Abstract
The serodiagnosis of Strongyloides stercoralis infection by enzyme-linked immunosorbent assays based on crude antigen (CrAg-ELISA), while useful, has been limited by the reliance on crude parasite extracts. Newer techniques such as the luciferase immunoprecipitation system assay (LIPS), based on a 31-kDa recombinant antigen (termed NIE) from S. stercoralis and/or the recombinant antigen S. stercoralis immunoreactive antigen (SsIR), or the NIE-ELISA have shown promise in controlled settings. We compared each of these serologic assays in individuals from both regions of the world in which S. stercoralis is endemic and those in which it is not. A comprehensive stool evaluation (sedimentation concentration, Baermann concentration with charcoal cultures, agar plate, and Harada-Mori) and four different serologic techniques using CrAg-ELISA or recombinant NIE-ELISA as well as LIPS using NIE alone or in combination with a second recombinant antigen (NIE/SsIR-LIPS) were compared among individuals with parasitologically proven infection (n = 251) and healthy controls from regions of the world in which the infection is nonendemic (n = 11). Accuracy was calculated for each assay. The prevalence of S. stercoralis infection was 29.4% among Argentinean stool samples (n = 228). Sedimentation concentration and Baermann were the most sensitive stool-based methods. NIE-LIPS showed the highest sensitivity (97.8%) and specificity (100%) of the serologic assays. The calculated negative predictive value was highest for both the NIE-LIPS and CrAg-ELISA (>97%) irrespective of disease prevalence. No cross-reactivity with soil-transmitted helminths was noted. NIE-LIPS compares favorably against the current CrAg-ELISA and stool evaluation, providing additional accuracy and ease of performance in the serodiagnosis of S. stercoralis infections irrespective of disease prevalence.
Strongyloides stercoralis is an intestinal nematode endemic to the tropics and subtropics and affects an estimated 30 to 100 million people worldwide (1, 2). As opposed to infections with other soil-transmitted helminthiases (STHs), chronic infections acquired in areas where S. stercoralis is endemic may be maintained asymptomatically for decades through the autoinfective cycle, which highlights the need for more sensitive diagnostic methods, particularly in patients with low-level infections and those who are immunocompromised (10, 13, 20, 25). Although chronically infected individuals may be asymptomatic, hyperinfection syndrome or disseminated disease can develop following the administration of corticosteroids and are clinical entities associated with a high (87%) mortality if untreated (22).
Identification of S. stercoralis larvae in the stool remains the gold standard for diagnosis of strongyloidiasis. Based on the particular biologic features of this STH, diagnostic approaches must take into consideration the absence of egg output, intermittent larval elimination, and low larval output in the chronic, asymptomatic stage (7-9). Although a variety of techniques have been evaluated for improving the direct detection of larvae in the stool, reportedly more sensitive techniques can be time-consuming and impractical (9, 25). Sensitivity of stool analysis improves slightly with multiple stool examinations (18). Given the challenges to direct larval detection, serologic approaches to the diagnosis of S. stercoralis infection have been developed. Of the serologic assays developed to date, the S. stercoralis enzyme-linked immunosorbent assay based on crude larval antigen (CrAg-ELISA) has been shown to have the highest sensitivity (94.6%) and is currently in use at the Centers for Disease Control and Prevention (CDC) (Atlanta, GA) in the United States and elsewhere (16). Despite its diagnostic utility, cross-reactivity with other helminth infections, including filarial infections, Ascaris lumbricoides infection, and acute schistosome infections, has been reported with the EIA (8). Reliance on crude larval antigen requires a constant supply of infective-stage larvae collected from the stools of experimentally infected animals, a process which is laborious, time-consuming, and expensive (16).
A recent major advance in the diagnosis of S. stercoralis infections has been the development of the luciferase immunoprecipitation system (LIPS) assay based on a 31-kDa recombinant antigen (termed NIE) derived from a S. stercoralis L3 cDNA library and/or the recombinant antigen S. stercoralis immunoreactive antigen (SsIR), which, unlike crude antigen, can be purified easily and produced in large amounts (21, 23). These assays based on NIE antigen have shown to be non-cross-reactive in filaria-infected individuals without S. stercoralis infection in over 80 samples coming from individuals infected with other STH, filariae, or uninfected controls (21). A rapid format of the LIPS assay (called QLIPS) can be can be performed in relatively little time (less than 15 min) (5).
Given the significant strengths of the assays based on recombinant-based antigens, we sought to determine how they compare with other serologic and stool-based methods in the diagnosis of S. stercoralis infection. The primary objective of this study was to assess the utility of newer serological techniques for Strongyloides stercoralis in estimating the true prevalence of infection in communities in Argentina in which the infection is highly endemic. In so doing, we were also able to provide background information on demographic, clinical, and laboratory features of individuals with parasitologically proven S. stercoralis infection in such communities, where prevalences of strongyloidiasis of 50% in the general population and 83% in children have been reported (27, 28), and in Western Australia, where the prevalence ranged from 0.26 to 60% (12). Taken together, the data could enable decisions regarding the need for mass chemotherapy.
MATERIALS AND METHODS
Study populations.
Patients at the Instituto de Investigaciones de Enfermedades Tropicales, Orán, Argentina, were prospectively evaluated from March to November 2007. After providing written consent, each subject provided one fresh stool sample in a sterile container without preservatives within 24 h of collection. All studies were performed by a team of four experienced lab personnel, with the manager performing the final reading on all samples. Stool samples were coded and dated upon entering the laboratory. After the sample was received by the laboratory and before treatment, study personnel filled clinical and demographic forms, treatment history was recorded, and blood samples for complete blood counts and serologic studies were obtained.
Only subjects who had not received any anthelmintic treatment within the previous 6 months were included in the study. The study protocol and the informed consent form were approved by a local Independent Bioethics Committee. All participants diagnosed with helminth infections during the course of the study received treatment at no cost.
Samples from uninfected individuals from areas in which S. stercoralis infection was nonendemic were provided by CDC, which also provided a positive reference sample from one patient identified through stool analysis. Australian samples (n = 23) were collected in a group of five indigenous cases from an area of endemicity in West Australia (12) and from African refugees in Australia with S. stercoralis larvae in a direct fecal smear.
Stool evaluations.
Fresh stools in the Argentinean group were evaluated by four techniques: sedimentation concentration, agar plate culture, Harada-Mori filter paper culture, and Baermann concentration of charcoal-cultured fresh stool, as described elsewhere (9). Stool samples were also analyzed with a flotation saline-based technique and in a McMaster plaque for the diagnosis and quantitation of eggs (for the diagnosis of hookworms, Ascaris lumbricoides, Trichuris trichiura, and Hymenolepis nana) as described elsewhere (9). Each sample was analyzed separately, and all larvae and eggs identified were entered in a database. In the case of an insufficient amount of stools to perform all techniques, priority was given to sedimentation concentration due to the higher sensitivity of this test in our preliminary studies. A case was defined as infected if at least one S. stercoralis larva was identified in the stool regardless of technique used. Patients from areas of nonendemicity with no history of travel to areas of endemicity were defined as uninfected cases. Parasitologic and serologic tests were done in a manner that was blinded from other results.
Serologic techniques.
The crude larval antigen enzyme immunosorbent assay (CrAg-ELISA) is a quantitative immunoassay made with antigen extract purified from infective third-stage larvae of S. stercoralis (L3) obtained from infected dogs. The sensitivity and the specificity of this test have been previously established to be 92.9% and 97.6%, respectively (I. McAuliffe, unpublished data), respectively (16). The NIE-ELISA detects IgG antibodies to a bacterially produced NIE recombinant antigen as has been previously described (23). Patient sera were tested in triplicate and compared to a standard positive IgG curve based on a standard curve run on each plate.
The LIPS assay detects IgG antibodies to either NIE antigen or two antigens, NIE and SsIR, and was performed exactly as described previously (21) except that an input of 1 million luminometer units (LU) of the enzyme reporter Renilla luciferase (Ruc) containing antigen(s) was used. Briefly, patient sera were diluted 1:10 in assay buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1% Triton X-100) in a 96-well polypropylene microtiter plate (Nunc, Roskilde, Denmark) and were added to 50 μl of 1 × 106 LU of Ruc antigen in polypropylene plates. The plate was incubated for 5 min at room temperature, after which the material was added to 7 μl of a 30% suspension of protein A/G beads in phosphate-buffered saline (PBS) (Pierce Biotechnology, Rockford, IL) in a 96-well-filter high-throughput-screening (HTS) plate (catalog no. MSBVN1B50; Millipore, Bedford, MA). After 5 min, the filter plate containing the mixture was then applied to a vacuum manifold and washed twice in buffer A and eight times with PBS. After the final wash, all plates were processed on a Berthold LB 960 Centro microplate luminometer using a colenterazine substrate mix (Promega). All data were the averages of triplicate results, and the averages were corrected for background reactivity (no serum added).
Determination of “cutoffs” for recombinant antigen-based assays.
Cutoffs for recombinant antigen-based assays (NIE-ELISA, NIE-LIPS, NIE-SsIR-LIPS), separating positive and negative values have not been previously established. Cutoffs for these assays were defined based on a subgroup of parasitologically proven samples (positive by stool-based testing—i.e., stool positive—for S. stercoralis by any parasitologic technique) and normal, healthy controls (sera from North American individuals without a compatible exposure history). The optimal cutoff point for each assay was determined by plotting the sensitivity and specificity for various cutoff point values by means of the receiving operating characteristic (ROC) curves. The area under the curve was calculated for each assay.
Statistical analysis.
Groups were compared and the continuous variables were analyzed for statistical significance with the Mann-Whitney U test (two tailed), as the data were not distributed normally. Differences were considered statistically significant if the P value was less than 0.05. Correlations were analyzed using the Spearman rank test. Data analysis was performed with SPSS for Windows 15.0 (SPSS Inc., Chicago, IL). Positive and negative predictive values for each serologic assay were calculated at a variety of disease prevalence levels. Categorical variables were compared with Fisher's exact test (two tailed).
RESULTS
Baseline study population characteristics.
A total of 262 samples were included in the analysis from Argentina (n = 228), Australia (n = 23), and North America (n = 10). One known positive sample was included as a positive “control” for all assays, obtained from the CDC. Demographic information was available for 215 samples from Argentina. Patients from Argentina were mostly pediatric, with 89% (n = 191) less than 15 years of age. Females younger than 15 years represented 63% of that age group population, and females older than 15 years represented 47% of the Argentina patient group. Patients seen in Australia had a median age of 37 years old, and 70% (n = 16) of them were males.
A total of 228 stool samples from Argentina were evaluated for the presence of larvae of S. stercoralis through up to four techniques (agar plate, Harada-Mori, sedimentation concentration, and Baermann concentration) plus a flotation technique (for identification of eggs of other STHs) in single stool samples. Among the 67 samples (29.4%) that were positive for S. stercoralis, the sedimentation concentration technique was the most sensitive (88%), followed by Baermann (81%), agar plate (58%), and Harada-Mori (50%). A combination of sedimentation concentration and Baermann detected 66 of 67 samples (the remaining positive stool sample was diagnosed by Harada-Mori) (Table 1). Polyparasitism was frequent in this group, with 114 samples (50%) having ≥2 helminths in stools. Only 29 samples (12.7%) were negative for STHs. Hookworm, H. nana, and A. lumbricoides eggs were found in 75%, 33%, and 20% of the stool specimens, respectively. Trichuris trichiura eggs were not detected.
TABLE 1.
Comparative analysis of all stool and serologic diagnostic methods for S. stercoralisa
| Method | No. of samples testedb | No. positive for S. stercoralis | % positive for S. stercoralis | No. exclusively positive by one methodc |
|---|---|---|---|---|
| Stool | ||||
| Agar plate | 187 | 26 | 14 | 0 |
| Baermann | 188 | 44 | 23 | 7 |
| Sedimentation concn | 228 | 59 | 26 | 4 |
| Harada-Mori | 214 | 29 | 14 | 1 |
| Any method | 228 | 67 | 29 | |
| Serology | ||||
| CrAg-ELISA | 262 | 155 | 59 | 5 |
| NIE-ELISA | 262 | 140 | 53 | 10 |
| NIE-LIPS | 262 | 214 | 82 | 41 |
| NIE-SsIR- LIPS | 262 | 158 | 60 | 2 |
Subjects included 228 patients from Argentina, 23 patients from Australia, and 11 healthy individuals from North America.
Variations in the number of stool specimens tested by each method were due to differences in stool volume per patient. In cases where stool quantity was insufficient, the concentration method was made a priority.
Exclusivity was calculated by the type of techniques, either serology or stool.
Comparison of serologic assays based on ROC curve analysis.
ROC curves for serologic results based on serum samples from patients with either S. stercoralis in stools (67 from Argentina and 23 from Australia) or those not infected from areas of nonendemicity (10 samples) were constructed for each recombinant antigen-based serologic test (NIE-ELISA, CrAg-ELISA, NIE-LIPS, NIE-SsIR-LIPS). The calculated area under the curve for all four tests were comparable (CrAg-ELISA, 0.986; NIE-ELISA, 0.922; NIE-LIPS, 0.933; and NIE-SsIR-LIPS, 0.977) (Fig. 1). Table 2 describes the cutoff limits determined to classify samples and the sensitivity and specificity for each test. The criterion for the selection of the cutoffs was based on the values with the highest sensitivity among those with 100% specificity, in order to avoid the confounding effect of other STH while maintaining the highest possible sensitivity (Fig. 1). Predictive values were calculated for the selected sensitivity and specificity corresponding to the cutoff of each method; these positive and negative predictive values were calculated at a variety of disease prevalence levels, including those expected in areas of hyperendemicity, as in the study area in Argentina, and also at lower disease prevalence levels, like those expected to be encountered in areas of nonendemicity, in order to estimate the usefulness of these serologic assays in a variety of hypothetical situations (Table 2). In terms of negative predictive values, the NIE-ELISA had a sensitivity of 84% with a negative predictive value of 85.84% at a disease prevalence of 50%; this negative predictive value climbed to >99% with prevalence levels of 3% or less (Table 2). The best performances were with NIE-LIPS and CrAg-ELISA, which have sensitivities of 97.8 and 97%, respectively; both assays had calculated negative predictive values >97% at high prevalences and >99.9% at low prevalence levels (Table 2).
FIG. 1.
Comparison of receiver-operator characteristic (ROC) curves for all four serologic methods based on results from a selected group of sera from patients with parasitologically proven S. stercoralis infection (n = 101) and healthy North American subjects without a history of travel to an area of endemicity (n = 10). The area under the curve (AUC) for each technique was as follows: CrAg-ELISA, 0.986; NIE-ELISA, 0.922; NIE-LIPS, 0.933; and NIE-SsIR-LIPS, 0.977.
TABLE 2.
Results for four assays for diagnosis of S. stercoralis
| Test | Cutoffa | % Sensitivity | % Specificity | Predictive valueb at disease prevalence (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 |
20 |
3 |
1 |
0.1 |
0.001 |
||||||||||
| PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | ||||
| CrAg-ELISA | 1.7 U/ml−1 | 97 | 100 | 100 | 97.09 | 100 | 99.26 | 100 | 99.91 | 100 | 99.97 | 100 | 100 | 100 | 100 |
| NIE-ELISA | 119.04 U/ml−1 | 84 | 100 | 100 | 85.84 | 100 | 96.04 | 100 | 99.49 | 100 | 99.83 | 100 | 99.98 | 100 | 100 |
| NIE-LIPS | 37.89 LU | 97.8 | 100 | 100 | 97.85 | 100 | 99.45 | 100 | 99.93 | 100 | 99.98 | 100 | 100 | 100 | 100 |
| NIE-SsIR-LIPS | 1199 LU | 91.2 | 100 | 100 | 91.91 | 100 | 97.85 | 100 | 99.73 | 100 | 99.91 | 100 | 99.99 | 100 | 100 |
Cutoffs for NIE-LIPS, NIE-ELISA, NIE-SsIR-LIPS assays with corresponding sensitivity and specificity as calculated by ROC curve analysis. The cutoff for the CrAg-ELISA has been previously determined and was not determined by our analysis.
Calculated positive and negative predictive values (PPV and NPV, respectively) at a variety of prevalence levels based on the selected cutoff values.
LIPS-based assays using NIE or NIE-SsIR antigens accurately detected antibody titers from patients with stools positive for S. stercoralis. Spearman analysis demonstrated a significant correlation among all serologic techniques (P < 0.001). The highest levels of correlation were found between CrAg-ELISA and both LIPS NIE-SsIR (R2 = 0.810) and LIPS NIE (R2 = 0.780). The NIE-LIPS assay was negative in two samples from patients with parasitologically proven infection (n = 91), one from Argentina and the other from Australia. In this group of S. stercoralis-positive stool samples, eight samples were negative for LIPS-NIE-SsIR (cutoff, 1,199 LU). For CrAg-ELISA (based on a standard cutoff of 1.7 U/ml), five samples were negative (three from Argentina and two from Australia) among those positive by stool analysis. NIE-ELISA (cutoff 83 U/ml) was inferior at detecting positive samples, with 10 samples from Argentina and Australia classified as negative. Only 1 Australian sample of the 91 stool positive cases was negative by all serologic methods.
Serologic methods were positive for a significant number of samples without identifiable larvae of S. stercoralis in thoroughly studied stool samples. Among the 161 samples negative for S. stercoralis by stool analysis, between 64 and 125 were positive in one or all of the serological methods used, suggesting the superiority of these specific antibody-based assays in detecting infection prior to anthelmintic therapy (see Fig. 3). As seen, a direct comparison of serologic values between stool positive and negative samples from Argentina shows a statistically significant difference (P < 0.0001) for NIE-LIPS, as well as for the other serologic methods (Fig. 2).
FIG. 2.
Distribution and median values of IgG antibody determined by crude antigen ELISA (a), recombinant NIE antigen (b), and luciferase immunoprecipitation system assay (LIPS) using NIE antigen (c) or NIE and SsIR antigens (d) in 261 samples classified according to geographic origin and stool analysis results. Cutoffs are indicated by horizontal lines for each technique. Error bars indicate median ± interquartile range (IQR) per group. Samples were stool positive from Argentina (n = 67), stool negative from Argentina (n = 161), CDC normal (n = 10), and stool positive from Australia (n = 23). The CDC normal group included noninfected North Americans without travel to areas of endemicity. P values were calculated using the Mann-Whitney U test (two tailed).
Cross-reactivity with other helminths.
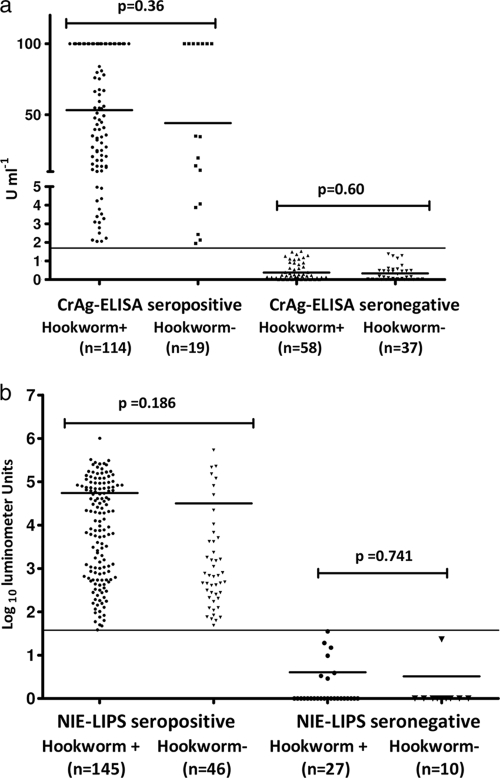
The impact of other STHs on S. stercoralis-specific serologies was evaluated with a particular focus on hookworms due to their high prevalence in the study population from Argentina. NIE-LIPS values were first stratified according to their S. stercoralis status and then compared between samples with and without hookworms as determined by stool analysis. In a post hoc analysis, the proportion of hookworm-positive stool samples was not different for patients with either CrAg-ELISA or NIE-LIPS positive or negative antibody titers (Fig. 3). Those that were seropositive by NIE-LIPS from Argentina (n = 191) were then compared for coinfection with hookworms and A. lumbricoides. No significant difference was seen between those who had just S. stercoralis in their stool and those coinfected with hookworms and/or A. lumbricoides.
FIG. 3.
Distribution and geometric mean values of IgG antibody titers to crude antigen determined by ELISA (a) and NIE determined by luciferase immunoprecipitation system assay (LIPS) (b) for 228 study samples from Argentina classified according to serologic results and the presence or absence of hookworms in the stool analyses. The serologic cutoff is marked as a thin horizontal line. Geometric means per group are shown as thick horizontal lines. P values were calculated using the Mann-Whitney U test (two tailed).
DISCUSSION
The diagnosis of chronic infections by the geohelminth S. stercoralis has been traditionally difficult and further complicated by the need for laborious techniques using fresh stools without preservative, requiring strong clinical suspicion for its diagnosis. As opposed to hyperinfection syndromes, where diagnosis is relatively straightforward, chronic, usually asymptomatic, infections are a diagnostic challenge (25). The analysis shown here, in a polyparasitized population and from diverse geographic regions, confirms that standard stool-based techniques are relatively insensitive in the chronic stage of this infection and that serologic assays provide a useful tool for the management of patients at risk for this infection and for the estimation of disease prevalence in a given population (11, 29). In this study, we confirmed the results of previous studies that the recombinant-antigen-based LIPS assay accurately differentiates between sera from patients with parasitologically proven S. stercoralis infection and healthy, uninfected controls, compared to the current best available serologic test (21). LIPS assays have been successfully applied to the characterization of antibody responses to multiple infections, including Pneumocystis jirovecii, HIV, hepatitis virus, and Loa loa and, more recently, to the diagnosis of infections due to S. stercoralis (4, 6, 21). As has been shown previously, the LIPS format was additionally found to be superior to an ELISA-based format using the same NIE recombinant antigen (21). The superior specificity of LIPS assays compared to the traditional ELISA can be explained, in part, by the use of mammalian COS1 cells, which produce antigens free of contaminating, cross-reacting bacterial proteins, as well as by the use of a solution-phase immunoprecipitation assay that allows detection of a large number of conformational epitopes. In addition, the LIPS assay (QLIPS method) can be performed in relatively little time (15 min) compared to a standard ELISA and requires only small amounts (1 μl) of human serum.
Cross-reactivity with other parasitic infections is a major concern in the development of any S. stercoralis serologic assay. In our study population, we found no association between seropositivity for S. stercoralis and infections with A. lumbricoides, hookworms, or H. nana. Since no other helminths were found in this population, this conclusion is limited to the mentioned STHs. The specificity of the serologic assays is also highlighted by the demonstration that the presence of other helminths in the stools did not affect the strongyloides-specific serology.
The highly accurate predictive values obtained with the cutoff values calculated for each serologic method would allow the use of both LIPS-based methods or the CrAg-ELISA at various clinical settings. In the case of community-based interventions in areas with high prevalence, each positive test could be trusted as positive with absolute confidence and negative tests would have predictive values above 97% for any prevalence level ≤20. It should be noted that with prevalence levels ≥20%, as in the population discussed here, false-negative values are of lesser clinical importance since mass treatments would be recommended regardless of the infection status and overtreatment rarely results in adverse events (19). An exception to this situation would be treatment of pre-school-age children (<2 years old), a group with safety aspects of anthelminthic therapy still unresolved (1). In the case of the management of individual cases in areas with low prevalence levels, the proposed cutoff values offer negative predictive values >99.5% with any of the four serologic methods.
The LIPS methodology used in this study differed from that used previously in that the input of Ruc antigen lysates was lower in this study (1 million LU rather than 10 million LU) (21). The use of a lower input of lysate has been suggested to further lower background signals, although it may result in a lower dynamic range (5). The rapid QLIPS format has not previously been applied to the diagnosis of S. stercoralis infection, although it performed well in this study.
The relatively low sensitivity of the stool-based methods compared to the serologic methods is underscored by the high proportion of seropositive samples among the negative stools from Argentina. The significant difference in median titers between stool positive and stool negative samples (Fig. 2), which remained significant when only the seropositive values were considered, could be secondary to the differential worm burden present in these groups, a concept that might explain the low sensitivity of the stool techniques in this population. Alternatively, seropositivity among stool negative individuals might represent past infections cleared through anthelmintic therapy or spontaneously (unlikely because of autoinfection). The time for “serologic cure” has been calculated to be 6 months for 90% of the cases but with wide variability and longer periods in other studies using larval-extract-based serologies (14-16). The study population in Argentina had not been treated with anthelmintics for a period of at least 6 months prior to the survey. Our data are in agreement with those from a survey in Peru in which an ELISA was more sensitive than a comprehensive single stool evaluation and in which sedimentation concentration is more sensitive than Baermann and agar plate tests (29), which have been reported as the most sensitive in several reports (7, 17, 24, 26).
The disadvantage of using a single stool specimen was in part controlled by the use of multiple techniques, which increased the overall sensitivity (3) but still could explain the presence of a significant number of seropositive samples among stool samples negative for S. stercoralis. This fact, despite not overcoming the presumed intermittent larval production by S. stercoralis, also underlines the constraint posed by the collection of multiple samples from each individual in a community program. The difficulty of this analysis coupled with the logistic impossibility of performing all stool tests in every sample due to scarce stool volume further highlights the limitations of these tools in pediatric populations.
Thus, the present study highlights the advantages and improved sensitivity of rapid, highly specific serologic assays for community-based assessment of S. stercoralis infection. Because of the potential of this infection to cause hyperinfection syndrome along with its general detrimental health effects on infected individuals, the identification of at-risk individuals rapidly and inexpensively becomes of paramount importance, along with the ability to look at the efficacy of treatment using serologic surrogates.
Footnotes
Published ahead of print on 25 August 2010.
REFERENCES
- 1.Albonico, M., H. Allen, L. Chitsulo, D. Engels, A. F. Gabrielli, and L. Savioli. 2008. Controlling soil-transmitted helminthiasis in pre-school-age children through preventive chemotherapy. PLoS Negl. Trop. Dis. 2:e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bethony, J., S. Brooker, M. Albonico, S. M. Geiger, A. Loukas, D. Diemert, and P. J. Hotez. 2006. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367:1521-1532. [DOI] [PubMed] [Google Scholar]
- 3.Brown, M., J. Bukusuba, P. Hughes, J. Nakiyingi, C. Watera, A. Elliott, and J. Whitworth. 2003. Screening for intestinal helminth infestation in a semi-urban cohort of HIV-infected people in Uganda: a combination of techniques may enhance diagnostic yield in the absence of multiple stool samples. Trop. Doct. 33:72-76. [DOI] [PubMed] [Google Scholar]
- 4.Burbelo, P. D., K. H. Ching, T. L. Mattson, J. S. Light, L. R. Bishop, and J. A. Kovacs. 2007. Rapid antibody quantification and generation of whole proteome antibody response profiles using LIPS (luciferase immunoprecipitation systems). Biochem. Biophys. Res. Commun. 352:889-895. [DOI] [PubMed] [Google Scholar]
- 5.Burbelo, P. D., H. P. Leahy, M. J. Iadarola, and T. B. Nutman. 2009. A four-antigen mixture for rapid assessment of Onchocerca volvulus infection. PLoS Negl. Trop. Dis. 3:e438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burbelo, P. D., R. Ramanathan, A. D. Klion, M. J. Iadarola, and T. B. Nutman. 2008. Rapid, novel, specific, high-throughput assay for diagnosis of Loa loa infection. J. Clin. Microbiol. 46:2298-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Kaminsky, R. G. 1993. Evaluation of three methods for laboratory diagnosis of Strongyloides stercoralis infection. J. Parasitol. 79:277-280. [PubMed] [Google Scholar]
- 8.Gam, A. A., F. A. Neva, and W. A. Krotoski. 1987. Comparative sensitivity and specificity of ELISA and IHA for serodiagnosis of strongyloidiasis with larval antigens. Am. J. Trop. Med. Hyg. 37:157-161. [DOI] [PubMed] [Google Scholar]
- 9.Garcia, L. S. 2001. Diagnostic medical parasitology, 4th ed., p. 786-801. ASM Press, Washington, DC.
- 10.Genta, R. M. 1989. Global prevalence of strongyloidiasis: critical review with epidemiologic insights into the prevention of disseminated disease. Rev. Infect. Dis. 11:755-767. [DOI] [PubMed] [Google Scholar]
- 11.Gyorkos, T. W., R. M. Genta, P. Viens, and J. D. MacLean. 1990. Seroepidemiology of Strongyloides infection in the Southeast Asian refugee population in Canada. Am. J. Epidemiol. 132:257-264. [DOI] [PubMed] [Google Scholar]
- 12.Johnston, F. H., P. S. Morris, R. Speare, J. McCarthy, B. Currie, D. Ewald, W. Page, and K. Dempsey. 2005. Strongyloidiasis: a review of the evidence for Australian practitioners. Aust. J. Rural Health 13:247-254. [DOI] [PubMed] [Google Scholar]
- 13.Keiser, P. B., and T. B. Nutman. 2004. Strongyloides stercoralis in the immunocompromised population. Clin. Microbiol. Rev. 17:208-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi, J., Y. Sato, H. Toma, M. Takara, and Y. Shiroma. 1994. Application of enzyme immunoassay for postchemotherapy evaluation of human strongyloidiasis. Diagn. Microbiol. Infect. Dis. 18:19-23. [DOI] [PubMed] [Google Scholar]
- 15.Lindo, J. F., N. S. Atkins, M. G. Lee, R. D. Robinson, and D. A. Bundy. 1996. Short report: long-term serum antibody isotype responses to Strongyloides stercoralis filariform antigens in eight patients treated with ivermectin. Am. J. Trop. Med. Hyg. 55:474-476. [DOI] [PubMed] [Google Scholar]
- 16.Loutfy, M. R., M. Wilson, J. S. Keystone, and K. C. Kain. 2002. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am. J. Trop. Med. Hyg. 66:749-752. [DOI] [PubMed] [Google Scholar]
- 17.Marchi Blatt, J., and G. A. Cantos. 2003. Evaluation of techniques for the diagnosis of Strongyloides stercoralis in human immunodeficiency virus (HIV) positive and HIV negative individuals in the city of Itajai, Brazil. Braz. J. Infect. Dis. 7:402-408. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen, P. B., and M. Mojon. 1987. Improved diagnosis of Strongyloides stercoralis by seven consecutive stool specimens. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 263:616-618. [DOI] [PubMed] [Google Scholar]
- 19.Peeling, R. W., P. G. Smith, and P. M. Bossuyt. 2006. A guide for diagnostic evaluations. Nat. Rev. Microbiol. 4:S2-S6. [DOI] [PubMed] [Google Scholar]
- 20.Pelletier, L. L., Jr., C. B. Baker, A. A. Gam, T. B. Nutman, and F. A. Neva. 1988. Diagnosis and evaluation of treatment of chronic strongyloidiasis in ex-prisoners of war. J. Infect. Dis. 157:573-576. [DOI] [PubMed] [Google Scholar]
- 21.Ramanathan, R., P. D. Burbelo, S. Groot, M. J. Iadarola, F. A. Neva, and T. B. Nutman. 2008. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. J. Infect. Dis. 198:444-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramanathan, R., and T. Nutman. 2008. Strongyloides stercoralis infection in the immunocompromised host. Curr. Infect. Dis. Rep. 10:105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravi, V., S. Ramachandran, R. W. Thompson, J. F. Andersen, and F. A. Neva. 2002. Characterization of a recombinant immunodiagnostic antigen (NIE) from Strongyloides stercoralis L3-stage larvae. Mol. Biochem. Parasitol. 125:73-81. [DOI] [PubMed] [Google Scholar]
- 24.Sato, Y., J. Kobayashi, H. Toma, and Y. Shiroma. 1995. Efficacy of stool examination for detection of Strongyloides infection. Am. J. Trop. Med. Hyg. 53:248-250. [DOI] [PubMed] [Google Scholar]
- 25.Siddiqui, A. A., and S. L. Berk. 2001. Diagnosis of Strongyloides stercoralis infection. Clin. Infect. Dis. 33:1040-1047. [DOI] [PubMed] [Google Scholar]
- 26.Steinmann, P., X. N. Zhou, Z. W. Du, J. Y. Jiang, L. B. Wang, X. Z. Wang, L. H. Li, H. Marti, and J. Utzinger. 2007. Occurrence of Strongyloides stercoralis in Yunnan Province, China, and comparison of diagnostic methods. PLoS Negl. Trop. Dis. 1:e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taranto, N. J., H. Bonomi de Filippi, and O. Orione. 1993. [Prevalence of Strongyloides stercoralis infection in childhood. Oran, Salta, Argentina]. Bol. Chil. Parasitol. 48:49-51. (In Spanish.) [PubMed] [Google Scholar]
- 28.Taranto, N. J., S. P. Cajal, M. C. De Marzi, M. M. Fernandez, F. M. Frank, A. M. Bru, M. C. Minvielle, J. A. Basualdo, and E. L. Malchiodi. 2003. Clinical status and parasitic infection in a Wichi Aboriginal community in Salta, Argentina. Trans. R. Soc. Trop. Med. Hyg. 97:554-558. [DOI] [PubMed] [Google Scholar]
- 29.Yori, P. P., M. Kosek, R. H. Gilman, J. Cordova, C. Bern, C. B. Chavez, M. P. Olortegui, C. Montalvan, G. M. Sanchez, B. Worthen, J. Worthen, F. Leung, and C. V. Ore. 2006. Seroepidemiology of strongyloidiasis in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 74:97-102. [PMC free article] [PubMed] [Google Scholar]