Abstract
Host macrophage migration inhibitory factor (MIF) has been implicated in the pathogenesis of malaria infections. Several Plasmodium parasite-derived MIFs were identified to have the potential to regulate host immune response. However, the role of Plasmodium MIFs in the immunopathogenesis of malaria infection and the relationships between these mediators and inflammatory cytokines remained unclear. In this study, we have investigated two Plasmodium MIFs in peripheral blood of uncomplicated malaria patients and analyzed their correlations with several major factors during malaria infection. We found that both Plasmodium falciparum MIF (PfMIF) and Plasmodium vivax MIF (PvMIF) levels in patients were positively correlated with parasitemia, tumor necrosis factor alpha, interleukin-10 (IL-10), and monocyte chemoattractant protein 1 but were not correlated with transforming growth factor β1 and IL-12. Of interest was that the PvMIF level was positively correlated with host body temperature and human MIF (HuMIF) concentrations. Moreover, multiple stepwise regression analysis also showed that parasitemia, IL-10, and HuMIF expression were significant predictors of Plasmodium MIF production. In addition, during antimalarial drug treatment, the decreasing of Plasmodium MIF concentrations was followed by parasitemia in most patients. Our results suggested that the Plasmodium MIF circulating level reflects the level of parasitemia and thus was closely correlated with disease severity in uncomplicated malaria. Therefore, this factor has the potential to be a promising disease predictor and is applicable in clinical diagnosis.
Malaria is one of the major global health problems, causing more than 300 million clinical cases and about one million deaths each year in tropic and subtropic areas. Among the four malaria parasite species that infect humans, Plasmodium falciparum and Plasmodium vivax are the most widely distributed ones, and the latter parasite is the dominating cause of malaria in southern China. Infection with P. falciparum or P. vivax provokes a complex immune response of the host, in which the cytokine network plays an essential role. One of the cytokines, macrophage migration inhibitory factor (MIF), has been considered a critical regulator in both innate and adaptive immune responses (5) and is involved in the pathogenesis of several parasitic infections (26, 32). In murine models of malaria, host MIF inhibited erythroid, multipotential, and granulocyte-macrophage progenitor-derived colony formation, and the circulating level of host MIF during Plasmodium chabaudi infection has been found to correlate with disease severity (21). Significantly, McDevitt et al. reported that the infection of MIF knockout mice with P. chabaudi was found to result in less-severe anemia, improved erythroid progenitor development, and increased survival compared to those of wild-type controls (24). In human malaria patients, MIF also was found to inhibit erythroid differentiation and hemoglobin production, further indicating that it plays a critical role in the pathogenesis of malarial anemia (24). Moreover, many reports have confirmed that MIF levels in malaria patients were altered compared to those of counterparts of noninfected individuals; however, the increase or decrease of MIF circulating levels was not consistent in these research studies, mostly due to their different sample sources and the extent of disease severity. For example, using child patient samples, Clark et al. found that children with cerebral malaria (CM) expressed very low MIF levels in blood vessel walls within the brain, whereas children with fatal P. falciparum malaria expressed high MIF levels in blood vessel walls within their peripheral tissue (9, 10). Awandare et al. reported that children with acute malaria or severe anemia showed a reduction in plasma MIF levels (3, 4). Women with malaria during pregnancy showed an increase in MIF levels in intervillous plasma and cultured intervillous blood mononuclear cells but not in the peripheral plasma (6-8). McDevitt et al. (24) and Femandes et al. (14) found an increase in plasma MIF levels in general patients with acute malaria; however, in experimental human P. falciparum malaria models, plasma MIF levels decreased significantly during early-blood-stage infection, which paralleled a similar decline in circulating lymphocytes (13). Collectively, those observations indicate that host MIF is a critical pathogenesis-associated cytokine manifested during malaria infection, and it is one that also participates in its immunopathology.
In recent years, several Plasmodium MIFs have been identified and subsequently confirmed to have potential in regulating host immune responses in vitro (2, 11, 30). However, the role of Plasmodium MIFs, especially in immunopathogenesis during malaria infection, still is obscure. Cordery et al. have shown an elevation of anti-P. falciparum MIF (PfMIF) IgG levels in patients with P. falciparum (11). Our group developed a monoclonal antibody (MAb 1B9) that specifically recognized PfMIF but not host MIF and other Plasmodium MIFs, and we also identified its exact epitope in the PfMIF sequence (33). By using this MAb, we have set up a specific sandwich enzyme-linked immunosorbent assay (ELISA) method for detecting and quantifying circulating PfMIF molecules in acute malaria patients (30). In this report, we describe another monoclonal antibody (MAb 3B4) that specifically recognized P. vivax MIF (PvMIF), and a specific sandwich ELISA method was established with 3B4 for PvMIF quantification. With these MAbs we investigated the circulating PfMIF and PvMIF levels in the plasma of malaria patients from the northern part of Burma, and we analyzed the correlations with some important mediators and inflammatory factors taking place during malaria infection. Additionally, the effects of individual HLA differences on Plasmodium MIF circulating levels were determined. Finally, we determined the alterations of Plasmodium MIF concentrations during antimalarial drug treatment. Collectively, these results will help us better understand the role of Plasmodium MIF during malaria infection.
MATERIALS AND METHODS
Study participants and laboratory evaluation.
This study was carried out in Burma in 2008. A total of 141 patients with P. falciparum (n = 74) and P. vivax (n = 67) malaria infections were enrolled in the study. Forty-eight healthy controls were from Beijing Union Medical College Hospital, Beijing, China. According to the criteria of the World Health Organization (WHO) (34), one P. falciparum patient had severe malarial anemia and one P. falciparum patient had hyperparasitemia; all other patients had uncomplicated malaria. About 80% of the patients mentioned that they had had at least one episode of malaria in the past year. On the admission of each patient, 4 ml of plasma and 2 ml of blood cells were collected, and a Giemsa-stained blood smear was prepared (on day 0). They then were treated promptly with the appropriate antimalarial and supportive therapy. A piperaquine and dihydroartemisinine combination therapy was used for P. falciparum-infected patients, whereas a standard 8-day course of chloroquine and pirmaquinum was used for P. vivax-infected patients. During drug therapy, 2 ml of plasma and a blood smear also were prepared on days 1, 3, and 7. Plasma was used for the detection of MIF, anti-MIF IgG, and other cytokines, and blood cells were used for HLA (A, B, DRB1, and DPB1) phenotyping; Giemsa-stained blood smears were used to determine parasitemia levels and the Plasmodium species. The level of parasitemia (asexual parasites/μl blood) was estimated by counting the number of asexual parasites against leukocytes, assuming each patient had 8,000 leukocytes/μl. Parasitemia (per μl) was calculated by using the following formula: parasitemia = number of parasites × (8,000/number of leukocytes). Patients were considered anemic when the hemoglobin level was less than or equal to 11g/dl of blood and were considered to have fever when the temperature was above 37.5°C. The proportion of patients lost to follow-up was <38% during antimalarial drug treatment (28/74 [37.8%] P. falciparum patients and 24/67 [35.8%] P. vivax patients by day 7).
Ethical approval was granted by the Institutional Review Board (IRB) of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, and written informed consent was obtained from the patients infected with P. falciparum and P. vivax malaria.
PvMIF gene cloning, protein expression, and purification.
To generate monoclonal antibodies, the PvMIF open reading frame was PCR amplified using PvMIF-specific primers PvHis1 (5′-GGAATTCCATATGCCGTGTTGCCAAGTCTCC-3′ with a 5′-NdeI restriction site) and PvHis2 (5′-CCGCTCGAGGCCGAAGAGCGATCCGTTGAAGG-3′ with a 5′-XhoI restriction site) from a P. vivax cDNA library (GenBank clone no. CV646628, CV637058, or CX020889), which was obtained from the Malaria Research and Reference Reagent Resource Center (MR4). The PCR product was cloned in frame into pET30a (Invitrogen) and predigested with NdeI and XhoI enzymes. Recombinant plasmid was transformed into Escherichia coli BL21(DE3), and the expression of the recombinant histidine-tagged PvMIF (rPvMIF-His) was induced by the addition of 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) to the bacterial culture and incubated for 4 h at 37°C. rPvMIF-His was purified using a nickel-agarose column (Ni-nitrilotriacetic acid agarose; Invitrogen).
To generate polyclonal antibodies, the PvMIF open reading frame was PCR amplified with primers PvGST1(5′-CGGGATCCATGCCGTGTTGCCAAGTCTCC-3′ with a 5′-BamHI restriction site) and PvGST2 (5′-CCGCTCGAGTTAGCCGAAGAGCGATCCGTTG-3′ with a 5′-XhoI restriction site). The PCR product then was cloned in frame into pGEX-4T-1 (GE Healthcare) using the BamHI and XhoI restriction sites. rPvMIF-GST was purified using glutathione Sepharose 4B (GE Healthcare). The protein concentrations were determined using the bicinchoninic acid protein assay kit (Pierce), and the purity exceeded 95%.
Antibody production.
According to standard protocols, anti-PvMIF monoclonal hybridoma cell lines were generated in BALB/c mice. BALB/c mice were immunized with three subcutaneous injections of 50 μg of rPvMIF-His every 2 weeks and with one intravenous tail injection of 100 μg of rPvMIF-His (without adjuvant). The initial injection was emulsified with Freund's complete adjuvant, with the following two injections emulsified with Freund's incomplete adjuvant. Hybridoma cells were obtained from splenocytes of immunized mice 3 days after the last boost injection and from myeloma SP2/0 cells. Positive hybridomas were cloned, and anti-PvMIF monoclonal antibody 3B4 (IgG1 isotype) was purified by the use of protein G Sepharose (Amersham Biosciences) from culture supernatants.
Polyclonal anti-PvMIF serum was generated by the immunization of New Zealand white rabbits with three subcutaneous injections of 200 μg of rPvMIF-GST every 2 weeks. Serum was harvested on day 50 after the initial injection, and polyclonal anti-PvMIF IgG was purified by the use of protein G Sepharose.
MIF-specific sandwich ELISA.
Plasma levels of PfMIF and PvMIF in the malaria-infected patients were measured by the MIF-specific sandwich ELISA method (30). Briefly, 96-well ELISA plates were coated with 1.5 μg of the anti-MIF MAb (anti-PfMIF MAb, 1B9, IgG1 isotype; anti-PvMIF MAb, 3B4, IgG1 isotype) in PBS overnight at room temperature. The plates were washed with Tris-buffered saline (TBS)-0.05% Tween 20 and blocked with TBS containing 2% goat serum for 2 h at 37°C. Plasma samples (100 μl) at no dilution were plated and incubated for 2 h at 37°C. After the incubation the plates were washed, and the anti-MIF polyclonal antibody (diluted 1:250) was plated and incubated for 1 h at 37°C. After being washed, alkaline phosphatase-conjugated goat anti-rabbit IgG (Beijing Zhongshan Goldenbridge Biotechnology Co., Ltd.) was added at a 1:1,000 dilution for 1 h at 37°C. Finally, the substrate p-nitrophenyl phosphate (pNPP) in ethanolamine solution was added and positive signals were read at 405 nm. MIF concentrations were determined using a standard curve obtained from known concentrations of recombinant MIF (24.4 to 50,000 pg/ml) included in each assay plate. The lower limit of detection was 100 pg/ml.
Anti-MIF IgG ELISA.
To determine plasma anti-PfMIF IgG, the ELISA plates were coated with 2 mg/ml PfMIF-specific peptide at room temperature overnight. The peptide is the epitope of the anti-PfMIF MAb 1B9, and its sequence is 36LGYIMSNYDYQKNLRFGGSNEA57 (33). For the detection of anti-HuMIF IgG and anti-PvMIF IgG, the ELISA plates were coated with rHuMIF-his and rPvMIF-his, respectively. The wells were blocked with TBS containing 2% goat serum for 2 h at 37°C, followed by the addition of patient plasma diluted 1:100 in blocking buffer for 2 h at 37°C. After being washed, alkaline phosphatase-conjugated rabbit anti-human IgG was added at a 1:1,000 dilution for 1 h at 37°C. pNPP then was added, and samples were read at 405 nm.
Cytokine and cytokine measurements.
Plasma inflammatory cytokine levels were measured by ELISA using reagents from R&D Systems (Minneapolis, MN). Briefly, the ELISA kits for tumor necrosis factor alpha (TNF-α; no. DY210), transforming growth factor β1 (TGF-β1; no. DY240), interleukin-10 (IL-10; no. DY 217B), IL-12 (clone 24945.11), MIF (clone 12302.2), and monocyte chemoattractant protein 1 (MCP-1; no. DY 279) were used. The sensitivities for the assays were the following: TNF-α, ≥15.6 pg/ml; TGF-β1, IL-10, and MCP-1, ≥31.2 pg/ml; IL-12, ≥0.078 pg/ml; and MIF, ≥100 pg/ml. To exclude TGF-β1 released from platelets, a separate aliquot of platelet-poor plasma was generated by centrifugation at 10,000 × g for 15 min. The resulting plasma samples were activated by incubation with 2.5 N acetic acid-10 N urea for 10 min, followed by neutralization with 2.7 N NaOH-1 M HEPES for 10 min before the determination of TGF-β1 levels.
HLA typing.
Genomic DNA was extracted from blood cells of the patients. Low- to moderate-resolution, PCR-based HLA typing was performed with the AB/DR SSP Uni-Tray AB/DR SSO Accuplex (Invitrogen Carlsbad, CA) for HLA-A, HLA-B, and HLA-DRB1 typing and the direct to high-res HLA-DPB1 SSP Uni-Tray (Invitrogen) for HLA-DPB1 typing. The results were validated by Beijing Siercheng Search Biotech Co., Ltd.
Statistical analysis.
Continuous variables were presented as the medians within interquartile ranges when nonnormally distributed. Continuous variables were compared using the Student's t test, and noncontinuous variables were compared using the χ2 test. Differences in cytokine levels between groups were analyzed by the nonparametric Mann-Whitney U test. For examining the correlations of MIF levels with cytokine levels, age, parasitemia, body temperature, and hemoglobin concentration, both Pearson's correlation test and multiple stepwise regression analysis were used. MIF levels, parasitemia, and IL-12 levels were log transformed to normalize their distribution prior to analysis. All data analyses were performed using SAS 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
Sequence analysis of PvMIF and development of its specific monoclonal antibody, 3B4.
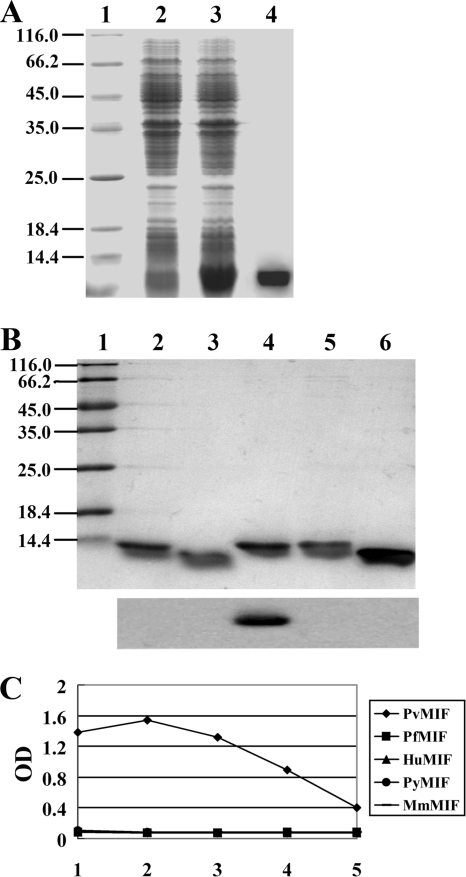
The Pvmif gene was amplified with the P. vivax cDNA library (GenBank clone no. CV646628, CV637058, or CX020889) as the template by specific primers. The sequence of Pvmif consists of 116 amino acids and is 32.48% identical to the sequence of human MIF (HuMIF) and 71.55% identical to that of PfMIF. The GenBank accession number of Pvmif mRNA is GU319966. For determining the presence of PvMIF in a patient's circulation, we have developed a monoclonal antibody, 3B4, that is specific to PvMIF. This antibody showed no cross-reactivity with recombinant HuMIF, PfMIF, murine MIF (MmMIF), and P. yoelii-derived MIF (PyMIF) in a Western blot assay or by ELISA (Fig. 1).
FIG. 1.
Purification of recombinant PvMIF and specificity identification of monoclonal antibody 3B4. (A) Protein extracted from preinduced (lane 2) and induced (lane 3) bacteria carrying pET 30a-Pvmif are shown. The induced extract was passed over a nickel affinity column, and the 13.4-kDa rPvMIF-His fusion protein was eluted with imidazole (lane 4). (B) Purified recombinant PyMIF (rPyMIF) (lane 2), rMmMIF (lane 3), rPvMIF (lane 4), rPfMIF (lane 5), and rHuMIF (lane 6) proteins are shown. All of the proteins were transferred to a polyvinyl difluoride membrane and immunostained with MAb 3B4 (bottom). All of the extracts and proteins were separated by 15% SDS-PAGE, and a low-molecule-weight protein marker is shown (lane 1). (C) Specificity detection of MAb 3B4 by ELISA. Purified rPvMIF, rPfMIF, rHuMIF, rPyMIF, and rMmMIF were coated. The dilutions of MAb 3B4 were the following: lane 1, 1:500; 2, 1:2,500; 3, 1:12,500; 4, 1:62,500; 5, 1:312,500.
Plasmodium-derived MIF could be detected in most of the acute malaria patients.
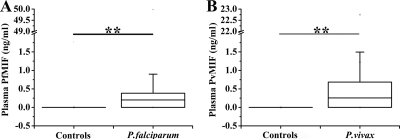
Among P. falciparum-infected patients, PfMIF was detectable in 47 of 74 individuals (63.5%), and its median level (interquartile range) was 0.202 ng/ml (0 to 0.385 ng/ml) (Fig. 2 A). PvMIF was detected in 47 of 67 P. vivax-infected patients (70.1%) with a median level (interquartile range) of 0.255 ng/ml (0 to 0.683 ng/ml) (Fig. 2B). No Plasmodium MIF was detectable in any of the healthy controls (Fig. 2A and B).
FIG. 2.
Plasmodium-derived MIF concentrations in healthy controls and patients with acute uncomplicated P. falciparum (A) or acute uncomplicated P. vivax (B) malaria infection. P values were determined by nonparametric Mann-Whitney U tests. **, P < 0.0001.
The circulation levels of Plasmodium-derived MIFs were positively correlated with parasitemia, TNF-α, IL-10, and MCP-1 levels.
For analyzing the relationship between Plasmodium MIF and the extent of malaria infection, we examined a series of inflammatory cytokines and disease-associated factors and analyzed their correlations with two Plasmodium MIFs.
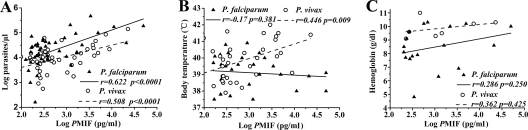
As shown in Table 1, the levels of parasitemia and hemoglobin as well as the body temperature of all infected patients were determined. The median parasitemia was significantly higher in P. falciparum-infected patients (11,550 parasites/μl) than in P. vivax-infected (7,161 parasites/μl) patients (P = 0.024). Conversely, the median hemoglobin concentration (g/dl) and body temperature (°C) were significantly lower in P. falciparum patients (12.2 versus 13.0 g/dl [P = 0.005] and 37.7 versus 38.5°C [P = 0.008], respectively). The correlation analysis demonstrated that both PfMIF and PvMIF levels in plasma showed significant positive correlations with parasitemia (r = 0.622 [P < 0.0001] for P. falciparum patients; r = 0.508 [P < 0.0001] for P. vivax patients) (Fig. 3 A) and no correlation with hemoglobin (r = 0.286 [P = 0.250] for P. falciparum patients; r = 0.362 [P = 0.425] for P. vivax patients) (Fig. 3C). In addition, only the logPvMIF (pg/ml) value showed a significant positive correlation with body temperature (r = 0.446 [P = 0.009]) (Fig. 3B).
TABLE 1.
Clinical, parasitological, and laboratory characteristics of patients with P. falciparum and P. vivax malaria infection
| Characteristic | P. falciparum malaria (n = 74) | P. vivax malaria (n = 67) | P |
|---|---|---|---|
| Age (yr) | |||
| 4-14, n (%) | 12 (16.2) | 21 (31.3) | 0.042b |
| 15-28, n (%) | 41 (55.4) | 36 (53.7) | |
| 29-56, n (%) | 21 (28.4) | 10 (14.9) | |
| Sex | |||
| Male, n (%) | 54 (73.0) | 56 (83.6) | 0.129b |
| Female, n (%) | 20 (27.0) | 11 (16.4) | |
| Hemoglobin,a g/dl | 12.2 (4.8-17.4) | 13.0 (9.1-17.8) | 0.005c |
| Parasitemia,a parasites/μl | 11,550 (160-1,300,000) | 7,161 (560-141,840) | 0.024c |
| Temperature,a °C | 37.7 (35.7-40.9) | 38.5 (36-42) | 0.008c |
Data shown are medians (ranges).
Significant value as determined by χ2 test with significance set at P < 0.05.
Significant value as determined by Student's t test with significance set at P < 0.05.
FIG. 3.
Correlation analysis of Plasmodium-derived MIF (PMIF) levels with parasitemia (n = 47 for P. falciparum patients and n = 47 for P. vivax patients) (A), fever (n = 29 and 33, respectively) (B), and anemia (n = 18 and 7, respectively) (C) in patients with acute uncomplicated malaria infection. Parasitemia and PMIF levels were log transformed. Statistical associations were determined by Pearson's correlation tests.
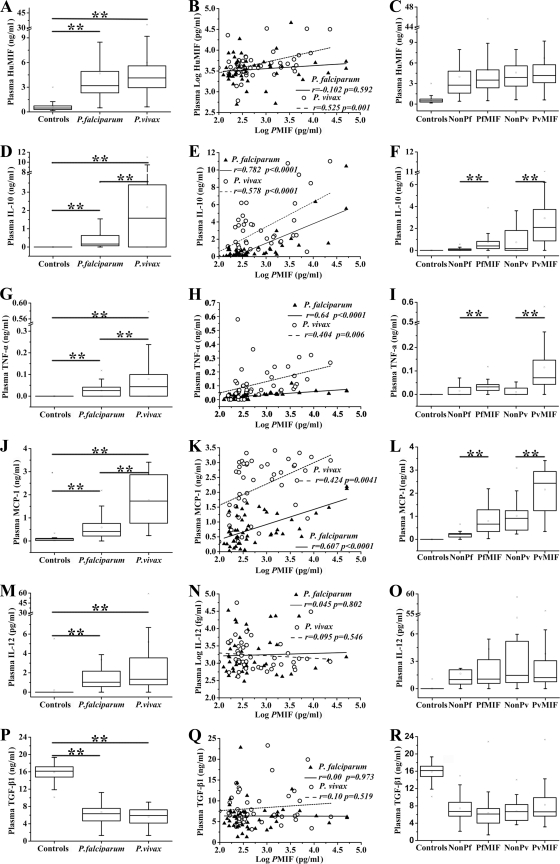
In cytokine detection analyses, HuMIF levels (shown as medians and interquartile ranges in ng/ml) in patients with P. falciparum and P. vivax malaria infection and healthy controls were 3.189 (interquartile range, 2.319 to 4.923), 4.131 (2.943 to 5.592), and 0.481 (0.300 to 0.728); IL-10 levels were 0.168 (0.062 to 0.633), 1.575 (0 to 3.400), and 0; TNF-α levels were 0.027 (0 to 0.042), 0.044 (0 to 0.100), and 0; MCP-1 levels were 0.439 (0.203 to 0.773), 1.728 (0.770 to 2.868), and 0.090 (0.051 to 0.110); TGF-β1 levels were 6.328 (4.643 to 7.564), 6.661 (5.454 to 9.573), and 16.139 (14.788 to 17.157); IL-12 levels (shown as medians and interquartile ranges in pg/ml) were 1.037 (0.576 to 2.181), 1.336 (0.770 to 3.433), and 0 (Fig. 4 A, D, G, J, M, and P). Both P. vivax- and P. falciparum-infected patients had significantly higher HuMIF, IL-10, TNF-α, MCP-1, and IL-12 plasma concentrations (P < 0.0001 for all analytic comparisons) (Fig. 4A, D, G, J, and M) and significantly lower TGF-β1 concentration levels in infected patients (P < 0.0001) (Fig. 4P) than healthy controls. Moreover, IL-10, TNF-α, and MCP-1 concentrations were significantly higher in P. vivax-infected patients than in P. falciparum-infected patients (P = 0.003 for IL-10 and P < 0.0001 for TNF-α and MCP-1, respectively) (Fig. 4D, G, and J). There were significant positive correlations between both of the Plasmodium MIFs and IL-10 (r = 0.782 [P < 0.0001] for P. falciparum patients; r = 0.578 [P < 0.0001] for P. vivax patients) (Fig. 4E), TNF-α (r = 0.640 [P < 0.0001] for P. falciparum patients; r = 0.404 [P = 0.006] for P. vivax patients) (Fig. 4H), and MCP-1 (r = 0.607 [P < 0.0001] for P. falciparum patients; r = 0.424 [P = 0.0041] for P. vivax patients) (Fig. 4K). Moreover, significant positive correlation also was observed between PvMIF and HuMIF plasma levels (r = 0.525 [P = 0.001]) (Fig. 4B). Furthermore, when we divided the patients into two groups, the Plasmodium MIF-detectable group and Plasmodium MIF-undetectable group, we found that the concentrations of IL-10, TNF-α, and MCP-1 were significantly higher in the Plasmodium MIF-detectable group than those in the Plasmodium MIF-undetectable group (Fig. 4F, I, and L), and these results were identical in P. vivax- and P. falciparum-infected patients (P < 0.0001 for all such comparisons).
FIG. 4.
HuMIF, IL-10, TNF-α, MCP-1, IL-12, and TGF-β1 levels in patients with acute uncomplicated malaria infection and healthy controls and the correlation analysis of Plasmodium-derived MIFs (PMIFs) and these cytokines. (A, D, G, J, M, P) Inflammatory cytokine levels in patients with uncomplicated malaria infection and healthy controls. (B, E, H, K, N, Q) Correlations between PMIF concentrations and cytokine levels. PMIF levels and the IL-12 level were log transformed. Pearson's correlation test was used to examine statistical correlations. (C, F, I, L, O, R) Inflammatory cytokine levels in groups in which PMIF is undetectable (nonPf and nonPv) or detectable (PfMIF and PvMIF). Data shown are medians and interquartile ranges. P values were determined by nonparametric Mann-Whitney U tests. *, P < 0.01; **, P < 0.0001.
In multiple stepwise regression analysis, PfMIF or PvMIF served as the criterion variable, and the predictors were parasitemia, HuMIF, TNF-α, IL-12, IL-10, MCP-1, TGF-β1, body temperature, age, and hemoglobin. The analytic results showed that parasitemia, IL-10, and HuMIF were significant predictors of PfMIF, with standardized estimate coefficients of 0.44, 0.65, and −0.19 (P < 0.0001, P < 0.0001, and P = 0.0018, respectively). Moreover, these three factors were also the significant predictors of PvMIF, with standardized estimate coefficients of 0.56, 0.36, and 0.18 (P < 0.0001, P = 0.0001, and P = 0.063, respectively) (Table 2).
TABLE 2.
Relationships between Plasmodium MIF and parasitemia, IL-10, and HuMIF assessed by multiple stepwise regression analysis
| Predictorb | PfMIFa |
PvMIFa |
||||
|---|---|---|---|---|---|---|
| Parameter estimate | Pc | Standardized estimate | Parameter estimate | Pc | Standardized estimate | |
| Parasitemia | 0.00002301 | <0.0001 | 0.44 | 0.00007727 | <0.0001 | 0.56 |
| IL-10 | 3.66 | <0.0001 | 0.65 | 0.48 | 0.0001 | 0.36 |
| HuMIF | −0.30 | 0.0018 | −0.19 | 0.09 | 0.063 | 0.18 |
PfMIF and PvMIF served as the criterion variables.
Parasitemia, IL-10, and HuMIF were significant predictors assessed by multiple stepwise regression analysis.
All P values shown were <0.10.
Analysis of the effect of individual HLA differences on Plasmodium MIF plasma levels.
We have determined the HLA typing, including HLA-A, HLA-B, HLA-DRB1, and HLA-DPB1, of all P. falciparum and P. vivax patient samples. We observed no correlation between HLA typing and PvMIF plasma levels. However, in P. falciparum patient samples, several HLA alleles were found to be correlated with PfMIF detectability. Four of 70 P. falciparum-infected patients were determined to have HLA-A*2402 and HLA-A*3301 alleles, and none of them had any detectable PfMIF. Further, 9 of 68 P. falciparum-infected patients had HLA-B*3802 in one allele, and 8 of 9 of these patients had no detectable PfMIF (a total of 70 samples and 68 samples have been determined to have the HLA-A and HLA-B phenotype in P. falciparum patients, respectively). In addition, these HLA alleles (HLA-A*2402, HLA-A*3301, and HLA-B*3802) showed no correlations with parasitemia (data not shown).
Alteration of Plasmodium MIF circulating level during antimalarial drug treatment.
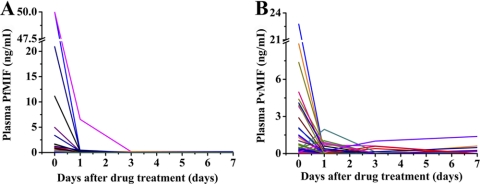
After being treated by antimalarial drugs, parasitemia in all malaria patients decreased quickly and almost disappeared by day 3. Not surprisingly, at the same time, clinical symptoms also disappeared. PfMIF plasma concentrations declined and vanished following the decline in parasitemia. However, PvMIF levels in some patients were noted to be fluctuating, and some even increased after both parasitemia and clinical symptoms disappeared (Fig. 5).
FIG. 5.
Plasmodium MIF level tracing in uncomplicated P. falciparum-infected (A) and uncomplicated P. vivax-infected (B) patients during antimalarial drug treatment. Each line with one type of color represents the Plasmodium MIF level change obtained from one patient tracking at four points: before treatment (day 0), 1 day after drug treatment (day 1), day 3, and day 7.
Anti-Plasmodium MIF response in patients.
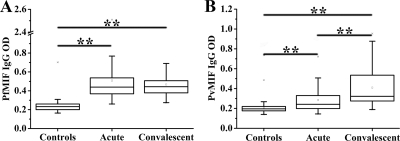
Antibody responses were examined in acute malaria patient samples (and the same patients during convalescence) and healthy controls. There were significantly higher median (interquartile range) anti-PfMIF antibody levels in the acute (0.439; 0.366 to 0.541) and convalescent samples (0.443; 0.374 to 0.515) than in the healthy control samples (0.232; 0.200 to 0.260) (P < 0.0001 for both comparisons) (Fig. 6 A). A similar result was observed in anti-PvMIF antibody detection (0.242 [0.199 to 0.330] for the acute malaria group, 0.327 [0.274 to 0.540] for the convalescent group, and 0.196 [0.174 to 0.221] for the healthy control group) (P < 0.0001 for both comparisons) (Fig. 6B). In addition, unlike the case for anti-PfMIF antibodies, the anti-PvMIF antibody level was significantly higher in convalescent samples than in acute samples (P < 0.0001) (Fig. 6B).
FIG. 6.
Antibody response to Plasmodium MIFs in patients with uncomplicated P. falciparum (A) or uncomplicated P. vivax (B) malaria infection. Samples were taken from acute uncomplicated malaria patients, convalescent patients, and healthy controls. Data shown are medians and interquartile ranges. P values were determined by nonparametric Mann-Whitney U tests. **, P < 0.0001.
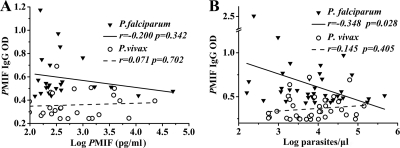
In addition, anti-PfMIF antibody in plasma showed a significant negative correlation with parasitemia (r = −0.348 [P = 0.028]), but anti-PvMIF antibody did not (r = 0.145 [P = 0.405]) (Fig. 7 B). Both anti-PfMIF antibody and anti-PvMIF antibodies showed no correlations with PfMIF or PvMIF (r = −0.200 [P = 0.342] for P. falciprum patients; r = 0.071 [P = 0.702] for P. vivax patients) (Fig. 7A), TNF-α (r = 0.071 [P = 0.663] for P. falciprum patients; r = 0.00 [P = 0.997] for P. vivax patients), and IL-10 (r = 0.114 [P = 0.479] for P. falciparum patients; r = 0.077 [P = 0.659] for P. vivax patients).
FIG. 7.
Correlation analysis of anti-Plasmodium MIF (PMIF) IgG with Plasmodium MIF (PMIF) levels (A) (n = 24 for P. falciparum patients and n = 25 for P. vivax patients) and parasitemia (B) (n = 40 and 35, respectively). Pearson's correlation test was used to examine statistical correlations.
DISCUSSION
Host cytokines were found to play important roles in malarial immunopathology. During malaria infection, TNF-α and IL-10 were confirmed to be significantly correlated with disease severity. Elevated IL-10 levels were found in P. falciparum hyperparasitemia and had a positive correlation with parasite numbers in patients infected with P. vivax (12, 18, 35). Additionally, IL-10 levels correlated well with high fever in infected patients (27, 35). In severe malaria, anti-inflammatory IL-10 and proinflammatory IL-6, IL-12, and TNF-α levels all were found to be elevated (20). The TNF-α level was associated with hyperparasitemia, severe anemia, and hypoglycemia, and it was linked to disease severity and subsequent complications (16, 19, 28). The results of our experiments showed that the levels of Plasmodium MIFs were positively correlated with parasitemia, TNF-α, and IL-10, which further suggested that Plasmodium MIF was correlated with the course of malaria infection and some clinical symptoms.
From drug treatment tracking studies, we have observed that PfMIF levels decrease as parasitemia decreases. Whereas PvMIF levels in some patients appeared to fluctuate or even increased after parasitemia disappeared with no clinical symptoms, PvMIF levels in most of the treated patients declined with parasitemia. In a previous animal model experiment, we also found the PyMIF concentration in the peripheral blood of mice to be decreased after drug treatment and basically followed the decline of parasitemia. Therefore, although we still do not know the exact reason why PvMIF concentrations fluctuated in a few of these patients, the Plasmodium MIF levels on the whole that are present in peripheral blood of the patients can be considered a reflection of parasitemia and the course of malaria infection.
In this study, we found that not all patient plasma samples contain detectable Plasmodium MIF. Plasmodium MIF could be detected in only 63.5% of P. falciparum-infected patients and 70.1% of P. vivax-infected patients. There is some evidence that specific HLA alleles associated with resistance or susceptibility to malaria and influenced the immune response. HLA-B*5301 and HLA-DRB1*1302 were independently associated with resistance to severe malaria in children from the Gambia (17). DRB1*04 and DBP1*1701 were associated with severe malaria in children from Gabon (23). HLA-DQB1*0501 was associated with a decreased risk of reinfection and anemia (22). In a case-control study, HLA-A3, B27, B49, DRB1*04, and DRB1*0809 significantly increased in P. falciparum patients from western India; moreover, HLA-B49 and DRB1*0809 were found to be positively associated with the patients with severe malaria (29). HLA-A*0201 and HLA-A2 could restrict CD8+T-lymphocyte responses to P. falciparum and P. vivax (1, 15). We speculated that individual HLA differences may influence Plasmodium MIF detection. To examine the influence of individual differences, we analyzed some loci in the HLA genotypes. In HLA typing results, although two loci were found to exhibit a certain correlation whether or not PfMIF could be detected, there is no reasonable explanation for all of the samples in which no PfMIF was detected. Moreover, no locus in the HLA genotype was found to be strongly correlated with PvMIF detection. In addition, this work in humans expands the studies of our previous work, where we found that PyMIF could be detected in all infected mice having different genetic backgrounds, such as BALB/c, C57BL/6, and Kunming mice (31). Therefore, we thought that individual genetic difference was not a reason for Plasmodium MIF being detectable in infected subjects. Significantly, we noticed that about 60% of patients (59.3% P. falciparum patients and 63.6% P. vivax patients) who have had no detectable Plasmodium MIF did have significant antibody responses, indicating the presence of Plasmodium MIF in those patients. Therefore, all of these indications suggest that individual differences do not determine Plasmodium MIF detection.
Many research papers have reported HuMIF levels in the plasma of healthy individuals and in patients with all kinds of diseases. However, there are no reports of any non-HuMIF detection, and the concentration of HuMIF generally is found to be higher than 1 ng/ml. Similar results have been confirmed in our experiments. However, it should be noted that the median concentration of Plasmodium MIF that we detected was about 200 pg/ml, and most of the Plasmodium MIF concentrations were well below 1 ng/ml, which is far lower than that normally observed for HuMIF. This disparity might easily be explained by the significant differences in numbers between host cells and parasite cells. Moreover, we noticed that the median parasitemia in P. falciparum- and P. vivax-infected patients were 0.29 and 0.18%, respectively. However, the median parasitemia in P. yoelii-infected mice could reach 30 to 40%, which accounted for 100% detection of PyMIF in all infected mice. Therefore, according to the assessment described above and the results of the positive correlation noted between Plasmodium MIF concentration and parasitemia, we can deduce that low levels of parasitemia result in low concentrations of Plasmodium MIF in plasma, and that some lower concentrations were beyond the limit of the Plasmodium MIF-specific sandwich ELISA methods currently being used.
Although the detection limit (100 pg/ml) of our Plasmodium MIF-specific sandwich ELISA is comparable to that of most commercial cytokine detection kits, the Plasmodium MIF concentration is much lower than that of most of the cytokine concentrations found in human plasma. We are trying to further optimize antibody pairs to be used for Plasmodium MIF capturing and detection with increased sensitivity. Moreover, Meyer-Siegler et al. reported that a significant amount of HuMIF complexes existed in human serum and did not bind to the MIF capture antibody in ELISA experiments, suggesting that the epitope on the surface of the HuMIF complex is not accessible to the capture antibody that is used in routine experiments (25). In a previous experiment, we also noticed a large-molecular-weight form of PyMIF in the sera of P. yoelii-infected mice (31). We speculated that there is a high-polymeric form of Plasmodium MIF, a complex of Plasmodium MIF with some other protein molecules, or even a complex of Plasmodium MIF with its antibodies (IgG or IgM) in the peripheral blood, and these high-polymeric or complex forms would influence the detection of MIF by ELISA. If this hypothesis is true, the actual plasma concentrations of Plasmodium MIF could be greater than what is now being detected. Therefore, a better method to disaggregate MIF polymer or MIF complex in plasma would be useful for improving detection, and Plasmodium MIF may become more readily detected in malaria-infected patients.
In summary, we confirmed the existence of PfMIF and PvMIF in the circulation of malaria parasite-infected patients, and we found that parasitemia and some selected cytokines were positively correlated with plasma concentrations of PfMIF and PvMIF. Multiple stepwise regression analysis showed that parasitemia and IL-10 were good correlative predictors of Plasmodium MIF concentrations. All of our experimental data supported the fact that circulating levels of Plasmodium MIF reflected malaria parasitemia in patients and closely correlated with the course of malaria infection and disease in uncomplicated malaria. Therefore, our results provided important data relevant to Plasmodium MIFs in human patients and will be helpful for understanding the role of these enigmatic factors during infection. Our results also indicated that Plasmodium MIF was a promising predictor that is applicable in clinical diagnosis.
Acknowledgments
This work was supported by research grants from the National Basic Research Program of China (973 Program; 2007CB513100), the Natural Science Foundation of China (30700761), and an intramural grant from the Institute of Pathogen Biology, Chinese Academy of Medical Sciences (2007IPB013).
We thank Wenhui Li for his helpful advice in revising the manuscript. We thank Ying Huang for providing blood samples of healthy controls. We thank Shi-ping Chen for technical assistance.
We have no conflicting financial interests in the studies relating to the manuscript.
Footnotes
Published ahead of print on 11 August 2010.
REFERENCES
- 1.Arévalo-Herrera, M., A. Z. Valencia, J. Vergara, A. Bonelo, K. Fleischhauer, J. M. Gonzalez, J. C. Restrepo, J. A. Lopez, D. Valmori, G. Corradin, and S. Herrera. 2002. Identification of HLA-A2 restricted CD8(+) T-lymphocyte responses to Plasmodium vivax circumsporozoite protein in individuals naturally exposed to malaria. Parasite Immunol. 24:161-169. [DOI] [PubMed] [Google Scholar]
- 2.Augustijn, K. D., R. Kleemann, J. Thompson, T. Kooistra, C. E. Crawford, S. E. Reece, A. Pain, A. H. Siebum, C. J. Janse, and A. P. Waters. 2007. Functional characterization of the Plasmodium falciparum and P. berghei homologues of macrophage migration inhibitory factor. Infect. Immun. 75:1116-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awandare, G. A., J. B. Hittner, P. G. Kremsner, D. O. Ochiel, C. C. Keller, J. B. Weinberg, I. A. Clark, and D. J. Perkins. 2006. Decreased circulating macrophage migration inhibitory factor (MIF) protein and blood mononuclear cell MIF transcripts in children with Plasmodium falciparum malaria. Clin. Immunol. 119:219-225. [DOI] [PubMed] [Google Scholar]
- 4.Awandare, G. A., Y. Ouma, C. Ouma, T. Were, R. Otieno, C. C. Keller, G. C. Davenport, J. B. Hittner, J. Vulule, R. Ferrell, J. M. Ong'echa, and D. J. Perkins. 2007. Role of monocyte-acquired hemozoin in suppression of macrophage migration inhibitory factor in children with severe malarial anemia. Infect. Immun. 75:201-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calandra, T., and T. Roger. 2003. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat. Rev. Immunol. 3:791-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaisavaneeyakorn, S., N. Lucchi, C. Abramowsky, C. Othoro, S. C. Chaiyaroj, Y. P. Shi, B. L. Nahlen, D. S. Peterson, J. M. Moore, and V. Udhayakumar. 2005. Immunohistological characterization of macrophage migration inhibitory factor expression in Plasmodium falciparum-infected placentas. Infect. Immun. 73:3287-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaisavaneeyakorn, S., J. M. Moore, C. Othoro, J. Otieno, S. C. Chaiyaroj, Y. P. Shi, B. L. Nahlen, A. A. Lal, and V. Udhayakumar. 2002. Immunity to placental malaria. IV. Placental malaria is associated with up-regulation of macrophage migration inhibitory factor in intervillous blood. J. Infect. Dis. 186:1371-1375. [DOI] [PubMed] [Google Scholar]
- 8.Chaiyaroj, S. C., A. S. Rutta, K. Muenthaisong, P. Watkins, M. Na Ubol, and S. Looareesuwan. 2004. Reduced levels of transforming growth factor-beta1, interleukin-12 and increased migration inhibitory factor are associated with severe malaria. Acta Trop. 89:319-327. [DOI] [PubMed] [Google Scholar]
- 9.Clark, I. A., M. M. Awburn, R. O. Whitten, C. G. Harper, N. G. Liomba, M. E. Molyneux, and T. E. Taylor. 2003. Tissue distribution of migration inhibitory factor and inducible nitric oxide synthase in falciparum malaria and sepsis in African children. Malar. J. 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark, I. A., and W. B. Cowden. 2003. The pathophysiology of falciparum malaria. Pharmacol. Ther. 99:221-260. [DOI] [PubMed] [Google Scholar]
- 11.Cordery, D. V., U. Kishore, S. Kyes, M. J. Shafi, K. R. Watkins, T. N. Williams, K. Marsh, and B. C. Urban. 2007. Characterization of a Plasmodium falciparum macrophage-migration inhibitory factor homologue. J. Infect. Dis. 195:905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Day, N. P., T. T. Hien, T. Schollaardt, P. P. Loc, L. V. Chuong, T. T. Chau, N. T. Mai, N. H. Phu, D. X. Sinh, N. J. White, and M. Ho. 1999. The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J. Infect. Dis. 180:1288-1297. [DOI] [PubMed] [Google Scholar]
- 13.De Mast, Q., F. C. Sweep, M. McCall, A. Geurts-Moespot, C. Hermsen, T. Calandra, M. G. Netea, R. W. Sauerwein, and A. J. van der Ven. 2008. A decrease of plasma macrophage migration inhibitory factor concentration is associated with lower numbers of circulating lymphocytes in experimental Plasmodium falciparum malaria. Parasite Immunol. 30:133-138. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes, A. A., L. J. Carvalho, G. M. Zanini, A. M. Ventura, J. M. Souza, P. M. Cotias, I. L. Silva-Filho, and C. T. Daniel-Ribeiro. 2008. Similar cytokine responses and degrees of anemia in patients with Plasmodium falciparum and Plasmodium vivax infections in the Brazilian Amazon region. Clin. Vaccine Immunol. 15:650-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González, J. M., K. Peter, F. Esposito, I. Nebie, J. M. Tiercy, A. Bonelo, M. Arevalo-Herrera, D. Valmori, P. Romero, S. Herrera, G. Corradin, and J. A. Lopez. 2000. HLA-A*0201 restricted CD8+ T-lymphocyte responses to malaria: identification of new Plasmodium falciparum epitopes by IFN-gamma ELISPOT. Parasite Immunol. 22:501-514. [DOI] [PubMed] [Google Scholar]
- 16.Grau, G. E., T. E. Taylor, M. E. Molyneux, J. J. Wirima, P. Vassalli, M. Hommel, and P. H. Lambert. 1989. Tumor necrosis factor and disease severity in children with falciparum malaria. N. Engl. J. Med. 320:1586-1591. [DOI] [PubMed] [Google Scholar]
- 17.Hill, A. V., C. E. Allsopp, D. Kwiatkowski, N. M. Anstey, P. Twumasi, P. A. Rowe, S. Bennett, D. Brewster, A. J. McMichael, and B. M. Greenwood. 1991. Common west African HLA antigens are associated with protection from severe malaria. Nature 352:595-600. [DOI] [PubMed] [Google Scholar]
- 18.Kurtzhals, J. A., V. Adabayeri, B. Q. Goka, B. D. Akanmori, J. O. Oliver-Commey, F. K. Nkrumah, C. Behr, and L. Hviid. 1998. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet 351:1768-1772. [DOI] [PubMed] [Google Scholar]
- 19.Kwiatkowski, D., A. V. Hill, I. Sambou, P. Twumasi, J. Castracane, K. R. Manogue, A. Cerami, D. R. Brewster, and B. M. Greenwood. 1990. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet 336:1201-1204. [DOI] [PubMed] [Google Scholar]
- 20.Lyke, K. E., R. Burges, Y. Cissoko, L. Sangare, M. Dao, I. Diarra, A. Kone, R. Harley, C. V. Plowe, O. K. Doumbo, and M. B. Sztein. 2004. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect. Immun. 72:5630-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martiney, J. A., B. Sherry, C. N. Metz, M. Espinoza, A. S. Ferrer, T. Calandra, H. E. Broxmeyer, and R. Bucala. 2000. Macrophage migration inhibitory factor release by macrophages after ingestion of Plasmodium chabaudi-infected erythrocytes: possible role in the pathogenesis of malarial anemia. Infect. Immun. 68:2259-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.May, J., B. Lell, A. J. Luty, C. G. Meyer, and P. G. Kremsner. 2001. HLA-DQB1*0501-restricted Th1 type immune responses to Plasmodium falciparum liver stage antigen 1 protect against malaria anemia and reinfections. J. Infect. Dis. 183:168-172. [DOI] [PubMed] [Google Scholar]
- 23.May, J., C. G. Meyer, J. F. Kun, B. Lell, D. Luckner, A. K. Dippmann, U. Bienzle, and P. G. Kremsner. 1999. HLA class II factors associated with Plasmodium falciparum merozoite surface antigen allele families. J. Infect. Dis. 179:1042-1045. [DOI] [PubMed] [Google Scholar]
- 24.McDevitt, M. A., J. Xie, G. Shanmugasundaram, J. Griffith, A. Liu, C. McDonald, P. Thuma, V. R. Gordeuk, C. N. Metz, R. Mitchell, J. Keefer, J. David, L. Leng, and R. Bucala. 2006. A critical role for the host mediator macrophage migration inhibitory factor in the pathogenesis of malarial anemia. J. Exp. Med. 203:1185-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer-Siegler, K. L., K. A. Iczkowski, and P. L. Vera. 2005. Further evidence for increased macrophage migration inhibitory factor expression in prostate cancer. BMC Cancer 5:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satoskar, A. R., M. Bozza, M. Rodriguez Sosa, G. Lin, and J. R. David. 2001. Migration-inhibitory factor gene-deficient mice are susceptible to cutaneous Leishmania major infection. Infect. Immun. 69:906-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seoh, J. Y., M. Khan, S. H. Park, H. K. Park, M. H. Shin, E. H. Ha, B. E. Lee, K. Yoo, H. S. Han, S. Oh, J. H. Wi, C. K. Hong, C. H. Oh, Y. A. Kim, and J. W. Park. 2003. Serum cytokine profiles in patients with Plasmodium vivax malaria: a comparison between those who presented with and without hyperpyrexia. Am. J. Trop. Med. Hyg. 68:102-106. [PubMed] [Google Scholar]
- 28.Shaffer, N., G. E. Grau, K. Hedberg, F. Davachi, B. Lyamba, A. W. Hightower, J. G. Breman, and N. D. Phuc. 1991. Tumor necrosis factor and severe malaria. J. Infect. Dis. 163:96-101. [DOI] [PubMed] [Google Scholar]
- 29.Shankarkumar, U., J. P. Devaraj, K. Ghosh, D. Karnad, K. Anand, and D. Mohanty. 2002. HLA associations in P. falciparum malaria patients from Mumbai, western India. Indian J. Malariol. 39:76-82. [PubMed] [Google Scholar]
- 30.Shao, D., Z. Han, Y. Lin, L. Zhang, X. Zhong, M. Feng, Y. Guo, and H. Wang. 2008. Detection of Plasmodium falciparum derived macrophage migration inhibitory factor homologue in the sera of malaria patients. Acta Tropica 106:9-15. [DOI] [PubMed] [Google Scholar]
- 31.Shao, D., X. Zhong, Y. F. Zhou, Z. Han, Y. Lin, Z. Wang, L. Bu, L. Zhang, X. D. Su, and H. Wang. Structural and functional comparison of MIF ortholog from Plasmodium yoelii with MIF from its rodent host. Mol. Immunol. 47:726-737. [DOI] [PubMed]
- 32.Stavitsky, A. B., C. Metz, S. Liu, J. Xianli, and R. Bucala. 2003. Blockade of macrophage migration inhibitory factor (MIF) in Schistosoma japonicum-infected mice results in an increased adult worm burden and reduced fecundity. Parasite Immunol. 25:369-374. [DOI] [PubMed] [Google Scholar]
- 33.Wang, Z., D. Shao, X. Zhong, C. Han, P. Cai, and H. Wang. 2009. Epitope mapping of monoclonal antibody 1B9 against plasmodium falciparum-derived macrophage migration inhibitory factor. Immunol. Investig. 38:422-433. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. 2000. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl. 1):S1-S90. [PubMed] [Google Scholar]
- 35.Zeyrek, F. Y., M. A. Kurcer, D. Zeyrek, and Z. Simsek. 2006. Parasite density and serum cytokine levels in Plasmodium vivax malaria in Turkey. Parasite Immunol. 28:201-207. [DOI] [PubMed] [Google Scholar]