Abstract
Enterovirus (EV) is an RNA virus that has circulated with different serotypes and genotypes worldwide. Enterovirus 71 (EV71) is a major neurotropic virus that causes severe brain stem encephalitis (BE) in infants and young children. The most vulnerable age for fatal infection is 6 to 11 months. This is associated with the coincident decline in maternal antibodies. The current report describes our finding that EV71 can infect human peripheral blood monocytes. We were able to show that EV71 infection is enhanced in the monocytic cell line THP-1 by the presence of subneutralizing concentrations of anti-EV71 antibodies. We also found that antibody-dependent enhancement (ADE) is mediated in part by Fcγ receptors. These observations support the concept that ADE augments the infectivity of EV71 for human monocytes and contributes to the age-dependent pathogenesis of EV71-induced disease. The ADE phenomenon must be considered during the development of an EV71 vaccine.
Enterovirus 71 (EV71) is a member of the family Picornaviridae. It was first recognized in California in 1969 (7, 37) and subsequently throughout the world (1, 26, 33, 35, 42). EV71 is associated with a wide variety of clinical syndromes, including hand-foot-and-mouth disease (HFMD), herpangina, aseptic meningitis, poliomyelitis-like paralysis, and brain stem encephalitis (BE). Several large epidemics of enterovirus infections have occurred in Taiwan during the past decade (1, 26, 42). The most frequently isolated enteroviruses are EV71, echovirus 30, coxsackievirus B4 (CB4), coxsackievirus A 16 (CA16), and CA10. We reported that patients with EV71 BE complicated by pulmonary edema (PE) had lower absolute monocyte counts than those with autonomic nerve system (ANS) dysregulation and uncomplicated BE (43). CD4+ T, CD8+ T, and NK cell counts also were significantly decreased (43). These observations suggest that immune cells are targets for EV71.
The Enterovirus genus is subdivided into four species: human enterovirus A (HEV-A), HEV-B, HEV-C, and HEV-D. EV71 belongs to the HEV-A group along with coxsackievirus A2 to A8, A10, A12, A14, A16, EV76, and EV89 to EV92 (44). Many genotypes of EV71 containing intra- and interserotypic recombinants have been shown to circulate in Taiwan and other Asian countries (20, 22). There is considerable cross-antigenicity among different subgenogroups from areas where EV71 is endemic (29). The highest rates of infection by enteroviruses occur in young children (<4 years of age). The peak incidence of infection is at about 1 year of age. The highest mortality rate occurs at 6 months to <1 year (2, 6, 18). Luo et al. reported that 63% of the pregnant women in Taiwan and 51% of their neonates have EV71 antibodies (27). The most vulnerable age for mortality, 6 to 11 months, coincides with the time that maternal antibodies decline. These observations raise the possibility that antibodies to cross-reacting prevalent enteroviruses and/or declining concentrations of maternal antibodies to EV71 might augment the infectivity of EV71 by antibody-dependent enhancement (ADE).
The ADE concept is based on the notion that heterotypic, nonneutralizing antibodies, derived from a maternal or a previous primary infection, bind to the virion and enhance viral entry through the interaction between virus-antibody complexes and Fcγ receptors (FcγR) on FcγR-bearing cells, particularly on monocytes. This phenomenon has been described for dengue virus (12, 14, 15, 24, 39), influenza virus (32), human immunodeficiency virus type 1 (36), CB, and other Picornaviridae viruses (13, 17). In the current study, we found that EV71 can infect human monocytes. This stimulated us to establish an in vitro model of the infection of EV71 using a monocytic cell line (THP-1). We found that the Fcγ receptor augments the ability of subneutralizing antibodies to enhance EV71 infection in this cell line.
(Presented in part at the 49th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 12 to 15 September 2009 [43a].)
MATERIALS AND METHODS
Virus.
A strain of EV71 (Taiwan/4643/98), isolated from a child who died from EV71 infection, was provided by the Virology Laboratory of National Cheng Kung University Hospital. The virus was propagated in rhabdomyosarcoma (RD) cells with Dulbecco's modified Eagle's medium (DMEM) supplemented with heat-inactivated 2% fetal bovine serum (FBS) and antibiotics. Virus cell cultures were frozen and thawed three times to release intracellular virus and centrifuged at 800 × g for 10 min at 4°C to remove the cell debris. The supernatant was stored in aliquots at −70°C. Virus titration was performed by plaque assay in RD cells.
Cell culture.
THP-1 (human monocytic cell line) were cells maintained in RPMI-1640 containing 2 mM l-glutamine, 10 mM HEPES (Sigma), 1.0 mM sodium pyruvate, 0.05 mM 2-mercaptoethanol, 10% heat-inactivated FBS, and 1% gentamicin. Cells were grown at 37°C in a 5% atmosphere of CO2.
Isolation of human PBMCs.
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood from adult volunteers and separated by the Ficoll-Hypaque method. The cells were washed three times by centrifugation in RPMI 1640. They were adjusted to a concentration of 2 ×106 cells/ml in RPMI 1640 supplemented with 10% FCS, 1% l-glutamine, 1.0 mM sodium pyruvate, and 1% gentamicin and distributed as 0.1-ml aliquots into 96-well tissue culture plates. To separate different subsets of mononuclear cells, the PBMCs were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-human CD4, CD8, CD20, and CD14 antibody for 30 min at 4°C and then purified by a FACSAria flow cytometer (Becton Dickinson Bioscience, San Jose, CA).
The Clinical Research Ethics Committee of the National Cheng Kung University Hospital approved the study protocol. Informed consent was obtained from the participants.
Immunofluorescent staining.
EV71-infected and mock-infected control cells were added to glass-chamber slides and fixed in 3.7% paraformaldehyde for 10 min at room temperature. The slides were washed with RPMI 1640 and cytocentrifuged. The cells were incubated for 30 min at room temperature with a 1:1,000 dilution of monoclonal antibody (MAb) against EV71 (MAb979; Chemicon International, Temecula, CA). A 1:200 dilution of FITC-conjugated secondary antibodies (Jackson ImmunoResearch Laboratory, Baltimore, PA) was added and counterstained with Hoechst 33258 (Sigma, St. Louis, MO), after which the cells were counted with a fluorescence microscope. The cells were washed three times with sterile phosphate-buffered saline (PBS) prior to microscopy.
Flow-cytometric analysis.
After being washed with staining buffer, EV71-infected cells and mock-infected cells (2 × 105 cells/ml) were fixed with 70% alcohol for 30 min at −20°C. The cells then were incubated with monoclonal antibody against EV71 (MAb 979) at a 1:500 dilution for 30 min at 4°C. FITC-conjugated secondary antibodies at a 1:200 dilution were added and incubated for 30 min in the dark at 4°C. This was followed by three washes with PBS before the cells were resuspended in 0.5 ml of PBS for flow-cytometric analysis.
Plaque assay.
RD cells (1 × 105) in 200 μl RPMI 1640 were seeded in each well of a 24-well plate. Serial dilutions of the different viral suspensions were added to the wells for 18 h of incubation. After absorption for 1 h at 37°C, the virus supernatant was replaced with DMEM containing 2% FBS and 0.8% methylcellulose for 72 h. After the removal of the medium and a 15-min fixation step with 4% formalin in PBS, the plaques were read by being stained with 1% crystal violet. Counts were expressed as PFU per milliliter.
Antibody-dependent enhancement assays.
THP-1 cells were distributed into 96-well tissue culture plates with heat-inactivated 10% fetal bovine serum as described above. Virus-antibody complexes were prepared by incubating plasma obtained from EV71-infected patients or a control group. The plasma fractions were serially diluted and mixed with the inoculum of EV71 for 30 min at 37°C. The EV71 and EV71-antibody complexes were added to cells at a multiplicity of infection (MOI) of 10 before incubation for 1 h at 4°C. Cells were washed three times in PBS and then incubated in RPMI 1640 (24 h at 37°C, 5% CO2 atmosphere). After incubation, cells were harvested and the EV71 infectivity was determined by staining with anti-VP1 antibody for flow cytometry.
Statistical analysis.
Proportional data were tested using χ2 analyses or Fisher's exact test. Continuous data were tested using the t test or the Mann-Whitney U test. All analyses were performed using SPSS software (version 11.5; Chicago, IL). Differences were considered significant at P < 0.05.
RESULTS
EV71 infection of PBMCs.
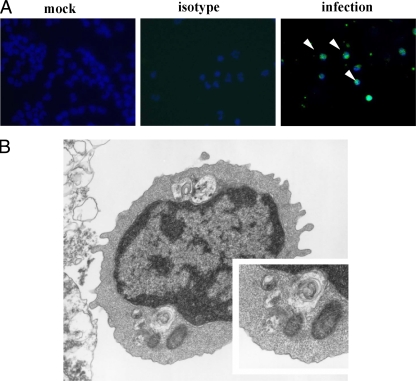
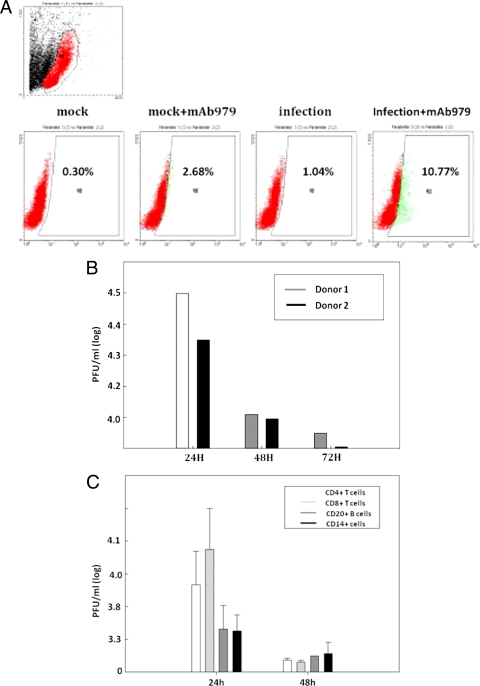
PBMCs from healthy subjects were cocultured with EV71 at an MOI of 10. The cells were stained with anti-EV71 monoclonal antibody at 24 h postinfection. The EV71 VP1+ capsid proteins were detected with anti-VP1 Ab by immunofluorescent microscopy. VP1+ proteins were observed on PBMCs inoculated with EV71 but not on mock-infected controls (Fig. 1 A). Transmission electronic microscopy revealed virus-like EV71 particles within the PBMCs (Fig. 1B). They were enclosed by double-membrane vesicles in the cytoplasm (19). Flow cytometry was used to quantitate virus replication in EV71-infected cells. VP1 was detected on EV71-infected PBMC (Fig. 2 A). Virus production determined by the plaque assay was detected in PBMCs from two donors (Fig. 2B). The human PBMCs were separated into CD3+ T cells, CD20+ B cells, and CD14+ monocytes by FACSAria analysis with specific antibody. Both lymphocytes and monocytes were shown to produce virus by plaque assay (Fig. 2C). These findings demonstrate that human monocytes can be infected by EV71.
FIG. 1.
EV71 infection of human PBMCs. PBMCs were infected with EV71 4643 stain at an MOI of 10 for 24 h. (A) Immunofluorescent staining of EV71-infected cells by anti-VP1 MAb. (B) Electron microscopic observation of EV71-infected monocytes.
FIG. 2.
EV71 infection of human monocytes. PBMCs were infected with EV71 4643 stain at an MOI of 10 for 24 h. (A) The EV71-infected cells were determined by anti-VP1 MAb by flow cytometry. (B) The virus production was determined from PBMC from two donors. (C) CD4+, CD8+, CD20+, and CD14+ cells were isolated by a FACSAria cell sorter. The virus production was determined at an MOI of 10 for 24 h.
Enhancement of EV71 infectivity at subneutralizing antibody levels.
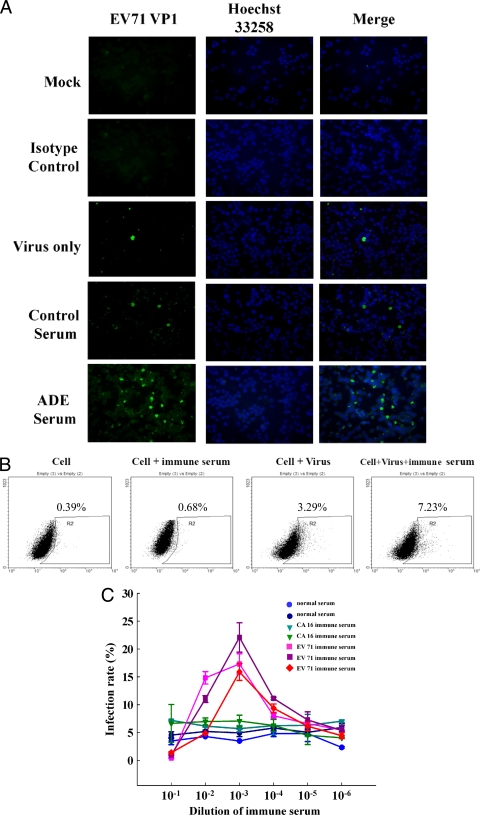
To characterize the effect of ADE on EV71 infection, the THP-1 monocytic cell line was used as the model cell. Various concentrations of EV71 immune serum were incubated with EV71 virus. High levels of immune serum prevented EV71 infection. In contrast, low levels (10−3 dilution) of anti-EV71 immune serum enhanced infectivity (Fig. 3). These effects were noted by the immunofluorescent and flow-cytometric staining of the VP1-positive cells (Fig. 3A and B) in a dose-dependent manner (Fig. 3C). Three representative immune sera obtained from EV71-infected patients demonstrated enhancing activities. EV71-neutralizing titers at about 1:512 dilution inhibited EV71 infection, whereas sera diluted to subneutralizing levels (5- to 0.5-fold) enhanced EV71 infectivity on THP-1 cells. CA16 immune serum and control sera did not enhance EV71 infection.
FIG. 3.
Anti-EV71 immune serum can enhance EV71 infection on THP-1 cells at subneutralizing concentrations. THP-1 cells were infected with EV71 4643 stain at an MOI of 10 for 24 h with or without the presence of immune serum. The EV71 infection was detected by immunofluorescent microscopy or determined by flow cytometry at 24 h postinfection. (A) Immunofluorescence staining of EV71 VP1+ on THP-1 cells by immune serum at a 10−3 dilution. (B) Flow-cytometric analysis of EV71 VP1+ on THP-1 cells by immune serum at a 10−3 dilution. (C) The dose range of enhancement of EV71 infection on THP-1 cells by anti-EV71 immune serum. The sera were obtained from EV71-infected patients and diluted to various concentrations. The dilution at 10−3 shows the highest enhancement. Coxsackievirus A16 (CA16) immune serum was collected from CA16-infected patients and used as an antibody specificity control. The neutralization titer of immune serum to EV71 is 512.
Human IVIG is able to neutralize or enhance EV71 infection in THP-1 cells in a concentration-dependent manner.
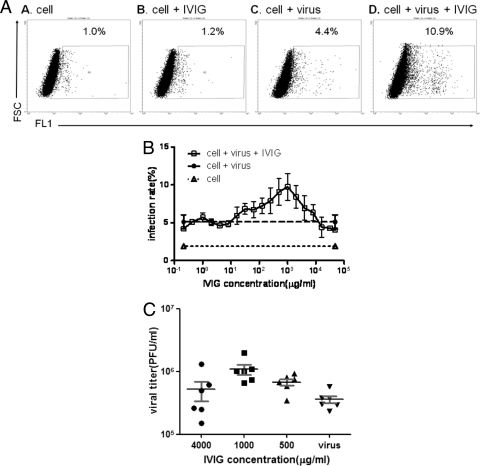
Human intravenous immunoglobulin (IVIG) sometimes is used for the treatment of severe EV71 infection. Therefore, we wished to determine whether it could neutralize or enhance EV71 infection in our model systems. Most commercial IVIGs can neutralize EV71 at a titer of 1:128. IVIG (Gamimune N; Bayer) was tested at a dose range of 0.2 to 5 × 104 μg/ml. Concentrations of 16 × 103 to 8 × 103 μg/ml were found to enhance EV71 infection by flow-cytometric analysis (Fig. 4 A and B). Virion production was determined by plaque assay. As shown in Fig. 4C, the amount of infectious virus particles in the supernatants was increased at concentrations of 500 μg/ml (6.76 × 105 ± 2 × 105; P = 0.015) and 1,000 μg/ml (1.08 × 106 ± 4.71 × 105; P = 0.002) compared to those of virus alone (3.58 × 105 ± 1.16 × 105). The dose of 4,000 μg/ml fell into a range between neutralization and enhancement.
FIG. 4.
Intravenous immunoglobulin (IVIG) can enhance the EV71 infection on THP-1 cells at subneutralization doses. THP-1 cells were infected with EV71 4643 stain at an MOI of 10 for 24 h alone or in the presence of various doses of IVIG. (A) The EV71 infection was detected by flow cytometry at 24 h postinfection. (B) The dose range of enhancement of EV71 infection on THP-1 cells by IVIG. (C) The virus titer was determined at 24 h postinfection by plaque assay. The neutralization titer of IVIG used with EV71 is 128. EV71 virus replication in THP-1 cells was enhanced at 500- and 1,000-μg/ml concentrations of IVIG.
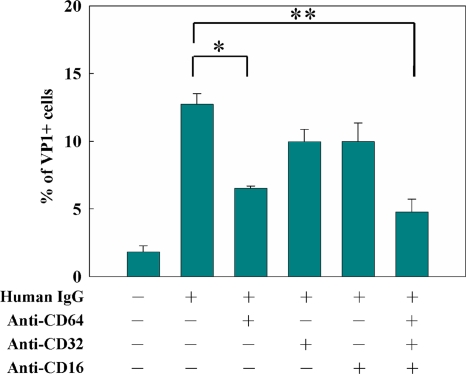
The role of Fcγ receptors in EV71-ADE.
To determine whether Fcγ receptors participate in the enhancement of EV71 infection by anti-EV71 antibody, anti-FcγR antibodies were used to block Fcγ receptors prior to EV71 infection. We found that patients' immune sera enhanced the EV71 infection of THP-1 cells by 3 to 13%. The addition of anti-FcγRI (CD64), but not anti-FcRγII (CD32) and anti-FcγRIII (CD16), significantly inhibited immune serum-mediated ADE infection (Fig. 5). These findings support the notion that Fcγ receptors play a role in EV71-ADE.
FIG. 5.
Anti-FcγR antibodies can inhibit the EV71 ADE phenomenon on THP-1 cells. THP-1 cells were incubated with different anti-FcγR (anti-FcγRI [CD64], anti-FcγRII [CD32], anti-FcγRIII [CD16]) antibodies for 15 min and then infected by EV71 alone or EV71 and patient immune serum (10−3 dilution) at an MOI of 10. The EV71 VP1+ cells were analyzed by flow cytometry 24 h postinfection. The anti-FcγRI (CD64) antibody can significantly inhibit the immune serum-mediated ADE. (* and **, P < 0.05).
DISCUSSION
We found that EV71 is capable of infecting human monocytes, that infectivity was enhanced by subneutralizing levels of immune sera or IVIG, and that Fcγ receptors participate in this phenomenon. These findings support the concept that EV71-ADE has important implications not only for the pathogenesis of EV71 infection but also for the development of a sufficiently immunogenic EV71 vaccine.
Enteroviruses can be isolated from the leukocytes of infected patients (8, 11, 25, 34, 41). CB3 can replicate in lymphocytes or monocytes (41). Poliovirus 1-infected PBMCs trigger the production of alpha interferon (IFN-α) (34). Kung et al. reported that human PBMCs were permissible for EV71 infection (25). We reported that Jurkat T cells infected with EV71 enhance FasL expression and might contribute to T-cell apoptosis during EV71 infection (5). These observations may explain the profound leukopenia noted in patients with severe EV71 BE (43). Moreover, infection and replication in monocytes might contribute to ADE. The ADE phenomenon has been described in many viral infections. The proposed mechanisms include FcγR dependent on an FcγR-bearing target; a complement-dependent effect on CR- or C1qR-bearing cells; antibody-induced conformational changes that facilitate membrane fusion; and the suppression of the cellular antiviral response (30, 38). The most commonly accepted concept is that ADE works through an FcγR-dependent mechanism. In the current study, we have shown that the FcγR is involved. We excluded the complement hypothesis by using heat-inactivated fetal calf serum.
THP-1 is a leukemia cell line of macrophage lineage with distinct monocytic markers. It has Fc and C3b receptors (10, 40). Using antibody against three receptors, FcγRI (CD64), FcγRII (CD32), and FcγRIII (CD16) (9), we found that only anti-FcγRI was able to block EV71-ADE on THP-1 cells. It appears that the EV71 virion-anti-EV71 antibody complex binds to the cell surface at the FcγRI to enhance entry and replication. Two additional EV71 receptors (P-selectin glycoprotein ligand-1 and scavenger receptor B2) have been described recently (31, 45). It is not known whether these or other as-yet unidentified receptors interact with the FcγR and participate in EV71 ADE. This will require further investigation.
Cytokine production has been shown to be increased by the enhancing antibody. In dengue virus infection, the anti-inflammatory cytokines interleukin-6 (IL-6) and IL-10 are enhanced and the Th1 cell-promoting cytokines (IL-12 and IFN-γ) are blocked (3). In respiratory syncytial virus infection of macrophages, ADE is associated with the increased expression of tumor necrosis factor α and IL-6 in BALB/c mice (16). The CB4-induced synthesis of IFN-α by PBMCs is enhanced in an ADE manner (4). In Ross River virus infection, virus-induced IL-10 is enhanced by ADE after FcγR ligation (28). We found increased systemic levels of IL-10, IL-13, and IFN-γ in patients with severe EV71 disease (43). The potential role of ADE in the expression of cytokine production in the immunopathogenesis of EV71 in humans currently is under investigation in our laboratory.
EV71 infection has become an important emerging infectious disease in children in Asia (2, 6, 18, 44, 46). It was previously thought to occur only episodically, but it is now endemic in the West Pacific area. Because of its considerable potential to cause severe central nervous system infections and its high secondary transmission among young children, there is considerable interest in developing a vaccine for this disease (23). The ADE phenomenon needs to be considered in the development of a safe and effective viral vaccine (21). In summary, the current findings indicate that ADE is involved in the pathogenesis of EV71 infection. The mechanism involves virion-antibody complexes mediated by nonneutralizing antibodies augmented by FcγR on monocytes.
Acknowledgments
We thank Calvin M. Kunin for his critical review of the manuscript.
This study was supported by grants from the National Science Council, Taiwan (NSC-95-2314-B-006-109 and NSC 96-2314-B-006-033-MY2), Multidisciplinary Center of Excellence for Clinical Trial and Research (DOH99-TD-B-111-102), Department of Health, Executive Yuan, Taiwan, and from the National Cheng Kung University Hospital (NCKUH no. 95-001).
Footnotes
Published ahead of print on 4 August 2010.
REFERENCES
- 1.Centers for Disease Control and Prevention. 1998. Deaths among children during an outbreak of hand, foot, and mouth disease-Taiwan, Republic of China, April-July 1998. MMWR Morb. Mortal. Wkly. Rep. 47:629-632. [PubMed] [Google Scholar]
- 2.Chang, L. Y., C. C. King, K. H. Hsu, et al. 2002. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics 109:e88. [DOI] [PubMed] [Google Scholar]
- 3.Chareonsirisuthigul, T., S. Kalayanarooj, and S. Ubol. 2007. Dengue virus (DENV) antibody-dependent enhancement of infection upregulates the production of anti-inflammatory cytokines, but suppresses anti-DENV free radical and pro-inflammatory cytokine production, in THP-1 cells. J. Gen. Virol. 88:365-375. [DOI] [PubMed] [Google Scholar]
- 4.Chehadeh, W., A. Bouzidi, G. Alm, P. Wattre, and D. Hober. 2001. Human antibodies isolated from plasma by affinity chromatography increase the coxsackievirus B4-induced synthesis of interferon-α by human peripheral blood mononuclear cells in vitro. J. Gen. Virol. 82:1899-1907. [DOI] [PubMed] [Google Scholar]
- 5.Chen, L. C., H. W. Shyu, S. H. Chen, H. Y. Lei, C. K. Yu, and T. M. Yeh. 2006. Enterovirus 71 infection induces Fas ligand expression and apoptosis of Jurkat cells. J. Med. Virol. 78:780-786. [DOI] [PubMed] [Google Scholar]
- 6.Chen, S. C., H. L. Chang, T. R. Yan, Y. T. Cheng, and K. T. Chen. 2007. An eight-year study of epidemiologic features of enterovirus 71 infection in Taiwan. Am. J. Trop. Med. Hyg. 77:188-191. [PubMed] [Google Scholar]
- 7.Cherry, J. D. 2004. Enteroviruses and parechoviruses. Textbook of pediatric infectious diseases, p. 1984-2041. In R. D. Feigen and J. D. Cherry (ed.), Textbook of pediatric infectious diseases, 5th ed. Saunders, Philadephia, PA.
- 8.Dagan, R., J. A. Jenista, S. L. Prather, K. R. Powell, and M. A. Menegus. 1985. Viremia in hospitalized children with enterovirus infections. J. Pediatr. 106:397-401. [DOI] [PubMed] [Google Scholar]
- 9.Fanger, N. A., K. Wardwell, L. Shen, T. F. Tedder, and P. M. Guyre. 1996. Type I (CD64) and type II (CD32) Fc gamma receptor-mediated phagocytosis by human blood dendritic cells. J. Immunol. 157:541-548. [PubMed] [Google Scholar]
- 10.Fleit, H. B., and C. D. Kobasiuk. 1991. The human monocyte-like cell line THP-1 expresses FcγRI and FcγRII. J. Leuk. Biol. 49:556-565. [DOI] [PubMed] [Google Scholar]
- 11.Freistadt, M. S., and K. E. Eberle. 1996. Correlation between poliovirus type 1 mahoney replication in blood cells and neurovirulence. J. Virol. 70:6486-6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerber, J. S., and D. M. Mosser. 2001. Reversing lipopolysaccharide toxicity by ligating the macrophage Fcg receptors. J. Immunol. 166:6861-6868. [DOI] [PubMed] [Google Scholar]
- 13.Girn, J., M. Kavoosi, and J. Chantler. 2002. Enhancement of coxsackievirus B3 infection by antibody to a different coxsackievirus strain. J. Gen. Virol. 83:351-358. [DOI] [PubMed] [Google Scholar]
- 14.Halstead, S. B., and E. J. O'Rourke. 1977. Antibody-enhanced dengue virus infection in primate leukocytes. Nature 265:739-741. [DOI] [PubMed] [Google Scholar]
- 15.Hawkes, R. A. 1964. Enhancement of the infectivity of arboviruses by specific antisera produced in domestic fowls. Aust. J. Exp. Biol. Med. Sci. 42:465-482. [DOI] [PubMed] [Google Scholar]
- 16.Hayes, P. J., R. Scott, and J. Wheeler. 1994. In vivo production of tumour necrosis factor-a and interleukin-6 in BALB/c mice inoculated intranasally with a high dose of respiratory syncytial virus. J. Med. Virol. 42:323-329. [DOI] [PubMed] [Google Scholar]
- 17.Hober, D., W. Chehadeh, A. Bouzidi, and P. Wattré. 2001. Antibody-dependent enhancement of coxsackievirus B4 infectivity of human peripheral blood mononuclear cells results in increased interferon-α synthesis. J. Infect. Dis. 184:1098-1108. [DOI] [PubMed] [Google Scholar]
- 18.Ho, M., E. R. Chen, K. H. Hsu, et al. 1999. An epidemic of enterovirus 71 infection in Taiwan. N. Engl. J. Med. 341:929-935. [DOI] [PubMed] [Google Scholar]
- 19.Huang, S. C., C. L. Chang, P. S. Wang, Y. Tsai, and H. S. Liu. 2009. Enterovirus 71-induced autophagy detected in vitro and in vivo promotes viral replication. J. Med. Virol. 81:1241-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, S. W., Y. W. Hsu, D. J. Smith, et al. 2009. Reemergence of enterovirus 71 in 2008 in Taiwan: dynamics of genetic and antigenic evolution from 1998 to 2008. J. Clin. Microbiol. 47:3653-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu, B. M., C. H. Chen, and M. T. Wan. 2007. Genetic diversity of epidemic enterovirus 71 strains recovered from clinical and environmental samples in Taiwan. Virus Res. 126:69-75. [DOI] [PubMed] [Google Scholar]
- 22.Huisman, W., B. E. E. Martina, G. F. Rimmelzwaan, R. A. Gruters, and A. D. M. E. Osterhaus. 2009. Vaccine-induced enhancement of viral infections. Vaccine 27:505-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juan, S., Y. Qian, S. Wang, J. M. G. Serrano, W. Li, Z. Huang, and S. Lu. 2010. EV71: an emerging infectious disease vaccine target in the Far East? Vaccine doi: 10.1016/j.vaccine.2010.03.003. [DOI] [PubMed]
- 24.Kliks, S. C., S. Nimmanitya, A. Nisalak, and D. S. Burke. 1988. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am. J. Trop. Med. Hyg. 38:411-419. [DOI] [PubMed] [Google Scholar]
- 25.Kung, C. M., C. C. King, C. N. Lee, L. M. Huang, P. I. Lee, and C. L. Kao. 2007. Differences in replication capacity between enterovirus 71 isolates obtained from patients with encephalitis and those obtained from patients with herpangina in Taiwan. J. Med. Virol. 79:60-68. [DOI] [PubMed] [Google Scholar]
- 26.Liu, C. C., H. W. Tseng, S. M. Wang, J. R. Wang, and I. J. Su. 2000. An outbreak of enterovirus 71 infection in Taiwan, 1998: epidemiologic and clinical manifestations. J. Clin. Virol. 17:23-30. [DOI] [PubMed] [Google Scholar]
- 27.Luo, S. T., P. S. Chiang, A. S. Chao, et al. 2009. Enterovirus 71 maternal antibodies in infants, Taiwan. Emerg. Infect. Dis. 15:581-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahalingam, S., and B. A. Lidbury. 2002. Suppression of lipopolysaccharide-induced antiviral transcription factor (STAT-1 and NF-kB) complexes by antibody-dependent enhancement of macrophage infection by Ross River virus. Proc. Natl. Acad. Sci. U. S. A. 99:13819-13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizuta, K., Y. Aoki, A. Suto, et al. 2009. Cross-antigenicity among EV71 strains from different genogroups isolated in Yamagata, Japan, between 1990 and 2007. Vaccine 27:3153-3158. [DOI] [PubMed] [Google Scholar]
- 30.Morens, D. M. 1994. Antibody-dependent enhancement of infection and the pathogenesis of viral disease. Clin. Infect. Dis. 19:500-512. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura, Y., M. Shimojima, Y. Tano, T. Miyamura, T. Wakita, and H. Shimizu. 2009. Tatsuo human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71. Nat. Med. 15:794-798. [DOI] [PubMed] [Google Scholar]
- 32.Ochiai, H., M. Kurokawa, K. Hayashi, and S. Niwayama. 1988. Antibody mediated growth of influenza A NWS virus in macrophagelike cell line P388D1. J. Virol. 62:20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ooi, M. H., S. C. Wong, Y. Podin, W. Akin, S. Sel, A. Mohan, C. H. Chieng, et al. 2007. Human enterovirus 71 disease in Sarawak, Malaysia: a prospective clinical, virological, and molecular epidemiological study. Clin. Infect. Dis. 44:646-656. [DOI] [PubMed] [Google Scholar]
- 34.Palmer, P., B. Charley, B. Rombaut, M. DaeÈron, and P. Lebon. 2000. Antibody-dependent induction of type I interferons by poliovirus in human mononuclear blood cells requires the type II Fcg receptor (CD32). Virology 278:86-94. [DOI] [PubMed] [Google Scholar]
- 35.Pérez-Vélez, C. M., M. S. Anderson, C. C. Robinson, et al. 2007. Outbreak of neurologic enterovirus type 71 disease: a diagnostic challenge. Clin. Infect. Dis. 45:950-957. [DOI] [PubMed] [Google Scholar]
- 36.Robinson, W. E., Jr., D. C. Montefiori, and W. M. Mitchell. 1988. Antibody dependent enhancement of human immunodeficiency virus type 1 infection. Lancet i:790-794. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt, N. J., E. H. Lennett, and H. H. Ho. 1974. An apparently new enterovirus isolated from patients with disease of central nervous system. J. Infect. Dis. 129:304-309. [DOI] [PubMed] [Google Scholar]
- 38.Takada, A., and Y. Kawaoka. 2003. Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev. Med. Virol. 13:387-398. [DOI] [PubMed] [Google Scholar]
- 39.Tirado, S. M., and K. J. Yoon. 2003. Antibody dependent enhancement of virus infection and disease. Viral Immunol. 16:69-86. [DOI] [PubMed] [Google Scholar]
- 40.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 41.Vuorinen, T., R. Vainionpaa, J. Heino, and T. Hyypia. 1999. Enterovirus receptors and virus replication in human leukocytes. J. Gen. Virol. 80:921-927. [DOI] [PubMed] [Google Scholar]
- 42.Wang, S. M., C. C. Liu, H. W. Tseng, et al. 1999. Clinical spectrum of enterovirus 71 infection of children in southern Taiwan, with an emphasis on the neurological complications. Clin. Infect. Dis. 29:184-190. [DOI] [PubMed] [Google Scholar]
- 43.Wang, S. M., H. Y. Lei, K. J. Huang, et al. 2003. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: the roles of cytokines and cellular immune activation in patients with pulmonary edema. J. Infect. Dis. 188:564-570. [DOI] [PubMed] [Google Scholar]
- 43a.Wang, S., H. Lei, and C. Liu. 2009. Antibody-dependent enhancement of enterovirus 71 infectivity of mononuclear cells. Abstr. 49th. Intersci. Conf. Antimicrob. Agents. Chemother., abstr. V-1751a, p. 385.
- 44.Wong, S. S. Y., C. C. Y. Yip, S. K. P. Lau, and K. Y. Yuen. 2010. Human enterovirus 71 and hand, foot and mouth disease. Epidemiol. Infect. doi: 10.1017/S0950268809991555. [DOI] [PubMed]
- 45.Yamayoshi, S., Y. Yamashita, J. Li, N. Hanagata, T. Minowa, T. Takemura, and S. Koike. 2009. Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nat. Med. 15:798-801. [DOI] [PubMed] [Google Scholar]
- 46.Yang, F., L. Ren, Z. Xiong, et al. 2009. Enterovirus 71 outbreak in the People's Republic of China in 2008. J. Clin. Microbiol. 47:2351-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]