Abstract
Diagnosis of human arboviral infections relies heavily on serological techniques such as the immunoglobulin M (IgM) antibody capture enzyme-linked immunosorbent assay (MAC-ELISA) and the indirect IgG ELISA. Broad application of these assays is hindered by the lack of standardized positive human control sera that react with a wide variety of flaviviruses (e.g., dengue, West Nile, yellow fever, Japanese encephalitis, Saint Louis encephalitis, and Powassan viruses), or alphaviruses (e.g., Eastern equine encephalitis, Western equine encephalitis, Venezuelan equine encephalitis, and chikungunya viruses) that can cause human disease. We have created human-murine chimeric monoclonal antibodies (cMAbs) by combining the variable regions of flavivirus (6B6C-1) or alphavirus (1A4B-6) broadly cross-reactive murine MAbs (mMAbs) with the constant region of human IgG1. These cMAbs may be used as standardized reagents capable of replacing human infection-immune-positive control sera in indirect IgG ELISA for diagnosis of all human flaviviral or alphaviral infections. The IgG cMAbs secreted from plasmid-transformed Sp2/0-Ag14 cells had serological activity identical to that of the parent mMAbs, as measured by ELISA using multiple flaviviruses or alphaviruses.
Arthropod-borne viruses (arboviruses) are responsible for a number of medically important human diseases. These viruses are maintained in nature through biological transmission between susceptible vertebrate hosts by blood-feeding arthropods, primarily mosquitoes and ticks. Although over 150 arboviruses are known to cause disease in humans, the majority of medically important arboviruses are found in three separate families, the Flaviviridae, the Togaviridae (genus Alphavirus), and the Bunyaviridae (24). Transmission of arboviruses can vary by season, a consequence of the feeding patterns of their respective arthropod vectors, as well as by specific geographic location, as is seen for dengue fever virus (DENV) and Japanese encephalitis virus (JEV) (20, 24). The primary clinical manifestation of arboviral disease in North America is encephalitis, although some arboviruses, such as yellow fever virus (YFV) are capable of causing severe hemorrhagic disease as well. Prior to the 1999 outbreak of West Nile virus (WNV) encephalitis in New York City, St. Louis encephalitis virus (SLEV) was the most important agent of epidemic viral encephalitis in North America, last causing a major epidemic in the mid-1970s (26, 28, 33). Since 1999, the distribution of WNV has rapidly expanded from New York to the rest of the United States and into Canada, Central America, and South America. As of April 2009, a total of 29,598 human WNV cases in the United States had been reported to the Centers for Disease Control and Prevention, of which 1,159 resulted in death (http://www.cdc.gov/ncidod/dvbid/westnile/surv&control.htm).
Given the globalization of commerce and travel, virus-infected people, animals, and arthropod vectors are able to move more easily between locations with great speed (16). Thus, it is likely that other arboviruses will follow the example of WNV, resulting in new or novel disease outbreaks in regions of the world outside their normal geographic ranges. Therefore, a rapid and standardized approach to identification of arboviral infections is needed worldwide for the diagnosis and tracking of current and reemerging arboviral diseases.
In the past, identification of antiviral antibody relied on four tests: the hemagglutination inhibition test, the complement fixation test, the plaque reduction neutralization test, and the indirect fluorescent antibody (IFA) test. Positive identification of a viral infection required a 4-fold increase in titer between acute- and convalescent-phase serum samples in these assays (20). Rapid serologic assays, such as the IgM capture enzyme-linked immunosorbent assay (MAC-ELISA) and IgG ELISA are now routinely used in diagnosis soon after infection. Early in infection, IgM antibody is more specific, while later in infection, IgG antibody is more cross-reactive. Inclusion of murine monoclonal antibodies (mMAbs) with defined virus specificities in these solid-phase assays has permitted a level of assay standardization that was not previously possible (30). In the diagnostic laboratory, the MAC-ELISA and the IgG ELISA are often used in tandem to identify positive specimens based on a 4-fold increase in titer between acute- and convalescent-phase serum samples and have replaced the more time-consuming and labor-intensive assays (11, 16, 21).
Application of the ELISA in serodiagnosis of arboviral infection is most hampered by the limited availability of human infection-immune sera for use as virus-reactive, antibody-positive control specimens. For the most part, antibody-positive control sera are derived by pooling small volumes of antibody-positive diagnostic serum specimens. The specimens are typically obtained for only the most prevalent arboviral agents (20, 21). Lot-to-lot variability of these serum pools can be high, and constant recollection and recalibration of antibody-positive and -negative control sera are necessary to ensure that test parameters remain valid (10, 21). Of even greater concern is the lack of antibody-positive control sera that can be used in diagnostic ELISAs to identify arboviruses that currently cause rare or infrequent human infections (20).
The replacement of variably reactive human control sera with group-specific human IgG antibodies would be a tremendous asset in the serological diagnosis of arboviral infections. Although a number of mMAbs demonstrating flaviviral, alphaviral, or bunyaviral group reactivity exist, they are unsuitable for use as positive serum controls in ELISAs designed to detect the presence of human antibodies. Moreover, the capture or detector antibodies used in these assays are often designed to react with other murine components of the ELISA, leading to an overwhelming false-positive response if mMAbs are employed as positive controls.
Fortunately, advances in the humanization of mMAbs have made it possible to overcome these limitations (31). One such method involves the incorporation of the heavy (H)- and light (L)-chain variable (V) regions of a given mMAb into an expression plasmid containing the constant (Cμ) region of human IgM (10). Upon transfection of cells, the resulting plasmid construct expresses a human-murine hybrid IgM chimeric MAb (cMAb) molecule that retains the specificity of the “parent” mMAb but reacts like human IgM in the MAC-ELISA (10, 12, 32).
We have previously reported on the construction and evaluation of an IgM cMAb with the specificity of the broadly flavivirus cross-reactive mMAb 6B6C-1. The 6ME2 IgM cMAb reacted with each flaviviral suckling mouse brain (SMB) or virus-like particle (VLP) antigen tested in the MAC-ELISA and displayed a strong preference for the WNV VLP antigen. The use of cell culture viral seed in place of the SMB or VLP antigens in the MAC-ELISA format resulted in enhanced reactivity, as measured by the maximum dilution of cMAb yielding a positive P/N value (positive/negative ratio; see below for details) against WNV, SLEV, DENV serotype 2 (DENV-2), and YFV. In this report we describe the development and characterization of two new IgG cMAbs for use in the indirect IgG ELISA. These cMAbs were created by incorporating the V regions of 6B6C-1 or the alphavirus group-specific mMAb 1A4B-6 into a plasmid construct containing the human IgG γ1 chain. The alpha- or flaviviral group reactivity of each cMAb was confirmed and subsequently evaluated in the standard indirect IgG ELISA. The cMAb demonstrating alphaviral (1GD5) or flaviviral (6GF4) group reactivities were selected for further use and were satisfactory replacements for antibody-positive human control sera against all alphaviruses or flaviviruses tested.
MATERIALS AND METHODS
Cell lines.
The Sp2/0-Ag14 (Sp2) murine myeloma, 6B6C-1 murine hybridoma, and 1A4B-6 murine hybridoma cell lines have been previously described (9, 12, 14, 25, 27, 32). Cells were propagated in hybridoma growth medium (HGM [high-glucose Dulbecco minimal essential medium containing l-glutamine supplemented with 20% fetal bovine serum, 1 mM sodium pyruvate, 2 mM l-glutamine, 0.15% sodium bicarbonate, 100 U/ml penicillin G sodium, 100 μg/ml streptomycin sulfate, and 0.1 mM nonessential amino acids]) unless noted otherwise. Cell culture viral seeds for WNV, YFV, JEV, SLEV, and DENV-2 were prepared as previously described (32).
Isolation of immunoglobulin V regions.
A Pharmacia QuickPrep mRNA purification kit (Amersham Pharmacia, Piscataway, NJ) was used according to the manufacturer's specifications to isolate mRNA from 5 × 107 hybridoma cells. PCR cloning of mMAb 6B6C-1 and 1A4B-6 V regions was performed as previously described (10, 32) using the first-round PCR primers listed in Table 1. For heavy-chain 3′ primers, M-IgG2a was used for mMAb 6B6C-1 and M-IgG2b was used for mMAb 1A4B-6. Primers were provided by the CDC Biotechnology Core Facility (Atlanta, GA). PCR-derived products were isolated by the QIAquick PCR purification kit (Qiagen Inc., Valencia, CA), cloned into pCR2.1-TOPO (Invitrogen, Baltimore, MD), and subsequently used to chemically transform TOP10 competent Escherichia coli (Invitrogen) in accordance with the manufacturer's protocol.
TABLE 1.
Oligonucleotide primers for isolation and modification of V regions
| Primer type and name | Sequencea |
|---|---|
| First-round primers | |
| M-IgG2a | 5′ ATTCGGATAGATCTAGTGGATAGACCGATGG 3′ |
| M-IgG2b | 5′ ATTCGGATAGATCTAGTGGATAGACTGATGG 3′ |
| MK-REV | 5′ ATTCGGATAGATCTTGGATGGTGGGAAGATG 3′ |
| MHV-7 (1A4B-6) | 5′ ACTAGTCGACATGGGATGGAGCGGGATCTTTCTCTT 3′ |
| MHV-9 (6B6C-1) | 5′ ACTAGTCGACATGGATTGGGTGTGGACCTGCTATTCCTG 3′ |
| MKV-1 (1A4B-6) | 5′ ACTAGTCGACATGAAGTTGCCTGTTAGGCTGTTGGTGCTG 3′ |
| MKV-9 (6B6C-1) | 5′ ACTAGTCGACATGGTATCCACACCTCAGTTCCTTG 3′ |
| Second-round primers | |
| VK-5′ (1A4B-6) | 5′ TCACGAAGTCTAGACCTCAAATGAAGTTGCCTGTTAGGCTGTTGGTGC 3′ |
| VK-5′ (6B6C-1) | 5′ TCACGAAGTCTAGACTGGACATGGTATCCCACTCAGTTCCTTG 3′ |
| VK-3′ (1A4B-6) | 5′ GAATCTATGGATCCTGACACACTTACGTTTGATTTCCAGCTTGGTGCCTCC 3′ |
| VK-3′ (6B6C-1) | 5′ GAATCTATGGATCCTGACACACTTACGTTTCAGCTCCAGCTTGGTCCCAGC 3′ |
| VH-5′ (1A4B-6) | 5′ ACACTATACTCGAGACTCCAACCATGGGATGGAGCGGGATCTTTCTCTT 3′ |
| VH-5′ (6B6C-1) | 5′ ACACTATACTCGAGACATCATGGCTTGGGTGTGGACCTTGCTAT 3′ |
| VH-3′ (1A4B-6) | 5′ TTCAGATCAAGCTTGACACACTTACCTGAGGAGACGGTGACTGAGGTTCC 3′ |
| VH-3′ (6B6C-1) | 5′ TTCAGATCAAGCTTGACACACTTACCTGAGGAGACGGTGACTGAGGTTCCT 3′ |
Restriction endonuclease sites are underlined.
Modification of V regions.
Variable light (kappa) (VK) and heavy (VH) regions of mMAbs 6B6C-1 and 1A4B-6 were further modified by a second round of PCR (Table 1) in order to add partial 5′ leader sequences, 3′ splice donor junctions, and appropriate restriction sites as previously described (10, 32). PCR-derived products were isolated and cloned into pCR2.1-TOPO as described above.
Assembly of human-murine chimeric IgG plasmid constructs.
VH and VK regions of mMAbs 6B6C-1 and 1A4B-6 were incorporated separately into the human IgG expression construct pdHL2 (Abbott Laboratories, Abbott Park, IL) by ligation as previously described (10, 32), generating plasmids pdHL2-6G (6B6C-1:IgG) and pdHL2-1G (1A4B-6:IgG). Plasmids pdHL2-6G and pdHL2-1G were used to transform E. coli DH5αE (Invitrogen) by electroporation in accordance with the manufacturer's protocol.
Sequencing.
V regions were sequenced in triplicate to ensure sequence fidelity after initial isolation and again after PCR modification. Sequencing reactions were performed using the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA), and sequence data were analyzed using the ABI 3130xl genetic analyzer (Applied Biosystems).
Transfection of cells with human-murine chimeric IgG plasmid constructs.
Sp2 cells were prepared and transfected with either plasmid pdHL2-6G or pdHL2-1G as previously described (32). Transfected cells were expanded and screened for human-murine chimeric IgG (cIgG) production by ELISA.
Detection of IgG cMAbs in cell culture supernatant.
Culture supernatants of cells transfected with pdHL2-6G or pdHL2-1G were analyzed for the presence of IgG cMAbs by ELISA using the protocol described by Thibodeaux and Roehrig (32) with the following IgG-specific reagents used in place of those originally used to detect chimeric IgM. AffiniPure goat anti-human IgG (H+L; Jackson Immunoresearch, West Grove, PA) diluted 1:1,000 in coating buffer (0.015 M sodium carbonate, 0.035 M sodium bicarbonate, pH 9.6) was used as the capture antibody. Secondary antibody consisted of alkaline phosphatase-conjugated goat anti-human IgG (heavy chain; Jackson Immunoresearch) diluted 1:5,000 in wash buffer (PBS-T; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, 0.5% Tween 20 [pH 7.2]). Purified human IgG (ChromPure human IgG, whole molecule; Jackson Immunoresearch) was used as a positive control.
Antibody purification.
Transfected Sp2 cells that tested positive for production of IgG cMAbs were expanded and passaged a total of three times in HGM with methotrexate (0.1 μM) followed by one additional passage in HGM without methotrexate. Supernatants from each passage were tested for anti-Eastern equine encephalitis virus (anti-EEEV) or anti-SLEV activity using the indirect IgG ELISA described below. Transfected cell lines that exhibited consistently high levels of antiflaviviral (6GF4 IgG) or antialphaviral (1GD5 IgG) IgG cMAb were sent to QED Bioscience Inc. (San Diego, CA) for ascites fluid production and antibody purification. Antibodies were purified from ascites fluid by protein G affinity chromatography and eluted in phosphate-buffered saline (PBS) at concentrations of 0.72 mg/ml (1GD5 IgG) and 0.98 mg/ml (6GF4 IgG).
Antigens and human control sera for indirect IgG ELISA.
SMB and VLP antigens for multiple flavi- and alphaviruses as well as corresponding normal antigens (normal SMB or COS-1 antigens) were obtained from the CDC Diagnostic and Reference Laboratory (CDC DRL, Arbovirus Diseases Branch, Division of Vector-Borne Diseases, CDC, Fort Collins, CO) and have been previously described (1-4, 13, 16, 21). SLEV (strain TBH-28), YFV (strain 17D), DENV-2 (strain New Guinea C), Powassan virus (POWV; strain LB), chikungunya virus (CHIKV; strain S27), Western equine encephalitis virus (WEEV; McMillan strain), EEEV (strain NJ/60), and Venezuelan equine encephalitis virus (VEEV; strain TC-83) SMB antigens were prepared as β-propiolactone-inactivated sucrose-acetone extracts of infected murine brain tissue for use in the indirect IgG ELISA. Lyophilized preparations of WNV and JEV VLPs, partially purified from COS-1 cell culture, were also used as antigens in the indirect IgG ELISA format. Flaviviral cell culture seeds of WNV (NY99), DENV-2 (30 PA), SLEV (MSI-7), JEV (14-14-2), and YFV (17D) at titers of 1 × 107 PFU/ml were also used as viral antigens in some ELISAs as described previously (32). Antigens were independently box titrated against appropriate antibody-positive control serum samples obtained from the CDC DRL; positive and negative serum samples were selected on the basis of a positive or negative reaction in previous serologic testing performed by the CDC DRL.
Indirect IgG ELISA for detection of flavivirus- or alphavirus-specific IgG cMAbs.
To detect the presence of antiviral IgG antibody in samples of cell culture supernatants, purified cMAb, or human sera, a modification of the indirect IgG ELISA protocol, originally described by Johnson et al. (16), was used. Briefly, Immulon II HB flat-bottomed 96-well plates were coated at 4°C overnight with 75 μl of clarified ascitic fluid containing genus-specific broadly cross-reactive mMAbs D14G2415 (4G2, flavivirus) or 1A4B-6 (alphavirus) diluted 1:8,000 or 1:20,000, respectively, in coating buffer (19, 25). Wells received multiple (five) washes with PBS-T between each step in the ELISA. Plates were blocked with 200 μl of blocking buffer per well for 1 h at 25°C. Flavi- or alphaviral antigens (VLP or SMB) diluted in PBS-T were added to each well (50 μl/well) and incubated overnight at 4°C. Alternatively, flaviviral cell culture seeds were used in place of VLP or SMB antigens where noted in Results. Human serum controls, purified 6GF4 IgG, or purified 1GD5 IgG was diluted (as detailed below) in PBS-T buffer, added to wells (50 μl/well), and subsequently incubated at 37°C for 1.5 h. Positive control sera for each viral antigen were used at empirically determined dilutions based on the results of box titration against appropriate antigens; negative human control sera were diluted to the same degree as positive control sera for each antigen. Samples of purified IgG cMAbs and cell culture supernatants containing IgG cMAbs were serially diluted 2-fold in PBS-T before being dispensed into appropriate wells; in some cases, purified cMAb was diluted 1:10 or 1:100 in PBS-T prior to serial dilution in order to better visualize titration curves. Secondary antibody consisting of alkaline phosphatase-conjugated goat anti-human IgG (heavy chain; Jackson Immunoresearch) diluted 1:5,000 in PBS-T was added to each well (50 μl/well) and allowed to incubate for 1 h at 37°C. Substrate (Sigma Fast p-nitrophenyl phosphate tablet sets) was added to each well (75 μl/well) and allowed to incubate for 30 to 60 min at 25°C. Absorbance values at 405 nm (A405) were read using an ELx808 absorbance microplate reader (BioTek Instruments Inc., Winooski, VT). All ELISAs were performed in triplicate.
Test validation and calculation of P/N values.
Test validation and positive-to-negative ratio (P/N) values were determined according to the procedure of Martin et al. (21) using internal positive and negative serum controls. The N value for each viral antigen was defined as the average A405 nm value for normal human serum reacted with a given viral antigen. The P value of 6GF4 or 1GD5 IgG cMAb for each viral antigen was determined to be the average A405 nm value at the maximum dilution at which a P/N value of 3 or greater was obtained for a given viral antigen. The Pmax/N value for positive human serum (PHS) or cMAb was determined as the A405 measurement at a given dilution of serum or antibody reacted with a viral antigen divided by the A405 value of negative human serum (NHS) reacted with that antigen.
CDR analysis.
Complementarity-determining-region (CDR) analysis was performed using the method described by Johnson and Wu (17).
Nucleotide sequence accession numbers.
The 6B6C-1 V region sequences have previously been published and assigned GenBank accession numbers as follows: for 6B6C-1 VK, accession number FJ234927; for 6B6C-1 VH, accession number FJ234928 (32). The following V region sequences for 1A4B-6 were submitted to GenBank and assigned the indicated accession numbers: 1A4B-6 VK, accession number GU724342; 1A4B-6 VH, accession number GU724341.
RESULTS
Cloning and sequencing of mMAb 1A4B-6 variable regions.
In order to generate alphavirus or flavivirus group-specific IgG cMAbs, hybridomas of mMAbs with group-specific activities for flaviviruses (6B6C-1) or alphaviruses (1A4B-6) were grown in cell culture. Both 6B6C-1 and 1A4B-6 are used regularly by the CDC DRL for ELISA-based serodiagnosis of arboviral infection (15, 16, 21, 26). The cloning and sequencing of variable regions from the 6B6C-1 mMAb have been previously documented in a related study involving the development of a flavivirus group-specific human-murine chimeric IgM for use in the flavivirus MAC-ELISA (32). For 1A4B-6, the heavy- and kappa-chain variable regions (VH and VK, respectively) were cloned by reverse transcription-PCR using a combination of degenerate primers that annealed to conserved VH and VK gene leader sequences and C region-specific primers (10). Multiple clones of each V gene product were sequenced to ensure against possible DNA polymerase-induced errors.
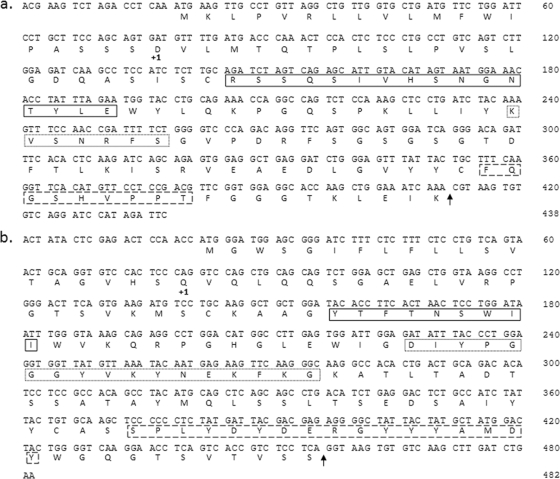
The 1A4B-6 VH cDNA and VK cDNA were sequenced, and a consensus of multiple sequence determinations was derived (Fig. 1a and b, respectively).
FIG. 1.
Nucleotide and deduced amino acid sequences of the pdHL2-1G V regions. +1, denotes start of mature protein; solid-line box, CDR 1; dotted-line box, CDR 2; dashed-line box, CDR 3. (a) pdHL2-1G VK region derived from 1A4B-6; (b) pdHL2-1G VH region derived from 1A4B-6.
Assembly of the 6B6C-1 and 1A4B-6 IgG cMAb plasmid constructs (pdHL2-6G or pdHL2-1G).
The 6B6C-1 and 1A4B-6 V regions were further modified by a second round of PCR to prepare VK and VH cDNA for insertion into the human IgG expression plasmid pdHL2. Primers used in this second-round PCR were designed to incorporate 3′ splice donor junctions in order to ensure correct expression of the 6B6C-1 and 1A4B-6 murine V regions with the human C region functional splice acceptor sites located in pdHL2. The second-round PCR primers also added appropriate restriction endonuclease sites at either end of each V region to permit subsequent cloning of the finished VK and VH inserts into pdHL2. The pdHL2 plasmid contains genomic clones of the human kappa (CΚ) and IgG (Cγ1) C region genes, both of which are controlled by a metallothionein I promoter and a mouse immunoglobulin H chain enhancer. Plasmid pdHL2 also contains an altered dihydrofolate reductase gene that allows for selective growth in media containing methotrexate. The 1A4B-6 V regions were cloned into pdHL2, forming plasmid pdHL2-1G; the 6B6C-1 V regions were cloned into pdHL2, forming plasmid pdHL2-6G.
Expression and purification of cMAbs.
Cell-free supernatants of Sp2 cells transfected with either pdHL2-1G or pdHL2-6G were analyzed by ELISA for the presence of cMAbs approximately 2 weeks following transfection. A total of 7 separate wells (out of 90) containing Sp2 cells transfected with pdHL2-1G tested positive for human IgG when evaluated by ELISA; of those transfected with plasmid pdHL2-6G, 2 of 90 were positive for human IgG. These nine transfectants were next tested for specific antiflaviviral or antialphaviral activity by indirect IgG ELISA using either SLEV or EEEV SMB antigens, respectively. Of the seven pdHL2-1G transfectants, five demonstrated specific antialphaviral activity; both pdHL2-6G transfectants were positive for antiflaviviral activity. Multiple passages of those transfectants demonstrating antiflaviviral or antialphaviral activity were analyzed by indirect IgG ELISA to ensure that plasmid retention over repeated cell culture passage was maintained and also allowed for the selection of those cells that consistently produced the highest antiflaviviral (6GF4 IgG) or antialphaviral (1GD5 IgG) activity as measured by ELISA. Sp2 cells secreting 6GF4 or 1GD5 were sent to QED Bioscience, Inc., for further purification. Purified lots of each human-murine chimeric antibody were received from QED Bioscience and subsequently evaluated for activity against multiple alphaviruses or flaviviruses by indirect IgG ELISA.
Arboviral group reactivity of cMAbs.
The 6GF4 and 1GD5 cMAbs were assayed for specific group reactivity by indirect IgG ELISA using SMB or VLP antigens of prominent members of the family Flaviviridae or Alphaviridae, respectively. The 6GF4 IgG, at a dilution of ≥1/256, reacted positively (P/N value of >3.0) with all flaviviral antigens tested, with the exception of the SLE SMB antigen, which required a higher concentration of cMAb (1:160 dilution) to achieve a P/N value greater than 3.0 (Table 2). The 6GF4 IgG reacted exceptionally well with those antigens demonstrating high Pmax/N values: YFV (SMB), WNV (VLP), and JEV (VLP). Against these antigens, the 6GF4 IgG, at dilutions of ≥1:25,600, gave P/N values of >3.0. When flaviviral VLP or SMB antigens were replaced with cell culture viral seed in the indirect IgG ELISA, Pmax/N values of 6GF4 IgG for each flavivirus changed to the following: for WNV, 3.50; DENV-2, 3.14; YFV, 3.33; SLEV, 4.65; JEV, 5.87. Cell culture viral seed for POWV was unavailable at the time of testing.
TABLE 2.
Reactivity of 6GF4 IgG with flavivirus SMB or VLP antigens in indirect IgG ELISA
| Virus | NHS and PHS dilution |
A405 |
Pmax/N |
Dilution of 6GF4 at: |
|||
|---|---|---|---|---|---|---|---|
| NHS | PHS | PHS | 6GF4 | Pmax/N | P/N > 3 | ||
| WNV | 100 | 0.404 | 2.148 | 5.31 | 5.76 | 1:200 | >1:25,600 |
| YFV | 400 | 0.295 | 0.581 | 1.97 | 7.18 | 1:200 | >1:25,600 |
| JEV | 400 | 0.326 | 2.208 | 6.77 | 6.53 | 1:200 | >1:25,600 |
| SLEV | 800 | 0.363 | 1.754 | 4.83 | 3.38 | 1:20 | 1:160 |
| DENV-2 | 400 | 0.283 | 0.800 | 2.83 | 5.46 | 1:1 | >1:256 |
| POWV | 400 | 0.095 | 0.439 | 4.62 | 3.95 | 1:8 | 1:256 |
For the alphavirus group-specific 1GD5 IgG, dilutions of 1:64 or greater were able to yield positive ELISA reactions (P/N > 3.0) with all alphaviral antigens assayed (Table 3). Although the 1GD5 IgG produced the highest overall Pmax/N values against the WEEV and VEEV SMB antigens, the 1GD5 IgG also reacted very well with the EEEV SMB antigen (P/N, >3 at a dilution of 1:320). Overall, there appeared to be less variability in the reactivity of the 1GD5 IgG among the alphaviral antigens than in that demonstrated by the 6FG4 IgG with flaviviral antigens, which showed a broad range of activity.
TABLE 3.
Reactivity of 1GD5 IgG with alphavirus SMB antigens in indirect IgG ELISA
| Virus | NHS and PHS dilution |
A405 |
Pmax/N |
Dilution of 1GD5 at: |
|||
|---|---|---|---|---|---|---|---|
| NHS | PHS | PHS | 1GD5 | Pmax/N | P/N> 3 | ||
| EEEV | 400 | 0.283 | 1.777 | 6.29 | 6.77 | 1:1 | 1:320 |
| VEEV | 400 | 0.187 | 1.755 | 9.40 | 9.10 | 1:1 | 1:256 |
| WEEV | 400 | 0.155 | NAa | NA | 12.08 | 1:1 | 1:64 |
| CHIKV | 400 | 0.174 | 1.603 | 3.06 | 4.74 | 1:2 | 1:128 |
NA, not applicable.
Samples of human infection-immune-positive control serum were included in each indirect IgG ELISA performed, and the resulting Pmax/N values were compared to the Pmax/N values obtained with the 6GF4 or 1GD5 IgG cMAbs. For the most part, the Pmax/N values produced by the chimeric IgG antibodies were comparable to those obtained using positive human control sera at previously determined optimum dilutions (Table 2 and Table 3). In the case of YFV and DENV-2, the positive human sera were unable to achieve Pmax/N values greater than 3.0; a sample of human serum positive for WEEV was unavailable at the time of these experiments. Absorbance values of purified chimeric IgG (at maximum dilutions yielding P/N values of >3 against viral antigens) against normal mouse brain antigen or normal COS antigens were at least 3-fold lower than those measured against viral antigens at identical antibody dilutions.
DISCUSSION
Antibody-positive human control sera for use in the MAC and indirect IgG ELISA are derived from small volumes of serum specimens submitted to the CDC for diagnostic purposes. Not surprisingly, these specimens are typically collected only from the most prevalent arboviral infections, thus limiting the application of ELISA in arboviral surveillance to the narrow range of diseases currently being diagnosed or tracked by disease surveillance systems at any given time. Furthermore, positive control serum pools prepared from these specimens suffer high lot-to-lot variability that necessitates constant recalibration of serological potency and coverage. Of even greater concern is the lack of antibody-positive control sera that can be used in these ELISAs for the identification of infrequent or emerging arboviruses (20, 21). In this study, we have demonstrated that IgG cMAbs expressing the variable region specificity of the mMAbs 6B6C-1 (cMAb 6GF4) or 1A4B-6 (cMAb 1GD5) can serve as suitable replacements for human control sera in the indirect IgG ELISA used in the serodiagnosis of arboviral disease. These cMAbs offer diagnostic laboratories an unlimited supply of control reagents of a set affinity and specificity, in known quantities that should facilitate diagnostic testing and lab-to-lab comparative evaluations.
A number of techniques have recently been described for engineering human antibodies. Transgenic mouse strains carrying human heavy- and light-chain loci, the immortalization of human B cells through viral transformation, and production of human hybridomas using new human fusion partner cell lines are all methods capable of producing human monoclonal antibodies (6, 18, 24). Unfortunately, these methods do not facilitate the design of human MAbs of a defined specificity. A considerable amount of additional screening would be required to identify specific group-reactive antibodies. An alternative to producing fully human MAbs is humanizing existing murine MAbs of known specificity. The flavivirus group-specific 6B6C-1 mMAb was originally raised against SLEV and is specific for the flaviviral envelope (E) protein (22, 27); mMAb 1A4B-6 reacts specifically with the E1d domain of the EEEV E1 glycoprotein and is alphavirus group reactive (14, 25). Both 6B6C-1 and 1A4B-6 are regularly used in serological assays as capture antibodies and antibody-enzyme conjugate detectors and were likely candidates for humanization (15, 16, 20, 21, 23, 28, 29). Using the pdHL2 IgG expression vector along with RNA purified from the 6B6C-1 and 1A4B-6 hybridomas, we prepared IgG cMAbs 6GF4 and 1GD5 for use in the indirect IgG ELISA.
The 6GF4 IgG cMAb was able to achieve positive P/N values with each flaviviral antigen tested in the indirect IgG ELISA. 6GF4 demonstrated a strong preference for the YFV, WNV, and JEV antigens compared to the relatively weaker reactions with DENV-2, POWV, or SLEV antigens. The 1GD5 IgG cMAb similarly reacted positively with all alphaviruses tested, and there appeared to be much less variability in the reactivity of 1GD5 when assayed against the representative members of the alphavirus family.
Although it was somewhat surprising to see that the 6GF4 cMAb reacted weakly with SLEV, the virus initially used to generate the 6B6C-1 mMAb, it is not all that alarming considering that the reactivity and quality of SMB and VLP antigens can vary greatly from one lot of antigen to the next. Furthermore, when tested against viral seed antigens instead of the SMB or VLP antigens in the indirect IgG ELISA format, the 6GF4 cMAb reacted better with the SLEV seed antigen, generating the second-highest overall Pmax/N value (4.65) of all flavivirus viral seed antigens tested. The 6ME2 IgM cMAb described in a previous publication also demonstrated a relatively low reactivity with the SLEV SMB antigen and reacted strongly with the SLE viral seed antigen (32). This disparity between the viral SMB antigen and the viral seed antigen might be attributed to differences inherent in the SLEV strains used and/or differences in the preparation of each antigen; the SLEV SMB antigen used in both the IgG and IgM ELISA studies was prepared from strain TBH-28, an isolate taken postmortem from cases of fatal SLE in the Tampa Bay area of Florida in 1962, while the viral seed antigen was prepared from Vero cells infected with SLEV strain MSI-7, the same SLE strain that was originally used to generate the 6B6C-1 mMAb. However, given the homogeneity of the 6B6C-1 epitope among all flaviviruses (5), the variable reactivity of 6GF4 with different flaviviral antigens is most likely due to the quality and concentration of the specific antigen lots available from the CDC DRL used in this assay rather than the preference of the chimeric antibody for a specific flavivirus.
The viral antigens included in these ELISAs are representative of the major flavivirus or alphavirus antigen complexes with members causing human disease. Therefore, it is reasonable to assume that these chimeric antibodies would be useful as positive controls in the serodiagnosis of all flaviviral or alphaviral specimens. One possible complication with using a MAb-derived chimeric antibody as a positive control reagent would be the occurrence of an arbovirus with an altered E glycoprotein (flavivirus) or E1 glycoprotein (alphavirus) rendering it nonreactive with the group-specific activities of 6B6C-1 or 1A4B-6. The epitope defined by 6B6C-1 and other E protein-specific flavivirus group-reactive mMAbs has recently been mapped to the E protein fusion loop (5). This sequence is highly conserved among all flaviviruses, probably because of its critical interaction with cell membranes during virus replication. The alphavirus E1 glycoprotein is also reported to be responsible for membrane fusion, and although no epitope map yet exists for the alphavirus E1 glycoprotein, the crystal structure of E1 has been determined and was found to share significant homology and identical topology with the flavivirus E glycoprotein (7, 8). Furthermore, we have shown that a flavivirus transmitted by a vector other than the mosquito, POWV, reacts with the 6GF4 IgG cMAb. This finding agrees with those previously reported by the CDC DRL that both tick-borne encephalitis virus and POWV react with the 6B6C-1 mMAb in the MAC-ELISA format (32).
Given the respective group reactivities of 6B6C-1 and 1A4B-6, the discovery of a nonreactive flavivirus or alphavirus is unlikely. If such a virus was isolated, however, one solution would be to develop a combination of two different chimeric antibodies sharing group reactivity but for separate epitopes. Additionally, since the current indirect IgG-ELISA formats utilize a 6B6C-1-enzyme conjugate detector in flavivirus serodiagnosis and a 1A4B-6 capture antibody in alphaviral serodiagnosis, any arbovirus expressing a mutated or nonreactive glycoprotein would not likely be detected by the currently employed assays.
The incorporation of cMAbs in immunoassays that rely on variably reactive human sera as controls will provide diagnostic laboratories with an unlimited supply of control reagents of a set affinity and specificity. Also, the use of a positive control with the specificity of a MAb allows for better characterization of unknown specimens than that of polyclonal sera with heterogeneous reactivities. The 6GF4 and 1GD5 IgG cMAbs should offer viable alternatives to positive human serum controls for flavivirus or alphavirus detection via the indirect IgG ELISA. Together with the previously described 6ME2 IgM cMAb, we now possess positive controls capable of evaluating both acute- and convalescent-phase sera for possible flavivirus infection. An IgM cMAb with alphavirus group specificity has also been developed, is currently being characterized for utilization in the MAC-ELISA for serodiagnosis of alphaviral disease, and will complete the set of cMAbs necessary to identify both classes of human antibodies developed in a wide variety of human arboviral infections.
Acknowledgments
This work was supported, in part, by a postdoctoral fellowship awarded to B.A.T. by the American Society for Microbiology and the Coordinating Center for Infectious Diseases.
We thank Nathan Liss for his assistance with the ELISAs in this study.
Footnotes
Published ahead of print on 25 August 2010.
REFERENCES
- 1.Burke, D. S., A. Nisalak, D. E. Johnson, and R. M. Scott. 1988. A prospective study of dengue infections in Bangkok. Am. J. Trop. Med. Hyg. 38:172-180. [DOI] [PubMed] [Google Scholar]
- 2.Calisher, C. H., N. Karabatsos, J. S. Lazuick, T. P. Monath, and K. L. Wolff. 1988. Reevaluation of the western equine encephalitis antigenic complex of alphaviruses (family Togaviridae) as determined by neutralization tests. Am. J. Trop. Med. Hyg. 38:447-452. [DOI] [PubMed] [Google Scholar]
- 3.Chang, G. J., A. R. Hunt, and B. Davis. 2000. A single intramuscular injection of recombinant plasmid DNA induces protective immunity and prevents Japanese encephalitis in mice. J. Virol. 74:4244-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, G. J., A. R. Hunt, D. A. Holmes, T. Springfield, T. S. Chiueh, J. T. Roehrig, and D. J. Gubler. 2003. Enhancing biosynthesis and secretion of premembrane and envelope proteins by the chimeric plasmid of dengue virus type 2 and Japanese encephalitis virus. Virology 306:170-180. [DOI] [PubMed] [Google Scholar]
- 5.Crill, W. D., and G. J. Chang. 2004. Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes. J. Virol. 78:13975-13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, G. C., M. L. Gallo, and J. R. F. Corvalan. 1999. Transgenic mice as a source of fully human antibodies for the treatment of cancer. Cancer Metastasis Rev. 18:421-425. [DOI] [PubMed] [Google Scholar]
- 7.Drummer, H. E., I. Boo, and P. Poumbourios. 2007. Mutagenesis of a conserved fusion peptide-like motif and membrane-proximal heptad-repeat region of hepatitis C virus glycoprotein E1. J. Gen. Virol. 88:1144-1148. [DOI] [PubMed] [Google Scholar]
- 8.Gibbons, D. L., M.-C. Vaney, A. Roussel, A. Vigouroux, B. Reilly, J. Lepault, M. Kielian, and F. A. Rey. 2004. Conformational change and protein-protein interactions of the fusion protein of Semliki Forest virus. Nature 427:320-325. [DOI] [PubMed] [Google Scholar]
- 9.Hackett, J., Jr., J. Hoff-Velk, A. Golden, C. Dealwis, D. Ostrow, and W. Mandecki. 1997. The effect of site-specific mutagenesis of a cysteine residue on the stability of a monoclonal antibody, p. 133-157. In W. Hori (ed.), Antibody engineering II, vol. 2. New technology, application and commercialization. International Business Communications, Inc., Southborough, Mass. [Google Scholar]
- 10.Hackett, J., Jr., J. Hoff-Velk, A. Golden, J. Brashear, J. Robinson, M. Rapp, M. Klass, D. H. Ostrow, and W. Mandecki. 1998. Recombinant mouse-human chimeric antibodies as calibrators in immunoassays that measure antibodies to Toxoplasma gondii. J. Clin. Microbiol. 36:1277-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes, D. A., D. E. Purdy, D. Y. Chao, A. J. Noga, and G. J. Chang. 2005. Comparative analysis of immunoglobulin M (IgM) capture enzyme-linked immunosorbent assay using virus-like particles or virus-infected mouse brain antigens to detect IgM antibody in sera from patients with evident flaviviral infections. J. Clin. Microbiol. 43:3227-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, T. J., M. E. Reid, G. R. Halverson, and K. Yazdanbakhsh. 2003. Production of recombinant murine-human chimeric IgM and IgG anti-Jsb for use in the clinical laboratory. Transfusion. 43:758-764. [DOI] [PubMed] [Google Scholar]
- 13.Hunt, A. R., C. B. Cropp, and G. J. Chang. 2001. A recombinant particulate antigen of Japanese encephalitis virus produced in stably-transformed cells is an effective noninfectious antigen and subunit immunogen. J. Virol. Methods. 97:133-149. [DOI] [PubMed] [Google Scholar]
- 14.Hunt, A. R., and J. T. Roehrig. 1985. Biochemical and biological characteristics of epitopes on the E1 glycoprotein of Western equine encephalitis virus. Virology 142:334-346. [DOI] [PubMed] [Google Scholar]
- 15.Hunt, A. R., R. A. Hall, A. J. Kerst, R. S. Nasci, H. M. Savage, N. A. Panella, K. L. Gottfried, K. L. Burkhalter, and J. T. Roehrig. 2002. Detection of West Nile virus antigen in mosquitoes and avian tissues by a monoclonal antibody-based capture enzyme immunoassay. J. Clin. Microbiol. 40:2023-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, A. J., D. A. Martin, N. Karabatsos, and J. T. Roehrig. 2000. Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. J. Clin. Microbiol. 38:1827-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, G., and T. T. Wu. 2000. Kabat database and its applications: 30 years after the first variability plot. Nucleic Acids Res. 28:214-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalantarov, G. F., S. A. Rudchenko, L. Lobel, and I. Trakht. 2002. Development of a fusion partner cell line for efficient production of human monoclonal antibodies from peripheral blood lymphocytes. Hum. Antibodies 11:85-96. [PubMed] [Google Scholar]
- 19.Kaufman, B. M., P. L. Summers, D. R. Dubois, and K. H. Eckels. 1987. Monoclonal antibodies against dengue 2 virus e-glycoprotein protect mice against lethal dengue infection. Am. J. Trop. Med. Hyg. 36:427-434. [DOI] [PubMed] [Google Scholar]
- 20.Lanciotti, R. S., and J. T. Roehrig. 2006. Arboviruses, p. 757-765. In B. Detrick, R. G. Hamilton, and J. D. Folds (ed.), Manual of molecular and clinical laboratory immunology, 7th ed. ASM Press, Washington, DC.
- 21.Martin, D. A., D. A. Muth, T. Brown, A. J. Johnson, N. Karabatsos, and J. T. Roehrig. 2000. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J. Clin. Microbiol. 38:1823-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathews, J. H., and J. T. Roehrig. 1984. Elucidation of the topography and determination of the protective epitopes on the E glycoprotein of Saint Louis encephalitis virus by passive transfer with monoclonal antibodies. J. Immunol. 132:1533-1537. [PubMed] [Google Scholar]
- 23.Purdy, D. E., A. J. Noga, and G.-J. Chang. 2004. Noninfectious recombinant antigen for detection of St. Louis encephalitis virus-specific antibodies in serum by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 42:4709-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roehrig, J. T. 2000. Arboviruses, p. 356-373. In S. Specter, R. L. Hodinka, and S. A. Young (ed.), Clinical virology manual, 3rd ed. ASM Press, Washington, DC.
- 25.Roehrig, J. T., A. R. Hunt, G.-J. Chang, B. Sheik, R. A. Bolin, T. F. Tsai, and D. W. Trent. 1990. Identification of monoclonal antibodies capable of differentiating antigenic varieties of Eastern equine encephalitis viruses. Am. J. Trop. Med. Hyg. 42:394-398. [DOI] [PubMed] [Google Scholar]
- 26.Roehrig, J. T., D. Nash, B. Maldin, A. Labowitz, D. A. Martin, R. S. Lanciotti, and G. L. Campbell. 2003. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg. Infect. Dis. 9:376-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roehrig, J. T., J. H. Mathews, and D. W. Trent. 1983. Identification of epitopes on the E glycoprotein of Saint Louis encephalitis virus using monoclonal antibodies. Virology 128:118-126. [DOI] [PubMed] [Google Scholar]
- 28.Roehrig, J. T., M. Layton, P. Smith, G. L. Campbell, R. Nasci, and R. S. Lanciotti. 2002. The emergence of West Nile virus in North America: ecology, epidemiology, and surveillance. Curr. Top. Microbiol. Immunol. 267:223-240. Rev. [DOI] [PubMed] [Google Scholar]
- 29.Roehrig, J. T., and R. A. Bolin. 1997. Monoclonal antibodies capable of distinguishing epizootic from enzootic varieties of subtype 1 Venezuelan equine encephalitis viruses in a rapid indirect immunofluorescence assay. J. Clin. Microbiol. 35:1887-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roehrig, J. T., T. M. Brown, A. J. Johnson, N. Karabatsos, D. A. Martin, C. J. Mitchell, and R. S. Nasci. 1998. Alphaviruses, p. 7-18. In J. R. Stephenson and A. Warnes (ed.), Diagnostic virology protocols. Humana Press, Totowa, NJ.
- 31.Shin, S. U., and S. L. Morrison. 1989. Production and properties of chimeric antibody molecules. Methods Enzymol. 178:459-476. [DOI] [PubMed] [Google Scholar]
- 32.Thibodeaux, B. A., and J. T. Roehrig. 2009. Development of a human-murine chimeric immunoglobulin M antibody for use in the serological detection of human flavivirus antibodies. Clin. Vaccine Immunol. 16:679-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai, T. F., and T. P. Monath. 1987. Viral diseases in North American transmitted by arthropods from vertebrate reservoirs, p. 1417-1456. In R. D. Feigin and J. D. Cherry, (ed.), Textbook of pediatric infectious diseases. The W. B. Saunders Co., Philadelphia, PA.