Abstract
Tuberculosis (TB) is a chronic infectious disease caused by Mycobacterium tuberculosis, with several million new cases detected each year. Current methods of diagnosis are time-consuming and/or expensive or have a low level of accuracy. Therefore, new diagnostics are urgently needed to address the global tuberculosis burden and to improve control programs. Serological assays remain attractive for use in resource-limited settings because they are simple, rapid, and inexpensive and offer the possibility of detecting cases often missed by routine sputum smear microscopy. The aim of this study was to identify M. tuberculosis seroreactive antigens from a panel of 103 recombinant proteins selected as diagnostic candidates. Initial library screening by protein array analysis and enzyme-linked immunosorbent assay (ELISA) identified 42 antigens with serodiagnostic potential. Among these, 25 were novel proteins. The reactive antigens demonstrated various individual sensitivities, ranging from 12% to 78% (specificities, 76 to 100%). When the antigens were analyzed in combinations, up to 93% of antibody responders could be identified among the TB patients. Selected seroreactive proteins were used to design 3 new polyepitope fusion proteins. Characterization of these antigens by multiantigen print immunoassay (MAPIA) revealed that the vast majority of the TB patients (90%) produced antibody responses. The results confirmed that due to the remarkable variation in immune recognition patterns, an optimal multiantigen cocktail should be designed to cover the heterogeneity of antibody responses and thus achieve the highest possible test sensitivity.
Tuberculosis (TB) is a chronic infectious disease caused by Mycobacterium tuberculosis and is one of the leading causes of mortality due to infectious disease worldwide (9). Nearly one-third of the world's population is believed to be infected, with approximately 8.8 million new cases detected each year (30, 45). The World Health Organization (WHO) cites TB as the single most important fatal infection, with over 1.6 million deaths per year, the majority (95%) of which are in developing countries (45).
Because of logistical and technical shortcomings, human TB testing in most countries is limited to clinical evaluation of symptomatic individuals and screening of high-risk populations. Compounding the severity of TB is the realization that a leading cause of death among HIV-positive people is concomitant TB, accounting for about one-third of AIDS-related deaths. It is estimated that a rapid and widely available diagnostic with 85% sensitivity and 95% specificity would result in 400,000 fewer deaths each year and would greatly reduce the global health cost of TB (18).
Existing TB diagnostic methods are either too time-consuming, too complex and labor-intensive, too inaccurate, or too expensive for routine use in resource-limited settings (2, 36). For active pulmonary disease, sputum smear microscopy, culture, and/or PCR-based probes can be used to support X-ray findings and/or clinical observations suggestive of TB. Of these, microscopic examination of sputum is the only rapid, relatively simple, and inexpensive test for TB. The reported sensitivity of Ziehl-Neelsen staining of unprocessed sputum smears from immunocompetent adults is only 40 to 70% (19, 21), and it may be significantly lower for children and/or HIV-infected patients (12). A delayed or missed TB diagnosis certainly contributes to M. tuberculosis transmission and increased TB mortality (22, 27).
Mycobacterial culture is the gold standard method of TB diagnosis. However, it requires up to 8 weeks for the isolation of M. tuberculosis from a clinical specimen, and importantly, in 10 to 20% of positive cases, the bacillus is not successfully cultured (3). Culture is more expensive than microscopy and requires a high standard of technical expertise. Therefore, a sensitive and specific point-of-care test for the rapid diagnosis of patients with active TB would facilitate early treatment and reduce M. tuberculosis transmission.
An antibody test for TB has long been sought. Serologic assays remain attractive for use in resource-limited settings because they generally are simple, rapid, and relatively inexpensive compared to other methods. For TB, serological tests may also offer the possibility of detecting cases that are usually missed by routine sputum smear microscopy, such as extrapulmonary disease and pediatric TB. Numerous in-house serological assays for TB, using a variety of antigens to detect circulating antibodies, have been developed over the years, including complement fixation tests, hemagglutination tests, radioimmunoassays, and enzyme-linked immunosorbent assays (ELISAs) (11, 38-40). Both lateral-flow and enzyme immunoassay formats have been developed and are currently available commercially, but so far none has demonstrated adequate sensitivity and specificity (7, 13, 31, 38).
In this study, we assessed a large panel of recombinant TB antigens for their serodiagnostic potential. From an initial screen of 103 recombinant proteins by protein microarray analysis and ELISA, 42 previously known and novel TB antigens were found to elicit specific antibody responses in TB patients. Several fusion proteins comprised of tandem arrangements of the selected antigens were made and serologically characterized by ELISA and multiple-antigen print immunoassays (MAPIA). The antigens identified hold promise for the development of a rapid and highly sensitive serodiagnostic test for TB.
MATERIALS AND METHODS
Study populations.
Serum samples from individuals who had pulmonary tuberculosis (culture and/or acid-fast bacterium [AFB] smear positive), collected prior to treatment, were obtained previously from Brazil (n = 92) (Roberto Badaro, Federal University of Bahia, Salvador, Brazil) (16). Serum samples obtained in India (from sputum smear-positive patients [n = 36] and sputum smear- and culture-negative controls from an area of malaria endemicity [endemic controls {EC}] [n = 20]) were obtained from the World Health Organization TB Specimen Bank. Samples from healthy U.S. blood donors (nonendemic controls [NEC] [n = 46]) were obtained from Boston Biomedica (West Bridgewater, MA). In all cases, drawing of blood was carried out with informed consent and the approval of the local ethics committee in the relevant country.
Antigen identification, cloning, and purification.
M. tuberculosis genes were selected as previously described (5). Briefly, M. tuberculosis genes included those previously identified by serological expression cloning and T-cell expression cloning methodologies at IDRI (23), those identified by proteomics as encoding secreted or membrane-associated proteins by two-dimensional PAGE (2D-PAGE) and mass spectrometry analysis (28; http://www.mpiib-berlin.mpg.de/2D-PAGE/) or as containing putative secretion signals, genes required for growth in macrophages (34), genes that were up- or downregulated in response to oxygen and carbon limitation (35), and mycobacterium-specific genes within the known immunogenic classes EsX and PE/PPE, based on Tuberculist (http://genolist.pasteur.fr/TubercuList/index.html). All targets were subjected to N-terminal signal sequence analysis and membrane-spanning region analysis, using the SignalP (http://www.cbs.dtu.dk/services/SignalP/) and TMPred (http://www.ch.embnet.org/software/TMPRED_form.html) programs. Predicted proteins were chosen as those containing fewer than three transmembrane regions and a molecular mass of 6 to 80 kDa.
DNAs encoding selected M. tuberculosis genes were PCR amplified from H37Rv genomic DNA by use of Pfx DNA polymerase (Invitrogen, Carlsbad, CA). PCR primers were designed to incorporate specific restriction enzyme sites 5′ and 3′ of the gene of interest and excluded in the target gene for directional cloning into the expression vector pET17b or pET28a (Novagen, Madison, WI). After PCR amplification, purified PCR products were digested with restriction enzymes, ligated into pET28a by use of T4 DNA ligase (NEB), and transformed into XL10G cells (Stratagene). Recombinant plasmid DNAs were recovered from individual colonies grown on LB agar plates containing appropriate antibiotics and were sequenced to confirm the correctly cloned coding sequence. The recombinant clones contained an N-terminal six-histidine tag followed by a thrombin cleavage site (pET28a) and the M. tuberculosis gene of interest.
The three fusion proteins described in this work (DID90A, DID90B, and DID104) were designed to incorporate specific restriction enzyme sites 5′ and 3′ of the gene of interest, with primer sequences as follows: Rv0934mat-5′HindIII, CAATTAAAGCTTTGTGGCTCGAAACCACCGAGC; Rv0934-3′SacI, CAATTAGAGCTCGCTGGAAATCGTCGCGATCAA; Rv2032-5′SacI, CAATTAGAGCTCATGCCGGACACCATGGTGACC; Rv2032-3′XhoI, CAATTACTCGAGCTACCGGTGATCCTTAGCCCG; Rv2031-5′NdeI-6his, CAATTACATATGCATCACCATCACCATCACATGGCCACCACCCTTCCCGTTC; Rv2031-3′HindIII, CAATTAAGCTTGTTGGTGGACCGGATCTGAATG; Rv2875mat-5′NdeI, CAATTACATATGCATCACCATCACCATCACGGCGATCTGGTGGGCCCG; Rv2875-3′HindIII, CAATTAAAGCTTCGCCGGAGGCATTAGCACGCT; Rv0831-5′NdeI-6his, CAGTTCCATATGCATCACCATCATCACCACATGCTCCCCGAGACAAATCAG; and Rv0831-3′HindIII, CTAGTCAAGCTTCTGGCGAAGCAGCTCATCTTTC. The Rv0934 and Rv2032 genes were PCR amplified from pET plasmid template DNA (30 cycles of 94°C for 30 s, 58°C for 30 s, and 58°C for 1 min 30 s). Rv0934 was restriction enzyme digested with HindIII and SacI and then cloned into the pET29a vector. The Rv2032 PCR product was digested with SacI and XhoI and ligated into the pEt29a-Rv0934 vector to create pET29a-Rv0934-Rv2032. Rv2031 was digested with NdeI and HindIII and cloned into the pET29a-Rv0934-Rv2032 vector. The resulting plasmid was sequence verified to contain the fusion gene construct DID90A (Rv2031-Rv0934-Rv2032). The pET29a-DID90A plasmid carries the coding sequence for a 90-kDa protein containing an N-terminal six-histidine tag, followed by the M. tuberculosis genes Rv2031, Rv0934 (C24 to S374), and Rv2032, separated by restriction site linkers. The Rv2875mat PCR product was digested with NdeI and HindIII, ligated into digested pET29a-DID90A vector, and sequence verified to generate pET29a-DID90B (Rv2875-Rv0934-Rv2032), carrying the coding sequence for a 91-kDa protein containing an N-terminal six-histidine tag, followed by the M. tuberculosis genes Rv2875 (G31-A193), Rv0934 (C24-S374), and Rv2032, separated by restriction site linkers. Rv0831 was digested with NdeI and HindIII and cloned into the digested pET29a-DID90A vector. The resulting plasmid was sequence verified to contain the fusion gene construct DID104 (Rv0831-Rv0934-Rv2032). The pET29a-DID104 plasmid carries the coding sequence for a 104-kDa protein containing an N-terminal six-histidine tag, followed by the M. tuberculosis genes Rv0831, Rv0934 (C24 to S374), and Rv2032, separated by restriction site linkers.
Recombinant plasmids were transformed into the Escherichia coli BL21 derivative Rosetta2(DE3)(pLysS) (Novagen). Recombinant strains were cultured overnight at 37°C in 2× yeast tryptone broth containing appropriate antibiotics, diluted 1/25 in fresh culture medium, grown to mid-log phase (optical density at 600 nm [OD600] of 0.5 to 0.7), and induced by the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). Cultures were grown for an additional 3 to 4 h, cells were harvested by centrifugation, and the bacterial pellets were stored at −20°C. Bacterial pellets were thawed and disrupted by sonication in 20 mM Tris (pH 8.0), 150 mM NaCl, and 1 mM phenylmethylsulfonyl fluoride (PMSF), followed by centrifugation to fractionate the soluble and insoluble material. Recombinant His-tagged protein products were isolated under native (soluble recombinant proteins) or denaturing (8 M urea) conditions, using Ni-nitrilotriacetic acid metal-ion-affinity chromatography according to the manufacturer's instructions (Qiagen, Valencia, CA), followed by ion-exchange chromatography (Bio-Rad, Hercules, CA) when necessary. Protein fractions were eluted with an increasing imidazole gradient and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Affinity-purified protein fractions were combined, dialyzed against 20 mM Tris, pH 8.0, concentrated using Amicon Ultra 10-kDa-cutoff centrifugal filters (Millipore, Billerica, MA), and quantified using the BCA protein assay (Pierce, Rockford, IL). Lipopolysaccharide (LPS) contamination was evaluated by the Limulus amoebocyte lysate assay (Cambrex Corp., East Rutherford, NJ). All of the recombinant proteins used in this study showed residual endotoxin levels of <100 endotoxin units (EU)/mg of protein.
Protein array serological screening.
Glass-based chips were fabricated with duplicate sets of a total of 79 recombinant M. tuberculosis proteins by Full Moon Biosystems, Sunnyvale, CA. Human IgG1 and EbaN1 were included as positive-control proteins to verify array development, and buffer alone was included as a background negative control. Sera were diluted 1/100 with blocking buffer and incubated with each slide at room temperature for 2 h. After being washed, slides were incubated with biotin-conjugated mouse anti-human IgG(H+L) (Jackson ImmunoResearch, West Grove, PA), washed, and then developed with Cy5-conjugated streptavidin (Martek Biosciences, Columbia, MD). Slides were scanned at 635 nm, using GenePix Pro 6.0 (Molecular Devices, Sunnyvale, CA). The signal intensity of binding of each antigen for each individual serum was normalized to the buffer-only spots for each individual serum to derive a fold-over-control (FOC) value. Positive TB reactivity was determined for individual proteins as a mean signal intensity of at least 3-fold above the mean for the control sera. Data tables were analyzed in MS Excel (Microsoft, Redmond, WA).
Antibody detection by ELISA.
Polysorp 96-well plates (Nunc, Rochester, NY) were coated with 50 μl of 2-μg/ml recombinant antigen in 0.1 M sodium bicarbonate, pH 9.6, overnight at 4°C and then blocked for 2 h at room temperature with phosphate-buffered saline plus 0.05% Tween 20 (PBST)-1% (wt/vol) bovine serum albumin (BSA) on a plate shaker. Sera were diluted 1:100 in PBST-0.1% BSA in duplicate and added to each well. Plates were incubated at room temperature for 2 h with shaking and then washed with PBST with 0.1% BSA, and then horseradish peroxidase (HRP)-conjugated anti-human IgG (Sigma, St. Louis, MO), diluted 1:10,000 in PBST with 0.1% BSA, was added to each well and incubated at room temperature for 60 min with shaking. After being washed, plates were developed with peroxidase color substrate (KPL, Baltimore, MD), and the reaction was quenched by addition of 1 N H2SO4 after 15 min. The corrected optical density of each well at 450 to 570 nm was read using a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA). Positive ELISA responses were defined as optical density readings of >2-fold above the mean for the control sera, with a minimum defined optical density cutoff of 0.2.
Antigen evaluation by MAPIA.
MAPIA was performed as previously described (26). Briefly, a semiautomatic microaerosolization device (Linomat IV; Camag Scientific Inc., Wilmington, DE) was used to spray antigens at a range of concentrations (between 0.02 mg/ml and 0.1 mg/ml) through a syringe needle onto nitrocellulose membranes (Schleicher & Schuell, Inc., Keene, NH) to generate parallel bands. After antigen printing, the membrane was cut into 3-mm-wide strips perpendicular to the antigen bands. The strips were blocked for 1 h with 1% nonfat milk in PBST and then incubated with individual serum samples diluted 1:50 in blocking solution for 1 h at room temperature. The strips were washed five times with PBST and incubated for 1 h with alkaline phosphatase-conjugated anti-human IgG diluted 1:5,000 (Sigma, St. Louis, MO). The strips were washed with PBST as described above, and the human IgG antibodies bound to immobilized antigens were visualized with 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium substrate (KPL). MAPIA results were scored by two independent operators who were unaware of the sample status. The appearance of any band of any intensity was read as a positive reaction.
RESULTS
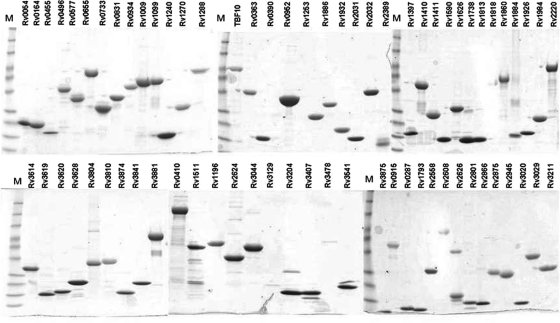
M. tuberculosis protein array screening for seroreactivity.
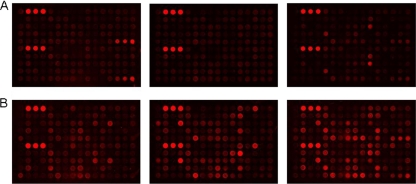
In previous work, we described the selection of a large body of M. tuberculosis antigens by use of data mining techniques to define new antigens with T-cell reactivity and vaccine potential (5). In this study, we examined the humoral immune response to these antigens by protein array analysis and ELISA to identify antigens with diagnostic potential. Throughout this study, a total of 103 recombinant M. tuberculosis proteins were produced in E. coli, with the majority achieving >95% purity according to visualization of SDS-PAGE gels (Fig. 1). We fabricated glass-based protein arrays to comprehensively analyze the diagnostic potential of a large number of M. tuberculosis proteins in a consistent and comparable fashion. A total of 79 M. tuberculosis proteins were expressed, immobilized on glass-based arrays, and tested with 32 sera from sputum-positive TB patients and with 16 NEC sera. Several proteins were recognized and bound by IgG within serum samples and could be grouped as (i) TB sensitive but lacking specificity (i.e., binding TB patient sera but also binding some NEC sera) or (ii) TB specific (i.e., binding specific patient sera but not NEC sera). A total of 28 M. tuberculosis proteins displayed TB-specific reactivity, with a mean signal intensity of at least 3-fold above the control level (Fig. 2 and Table 1).
FIG. 1.
SDS-PAGE analysis of purified recombinant M. tuberculosis proteins. Individual M. tuberculosis proteins are listed by their H37Rv gene numbers. Two to 5 μg of each antigen was run in a 4 to 20% SDS-PAGE gel and stained with Coomassie blue to determine its relative purity. Lanes M, molecular size standards (160, 120, 80, 60, 40, 25, 20, 15, and 10 kDa).
FIG. 2.
Serum reactivity in recombinant M. tuberculosis protein arrays. Seventy-nine M. tuberculosis proteins were printed in duplicate, incubated with TB-positive or NEC sera, developed, and scanned. Representative images of 3 control sera (A) and 3 TB-positive sera (B) on protein arrays are shown.
TABLE 1.
Serologic responses to M. tuberculosis proteins in protein array and ELISA screens
| H37Rv locus tag | Gene name | Molecular mass (kDa) | Reference for previously described immune function | Functional categorya | Initial target selectionb | Protein array FOC value | ELISA antibody responsec |
|---|---|---|---|---|---|---|---|
| Rv0054 | ssb | 17.3 | 2 | S | 4 | + | |
| Rv0164 | TB18.5 | 17.7 | 10 | S | 3 | ++ | |
| Rv0410 | pnkG | 81.6 | 9 | S | 3 | + | |
| Rv0455c | Hypothetical | 16.6 | 10 | S | 1 | + | |
| Rv0655 | mkl | 39.3 | 3 | M | 4 | ++ | |
| Rv0831c | Hypothetical | 30.2 | 10 | S | 2 | +++ | |
| Rv0934 | pstS1 | 38.2 | 17 | 3 | S | 4 | +++ |
| Rv0952 | sucD | 31.2 | 7 | B | 3 | − | |
| Rv1009 | rpfB | 38 | 3 | M | 4 | + | |
| Rv1099 | Hypothetical | 34.6 | 10 | M | 3 | ++ | |
| Rv1240 | mdh | 34.3 | 7 | H | ND | + | |
| Rv1288 | Hypothetical | 49.6 | 10 | B | 3 | + | |
| Rv1410c | p55 | 54.7 | 3 | M | 3 | + | |
| Rv1569 | bioF1 | 40 | 7 | M | ND | ++ | |
| Rv1789 | PPE26 | 38.6 | 6 | P/E | 3 | ++ | |
| Rv1813c | Hypothetical | 15 | 4 | 10 | H | 3 | − |
| Rv1827 | cfp17 | 17.2 | 43 | 10 | EC | ND | + |
| Rv1837 | mtb81 | 80.7 | 14 | 10 | M | ND | ++ |
| Rv1860 | apa | 32.7 | 8 | 3 | S | 3 | + |
| Rv1886c | fpbB | 34.6 | 42 | 1 | S | 3 | +++ |
| Rv1908 | katG | 80.6 | 46 | 0 | M | ND | + |
| Rv1984c | cfp21 | 21.8 | 44 | 3 | S | 3 | −/+ |
| Rv2031 | acr | 16.2 | 20 | 0 | S | 3 | + |
| Rv2032 | acg | 36.6 | 10 | H | 5 | +++ | |
| Rv2220 | glnA1 | 53.5 | 41 | 7 | S | 1 | ++ |
| Rv2450 | rpfE | 17.4 | 3 | B | 4 | −/+ | |
| Rv2608 | PPE42 | 59.7 | 6 | 6 | P/E | 2 | +++ |
| Rv2623 | TB31.7 | 31.7 | 10 | H | 4 | + | |
| Rv2866 | Hypothetical | 10.2 | 10 | H | 7 | + | |
| Rv2873 | mpt83 | 20 | 15 | 3 | S | 3 | ++ |
| Rv2875 | mpt70 | 19.1 | 32 | 3 | S | 4 | +++ |
| Rv3020 | esxS | 9.8 | 3 | P/E | 3 | −/+ | |
| Rv3044 | fecB | 36.9 | 3 | H | 1 | + | |
| Rv3310 | sapM | 31.8 | 3 | S | ND | ++ | |
| Rv3407 | Hypothetical | 11 | 29 | 0 | B | 3 | −/+ |
| Rv3611 | Hypothetical | 23.8 | 16 | M | 3 | − | |
| Rv3614 | Hypothetical | 19.8 | 10 | M | 5 | ++ | |
| Rv3619 | esxI | 9.8 | 3 | P/E | 6 | + | |
| Rv3628 | ppa | 18.3 | 7 | S | 3 | −/+ | |
| Rv3841 | bfrB | 20.4 | 33 | 7 | EC | ND | ++ |
| Rv3874 | cfp10 | 10.8 | 10 | 3 | P/E | ND | ++ |
| Rv3881 | mtb48 | 47.6 | 24 | 10 | S | ND | ++ |
As defined by Tuberculist (http://genolist.pasteur.fr/TubercuList/index.html). 0, virulence, detoxification, adaptation; 1, lipid metabolism; 2, information pathways; 3, cell wall and cell processes; 6, PE/PPE proteins; 7, intermediary metabolism; 8, unknown; 9, regulatory proteins; 10, conserved hypothetical proteins; 16, conserved hypothetical proteins with an ortholog in M. bovis.
EC, expression cloning; S, secreted proteins; P/E: PE, PPE, and EsX proteins; M, macrophage growth required; H, hypoxic response; B, other database searches.
−, <2-fold mean difference between TB-positive and -negative controls; −/+, near mean cutoff with fewer than 12% of the TB-positive serum samples; +, ++, and +++, percentages of TB-positive responders (12 to 28%, 29 to 43%, and >44%, respectively).
M. tuberculosis antigen characterization by ELISA.
To confirm the protein array results and to further test M. tuberculosis recombinant proteins not available at the time of protein array generation (n = 24), ELISA screening was performed using the same serum set used for the protein arrays. TB-positive antibody responses were observed by ELISA for 34 proteins, including 20 of the 28 antigens that were positive in the protein array screen. Antibody responses observed for 5 proteins were below the FOC cutoff of 3-fold by protein array analysis, as were those for 9 additional proteins not present on the arrays (Table 1). A total of 42 proteins were found to bind antibodies in the sera of TB patients by either protein array or ELISA screening, as shown in Table 1. These included the following 17 previously described immunogenic M. tuberculosis proteins: Rv0934 (38 kDa) (17), Rv1813 (4), Rv1827 (Cfp17) (43), Rv1837 (GlcB) (14), Rv1860 (DPEP) (8), Rv1886 (Ag85b) (42), Rv1908 (KatG) (46), Rv1984 (44), Rv2031 (α-crystallin) (20), Rv2220 (GlnA1) (41), Rv2608 (PPE42) (6), Rv2873 (mpt83) (15), Rv2875 (mpt70) (32), Rv3407(29), Rv3841 (Bfrb) (33), Rv3874 (Cfp10) (10), and Rv3881 (Mtb48) (24). They also included 25 previously uncharacterized M. tuberculosis antigens (Rv0054, Rv0164, Rv0410, Rv0455, Rv0655, Rv0831, Rv0952, Rv1009, Rv1099, Rv1240, Rv1288, Rv1410, Rv1569, Rv1789, Rv2032, Rv2450, Rv2623, Rv2866, Rv3020, Rv3044, Rv3310, Rv3611, Rv3614, Rv3619, and Rv3628). The remainder of the recombinant antigens tested either failed to elicit significant antibody responses by this serum set or showed nonspecific binding with the control serum samples and therefore were excluded from further analysis.
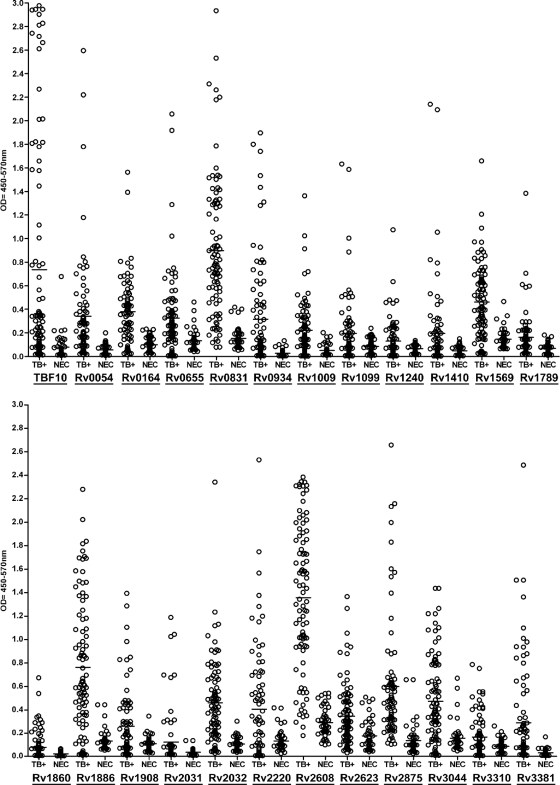
The antigens eliciting specific antibody responses in the initial screen by ELISA were further characterized with a larger panel of 92 serum samples from sputum-positive TB patients from Brazil and 46 control sera. Representative ELISA results are summarized in Fig. 3. TBF10, a previously characterized fusion consisting of three proteins (Rv0379, Rv0934, and Rv3874), was used as a reference antigen (16). TBF10 elicited antibody responses in 53 of the 92 TB sera (sensitivity, 58%; specificity, 89%). The recombinant antigens demonstrated various sensitivities in ELISA, ranging from 12% to 76%, with low or no reactivity with NEC sera (specificity, 70 to 100%). Several antigens had individual sensitivities and specificities exceeding those of TBF10. These were Rv0831 (76% sensitivity and 89% specificity), Rv2875 (74% sensitivity and 91% specificity), Rv1886 (74% sensitivity and 87% specificity), and Rv2032 (70% sensitivity and 96% specificity). The Rv2608 antigen appeared to recognize a large proportion of the TB sera but had high levels of background binding (specificity, 70%). When antigen profiles of individual serum reactivities were analyzed, the combination of Rv2875, Rv2031, Rv2032, Rv0831, and TBF10 was able to elicit antibody responses in 86 of the 92 Brazilian TB samples (93% sensitivity), while 6 of 46 NEC samples (87% specificity) reacted with one or more of these antigens. The 6 remaining TB-positive samples failed to elicit antibody responses to any of the antigens or to a preparation of M. tuberculosis whole-cell lysate (data not shown).
FIG. 3.
ELISA results for recombinant M. tuberculosis proteins. TB+, confirmed sputum smear-positive pulmonary TB samples (n = 92) from Brazil; NEC, negative, nonendemic (U.S.) control sera (n = 46). Representative data for 24 recombinant M. tuberculosis antigens are shown. The median OD is represented by a horizontal line. Individual antigens are listed at the bottom of each graph, with positive samples determined as those samples giving ELISA readings 2-fold above the mean of the negative controls, with an OD450 of >0.2.
Design of polyprotein fusions.
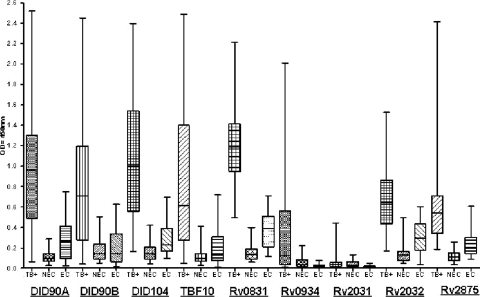
Due to the heterogeneity of the antibody responses observed in TB patients, multiple antigens are necessary to increase the sensitivity of serodiagnostic tests. Based on the above ELISA antigen recognition patterns, we developed a series of fusion proteins, designated DID90A (Rv2031-Rv0934-Rv2032), DID90B (Rv2875-Rv0934-Rv2032), and DID104 (Rv0831-Rv0934-Rv2032), to assess the ability of these antigens to complement each other when arranged in tandem. The antigen fusions and individual antigen components were assessed by ELISA, using a panel of 36 sputum smear-positive samples and 20 EC samples from India, with comparison to 20 NEC sera. As shown in Fig. 4, the DID90A and DID90B fusion proteins demonstrated reactivity profiles with the Indian TB and EC samples similar to that obtained for the TBF10 antigen (61% sensitivity and 85% specificity), with the DID104 fusion performing slightly better (69% sensitivity and 85% specificity). Some differences among the individual antigens were observed within the Brazilian and Indian serum cohorts. Among the Indian TB-positive samples, Rv0831 had increased sensitivity (83%), but it also cross-reacted with the EC sera (70% sensitivity). Rv2875 (55% sensitivity and 90% specificity) and Rv2032 (53% sensitivity and 85% specificity) had slight decreases in sensitivity but similar specificities. The Rv0934 antigen exhibited similar reactivity profiles in both the Brazilian and Indian cohorts (41% sensitivity and 95% specificity), while Rv2031 was poorly recognized within the Indian serum samples.
FIG. 4.
Fusion protein serum ELISA. Results for DID90A, DID90B, DID104, TBF10, and the individual component antigens are displayed as box plots. TB+, confirmed pulmonary TB samples (n = 36) from India; NEC, negative, nonendemic (U.S.) control sera (n = 30); EC, negative, endemic (Indian) control sera (n = 20). Each box represents data from the 25th to the 75th percentile, the median is represented by a horizontal line, and the whiskers extend to the lowest and highest values.
Characterization of M. tuberculosis antigens by MAPIA.
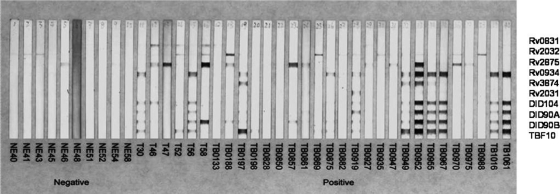
We used MAPIA to validate the selected antigens and fusion molecules most suitable for developing rapid lateral-flow assays. MAPIA involves the immobilization of multiple antigens on nitrocellulose membranes and provides a valuable means to characterize individual recognition patterns. We previously found that the serological performance of antigens in MAPIA is a good predictor of their performance in other membrane-based assays (26). The four fusion proteins, along with the single component antigens, were evaluated by MAPIA. Figure 5 demonstrates the presence in most TB sera of IgG antibodies against several single antigens and fusion proteins. Antibody responses to at least one antigen could be detected in 27 of 30 TB-positive serum samples, while no or very weak bands were observed for the negative-control group.
FIG. 5.
M. tuberculosis antigen reactivity in MAPIA. The 4 fusion polyproteins and 6 single antigens were printed on nitrocellulose membranes (listed on the right), and the assay was performed as described in Materials and Methods. Each strip represents one serum sample and displays the antigen reactivity pattern for that sample. Results are shown for 10 negative-control sera (left) and 30 sera from TB patients, including 6 from India and 24 from Brazil (right).
DISCUSSION
It has been suggested that implementation of rapid serological tests would be useful in combination with other methods for diagnosis of active TB in settings where bacterial culture is not routinely available (2). However, so far, no rapid serodiagnostic test has proven reproducibly accurate, preventing widespread application of such tests. Antibody responses in TB are directed against a broad set of antigens, with remarkable patient-to-patient variation in antigen recognition (1). Even taking this variation into account, the sensitivities of tests have generally been poor (1, 3, 25). The low specificities of antibody-based tests evaluated to date may result from the presence of antibodies in response to any of the following circumstances: latent TB, inactive (treated) disease, prior vaccination with Mycobacterium bovis bacillus Calmette-Guérin (BCG), or exposure to non-M. tuberculosis mycobacteria. Since these conditions may influence the performance of serological assays, reported results obtained in different clinical settings vary significantly (9), with test sensitivities ranging from 10 to 90% and specificities ranging from 47 to 100% (31, 38, 40). Higher test sensitivities are typically associated with lower specificities, and vice versa; no commercial serologic test is currently available that meets acceptable levels of sensitivity and specificity (38). Despite these limitations, the interest in developing simple formats for rapid TB diagnosis remains high for field implementation in resource-limited settings.
We expressed and purified over 100 potential M. tuberculosis proteins selected by genome and database mining. We previously described the T-cell reactivity and vaccine potential of some of these antigens (5). The present study examined the serodiagnostic value of the candidate molecules by protein array analysis, ELISA, and MAPIA. As expected, many of the proteins were nonreactive with TB patient sera, while others reacted with both TB patient and control sera. Such proteins were excluded from further analyses. From the initial protein array and ELISA screens, 42 antigens demonstrated various degrees of reactivity with TB patient sera. Among these, 17 antigens were previously reported (4, 6, 8, 10, 14-17, 20, 24, 29, 32, 33, 41-44, 46), while the remaining 25 proteins were previously uncharacterized. These antigens included 16 presumptively secreted or membrane-associated antigens, 8 antigens expressed from genes required for growth in macrophages, 6 antigens induced by hypoxia, 5 antigens associated with virulence, from the PE/PPE and EsX classes, and 4 antigens from other database searches. While there was generally good concordance between the assays, with 20 of 28 proteins positive for specific TB seroreactivity, some differences were observed. Eight antigens positive by protein array (Rv0952, Rv1813, Rv1984, Rv2450, Rv3020, Rv3407, and Rv3611) showed low responses by ELISA or lacked specificity within the serum subsets; conversely, 5 proteins positive by ELISA (Rv0455, Rv0831, Rv2220, Rv2608, and Rv3044) failed to demonstrate responses above the cutoff values in protein arrays. These discrepancies may be due to variable coating efficiencies of antigens on the different immobilization surfaces or to differences between assays in calculating cutoff values.
The seroreactive TB antigens were analyzed for responses to a larger panel of TB serum samples from sputum smear-positive patients and to NEC sera to further reduce the antigen complexity down to those most useful at diagnosing active TB. The antigens demonstrated various individual sensitivities, ranging from 12% to 78%, with generally low background binding (specificity, ∼76 to 100%). Typically, antigens with low sensitivities had high specificities (for Rv1860, 12% sensitivity and 100% specificity; for Rv3874, 16% sensitivity and 100% specificity), while increasing sensitivity resulted in decreased specificity (for Rv2608, 78% sensitivity and 76% specificity; for Rv1886, 74% sensitivity and 87% specificity). Based on additive responses among individual serum samples, Rv0934, Rv3874, Rv2875, Rv2031, Rv2032, and Rv0831 defined the minimal subset of antigens necessary to provide the greatest overall sensitivity. When these seroreactive antigens were analyzed in combinations, 93% of antibody responders could be identified among the TB patients. A number of the antigens described (Rv0455, Rv3619, Rv3310, Rv1410, and Rv1240) had redundant patterns of reactivity with other antigens and therefore did not increase the overall sensitivity.
The generation of fusion proteins has been used as a means to reduce the cost and complexity of antigen cocktails in rapid lateral-flow formats and to increase sensitivity and specificity (16, 37). We generated a series of related fusion proteins and tested them by ELISA, along with the individual antigen components. The three new fusions demonstrated similar sensitivities and specificities with a serum panel from India and were comparable to the previously reported reference antigen TBF10 (16). Some differences in ELISA reactivity were observed between the NEC sera from the United States and the EC sera from India, with slight increases in reactivity noted for all fusions and individual proteins with the endemic controls (Fig. 4). The Indian endemic control samples obtained were defined as TB sputum smear and culture negative, though the presence of latent TB or other mycobacterial infection was not assessed and cannot be ruled out as the basis of the reactivity difference. Note that few of the proteins described in this study are considered M. tuberculosis specific and that they may have regions of homology with environmental mycobacteria. Nevertheless, we observed good discrimination between the Indian TB-positive serum set and the EC serum set.
The remarkable variation in immune recognition patterns for TB requires multiantigen cocktails to cover the heterogeneous antibody responses and thus achieve the highest possible test sensitivity. MAPIA using the fusion antigens and selected individual components also demonstrated that the vast majority of the TB patients (90%) produced antibody responses to one or more antigens, with a combination of 6 proteins (Rv0831, Rv2031, Rv2032, Rv2875, Rv0934, and Rv3874) needed for the greatest sensitivity. Refinement of antigen cocktails and/or the production of fusion molecules comprised of antigens described herein may lead to improved sensitivity and specificity for the development of a rapid, accurate, and inexpensive point-of-care diagnostic test. Studies to achieve this goal are in progress.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants AI-044373 and AI-067251 to S.G.R. and AI079884-01 to K.P.L.
We thank Yasuyuki Goto, Wakako Goto, Jacqueline Whittle, and Rhea Coler for technical assistance and critical comments for manuscript preparation.
Footnotes
Published ahead of print on 18 August 2010.
REFERENCES
- 1.Abebe, F., C. Holm-Hansen, H. G. Wiker, and G. Bjune. 2007. Progress in serodiagnosis of Mycobacterium tuberculosis infection. Scand. J. Immunol. 66:176-191. [DOI] [PubMed] [Google Scholar]
- 2.Al Zahrani, K., H. Al Jahdali, L. Poirier, P. Rene, M. L. Gennaro, and D. Menzies. 2000. Accuracy and utility of commercially available amplification and serologic tests for the diagnosis of minimal pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 162:1323-1329. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin, S. L., S. Bertholet, M. Kahn, I. Zharkikh, G. C. Ireton, T. S. Vedvick, S. G. Reed, and R. N. Coler. 2009. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine 27:3063-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertholet, S., G. C. Ireton, M. Kahn, J. Guderian, R. Mohamath, N. Stride, E. M. Laughlin, S. L. Baldwin, T. S. Vedvick, R. N. Coler, and S. G. Reed. 2008. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J. Immunol. 181:7948-7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakhaiyar, P., Y. Nagalakshmi, B. Aruna, K. J. Murthy, V. M. Katoch, and S. E. Hasnain. 2004. Regions of high antigenicity within the hypothetical PPE major polymorphic tandem repeat open-reading frame, Rv2608, show a differential humoral response and a low T cell response in various categories of patients with tuberculosis. J. Infect. Dis. 190:1237-1244. [DOI] [PubMed] [Google Scholar]
- 7.Chan, E. D., L. Heifets, and M. D. Iseman. 2000. Immunologic diagnosis of tuberculosis: a review. Tuber. Lung Dis. 80:131-140. [DOI] [PubMed] [Google Scholar]
- 8.Chanteau, S., V. Rasolofo, T. Rasolonavalona, H. Ramarokoto, C. Horn, G. Auregan, and G. Marchal. 2000. 45/47 kilodalton (APA) antigen capture and antibody detection assays for the diagnosis of tuberculosis. Int. J. Tuber. Lung Dis. 4:377-383. [PubMed] [Google Scholar]
- 9.Corbett, E. L., C. J. Watt, N. Walker, D. Maher, B. G. Williams, M. C. Raviglione, and C. Dye. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009-1021. [DOI] [PubMed] [Google Scholar]
- 10.Dillon, D. C., M. R. Alderson, C. H. Day, T. Bement, A. Campos-Neto, Y. A. Skeiky, T. Vedvick, R. Badaro, S. G. Reed, and R. Houghton. 2000. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J. Clin. Microbiol. 38:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinnes, J., J. Deeks, H. Kunst, A. Gibson, E. Cummins, N. Waugh, F. Drobniewski, and A. Lalvani. 2007. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol. Assess. 11:1-196. [DOI] [PubMed] [Google Scholar]
- 12.Elliott, A. M., K. Namaambo, B. W. Allen, N. Luo, R. J. Hayes, J. O. Pobee, and K. P. McAdam. 1993. Negative sputum smear results in HIV-positive patients with pulmonary tuberculosis in Lusaka, Zambia. Tuber. Lung Dis. 74:191-194. [DOI] [PubMed] [Google Scholar]
- 13.Gounder, C., F. C. De Queiroz Mello, M. B. Conde, W. R. Bishai, A. L. Kritski, R. E. Chaisson, and S. E. Dorman. 2002. Field evaluation of a rapid immunochromatographic test for tuberculosis. J. Clin. Microbiol. 40:1989-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendrickson, R. C., J. F. Douglass, L. D. Reynolds, P. D. McNeill, D. Carter, S. G. Reed, and R. L. Houghton. 2000. Mass spectrometric identification of mtb81, a novel serological marker for tuberculosis. J. Clin. Microbiol. 38:2354-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hewinson, R. G., S. L. Michell, W. P. Russell, R. A. McAdam, and W. R. Jacobs, Jr. 1996. Molecular characterization of MPT83: a seroreactive antigen of Mycobacterium tuberculosis with homology to MPT70. Scand. J. Immunol. 43:490-499. [DOI] [PubMed] [Google Scholar]
- 16.Houghton, R. L., M. J. Lodes, D. C. Dillon, L. D. Reynolds, C. H. Day, P. D. McNeill, R. C. Hendrickson, Y. A. Skeiky, D. P. Sampaio, R. Badaro, K. P. Lyashchenko, and S. G. Reed. 2002. Use of multiepitope polyproteins in serodiagnosis of active tuberculosis. Clin. Diagn. Lab. Immunol. 9:883-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadival, G. V., S. D. Chaparas, and D. Hussong. 1987. Characterization of serologic and cell-mediated reactivity of a 38-kDa antigen isolated from Mycobacterium tuberculosis. J. Immunol. 139:2447-2451. [PubMed] [Google Scholar]
- 18.Keeler, E., M. D. Perkins, P. Small, C. Hanson, S. Reed, J. Cunningham, J. E. Aledort, L. Hillborne, M. E. Rafael, F. Girosi, and C. Dye. 2006. Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature 444(Suppl. 1):49-57. [DOI] [PubMed] [Google Scholar]
- 19.Kim, T. C., R. S. Blackman, K. M. Heatwole, T. Kim, and D. F. Rochester. 1984. Acid-fast bacilli in sputum smears of patients with pulmonary tuberculosis. Prevalence and significance of negative smears pretreatment and positive smears post-treatment. Am. Rev. Respir. Dis. 129:264-268. [PubMed] [Google Scholar]
- 20.Lee, B. Y., S. A. Hefta, and P. J. Brennan. 1992. Characterization of the major membrane protein of virulent Mycobacterium tuberculosis. Infect. Immun. 60:2066-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy, H., C. Feldman, H. Sacho, H. van der Meulen, J. Kallenbach, and H. Koornhof. 1989. A reevaluation of sputum microscopy and culture in the diagnosis of pulmonary tuberculosis. Chest 95:1193-1197. [DOI] [PubMed] [Google Scholar]
- 22.Lienhardt, C., J. Rowley, K. Manneh, G. Lahai, D. Needham, P. Milligan, and K. P. McAdam. 2001. Factors affecting time delay to treatment in a tuberculosis control programme in a sub-Saharan African country: the experience of The Gambia. Int. J. Tuber. Lung Dis. 5:233-239. [PubMed] [Google Scholar]
- 23.Lodes, M. J., D. C. Dillon, R. L. Houghton, and Y. A. Skeiky. 2004. Expression cloning. Methods Mol. Med. 94:91-106. [DOI] [PubMed] [Google Scholar]
- 24.Lodes, M. J., D. C. Dillon, R. Mohamath, C. H. Day, D. R. Benson, L. D. Reynolds, P. McNeill, D. P. Sampaio, Y. A. Skeiky, R. Badaro, D. H. Persing, S. G. Reed, and R. L. Houghton. 2001. Serological expression cloning and immunological evaluation of MTB48, a novel Mycobacterium tuberculosis antigen. J. Clin. Microbiol. 39:2485-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyashchenko, K., R. Colangeli, M. Houde, H. Al Jahdali, D. Menzies, and M. L. Gennaro. 1998. Heterogeneous antibody responses in tuberculosis. Infect. Immun. 66:3936-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyashchenko, K. P., M. Singh, R. Colangeli, and M. L. Gennaro. 2000. A multi-antigen print immunoassay for the development of serological diagnosis of infectious diseases. J. Immunol. Methods 242:91-100. [DOI] [PubMed] [Google Scholar]
- 27.MacIntyre, C. R., A. J. Plant, J. Hulls, J. A. Streeton, N. M. Graham, and G. J. Rouch. 1995. High rate of transmission of tuberculosis in an office: impact of delayed diagnosis. Clin. Infect. Dis. 21:1170-1174. [DOI] [PubMed] [Google Scholar]
- 28.Mattow, J., U. E. Schaible, F. Schmidt, K. Hagens, F. Siejak, G. Brestrich, G. Haeselbarth, E. C. Muller, P. R. Jungblut, and S. H. Kaufmann. 2003. Comparative proteome analysis of culture supernatant proteins from virulent Mycobacterium tuberculosis H37Rv and attenuated M. bovis BCG Copenhagen. Electrophoresis 24:3405-3420. [DOI] [PubMed] [Google Scholar]
- 29.Mollenkopf, H. J., L. Grode, J. Mattow, M. Stein, P. Mann, B. Knapp, J. Ulmer, and S. H. Kaufmann. 2004. Application of mycobacterial proteomics to vaccine design: improved protection by Mycobacterium bovis BCG prime-Rv3407 DNA boost vaccination against tuberculosis. Infect. Immun. 72:6471-6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perkins, M. D., and J. Cunningham. 2007. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. J. Infect. Dis. 196(Suppl. 1):S15-S27. [DOI] [PubMed] [Google Scholar]
- 31.Pottumarthy, S., V. C. Wells, and A. J. Morris. 2000. A comparison of seven tests for serological diagnosis of tuberculosis. J. Clin. Microbiol. 38:2227-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roche, P. W., J. A. Triccas, D. T. Avery, T. Fifis, H. Billman-Jacobe, and W. J. Britton. 1994. Differential T cell responses to mycobacteria-secreted proteins distinguish vaccination with bacille Calmette-Guerin from infection with Mycobacterium tuberculosis. J. Infect. Dis. 170:1326-1330. [DOI] [PubMed] [Google Scholar]
- 33.Sartain, M. J., R. A. Slayden, K. K. Singh, S. Laal, and J. T. Belisle. 2006. Disease state differentiation and identification of tuberculosis biomarkers via native antigen array profiling. Mol. Cell. Proteomics 5:2102-2113. [DOI] [PubMed] [Google Scholar]
- 34.Sassetti, C. M., and E. J. Rubin. 2003. Genetic requirements for mycobacterial survival during infection. Proc. Natl. Acad. Sci. U. S. A. 100:12989-12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherman, D. R., M. Voskuil, D. Schnappinger, R. Liao, M. I. Harrell, and G. K. Schoolnik. 2001. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc. Natl. Acad. Sci. U. S. A. 98:7534-7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, M. B., J. S. Bergmann, M. Onoroto, G. Mathews, and G. L. Woods. 1999. Evaluation of the enhanced amplified Mycobacterium tuberculosis direct test for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. Arch. Pathol. Lab. Med. 123:1101-1103. [DOI] [PubMed] [Google Scholar]
- 37.Steingart, K. R., N. Dendukuri, M. Henry, I. Schiller, P. Nahid, P. C. Hopewell, A. Ramsay, M. Pai, and S. Laal. 2009. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin. Vaccine Immunol. 16:260-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steingart, K. R., M. Henry, S. Laal, P. C. Hopewell, A. Ramsay, D. Menzies, J. Cunningham, K. Weldingh, and M. Pai. 2007. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med. 4:e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steingart, K. R., M. Henry, S. Laal, P. C. Hopewell, A. Ramsay, D. Menzies, J. Cunningham, K. Weldingh, and M. Pai. 2007. A systematic review of commercial serological antibody detection tests for the diagnosis of extrapulmonary tuberculosis. Thorax 62:911-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steingart, K. R., A. Ramsay, and M. Pai. 2007. Optimizing sputum smear microscopy for the diagnosis of pulmonary tuberculosis. Expert Rev. Anti Infect. Ther. 5:327-331. [DOI] [PubMed] [Google Scholar]
- 41.Tullius, M. V., G. Harth, and M. A. Horwitz. 2003. Glutamine synthetase GlnA1 is essential for growth of Mycobacterium tuberculosis in human THP-1 macrophages and guinea pigs. Infect. Immun. 71:3927-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Vooren, J. P., A. Drowart, M. De Cock, A. Van Onckelen, M. H. D'Hoop, J. C. Yernault, C. Valcke, and K. Huygen. 1991. Humoral immune response of tuberculous patients against the three components of the Mycobacterium bovis BCG 85 complex separated by isoelectric focusing. J. Clin. Microbiol. 29:2348-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weldingh, K., and P. Andersen. 1999. Immunological evaluation of novel Mycobacterium tuberculosis culture filtrate proteins. FEMS Immunol. Med. Microbiol. 23:159-164. [DOI] [PubMed] [Google Scholar]
- 44.Weldingh, K., I. Rosenkrands, S. Jacobsen, P. B. Rasmussen, M. J. Elhay, and P. Andersen. 1998. Two-dimensional electrophoresis for analysis of Mycobacterium tuberculosis culture filtrate and purification and characterization of six novel proteins. Infect. Immun. 66:3492-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.WHO. 2009. Global tuberculosis control: surveillance, planning, financing, vol. 376. World Health Organization, Geneva, Switzerland.
- 46.Zhang, Y., B. Heym, B. Allen, D. Young, and S. Cole. 1992. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 358:591-593. [DOI] [PubMed] [Google Scholar]