Abstract
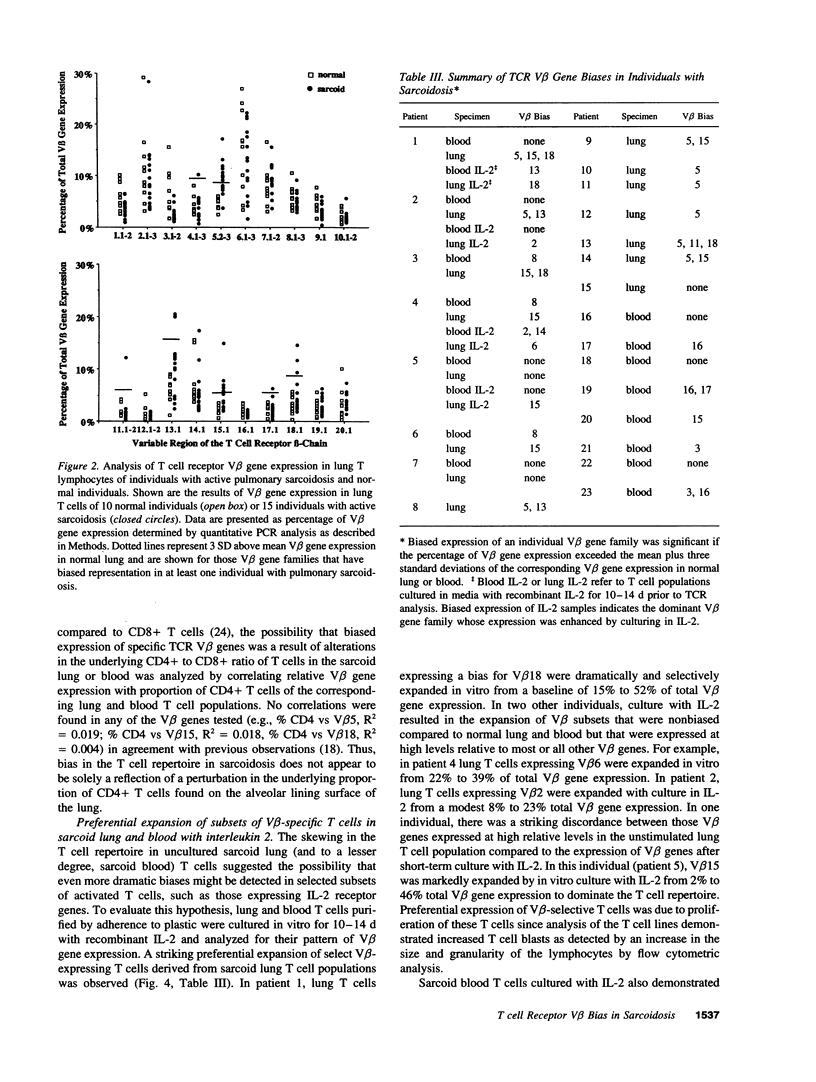
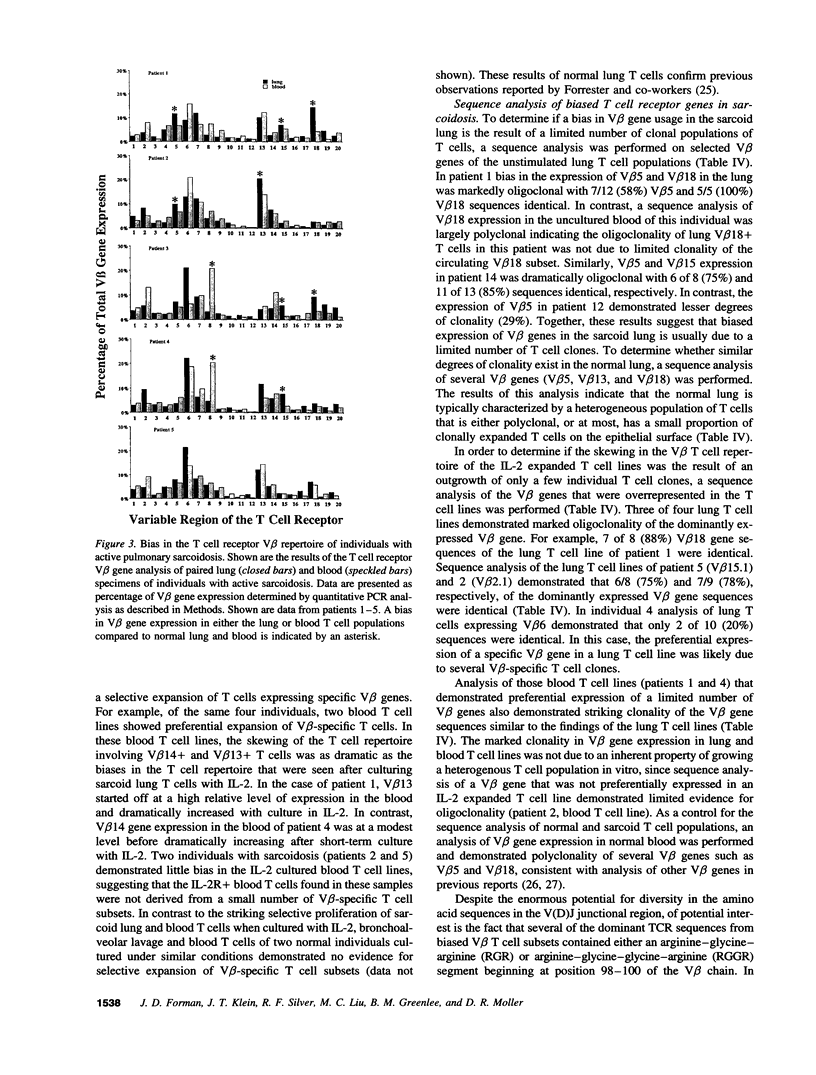
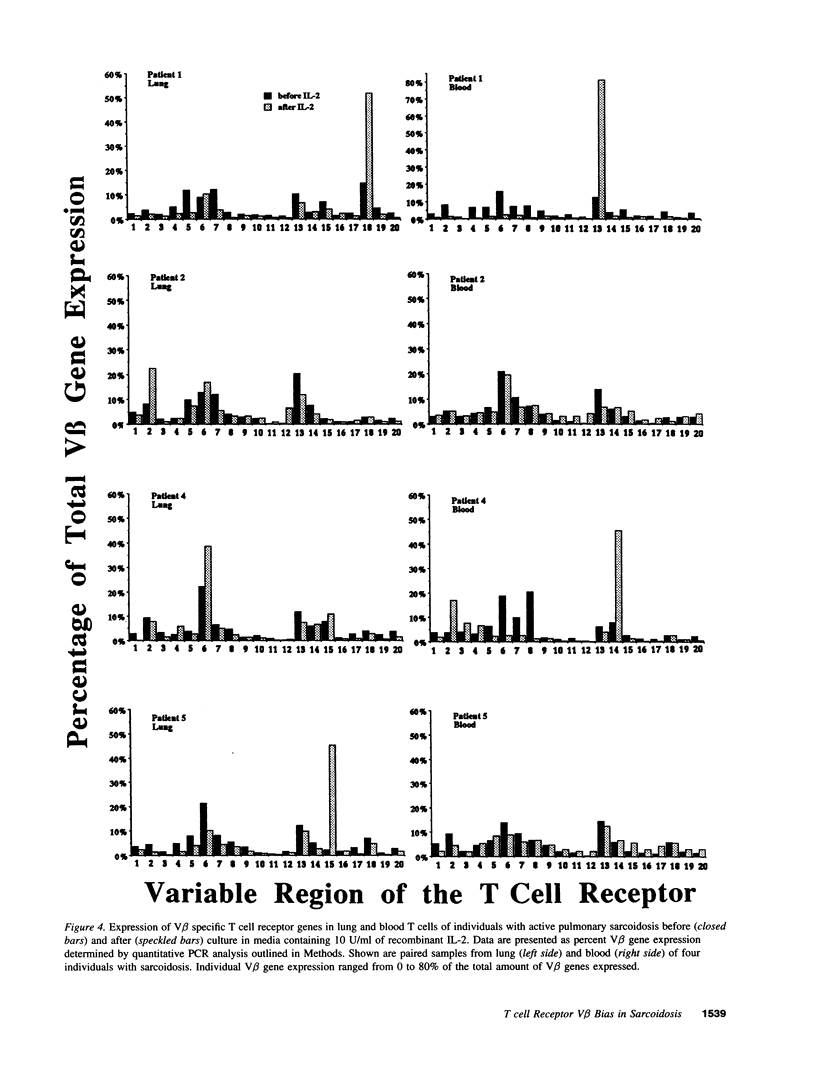
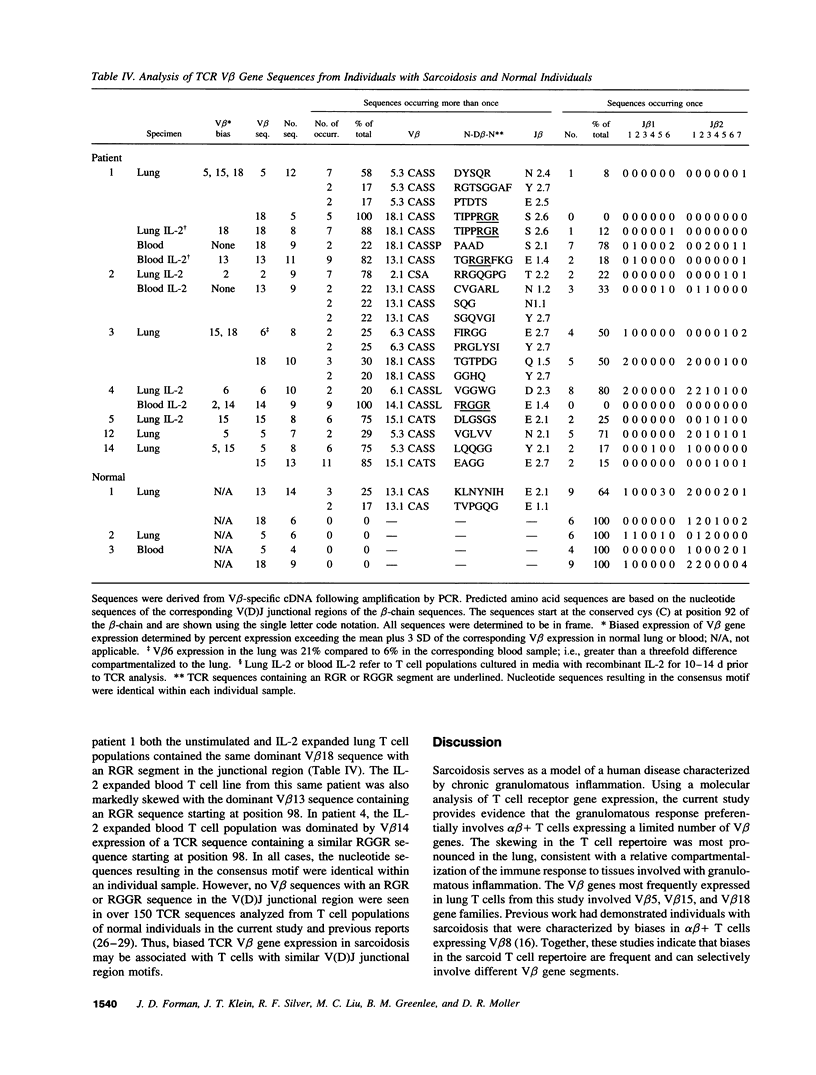
Sarcoidosis is a granulomatous disease in which activated T cells, responding to an unidentified stimulus, accumulate at sites of disease such as the lung. To evaluate the hypothesis that active sarcoidosis is characterized by a selective activation and expansion of a limited repertoire of T cell receptor (TCR) specific T cells, we analyzed TCR V beta gene expression in lung and blood T cells of patients with active sarcoidosis and, for comparison, normal individuals using polymerase chain reaction amplification of 20 V beta gene families. Analysis of normal bronchoalveolar lavage T cells revealed TCR V beta distributions similar to that of normal blood, providing evidence for a lack of generalized skewing of the T cell repertoire in the normal, noninfected lung. Compared to normal lung and blood, subgroups of individuals with sarcoidosis demonstrated biased expression of one or more V beta genes in either the lung or blood. Five V beta gene families (V beta 5, V beta 8, V beta 15, V beta 16, and V beta 18) were most frequently utilized in a biased fashion by sarcoid lung or blood T cells. Furthermore, dramatic skewing of the T cell repertoire was apparent when sarcoid lung and blood T cells were expanded by short-term culture with IL-2. Sequence analysis demonstrated a bias in V beta gene expression was usually due to expansion of select V beta-specific clones, some of which contained a similar V(D)J junctional region motif. These observations provide evidence for a selective activation and accumulation of antigen-specific V beta-expressing T cells in sarcoidosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuto O., Campen T. J., Royer H. D., Hussey R. E., Poole C. B., Reinherz E. L. Molecular analysis of T cell receptor (Ti) variable region (V) gene expression. Evidence that a single Ti beta V gene family can be used in formation of V domains on phenotypically and functionally diverse T cell populations. J Exp Med. 1985 Jun 1;161(6):1326–1343. doi: 10.1084/jem.161.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbi B., Moller D. R., Kirby M., Holroyd K. J., Crystal R. G. Increased numbers of T lymphocytes with gamma delta-positive antigen receptors in a subgroup of individuals with pulmonary sarcoidosis. J Clin Invest. 1990 May;85(5):1353–1361. doi: 10.1172/JCI114579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S. P., Ebert E. C., Blumenthal R. L., McDermott F. V., Wucherpfennig K. W., Landau S. B., Blumberg R. S. Oligoclonal expansion and CD1 recognition by human intestinal intraepithelial lymphocytes. Science. 1991 Sep 20;253(5026):1411–1415. doi: 10.1126/science.1716785. [DOI] [PubMed] [Google Scholar]
- Blumberg R. S., Yockey C. E., Gross G. G., Ebert E. C., Balk S. P. Human intestinal intraepithelial lymphocytes are derived from a limited number of T cell clones that utilize multiple V beta T cell receptor genes. J Immunol. 1993 Jun 1;150(11):5144–5153. [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C., Boswell D. R., Lesk A. M. The outline structure of the T-cell alpha beta receptor. EMBO J. 1988 Dec 1;7(12):3745–3755. doi: 10.1002/j.1460-2075.1988.tb03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- DerSimonian H., Band H., Brenner M. B. Increased frequency of T cell receptor V alpha 12.1 expression on CD8+ T cells: evidence that V alpha participates in shaping the peripheral T cell repertoire. J Exp Med. 1991 Sep 1;174(3):639–648. doi: 10.1084/jem.174.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink P. J., Matis L. A., McElligott D. L., Bookman M., Hedrick S. M. Correlations between T-cell specificity and the structure of the antigen receptor. Nature. 1986 May 15;321(6067):219–226. doi: 10.1038/321219a0. [DOI] [PubMed] [Google Scholar]
- Forrester J. M., Newman L. S., Wang Y., King T. E., Jr, Kotzin B. L. Clonal expansion of lung V delta 1+ T cells in pulmonary sarcoidosis. J Clin Invest. 1993 Jan;91(1):292–300. doi: 10.1172/JCI116184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy J. J., Oppitz U., Weyand C. M. Clonal heterogeneity of superantigen reactivity in human V beta 6+ T cell clones. Limited contributions of V beta sequence polymorphisms. J Immunol. 1992 Jan 15;148(2):604–611. [PubMed] [Google Scholar]
- Grunewald J., Janson C. H., Eklund A., Ohrn M., Olerup O., Persson U., Wigzell H. Restricted V alpha 2.3 gene usage by CD4+ T lymphocytes in bronchoalveolar lavage fluid from sarcoidosis patients correlates with HLA-DR3. Eur J Immunol. 1992 Jan;22(1):129–135. doi: 10.1002/eji.1830220120. [DOI] [PubMed] [Google Scholar]
- Grunewald J., Janson C. H., Wigzell H. Biased expression of individual T cell receptor V gene segments in CD4+ and CD8+ human peripheral blood T lymphocytes. Eur J Immunol. 1991 Mar;21(3):819–822. doi: 10.1002/eji.1830210342. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. L., Esser U., Fazekas de St Groth B., Reay P. A., Davis M. M. Mapping T-cell receptor-peptide contacts by variant peptide immunization of single-chain transgenics. Nature. 1992 Jan 16;355(6357):224–230. doi: 10.1038/355224a0. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Heat shock proteins and the immune response. Immunol Today. 1990 Apr;11(4):129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- Konishi K., Moller D. R., Saltini C., Kirby M., Crystal R. G. Spontaneous expression of the interleukin 2 receptor gene and presence of functional interleukin 2 receptors on T lymphocytes in the blood of individuals with active pulmonary sarcoidosis. J Clin Invest. 1988 Sep;82(3):775–781. doi: 10.1172/JCI113678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzin B. L., Karuturi S., Chou Y. K., Lafferty J., Forrester J. M., Better M., Nedwin G. E., Offner H., Vandenbark A. A. Preferential T-cell receptor beta-chain variable gene use in myelin basic protein-reactive T-cell clones from patients with multiple sclerosis. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9161–9165. doi: 10.1073/pnas.88.20.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann P. V., Forsthuber T., Miller A., Sercarz E. E. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992 Jul 9;358(6382):155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- Malhotra U., Spielman R., Concannon P. Variability in T cell receptor V beta gene usage in human peripheral blood lymphocytes. Studies of identical twins, siblings, and insulin-dependent diabetes mellitus patients. J Immunol. 1992 Sep 1;149(5):1802–1808. [PubMed] [Google Scholar]
- Moller D. R., Konishi K., Kirby M., Balbi B., Crystal R. G. Bias toward use of a specific T cell receptor beta-chain variable region in a subgroup of individuals with sarcoidosis. J Clin Invest. 1988 Oct;82(4):1183–1191. doi: 10.1172/JCI113715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel P. A., Livingstone A. M., Fathman C. G. Correlation of T cell receptor V beta gene family with MHC restriction. J Exp Med. 1987 Aug 1;166(2):583–588. doi: 10.1084/jem.166.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss P. A., Moots R. J., Rosenberg W. M., Rowland-Jones S. J., Bodmer H. C., McMichael A. J., Bell J. I. Extensive conservation of alpha and beta chains of the human T-cell antigen receptor recognizing HLA-A2 and influenza A matrix peptide. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8987–8990. doi: 10.1073/pnas.88.20.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksenberg J. R., Panzara M. A., Begovich A. B., Mitchell D., Erlich H. A., Murray R. S., Shimonkevitz R., Sherritt M., Rothbard J., Bernard C. C. Selection for T-cell receptor V beta-D beta-J beta gene rearrangements with specificity for a myelin basic protein peptide in brain lesions of multiple sclerosis. Nature. 1993 Mar 4;362(6415):68–70. doi: 10.1038/362068a0. [DOI] [PubMed] [Google Scholar]
- Paliard X., West S. G., Lafferty J. A., Clements J. R., Kappler J. W., Marrack P., Kotzin B. L. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991 Jul 19;253(5017):325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- Rosenberg W. M., Moss P. A., Bell J. I. Variation in human T cell receptor V beta and J beta repertoire: analysis using anchor polymerase chain reaction. Eur J Immunol. 1992 Feb;22(2):541–549. doi: 10.1002/eji.1830220237. [DOI] [PubMed] [Google Scholar]
- Saltini C., Spurzem J. R., Lee J. J., Pinkston P., Crystal R. G. Spontaneous release of interleukin 2 by lung T lymphocytes in active pulmonary sarcoidosis is primarily from the Leu3+DR+ T cell subset. J Clin Invest. 1986 Jun;77(6):1962–1970. doi: 10.1172/JCI112525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura N., Holroyd K. J., Banks T., Kirby M., Okayama H., Crystal R. G. Diversity in junctional sequences associated with the common human V gamma 9 and V delta 2 gene segments in normal blood and lung compared with the limited diversity in a granulomatous disease. J Exp Med. 1990 Jul 1;172(1):169–181. doi: 10.1084/jem.172.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. D., Hunninghake G. W. Current concepts of the pathogenesis of sarcoidosis. Am Rev Respir Dis. 1987 Mar;135(3):747–760. doi: 10.1164/arrd.1987.135.3.747. [DOI] [PubMed] [Google Scholar]
- Toyonaga B., Yoshikai Y., Vadasz V., Chin B., Mak T. W. Organization and sequences of the diversity, joining, and constant region genes of the human T-cell receptor beta chain. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8624–8628. doi: 10.1073/pnas.82.24.8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnacliffe A., Kefford R., Milstein C., Forster A., Rabbitts T. H. Sequence and evolution of the human T-cell antigen receptor beta-chain genes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5068–5072. doi: 10.1073/pnas.82.15.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyemura K., Ohmen J. D., Grisso C. L., Sieling P. A., Wyzykowski R., Reisinger D. M., Rea T. H., Modlin R. L. Limited T-cell receptor beta-chain diversity of a T-helper cell type 1-like response to Mycobacterium leprae. Infect Immun. 1992 Nov;60(11):4542–4548. doi: 10.1128/iai.60.11.4542-4548.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. H., Ohmen J. D., Uyemura K., Rea T. H., Kronenberg M., Modlin R. L. Selection of T lymphocytes bearing limited T-cell receptor beta chains in the response to a human pathogen. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):188–192. doi: 10.1073/pnas.90.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Ota K., Endo N., Seidman J. G., Rosenzweig A., Weiner H. L., Hafler D. A. Shared human T cell receptor V beta usage to immunodominant regions of myelin basic protein. Science. 1990 May 25;248(4958):1016–1019. doi: 10.1126/science.1693015. [DOI] [PubMed] [Google Scholar]
- van Schooten W. C., Ko J. L., van der Stoep N., Haanen J. B., Pickering L., de Vries R. R., van den Elsen P. T-cell receptor beta-chain gene usage in the T-cell recognition of Mycobacterium leprae antigens in one tuberculoid leprosy patient. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11244–11248. doi: 10.1073/pnas.89.23.11244. [DOI] [PMC free article] [PubMed] [Google Scholar]