Abstract
The flux of terrestrially derived pathogens to coastal waters presents a significant health risk to marine wildlife, as well as to humans who utilize the nearshore for recreation and seafood harvest. Anthropogenic changes in natural habitats may result in increased transmission of zoonotic pathogens to coastal waters. The objective of our work was to evaluate how human-caused alterations of coastal landscapes in California affect the transport of Toxoplasma gondii to estuarine waters. Toxoplasma gondii is a protozoan parasite that is excreted in the feces of infected felids and is thought to reach coastal waters in contaminated runoff. This zoonotic pathogen causes waterborne toxoplasmosis in humans and is a significant cause of death in threatened California sea otters. Surrogate particles that mimic the behavior of T. gondii oocysts in water were released in transport studies to evaluate if the loss of estuarine wetlands is contributing to an increased flux of oocysts into coastal waters. Compared to vegetated sites, more surrogates were recovered from unvegetated mudflat habitats, which represent degraded wetlands. Specifically, in Elkhorn Slough, where a large proportion of otters are infected with T. gondii, erosion of 36% of vegetated wetlands to mudflats may increase the flux of oocysts by more than 2 orders of magnitude. Total degradation of wetlands may result in increased Toxoplasma transport of 6 orders of magnitude or more. Destruction of wetland habitats along central coastal California may thus facilitate pathogen pollution in coastal waters with detrimental health impacts to wildlife and humans.
Estuaries are recognized as being critically endangered worldwide. Pollution of estuarine waters is a significant threat to the health of aquatic life, as well as to humans who depend on coastal habitats (23). Contamination of nearshore waters with terrestrially derived, zoonotic pathogens has received little attention in the field of marine water pollution, which has primarily focused on chemical and nutrient pollutants (22, 42, 46, 55). Yet, studies have documented the presence of fecal pathogens from terrestrial animals in coastal waters and filter-feeding shellfish (7, 37, 48), as well as infections and deaths in aquatic wildlife and humans who become exposed through recreation activities or seafood (4, 18, 39). The zoonotic parasite Toxoplasma gondii is emerging as an important waterborne pathogen in both human and marine wildlife populations (2, 3, 6, 11, 15, 38). Consumption of raw oysters, clams, or mussels has recently been determined to be a risk factor for human exposure to T. gondii (24). Moreover, this parasite is an important cause of death in threatened Southern sea otters (Enhydra lutris nereis) (10, 29). Sea otter infection appears most likely to result from ingestion of environmentally resistant T. gondii oocysts that reach coastal waters in contaminated freshwater runoff (35, 36). These oocysts are shed in the feces of infected wild and domestic felids, with an individual cat capable of shedding up to 1 billion oocysts over several days postinfection (12).
Elkhorn Slough, within Monterey Bay in California, is one of the high-risk sites for sea otter infection with T. gondii, with seroprevalence rates of 79% in otters sampled in this area (35). To date, the reasons for the high sea otter prevalence of infections with T. gondii at this site remain unknown. This estuarine habitat has been extensively altered by human activities and is listed as an impaired body of water by the State of California (9). Specifically, extensive degradation has been observed in the slough, with over one-third of vegetated wetlands converted to mudflats due to erosion (49). While the effect of this landscape alteration on the transport of waterborne pathogens is not currently known, such degradation may facilitate contamination of nearshore waters with T. gondii.
Wetland habitats provide valuable ecosystem services, including improvement of effluent water quality characteristics through removal of a variety of pollutants (28, 50, 57). Artificially constructed wetlands are now used globally in water treatment facilities to remove nutrients, chemical pollutants, and fecal pathogens from contaminated waters before discharge into receiving water bodies (8, 17, 21, 26, 27). However, compared with freshwater and constructed wetlands, significantly less research has focused on the effects of natural, estuarine wetlands on water quality. In the few studies that investigated the impact of saltwater marshes on marine water quality, these habitats were shown to reduce concentrations of chemicals and nutrients that reach coastal waters in contaminated overland runoff (5, 51). In addition, the percentage of watershed-impervious surface coverage and reduction of natural coastal habitats due to anthropogenic changes has been associated with increased coastal water pollution (33, 34). Despite previous research suggesting a link between wetland degradation and coastal pathogen pollution (5, 33, 34, 51), the role estuarine wetlands play in the transport of terrestrial pathogens from land to sea has not been previously investigated.
The overall goal of our research was to evaluate the effect of coastal wetland degradation on contamination of estuarine and coastal waters with terrestrially derived, zoonotic pathogens. Specifically, the objective of this study was to measure T. gondii oocyst transport through vegetated estuarine wetlands and nonvegetated mudflats to quantify the effect of vegetation loss on the flux of this zoonotic pathogen to coastal waters. Due to the biohazard risks associated with the release of environmentally resistant oocysts, experiments used previously validated surrogate microspheres and a specially designed flume that was deployed in vegetated and mudflat (nonvegetated) estuarine wetland habitats. The flume-in-field study design allowed for replication of experiments using specific hydrological parameters while conducting the study within a natural estuarine environment with in situ vegetation, substrate, and water. The two autofluorescent microspheres used in this study have similar physical and surface chemistry properties to T. gondii oocysts and have been previously evaluated as surrogate particles for this protozoan parasite (44). Our results provide novel insights into the consequences of changes in coastal habitat on the ecology of zoonotic infectious disease organisms in coastal marine ecosystems.
MATERIALS AND METHODS
Surrogate microspheres.
Two types of autofluorescent, carboxylate-modified polystyrene microspheres, previously evaluated as T. gondii surrogate particles (44), were used in this study: Dragon Green (DG) microspheres (10.35-μm diameter; density, 1.06 specific gravity; COOH, 1.0 μEq/g titration) and Glacial Blue (GB) microspheres (8.6-μm diameter; density, 1.06. specific gravity; COOH, 800 μEq/g) were obtained from Bangs Laboratory, Fishers, IN (product numbers FC07F/5493 and PC06N/8319, respectively).
Field measurements.
To conduct flume experiments that simulated realistic hydrological parameters of estuarine wetlands, field measurements, including current velocity and water depth, were collected during several ebb tide cycles within mudflats and vegetated wetland habitats. Water depth measurements were obtained using HOBO water level data loggers (ONSET, Pocasset, MA) that were deployed for 24 to 72 h at both habitat types simultaneously; a total of nine deployments were conducted. During the ebb tide cycle, current velocity profiles were measured within mudflats and vegetated sites by using a FlowTracker handheld acoustic Doppler velocimeter (SonTek/YSI, San Diego, CA). Velocity profiles were measured by obtaining a current velocity reading at 2-cm increments within the water column. Measurements were conducted on five different tide cycles, and at least three profiles were obtained during the same tide cycle for each habitat type. The current velocity and water depth measurements collected under natural ebb tide conditions are reported as supplemental data (see Table S1 in the supplemental material).
Experimental setup.
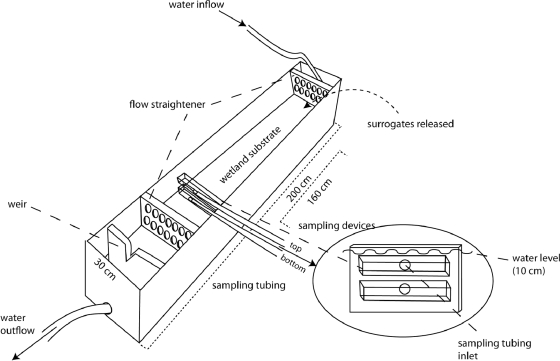
Transport experiments were conducted in the Doran Park coastal wetlands in Bodega Harbor (CA), within a bottomless polypropylene flume (200 cm long, 30 cm wide, 25 cm tall) that was placed in either mudflat or vegetated wetland sites (Fig. 1). Estuarine wetlands in this location are pickleweed (Salicornia virginica)-dominated marshes that include Jaumea (Jaumea carnosa) and salt grass (Distichlis spicata) and are similar to wetland vegetation present in Elkhorn Slough (16a). Reported water quality parameters from Elkhorn Slough (including temperature, pH, turbidity, and salinity) were also within the range that was recorded for the water pumped from the tidal channel (Doran Park) during the field transport experiments (Table 1). The flume was pressed into the ground to prevent leakage, and water from a nearby tidal creek was pumped using a WX10 four-stroke water pump (Honda, Tokyo, Japan) into the inflow end of the flume at a discharge rate that would yield current velocities representing natural ebb tide conditions. A discharge hose was connected at the distal end of the flume to drain water away from the flume. To reduce artificial turbulence, two flow-straightener devices were used within the flume, one immediately downstream of the inflow hose and the second positioned behind the water sampling device at the outflow end. A weir was inserted downstream of the second straightener device and was used in conjunction with the water pump settings to achieve a constant depth of 10 cm concurrent with desired velocities for all experiments. The device for sampling water consisted of two separate rectangular orifices (15 cm by 3 cm by 2 cm) designed to remove fluid in a way that minimally altered the ambient flow (i.e., sample velocity was matched to ambient velocity; the proportion of the flow removed was small). These orifices were connected to two peristaltic pumps (SP100; Global Water, Gold River, CA) that continuously suctioned water from the bottom and top levels of the water column. Once the flume was positioned in the selected habitat type, the water depth and velocity were adjusted to simulate ebb tide flow conditions (and confirmed with velocity profiles from the FlowTracker velocimeter, as described above for field measurements).
FIG. 1.
Schematic diagram of the bottomless flume used in Toxoplasma gondii surrogate transport studies. The flume was placed in either mudflat or vegetated wetland habitats, and water was pumped from the nearby tidal creek into the inflow end at a rate that achieved desired current velocities, while the weir at the outflow end was used to set the water depth to 10 cm for all experiments. Two flow straighteners were used at either end of the flume to reduce turbulence associated with inflow and outflow features. Surrogates were released downstream of the inflow-end flow straightener, and water samples were collected 160 cm downstream from the release point by using two peristaltic pumps that suctioned water separately from the top and bottom depths. The figure is not drawn to scale. (Courtesy of Alison Kent.)
TABLE 1.
Water quality parameters in the Toxoplasma gondii surrogate microspheres transport studiesa
| Exptl condition | Replicate | Salinity (ppt) | pH | Turbidity (NTU) | DOC (mg/liter) | TSS (mg/liter) | TSS-C (mg/liter) | TSS-N (mg/liter) |
|---|---|---|---|---|---|---|---|---|
| Mudflat | 1 | 37 | 7.90 | 12 | 6.5 | 80 | 1.00 | 0.20 |
| 2 | 35 | 7.86 | 14 | 1.9 | 21 | 0.83 | 0.09 | |
| Mudflat slow | 1 | 35 | 7.99 | 9 | 2.6 | 14 | 0.86 | 0.08 |
| 2 | 32 | 8.32 | 14 | 1.3 | 157 | 0.75 | 0.09 | |
| Vegetated | 1 (Fall) | 36 | 7.87 | 9 | 4.2 | 57 | 0.85 | 0.08 |
| 2 (Spring) | 29 | 8.05 | 15 | 3.4 | 161 | 0.63 | 0.10 |
NTU, nephelometric turbidity units; DOC, dissolved organic carbon; TSS-C, TSS carbon component; TSS-N, TSS nitrogen component.
In each experiment, 4.8 × 106 DG and GB surrogate microspheres were released immediately downstream of the inflow straightener device. This number corresponds to a concentration of 100,000 microspheres/liter of effective volume through which particles traveled, based on sampling distance (160 cm), flume width (30 cm), and water height level (10 cm). Surrogates were diluted in 1 liter of in situ environmental water and released from a Teflon-coated 20-cm-wide rectangular pan that evenly distributed the particles across the width of the flume. Water samples were collected separately from the top and bottom sampling ports in 500-ml polystyrene bottles every 30 s for the first 3 min, every 1 min between 3 and 10 min, and every 5 min between 10 and 60 min after microspheres were released, resulting in a total of 48 water samples per release experiment. Bottles were placed in coolers and transported back to the laboratory for analysis. Immediately prior to and following the microsphere release experiment, a velocity profile was taken as described above, and total mass flux measurements were taken at the inflow and outflow locations. Prior to releasing the surrogates, a negative-control (10-liter) water sample was collected for water quality analyses. Parameters tested included salinity (Sybon refractometer [Bethesda, MD]); pH (Accumet pH meter; Fisher Scientific, Pittsburgh, PA); dissolved organic carbon (Shimadzu TOC/TN analyzer [Columbia, MD]); total suspended solids (TSS), TSS-nitrogen, and TSS-carbon (Carlo Erba NC1500; Interscience BV, Breda, Netherlands); and total dissolved solids (TDS) and turbidity (Micro 100 turbidimeter; HF Scientific Inc., Fort Myers, FL).
Surrogate microsphere recovery.
Surrogate microspheres were quantified using the membrane filtration technique as previously described (45). Briefly, water samples were well mixed, and a 100-ml aliquot was vacuum suctioned onto mixed cellulose membranes using a MicroFil filtration funnel (Millipore Corp., Billerica, MA). Two membranes were processed for each sample and scanned using a Zeiss Axioskop epifluorescence microscope equipped with a UV emission filter set (emitter, 460/50-nm band-pass filter; Chroma 11000 v3) at 100×, and the numbers of DG and GB microspheres were enumerated. In addition, for each experiment a negative-control water sample was spiked with known numbers of DG and GB microspheres and processed in an identical way as the samples to account for differential fluorescence intensities of the two microsphere types, as well as to correct for potential variability in the detection of microspheres in different water samples between experiments. For each experiment, correction coefficients were established to account for the brighter fluorescence of DG microspheres compared with GB microspheres (45).
To calculate the recovery of surrogate microspheres, the water fluxes (in liters/min) corresponding to top and bottom concentration measurements were estimated by obtaining top and bottom mean current velocities from current velocity profile measurements averaged over the uppermost and lowermost 5 cm of the water column. Water flux (in cm3/s) was then obtained from the equation Qw = u·A, where u is velocity (in cm/s) and A is the cross-sectional area of the water body within the flume for the top and bottom sections (150 cm2). The flux of microspheres, Qm (number/min) for each sampling level and time was then calculated from the equation Qm = C·Qw, where C is the concentration of the microspheres in each sample (number/liter) and Qw is the corresponding water flux (liters/min). For each microsphere release experiment, the top and bottom fluxes of DG and GB microspheres were plotted over time, curves were interpolated from the data points, and the areas under the curves were integrated to obtain a total number of microspheres recovered from the top and bottom sampling ports (Origin 7.0 Software, Northampton, MA). The percent recovery of surrogate microspheres for each experiment was calculated from the proportion of beads recovered versus beads released for each surrogate type.
Statistical analyses.
To evaluate whether vegetation, water current velocity, or both were significant predictors for surrogate recovery, a multivariate negative binomial regression model was fit to the flux of DG and GB surrogates (STATA Software, College Station, TX). The results for each release experiment were set as a group effect to adjust for repeated sampling within experiments, and the sampling time was incorporated as an exposure variable to compare flux rates for each time point between experiments.
RESULTS
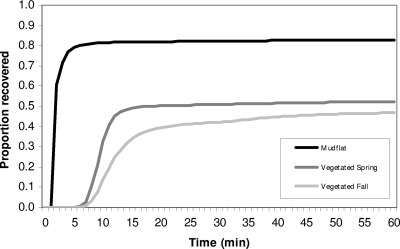
The recoveries of Toxoplasma gondii surrogate microspheres in field transport experiments conducted in mudflat habitats were higher than in vegetated wetlands (Fig. 2). The cumulative proportions of DG microspheres that were recovered over time in the mudflat and vegetated wetland experiments are presented in Fig. 2 (similar curves were obtained for cumulative recoveries of GB microspheres). In mudflat experiments, 90% of recovered microspheres reached the sampling device 1 to 3 min after time of release, producing a steep breakthrough curve. In contrast, surrogate microspheres were not detected during the vegetated wetland experiments until 7 min after surrogate release and had slower breakthrough curves, with 90% of recovered surrogates obtained by 10 min and 16 min for the Spring and Fall experiments, respectively.
FIG. 2.
Cumulative proportion of Toxoplasma gondii surrogate microspheres (Dragon Green) recovered in transport studies within vegetated and mudflat wetland habitats. Vegetated wetland experiments were performed in the Fall (dense vegetation) and in the Spring (sparse vegetation). The curve for the mudflat habitat represents the average of duplicate experiments (see Table 2).
Substrate type and current velocity were both significant predictors of surrogate recovery as determined by the statistically significant coefficients of these variables for estimating flux of microspheres based on negative binomial regression. The flux of DG and GB microspheres released in mudflats was 4.54 times (95% confidence interval [CI], 2.59 to 7.97) and 4.14 times (95% CI, 1.94 to 8.82) greater than microspheres released in vegetated sites, respectively (P < 0.001). Further, the flux of DG and GB microspheres was 5.18 times (95% CI, 3.64 to 7.37) and 5.21 times (95% CI, 3.75 to 7.24) greater under fast current velocity conditions present in mudflats than with the slower velocities present in pickleweed marsh (P < 0.001).
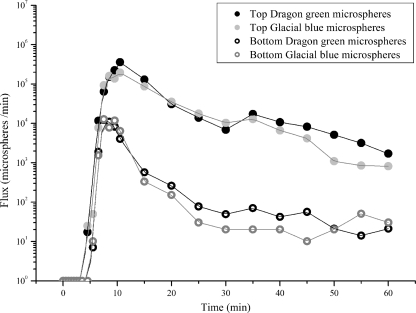
The physical and chemical water quality parameters of in situ water used in the transport studies are presented in Table 1, and the current velocities and flow settings used for the three wetland conditions are presented in Table 2. All transport experiments were conducted within a bottomless flume that was placed within either mudflat or vegetated marsh habitats and in which the water flow regimen was adjusted to simulate natural hydrodynamic parameters measured under ebb tide conditions (Fig. 1). For each surrogate release experiment, the total recovery of microspheres was calculated by measuring the flux of DG and GB microspheres per minute and integrating over time (area under the curve) (Fig. 3). Curves were constructed separately for each surrogate type and top and bottom sampling ports, as shown for one of the duplicate vegetated release experiments in Fig. 3. In all experiments, microspheres reached the top port 1 to 2 min before they were detected through the bottom port, and their flux peaked at 1 to 2 orders of magnitude higher than the surrogate flux from the bottom sampling port.
TABLE 2.
Experimental conditions and recovery rates of Toxoplasma gondii surrogate microspheres in wetland transport studies
| Exptl condition | Replicate no. | Velocity (cm/s) |
Discharge (liters/min) |
Surrogate recovery (%)a |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dragon Green |
Glacial Blue |
||||||||||
| Top | Bottom | Top | Bottom | Top | Bottom | Total | Top | Bottom | Total | ||
| Mudflat | 1 | 1.99 | 0.63 | 17.91 | 5.70 | 79.68 | 9.10 | 88.78 | 83.06 | 8.10 | 91.17 |
| 2 | 1.99 | 0.54 | 17.91 | 4.84 | 79.68 | 10.04 | 80.86 | 68.70 | 9.12 | 77.82 | |
| Mudflat slow | 1 | 0.29 | 0.15 | 2.60 | 1.33 | 58.85 | 14.11 | 72.96 | 49.42 | 12.33 | 61.76 |
| 2 | 0.34 | 0.17 | 3.05 | 1.50 | 37.39 | 21.08 | 58.47 | 38.13 | 20.76 | 58.88 | |
| Vegetated | 1 (Fall) | 0.24 | 0.10 | 2.16 | 0.88 | 47.24 | 0.87 | 48.12 | 36.57 | 0.95 | 37.52 |
| 2 (Spring) | 0.29 | 0.15 | 2.60 | 1.33 | 48.08 | 6.19 | 54.27 | 46.50 | 5.90 | 52.41 | |
The number of surrogate microspheres recovered was calculated for top and bottom samples based on the product of the discharge rate and sphere concentration for each level; the percent recovery is the proportion of spheres recovered versus spheres released (see Materials and Methods).
FIG. 3.
Flux of Toxoplasma gondii surrogate microspheres recovered in a transport experiment representative of a vegetated estuarine wetland habitat in Fall. Microspheres were sampled separately in the upper and lower water columns (top and bottom sampling devices [Fig. 1]), and fluxes were calculated for the “top” and “bottom” halves of the water column by using measured current velocities as described in Materials and Methods.
In the duplicate release experiments conducted within mudflat sites under simulated natural flow conditions, the recoveries of both DG and GB microspheres averaged 85%. The experiments in vegetated sites were conducted once in the Fall, when vegetation was dense, and once in the Spring, when marsh foliage was sparse. Recoveries of DG and GB microspheres in the Fall experiment were 48 and 38%, respectively, and in the Spring vegetated experiment recoveries were 54 and 52%, respectively (Table 2).
Because the lower recoveries of surrogates from vegetated sites may be due to the presence of the vegetation or due to the naturally slower flow velocity, transport experiments were also conducted in mudflats under artificial flow conditions that mimicked the slower current velocities present in vegetated sites. This experimental setup was designed to further evaluate if current velocity, presence of vegetation, or both, were important variables that affect pathogen transport through estuarine environments. The mean recoveries of surrogate microspheres from these slow-velocity, mudflat experiments were 66 and 60% for DG and GB surrogate microspheres, respectively (Table 2). These recoveries appear to be lower than those obtained under natural flow conditions in mudflats and higher than surrogate recoveries obtained from vegetated wetlands with comparable velocities.
DISCUSSION
The reduction of T. gondii surrogates recovered from the flow through estuarine vegetated wetlands compared to mudflats provides compelling evidence that wetlands play a critical role in reducing the transport of this zoonotic pathogen from land to sea. Increased contamination of coastal marine ecosystems with T. gondii due to degradation of coastal wetlands has significant implications to marine wildlife as well as human public health. Because the only known definitive hosts of T. gondii are terrestrial (felids) and the oocyst stage cannot reproduce in the environment, this pathogen offers a unique model to better understand the transport of land-based pathogens to coastal waters. While the surrogate microspheres used in this study were selected based on the similar surface properties they share with T. gondii oocysts, the implications of our findings may extend to other waterborne pathogens. Like the carboxylated microspheres used here as surrogates, many pathogens (including some viruses, bacteria, and protozoa) are negatively charged and relatively buoyant in water (19). Optimal removal of different classes of pathogens by wetlands can differ due to their unique physical properties and the diversity of the habitat types being evaluated; however, some reduction of pathogen concentrations has been observed in effluent waters regardless of the specific physiochemical and biological properties of the wetlands (40). Thus, landscape changes in coastal habitats that result in the loss of vegetated estuarine wetlands may facilitate the contamination of nearshore waters with waterborne pathogens that are infective for humans and marine mammals, such as the threatened Southern sea otter population.
The recoveries of T. gondii surrogate microspheres from mudflat habitats appeared to be higher than recoveries from vegetated wetlands. In addition, within wetland sites, a seasonal effect may be influencing particle recoveries due to vegetation density differences that were present between the Fall and Spring experiments. In mudflat habitats where natural flow conditions were simulated, an average of 85% of both the DG and GB surrogate microspheres was recovered during transport experiments. In comparison, only 48% of DG and 38% of GB microspheres were recovered from the vegetated marsh experiment conducted in the Fall (October 2009), when vegetation foliage was thick. As expected, when vegetation was less dense in early Spring (March 2009), higher recoveries were observed, with 54% for DG and 52% for GB microspheres. The seasonal differences in surrogate recoveries suggest that the effect of vegetated wetlands on transport of pathogens from land to sea may vary over the course of the wet season, when rainfall drives runoff events from terrestrial sources to coastal waters. Highest concentrations of contaminants in overland runoff typically occur during a “first flush” event (31), which in California occurs following heavy rains in fall or early winter, following a long, dry summer. After the ground has been saturated by precipitation, overland runoff flushes contaminants that have accumulated on land surfaces during the dry season into waterways and through estuaries to the ocean (1). The pathogen retention ability of vegetated wetlands is greatest when their foliage is thick, which in California estuaries occurs from late spring to late fall months (25). Depending on when the first heavy rains occur, the dense foliage in estuarine marshes during the Fall may maximize pathogen retention in wetlands during the first-flush event, i.e., when the flux of pathogens from watersheds is highest. Following rain events in late winter and early spring months, a relatively higher proportion of pathogens present in the water column is expected to move through the less densely vegetated wetlands, although the flux of pathogens is still expected to be lower than the flux through mudflat habitats, based on T. gondii surrogate experiment results.
The use of appropriate surrogates to evaluate the transport of T. gondii oocysts in field-based experiments was essential, as releasing oocysts in the environment is prohibited. Toxoplasma gondii oocysts can survive in water and soil for months to years (20, 32, 56). Therefore, the release of oocysts into wetlands would pose an unacceptable risk of infection to both people and animals. While inactivation of oocysts would reduce the risk, most chemical and physical methods of inactivation have proved inadequate to reliably and completely destroy oocyst viability (16, 52-54). Inactivation may also alter the surface properties of nonviable compared to viable oocysts and thus impact their transport behavior, as previously shown (30). Smaller-scale, laboratory-based experiments can be useful for comparing the transport behavior of oocysts and surrogate microspheres. However, tank experiments with infective oocysts still pose biohazard risks and preclude the ability to use in situ substrate, plants, and water, which may also impact the study results. The two microsphere types used in this study were previously selected as surrogate particles for oocysts based on the similar surface properties they share with T. gondii oocysts, including their size, specific gravity, shape, surface charge (electrophoretic mobility), and hydrophobicity characteristics (44). While neither microsphere type is identical to oocysts, the two surrogates closely bracket T. gondii in surface characteristics, which suggests that their joint use in transport studies will bracket the transport potential of T. gondii oocysts under similar environmental conditions.
At Elkhorn Slough, where a high proportion of sea otters are seropositive for T. gondii (35), 36% of vegetated wetlands eroded to mudflats following the dredging of Moss Landing harbor in 1947 (49). The effect of this anthropogenic coastal landscape change on the transport of T. gondii from land to sea can be estimated using the recoveries of surrogates obtained in our study. In the Fall vegetated site experiment, the average recovery of DG and GB surrogates from the point of microsphere release to the sampling device (1.6 m downstream) was 43%. Based on this experiment, the proportion of oocysts that are expected to be transported through each 1.6 m of vegetated marsh is 0.43, and thus the proportion transported through a continuous vegetated marsh of length L is likely to be (0.43)L/1.6. Similarly, one expects (0.85)L/1.6 to be transported through a mudflat habitat. Considering a 30-m wetland in Elkhorn Slough prior to vegetation loss, one expects (0.43)30/1.6 oocysts to pass through the wetland and enter coastal waters. If that land plot experienced the observed average of 36% vegetation loss, 11 m of the 30-m marsh would erode to mudflat, increasing the oocyst flux to (0.43)19/1.6 (0.85)11/1.6. Putting this into context, one bowel movement from an infected domestic cat that is shedding oocysts can contain more than 100 million T. gondii oocysts, as documented in oocyst production experiments in our laboratory and elsewhere (12). Based on studies with the related protozoan Cryptosporidium (47), it is conceivable that 10 million (10%) oocysts would become entrained in runoff following a rainfall event. If that feline scat were deposited in proximity to a vegetated marsh, only a single oocyst would escape into coastal waters, whereas under the same scenario, 128 oocysts would escape from a 36% devegetated wetland. Thus, the observed 36% decrease in vegetated estuarine wetlands corresponds to a 2-orders of magnitude increase in oocyst transport through estuarine wetlands to coastal waters. Considering that infection with T. gondii can occur following ingestion of a single oocyst (13, 14), this represents a significant rise in the risk of infection to susceptible hosts following exposure to oocysts in coastal habitats. Furthermore, in some areas of Elkhorn Slough, nearly all historically vegetated habitats have undergone erosion (49). In locations where complete erosion has occurred, the oocyst flux through every 10 m of previously vegetated habitat increases by approximately 2 orders of magnitude. Considering 10 million T. gondii oocysts present in the runoff (as described above) and a 30-m stretch of densely vegetated wetland habitat that has been completely eroded to mudflat, the single oocyst that escaped pre-1947 increases to 500,000 oocysts delivered to coastal waters.
The presence of vegetation in estuarine wetlands is expected to have two effects on the concentration of oocysts in the water: (i) vegetation slows flow velocities, increasing settling due to increased transport time; (ii) vegetation provides structures in the water that can remove oocysts through straining and adhesion processes, such as attachment to biofilms (43). To separate these effects, a “mud-slow” experiment was conducted using slow velocities typical of vegetated marsh habitats but measured over a bare mud substrate. The recoveries of surrogate microspheres from the slow mudflat experiments were higher than for vegetated conditions but lower than mudflat experiments using current velocities typically measured over mudflats. These results suggest that the lower recovery of surrogates from vegetated wetland sites is due to the combination of slower currents and the direct effect of vegetation structure, as supported by the significance of both variables for predicting surrogate flux (negative binomial model [see Results]). Also, the results suggest that increased tidal velocity over preexisting mudflats will further increase the flux of T. gondii to open coastal waters used by otters and other susceptible species. The ongoing loss of vegetated wetland habitats in Elkhorn Slough is a result of tidal scour of marshland, caused by increased tidal velocities, tidal amplitude, and duration time of land inundation (49). Indeed, there is a general loss of wetlands in California and elsewhere, with many vegetated wetlands converted to pavement and with runoff routed to storm drains, from which it is expected there would be close to 100% recovery of oocysts in a 1.6-m experimental flume. Taken together, this loss of vegetated wetlands is likely to account for an increase in oocyst flux to open coastal waters of several orders of magnitude.
The slow recovery of the threatened California sea otter population has been attributed in part to high mortality of prime reproductive age adults from infectious diseases, with terrestrially derived protozoal infections a leading cause of death in this species (29). Identification of factors that facilitate exposure of otters to terrestrial, waterborne pathogens can provide scientific-based guidelines to assist conservation, management, and policy decisions that aim to reduce further contamination of coastal ecosystems. The results presented here highlight the beneficial role estuarine wetlands provide in reducing the flow of zoonotic pathogens to marine waters and suggest that wetland conservation and restoration can contribute to a management plan designed to reduce exposure of sea otters and other marine mammals to terrestrial pathogens.
In addition to the numerous ecosystem services wetlands are known to provide, this study offers quantitative data that support the crucial role vegetated estuarine wetlands play in improving water quality by removal of terrestrially derived pathogens from effluent waters. The loss of estuarine wetlands is a global phenomenon; 67% of wetland habitats along estuaries and coastal seas have been lost (23). Rising sea levels resulting from global climate change are also expected to lead to inundation and subsequent loss of estuarine wetlands in locations where the marshland cannot retreat inland due to urbanization or agricultural practices (41). Management decisions for land can thus have a substantial impact on the water quality of our oceans. The degradation, removal, and replacement of estuarine wetlands contribute to increased concentrations of zoonotic pathogens in coastal waters, where they can accumulate and pose significant health risks to wildlife and humans.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation, Ecology of Infectious Disease grant 0525765, and through support provided by the Wildlife Health Center, School of Veterinary Medicine, University of California, Davis.
We thank Aiko Adell Nakashima and David Dann for assistance with field experiments and sample analyses. We also thank Bill Newman for constructing the flume, Alison Kent for drafting the flume diagram, and Tad (Timothy) Doane for conducting the water quality analyses.
This publication is a contribution of the Bodega Marine Laboratory, University of California, Davis.
Footnotes
Published ahead of print on 27 August 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Asaf, L., R. Nativ, D. Shain, M. Hassan, and S. Geyer. 2004. Controls on the chemical and isotopic compositions of urban stormwater in a semiarid zone. J. Hydrol. 294:270-293. [Google Scholar]
- 2.Bahia-Oliveira, L. M., J. L. Jones, J. Azevedo-Silva, C. C. Alves, F. Orefice, and D. G. Addiss. 2003. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerg. Infect. Dis. 9:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benenson, M. W., E. T. Takafuji, S. M. Lemon, R. L. Greenup, and A. J. Sulzer. 1982. Oocyst-transmitted toxoplasmosis associated with ingestion of contaminated water. N. Engl. J. Med. 307:666-669. [DOI] [PubMed] [Google Scholar]
- 4.Bogomolni, A., R. Gast, J. Ellis, M. Dennett, K. Pugliares, B. Lentell, and M. Moore. 2008. Victims or vectors: a survey of marine vertebrate zoonoses from coastal waters of the Northwest Atlantic. Dis. Aquat. Organ. 81:13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowen, J. L., and I. Valiela. 2004. Nitrogen loads to estuaries: using loading models to assess the effectiveness of management options to restore estuarine water quality. Estuaries 27:482-500. [Google Scholar]
- 6.Bowie, W. R., A. S. King, D. H. Werker, J. L. Isaac-Renton, A. Bell, S. B. Eng., and S. A. Marion. 1997. Outbreak of toxoplasmosis associated with municipal drinking water. The BC Toxoplasma Investigation Team. Lancet 350:173-177. [DOI] [PubMed] [Google Scholar]
- 7.Brands, D. A., A. E. Inman, C. P. Gerba, C. J. Mare, S. J. Billington, L. A. Saif, J. F. Levine, and L. A. Joens. 2005. Prevalence of Salmonella spp. in oysters in the United States. Appl. Environ. Microbiol. 71:893-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brix, H. 1994. Use of constructed wetlands in water pollution control: historical development, present status, and future perspectives. Water Sci. Technol. 30:209-223. [Google Scholar]
- 9.California Environmental Protection Agency. September 2009, posting date. California's 2006 Clean Water Act Section 303(d) list of water quality limited segments requiring TMDLS. State Water Resources Control Board, Sacramento, CA. http://www.swrcb.ca.gov/water_issues/programs/tmdl/docs/303dlists2006/epa/r3_06_303d_reqtmdls.pdf.
- 10.Conrad, P. A., M. A. Miller, C. Kreuder, E. R. James, J. Mazet, H. Dabritz, D. A. Jessup, F. Gulland, and M. E. Grigg. 2005. Transmission of Toxoplasma: clues from the study of sea otters as sentinels of Toxoplasma gondii flow into the marine environment. Int. J. Parasitol. 35:1155-1168. [DOI] [PubMed] [Google Scholar]
- 11.Darde, M. L., I. Villena, J. M. Pinon, and I. Beguinot. 1998. Severe toxoplasmosis caused by a Toxoplasma gondii strain with a new isoenzyme type acquired in French Guyana. J. Clin. Microbiol. 36:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubey, J. P., and J. K. Frenkel. 1972. Cyst-induced toxoplasmosis in cats. J. Protozool. 19:155-177. [DOI] [PubMed] [Google Scholar]
- 13.Dubey, J. P., J. K. Lunney, S. K. Shen, O. C. Kwok, D. A. Ashford, and P. Thulliez. 1996. Infectivity of low numbers of Toxoplasma gondii oocysts to pigs. J. Parasitol. 82:438-443. [PubMed] [Google Scholar]
- 14.Dubey, J. P., C. A. Speer, S. K. Shen, O. C. Kwok, and J. A. Blixt. 1997. Oocyst-induced murine toxoplasmosis: life cycle, pathogenicity, and stage conversion in mice fed Toxoplasma gondii oocysts. J. Parasitol. 83:870-882. [PubMed] [Google Scholar]
- 15.Dubey, J. P., R. Zarnke, N. J. Thomas, S. K. Wong, W. Van Bonn, M. Briggs, J. W. Davis, R. Ewing, M. Mense, O. C. Kwok, S. Romand, and P. Thulliez. 2003. Toxoplasma gondii, Neospora caninum, Sarcocystis neurona, and Sarcocystis canis-like infections in marine mammals. Vet. Parasitol. 116:275-296. [DOI] [PubMed] [Google Scholar]
- 16.Dumetre, A., C. Le Bras, M. Baffet, P. Meneceur, J. P. Dubey, F. Derouin, J. P. Duguet, M. Joyeux, and L. Moulin. 2008. Effects of ozone and ultraviolet radiation treatments on the infectivity of Toxoplasma gondii oocysts. Vet. Parasitol. 153:209-213. [DOI] [PubMed] [Google Scholar]
- 16a.Elkhorn Slough Tidal Wetland Project Team. 2007. Elkhorn Slough tidal wetland strategic plan. Elkhorn Slough Foundation, Watsonville, CA. http://library.elkhornslough.org/twp/ESTWP/ESTWP_PLAN_050207_lres.pdf.
- 17.Falabi, J. A., C. P. Gerba, and M. M. Karpiscak. 2002. Giardia and Cryptosporidium removal from waste-water by a duckweed (Lemna gibba L.) covered pond. Lett. Appl. Microbiol. 34:384-387. [DOI] [PubMed] [Google Scholar]
- 18.Fayer, R., J. P. Dubey, and D. S. Lindsay. 2004. Zoonotic protozoa: from land to sea. Trends Parasitol. 20:531-536. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson, C., A. Husman, N. Altavilla, D. Deere, and N. Ashbolt. 2003. Fate and transport of surface water pathogens in watersheds. Crit. Rev. Environ. Sci. Technol. 33:299-361. [Google Scholar]
- 20.Frenkel, J. K., A. Ruiz, and M. Chinchilla. 1975. Soil survival of Toxoplasma oocysts in Kansas and Costa Rica. Am. J. Trop. Med. Hyg. 24:439-443. [DOI] [PubMed] [Google Scholar]
- 21.Gerba, C. P., J. A. Thurston, J. A. Falabi, P. M. Watt, and M. M. Karpiscak. 1999. Optimization of artificial wetland design for removal of indicator microorganisms and pathogenic protozoa. Water Sci. Technol. 40:363-368. [Google Scholar]
- 22.Howarth, R., A. Sharpley, and D. Walker. 2002. Sources of nutrient pollution to coastal waters in the United States: implications for achieving coastal water quality goals. Estuaries 25:656-676. [Google Scholar]
- 23.Jackson, J. 2008. Ecological extinction and evolution in the brave new ocean. Proc. Natl. Acad. Sci. U. S. A. 105:11458-11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, J. L., V. Dargelas, J. Roberts, C. Press, J. S. Remington, and J. G. Montoya. 2009. Risk factors for Toxoplasma gondii infection in the United States. Clin. Infect. Dis. 49:878-884. [DOI] [PubMed] [Google Scholar]
- 25.Josselyn, M. 1983. The ecology of San Francisco Bay tidal marshes: a community profile, vol. FWS/OBS-83/23. U.S. Fish and Wildlife Service, Washington, DC.
- 26.Kao, C. M., J. Y. Wang, H. Y. Lee, and C. K. Wen. 2001. Application of a constructed wetland for non-point source pollution control. Water Sci. Technol. 44:585-590. [PubMed] [Google Scholar]
- 27.Kao, C. M., J. Y. Wang, and M. J. Wu. 2001. Evaluation of atrazine removal processes in a wetland. Water Sci. Technol. 44:539-544. [PubMed] [Google Scholar]
- 28.Knox, A., R. Dahgren, K. Tate, and E. Atwill. 2008. Efficacy of natural wetlands to retain nutrient, sediment and microbial pollutants. J. Environ. Qual. 37:1837-1846. [DOI] [PubMed] [Google Scholar]
- 29.Kreuder, C., M. A. Miller, D. A. Jessup, L. J. Lowenstine, M. D. Harris, J. A. Ames, T. E. Carpenter, P. A. Conrad, and J. A. Mazet. 2003. Patterns of mortality in southern sea otters (Enhydra lutris nereis) from 1998-2001. J. Wildl. Dis. 39:495-509. [DOI] [PubMed] [Google Scholar]
- 30.Kuznar, Z. A., and M. Elimelech. 2005. Role of surface proteins in the deposition kinetics of Cryptosporidium parvum oocysts. Langmuir 21:710-716. [DOI] [PubMed] [Google Scholar]
- 31.Lee, J., K. Bang, L. Ketchum, J. Choe, and M. Yu. 2002. First flush analysis of urban storm runoff. Sci. Total Environ. 293:163-175. [DOI] [PubMed] [Google Scholar]
- 32.Lindsay, D. S., and J. P. Dubey. 2009. Long-term survival of Toxoplasma gondii sporulated oocysts in seawater. J. Parasitol. 95:1019-1020. [DOI] [PubMed] [Google Scholar]
- 33.Mallin, M., S. Ensign, M. McIver, G. Shank, and P. Fowler. 2001. Demographic, landscape, and meteorological factors controlling the microbial pollution of coastal waters. Hydrobiologia 460:185-193. [Google Scholar]
- 34.Mallin, M., K. Williams, E. Esham, and R. Lowe. 2000. Effect of human development on bacteriological water quality in coastal watersheds. Ecol. Appl. 10:1047-1056. [Google Scholar]
- 35.Miller, M. A., I. A. Gardner, C. Kreuder, D. M. Paradies, K. R. Worcester, D. A. Jessup, E. Dodd, M. D. Harris, J. A. Ames, A. E. Packham, and P. A. Conrad. 2002. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis). Int. J. Parasitol. 32:997-1006. [DOI] [PubMed] [Google Scholar]
- 36.Miller, M. A., W. A. Miller, P. A. Conrad, E. R. James, A. C. Melli, C. M. Leutenegger, H. A. Dabritz, A. E. Packham, D. Paradies, M. Harris, J. Ames, D. A. Jessup, K. Worcester, and M. E. Grigg. 2008. Type X Toxoplasma gondii in a wild mussel and terrestrial carnivores from coastal California: new linkages between terrestrial mammals, runoff and toxoplasmosis of sea otters. Int. J. Parasitol. 38:1319-1328. [DOI] [PubMed] [Google Scholar]
- 37.Miller, W., M. Miller, I. Gardner, E. Atwill, M. Harris, J. Ames, D. Jessup, A. Melli, D. Paradies, K. Worcester, P. Olin, N. Barnes, and P. Conrad. 2005. New genotypes and factors associated with Cryptosporidium detection in mussels (Mytilus spp.) along the California coast. Int. J. Parasitol. 35:1103-1113. [DOI] [PubMed] [Google Scholar]
- 38.Palanisamy, M., B. Madhavan, M. B. Balasundaram, R. Andavar, and N. Venkatapathy. 2006. Outbreak of ocular toxoplasmosis in Coimbatore, India. Indian J. Ophthalmol. 54:129-131. [DOI] [PubMed] [Google Scholar]
- 39.Pancorbo, O., and H. Barnhart. 1992. Microbioal pathogens and indicators in estuarine environments and shellfish: critical need for better indicator(s) of human-specific fecal pollution. J. Environ. Health 54:57-63. [Google Scholar]
- 40.Reinoso, R., L. Torres, and E. Becares. 2008. Efficiency of natural systems for removal of bacteria and pathogenic parasites from wastewater. Sci. Total Environ. 395:80-86. [DOI] [PubMed] [Google Scholar]
- 41.Scavia, D., J. Field, D. Boesch, R. Buddemeier, V. Burkett, D. Cayan, M. Fogarty, M. Harwell, R. Howarth, C. Mason, D. Reed, T. Royer, A. Sallenger, and J. Titus. 2002. Climate change impacts on US coastal and marine ecosystems. Estuaries 25:149-164. [Google Scholar]
- 42.Schiedek, D., B. Sundelin, J. Readman, and R. Macdonald. 2007. Interactions between climate change and contaminants. Mar. Pollut. Bull. 54:1845-1856. [DOI] [PubMed] [Google Scholar]
- 43.Searcy, K. E., A. I. Packman, E. R. Atwill, and T. Harter. 2006. Capture and retention of Cryptosporidium parvum oocysts by Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 72:6242-6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shapiro, K., J. Largier, J. A. Mazet, W. Bernt, J. R. Ell, A. C. Melli, and P. A. Conrad. 2009. Surface properties of Toxoplasma gondii oocysts and surrogate microspheres. Appl. Environ. Microbiol. 75:1185-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shapiro, K., J. A. Mazet, A. Schriewer, S. Wuertz, H. Fritz, W. A. Miller, J. Largier, and P. A. Conrad. 2010. Detection of Toxoplasma gondii oocysts and surrogate microspheres in water using ultrafiltration and capsule filtration. Water Res. 44:893-903. [DOI] [PubMed] [Google Scholar]
- 46.Smith, V. 2003. Eutrophication of freshwater and coastal marine ecosystems: a global problem. Environ. Sci. Pollut. Res. 10:126-139. [DOI] [PubMed] [Google Scholar]
- 47.Tate, K., M. Pereira, and E. Atwill. 2004. Efficacy of vegetated buffer strips for retaining Cryptosporidium parvum. J. Environ. Qual. 33:2243-2251. [DOI] [PubMed] [Google Scholar]
- 48.Touron, A., T. Berthe, G. Gargala, M. Fournier, M. Ratajczak, P. Servais, and F. Petit. 2007. Assessment of faecal contamination and the relationship between pathogens and faecal bacterial indicators in an estuarine environment (Seine, France). Mar. Pollut. Bull. 54:1441-1450. [DOI] [PubMed] [Google Scholar]
- 49.Van Dyke, E., and K. Wasson. 2005. Historical ecology of a central California estuary: 150 years of habitat change. Estuaries 28:173-189. [Google Scholar]
- 50.Verhoeven, J. T. A., B. Arheimer, C. Q. Yin, and M. M. Hefting. 2006. Regional and global concerns over wetlands and water quality. Trends Ecol. Evol. 21:96-103. [DOI] [PubMed] [Google Scholar]
- 51.Villar, C., J. Stripeikis, M. Tudino, L. d'Huicque, O. Troccoli, and C. Bonetto. 1999. Trace metal concentrations in coastal marshes of the Lower Parana River and the Rio de la Plata Estuary. Hydrobiologia 397:187-195. [Google Scholar]
- 52.Wainwright, K. E., M. Lagunas-Solar, M. A. Miller, B. C. Barr, I. A. Gardner, C. Pina, A. C. Melli, A. E. Packham, N. Zeng, T. Truong, and P. A. Conrad. 2007. Physical inactivation of Toxoplasma gondii oocysts in water. Appl. Environ. Microbiol. 73:5663-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wainwright, K. E., M. Lagunas-Solar, M. A. Miller, B. C. Barr, A. C. Melli, A. E. Packham, N. Zeng, T. Truong, and P. A. Conrad. 2010. Radiofrequency- induced thermal inactivation of Toxoplasma gondii oocysts in water. Zoonoses Public Health 57:74-81. [DOI] [PubMed] [Google Scholar]
- 54.Wainwright, K. E., M. A. Miller, B. C. Barr, I. A. Gardner, A. C. Melli, T. Essert, A. E. Packham, T. Truong, M. Lagunas-Solar, and P. A. Conrad. 2007. Chemical inactivation of Toxoplasma gondii oocysts in water. J. Parasitol. 93:925-931. [DOI] [PubMed] [Google Scholar]
- 55.Wurl, O., and J. Obbard. 2004. A review of pollutants in the sea-surface microlayer (SML): a unique habitat for marine organisms. Mar. Pollut. Bull. 48:1016-1030. [DOI] [PubMed] [Google Scholar]
- 56.Yilmaz, S. M., and S. H. Hopkins. 1972. Effects of different conditions on duration of infectivity of Toxoplasma gondii oocysts. J. Parasitol. 58:938-939. [PubMed] [Google Scholar]
- 57.Zedler, J. B., and S. Kercher. 2005. Wetland resources: status, trends, ecosystem services, and restorability. Annu. Rev. Environ. Resour. 30:39-74. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.