Abstract
We determined the seroprevalence of protective antibodies against Hib in Mexican children under the age of five using a standardized enzyme-linked immunosorbent assay. Hib antibodies (≥0.15 μg/ml) were present in 95.34% (±1.14% [seroprevalence ± standard error]) of samples. Fewer children aged 30 to 47 months had protective Hib antibody levels (91.45% ± 2.60%) than children from 12 to 29 and 48 to 59 months (97.3% ± 1.34% and 97.44% ± 1.80%, respectively).
Infection caused by Haemophilus influenzae type b (Hib) remains one of the leading causes of invasive bacterial infection in children less than 5 years of age in countries where the Hib vaccine is not used (28). Due to the availability of Hib conjugate vaccines (Hib-CV), universal routine infant Hib immunization has proven to reduce Hib invasive disease (1, 10, 16, 18, 27). This protection is afforded through direct vaccine protection and indirectly through herd protection produced by decreased nasopharyngeal carriage of Hib in the community (10, 11, 15, 26).
In Latin America, Uruguay became the first country, in 1994, to introduce Hib-CV in their routine immunization program, followed by Chile in 1996 (6). By 1998, with the support of the Pan-American Health Organization (PAHO) revolving fund for joint purchases of vaccine, more than 15 other countries in Latin America and the Caribbean integrated the Hib vaccine. In 1999, Mexico was the first country to introduce Hib vaccination using the pentavalent combination (DTP-HB/Hib) vaccine in a three-dose schedule alone (2, 4, and 6 months of age) (5, 9, 22).
In 2006, the World Health Organization (WHO) put forward a position paper demonstrating that the administration of a Hib booster dose during the child's second year of life may provide additional protection (28). PAHO is currently assisting four countries to determine the usefulness of a booster dose in order to adapt routine immunization programs in Latin America and the Caribbean (6).
Given the increasing reemergence of invasive disease due to Hib in some settings, we were interested in assessing the persistence of protective titers against Hib in sera from children younger than 5 years of age born after the introduction of Hib-CV into the universal immunization schedule in Mexico in 1999.
Thus, we employed sera obtained from the nationally representative cross-sectional Mexican Health and Nutrition Survey 2006 (17). To assess the seroprevalence of protective antibody titers among children in the sample, a single blood sample was drawn from participants, and the serum was frozen at −150°C until analysis. From a total of 2,473 available sera from children between 12 and 60 months of age, we selected 343 samples (95% confidence interval [CI], ±5%), considering a minimum of 73 sera for each age stratum (12 to 23, 24 to 35, 36 to 47, and 48 to 59 months). The first stratum was overrepresented in order to evaluate children 12 to 17 months and 18 to 23 months of age separately. The complete-3-dose-schedule vaccine coverage was 92%. Hib anti-polyribosylribitol phosphate-specific antibody (anti-PRP antibody) IgG classes were quantified using a standardized enzyme-linked immunosorbent assay (ELISA) at the Immunology Laboratory, Hospital Dr. Hernán Henríquez Aravena, in Temuco, Chile (7). The sera were titrated against an international Hib reference serum with a known antibody concentration (lot 1983; Center for Biologics and Evaluation Research, U.S. FDA). The specific HbOHA antigen for ELISA was kindly donated by M. Nahm from the WHO laboratory at the University of Alabama, Birmingham.
The outcome was the Hib antibody titers at the various serum-sampling time points and whether these values were at least 0.15 μg/ml (the putative protective level) or more than 1.00 μg/ml, which is considered predictive of longer-term protection (11). The Hib antibody concentrations were stratified into four categories: <0.15 μg/ml, 0.15 to 1.0 μg/ml, 1.01 to 5.0 μg/ml, and >5.01 μg/ml. The groups of children were stratified according to age in months. Eight groups were constructed for the analysis (Table 1). The analysis was carried out by estimating the seroprevalence and standard error at 95% (±SE). Age groups were compared using the chi-squared test (95% confidence interval and one tail). Statistical analyses were executed using SPSS 15.0. The study was approved by the Institutional Review Board of the National Institute of Public Health, Cuernavaca, Mexico.
TABLE 1.
Hib anti-polyribosylribitol phosphate-specific antibody levels among 343 children stratified by age group, using sera from the Mexican Nutritional Survey conducted in 2006
| Age (mo) | No. in group | No. (%) with antibody level (μg/ml) of: |
|||
|---|---|---|---|---|---|
| <0.15 | 0.15-1.0 | 1.01-5.0 | >5.0 | ||
| 12-17 | 46 | 1 (2.2) | 22 (47.8) | 14 (30.4) | 9 (19.6) |
| 18-23 | 62 | 2 (3.2) | 35 (56.5) | 14 (22.6) | 11 (17.7) |
| 24-29 | 40 | 1 (2.5) | 23 (57.5) | 12 (30.0) | 4 (10.0) |
| 30-35 | 37 | 3 (8.1) | 22 (59.5) | 8 (21.6) | 4 (10.8) |
| 36-41 | 32 | 2 (8.3) | 21 (65.6) | 6 (18.8) | 3 (9.4) |
| 42-47 | 48 | 5 (10.4) | 23 (47.9) | 15 (31.3) | 5 (10.4) |
| 48-53 | 48 | 1 (2.1) | 31 (64.6) | 11 (22.9) | 5 (10.4) |
| 54-59 | 30 | 1 (3.3) | 14 (46.7) | 12 (40.0) | 3 (10.0) |
| Total | 343 | 16 (4.7) | 191 (55.7) | 92 (26.8) | 44 (12.8) |
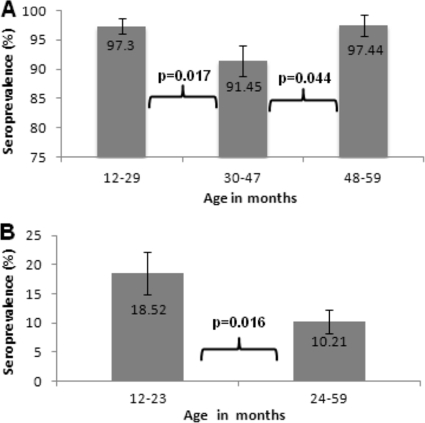
Among the children studied, the overall Hib seroprevalence of protective antibody titers (≥0.15 μg/ml) was 95.34% (±1.14%), with 16 children (4.66% ± 1.14%) being seronegative for Hib. Table 1 depicts the Hib antibody titers stratified by all age groups. The groups aged 30 to 35, 36 to 41, and 42 to 47 months had the largest proportions of children seronegative to Hib (8.1, 8.3, and 10.4%, respectively). When all children in the study were grouped into three different age stratums, 12 to 29, 30 to 47 and 48 to 59 months, the group aged 30 to 47 months had a statistically significantly smaller proportion with Hib-protective antibody titers (91.45% ± 2.60%) compared to the groups aged 12 to 29 and 48 to 59 months (97.3% ± 1.34% and 97.44% ± 1.80%, respectively) (Fig. 1 A).
FIG. 1.
(A) Hib anti-polyribosylribitol phosphate-specific antibody levels (≥0.15 μg/ml) in children stratified into three different age groups. (B) High Hib antibody levels (≥5.01 μg/ml) among 343 children in two age groups, using sera from the Mexican Health and Nutrition Survey conducted in 2006. Error bars show standard errors.
The highest Hib antibody concentrations (>5.0 μg/ml) were found among 44 children (12.8% ± 1.81%) from the entire population evaluated. The groups that were 12 to 17 months of age and 18 to 23 months of age demonstrated proportions of 19.6% and 17.7%, respectively, that had the highest antibody titers (Table 1). When we analyzed the entire group of children by dividing them into two groups, aged 12 to 23 months and 24 to 59 months, we found that 18.52% of the 12- to 23-month-old group had the highest antibody titers (>5.0 μg/ml; P = 0.0163), statistically significant compared to 10.21% in the 24- to 59-month-old group (Fig. 1B).
Hib-CV, when incorporated into routine infant immunization schedules, has been demonstrated to have a major impact in different settings in decreasing the rates of invasive disease (1, 10, 16, 18, 27). A booster dose may offer the opportunity to have effector memory ready to offer protection for infectious diseases with short incubation periods, such as that caused by Hib, and where central memory responses require at least 7 days to be detected (2).
In Mexico, the decision to use the combination pentavalent vaccine, beginning in 1999, was reached after documenting that high anti-PRP antibody concentrations were reached 1 month after vaccination, with 100% of subjects considered to be seroprotected in the combined vaccination group (antibody concentrations of ≥0.15 μg/ml), versus 99.4% in the separate-injection vaccination group (9, 26). The use of other combination formulations of the Hib vaccine, including the pentavalent formulation with acellular pertussis (aP) and inactivated polio vaccine (IPV), known in Mexico as acellular pentavalent (DTaP-IPV/Hib), has been demonstrated to be effective in other settings as well (3, 4, 12-14).
However, this study showed that, despite the high reported Hib vaccination coverage in Mexico, there is a significant reduction of vaccine-induced seroprotection against Hib in the group aged 24 to 59 months, particularly among those children 30 to 47 months of age. This reduction in seroprotective titers suggests the potential need for providing one booster dose to prevent the threat of reemergence of Hib in children from 2 to 5 years of age, as has occurred elsewhere (5, 6, 8, 19, 23-25). The risk of Hib invasive disease occurs mostly during the first 2 years of life, but the risk remains for those between the ages of 24 and 59 months (9). Our findings support reports demonstrating decreased vaccine effectiveness associated with waning protective titers against Hib (20). The increasing use of Hib-CV and the removal of opportunities for natural boosting mean that maintaining long-lived immunity to Hib remains a significant challenge. These were some of the reasons behind the 2005 recommendation from the National Vaccination Council in Mexico to move from a three-dose regimen to a 4th-dose Hib schedule through an acellular pentavalent vaccine (DTaP-IPV/Hib), starting its implementation in 2007 (21).
Footnotes
Published ahead of print on 18 August 2010.
REFERENCES
- 1.Adegbola, R. A., O. Secka, G. Lahai, N. Lloyd-Evans, A. Njie, S. Usen, C. Oluwalana, S. Obaro, M. Weber, T. Corrah, K. Mulholland, K. McAdam, B. Greenwood, and P. J. Milligan. 2005. Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunisation with a Hib conjugate vaccine: a prospective study. Lancet 366:144-150. [DOI] [PubMed] [Google Scholar]
- 2.Amanna, I. J., N. E. Carlson, and M. K. Slifka. 2007. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357:1903-1915. [DOI] [PubMed] [Google Scholar]
- 3.Capeding, M. R., J. Cadorna-Carlos, M. Book-Montellano, and E. Ortiz. 2008. Immunogenicity and safety of a DTaP-IPV//PRP∼T combination vaccine given with hepatitis B vaccine: a randomized open-label trial. Bull. World Health Organ. 86:443-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlsson, R. M., B. A. Claesson, U. Selstam, E. Fagerlund, M. Granstrom, C. Blondeau, and A. Hoffenbach. 1998. Safety and immunogenicity of a combined diphtheria-tetanus-acellular pertussis-inactivated polio vaccine-Haemophilus influenzae type b vaccine administered at 2-4-6-13 or 3-5-12 months of age. Pediatr. Infect. Dis. J. 17:1026-1033. [DOI] [PubMed] [Google Scholar]
- 5.Cruces, R. P., F. A. Donoso, A. J. Camacho, and H. M. Llorente. 2006. Invasive infections caused by Haemophilus influenzae type b after the institution of the conjugated vaccine on the expanded program on immunization in Chile. Rev. Chilena Infectol. 23:50-54. [DOI] [PubMed] [Google Scholar]
- 6.Danovaro-Holliday, M. C., S. Garcia, C. de Quadros, G. Tambini, and J. K. Andrus. 2008. Progress in vaccination against Haemophilus influenzae type b in the Americas. PLoS Med. 5:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez, J., S. Balter, J. Feris, E. Gomez, Z. Garib, P. L. Castellanos, J. Sanchez, S. Romero-Steiner, and O. S. Levine. 2000. Randomized trial of the immunogenicity of fractional dose regimens of PRP-T Haemophilus influenzae type b conjugate vaccine. Am. J. Trop. Med. Hyg. 62:485-490. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald, M., M. Canny, and D. O'Flanagan. 2005. Vaccination catch-up campaign in response to recent increase in Hib infection in Ireland. Euro Surveill. 10:E050929. [DOI] [PubMed] [Google Scholar]
- 9.Franco-Paredes, C., L. Lammoglia, I. Hernandez, and J. I. Santos-Preciado. 2008. Epidemiology and outcomes of bacterial meningitis in Mexican children: 10-year experience (1993-2003). Int. J. Infect. Dis. 12:380-386. [DOI] [PubMed] [Google Scholar]
- 10.Heath, P. T., and J. McVernon. 2002. The UK Hib vaccine experience. Arch. Dis. Child. 86:396-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hviid, A., and M. Melbye. 2004. Impact of routine vaccination with a conjugate Haemophilus influenzae type b vaccine. Vaccine 22:378-382. [DOI] [PubMed] [Google Scholar]
- 12.Kalies, H., T. Verstraeten, V. Grote, N. Meyer, A. Siedler, H. J. Schmitt, T. Breuer, L. H. Moulton, and R. von Kries. 2004. Four and one-half-year follow-up of the effectiveness of diphtheria-tetanus toxoids-acellular pertussis/Haemophilus influenzae type b and diphtheria-tetanus toxoids-acellular pertussis-inactivated poliovirus/H. influenzae type b combination vaccines in Germany. Pediatr. Infect. Dis. J. 23:944-950. [DOI] [PubMed] [Google Scholar]
- 13.Lagos, R., K. Kotloff, A. Hoffenbach, O. San Martin, P. Abrego, A. M. Ureta, E. Pines, C. Blondeau, F. Bailleux, and M. M. Levine. 1998. Clinical acceptability and immunogenicity of a pentavalent parenteral combination vaccine containing diphtheria, tetanus, acellular pertussis, inactivated poliomyelitis and Haemophilus influenzae type b conjugate antigens in two-, four- and six-month-old Chilean infants. Pediatr. Infect. Dis. J. 17:294-304. [DOI] [PubMed] [Google Scholar]
- 14.Mallet, E., P. Fabre, E. Pines, H. Salomon, T. Staub, F. Schodel, P. Mendelman, L. Hessel, G. Chryssomalis, E. Vidor, and A. Hoffenbach. 2000. Immunogenicity and safety of a new liquid hexavalent combined vaccine compared with separate administration of reference licensed vaccines in infants. Pediatr. Infect. Dis. J. 19:1119-1127. [DOI] [PubMed] [Google Scholar]
- 15.Murphy, T. V., P. Pastor, F. Medley, M. T. Osterholm, and D. M. Granoff. 1993. Decreased Haemophilus colonization in children vaccinated with Haemophilus influenzae type b conjugate vaccine. J. Pediatr. 122:517-523. [DOI] [PubMed] [Google Scholar]
- 16.Murphy, T. V., K. E. White, P. Pastor, L. Gabriel, F. Medley, D. M. Granoff, and M. T. Osterholm. 1993. Declining incidence of Haemophilus influenzae type b disease since introduction of vaccination. JAMA 269:246-248. [PubMed] [Google Scholar]
- 17.Olaiz-Fernandez, G., J. Rivera-Domarco, T. Shamah-Levy, R. Rojas, S. Villalpando-Hernandez, M. Hernández-Avila, and J. Sepulveda-Amor. 2006. Encuesta nacional de salud y nutrición 2006. Instituto Nacional de Salud Pública Press, Cuernavaca, Mexico.
- 18.Pan American Health Organization. 1996. Impact of Uruguay's introduction of the Haemophilus influenzae type b (Hib) vaccine. EPI Newsl. 18:6. [PubMed] [Google Scholar]
- 19.Peltola, H., E. Salo, and H. Saxen. 2005. Incidence of Haemophilus influenzae type b meningitis during 18 years of vaccine use: observational study using routine hospital data. BMJ 330:18-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramsay, M. E., J. McVernon, N. J. Andrews, P. T. Heath, and M. P. Slack. 2003. Estimating Haemophilus influenzae type b vaccine effectiveness in England and Wales by use of the screening method. J. Infect. Dis. 188:481-485. [DOI] [PubMed] [Google Scholar]
- 21.Rentería, A. 2007. La cartilla nacional de vacunación de México para 2007. Arch. Invest. Pediatr. Mex. 10:3-4. [Google Scholar]
- 22.Romanin, V., L. Chiavetta, M. C. Salvay, M. J. Chiolo, M. Regueira, A. Barrios, and G. Califano. 2007. Vacuna anti-Haemophilus influenzae de tipo b (Hib) en el calendario nacional de Argentina: portación nasofaríngea de Hib tras 8 años de su introducción. Arch. Argent Pediatr. 105:498-505. [Google Scholar]
- 23.Schouls, L. M., A. van der Ende, I. van de Pol, C. Schot, L. Spanjaard, P. Vauterin, D. Wilderbeek, and S. Witteveen. 2005. Increase in genetic diversity of Haemophilus influenzae serotype b (Hib) strains after introduction of Hib vaccination in The Netherlands. J. Clin. Microbiol. 43:2741-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slack, M. H., D. Schapira, R. J. Thwaites, M. Burrage, J. Southern, D. Goldblatt, and E. Miller. 2004. Responses to a fourth dose of Haemophilus influenzae type B conjugate vaccine in early life. Arch. Dis. Child. Fetal Neonatal Ed. 89:F269-F271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spanjaard, L., S. van den Hof, H. E. de Melker, P. E. Vermeer-de Bondt, A. van der Ende, and G. T. Rijkers. 2005. Increase in the number of invasive Haemophilus influenzae type b infections. Ned. Tijdschr. Geneeskd. 149:2738-2742. (In Dutch.) [PubMed] [Google Scholar]
- 26.Takala, A. K., J. Eskola, M. Leinonen, H. Kayhty, A. Nissinen, E. Pekkanen, and P. H. Makela. 1991. Reduction of oropharyngeal carriage of Haemophilus influenzae type b (Hib) in children immunized with an Hib conjugate vaccine. J. Infect. Dis. 164:982-986. [DOI] [PubMed] [Google Scholar]
- 27.Wenger, J. D., J. DiFabio, J. M. Landaverde, O. S. Levine, and T. Gaafar. 1999. Introduction of Hib conjugate vaccines in the non-industrialized world: experience in four “newly adopting” countries. Vaccine 18:736-742. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. 2006. WHO position paper on Haemophilus influenzae type b conjugate vaccines, p. 445-452. World Health Organization, Geneva, Switzerland.