Abstract
New or improved vaccines against dengue virus types 1 to 4 (DENV1 to DENV4) and Japanese encephalitis virus (JEV), the causative agents of dengue fever and Japanese encephalitis (JE), respectively, are urgently required. The use of noninfectious subviral extracellular particles (EPs) is an inexpensive and safe strategy for the production of protein-based flavivirus vaccines. Although coexpression of premembrane (prM) and envelope (E) proteins has been demonstrated to produce EPs in mammalian cells, low yields have hindered their commercial application. Therefore, we used an insect cell expression system with Spodoptera frugiperda-derived Sf9 cells to investigate high-level production of DENV2 and JEV EPs. Sf9 cells transfected with the prM and E genes of DENV2 or JEV secreted corresponding viral antigens in a particulate form that were biochemically and biophysically equivalent to the authentic antigens obtained from infected C6/36 mosquito cells. Additionally, equivalent neutralizing antibody titers were induced in mice immunized either with EPs produced by transfected Sf9 cells or with EPs produced by transfected mammalian cells, in the context of coimmunization with a DNA vaccine that expresses EPs. Furthermore, the results of an enzyme-linked immunosorbent assay (ELISA) using an EP antigen derived from Sf9 cells correlated significantly with the results obtained by a neutralization test and an ELISA using an EP antigen derived from mammalian cells. Finally, Sf9 cells could produce 10- to 100-fold larger amounts of E antigen than mammalian cells. These results indicate the potential of Sf9 cells for high-level production of flavivirus protein vaccines and diagnostic antigens.
Dengue virus types 1 to 4 (DENV1-4) and Japanese encephalitis virus (JEV), the causative agents of dengue fever and Japanese encephalitis (JE), respectively, are globally important human pathogens (10) for which new or improved vaccines are urgently required. DENV1-4 cause dengue fever and dengue hemorrhagic fever in tropical areas and many subtropical areas. An estimated 50 million to 100 million dengue cases occur annually, with 2.5 billion people at risk of infection (11). However, there is no approved vaccine for dengue diseases, and the development of such a vaccine is urgently needed (12). JEV is the single largest cause of childhood viral encephalitis in the world, with an estimated 50,000 cases annually. Mortality rates can reach 30% among confirmed cases, and as many as one-third of survivors suffer from permanent and severe psychoneurological sequelae (13, 39). Although inactivated vaccines are used internationally for JE, they are too expensive for widespread use in most developing countries (3), and therefore, more cost-effective alternatives are needed.
Neutralizing antibodies are important in host protection against dengue diseases and JE (10, 34). For JE, previously used mouse brain-derived (16, 44) and more recently used Vero cell-derived (20, 25, 35) inactivated vaccines can efficiently induce neutralizing antibody responses. However, these protein-based vaccines are produced from infectious agents, and their production therefore requires biosafety level 2 or 3 containment facilities and complex purification protocols, thus increasing the cost of the vaccine. Vaccine production without infective procedures can be achieved using genetic engineering techniques (29, 55).
DENV1-4 and JEV are members of the genus Flavivirus in the family Flaviviridae (37). The envelope (E) protein is the major component of the envelopes of flavivirus virion particles and possesses most of the neutralizing epitopes (46). The other protein on the envelopes of mature virions is the membrane (M) protein, which is synthesized as the precursor membrane (prM) protein in infected cells. Cells expressing flavivirus prM and E proteins are known to secrete nucleocapsid-free subviral extracellular particles (EPs), which are similar to slowly sedimenting hemagglutinin (SHA) particles secreted from flavivirus-infected cells (47). EPs of JEV synthesized in mammalian expression systems have been evaluated for their immunogenicity and/or protective efficacy in mice (15, 24, 43). Two of these studies (24, 43) demonstrated that the EPs induced neutralizing antibodies at levels comparable to those induced by an inactivated JE vaccine. In our laboratory, mammalian cell lines continuously expressing EPs of dengue type 2 virus (DENV2) (26) or JEV (27) have been generated and designated D cells and F cells, respectively. The EPs contained an E protein that was antigenically and biochemically equivalent to the authentic E protein, and the EPs were immunogenic and protective in mice. However, the yields of viral antigens produced from D and F cells were low and would not meet the requirements for commercial vaccine production. Increasing the levels of viral antigen production from transfected cells would reduce the cost of vaccine preparation.
Recently, insect cell expression systems have been increasingly used in various fields of medical sciences (1, 6, 17, 53). In general, insect cells are easier to cultivate than mammalian cells, because they often do not require serum supplementation in the culture medium or incubation under CO2. In addition, insect cells can be adapted to suspension culture, allowing cultures to be simply scaled up. Furthermore, various techniques have been developed for high-density culture of insect cells; for example, in one study, the immobilization of insect cells within biomass support particles achieved a density of approximately 3 × 107 cells/cm3 (50). Thus, the insect cell expression system can be a simple and inexpensive strategy for vaccine antigen production.
In addition to their use as vaccine antigens, EPs derived from mammalian cells could be used as serodiagnostic antigens (27, 32). The production of serodiagnostic antigens may also encounter problems when the antigens are sourced directly from infectious agents. Currently, numerous commercial assays utilizing several different formats, such as the immunochromatography test and the IgM capture enzyme-linked immunosorbent assay (ELISA), are available for the diagnosis of DENV and JEV infections (4, 49). These commercial tests use viral antigens derived from transfected or infected cultured cells. Thus, the application of insect cell-derived EPs as diagnostic antigens would be an attractive alternative.
In this study, we produced EPs of DENV2 and JEV in a transient expression system using the Sf9 cell line, which was derived from the pupal ovarian tissue of the fall armyworm, Spodoptera frugiperda. These proteins were evaluated for vaccine and diagnostic antigens, mainly by direct comparison with mammalian-cell-derived EPs. The EPs produced from Sf9 cells were immunogenic in mice and useful as antigens for ELISA. In addition, Sf9 cells produced larger amounts of antigen than CHO cells, suggesting the potential applicability of insect cells for the production of DENV2 and JEV antigens for vaccines and serodiagnostic tests.
MATERIALS AND METHODS
Cells.
Sf9 cells were purchased from Novagen (Darmstadt, Germany) and cultivated in BacVector-insect cell medium (Novagen). A mosquito cell line, C6/36, and mammalian cell lines, Vero and CHO-K1, have been described previously (27). Briefly, the growth medium was Eagle's minimal essential medium (MEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and kanamycin (60 μg/ml); for C6/36 and CHO-K1 cells, nonessential amino acids were also added. D and F cells, which are CHO-K1 cells genetically modified to continuously express EPs of DENV2 or JEV, respectively (26, 27), were grown in the same medium as CHO-K1 cells. Cells were cultivated at 28°C for insect cells or at 37°C for mammalian cells under a humidified atmosphere of 5% CO2. The maintenance medium used for mammalian and C6/36 cells following transfection or infection was the growth medium containing 0.1% bovine serum albumin (BSA) in place of 10% FBS.
Viruses.
The New Guinea C (NGC) strain of DENV2 (26) and the Nakayama strain of JEV (41) were used. Culture fluids harvested from C6/36 cells infected with each of these viruses were used for biochemical characterizations and neutralization tests. The virulent Beijing P3 strain of JEV, in the form of a 10% suckling mouse brain homogenate, was used for mouse challenge experiments (41).
Plasmids.
The pcDNA3-based plasmids encoding the prM and E proteins of DEN2V strain NGC (designated pcD2ME [26]) or JEV strain Nakayama (designated pcJEME [27]) have been described previously. To construct appropriate expression plasmids for the insect cells, the prM and E genes were excised from the pcD2ME plasmid and were inserted into the pIB/V5His vector (Invitrogen, San Diego, CA) using the EcoRV and XhoI sites; this construct was designated pIBD2ME. Similarly, the prM and E genes were excised from the pcJEME plasmid and were inserted into the pIB/V5His vector using the EcoRI and XhoI sites; this construct was designated pIBJEME. Plasmid inserts were confirmed by sequencing. All plasmid DNAs (pcDNA3, pcD2ME, pcJEME, pIBD2ME, and pIBJEME) were purified using a Qiagen plasmid kit (Qiagen, Hilden, Germany).
Antibodies.
Rabbit polyclonal antibodies to DENV2 (26) or JEV (21) have been described previously. Briefly, these were obtained by repeated immunization of a rabbit with a virion fraction that was dissociated with 0.05% Triton X-100. Mouse monoclonal antibodies reactive with JEV antigens were JE-10B4 (specific for JEV E) (30) and J2-2F1 (specific for JEV prM/M) (40) (provided by Mary K. Gentry of the Walter Reed Army Institute of Research, Washington, DC), while those reactive with dengue virus antigens were D3-2H2 (specific for prM) (14), D2-3H5 (specific for E) (9), and D1-4G2 (specific for E) (14) (obtained from hybridomas purchased from the American Type Culture Collection, Manassas, VA).
Sera.
The serum samples used for evaluating EPs as ELISA antigens were those previously obtained from mice immunized with pcD2ME or pcJEME (28).
Transfection.
Sf9 cells were transfected with 1 to 4 μg of pIBD2ME or pIBJEME by using the FuGENE HD transfection reagent (Roche, Mannheim, Germany), according to the manufacturer's instructions. Similarly, CHO-K1 cells were transfected with 1 to 4 μg of pcD2ME or pcJEME using the same transfection reagent. For investigation of the yield, these cells were then incubated in growth medium. For the preparation of immunogens, transfected cells were incubated in the maintenance medium.
Immunochemical staining.
Immunochemical staining was performed essentially as described previously (27). Briefly, cells were fixed with a mixture of cold methanol and acetone (1:1) and were then dried. These cells were then incubated serially with monoclonal antibodies to JEV or DENV2, biotinylated anti-mouse IgG, ABC (avidin-biotinylated peroxidase complex) reagents, and the VIP substrate (Vector Laboratories, Inc., Burlingame, CA).
Sedimentation analysis.
Samples were applied to a 10 to 40% (wt/wt) or a 10 to 50% continuous sucrose density gradient prepared in TN buffer (10 mM Tris-HCl [pH 7.5], 100 mM NaCl). Following centrifugation at 55,000 rpm for 90 min at 4°C in the S55S rotor of a Himac CS100GX micro-ultracentrifuge (Hitachi Koki, Ibaraki, Japan), fractions were collected from the bottom of the tube. Each fraction was tested for the level of E antigen by ELISA (see below) and for infectivity by using a plaque assay on Vero cell monolayers.
Purification of extracellular particles.
EPs were purified based on a method described previously (27). Briefly, culture supernatants harvested from transfected cells were clarified and precipitated with 10% polyethylene glycol (PEG; molecular mass, approximately 6,000 Da). Following centrifugation, the pellets were suspended in TN buffer and were applied to a sucrose density gradient (see above). The collected fractions were examined for E antigen levels by ELISA (see below). The fractions containing the highest and the second highest levels of E antigen were used as purified EPs. EPs obtained from Sf9 cells transfected with pIBD2ME or pIBJEME were designated D2EP-Sf9 or JEEP-Sf9, respectively. Similarly, those obtained from CHO-K1 cells transfected with pcD2ME or pcJEME were designated D2EP-CHO or JEEP-CHO, respectively. Those obtained with D cells and F cells were designated D2EP-D and JEEP-F, respectively.
ELISA for quantification of E antigen.
The DENV2 and JEV E antigens were quantified by a sandwich ELISA as previously described (27). Briefly, 96-well microplates sensitized with a rabbit polyclonal antibody were serially incubated with samples at a dilution of 1:10 (for sucrose gradient fractions) or at serial 2-fold dilutions (for culture fluid samples), a monoclonal anti-E antibody (D2-3H5 or JE-10B4), alkaline phosphatase-conjugated anti-mouse IgG, and p-nitrophenyl phosphate. Antigen levels were calculated from the absorbance values obtained with the sample and a reference standard. The reference standard was prepared with D2EP-D or JEEP-F fractions, and the amount of E protein contained in the reference standard was estimated by comparison of an E preparation concentrated from the reference standard with the BSA samples on silver-stained gels.
Endoglycosidase treatment.
N-Glycosidase F (PNGase F; Roche Diagnostics, Basel, Switzerland) was used according to the manufacturer's instructions, with some modifications. Purified EPs were boiled for 5 min with sodium dodecyl sulfate (SDS) at a final concentration of 1%. After cooling, the sample was divided into two equal aliquots and was mixed with the “reaction” buffer to make final concentrations of 64 mM phosphate buffer (pH 6.4), 50 mM EDTA, and 1% Triton X-100. One unit of PNGase F was then added to one of the two aliquots. Both were incubated at 37°C for 24 h. For inactivation of PNGase F, the samples were incubated at 65°C for 20 min.
Immunoprecipitation.
The dengue viral antigens were immunoprecipitated with antibody D1-4G2 by using protein A-agarose (Life Technologies, Gaithersburg, MD) according to the manufacturer's instructions. The precipitated proteins were eluted in electrophoresis buffer, separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and detected by Western blotting (see below).
Western blotting.
Western blotting was performed essentially as described previously (33). Briefly, samples were run on standard Laemmli gels under nonreducing conditions, transferred to polyvinylidene difluoride membranes (Millipore Corporation, Bedford, MA), and then detected by monoclonal antibodies to the E or prM protein of DENV2 or JEV.
Mouse experiment.
Groups of five or six 4-week-old male ICR mice (CLEA Japan, Tokyo, Japan) were inoculated twice or three times with purified EPs or with EPs mixed with DNA plasmids by using a spring-powered needle-free jet injector (ShimaJET; Shimadzu Corp., Kyoto, Japan). The DNA plasmids used were pcDNA3, pcD2ME, and pcJEME. Mice were bled retro-orbitally, and individual sera were examined for neutralizing antibodies, unless otherwise specified. For protection experiments, immunized mice were challenged intraperitoneally with 100 50% lethal doses (LD50) of the Beijing P3 strain of JEV 10 weeks after the first immunization. The challenged mice were observed for survival daily for 3 weeks. All of the animal experiments were conducted according to the Guidelines for Animal Experimentation at the Kobe University Graduate School of Medicine.
Neutralization test.
Neutralizing antibody titers were determined essentially as described previously, by using plaque reduction assays performed with DENV2 strain NGC or JEV strain Nakayama in the presence of rabbit complement (19). The plaques were visualized by immunochemical staining (see above). The neutralizing antibody titers were expressed as the maximum serum dilution yielding a 70% reduction in plaque numbers.
ELISA for quantification of antibodies to DENV2 or JEV.
An ELISA to quantify IgG antibodies to DENV2 or JEV was performed as described previously (31). Briefly, microplates were sensitized by incubation first with rabbit hyperimmune sera against either DENV2 or JEV and then with a DENV2 or JEV antigen. Subsequently, the microplates were incubated serially with test sera (dilution, 1:100), alkaline phosphatase-conjugated anti-mouse IgG, and p-nitrophenyl phosphate. The antigens—D2EP-Sf9, D2EP-D, JEEP-Sf9, and JEEP-F—were used at 5 ng/well. To eliminate nonspecific reactions, the absorbances obtained with nonsensitized wells were subtracted from those obtained with antigen-sensitized wells. The cutoff value, differentiating positive from negative sera, was determined by the mean plus 3 standard deviations (SDs) obtained with 14 naïve mouse sera.
Statistical analysis.
Statistical analysis was performed using Student's t test, Fisher's exact test, or Pearson's correlation coefficient test. Probability levels (P) of <0.05 were considered significant.
RESULTS
In vitro expression of pIBD2ME and pIBJEME in Sf9 cells.
Following transfection with pIBD2ME or pIBJEME, 10 to 30% of cells were stained with the monoclonal antibodies specific for E or prM proteins (data not shown). This result indicated that pIBD2ME and pIBJEME are able to express E and prM proteins in Sf9 cells.
Biophysical/biochemical characterization of E antigens produced by Sf9 cells.
Sedimentation analyses of the antigens released from pIBD2ME- or pIBJEME-transfected Sf9 cells were performed. Although these data are not shown, these analyses identified a peak corresponding to the E antigen in the Sf9 samples which coincided with that detected in pcD2ME- or pcJEME-transfected CHO cells and with the SHA particles obtained from DENV2- or JEV-infected C6/36 cells. Western blot analyses of the extracellular antigens indicated that for both DENV2 and JEV, the E protein contained in the Sf9 samples comigrated in SDS gels with the same component found in the transfected CHO or infected C6/36 samples, when these E protein samples were treated with PNGase F (data not shown). These results indicated that DENV2 and JEV antigens produced by Sf9 cells were produced in a particulate form and were equivalent to those produced by mammalian cells (CHO) and the cells of another insect (C6/36).
Immunogenicity of EPs produced by Sf9 cells.
To evaluate the potential of insect cell-derived EPs to be used as vaccine antigens, these EPs were examined for their ability to induce neutralizing antibodies in mice. Two groups of six mice were immunized twice, with an interval of 6 weeks between immunizations, with 1 μg of JEEP-Sf9 either alone or mixed with 7.3 μg of pcDNA3, used as a CpG adjuvant. Three weeks after the second immunization, mice developed neutralizing antibody titers of 1:80 (without adjuvant) and 1:320 (with adjuvant), as determined from pooled sera (data not shown). Another experiment was performed with a group of six mice immunized three times at intervals of 2 weeks with a mixture of 100 ng of D2EP-Sf9 and 36.5 μg of pcDNA3. One week after the third immunization, sera pooled from these mice showed a neutralizing antibody titer of 1:20 (data not shown). Despite the limited number of animals used in these experiments, these results indicated the ability of EPs derived from insect cells to elicit neutralizing antibodies in mice.
To further evaluate the vaccine potential of insect cell-derived EPs, their ability to induce neutralizing antibodies was compared with that of mammalian-cell-derived EPs. For this evaluation, EPs were coinoculated with DNA vaccines: either pcD2ME or pcJEME. Specifically, groups of five mice were immunized twice, with a 6-week interval between immunizations, with either 100 ng of D2EP-Sf9 mixed with 50 μg of pcD2ME or 1 μg of JEEP-Sf9 mixed with 10 μg of pcJEME. In addition, the same amounts of EPs derived from CHO cells transiently or continuously expressing EPs (D2EP-CHO, D2EP-D, JEEP-CHO, JEEP-F) were used in place of insect cell-derived EPs. For reference, groups of five mice were immunized with pcD2ME or pcJEME alone. Furthermore, six mice were inoculated with phosphate-buffered saline (PBS) as a control and were used in the subsequent challenge experiment.
The time course of the mean antibody titers obtained by coimmunization of pcD2ME with D2EP-Sf9 was comparable to those obtained with D2EP-D and D2EP-CHO (Table 1). The titers at weeks 7 and 9 were approximately 8- to 16-fold higher than those obtained by immunization with pcD2ME alone, and the differences were significant (P, <0.001). Similarly, neutralizing antibody titers induced by coimmunization with pcJEME and JEEP-Sf9 were equivalent to those induced by coimmunization with pcJEME and either JEEP-F or JEEP-CHO. The mean antibody titers at week 9 were approximately 8-fold higher than that obtained by immunization with pcJEME alone. Significant differences in titers between mice immunized with DNA alone and those coimmunized with any EP preparation, except for mice coimmunized with pcJEME and JEEP-CHO, were detected at weeks 7 and 9. These results indicated that EPs produced by Sf9 cells can induce neutralizing antibodies in mice at levels similar to those induced by EPs produced by mammalian cells, when assessed in the context of coimmunization with DNA vaccines.
TABLE 1.
Evaluation of Sf9 cell-derived EPs for immunogenicity and protective capacity in micea
| Expt no. | Immunogenb |
No. of mice | Neutralizing antibody titerc at: |
% Survivale (no. alive/total no.) | ||||
|---|---|---|---|---|---|---|---|---|
| EPs | DNA | Wk 3d | Wk 5 | Wk 7 | Wk 9 | |||
| 1 | D2EP-Sf9 | pcD2ME | 5 | <1:10 | <1:10 | 1:226*** | 1:160*** | — |
| D2EP-CHO | pcD2ME | 5 | <1:10 | <1:10 | 1:453*** | 1:320*** | — | |
| D2EP-D | pcD2ME | 5 | <1:10 | <1:10 | 1:320*** | 1:453*** | — | |
| pcD2ME | 5 | <1:10 | <1:10 | 1:20 | 1:20 | — | ||
| 2 | JEEP-Sf9 | pcJEME | 5 | 1:10 | 1:10 | 1:844*** | 1:844* | 100 (5/5)* |
| JEEP-CHO | pcJEME | 5 | <1:10 | 1:11 | 1:184 | 1:735 | 100 (5/5)* | |
| JEEP-F | pcJEME | 5 | <1:10 | 1:20 | 1:557** | 1:1,110* | 100 (5/5)* | |
| pcJEME | 5 | 1:15 | <1:10 | 1:106 | 1:121 | — | ||
| PBS | 6 | <1:10 | <1:10 | <1:10 | <1:10 | 16.7 (1/6) | ||
Groups of 4-week-old male ICR mice were immunized twice, with an interval of 6 weeks between immunizations, with the indicated immunogens. As a control for the challenge experiment, mice were inoculated with PBS (Expt 2).
The doses of EPs (E amounts) were 100 ng for D2EPs and 1 μg for JEEPs. The doses of DNA vaccines were 50 μg for pcD2ME and 10 μg for pcJEME.
Represented as the maximum serum dilution yielding a 70% reduction in plaque number. Neutralizing antibody titers against DENV2 were obtained with pooled sera and are represented as the geometric mean titer (GMT) obtained from two separate assays (Expt 1). Neutralizing antibody titers against JEV were obtained with individual sera and are represented as the GMT (Expt 2). When GMTs were calculated, titers below the detection limit (<1:10) were assigned the value of 1:5. Asterisks indicate significant differences from antibody levels induced by DNA alone (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
wk, weeks after the first immunization.
Survival 3 weeks after challenge with 100 LD50 of the Beijing P3 strain of JEV. —, not done. Asterisks indicate significant differences from the survival rate of the PBS-inoculated group (P < 0.05).
The protective capacity of induced neutralizing antibodies was investigated by a challenge experiment (Table 1). For this experiment, mice immunized with a mixture of JEV-EPs and pcJEME were used 10 weeks after the first immunization. All of the immunized mice were fully protected from lethal JEV challenge. Although this challenge dose resulted in one survivor in the PBS-inoculated group, the survival rates were significantly different between any vaccinated group (100%) and the PBS-inoculated group (16.7%: P, <0.05 by Fisher's exact test). These results indicated that neutralizing antibodies induced in mice by Sf9-derived EPs showed a level of protection equal to that of EPs produced by mammalian cells under our experimental conditions.
Applicability of Sf9 cell-derived EP antigens to ELISA.
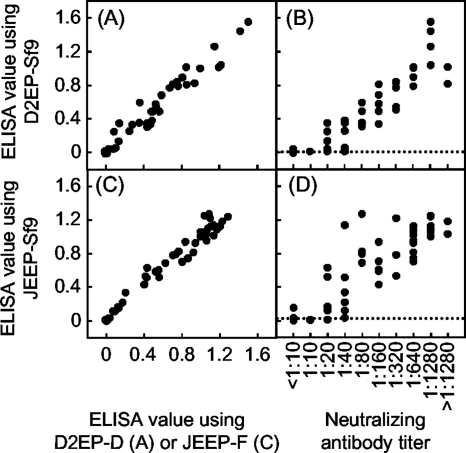
To evaluate the applicability of EPs produced by insect cells as antigens in immunodiagnostic tests, Sf9 cell-derived EP antigens were tested in a conventional ELISA and compared with EP antigens derived from mammalian cells (Fig. 1). For each of the antigens, D2EP-Sf9 or JEEP-Sf9, 50 mouse sera with known neutralizing antibody titers were used. Comparison of the ELISA reactivities against D2EP-Sf9 and D2EP-D provided a high correlation coefficient of 0.979 (P, <0.001). Also, a high correlation coefficient of 0.984 (P, <0.001) was obtained between ELISA values against the JEEP-Sf9 and JEEP-F antigens. These results indicated that EPs derived from Sf9 cells were antigenically equivalent to those derived from mammalian cells. Furthermore, ELISA values obtained with D2EP-Sf9 or JEEP-Sf9 antigens were significantly correlated with the titers of neutralizing antibodies against DENV2 and JEV, with correlation coefficients of 0.914 (P, <0.001) and 0.846 (P, <0.001), respectively. The consistency of the results obtained by ELISA using D2EP-Sf9 with those obtained by the neutralization test was 92.2%, with the ELISA showing 94.9% sensitivity and 88.0% specificity by comparison with the neutralization test. A similar analysis using JEEP-Sf9 antigens showed 92.2% consistency, with 93.3% sensitivity and 89.5% specificity. Although our ELISA was slightly less sensitive than the neutralization test under the present assay conditions, the significantly high correlation and consistency with this representative serodiagnostic method indicated the potential applicability of the Sf9-cell-derived EPs in immunodiagnostic tests.
FIG. 1.
Evaluation of EPs derived from Sf9 cells for use as ELISA antigens. The ELISA using Sf9 cell-derived EP antigens was compared with the ELISA using CHO cell-derived EP antigens (A and C) and with the neutralization test (B and D), using 50 mouse sera. The dotted lines indicate the cutoff levels in the ELISA to differentiate positive from negative samples: 0.008 for DENV2 and 0.014 for JEV. This cutoff level was calculated from ELISA absorbance values obtained using 14 naïve mouse sera (mean plus 3 SDs).
Yield of EPs from transiently transfected Sf9 cells.
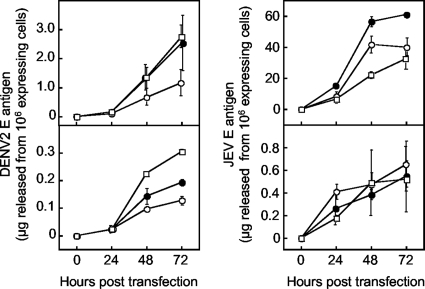
Since one of the advantages of using insect cells is that they are able to produce recombinant proteins in larger amounts than mammalian cells, the yields of E antigen from Sf9 cells were compared with those obtained from CHO cells in a transient expression system. Following transfection with varying amounts (1 to 4 μg) of plasmids, culture supernatant samples were collected at 24, 48, and 72 h, and the amount of E antigen in the culture fluids was determined by ELISA. As shown in Fig. 2, Sf9 cells produced roughly 10-fold-higher levels of DENV2 EPs than CHO cells at 72 h posttransfection. For JEV EPs, the levels of production of EPs by Sf9 cells were markedly higher than those by CHO cells. At 72 h posttransfection, the highest yield obtained from Sf9 cells (transfected with 2 μg of pIBJEME) was approximately 100-fold higher than that from CHO cells (transfected with 1 μg of pcJEME). The expression level of intracellular E protein was approximately 25-fold higher than that of extracellular E protein in pIBD2ME-transfected Sf9 cells, whereas it was only 2-fold higher in pcD2ME-transfected CHO cells under the same conditions, at 24 h posttransfection (data not shown).
FIG. 2.
Yields of E antigens from transiently transfected Sf9 and CHO cells. Sf9 cells (top) were transfected with pIBD2ME (left) or pIBJEME (right), while CHO cells (bottom) were transfected with pcD2ME (left) or pcJEME (right). Cells grown in 35-mm-diameter dishes were transfected with 1 (open circles), 2 (filled circles), or 4 (open squares) μg of each plasmid and were maintained in 2 ml of BacVector (Sf9 cells) or MEM containing 10% FBS (CHO cells). Three dishes were used for each experimental condition. At daily intervals, up to 3 days after transfection, 250-μl portions of culture fluids were sampled from two of the three dishes and were examined for E antigen levels by ELISA. The amounts of E antigen obtained from the two dishes were averaged. Cells in the other dishes were fixed and stained with antibody D1-4G2 or JE-10B4 at 24 h posttransfection in order to count the number of E-expressing cells. The ordinate indicates the amount of E antigen adjusted to that released from 106 E-expressing cells. The experiments were repeated twice. Each plot shows means and SDs (indicated by error bars) obtained from the two experiments.
DISCUSSION
Insect cell expression systems have been well characterized for the production of recombinant proteins and have been successfully applied in life science research and the production of pharmaceutical agents (1, 6, 17, 53). The cultivation of insect cells does not require fetal bovine serum or other components of animal origin, reducing the risk of possible contamination of the vaccine product with animal pathogens, such as transmissible spongiform encephalopathy prions. Additionally, since insects are phylogenetically very distant from humans, insect-derived cells are generally considered to be less likely to be susceptible to human-pathogenic viruses than mammalian cells. Furthermore, various posttranslational modifications, such as glycosylation, can be achieved during the biosynthesis of recombinant proteins in insect cells, since the secretion pathway in insect cells resembles that of mammalian cells. Finally, it is speculated that the cytotoxicity of viral proteins against insect cells may be lower than that against mammalian cells, since DENV2 and JEV are insect-borne viruses. Thus, insect cells are considered more favorably than mammalian cells in terms of safety and high expression efficiency for the large-scale production of flavivirus vaccines and diagnostic antigens. In this study, we used Sf9 cells to evaluate the suitability of insect cells for the production of viral antigens. Since an Sf9 cell-derived human papillomavirus vaccine has already been licensed in many countries (45), Sf9 cells are a promising candidate for vaccine production.
Various subunit vaccines to prevent flavivirus diseases have been developed using several strategies. Our previous studies have demonstrated that the prM and E proteins expressed in mammalian cells using a recombinant vaccinia virus expression system (32, 33) or a continuous expression system (26, 27) were able to form EPs, which were useful both in vaccination and in serological diagnosis. Other laboratories have established high-yield expression systems for EPs of JEV or West Nile virus using the mammalian cell lines COS-1 (5, 15), RK-13 (24, 43), and CHO-K1 (52). In addition to mammalian cells, bacterial (2, 42, 54, 57), yeast (7, 8, 38), and insect (23, 36, 48, 51, 56) cells have been used to produce flavivirus vaccine candidates; most of these studies have focused on a truncated E protein or domain III of the E protein. These vaccine candidates were able to induce neutralizing antibodies and/or protection in animals. However, the E antigen produced in these expression systems appeared to be poorly immunogenic, since relatively large doses (20 to 100 μg) of the purified antigens were generally needed to induce neutralizing antibodies in mice (2, 7, 36, 38, 42, 48, 54, 57).
The baculovirus expression system is the most frequently used system for producing vaccine candidates from insect cells, including those against flaviviruses (23, 48, 51, 56). Two studies that used the baculovirus expression system to investigate EP vaccines have been reported (48, 56). Although this virus-based system achieved high-level expression and the correct formation of flavivirus E proteins, the disadvantage is that it is difficult to produce and purify antigens on a large scale, because proteases contained in the cell lysates can cause the degradation of the viral proteins of interest. In addition, contamination by a large amount of unneeded antigens and/or infectious particles derived from the vector virus is an unavoidable problem in this system targeting intracellular antigens. On the other hand, the plasmid-based EP expression system does not use infectious virus and does not lyse cells.
In this study, the immunogenicity of insect cell-derived EPs was evaluated mainly by using mice in the context of coimmunization with DNA vaccines. Coimmunization with protein (EPs) and gene (DNA) vaccines is an effective strategy for enhancing their immunogenicity. It has been demonstrated that the ability of one type of vaccine to induce neutralizing antibodies was synergistically increased by simultaneous immunization with another type of vaccine, allowing reductions in the doses of both vaccines and consequently reducing the cost (18, 19, 22). In this study, mice immunized twice with EPs obtained from transfected Sf9 cells in combination with DNA vaccines produced neutralizing antibodies, and the antibody titers were comparable to those elicited by EPs derived from mammalian cells, indicating that the immunogenicities of EPs derived from insect and mammalian cells are equivalent.
Sf9 cells produced up to 100-fold-larger amounts of JEV EPs than CHO cells under transient-expression conditions. The highest yield of JEV E antigen, approximately 60 μg, was obtained from 106 Sf9 cells at 72 h posttransfection. Assuming that high-density culture techniques, such as biomass support particles that allow 3 × 107 cells/cm3, are used, 72-h cultivation using 1 liter of biomass support particles should yield 180 mg of JEV E antigen. Although we obtained only 10-fold higher levels of DENV2 E antigen in Sf9 cells than in CHO cells, high-level intracellular E antigen expression was observed. This result confirmed the ability of Sf9 cells to produce E antigen at a high level, even for DENV2, and indicated the potential for a further increase in the yield of extracellular E antigen.
In conclusion, Sf9 cells produced EPs of DENV2 and JEV that contained E antigens biochemically and antigenically equivalent to those expressed in mammalian cells. Additionally, EPs derived from Sf9 cells displayed immunogenicity and antigenicity equivalent to those of EPs derived from mammalian cells. Thus, the Sf9 cell expression system using a plasmid vector may be useful for the production of E antigens. DENV2 and JEV EPs obtained from this system are potentially safe, effective, low-cost candidates for the next generation of DNEV2 or JEV subunit vaccines.
Acknowledgments
This research project received financial support in part from the Research for Promoting Technological Seeds (Japan Science and Technology Agency), the Research on Publicly Essential Drugs and Medical Devices (Japan Health Sciences Foundation), and the Research on Emerging and Re-emerging Infectious Diseases (Ministry of Health, Labor, and Welfare of Japan [H21-shinkou-ippan-005]).
Footnotes
Published ahead of print on 28 July 2010.
REFERENCES
- 1.Altmann, F., E. Staudacher, I. B. Wilson, and L. März. 1999. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj. J. 16:109-123. [DOI] [PubMed] [Google Scholar]
- 2.Babu, J. P., P. Pattnaik, N. Gupta, A. Shrivastava, M. Khan, and P. V. Lakshmana Rao. 2008. Immunogenicity of a recombinant envelope domain III protein of dengue virus type-4 with various adjuvants in mice. Vaccine 26:4655-4663. [DOI] [PubMed] [Google Scholar]
- 3.Bharati, K., and S. Vrati. 2006. Japanese encephalitis: development of new candidate vaccines. Expert Rev. Anti Infect. Ther. 4:313-324. [DOI] [PubMed] [Google Scholar]
- 4.Cuzzubbo, A. J., T. P. Endy, A. Nisalak, S. Kalayanarooj, D. W. Vaughn, S. A. Ogata, D. E. Clements, and P. L. Devine. 2001. Use of recombinant envelope proteins for serological diagnosis of dengue virus infection in an immunochromatographic assay. Clin. Diagn. Lab. Immunol. 8:1150-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis, B. S., G. J. Chang, B. Cropp, J. T. Roehrig, D. A. Martin, C. J. Mitchell, R. Bowen, and M. L. Bunning. 2001. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J. Virol. 75:4040-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douris, V., L. Swevers, V. Labropoulou, E. Andronopoulou, Z. Georgoussi, and K. Iatrou. 2006. Stably transformed insect cell lines: tools for expression of secreted and membrane-anchored proteins and high-throughput screening platforms for drug and insecticide discovery. Adv. Virus Res. 68:113-156. [DOI] [PubMed] [Google Scholar]
- 7.Etemad, B., G. Batra, R. Raut, S. Dahiya, S. Khanam, S. Swaminathan, and N. Khanna. 2008. An envelope domain III-based chimeric antigen produced in Pichia pastoris elicits neutralizing antibodies against all four dengue virus serotypes. Am. J. Trop. Med. Hyg. 79:353-363. [PubMed] [Google Scholar]
- 8.Ge, F. F., Y. F. Qiu, X. Y. Gao, Y. W. Yang, and P. Y. Chen. 2006. Fusion expression of major antigenic segment of JEV E protein-hsp70 and the identification of domain acting as adjuvant in hsp70. Vet. Immunol. Immunopathol. 113:288-296. [DOI] [PubMed] [Google Scholar]
- 9.Gentry, M. K., E. A. Henchal, J. M. McCown, W. E. Brandt, and J. M. Dalrymple. 1982. Identification of distinct antigenic determinants on dengue-2 virus using monoclonal antibodies. Am. J. Trop. Med. Hyg. 31:548-555. [DOI] [PubMed] [Google Scholar]
- 10.Gubler, D. J., G. Kuno, and L. Markoff. 2007. Flaviviruses, p 1153-1252. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 11.Halstead, S. B. 2007. Dengue. Lancet 370:1644-1652. [DOI] [PubMed] [Google Scholar]
- 12.Halstead, S. B., and D. W. Vaughn. 2008. Dengue vaccines, p. 1155-1161. In S. A. Plotkin, W. A. Orenstein, and P. A. Offit (ed.), Vaccines, 5th ed. Saunders Elsevier, Maryland Heights, MO.
- 13.Halstead, S. B., and J. Jacobsen. 2008. Japanese encephalitis vaccines, p 311-352. In S. A. Plotkin, W. A. Orenstein, and P. A. Offit (ed.), Vaccines, 5th ed. Saunders Elsevier, Maryland Heights, MO.
- 14.Henchal, E. A., J. M. McCown, D. S. Burke, M. C. Seguin, and W. E. Brandt. 1985. Epitopic analysis of antigenic determinants on the surface of dengue-2 virions using monoclonal antibodies. Am. J. Trop. Med. Hyg. 34:162-169. [DOI] [PubMed] [Google Scholar]
- 15.Hunt, A. R., C. B. Cropp, and G. J. Chang. 2001. A recombinant particulate antigen of Japanese encephalitis virus produced in stably-transformed cells is an effective noninfectious antigen and subunit immunogen. J. Virol. Methods 97:133-149. [DOI] [PubMed] [Google Scholar]
- 16.Igarashi, A. 2002. Control of Japanese encephalitis in Japan: immunization of humans and animals, and vector control. Curr. Top. Microbiol. Immunol. 267:139-152. [DOI] [PubMed] [Google Scholar]
- 17.Ikonomou, L., Y. J. Schneider, and S. N. Agathos. 2003. Insect cell culture for industrial production of recombinant proteins. Appl. Microbiol. Biotechnol. 62:1-20. [DOI] [PubMed] [Google Scholar]
- 18.Imoto, J., and E. Konishi. 2005. Needle-free jet injection of a mixture of Japanese encephalitis DNA and protein vaccine in a murine model. Viral Immunol. 18:205-212. [DOI] [PubMed] [Google Scholar]
- 19.Imoto, J., and E. Konishi. 2007. Dengue tetravalent DNA vaccine increases its immunogenicity in mice when mixed with a dengue type 2 subunit vaccine or an inactivated Japanese encephalitis vaccine. Vaccine 25:1076-1084. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa, T. 2005. Development of new Japanese encephalitis vaccine. Nippon Rinsho 63:2133-2137. (In Japanese.) [PubMed] [Google Scholar]
- 21.Ishikawa, T., and E. Konishi. 2006. Mosquito cells infected with Japanese encephalitis virus release slowly-sedimenting hemagglutinin particles in association with intracellular formation of smooth membrane structures. Microbiol. Immunol. 50:211-223. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa, T., T. Takasaki, I. Kurane, S. Nukuzuma, T. Kondo, and E. Konishi. 2007. Co-immunization with West Nile DNA and inactivated vaccines provides synergistic increases in their immunogenicities in mice. Microbes Infect. 9:1089-1095. [DOI] [PubMed] [Google Scholar]
- 23.Kelly, E. P., J. J. Greene, A. D. King, and B. L. Innis. 2000. Purified dengue 2 virus envelope glycoprotein aggregates produced by baculovirus are immunogenic in mice. Vaccine 18:2549-2559. [DOI] [PubMed] [Google Scholar]
- 24.Kojima, A., A. Yasuda, H. Asanuma, T. Ishikawa, A. Takamizawa, K. Yasui, and T. Kurata. 2003. Stable high-producer cell clone expressing virus-like particles of the Japanese encephalitis virus E protein for a second-generation subunit vaccine. J. Virol. 77:8745-8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kollaritsch, H., M. Paulke-Korinek, and K. Dubischar-Kastner. 2009. IC51 Japanese encephalitis vaccine. Expert Opin. Biol. Ther. 9:921-931. [DOI] [PubMed] [Google Scholar]
- 26.Konishi, E., and A. Fujii. 2002. Dengue type 2 virus subviral extracellular particles produced by a stably transfected mammalian cell line and their evaluation for a subunit vaccine. Vaccine 20:1058-1067. [DOI] [PubMed] [Google Scholar]
- 27.Konishi, E., A. Fujii, and P. W. Mason. 2001. Generation and characterization of a mammalian cell line continuously expressing Japanese encephalitis virus subviral particles. J. Virol. 75:2204-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konishi, E., A. Terazawa, and A. Fujii. 2003. Evidence for antigen production in muscles by dengue and Japanese encephalitis DNA vaccines and a relation to their immunogenicity in mice. Vaccine 21:3713-3720. [DOI] [PubMed] [Google Scholar]
- 29.Konishi, E., I. Kurane, and P. W. Mason. 2000. Immune response to traditional and genetically engineered Japanese encephalitis vaccines. Recent Res. Dev. Virol. 2:1-21. [Google Scholar]
- 30.Konishi, E., K. Yagawa, and A. Yamanaka. 2008. Vero cells infected with vaccinia viruses expressing Japanese encephalitis virus envelope protein induce polykaryocyte formation under neutral conditions. Jpn. J. Infect. Dis. 61:410-411. [PubMed] [Google Scholar]
- 31.Konishi, E., M. Shoda, N. Ajiro, and T. Kondo. 2004. Development and evaluation of an enzyme-linked immunosorbent assay for quantifying antibodies to Japanese encephalitis virus nonstructural 1 protein to detect subclinical infections in vaccinated horses. J. Clin. Microbiol. 42:5087-5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konishi, E., P. W. Mason, and R. E. Shope. 1996. Enzyme-linked immunosorbent assay using recombinant antigens for serodiagnosis of Japanese encephalitis. J. Med. Virol. 48:76-79. [DOI] [PubMed] [Google Scholar]
- 33.Konishi, E., S. Pincus, E. Paoletti, R. E. Shope, T. Burrage, and P. W. Mason. 1992. Mice immunized with a subviral particle containing the Japanese encephalitis virus prM/M and E proteins are protected from lethal JEV infection. Virology 188:714-720. [DOI] [PubMed] [Google Scholar]
- 34.Kurane, I. 2002. Immune responses to Japanese encephalitis virus. Curr. Top. Microbiol. Immunol. 267:91-103. [DOI] [PubMed] [Google Scholar]
- 35.Kuzuhara, S., H. Nakamura, K. Hayashida, J. Obata, M. Abe, K. Sonoda, K. Nishiyama, K. Sugawara, K. Takeda, T. Honda, H. Matsui, T. Shigaki, Y. Kino, H. Mizokami, M. Tanaka, K. Mizuno, and K. Ueda. 2003. Non-clinical and phase I clinical trials of a Vero cell-derived inactivated Japanese encephalitis vaccine. Vaccine 21:4519-4526. [DOI] [PubMed] [Google Scholar]
- 36.Ledizet, M., K. Kar, H. G. Foellmer, T. Wang, S. L. Bushmich, J. F. Anderson, E. Fikrig, and R. A. Koski. 2005. A recombinant envelope protein vaccine against West Nile virus. Vaccine 23:3915-3924. [DOI] [PubMed] [Google Scholar]
- 37.Lindenbach, B. D., H.-J. Thiel, and C. M. Rice. 2007. Flaviviridae: the viruses and their replication, p. 1101-1152. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 38.Liu, W., H. Jiang, J. Zhou, X. Yang, Y. Tang, D. Fang, and L. Jiang. 2010. Recombinant dengue virus-like particles from Pichia pastoris: efficient production and immunological properties. Virus Genes 40:53-59. [DOI] [PubMed] [Google Scholar]
- 39.Mackenzie, J. S. 2005. Emerging zoonotic encephalitis viruses: lessons from Southeast Asia and Oceania. J. Neurovirol. 11:434-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mason, P. W., J. M. Dalrymple, M. K. Gentry, J. M. McCown, C. H. Hoke, D. D. Burke, M. J. Fournier, and T. L. Mason. 1989. Molecular characterization of a neutralizing domain of the Japanese encephalitis virus structural glycoprotein. J. Gen. Virol. 70:2037-2049. [DOI] [PubMed] [Google Scholar]
- 41.Mason, P. W., S. Pincus, M. J. Fournier, T. L. Mason, R. E. Shope, and E. Paoletti. 1991. Japanese encephalitis virus-vaccinia recombinants produce particulate forms of the structural membrane proteins and induce high levels of protection against lethal JEV infection. Virology 180:294-305. [DOI] [PubMed] [Google Scholar]
- 42.McDonald, W. F., J. W. Huleatt, H. G. Foellmer, D. Hewitt, J. Tang, P. Desai, A. Price, A. Jacobs, V. N. Takahashi, Y. Huang, V. Nakaar, L. Alexopoulou, E. Fikrig, and T. J. Powell. 2007. A West Nile virus recombinant protein vaccine that coactivates innate and adaptive immunity. J. Infect. Dis. 195:1607-1617. [DOI] [PubMed] [Google Scholar]
- 43.Mutoh, E., T. Ishikawa, A. Takamizawa, T. Kurata, T. Sata, and A. Kojima. 2004. Japanese encephalitis subunit vaccine composed of virus-like envelope antigen particles purified from serum-free medium of a high-producer J12#26 cell clone. Vaccine 22:2599-2608. [DOI] [PubMed] [Google Scholar]
- 44.Oya, A., and I. Kurane. 2007. Japanese encephalitis for a reference to international travelers. J. Travel Med. 14:259-268. [DOI] [PubMed] [Google Scholar]
- 45.Paavonen, J., D. Jenkins, F. X. Bosch, P. Naud, J. Salmerón, C. M. Wheeler, S. N. Chow, D. L. Apter, H. C. Kitchener, X. Castellsague, N. S. de Carvalho, S. R. Skinner, D. M. Harper, J. A. Hedrick, U. Jaisamrarn, G. A. Limson, M. Dionne, W. Quint, B. Spiessens, P. Peeters, F. Struyf, S. L. Wieting, M. O. Lehtinen, and G. Dubin; HPV PATRICIA study group. 2007. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 369:2161-2170. [DOI] [PubMed] [Google Scholar]
- 46.Pierson, T. C., and M. S. Diamond. 2008. Molecular mechanisms of antibody-mediated neutralisation of flavivirus infection. Expert Rev. Mol. Med. 10:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Putnak, R., K. Porter, and C. Schmaljohn. 2003. DNA vaccines for flaviviruses. Adv. Virus. Res. 61:445-468. [DOI] [PubMed] [Google Scholar]
- 48.Qiao, M., M. Ashok, K. A. Bernard, G. Palacios, Z. H. Zhou, W. I. Lipkin, and T. J. Liang. 2004. Induction of sterilizing immunity against West Nile Virus (WNV), by immunization with WNV-like particles produced in insect cells. J. Infect. Dis. 190:2104-2108. [DOI] [PubMed] [Google Scholar]
- 49.Ravi, V., A. Desai, M. Balaji, M. P. Apte, L. Lakshman, D. K. Subbakrishna, G. Sridharan, T. N. Dhole, and B. V. Ravikumar. 2006. Development and evaluation of a rapid IgM capture ELISA (JEV-Chex) for the diagnosis of Japanese encephalitis. J. Clin. Virol. 35:429-434. [DOI] [PubMed] [Google Scholar]
- 50.Shishido, T., M. Muraoka, H. Yamaji, A. Kondo, and H. Fukuda. 2007. Production of bionanocapsules in immobilized insect cell culture using porous biomass support particles. J. Biosci. Bioeng. 103:572-574. [DOI] [PubMed] [Google Scholar]
- 51.Staropoli, I., M. P. Frenkiel, F. Mégret, and V. Deubel. 1997. Affinity-purified dengue-2 virus envelope glycoprotein induces neutralizing antibodies and protective immunity in mice. Vaccine 15:1946-1954. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi, H., N. Ohtaki, M. Maeda-Sato, M. Tanaka, K. Tanaka, H. Sawa, T. Ishikawa, A. Takamizawa, T. Takasaki, H. Hasegawa, T. Sata, W. W. Hall, T. Kurata, and A. Kojima. 2009. Effects of the number of amino acid residues in the signal segment upstream or downstream of the NS2B-3 cleavage site on production and secretion of prM/M-E virus-like particles of West Nile virus. Microbes Infect. 11:1019-1028. [DOI] [PubMed] [Google Scholar]
- 53.Verma, R., E. Boleti, and A. J. George. 1998. Antibody engineering: comparison of bacterial, yeast, insect and mammalian expression systems. J. Immunol. Methods 216:165-181. [DOI] [PubMed] [Google Scholar]
- 54.Verma, S. K., S. Kumar, N. Gupta, S. Vedi, S. M. Bhattacharya, and P. V. Lakshmana Rao. 2009. Bacterially expressed recombinant envelope protein domain III of Japanese encephalitis virus (rJEV-DIII) elicits Th1 type of immune response in BALB/c mice. Vaccine 27:6905-6909. [DOI] [PubMed] [Google Scholar]
- 55.Widman, D. G., I. Frolov, and P. W. Mason. 2008. Third-generation flavivirus vaccines based on single-cycle, encapsidation-defective viruses. Adv. Virus Res. 72:77-126. [DOI] [PubMed] [Google Scholar]
- 56.Yang, D. K., C. H. Kweon, B. H. Kim, S. I. Lim, J. H. Kwon, S. H. Kim, J. Y. Song, and H. R. Han. 2005. Immunogenicity of baculovirus expressed recombinant proteins of Japanese encephalitis virus in mice. J. Vet. Sci. 6:125-133. [PubMed] [Google Scholar]
- 57.Zhang, Z. S., Y. S. Yan, Y. W. Weng, H. L. Huang, S. Q. Li, S. He, and J. M. Zhang. 2007. High-level expression of recombinant dengue virus type 2 envelope domain III protein and induction of neutralizing antibodies in BALB/c mice. J. Virol. Methods 143:125-131. [DOI] [PubMed] [Google Scholar]