Abstract
The use of natural compounds from plants can provide an alternative approach against food-borne pathogens. The mechanisms of action of most plant extracts with antimicrobial activity have been poorly studied. In this work, changes in membrane integrity, membrane potential, internal pH (pHin), and ATP synthesis were measured in Vibrio cholerae cells after exposure to extracts of edible and medicinal plants. A preliminary screen of methanolic, ethanolic, and aqueous extracts of medicinal and edible plants was performed. Minimal bactericidal concentrations (MBCs) were measured for extracts showing high antimicrobial activity. Our results indicate that methanolic extracts of basil (Ocimum basilicum L.), nopal cactus (Opuntia ficus-indica var. Villanueva L.), sweet acacia (Acacia farnesiana L.), and white sagebrush (Artemisia ludoviciana Nutt.) are the most active against V. cholera, with MBCs ranging from 0.5 to 3.0 mg/ml. Using four fluorogenic techniques, we studied the membrane integrity of V. cholerae cells after exposure to these four extracts. Extracts from these plants were able to disrupt the cell membranes of V. cholerae cells, causing increased membrane permeability, a clear decrease in cytoplasmic pH, cell membrane hyperpolarization, and a decrease in cellular ATP concentration in all strains tested. These four plant extracts could be studied as future alternatives to control V. cholerae contamination in foods and the diseases associated with this microorganism.
The search for natural antimicrobials to use in foods is encouraged by the high prevalence of food-borne diseases and the current popular preference of consuming only natural foods (31). Furthermore, the resistance of microorganisms to common and novel antibiotics is on the rise (38).
Some plant products have been historically used as natural antimicrobials to extend the shelf life of foods and as therapeutics used in folk medicine to treat diseases caused by pathogens (1). Currently, plant products are considered to be important alternative sources of new antimicrobial drugs against antibiotic-resistant microorganisms (38) and as preservatives of food (33). According to this trend, the use of natural compounds derived from plants for the prevention of pathogenic and spoilage microorganisms in foods has been extensively reported (31).
Because a large number of plant species still need to be analyzed for their antimicrobial activity against diverse bacteria, it is critical to develop simple systems for rapid antimicrobial screening. Toward this end, several methods have been described, including those based on the use of membrane-impermeable fluorescent probes (31). In these assays, probes may be found to passively diffuse through the cell wall of bacteria, acting as an indicator of a loss of membrane integrity, which frequently is taken as an indicator of cell viability (22).
Bacteria use two forms of metabolic energy: energy-rich phosphate bonds, such as ATP, and electrochemical energy provided by ion gradients (6). Measurements of these forms of energy, such as membrane potential, cytoplasmic pH, and ATP synthesis, can be used as indicators of loss of cell viability. Changes in membrane potential are an early indication of injury in bacteria, and the ability of a cell to maintain a stable membrane potential can be determined by probe uptake or exclusion (26). On the other hand, ATP plays a fundamental role in cellular energetics, metabolic regulation, and cellular signaling (4). An increase in cytosolic ATP concentration is a key event in the membrane depolarization of ATP-dependent K+ channels (12). A common method used to measure this compound employs bioluminescence to measure cellular ATP levels (45).
Membrane integrity is fundamental for the control of cytoplasmic pH in bacteria, which is essential for many physiological activities (28). The capacity of cells to maintain a pH gradient (higher pH inside than outside the cell) may also supply information about cellular viability (9). In principle, these bacterial vital signs (intracellular pH, ATP production, and membrane potential and integrity) could form the basis of a rapid search for novel antimicrobial agents.
Scientific validation of the antimicrobial properties of plants has been extensively reported (10). However, little information is available about the mechanisms of action of antimicrobial compounds in bacteria. Several proposed mechanisms include membrane damage, changes in intracellular pH, membrane potential, and ATP synthesis (22, 42). In this work, we demonstrate that damage to membrane integrity, as well as changes in membrane potential, internal pH (pHin), and synthesis of ATP, occurs in Vibrio cholerae after exposure to particular extracts of plants.
MATERIALS AND METHODS
Preparation of plant extracts.
Plant specimens (n = 27) were obtained from local supermarkets or collected in gardens of the Universidad Autónoma de Nuevo León. Voucher samples were deposited at the herbarium of the Botanical Department of Biological Science Faculty, Universidad Autónoma de Nuevo León, for identification (Table 1).
TABLE 1.
Plants used in this work and growth inhibition of Vibrio cholerae strains by aqueous, ethanolic, and methanolic extracts
| Common name (part used) | Scientific name | Inhibition zone (cm)a for indicated solvent and V. cholerae strain |
|||||
|---|---|---|---|---|---|---|---|
| Aqueous |
Ethanol |
Methanol |
|||||
| 1837 | 569-B | 1837 | 569-B | 1837 | 569-B | ||
| Basil (whole plant) | Ocimum basilicum (L.) | NI | NI | 1.3 ± 0.1 | 1.4 ± 0.2 | 2.2 ± 0.2 | 2.1 ± 0.1 |
| Artichoke (whole plant) | Cynara scolymus (L.) | NI | NI | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.1 | 1.6 ± 0.1 |
| Peanut (fruit) | Arachis hypogaea (L.) | NI | NI | NI | NI | NI | NI |
| Pumpkin (fruit) | Cucurbita pepo (L.) | NI | NI | NI | NI | NI | NI |
| Sweet potato (fruit) | Ipomoea batatas (L.) | NI | NI | NI | NI | NI | NI |
| Naseberry (fruit) | Manilkara zapota (L.) | NI | NI | NI | NI | 1.8 ± 0.1 | 2 ± 0.1 |
| Nopal cactus (cladode) | Opuntia ficus-indica (L.) | NI | NI | 1.3 ± 0.1 | 1.4 ± 0.1 | 2.4 ± 0.2 | 2.6 ± 0.1 |
| Japanese plum (fruit) | Prunus salicina (Lindl.) | NI | NI | NI | NI | 1.3 ± 0.2 | 1.3 ± 0.2 |
| Coconut (fruit) | Cocos nucifera (L.) | NI | NI | NI | NI | NI | NI |
| Summer rape (root) | Brassica napus (L.) | NI | NI | NI | NI | NI | NI |
| Cranberry (fruit) | Vaccinium macrocarpon (Ait.) | NI | NI | NI | NI | NI | NI |
| Peach (fruit) | Prunus persica (L.) | NI | NI | NI | NI | 1.1 ± 0.1 | 1.3 ± 0.2 |
| Asparagus (fruit) | Asparagus officinalis (L.) | NI | NI | NI | NI | 1.2 ± 0.1 | 1.5 ± 0.2 |
| White sagebrush (whole plant) | Artemisia ludoviciana (Nutt.) | NI | NI | 1.2 ± 0.2 | 1.2 ± 0.3 | 2.4 ± 0.1 | 2.2 ± 0.2 |
| Raspberry (fruit) | Rubus idaeus (L.) | NI | NI | NI | NI | 1.7 ± 0.2 | 1.9 ± 0.1 |
| Spearmint (whole plant) | Mentha spicata (L.) | NI | NI | NI | NI | NI | NI |
| Irish lace (fruit) | Tagetes filifolia (Lag.) | NI | NI | NI | NI | NI | NI |
| Sweet acacia (bark) | Acacia farneciana (L.) Willd. | NI | NI | 1.4 ± 0.1 | 1.5 ± 0.1 | 2.7 ± 0.3 | 2.4 ± 0.2 |
| Kiwi fruit (fruit) | Actinidia chinensis (Planch.) | NI | NI | NI | NI | 1.6 ± 0.1 | 2.5 ± 0.2 |
| Mango (fruit) | Mangifera indica (L.) | 0.9 ± 0.01 | 0.8 ± 0.01 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.5 ± 0.2 | 2 ± 0.1 |
| Cantaloupe (fruit) | Cucumis melo (L.) | NI | NI | NI | NI | NI | NI |
| Honey mesquite (bark) | Prosopis glandulosa (Torr.) | NI | NI | 1.4 ± 0.1 | 1.2 ± 0.1 | 1.8 ± 0.1 | 1.3 ± 0.1 |
| Pineapple (fruit) | Ananas comosus (L.) Merr. | 0.8 ± 0.01 | 0.7 ± 0.01 | 0.9 ± 0.1 | 1.1 ± 0.1 | 1.6 ± 0.1 | 1.8 ± 0.2 |
| Catawba grape (fruit) | Vitis labrusca (L.) | 0.7 ± 0.01 | 0.7 ± 0.01 | NI | NI | NI | NI |
| Golden sword (flower) | Yucca filifera (Chabaud.) | NI | NI | NI | NI | NI | NI |
| Lemon grass (leaves) | Cymbopogon citratus (Stapf.) | NI | NI | NI | NI | 1.8 ± 0.2 | 1.7 ± 0.2 |
| Poblano pepper (fruit) | Capsicum annuum (L.) | NI | NI | NI | NI | 1.7 ± 0.2 | 1.5 ± 0.06 |
Values are means ± standard deviations. NI, no inhibition.
Dried and milled plant materials (100 g) were macerated with 500 ml of distilled water, ethanol (96%), or methanol (absolute) in tightly sealed vessels either at 4°C for 16 h for aqueous extraction or at room temperature overnight for alcoholic extraction. The macerate was then centrifuged at 3,000 × g for 20 min and filtered through Whatman no. 1 filter paper. Filtrates were concentrated in a rotary evaporator (R3000; Buchi) under low pressure and temperature until completely dry. The dried extracts were subsequently resuspended in 10 ml of the solvent used for primary extraction. Extracts were filter sterilized using nitrocellulose membranes (0.45-μm pore size; Millipore). All extracts were stored at 4°C in the dark until needed (usually no more than 12 weeks); during that period, the activity of the extract was evaluated monthly.
Bacterial strains and culture conditions.
V. cholerae classical O1 strain 569-B (Inaba) and O139 strain AI-1837 (El Tor) were kindly provided by Elisa Elliot from the Food and Drug Administration, Washington DC. Strains were maintained in Luria-Bertani (LB) agar (Difco) at room temperature with periodical subculturing every 2 months. Active cultures were obtained for use in assays by inoculation of a loopful of each strain into 5 ml LB broth (Difco) and incubated for 18 h at 37°C.
Preliminary antimicrobial test.
Preliminary antimicrobial screening was performed using the agar well diffusion bioassay (13). Tubes containing 5 ml LB broth were inoculated from an overnight culture of V. cholerae. After 3 h of incubation, 100 μl of each microorganism (approximately 106 CFU) was spread separately onto the surface of LB agar. Wells were made in the agar by using an inverted sterile Durham tube (6 mm in diameter), and 100 μl of each extract was deposited in the well. Plates were incubated at 37°C for 24 h. Antimicrobial activity was detected by the presence of a growth inhibition zone surrounding the well. The diameter of this zone was measured and recorded. Solvents used for each extract were employed as controls.
MBC determinations.
Of the 27 plants (81 extracts) tested, only those that showed antibacterial activity against V. cholerae in the preliminary screening were selected for further analysis, and their minimal bactericidal concentrations (MBCs) were determined using a dilution method (17). For MBC determinations, different concentrations of plant extracts were added to tubes with 2 ml LB medium plus 20 μl cell culture (2 × 106 CFU/ml). After incubation (37°C for 24 h), an aliquot (100 μl) of each sample that did not show visible growth was inoculated onto plates containing LB agar. Plates were incubated for 24 h at 37°C and then examined for microbial growth. Ethanol, methanol, water, and tetracycline (Sigma-Aldrich, Mexico City, Mexico) were used as controls. The MBC was defined as the lowest extract concentration at which no microbial growth was detected. The extracts with MBCs lower than 5 mg/ml were selected for further experiments.
Bacterial membrane integrity assay.
Bacterial cell membrane integrity was determined using the LIVE/DEAD BacLight kit (Molecular Probes, Eugene, OR). To ensure sufficient antimicrobial activity and detection of the changes in a short time, several concentrations equal to or higher than the MBC were analyzed and the membrane disruption determined. The reagents (Syto9 and propidium iodide) were prepared according to the manufacturer's instructions and mixed in equal proportions. The mixture was then applied to separate cultures (1 ml) in LB broth previously treated with plant extracts (1×, 5×, 10×, and 20× the MBC for 15 min) and incubated for an additional 15 min in the dark. Cultures containing methanol but no plant extract were used as controls. Cells were visualized under an epifluorescence microscope (Axioskop 40; Carl Zeiss) equipped with a filter block that simultaneously detected the two fluorogens in the mixture (36).
Determination of cytoplasmic pH (pHin).
For loading of cells with fluorescent probe, LB broth (5 ml) was inoculated (1%) with activated overnight cultures of Vibrio and incubated at 37°C for 3 h. Cells were then harvested by centrifugation (1,500 × g, 10 min) and washed twice with 50 mM HEPES buffer with 5 mM EDTA, pH 8. The cell pellet was resuspended in 10 ml of this buffer plus 1.0 μM fluorescent probe, carboxyfluorescein diacetate succinimidyl ester (cFDA-SE; Molecular Probes, Eugene, OR). Cells were then incubated for 10 min at 37°C, washed once in 50 mM potassium phosphate buffer with 10 mM MgCl2, pH 7.0, and resuspended in 10 ml buffer. To eliminate nonconjugated cFDA-SE, glucose (10 mM, final concentration) was added and cells were incubated for an additional 30 min at 37°C. Cells were then washed twice, resuspended in 50 mM phosphate buffer (pH 7), and placed on ice until needed (7).
pHin assay.
The pHin of V. cholerae was analyzed according to the method described by Breeuwer et al. (7), with some modifications. An aliquot of fluorescently labeled (3 μl of a 1 × 107 CFU/ml cell suspension) was dispensed in a 5-ml spectrophotometer cuvette (polystyrene; VWR, West Chester, PA). Extract (10× the MBC) was added after the cuvette was placed in the spectrofluorometer (VersaFluor; Bio-Rad, Hercules, CA). Fluorescence intensities were measured immediately and every minute for 10 min using an excitation wavelength of 490 nm and emission wavelength of 520 nm. The excitation and emission slit widths were 5 and 10 nm, respectively. During the assay, the system was maintained at room temperature (25°C). Fluorescence of the cell-free filtrate (background fluorescence) was measured after the 10-min assay. In this case, treated suspensions were filtered (0.45-μm pore size), and the fluorescence of the cell-free supernatant was measured and deducted from values for the treated suspensions.
Calibration curves were determined for cFDA-SE-loaded cells (without extract or methanol but with buffers of various pHs). Controls used included the following buffers: glycine (50 mM), citric acid (50 mM), Na2HPO4·2H2O (50 mM), and KCl (50 mM) adjusted to various pH values (4, 5, 6, 7, 8, 9, and 10). The fluorescence intensity was measured at 25°C after equilibrating the pHin and pHout by addition of valinomycin (1 μmol liter−1; Sigma-Aldrich, Mexico City, Mexico) and nigericin (1 μmol liter−1; Sigma-Aldrich, Mexico City, Mexico). In this assay, a drop in relative fluorescence occurs when the cytoplasmic pH decreases (7).
Fluorometric assay for membrane potential.
The method described by Pag et al. (29) was followed, with minor exceptions, to measure changes in membrane polarity caused by plant extracts. V. cholerae cells were grown in 3 ml of 0.5× Mueller-Hinton broth (Difco) at 37°C to an optical density at 610 nm (OD610) of 0.5 (approximately 1 × 107 CFU/ml). Then, 1 μM membrane potential-sensitive fluorescent probe bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC4 [3]; Molecular Probes, Eugene, OR] was added for 5 min, followed by the addition of extract (10× the MBC). After 5 min, fluorescence was measured at the excitation and emission wavelengths of 492 and 515 nm, respectively, using the spectrofluorometer referred to above. Background fluorescence resulting from the extracts added to the medium was determined and the results corrected.
Bioluminescence assay for ATP determination.
The level of cellular ATP is an important parameter for evaluating the available energy in a microorganism. To measure this, a method described by Yuroff et al. (45) was followed with some modifications. ATP generation was detected using the Enliten ATP detection kit (Promega, Madison, WI).
Activated cultures of Vibrio strains were dispensed into 5 ml LB broth and incubated for 3.5 h to an OD610 of 0.5 (approximate 1 × 107 CFU/ml). The cultures were treated with different plant extracts (10× the MBC) and incubated for 15 min. To extract the ATP from cell suspensions, 250 μl of ice-cold 24% (vol/vol) perchloric acid was added to 500 μl of treated suspension. The mixtures were held on ice for 20 min and centrifuged (10,000 × g for 5 min). Supernatants (500 μl) were neutralized with 125 μl of 4 M KOH, held on ice for 30 min, and centrifuged as described above. To quantify ATP supernatants, 50 μl was placed in white opaque 96-well microtiter plates (Nunc, Copenhagen, Denmark), and 100 μl of Enliten luciferase/luciferin reagent (Promega, Madison, WI) plus 100 μl of 10 mM Tris (pH 8.0) were added. After 10 min of incubation, light emission (bioluminescence) was determined using a multimode detector (Beckman Coulter DTX 880; CA). ATP values were expressed as relative units, which were defined as the amount of light emitted per unit of cell density.
Statistical analyses.
All experiments were performed in triplicate at least twice. Statistical analyses were performed using SPSS software (version 10.0; SPSS, Inc., Chicago, IL). Results were analyzed with an analysis of variance test, and the mean comparison was used for the analysis. Differences between means were considered significant at P values of ≤0.05.
RESULTS
Preliminary screening and MBC determinations.
A total of 81 extracts were prepared from the 27 plants evaluated for antimicrobial activity against V. cholera. In the preliminary assay, 16 methanolic extracts showed good activity against both strains tested and were selected for further MBC analysis (Table 1). In addition, eight ethanolic extracts were effective against V. cholerae strains, while only three aqueous extracts displayed growth-inhibitory activities (Table 1).
MBC analysis indicated that methanolic extracts of basil (Ocimum basilicum L.), nopal cactus (Opuntia ficus-indica var. Villanueva L.), sweet acacia (Acacia farnesiana L.), and white sagebrush (Artemisia ludoviciana Nutt) exhibited the highest antimicrobial activities. The MBC of nopal cactus extracts was 3 mg/ml, and for basil the MBC ranged from 2 to 3 mg/ml, whereas sweet acacia and white sagebrush exhibited the lowest MBCs (0.5 to 1.0 mg/ml) against both bacterial strains (Table 2). The MBC of the other extracts was greater than 10 mg/ml, and these extracts were not examined further.
TABLE 2.
MBCs of selected methanolic extracts against two V. cholerae strains
| Common name | Scientific name | MBC (mg/ml) for V. cholerae straina: |
|
|---|---|---|---|
| 1837 | 569-B | ||
| Basil | Ocimum basilicum | 2 ± 0.6 | 3 ± 0.5 |
| Nopal cactus | Opuntia ficus-indica | 3 ± 0.05 | 3 ± 0.1 |
| Sweet acacia | Acacia farnesiana | 0.5 ± 0.1 | 0.9 ± 0.1 |
| White sagebrush | Artemisia ludoviciana | 0.7 ± 0.2 | 1.0 ± 0.3 |
| Tetracycline | 2 × 10−4 ± 7 × 10−5 | 5 × 10−4 ± 3 × 10−5 | |
Values are means ± standard deviations.
Effects of plant extracts on membrane integrity.
The LIVE/DEAD BacLight system used to analyze membrane integrity after addition of methanolic extracts was effective at demonstrating the antimicrobial activities of the extracts. Epifluorescence microscopic observations revealed that the cells treated with extracts at 10× or 20× the MBCs were stained (100%) by propidium iodide, a characteristic of cells with membrane damage (data not shown). The MBCs of the four extracts against the two strains resulted in 1 to 14% stained cells, and 5× the MBC resulted in 8 to 41% stained cells. Non-membrane-damaged cells (stained green with Syto9 dye) appeared (<1%) in controls (with methanol in the absence of extracts).
Effects of plant extracts on pHin.
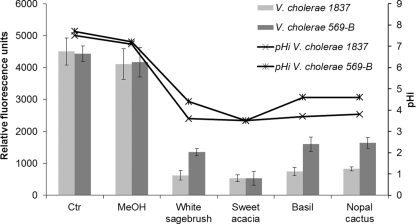
A clear change in cytoplasmic pH was observed after addition of methanolic extracts (Fig. 1). Exposure of V. cholerae strains to all extracts tested decreased the pHin significantly (P ≤ 0.05), from 7.2 to 3.9 ± 0.5 (standard deviation). Sweet acacia had the strongest effect on both V. cholerae strains; however, V. cholerae strain 1837 was more sensitive (P ≤ 0.05) to all the extracts tested except for sweet acacia.
FIG. 1.
Effects of methanolic plant extracts on the intracellular pH of two V. cholerae strains. Ctr, control; MeOH, methanol control.
Effects of selected extracts on membrane potential.
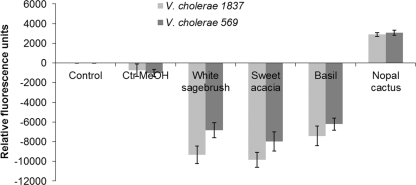
DiBAC4 (3), a fluorescent dye used for monitoring changes in the membrane potential of the V. cholerae strains, demonstrated that cells treated with methanolic extracts of basil, white sagebrush, and sweet acacia displayed cell membrane hyperpolarization, as evidenced by a decrease in fluorescence (negative values). On the other hand, nopal cactus treatment caused rapid depolarization, as evidenced by an increase in fluorescence (Fig. 2).
FIG. 2.
Effects of selected extracts on the membrane potentials of V. cholerae strains. Negative (hyperpolarization) and positive (depolarization) values produce a loss of cellular homeostasis. Ctr-MeOH, methanol control.
Effects of selected extracts on total ATP concentration.
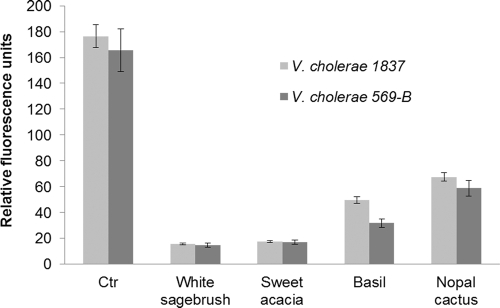
Our results indicated that all extracts provoked significant decreases (P ≤ 0.05) in cellular ATP concentrations in the two strains tested (Fig. 3). White sagebrush and sweet acacia had the strongest effects in both V. cholerae strains. Basil was less active, followed by nopal cactus.
FIG. 3.
Effects of methanolic plant extracts on ATP production by two V. cholerae strains. Ctr, control.
DISCUSSION
The antimicrobial properties of plants have been recognized for a long time; however, in many cases, the mechanisms of action are poorly understood (15). In this work, the antimicrobial properties of aqueous, ethanolic, and methanolic extracts were evaluated using the well diffusion technique (31). From 81 extracts analyzed, 4 (basil, nopal cactus, sweet acacia, and white sagebrush) possessed the highest detectable anti-Vibrio activities. In all cases, the methanolic extracts were more active than the ethanolic or aqueous extracts. Methanolic extracts from plants consistently provide more antimicrobial activity than those extracted in ethanol or other more polar substances (10, 27). The higher antibacterial activity of methanol extracts is hypothesized to be due to the polarity of the solvent and to the capability to dissolve or diffuse into the medium used in the assays (10).
Of the four most active plants extracts, basil and nopal cactus have been commonly used as edible plants, while sweet acacia and white sagebrush have been used as medicinal plants. Basil has been used principally as a culinary herb, to add flavor to soups and sauces. The leaves are generally used fresh in salads, but these can also be dried to make a refreshing tea (25). Medicinal uses have also been reported, including antibacterial, digestive, and gastricuses, for the treatment of colds and influenza disease, and for nausea and gastroenteritis (30).
Nopal cactus is a crop with multiple uses. It plays an ecological role in soil conservation, as well as in the production of edible fruits, vegetables, and other value-added products. Plant cladodes or cactus stems are used as fresh green vegetables (nopalitos and cactus) for human consumption. The fruit as well as the cactus stem are used to prepare value-added products, such as jam, squash, wine, pickles, body lotions, shampoos, and creams. Nopal cactus also has several medicinal and industrial uses (37).
Phytochemical analysis of these edible plants has identified several compounds that could be responsible for the antimicrobial activities observed in this study. Hussain et al. (19) reported that basil contains essential oils (principally linalool), oxygenated monoterpenes, and sesquiterpene hydrocarbons. On the other hand, Lee et al. (23) found flavonoids and terpenes in raw cladodes of nopal cactus.
White sagebrush is a medicinal plant used to treat stomach problems, coughs, colds, and headaches. It has also been demonstrated to give symptomatic relief of diarrhea. The tea made with this plant has been used in traditional Mexican medicine to alleviate intestinal pain (32). On the other hand, sweet acacia has many traditional uses: the flowers are used as headache remedies and for the treatment of indigestion; the fruit is used to treat dysentery and skin inflammations (40). The MBCs obtained with white sagebrush and sweet acacia were relatively low, ranging from 0.3 to 0.9 mg/ml. Garcia et al. (14) reported that ethanolic extracts of Acacia farnesiana and Acacia ludoviciana had MBCs ranging from 4 to 7 mg/ml against three strains of V. cholerae. These concentrations slightly differ, but the discrepancy could depend on factors such as plant source, time of collection, bacterial strain, and solvent used for extraction. Although tetracycline had a lower MBC (Table 2) than the extracts, the purified active compounds of the plant extracts could exhibit MBCs lower than the whole extracts.
Ruiz-Cancino et al. (32) identified sesquiterpene lactones and flavonoids in the aerial parts of white sagebrush. Sweet acacia has been shown to contain terpenes, hydrolyzable and condensed tannins, flavonoids, and alkaloids (34). These types of compounds have been reported to be antimicrobial and may be responsible the activities of these plants.
Prior investigations into the mechanism of action of natural antimicrobials have focused on their effects on cellular membranes (22, 39, 43). In this work, several fluorometric techniques were used to measure alteration of the dissipation of two components of the proton motive force, the pHin and the electrical potential (35). Direct labeling of cells with fluorescent dyes has advantages, since plate counting, a very popular method, may not account for all viable cells, particularly those that are in a viable but not cultivable stage (21).
To detect damage to cell membranes, the BacLight LIVE/DEAD viability kit was used. This system has been successfully used in various bacterial species in pure culture and in different environments and food products (3, 24, 36). This simple and rapid method showed that the four methanolic extracts tested disrupted the cell membrane of V. cholerae, causing an increased permeabilization, which was detected as an increase in nuclear propidium iodide staining.
The cFSE technique for measuring the pHin of bacteria is based on intracellular conjugation of the succinimidyl group of cFSE with the aliphatic amines of intracellular proteins and subsequent elimination of free probe by a short incubation in the presence of glucose (18). The method has been reported to be effective for Gram-positive and Gram-negative bacteria; however, some interference can occur with Gram-negative bacteria, because of the inability of cFDA-SE to cross the outer membrane of Gram-negative microorganisms. Preincubation of bacteria with EDTA has been suggested to circumvent this problem (7). In this work, all the extracts induced changes in the pHin, indicating that membrane damage had occurred. Reduction in the internal pH in other organisms, such as Staphylococcus aureus, has been observed to occur as the result of treatment with oregano essential oil, thymol, or carvacrol (22).
DiBAC4 (3) is a fluorescent membrane potential dye that has been used as an indicator of changes in membrane polarization (41). The fluorescence emitted by the dye is enhanced when the dye crosses the cell membrane as a result of membrane depolarization (less negative charge inside the cell) (44). Three of the methanolic extracts analyzed (basil, white sagebrush, and sweet acacia) caused a hyperpolarization (more negative charge inside the cell) of cellular membranes. Although a depolarization effect was expected, hyperpolarization has also been reported as an important type of membrane damage (45). Recent studies of this phenomenon have concluded that hyperpolarization occurs primarily due to a pH change (from acidic to neutral) or by increasing movement of ions, specifically K+, which diffuses out of the cell membrane through K+ channels and affects cellular homeostasis (5). Extracts of nopal cactus increased fluorescence in the treated cells due to membrane depolarization (less negative inside the cell) as a result of a decrease of the membrane potential (20).
When metabolic ATP was measured in the presence of methanolic plant extracts, the results showed that all four extracts caused a decrease in the cytoplasmic ATP concentration of V. cholerae. Although a direct effect of the extracts on increased ATPase activity cannot be ruled out, it has been demonstrated that natural products present in plant extracts affect the permeability of Listeria monocytogenes membranes, leading to a subsequent decrease in cytoplasmic ATP as a consequence of envelope damage (8). Gonzalez et al. (16) reported that diminished ATP concentrations could be caused by release of cytoplasmic ATP and unabated hydrolysis by the proton-pumping ATPase, which results in rapid depletion of the intracellular ATP pool. Two mechanisms have been proposed to explain the ATP hydrolysis: (i) a shift in the equilibrium of the ATP hydrolysis reaction as a consequence of inorganic phosphate loss through the membrane due to impaired permeability, and (ii) depletion of the intracellular ATP pool and dissipation of proton motive force components (42). The effect of antimicrobial compounds on the diminishing proton motive force is strongly correlated with the leakage of specific ions (2). Several authors have reported that natural preservatives, including essential oils, phenols, and bacteriocins, can promote the loss of cellular components, such as ions, ATP, nucleic acids, amino acids, etc. (11, 22, 42).
The cell membrane is an active structure that acts as a barrier between the cytoplasm and the extracellular medium. It is essential for maintaining optimal internal conditions for metabolism and energy transduction. The plant extracts studied here were able to disrupt the cell membrane, resulting in increased permeabilization, changes in the pHin and the membrane potential, and a decrease in the cytoplasmic ATP concentration, which together resulted in bactericidal activity. Secondary effects that may be involved and further decrease viability include the inhibition of several enzymes caused by leakage of essential ions, loss of turgor pressure, and alterations in macromolecular synthesis and other processes in the bacterial cell.
We conclude that the extracts of two edible plants (basil and nopal catcus) and two medicinal plants (white sagebrush and sweet acacia) cause damage to the membrane of V. cholerae, exerting profound physiological changes that lead to bacterial death. Additional experiments will be needed to provide information about other microbial sites or aspects affected by these extracts. Experiments are in progress to determine the specific compounds responsible for the antimicrobial activity of each extract. These four plant extracts could be studied as future alternatives to control V. cholerae contamination in foods and the diseases associated with this microorganism. However, for application in foods, appropriate methods for the nontoxic extraction of the active compounds would have to be developed. Also, the effect of active compounds on the beneficial and normal microbial flora in the human body would have to be determined, as well as the risks and benefits of potential applications in humans, including toxicity studies.
Acknowledgments
This research was supported by the Consejo Nacional de Ciencia y Tecnología de México (CONACYT) and PAICYT-UANL. Eduardo Sánchez was supported by a scholarship from CONACYT.
We thank Ronald Labbe for his helpful discussions.
Footnotes
Published ahead of print on 27 August 2010.
REFERENCES
- 1.Adiguzel, A., H. Ozer, M. Sokmen, M. Gulluce, A. Sokmen, H. Kilic, F. Sahin, and O. Baris. 2009. Antimicrobial and antioxidant activity of the essential oil and methanol extract of Nepeta cataria. Pol. J. Microbiol. 58:69-76. [PubMed] [Google Scholar]
- 2.Bakker, E., and W. E. Mangerich. 1981. Interconversion of components of the bacterial proton motive force by electrogenic potassium transport. J. Bacteriol. 147:820-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berney, M., F. Hammes, F. Bosshard, H.-U. Weilenmann, and T. Egil. 2007. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight kit in combination with flow cytometry. Appl. Environ. Microbiol. 73:3283-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biteau, B., J. Labarre, and M. B. Toledano. 2003. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature 425:980-984. [DOI] [PubMed] [Google Scholar]
- 5.Bot, C., and C. Prodan. 2009. Probing the membrane potential of living cells by dielectric spectroscopy. Eur. Biophys. J. 38:1049-1059. [DOI] [PubMed] [Google Scholar]
- 6.Breeuwer, P., and T. Abee. 2004. Assessment of the membrane potential, intracellular pH and respiration of bacteria employing fluorescence techniques. Mol. Microb. Ecol. Man. 8:1563-1580. [Google Scholar]
- 7.Breeuwer, P., J. L. Drocourt, F. M. Rombouts, and T. Abee. 1996. A novel method for continuous determination of the intracellular pH in bacteria with the internally conjugated fluorescent probe 5 (and 6)-carboxyfluorescein succinimidyl ester. Appl. Environ. Microbiol. 62:178-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caillet, S., and M. Lacroix. 2006. Effect of gamma radiation and oregano essential oil on murein and ATP concentration of Listeria monocytogenes. J. Food Prot. 69:2961-2969. [DOI] [PubMed] [Google Scholar]
- 9.Chitarra, L. G., P. Breeuwer, R. W. van den Bulk, and T. Abee. 2000. Rapid fluorescence assessment of intracellular pH as a viability indicator of Clavibacter michiganensis subsp. michiganensis. J. Appl. Microbiol. 88:809-816. [DOI] [PubMed] [Google Scholar]
- 10.Cowan, M. M. 1999. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12:564-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox, S. D., J. E. Gustafson, C. M. Mann, J. L. Markham, Y. C. Liew, R. P. Hartland, H. C. Bell, J. R. Warmington, and S. G. Wyllie. 1998. Tea tree oil causes K+ leakage and inhibits respiration in Escherichia coli. Lett. Appl. Microbiol. 26:355-358. [DOI] [PubMed] [Google Scholar]
- 12.Das, A., and L. D. Ljungdahl. 2003. Clostridium pasteurianum F1F0 ATP synthase: operon, composition, and some properties. J. Bacteriol. 185:5527-5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia, S., G. Alarcón, M. Gómez, and N. Heredia. 2005. Haematoxylon brasiletto extracts inhibit growth, enterotoxin production, and adhesion of Vibrio cholerae. Food Biotechnol. 19:15-26. [Google Scholar]
- 14.Garcia, S., G. Alarcon, C. Rodriguez, and N. Heredia. 2006. Extracts of Acacia farnesiana and Artemisia ludoviciana inhibit growth, enterotoxin production and adhesion of Vibrio cholerae. World J. Microbiol. Biotechnol. 22:669-674. [Google Scholar]
- 15.Garcia-Alvarado, J. S., M. J. Verde-Star, and N. L. Heredia. 2001. Traditional uses and scientific knowledge of medicinal plants from Mexico and Central America. A review. J. Herbs Spices Med. Plants 8:37-89. [Google Scholar]
- 16.Gonzalez, B., E. Glaasker, E. R. S. Kunji, A. J. M. Driessen, J. E. Suarez, and W. N. Konings. 1996. Bactericidal mode of action of plantaricin C. Appl. Environ. Microbiol. 62:2701-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heredia, N., M. Escobar, C. Rodríguez, and S. García. 2005. Extracts of Haematoxilon brasiletto inhibit growth, verotoxin production, and adhesion of enterohemorragic E. coli O157:H7 to HeLa cells. J. Food Prot. 68:1346-1351. [DOI] [PubMed] [Google Scholar]
- 18.Holyoak, C. D., M. Stratford, Z. McMullin, M. B. Cole, K. Crimmins, A. J. P. Brown, and P. J. Coote. 1996. Activity of the plasma membrane H+-ATPase and optimal glucolytic flux are required for rapid adaptation and growth of Saccharomyces cerevisiae in the presence of the weak-acid preservative sorbic acid. Appl. Environ. Microbiol. 62:3158-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussain, A. I., F. Anwar, S. T. H. Sherazi, and R. Przybylski. 2008. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 108:986-995. [DOI] [PubMed] [Google Scholar]
- 20.Jepras, R. I., F. E. Paul, S. C. Pearson, and M. J. Wilkinson. 1997. Rapid assessment of antibiotic effects on Escherichia coli by bis-(1,3-dibutylbarbituric acid) trimethine oxonol and flow cytometry. Antimicrob. Agents Chemother. 41:2001-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahtinen, S. J., M. Gueimonde, A. C. Ouwehand, J. P. Reinikainen, and S. J. Salminen. 2005. Probiotic bacteria may become dormant during storage. Appl. Environ. Microbiol. 71:1662-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert, R. J. W., P. N. Skandamis, P. Coote, and G. J. E. Nychas. 2001. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 91:453-462. [DOI] [PubMed] [Google Scholar]
- 23.Lee, E., H. Kim, Y. Song, C. Jin, K. Lee, J. Cho, and Y. Lee. 2003. Constituents of the stems and fruits of Opuntia ficus-indica var. saboten. Arch. Pharmacol. Res. 26:1018-1023. [DOI] [PubMed] [Google Scholar]
- 24.Leuko, S., A. Legat, S. Fendrihan, and H. Stan-Lotter. 2004. Evaluation of the LIVE/DEAD BacLight kit for detection of extermophilic Archae and visualization of microorganisms in environmental hypersaline samples. Appl. Environ. Microbiol. 70:6884-6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makinen, S. M., and K. K. Paakkonen. 1999. Processing and use of basil in foodstuffs, beverages and in food preparation, p. 137-152. In R. Hiltunen and Y. Holm (ed.), Basil: the genus Ocimum. Harwood Academic Publishers, Amsterdam, Netherlands.
- 26.Mason, D. J., R. Lopez-Amoros, R. Allman, J. M. Stark, and D. Lloyd. 1995. The ability of membrane potential dyes and calcafluor white to distinguish between viable and non-viable bacteria. J. Appl. Bacteriol. 78:309-315. [DOI] [PubMed] [Google Scholar]
- 27.Nickavar, B., G. Amin, and P. Ghavamian. 2002. Antimicrobial activity of Pulicaria dysenterica L. Iran. J. Pharm. Res. 1:31-32. [Google Scholar]
- 28.Olson, E. R. 1993. Influence of pH on bacterial gene expression. Mol. Microbiol. 8:5-14. [DOI] [PubMed] [Google Scholar]
- 29.Pag, U., M. Oedenkoven, N. Papo, Z. Oren, Y. Shai, and H.-G. Sahl. 2004. In vitro activity and mode of action of diastereomeric antimicrobial peptides against bacterial clinical isolates. J. Antimicrob. Chemother. 53:230-239. [DOI] [PubMed] [Google Scholar]
- 30.Prabuseenivasan, S., M. Jayakumar, and S. Ignacimuthu. 2006. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasooli, I. 2007. Food preservation: a biopreservative approach. Food 1:111-136. [Google Scholar]
- 32.Ruiz-Cancino, A., A. E. Cano, and G. Delgado. 1993. Sesquiterpene lactones and flavonoids from Artemisia ludoviciana ssp. mexicana. Phytochemistry 33:1113-1115. [Google Scholar]
- 33.Schuenzel, K. M., and M. A. Harrison. 2002. Microbial antagonists of foodborne pathogens on fresh minimally processed vegetables. J. Food Prot. 65:1909-1915. [DOI] [PubMed] [Google Scholar]
- 34.Seigler, D. S. 2003. Phytochemistry of Acacia-sensu lato. Biochem. Syst. Ecol. 31:845-873. [Google Scholar]
- 35.Sikkema, J., J. A. M. De Bont, and B. Poolman. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva, S., P. Teixeira, R. Oliveira, and J. Azeredo. 2008. Adhesion to and viability of Listeria monocytogenes on food contact surfaces. J. Food Prot. 71:1379-1385. [DOI] [PubMed] [Google Scholar]
- 37.Singh, G. B., and P. Felker. 1998. Cacti: a new world food. Indian Hortic. 43:26-29. [Google Scholar]
- 38.Sittiwet, C., and D. Puangpronpitag. 2009. Antimicrobial properties of Derris scandens aqueous extract. J. Biol. Sci. 9:607-611. [Google Scholar]
- 39.Skandamis, P., K. Koutsoumanis, K. Fasseas, and G.-J. E. Nychas. 2001. Inhibition of oregano essential oil and EDTA on Escherichia coli O157:H7. Ital. J. Food Sci. 13:65-75. [Google Scholar]
- 40.Standley, P. C. Contributions from the National Herbarium, vol. 23. Trees and shrubs of Mexico. Smithsonian Institution, Washington, DC.
- 41.Suzuki, H., Z.-Y. Wang, M. Yamakoshi, M. Kobayashi, and T. Nozawa. 2003. Probing the transmembrane potential of bacterial cells by voltage-sensitive dyes. Anal. Sci. 19:1239-1242. [DOI] [PubMed] [Google Scholar]
- 42.Ultee, A., E. P. W. Kets, and E. J. Smid. 1999. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 65:4606-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ultee, A., M. H. J. Bennink, and R. Moezelaar. 2002. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 68:1561-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whiteaker, K. L., S. M. Gopalakrishnan, D. Groebe, C.-C. Shieh, U. Warrior, D. J. Burns, M. J. Coghlan, V. E. Scott, and M. Gopalakrishnan. 2001. Validation of FLIPR membrane potential dye for high throughput screening of potassium channel modulators. J. Biomol. Screen. 6:305-312. [DOI] [PubMed] [Google Scholar]
- 45.Yuroff, A. S., G. Sbat, and W. J. Hickey. 2003. Transporter-mediated uptake of 2-chloro and 2-hydroxibenzoato by Pseudomonas huttiensis strain D1. Appl. Environ. Microbiol. 69:7401-7408. [DOI] [PMC free article] [PubMed] [Google Scholar]