Abstract
In developing countries, the conventional test and slaughter strategy for the control of bovine tuberculosis is prohibitively expensive, and alternative control methods such as vaccination are urgently required. In this study, the efficacy of Mycobacterium bovis bacillus Calmette-Guérin (BCG) for protection against bovine tuberculosis (bTB) was evaluated in Holstein calves under field conditions in Ethiopia. Thirteen neonatally vaccinated and 14 control calves were exposed for 10 to 23 months to skin test reactor cows. Gamma interferon (IFN-γ) testing, comparative intradermal tuberculin testing, postmortem examination, and bacteriological culture were used for the evaluation of BCG efficacy. The overall mean pathology score was significantly (P < 0.05) higher in control calves than in vaccinated calves. Culture positivity for Mycobacterium bovis was higher in the control calves than in the vaccinated calves, and significantly more BCG-vaccinated animals would have passed a standard meat inspection (P = 0.021). Overall, the protective efficacy of BCG was between 56% and 68%, depending on the parameters selected. Moreover, by measuring gamma interferon responses to the antigens ESAT-6 and CFP-10, which are present in M. bovis but absent from BCG, throughout the experiment, we were able to distinguish between vaccinated animals that were protected against bTB and those animals that were not protected. In conclusion, the present trial demonstrated an encouraging protective effect of BCG against bTB in a natural transmission setting in Ethiopia.
Bovine tuberculosis (bTB), caused mainly by Mycobacterium bovis, is characterized by the formation of granulomatous lesions primarily in the lymph nodes (LNs) and lungs. More than 50 million cattle are infected with M. bovis, resulting in economic losses of approximately $3 billion annually worldwide (19), and in developing countries, M. bovis infection is thought to account for 5 to 15% of human TB (2). Conventionally, the control of bTB is based on a test and slaughter strategy, which has reduced the incidence and prevalence of the disease in developed countries, except in those with wildlife reservoirs such as the United Kingdom and New Zealand. However, this method is too costly to be applicable to most of the developing world. Vaccination of cattle represents an alternative intervention strategy to reduce the impact of bTB on livestock productivity and human health in developing countries. The only currently available vaccine against tuberculosis is the human vaccine M. bovis bacillus Calmette-Guérin (BCG). BCG has been used in cattle in a large number of experiments and trials with variable efficacies (reviewed by Buddle et al. [8]). A series of field trials in East Germany and Malawi found no evidence of protection (3, 4, 11). In contrast, field trials in Malawi and Madagascar reported protective efficacies of 25% and 45%, respectively (9, 23), and other workers have reported 50% protection in field trials in Malawi and the United Kingdom, as well as New Zealand (10, 25). Higher levels of protection have been reported in the United Kingdom by Vordermeier et al. (20). Experimental infection studies over the last 15 years using intratracheal or aerosol challenge routes have optimized the use of BCG in cattle and reaffirmed the ability of BCG to protect cattle against bTB (5, 6, 24). However, few recent studies have evaluated the ability of BCG to protect cattle in a more natural cattle-to-cattle transmission setting using naturally infected donor cattle as the source of infection.
In this study, we assessed the efficacy of neonatal BCG vaccination in a natural transmission setting by introducing BCG-vaccinated and control calves into a herd composed of tuberculin skin test reactor animals in a farm in central Ethiopia. The farm was managed under routine Ethiopian intensive husbandry conditions, and protective efficacy was determined after 10 to 23 months in contact with infected animals by postmortem examination and M. bovis culture. We also evaluated whether prototype reagents for the differential diagnosis of infected and vaccinated animals (DIVA reagents) were able to distinguish vaccinated and protected animals from those that were vaccinated but not protected.
MATERIALS AND METHODS
Study neonates and their sources.
The experiment was conducted at a high-intensity farm in central Ethiopia. The cattle in this farm (all purebred Holstein) were tested for bTB in October 2002; 48% of the herd was positive by the comparative intradermal tuberculin test (CIDT). On the basis of this result, the herd was separated into CIDT-positive and CIDT-negative cohorts, which were physically separated and managed independently. This was followed by three more rounds of CIDTs on the “negative” herd and removal of reactors to the positive herd, reducing the incidence of bTB to below 1% a year. Since this action, the farm has been continuously tested for the control of bTB in the negative herd and the prevalence of bTB has remained low. Neonates for the present study were recruited from the negative herd. In addition, they were tested for bTB using the gamma interferon (IFN-γ) test before their recruitment into the experiment. The observation that all unvaccinated control calves were CIDT negative at the time they were brought into contact with the infected donors further demonstrated that the calves used in this study were free from infection with M. bovis. Before this experiment was undertaken, it was approved both by the local Ethics Committee and the Ethical Review Board of the Veterinary Laboratories Agency.
Vaccination schedule of the neonates.
This experiment was undertaken on 27 neonates, of which 13 were vaccinated within 2 weeks of birth by subcutaneous injection with 1 × 106 CFU BCG Danish (Serum Staten Institute [SSI], Copenhagen, Denmark), which was supplied as freeze-dried preparation and reconstituted in Sauton's medium as per the supplier's instructions. Fourteen calves were used as nonvaccinated controls. As the delivery of the cows was not synchronized, the 27 neonates were recruited at different times and randomly allocated to control and vaccinated groups. Until calves were introduced into the reactor herd, at 2 months of age, the neonates were kept in individual calf pens, which were situated in the negative herd, and fed with milk, concentrate, and grass. At around 6 weeks postvaccination (when the animals were about 2 months old), the calves were moved to the positive herd and kept in contact with reactor animals. During the course of the experiment, the farm followed a government protocol for progressive removal of CIDT reactor animals, and the number of positive animals in the positive herd declined from 120 at the beginning of the experiment to 42 at the end.
Comparative intradermal tuberculin test.
The CIDTs were performed by a veterinarian who was in charge of the farm and did not know the vaccination status of each calf. The tests were performed twice: prior to exposure to infected cows and immediately before slaughter. On each occasion, 2,500 IU/ml each of bovine purified protein derivative (PPD-B) and avian PPD (PPD-A) (both supplied by the Veterinary Laboratories Agency, Addlestone, Surrey, United Kingdom) was injected, and the increase in skin thickness was measured 72 h later. Results were interpreted according to the recommendations of the World Organization for Animal Health (OIE) (16). Briefly, when the change in skin thickness was greater at the avian PPD injection site, the animal was considered positive for mycobacterial species other than the mammalian type (Mycobacterium tuberculosis and M. bovis). When increases were observed at both injection sites, the difference between the two reaction sizes was considered. If the increase in skin thickness at the injection site for PPD-B (B) was greater than the increase in skin thickness at the injection site for PPD-A (A), and B − A was less than 2 mm, between 2 and 4 mm, or 4 mm and above, the animal was classified as negative, suspect, or positive for bTB, respectively.
Whole-blood culture and IFN-γ test.
An IFN-γ test was performed on a regular basis following vaccination as well as following exposure. Blood samples were collected from the jugular vein into heparinized Vacutainers and transported to the laboratory within 8 h of collection. Whole blood was dispensed in duplicate in 250 μl/well of 96-well flat-bottom culture plates. Antigens were added in 25-μl aliquots to give the following final assay concentrations: avian PPD, 10 μg/ml; bovine PPD, 10 μg/ml (both tuberculins were obtained from the Veterinary Laboratories Agency, Weybridge, United Kingdom); and ESAT6-CFP10 protein cocktail, each protein at 5 μg/ml (kindly provided by M. Singh, Braunschweig, Germany). Phytohemagglutinin (PHA; 5 μg/ml) and saline (25 μl) were used as positive and negative controls, respectively. Cultures were incubated at 37°C in a humid 5% CO2 atmosphere for 48 h, and supernatants were harvested and frozen. Levels of IFN-γ in the supernatants were measured by an enzyme-linked immunosorbent assay with the bovine IFN-γ (Bovigam) test kit (Prionics, Schlieren, Switzerland) in accordance with the manufacturer's instructions.
Postmortem examination and pathology scoring.
At the end of the exposure period, the calves were shipped to the Addis Ababa Abattoir and then slaughtered. The meat inspection was performed by the meat inspector working in the Addis Ababa Abattoir, while detailed postmortem examinations were performed on each of them by a veterinarian who did not know the vaccination status of the calves. The lungs and lymph nodes (LNs) were removed for the investigation of TB lesions. The seven lobes of the two lungs were inspected externally and palpated for the presence of TB lesions. Each lobe was then sectioned into 2-cm-thick slices to facilitate the detection of lesions. Similarly, LNs, including mandibular LNs, medial retropharyngeal LNs, cranial and caudal mediastinal LNs, left and right bronchial LNs, hepatic LNs, and mesenteric LNs were sliced into thin sections (2 mm thick) and inspected for the presence of visible lesions (VL). When gross lesions suggestive of bTB were found in any of the tissues examined, the animal was classified as lesioned. Animals in which suspicious lesions were not found were classified as nonlesioned (no visible lesions [NVL]).
The severity of the gross lesions was scored by applying the semiquantitative procedure developed by Vordermeier et al. (20), with minor modifications to facilitate performance under field conditions (1). Briefly, lesions in the lobes of the lungs were scored separately as follows: 0, no visible lesions; 1, no gross lesions but lesions apparent on slicing of the lobe; 2, fewer than five gross lesions; 3, more than five gross lesions; and 4, gross coalescing lesions. The scores of the individual lobes were added up to calculate the lung score. Similarly, the severity of gross lesions in individual lymph nodes was scored as follows: 0, no gross lesion; 1, a small lesion at one focus (just starting); 2, small lesions at more than one focus; and 3, extensive necrosis. Individual lymph node scores were added up to calculate the lymph node score. Finally, both lymph node and lung pathology scores were added up to determine the total pathology score per animal. Using the pathology data obtained, a theoretical meat inspection was also undertaken to determine the proportion of animals that would have been condemned at an abattoir. The meat inspection criteria used in Great Britain were applied (http://www.food.gov.uk/multimedia/pdfs/mhsmanualch2part4rev33.pdf).
Isolation of mycobacteria.
Isolation of mycobacteria from tissue was performed in accordance with OIE protocols (16). Briefly, tissue specimens from the lymph nodes and lungs were collected into sterile universal bottles in 5 ml of a 0.9% saline solution and then transported to the laboratory for bacterial isolation. Individual lymph node and lung samples were cultured; if lesions were visible, lesioned material was cultured. If no lesions were visible, a selection of tissue was cultured from each lymph node representing a cross section of each node. Thereafter, they were sectioned into pieces with sterile blades and then homogenized with a pestle and a mortar. The homogenate was decontaminated by adding an equal volume of 4% NaOH and centrifuged at 3,000 rpm for 15 min. The supernatant was discarded, while the sediment was neutralized with 1% (0.1 N) HCl with phenol red as an indicator. Neutralization was achieved when the color of the solution changed from purple to yellow. Thereafter, 0.1 ml of suspension from each sample was spread onto duplicate slants of Lowenstein-Jensen medium; one was enriched with sodium pyruvate, while the other was enriched with glycerol. Cultures were incubated aerobically at 37°C for about 5 to 8 weeks with weekly observation for growth of colonies.
Statistical analysis.
TB status of the study calves was defined by the detection of typical lesions in the tissues, by culture positivity for M. bovis, or by both criteria. To estimate the incidence of TB per group, the number of calves that developed TB was divided by the total number of calves in that group. Vaccine efficacy was estimated using the formula described by Orestein et al. (17), which considers the incidence rates of disease in the vaccinated and unvaccinated calves; i.e., vaccine efficacy is the percentage reduction in the incidence rate of disease among vaccinated calves compared to the unvaccinated calves. The formula used for calculating vaccine efficacy (VE) in this study was VE = ARU − ARV/ARU × 100%, where ARU is attack rate in the unvaccinated group and ARV is the attack rate in the vaccinated group. Comparisons of gross pathology, bacteriology, and extent of organ condemnation due to TB between the vaccinated and control calves were made using Fisher's exact test. The risk of TB infection was estimated by relative risk analysis (14). Means of optical density at 450 nm (OD450) values for the IFN-γ responses in the vaccinated and control groups were compared using Student's t test. Pathology scores for vaccinated and control groups were compared using the Mann-Whitney U test. Poisson's correlation coefficient, r, was used to estimate the association between different variables. Statistical significance was fixed at P < 0.05.
RESULTS
Skin test.
Table 1 shows the result of the CIDTs performed on the experimental calves 6 weeks postvaccination and immediately before their slaughter. Six weeks postvaccination (i.e., before exposure to the infected herd), all vaccinated calves were CIDT positive, while all control calves were CIDT negative. In contrast, after completion of the exposure period, 79% (11/14) of the control calves were CIDT positive, while less than half (5/13) of the vaccinated calves were CIDT positive.
TABLE 1.
Comparative intradermal tuberculin test results from experimental neonatal calves upon exposure to an infected herd and at completion of the exposure time
| Test group | No. of results: |
|||
|---|---|---|---|---|
| Negative | Positive | Doubtful | Total | |
| Skin test upon exposure | ||||
| Control | 14 | 0 | 14 | |
| Vaccinated | 0 | 12 | 1 | 13 |
| Total | 14 | 13 | 27 | |
| Skin test upon slaughter | ||||
| Control | 3 | 11 | 14 | |
| Vaccinated | 6 | 5 | 2 | 13 |
| Total | 9 | 16 | 27 | |
Protective efficacy of BCG.
The protective efficacy of BCG against bTB was evaluated by postmortem examination and culture of M. bovis from lymph nodes and lung tissues. The results of these investigations are presented in Table 2. A high transmission rate of pulmonary TB was observed in control calves, where 86% (12/14) presented with visible lesions and M. bovis could be cultured from the tissues of 79% (11/14). In contrast, only 39% (5/13) and 31% (4/13) of the BCG-vaccinated animals presented visible lesions or were culture positive, respectively. These represent protective efficacies of 56 and 61%, respectively (Table 2). Next we determined the proportion of vaccinated and control animals that would have been condemned at a standard meat inspection. While 71% (10/14) of control animals would have failed such an inspection, with the carcasses fully condemned, only 23% (3/13) of BCG-vaccinated cows would have been fully condemned, which constitutes a 68% protection rate (Table 2). Disease had spread in 3/14 unvaccinated animals to organ systems outside the head and lung regions. In contrast, no such dissemination was observed in the BCG vaccinates, although this difference is not statistically significant (Table 2). Taking these parameters into account, we calculated the relative risk of an unvaccinated animal contracting bTB compared to that of a BCG-vaccinated animal as between 2.23 and 3.1 (Table 2).
TABLE 2.
Protective efficacy of BCG against bTB in vaccinates and nonvaccinated calves after 10 to 23 months of exposure to the infected herd
| Parameter | Overall pathology | M. bovis culture positivity | Spread outside head and lung regions | Condemned at meat inspection |
|---|---|---|---|---|
| No. (%) of control calves (n = 14) | 12 (86) | 11 (79) | 3 (21) | 10 (71) |
| No. (%) of vaccinated calves (n = 13) | 5 (39) | 4 (31) | 1 (8) | 3 (23) |
| P value (Fisher's exact test) | 0.018 | 0.021 | NS | 0.021 |
| Protective efficacy (%) | 56 | 61 | 63 | 68 |
| Relative risk | 2.23 | 2.55 | 2.62 | 3.10 |
Next, a quantitative evaluation of the pathological changes found during the postmortem examination was performed by applying a standard pathology scoring system developed for evaluating vaccine protection in an experimental model of bovine tuberculosis (1, 20). As shown in Table 3, BCG vaccination significantly reduced the number of lesioned lymph nodes/animal from 1.9 in controls to 0.5 in vaccinated animals (P = 0.019), as well as the mean severity in the lymph nodes expressed as pathology scores (controls versus vaccinated, 6.4 versus 1.3, respectively; P = 0.006). Although we also observed a reduction in the number of lesioned lung lobes/animal and reduction of the mean lung pathology scores in the BCG-vaccinated animals, these differences were not statistically significant (P > 0.05). Lastly, the total pathology scores, which combine lymph node and lung scores, were also significantly reduced in vaccinated animals (from 14.1 in controls to 4.6 in vaccinated calves; P = 0.014) (Table 3).
TABLE 3.
Mean pathology scores of lung and lymph nodes in vaccinates and controls
| Group or parameter | Mean no. of lesioned LNs/animal (95% CI)a | Mean LN score (95% CI) | Mean no. of lesioned lung lobes/animal (95% CI) | Mean lung score (95% CI) | Mean total pathology score (95% CI) |
|---|---|---|---|---|---|
| Control (n = 14) | 1.9 (0.9-3.0) | 6.4 (3.4-9.5) | 1.6 (1.4-3.5) | 7.6 (0-16.2) | 14.1 (2.5-24.6) |
| Vaccinated (n = 13) | 0.5 (0-1.1) | 1.3 (0- 3.3) | 1.1 (0-2.7) | 3.3 (0-7.9) | 4.6 (0-10.5) |
| Mann-Whitney U test (P value) | 0.019 | 0.006 | NS | NS | 0.014 |
95% CI, 95% confidence interval.
Blood IFN-γ responses after vaccination and challenge.
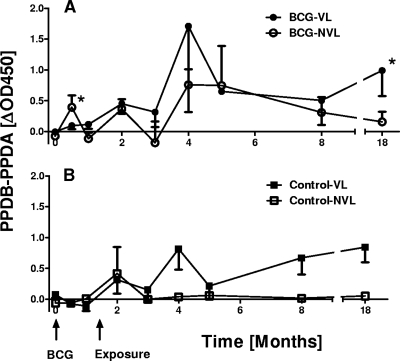
The production of IFN-γ after in vitro stimulation with PPD-B and PPD-A was determined regularly up to 18 months postvaccination and postchallenge. The results representing PPD-B-biased responses (PPD-B responses − PPD-A responses) are shown in Fig. 1. Following vaccination, modest PPD-B-biased IFN-γ responses were detected 2 weeks after vaccination only in BCG-vaccinated calves that showed no lesions at postmortem (BCG NVL [i.e., no visible lesions]; Fig. 1A). In contrast, no increases were found in the lesioned, unprotected BCG-vaccinated animals (BCG VL [i.e., visible lesions]; Fig. 1A) or in the unvaccinated controls (control VL and NVL; Fig. 1B). After challenge, IFN-γ responses developed rapidly in all groups, peaking 4 months postvaccination (2.5 months after animals were put in contact with the infected donor cattle) in BCG vaccinates and in the VL control calves (Fig. 1A and B). The early onset of IFN-γ responses postcontact suggests that transmission from donors to sentinels occurred within weeks of contact. NVL control calves showed a mild initial increase in PPD-B-biased response 2 weeks postcontact only and then remained negative throughout the remainder of the experimentation period. This may indicate that they were exposed to M. bovis early postcontact but that disease did not develop subsequently. Third, after 3.5 months of contact with infected donors, mean IFN-γ levels stabilized in both VL BCG-vaccinated and VL control groups (5 months postvaccination) but increased again toward the end of the in-contact period (Fig. 1A and B, 18 months postvaccination). Interestingly, the level of IFN-γ production in protected BCG NVL calves decreased during the in-contact period (Fig. 1A). These observations were supported by further analysis that integrated the in vitro IFN-γ production over the experimental period by calculating areas under the curves (AUC). First, only protected BCG-vaccinated calves produced IFN-γ following vaccination (AUC BCG NVL: 0.16 compared to 0.07 in BCG-VL and control groups). Second, following challenge, the IFN-γ produced as measured by AUC was similar in unprotected vaccinates and lesioned controls (AUC for BCG VL and control VL, 11.8 and 10.1, respectively), while protected BCG NVL calves presented with a considerably smaller AUC: 5.1. This might reflect the previous observation that in vitro IFN-γ production can correlate to some degree with the degree of pathology and bacterial loads (13, 20).
FIG. 1.
In vitro IFN-γ responses following vaccination and challenge. (A) Responses of BCG vaccinates; (B) responses of control animals. Results are expressed as mean ΔOD450 responses (OD450 values induced by PPD-A subtracted from PPD-B values). Time is shown as months postvaccination. VL, animals with visible lesions, NVL, animals with no visible lesions. Please note that NVL BCG-vaccinated animals and controls were also M. bovis culture negative.*, P < 0.05 between BCG-vaccinated VL and BCG-vaccinated NVL (Student's t test).
Duration of exposure and severity of pathology.
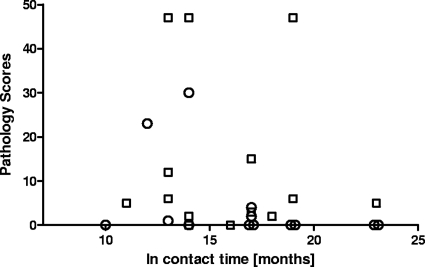
To test whether differences in the extent of pathology between the two groups reflected different periods of exposure, we compared the pathology scores found at postmortem with the time period that individual animals were in contact with the reactor herd of infected cattle. As Fig. 2 demonstrates, there is no correlation between the exposure time and disease severity in vaccinated calves. Indeed, the animals in contact the longest (10 and 23 months) were fully protected. In contrast, unvaccinated control animals presented with visible pathology independent of the in-contact period (Fig. 2).
FIG. 2.
Association between the severity of pathology of bTB in experimental calves and the duration of exposure to the infected herd. Circles, BCG-vaccinated calves; squares, control animals.
Performance of a DIVA reagent based on ESAT-6 and CFP-10.
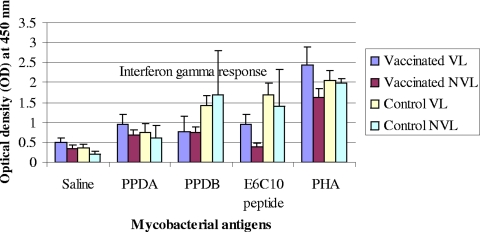
DIVA reagents based on several antigens that are not recognized immunologically by BCG-vaccinated animals have been developed (7, 18). We took the opportunity in the present study to establish whether a prototype DIVA reagent based on the antigens ESAT-6 and CFP-10 was capable of distinguishing not only BCG-vaccinated from infected animals but also calves that were vaccinated and fully protected (i.e., without visible lesions: NVL/M. bovis culture negative) from those that were vaccinated but developed visible pathology (VL). Therefore, at the last bleed before slaughter, whole blood was collected and stimulated in vitro with PPD-A, PPD-B, and a cocktail of ESAT-6 and CFP-10 in the form of recombinant proteins. The IFN-γ produced was then measured, using the Bovigam IFN-γ assay. The results in Fig. 3 demonstrate that ESAT-6 and CFP-10 induced strong responses in nonvaccinated cattle and in vaccinates that presented with visible lesions, but not in the BCG-vaccinated cows without lesions (NVL; Fig. 3). In contrast, PPD-B did not allow this distinction (Fig. 3). Therefore, these data suggest that ESAT-6 and CFP-10 when used as DIVA reagents not only will distinguish between BCG-vaccinated and infected cattle but also will differentiate between vaccinated, protected and vaccinated, unprotected, or partially protected individuals.
FIG. 3.
IFN-γ responses to mycobacterial antigens in vaccinated and control neonatal calves with visible lesions (VL) or no visible lesions (NVL). IFN-γ response was measured upon slaughter just immediately before completion of the exposure time. IFN-γ responses to bovine PPD and ESAT-6-CFP-10 peptide cocktail were greater (P < 0.05) in control calves than in vaccinated calves. E6C10 is a cocktail of ESAT-6 and CFP-10 recombinant proteins. The abbreviations of the different animal groups are defined in the legend to Fig. 1.
DISCUSSION
In the present trial, the efficacy of BCG against bTB was evaluated in a natural transmission setting using bovine neonates in Ethiopia. To avoid any interference with environmental mycobacteria in the development of BCG-induced immunity, we vaccinated calves neonatally. This was demonstrated by very low PPD-A responses before vaccination (data not shown). To our knowledge, this is one of the few recent studies in which BCG activity was determined in a natural cattle-to-cattle transmission setting on a farm managed under routine husbandry conditions. For transmission studies to result in statistically robust data, a high transmission rate in the control animals is required. This was achieved in the present study (12/14 diseased control animals) by having a high-prevalence donor herd (100% CIDT positive) and a high donor/sentinel ratio. Our results demonstrate that BCG is a potent vaccine against bTB, confirming expectations from a number of recent studies using experimental challenge via intranasal, intratracheal, or aerosol routes (8, 21). However, in contrast to the findings from experimental studies in which BCG vaccination results in significantly reduced pathology but rarely in full protection (NVL/culture-negative), we found that under conditions of natural transmission, BCG vaccination significantly reduced the number of lesioned and culture-positive animals despite continued exposure to M. bovis over 10 to 23 months. This may be due to exposure of animals to a higher infectious dose in challenge compared to natural transmission experiments. This is an encouraging result and highlights the potential utility of BCG vaccination in a bTB control program, particularly in developing countries where testing and culling are prohibitively expensive.
Detailed postmortem examination and bacteriology were employed for estimation of the efficacy of BCG in the present experiment, and the efficacy of BCG recorded in the present trial was similar to the efficacy of BCG (around 60%) reported in Mexico by Lopez-Valencia et al., using a similar experimental design on the basis of in vitro tests (12), although they were unable to perform postmortem examinations on all animals. Thus, the advantage of our experiment was that the natural transmission was effective, and as a result, lesions of various severities including disseminated types developed in the experimental calves. Lopez-Valencia and coworkers concluded that BCG protection waned at around 1 year postvaccination. In contrast, our data suggest that protective immunity could be maintained for up to 23 months. A key finding is that significantly more vaccinated animals would have passed slaughterhouse meat inspection than the control calves. This is particularly important in the context of developing countries, in which the major economic impact of bTB is in reduced productivity and carcass condemnation, in contrast to the emphasis in developed countries on trade regulations based on skin test reactivity rather than extent of disease.
Based on PCR analysis of nasal exudates, Lopez-Valencia et al. (12) demonstrated a significant reduction in shedding of M. bovis by BCG-vaccinated animals: i.e., 21.7% of the control animals shed bacilli into nasal secretions, whereas no shedding was detected in the BCG-vaccinated calves (12). While for logistical reasons we did not directly test shedding in our study, the reduction in the number of transmission-associated thoracic lymph nodes and lung lesions (15) also supports the notion that BCG vaccination will reduce bTB transmission. Future trials to measure the impact of BCG on transmission will be important in assessing the potential economic benefits of vaccination.
As it proved impossible to source sufficient calves from disease-free mothers to initiate the experiment fully at one time point, vaccinated and unvaccinated sentinels had to be introduced into the experiment when they became available over a 12-month period. Furthermore, despite our plan to continue the experiment until all calves would have been in the donor herd for the same period, the herd owner significantly accelerated the depopulation of the infected herd. This meant that we had to terminate the experiment with some sentinels being in contact for only 10 months. All sentinels animals were therefore killed at the same time. This could have affected the transmission rate or protective efficacy due to some calves being in contact for shorter periods and the gradual reduction in the number of infected donor cattle. However, as shown in Fig. 2, there was no relationship between duration of the in-contact period or the number of donor animals and protection, transmission rate, or the degree of pathology. Such a gradual removal of infected animals in conjunction with restocking with BCG-vaccinated calves may constitute a practical control strategy in high-value herds in developing countries that cannot afford large-scale whole-herd depopulation. Our study could form the basis of a cost-benefit analysis of this approach in a developing country setting.
We also studied cell-mediated immune responses before and after exposure by measuring CIDT (skin test) and in vitro IFN-γ responses. As expected, all BCG-vaccinated animals were skin test positive at about 6 weeks after vaccination. They were also producing IFN-γ on in vitro stimulation, although this was restricted to calves that were fully protected (NVL). This is an important observation as it suggests that skin test responses did not correlate with or predict protection, while in vitro blood responses did. Interestingly, at the end of the experiment, after 10 to 23 months of exposure to infected cattle, only 5 BCG vaccinates tested skin test negative. As this test negativity correlated with the NVL animals, it is therefore possible to continue with a tuberculin skin test-based test and control strategy even in the context of BCG vaccination if one adheres to a defined period postvaccination when skin testing cannot be applied. However, this skin test exclusion period has to be defined more carefully. We also demonstrated that prototype DIVA reagents based on ESAT-6 and CFP-10 applied in the Bovigam IFN-γ assay can be used to distinguish between BCG-vaccinated and infected animals, as observed earlier (22). Furthermore, the results from this study demonstrate that these DIVA reagents also allow the discrimination of vaccinated and fully protected cattle from those vaccinated but only partially or not protected. This outcome has been suggested previously from results obtained after intratracheal challenge experiments (20), and it is encouraging that these observations were confirmed in this natural transmission experiment. It should be noted that unprotected (VL) vaccinated animals showed a PPD-B bias at almost every time point postinfection (Fig. 1). However, on several occasions—for example, as shown in Fig. 3—PPD-A responses were stronger than PPD-B responses. These animals would therefore have tested negative for bovine tuberculosis. In contrast, they responded to ESAT-6 and CFP-10, which further highlights the advantages of using defined antigens like ESAT-6 and CFP-10.
In conclusion, the present study has demonstrated the efficacy of BCG in an Ethiopian transmission setting in a farm managed under routine conditions. This control strategy may be particularly applicable to the type of farms that are found in and around Addis Ababa, where dairy cattle consisting of Holsteins and their crosses with zebu are kept under intensive husbandry with a consequent high risk of bTB. Further studies will be required to assess the impact of BCG in the more resistant zebu breeds.
BCG vaccination could have a major impact on animal and human health as well as reducing economic losses in developing countries such as Ethiopia. Further studies of this nature, measuring the effect of vaccination on both disease development and onward transmission, are required in order to produce economic impact studies to determine if BCG vaccination represents a cost-effective control strategy.
Acknowledgments
We acknowledge the Wellcome Trust for financially supporting this project.
Furthermore, we thank the Federal Ministry of Agriculture of Ethiopia for facilitating the execution of this experiment on its farm.
Footnotes
Published ahead of print on 18 August 2010.
REFERENCES
- 1.Ameni, G., A. Aseffa, H. Engers, D. B. Young, G. R. Hewinson, and H. M. Vordermeier. 2006. Cattle husbandry is a predominant factor affecting the pathology of bovine tuberculosis and gamma interferon responses to mycobacterial antigens. Clin. Vaccine Immunol. 13:1030-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashford, D. A., E. Whintey, P. Raghunathan, and P. Cosivi. 2001. Epidemiology of selected mycobacteria that infect humans and other animals. Rev. Sci. Tech. 20:325-337. [DOI] [PubMed] [Google Scholar]
- 3.Berggren, S. A. 1977. Incidence of tuberculosis in BCG vaccinated and control cattle in relation to age distribution in Malawi. Br. Vet. J. 133:490-494. [DOI] [PubMed] [Google Scholar]
- 4.Berggren, S. A. 1981. Field experiment with BCG vaccine in Malawi. Br. Vet. J. 137:88-94. [DOI] [PubMed] [Google Scholar]
- 5.Buddle, B. M., G. W. de Lisle, A. Pfeffer, and F. E. Aldwell. 1995. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine 13:1123-1130. [DOI] [PubMed] [Google Scholar]
- 6.Buddle, B. M., D. Keen, A. Thomson, G. Jowett, A. R. McCarthy, J. Heslop, G. W. de Lisle, J. L. Stanford, and F. E. Aldwell. 1995. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route but not by vaccination with killed Mycobacterium vaccae. Res. Vet. Sci. 59:10-16. [DOI] [PubMed] [Google Scholar]
- 7.Buddle, B. M., M. A. Skinner, and M. A. Chambers. 2000. Immunological approaches to the control of tuberculosis in wildlife reservoirs. Vet. Immunol. Immunopathol. 74:1-16. [DOI] [PubMed] [Google Scholar]
- 8.Buddle, B. M., D. N. Wedlock, M. Denis, and M. A. Skinner. 2005. Identification of immune response correlates for protection against bovine tuberculosis. Vet. Immunol. Immunopathol. 108:45-51. [DOI] [PubMed] [Google Scholar]
- 9.Cheneau, Y., and J. Blancou. 1975. Comparative values of live or killed BCG and trypsinized Koch's bacillus in the immunization of zebu against tuberculosis. Rev. Elev. Med. Vet. Pays Trop. 28:1-7. [PubMed] [Google Scholar]
- 10.Ellwood, D. C., and F. G. Waddington. 1972. A second experiment to challenge the resistance to tuberculosis in B.C.G. vaccinated cattle in Malawi. Br. Vet. J. 128:619-626. [DOI] [PubMed] [Google Scholar]
- 11.Hubrig, T., and W. Kruger. 1958. Untersuchungen zur Tuberkuloseschutzimpfung bei Rindern. Monatsh. Vetmed. 13:513-519. [Google Scholar]
- 12.Lopez-Valencia, G., T. Renteria-Evangelista, J. de Jesus Williams, A. Licea-Navorro, A. De la Mora-Valle, and G. Medina-Basulto. 2010. Field evaluation of the protective efficacy of Mycobacterium bovis BCG vaccine against bovine tuberculosis. Res. Vet. Sci. 88:44-49. [DOI] [PubMed] [Google Scholar]
- 13.Lyashchenko, K., A. Whelam, R. Greenwald, J. M. Pollock, P. Andersen, R. G. Hewinsen, and H. M. Vordermeier. 2004. Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with Mycobacterium bovis BCG and infected with M. bovis. Infect. Immun. 72:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin, S. W., H. A. Meek, and P. Willerberg. 1987. Veterinary epidemiology: principles and methods, p. 63-64. Iowa State University Press, Ames, IA.
- 15.Neill, S. D., D. G. Bryson, and J. M. Pollock. 2001. Pathogenesis of tuberculosis in cattle. Tuberculosis 81:79-86. [DOI] [PubMed] [Google Scholar]
- 16.OIE. 2007. Bovine tuberculosis. Manual of diagnostic tests and vaccines for terrestrial animals, part 2, section 2.3, chapter 2.3.3. World Organisation for Animal Health, Paris, France.
- 17.Orenstein, W. A., R. H. Bernier, T. J. Dondero, A. R. Hinman, J. S. Marks, K. J. Bart, and B. Sirotkin. 1985. Field evaluation of vaccine efficacy. Bull. World Health Organ. 63:1055-1068. [PMC free article] [PubMed] [Google Scholar]
- 18.Sidders, B., C. Pirson, P. J. Hogarth, R. G. Hewinson, N. G. Stoker, H. M. Vordermeier, and K. Ewer. 2008. Screening of highly expressed mycobacterial genes identifies Rv3615c as a useful differential diagnostic antigen for Mycobacterium tuberculosis complex. Infect. Immun. 76:3932-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steele, J. H. 1995. Regional and country status report, p. 169-172. In C. O. Thoen and J. H. Steele (ed.), Mycobacterium bovis infection in animals and humans. Iowa State University Press, Ames, IA.
- 20.Vordermeier, H. M., M. A. Chambers, P. J. Cockle, A. O. Whelan, J. Simons, and R. J. Hewinson. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vordermeier, H. M., K. Huygen, M. Singh, R. G. Hewinson, and Z. Xing. 2006. Immune response induced in cattle by vaccination with recombinant adenovirus expressing mycobacterial antigen 85A and Mycobacterium bovis BCG. Infect. Immun. 74:1416-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waddington, F. G., and D. C. Ellwood. 1972. An experiment to challenge the resistance to tuberculosis in BCG vaccinated cattle in Malawi. Br. Vet. J. 128:541-551. [DOI] [PubMed] [Google Scholar]
- 24.Waters, W. R., M. V. Palmer, B. J. Nonnecke, T. C. Thacker, C. F. C. Scherer, D. M. Estes, R. G. Hewinson, H. M. Vordermeier, S. W. Barnes, G. C. Federe, J. R. Walker, R. J. Glynne, T. Hsu, B. Weinrick, K. Biermann, M. H. Larsen, and W. R. Jacobs. 2009. Efficacy and immunogenicity of Mycobacterium bovis DeltaRD1 against aerosol M. bovis infection in neonatal calves. Vaccine 27:1201-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuckerman, L. 1980. Badgers, cattle, and tuberculosis. Her Majesty's Stationery Office, London, United Kingdom.