkx, H. H., E. J. Kuijper, C. A. Fijen, M. Jak, J. Dankert, and S. J. van Deventer. 1995. Inherited complement deficiency in children surviving fulminant meningococcal septic shock. Eur. J. Pediatr. 154:735-738. [DOI] [PubMed] [Google Scholar]
- 95.Djibo, S., P. Nicolas, J. M. Alonso, A. Djibo, D. Couret, J. Y. Riou, and J. P. Chippaux. 2003. Outbreaks of serogroup X meningococcal meningitis in Niger 1995-2000. Trop. Med. Int. Health 8:1118-1123. [DOI] [PubMed] [Google Scholar]
- 96.Dodds, A. W., X. D. Ren, A. C. Willis, and S. K. Law. 1996. The reaction mechanism of the internal thioester in the human complement component C4. Nature 379:177-179. [DOI] [PubMed] [Google Scholar]
- 97.Donders, G. G., O. Babula, G. Bellen, I. M. Linhares, and S. S. Witkin. 2008. Mannose-binding lectin gene polymorphism and resistance to therapy in women with recurrent vulvovaginal candidiasis. BJOG 115:1225-1231. [DOI] [PubMed] [Google Scholar]
- 98.Dorfman, R., A. Sandford, C. Taylor, B. Huang, D. Frangolias, Y. Wang, R. Sang, L. Pereira, L. Sun, Y. Berthiaume, L. C. Tsui, P. D. Pare, P. Durie, M. Corey, and J. Zielenski. 2008. Complex two-gene modulation of lung disease severity in children with cystic fibrosis. J. Clin. Invest. 118:1040-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Doyle, H. A., J. Yan, B. Liang, and M. J. Mamula. 2001. Lupus autoantigens: their origins, forms, and presentation. Immunol. Res. 24:131-147. [DOI] [PubMed] [Google Scholar]
- 100.Drickamer, K., and M. E. Taylor. 1993. Biology of animal lectins. Annu. Rev. Cell Biol. 9:237-264. [DOI] [PubMed] [Google Scholar]
- 101.Drogari-Apiranthitou, M., C. A. Fijen, D. Van De Beek, E. F. Hensen, J. Dankert, and E. J. Kuijper. 2000. Development of antibodies against tetravalent meningococcal polysaccharides in revaccinated complement-deficient patients. Clin. Exp. Immunol. 119:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Drumm, M. L., M. W. Konstan, M. D. Schluchter, A. Handler, R. Pace, F. Zou, M. Zariwala, D. Fargo, A. Xu, J. M. Dunn, R. J. Darrah, R. Dorfman, A. J. Sandford, M. Corey, J. Zielenski, P. Durie, K. Goddard, J. R. Yankaskas, F. A. Wright, and M. R. Knowles. 2005. Genetic modifiers of lung disease in cystic fibrosis. N. Engl. J. Med. 353:1443-1453. [DOI] [PubMed] [Google Scholar]
- 103.Dzwonek, A., V. Novelli, M. Bajaj-Elliott, M. Turner, M. Clapson, and N. Klein. 2006. Mannose-binding lectin in susceptibility and progression of HIV-1 infection in children. Antivir. Ther. 11:499-505. [PubMed] [Google Scholar]
- 104.Edwards, M. S., D. L. Kasper, H. J. Jennings, C. J. Baker, and A. Nicholson-Weller. 1982. Capsular sialic acid prevents activation of the alternative complement pathway by type III group B streptococci. J. Immunol. 128:1278-1283. [PubMed] [Google Scholar]
- 105.Edwards, M. S., A. Nicholson-Weller, C. J. Baker, and D. L. Kasper. 1980. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J. Exp. Med. 151:1275-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eisen, D. P., M. M. Dean, M. A. Boermeester, K. J. Fidler, A. C. Gordon, G. Kronborg, J. F. Kun, Y. L. Lau, A. Payeras, H. Valdimarsson, S. J. Brett, W. K. Ip, J. Mila, M. J. Peters, S. Saevarsdottir, J. W. van Till, C. J. Hinds, and E. S. McBryde. 2008. Low serum mannose-binding lectin level increases the risk of death due to pneumococcal infection. Clin. Infect. Dis. 47:510-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ejstrud, P., B. Kristensen, J. B. Hansen, K. M. Madsen, H. C. Schonheyder, and H. T. Sorensen. 2000. Risk and patterns of bacteraemia after splenectomy: a population-based study. Scand. J. Infect. Dis. 32:521-525. [DOI] [PubMed] [Google Scholar]
- 108.Ekdahl, K., L. Truedsson, A. G. Sjoholm, and J. H. Braconier. 1995. Complement analysis in adult patients with a history of bacteremic pneumococcal infections or recurrent pneumonia. Scand. J. Infect. Dis. 27:111-117. [DOI] [PubMed] [Google Scholar]
- 109.Ellison, R. T., III, J. G. Curd, P. F. Kohler, L. B. Reller, and F. N. Judson. 1987. Underlying complement deficiency in patients with disseminated gonococcal infection. Sex. Transm. Dis. 14:201-204. [DOI] [PubMed] [Google Scholar]
- 110.Ellison, R. T., III, S. R. Mason, P. F. Kohler, J. G. Curd, and L. B. Reller. 1986. Meningococcemia and acquired complement deficiency. Association in patients with hepatic failure. Arch. Intern. Med. 146:1539-1540. [PubMed] [Google Scholar]
- 111.Ellison, R. T., III., P. F. Kohler, J. G. Curd, F. N. Judson, and L. B. Reller. 1983. Prevalence of congenital or acquired complement deficiency in patients with sporadic meningocococcal disease. N. Engl. J. Med. 308:913-916. [DOI] [PubMed] [Google Scholar]
- 112.El Sahly, H. M., R. A. Reich, S. J. Dou, J. M. Musser, and E. A. Graviss. 2004. The effect of mannose binding lectin gene polymorphisms on susceptibility to tuberculosis in different ethnic groups. Scand. J. Infect. Dis. 36:106-108. [DOI] [PubMed] [Google Scholar]
- 113.Endeman, H., B. L. Herpers, B. A. de Jong, G. P. Voorn, J. C. Grutters, H. van Velzen-Blad, and D. H. Biesma. 2008. Mannose-binding lectin genotypes in susceptibility to community-acquired pneumonia. Chest 134:1135-1140. [DOI] [PubMed] [Google Scholar]
- 114.Eng., R. H. 1980. Bactericidal screening test for late complement component deficiencies or defects. J. Clin. Microbiol. 11:631-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Estabrook, M. M., J. M. Griffiss, and G. A. Jarvis. 1997. Sialylation of Neisseria meningitidis lipooligosaccharide inhibits serum bactericidal activity by masking lacto-N-neotetraose. Infect. Immun. 65:4436-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ezekowitz, R. A., M. Kuhlman, J. E. Groopman, and R. A. Byrn. 1989. A human serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. J. Exp. Med. 169:185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Faber, J., T. Schuessler, A. Finn, C. Murdoch, W. Zenz, P. Habermehl, C. U. Meyer, B. U. Zabel, H. Schmitt, F. Zepp, and M. Knuf. 2007. Age-dependent association of human mannose-binding lectin mutations with susceptibility to invasive meningococcal disease in childhood. Pediatr. Infect. Dis. J. 26:243-246. [DOI] [PubMed] [Google Scholar]
- 118.Falletta, J. M., G. M. Woods, J. I. Verter, G. R. Buchanan, C. H. Pegelow, R. V. Iyer, S. T. Miller, C. T. Holbrook, T. R. Kinney, E. Vichinsky, et al. 1995. Discontinuing penicillin prophylaxis in children with sickle cell anemia. Prophylactic Penicillin Study II. J. Pediatr. 127:685-690. [DOI] [PubMed] [Google Scholar]
- 119.Farhoudi, A. H. 1988. Recurrent pneumococcal meningitis associated with C3 deficiency. Presse Med. 17:696. [PubMed] [Google Scholar]
- 120.Fasano, M. B., P. Densen, R. H. McLean, and J. A. Winkelstein. 1990. Prevalence of homozygous C4B deficiency in patients with deficiencies of terminal complement components and meningococcemia. J. Infect. Dis. 162:1220-1221. [DOI] [PubMed] [Google Scholar]
- 121.Fearon, D. T. 1978. Regulation by membrane sialic acid of beta1H-dependent decay—dissociation of amplification C3 convertase of the alternative complement pathway. Proc. Natl. Acad. Sci. U. S. A. 75:1971-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fearon, D. T., and K. F. Austen. 1975. Properdin: binding to C3b and stabilization of the C3b-dependent C3 convertase. J. Exp. Med. 142:856-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feliciano, R., W. Swedler, and J. Varga. 1999. Infection with uncommon subgroup Y Neisseria meningitidis in patients with systemic lupus erythematosus. Clin. Exp. Rheumatol. 17:737-740. [PubMed] [Google Scholar]
- 124.Fernie, B. A., and M. J. Hobart. 1998. Complement C7 deficiency: seven further molecular defects and their associated marker haplotypes. Hum. Genet. 103:513-519. [DOI] [PubMed] [Google Scholar]
- 125.Fernie, B. A., A. Orren, G. Sheehan, M. Schlesinger, and M. J. Hobart. 1997. Molecular bases of C7 deficiency: three different defects. J. Immunol. 159:1019-1026. [PubMed] [Google Scholar]
- 126.Fernie, B. A., R. Wurzner, A. Orren, B. P. Morgan, P. C. Potter, A. E. Platonov, I. V. Vershinina, G. A. Shipulin, P. J. Lachmann, and M. J. Hobart. 1996. Molecular bases of combined subtotal deficiencies of C6 and C7: their effects in combination with other C6 and C7 deficiencies. J. Immunol. 157:3648-3657. [PubMed] [Google Scholar]
- 127.Ferreira, V. P., C. Cortes, and M. K. Pangburn. 2010. Native polymeric forms of properdin selectively bind to targets and promote activation of the alternative pathway of complement. Immunobiology [Epub ahead of print.] doi: 10.1016/j.imbio.2010.02.002. [DOI] [PMC free article] [PubMed]
- 128.Figueroa, J. E., and P. Densen. 1991. Infectious diseases associated with complement deficiencies. Clin. Microbiol. Rev. 4:359-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fijen, C. A., B. H. Derkx, E. J. Kuijper, M. Mannens, S. R. Poort, M. Peters, M. R. Daha, and J. Dankert. 1995. Fulminant meningococcal septic shock in a boy with combined inherited properdin and protein C deficiency. Clin. Exp. Immunol. 102:290-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fijen, C. A., E. J. Kuijper, J. Dankert, M. R. Daha, and D. A. Caugant. 1998. Characterization of Neisseria meningitidis strains causing disease in complement-deficient and complement-sufficient patients. J. Clin. Microbiol. 36:2342-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fijen, C. A., E. J. Kuijper, M. Drogari-Apiranthitou, Y. Van Leeuwen, M. R. Daha, and J. Dankert. 1998. Protection against meningococcal serogroup ACYW disease in complement-deficient individuals vaccinated with the tetravalent meningococcal capsular polysaccharide vaccine. Clin. Exp. Immunol. 114:362-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fijen, C. A., E. J. Kuijper, A. J. Hannema, A. G. Sjoholm, and J. P. van Putten. 1989. Complement deficiencies in patients over ten years old with meningococcal disease due to uncommon serogroups. Lancet ii:585-588. [DOI] [PubMed] [Google Scholar]
- 133.Fijen, C. A., E. J. Kuijper, M. Te Bulte, M. M. van de Heuvel, A. C. Holdrinet, R. B. Sim, M. R. Daha, and J. Dankert. 1996. Heterozygous and homozygous factor H deficiency states in a Dutch family. Clin. Exp. Immunol. 105:511-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fijen, C. A., E. J. Kuijper, M. T. te Bulte, M. R. Daha, and J. Dankert. 1999. Assessment of complement deficiency in patients with meningococcal disease in The Netherlands. Clin. Infect. Dis. 28:98-105. [DOI] [PubMed] [Google Scholar]
- 135.Fijen, C. A., E. J. Kuijper, H. G. Tjia, M. R. Daha, and J. Dankert. 1994. Complement deficiency predisposes for meningitis due to nongroupable meningococci and Neisseria-related bacteria. Clin. Infect. Dis. 18:780-784. [DOI] [PubMed] [Google Scholar]
- 136.Findlow, H., U. Vogel, J. E. Mueller, A. Curry, B. M. Njanpop-Lafourcade, H. Claus, S. J. Gray, S. Yaro, Y. Traore, L. Sangare, P. Nicolas, B. D. Gessner, and R. Borrow. 2007. Three cases of invasive meningococcal disease caused by a capsule null locus strain circulating among healthy carriers in Burkina Faso. J. Infect. Dis. 195:1071-1077. [DOI] [PubMed] [Google Scholar]
- 137.Finne, J., M. Leinonen, and P. H. Makela. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355-357. [DOI] [PubMed] [Google Scholar]
- 138.Fox, A. J., D. M. Jones, S. M. Scotland, B. Rowe, A. Smith, M. R. Brown, R. G. Fitzgeorge, A. Baskerville, N. J. Parsons, J. A. Cole, et al. 1989. Serum killing of meningococci and several other gram-negative bacterial species is not decreased by incubating them with cytidine 5′-monophospho-N-acetyl neuraminic acid. Microb. Pathog. 7:317-318. (Letter.) [DOI] [PubMed] [Google Scholar]
- 139.Frakking, F. N., N. Brouwer, M. D. van de Wetering, I. K. Budde, P. F. Strengers, A. D. Huitema, I. Laursen, G. Houen, H. N. Caron, K. M. Dolman, and T. W. Kuijpers. 2009. Safety and pharmacokinetics of plasma-derived mannose-binding lectin (MBL) substitution in children with chemotherapy-induced neutropaenia. Eur. J. Cancer 45:505-512. [DOI] [PubMed] [Google Scholar]
- 140.Fransen, F., S. G. Heckenberg, H. J. Hamstra, M. Feller, C. J. Boog, J. P. van Putten, D. van de Beek, A. van der Ende, and P. van der Ley. 2009. Naturally occurring lipid A mutants in Neisseria meningitidis from patients with invasive meningococcal disease are associated with reduced coagulopathy. PLoS Pathog. 5:e1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fredrikson, G. N., J. Westberg, E. J. Kuijper, C. C. Tijssen, A. G. Sjoholm, M. Uhlen, and L. Truedsson. 1996. Molecular characterization of properdin deficiency type III: dysfunction produced by a single point mutation in exon 9 of the structural gene causing a tyrosine to aspartic acid interchange. J. Immunol. 157:3666-3671. [PubMed] [Google Scholar]
- 142.Fremeaux-Bacchi, V., A. Le Coustumier, J. Blouin, M. D. Kazatchkine, and L. Weiss. 1995. Partial properdin deficiency revealed by a septicemia caused by Neisseria meningitidis. Presse Med. 24:1305-1307. [PubMed] [Google Scholar]
- 143.Frosch, M., and U. Vogel. 2006. Structure and genetics of the meningococcal capsule, p. 145-162. In M. Frosch and M. C. J. Maiden (ed.), Handbook of meningococcal disease. Wiley-VCH, Weinheim, Germany.
- 144.Fukumori, Y., K. Yoshimura, S. Ohnoki, H. Yamaguchi, Y. Akagaki, and S. Inai. 1989. A high incidence of C9 deficiency among healthy blood donors in Osaka, Japan. Int. Immunol. 1:85-89. [DOI] [PubMed] [Google Scholar]
- 145.Gadjeva, M., S. Thiel, and J. C. Jensenius. 2001. The mannan-binding-lectin pathway of the innate immune response. Curr. Opin. Immunol. 13:74-78. [DOI] [PubMed] [Google Scholar]
- 146.Gagneux, S. P., A. Hodgson, T. A. Smith, T. Wirth, I. Ehrhard, G. Morelli, B. Genton, F. N. Binka, M. Achtman, and G. Pluschke. 2002. Prospective study of a serogroup X Neisseria meningitidis outbreak in northern Ghana. J. Infect. Dis. 185:618-626. [DOI] [PubMed] [Google Scholar]
- 147.Galloway, Y., P. Stehr-Green, A. McNicholas, and J. O'Hallahan. 2009. Use of an observational cohort study to estimate the effectiveness of the New Zealand group B meningococcal vaccine in children aged under 5 years. Int. J. Epidemiol. 38:413-418. [DOI] [PubMed] [Google Scholar]
- 148.Garcia-Laorden, M. I., M. J. Pena, J. A. Caminero, A. Garcia-Saavedra, M. I. Campos-Herrero, A. Caballero, and C. Rodriguez-Gallego. 2006. Influence of mannose-binding lectin on HIV infection and tuberculosis in a Western-European population. Mol. Immunol. 43:2143-2150. [DOI] [PubMed] [Google Scholar]
- 149.Garred, P., M. Harboe, T. Oettinger, C. Koch, and A. Svejgaard. 1994. Dual role of mannan-binding protein in infections: another case of heterosis? Eur. J. Immunogenet. 21:125-131. [DOI] [PubMed] [Google Scholar]
- 150.Garred, P., H. O. Madsen, U. Balslev, B. Hofmann, C. Pedersen, J. Gerstoft, and A. Svejgaard. 1997. Susceptibility to HIV infection and progression of AIDS in relation to variant alleles of mannose-binding lectin. Lancet 349:236-240. [DOI] [PubMed] [Google Scholar]
- 151.Garred, P., T. E. Michaelsen, G. Bjune, S. Thiel, and A. Svejgaard. 1993. A low serum concentration of mannan-binding protein is not associated with serogroup B or C meningococcal disease. Scand. J. Immunol. 37:468-470. [DOI] [PubMed] [Google Scholar]
- 152.Garred, P., T. Pressler, S. Lanng, H. O. Madsen, C. Moser, I. Laursen, F. Balstrup, C. Koch, and C. Koch. 2002. Mannose-binding lectin (MBL) therapy in an MBL-deficient patient with severe cystic fibrosis lung disease. Pediatr. Pulmonol. 33:201-207. [DOI] [PubMed] [Google Scholar]
- 153.Garred, P., T. Pressler, H. O. Madsen, B. Frederiksen, A. Svejgaard, N. Hoiby, M. Schwartz, and C. Koch. 1999. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J. Clin. Invest. 104:431-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Garred, P., C. Richter, A. B. Andersen, H. O. Madsen, I. Mtoni, A. Svejgaard, and J. Shao. 1997. Mannan-binding lectin in the sub-Saharan HIV and tuberculosis epidemics. Scand. J. Immunol. 46:204-208. [DOI] [PubMed] [Google Scholar]
- 155.Garty, B. Z., M. Nitzan, and Y. L. Danon. 1993. Systemic meningococcal infections in patients with acquired complement deficiency. Pediatr. Allergy Immunol. 4:6-9. [DOI] [PubMed] [Google Scholar]
- 156.Gaston, M. H., J. I. Verter, G. Woods, C. Pegelow, J. Kelleher, G. Presbury, H. Zarkowsky, E. Vichinsky, R. Iyer, J. S. Lobel, et al. 1986. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N. Engl. J. Med. 314:1593-1599. [DOI] [PubMed] [Google Scholar]
- 157.Genel, F., F. Atlihan, N. Gulez, A. G. Sjoholm, L. Skattum, and L. Truedsson. 2006. Properdin deficiency in a boy with fulminant meningococcal septic shock. Acta Paediatr. 95:1498-1500. [DOI] [PubMed] [Google Scholar]
- 158.Ghannam, A., M. Pernollet, J. L. Fauquert, N. Monnier, D. Ponard, M. B. Villiers, J. Peguet-Navarro, A. Tridon, J. Lunardi, D. Gerlier, and C. Drouet. 2008. Human C3 deficiency associated with impairments in dendritic cell differentiation, memory B cells, and regulatory T cells. J. Immunol. 181:5158-5166. [DOI] [PubMed] [Google Scholar]
- 159.Gilliam, B. E., A. E. Wolff, and T. L. Moore. 2007. Partial C4 deficiency in juvenile idiopathic arthritis patients. J. Clin. Rheumatol. 13:256-260. [DOI] [PubMed] [Google Scholar]
- 160.Giraldo, P. C., O. Babula, A. K. Goncalves, I. M. Linhares, R. L. Amaral, W. J. Ledger, and S. S. Witkin. 2007. Mannose-binding lectin gene polymorphism, vulvovaginal candidiasis, and bacterial vaginosis. Obstet. Gynecol. 109:1123-1128. [DOI] [PubMed] [Google Scholar]
- 161.Giuliani, M. M., J. Adu-Bobie, M. Comanducci, B. Arico, S. Savino, L. Santini, B. Brunelli, S. Bambini, A. Biolchi, B. Capecchi, E. Cartocci, L. Ciucchi, F. Di Marcello, F. Ferlicca, B. Galli, E. Luzzi, V. Masignani, D. Serruto, D. Veggi, M. Contorni, M. Morandi, A. Bartalesi, V. Cinotti, D. Mannucci, F. Titta, E. Ovidi, J. A. Welsch, D. Granoff, R. Rappuoli, and M. Pizza. 2006. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. U. S. A. 103:10834-10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Glass, D., D. Raum, D. Gibson, J. S. Stillman, and P. H. Schur. 1976. Inherited deficiency of the second component of complement. Rheumatic disease associations. J. Clin. Invest. 58:853-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Goldblatt, D., R. Borrow, and E. Miller. 2002. Natural and vaccine-induced immunity and immunologic memory to Neisseria meningitidis serogroup C in young adults. J. Infect. Dis. 185:397-400. [DOI] [PubMed] [Google Scholar]
- 164.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 129:1327-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Granoff, D. M., R. K. Gupta, R. B. Belshe, and E. L. Anderson. 1998. Induction of immunologic refractoriness in adults by meningococcal C polysaccharide vaccination. J. Infect. Dis. 178:870-874. [DOI] [PubMed] [Google Scholar]
- 167.Greenwood, B. M., A. K. Bradley, and R. A. Wall. 1985. Meningococcal disease and season in sub-Saharan Africa. Lancet ii:829-830. [DOI] [PubMed] [Google Scholar]
- 168.Griffiss, J. M. 1975. Bactericidal activity of meningococcal antisera. Blocking by IgA of lytic antibody in human convalescent sera. J. Immunol. 114:1779-1784. [PubMed] [Google Scholar]
- 169.Griffiss, J. M., and M. A. Bertram. 1977. Immunoepidemiology of meningococcal disease in military recruits. II. Blocking of serum bactericidal activity by circulating IgA early in the course of invasive disease. J. Infect. Dis. 136:733-739. [DOI] [PubMed] [Google Scholar]
- 170.Grossman, N., K. A. Joiner, M. M. Frank, and L. Leive. 1986. C3b binding, but not its breakdown, is affected by the structure of the O-antigen polysaccharide in lipopolysaccharide from salmonellae. J. Immunol. 136:2208-2215. [PubMed] [Google Scholar]
- 171.Grossman, N., M. A. Schmetz, J. Foulds, E. N. Klima, V. E. Jimenez-Lucho, L. L. Leive, K. A. Joiner, and V. Jiminez. 1987. Lipopolysaccharide size and distribution determine serum resistance in Salmonella montevideo. J. Bacteriol. 169:856-863. (Erratum, 169:2911.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Grumach, A. S., M. M. Vilela, C. H. Gonzalez, N. Starobinas, A. B. Pereira, W. Dias-da-Silva, and M. M. Carneiro-Sampaio. 1988. Inherited C3 deficiency of the complement system. Braz. J. Med. Biol. Res. 21:247-257. [PubMed] [Google Scholar]
- 173.Guttman, R. M., and B. A. Waisbren. 1975. Bacterial blocking activity of specific IgG in chronic Pseudomonas aeruginosa infection. Clin. Exp. Immunol. 19:121-130. [PMC free article] [PubMed] [Google Scholar]
- 174.Hackett, S. J., M. Guiver, J. Marsh, J. A. Sills, A. P. Thomson, E. B. Kaczmarski, and C. A. Hart. 2002. Meningococcal bacterial DNA load at presentation correlates with disease severity. Arch. Dis. Child. 86:44-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Halle, D., D. Elstein, D. Geudalia, A. Sasson, E. Shinar, M. Schlesinger, and A. Zimran. 2001. High prevalence of complement C7 deficiency among healthy blood donors of Moroccan Jewish ancestry. Am. J. Med. Genet. 99:325-327. [DOI] [PubMed] [Google Scholar]
- 176.Hansen, S., S. Thiel, A. Willis, U. Holmskov, and J. C. Jensenius. 2000. Purification and characterization of two mannan-binding lectins from mouse serum. J. Immunol. 164:2610-2618. [DOI] [PubMed] [Google Scholar]
- 177.Haralambous, E., S. O. Dolly, M. L. Hibberd, D. J. Litt, I. A. Udalova, C. O'Dwyer, P. R. Langford, J. Simon Kroll, and M. Levin. 2006. Factor H, a regulator of complement activity, is a major determinant of meningococcal disease susceptibility in UK Caucasian patients. Scand. J. Infect. Dis. 38:764-771. [DOI] [PubMed] [Google Scholar]
- 178.Hardin, J. A., and J. E. Craft. 1987. Patterns of autoimmunity to nucleoproteins in patients with systemic lupus erythematosus. Rheum. Dis. Clin. North Am. 13:37-46. [PubMed] [Google Scholar]
- 179.Harriman, G. R., A. F. Esser, E. R. Podack, A. C. Wunderlich, A. I. Braude, T. F. Lint, and J. G. Curd. 1981. The role of C9 in complement-mediated killing of Neisseria. J. Immunol. 127:2386-2390. [PubMed] [Google Scholar]
- 180.Hart, M. L., M. Saifuddin, K. Uemura, E. G. Bremer, B. Hooker, T. Kawasaki, and G. T. Spear. 2002. High mannose glycans and sialic acid on gp120 regulate binding of mannose-binding lectin (MBL) to HIV type 1. AIDS Res. Hum. Retroviruses 18:1311-1317. [DOI] [PubMed] [Google Scholar]
- 181.Hartshorn, K. L., K. Sastry, M. R. White, E. M. Anders, M. Super, R. A. Ezekowitz, and A. I. Tauber. 1993. Human mannose-binding protein functions as an opsonin for influenza A viruses. J. Clin. Invest. 91:1414-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hausdorff, W. P., J. Bryant, P. R. Paradiso, and G. R. Siber. 2000. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin. Infect. Dis. 30:100-121. [DOI] [PubMed] [Google Scholar]
- 183.Hayama, K., N. Sugai, S. Tanaka, S. Lee, H. Kikuchi, J. Ito, J. Suzuki, Y. Nagata, H. Kondo, O. Harayama, et al. 1989. High-incidence of C9 deficiency throughout Japan: there are no significant differences in incidence among eight areas of Japan. Int. Arch. Allergy Appl. Immunol. 90:400-404. [DOI] [PubMed] [Google Scholar]
- 184.Hazlewood, M. A., D. S. Kumararatne, A. D. Webster, M. Goodall, P. Bird, and M. Daha. 1992. An association between homozygous C3 deficiency and low levels of anti-pneumococcal capsular polysaccharide antibodies. Clin. Exp. Immunol. 87:404-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Heidelberger, M. 1941. Quantitative chemical studies on complement or alexin. J. Exp. Med. 73:691-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Herwaldt, B., D. H. Persing, E. A. Precigout, W. L. Goff, D. A. Mathiesen, P. W. Taylor, M. L. Eberhard, and A. F. Gorenflot. 1996. A fatal case of babesiosis in Missouri: identification of another piroplasm that infects humans. Ann. Intern. Med. 124:643-650. [DOI] [PubMed] [Google Scholar]
- 187.Hibberd, M. L., M. Sumiya, J. A. Summerfield, R. Booy, and M. Levin. 1999. Association of variants of the gene for mannose-binding lectin with susceptibility to meningococcal disease. Meningococcal Research Group. Lancet 353:1049-1053. [DOI] [PubMed] [Google Scholar]
- 188.Hiemstra, P. S., E. Langeler, B. Compier, Y. Keepers, P. C. Leijh, M. T. van den Barselaar, D. Overbosch, and M. R. Daha. 1989. Complete and partial deficiencies of complement factor D in a Dutch family. J. Clin. Invest. 84:1957-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hirsch, R. L., D. E. Griffin, and J. A. Winkelstein. 1981. Host modification of Sindbis virus sialic acid content influences alternative complement pathway activation and virus clearance. J. Immunol. 127:1740-1743. [PubMed] [Google Scholar]
- 190.Hoal-Van Helden, E. G., J. Epstein, T. C. Victor, D. Hon, L. A. Lewis, N. Beyers, D. Zurakowski, A. B. Ezekowitz, and P. D. Van Helden. 1999. Mannose-binding protein B allele confers protection against tuberculous meningitis. Pediatr. Res. 45:459-464. [DOI] [PubMed] [Google Scholar]
- 191.Hoang, L. M., E. Thomas, S. Tyler, A. J. Pollard, G. Stephens, L. Gustafson, A. McNabb, I. Pocock, R. Tsang, and R. Tan. 2005. Rapid and fatal meningococcal disease due to a strain of Neisseria meningitidis containing the capsule null locus. Clin. Infect. Dis. 40:e38-42. [DOI] [PubMed] [Google Scholar]
- 192.Hoare, S., O. El-Shazali, J. E. Clark, A. Fay, and A. J. Cant. 2002. Investigation for complement deficiency following meningococcal disease. Arch. Dis. Child. 86:215-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hobart, M. J., B. A. Fernie, K. A. Fijen, and A. Orren. 1998. The molecular basis of C6 deficiency in the western Cape, South Africa. Hum. Genet. 103:506-512. [DOI] [PubMed] [Google Scholar]
- 194.Holmberg, V., F. Schuster, E. Dietz, J. C. Sagarriga Visconti, S. D. Anemana, U. Bienzle, and F. P. Mockenhaupt. 2008. Mannose-binding lectin variant associated with severe malaria in young African children. Microbes Infect. 10:342-348. [DOI] [PubMed] [Google Scholar]
- 195.Holst, J., B. Feiring, J. E. Fuglesang, E. A. Hoiby, H. Nokleby, I. S. Aaberge, and E. Rosenqvist. 2003. Serum bactericidal activity correlates with the vaccine efficacy of outer membrane vesicle vaccines against Neisseria meningitidis serogroup B disease. Vaccine 21:734-737. [DOI] [PubMed] [Google Scholar]
- 196.Holst, J., B. Feiring, L. M. Naess, G. Norheim, P. Kristiansen, E. A. Hoiby, K. Bryn, P. Oster, P. Costantino, M. K. Taha, J. M. Alonso, D. A. Caugant, E. Wedege, I. S. Aaberge, R. Rappuoli, and E. Rosenqvist. 2005. The concept of “tailor-made,” protein-based, outer membrane vesicle vaccines against meningococcal disease. Vaccine 23:2202-2205. [DOI] [PubMed] [Google Scholar]
- 197.Homann, C., K. Varming, K. Hogasen, T. E. Mollnes, N. Graudal, A. C. Thomsen, and P. Garred. 1997. Acquired C3 deficiency in patients with alcoholic cirrhosis predisposes to infection and increased mortality. Gut 40:544-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hummelshoj, T., L. Munthe-Fog, H. O. Madsen, T. Fujita, M. Matsushita, and P. Garred. 2005. Polymorphisms in the FCN2 gene determine serum variation and function of Ficolin-2. Hum. Mol. Genet. 14:1651-1658. [DOI] [PubMed] [Google Scholar]
- 199.Hussain, A., K. S. Prasad, D. Bhattacharyya, and K. El-Bouri. 2007. C2 deficiency primary meningococcal arthritis of the elbow by Neisseria meningitidis serogroup Y in a 12-year old girl. Infection 35:287-288. [DOI] [PubMed] [Google Scholar]
- 200.Hynes, R. O. 1987. Integrins: a family of cell surface receptors. Cell 48:549-554. [DOI] [PubMed] [Google Scholar]
- 201.Inaba, S., K. Okochi, K. Fukada, S. Kinoshita, Y. Maeda, and M. Yoshinari. 1987. The occurrence of precipitating antibodies in transfused Japanese patients with hereditary ninth component of complement deficiency and frequency of C9 deficiency. Transfusion 27:475-477. [DOI] [PubMed] [Google Scholar]
- 202.Ingwer, I., B. H. Petersen, and G. Brooks. 1978. Serum bactericidal action and activation of the classic and alternate complement pathways by Neisseria gonorrhoeae. J. Lab Clin. Med. 92:211-220. [PubMed] [Google Scholar]
- 203.Ip, W. K., Y. F. To, S. K. Cheng, and Y. L. Lau. 2004. Serum mannose-binding lectin levels and mbl2 gene polymorphisms in different age and gender groups of southern Chinese adults. Scand. J. Immunol. 59:310-314. [DOI] [PubMed] [Google Scholar]
- 204.Israeli, A., M. Shapiro, and M. A. Ephros. 1987. Plasmodium falciparum malaria in an asplenic man. Trans. R. Soc. Trop. Med. Hyg. 81:233-234. [DOI] [PubMed] [Google Scholar]
- 205.Ivemark, B. I. 1955. Implications of agenesis of the spleen on the pathogenesis of conotruncus anomalies in childhood; an analysis of the heart malformations in the splenic agenesis syndrome, with fourteen new cases. Acta Paediatr. Suppl. 44:7-110. [PubMed] [Google Scholar]
- 206.Jarvis, G. A., and J. M. Griffiss. 1991. Human IgA1 blockade of IgG-initiated lysis of Neisseria meningitidis is a function of antigen-binding fragment binding to the polysaccharide capsule. J. Immunol. 147:1962-1967. [PubMed] [Google Scholar]
- 207.Jarvis, G. A., and N. A. Vedros. 1987. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect. Immun. 55:174-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jennings, H. J., A. K. Bhattacharjee, D. R. Bundle, C. P. Kenny, A. Martin, and I. C. Smith. 1977. Strucutres of the capsular polysaccharides of Neisseria meningitidis as determined by 13C-nuclear magnetic resonance spectroscopy. J. Infect. Dis. 136(Suppl.):S78-S83. [DOI] [PubMed] [Google Scholar]
- 209.Jensenius, J. C., P. H. Jensen, K. McGuire, J. L. Larsen, and S. Thiel. 2003. Recombinant mannan-binding lectin (MBL) for therapy. Biochem. Soc. Trans. 31:763-767. [DOI] [PubMed] [Google Scholar]
- 210.Jimenez-Lucho, V. E., K. A. Joiner, J. Foulds, M. M. Frank, and L. Leive. 1987. C3b generation is affected by the structure of the O-antigen polysaccharide in lipopolysaccharide from salmonellae. J. Immunol. 139:1253-1259. [PubMed] [Google Scholar]
- 211.Joiner, K. A., C. H. Hammer, E. J. Brown, R. J. Cole, and M. M. Frank. 1982. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J. Exp. Med. 155:797-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Joiner, K. A., C. H. Hammer, E. J. Brown, and M. M. Frank. 1982. Studies on the mechanism of bacterial resistance to complement-mediated killing. II. C8 and C9 release C5b67 from the surface of Salmonella minnesota S218 because the terminal complex does not insert into the bacterial outer membrane. J. Exp. Med. 155:809-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jones, D. M., R. Borrow, A. J. Fox, S. Gray, K. A. Cartwright, and J. T. Poolman. 1992. The lipooligosaccharide immunotype as a virulence determinant in Neisseria meningitidis. Microb. Pathog. 13:219-224. [DOI] [PubMed] [Google Scholar]
- 214.Jongerius, I., J. Kohl, M. K. Pandey, M. Ruyken, K. P. van Kessel, J. A. van Strijp, and S. H. Rooijakkers. 2007. Staphylococcal complement evasion by various convertase-blocking molecules. J. Exp. Med. 204:2461-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jonsson, G., V. A. Oxelius, L. Truedsson, J. H. Braconier, G. Sturfelt, and A. G. Sjoholm. 2006. Homozygosity for the IgG2 subclass allotype G2M(n) protects against severe infection in hereditary C2 deficiency. J. Immunol. 177:722-728. [DOI] [PubMed] [Google Scholar]
- 216.Jonsson, G., L. Truedsson, G. Sturfelt, V. A. Oxelius, J. H. Braconier, and A. G. Sjoholm. 2005. Hereditary C2 deficiency in Sweden: frequent occurrence of invasive infection, atherosclerosis, and rheumatic disease. Medicine (Baltimore) 84:23-34. [DOI] [PubMed] [Google Scholar]
- 217.Kakkanaiah, V. N., G. Q. Shen, E. A. Ojo-Amaize, and J. B. Peter. 1998. Association of low concentrations of serum mannose-binding protein with recurrent infections in adults. Clin. Diagn. Lab. Immunol. 5:319-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kang, Y. S., S. Yamazaki, T. Iyoda, M. Pack, S. A. Bruening, J. Y. Kim, K. Takahara, K. Inaba, R. M. Steinman, and C. G. Park. 2003. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int. Immunol. 15:177-186. [DOI] [PubMed] [Google Scholar]
- 219.Karp, C. L., M. Wysocka, L. M. Wahl, J. M. Ahearn, P. J. Cuomo, B. Sherry, G. Trinchieri, and D. E. Griffin. 1996. Mechanism of suppression of cell-mediated immunity by measles virus. Science 273:228-231. [DOI] [PubMed] [Google Scholar]
- 220.Katschke, K. J., Jr., K. Y. Helmy, M. Steffek, H. Xi, J. Yin, W. P. Lee, P. Gribling, K. H. Barck, R. A. Carano, R. E. Taylor, L. Rangell, L. Diehl, P. E. Hass, C. Wiesmann, and M. van Lookeren Campagne. 2007. A novel inhibitor of the alternative pathway of complement reverses inflammation and bone destruction in experimental arthritis. J. Exp. Med. 204:1319-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kaufmann, T., G. Hansch, C. Rittner, P. Spath, F. Tedesco, and P. M. Schneider. 1993. Genetic basis of human complement C8 beta deficiency. J. Immunol. 150:4943-4947. [PubMed] [Google Scholar]
- 222.Kaur, S., V. K. Gupta, A. Shah, S. Thiel, P. U. Sarma, and T. Madan. 2006. Elevated levels of mannan-binding lectin [corrected] (MBL) and eosinophilia in patients of bronchial asthma with allergic rhinitis and allergic bronchopulmonary aspergillosis associate with a novel intronic polymorphism in MBL. Clin. Exp. Immunol. 143:414-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kavai, M. 2008. Immune complex clearance by complement receptor type 1 in SLE. Autoimmun. Rev. 8:160-164. [DOI] [PubMed] [Google Scholar]
- 224.Kazatchkine, M. D., D. T. Fearon, and K. F. Austen. 1979. Human alternative complement pathway: membrane-associated sialic acid regulates the competition between B and beta1 H for cell-bound C3b. J. Immunol. 122:75-81. [PubMed] [Google Scholar]
- 225.Keiser, H. D. 1997. Recurrent disseminated gonococcal infection in a patient with hypocomplementemia and membranoproliferative glomerulonephritis. J. Clin. Rheumatol. 3:286-289. [DOI] [PubMed] [Google Scholar]
- 226.Kelly, P., D. L. Jack, A. Naeem, B. Mandanda, R. C. Pollok, N. J. Klein, M. W. Turner, and M. J. Farthing. 2000. Mannose-binding lectin is a component of innate mucosal defense against Cryptosporidium parvum in AIDS. Gastroenterology 119:1236-1242. [DOI] [PubMed] [Google Scholar]
- 227.Kemper, C., A. C. Chan, J. M. Green, K. A. Brett, K. M. Murphy, and J. P. Atkinson. 2003. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature 421:388-392. [DOI] [PubMed] [Google Scholar]
- 228.Kimura, Y., M. Madhavan, M. K. Call, W. Santiago, P. A. Tsonis, J. D. Lambris, and K. Del Rio-Tsonis. 2003. Expression of complement 3 and complement 5 in newt limb and lens regeneration. J. Immunol. 170:2331-2339. [DOI] [PubMed] [Google Scholar]
- 229.Kishimoto, T. K., K. O'Conner, and T. A. Springer. 1989. Leukocyte adhesion deficiency. Aberrant splicing of a conserved integrin sequence causes a moderate deficiency phenotype. J. Biol. Chem. 264:3588-3595. [PubMed] [Google Scholar]
- 230.Kishore, U., and K. B. Reid. 2000. C1q: structure, function, and receptors. Immunopharmacology 49:159-170. [DOI] [PubMed] [Google Scholar]
- 231.Klabunde, J., A. C. Uhlemann, A. E. Tebo, J. Kimmel, R. T. Schwarz, P. G. Kremsner, and J. F. Kun. 2002. Recognition of Plasmodium falciparum proteins by mannan-binding lectin, a component of the human innate immune system. Parasitol. Res. 88:113-117. [DOI] [PubMed] [Google Scholar]
- 232.Konradsen, H. B., V. A. Oxelius, M. Hahn-Zoric, and L. A. Hanson. 1994. The importance of G1m and 2 allotypes for the IgG2 antibody levels and avidity against pneumococcal polysaccharide type 1 within mono- and dizygotic twin-pairs. Scand. J. Immunol. 40:251-256. [DOI] [PubMed] [Google Scholar]
- 233.Kopf, M., B. Abel, A. Gallimore, M. Carroll, and M. F. Bachmann. 2002. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat. Med. 8:373-378. [DOI] [PubMed] [Google Scholar]
- 234.Korb, L. C., and J. M. Ahearn. 1997. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J. Immunol. 158:4525-4528. [PubMed] [Google Scholar]
- 235.Kotwal, G. J., and B. Moss. 1988. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature 335:176-178. [DOI] [PubMed] [Google Scholar]
- 236.Kraiczy, P., and R. Wurzner. 2006. Complement escape of human pathogenic bacteria by acquisition of complement regulators. Mol. Immunol. 43:31-44. [DOI] [PubMed] [Google Scholar]
- 237.Krause, P. J., B. E. Gewurz, D. Hill, F. M. Marty, E. Vannier, I. M. Foppa, R. R. Furman, E. Neuhaus, G. Skowron, S. Gupta, C. McCalla, E. L. Pesanti, M. Young, D. Heiman, G. Hsue, J. A. Gelfand, G. P. Wormser, J. Dickason, F. J. Bia, B. Hartman, S. R. Telford III, D. Christianson, K. Dardick, M. Coleman, J. E. Girotto, and A. Spielman. 2008. Persistent and relapsing babesiosis in immunocompromised patients. Clin. Infect. Dis. 46:370-376. [DOI] [PubMed] [Google Scholar]
- 238.Krause, P. J., T. Lepore, V. K. Sikand, J. Gadbaw, Jr., G. Burke, S. R. Telford III, P. Brassard, D. Pearl, J. Azlanzadeh, D. Christianson, D. McGrath, and A. Spielman. 2000. Atovaquone and azithromycin for the treatment of babesiosis. N. Engl. J. Med. 343:1454-1458. [DOI] [PubMed] [Google Scholar]
- 239.Kristensen, K. 1992. Antibody response to a Haemophilus influenzae type b polysaccharide tetanus toxoid conjugate vaccine in splenectomized children and adolescents. Scand. J. Infect. Dis. 24:629-632. [DOI] [PubMed] [Google Scholar]
- 240.Kronborg, G., N. Weis, H. O. Madsen, S. S. Pedersen, C. Wejse, H. Nielsen, P. Skinhoj, and P. Garred. 2002. Variant mannose-binding lectin alleles are not associated with susceptibility to or outcome of invasive pneumococcal infection in randomly included patients. J. Infect. Dis. 185:1517-1520. [DOI] [PubMed] [Google Scholar]
- 241.Kuipers, S., P. C. Aerts, O. J. Cluysenaer, A. K. Bartelink, R. A. Ezekowitz, W. A. Bax, M. Salimans, and H. Vandyk. 2003. A case of familial meningococcal disease due to deficiency in mannose-binding lectin (MBL). Adv. Exp. Med. Biol. 531:351-355. [DOI] [PubMed] [Google Scholar]
- 242.Kurtz, C. B., E. O'Toole, S. M. Christensen, and J. H. Weis. 1990. The murine complement receptor gene family. IV. Alternative splicing of Cr2 gene transcripts predicts two distinct gene products that share homologous domains with both human CR2 and CR1. J. Immunol. 144:3581-3591. [PubMed] [Google Scholar]
- 242a.Kyaw, M. H., E. M. Holmes, F. Toolis, B. Wayne, J. Chalmers, I. G. Jones, and H. Campbell. 2006. Evaluation of severe infection and survival after splenectomy. Am. J. Med. 119:276.e1-276.e7. [DOI] [PubMed] [Google Scholar]
- 243.Lambourne, J., D. Agranoff, R. Herbrecht, P. F. Troke, A. Buchbinder, F. Willis, V. Letscher-Bru, S. Agrawal, S. Doffman, E. Johnson, P. L. White, R. A. Barnes, G. Griffin, J. A. Lindsay, and T. S. Harrison. 2009. Association of mannose-binding lectin deficiency with acute invasive aspergillosis in immunocompromised patients. Clin. Infect. Dis. 49:1486-1491. [DOI] [PubMed] [Google Scholar]
- 244.Lapeyssonnie, L. 1963. Cerebrospinal meningitis in Africa. Bull. World Health Organ. 28(Suppl.):1-114. [PMC free article] [PubMed] [Google Scholar]
- 245.Laursen, S. B., T. S. Dalgaard, S. Thiel, B. L. Lim, T. V. Jensen, H. R. Juul-Madsen, A. Takahashi, T. Hamana, M. Kawakami, and J. C. Jensenius. 1998. Cloning and sequencing of a cDNA encoding chicken mannan-binding lectin (MBL) and comparison with mammalian analogues. Immunology 93:421-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Law, S. K., N. A. Lichtenberg, and R. P. Levine. 1980. Covalent binding and hemolytic activity of complement proteins. Proc. Natl. Acad. Sci. U. S. A. 77:7194-7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Leggiadro, R. J., and J. A. Winkelstein. 1987. Prevalence of complement deficiencies in children with systemic meningococcal infections. Pediatr. Infect. Dis. J. 6:75-76. [DOI] [PubMed] [Google Scholar]
- 248.Lehner, P. J., K. A. Davies, M. J. Walport, A. P. Cope, R. Wurzner, A. Orren, B. P. Morgan, and J. Cohen. 1992. Meningococcal septicaemia in a C6-deficient patient and effects of plasma transfusion on lipopolysaccharide release. Lancet 340:1379-1381. [DOI] [PubMed] [Google Scholar]
- 249.Le Moal, G., C. Landron, G. Grollier, R. Robert, and C. Burucoa. 2003. Meningitis due to Capnocytophaga canimorsus after receipt of a dog bite: case report and review of the literature. Clin. Infect. Dis. 36:e42-e46. [DOI] [PubMed] [Google Scholar]
- 250.Levy, M., L. Halbwachs-Mecarelli, M. C. Gubler, G. Kohout, A. Bensenouci, P. Niaudet, G. Hauptmann, and P. Lesavre. 1986. H deficiency in two brothers with atypical dense intramembranous deposit disease. Kidney Int. 30:949-956. [DOI] [PubMed] [Google Scholar]
- 251.Lewis, L. A., B. Choudhury, J. T. Balthazar, L. E. Martin, S. Ram, P. A. Rice, D. S. Stephens, R. Carlson, and W. M. Shafer. 2009. Phosphoethanolamine substitution of lipid A and resistance of Neisseria gonorrhoeae to cationic antimicrobial peptides and complement-mediated killing by normal human serum. Infect. Immun. 77:1112-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lin, J. S., S. P. Donegan, T. C. Heeren, M. Greenberg, E. E. Flaherty, R. Haivanis, X. H. Su, D. Dean, W. J. Newhall, J. S. Knapp, S. K. Sarafian, R. J. Rice, S. A. Morse, and P. A. Rice. 1998. Transmission of Chlamydia trachomatis and Neisseria gonorrhoeae among men with urethritis and their female sex partners. J. Infect. Dis. 178:1707-1712. [DOI] [PubMed] [Google Scholar]
- 253.Lingappa, J. R., A. M. Al-Rabeah, R. Hajjeh, T. Mustafa, A. Fatani, T. Al-Bassam, A. Badukhan, A. Turkistani, S. Makki, N. Al-Hamdan, M. Al-Jeffri, Y. Al Mazrou, B. A. Perkins, T. Popovic, L. W. Mayer, and N. E. Rosenstein. 2003. Serogroup W-135 meningococcal disease during the Hajj, 2000. Emerg. Infect. Dis. 9:665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lint, T. F., H. J. Zeitz, and H. Gewurz. 1980. Inherited deficiency of the ninth component of complement in man. J. Immunol. 125:2252-2257. [PubMed] [Google Scholar]
- 255.Lion, C., F. Escande, and J. C. Burdin. 1996. Capnocytophaga canimorsus infections in human: review of the literature and cases report. Eur. J. Epidemiol. 12:521-533. [DOI] [PubMed] [Google Scholar]
- 256.Litzman, J., T. Freiberger, D. Bartonkova, M. Vlkova, V. Thon, and J. Lokaj. 2003. Early manifestation and recognition of C2 complement deficiency in the form of pyogenic infection in infancy. J. Paediatr. Child Health 39:274-277. [DOI] [PubMed] [Google Scholar]
- 257.Liu, F., Q. Liao, and Z. Liu. 2006. Mannose-binding lectin and vulvovaginal candidiasis. Int. J. Gynaecol. Obstet. 92:43-47. [DOI] [PubMed] [Google Scholar]
- 258.Liu, W., F. Zhang, Z. T. Xin, Q. M. Zhao, X. M. Wu, P. H. Zhang, S. de Vlas, J. H. Richardus, J. D. Habbema, H. Yang, and W. C. Cao. 2006. Sequence variations in the MBL gene and their relationship to pulmonary tuberculosis in the Chinese Han population. Int. J. Tuber. Lung Dis. 10:1098-1103. [PubMed] [Google Scholar]
- 259.Looareesuwan, S., P. Suntharasamai, H. K. Webster, and M. Ho. 1993. Malaria in splenectomized patients: report of four cases and review. Clin. Infect. Dis. 16:361-366. [DOI] [PubMed] [Google Scholar]
- 260.Lubinski, J. M., L. Wang, A. M. Soulika, R. Burger, R. A. Wetsel, H. Colten, G. H. Cohen, R. J. Eisenberg, J. D. Lambris, and H. M. Friedman. 1998. Herpes simplex virus type 1 glycoprotein gC mediates immune evasion in vivo. J. Virol. 72:8257-8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Luty, A. J., J. F. Kun, and P. G. Kremsner. 1998. Mannose-binding lectin plasma levels and gene polymorphisms in Plasmodium falciparum malaria. J. Infect. Dis. 178:1221-1224. [DOI] [PubMed] [Google Scholar]
- 262.Lynch, A. M., and R. Kapila. 1996. Overwhelming postsplenectomy infection. Infect. Dis. Clin. North Am. 10:693-707. [DOI] [PubMed] [Google Scholar]
- 263.Maas, J., A. M. de Roda Husman, M. Brouwer, A. Krol, R. Coutinho, I. Keet, R. van Leeuwen, and H. Schuitemaker. 1998. Presence of the variant mannose-binding lectin alleles associated with slower progression to AIDS. Amsterdam Cohort Study. AIDS 12:2275-2280. [DOI] [PubMed] [Google Scholar]
- 264.MacDonald, N. E., S. A. Halperin, B. J. Law, B. Forrest, L. E. Danzig, and D. M. Granoff. 1998. Induction of immunologic memory by conjugated versus plain meningococcal C polysaccharide vaccine in toddlers: a randomized controlled trial. JAMA 280:1685-1689. [DOI] [PubMed] [Google Scholar]
- 265.Machtinger, L., S. R. Telford III, C. Inducil, E. Klapper, S. H. Pepkowitz, and D. Goldfinger. 1993. Treatment of babesiosis by red blood cell exchange in an HIV-positive, splenectomized patient. J. Clin. Apher. 8:78-81. [DOI] [PubMed] [Google Scholar]
- 266.MacLennan, J., S. Obaro, J. Deeks, D. Williams, L. Pais, G. Carlone, R. Moxon, and B. Greenwood. 1999. Immune response to revaccination with meningococcal A and C polysaccharides in Gambian children following repeated immunisation during early childhood. Vaccine 17:3086-3093. [DOI] [PubMed] [Google Scholar]
- 267.Madan, T., S. Kaur, S. Saxena, M. Singh, U. Kishore, S. Thiel, K. B. Reid, and P. U. Sarma. 2005. Role of collectins in innate immunity against aspergillosis. Med. Mycol. 43(Suppl. 1):S155-S163. [DOI] [PubMed] [Google Scholar]
- 268.Madico, G., J. Ngampasutadol, S. Gulati, U. Vogel, P. A. Rice, and S. Ram. 2007. Factor H binding and function in sialylated pathogenic neisseriae is influenced by gonococcal, but not meningococcal, porin. J. Immunol. 178:4489-4497. [DOI] [PubMed] [Google Scholar]
- 269.Madico, G., J. A. Welsch, L. A. Lewis, A. McNaughton, D. H. Perlman, C. E. Costello, J. Ngampasutadol, U. Vogel, D. M. Granoff, and S. Ram. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177:501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Madsen, H. O., P. Garred, J. A. Kurtzhals, L. U. Lamm, L. P. Ryder, S. Thiel, and A. Svejgaard. 1994. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics 40:37-44. [DOI] [PubMed] [Google Scholar]
- 271.Madsen, H. O., P. Garred, S. Thiel, J. A. Kurtzhals, L. U. Lamm, L. P. Ryder, and A. Svejgaard. 1995. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J. Immunol. 155:3013-3020. [PubMed] [Google Scholar]
- 272.Maiden, M. C., A. B. Ibarz-Pavon, R. Urwin, S. J. Gray, N. J. Andrews, S. C. Clarke, A. M. Walker, M. R. Evans, J. S. Kroll, K. R. Neal, D. A. Ala'aldeen, D. W. Crook, K. Cann, S. Harrison, R. Cunningham, D. Baxter, E. Kaczmarski, J. Maclennan, J. C. Cameron, and J. M. Stuart. 2008. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J. Infect. Dis. 197:737-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Malik, S., M. Arias, C. Di Flumeri, L. F. Garcia, and E. Schurr. 2003. Absence of association between mannose-binding lectin gene polymorphisms and HIV-1 infection in a Colombian population. Immunogenetics 55:49-52. [DOI] [PubMed] [Google Scholar]
- 274.Manderson, A. P., M. Botto, and M. J. Walport. 2004. The role of complement in the development of systemic lupus erythematosus. Annu. Rev. Immunol. 22:431-456. [DOI] [PubMed] [Google Scholar]
- 275.Mandrell, R. E., and M. A. Apicella. 1993. Lipo-oligosaccharides (LOS) of mucosal pathogens: molecular mimicry and host-modification of LOS. Immunobiology 187:382-402. [DOI] [PubMed] [Google Scholar]
- 276.Mandrell, R. E., J. M. Griffiss, and B. A. Macher. 1988. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J. Exp. Med. 168:107-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Mandrell, R. E., R. McLaughlin, Y. Aba Kwaik, A. Lesse, R. Yamasaki, B. Gibson, S. M. Spinola, and M. A. Apicella. 1992. Lipooligosaccharides (LOS) of some Haemophilus species mimic human glycosphingolipids, and some LOS are sialylated. Infect. Immun. 60:1322-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Mangano, A., C. Rocco, S. M. Marino, D. Mecikovsky, F. Genre, P. Aulicino, R. Bologna, and L. Sen. 2008. Detrimental effects of mannose-binding lectin (MBL2) promoter genotype XA/XA on HIV-1 vertical transmission and AIDS progression. J. Infect. Dis. 198:694-700. [DOI] [PubMed] [Google Scholar]
- 279.Manuel, O., P. E. Tarr, J. P. Venetz, M. Trendelenburg, P. R. Meylan, and M. Pascual. 2007. Meningococcal disease in a kidney transplant recipient with mannose-binding lectin deficiency. Transpl. Infect. Dis. 9:214-218. [DOI] [PubMed] [Google Scholar]
- 280.Marc LaForce, F., N. Ravenscroft, M. Djingarey, and S. Viviani. 2009. Epidemic meningitis due to group A Neisseria meningitidis in the African meningitis belt: a persistent problem with an imminent solution. Vaccine 27(Suppl. 2):B13-B19. [DOI] [PubMed] [Google Scholar]
- 281.Marder, R. J., F. X. Burch, F. R. Schmid, C. R. Zeiss, and H. Gewurz. 1978. Low molecular weight C1q-precipitins in hypocomplementemic vasculitis-urticaria syndrome: partial purification and characterization as immunoglobulin. J. Immunol. 121:613-618. [PubMed] [Google Scholar]
- 282.Mardiney, M. R., Jr., H. J. Muller-Eberhard, and J. D. Feldman. 1968. Ultrastructural localization of the third and fourth components of complement on complement-cell complexes. Am. J. Pathol. 53:253-261. [PMC free article] [PubMed] [Google Scholar]
- 283.Markiewski, M. M., D. Mastellos, R. Tudoran, R. A. DeAngelis, C. W. Strey, S. Franchini, R. A. Wetsel, A. Erdei, and J. D. Lambris. 2004. C3a and C3b activation products of the third component of complement (C3) are critical for normal liver recovery after toxic injury. J. Immunol. 173:747-754. [DOI] [PubMed] [Google Scholar]
- 284.Mascart-Lemone, F., G. Hauptmann, J. Goetz, J. Duchateau, G. Delespesse, B. Vray, and I. Dab. 1983. Genetic deficiency of C4 presenting with recurrent infections and a SLE-like disease. Genetic and immunologic studies. Am. J. Med. 75:295-304. [DOI] [PubMed] [Google Scholar]
- 285.Masignani, V., M. Comanducci, M. M. Giuliani, S. Bambini, J. Adu-Bobie, B. Arico, B. Brunelli, A. Pieri, L. Santini, S. Savino, D. Serruto, D. Litt, S. Kroll, J. A. Welsch, D. M. Granoff, R. Rappuoli, and M. Pizza. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197:789-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Mastellos, D., and J. D. Lambris. 2002. Complement: more than a ‘guard’ against invading pathogens? Trends Immunol. 23:485-491. [DOI] [PubMed] [Google Scholar]
- 287.Mastellos, D., J. C. Papadimitriou, S. Franchini, P. A. Tsonis, and J. D. Lambris. 2001. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J. Immunol. 166:2479-2486. [DOI] [PubMed] [Google Scholar]
- 288.McEllistrem, M. C., J. A. Kolano, M. A. Pass, D. A. Caugant, A. B. Mendelsohn, A. G. Fonseca Pacheco, K. A. Shutt, J. Razeq, and L. H. Harrison. 2004. Correlating epidemiologic trends with the genotypes causing meningococcal disease, Maryland. Emerg. Infect. Dis. 10:451-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.McNearney, T. A., C. Odell, V. M. Holers, P. G. Spear, and J. P. Atkinson. 1987. Herpes simplex virus glycoproteins gC-1 and gC-2 bind to the third component of complement and provide protection against complement-mediated neutralization of viral infectivity. J. Exp. Med. 166:1525-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.McWhinney, P. H., P. Langhorne, W. C. Love, and K. Whaley. 1991. Disseminated gonococcal infection associated with deficiency of the second component of complement. Postgrad. Med. J. 67:297-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Meerveld-Eggink, A., O. de Weerdt, G. T. Rijkers, H. van Velzen-Blad, and D. H. Biesma. 2008. Vaccination coverage and awareness of infectious risks in patients with an absent or dysfunctional spleen in the Netherlands. Vaccine 26:6975-6979. [DOI] [PubMed] [Google Scholar]
- 292.Meri, S., and M. K. Pangburn. 1990. Discrimination between activators and nonactivators of the alternative pathway of complement: regulation via a sialic acid/polyanion binding site on factor H. Proc. Natl. Acad. Sci. U. S. A. 87:3982-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Merino, S., S. Camprubi, S. Alberti, V. J. Benedi, and J. M. Tomas. 1992. Mechanisms of Klebsiella pneumoniae resistance to complement-mediated killing. Infect. Immun. 60:2529-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Meyer, O., G. Hauptmann, G. Tappeiner, H. D. Ochs, and F. Mascart-Lemone. 1985. Genetic deficiency of C4, C2 or C1q and lupus syndromes. Association with anti-Ro (SS-A) antibodies. Clin. Exp. Immunol. 62:678-684. [PMC free article] [PubMed] [Google Scholar]
- 295.Michalek, M. T., E. G. Bremer, and C. Mold. 1988. Effect of gangliosides on activation of the alternative pathway of human complement. J. Immunol. 140:1581-1587. [PubMed] [Google Scholar]
- 296.Miguelez Morales, M., M. Linares Feria, and M. del Carmen Mesa. 1995. Haemophagocytic syndrome due to babesiosis in a splenectomized patient. Br. J. Haematol. 91:1033. [DOI] [PubMed] [Google Scholar]
- 297.Milanese, M., L. Segat, F. De Seta, D. Pirulli, A. Fabris, M. Morgutti, and S. Crovella. 2008. MBL2 genetic screening in patients with recurrent vaginal infections. Am. J. Reprod Immunol. 59:146-151. [DOI] [PubMed] [Google Scholar]
- 298.Misra, A., A. Peethambaram, and A. Garg. 2004. Clinical features and metabolic and autoimmune derangements in acquired partial lipodystrophy: report of 35 cases and review of the literature. Medicine (Baltimore) 83:18-34. [DOI] [PubMed] [Google Scholar]
- 299.Mitchell, L., and D. Hourcade. 2008. Inhibition of properdin-directed complement activation by serum amyloid P component. Mol. Immunol. 45:4103. [Google Scholar]
- 300.Mitchell, S. R., P. Q. Nguyen, and P. Katz. 1990. Increased risk of neisserial infections in systemic lupus erythematosus. Semin. Arthritis Rheum. 20:174-184. [DOI] [PubMed] [Google Scholar]
- 301.Mogues, T., T. Ota, A. I. Tauber, and K. N. Sastry. 1996. Characterization of two mannose-binding protein cDNAs from rhesus monkey (Macaca mulatta): structure and evolutionary implications. Glycobiology 6:543-550. [DOI] [PubMed] [Google Scholar]
- 302.Mold, C., B. M. Bradt, G. R. Nemerow, and N. R. Cooper. 1988. Epstein-Barr virus regulates activation and processing of the third component of complement. J. Exp. Med. 168:949-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Molina, H., T. Kinoshita, K. Inoue, J. C. Carel, and V. M. Holers. 1990. A molecular and immunochemical characterization of mouse CR2. Evidence for a single gene model of mouse complement receptors 1 and 2. J. Immunol. 145:2974-2983. [PubMed] [Google Scholar]
- 304.Moller, A. S., A. Bjerre, B. Brusletto, G. B. Joo, P. Brandtzaeg, and P. Kierulf. 2005. Chemokine patterns in meningococcal disease. J. Infect. Dis. 191:768-775. [DOI] [PubMed] [Google Scholar]
- 305.Mombo, L. E., C. Y. Lu, S. Ossari, I. Bedjabaga, L. Sica, R. Krishnamoorthy, and C. Lapoumeroulie. 2003. Mannose-binding lectin alleles in sub-Saharan Africans and relation with susceptibility to infections. Genes Immun. 4:362-367. [DOI] [PubMed] [Google Scholar]
- 306.Moran, A. P., M. M. Prendergast, and B. J. Appelmelk. 1996. Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease. FEMS Immunol. Med. Microbiol. 16:105-115. [DOI] [PubMed] [Google Scholar]
- 307.Moulds, J. M., L. Kassambara, J. J. Middleton, M. Baby, I. Sagara, A. Guindo, S. Coulibaly, D. Yalcouye, D. A. Diallo, L. Miller, and O. Doumbo. 2000. Identification of complement receptor one (CR1) polymorphisms in west Africa. Genes Immun. 1:325-329. [DOI] [PubMed] [Google Scholar]
- 308.Moulds, J. M., M. W. Nickells, J. J. Moulds, M. C. Brown, and J. P. Atkinson. 1991. The C3b/C4b receptor is recognized by the Knops, McCoy, Swain-Langley, and York blood group antisera. J. Exp. Med. 173:1159-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Mullick, J., A. K. Singh, Y. Panse, V. Yadav, J. Bernet, and A. Sahu. 2005. Identification of functional domains in kaposica, the complement control protein homolog of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8). J. Virol. 79:5850-5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Munthe-Fog, L., T. Hummelshoj, C. Honore, H. O. Madsen, H. Permin, and P. Garred. 2009. Immunodeficiency associated with FCN3 mutation and ficolin-3 deficiency. N. Engl. J. Med. 360:2637-2644. [DOI] [PubMed] [Google Scholar]
- 311.Munthe-Fog, L., T. Hummelshoj, Y. J. Ma, B. E. Hansen, C. Koch, H. O. Madsen, K. Skjodt, and P. Garred. 2008. Characterization of a polymorphism in the coding sequence of FCN3 resulting in a Ficolin-3 (Hakata antigen) deficiency state. Mol. Immunol. 45:2660-2666. [DOI] [PubMed] [Google Scholar]
- 312.Musher, D. M. 2006. Pneumococcal serotypes and virulence. J. Infect. Dis. 193:477. (Author reply, 193:477-478.) [DOI] [PubMed] [Google Scholar]
- 313.Nagata, M., T. Hara, T. Aoki, Y. Mizuno, H. Akeda, S. Inaba, K. Tsumoto, and K. Ueda. 1989. Inherited deficiency of ninth component of complement: an increased risk of meningococcal meningitis. J. Pediatr. 114:260-264. [DOI] [PubMed] [Google Scholar]
- 314.Nassif, X., S. Bourdoulous, E. Eugene, and P. O. Couraud. 2002. How do extracellular pathogens cross the blood-brain barrier? Trends Microbiol. 10:227-232. [DOI] [PubMed] [Google Scholar]
- 315.Nauta, A. J., B. Bottazzi, A. Mantovani, G. Salvatori, U. Kishore, W. J. Schwaeble, A. R. Gingras, S. Tzima, F. Vivanco, J. Egido, O. Tijsma, E. C. Hack, M. R. Daha, and A. Roos. 2003. Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur. J. Immunol. 33:465-473. [DOI] [PubMed] [Google Scholar]
- 316.Nelson, R. A., Jr. 1953. The immune-adherence phenomenon; an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis. Science 118:733-737. [DOI] [PubMed] [Google Scholar]
- 317.Neth, O., I. Hann, M. W. Turner, and N. J. Klein. 2001. Deficiency of mannose-binding lectin and burden of infection in children with malignancy: a prospective study. Lancet 358:614-618. [DOI] [PubMed] [Google Scholar]
- 318.Neth, O., D. L. Jack, A. W. Dodds, H. Holzel, N. J. Klein, and M. W. Turner. 2000. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect. Immun. 68:688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Newman, S. L., L. B. Vogler, R. D. Feigin, and R. B. Johnston, Jr. 1978. Recurrent septicemia associated with congenital deficiency of C2 and partial deficiency of factor B and the alternative complement pathway. N. Engl. J. Med. 299:290-292. [DOI] [PubMed] [Google Scholar]
- 320.Nielsen, H. E., K. C. Christensen, C. Koch, B. S. Thomsen, N. H. Heegaard, and J. Tranum-Jensen. 1989. Hereditary, complete deficiency of complement factor H associated with recurrent meningococcal disease. Scand. J. Immunol. 30:711-718. [DOI] [PubMed] [Google Scholar]
- 321.Nielsen, H. E., C. Koch, P. Magnussen, and I. Lind. 1989. Complement deficiencies in selected groups of patients with meningococcal disease. Scand. J. Infect. Dis. 21:389-396. [DOI] [PubMed] [Google Scholar]
- 322.Nielsen, S. L., P. L. Andersen, C. Koch, J. C. Jensenius, and S. Thiel. 1995. The level of the serum opsonin, mannan-binding protein in HIV-1 antibody-positive patients. Clin. Exp. Immunol. 100:219-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Nimmerjahn, F., and J. V. Ravetch. 2008. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8:34-47. [DOI] [PubMed] [Google Scholar]
- 324.Nishizaka, H., T. Horiuchi, Z. B. Zhu, Y. Fukumori, K. Nagasawa, K. Hayashi, R. Krumdieck, C. G. Cobbs, M. Higuchi, S. Yasunaga, Y. Niho, and J. E. Volanakis. 1996. Molecular bases for inherited human complement component C6 deficiency in two unrelated individuals. J. Immunol. 156:2309-2315. [PubMed] [Google Scholar]
- 325.Nolte, M. T., B. Pirofsky, G. A. Gerritz, and B. Golding. 1979. Intravenous immunoglobulin therapy for antibody deficiency. Clin. Exp. Immunol. 36:237-243. [PMC free article] [PubMed] [Google Scholar]
- 326.O'Hara, A. M., B. A. Fernie, A. P. Moran, Y. E. Williams, J. J. Connaughton, A. Orren, and M. J. Hobart. 1998. C7 deficiency in an Irish family: a deletion defect which is predominant in the Irish. Clin. Exp. Immunol. 114:355-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.O'Hara, A. M., A. P. Moran, R. Wurzner, and A. Orren. 2001. Complement-mediated lipopolysaccharide release and outer membrane damage in Escherichia coli J5: requirement for C9. Immunology 102:365-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Olesen, H. V., J. C. Jensenius, R. Steffensen, S. Thiel, and P. O. Schiotz. 2006. The mannan-binding lectin pathway and lung disease in cystic fibrosis—disfunction of mannan-binding lectin-associated serine protease 2 (MASP-2) may be a major modifier. Clin. Immunol. 121:324-331. [DOI] [PubMed] [Google Scholar]
- 329.Orren, A., D. A. Caugant, C. A. Fijen, J. Dankert, E. J. van Schalkwyk, J. T. Poolman, and G. J. Coetzee. 1994. Characterization of strains of Neisseria meningitidis recovered from complement-sufficient and complement-deficient patients in the Western Cape Province, South Africa. J. Clin. Microbiol. 32:2185-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Orren, A., P. C. Potter, R. C. Cooper, and E. du Toit. 1987. Deficiency of the sixth component of complement and susceptibility to Neisseria meningitidis infections: studies in 10 families and five isolated cases. Immunology 62:249-253. [PMC free article] [PubMed] [Google Scholar]
- 331.Orren, A., R. E. Warren, P. C. Potter, A. M. Jones, P. J. Lachmann, and J. T. Poolman. 1992. Antibodies to meningococcal class 1 outer membrane proteins in South African complement-deficient and complement-sufficient subjects. Infect. Immun. 60:4510-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Orren, A., R. Wurzner, P. C. Potter, B. A. Fernie, S. Coetzee, B. P. Morgan, and P. J. Lachmann. 1992. Properties of a low molecular weight complement component C6 found in human subjects with subtotal C6 deficiency. Immunology 75:10-16. [PMC free article] [PubMed] [Google Scholar]
- 333.Oxelius, V. A. 1993. Serum IgG and IgG subclass contents in different Gm phenotypes. Scand. J. Immunol. 37:149-153. [DOI] [PubMed] [Google Scholar]
- 334.Ozbas-Gerceker, F., I. Tezcan, A. I. Berkel, S. Ozkara, A. Ozcan, F. Ersoy, O. Sanal, and M. Ozguc. 2003. The effect of mannose-binding protein gene polymorphisms in recurrent respiratory system infections in children and lung tuberculosis. Turk. J. Pediatr. 45:95-98. [PubMed] [Google Scholar]
- 335.Pangburn, M. K. 1989. Analysis of the natural polymeric forms of human properdin and their functions in complement activation. J. Immunol. 142:202-207. [PubMed] [Google Scholar]
- 336.Pangburn, M. K., and H. J. Muller-Eberhard. 1978. Complement C3 convertase: cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc. Natl. Acad. Sci. U. S. A. 75:2416-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Pangburn, M. K., and N. Rawal. 2002. Structure and function of complement C5 convertase enzymes. Biochem. Soc. Trans. 30:1006-1010. [DOI] [PubMed] [Google Scholar]
- 338.Parham, K. L., A. Roberts, A. Thomas, R. Wurzner, H. E. Henderson, P. C. Potter, B. P. Morgan, and A. Orren. 2007. Prevalence of mutations leading to complete C6 deficiency (C6Q0) in the Western Cape, South Africa and detection of novel mutations leading to C6Q0 in an Irish family. Mol. Immunol. 44:2756-2760. [DOI] [PubMed] [Google Scholar]
- 339.Pastinen, T., K. Liitsola, P. Niini, M. Salminen, and A. C. Syvanen. 1998. Contribution of the CCR5 and MBL genes to susceptibility to HIV type 1 infection in the Finnish population. AIDS Res. Hum. Retroviruses 14:695-698. [DOI] [PubMed] [Google Scholar]
- 340.Peleg, D., H. Harit-Bustan, Y. Katz, S. Peller, M. Schlesinger, and S. Schonfeld. 1992. Inherited C3 deficiency and meningococcal disease in a teenager. Pediatr. Infect. Dis. J. 11:401-404. [DOI] [PubMed] [Google Scholar]
- 341.Peltola, H. 1983. Meningococcal disease: still with us. Rev. Infect. Dis. 5:71-91. [DOI] [PubMed] [Google Scholar]
- 342.Pers, C., B. Gahrn-Hansen, and W. Frederiksen. 1996. Capnocytophaga canimorsus septicemia in Denmark, 1982-1995: review of 39 cases. Clin. Infect. Dis. 23:71-75. [DOI] [PubMed] [Google Scholar]
- 343.Petersen, K. A., F. Matthiesen, T. Agger, L. Kongerslev, S. Thiel, K. Cornelissen, and M. Axelsen. 2006. Phase I safety, tolerability, and pharmacokinetic study of recombinant human mannan-binding lectin. J. Clin. Immunol. 26:465-475. [DOI] [PubMed] [Google Scholar]
- 344.Pfarr, N., D. Prawitt, M. Kirschfink, C. Schroff, M. Knuf, P. Habermehl, W. Mannhardt, F. Zepp, W. Fairbrother, M. Loos, C. B. Burge, and J. Pohlenz. 2005. Linking C5 deficiency to an exonic splicing enhancer mutation. J. Immunol. 174:4172-4177. [DOI] [PubMed] [Google Scholar]
- 345.Pfeiffer, F., and R. Issaeff. 1894. Uber die Specifishche der Bedeutung der Choleraimmunitat. Z. Hyg. Infecktionskr. 17:355. [Google Scholar]
- 346.Pickering, M. C., M. Botto, P. R. Taylor, P. J. Lachmann, and M. J. Walport. 2000. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv. Immunol. 76:227-324. [DOI] [PubMed] [Google Scholar]
- 347.Pickering, M. C., and H. T. Cook. 2008. Translational mini-review series on complement factor H: renal diseases associated with complement factor H: novel insights from humans and animals. Clin. Exp. Immunol. 151:210-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Pickering, M. C., and M. J. Walport. 2000. Links between complement abnormalities and systemic lupus erythematosus. Rheumatology (Oxford) 39:133-141. [DOI] [PubMed] [Google Scholar]
- 349.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Arico, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816-1820. [DOI] [PubMed] [Google Scholar]
- 350.Platonov, A. E., V. B. Beloborodov, L. I. Pavlova, I. V. Vershinina, and H. Kayhty. 1995. Vaccination of patients deficient in a late complement component with tetravalent meningococcal capsular polysaccharide vaccine. Clin. Exp. Immunol. 100:32-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Platonov, A. E., V. B. Beloborodov, and I. V. Vershinina. 1993. Meningococcal disease in patients with late complement component deficiency: studies in the U.S.S.R. Medicine (Baltimore) 72:374-392. [DOI] [PubMed] [Google Scholar]
- 352.Platonov, A. E., I. V. Vershinina, E. J. Kuijper, R. Borrow, and H. Kayhty. 2003. Long term effects of vaccination of patients deficient in a late complement component with a tetravalent meningococcal polysaccharide vaccine. Vaccine 21:4437-4447. [DOI] [PubMed] [Google Scholar]
- 353.Platonov, A. E., R. Wurzner, B. Beloborodov, A. M. Jones, D. V. Troshansky, I. V. Vershinina, P. J. Lachmann, and A. Orren. 1994. Paradoxical reconstitution of complement activity following plasma transfusion of an individual with deficiency of the seventh component of complement. Immunology 81:142-148. [PMC free article] [PubMed] [Google Scholar]
- 354.Plummer, F. A., H. Chubb, J. N. Simonsen, M. Bosire, L. Slaney, I. Maclean, J. O. Ndinya-Achola, P. Waiyaki, and R. C. Brunham. 1993. Antibody to Rmp (outer membrane protein 3) increases susceptibility to gonococcal infection. J. Clin. Invest. 91:339-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Podack, E. R., G. Biesecker, and H. J. Muller-Eberhard. 1979. Membrane attack complex of complement: generation of high-affinity phospholipid binding sites by fusion of five hydrophilic plasma proteins. Proc. Natl. Acad. Sci. U. S. A. 76:897-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Pongponratn, E., P. Viriyavejakul, P. Wilairatana, D. Ferguson, U. Chaisri, G. Turner, and S. Looareesuwan. 2000. Absence of knobs on parasitized red blood cells in a splenectomized patient in fatal falciparum malaria. Southeast Asian J. Trop. Med. Public Health 31:829-835. [PubMed] [Google Scholar]
- 357.Porteu, F., A. Fischer, B. Descamps-Latscha, and L. Halbwachs-Mecarelli. 1986. Defective complement receptors (CR1 and CR3) on erythrocytes and leukocytes of factor I (C3b-inactivator) deficient patients. Clin. Exp. Immunol. 66:463-471. [PMC free article] [PubMed] [Google Scholar]
- 358.Potter, P. C., C. E. Frasch, W. J. van der Sande, R. C. Cooper, Y. Patel, and A. Orren. 1990. Prophylaxis against Neisseria meningitidis infections and antibody responses in patients with deficiency of the sixth component of complement. J. Infect. Dis. 161:932-937. [DOI] [PubMed] [Google Scholar]
- 359.Preissner, K. T., E. R. Podack, and H. J. Muller-Eberhard. 1985. The membrane attack complex of complement: relation of C7 to the metastable membrane binding site of the intermediate complex C5b-7. J. Immunol. 135:445-451. [PubMed] [Google Scholar]
- 360.Price, V. E., S. Dutta, V. S. Blanchette, S. Butchart, M. Kirby, J. C. Langer, and E. L. Ford-Jones. 2006. The prevention and treatment of bacterial infections in children with asplenia or hyposplenia: practice considerations at the Hospital for Sick Children, Toronto. Pediatr. Blood Cancer 46:597-603. [DOI] [PubMed] [Google Scholar]
- 361.Prohaszka, Z., S. Thiel, E. Ujhelyi, J. Szlavik, D. Banhegyi, and G. Fust. 1997. Mannan-binding lectin serum concentrations in HIV-infected patients are influenced by the stage of disease. Immunol. Lett. 58:171-175. [DOI] [PubMed] [Google Scholar]
- 362.Provost, T. T., F. C. Arnett, and M. Reichlin. 1983. Homozygous C2 deficiency, lupus erythematosus, and anti-Ro (SSA) antibodies. Arthritis Rheum. 26:1279-1282. [DOI] [PubMed] [Google Scholar]
- 363.Rasmussen, J. M., I. Brandslund, B. Teisner, H. Isager, S. E. Svehag, L. Maarup, L. Willumsen, J. O. Ronne-Rasmussen, H. Permin, P. L. Andersen, et al. 1987. Screening for complement deficiencies in unselected patients with meningitis. Clin. Exp. Immunol. 68:437-445. [PMC free article] [PubMed] [Google Scholar]
- 364.Reca, R., D. Mastellos, M. Majka, L. Marquez, J. Ratajczak, S. Franchini, A. Glodek, M. Honczarenko, L. A. Spruce, A. Janowska-Wieczorek, J. D. Lambris, and M. Z. Ratajczak. 2003. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood 101:3784-3793. [DOI] [PubMed] [Google Scholar]
- 365.Reis, E. S., D. A. Falcao, and L. Isaac. 2006. Clinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor H. Scand. J. Immunol. 63:155-168. [DOI] [PubMed] [Google Scholar]
- 366.Rice, P. A., H. Vayo, M. Tam, and M. S. Blake. 1986. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J. Exp. Med. 164:1735-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Richmond, P., E. Kaczmarski, R. Borrow, J. Findlow, S. Clark, R. McCann, J. Hill, M. Barker, and E. Miller. 2000. Meningococcal C polysaccharide vaccine induces immunologic hyporesponsiveness in adults that is overcome by meningococcal C conjugate vaccine. J. Infect. Dis. 181:761-764. [DOI] [PubMed] [Google Scholar]
- 368.Roantree, R. J., and L. A. Rantz. 1960. A study of the relationship of the normal bactericidal activity of human serum to bacterial infection. J. Clin. Invest. 39:72-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Rooijakkers, S. H., and J. A. van Strijp. 2007. Bacterial complement evasion. Mol. Immunol. 44:23-32. [DOI] [PubMed] [Google Scholar]
- 370.Rose, K. L., D. Paixao-Cavalcante, J. Fish, A. P. Manderson, T. H. Malik, A. E. Bygrave, T. Lin, S. H. Sacks, M. J. Walport, H. T. Cook, M. Botto, and M. C. Pickering. 2008. Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J. Clin. Invest. 118:608-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, L. Lefkowitz, M. L. Cartter, R. Danila, P. Cieslak, K. A. Shutt, T. Popovic, A. Schuchat, L. H. Harrison, and A. L. Reingold. 1999. The changing epidemiology of meningococcal disease in the United States, 1992-1996. J. Infect. Dis. 180:1894-1901. [DOI] [PubMed] [Google Scholar]
- 372.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, T. Popovic, and J. M. Hughes. 2001. Meningococcal disease. N. Engl. J. Med. 344:1378-1388. [DOI] [PubMed] [Google Scholar]
- 373.Rosner, F., M. H. Zarrabi, J. L. Benach, and G. S. Habicht. 1984. Babesiosis in splenectomized adults. Review of 22 reported cases. Am. J. Med. 76:696-701. [DOI] [PubMed] [Google Scholar]
- 374.Ross, S. C., and P. Densen. 1984. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore) 63:243-273. [PubMed] [Google Scholar]
- 375.Ross, S. C., P. J. Rosenthal, H. M. Berberich, and P. Densen. 1987. Killing of Neisseria meningitidis by human neutrophils: implications for normal and complement-deficient individuals. J. Infect. Dis. 155:1266-1275. [DOI] [PubMed] [Google Scholar]
- 376.Rottem, M., D. Miron, E. Shiloah, Y. Horovitz, and M. Schlezinger. 1998. Properdin deficiency: rare presentation with meningococcal bone and joint infections. Pediatr. Infect. Dis. J. 17:356-358. [DOI] [PubMed] [Google Scholar]
- 377.Roumenina, L. T., M. M. Ruseva, A. Zlatarova, R. Ghai, M. Kolev, N. Olova, M. Gadjeva, A. Agrawal, B. Bottazzi, A. Mantovani, K. B. Reid, U. Kishore, and M. S. Kojouharova. 2006. Interaction of C1q with IgG1, C-reactive protein and pentraxin 3: mutational studies using recombinant globular head modules of human C1q A, B, and C chains. Biochemistry 45:4093-4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Rowe, J. A., J. M. Moulds, C. I. Newbold, and L. H. Miller. 1997. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature 388:292-295. [DOI] [PubMed] [Google Scholar]
- 379.Rowe, P. C., R. H. McLean, R. A. Wood, R. J. Leggiadro, and J. A. Winkelstein. 1989. Association of homozygous C4B deficiency with bacterial meningitis. J. Infect. Dis. 160:448-451. [DOI] [PubMed] [Google Scholar]
- 380.Rowin, K. S., H. B. Tanowitz, A. Rubinstein, M. Kunkel, and M. Wittner. 1984. Babesiosis in asplenic hosts. Trans. R. Soc. Trop. Med. Hyg. 78:442-444. [DOI] [PubMed] [Google Scholar]
- 381.Roy, S., K. Knox, S. Segal, D. Griffiths, C. E. Moore, K. I. Welsh, A. Smarason, N. P. Day, W. L. McPheat, D. W. Crook, and A. V. Hill. 2002. MBL genotype and risk of invasive pneumococcal disease: a case-control study. Lancet 359:1569-1573. [DOI] [PubMed] [Google Scholar]
- 382.Rua Figueroa, I., E. Loza Cortina, S. Gonzalez Suarez, C. E. Arruabarrena, and J. L. Pena Sagredo. 1993. Disseminated gonococcal infection in an elderly patient. Ann. Rheum. Dis. 52:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Rune Andersen, S., J. Kolberg, E. A. Hoiby, E. Namork, D. A. Caugant, L. Oddvar Froholm, E. Jantzen, and G. Bjune. 1997. Lipopolysaccharide heterogeneity and escape mechanisms of Neisseria meningitidis: possible consequences for vaccine development. Microb. Pathog. 23:139-155. [DOI] [PubMed] [Google Scholar]
- 384.Rupert, K. L., J. M. Moulds, Y. Yang, F. C. Arnett, R. W. Warren, J. D. Reveille, B. L. Myones, C. A. Blanchong, and C. Y. Yu. 2002. The molecular basis of complete complement C4A and C4B deficiencies in a systemic lupus erythematosus patient with homozygous C4A and C4B mutant genes. J. Immunol. 169:1570-1578. [DOI] [PubMed] [Google Scholar]
- 385.Saifuddin, M., M. L. Hart, H. Gewurz, Y. Zhang, and G. T. Spear. 2000. Interaction of mannose-binding lectin with primary isolates of human immunodeficiency virus type 1. J. Gen. Virol. 81:949-955. [DOI] [PubMed] [Google Scholar]
- 386.Sanal, O., M. Loos, F. Ersoy, G. Kanra, G. Secmeer, and I. Tezcan. 1992. Complement component deficiencies and infection: C5, C8 and C3 deficiencies in three families. Eur. J. Pediatr. 151:676-679. [DOI] [PubMed] [Google Scholar]
- 387.Sanal, O., L. Yel, I. Tezcan, F. Ersoy, and A. I. Berkel. 1996. Homozygous C2 deficiency: association with defective alternative pathway function and immunoglobulin deficiency. Int. Arch. Allergy Immunol. 110:195-198. [DOI] [PubMed] [Google Scholar]
- 388.Sarvas, H., N. Rautonen, H. Kayhty, M. Kallio, and O. Makela. 1990. Effect of Gm allotypes on IgG2 antibody responses and IgG2 concentrations in children and adults. Int. Immunol. 2:317-322. [DOI] [PubMed] [Google Scholar]
- 389.Schejbel, L., V. Rosenfeldt, H. Marquart, N. H. Valerius, and P. Garred. 2009. Properdin deficiency associated with recurrent otitis media and pneumonia, and identification of male carrier with Klinefelter syndrome. Clin. Immunol. [DOI] [PubMed]
- 390.Schifferli, J. A., Y. C. Ng, J. Estreicher, and M. J. Walport. 1988. The clearance of tetanus toxoid/anti-tetanus toxoid immune complexes from the circulation of humans. Complement- and erythrocyte complement receptor 1-dependent mechanisms. J. Immunol. 140:899-904. [PubMed] [Google Scholar]
- 391.Schlesinger, M., R. Greenberg, J. Levy, H. Kayhty, and R. Levy. 1994. Killing of meningococci by neutrophils: effect of vaccination on patients with complement deficiency. J. Infect. Dis. 170:449-453. [DOI] [PubMed] [Google Scholar]
- 392.Schlesinger, M., U. Mashal, J. Levy, and Z. Fishelson. 1993. Hereditary properdin deficiency in three families of Tunisian Jews. Acta Paediatr. 82:744-747. [DOI] [PubMed] [Google Scholar]
- 393.Schlesinger, M., Z. Nave, Y. Levy, P. E. Slater, and Z. Fishelson. 1990. Prevalence of hereditary properdin, C7 and C8 deficiencies in patients with meningococcal infections. Clin. Exp. Immunol. 81:423-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Schneider, M. C., R. M. Exley, H. Chan, I. Feavers, Y. H. Kang, R. B. Sim, and C. M. Tang. 2006. Functional significance of factor H binding to Neisseria meningitidis. J. Immunol. 176:7566-7575. [DOI] [PubMed] [Google Scholar]
- 395.Schneider, M. C., R. M. Exley, S. Ram, R. B. Sim, and C. M. Tang. 2007. Interactions between Neisseria meningitidis and the complement system. Trends Microbiol. 15:233-240. [DOI] [PubMed] [Google Scholar]
- 396.Schneider, P. M., M. C. Carroll, C. A. Alper, C. Rittner, A. S. Whitehead, E. J. Yunis, and H. R. Colten. 1986. Polymorphism of the human complement C4 and steroid 21-hydroxylase genes. Restriction fragment length polymorphisms revealing structural deletions, homoduplications, and size variants. J. Clin. Invest. 78:650-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Schoolnik, G. K., T. M. Buchanan, and K. K. Holmes. 1976. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J. Clin. Invest. 58:1163-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Schwertz, R., E. Esser, R. A. Seger, A. Rubinstein, G. Hauptmann, and V. Wahn. 1991. Defective activation of the alternative pathway of complement in patients with homozygous C2 deficiency: studies in two unrelated families. Eur. J. Pediatr. 150:647-651. [DOI] [PubMed] [Google Scholar]
- 399.Selander, B., H. Kayhty, E. Wedege, E. Holmstrom, L. Truedsson, C. Soderstrom, and A. G. Sjoholm. 2000. Vaccination responses to capsular polysaccharides of Neisseria meningitidis and Haemophilus influenzae type b in two C2-deficient sisters: alternative pathway-mediated bacterial killing and evidence for a novel type of blocking IgG. J. Clin. Immunol. 20:138-149. [DOI] [PubMed] [Google Scholar]
- 400.Seppanen, M., M. L. Lokki, T. Timonen, M. Lappalainen, H. Jarva, A. Jarvinen, S. Sarna, V. Valtonen, and S. Meri. 2001. Complement C4 deficiency and HLA homozygosity in patients with frequent intraoral herpes simplex virus type 1 infections. Clin. Infect. Dis. 33:1604-1607. [DOI] [PubMed] [Google Scholar]
- 401.Seppanen, M., S. Meri, I. L. Notkola, I. J. Seppala, E. Hiltunen-Back, H. Sarvas, M. Lappalainen, H. Valimaa, A. Palikhe, V. V. Valtonen, and M. L. Lokki. 2006. Subtly impaired humoral immunity predisposes to frequently recurring genital herpes simplex virus type 2 infection and herpetic neuralgia. J. Infect. Dis. 194:571-578. [DOI] [PubMed] [Google Scholar]
- 402.Setty, S., Z. Khalil, P. Schori, M. Azar, and P. Ferrieri. 2003. Babesiosis. Two atypical cases from Minnesota and a review. Am. J. Clin. Pathol. 120:554-559. [DOI] [PubMed] [Google Scholar]
- 403.Siber, G. R., P. H. Schur, A. C. Aisenberg, S. A. Weitzman, and G. Schiffman. 1980. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N. Engl. J. Med. 303:178-182. [DOI] [PubMed] [Google Scholar]
- 404.Sierra, G. V., H. C. Campa, N. M. Varcacel, I. L. Garcia, P. L. Izquierdo, P. F. Sotolongo, G. V. Casanueva, C. O. Rico, C. R. Rodriguez, and M. H. Terry. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 14:195-207, 208-210. [PubMed] [Google Scholar]
- 405.Sim, R. B., T. M. Twose, D. S. Paterson, and E. Sim. 1981. The covalent-binding reaction of complement component C3. Biochem. J. 193:115-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Singh, K. K., A. Lieser, P. K. Ruan, T. Fenton, and S. A. Spector. 2008. An age-dependent association of mannose-binding lectin-2 genetic variants on HIV-1-related disease in children. J. Allergy Clin. Immunol. 122:173-180, 180.e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Skattum, L., B. Gullstrand, E. Holmstrom, V. A. Oxelius, and L. Truedsson. 2008. Serum bactericidal activity against Neisseria meningitidis in patients with C3 nephritic factors is dependent on IgG allotypes. Clin. Immunol. 129:123-131. [DOI] [PubMed] [Google Scholar]
- 408.Skattum, L., U. Martensson, and A. G. Sjoholm. 1997. Hypocomplementaemia caused by C3 nephritic factors (C3 NeF): clinical findings and the coincidence of C3 NeF type II with anti-C1q autoantibodies. J. Intern. Med. 242:455-464. [DOI] [PubMed] [Google Scholar]
- 409.Skjoedt, M. O., Y. Palarasah, L. Munthe-Fog, Y. Jie Ma, G. Weiss, K. Skjodt, C. Koch, and P. Garred. 2009. MBL-associated serine protease-3 circulates in high serum concentrations predominantly in complex with Ficolin-3 and regulates Ficolin-3 mediated complement activation. Immunobiology [Epub ahead of print.] doi: 10.1016/j.imbio.2009.10.006. [DOI] [PubMed]
- 410.Slovut, D. P., E. Benedetti, and A. J. Matas. 1996. Babesiosis and hemophagocytic syndrome in an asplenic renal transplant recipient. Transplantation 62:537-539. [DOI] [PubMed] [Google Scholar]
- 411.Smets, F., A. Bourgois, C. Vermylen, B. Brichard, P. Slacmuylders, S. Leyman, and E. Sokal. 2007. Randomised revaccination with pneumococcal polysaccharide or conjugate vaccine in asplenic children previously vaccinated with polysaccharide vaccine. Vaccine 25:5278-5282. [DOI] [PubMed] [Google Scholar]
- 412.Snyderman, R., D. T. Durack, G. A. McCarty, F. E. Ward, and L. Meadows. 1979. Deficiency of the fifth component of complement in human subjects. Clinical, genetic and immunologic studies in a large kindred. Am. J. Med. 67:638-645. [DOI] [PubMed] [Google Scholar]
- 413.Soborg, C., H. O. Madsen, A. B. Andersen, T. Lillebaek, A. Kok-Jensen, and P. Garred. 2003. Mannose-binding lectin polymorphisms in clinical tuberculosis. J. Infect. Dis. 188:777-782. [DOI] [PubMed] [Google Scholar]
- 414.Soderstrom, C., J. H. Braconier, D. Danielsson, and A. G. Sjoholm. 1987. Bactericidal activity for Neisseria meningitidis in properdin-deficient sera. J. Infect. Dis. 156:107-112. [DOI] [PubMed] [Google Scholar]
- 415.Soderstrom, C., J. H. Braconier, H. Kayhty, A. G. Sjoholm, and B. Thuresson. 1989. Immune response to tetravalent meningococcal vaccine: opsonic and bactericidal functions of normal and properdin deficient sera. Eur. J. Clin. Microbiol. Infect. Dis. 8:220-224. [DOI] [PubMed] [Google Scholar]
- 416.Soothill, J. F., and B. A. Harvey. 1976. Defective opsonization. A common immunity deficiency. Arch. Dis. Child. 51:91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Spath, P. J., A. G. Sjoholm, G. N. Fredrikson, G. Misiano, R. Scherz, U. B. Schaad, B. Uhring-Lambert, G. Hauptmann, J. Westberg, M. Uhlen, C. Wadelius, and L. Truedsson. 1999. Properdin deficiency in a large Swiss family: identification of a stop codon in the properdin gene, and association of meningococcal disease with lack of the IgG2 allotype marker G2m(n). Clin. Exp. Immunol. 118:278-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Spear, G. T., M. R. Zariffard, J. Xin, and M. Saifuddin. 2003. Inhibition of DC-SIGN-mediated trans infection of T cells by mannose-binding lectin. Immunology 110:80-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Spiller, O. B., L. Mark, C. E. Blue, D. G. Proctor, J. A. Aitken, A. M. Blom, and D. J. Blackbourn. 2006. Dissecting the regions of virion-associated Kaposi's sarcoma-associated herpesvirus complement control protein required for complement regulation and cell binding. J. Virol. 80:4068-4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Spitzer, D., L. M. Mitchell, J. P. Atkinson, and D. E. Hourcade. 2007. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J. Immunol. 179:2600-2608. [DOI] [PubMed] [Google Scholar]
- 421.Springer, T. A., W. S. Thompson, L. J. Miller, F. C. Schmalstieg, and D. C. Anderson. 1984. Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. J. Exp. Med. 160:1901-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Sprong, T., D. Roos, C. Weemaes, C. Neeleman, C. L. Geesing, T. E. Mollnes, and M. van Deuren. 2006. Deficient alternative complement pathway activation due to factor D deficiency by 2 novel mutations in the complement factor D gene in a family with meningococcal infections. Blood 107:4865-4870. [DOI] [PubMed] [Google Scholar]
- 423.Stanford, E., F. Print, M. Falconer, K. Lamden, S. Ghebrehewet, N. Phin, D. Baxter, M. Helbert, R. McCann, N. Andrews, P. Balmer, R. Borrow, and E. Kaczmarski. 2009. Immune response to pneumococcal conjugate vaccination in asplenic individuals. Hum. Vaccin. 5:85-91. [DOI] [PubMed] [Google Scholar]
- 424.Steffensen, R., S. Thiel, K. Varming, C. Jersild, and J. C. Jensenius. 2000. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J. Immunol. Methods 241:33-42. [DOI] [PubMed] [Google Scholar]
- 425.Stephens, D. S. 1999. Uncloaking the meningococcus: dynamics of carriage and disease. Lancet 353:941-942. [DOI] [PubMed] [Google Scholar]
- 426.Stephens, D. S., P. A. Spellman, and J. S. Swartley. 1993. Effect of the (alpha 2→8)-linked polysialic acid capsule on adherence of Neisseria meningitidis to human mucosal cells. J. Infect. Dis. 167:475-479. [DOI] [PubMed] [Google Scholar]
- 427.Stevens, B., N. J. Allen, L. E. Vazquez, G. R. Howell, K. S. Christopherson, N. Nouri, K. D. Micheva, A. K. Mehalow, A. D. Huberman, B. Stafford, A. Sher, A. M. Litke, J. D. Lambris, S. J. Smith, S. W. John, and B. A. Barres. 2007. The classical complement cascade mediates CNS synapse elimination. Cell 131:1164-1178. [DOI] [PubMed] [Google Scholar]
- 428.Stiegler, D., J. D. Gilbert, M. S. Warner, and R. W. Byard. 2010. Fatal dog bite in the absence of significant trauma: Capnocytophaga canimorsus infection and unexpected death. Am. J. Forensic Med. Pathol. 31:198-199. [DOI] [PubMed] [Google Scholar]
- 429.Sumiya, M., M. Super, P. Tabona, R. J. Levinsky, T. Arai, M. W. Turner, and J. A. Summerfield. 1991. Molecular basis of opsonic defect in immunodeficient children. Lancet 337:1569-1570. [DOI] [PubMed] [Google Scholar]
- 430.Summerfield, J. A., S. Ryder, M. Sumiya, M. Thursz, A. Gorchein, M. A. Monteil, and M. W. Turner. 1995. Mannose binding protein gene mutations associated with unusual and severe infections in adults. Lancet 345:886-889. [DOI] [PubMed] [Google Scholar]
- 431.Super, M., S. Thiel, J. Lu, R. J. Levinsky, and M. W. Turner. 1989. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet ii:1236-1239. [DOI] [PubMed] [Google Scholar]
- 432.Sutterwala, F. S., G. J. Noel, R. Clynes, and D. M. Mosser. 1997. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J. Exp. Med. 185:1977-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Takahashi, M., Y. Ishida, D. Iwaki, K. Kanno, T. Suzuki, Y. Endo, Y. Homma, and T. Fujita. 2010. Essential role of mannose-binding lectin-associated serine protease-1 in activation of the complement factor D. J. Exp. Med. 207:29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Tan, E. M. 1997. Autoantibodies and autoimmunity: a three-decade perspective. A tribute to Henry G. Kunkel. Ann. N. Y. Acad. Sci. 815:1-14. [DOI] [PubMed] [Google Scholar]
- 435.Tan, L. K., G. M. Carlone, and R. Borrow. 2010. Advances in the development of vaccines against Neisseria meningitidis. N. Engl. J. Med. 362:1511-1520. [DOI] [PubMed] [Google Scholar]
- 436.Taylor, P. W. 1972. An antibactericidal factor in the serum of two patients with infections of the upper urinary tract. Clin. Sci. 43:23-30. [DOI] [PubMed] [Google Scholar]
- 437.Tedesco, F., L. Roncelli, B. H. Petersen, V. Agnello, and J. M. Sodetz. 1990. Two distinct abnormalities in patients with C8 alpha-gamma deficiency. Low level of C8 beta chain and presence of dysfunctional C8 alpha-gamma subunit. J. Clin. Invest. 86:884-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Teeranaipong, P., J. Ohashi, J. Patarapotikul, R. Kimura, P. Nuchnoi, H. Hananantachai, I. Naka, C. Putaporntip, S. Jongwutiwes, and K. Tokunaga. 2008. A functional single-nucleotide polymorphism in the CR1 promoter region contributes to protection against cerebral malaria. J. Infect. Dis. 198:1880-1891. [DOI] [PubMed] [Google Scholar]
- 439.Tesh, V. L., R. L. Duncan, Jr., and D. C. Morrison. 1986. The interaction of Escherichia coli with normal human serum: the kinetics of serum-mediated lipopolysaccharide release and its dissociation from bacterial killing. J. Immunol. 137:1329-1335. [PubMed] [Google Scholar]
- 440.Thathy, V., J. M. Moulds, B. Guyah, W. Otieno, and J. A. Stoute. 2005. Complement receptor 1 polymorphisms associated with resistance to severe malaria in Kenya. Malar. J. 4:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Thiel, S., P. D. Frederiksen, and J. C. Jensenius. 2006. Clinical manifestations of mannan-binding lectin deficiency. Mol. Immunol. 43:86-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Thiel, S., U. Holmskov, L. Hviid, S. B. Laursen, and J. C. Jensenius. 1992. The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin. Exp. Immunol. 90:31-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Thomas, L., and J. H. Dingle. 1943. Investigations of meningococcal infection. III. Bactericidal action of normal and immune sera for the meningococcus. J. Clin. Invest. 22:375-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Thomas, L., H. W. Smith, and J. H. Dingle. 1943. Investigations of meningococcal infection. II. Immunological aspects. J. Clin. Invest. 22:361-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Thompson, R. A., and M. H. Winterborn. 1981. Hypocomplementaemia due to a genetic deficiency of beta 1H globulin. Clin. Exp. Immunol. 46:110-119. [PMC free article] [PubMed] [Google Scholar]
- 446.Tikhomirov, E. 1987. Meningococcal meningitis: global situation and control measures. World Health Stat. Q. 40:98-109. [PubMed] [Google Scholar]
- 447.Tikhomirov, E., M. Santamaria, and K. Esteves. 1997. Meningococcal disease: public health burden and control. World Health Stat. Q. 50:170-177. [PubMed] [Google Scholar]
- 448.Tikly, M., M. Diese, N. Zannettou, and R. Essop. 1997. Gonococcal endocarditis in a patient with systemic lupus erythematosus. Br. J. Rheumatol. 36:270-272. [DOI] [PubMed] [Google Scholar]
- 449.Tomas, J. M., S. Camprubi, S. Merino, M. R. Davey, and P. Williams. 1991. Surface exposure of O1 serotype lipopolysaccharide in Klebsiella pneumoniae strains expressing different K antigens. Infect. Immun. 59:2006-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Tomlinson, S., L. C. Pontes de Carvalho, F. Vandekerckhove, and V. Nussenzweig. 1994. Role of sialic acid in the resistance of Trypanosoma cruzi trypomastigotes to complement. J. Immunol. 153:3141-3147. [PubMed] [Google Scholar]
- 451.Totan, M. 2002. Recurrent pneumococcal meningitis in homozygous C3 deficiency. Indian J. Pediatr. 69:625-626. [DOI] [PubMed] [Google Scholar]
- 452.Trotter, C., R. Borrow, N. Andrews, and E. Miller. 2003. Seroprevalence of meningococcal serogroup C bactericidal antibody in England and Wales in the pre-vaccination era. Vaccine 21:1094-1098. [DOI] [PubMed] [Google Scholar]
- 453.Trotter, C., J. Findlow, P. Balmer, A. Holland, R. Barchha, N. Hamer, N. Andrews, E. Miller, and R. Borrow. 2007. Seroprevalence of bactericidal and anti-outer membrane vesicle antibodies to Neisseria meningitidis group B in England. Clin. Vaccine Immunol. 14:863-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Truedsson, L., A. A. Bengtsson, and G. Sturfelt. 2007. Complement deficiencies and systemic lupus erythematosus. Autoimmunity 40:560-566. [DOI] [PubMed] [Google Scholar]
- 455.Truedsson, L., B. Gullstrand, T. Jonsson, and C. Klint. 1995. Serum concentrations of C4 isotypes and factor B in type I C2 deficiency suggest haplotype-dependent quantitative expression of MHC class III complement genes. Exp. Clin. Immunogenet. 12:66-73. [PubMed] [Google Scholar]
- 456.Tsai, C. M. 2001. Molecular mimicry of host structures by lipooligosaccharides of Neisseria meningitidis: characterization of sialylated and nonsialylated lacto-N-neotetraose (Galbeta1-4GlcNAcbeta1-3Galbeta1-4Glc) structures in lipooligosaccharides using monoclonal antibodies and specific lectins. Adv. Exp. Med. Biol. 491:525-542. [DOI] [PubMed] [Google Scholar]
- 457.Turner, M. W. 1998. Mannose-binding lectin (MBL) in health and disease. Immunobiology 199:327-339. [DOI] [PubMed] [Google Scholar]
- 458.Tzeng, Y. L., and D. S. Stephens. 2000. Epidemiology and pathogenesis of Neisseria meningitidis. Microbes Infect. 2:687-700. [DOI] [PubMed] [Google Scholar]
- 459.Uria, M. J., Q. Zhang, Y. Li, A. Chan, R. M. Exley, B. Gollan, H. Chan, I. Feavers, A. Yarwood, R. Abad, R. Borrow, R. A. Fleck, B. Mulloy, J. A. Vazquez, and C. M. Tang. 2008. A generic mechanism in Neisseria meningitidis for enhanced resistance against bactericidal antibodies. J. Exp. Med. 205:1423-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Valdimarsson, H. 2003. Infusion of plasma-derived mannan-binding lectin (MBL) into MBL-deficient humans. Biochem. Soc. Trans. 31:768-769. [DOI] [PubMed] [Google Scholar]
- 461.Valdimarsson, H., T. Vikingsdottir, P. Bang, S. Saevarsdottir, J. E. Gudjonsson, O. Oskarsson, M. Christiansen, L. Blou, I. Laursen, and C. Koch. 2004. Human plasma-derived mannose-binding lectin: a phase I safety and pharmacokinetic study. Scand. J. Immunol. 59:97-102. [DOI] [PubMed] [Google Scholar]
- 462.Vallinoto, A. C., M. R. Menezes-Costa, A. E. Alves, L. F. Machado, V. N. de Azevedo, L. L. Souza, O. Ishak Mde, and R. Ishak. 2006. Mannose-binding lectin gene polymorphism and its impact on human immunodeficiency virus 1 infection. Mol. Immunol. 43:1358-1362. [DOI] [PubMed] [Google Scholar]
- 463.Vallinoto, A. C., N. A. Muto, A. E. Alves, L. F. Machado, V. N. Azevedo, L. L. Souza, M. O. Ishak, and R. Ishak. 2008. Characterization of polymorphisms in the mannose-binding lectin gene promoter among human immunodeficiency virus 1 infected subjects. Mem. Inst. Oswaldo Cruz 103:645-649. [DOI] [PubMed] [Google Scholar]
- 464.van Deuren, M., J. van der Ven-Jongekrijg, A. K. Bartelink, R. van Dalen, R. W. Sauerwein, and J. W. van der Meer. 1995. Correlation between proinflammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. J. Infect. Dis. 172:433-439. [DOI] [PubMed] [Google Scholar]
- 465.van Emmerik, L. C., E. J. Kuijper, C. A. Fijen, J. Dankert, and S. Thiel. 1994. Binding of mannan-binding protein to various bacterial pathogens of meningitis. Clin. Exp. Immunol. 97:411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.van Till, J. W., P. W. Modderman, M. de Boer, M. H. Hart, M. G. Beld, and M. A. Boermeester. 2008. Mannose-binding lectin deficiency facilitates abdominal Candida infections in patients with secondary peritonitis. Clin. Vaccine Immunol. 15:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Virji, M. 2009. Pathogenic neisseriae: surface modulation, pathogenesis and infection control. Nat. Rev. Microbiol. 7:274-286. [DOI] [PubMed] [Google Scholar]
- 468.Vogel, U., H. Claus, L. von Muller, D. Bunjes, J. Elias, and M. Frosch. 2004. Bacteremia in an immunocompromised patient caused by a commensal Neisseria meningitidis strain harboring the capsule null locus (cnl). J. Clin. Microbiol. 42:2898-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Vogel, U., and M. Frosch. 1999. Mechanisms of neisserial serum resistance. Mol. Microbiol. 32:1133-1139. [DOI] [PubMed] [Google Scholar]
- 470.Waage, A., P. Brandtzaeg, A. Halstensen, P. Kierulf, and T. Espevik. 1989. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J. Exp. Med. 169:333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Waghorn, D. J. 2001. Overwhelming infection in asplenic patients: current best practice preventive measures are not being followed. J. Clin. Pathol. 54:214-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Waisbren, B. A., and I. Brown. 1966. A factor in the serum of patients with persisting infection that inhibits the bactericidal activity of normal serum against the organism that is causing the infection. J. Immunol. 97:431-437. [PubMed] [Google Scholar]
- 473.Walport, M. J. 2002. Complement and systemic lupus erythematosus. Arthritis Res. 4(Suppl. 3):S279-S293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Walport, M. J., K. A. Davies, and M. Botto. 1998. C1q and systemic lupus erythematosus. Immunobiology 199:265-285. [DOI] [PubMed] [Google Scholar]
- 475.Wang, X., D. T. Fleischer, W. T. Whitehead, D. L. Haviland, S. I. Rosenfeld, J. P. Leddy, R. Snyderman, and R. A. Wetsel. 1995. Inherited human complement C5 deficiency. Nonsense mutations in exons 1 (Gln1 to Stop) and 36 (Arg1458 to Stop) and compound heterozygosity in three African-American families. J. Immunol. 154:5464-5471. [PubMed] [Google Scholar]
- 476.Weis, W. I., K. Drickamer, and W. A. Hendrickson. 1992. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature 360:127-134. [DOI] [PubMed] [Google Scholar]
- 477.Weiss, S. J., A. E. Ahmed, and V. R. Bonagura. 1998. Complement factor D deficiency in an infant first seen with pneumococcal neonatal sepsis. J. Allergy Clin. Immunol. 102:1043-1044. [DOI] [PubMed] [Google Scholar]
- 478.Wessels, M. R., and D. L. Kasper. 1989. Antibody recognition of the type 14 pneumococcal capsule. Evidence for a conformational epitope in a neutral polysaccharide. J. Exp. Med. 169:2121-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Westberg, J., G. N. Fredrikson, L. Truedsson, A. G. Sjoholm, and M. Uhlen. 1995. Sequence-based analysis of properdin deficiency: identification of point mutations in two phenotypic forms of an X-linked immunodeficiency. Genomics 29:1-8. [DOI] [PubMed] [Google Scholar]
- 480.White, D. J., J. Talarico, H. G. Chang, G. S. Birkhead, T. Heimberger, and D. L. Morse. 1998. Human babesiosis in New York State: review of 139 hospitalized cases and analysis of prognostic factors. Arch. Intern. Med. 158:2149-2154. [DOI] [PubMed] [Google Scholar]
- 481.White, M. R., E. Crouch, D. Chang, K. Sastry, N. Guo, G. Engelich, K. Takahashi, R. A. Ezekowitz, and K. L. Hartshorn. 2000. Enhanced antiviral and opsonic activity of a human mannose-binding lectin and surfactant protein D chimera. J. Immunol. 165:2108-2115. [DOI] [PubMed] [Google Scholar]
- 482.WHO. 2006. WHO position paper on Haemophilus influenzae type b conjugate vaccines (replaces WHO position paper on Hib vaccines previously published in the Weekly Epidemiological Record). Wkly. Epidemiol. Rec 81:445-452. [PubMed] [Google Scholar]
- 483.Wiesmann, C., K. J. Katschke, J. Yin, K. Y. Helmy, M. Steffek, W. J. Fairbrother, S. A. McCallum, L. Embuscado, L. DeForge, P. E. Hass, and M. van Lookeren Campagne. 2006. Structure of C3b in complex with CRIg gives insights into regulation of complement activation. Nature 444:217-220. [DOI] [PubMed] [Google Scholar]
- 484.Witzel-Schlomp, K., P. J. Spath, M. J. Hobart, B. A. Fernie, C. Rittner, T. Kaufmann, and P. M. Schneider. 1997. The human complement C9 gene: identification of two mutations causing deficiency and revision of the gene structure. J. Immunol. 158:5043-5049. [PubMed] [Google Scholar]
- 485.Wong, S. H., D. R. Lennon, C. M. Jackson, J. M. Stewart, S. Reid, E. Ypma, J. M. O'Hallahan, P. Oster, K. Mulholland, and D. R. Martin. 2009. Immunogenicity and tolerability in infants of a New Zealand epidemic strain meningococcal B outer membrane vesicle vaccine. Pediatr. Infect. Dis. J. 28:385-390. [DOI] [PubMed] [Google Scholar]
- 486.Wurzner, R. 1999. Evasion of pathogens by avoiding recognition or eradication by complement, in part via molecular mimicry. Mol. Immunol. 36:249-260. [DOI] [PubMed] [Google Scholar]
- 487.Wurzner, R., M. J. Hobart, B. A. Fernie, D. Mewar, P. C. Potter, A. Orren, and P. J. Lachmann. 1995. Molecular basis of subtotal complement C6 deficiency. A carboxy-terminally truncated but functionally active C6. J. Clin. Invest. 95:1877-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Wurzner, R., K. Tokunaga, R. Nitze, O. Gotze, and P. J. Lachmann. 1991. C7*N is a hypomorphic allele of the human complement c7 M/N protein polymorphism. Exp. Clin. Immunogenet. 8:177-183. [PubMed] [Google Scholar]
- 489.Wurzner, R., K. Witzel-Schlomp, K. Tokunaga, B. A. Fernie, M. J. Hobart, and A. Orren. 1998. Reference typing report for complement components C6, C7 and C9 including mutations leading to deficiencies. Exp. Clin. Immunogenet. 15:268-285. [DOI] [PubMed] [Google Scholar]
- 490.Xu, W., S. P. Berger, L. A. Trouw, H. C. de Boer, N. Schlagwein, C. Mutsaers, M. R. Daha, and C. van Kooten. 2008. Properdin binds to late apoptotic and necrotic cells independently of C3b and regulates alternative pathway complement activation. J. Immunol. 180:7613-7621. [DOI] [PubMed] [Google Scholar]
- 491.Yang, Y., E. K. Chung, Y. L. Wu, S. L. Savelli, H. N. Nagaraja, B. Zhou, M. Hebert, K. N. Jones, Y. Shu, K. Kitzmiller, C. A. Blanchong, K. L. McBride, G. C. Higgins, R. M. Rennebohm, R. R. Rice, K. V. Hackshaw, R. A. Roubey, J. M. Grossman, B. P. Tsao, D. J. Birmingham, B. H. Rovin, L. A. Hebert, and C. Y. Yu. 2007. Gene copy-number variation and associated polymorphisms of complement component C4 in human systemic lupus erythematosus (SLE): low copy number is a risk factor for and high copy number is a protective factor against SLE susceptibility in European Americans. Am. J. Hum. Genet. 80:1037-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Yang, Y., E. K. Chung, B. Zhou, K. Lhotta, L. A. Hebert, D. J. Birmingham, B. H. Rovin, and C. Y. Yu. 2004. The intricate role of complement component C4 in human systemic lupus erythematosus. Curr. Dir. Autoimmun. 7:98-132. [DOI] [PubMed] [Google Scholar]
- 493.Yarden, J., D. Radojkovic, K. De Boeck, M. Macek, Jr., D. Zemkova, V. Vavrova, R. Vlietinck, J. J. Cassiman, and H. Cuppens. 2004. Polymorphisms in the mannose binding lectin gene affect the cystic fibrosis pulmonary phenotype. J. Med. Genet. 41:629-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Yazdankhah, S. P., and D. A. Caugant. 2004. Neisseria meningitidis: an overview of the carriage state. J. Med. Microbiol. 53:821-832. [DOI] [PubMed] [Google Scholar]
- 495.Ying, S. C., A. T. Gewurz, H. Jiang, and H. Gewurz. 1993. Human serum amyloid P component oligomers bind and activate the classical complement pathway via residues 14-26 and 76-92 of the A chain collagen-like region of C1q. J. Immunol. 150:169-176. [PubMed] [Google Scholar]
- 496.Yu, C. Y., G. Hauptmann, Y. Yang, Y. L. Wu, D. J. Birmingham, B. H. Rovin, and L. A. Hebert. 2007. Complement deficiencies in human systemic lupus erythematosus (SLE) and SLE nephritis: epidemiology and pathogenesis, p. 203-213. In G. C. Tsokos (ed.), Systemic lupus erythematosus: a companion to rheumatology. Elsevier, Philadelphia, PA.
- 497.Zhu, Z. B., K. Totemchokchyakarn, T. P. Atkinson, and J. E. Volanakis. 1998. Molecular defects leading to human complement component C6 deficiency in an African-American family. Clin. Exp. Immunol. 111:91-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Zimmerman, P. A., J. Fitness, J. M. Moulds, D. T. McNamara, L. J. Kasehagen, J. A. Rowe, and A. V. Hill. 2003. CR1 Knops blood group alleles are not associated with severe malaria in the Gambia. Genes Immun. 4:368-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Zinneman, H. H., H. Glenchur, and W. H. Hall. 1959. The nature of blocking antibodies in human brucellosis. J. Immunol. 83:206-212. [PubMed] [Google Scholar]