Abstract
A54145 factors are calcium-dependent lipopeptide antibiotics produced by Streptomyces fradiae NRRL 18160. A54145 is structurally related to the clinically important daptomycin, and as such may be a useful scaffold for the development of a novel lipopeptide antibiotic. We developed methods to genetically manipulate S. fradiae by deletion mutagenesis and conjugal transfer of plasmids from Escherichia coli. Cloning the complete pathway on a bacterial artificial chromosome (BAC) vector and the construction of ectopic trans-complementation with plasmids utilizing the φC31 or φBT1 site-specific integration system allowed manipulation of A54145 biosynthesis. The BAC clone pDA2002 was shown to harbor the complete A54145 biosynthesis gene cluster by heterologous expression in Streptomyces ambofaciens and Streptomyces roseosporus strains in yields of >100 mg/liter. S. fradiae mutants defective in LptI methyltransferase function were constructed, and they produced only A54145 factors containing glutamic acid (Glu12), at the expense of factors containing 3-methyl-glutamic acid (3mGlu12). This provided a practical route to produce high levels of pure Glu12-containing lipopeptides. A suite of mutant strains and plasmids was created for combinatorial biosynthesis efforts focused on modifying the A54145 peptide backbone to generate a compound with daptomycin antibacterial activity and activity in Streptococcus pneumoniae pulmonary infections.
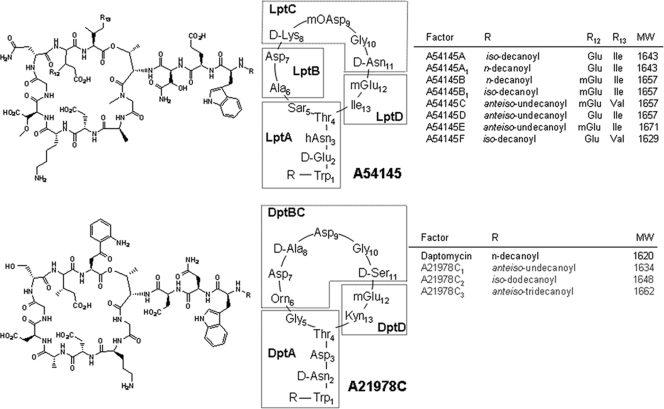
The calcium-dependent cyclic acidic lipodepsipeptide antibiotics were first reported in the 1980s and 1990s (8). These include A21978C, produced by Streptomyces roseosporus (17, 18), calcium-dependent antibiotic (CDA), produced by Streptomyces coelicolor (26), and A54145, produced by Streptomyces fradiae NRRL 18160 (11, 12, 23). A21978C (Fig. 1) has been of particular interest because the N-decanoyl lipid tail derivative of the A21978C peptide is daptomycin (8), which is approved for the treatment of complicated skin and skin structure infections caused by Gram-positive bacteria (2) and for bacteremia and right-sided endocarditis caused by Staphylococcus aureus, including strains resistant to methicillin (MRSA) (21). Daptomycin lacks efficacy in community-acquired pneumonia (CAP) infections, even though it is very active in vitro against the predominant pathogen, Streptococcus pneumoniae (8, 43). In vitro studies have shown that daptomycin becomes sequestered in bovine pulmonary surfactant, most likely in the lipid component, and has decreased antibacterial potency against Gram-positive pathogens (46); this may be a significant factor contributing to the poor clinical efficacy in CAP. Attempts to improve the efficacy of daptomycin through chemical modifications of the lipid side chain or additions to the δ-amino group of ornithine (Orn6) (reviewed in reference 8), or by molecular engineering of peptide assembly (4, 13, 25, 37-39), have not generated a lead molecule with sufficient in vivo efficacy in a mouse pneumonia model for S. pneumoniae.
FIG. 1.
Structures of the lipopeptide antibiotics and NRPS protein subunit relationships. (Top) A54145 factors normally produced by S. fradiae. Note that factors A, A1, D, and F have Glu at position 12, and factors B, B1, C, and E have 3mGlu at position 12. (Bottom) A21978C factors normally produced by S. rosesosporus and daptomycin.
A54145 factors share a number of features in common with daptomycin, but they differ at several amino acid positions (Fig. 1). The most biologically active A54145 factors against S. aureus contain four modified amino acids, l-hydroxy-Asn2 (hAsn2), sarcosine5 (Sar5), l-methoxy-Asp9 (mOAsp9), and l-3-methyl-Glu12 (3mGlu12) (14). During a standard fermentation, multiple A54145 factors are produced as the result of natural variation at position 12 (3mGlu or Glu), at position 13 (Ile or Val) and at the lipid tail attached to the peptide core. The A54145 factors A, A1, and D (collectively designated the A-core) have the identical peptide containing Glu12 and Ile13 but have different lipid tails, whereas factors B, B1, and E (the B-core) contain 3mGlu12 and Ile13. During fermentation of S. fradiae, factor A accumulates as a major component but plateaus early, and factor B1 accumulates preferentially late in the fermentation (11, 12). In studies at Eli Lilly and Company, it was shown that the B-core factors were slightly more potent antibiotics, but factor B was substantially more toxic than its Glu12-containing counterpart, factor A1 (14).
During the development of molecular engineering approaches to modify daptomycin biosynthesis, the genes for A54145 lipopeptide biosynthesis (lpt) were cloned and sequenced to provide nonribosomal peptide synthetase (NRPS) modules and subunits to exchange with those of daptomycin (37). Since some of the A54145 A-core factors were shown to be much less inhibited by bovine surfactant than daptomycin (40), the A54145 A-core lipopeptides should be useful starting points for both chemical and molecular engineering modification studies. We initiated a program to develop molecular genetics methods, with plasmids and host cloning strains to facilitate molecular engineering of A54145 biosynthesis in S. fradiae.
In this report, we describe the engineering of a bacterial artificial chromosome (BAC) containing the A54145 biosynthesis genes by using λ-Red-mediated recombination in Escherichia coli and expression of the A54145 biosynthesis pathway in heterologous streptomycetes. The development of S. fradiae strains deleted for multiple A54145 genes and the construction of plasmid vectors with conjugation and site-specific integration functions for ectopic expression of sets of A54145 biosynthesis genes in S. fradiae and combinatorial biosynthesis (40) are discussed. This genetic system was used to generate a strain with deletion of lptI, a gene that encodes a methyltransferase involved in the biosynthesis of 3mGlu12, and the mutant produced the desired A-core lipopeptides containing Glu12, which are important starting materials for medicinal chemistry approaches to produce novel lipopeptides.
MATERIALS AND METHODS
Strains, plasmids, and general methods.
Key bacterial strains and plasmids are listed in Table 1. Chemicals were purchased from Sigma-Aldrich Co. (Milwaukee, WI) unless noted otherwise. E. coli strains were grown in LB broth or on LB agar (45) at 37°C, except when the temperature-sensitive plasmid pKD119, used for λ-Red-mediated recombination in BW25113 (16), was present. Where appropriate, media were supplemented with ampicillin (Amp; 100 μg/ml), apramycin (Am; 100 μg/ml), chloramphenicol (Cm; 25 μg/ml), tetracycline (Tc; 12.5 μg/ml), streptomycin (Sm; 100 μg/ml), or hygromycin B (Hm; 50 μg/ml), unless stated otherwise. Hm was purchased from Roche Applied Science (Indianapolis, IN). Plasmids were introduced by electroporation into E. coli; strain DH10B (Invitrogen, Carlsbad, CA) was used for general cloning, and ML22 (13) was used as the donor in conjugations. λ-Red-mediated recombination was carried out as described previously (16). Oligonucleotide primer sequences (“primers”) are listed in Table S1 of the supplemental material. Streptomycete mycelial cultures were preserved in 20% (wt/vol) glycerol at −80°C. S. fradiae spores generated on modified R2YE (mR2YE) agar plates (29) were stored in centrifuge tubes as 1-ml aliquots in 10% (wt/vol) glycerol plus 0.1% (wt/vol) Tween 20 at −80°C.
TABLE 1.
Key strains and plasmids in this study
| Strain or plasmid (reference) | Relevant characteristicsa |
|---|---|
| S. fradiae strains | |
| NRRL 18160 (11) | A54145 factor high producer |
| XH25 | NRRL 18160 Smr (rpsL K88R) |
| JR397 | XH25 ΔlptI |
| DA613 | XH25 ΔlptI::tsr |
| DA728 | XH25 ΔlptD |
| DA740 | XH25 ΔlptBCD |
| DA895 | XH25 ΔlptD ΔlptI::tsr |
| DA901 | XH25 ΔlptBCD ΔlptI::tsr |
| DA1187 | XH25 ΔlptEF-I::tsr |
| DA1077 | XH25 ΔlptI::tsr + ermEp* lptI (pDA1660) |
| DA1087 | XH25 ΔlptI::tsr + ermEp* dptI (pDA1658) |
| DA1096 | XH25 ΔlptI::tsr + lptI (pCV16) |
| DA1102 | XH25 ΔlptD + lptD-I (pDA2016) |
| DA1106 | XH25 ΔlptD + lptB-I (pDA2012) |
| DA1109 | XH25 ΔlptBCD + lptB-I (pDA2012) |
| DA1204 | XH25 ΔlptBCD ΔlptI::tsr + lptB-I (pDA2012) |
| DA1206 | XH25 ΔlptBCD ΔlptI::tsr + lptB-H (pDA2048) |
| DA1444 | XH25 ΔlptBCD ΔlptI::tsr + lptBC (pDA2040) + lptD-I (pDA2106) |
| DA1324 | XH25 ΔlptEF-I::tsr + lptEF-I (pJR153) |
| DA1674 | XH25 ΔlptEF-I::tsr + lptEF-P (pDA2054) |
| DA1676 | XH25 ΔlptEF-I::tsr + lptEF-I (pDA2002) |
| S. roseosporus strains | |
| UA431 (39) | Daptomycin nonproducer, ΔdptEF-J::tsr (“ΔdptEF-J::tsr”) |
| DA1155 | UA431 + lptEF-I (pDA2002) |
| DA1156 | UA431 + lptEF-I (pDA2002) |
| DA1160 | UA431 + pV89 (vector control) |
| S. ambofaciens strains | |
| BES2074 (44) | Spiramycin nonproducer, srmR7::Tn10 Nm |
| DA1163 | BES2074 + lptEF-I (pDA2002) |
| DA1164 | BES2074 + lptEF-I (pDA2002) |
| DA1168 | BES2074 + pV89 (vector control) |
| Plasmids | |
| pRHB538 (28) | rep(Ts) Am oriT |
| pJC06 | pRHB538::ΔlptD |
| pJC07 | pRHB538::ΔlptBCD |
| pJC11 | pRHB538::ΔlptI |
| pJC12 | pRHB538::ΔlptI::tsr |
| pDA1715 | pRHB538::ΔlptEF-I |
| pDA1716 | pRHB538::ΔlptEF-I::tsr |
| pDA1600 | pNEB193::Tsrr cassette |
| pDA1650 | Modified pIJ8660 (multiple cloning site) |
| pDA1652 | pDA1650::ermE*p |
| pDA1658 | pDA1652::ermE*p::lptI |
| pDA1660 | pDA1652::ermE*p::dptI |
| pDA1800 | pBR322::pECBAC1-HindIII + φC31 cassette + orf21 |
| pDA1803 | pBR322::pECBAC1-HindIII + φBT1 cassette + orf21 |
| pDA1811 | pBR322::pECBAC1 + fC31 cassette + ermE*p::lptB |
| pDA1814 | pBR322::pECBAC1 + φC31 cassette + ermE*p::lptD |
| pDA1834 | pBR322::pECBAC1 + φBT1 cassette + ermE*p::lptD |
| pCB01 | pECBAC1::lpt gene cluster |
| pJR153 | pCB01::φBT1 cassette at orf21 |
| pDA2002 | pCB01::φC31 cassette at orf21 |
| pDA2012 | pCB01::φC31 cassette + ermE*p::lptB |
| pDA2016 | pCB01::φC31 cassette + ermE*p::lptD |
| pDA1836 | pOJ260 + to terminator cassette |
| pDA2040 | pCB01::φC31 cassette + ermE*p::lptB, to terminator cassette in lptD (pDA2012) |
| pDA2048 | pCB01::φC31 cassette + ermE*p::lptB, to terminator cassette in lptJ (pDA2012) |
| pDA2054 | pCB01::φC31 cassette at orf21, to terminator cassette in lptI (pDA2002) |
| pDA2106 | pCB01::φBT1 cassette + ermE*p::lptD |
| pV89 | pNEB193::φC31 cassette |
| pCV16 (35) | pStreptoCos with partial lpt gene cluster including lptI, φC31 cassette |
lptD-I, lptDGHJKLMNPI; lptB-I, lptBCDGHJKLMNPI; lptB-H, lptBCDGH; lptEF-I, lptEFABCDGHJKLMNPI; lptEF-P, lptEFABCDGHJKLMNP; dptEF-J, dptEFABCDGHJ.
Isolation of rpsL mutants and testing of the selection method.
A positive selection system that exploits the recessive nature of rpsL mutations associated with streptomycin resistance (Smr) in streptomycetes (28) was tested during the initial work with S. fradiae. In S. roseosporus, a wild-type rpsL+ allele (Sms) on the clone and delivery vector pRHB538 used to introduce in vitro-modified DNA sequences into an rpsL (Smr) host by allelic exchange renders merodiploids or intermediates with a single-crossover insertion of the plasmid into the chromosome inviable on Sm-containing medium. Smr recombinants can be recovered if there is a second crossover, because the desired homologous replacement at the target locus also leads to loss of the rpsL+ vector. To isolate an rpsL (Smr) strain, spores of S. fradiae NRRL18160 were plated onto AS-1 agar containing 50 or 100 μg/ml Sm. Presumptive Smr mutants were retested on AS-1 agar (3) plus 100 μg/ml Sm, and the rpsL gene from each strain was PCR amplified and sequenced using primers P1 and P2. The wild-type rpsL gene sequence has been deposited in GenBank as HM043809. Construction of the lptI deletion strain, DA613(ΔlptI::tsr), by utilizing pJC12 was employed to test the system in the rpsL mutant, XH25. A Tsrr cassette between 2-kb fragments homologous to chromosomal regions flanking lptI was introduced into S. fradiae, and two Smr exconjugants were grown in the presence of thiostrepton (Tsr), shifted to a nonpermissive temperature (38°C) for 48 h, and plated on medium with Tsr or Tsr plus Sm at 38°C. Putative double-crossover mutants (Sms Tsrr) were confirmed by growth on agar medium plates with appropriate antibiotic amendments and by PCR of the lptI region. The frequency of mutants was <5% and nearly equal for medium with or without Sm, suggesting that the rpsL counterselection alone was not sufficiently stringent in the S. fradiae XH25 background to kill plasmid-containing strains. Nonetheless, the other elements of the strategy remained effective, so we continued to use the method for other constructions.
Construction of lptI deletion plasmids (pJC11 and pJC12).
Fragments of 2 kb flanking lptI were amplified from cosmid pCV16 (36) with primers P3 (with a HindIII recognition site extension) and P4 (XbaI) for the upstream fragment and with primers P5 (XbaI) and P6 (EcoRI) for the downstream fragment. The amplicons were digested with the appropriate restriction enzymes and inserted into EcoRI- and HindIII-digested pRHB538 (28). The resulting plasmid, pJC11, contains a 4-kb insert with an in-frame deletion of lptI that retains 30 bp of the coding sequence from the start of translation and 24 bp from the stop and is centered on the unique XbaI site. To generate pJC12, a SmaI fragment containing a Tsr and Cm resistance gene cassette (Tsrr cassette) was removed from pDA1600 and inserted into pJC11, after the latter was digested with XbaI and end filled by Klenow polymerase.
Construction of lptEFABCDGHJKLMNPI, lptD, and lptBCD deletion plasmids (pDA1716, pJC06, and pJC07).
A 3.6-kb fragment upstream of lptEF (lpt bp 39600 to 43259) was subcloned from S. fradiae cosmid 12G11 (36) into pNEB193 (New England Biolabs, Ipswich, MA) and excised as an EcoRI-XbaI fragment to replace the EcoRI-XbaI upstream lptI region in pJC11 to create pDA1715 (ΔlptEFABCDGHJKLMNPI). Insertion of a Tsrr cassette at the XbaI site then generated pDA1716 (ΔlptEFABCDGHJKLMNPI::tsr).
To generate pJC06 (ΔlptD), 3.0-kb regions flanking lptD were amplified from pCV15 (36) with primers P11 (HindIII) and P12 (XbaI) for the upstream fragment and primers P9 (XbaI) and P10 (BamHI) for the downstream fragment and then cloned as blunt-ended amplicons into EcoRV-digested pZERO (Invitrogen). The upstream fragment (lptC to its stop codon ending in the XbaI site), excised with HindIII and XbaI, was then ligated with the downstream fragment (beginning at the putative ribosome binding site and start codon for lptG), excised with XbaI and BamHI, into BamHI- and HindIII-digested pRHB538. For pJC07 (ΔlptBCD), a 2.0-kb fragment upstream of lptB was amplified from cosmid pCV14 (36) using primers P7 (EcoRI) and P8 (BamHI), cloned as a blunt-ended fragment into pZERO, and then subcloned as an EcoRI-BamHI fragment into compatibly cut pBluescript SK (Stratagene). This fragment (lptA to the lptA stop codon ending with BamHI) was then released by digestion as a HindIII-XbaI fragment and ligated to the same downstream fragment used in the construction of pJC06.
Construction of lptI complementation plasmids.
Plasmid pIJ8660 (48) was modified to enable heterologous gene expression under the control of the ermE* promoter (9). In pDA1650, the BamHI-to-PacI region of the pIJ8660 multiple cloning site was replaced by EcoRI, BamHI, and PacI sites, and the NotI site upstream of the terminator was converted to an XbaI site (see construction details and the plasmid map in Fig. S1 of the supplemental material). The ermE* promoter was removed from pIJ4070 (29) as an EcoRI and BamHI fragment and subcloned into pDA1650 to generate pDA1652. The lptI gene was amplified from pCV16 using primers P39 (NdeI) and P40 (XbaI), digested with NdeI and XbaI, and subcloned into pDA1652 digested with the same enzymes to generate pDA1658. Similarly, dptI from S. roseosporus (dpt bp 99447 to 100466; GenBank accession number AY787762) was amplified from pCV1 (35) using primers P41 (NdeI) and P42 (XbaI) and subcloned into pDA1652 to generate pDA1660.
Construction of integrative BACs carrying the lpt cluster (pDA2002 and pJR153).
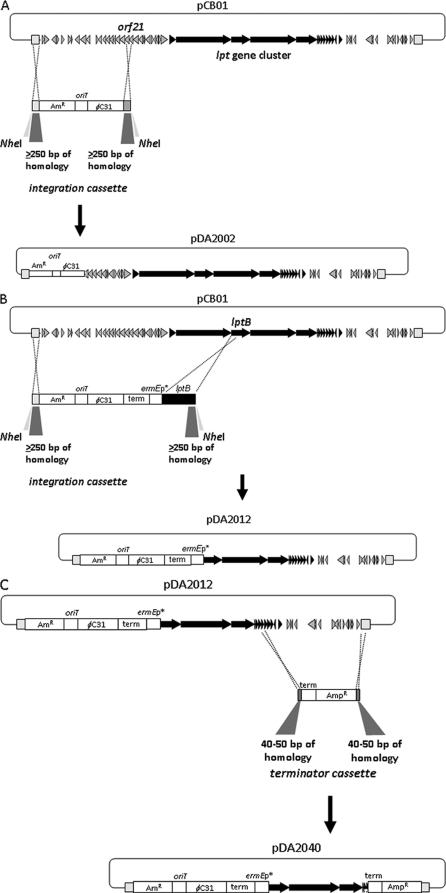
A BAC library (Amplicon Express, Pullman, WA) constructed in the E. coli vector pECBAC1 (22) from a Sau3AI partial digest of genomic DNA from S. fradiae NRRL 18160 protoplasts was screened by PCR with primers P13 and P14 for orf21, upstream of the lpt cluster, and with primers P5 and P6 for lptI, at the end of the cluster, to identify BACs carrying the entire lpt cluster, e.g., pCB01. A Streptomyces integration cassette containing Amr, oriT, and φC31 attP/int functions (φC31 cassette) was subcloned from pStreptoBAC V (35) as a DraI-AatII fragment into the SmaI site of pNEB193 (generating pV89) and then excised via KpnI and BamHI sites in the vector. Flanking DNA fragments needed to target and recombine this cassette into pCB01 were generated by PCR: a 261-bp upstream fragment from the HindIII side of the pECBAC1 backbone was amplified using primers P15 (EcoRI and NheI) and P16 (KpnI) and digested with EcoRI and KpnI; a 1.4-kb downstream product including part of orf21 was amplified using P19 (BamHI) and P20 (XbaI) and digested with BamHI and XbaI. To assemble and clone these fragments, a 2.6-kb portion of pBR322 (GenBank accession number number J01749; pBR322 bp 1692 to 4300, containing the replication origin and Ampr gene) was amplified using primers P21 (XbaI) and P22 (EcoRI and NheI) and digested with XbaI and EcoRI. The φC31 cassette was ligated with the upstream fragment, the downstream fragment, and the pBR322 backbone to generate pDA1800 (pECBAC1-HindIII). The φC31 cassette was removed from pDA1800 as a KpnI-BamHI fragment and replaced with a 4.8-kb KpnI-SpeI fragment containing Hmr, oriT, and φBT1 att/int functions (φBT1 cassette) from MS82 (24) to generate pDA1803. The ∼7-kb NheI fragments comprised of the φC31 cassette, pECBAC1, and orf21 regions were removed from pDA1800 and pDA1803 and electroporated into λ-Red gene-induced E. coli BW25113 carrying pKD119 (16) and pCB01 (previously introduced by electroporation) for λ-Red-mediated recombination. Strains with recombinant BACs were selected using Cmr and Amr (φC31 cassette) or Cmr and HmR (φBT1 cassette), and insertions of the cassettes at orf21 in pCB01 were confirmed by restriction and PCR analyses, yielding pDA2002 and pJR153, respectively.
Construction of integrative, ermE*p-containing BACs (pDA2012, pDA2016, and pDA2106).
Derivatives of pCB01 with the ermE* promoter inserted immediately upstream of the start codon of lptB or lptD were constructed by amplifying either a 1.2-kb fragment with P23 (NdeI) and P24 (XbaI) (for lptB) or an ∼0.4-kb fragment with P25 (NdeI) and P26 (XbaI) (for lptD), digesting each with NdeI and XbaI, and subcloning into a holding vector. The ermE* promoter and adjacent fd terminator upstream were excised as a 0.9-kb SphI-NdeI fragment from pDA1652 and ligated with an NdeI-XbaI-excised lptB fragment to replace the orf21 region (excised with SphI and XbaI) of pDA1800, resulting in pDA1811. Subsequent replacement of the lptB fragment in pDA1811 with the NdeI-XbaI-excised lptD fragment generated pDA1814. Finally, replacement of the φC31 cassette in pDA1814 with the φBT1 cassette resulted in pDA1834. The ∼6- to 7-kb NheI fragments from these plasmids containing the φC31 or φBT1 cassette and flanking regions were excised from the plasmids and used for λ-Red-mediated recombination into pCB01 to generate the BACs pDA2012 (φC31, ermE*p preceding lptB) and pDA2016 (φC31, ermE*p preceding lptD), after selection for Amr and Cmr, and pDA2106 (φBT1, ermE*p preceding lptD) after selection for Hmr and Cmr.
Construction of BACs with to terminator-Ampr cassette (pDA2048, pDA2040, and pDA2054).
A 272-bp PCR product containing the λ to terminator was amplified from pIJ8660 using the primers P27 (EcoRI) and P28 (NotI), and the Ampr gene was amplified from pBR322 as a 1.3-kb PCR product using the primers P29 (NotI) and P30 (HindIII plus P1 primer binding site) (pBR322 bp 3024 to 4225). These amplicons were digested with the appropriate restriction enzymes, and the plasmid pOJ260 (10) was digested with EcoRI and HindIII; all three DNA fragments were ligated together to generate pDA1836.
The to terminator-Ampr cassette was amplified from pDA1836 for λ-Red recombination by using one primer containing a terminal 45 to 50 nucleotides (nt) of gene-specific sequence for targeted recombination on the BAC insert and 23 nt of proximal vector sequence (AGCGGATAACAATTTCACACAGGA) upstream of to and a second primer with a terminal 45 to 50 nt of gene-specific sequence for targeted recombination and a proximal 21 nt (TGTGTAGGCTGGAGCTGCTTC) downstream of Ampr. Templates made in this manner were used to modify pDA2012 and make pDA2048 and pDA2040 (deleting lptJ or lptD and downstream DNA, respectively, from pCB01 derivatives) and to modify pDA2002 to make pDA2054 (deleting lptI and other downstream DNA).
Conjugal transfer of plasmids from E. coli to streptomycetes.
Thawed S. fradiae spore suspensions were centrifuged (typically at 800 × g for 10 min), and the pelleted spores were washed, resuspended in 0.5 ml spore germination medium (29), heated at 50°C for 10 min, and then gently shaken in 10 ml germination medium at 30°C for 2 to 4 h. The germinated spores were collected by centrifugation, washed, and resuspended in 10 ml LB. Overnight cultures of E. coli donor strains were diluted 1:40 into 10 ml LB plus appropriate antibiotics, grown for 2 to 3 h, then pelleted by centrifugation, washed, and resuspended in 2 ml LB. Aliquots (0.1 ml) of E. coli and S. fradiae cells mixed at 1:4 and 4:1 (vol/vol) were spread on ISP4 agar (Difco, Sparks, MD) with 10 mM MgCl2 and incubated at 30°C. After 18 to 24 h, plates were overlaid with 3 ml R2 soft agar (7) containing antibiotics to the following final plate concentrations: trimethoprim (Tmp; 50 μg/ml), to select against E. coli donor cells, and either Tsr (10 μg/ml), Am (10 μg/ml), or Hm (150 μg/ml) to select for plasmid-containing S. fradiae exconjugants. Colonies that appeared in 3 to 5 days were patched onto mR2YE agar containing appropriate antibiotics.
For gene replacements in S. fradiae XH25 and derivatives using pRHB538-based plasmids that carried a Tsrr cassette, exconjugants (Amr Tsrr) carrying plasmids were grown with Tsr at 30°C for 24 h, homogenized, and inoculated into fresh CSM (27) plus Tsr at 38°C for 48 h. Then, the cultures were homogenized, diluted, and grown for 48 h on mR2YE agar with Tsr or Tsr plus Sm at 38°C and finally plated and grown for 48 h on media with Tsr or Tsr plus Sm at 38°C. Putative double-crossover mutants (Ams Tsrr) were confirmed by patching colonies onto plates with appropriate antibiotic supplements and by PCR analysis of the appropriate chromosomal locus for the expected changes in amplicon size or sequence analysis or both. For markerless gene replacements into S. fradiae XH25 derivatives carrying a Tsrr cassette at the target locus, the Amr initial exconjugants were screened for loss of the plasmid conferring Amr and loss of the Tsrr cassette by patching mycelia onto mR2YE agar plates containing Tsr, Am, or no antibiotic and confirmed by PCR.
S. ambofaciens and S. roseosporus strains grown for 24 to 48 h in trypticase soy broth (TSB) were homogenized, pelleted by centrifugation, washed, and resuspended in TSB. E. coli donors were prepared and mixed as described above with each streptomycete and plated onto AS-1 agar supplemented with 10 mM MgCl2 (10) for S. roseosporus or with 10 mM MgCl2 and 20 mM N-[Tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid (TES; pH 7.6) (1) for S. ambofaciens. After 18 to 24 h at 30°C, plates were overlaid with 2.5 ml of soft nutrient agar (29) containing nalidixic acid (Nal; 50 μg/ml) to select against E. coli donor cells and Am (50 μg/ml) to select for streptomycetes containing plasmid. Amr colonies were patched onto mR2YE agar plates containing Am and Nal.
Fermentation and measurement of lipopeptide production.
S. fradiae strains were grown for 24 h in CSM broth (27) containing the appropriate antibiotics to generate liquid starter cultures. Starter culture aliquots (1 ml) were transferred to a 125-ml baffled flask containing 25 ml of seed medium A355 (35) and grown at 30°C for 24 h at 200 rpm (5-cm orbit). Seed culture (2 ml) was transferred to a 250-ml baffled flask containing 50 ml of DSF production medium (12) plus 0.79% (wt/vol) l-Ile (DSF-Ile) unless otherwise noted. For DSF medium, dextrose was purchased from Cargill (Minneapolis, MN), nutrisoy was from ADM (Decatur, IL), molasses was from SVG (Bologna, Italy), and all other reagents were from Sigma. Production cultures were grown at 30°C for 6 days at 200 rpm, 5-cm orbit, S. roseosporus and S. ambofaciens strains were grown for 48 h in TSB as starter cultures and for 48 h in A355, and fermentations were carried out in DSF-Ile. Culture supernatants were harvested by centrifugation of whole culture broth at 24,000 × g for 15 min at 4°C, followed by a second identical centrifugation step. Production of A54145 factors and hybrid lipopeptides was analyzed with an Agilent 1100 high-performance liquid chromatography (HPLC) system with a Waters Symmetry C8 column (4.6 by 250 mm; 5 μm; Waters, Milford, MA) with a Waters C8 Guard column. Mobile solvent systems were water buffered with 0.01% (vol/vol) trifluoroacetic acid (TFA) (solvent A) and acetonitrile buffered with 0.01% (vol/vol) TFA (solvent B). Solvent B was increased linearly from 30% to 45% over 14 min at a flow rate of 1.5 ml/min. UV detection was performed at 224 nm. Lipopeptide concentrations were determined by comparison with reference A54145 factor D purified from S. fradiae fermentations and determined to be ≥98% pure (J.-Q. Gu, personal communication). Total lipopeptide values reported are from triplicate production flasks, except where replicate strains were each fermented only once in the heterologous complementation experiments. For LC-mass spectrometry (MS) characterization of the lipopeptide compounds, separation was performed on a Waters Symmetry C8 column (4.6 by 250 mm). Acetonitrile-water containing 0.01% TFA was used as the mobile phase under a linear gradient elution mode (acetonitrile, 0 to 90%; 32.5 min) at a flow rate of 1.5 ml/min. UV detection was performed at 224 nm. The mass spectrometer used was an LCQ DECA XP (Thermo, Waltham, MA). Mass spectra were determined using Q1 as the scanning quadrupole at an electrospray ionization voltage of 5 kV and capillary temperature of 225°C. A mass range of m/z 50 to 2,000 was covered with a scan time of 1 s, and data were collected in the positive ion mode using an electron multiplier voltage of 1,100 V.
A54145 factor production in shake flask cultures was assessed using an S. aureus ATCC 29213 well bioassay as described previously (13). An S. aureus seeded soft agar overlay (5 mM CaCl2) poured over 48- to 72-h-old S. fradiae colonies patched onto AS-1 agar plates (34) was also effective for detecting bioactivity by growth inhibition.
RESULTS AND DISCUSSION
Validation of the lpt gene cluster by heterologous expression.
To facilitate the rapid engineering of the A54145 biosynthesis gene cluster in E. coli, it was necessary to have the complete pathway cloned in a single vector. As none of the previously reported S. fradiae cosmid library clones contained the entire pathway (36), a BAC library based on the E. coli vector pECBAC1 was constructed. PCR screening was used to identify multiple candidates containing all of the contiguous loci of the A54145 biosynthesis pathway. The BAC pCB01, with a >100-kb insert, was chosen for further work.
Before pCB01 could be introduced into Streptomyces, an integration cassette comprised of an origin of transfer (oriT from RP4/RK2) for conjugation and the Amr bifunctional marker for selection in E. coli and Streptomyces as well as the φC31 attP/int functions for Streptomyces site-specific integration (altogether referred to as the φC31 cassette) was added. Flanking regions of homology from pCB01, represented by amplicons from the vector backbone and from orf21, 12 kb upstream from the lpt biosynthesis loci (and presumably well beyond the region required for A54145 biosynthesis) were added to each side of the cassette and inserted into pCB01 by λ-Red-mediated recombination (Fig. 2 A). This not only added the φC31 cassette into the plasmid but also removed non-lpt DNA upstream of orf21.
FIG. 2.
Examples of using λ-Red-mediated recombination in E. coli to engineer BAC pCB01 and derivatives. (A) Insertion of a cassette to drive conjugation from E. coli and site-specific integration in Streptomyces species, generating pDA2002. (B) Integration of the conjugation/insertion promoter cassette and deletion of the genes upstream of lptB to generate pDA2012. (C) Further deletion of genes from pDA2012 and insertion of a terminator cassette to generate pDA2040.
To confirm that the complete set of A54145 biosynthesis genes was contained on the BAC, pDA2002 was introduced into S. ambofaciens BES2074, a spiramycin-nonproducing strain (20, 44), and S. roseosporus UA431, a daptomycin-nonproducing strain deleted for the entire dpt gene cluster (39). These two strains were derived in research programs for other natural products and are amenable to genetic manipulation (15, 30, 32-34); they have been previously engineered to eliminate production of their major secondary metabolites. Table 2 shows that S. ambofaciens and S. roseosporus exconjugants expressing the lpt gene cluster from the φC31 integration site produced A54145 lipopeptide factors, while strains containing an empty vector produced no lipopeptides. Both sets of heterologous strains were more efficient than S. fradiae at producing the fully modified A54145 B-core lipopeptides. The yields in S. roseosporus were about 100 mg/liter (24% of the S. fradiae high-producer control), whereas the yields in S. ambofaciens averaged about 385 mg/liter (92% of control). The relatively high yields may be related to the history of the hosts, in that their progenitors were originally developed for robust performance in fermentations for production of secondary metabolites (>250 mg/liter for A21978C factors [39] and 1.7 g/liter for spiramycin [20]). The production of A54145 in S. ambofaciens was particularly striking and suggests that BES2074 may be a more universal host for the expression of both macrolide and other secondary metabolite biosynthesis gene clusters, including cryptic pathways identified in genomics programs (5). The chemical analyses were consistent with S. aureus well bioassays in which fermentation broth from pDA2002-containing strains gave large zones of inhibition while strains containing an empty vector at φC31 attB showed no zones of inhibition.
TABLE 2.
Production of A54145 factors in S. ambofaciens BES2074 and S. roseosporus UA431
| Strain | Plasmid | Lipopeptide yield (mg/liter) | % of controla | A-core (%) |
|---|---|---|---|---|
| S. fradiae NRRL 18160 | 423 ± 11 | 100 | 63 | |
| S. ambofaciens DA1163 | pDA2002 | 362 | 86 | 22 |
| S. ambofaciens DA1164 | pDA2002 | 410 | 97 | 27 |
| S. ambofaciens DA1168 | pV89 | 0 | 0 | 0 |
| S. roseosporus DA1155 | pDA2002 | 106 | 25 | 22 |
| S. roseosporus DA1156 | pDA2002 | 95 | 22 | 2 |
| S. roseosporus DA1160 | pV89 | 0 | 0 | 0 |
Results for the control, S. fradiae NRRL18160, are shown here and in Table 3. The lipopeptide yields from heterologous expression strains were determined from single fermentations in a separate experiment.
These results not only confirm that all of the genes required for A54145 biosynthesis and transport are present in pDA2002, but also they may shed light on the mechanism of selection of lipopeptide side chains during lipopeptide assembly. Both recombinants produced the major lipopeptides normally produced by S. fradiae. This implies that the fused LptEF enzyme (acyl coenzyme A ligase and acyl carrier protein) chooses iso-decanoyl, n-decanoyl, and anteiso-undecanoyl over other available lipid precursors. In contrast, the DptE and DptF proteins expressed in S. roseosporus choose a different set of lipid precursors that are on average two carbon units longer (anteiso-undecanoyl, iso-dodecanoyl, and anteiso-tridecanoyl) when producing A21978C. Also, it was shown previously that Streptomyces lividans containing the cloned daptomycin biosynthesis genes produced the three most common A21978C lipopeptides normally produced in S. roseosporus (6, 42). These observations support the notion that the fused LptEF enzyme and the separate DptE and DptF enzymes have modestly different lipid substrate specificities that override any differences in lipid pools in the four Streptomyces species used in expression studies (6).
System for genetic engineering of A54145 biosynthesis.
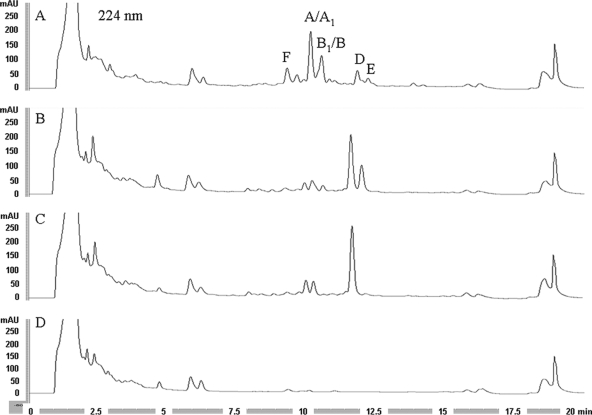
Acquiring a complete, validated lpt pathway in one clone launched the development of several generations of modified hosts and complementation plasmids aimed at engineering A54145 biosynthesis in vivo (40). The starting strain for modifications at the lpt locus was S. fradiae XH25. This strain, bearing a spontaneous mutation in rpsL causing the amino acid substitution K88R, was one of several tested during evaluation of a positive selection system (see Materials and Methods) and was used here because of its reliable and robust production of A54145 factors in shake flasks (112% of control) (Table 3). In some streptomycetes, Smr strains with mutations in rpsL exhibit elevated production of secondary metabolites; in S. coelicolor and S. lividans, K88E substitutions are known to enhance antibiotic production (41). In S. fradiae, a mutant expressing a K88E substitution produced 88% of control antibiotic production (Table 3). Fermentations of XH25 and derivatives were conducted using the optimized DSF-Ile medium developed in this study. This modification did not affect the overall titer but simplified HPLC analysis by driving production toward the Ile13-containing factors that are measured to represent total lipopeptide (Fig. 3 A and B). Because L-Ile feeding enriches for factors containing Ile13 and anteiso-undecanoyl side chains derived from precursors generated by Ile catabolism (12), production of factors D and E by XH25 increased from 20% to 75% of total lipopeptides.
TABLE 3.
Production of lipopeptides by Smr mutants of S. fradiae
| S. fradiae strain | RpsL substitution (rpsL codon change)a | Lipopeptide yield (mg/liter) | % of control | A-core (%) |
|---|---|---|---|---|
| NRRL 18160 | 423 ± 11 | 100 | 64 | |
| XH3 | K43T (ACG) | 114 ± 4 | 27 | 53 |
| XH7 | K43N (AAT) | 459 ± 24 | 109 | 68 |
| XH9 | K43R (AGG) | 407 ± 43 | 96 | 57 |
| XH10 | K88E (GAG) | 371 ± 17 | 88 | 52 |
| XH25 | K88R (AGG) | 472 ± 29 | 112 | 62 |
The wild-type codons in the S. fradiae NRRL 18160 rpsL gene encoding K43 and K88 were both AAG.
FIG. 3.
Results from HPLC analysis of fermentation broths. The A54145 factors A, A1, and D (A-core lipopeptides) have Glu12 and Ile13, factors B, B1, and E (B-core) contain 3mGlu12 and Ile13, and factor F contains Glu12 and Val13. (A) S. fradiae XH25 in DSF roduction medium; (B) XH25 in DSF-Ile; (C) S. fradiae DA613 (ΔlptI::tsr) in DSF-Ile; (D) S. fradiae DA728 (ΔlptD) in DSF-Ile.
Host strains with defined deletions of different sets of lpt genes that could be used for homologous and heterologous trans-complementation studies (Fig. 4 A) were constructed in XH25 or derivatives by one or more gene replacements using the vector pRHB538 (28), which has Amr for selection and a temperature-sensitive origin of replication in streptomycetes. Typically, chromosomal deletions were obtained by subcloning 2- to 3-kb upstream and downstream regions flanking the locus of interest together in pRHB538 to create “deletion plasmids,” introducing them via the conjugative host E. coli ML22 into XH25 and, after allowing for homologous recombination in the latter, finally selecting among the strains for those with the appropriate antibiotic resistance phenotypes. A thiostrepton resistance gene (Tsrr cassette) was inserted between the cloned regions to facilitate selection in some cases, but in others the deletion was in frame, leaving a segment of DNA encoding a small peptide derived from the first and last few amino acids of the original protein. The latter was a consideration to avoid potential polarity effects on downstream genes after deletion of the NRPS genes, as these appear to be arranged as an operon and may be expressed as a polycistronic transcript starting at lptEF (36). Five single-deletion strains, including two at lptI and three at NRPS genes, and two double mutants, combining changes at lptI and NRPS genes, were made (Table 1). Whole broth bioassays against S. aureus as well as HPLC analysis were used to verify the complete loss of A54145 factor production in the NRPS deletion hosts (Fig. 3D).
FIG. 4.
Ectopic trans-complementation system for engineering A54145 biosynthesis in S. fradiae. (A) The top line shows the organization of A54145 biosynthesis genes cloned in the BAC pCB01. The next series of lines show the extent of deletions (dotted lines) with or without marker insertions (solid boxes) in various S. fradiae mutants. (B) Plasmids derived from pCB01 useful for trans-complementation and genetic engineering of lipopeptide biosynthesis.
Plasmids capable of expressing one or more lpt genes from ectopic locations to complement the deletion hosts were designed to complete the system. Further modification of pDA2002 or derivatives by λ-Red-mediated recombination generated the series of BACs shown in Fig. 4B. The apparent organization of the A54145 NRPS genes in an operon required addition of a promoter (ermE*) in front of any NRPS genes separated from lptEF and a to terminator to stop transcription of the biosynthesis genes, if the end of the natural cluster was removed (Fig. 2B and C). The ermE* promoter was selected because it has been utilized successfully for expression of secondary metabolite biosynthesis genes in other Streptomyces spp., including the expression of daptomycin NRPS and other genes in S. roseosporus (13, 19, 37, 39). To develop an ectopic trans-complementation system for combinatorial biosynthesis, it was desirable to be able to express the four NRPS genes from three loci. For reasons not understood, an IS117-containing vector does not work in S. fradiae (unpublished data), even though this was used successfully in other daptomycin molecular engineering studies (39), and so two other integration systems, those of φC31 and φBT1 (24), and their respective attB sites in S. fradiae were used. The φC31 system functions well in many streptomycetes (29), and the φBT1 integration system is as efficient as that of φC31 in S. lividans (24); our results (below) extend this observation to S. fradiae. The ability to use the φBT1 integration system expands the tools available to the combinatorial biosynthesis platform in S. fradiae, making it as accommodating as that previously developed in S. roseosporus for manipulation of daptomycin biosynthesis (39).
Identification of lptI as a methyltransferase gene by deletion analysis.
The first application of the engineering system was to construct a mutant defective in the conversion of Glu to 3mGlu to facilitate medicinal chemistry studies directed at making modifications of the lipid side chain. This goal of generating a strain that produces A54145 A-core compounds exclusively was achieved by deleting the lptI gene, thus eliminating the production of toxic B-core factors and extensive downstream processing.
LptI has two distantly related homologues, GlmT and DptI from the CDA and daptomycin pathways, that have been demonstrated to be methyltransferases involved in the formation of 3mGlu in lipopeptides (31, 38). GlmT methyltransferase from S. coelicolor converts α-ketoglutarate to a (2S,3R)-3-methylglutamate intermediate that is further converted to 3mGlu by a transaminase from primary metabolism (31). Fermentation of two strains deleted for lptI, DA613 (ΔlptI::tsr) and JR397 (ΔlptI), showed 40% improved total lipopeptide titers over the XH25 control (Table 4). Notably, production was confined to A-core factors A, A1, and D, as shown by HPLC (Fig. 3C) and LC-MS analyses. These observations are consistent with a proposed role for LptI as a methyltransferase involved in the formation of 3mGlu (37).
TABLE 4.
Production of lipopeptides by mutants defective in lptI methyltransferase and results of trans-complementation with lptI and dptI genes
| Strain | Deletion | Complementation vector | Gene(s) on vector | Lipopeptide yield (mg/liter) | % of controlb | A-core (%) |
|---|---|---|---|---|---|---|
| XH25 | 472 ± 29 | 100 | 62 | |||
| DH613 | ΔlptI::tsr | 677 ± 24 | 141 | 100 | ||
| JR397 | ΔlptI | 692 ± 41 | 138 | 100 | ||
| DA1096 | ΔlptI::tsr | pCV16 | lptIa | 550 ± 18 | 115 | 76 |
| DA1087 | ΔlptI::tsr | pDA1658 | ermE*p-lptI | 556 ± 16 | 116 | 83 |
| DA1077 | ΔlptI::tsr | pDA1660 | ermE*p-dptI | 413 ± 40 | 86 | 15 |
The unanticipated yield enhancement over the simple channeling of lipopeptide biosynthesis into A-core lipopeptides suggested that Glu may be incorporated more efficiently than 3mGlu at position 12 during A54145 biosynthesis, or that the nascent Glu-containing peptide may be further processed more efficiently. This notion was further supported by the results of an experiment in which DA613 (ΔlptI::tsr) was complemented in trans by integration at the φC31 locus of a copy of the lptI gene under the control of its native promoter (on cosmid pCV16) or that of ermE*p (pDA1658). The complemented strains, DA1096 and DA1087, produced a mixture of lipopeptides containing 76% and 83% A-core factors, respectively, at titers of ∼80% of DA613, while a control plasmid lacking lptI or dptI did not restore the production of B-core lipopeptides. When the dptI methyltransferase gene expressed from ermE*p was used to complement the lptI mutation in DA613, the recombinant strain (DA1077) incorporated 3mGlu much more efficiently, and the total lipopeptide yield was reduced to 61% of that produced by DA613. These results confirm the role of LptI as a methyltransferase involved in the biosynthesis of 3mGlu and indicate that Glu is the preferred substrate for efficient lipopeptide production. This contrasts with A21978C biosynthesis in S. roseosporus, in which the deletion of the dptI gene caused ∼50% reduced production of lipopeptide analogues containing Glu12 (38), suggesting a preference for 3mGlu incorporation or further processing at position 12 during daptomycin biosynthesis. The highly efficient production of A54145 A-core lipopeptides by the S. fradiae ΔlptI mutants has greatly facilitated the production of many A54145 derivatives containing different lipid tails via medicinal chemistry approaches (37a, 41a).
Complementation of S. fradiae host strains defective in A54145 biosynthesis.
Table 5 shows the results of trans-complementation experiments that addressed the efficiency of reconstructing the lpt pathway from genes transplanted from the native locus. The simplest (single-site) reconstructions were in strains DA1676 and DA1324, where the complete native lpt pathway was reinserted into the φC31 and φBT1 attB sites, respectively, under the control of the native promoters, using pDA2002 and pJR153, an analogous derivative of pCB01 carrying a φBT1 cassette. The strains produced 513 mg/liter and 583 mg/liter, respectively, representing 109% and 124% of the XH25 parent production. Both produced similar percentages (∼60%) of A-core lipopeptides. These data indicate that both insertion sites are suitable for the high-level expression of lpt genes. In strain DA1674, the lpt pathway deletion was complemented by pDA2054, containing all of the lpt genes except lptI, inserted at the φC31 attB site. This strain produced 524 mg/liter of 100% A-core lipopeptides.
TABLE 5.
Lipopeptide production by ectopically trans-complemented lpt deletion mutantsb
| Strain | Deletion(s) | Insertion via φC31 | Insertion via φBT1 | Gene changea | Yield (mg/liter) | % of controlb | A-core (%) |
|---|---|---|---|---|---|---|---|
| XH25 | 472 ± 29 | 100 | 62 | ||||
| DA1676 | ΔlptEF-I | lptEF-I | 513 ± 16 | 109 | 60 | ||
| DA1324 | ΔlptEF-I | lptEF-I | 583 ± 95 | 124 | 61 | ||
| DA1674 | ΔlptEF-I | lptEF-P | ΔlptI | 524 ± 19 | 112 | 100 | |
| DA1204 | ΔlptBCD ΔlptI | lptB-I | lptG-P | 395 ± 11 | 84 | 52 | |
| DA1206 | ΔlptBCD ΔlptI | lptB-H | ΔlptI, lptGH | 419 ± 39 | 89 | 100 | |
| DA1444 | ΔlptBCD ΔlptI | lptBC | lptD-I | lptG-I | 408 ± 48 | 87 | 36 |
| DA1102 | ΔlptD | lptD-I | lptG-I | 478 ± 26 | 101 | 32 | |
| DA1106 | ΔlptD | lptB-I | lptBC, lptG-I | 486 ± 80 | 103 | 38 | |
| DA1109 | ΔlptBCD | lptB-I | lptG-I | 444 ± 18 | 94 | 20 |
lpt genes missing (Δ) or duplicated (shown in bold) by the complementing plasmid are listed.
lptEF-I, lptEFABCDGHJKLMNPI; lptEF-P, lptEFABCDGHJKLMNP; lptB-I, lptBCDGHJKLMNPI; lptG-P, lptGHJKLMNP; lptB-H, lptBCDGH; lptD-I, lptDGHJKLMNPI; lptG-I, lptGHJKLMNPI.
A two-site reconstitution of the A54145 pathway was generated in strain DA1204, where all lpt NRPS genes beyond lptEFA were deleted from the native locus and, by using pDA2012, inserted into the φC31 attB site with lptB and some downstream genes under the transcriptional control of the ermE* promoter. Strain DA1204 produced 395 mg/liter (84% of control) and 52% A-core. Strain DA1206, carrying pDA2048 at the φC31 attB site, was similar to DA1204 except that it lacked lptI. This strain produced 419 mg/liter of 100% A-core lipopeptides.
A three-site reconstitution was made in strain DA1444. The lptEFA genes were at the native locus under the control of the native promoter(s), lptB and lptC were expressed as a dicistron under the control of ermE*p at the φC31 attB site (pDA2040), and lptD through lptI were expressed from the φBT1 attB site with the ermE* promoter inserted in front of lptD (pDA2106). DA1444 produced 408 mg/liter of lipopeptides (87% of control) and converted Glu to 3mGlu more efficiently than control (36% A-core).
Three other recombinants (DA1102, DA1106, and DA1109) were used to test the complementation of a single NRPS deletion (ΔlptD) or a triple NRPS deletion (ΔlptBCD) by vectors containing multiple genes, resulting in duplication of several lpt genes. All three produced lipopeptide yields in the range of 94% to 103% of control. All three had two copies of the lptI gene and incorporated higher percentages of 3mGlu at position 12 than the control.
Conclusion.
Previous work at Eli Lilly and Company and at Cubist Pharmaceuticals suggested that A54145 A-core lipopeptides might be good starting scaffolds to generate lipopeptide antibiotics active in pulmonary infections caused by S. pneumoniae (40). A mutant defective in the conversion of Glu to 3mGlu used to facilitate medicinal chemistry studies directed at making modifications of the lipid side chain and a genetic system to carry out combinatorial biosynthesis directed at modifying the tridecapeptide of A54145 were developed. The results of this work demonstrated the feasibility of generating A54145 lipopeptides by trans-complementation and coupling it with the ability to control 3mGlu12 incorporation. λ-Red-mediated recombination was an efficient way to disrupt or modify specific genes in the cloned lpt pathway and enabled the construction of a set of host strains and complementing plasmids of increased genetic complexity. Pathway reconstitution from plasmids inserted in ectopic locations in the chromosome resulted in yields similar to those of the original strain, which indicates that the production of useful amounts of hybrid A54145 lipopeptides is possible if components of heterologous pathways are tested. A combination of compatible functional site-specific integration systems and antibiotic resistance markers allows for multiple engineered plasmids to be used in a host strain at the same time for combinatorial biosynthesis. S. fradiae has already proven to be an excellent genetic host for combinatorial biosynthesis to produce novel lipopeptides when using the genetic tools described in this study (40).
Supplementary Material
Acknowledgments
We thank Maggie Smith of the University of Aberdeen for providing plasmid MS82, Mervyn Bibb of the John Innes Institute for plasmids pIJ8660 and pIJ4070, and R. N. Rao of Eli Lilly and Company for providing S. ambofaciens BES2074. We thank S. Doekel and J. Chapple and members in the Analytical Chemistry, Natural Products Chemistry, and Natural Products Fermentation departments for technical assistance.
This work was supported by Cubist Pharmaceuticals, Inc.
Footnotes
Published ahead of print on 27 August 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Alexander, D. C., D. J. Devlin, D. D. Hewitt, A. C. Horan, and T. J. Hosted. 2003. Development of the Micromonospora carbonacea var. africana ATCC 39149 bacteriophage pMLP1 integrase for site-specific integration in Micromonospora spp. Microbiology 149:2443-2453. [DOI] [PubMed] [Google Scholar]
- 2.Arbeit, R. D., D. Maki, F. P. Tally, E. Campanaro, and B. I. Eisenstein. 2004. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 38:1673-1681. [DOI] [PubMed] [Google Scholar]
- 3.Baltz, R. H. 1980. Genetic recombination by protoplast fusion in Streptomyces. Dev. Ind. Microbiol. 21:43-54. [Google Scholar]
- 4.Baltz, R. H. 2006. Molecular engineering approaches to peptide, polyketide and other antibiotics. Nat. Biotechnol. 24:1533-1540. [DOI] [PubMed] [Google Scholar]
- 5.Baltz, R. H. 2008. Renaissance in antibacterial discovery from actinomycetes. Curr. Opin. Pharmacol. 8:557-563. [DOI] [PubMed] [Google Scholar]
- 6.Baltz, R. H. 2008. Biosynthesis and genetic engineering of lipopeptide antibiotics related to daptomycin. Curr. Top. Med. Chem. 8:618-638. [DOI] [PubMed] [Google Scholar]
- 7.Baltz, R. H., and P. Matsushima. 1981. Protoplast fusion in Streptomyces: conditions for efficient genetic recombination and cell regeneration. J. Gen. Microbiol. 127:137-146. [DOI] [PubMed] [Google Scholar]
- 8.Baltz, R. H., V. Miao, and S. K. Wrigley. 2005. Natural products to drugs: daptomycin and related lipopeptide antibiotics. Nat. Prod. Rep. 22:717-741. [DOI] [PubMed] [Google Scholar]
- 9.Bibb, M. J., J. White, J. M. Ward, and G. R. Janssen. 1994. The mRNA for the 23S rRNA methylase encoded by the ermE gene of Saccharopolyspora erythraea is translated in the absence of a conventional ribosome-binding site. Mol. Microbiol. 14:533-545. [DOI] [PubMed] [Google Scholar]
- 10.Bierman, M., R. Logan, K. O'Brien, E. T. Seno, R. N. Rao, and B. E. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43-49. [DOI] [PubMed] [Google Scholar]
- 11.Boeck, L. D., H. R. Papiska, R. W. Wetzel, J. S. Mynderse, D. S. Fukuda, F. P. Mertz, and D. M. Berry. 1990. A54145, a new lipopeptide antibiotic complex: discovery, taxonomy, fermentation and HPLC. J. Antibiot. 43:587-593. [DOI] [PubMed] [Google Scholar]
- 12.Boeck, L. D., and R. W. Wetzel. 1990. A54145, a new lipopeptide antibiotic complex: factor control through precursor directed biosynthesis. J. Antibiot. 43:607-615. [DOI] [PubMed] [Google Scholar]
- 13.Coëffet-Le Gal, M.-F., L. Thurson, P. Rich, V. Miao, and R. H. Baltz. 2006. Complementation of daptomycin dptA and dptD deletion mutations in-trans and production of hybrid lipopeptide antibiotics. Microbiology 152:2993-3001. [DOI] [PubMed] [Google Scholar]
- 14.Counter, F. T., N. E. Allen, D. S. Fukuda, J. N. Hobbs, J. Ott, P. W. Ensminger, J. S. Mynderse, D. A. Preston, and C. Y. Wu. 1990. A54145, a new lipopeptide antibiotic complex: microbiological evaluation. J. Antibiot. 43:616-622. [DOI] [PubMed] [Google Scholar]
- 15.Cox, K. L., and R. H. Baltz. 1984. Restriction of bacteriophage plaque formation in Streptomyces spp. J. Bacteriol. 159:499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Datsenko, K. A., and B. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debono, M., M. Barnhart, C. B. Carrell, J. A. Hoffmann, J. L. Occolowitz, B. J. Abbott, D. S. Fukuda, R. L. Hamill, K. Biemann, and W. C. Herlihy. 1987. A21978C, a complex of new acidic peptide antibiotics: isolation, chemistry, and mass spectral structure elucidation. J. Antibiot. 40:761-777. [DOI] [PubMed] [Google Scholar]
- 18.Debono, M., B. J. Abbott, R. M. Molloy, D. S. Fukuda, A. H. Hunt, V. M. Daupert, F. T. Counter, J. L. Ott, C. B. Carrell, L. C. Howard, L. D. Boeck, and R. L. Hamill. 1988. Enzymatic and chemical modifications of lipopeptide antibiotic A21978C: the synthesis and evaluation of daptomycin (LY146032). J. Antibiot. 41:1093-1105. [DOI] [PubMed] [Google Scholar]
- 19.Doekel, S., M.-F. Coëffet-Le Gal, J.-Q. Gu, M. Chu, R. H. Baltz, and P. Brian. 2008. Non-ribosomal peptide synthetase module fusions to produce derivatives of daptomycin in Streptomyces roseosporus. Microbiology 154:2872-2880. [DOI] [PubMed] [Google Scholar]
- 20.Ford, L. M., T. E. Eaton, and O. W. Godfrey. 1990. Selection for Streptomyces ambofaciens mutants that produce large quantities of spiramycin and determination of optimal conditions for spiramycin production. Appl. Environ. Microbiol. 56:3511-3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowler, V. G., H. W. Boucher, G. R. Corey, E. Abrutyn, A. W. Karchmer, M. E. Rupp, D. P. Levine, H. F. Chambers, F. P. Tally, G. A. Vigliani, C. H. Cabell, A. S. Link, I. DeMeyer, G. S. Filler, M. Zervos, P. Cook, J. Parsonnet, J. M. Bernstein, C. S. Price, G. N. Forrest, G. Fätkenheure, M. Gareca, S. J. Rehm, H. R. Brodt, A. Tice, S. E. Cosgrove, and the S. aureus Endocarditis and Bacteremia Study Group. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355:653-655. [DOI] [PubMed] [Google Scholar]
- 22.Frijters, A. C. J., Z. Zhang, M. van Damme, G.-L. Wang, P. C. Ronald, and R. W. Michelmore. 1997. Construction of a bacterial artificial chromosome library containing large EcoRI and HindIII genomic fragments of lettuce. Theor. Appl. Genet. 94:390-399. [Google Scholar]
- 23.Fukuda, D. S., M. Debono, R. M. Molloy, and J. S. Mynderse. 1990. A54145, a new lipopeptide antibiotic complex: microbial and chemical modification. J. Antibiot. 43:601-606. [DOI] [PubMed] [Google Scholar]
- 24.Gregory, M. A., R. Till, and M. C. M. Smith. 2003. Integration site for Streptomyces phage φBT1 and development of site-specific integrating vectors. J. Bacteriol. 185:5320-5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu, J.-Q., K. T. Nguyen, C. Gandhi, V. Rajgarhia, R. H. Baltz, P. Brian, and M. Chu. 2007. Structural characterization of daptomycin analogues A21978C1-3(D-Asn11) produced by a recombinant Streptomyces roseosporus strain. J. Nat. Prod. 70:233-240. [DOI] [PubMed] [Google Scholar]
- 26.Hojati, Z., C. Milne, B. Harvey, L. Gordon, M. Borg, F. Flett, B. Wilkinson, P. J. Sidebottom, B. A. M. Rudd, M. A. Hayes, C. P. Smith, and J. Micklefield. 2002. Structure, biosynthetic origin, and engineered biosynthesis of calcium-dependent antibiotics from Streptomyces coelicolor. Chem. Biol. 9:1175-1187. [DOI] [PubMed] [Google Scholar]
- 27.Hosted, T. J., and R. H. Baltz. 1996. Mutants of Streptomyces roseosporus that express enhanced recombination within partially homologous genes. Microbiology 142:2803-2813. [DOI] [PubMed] [Google Scholar]
- 28.Hosted, T. J., and R. H. Baltz. 1997. Use of rpsL for dominance selection and gene replacement in Streptomyces roseosporus. J. Bacteriol. 179:180-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. John Innes Foundation, Norwich, England.
- 30.Kuhstoss, S., M. Huber, J. R. Turner, J. W. Paschal, and R. N. Rao. 1996. Production of a novel polyketide through the construction of a hybrid polyketide synthase. Gene 183:231-236. [DOI] [PubMed] [Google Scholar]
- 31.Mahlert, C., F. Kopp, J. Thirlway, J. Micklefield, and M. A. Marahiel. 2007. Stereospecific enzymatic transformation of α-ketoglutarate to (2S,3R)-3-methyl glutamate during acidic lipopeptide biosynthesis. J. Am. Chem. Soc. 129:12011-12018. [DOI] [PubMed] [Google Scholar]
- 32.Matsushima, P., and R. H. Baltz. 1985. Efficient plasmid transformation of Streptomyces ambofaciens and Streptomyces fradiae protoplasts. J. Bacteriol. 163:180-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McHenney, M. A., and R. H. Baltz. 1996. Gene transfer and transposition mutagenesis in Streptomyces roseosporus: mapping of insertions that influence daptomycin of pigment production. Microbiology 142:2363-2373. [DOI] [PubMed] [Google Scholar]
- 34.McHenney, M. A., T. J. Hosted, B. S. Dehoff, P. R. Rosteck, and R. H. Baltz. 1998. Molecular cloning and physical mapping of the daptomycin gene cluster from Streptomyces roseosporus. J. Bacteriol. 180:143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miao, V., M.-F. Coëffet-Le Gal, P. Brian, R. Brost, J. Penn, A. Whiting, S. Martin, R. Ford, I. Parr, M. Bouchard, C. J. Silva, S. K. Wrigley, and R. H. Baltz. 2005. Daptomycin biosynthesis in Streptomyces roseosporus: cloning and analysis of the gene cluster and revision of peptide stereochemistry. Microbiology 151:1507-1523. [DOI] [PubMed] [Google Scholar]
- 36.Miao, V., R. Brost, J. Chapple, K. She, M.-F. Coëffet-Le Gal, and R. H. Baltz. 2006. The lipopeptide antibiotic A54145 biosynthetic gene cluster from Streptomyces fradiae. J. Ind. Microbiol. Biotechnol. 33:129-140. [DOI] [PubMed] [Google Scholar]
- 37.Miao, V., M.-F. Coëffet-Le Gal, K. Nguyen, P. Brian, J. Penn, A. Whiting, J. Steele, D. Kau, S. Martin, R. Ford, T. Gibson, M. Bouchard, S. K. Wrigley, and R. H. Baltz. 2006. Genetic engineering in Streptomyces roseoporus to produce hybrid lipopeptide antibiotics. Chem. Biol. 13:269-276. [DOI] [PubMed] [Google Scholar]
- 37a.Morton, L. I., S. Zhang, A. Arya, A. D. G. Van Praagh, L. Chuong, C. Kang, X. Zhang, and T. Li. 2009. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F1-2030. American Society for Microbiology, Washington, DC.
- 38.Nguyen, K., D. Kau, J.-Q. Gu, P. Brian, S. K. Wrigley, R. H. Baltz, and V. Miao. 2006. A glutamic acid 3-methyltransferase encoded by an accessory gene locus important for daptomycin biosynthesis in Streptomyces roseosporus. Mol. Microbiol. 61:1294-1307. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen, K., D. Ritz, J.-Q. Gu, D. Alexander, M. Chu, V. Miao, P. Brian, and R. H. Baltz. 2006. Combinatorial biosynthesis of lipopeptide antibiotics related to daptomycin. Proc. Natl. Acad. Sci. U. S. A. 103:17462-17467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen, K., X. He, D. C. Alexander, C. Li, J.-Q. Gu, C. Mascio, A. Van Praagh, L. Mortin, M. Chu, J. A. Silverman, P. Brian, and R. H. Baltz. 2010. Genetically engineered lipopeptide antibiotics related to A54145 and daptomycin with improved properties. Antimicrob. Agents Chemother. 54:1404-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ochi, K. 2007. From microbial differentiation to ribosome engineering. Biosci. Biotechnol. Biochem. 71:1373-1386. [DOI] [PubMed] [Google Scholar]
- 41a.Pang, W. K., A. Pearson, L. Martel, Y. Zhang, V. Kulkarni, P. Herradura, C. Leitheiser, I. Parr, C. Mascio, N. Cotroneo, J. Silverman, A. D. G. Van Praagh, L. I. Mortin, and D. Keith. 2009. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F1-2028. American Society for Microbiology, Washington, DC.
- 42.Penn, J., A. Whiting, S. W. Wrigley, M. Latif, T. Gibson, C. J. Silva, X. Li, V. Miao, P. Brian, and R. H. Baltz. 2006. Heterologous production of daptomycin in Streptomyces lividans. J. Ind. Microbiol. Biotechnol. 33:121-128. [DOI] [PubMed] [Google Scholar]
- 43.Pertel, P. E., P. Bernardo, C. Fogerty, P. Matthews, R. Northland, M. Benvenuto, G. M. Thorne, S. A. Luperchio, R. D. Arbeit, and J. Alder. 2008. Effects of prior effective therapy on the efficacy of daptomycin and cephtriaxone for the treatment of community-acquired pneumonia. Clin. Infect. Dis. 46:1142-1151. [DOI] [PubMed] [Google Scholar]
- 44.Richardson, M. A., S. Kuhstoss, M. L. B. Huber, L. Ford, O. Godfrey, J. R. Turner, and R. N. Rao. 1990. Cloning of spiramycin biosynthetic genes and their use in constructing Streptomyces ambofaciens mutants defective in spiramycin biosynthesis. J. Bacteriol. 172:3790-3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 46.Silverman, J. A., L. I. Morton, A. D. Vanpraagh, T. Li, and J. Alder. 2005. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J. Infect. Dis. 191:2149-2152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.