Abstract
Mycobacterium bovis bacillus Calmette-Guérin (BCG) is the only tuberculosis (TB) vaccine currently available, but its efficacy against adult pulmonary TB remains controversial. BCG induces specific immune responses to mycobacterial antigens and may elicit protective immunity against TB. TB remains a major public health problem, especially among the elderly, yet the efficacy of BCG in the elderly is unknown. We investigated the ability of BCG vaccination to prevent TB in young (6-week-old), middle-aged (18-month-old), and old (60-month-old) guinea pigs. BCG-Tokyo vaccination reduced the growth of Mycobacterium tuberculosis H37Rv in all three groups. By use of an enzyme-linked immunospot (ELISPOT) assay, antigen-specific gamma interferon (IFN-γ)-producing cells were detected in the 60-month-old guinea pigs after a booster vaccination with BCG-Tokyo. Our findings suggest that BCG-Tokyo has a protective effect against tuberculosis infection regardless of age.
Tuberculosis (TB) remains a major public health problem, especially among elderly people. Patients ≥60 years of age account for ≥50% of new cases in Japan (29). The increasing susceptibility of the elderly to Mycobacterium tuberculosis is generally thought to be associated with age-related changes in immune system function, especially losses or delays in antigen-specific CD4+ T-cell function (14). Compromised antigen-specific CD4+ T-cell responses may contribute to increased susceptibility to M. tuberculosis infection in mice (27).
Mycobacterium bovis bacillus Calmette-Guérin (BCG) is the only TB vaccine currently available. BCG has been used for more than 80 years (41), and vaccination with BCG is the standard for TB prevention in most countries. BCG induces specific immune responses to mycobacterial antigens and may elicit protective immunity against tuberculosis. BCG provides efficient protection against severe and disseminated TB, such as tuberculosis meningitis and miliary tuberculosis, in children (33, 34, 40). Although the long-term efficacy of BCG has been documented (3, 6), with several reports indicating efficient protection against disseminated TB in newborns and children, it appears to have less efficacy against adult pulmonary TB (2). In fact, its efficacy against pulmonary TB in both adults and the elderly is controversial, as is the efficacy of revaccination (5).
In the present study, we examined the efficacy of BCG against TB at different ages in a common guinea pig model (15, 25, 30). We used three age-segregated groups—young (6 weeks old), middle-aged (18 months old), and old (60 months old)—and we measured the number of antigen-specific gamma interferon (IFN-γ)-producing cells as an indicator of the efficacy of the vaccine against TB.
MATERIALS AND METHODS
Animals.
Female pathogen-free outbred Hartley guinea pigs were purchased from Japan SLC (Shizuoka, Japan). The guinea pigs were divided into the three groups described above and were housed in accordance with the guidelines for animal experimentation of the Japanese Association for Laboratory Animal Science (1987) and in full compliance with the Law for the Humane Treatment and Management of Animals (Japan). The guinea pigs were fed and maintained in accordance with the guidelines set forth by the Institutional Animal Care and Use Committee of the National Institute of Infectious Diseases (NIID), Japan. Once approved by an institutional committee for animal experiments, these studies were conducted at the Animal Facility of Toyama Campus, NIID, Japan, in accordance with the requirements specifically stated in the Laboratory Biosafety Manual of the World Health Organization.
BCG vaccination.
The guinea pigs were vaccinated with 5 × 105 CFU of BCG (strain Tokyo 172) injected subcutaneously into the left or right inguinal region. The vaccination schedules were as follows. The old guinea pigs were vaccinated with BCG, maintained for 60 months, and then revaccinated with BCG 6 weeks before M. tuberculosis infection (group 1; n, 2). The middle-aged guinea pigs were vaccinated either 18 months or 6 weeks before the infection (groups 2 and 3, respectively; n, 3). The young animals either were vaccinated 6 weeks before the infection (group 4; n, 3) or were not vaccinated (group 5; n, 4) (Fig. 1).
FIG. 1.
Experimental design. Guinea pigs in the old group were vaccinated with BCG at the age of 6 weeks and were then maintained for 60 months before revaccination with BCG 6 weeks before M. tuberculosis infection (group 1). Guinea pigs in the middle-aged groups either were vaccinated with BCG at the age of 6 weeks and were then maintained for 18 months before infection (group 2) or were not vaccinated with BCG until 6 weeks before infection (group 3). Guinea pigs in the young groups either were vaccinated 6 weeks before infection (group 4) or were not vaccinated (group 5).
Aerosol challenge with M. tuberculosis H37Rv.
Virulent M. tuberculosis H37Rv (NIHJ1633) was grown in Middlebrook 7H9 broth (Difco, Detroit, MI) supplemented with albumin dextrose catalase (ADC) enrichment and 0.05% Tween 80 for 14 to 21 days at 37°C. The bacilli were subjected to gentle sonication in order to obtain a single-cell suspension and were frozen at −80°C until use. Thawed aliquots were diluted in phosphate-buffered saline (PBS) containing 0.05% Tween 20 to the desired inoculum concentration. BCG-vaccinated and unvaccinated guinea pigs were exposed to 2.5 ml of M. tuberculosis H37Rv at 5 × 104 CFU/ml by using an inhalation exposure system, model 4212 (Glas-Col, Terre Haute, IN). Then BCG-vaccinated and unvaccinated guinea pigs were infected with approximately 10 CFU of virulent M. tuberculosis H37Rv via the respiratory route. The animals were housed under biosafety level 3 conditions in a manner consistent with the international animal care and use guidelines of the National Institute of Infectious Diseases of Japan.
DTH skin test.
To investigate delayed-type hypersensitivity (DTH) skin reactions, 0.2 μg of tuberculin purified protein derivative (PPD) was injected intradermally into BCG-vaccinated and unvaccinated guinea pigs, and the skin reactions were measured after 24 h.
Microbial enumeration.
At 5 weeks postchallenge, specimens from the lungs, tracheal lymph node, and spleen from each (BCG-vaccinated or unvaccinated) aerosol-challenged guinea pig were removed aseptically and were homogenized separately in 1 ml of sterile saline using a Stomacher-80T instrument (Organo, Tokyo, Japan). Appropriate dilutions were inoculated onto 1% Ogawa medium (Kyokuto, Tokyo, Japan) and were incubated at 37°C for 3 weeks. The number of M. tuberculosis H37Rv colonies on the medium was counted and expressed as the mean log10 CFU per tissue.
Histopathology.
The dissected lung samples from each guinea pig were fixed with 10% neutral-buffered formalin and were embedded in paraffin wax. The sections from these tissues were 4 μm thick and were stained with hematoxylin and eosin (H&E) or with Ziehl-Neelsen stain for acid-fast bacilli.
Preparation of cells.
Mononuclear cells were isolated from the peripheral blood of the guinea pigs. Approximately 10 ml of blood was harvested from the animals by cardiac puncture at 0, 6, and 11 weeks after BCG vaccination. Before blood collection, the animals were anesthetized with ketamine (44 mg/kg). Peripheral blood mononuclear cells (PBMCs) were prepared with Lymphosepar (IBL Co., Ltd., Gunma, Japan) and were then adjusted to 1 × 106/ml in complete medium (RPMI 1640 supplemented with 10% fetal bovine serum). Cell viability was determined by a trypan blue dye exclusion test. Single-cell suspensions were cultured with or without PPD (10 μg/ml) at 37°C in a humidified 5% CO2 environment for 40 h. Phytohemagglutinin (PHA) (1 μg/ml) was used as a positive control to stimulate whole T cells.
IFN-γ ELISPOT assay.
IFN-γ-secreting cells were assessed by an enzyme-linked immunospot (ELISPOT) assay that was modified and improved to detect guinea pig IFN-γ-producing cells. Due to the low cross-reactivity of murine, rat, and guinea pig IFN-γ, we obtained a novel rabbit polyclonal anti-guinea pig IFN-γ antibody and developed a guinea pig IFN-γ ELISPOT assay system. Briefly, based on the predicted amino acid sequence of guinea pig IFN-γ described previously(16), we synthesized peptides (GG-1, GG-2, GG-3, GG-4, and GG-5). The immunogen (100 mg of peptide) was injected subcutaneously into rabbits, and 7 weeks after immunization, the rabbits were bled from the ear artery (50 to 100 ml). Antibodies were purified from antisera by affinity chromatography with immobilized synthetic peptides. Immunoblot analysis showed that only antisera against GG-2 and GG-5 reacted with a protein band of about 20 kDa, which is the putative molecular mass of guinea pig IFN-γ. Furthermore, the binding affinities of the purified antibodies for recombinant guinea pig IFN-γ were assessed. Recombinant guinea pig IFN-γ was prepared using the baculovirus system. Guinea pig IFN-γ cDNA was transfected into a baculovirus genome (Abv baculovirus; Katakura Industries Co. Ltd., Tokyo, Japan) with the pYNG transfer vector. Recombinant guinea pig IFN-γ was purified from the culture supernatant by an immunoaffinity column, and its bioactivity was measured based on the inhibition of the cytopathic effect of encephalomyocarditis virus (EMCV) on 104C1 guinea pig fibroblasts. We modified a previously described IFN-γ ELISPOT assay protocol (18) to detect guinea pig IFN-γ-producing cells. A polyclonal rabbit antibody to a guinea pig IFN-γ peptide was allowed to adhere overnight at 4°C to a 96-well nitrocellulose plate (MultiScreen-HA; Millipore, Billerica, MA) at a concentration of 5 μg/ml. The plate was washed with PBS-0.05% Tween 20 (PBST) and was then blocked with PBS supplemented with 1% bovine serum albumin (BSA) for 2 h at room temperature. Guinea pig PBMCs were transferred to the antibody-coated 96-well nitrocellulose plate in triplicate at an input cell number of 1 × 105 per well and were then incubated for 5 h at 37°C in a humidified 5% CO2 environment. After 5 h of culturing, the plate was washed with PBST to remove cells and was then incubated with a biotinylated rabbit anti-IFN-γ secondary antibody at a concentration of 5 μg/ml for 2 h at room temperature. After a wash with PBST, the plate was treated with streptavidin-alkaline phosphatase and the substrate 5-bromo-4-chloro-3-indolylphosphate (BCIP)-nitroblue tetrazolium (NBT) (ELISPOT blue color module; R&D Systems, Minneapolis, MN). Spot-forming cells (SFCs) were quantified using the KS ELISPOT compact system (Carl Zeiss Japan, Tokyo, Japan).
Statistical analysis.
The data were analyzed using the Tukey-Kramer test and Pearson's correlation coefficient test using Statcel 2 software. Differences between treatments were determined by the least-squares significant-difference multiple-comparison method. A probability level of 5% (P, <0.05) was considered statistically significant.
RESULTS
DTH skin responses of guinea pigs to PPD.
DTH was assessed on the skin of guinea pigs 6 or 11 weeks after BCG inoculation both before and after challenge with M. tuberculosis. At week 6, significant DTH responses to PPD were detected in all of the guinea pigs vaccinated with BCG, while no response to PPD was detected in unvaccinated guinea pigs. The mean diameters of the indurations were as follows: 17.0 ± 4.2 mm (group 1), 20.0 ± 0.6 mm (group 2), 18.0 ± 1.7 mm (group 3), 15.3 ± 2.1 mm (group 4), and 5.5 ± 1.3 mm (group 5). No significant difference was observed among the groups vaccinated with BCG. DTH responses were detected in all groups at 5 weeks after the challenge with M. tuberculosis (Fig. 2).
FIG. 2.
PPD skin reactions of the guinea pigs. DTH was assessed on the basis of the skin reactions of guinea pigs 6 or 11 weeks (6w or 11w) after BCG inoculation both before and after challenge with M. tuberculosis. Error bars represent standard deviations. No significant difference among the groups vaccinated with BCG was observed.
BCG-induced PPD-specific T-cell responses.
We examined the stimulation by PPD of IFN-γ production by the PBMCs of the guinea pigs. To investigate T-cell functions specific for PPD, an IFN-γ ELISPOT assay was performed for groups 1, 4, and 5. The old guinea pigs of group 1 were inoculated with BCG 60 months before the infection and at week zero, and the young guinea pigs of group 4 were inoculated with BCG at week zero. Another group of young guinea pigs, group 5, was not inoculated. IFN-γ production by PBMCs was examined in each group at weeks zero, 6, and 11. At week 6 after the BCG vaccination, significant and specific IFN-γ responses to PPD were detected in groups 1 and 4 (Fig. 3). The mean numbers of SFCs among the PBMCs from the animals in groups 1 and 4 were 216.25 ± 24.50 and 108.75 ± 9.57, respectively. No significant IFN-γ production was detected in group 5. PPD-specific IFN-γ-secreting cells were more frequent among the PBMCs from group 1 than among those from group 4. Five weeks after the challenge with M. tuberculosis (at week 11 after the BCG vaccination), a significant increase in IFN-γ production by PBMCs following stimulation with PPD was observed in groups 1 and 4, although there was no difference between the groups in the mean frequency of cells responding specifically to PPD. The number of SFCs was also higher in group 5 after the challenge with M. tuberculosis. However, the number of SFCs was significantly lower than those in the BCG-vaccinated groups (P, < 0.01). At week zero of BCG vaccination, no increase in IFN-γ production was detected by the ELISPOT assay in group 1 in spite of the early BCG vaccination; groups 4 and 5 also showed no increase.
FIG. 3.
BCG-induced PPD-specific T-cell responses. To investigate T-cell functions specific for PPD in groups 1, 4, and 5, an IFN-γ ELISPOT assay was performed at weeks zero, 6, and 11 after BCG vaccination. Error bars represent standard deviations. Asterisks indicate that the mean numbers of IFN-γ SFCs were significantly different. **, P < 0.01, as determined by analysis of variance (ANOVA) followed by a posthoc Tukey-Kramer test. There was no difference in IFN-γ SFCs between the old and young guinea pigs vaccinated with BCG.
Effect of BCG vaccination on bacterial growth in young, middle-aged, and old guinea pigs challenged with M. tuberculosis H37Rv.
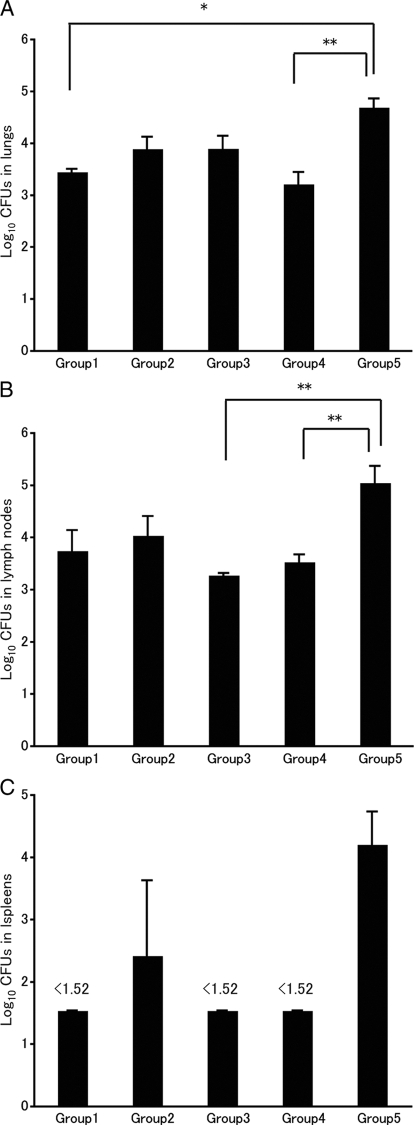
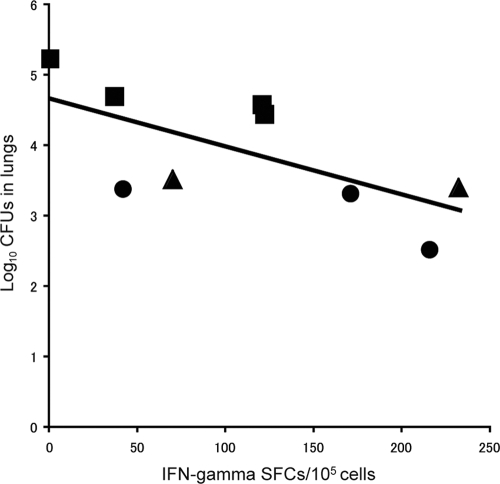
To determine the impact of BCG vaccination on bacterial growth in young, middle-aged, and old guinea pigs, bacterial replication in the lungs (Fig. 4 A), tracheal lymph nodes (Fig. 4B), and spleen (Fig. 4C) was examined for each group. In all cases, those animals vaccinated with BCG showed less bacterial growth in the lungs and tracheal lymph nodes than unvaccinated animals. In the spleen, no bacterial replication was detected except in groups 2 and 5. In group 2, which received BCG vaccination 18 months before the M. tuberculosis challenge, the effect of BCG may have been attenuated. Group 1, which was revaccinated with BCG before the challenge, showed significantly less bacterial growth in the lungs than the unvaccinated guinea pigs (P, <0.05). In the tracheal lymph nodes, bacterial growth was also reduced, but the difference was not significant. We also found a significant negative correlation between the number of IFN-γ SFCs and the residual number of bacteria in the lungs (expressed in log10 CFU) 5 weeks after M. tuberculosis challenge (r, −0.6696; P, 0.04852) in groups 1, 4, and 5 (Fig. 5). This finding suggests that PPD-specific T-cell responses induced by BCG are crucial for the host defense against M. tuberculosis infection. Thus, BCG appears to have a protective effect in guinea pigs at all ages.
FIG. 4.
Effects of BCG vaccination on bacterial growth in young, middle-aged, and old guinea pigs challenged with M. tuberculosis H37Rv. To determine the impact of BCG vaccination on bacterial growth, bacterial replication in lung (A), tracheal lymph node (B), and spleen (C) specimens from each guinea pig was examined. The minimum detectable level of bacilli in the tissue homogenate was 1.52 log10 CFU. Error bars represent standard deviations. Asterisks indicate that the mean numbers of M. tuberculosis CFU in an organ were significantly different. *, P < 0.05, and **, P < 0.01, as determined by analysis of variance (ANOVA) followed by a posthoc Tukey-Kramer test.
FIG. 5.
Correlation between IFN-γ production and bacterial growth in the lungs. There was a statistically significant negative correlation between the number of IFN-γ SFCs detected 5 weeks after M. tuberculosis challenge and bacterial growth in the lungs (r, −0.6696; P, 0.04852 as determined by Pearson's correlation coefficient test). Circles, group 4 (young, vaccinated); squares, group 5 (young, unvaccinated); triangles: group 1 (old, revaccinated).
Histopathology.
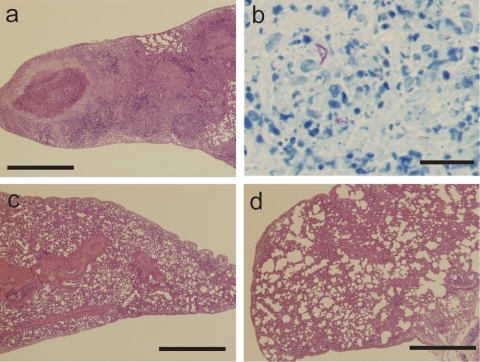
Figure 6 shows histopathological images of the lungs of young unvaccinated guinea pigs (Fig. 6a and b) and BCG-vaccinated guinea pigs (Fig. 6c and d) 5 weeks after M. tuberculosis challenge. Figure 6c shows a lung from a young BCG-vaccinated guinea pig (group 4), and Fig. 6d shows a lung from an old BCG-revaccinated guinea pig (group 1). In the lungs from unvaccinated guinea pigs, large granuloma nodules with central necrosis were predominant and consisted of epithelioid cells. Acid-fast bacilli were detected in the granulomas by Ziehl-Neelsen staining (Fig. 6b). Although granuloma nodules were also observed in the lungs of vaccinated guinea pigs (Fig. 6c and d), vaccination with BCG reduced granuloma nodule formation in the lungs of both young and old guinea pigs, and no acid-fast bacilli were detected in the granulomas by Ziehl-Neelsen staining (data not shown).
FIG. 6.
Histopathology of lungs from guinea pigs infected with M. tuberculosis H37Rv. Shown are histopathological observations in the lungs of young unvaccinated (a and b), young BCG-vaccinated (c), and old BCG-revaccinated (d) guinea pigs 5 weeks after M. tuberculosis challenge. Bars, 1 mm (a, c, and d) and 50 μm (b).
DISCUSSION
BCG is the only TB vaccine currently available, and it has been used since 1921. It is inexpensive and safe, with few complications reported in infants. BCG provides efficient protection against severe and disseminated TB, such as tuberculosis meningitis and miliary TB, in children (33, 34, 40). However, its efficacy at preventing pulmonary TB in adults is controversial. Aronson et al. reported that BCG vaccination had long-term efficacy for American Indians and Alaskan natives (3), suggesting that a single dose offered protection for 50 to 60 years. The long-term efficacy estimates from clinical trials, observational case-control studies, and contact studies range from 0 to 80% (7), although the efficacy for the elderly is unknown. In the present study, we demonstrated that revaccination of elderly guinea pigs with BCG-Tokyo reduced bacterial replication in the lungs, alveolar lymph nodes, and spleen. In addition, in 60-month-old guinea pigs, PPD-specific IFN-γ responses were observed after the BCG-Tokyo booster vaccination. These findings suggest that BCG-Tokyo has a protective effect at all ages.
However, the efficacy of BCG revaccination is a matter of international debate (5). Several studies have shown that BCG revaccination had no protective efficacy against TB (19, 28, 32). Fjällbrant et al. reported that both primary vaccination and revaccination of tuberculin skin test-negative young adults caused a significant increase in the T-helper type 1 (Th1) immune response (12), a result consistent with the present findings in the old guinea pig model. This result suggests that BCG revaccination has a protective effect against TB. However, other factors that determine the efficacy of BCG revaccination, including age, duration of vaccination, and the influence of environmental mycobacteria, must be considered.
Cell-mediated immune responses play an essential role in the control of M. tuberculosis infection and TB. In particular, CD4+ and CD8+ T-cell subsets are considered to play important roles in the production of cytokines such as IFN-γ and tumor necrosis factor alpha (TNF-α). These cytokines are involved in inflammatory processes, including macrophage activation, control of M. tuberculosis replication, and granuloma formation (1, 10, 13). Using guinea pig models, Jeevan et al. suggested that BCG vaccination induces upregulation of IFN-γ and TNF-α after M. tuberculosis challenge (16). In the present study, we investigated IFN-γ responses by using an ELISPOT assay. To the best of our knowledge, this is the first study that used a guinea pig model together with an antigen-specific ELISPOT assay to show that BCG induces PPD-stimulated IFN-γ responses. The secretion of PPD-specific IFN-γ was observed in both young and old BCG-vaccinated guinea pigs. The number of PPD-specific IFN-γ-secreting cells was greater in 60-month-old vaccinated guinea pigs (group 1) than in young vaccinated guinea pigs (group 4). Because this was the second BCG vaccination for group 1, a booster effect may have occurred. Bacterial growth in the lungs, lymph nodes, and spleen was higher in unvaccinated guinea pigs (group 5) than in the vaccinated groups. The number of PPD-specific IFN-γ-producing PBMCs in the unvaccinated guinea pigs was significantly lower than that in the vaccinated guinea pigs. The results of our M. tuberculosis aerosol infection experiment suggest that the number of PPD-specific IFN-γ-producing PBMCs correlates with the level of protection against M. tuberculosis. While TNF-α is another important cytokine that protects against M. tuberculosis, BCG vaccination appears to modulate the potentially harmful effects of TNF-α and to reduce M. tuberculosis replication (26, 42). Recent studies have shown that general immune responses are important for resistance to M. tuberculosis. Interleukin-12 (IL-12) is required for dendritic cell migration (22), maintenance of pulmonary Th1 cells (11), and macrophage activation and subsequent production of IFN-γ. IL-27 has both proinflammatory and anti-inflammatory properties. IL-12 and/or M. tuberculosis-induced IL-27 gene expression in human macrophages may regulate macrophage function during M. tuberculosis infection (31). In addition, the importance of Th17 responses, including IL-17 and IL-23, in the pathophysiology of M. tuberculosis infection has been reported recently (4, 20, 21). M. tuberculosis-specific Th1 (IL-12 and IFN-γ) and Th17 responses play roles in the increased expression of cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed death-1 (PD-1), while IL-23 induces IFN-γ and supports the IL-17 response in the lungs. McMurray and colleagues, using a laser capture microdissection (LCM) technique, reported cytokine mRNA responses in situ in the pulmonary granulomas of nonvaccinated and BCG-vaccinated guinea pigs (23, 24). TNF-α mRNA was dominant in primary lesions microdissected from nonvaccinated guinea pigs at both 3 and 6 weeks postinfection, while the cytokine profiles of granulomas from BCG-vaccinated guinea pigs shifted from type 1 cytokine mRNA (IFN-γ and IL-12p40) at 3 weeks to a predominantly anti-inflammatory profile dominated by transforming growth factor-β (TGF-β) at 6 weeks (23, 24). These results suggest that BCG vaccination modulates cytokine responses in the lungs to promote antimycobacterial functions while controlling the potentially damaging inflammatory response.
A DTH skin test for PPD has been employed in the diagnosis of TB. While the test is highly sensitive for PPD, its specificity in the diagnosis of TB infection is controversial, because after BCG vaccination, a DTH response is detected. In the present study, DTH responses to PPD were detected in all of the guinea pigs vaccinated with BCG, and no significant difference was observed among the age groups. However, IFN-γ production by PBMCs was significantly different between the groups, and the number of PPD-specific IFN-γ-producing PBMCs correlated with the degree of protection against M. tuberculosis. These results suggest that ELISPOT assays that detect TB-specific immune responses may be the most accurate means of monitoring immunity against TB.
In Japan, individuals 65 years old or older represented 22.1% of the population in 2008, and this age group is expected to grow to one-third of the population by 2035 (8). This trend is seen in other countries as well. Currently, in Japan, more than 50% of the new cases of TB occur in patients ≥60 years old (29). The increasing susceptibility of the elderly to M. tuberculosis is generally thought to be associated with immune senescence, the most significant change being the loss or delayed production of antigen-specific CD4+ T cells (14). In mice, an inadequate antigen-specific CD4+ T-cell response is thought to contribute to increased susceptibility to M. tuberculosis infection (27). However, the mouse model has revealed that old mice express early resistance to pulmonary tuberculosis infection (9, 39). CD8+ T cells contribute to TB resistance via IL-12p70-dependent production of IFN-γ (35-38). However, this innate immune response is antigen independent, and the early resistance cannot be sustained. The bacterial load in the lungs of old mice increases about 90 days after infection (9), and the lungs of old mice are eventually more susceptible to bacterial growth (39). In the present study, antigen-specific IFN-γ production was observed after BCG revaccination of 60-month-old guinea pigs. In humans, the elderly have more preexisting diseases, such as diabetes mellitus (DM) and hypertension, some of which may be associated with an increased risk of TB (17). Clearly, further evaluation of BCG in the elderly is necessary.
In conclusion, we found that vaccination of elderly guinea pigs with BCG-Tokyo reduces bacterial replication in the lungs, alveolar lymph nodes, and spleens of infected animals. In addition, PPD-specific IFN-γ responses were observed after the second BCG-Tokyo vaccination. These findings suggest that BCG-Tokyo has a protective effect at all ages.
Acknowledgments
We thank E. H. Jego and J. Khoh (Office of Medical Education, Nihon University School of Medicine) for revising the English of the manuscript.
Footnotes
Published ahead of print on 4 August 2010.
REFERENCES
- 1.Aktas, E., F. Ciftci, S. Bilgic, O. Sezer, E. Bozkanat, O. Deniz, U. Citici, and G. Deniz. 2009. Peripheral immune response in pulmonary tuberculosis. Scand. J. Immunol. 70:300-308. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, P. 2007. Tuberculosis vaccines—an update. Nat. Rev. Microbiol. 5:484-487. [DOI] [PubMed] [Google Scholar]
- 3.Aronson, N. E., M. Santosham, G. W. Comstock, R. S. Howard, L. H. Moulton, E. R. Rhoades, and L. H. Harrison. 2004. Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: a 60-year follow-up study. JAMA 291:2086-2091. [DOI] [PubMed] [Google Scholar]
- 4.Babu, S., S. Q. Bhat, N. P. Kumar, S. Jayantasri, S. Rukmani, P. Kumaran, P. G. Gopi, C. Kolappan, V. Kumaraswami, and T. B. Nutman. 2009. Human type 1 and 17 responses in latent tuberculosis are modulated by coincident filarial infection through cytotoxic T lymphocyte antigen-4 and programmed death-1. J. Infect. Dis. 200:288-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barreto, M. L., S. M. Pereira, and A. A. Ferreira. 2006. BCG vaccine: efficacy and indications for vaccination and revaccination. J. Pediatr. (Rio J.) 82:S45-S54. [DOI] [PubMed] [Google Scholar]
- 6.Black, G. F., R. E. Weir, S. Floyd, L. Bliss, D. K. Warndorff, A. C. Crampin, B. Ngwira, L. Sichali, B. Nazareth, J. M. Blackwell, K. Branson, S. D. Chaguluka, L. Donovan, E. Jarman, E. King, P. E. Fine, and H. M. Dockrell. 2002. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 359:1393-1401. [DOI] [PubMed] [Google Scholar]
- 7.Brewer, T. F. 2000. Preventing tuberculosis with bacillus Calmette-Guerin vaccine: a meta-analysis of the literature. Clin. Infect. Dis. 31(Suppl 3):S64-S67. [DOI] [PubMed] [Google Scholar]
- 8.Cabinet Office, Government of Japan. 2008. Annual report on the aging society. http://www8.cao.go.jp/kourei/english/annualreport/index-wh.html.
- 9.Cooper, A. M., J. E. Callahan, J. P. Griffin, A. D. Roberts, and I. M. Orme. 1995. Old mice are able to control low-dose aerogenic infections with Mycobacterium tuberculosis. Infect. Immun. 63:3259-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper, A. M., and S. A. Khader. 2008. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol. Rev. 226:191-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng, C. G., D. Jankovic, M. Kullberg, A. Cheever, C. A. Scanga, S. Hieny, P. Caspar, G. S. Yap, and A. Sher. 2005. Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL-12 production. J. Immunol. 174:4185-4192. [DOI] [PubMed] [Google Scholar]
- 12.Fjällbrant, H., M. Ridell, and L. O. Larsson. 2007. Primary vaccination and revaccination of young adults with BCG: a study using immunological markers. Scand. J. Infect. Dis. 39:792-798. [DOI] [PubMed] [Google Scholar]
- 13.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman, A., J. Turner, and B. Szomolay. 2008. A model on the influence of age on immunity to infection with Mycobacterium tuberculosis. Exp. Gerontol. 43:275-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta, U. D., and V. M. Katoch. 2005. Animal models of tuberculosis. Tuberculosis (Edinb.) 85:277-293. [DOI] [PubMed] [Google Scholar]
- 16.Jeevan, A., T. Yoshimura, K. E. Lee, and D. N. McMurray. 2003. Differential expression of gamma interferon mRNA induced by attenuated and virulent Mycobacterium tuberculosis in guinea pig cells after Mycobacterium bovis BCG vaccination. Infect. Immun. 71:354-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon, C. Y., and M. B. Murray. 2008. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 5:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanekiyo, M., K. Matsuo, M. Hamatake, T. Hamano, T. Ohsu, S. Matsumoto, T. Yamada, S. Yamazaki, A. Hasegawa, N. Yamamoto, and M. Honda. 2005. Mycobacterial codon optimization enhances antigen expression and virus-specific immune responses in recombinant Mycobacterium bovis bacille Calmette-Guérin expressing human immunodeficiency virus type 1 Gag. J. Virol. 79:8716-8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karonga Prevention Trial Group. 1996. Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet 348:17-24. [PubMed] [Google Scholar]
- 20.Khader, S. A., G. K. Bell, J. E. Pearl, J. J. Fountain, J. Rangel-Moreno, G. E. Cilley, F. Shen, S. M. Eaton, S. L. Gaffen, S. L. Swain, R. M. Locksley, L. Haynes, T. D. Randall, and A. M. Cooper. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8:369-377. [DOI] [PubMed] [Google Scholar]
- 21.Khader, S. A., and A. M. Cooper. 2008. IL-23 and IL-17 in tuberculosis. Cytokine 41:79-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khader, S. A., S. Partida-Sanchez, G. Bell, D. M. Jelley-Gibbs, S. Swain, J. E. Pearl, N. Ghilardi, F. J. Desauvage, F. E. Lund, and A. M. Cooper. 2006. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J. Exp. Med. 203:1805-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ly, L. H., M. I. Russell, and D. N. McMurray. 2008. Cytokine profiles in primary and secondary pulmonary granulomas of guinea pigs with tuberculosis. Am. J. Respir. Cell Mol. Biol. 38:455-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ly, L. H., M. I. Russell, and D. N. McMurray. 2007. Microdissection of the cytokine milieu of pulmonary granulomas from tuberculous guinea pigs. Cell. Microbiol. 9:1127-1136. [DOI] [PubMed] [Google Scholar]
- 25.McMurray, D. N. 2001. Disease model: pulmonary tuberculosis. Trends Mol. Med. 7:135-137. [DOI] [PubMed] [Google Scholar]
- 26.McMurray, D. N., S. S. Allen, A. Jeevan, T. Lasco, H. Cho, T. Skwor, T. Yamamoto, C. McFarland, and T. Yoshimura. 2005. Vaccine-induced cytokine responses in a guinea pig model of pulmonary tuberculosis. Tuberculosis (Edinb.) 85:295-301. [DOI] [PubMed] [Google Scholar]
- 27.Orme, I. M., J. P. Griffin, A. D. Roberts, and D. N. Ernst. 1993. Evidence for a defective accumulation of protective T cells in old mice infected with Mycobacterium tuberculosis. Cell. Immunol. 147:222-229. [DOI] [PubMed] [Google Scholar]
- 28.Rahman, M., M. Sekimoto, K. Hira, H. Koyama, Y. Imanaka, and T. Fukui. 2002. Is bacillus Calmette-Guérin revaccination necessary for Japanese children? Prev. Med. 35:70-77. [DOI] [PubMed] [Google Scholar]
- 29.Research Institute of Tuberculosis/JATA, Tuberculosis Surveillance Center. 2009. Annual reports 2008. http://jata.or.jp/rit/ekigaku/en/index.php?annual%20report.
- 30.Ritz, N., W. A. Hanekom, R. Robins-Browne, W. J. Britton, and N. Curtis. 2008. Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol. Rev. 32:821-841. [DOI] [PubMed] [Google Scholar]
- 31.Robinson, C. M., and G. J. Nau. 2008. Interleukin-12 and interleukin-27 regulate macrophage control of Mycobacterium tuberculosis. J. Infect. Dis. 198:359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodrigues, L. C., S. M. Pereira, S. S. Cunha, B. Genser, M. Y. Ichihara, S. C. de Brito, M. A. Hijjar, I. Dourado, A. A. Cruz, C. Sant'Anna, A. L. Bierrenbach, and M. L. Barreto. 2005. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet 366:1290-1295. [DOI] [PubMed] [Google Scholar]
- 33.Soysal, A., K. A. Millington, M. Bakir, D. Dosanjh, Y. Aslan, J. J. Deeks, S. Efe, I. Staveley, K. Ewer, and A. Lalvani. 2005. Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: a prospective community-based study. Lancet 366:1443-1451. [DOI] [PubMed] [Google Scholar]
- 34.Trunz, B. B., P. Fine, and C. Dye. 2006. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367:1173-1180. [DOI] [PubMed] [Google Scholar]
- 35.Turner, J., A. A. Frank, and I. M. Orme. 2002. Old mice express a transient early resistance to pulmonary tuberculosis that is mediated by CD8 T cells. Infect. Immun. 70:4628-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vesosky, B., D. K. Flaherty, E. K. Rottinghaus, G. L. Beamer, and J. Turner. 2006. Age dependent increase in early resistance of mice to Mycobacterium tuberculosis is associated with an increase in CD8 T cells that are capable of antigen independent IFN-γ production. Exp. Gerontol. 41:1185-1194. [DOI] [PubMed] [Google Scholar]
- 37.Vesosky, B., D. K. Flaherty, and J. Turner. 2006. Th1 cytokines facilitate CD8-T-cell-mediated early resistance to infection with Mycobacterium tuberculosis in old mice. Infect. Immun. 74:3314-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vesosky, B., E. K. Rottinghaus, C. Davis, and J. Turner. 2009. CD8 T cells in old mice contribute to the innate immune response to Mycobacterium tuberculosis via interleukin-12p70-dependent and antigen-independent production of gamma interferon. Infect. Immun. 77:3355-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vesosky, B., and J. Turner. 2005. The influence of age on immunity to infection with Mycobacterium tuberculosis. Immunol. Rev. 205:229-243. [DOI] [PubMed] [Google Scholar]
- 40.Walker, V., G. Selby, and I. Wacogne. 2006. Does neonatal BCG vaccination protect against tuberculous meningitis? Arch. Dis. Child. 91:789-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto, S., and T. Yamamoto. 2007. Historical review of BCG vaccine in Japan. Jpn. J. Infect. Dis. 60:331-336. [PubMed] [Google Scholar]
- 42.Yamamoto, T., T. M. Lasco, K. Uchida, Y. Goto, A. Jeevan, C. McFarland, L. Ly, S. Yamamoto, and D. N. McMurray. 2007. Mycobacterium bovis BCG vaccination modulates TNF-α production after pulmonary challenge with virulent Mycobacterium tuberculosis in guinea pigs. Tuberculosis (Edinb.) 87:155-165. [DOI] [PubMed] [Google Scholar]