Abstract
Many proteins of Trypanosoma cruzi, the causative agent of Chagas' disease, contain characteristic arrays of highly repetitive immunogenic amino acid motifs. Diagnostic tests using these motifs in monomeric or dimeric form have proven to provide markedly improved specificity compared to conventional tests based on crude parasite extracts. However, in many cases the available tests still suffer from limited sensitivity. In this study we produced stable synthetic genes with maximal codon variability for the four diagnostic antigens, B13, CRA, TcD, and TcE, each containing between three and nine identical amino acid repeats. These genes were combined by linker sequences encoding short proline-rich peptides, giving rise to a 24-kDa fusion protein which was used as a novel diagnostic antigen in an enzyme-linked immunosorbent assay setup. Validation of the assay with a large number of well-characterized patient sera from Bolivia and Brazil revealed excellent diagnostic performance. The high sensitivity of the new test may allow future studies to use blood collected by finger prick and dried on filter paper, thus dramatically reducing the costs and effort for the detection of T. cruzi infection.
Chagas' disease is called a silent disease because most people do not realize they are infected with the parasite Trypanosoma cruzi until late in life, if ever. However, 20 to 30% of those infected will suffer from irreversible damage to the heart, esophagus, or colon, decades after acquisition of the parasite (5, 40). Heart failure is a common cause of sudden death early in life (26). In spite of intense attempts to eradicate the insect vector in past years, the disease still affects over 8 million persons in Latin America, and 75 million people are at risk of infection (40). In some areas of Bolivia, such as Tarija or in peripheral urban districts of Cochabamba, the infection rate among children was found to be as high as 28% (28), and the disease may account for 13% of all deaths in this nation (27).
This unbearable situation could be changed by diagnostic screening of the population at risk at regular intervals followed by therapy of positive cases. Unfortunately, the available drugs, nifurtimox and benznidazole, are only effective during the early stage of the infection. When used for the treatment of the later stages of the disease, parasite eradication is markedly less effective and the drugs frequently induce severe side effects (1). Treatment of adults, therefore, has to be considered with caution. However, treatment of all infected children and young adults up to 15 to 16 years of age appears to be a reasonable policy. This strategy combined with rigorous vector control could significantly reduce the infection rate of the whole population in the long term.
Different serologic assays are available for testing clinical and donor specimens for T. cruzi infection. The most widely used procedures are an enzyme-linked immunosorbent assay (ELISA) and indirect hemagglutination (IHA). Most assays use crude lysates of the parasite as antigen, but more recent tests are based on recombinant proteins (3, 4, 7, 8, 14, 19, 22, 25, 29-31, 35-37). Most use a collection of short recombinant peptides as antigens. These peptides correspond to repetitive amino acid sequences that occur in high copy numbers in different parasite proteins. The sera of infected individuals frequently contain high titers of antibodies against these repetitive motifs. (9, 16, 35). Recently developed diagnostic tests contain combinations of monomers or dimers of these repeats. Even though tests based on recombinant antigens are generally highly specific, many yield only suboptimal sensitivity rates (12, 21, 32).
In this report, we describe the production of several such repetitive structures in higher oligomeric form and their performance in immunoassays. Oligomeric antigens had high reactivities with patient sera, especially when presented as a fusion of several different oligomers. A fusion of the antigens B13, CRA, TcD, and TcE, called TcBCDE, was found to be highly specific for T. cruzi, whereas some other known antigens led to cross-reactions with sera from leishmaniasis patients.
MATERIALS AND METHODS
Serum samples.
Serum samples from patients with Chagas' disease were collected in Bolivia from patients attending the health service center SEDES (Servicio Departmental de Salud) in Cochabamba, the Laboratory of Parasitology at INLASA, La Paz, as well as Percy Boland Maternal Hospital, Santa Cruz. In Brazil samples were collected at the Hospital das Clinicas, Universidade Federal de Goiás, Goiania. Finally, samples from Latin American immigrants in Spain at different health centers were delivered to the Laboratory of Parasitology at the Universidad de Barcelona for research purposes. All sera included in this study were classified as positive or negative by two or three different tests as specified below for the individual trial results. Sera from patients with visceral or cutaneous leishmaniasis were either collected from patients at the Universitat de Barcelona, Barcelona, Spain, or were donated by Abdullatif Ali, University of Dhamar, Dhamar, Yemen. Leishmaniasis had been confirmed by identification of the parasites in smears of skin lesions by microscopy and/or PCR analysis (C. Riera, unpublished results). Uninfected human serum samples were obtained from blood donors at the Academic Hospital at the University of Giessen, Giessen, Germany. Sera from Mongolian patients with syphilis and brucellosis were donated by Zandraa Jamba, Health Science University of Mongolia, Ulaanbaatar, Mongolia.
Synthetic gene construction.
Oligonucleotides designed by using DNA-Works 3.1 software (17) were assembled to complete DNA fragments by a PCR method essentially as described previously (39). By this way, genes encoding up to nine identical amino acid repeats were constructed for the antigens MAP (20), JL8 (24), CRA (22), B13 (14), TcD (3), TcE (19), SAPA (34), and TcMyo. The two versions of the FRA antigen were bought as synthetic DNA from a commercial supplier (ATG:biosynthetics GmbH, Merchausen, Germany). For easy purification of the resulting translation products by metal affinity chromatography, the open reading frames, on average 400 to 500 bp in length, were fused with a hexahistidine affinity tag contained in the expression vector pQE-30 (Qiagen, Hilden, Germany) via a BamHI site at the 5′ end and a HindIII site at the 3′ end. The primary structures of the antigens resulting from the different synthetic genes are listed in Table 1.
TABLE 1.
Synthetic tandem repeat antigens of T. cruzi
| Antigen | Amino acid sequence | No. of repeats in genome | Similarity with Leishmaniaspp. |
|---|---|---|---|
| MAP | PRHVDPDHFRSTTQDAYRPVDPSAYKRALPLEEEEDVG PRHVDPDHFRSTTQDAYRPVDPSAYKRALPLEEEEDVG PRHVDPDHFRSTTQDAYRPVDPSAYKRALPQEEEEDVG | 135 | 40% (L. infantum), 52% (L. braziliensis) |
| JL8 | AAEATKVAEAEKQR AAEATKAVEAEKQR AAEATKVAEAEKQK | 140 | Very weak similarity |
| CRA | KVAEAEKQKAAEAT KVAEAEKQKAAEAT KVAEAEKQKAAEAT | 130 | No similarity |
| B13 | PFGQAAAGDKPS PFGQAAAGDKPS PFGQAAAGDKPK | 103 | No similarity |
| FRA | AFLDQKPEGVPLRELPLDDDSDFVAMEQERRQLLEKDPRRNAREIAAL EESMNARAQELAREKKLADR AFLDQKPEGVPLRELPLDDDSDFVAME QERRQLLEKDPRRNAKEIAALEESMNARAQELAREKKLADR AFLDQK | 14 | 70% (L. infantum), 63% (L. braziliensis) |
| FRA2a | MEQERRQLLEKDPRRNAREIAALEESMNARAQELAREKKLADRAFPDSPNSMEQERRQLLEKDPRRNAREIAALEESMNARAQELAREKKLADRAFPNSPDMEQERRQLLEKDPRRNAREIAALEESMNARAQELAREKKLADRAF | 14 | 55% (with both L. infantumand L. braziliensis) |
| TcD | PKPAE PKPAE PKPAE PKPAE PKPAE PKPAE PKPAE PKPAE PKPAE | 430 | No similarity |
| TcE | PAKAAA PPAKAAA PPAKAAA PPAKAAA PPAKAAA PPAKAAAP | 70 | No similarity |
| SAPA | PVDSSAHGTPST PVDSSAHGTPST PVDSSAHSTPST PVDSSAHSTPST PADSSAHSTPST | >130 | No similarity |
| TcMyo | LAQREADNEKLAED LAQREADNEKLAEE LAQREADNEKLTED LAQREADNEKLAED | >170 | No similarity |
Underlined residues indicate introduced proline-rich spacer sequences.
In a second step, the recombinant genes were fused as follows. The 5′ end of CRA was ligated to a BamHI site near the 3′ end of B13. The TcD and TcE antigens were fused with HindIII-BamHI adapter oligonucleotides (5′-AGCTGCCGAGCCTGAGCA and 5′-GATCTGCTCAGGCTCGGC) that encode a peptide with two proline residues as a hinge region to facilitate folding of the individual antigens in an optimal conformation. Finally, the two resulting dimeric sequences were connected to a single DNA construct by using another HindIII-BamHI adapter (5′-AGCTCTCTCCGCTGCCGA and 5′-GATCTCGGCAGCGGAGAG). The amino acid sequence of the biggest fusion, B13-CRA-TcD-TcE (TcBCDE), including the vector-derived histidine tag, is MRGSHHHHHH GS PSPFGQAAAGDK PSPFGQAAAGDK PSPFGQAAAGDKP GS KVAEAEKQKAAEATKVAEAEKQKAAEAT KVAEAEKQKAAEAT KLPSLSRS PKPAE PKPAE PKPAE PKPAE PKPAE PKPAE PKPAE PKPAE PKPAE KLPSLSRS PAKAAAP PAKAAAP PAKAAAP PAKAAAP PAKAAAP PAKAAAP PAKAAAP KL. The individual elements of the repeats as well as the residues deriving from restriction sites and adapter sequences (underlined) used for the fusion of the fragments are separated by short gaps.
Expression and purification of recombinant antigens.
Escherichia coli XL1-Blue/pREP cells transformed with the respective plasmid constructs were induced for protein expression by using isopropyl-β-d-thiogalactopyranoside (Gerbu, Heidelberg, Germany) and harvested by centrifugation, and the proteins were purified under denaturing conditions using TALON metal affinity resin (BD Biosciences, Palo Alto, CA) as recommended by the supplier. Protein concentrations were determined according to the methods of Bradford (2).
Immunoblot assays.
To determine sensitivity and specificity, the recombinant antigens were serially diluted in 10 mM Tris-HCl (pH 7.5)-150 mM NaCl (Tris-buffered saline [TBS]), 10% glycerol and applied to nitrocellulose sheets as a line (10 μl/cm). Nonspecific binding sites were blocked by a solution of 1% Tween 20 in TBS. The sheets were then cut perpendicularly to the antigen lines in 0.4-mm strips and incubated with human serum diluted 1:200 in TBS and 1% bovine serum albumin for 1 h at room temperature on a shaker. The strips were washed three times for 10 min each with TBS, 0.1% Tween 20, incubated for 1 h with anti-human IgG conjugated to alkaline phosphatase (Dianova, Hamburg, Germany), and stained with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium as described previously (23). Antigen concentrations that led to a clear positive signal with sera from patients with Chagas' disease but not with negative-control sera or sera from patients with syphilis or leishmaniasis were determined as optimal and used in further experiments. Optimal concentrations varied between 100 μg/ml and 10 ng/ml depending on the antigen.
TcBCDE ELISA.
Microtiter plates (Greiner Bio-One, Frickenhausen, Germany) were coated with the TcBCDE antigen at a concentration of 10 ng/ml. To prevent nonspecific adsorption of this tiny amount of protein to the walls of plastic tubes or pipette tips, the dilution buffer phosphate-buffered saline (PBS) contained 2 μg/ml of bovine serum albumin. The plates were processed essentially as described previously (18) using 1% fat-free milk powder (Roth, Karlsruhe, Germany) in PBS as blocking solution. Upon drying overnight at 50°C, the plates were sealed with an adhesive plastic foil and stored in dark plastic bags at ambient temperature. Prior to the conduct of the assay, the serum samples were diluted 1:100 in blocking solution, and specific antibodies were detected with a goat anti-human IgG horseradish peroxidase conjugate (Dianova, Hamburg, Germany) in combination with 3,3′,5,5′-tetramethylbenzidine as staining substrate (10). For evaluation of the results, the cutoff value was determined as the mean of all negative samples plus 3 standard deviations.
Other diagnostic tests.
The commercial Chagas' disease-specific tests Chagas Stat-Pak (Chembio Diagnostic Systems, Medford, NY), Bioelisa Chagas (Biokit, Barcelona, Spain), and Wiener Chagatest-ELISA Recombinante version 3.0 (Wiener Laboratorios, Santa Fé, Argentina), which are all based on recombinant antigens, and the Bios Chile ELISA para Chagas III (Bios Chile, Santiago, Chile), which is based on whole extracts of Trypanosoma cruzi strains Tulahuen and Mn as antigens, were used according to the manufacturers' instructions.
A Leishmania major-specific ELISA was prepared from a crude extract of cultivated promastigotes essentially as described by Zeinali et al. (42).
Nucleotide sequence accession number.
The nucleotide sequence for TcBCDE downstream of the histidine tag has been deposited in GenBank (accession number HM565960).
RESULTS
Production of oligomeric T. cruzi antigens.
A systematic in silico analysis of the T. cruzi genome revealed that many proteins contain highly conserved repetitive amino acid sequences in arrays of up to several hundred copies. The same repeats occur frequently in two or more related genes, possibly representing different alleles of the same gene in the heterozygous chromosomes of the diploid genome of T. cruzi (6, 13). As antibodies recognize these repeats most probably as defined tertiary structures, we sought to incorporate these structures in recombinant antigens by producing oligomeric forms of the amino acid repeats.
In order to predict the specificity of diagnostic antigens for T. cruzi, we searched in GeneDB for similar sequences in the genomes of Leishmania braziliensis, Leishmania infantum, and L. major by using the BLAST program. No sequences with significant similarity were detected for the antigens B13, CRA, TcD, TcE, and SAPA, whereas for other known tandem repeat antigens related sequences became clearly apparent. The antigens MAP and F8 occur with up to 50% identity, and the antigen FRA occurs with even 80% identity in different Leishmania species.
Oligomers of the coding sequences could be maintained stably only in E. coli when the repetitive character of the DNA was modified synthetically by incorporating different nucleotides in variable positions of codons. In this way, we succeeded in constructing 10 different oligomeric antigens, which are listed in Table 1. Details on the gene synthesis are described in Materials and Methods.
Immunoblot evaluation of recombinant antigens.
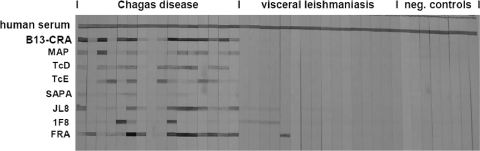
Purified antigens were tested in immunoblot assays as described in Materials and Methods. The most reactive antigen was the dimeric FRA repeat, leading at a concentration of 1 μg/ml to strong positive results with more than 95% of all infection sera. In contrast, TcMyo, a tetramer of a sequence occurring more than 170 times in the putative myosin gene of T. cruzi, had to be applied at a concentration of 200 μg/ml to lead to a significant reaction with only a few of the patient sera (data not shown). Immunogenicity is not necessarily correlated with the copy number of an antigen, but rather with the genetic disposition of the host. TcMyo may be highly immunogenic in one or more of the many other animal species infected with T. cruzi. In further immunoblotting experiments, the specificity of the antigens was determined using sera from leishmaniasis patients (Fig. 1). These tests revealed that not all of the antigens described in the literature are exclusively specific for T. cruzi, at least not if presented in oligomeric form. Some sera led to strong immunostaining of the synthetic FRA dimer and other sera interacted with 1F8 and JL8. A weak cross-reactivity was also observed with sera from cutaneous leishmaniasis patients (data not shown). As the leishmaniasis sera used in our study were from the Republic of Yemen, where Chagas' disease does not occur, coinfections can be excluded as a possible explanation of these results, pointing to the considerable similarity of the relevant antigens of Leishmania and T. cruzi.
FIG. 1.
Cross-reaction tests for the different recombinant antigens. The first line is a colorimetric reaction positive control (human serum). All antigens were tested with sera obtained from patients with Chagas' disease (lane 8 corresponds to a probably damaged hemolytic serum sample), visceral leishmaniasis, or with sera from healthy German persons as negative controls. Strong cross-reactions of the FRA and 1F8 antigens and weaker cross-reactions of JL8 antigen with visceral leishmaniasis patient sera are apparent.
Recombinant FRA antigen is used in several commercial diagnostic test kits for Chagas' disease, probably because of its high reactivity. As it was not clear to us whether the FRA antigen in such kits corresponds to the complete length of the repeat, or to subfragments only, we produced a trimer of the least conserved part of the repeat, named FRA2 (Table 1). The resulting antigen revealed strong reactivity with chagasic sera, but it was still cross-reactive with leishmaniasis sera, albeit to a lesser degree. For this reason we decided not to include recombinant FRA antigen in further tests.
Establishment of a specific and highly sensitive ELISA.
In contrast to the signal strength observed in immunoblot assays, reactivity in the ELISA appeared to be too weak for some of the antigens. Concentrations of 10 μg of protein/ml were needed for coating the microtiter plates with B13, TcD, and TcE in order to obtain clearly visible signals under standard test conditions. We supposed that the short polypeptides may not bind sufficiently strongly to the surface of the plastic plates, and therefore we constructed chimeric antigens of a bigger size. Only antigens which had proven not to cross-react with leishmaniasis sera were combined. Fusion of B13 with CRA on the one hand and TcD with TcE on the other hand led indeed to an increase of reactivity. Clear signals were obtained with protein concentrations of only 1 μg/ml each in the coating buffer. This finding might be explained by better adsorption of the antigens to the microtiter plate, by adoption of an improved conformation in the absence of the histidine tag adjacent to the second fusion partner, or by a combination of both effects.
Due to the unexpected increase of the reactivities of the B13-CRA and TcD-TcE fusion polypeptides, and in order to simplify antigen purification, we combined in the next step the two primary fusion proteins to a single polypeptide consisting of four antigenic repeat motifs. The final construct, designated TcBCDE, consisted of three repeats of the B13 antigen, three repeats of the CRA antigen, nine repeats of the TcD antigen, and six repeats of the TcE antigen. The fusion antigen showed excellent immunoreactivity with only 1 ng of antigen per well of a microtiter plate, i.e., a dilution of 1:105 of a 1-mg/ml stock solution was sufficient for a strong signal with Chagas' disease-positive serum.
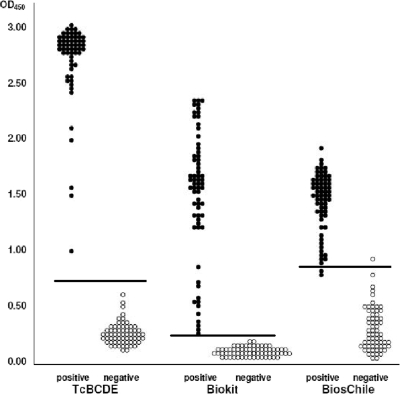
The diagnostic efficiency of TcBCDE was tested first with sera stored at SEDES, Cochabamba, Bolivia, which had been tested by IHA (Polychaco, Buenos Aires, Argentina) and an immunofluorescent antibody test. Only sera positive in both tests were included as positive samples. Sera which had been clearly negative in these tests were used as controls. The reactivity of the TcBCDE ELISA was compared with two commercial diagnostic tests, Bioelisa Chagas (recombinant antigen) and Bios-Chile Chagas ELISA (T. cruzi crude extract antigen). The same set of 65 T. cruzi-positive and 65 negative-control sera was analyzed using 5 μl of each serum sample. The results shown in Fig. 2 clearly indicate a higher reactivity of the TcBCDE antigen than to the antigens used in the two commercial test kits. Under the conditions used in this experiment, staining in the TcBCDE ELISA was very fast and had to be stopped after 5 min. To facilitate handling, 1 to 2 μl instead of 5 μl of patient serum was used in further tests.
FIG. 2.
Comparison of the TcBCDE ELISA with the Biokit Chagas ELISA and the Bios Chile Chagas ELISA. The same sets of 65 sera from T. cruzi-positive patients and of 65 negative-control sera from SEDES, Cochabamba, were tested under similar conditions (5 μl of serum each for the reaction with antigen, and peroxidase-conjugated anti-human IgG as secondary antibody). Calculated cutoff values are indicated by horizontal lines.
The specificity of the three different Chagas' disease-specific tests was evaluated with 35 serum samples from leishmaniasis patients from the Republic of Yemen and with 50 sera from Mongolian patients with syphilis. As neither Mongolia nor the Republic of Yemen are countries where Chagas' disease is endemic, cross-reactivity due to coinfections could be excluded. None of the leishmaniasis sera reacted in the TcBCDE ELISA or Bioelisa Chagas test, but five of them were positive in the Bios-Chile Chagas ELISA. The syphilis sera were negative in all three tests.
The issue of cross-reactivity was analyzed in more detail with 25 patient sera classified as inconclusive in Cochabamba, because they revealed either weakly positive results in the Bios-Chile Chagas ELISA or in alternative assays based on crude parasite extracts, such as IHA. We applied four different tests: the TcBCDE ELISA and Bioelisa Chagas employing recombinant antigens on the one hand, and Bios-Chile Chagas ELISA and an ELISA employing a self-prepared crude extract from L. major as antigen (42) on the other hand. While both of the assays that use recombinant antigen led to clearly negative results with all of these sera, 10 sera were positive in the Bios-Chile Chagas ELISA and 16 in the L. major ELISA (data not shown). As the reactivity with the two crude extract ELISAs appeared to be very similar, it can be assumed that many of the weakly positive results among the inconclusive samples may be caused by acute or treated Leishmania infections. Different forms of leishmaniasis occur in the area surrounding Cochabamba, even though there are no clear data on infection rates. Leishmania-specific tests are not performed in this area.
Validation of the new ELISA.
In order to validate the sensitivity and specificity of TcBCDE as a diagnostic antigen, ELISAs were performed with additional sera collected from chagasic patients at INLASA, La Paz, and at the Percy Boland Maternal Hospital, Santa Cruz, Bolivia, in parallel with other diagnostic kits based on recombinant antigens, including the Wiener Chagatest Recombinante, Bioelisa Chagas, and Chagas Stat-Pak tests. Altogether, more than 200 serum samples were analyzed. The assays were performed using 10 μl of serum (Wiener Chagatest and Bioelisa Chagas) or with 5 μl of serum (Chagas Stat-Pak), as recommended by the suppliers. For the TcBCDE ELISA, only 1 μl of serum was used. The results are summarized in the upper part of Table 2. With both sets of patient sera, one more sample each was classified as (weakly) positive by the TcBCDE ELISA. Assuming that the results with the other tests were correct, the TcBCDE ELISA must be considered at least equally sensitive and 99% specific. Alternatively, the results could be explained by a higher sensitivity of the TcBCDE ELISA.
TABLE 2.
Reactivity of the TcBCDE ELISA in comparison with different T. cruzi-specific immunoassays
| Test | No. of samples with indicated reaction (total no. of samples from site) |
||
|---|---|---|---|
| La Paz, Bolivia (n = 130) | Santa Cruz, Bolivia (n = 85) | Goiania, Brazil (n = 381) | |
| TcBCDE-ELISA | |||
| Positive | 77 | 65 | 164 |
| Negative | 53 | 20 | 217 |
| Wiener Chagatest | |||
| Positive | 76 | 64 | |
| Negative | 54 | 21 | |
| Bioelisa Chagas | |||
| Positive | 64 | ||
| Negative | 21 | ||
| Chagas Stat-Pak | |||
| Positive | 64 | ||
| Negative | 21 | ||
| Biomanguinhos crude extract ELISA | |||
| Positive | 165 | ||
| Negative | 216 | ||
| Immunofluorescence | |||
| Positive | 165 | ||
| Negative | 216 | ||
| Wiener hemagglutination | |||
| Positive | 165 | ||
| Negative | 216 | ||
In order to investigate the diagnostic potential of the TcBCDE ELISA for T. cruzi strains outside Bolivia, tests with several hundred sera collected from patients in Goiania, Brazil, were performed. These sera had been characterized thoroughly based on a T. cruzi crude extract ELISA (EIE Biomanguinhos; Fiocruz, Rio de Janeiro, Brazil), indirect hemagglutination (Wiener, Rosario, Argentina), and indirect immunofluorescence (bioMérieux conjugate with an in-house Y strain of T. cruzi) (A. O. Luquetti, unpublished results). A total of 165 samples positive in all three of these conventional serological assays and 216 samples negative in all three tests were analyzed with the TcBCDE ELISA. One of the samples positive in the conventional test was negative in the TcBCDE ELISA, corresponding to 99.3% sensitivity and 100% specificity (lower part of Table 2).
DISCUSSION
High immunoreactivity of the artificial TcBCDE antigen.
The TcBCDE antigen was found to be recognized by T. cruzi-specific human antibodies with remarkable affinity. Under the assumption that 0.1% of total IgG is specific for the antigen, the calculated interaction between 1 μl of serum (0.05 pmol specific antibody) and 1 ng of antigen (0.05 pmol) corresponds to a molar relation of 1:1. In other recombinant ELISAs for Chagas' disease, the relation of specific IgG to recombinant antigen is instead 1:20 at the molar level. In the Bioelisa Chagas assay (7), for example, 10 μl of serum (0.5 pmol specific antibody) is used with 75 ng of antigen (9 pmol).
Many of the commercial recombinant ELISAs for Chagas' disease are based on the fusion protein TcF (7). This antigen contains in part the same antigenic motifs as TcBCDE, but in a different context. TcF contains two repeats each of the antigens B13 (PEP2), TcD, TcE, and TcLo1.2 as direct fusions, i.e., not separated by spacer sequences as in TcBCDE. The CRA antigen is replaced by TcLo1.2, which occurs as a tandem repeat in related forms in five different proteins of T. cruzi. The major differences between TcBCDE and TcF are the number of repeats of the antigens, which is considerably higher in TcBCDE than in TcF, on the one hand and the presence of proline-rich spacer regions between the individual antigens on the other hand. Obviously, the immunoreactive epitopes of (some of) the antigens are more accessible to the antibodies when presented in a larger protein.
Sera with high levels of T. cruzi-specific antibodies are detected equally efficiently with existing recombinant tests and the TcBCDE ELISA. However, the latter test has a greater potency to discriminate weakly positive samples from background. This can be demonstrated statistically by calculating the quotient of measured optical density values divided by the cutoff values. In the results shown in Fig. 2, only 3 of the 65 positive samples had a quotient smaller than 2.0 in the TcBCDE ELISA, whereas in the Bioelisa Chagas 9 samples were below this value. This potency facilitates diagnosis, because weakly positive results routinely need to be confirmed by alternative assays.
Specificity of the TcBCDE antigen.
The high dilution of TcBCDE used for immunoassays minimizes contamination with bacterial antigens. This reduces greatly the cross-reactions with E. coli-specific antibodies, which are generally found in human serum (15, 41) and frequently cause background problems in immunoassays based on recombinant proteins. This may be an additional reason why the TcBCDE ELISA discriminates better between positive and negative results than alternative diagnostic methods available at present.
Cross-reaction of chagasic sera with other organisms is well known and occurs mainly for closely related members of the Trypanosomatidae family. While this can be tolerated for the analysis of donated blood—suspicious samples have to be eliminated anyway—misdiagnosis of patients may have severe consequences. Tests based on crude parasite extracts as antigens react frequently with sera from persons infected with different species of Leishmania, or with Trypanosoma rangeli (33).
Clear discrimination between Chagas' disease and leishmaniasis is a major problem, as the pathogens occur in parallel in many parts of Latin America. Leishmania strains found in the New World (L. braziliensis, L. chagasi, L. mexicana, and others) differ from the Old World strains L. infantum and L. major used in this study. However, with the exception of L. braziliensis, limited information is available for the genomic sequences of these New World species so far. Comparing T. cruzi sequences with L. braziliensis appears to be justified in any case, as up to 85% of all Leishmania infections in Bolivia are caused by L. braziliensis (11). Furthermore, Old World Leishmania and L. braziliensis revealed significant similarities in MAP and FRA antigens, as indicated in Table 1. Most probably, cross-reactivity can be avoided completely by selecting appropriate recombinant antigens for immunological tests. None of the antigens contained in TcBCDE (i.e., B13, CRA, TcD, and TcE) showed experimental cross-reactivity with sera from individuals infected with Leishmania or T. rangeli (37, 38).
Diagnosis at affordable costs.
At present, diagnosis of Chagas' disease is normally performed only with donated blood, or when patients suffer from clinical symptoms. However, when symptoms such as cardiac or digestive dysfunctions become apparent, the efficacy of etiological treatment with the available drugs is rather poor. On the other hand, therapy is more promising if the infection is recognized early, i.e., long before the onset of clinical symptoms. Therefore, the only way to detect the infection in time would depend on screening of the endangered population in regular intervals, which demands diagnosis on a large scale.
The procedures described in this work exemplify how public bioinformatic data can be used to approach medical problems of developing countries in an inexpensive way. Neither the numerous genomic data nor the software programs needed for this work had to be bought, nor were they available only for a restricted number of persons. The results show the way forward to produce recombinant antigen for millions of assays at very low costs. Moreover, the small amounts of serum needed for this test may allow the use of blood collected by finger prick and dried on filter paper, thus reducing costs even more.
In the future, the test could be adapted to the specific needs in economically developing areas. Because most medical facilities in areas at risk are poorly equipped, work on a simple lateral flow device with TcBCDE as antigen is in progress.
Acknowledgments
We thank Barbara Preiss, Rozangela Amaral Oliveira, and Suelene B. Nascimento Tavares for excellent technical assistance, Martin Llewellyn, London, and Montserrat Portús, Barcelona, for critically reading the manuscript, and Volga Iñiguez for generously allocating space, consumables, and mental support in her laboratory in La Paz.
The work is dedicated to the late Heinz Schaller, Heidelberg, who gave support by a generous donation. P.H. was temporarily supported by a grant from AECI, Spain.
Footnotes
Published ahead of print on 28 July 2010.
REFERENCES
- 1.Andrade, S. G., A. Rassi, J. B. Magalhaes, F. Ferriolli Filho, and A. O. Luquetti. 1992. Specific chemotherapy of Chagas disease: a comparison between the response in patients and experimental animals inoculated with the same strains. Trans. R. Soc. Trop. Med. Hyg. 86:624-626. [DOI] [PubMed] [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Burns, J. M., Jr., W. G. Shreffler, D. E. Rosman, P. R. Sleath, C. J. March, and S. G. Reed. 1992. Identification and synthesis of a major conserved antigenic epitope of Trypanosoma cruzi. Proc. Natl. Acad. Sci. U. S. A. 89:1239-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caballero, Z. C., O. E. Sousa, W. P. Marques, A. Saez-Alquezar, and E. S. Umezawa. 2007. Evaluation of serological tests to identify Trypanosoma cruzi infection in humans and determine cross-reactivity with Trypanosoma rangeli and Leishmania spp. Clin. Vaccine Immunol. 14:1045-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2007. Chagas disease. Detailed factsheet. Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/chagas/factsheets/detailed.html.
- 6.El-Sayed, N. M., P. J. Myler, D. Nilsson, G. Aggarwal, A. N. Tran, E. Ghedin, E. A. Worthey, A. L. Delcher, G. Blandin, S. J. Westenberger, E. Caler, G. C. Cerqueira, C. Branche, B. Haas, A. Anupama, E. Arner, L. Aslund, P. Attipoe, E. Bontempi, F. Bringaud, P. Burton, E. Cadag, D. A. Campbell, M. Carrington, J. Crabtree, H. Darban, J. F. da Silveira, P. de Jong, K. Edwards, P. T. Englund, G. Fazelina, T. Feldblyum, M. Ferella, A. C. Frasch, K. Gull, D. Horn, L. Hou, Y. Huang, E. Kindlund, M. Klingbeil, S. Kluge, H. Koo, D. Lacerda, M. J. Levin, H. Lorenzi, T. Louie, C. R. Machado, R. McCulloch, A. McKenna, Y. Mizuno, J. C. Mottram, S. Nelson, S. Ochaya, K. Osoegawa, G. Pai, M. Parsons, M. Pentony, U. Pettersson, M. Pop, J. L. Ramirez, J. Rinta, L. Robertson, S. L. Salzberg, D. O. Sanchez, A. Seyler, R. Sharma, J. Shetty, A. J. Simpson, E. Sisk, M. T. Tammi, R. Tarleton, S. Teixeira, S. Van Aken, C Vogt, P. N. Ward, B. Wickstead, J. Wortman, O. White, C. M. Fraser, K. D., Stuart, and B. Andersson. 2005. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science 309:409-415. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira, A. W., Z. R. Belem, E. A. Lemos, S. G. Reed, and A. Campos-Neto. 2001. Enzyme-linked immunosorbent assay for serological diagnosis of Chagas' disease employing a Trypanosoma cruzi recombinant antigen that consists of four different peptides. J. Clin. Microbiol. 39:4390-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco da Silveira, F. J., E. S. Umezawa, and A. O. Luquetti. 2001. Chagas' disease: recombinant Trypanosoma cruzi antigens for serological diagnosis.Trends Parasitol. 17:286-291. [DOI] [PubMed] [Google Scholar]
- 9.Frasch, A. C., J. J. Cazzulo, L. Aslund, and U. Pettersson. 1991. Comparison of genes encoding Trypanosoma cruzi antigens. Parasitol. Today 7:148-151. [DOI] [PubMed] [Google Scholar]
- 10.Frey, A., B. Meckelein, D. Externest, and M. A. Schmidt. 2000. A stable and highly sensitive 3,3′,5,5′-tetramethylbenzidine-based substrate reagent for enzyme-linked immunosorbent assays. J. Immunol. Methods 233:47-56. [DOI] [PubMed] [Google Scholar]
- 11.García, A. L., R. Parrado, E. Rojas, R. Delgado, J. C. Dujardin, and R. Reithinger. 2009. Leishmaniases in Bolivia: comprehensive review and current status. Am. J. Trop. Med. Hyg. 80:704-711. [PubMed] [Google Scholar]
- 12.Gomes, Y. M., V. M. Lorena, and A. O. Luquetti. 2009. Diagnosis of Chagas disease: what has been achieved? What remains to be done with regard to diagnosis and follow up studies? Mem. Inst. Oswaldo Cruz 104(Suppl. 1):115-121. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez, A., T. J. Lerner, M. Huecas, B. Sosa-Pineda, N. Nogueira, and P. M. Lizardi. 1985. Apparent generation of a segmented mRNA from two separate tandem gene families in Trypanosoma cruzi. Nucleic Acids Res. 13:5789-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruber, A., and B. Zingales. 1993. Trypanosoma cruzi: characterization of two recombinant antigens with potential application in the diagnosis of Chagas' disease. Exp. Parasitol. 76:1-12. [DOI] [PubMed] [Google Scholar]
- 15.Herías, M. V., I. Mattsby-Baltzer, J. R. Cruz, and L. A. Hanson. 1997. Antibodies to Escherichia coli and Shigella flexneri in milk from undernourished mothers: studies on sodium dodecyl sulfate-polyacrylamide gel electrophoresis-separated antigens. Pediatr. Res. 42:644-650. [DOI] [PubMed] [Google Scholar]
- 16.Hoft, D. F., K. S. Kim, K. Otsu, D. R. Moser, W. J. Yost, J. H. Blumin, J. E. Donelson, and L. V. Kirchhoff. 1989. Trypanosoma cruzi expresses diverse repetitive protein antigens. Infect. Immun. 57:1959-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoover, D. M., and J. Lubkowski. 2002. DNAWorks: an automated method for designing oligonucleotides for PCR-based gene synthesis. Nucleic Acids Res. 30:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hornbeck, P., S. E. Winston, and S. A. Fuller. 2001. Enzyme-linked immunosorbent assays (ELISA), unit 11.2. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology. John Wiley and Sons, Inc., Hoboken, NJ. [DOI] [PubMed]
- 19.Houghton, R. L., D. R. Benson, L. D. Reynolds, P. D. McNeill, P. R. Sleath, M. J. Lodes, Y. A. Skeiky, D. A. Leiby, R. Badaro, and S. G. Reed. 1999. A multi-epitope synthetic peptide and recombinant protein for the detection of antibodies to Trypanosoma cruzi in radioimmunoprecipitation-confirmed and consensus-positive sera. J. Infect. Dis. 179:1226-1234. [DOI] [PubMed] [Google Scholar]
- 20.Kerner, N., P. Ligeard, M. J. Levin, and M. Hontebeyrie-Joskowicz. 1991. Trypanosoma cruzi: antibodies to a MAP-like protein in chronic Chagas' disease cross-react with mammalian cytoskeleton. Exp. Parasitol. 73:451-459. [DOI] [PubMed] [Google Scholar]
- 21.Krieger, M. A., E. Almeida, W. Oelemann, J. J. Lafaille, J. B. Pereira, H. Krieger, M. R. Carvalho, and S. Goldenberg. 1992. Use of recombinant antigens for the accurate immunodiagnosis of Chagas' disease. Am. J. Trop. Med. Hyg. 46:427-434. [DOI] [PubMed] [Google Scholar]
- 22.Lafaille, J. J., J. Linss, M. A. Krieger, T. Souto-Padrón, W. de Souza, and S. Goldenberg. 1989. Structure and expression of two Trypanosoma cruzi genes encoding antigenic proteins bearing repetitive epitopes. Mol. Biochem. Parasitol. 35:127-136. [DOI] [PubMed] [Google Scholar]
- 23.Leary, J. J., D. J. Brigati, and D. C. Ward. 1983. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc. Natl. Acad. Sci. U. S. A. 80:4045-4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin, M. J., E. Mesri, R. Benarous, G. Levitus, A. Schijman, P. Levy-Yeyati, P. A. Chiale, A. M. Ruiz, A. Kahn, M. B. Rosenbaum, H. N. Torres, and E. L. Segura. 1989. Identification of major Trypanosoma cruzi antigenic determinants in chronic Chagas' heart disease. Am. J. Trop. Med. Hyg. 41:530-538. [DOI] [PubMed] [Google Scholar]
- 25.Luquetti, A. O., C. Ponce, E. Ponce, J. Esfandiari, A. Schijman, S. Revollo, N. Añez, B. Zingales, R. R. Aldao, A. Gonzalez, M. Levin, E. Umezawa, and J. Franco da Silveira. 2003. Chagas disease diagnosis: a multicentric evaluation of Chagas Stat-Pak, a rapid immunochromatographic assay with recombinant proteins of Trypanosoma cruzi. Diagn. Microbiol. Infect. Dis. 46:265-271. [DOI] [PubMed] [Google Scholar]
- 26.Lopes, E. R. 1999. Sudden death in patients with Chagas disease. Mem. Inst. Oswaldo Cruz 94(Suppl. I):321-324. [DOI] [PubMed] [Google Scholar]
- 27.Medecins sans Frontières. 2004. Bolivia. A killer that preys on the poor: Chagas disease. Medicins sans Frontières, Geneva, Switzerland. http://www.msf.org/source/actrep/2004/pdf/62-63.pdf.
- 28.Medrano-Mercado, N., R. Ugarte-Fernandez, V. Butrón, S. Uber-Busek, H. L. Guerra, T. C. Araújo-Jorge, and R. Correa-Oliveira. 2008. Urban transmission of Chagas disease in Cochabamba, Bolivia. Mem. Inst. Oswaldo Cruz 103:423-430. [DOI] [PubMed] [Google Scholar]
- 29.Moncayo, A., and A. O. Luquetti. 1990. Multicentre double blind study for evaluation of Trypanosoma cruzi defined antigens as diagnostic reagents. Mem. Inst. Oswaldo Cruz 85:489-495. [DOI] [PubMed] [Google Scholar]
- 30.Paranhos, G. S., P. C. Cotrim, R. A. Mortara, A. Rassi, R. Corral, H. L. Freilij, S. Grinstein, J. Wanderley, M. E. Camargo, and J. F. da Silveira. 1990. Trypanosoma cruzi: cloning and expression of an antigen recognized by acute and chronic human chagasic sera. Exp. Parasitol. 71:284-293. [DOI] [PubMed] [Google Scholar]
- 31.Peralta, J. M., M. G. Teixeira, W. G. Shreffler, J. B. Pereira, J. M. Burns, Jr., P. R. Sleath, and S. G. Reed. 1994. Serodiagnosis of Chagas' disease by enzyme-linked immunosorbent assay using two synthetic peptides as antigens. J. Clin. Microbiol. 32:971-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roddy, P., J. Goiri, L. Flevaud, P. P. Palma, S. Morote, N. Lima, L. Villa, F. Torrico, and P. Albajar-Viñas. 2008. Field evaluation of a rapid immunochromatographic assay for detection of Trypanosoma cruzi infection by use of whole blood. J. Clin. Microbiol. 46:2022-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saldaña, A., and O. E. Sousa. 1996. Trypanosoma rangeli: epimastigote immunogenicity and cross-reaction with Trypanosoma cruzi. J. Parasitol. 82:363-366. [PubMed] [Google Scholar]
- 34.Schneider, A., A. Hemphill, T. Wyler, and T. Seebeck. 1988. Large microtubule-associated protein of T. brucei has tandemly repeated, near-identical sequences. Science 241:459-462. [DOI] [PubMed] [Google Scholar]
- 35.Umezawa, E. S., S. F. Bastos, M. E. Camargo, L. M. Yamauchi, M. R. Santos, A. Gonzalez, B. Zingales, M. J. Levin, O. Sousa, R. Rangel-Aldao, and J. F. da Silveira. 1999. Evaluation of recombinant antigens for serodiagnosis of Chagas' disease in South and Central America. J. Clin. Microbiol. 37:1554-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Umezawa, E. S., S. F. Bastos, J. R. Coura, M. J. Levin, A. Gonzalez, R. Rangel-Aldao, B. Zingales, A. O. Luquetti, and J. Franco da Silveira. 2003. An improved serodiagnostic test for Chagas' disease employing a mixture of Trypanosoma cruzi recombinant antigens. Transfusion 43:91-97. [DOI] [PubMed] [Google Scholar]
- 37.Umezawa, E. S., A. O. Luquetti, G. Levitus, C. Ponce, E. Ponce, D. Henriquez, S. Revollo, B. Espinoza, O. Sousa, B. Khan, and J. F. da Silveira. 2004. Serodiagnosis of chronic and acute Chagas' disease with Trypanosoma cruzi recombinant proteins: results of a collaborative study in six Latin American countries. J. Clin. Microbiol. 42:449-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villa, L., S. Morote, O. Bernal, D. Bulla, and P. Albajar-Vinas. 2007. Access to diagnosis and treatment of Chagas disease/infection in endemic and non-endemic countries in the XXI century. Mem. Inst. Oswaldo Cruz 102:87-94. [DOI] [PubMed] [Google Scholar]
- 39.Withers-Martinez, C., E. P. Carpenter, F. Hackett, B. Ely, M. Sajid, M. Grainger, and M. J. Blackman. 1999. PCR-based gene synthesis as an efficient approach for expression of the A+T-rich malaria genome. Protein Eng. 12:1113-1120. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. 2005. Tropical disease research: progress 2003-2004: Seventeenth Programme Report of the UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases. World Health Organization, Geneva, Switzerland.
- 41.Yip, C. W., C. C. Hon, F. Zeng, K. Y. Chow, K. H. Chan, J. S. Peiris, and F. C. Leung. 2007. Naturally occurring anti-Escherichia coli protein antibodies in the sera of healthy humans cause analytical interference in a recombinant nucleocapsid protein-based enzyme-linked immunosorbent assay for serodiagnosis of severe acute respiratory syndrome. Clin. Vaccine Immunol. 14:99-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeinali, M., S. K. Ardestani, and A. Kariminia. 2007. Purification and biochemical characterization of two novel antigens from Leishmania major promastigotes. Korean J. Parasitol. 45:287-293. [DOI] [PMC free article] [PubMed] [Google Scholar]