Abstract
Multi-antimicrobial-resistant Salmonella enterica strains frequently carry resistance genes on plasmids. Recent studies focus heavily on large conjugative plasmids, and the role that small plasmids play in resistance gene transfer is largely unknown. To expand our previous studies in assessing the prevalence of the isolates harboring ColE1-like plasmids carrying the aph gene responsible for kanamycin resistance (Kanr) phenotypes, 102 Kanr Salmonella isolates collected through the National Antimicrobial Resistance Monitoring System (NARMS) in 2005 were screened by PCR using ColE1 primer sets. Thirty isolates were found to be positive for ColE1-like replicon. Plasmids from 23 isolates were able to propagate in Escherichia coli and were subjected to further characterization. Restriction mapping revealed three major plasmid groups found in three or more isolates, with each group consisting of two to three subtypes. The aph genes from the Kanr Salmonella isolates were amplified by PCR, sequenced, and showed four different aph(3′)-I genes. The distribution of the ColE1 plasmid groups in association with the aph gene, Salmonella serovar, and isolate source demonstrated a strong linkage of the plasmid with S. enterica serovar Typhimurium DT104. Due to their high copy number and mobility, the ColE1-like plasmids may play a critical role in transmission of antibiotic resistance genes among enteric pathogens, and these findings warrant a close monitoring of this plasmid incompatibility group.
Salmonella species account for an estimated 1.4 million cases of food-borne illnesses in the United States each year, resulting in ∼16,000 hospitalizations and 580 deaths (26). FoodNet 2008 surveillance indicated that 56.2% of the Salmonella isolates serotyped belong to three predominant serovars: Enteritidis (20.1%), Typhimurium (16.0%), and Newport (10.1%) (11).
Due to their resistance to treatments, multi-antimicrobial-resistant (MR) isolates pose an even greater threat to human and animal health. Resistance genes are often carried on mobile genetic elements, such as phage, transposons, and plasmids, and are subject to frequent horizontal transfers. Plasmids contain a replicon consisting of sequences that are necessary for self-replication in a host cell, including the origin of replication, replication functions, segregation, and control of initiation (5, 14). Because of the specificity of these sequences for replication and segregation machinery, one bacterial strain cannot maintain different plasmids that use the same mechanisms and are thus defined as incompatible. Plasmid incompatibility can be used to classify plasmids into incompatibility (Inc) types based on the sequences controlling these functions (14, 28). There are 27 known replicon types or Inc types in Enterobacteriaceae (6), and a series of multiplex PCR-based replicon typing assays has been developed to distinguish 18 of the types (7, 23).
Most studies on the prevalence of antimicrobial resistance plasmids have focused on large MR plasmids (≥60 kb) (6, 7, 8, 17, 18, 23, 25, 40). The distribution and prevalence of the low-molecular-weight R plasmids is largely unknown. The low-molecular-weight plasmids have been estimated to be present in ca. 10% of the Salmonella field strains and can carry genes encoding a wide variety of biological functions, such as restriction-modification systems, retron reverse transcriptase, O-antigen, and antibiotic resistance (32). Gregorova and coworkers sequenced some of the small native Salmonella plasmids and focused on the potential of plasmid profiling as a molecular typing tool for Salmonella species (20, 21, 33). However, complete sequence information and systematic analysis of the low-molecular-weight antibiotic resistance plasmids is still scarce.
Limited numbers of the ColE1-like plasmids carrying resistance genes in Salmonella serovars have been sequenced, including plasmids carrying quinolone resistance genes pSGI15 (FN428572, direct submission), pTPqnrS-1a (AM746977 [24]) and pST728/06-2 (EU715253 [42]), β-lactamase (bla) genes pA172 in serovar Newport (EU331425 [44]), and pAnkS in serovar Typhimurium (DQ916413, direction submission), as well as kanamycin resistance (Kanr) plasmids NTP16 (L05392 [4]), pU302S (AY333433 [12]) in serovar Typhimurium, and pSN11/00Kan in serovar Newport (GQ470395 [13]).
In the present study, we sought to gain a more comprehensive knowledge on the prevalence of Kanr ColE1-like plasmids in Salmonella serovars. The presence of such plasmids in Salmonella isolates resistant to kanamycin from sick animals and their environment in 2005 was determined by using PCR typing with primers specific for ColE1 plasmids. When possible, the ColE1-like plasmids identified were transformed into Escherichia coli and further characterized by restriction mapping and sequencing. Salmonella enterica isolates found to harbor these ColE1-like plasmids were also characterized for antimicrobial resistance, plasmid replicon types, and PFGE analysis.
MATERIALS AND METHODS
Salmonella strain collection and characterization.
The 102 Kanr Salmonella enterica isolates tested for the presence of ColE1-like plasmids in the present study were collected from samples submitted to veterinary diagnostic laboratories in 2005 in which Salmonella was identified as either the primary or the secondary etiologic agent associated with the illness, as previously described (25), and originated from all five regions in the United States as defined for the NARMS program (http://www.ars.usda.gov/Main/docs.htm?docid=6750). Veterinary diagnostic laboratories obtained isolates from the state in which they are located and then sent them to the National Veterinary Services Laboratory (NVSL) in Ames, IA (http://www.aphis.usda.gov/animal_health/lab_info_services/about_nvsl.shtml). The isolates used in the present study were obtained from the NVSL. All bacterial strains were stored at −80°C in LB Lennox (Hardy Diagnostics, Santa Maria, CA) with 15% glycerol or stored at room temperature on tryptic soy agar (Hardy Diagnostics, Santa Maria, CA) slants until use.
Isolates were tested for susceptibility to 15 antimicrobial drugs, analyzed by pulsed-field gel electrophoresis (PFGE) using XbaI, and cluster analysis was conducted as previously described (25). Briefly, susceptibility testing was conducted on each Salmonella enterica isolate to a panel of 15 antimicrobial drugs as defined by the NARMS program (http://www.ars.usda.gov/Main/docs.htm?docid_6750) using the Sensititer system (Trek Diagnostic Systems, Inc., Westlake, OH) that included amikacin (AMK), amoxicillin-clavulanic acid (AMOX/CA), ampicillin (AMPC), cefoxitin (CFOX), ceftiofur (TIO), ceftriaxone (CTRX), chloramphenicol (CHL), ciprofloxacin (CIPX), gentamicin (GEN), kanamycin (KAN), nalidixic acid (NAL), streptomycin (STR), sulfamethoxazole (SSS), tetracycline (TET), and trimethoprim-sulfamethoxazole (SMX/TMP). Each isolate was classified as being resistant, susceptible, or intermediate using Clinical and Laboratory Standards Institute (formerly the National Committee for Clinical Laboratory Standards) breakpoints when available; otherwise, breakpoint interpretations from the National Antimicrobial Resistance Monitoring System were used (27). S. enterica serovar Typhimurium isolates that were pentaresistant (i.e., resistant to AMPC, CHL, STR, sulfonamides, and TET [ACSSuT]) were phage typed. Serotype and phage type were determined by the National Veterinary Services Laboratories as previously described (15, 41). Any isolates that did not group as expected by PFGE were reserotyped.
All isolates were analyzed at the USDA VetNet Laboratory (Athens, GA) using a 24-h Salmonella PFGE protocol as described by PulseNet (9). Briefly, genomic DNA was digested with 10 U of XbaI (Roche Molecular Biochemicals, Indianapolis, IN) and separated with the CHEF-Mapper XA PFGE system (Bio-Rad, Hercules, CA) in 0.5× Tris-borate-EDTA buffer at 14°C at 6V for 18 h with a ramped pulse time of 2.16 to 63.8. The BioNumerics software program (Applied Maths Scientific Software Development, Sint-Martens-Latem, Belgium) was applied for cluster analysis using the Dice coefficient and the unweighted pair-group method (UPGMA).
Multiplex PCR for plasmid replicon type.
Salmonella isolates were examined by PCR using three multiplex primer panels for the presence of 18 plasmid replicons as described previously by Carattoli et al. (7, 8) and Johnson et al. (23). The exact procedure followed was described by Lindsey et al. (25). Positive controls for each replicon type assayed were used in the replicon typing procedure, these controls were originally created in the laboratory of Werner K. Maas (7, 14) and generously provided by Alessandra Carattoli (Istituto Superiore di Sanita, Rome, Italy). An isolate was considered to be positive for a particular replicon if an amplicon of the expected size was observed on the agarose gels.
PCR screening for ColE1-like plasmids.
Salmonella isolates were examined by PCR using the ColE1 typing primers CC7059F, CC7062R, and CC7063F designed for the present study (Table 1). The primers were originally designed for sequencing plasmids pU302S and pSN11/00Kan using Omiga (v.2.0; Genetics Computer Group, Inc., Madison, WI), or Primer3 (http://frodo.wi.mit.edu/primer3/). A subset of primers located within the RNA I/II and rom conserved regions based on the alignment of pU302S, pSN11/00Kan, ColE1, NTP16, and pColK-K235 were tested and selected for ColE1 typing purpose. The reference strains were Salmonella serovar Typhimurium G8430, Salmonella serovar Newport SN11/00, and E. coli DH5α carrying plasmids pU302S and pSN11/00Kan (12, 13). Primers were manufactured by Eurofins MWG Operon (Huntsville, AL). Template DNA for PCR from the 102 Salmonella isolates was prepared by using boiled cell lysates as previously described (25). Plasmid DNA from ColE1-postive Salmonella isolates was extracted from 2 ml of LB broth cultures grown overnight with KAN and processed with a QIAprep spin miniprep kit (Qiagen, Valencia, CA). PCRs were performed with a final volume of 25 μl containing 5 μl of boiled cell lysate (initial screening) or 1 μl (10 to 50 ng) of plasmid DNA (for confirmation), 1× ABI AmpliTaq buffer 1, 0.5 μM concentrations of each primer, 3 mM MgCl2, 0.2 mM concentrations of each deoxynucleoside triphosphate, and 1.25 U of AmpliTaq polymerase (Applied Biosystems, Foster City, CA) according to the polymerase manufacturer's instructions. The PCR products were visualized on 1× Tris-borate-EDTA containing 2% agarose. ColE1 typing primer pairs CC7063F/CC7062R and CC7059F/CC7062R generated 251- and 351-bp products, respectively. An isolate was considered positive if both amplicons of the expected size were observed.
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Sequence (5′-3′) |
|---|---|
| CC7059F | TTCGTGCACACAGCCCA |
| CC7062R | TGCGGTTATCCACAGAATCA |
| CC7063F | GCGGACAGGTATCCGGTAA |
| APH-F1 | ATGAGCCATATTCAACGGG |
| APH-R1 | AGAAAAACTCATCGAGCATC |
| APH-R2 | CGGTGAGAATGGCAAAAGC |
| APH-F4 | CGATTCCTGTTTGTAATTGTCC |
Plasmid purification, transformation, and restriction digestion.
Approximately 40 to 100 ng of plasmid DNA from Salmonella isolates was used for transformation of CaCl2-treated E. coli DH5α competent cells, and colonies were selected on LB agar supplemented with 50 μg of kanamycin A (Sigma-Aldrich, St. Louis, MO)/ml. Plasmids were prepared from the Kanr E. coli colonies by using the QIAprep spin miniprep kit and were subjected to restriction digestions using BglII, HindIII, KpnI, NdeI, SmaI, and XhoI, singularly or in combination. All restriction enzymes were purchased from New England BioLabs (Ipswich, MA), and digestions followed the manufacturer's recommendation.
Representative clones from each plasmid group or subgroup were subjected to further analyses using plasmids purified with the Qiagen plasmid midi kit. Approximately 100 ng of DNA was digested with NlaIII for 2 h at 37°C and separated on 2% GenePure agarose 3:1 (ISC BioExpress, Kaysville, UT) in 1× Tris-acetate-EDTA (TAE) buffer, 140 V·h, alongside a 100-bp ladder (Invitrogen, Carlsbad, CA). Agarose gels were stained with GelRed (Biotium, Inc., Hayward, CA) for 1 h and photographed by using the AlphaImager gel documentation system (Alpha Innotech, San Leandro, CA).
aph genes in Kanr ColE1+ Salmonella isolates.
The aph gene was amplified by using APH-F1 and APH-R1 primers designed in the present study (Table 1), spanning from the start codon to the first base of the stop codon. The PCRs contained 1.5 μl of DNA template (1:10 dilution of plasmid minipreps from Salmonella isolates), 20 μM concentrations of each primer, 25 μl of 2× Qiagen multiplex PCR master mix, 0.5× Q-Solution, and water in a 50-μl reaction volume. Amplification was performed on a GeneAmp PCR System 9700 (Applied Biosystems) with an initial activation step of 15 min at 95°C, followed by 35 cycles of 30 s at 94°C, 90 s at 57°C, and 60 s at 72°C, with a final extension step of 10 min at 72°C. The amplified products were separated on 1% TAE-agarose, stained with ethidium bromide, and photographed by using AlphaImager (Alpha Innotech). PCR products were cleaned up by using the QIAquick PCR purification kit (Qiagen) and sequenced with APH-F1, APH-F4, APH-R1, and APH-R2 primers (Table 1) using the BigDye Terminator v3.0 on an ABI 3730 sequence analyzer (Applied Biosystems) according to the manufacturer's recommendations. Nucleotide sequences were assembled by using the Sequencher program (Gene Codes, Ann Arbor, MI) and compared to GenBank database entries using the BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi [1, 43]). The best fit model for phlyogram generation was analyzed by using jModeltest (31), and the final tree was generated with the K80 algorithm (22) by using PAUP*4.1 (37). When multiple copies of the aph gene were suspected, the amplified products from select isolates were cloned into the TOPO TA cloning vector pCR2.1-TOPO (Invitrogen), and 8 to 16 individual clones were sequenced and analyzed as described above.
Statistical analysis.
Linkage disequilibrium (LD) was calculated as an extension of the Fisher exact probability test on contingency tables (35) implemented in the program Arlequin (16). Standard settings were used (10,000 steps in the Markov chain and 1,000 dememorization steps), and calculations of D, D′, and r2 coefficients were made with a significance level of 0.05.
GenBank accession number.
The complete sequence of the aph(ii) gene identified in the present study has been deposited into GenBank with accession number GQ426885.
RESULTS
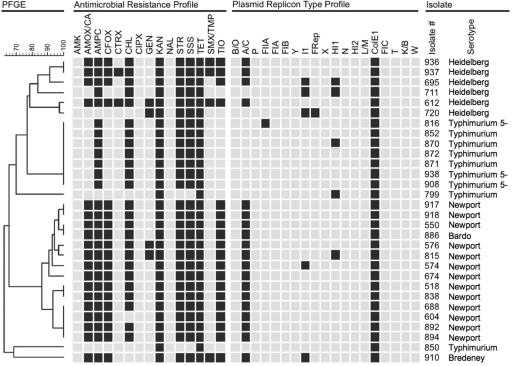
We previously characterized 437 Salmonella diagnostic isolates collected in 2005 for their antimicrobial resistance profiles and replicon types. A total of 170 of the 437 (39%) isolates were shown to be resistant to KAN, and 153 of the Kanr isolates were also TET resistant (Tetr) (90%) (25). For the present study, 102 of the Kanr isolates were randomly selected for PCR screening by using the ColE1 typing primer pairs CC7063F/CC7062R and CC7059F/CC7062R (Table 1); 30 isolates (29.4%) were found to be positive for ColE1-like replicon, representing five serovars from eight different animal sources, including (beef) cattle, chicken, dairy cattle, equine, reptile, swine, turkey, and miscellaneous mammal (an unspecified species), as well as from environmental samples (Table 2). PFGE analysis was conducted on all 30 ColE1-positive isolates. Cluster analysis based on PFGE patterns showed 17 unique types (Fig. 1). All 30 ColE1-positive isolates were resistant to TET. In addition, 28 (93%) of the strains were resistant to STR and SSS, 27 (90%) were resistant to AMPC, and 25 (83%) isolates were resistant to CHL. No isolate was resistant to AMK, CIPX, or NAL (Fig. 1). Nineteen isolates were resistant to eight or more antibiotics, eight were resistant to six antibiotics, one was resistant to five antibiotics, and two isolates were resistant to only KAN and TET (Fig. 1). Of the 30 ColE1-positive isolates, 19 were positive for the large Inc A/C plasmid, and 6 (all serovar Typhimurium) were not detected to have other replicons (Fig. 1).
TABLE 2.
Salmonella isolates used in this study
| ARS no. | Serovar | Source species | Regiona | Kanr ColE1 plasmid groupb | aph sequencec |
|---|---|---|---|---|---|
| 518 | Newport | Chicken | 2 | A2 | i |
| 550 | Newport | Chicken | 2 | B | i |
| 574 | Newport | Cattle | 3 | C3 | ii |
| 576 | Newport | Cattle | 3 | C | ii |
| 604 | Newport | Reptile | 4 | A | i |
| 612 | Heidelberg | Swine | 3 | N/T | iii |
| 674 | Newport | Cattle | 2 | B | i |
| 688 | Newport | Cattle | 3 | A | i |
| 695 | Heidelberg | Swine | 2 | N/T | i |
| 711 | Heidelberg | Swine | 3 | N/T | i+ii |
| 720 | Heidelberg | Turkey | 3 | N/T | i+ii |
| 799 | Typhimurium | Swine | 3 | N/T | iv |
| 815 | Newport | Cattle | 3 | C | ii |
| 816 | Typhimurium var. 5− (DT104) | Cattle | 1 | A | i |
| 838 | Newport | Swine | 1 | B | i |
| 850 | Typhimurium | Swine | 1 | N/T | i+ii |
| 852 | Typhimurium (DT104) | Dairy cattle | 1 | C | ii |
| 870 | Typhimurium (DT104) | Cattle | 1 | A | i |
| 871 | Typhimurium (DT104) | Swine | 1 | A | i |
| 872 | Typhimurium (DT104) | Environmental | 1 | A | i |
| 886 | Bardo | Dairy cattle | 1 | C2 | ii |
| 892 | Newport | Cattle | 4 | A | i |
| 894 | Newport | Cattle | 1 | A | i |
| 908 | Typhimurium var. 5− (DT104) | Cattle | 1 | A | i |
| 910 | Bredeney | Turkey | 1 | N/T | i+ii |
| 917 | Newport | Equine | 1 | B | i |
| 918 | Newport | Miscellaneous mammal | 1 | B | i |
| 936 | Heidelberg | Dairy cattle | 1 | B2 | i |
| 937 | Heidelberg | Dairy cattle | 1 | B2 | i |
| 938 | Typhimurium var. 5− (DT104) | Environmental | 1 | A | i |
Regions in the United States: region 1, the northeast (CT, DE, IN, MA, MD, ME, MI, NH, NJ, NY, OH, PA, RI, and VT); region 2, the southeast (AL, FL, GA, KY, NC, PR, SC, TN, VA, and WV); region 3, the midwest (IA, IL, KS, MN, MO, ND, NE, SD, and WI); and region 4, the southwest (AR, LA, MS, OK, and TX).
FIG. 1.
PFGE-based dendrogram, resistance profile, replicon typing, isolate number, and strain serovars. The dendrogram is based on PFGE analyses using BioNumerics software. Antimicrobial resistance phenotypes (columns 2 to 16): black indicates resistance, and gray indicates intermediate or susceptible. Replicon typing results (columns 17 to 28): black indicates that the trait was detected, and gray indicates that the trait was not detected. The isolate number (column 36) and serovar (column 37) follow. Abbreviations for antimicrobial resistance phenotypes are as follows: amoxicillin-clavulanic acid (AMOX/CA), ampicillin (AMPC), cefoxitin (CFOX), ceftiofur (TIO), ceftriaxone (CTRX), chloramphenicol (CHL), gentamicin (GEN), kanamycin (KAN), nalidixic acid (NAL), streptomycin (STR), sulfamethoxazole (SSS), tetracycline (TET), and trimethoprim-sulfamethoxazole (SMX/TMP).
Kanr ColE1-like plasmids.
Plasmids from 23 of the Kanr ColE1+ isolates were able to transform, replicate, and propagate in E. coli DH5α. Plasmid DNA from these E. coli clones was purified and characterized by restriction mapping with single or double digestions of SmaI, BglII + XhoI, HindIII + NdeI, and KpnI + NdeI. Three major Kanr ColE1-like plasmid pattern groups (A, B, and C), and four additional distinct patterns (A2, B2, C2, and C3) were identified. The four distinct patterns are shown to be variants of the three major groups, with one to five bands different from the archetype of the plasmid group by NlaIII digest (Fig. 2). The information on the Salmonella isolates, including serovar, source species, region, Kanr ColE1-like plasmid, and the aph gene sequence groups, is listed in Table 2. The most prevalent plasmid group was A/A2, which is identical or highly similar to pU302S (GenBank accession no. AY333433), and was found in 11 isolates, including 6 serovar Typhimurium and 5 serovar Newport strains; 66.7% (6/9) of the Typhimurium isolates carried the group A/A2 plasmids. Plasmid group B/B2, similar to the 5.7-kb plasmid pSN11/00Kan from S. Newport (GenBank accession no. GQ470395), was found in five Newport and two Heidelberg strains. Group C and its variants C2 and C3, were found in five isolates (three Newport, one Typhimurium, and one Bardo); all were from cattle or dairy cattle. The aph gene sequence (see below) and the preliminary plasmid sequence (data not shown) of the group C/C2/C3 plasmids are different from the Kanr ColE1 plasmid groups A/A2 and B/B2, suggesting that group C/C2/C3 plasmids may have evolved separately.
FIG. 2.
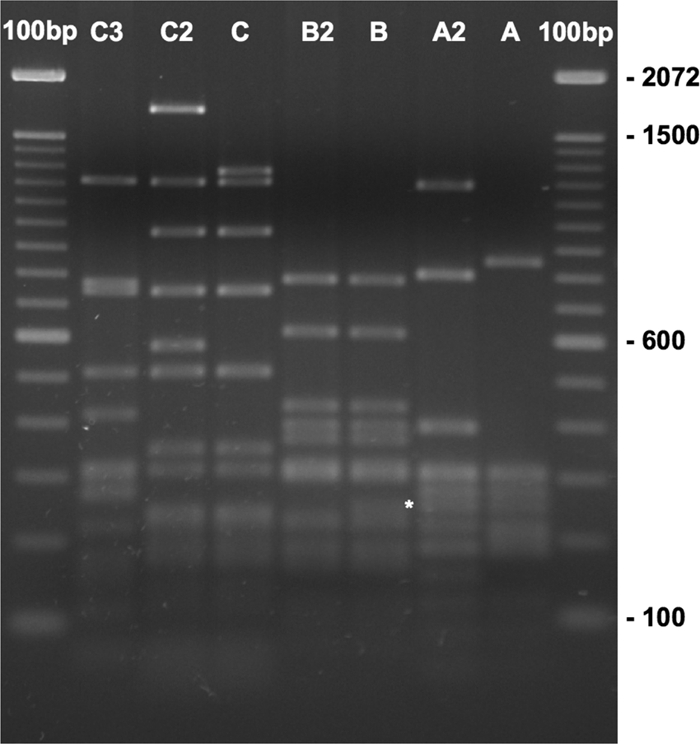
NlaIII digestion of representative Kanr ColE1-like plasmids. Plasmid DNAs were digested with NlaIII for 2 h and separated on 2% TAE-agarose 3:1/140 V·h. Gel was then stained with GelRed for 1 h and photographed. Lanes from left to right: 100-bp ladder, group C3 (ARS#574), group C2 (ARS#886), group C (ARS#852), group B2 (ARS#936), group B (ARS#550), group A2 (ARS#518), group A (ARS#688), and 100-bp ladder. The difference between B and B2 is marked by an asterisk.
DNA from seven isolates failed to transform E. coli DH5α using three independent preparations of plasmids. Two of the seven “nontransformable” isolates (“N/T” in Table 2) were from turkeys, and five were from swine (out of total seven swine isolates). DNA from four of the six Heidelberg isolates and both KAN+TET-resistant Typhimurium isolates were nontransformable.
aph genes in Kanr ColE1+ Salmonella isolates.
All 30 strains tested positive for aph gene by PCR. The 814-bp PCR products were sequenced and showed four different aph sequences, all belonging to the aph(3′)-Ia family (34), with 98.6% or higher nucleotide identity to each other. For the purpose of discussion in the present study, the four nearly identical aph genes are designated by the extensions i, ii, iii, and iv in the text and in Tables 2 and 3. Three of the aph sequences, aph(i), aph(iii), and aph(iv), show 100% matches to existing entries in the NCBI GenBank database by BLAST. The majority (23 of 30) of the isolates carried the aph(i) gene identical to the aph(3′)-I of pU302S (AY333433); the other three aph genes possessed up to 11 base substitutions compared to that of the aph(i). Five of the eleven substitutions occurred in all three sequence types [aph(ii)-(iv)] (Table 3). The only aph(iii) gene from swine isolate ARS#612 was identical to those of the Tn903 (V00359; designated aphA1) and pU302L [AY333434; designated aph(3′)-Ia]. Another swine isolate, ARS#799, carried the aph(iv) gene, which was identical to that of E. coli plasmid pAK33 (AY260546; designated aphA7). Both aph(iii) and aph(iv) genes were from the “N/T” isolates (Table 2). The newly identified aph(ii) gene (deposited into GenBank, accession no. GQ426885) showed 99% (807/814) nucleotide identity and 98% (267/271) amino acid identity to the aph gene on the Enterobacter cloacae plasmid pEC-IMP (EU855787). The aph(ii) gene was only found on the group C/C2/C3 Kanr ColE1 plasmids isolated from cattle or dairy cattle, with the exception of isolates carrying two copies of aph genes (see below).
TABLE 3.
Nucleotide substitution of different aph(3′)-I genes in this studya
| aph sequence | Nucleotide at position: |
GenBank accession no. | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 36 | 38 | 55 | 80 | 142 | 230 | 377 | 434 | 489 | 504 | 609 | 693 | 714 | 720 | 747 | 756 | ||
| i | G (Arg) | A (Gln) | C (Leu) | A (Lys) | G (Asp) | A (Glu) | C (Ala) | G (Arg) | G (Ala) | T (Asp) | T (Asn) | T (Ile) | A (Glu) | T (Ser) | A (Gln) | T (Gly) | AY333433 |
| ii | A | C (Pro) | C | G (Arg) | G | A | C | A (His) | G | C | C | C | G | A | T (His) | C | GQ426885 |
| iii | G | C (Pro) | A (Met) | A | G | A | T (Val) | G | G | C | C | C | G | T | A | T | V00359 |
| iv | G | C (Pro) | C | G (Arg) | A (Asn) | C (Ala) | T (Val) | G | T | C | C | C | G | T | A | T | AY260546 |
Nucleotide 1 of the aph gene is the “A” of the ATG start codon. The nucleotide sequence that is different from the aph(i) sequence is indicated in boldface. Only nonsynonymous substitutions that resulted in amino acid changes, compared to that of the aph(i), are indicated within parentheses for aph sequence types ii to iv.
Double peaks of similar intensity in sequencing chromatograms were observed at certain positions in the batch sequences of the aph PCR products from four isolates ARS#711, ARS#720, ARS#850, and ARS#910—two from turkeys and two from swine. Individual PCR fragments were cloned into the TOPO TA cloning vector pCR2.1-TOPO, and up to 16 clones were randomly chosen and sequenced. These four strains were confirmed to carry two copies of aph genes, aph(i) and aph(ii). However, plasmid preps from all four isolates failed to convert E. coli to Kanr by transformation; it is unclear whether these genes are located on the chromosome or other plasmids.
Distribution of ColE1-like replicon, pentaresistance, and phage type DT104 within serovar Typhimurium.
In the present study, of the 102 Kanr isolates there were 17 isolates of serovar Typhimurium and Typhimurium variant 5−, 10 of which were pentaresistant (ACSSuT) plus Kanr. Of the ten serovar Typhimurium ACSSuT+KAN-resistant isolates, seven isolates were ColE1 PCR(+) and three were not; moreover, eight of these isolates were phage type DT104, and two were untypeable (Tables 2 and 4). The prevalences of pentaresistance, DT104, and ColE1 PCR(+) in Typhimurium isolates are presented in Table 4.
TABLE 4.
Prevalence of pentaresistance, DT104, and ColE1 PCR(+) in Typhimurium isolates in this studya
| Characteristic |
No. (%) of isolates |
||||
|---|---|---|---|---|---|
| Pentaresistance | DT104 | ColE1 PCR | Serovar Typhimurium | Serovar Typhimurium variant 5− | Serovar Typhimurium and serovar Typhimurium variant 5− |
| + | − | − | 1 (12.5) | 1 (11.1) | 2 (11.8) |
| + | + | − | 0 (0) | 1 (11.1) | 1 (5.9) |
| + | + | + | 4 (50.0) | 3 (33.3) | 7 (41.2) |
| − | + | + | 0 (0) | 0 (0) | 0 (0) |
| − | − | + | 2 (25) | 0 (0) | 2 (11.8) |
| − | + | − | 0 (0) | 0 (0) | 0 (0) |
| + | − | + | 0 (0) | 0 (0) | 0 (0) |
| − | − | − | 1 (12.5) | 4 (44.4) | 5 (29.4) |
The three leftmost columns indicate the presence (+) or absence (−) of the phenotype. The remaining columns contain the numbers of positive individuals and the percentages of the total serovar population in parentheses. The column on the far right contains the combined totals of serovar Typhimurium and serovar Typhimurium variant 5− columns.
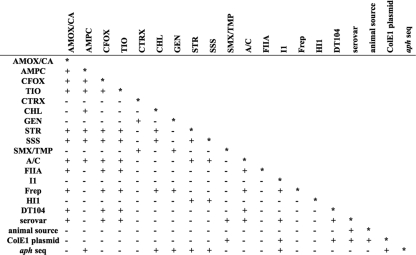
Linkage disequilibrium (LD) of the antibiotic resistance, plasmid replicon type, the presence or absence of DT104, serovar, source species, ColE1 plasmid group, and aph sequence (as listed in Table 2) was calculated and shown in Fig. 3. Pairwise LD between isolate serovar, resistance to the antimicrobials AMOX/CA, CFOX, TIO, and SMX/TMP, and replicon types Inc A/C and I1, as well as DT104, source species, and the Kanr ColE1 plasmid group, showed a linkage (P = 0.05) (Fig. 3). A nonrandom association was seen between S. enterica isolate serovar and resistance to the antimicrobials AMPC, CTRX, and SSS and replicon types Inc FIIA, Frep, and HI1 (Fig. 3). Pairwise LD between the Kanr ColE1 plasmid groups showed linkage (P = 0.05) to resistance to the antimicrobial SMX/TMP and replicon type Inc I1, as well as DT104 and the aph sequence (Fig. 3). The aph sequence of S. enterica isolates showed linkage (P = 0.05) with resistance to the antimicrobials AMPC, CHL, GEN, STR, and replicon type Inc I1 and the ColE1 plasmid groups. Pairwise LD between the source species showed linkage (P = 0.05) to serovar and ColE1 plasmid group. Linkage between serovar and isolate source species has been found elsewhere (2, 10; http://www.ars.usda.gov/Main/docs.htm?docid=17320). No association was seen between the source region and resistance to any antimicrobial, Inc group, DT104, source species, ColE1 plasmid group, or aph sequence.
FIG. 3.
Pairwise linkage disequilibrium among antibacterial resistance, replicon type, DT104 phage type, isolate serovar, animal source, ColE1 plasmid group and aph sequence. A “+” indicates a P value of ≤0.05, indicating significant linkage, and a “-” indicates a P value of >0.05. The abbreviations for antimicrobial resistance phenotype are the same as in Fig. 1.
DISCUSSION
The objective of the present study was to investigate the prevalence of Kanr ColE1-like plasmids in 102 Kanr Salmonella enterica isolates recovered from ill animals in 2005 using PCR typing with primers specific to these ColE1-like plasmids. The plasmids were characterized by using restriction mapping and determination of antimicrobial resistance profiles and replicon types. The bacterial host isolates were subjected to cluster analysis based on PFGE analysis, and linkage disequilibrium was determined for the analyzed traits.
Several interesting observations were made from the data collected. The plasmid group C/C2/C3 carried a specific aph gene that was unique to this group and was only present in beef and dairy cattle isolates. In addition, the “nontransformable” isolates were all from swine and turkeys; none were from cattle/dairy cattle or other source species. The Kanr ColE1-like plasmid group, animal source, and serovar all showed linkage to each other (P = 0.05). This suggests that serovar and animal source may play a role in the transmission of these plasmids. Also, in S. Newport, all three groups of Kanr ColE1-like plasmids were found. This, in addition to the wide variety of PFGE patterns in S. Newport suggests that this is a highly heterogeneous serovar. Group A/A2 plasmids were more prevalent and widely spread (found in most sources and in four regions in the United States). This plasmid group showed a high degree of sequence similarity to the Salmonella Kanr ColE1-like plasmid NTP16 (4) and was likely to have been prevalent in the Salmonella population for a long period of time.
Plasmid DNA from seven isolates, all from swine or turkeys, failed to convert E. coli DH5α to Kanr from three independent preparations of plasmids. NlaIII digestion of the plasmids from the original Salmonella strains showed the presence of low-molecular-weight, high-copy-number plasmids in three isolates (612, 711, and 910), and yet none of these patterns matched any of the groups/subgroups that were transformable into E. coli (data not shown). Replicon typing results showed that these isolates carried between zero and three additional plasmid replicons (Inc A/C, I1, HI1, or Frep) other than ColE1. It is possible that the visualized small plasmids are not ColE1-like plasmids or that the ColE1-like plasmids in these isolates did not carry the aph gene. The aph gene may be located on the chromosome or on other plasmid(s) in these “N/T” isolates. Further study is needed to characterize these isolates.
Replicon typing revealed 19 (63%) of the ColE1-positive isolates were also positive for the large (>150 kb) Inc A/C plasmid. The Inc A/C multidrug resistance plasmid is often found in this population of multiply antimicrobial resistant Salmonella (18, 25, 40). The PFGE cluster analysis showed two groups that may be considered to be epidemic, highly successful clones, which, for the sake of this discussion, were considered to be PFGE types (36) with three or more identical individual isolates. The largest PFGE clonal group contained isolates 816, 852, 870 to 872, 908, and 938, and all were from region 1, serovar Typhimurium or Typhimurium variant 5−, and phage type DT104 (Table 2 and Fig. 1). Isolate 852 was different from the other epidemic clones in that it carried Kanr ColE1 plasmid group C and aph sequence type ii. Two of these clones also showed diversity in plasmid replicon type: isolate 816 with FIIA and isolate 870 with HI1. It is interesting that none of these clones carried Inc A/C plasmids, and all were phage type DT104, as shown previously in a related Inc A/C study in which a negative correlation between Inc A/C and DT104 was found (25). Here, we found 100% of the ACSSuT+KAN-resistant, serovar Typhimurium or Typhimurium variant 5− and phage type DT104 isolates were ColE1 positive (Fig. 1, Tables 2 and 4). The second epidemic clonal group contained four isolates—604, 688, 892, and 894—that were all serovar Newport. All of these isolates had identical antimicrobial resistance and plasmid replicon type profiles except isolate 604, which was not resistant to CHL.
As previously seen in a larger Inc A/C study (25), it is interesting to compare the clades that contain serovar Typhimurium or Typhimurium variant 5− with the one that contains Newport. All individuals in the Newport clade were positive by PCR for both the ColE1 and the Inc A/C plasmids in the present study. The Typhimurium clade is more often associated with pentaresistance (ACSSuT resistance), and ColE1-like plasmids were prevalent, whereas Inc A/C plasmids were not observed in this clade. Serovar Typhimurium and pentaresistance is often associated with phage type DT104, a type that has multiple resistance genes located on a genomic island (3, 19, 38, 39). In the present study, all seven (100%) of the isolates that were serovar Typhimurium and serovar Typhimurium variant 5−, ACSSuT+KAN resistant, and positive for ColE1 by PCR were also associated with the DT104 phage type (Tables 2 and 4). It should be noted that although isolates for the present study were randomly selected from NARMS 2005 diagnostic isolates on the basis of KAN resistance 96% of the isolates are also Tetr. The presence of TET resistance may increase the chance of finding DT104 positive isolates in serovars Typhimurium and Typhimurium variant 5−. It would be unusual to find a population of Kanr Tets isolates in serovars Typhimurium and Typhimurium variant 5− that are DT104 positive. A 1997 study on Salmonella isolated in Canada from animals, foods of animal origins, and the environment of animal production found that phage type DT104 comprised of 29.5% of all Typhimurium (175/593); only 24% showed the classical ACSSuT pentaresistance pheotype. A total of 29.1% (51/175) of DT104 isolates were Kanr, and 28% (49/175) were ACSSuT+KAN resistant. KAN resistance was encoded by aphA-1 gene on 2.0-MDa plasmids (∼3 kb): 50 of the 51 Kanr isolates possessed the 2.0-MDa plasmid, and all strains possessing the 2.0-MDa plasmid were resistant to KAN. However, the report did not further characterize the plasmids by sequencing, and only four strains were tested by transformation. One of those that failed to transform was found to lack the 2.0-MDa plasmid, and the aph gene in this isolate was found to be located on the chromosome by Southern hybridization (30).
Concluding remarks.
Although the ColE1-like plasmids generally do not carry conjugal transfer genes, many carry mobilization genes that are capable of plasmid transfer in the presence of other conjugative plasmids (12, 29). The high copy number (50 or more copies per cell) of this plasmid family also makes it more likely to be transferred into naive strains, posing a high risk of transmitting the antibiotic resistance genes. The mobility and high copy number of the ColE1 plasmid family warrants close monitoring of its prevalence and transmission among food animals. The linkage shown here between ColE1 and DT104 needs to be studied further in a larger population of isolates to see whether it continues. Studying a population of isolates with less overall antibiotic resistance or a Kanr Tets isolates would also be interesting. The ColE1 typing primers designed in the present study successfully identified three groups of ColE1-like plasmids, including the new group C/C2/C3. We are currently sequencing representative plasmids from each group or subgroup identified here, with special emphasis on the group C/C2/C3 plasmids. With additional sequence information, we can further improve the ColE1 typing primers and incorporate them into the plasmid replicon typing sets (7, 8, 23) to expand the coverage of known plasmid replicon types used for routine characterization of Salmonella and other enteric pathogens.
Acknowledgments
We gratefully acknowledge Alessandra Carattoli for replicon typing control strains; David Needleman and Susan Lawler at the ERRC sequencing facility for providing sequencing service; and Tyler Wilcher, Tiffanie Woodley, Sandra House, Takiyah Ball, Cheryl Gresham, Carolina Hall, Beth McGlinchey, and Jovita Haro at the RRC for technical assistance. We also thank Pina Fratamico for critically reviewing the manuscript.
Any mention of trade names or commercial products is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Footnotes
Published ahead of print on 6 August 2010.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ARS-USDA. 2006. National Antimicrobial Resistance Monitoring System (NARMS): Enteric Bacteria 2006 Veterinary Isolates Final Report Slaughter Isolates. ARS-USDA, Washington, DC. http://www.ars.usda.gov/main/docs.htm?docid=17320.
- 3.Briggs, C. E., and P. M. Fratamico. 1999. Molecular characterization of an antibiotic resistance gene cluster of Salmonella typhimurium DT104. Antimicrob. Agents Chemother. 43:846-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannon, P. M., and P. Strike. 1992. Complete nucleotide sequence and gene organization of plasmid NTP16. Plasmid 27:220-230. [DOI] [PubMed] [Google Scholar]
- 5.Carattoli, A. 2001. Importance of integrons in the diffusion of resistance. Vet. Res. 32:243-259. [DOI] [PubMed] [Google Scholar]
- 6.Carattoli, A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob. Agents Chemother. 53:2227-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carattoli, A., A. Bertini, L. Villa, V. Falbo, K. L. Hopkins, and E. J. Threlfall. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219-228. [DOI] [PubMed] [Google Scholar]
- 8.Carattoli, A., V. Miriagou, A. Bertini, A. Loli, C. Colinon, L. Villa, J. M. Whichard, and G. M. Rossolini. 2006. Replicon typing of plasmids encoding resistance to newer beta-lactams. Emerg. Infect. Dis. 12:1145-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Dis. Control and Prevention (CDC). 2004. One-day (24-28 h) standardized laboratory protocol for molecular subtyping of Escherichia coli O157:H7, non-typhoidal Salmonella serovars, and Shigella sonnei by pulsed field gel electrophoresis (PFGE). Training manual. U.S. Department of Health and Human Services, Atlanta, GA.
- 10.Centers for Disease Control and Prevention. 2006. Public Health Laboratory Information System (PHLIS): Salmonella surveillance, annual summary. CDC, Atlanta, GA. http://www.cdc.gov/ncidod/dbmd/phlisdata/salmonella.htm.
- 11.Centers for Disease Control and Prevention. 2009. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food: 10 states, 2008. MMWR Morb. Mortal. Wkly. Rep. 58:333-337. [PubMed] [Google Scholar]
- 12.Chen, C.-Y., G. W. Nace, B. Solow, and P. Fratamico. 2007. Complete nucleotide sequences of 84.5- and 3.2-kb plasmids in the multi-antibiotic resistant Salmonella enterica serovar Typhimurium U302 strain G8430. Plasmid 57:29-43. [DOI] [PubMed] [Google Scholar]
- 13.Chen, C.-Y., T. P. Strobaugh, Jr., and J. G. Frye. 2010. Characterization of small ColE1-like plasmids conferring kanamycin resistance in Salmonella enterica subsp. enterica serovars Typhimurium and Newport. Plasmid 63:150-154. [DOI] [PubMed] [Google Scholar]
- 14.Couturier, M., F. Bex, P. L. Bergquist, and W. K. Maas. 1988. Identification and classification of bacterial plasmids. Microbiol. Rev. 52:375-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dargatz, D. A., P. J. Fedorka-Cray, S. R. Ladely, K. E. Ferris, A. L. Green, and M. L. Headrick. 2002. Antimicrobial susceptibility patterns of Salmonella isolates from cattle in feedlots. J. Am. Vet. Med. Assoc. 221:268-272. [DOI] [PubMed] [Google Scholar]
- 16.Excoffier, L., G. Laval, and S. Schneider. 2005. Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol. Bioinform. Online 1:47-50. [PMC free article] [PubMed] [Google Scholar]
- 17.Fricke, W. F., P. F. McDermott, M. K. Mammel, S. Zhao, T. J. Johnson, D. A. Rasko, P. J. Fedorka-Cray, A. Pedroso, M. Jean, J. M. Whichard, E. LeClerc, D. G. White, T. A. Cebula, and J. Ravel. 2009. Antimicrobial resistance-encoding APEC plasmids in Salmonella enterica serovar Kentucky from poultry. Appl. Environ. Microbiol. 75:5963-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fricke, W. F., T. J. Welch, P. F. McDermott, M. K. Mammel, J. F. LeClerc, D. G. White, T. A. Cebula, and J. Ravel. 2009. Comparative genomics of the IncA/C multidrug resistance plasmid family. J. Bacteriol. 191:4750-4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glynn, M. K., C. Bopp, W. Dewitt, P. Dabney, M. Mokhtar, and F. J. Angulo. 1998. Emergence of multidrug-resistant Salmonella enterica serovar Typhimurium DT104 infections in the United States. N. Engl. J. Med. 338:1333-1338. [DOI] [PubMed] [Google Scholar]
- 20.Gregorova, D., J. Matiasovicova, A. Sebkova, M. Faldynova, and I. Rychlik. 2004. Salmonella enterica subsp. enterica serovar Enteritidis harbours ColE1, ColE2, and rolling-circle-like replicating plasmids. Can. J. Microbiol. 50:107-112. [DOI] [PubMed] [Google Scholar]
- 21.Gregorova, D., M. Pravcova, R. Karpiskova, and I. Rychlik. 2002. Plasmid pC present in Salmonella enterica serovar Enteritidis PT14b strains encodes a restriction modification system. FEMS Microbiol. Lett. 214:195-198. [DOI] [PubMed] [Google Scholar]
- 22.Guindon, S., and O. Gascuel. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696-704. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, T. J., Y. M. Wannemuehler, S. J. Johnson, C. M. Logue, D. G. White, C. Doetkott, and L. K. Nolan. 2007. Plasmid replicon typing of commensal and pathogenic Escherichia coli isolates. Appl. Environ. Microbiol. 73:1976-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kehrenberg, C., K. L. Hopkins, E. J. Threlfall, and S. Schwarz. 2007. Complete nucleotide sequence of a small qnrS1-carrying plasmid from Salmonella enterica subsp. enterica Typhimurium DT193. J. Antimicrob. Chemother. 60:903-905. [DOI] [PubMed] [Google Scholar]
- 25.Lindsey, R. L., P. J. Fedorka-Cray, J. G. Frye, and R. J. Meinersmann. 2009. Inc A/C plasmids are prevalent in multidrug-resistant Salmonella enterica isolates. Appl. Environ. Microbiol. 75:1908-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing. 12th informational supplement M100-S12. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 28.Novick, R. P., and F. C. Hoppensteadt. 1978. On plasmid incompatibility. Plasmid 1:421-434. [DOI] [PubMed] [Google Scholar]
- 29.Osborn, A. M., and D. Böltner. 2002. When phage, plasmids and transposons collide: genomic islands, and conjugative- and mobilizable-transposons as a mosaic continuum. Plasmid 48:202-212. [DOI] [PubMed] [Google Scholar]
- 30.Poppe, C., K. Ziebell, L. Martin, and K. Allen. 2002. Diversity in antimicrobial resistance and other characteristics among Salmonella Typhimurium DT104 isolates. Microb. Drug Resist. 8:107-122. [DOI] [PubMed] [Google Scholar]
- 31.Posada, D. 2008. jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 25:1253-1256. [DOI] [PubMed] [Google Scholar]
- 32.Rychlik, I., D. Gregorova, and H. Hradecka. 2006. Distribution and function of plasmids in Salmonella enterica. Vet. Microbiol. 112:1-10. [DOI] [PubMed] [Google Scholar]
- 33.Rychlik, I., A. Sebkova, D. Gregorova, and R. Karpiskova. 2001. Low-molecular-weight plasmid of Salmonella enterica serovar Enteritidis codes for retron reverse transcriptase and influences phage resistance. J. Bacteriol. 183:2852-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw, K. J., P. N. Rather, R. S. Hare, and G. H. Miller. 1993. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 57:138-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slatkin, M. 1994. Linkage disequilibrium in growing and stable populations. Genetics 137:331-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, J. M., N. H. Smith, M. O'Rourke, and B. G. Spratt. 1993. How clonal are bacteria? Proc. Natl. Acad. Sci. U. S. A. 90:4384-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swafford, D. L. 2003. PAUP* v4.1: phylogenetic analysis using parsimony (*and other methods). Sinauer Associates, Sunderland, MA.
- 38.Threlfall, E. J. 2000. Epidemic Salmonella typhimurium DT 104: a truly international multiresistant clone. J. Antimicrob. Chemother. 46:7-10. [DOI] [PubMed] [Google Scholar]
- 39.Threlfall, E. J., J. A. Frost, L. R. Ward, and B. Rowe. 1994. Epidemic in cattle and humans of Salmonella typhimurium DT 104 with chromosomally integrated multiple drug resistance. Vet. Rec. 134:577. [DOI] [PubMed] [Google Scholar]
- 40.Welch, T. J., W. F. Fricke, P. F. McDermott, D. G. White, M. L. Rosso, D. A. Rasko, M. K. Mammel, M. Eppinger, M. J. Rosovitz, D. Wagner, L. Rahalison, J. E. LeClerc, J. M. Hinshaw, L. E. Lindler, T. A. Cebula, F. Carniel, and J. Ravel. 2007. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One 2:e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells, S. J., P. J. Fedorka-Cray, D. A. Dargatz, K. Ferris, and A. Green. 2001. Fecal shedding of Salmonella spp. by dairy cows on farm and at cull cow markets. J. Food Prot. 64:3-11. [DOI] [PubMed] [Google Scholar]
- 42.Wu, J. J., W. C. Ko, C. S. Chiou, H. M. Chen, L. R. Wang, and J. J. Yan. 2008. Emergence of Qnr determinants in human Salmonella isolates in Taiwan. J. Antimicrob. Chemother. 62:1269-1272. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, Z., S. Schwartz, L. Wagner, and W. Miller. 2000. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 7:203-214. [DOI] [PubMed] [Google Scholar]
- 44.Zioga, A., J. M. Whichard, S. D. Kotsakis, L. S. Tzouvelekis, E. Tzelepi, and V. Miriagou. 2009. CMY-31 and CMY-36 cephalosporinases encoded by ColE1-like plasmids. Antimicrob. Agents Chemother. 53:1256-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]