Abstract
Clostridium difficile is a major cause of antibiotic-associated diarrheal disease in many parts of the world. In recent years, distinct genetic variants of C. difficile that cause severe disease and persist within health care settings have emerged. Highly resistant and infectious C. difficile spores are proposed to be the main vectors of environmental persistence and host transmission, so methods to accurately monitor spores and their inactivation are urgently needed. Here we describe simple quantitative methods, based on purified C. difficile spores and a murine transmission model, for evaluating health care disinfection regimens. We demonstrate that disinfectants that contain strong oxidizing active ingredients, such as hydrogen peroxide, are very effective in inactivating pure spores and blocking spore-mediated transmission. Complete inactivation of 106 pure C. difficile spores on indicator strips, a six-log reduction, and a standard measure of stringent disinfection regimens require at least 5 min of exposure to hydrogen peroxide vapor (HPV; 400 ppm). In contrast, a 1-min treatment with HPV was required to disinfect an environment that was heavily contaminated with C. difficile spores (17 to 29 spores/cm2) and block host transmission. Thus, pure C. difficile spores facilitate practical methods for evaluating the efficacy of C. difficile spore disinfection regimens and bringing scientific acumen to C. difficile infection control.
Clostridium difficile is a Gram-positive, spore-forming, anaerobic bacterium that is a major cause of health care-acquired infections and antibiotic-associated diarrhea (2). In recent years, several genetic variants of C. difficile have emerged as important health care pathogens (6). Perhaps most notable is the “hypervirulent” variant, commonly referred to as PCR ribotype 027/restriction endonuclease analysis (REA) group BI, that produces elevated levels of toxins TcdA and TcdB (17, 19). Other virulent ribotypes that display extensive heterogeneity among their toxin protein sequences (26) and gene activities (8) have emerged. Using whole-genome sequencing, we demonstrated that there are broad genetic differences between the entire genomes of several common variants, including ribotype/REA group variants 012/R, 017/CF, and 027/BI used in this study (12, 27, 31). In contrast, phylogeographic analysis of 027/BI isolates from Europe and the United States demonstrates that this clade is extremely clonal and implies recent transcontinental spread of hypervirulent C. difficile (12).
C. difficile is distinct from many other health care pathogens because it produces highly infectious spores that are shed into the environment (25, 28). C. difficile spores can resist disinfection regimens that normally inactivate other health care pathogens, such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci, therefore challenging current infection control measures (2). A multifaceted approach is normally used to control C. difficile in health care facilities (32). Interventions include antimicrobial stewardship, increased clinical awareness, patient isolation (11), and enhanced environmental disinfection regimens based on hydrogen peroxide (H2O2) vapor (HPV) (4). While attempts to break the spore-mediated infection cycle and interrupt these efficient routes of transmission are important for infection control measures, there is little quantitative evidence indicating which interventions are most effective (7). Here we describe the exploitation of pure C. difficile spores (16) and a murine transmission model (15) in simple, practical methods to quantitatively monitor the impact of health care disinfection regimens on C. difficile viability. These methods can be used to optimize disinfection regimens targeted at C. difficile.
MATERIALS AND METHODS
C. difficile strains and spore purification.
C. difficile spores were purified as previously described (16) by using the human-virulent C. difficile strains 630 (tcdA+ tcdB+; ribotype 012/REA group R) (27), R20291 (tcdA+ tcdB+; ribotype 027/REA group BI) (31) and M68 (tcdA tcdB+; ribotype 017/REA group CF) (8). The genomes of these strains have been sequenced and are available at http://www.sanger.ac.uk/Projects/C_difficile/.
Spore inactivation assay.
The concentration of the spore preparation was determined by visual enumeration with a light microscope and culturing on brain heart infusion (BHI) broth supplemented with 0.25% taurocholate (16). To test for disinfectant susceptibility/resistance, ∼106 pure spores were placed in the indicated disinfectant in a total volume of 0.5 ml for 1, 10, or 20 min. The following disinfectants (Table 1 ) were prepared as described by the manufacturer and used at a 100% concentration unless otherwise indicated: 70% ethanol (VWR, United Kingdom), Spirigel (Ecolab Ltd., Leeds, United Kingdom), HiBiScrub (Reagent Medical, Manchester, United Kingdom), Flash (Proctor and Gamble, Surrey, United Kingdom), Steri-7 (Steri-7 Worldwide [Cyprus] Ltd., Surrey, United Kingdom), Virusolve (Amity International, Barnsley, United Kingdom), H2O2 (Sigma, United Kingdom), Virkon (Antec International, Suffolk, United Kingdom), Spor-Klenz (Steris Ltd., St. Louis, MO), sodium hypochlorite (Adams Healthcare, Leeds, United Kingdom), and Chlor-Clean (Guest Medical, Kent, United Kingdom). After treatment, spores were pelleted with centrifugation and washed 2 times in sterile water to remove the disinfectant. Viable spores were enumerated as previously described (16). To compare the efficacies of the treatments, the number of viable spore counts was logarithmically (base 10) transformed, and then the data were fitted to analysis of variance models containing both treatment and ribotype/REA type effects. Spores were processed and transmission electron microscopy (TEM) images taken as previously described (16).
TABLE 1.
Summary of health care disinfectants and active ingredients tested against pure C. difficile spores
| Disinfecting agent | Use(s) | Active ingredient | C. difficile spore inactivationa |
|---|---|---|---|
| 70% ethanol | Surface | Alcohol | N |
| Spirigel | Hand | Alcohol | N |
| HiBiScrub | Hand/preoperative | Chlorhexidine gluconate | N |
| Flash (no bleach) | Surface | Benzisothiazolinone | Moderate |
| Steri-7 | Surface | Isothiazolin-benzalkonium chloride | Moderate |
| Virusolve | Surface | Alkyl triamine/bromine | Y |
| 3% H2O2 | Surface | H2O2 | Y |
| 1% Virkon | Surface | Potassium peroxymonosulfate | Y |
| 10% H2O2 | Surface | H2O2 | Y |
| Spor-Klenz | Surface | H2O2-peroxyacetic acid | Y |
| 1% sodium hypochlorite | Surface | Sodium hypochlorite | Y |
| Chlor-Clean | Surface | Sodium dichloroisocyanurate | Y |
Based on data from Fig. 1a. N, no; Y, yes.
Spore strip assay.
Geobacillus stearothermophilus spore strips (106 spores/strip) were purchased from Raven Biological Laboratories, Inc. (Cheshire, United Kingdom). Pure C. difficle M68 spores were suspended in sterile phosphate-buffered saline (PBS) at 107 spores/ml. From this stock, 0.1 ml (106 spores) was absorbed onto a sterile piece of Whatman filter paper (4 cm by 1 cm) and aseptically placed in a sterile bijou tube (7 ml) and capped. HPV treatment was performed by placing the uncapped tubes with spore strips inside in a biosafety cabinet fitted with a Bioquel Claris HPV generator and an HPV detector. HPV was administered to the cabinet at a level of 400 ppm and maintained for the indicated time prior to purging of the cabinet to remove that HPV. After exposure to HPV, G. stearothermophilus was cultured from spore strips and test results were interpreted based on the manufacturers' protocols. The C. difficile spore strips were cultured in 4 ml BHI (Oxoid, Cambridge, United Kingdom) broth supplemented with 0.25% taurocholate under anaerobic conditions for 72 h. Tubes that became turbid were tested for C. difficile by streaking on CCEY agar plates (Bioconnections, Wetherby, United Kingdom) (scored positive), whereas tubes that remained clear were scored negative for C. difficile growth.
Murine experiments.
Transmission experiments and infective dose experiments were performed as previously described (15, 16). Cages were contaminated with the indicated numbers of pure M68 spores (infective dose experiment) or with feces from donor mice that were shedding high levels (>107 spores/gram feces) of C. difficile M68 spores (transmission experiment). Transmission occurred if mice were shedding C. difficile 4 days after exposure to a spore-contaminated environment (16). To estimate the environmental spore load after fecal contamination, 10 ml of sterile water was added to each cage and the cage bottoms were scrubbed with sterile cotton swabs. After extensive scrubbing, the water was collected, diluted, and plated on CCEY agar and incubated anaerobically at 37°C overnight as previously described (15). C. difficile colonies were counted and expressed as spores per cage or spores per cm2 (cage bottom is 800 cm2) in Fig. 3.
Surface disinfection was performed by adding 15 ml (covering the cage floor) of the indicated disinfectant to the contaminated cage for 10 min (see Fig. 3) as previously described (15). Disinfectants were then removed and the surface was patted dry with sterile paper towels before naïve mice were placed into the cage. HPV disinfection of contaminated cages (see Fig. 3) was performed as described above. All animal infections were performed in accordance with the United Kingdom Home Office Animals (Scientific Procedures) Act of 1986.
RESULTS
Oxidizing disinfectants effectively inactivate C. difficile spores.
We previously described a protocol to purify infectious and highly resistant C. difficile spores (16). The ability to prepare pure spores allowed us to accurately measure the sensitivity of C. difficile spores to disinfectants in the absence of contaminating vegetative cells and their extracellular products, such as an S-layer (9) and capsule (3), that can interfere with spore-disinfectant interactions (20). We tested pure C. difficile spores against a panel of disinfectants that are commonly used in health care facilities (Fig. 1 a; Table 1). The active ingredient and intended application for each disinfectant are provided in Table 1 because these products are often sold under different commercial names in different countries. We chose three wild-type strains of human-virulent C. difficile that represent the genotypes (PCR ribotype/REA type) 012/R, 017/CF, and 027/BI. The genome of each of these strains has been sequenced (12). In each experiment, we treated 106 spores, as complete inactivation of 106 microorganisms, commonly referred to as a six-log reduction, is considered a standard measure for stringent disinfection regimens (20).
FIG. 1.
Efficacy of health care disinfectants in inactivating pure C. difficile spores. (a) Viability of spores derived from distinct C. difficile genetic variants after exposure to various health care disinfectants. All C. difficile strains are virulent to humans. A summary of the disinfectants is listed in Table 1. Data are representative of 5 separate experiments. Error bars indicate standard deviations. The broken horizontal line indicates the detection limit of 2 CFU/ml. EtOH, ethyl alcohol. (b) TEM image of an untreated, viable spore. (c) TEM of a spore that was inactivated by 10% hydrogen peroxide exposure for 10 min.
C. difficile spores efficiently germinate and form colonies on nutrient agar supplemented with taurocholate (Fig. 1a) (30, 36). We found that exposure to 70% ethanol, Spirigel, and HiBiScrub had no obvious or reproducible effect on the viability of the C. difficile spores compared to those immersed in sterile H2O (F = 1.02; P = 0.41) (Fig. 1a). The commonly used commercial detergents Flash and Steri-7 similarly (F = 3.47; P = 0.10) reduced the spore viability ∼10-fold, which is statistically different from the value for the water treatment (F = 898.8; P < 10−16) (Fig. 1a). Virkon, 3% hydrogen peroxide, and Virusolve inactivated the majority of spores, whereas Spor-Klenz, sodium hypochlorite, Chlor-Clean, and 10% hydrogen peroxide completely inactivated the spores in a manner that was significantly better than those of the other treatments (F = 134.0; P < 10−16) (Fig. 1a). For example, we were not able to culture C. difficile (detection limit of 2 CFU/ml) when spores were treated with an appropriate concentration of Spor-Klenz, sodium hypochlorite, Chlor-Clean, or 10% hydrogen peroxide for 20 min, corresponding to a six-log reduction in viability (Fig. 1a). Importantly, spores derived from distinct genetic variants of C. difficile, specifically ribotypes 012, 017, and 027, responded in similar manners to each disinfectant.
Untreated, viable spores have an electron-dense outer spore coat with distinct striated layers that encompasses a lighter layer referred to as the cortex (Fig. 1b). Encased within the cortex is the core, defined by a continuous membrane that surrounds the nuclear DNA and ribosomes (Fig. 1b). After 10% H2O2 treatment, the spore coat showed significant decreases in intensity and banding patterns, while the inner layers appeared more separated and diffuse (Fig. 1c). The core staining was less uniform than seen in untreated spores, and this core staining appeared to be comparatively expanded (Fig. 1c). Therefore, C. difficile spores are resistant to many commonly used health care disinfectants but are inactivated and rendered nonviable by disinfectants that contain oxidizing active ingredients.
Kinetics of pure C. difficile spore inactivation by hydrogen peroxide.
The level of spore inactivation is likely to depend to a significant extent on the contact time for C. difficile spores and the disinfectant, as sufficient time is required to allow the disinfecting agent to access and damage the cellular target (20). Thus, we evaluated the kinetics of spore inactivation (106 spores) after independent exposure to two concentrations of a hydrogen peroxide solution (Fig. 2 a). Treatment of C. difficile spores with 1% hydrogen peroxide resulted in an immediate (i.e., 1-min) inactivation of 75% of the spores without subsequent inactivation over the following 19 min (Fig. 2a). In contrast, a 1-min exposure to 10% hydrogen peroxide resulted in inactivation of >99% of the spores, followed by a gradual reduction in viable spores over the next 19 min when the spores were fully inactivated (Fig. 2a).
FIG. 2.
C. difficile spores are inactivated by prolonged exposure to high-level hydrogen peroxide. (a) Viability of C. difficile M68 spores after exposure to 1% or 10% hydrogen peroxide for 1, 10, and 20 min. Data are representative of three separate experiments. Error bars indicate standard deviations. The broken horizontal line indicates the detection limit of 2 CFU/ml. (b) Spore strips containing either 106 C. difficile M68 or G. stearothermophilus spores were exposed to 400 ppm hydrogen peroxide vapor for 1, 5, 20, or 60 min prior to culture of the bacteria to determine the efficacy of disinfection. Positive growth strip indicates that the spores were not inactivated by HPV. Six spore strips were tested per time point.
Indicator organisms are commonly used to evaluate the efficacy of environmental decontamination regimens, particularly in environments such as purpose-built bacterial containment facilities. Geobacillus stearothermophilus spore strips are available as commercial kits and are considered the gold standard. Consequently, we exposed both standard G. stearothermophilus spore strips (106 spores) and custom-made C. difficile spore strips (106 pure spores on sterile filter paper) to 400 ppm hydrogen peroxide vapor for 1, 5, 20, and 60 min before attempting to culture the bacteria (Fig. 2b). G. stearothermophilus spore strips required a 20- to 60-min exposure to HPV to render the bacteria nonviable. In contrast, the C. difficile spore strips required a 5- to 20-min exposure to render the bacteria completely nonviable (Fig. 2b). Thus, prolonged exposure with HPV is required to achieve a six-log reduction of bacterial spores and C. difficile spores are significantly more sensitive to HPV than G. stearothermophilus spores.
Oxidizing disinfectants block spore-mediated transmission.
It is difficult to extrapolate in vitro disinfection results directly to an applied setting, as during host transmission, organic/fecal materials that can interfere with interactions between the disinfectant and spore may be present (20). Murine fecal-oral transmission models are valuable surrogates for the study of host and pathogen factors that are involved in transmission (14, 33, 35) and for the evaluation of the ability of disinfectants to block spore-mediated transmission (15). Here we used a murine transmission model to evaluate the abilities of various health care disinfectants to block spore-mediated transmission. With our transmission model, we found that mice that are exposed to an environment contaminated with pure C. difficile M68 spores for 1 h become colonized in a dose-dependent manner (Fig. 3), which is consistent with previous results from a study using C. difficile 630 spores (16). Based on these experiments, we are able to estimate that the environmental spore load required to infect 50% of those mice exposed for 1 h is 5 to 10 spores/cm2 (Fig. 3).
FIG. 3.
Environmental C. difficile spores are highly infective. Infective dose curves of pure and fecally derived C. difficile M68 spores in mice (4 per dose) after exposure to environmental spore contamination. Based on the Reed-Muench formulation (24), the environmental spore dose to infect 50% (ID50) of the mice with a 1-hour exposure is 5 spores/cm2 for fecal spores and 10 spores/cm2 for pure spores, as indicated by the gray vertical dashed lines and the black arrow below the graph. Data are representative of two independent experiments. As a reference, the vertical arrow below the graph labeled “spore contamination” indicates the level of spores that was retrieved from the cage after fecal contamination by the infected mice corresponding to Fig. 4. The vertical arrow labeled “106 spores/cage” indicates the level of spores that is equivalent to that on the indicator strips corresponding to Fig. 2.
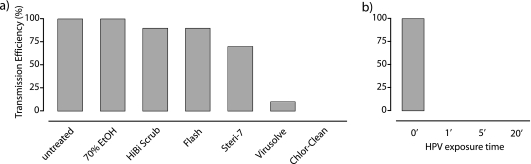
Heavy contamination of cages by mice shedding high levels of C. difficile M68 spores (average 23 ± 6.1 spores/cm2 of cage bottom; n = 4 cages) facilitates efficient spore-mediated transmission to naïve mice (Fig. 4 a). Next, we tested the efficiencies of various health care disinfectants in blocking C. difficile transmission. As expected, a 10-min surface disinfection with 70% alcohol, HiBiScrub, and the detergents Flash and Steri-7 had minimal to moderate impacts on the reduction of transmission (Fig. 4a). In contrast, the oxidizing disinfectants Virusolve and Chlor-Clean were the most effective treatments for reducing host-to-host transmission (Fig. 4a). Enhanced cleaning of hospital wards is routinely accomplished with HPV (5). We next tested the efficacy of HPV (400 ppm) in blocking spore-mediated transmission. HPV treatment proved to be dramatically efficient, with a 1-min pulse reducing environmental spore contamination sufficiently to completely eliminate transmission (Fig. 4b). Thus, oxidizing disinfectants effectively inactivate environmental spores below the dose required to infect mice.
FIG. 4.
Efficacies of health care disinfectants in reducing host transmission of C. difficile via environmental spore contamination. (a) Transmission efficiency of environmental C. difficile M68 spores to naïve mice after surface disinfection with the indicated agent (10 mice per agent) compared to that of the untreated group (18 mice untreated). (b) Effect of hydrogen peroxide vapor contact time on reducing transmission of C. difficile spores to naïve mice. Exposure time is defined as the time the HPV was at the peak level of 400 ppm. There were 10 mice corresponding to each time point (0, 1, 5, and 20 min). The estimated level of spore contamination was 23 ± 6 spores/cm2.
DISCUSSION
Disinfection of the hospital environment with hypochlorite solution (11, 18, 21, 34) and hydrogen peroxide dry mist or vapor (5, 29) reduces the incidence of C. difficile disease. However, it is difficult to evaluate the direct impact of these disinfectants alone, as during use of enhanced disinfection regimens, there is generally an increased sense of awareness among hospital staff that may also contribute to improved infection control (7, 34). Thus, the availability of pure C. difficile spores and a murine transmission model offer the unique opportunity to quantitatively evaluate and monitor the efficacy of C. difficile disinfection regimens in a controlled manner.
We showed that purified spores derived from distinct genetic variants of human-virulent C. difficile are effectively and equally inactivated by a variety of disinfectants that contain strong oxidizing agents. Therefore, the spore structures of these variants have not genetically acquired altered resistance to health care disinfectants. Overall, our observations are consistent with those from others who have successfully demonstrated that cultures of C. difficile containing a mixture of vegetative cells and spores are inactivated by chlorine-releasing disinfectants (10) and hydrogen peroxide dry mist (1). We also demonstrated that oxidation-based disinfectants efficiently block spore-mediated transmission between mice, whereas many commonly used health care disinfectants failed. The availability of a complete C. difficile spore proteome (16) will facilitate studies aimed at identifying the targets of the oxidizing disinfectants and could aid in the development of novel disinfectants that can be used on hands, potentially one of the most important mediators of transmission (23).
Environmental disinfection protocols with gaseous disinfectants (such as HPV or formaldehyde) commonly employ excessive treatment regimens to provide the greatest assurance of sterility. Generally, a six-log reduction of G. stearothermophilus spores on indicator strips is the desired endpoint (22). With our HPV disinfection protocol, it takes >20 min to fully inactivate 106 G. stearothermophilus spores but only 5 min to inactivate 106 C. difficile spores. Thus, G. stearothermophilus spores are likely to be inherently very resistant to disinfection (13). In contrast, we demonstrate that only a 1-min HPV treatment in a heavily contaminated environment was required to reduce viable C. difficile spore levels below the infectious dose and block transmission. Thus, disinfection regimens that completely inactivate either type of spore strip should indicate successful environmental C. difficile spore disinfection.
Since G. stearothermophilus spores are significantly more resistant than C. difficile spores, it may be prudent to include C. difficile spore strips to monitor the efficacy of disinfection with hydrogen peroxide vapor. This could be achieved by safely enclosing accurately measured quantities of C. difficile spores (possibly from a nontoxigenic strain) within strips that fully retain the spores but allow access of the inactivating vapors. Such an approach would facilitate the tailoring of HPV disinfection regimens to C. difficile and bring scientific acumen to C. difficile infection control.
Acknowledgments
We thank Nicola Goodwin for assistance with the animal experiments.
This work was funded by the Wellcome Trust and a Royal Society of London fellowship to T.D.L.
We have no conflict of interest.
Footnotes
Published ahead of print on 27 August 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Barbut, F., D. Menuet, M. Verachten, and E. Girou. 2009. Comparison of the efficacy of a hydrogen peroxide dry-mist disinfection system and sodium hypochlorite solution for eradication of Clostridium difficile spores. Infect. Control Hosp. Epidemiol. 30:507-514. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett, J. G. 2006. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann. Intern. Med. 145:758-764. [DOI] [PubMed] [Google Scholar]
- 3.Borriello, S. P. 1998. Pathogenesis of Clostridium difficile infection. J. Antimicrob. Chemother. 41(Suppl. C):13-19. [DOI] [PubMed] [Google Scholar]
- 4.Boyce, J. M. 2007. Environmental contamination makes an important contribution to hospital infection. J. Hosp. Infect. 65(Suppl. 2):50-54. [DOI] [PubMed] [Google Scholar]
- 5.Boyce, J. M., N. L. Havill, J. A. Otter, L. C. McDonald, N. M. Adams, T. Cooper, A. Thompson, L. Wiggs, G. Killgore, A. Tauman, and J. Noble-Wang. 2008. Impact of hydrogen peroxide vapor room decontamination on Clostridium difficile environmental contamination and transmission in a healthcare setting. Infect. Control Hosp. Epidemiol. 29:723-729. [DOI] [PubMed] [Google Scholar]
- 6.Brazier, J. S. 2008. Clostridium difficile: from obscurity to superbug. Br. J. Biomed. Sci. 65:39-44. [DOI] [PubMed] [Google Scholar]
- 7.Dancer, S. J. 2009. The role of environmental cleaning in the control of hospital-acquired infection. J. Hosp. Infect. 73:378-385. [DOI] [PubMed] [Google Scholar]
- 8.Drudy, D., N. Harnedy, S. Fanning, R. O'Mahony, and L. Kyne. 2007. Isolation and characterisation of toxin A-negative, toxin B-positive Clostridium difficile in Dublin, Ireland. Clin. Microbiol. Infect. 13:298-304. [DOI] [PubMed] [Google Scholar]
- 9.Fagan, R. P., D. Albesa-Jove, O. Qazi, D. I. Svergun, K. A. Brown, and N. F. Fairweather. 2009. Structural insights into the molecular organization of the S-layer from Clostridium difficile. Mol. Microbiol. 71:1308-1322. [DOI] [PubMed] [Google Scholar]
- 10.Fawley, W. N., S. Underwood, J. Freeman, S. D. Baines, K. Saxton, K. Stephenson, R. C. Owens, Jr., and M. H. Wilcox. 2007. Efficacy of hospital cleaning agents and germicides against epidemic Clostridium difficile strains. Infect. Control Hosp. Epidemiol. 28:920-925. [DOI] [PubMed] [Google Scholar]
- 11.Gerding, D. N., C. A. Muto, and R. C. Owens, Jr. 2008. Measures to control and prevent Clostridium difficile infection. Clin. Infect. Dis. 46(Suppl. 1):S43-S49. [DOI] [PubMed] [Google Scholar]
- 12.He, M., M. Sebaihia, T. D. Lawley, R. A. Stabler, L. F. Dawson, M. J. Martin, K. E. Holt, H. M. Seth-Smith, M. A. Quail, R. Rance, K. Brooks, C. Churcher, D. Harris, S. D. Bentley, C. Burrows, L. Clark, C. Corton, V. Murray, G. Rose, S. Thurston, A. van Tonder, D. Walker, B. W. Wren, G. Dougan, and J. Parkhill. 2010. Evolutionary dynamics of Clostridium difficile over short and long time scales. Proc. Natl. Acad. Sci. U. S. A. 107:7527-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokubo, M., T. Inoue, and J. Akers. 1998. Resistance of common environmental spores of the genus Bacillus to vapor hydrogen peroxide. PDA J. Pharm. Sci. Technol. 52:228-231. [PubMed] [Google Scholar]
- 14.Lawley, T. D., D. M. Bouley, Y. E. Hoy, C. Gerke, D. A. Relman, and D. M. Monack. 2008. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infect. Immun. 76:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawley, T. D., S. Clare, A. W. Walker, D. Goulding, R. A. Stabler, N. Croucher, P. Mastroeni, P. Scott, C. Raisen, L. Mottram, N. F. Fairweather, B. W. Wren, J. Parkhill, and G. Dougan. 2009. Antibiotic treatment of Clostridium difficile carrier mice triggers a supershedder state, spore-mediated transmission, and severe disease in immunocompromised hosts. Infect. Immun. 77:3661-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawley, T. D., N. J. Croucher, L. Yu, S. Clare, M. Sebaihia, D. Goulding, D. J. Pickard, J. Parkhill, J. Choudhary, and G. Dougan. 2009. Proteomic and genomic characterization of highly infectious Clostridium difficile 630 spores. J. Bacteriol. 191:5377-5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loo, V. G., L. Poirier, M. A. Miller, M. Oughton, M. D. Libman, S. Michaud, A. M. Bourgault, T. Nguyen, C. Frenette, M. Kelly, A. Vibien, P. Brassard, S. Fenn, K. Dewar, T. J. Hudson, R. Horn, P. Rene, Y. Monczak, and A. Dascal. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442-2449. [DOI] [PubMed] [Google Scholar]
- 18.Mayfield, J. L., T. Leet, J. Miller, and L. M. Mundy. 2000. Environmental control to reduce transmission of Clostridium difficile. Clin. Infect. Dis. 31:995-1000. [DOI] [PubMed] [Google Scholar]
- 19.McDonald, L. C., G. E. Killgore, A. Thompson, R. C. Owens, Jr., S. V. Kazakova, S. P. Sambol, S. Johnson, and D. N. Gerding. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433-2441. [DOI] [PubMed] [Google Scholar]
- 20.McDonnell, G., and A. D. Russell. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMullen, K. M., J. Zack, C. M. Coopersmith, M. Kollef, E. Dubberke, and D. K. Warren. 2007. Use of hypochlorite solution to decrease rates of Clostridium difficile-associated diarrhea. Infect. Control Hosp. Epidemiol. 28:205-207. [DOI] [PubMed] [Google Scholar]
- 22.Otter, J. A., and G. L. French. 2009. Survival of nosocomial bacteria and spores on surfaces and inactivation by hydrogen peroxide vapour (HPV). J. Clin. Microbiol. 47:205-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oughton, M. T., V. G. Loo, N. Dendukuri, S. Fenn, and M. D. Libman. 2009. Hand hygiene with soap and water is superior to alcohol rub and antiseptic wipes for removal of Clostridium difficile. Infect. Control Hosp. Epidemiol. 30:939-944. [DOI] [PubMed] [Google Scholar]
- 24.Ozanne, G. 1984. Estimation of endpoints in biological systems. Comput. Biol. Med. 14:377-384. [DOI] [PubMed] [Google Scholar]
- 25.Riggs, M. M., A. K. Sethi, T. F. Zabarsky, E. C. Eckstein, R. L. Jump, and C. J. Donskey. 2007. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin. Infect. Dis. 45:992-998. [DOI] [PubMed] [Google Scholar]
- 26.Rupnik, M. 2008. Heterogeneity of large clostridial toxins: importance of Clostridium difficile toxinotypes. FEMS Microbiol. Rev. 32:541-555. [DOI] [PubMed] [Google Scholar]
- 27.Sebaihia, M., B. W. Wren, P. Mullany, N. F. Fairweather, N. Minton, R. Stabler, N. R. Thomson, A. P. Roberts, A. M. Cerdeno-Tarraga, H. Wang, M. T. Holden, A. Wright, C. Churcher, M. A. Quail, S. Baker, N. Bason, K. Brooks, T. Chillingworth, A. Cronin, P. Davis, L. Dowd, A. Fraser, T. Feltwell, Z. Hance, S. Holroyd, K. Jagels, S. Moule, K. Mungall, C. Price, E. Rabbinowitsch, S. Sharp, M. Simmonds, K. Stevens, L. Unwin, S. Whithead, B. Dupuy, G. Dougan, B. Barrell, and J. Parkhill. 2006. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat. Genet. 38:779-786. [DOI] [PubMed] [Google Scholar]
- 28.Sethi, A. K., W. N. Al-Nassir, M. M. Nerandzic, G. S. Bobulsky, and C. J. Donskey. 2010. Persistence of skin contamination and environmental shedding of Clostridium difficile during and after treatment of C. difficile infection. Infect. Control Hosp. Epidemiol. 31:21-27. [DOI] [PubMed] [Google Scholar]
- 29.Shapey, S., K. Machin, K. Levi, and T. C. Boswell. 2008. Activity of a dry mist hydrogen peroxide system against environmental Clostridium difficile contamination in elderly care wards. J. Hosp. Infect. 70:136-141. [DOI] [PubMed] [Google Scholar]
- 30.Sorg, J. A., and A. L. Sonenshein. 2008. Bile salts and glycine as cogerminants for Clostridium difficile spores. J. Bacteriol. 190:2505-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stabler, R. A., M. He, L. Dawson, M. Martin, E. Valiente, C. Corton, T. D. Lawley, M. Sebaihia, M. A. Quail, G. Rose, D. N. Gerding, M. Gibert, M. R. Popoff, J. Parkhill, G. Dougan, and B. W. Wren. 2009. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol. 10:R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vonberg, R. P., E. J. Kuijper, M. H. Wilcox, F. Barbut, P. Tull, P. Gastmeier, P. J. van den Broek, A. Colville, B. Coignard, T. Daha, S. Debast, B. I. Duerden, S. van den Hof, T. van der Kooi, H. J. Maarleveld, E. Nagy, D. W. Notermans, J. O'Driscoll, B. Patel, S. Stone, and C. Wiuff. 2008. Infection control measures to limit the spread of Clostridium difficile. Clin. Microbiol. Infect. 14(Suppl. 5):2-20. [DOI] [PubMed] [Google Scholar]
- 33.Wijburg, O. L., T. K. Uren, K. Simpfendorfer, F. E. Johansen, P. Brandtzaeg, and R. A. Strugnell. 2006. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J. Exp. Med. 203:21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilcox, M. H., W. N. Fawley, N. Wigglesworth, P. Parnell, P. Verity, and J. Freeman. 2003. Comparison of the effect of detergent versus hypochlorite cleaning on environmental contamination and incidence of Clostridium difficile infection. J. Hosp. Infect. 54:109-114. [DOI] [PubMed] [Google Scholar]
- 35.Wiles, S., W. P. Hanage, G. Frankel, and B. Robertson. 2006. Modelling infectious disease—time to think outside the box? Nat. Rev. Microbiol. 4:307-312. [DOI] [PubMed] [Google Scholar]
- 36.Wilson, K. H., M. J. Kennedy, and F. R. Fekety. 1982. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J. Clin. Microbiol. 15:443-446. [DOI] [PMC free article] [PubMed] [Google Scholar]