Abstract
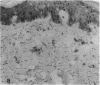
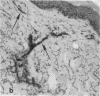
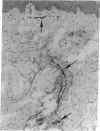
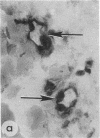
To better understand the events involved in the local migration of inflammatory cells into sites of allergic reactions, we studied expression of the cytokine inducible endothelial cell (EC) neutrophil adhesion molecule, endothelial-leukocyte adhesion molecule (ELAM-1), in sequential skin biopsies from patients with respiratory allergy during the late phase reaction (LPR) between 20 min and until 24 h after intradermal allergen (ragweed or dust mites) injection. In 7 of 7 atopic patients but in only 1 of 4 apparently normal controls, allergen induced appearance of ELAM-1 on EC. ELAM-1 expression occurred concurrently with the development of inflammatory cell infiltrates by 3-4 h after intradermal injection. Saline injected sites in all subjects were negative. Skin organ cultures demonstrated that allergen could produce the same EC changes in vitro whether allergen was injected in vivo 20 min before culture or added during skin culture. These EC changes in organ culture were inhibited by the presence of combined anti-sera to both TNF-alpha and IL-1, but not by antisera to either cytokine alone. We conclude that EC activation occurs in elicited LPR and suggest that cytokine-induced EC activation may play a role in the migration of inflammatory cells into allergic skin reactions. Furthermore, resident cells in the skin rather than infiltrating leukocytes appear to be the source of the cytokines that mediate endothelial activation.
Full text
PDF

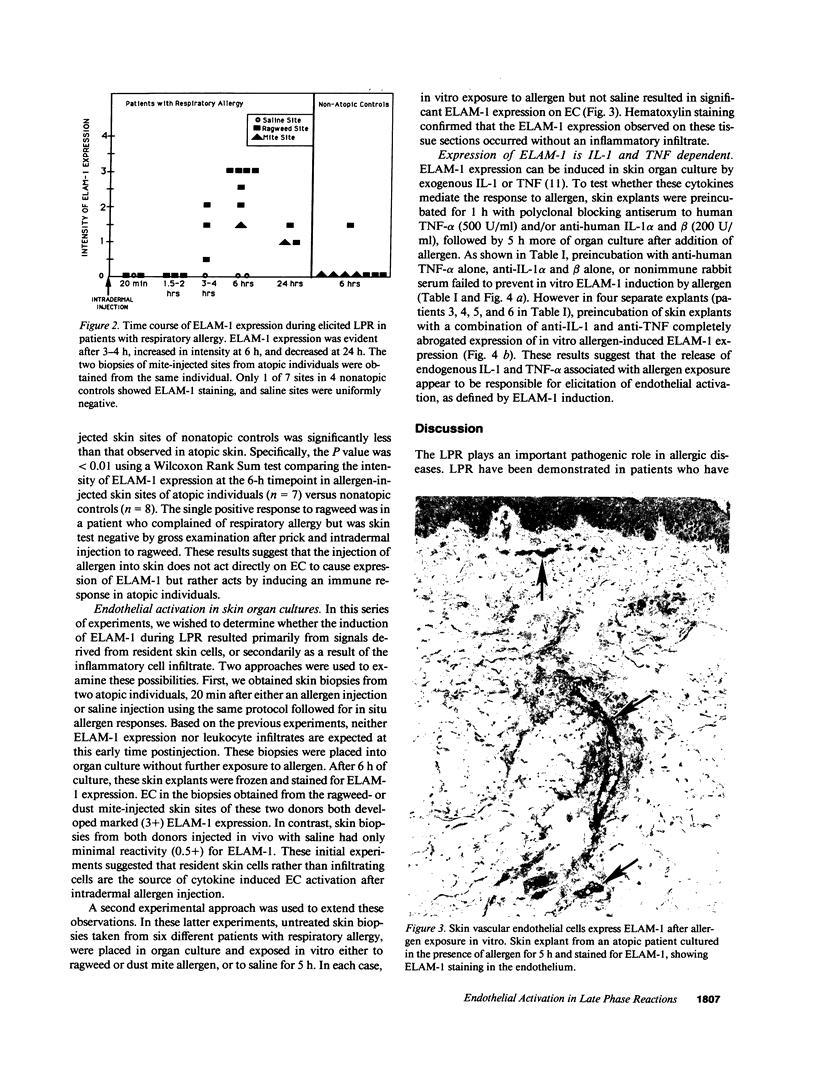


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bascom R., Wachs M., Naclerio R. M., Pipkorn U., Galli S. J., Lichtenstein L. M. Basophil influx occurs after nasal antigen challenge: effects of topical corticosteroid pretreatment. J Allergy Clin Immunol. 1988 Mar;81(3):580–589. [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd P. R., Rogers H. W., Gordon J. R., Martin C. A., Jayaraman S., Wilson S. D., Dvorak A. M., Galli S. J., Dorf M. E. Interleukin 3-dependent and -independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med. 1989 Jul 1;170(1):245–257. doi: 10.1084/jem.170.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier A., Thomson N. C., Frith P. A., Roberts R., Hargreave F. E. Allergen-induced increase in bronchial responsiveness to histamine: relationship to the late asthmatic response and change in airway caliber. J Allergy Clin Immunol. 1982 Sep;70(3):170–177. doi: 10.1016/0091-6749(82)90038-0. [DOI] [PubMed] [Google Scholar]
- Cockcroft D. W., Murdock K. Y. Comparative effects of inhaled salbutamol, sodium cromoglycate, and beclomethasone dipropionate on allergen-induced early asthmatic responses, late asthmatic responses, and increased bronchial responsiveness to histamine. J Allergy Clin Immunol. 1987 May;79(5):734–740. doi: 10.1016/0091-6749(87)90204-1. [DOI] [PubMed] [Google Scholar]
- Cotran R. S., Gimbrone M. A., Jr, Bevilacqua M. P., Mendrick D. L., Pober J. S. Induction and detection of a human endothelial activation antigen in vivo. J Exp Med. 1986 Aug 1;164(2):661–666. doi: 10.1084/jem.164.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolovich J., Hargreave F. E., Chalmers R., Shier K. J., Gauldie J., Bienenstock J. Late cutaneous allergic responses in isolated IgE-dependent reactions. J Allergy Clin Immunol. 1973 Jul;52(1):38–46. doi: 10.1016/0091-6749(73)90119-x. [DOI] [PubMed] [Google Scholar]
- Durham S. R., Lee T. H., Cromwell O., Shaw R. J., Merrett T. G., Merrett J., Cooper P., Kay A. B. Immunologic studies in allergen-induced late-phase asthmatic reactions. J Allergy Clin Immunol. 1984 Jul;74(1):49–60. doi: 10.1016/0091-6749(84)90086-1. [DOI] [PubMed] [Google Scholar]
- Gleich G. J. The late phase of the immunoglobulin E-mediated reaction: a link between anaphylaxis and common allergic disease? J Allergy Clin Immunol. 1982 Sep;70(3):160–169. doi: 10.1016/0091-6749(82)90037-9. [DOI] [PubMed] [Google Scholar]
- Klein L. M., Lavker R. M., Matis W. L., Murphy G. F. Degranulation of human mast cells induces an endothelial antigen central to leukocyte adhesion. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8972–8976. doi: 10.1073/pnas.86.22.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T. S. Production of cytokines by epithelial tissues. A new model for cutaneous inflammation. Am J Dermatopathol. 1989 Feb;11(1):69–73. doi: 10.1097/00000372-198902000-00011. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Fiers W., Pober J. S. Membrane interleukin 1 induction on human endothelial cells and dermal fibroblasts. J Immunol. 1987 Oct 1;139(7):2317–2324. [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Birinyi L. K., Auger K. R., Dinarello C. A. Inducible interleukin-1 gene expression in human vascular smooth muscle cells. J Clin Invest. 1986 Dec;78(6):1432–1438. doi: 10.1172/JCI112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messadi D. V., Pober J. S., Fiers W., Gimbrone M. A., Jr, Murphy G. F. Induction of an activation antigen on postcapillary venular endothelium in human skin organ culture. J Immunol. 1987 Sep 1;139(5):1557–1562. [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Cytokines and endothelial cell biology. Physiol Rev. 1990 Apr;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. The role of endothelial cells in inflammation. Transplantation. 1990 Oct;50(4):537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Warner J. O., Price J. F., Soothill J. F., Hey E. N. Controlled trial of hyposensitisation to Dermatophagoides pteronyssinus in children with asthma. Lancet. 1978 Oct 28;2(8096):912–915. doi: 10.1016/s0140-6736(78)91630-6. [DOI] [PubMed] [Google Scholar]
- Warner S. J., Libby P. Human vascular smooth muscle cells. Target for and source of tumor necrosis factor. J Immunol. 1989 Jan 1;142(1):100–109. [PubMed] [Google Scholar]