Abstract
An understanding of the mechanisms involved in the different steps of bacterial disease epidemiology is essential to develop new control strategies. Seeds are the passive carriers of a diversified microbial cohort likely to affect seedling physiology. Among seed-borne plant-pathogenic bacteria, seed carriage in compatible situations is well evidenced. The aims of our work are to determine the efficiency of pathogen transmission to seeds of a nonhost plant and to evaluate bacterial and plant behaviors at emergence. Bacterial transmission from flowers to seeds and from seeds to seedlings was measured for Xanthomonas campestris pv. campestris in incompatible interactions with bean. Transmissions from seeds to seedlings were compared for X. campestris pv. campestris, for Xanthomonas citri pv. phaseoli var. fuscans in compatible interactions with bean, and for Escherichia coli, a human pathogen, in null interactions with bean. The induction of defense responses was monitored by using reverse transcription and quantitative PCR (RT-qPCR) of genes representing the main signaling pathways and assaying defense-related enzymatic activities. Flower inoculations resulted in a high level of bean seed contamination by X. campestris pv. campestris, which transmitted efficiently to seedlings. Whatever the type of interaction tested, dynamics of bacterial population sizes were similar on seedlings, and no defense responses were induced evidencing bacterial colonization of seedlings without any associated defense response induction. Bacteria associated with the spermosphere multiply in this rich environment, suggesting that the colonization of seedlings relies mostly on commensalism. The transmission of plant-pathogenic bacteria to and by nonhost seeds suggests a probable role of seeds of nonhost plants as an inoculum source.
The process of the microbial colonization of germinating seeds and seedlings shapes traits that are important for successful biological control and has therefore been subjected to intense research (for a review, see reference 38). This contrasts strikingly with the situation for seed-borne plant-pathogenic bacteria and their hosts, for which little is known at this step, with efforts having been devoted mainly to interactions with fully developed plants. However, pathogen transmission is one of the most important parameters for fitness (16, 32). It combines the ability to survive outside the host prior to infection, multiply on the host, and disperse and transmit to new ecological niches, including host seeds. Seeds are passive carriers of pathogens that are transmitted when the seeds are sown and emerge (30). Seed accounts for the movement of plant pathogens across vast distances and may even be responsible for the emergence of diseases in new areas. Seed-borne bacterial pathogens are of particular concern because strategies for the management of bacterial diseases are limited, and none are efficient (20). Moreover, seeds harbor a diverse microbial community, including human pathogens such as Salmonella enterica serovars and Escherichia coli O157:H7 (24, 37). Information is lacking so far with regard to the ability of seed-borne plant-pathogenic bacteria to multiply on germinating nonhost seeds and colonize seedlings. Some humans pathogens establish themselves endophytically in plants and may be inherited from generation to generation (57).
Bacterial spermosphere-colonizing traits have been identified mainly for organisms used for biological disease control; in contrast, only few studies focused on interactions encountered in the spermosphere and seedling environments between plant-pathogenic bacteria and the plant. The colonization of germinating seeds and seedlings represents a critical step in the establishment of bacterial diseases. One observation made previously by Hirano et al. (23) suggested that Pseudomonas syringae pv. syringae does not behave as a parasite during bean germination, as its type III secretion system (T3SS) does not seem to play a major role during seed germination and seedling colonization. Indeed, similar dynamics of population sizes were observed for wild-type P. syringae pv. syringae and its T3SS mutants on seedlings in field trials.
The molecular bases of interactions between plant-pathogenic bacteria and plants have been studied extensively in the phyllosphere environment. The plant immune system is based on the recognition of pathogen-associated molecular patterns (PAMPs), resulting in the activation of defense responses (41). Three main pathways controlling the activation of plant defenses are described. The salicylic pathway controls defenses that are active against pathogens with a biotrophic lifestyle, whereas jasmonate and ethylene pathways control defenses that are active against necrotrophic pathogens (27). Most Gram-negative bacterial pathogens harbor a T3SS that allows the delivery of effectors directly into the plant cytoplasm, leading to a hypersensitive reaction (HR) in incompatible interactions and symptom development in compatible ones (5, 21, 35). In compatible interactions, defense responses are suppressed by T3SS effectors (3, 14). Alternatively, T3SS effectors such as AvrBS1 to AvrBS4 of Xanthomonas axonopodis pv. vesicatoria were also described to enhance epiphytic fitness and bacterial transmission in the field (63).
The floral transmission of bacteria to seeds is a permissive pathway compared to transmission through the vascular system (12). Floral transmission allows the contamination of seeds by a cohort of diverse bacteria, including biocontrol agents (17, 52). T3SS mutants of Xanthomonas citri pv. phaseoli var. fuscans are strongly impaired in their ability to transmit to seeds, but transmission through the floral pathway is not completely abolished for these strains (12). None of the five adhesins identified in the X. citri pv. phaseoli var. fuscans genome are involved in floral transmission to bean seeds. Conversely, all of them are involved in transmission through the vascular pathway, limiting or enhancing transmission efficiency and associated bacterial population sizes (11).
Xanthomonads are plant-pathogenic bacteria, and most of them are seed borne. Seed carriage is well documented for pathogens on their host, but information is lacking concerning carriage by a nonhost seed. Recently, Darsonval and colleagues (12) showed that plant-pathogenic bacteria may transmit to nonhost plant seeds. Indeed, following spray inoculation of the canopy of mother plants, bean seeds were contaminated by the incompatible bacterium X. campestris pv. campestris. However, the risk of generation of a primary inoculum focus at emergence associated with this nonhost carriage was not evaluated. Black rot, caused by the bacterium X. campestris pv. campestris, is considered the most serious disease of crucifer crops worldwide (46). Plants, such as bean, that are not in the crucifer family are not susceptible. Conversely, bean is a susceptible host for X. citri pv. phaseoli var. fuscans (1) (formerly Xanthomonas axonopodis pv. phaseoli var. fuscans [60] or X. fuscans subsp. fuscans [47]), which is responsible for the common bacterial blight of bean (62). Both bacteria are seed borne on their hosts, and in natural settings, their transmission by nonhost seeds had not yet been reported.
In this paper we aimed to understand colonization processes of germinating bean seeds and seedlings by X. citri pv. phaseoli var. fuscans, X. campestris pv. campestris, and Escherichia coli, representing a compatible, an incompatible, and a null interaction, respectively. In an attempt to evaluate the risk of primary inoculum generation associated with nonhost carriage, we monitored the efficiency of the transmission of incompatible bacteria from flowers to seeds and from seeds to seedlings. We also compared the dynamics of bacterial population sizes in regard to defense reactions developed by the plant during germination and seedling development. The role of the T3SS and adhesion in seedling colonization was examined with the use of mutants in structural and regulatory genes of the T3SS and in yapH, a gene encoding a nonfimbrial adhesin.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains used in this study are described in Table 1. The pathogenicity of wild-type strains on their respective susceptible hosts was routinely checked. Xanthomonad strains were grown at 28°C in 10% TSA medium (1.7 g liter−1 tryptone, 0.3 g liter−1 soybean peptone, 0.25 g liter−1 glucose, 0.5 g liter−1 NaCl, 0.5 g liter−1 K2HPO4, and 15 g liter−1 agar) supplemented with the appropriate antibiotics. Escherichia coli cells were cultivated at 37°C in LB medium (10 g liter−1 tryptone, 5 g liter−1 yeast extract, 5 g liter−1 NaCl, 15 g liter−1 agar) and, for in planta studies, on 100% TSA medium (17 g liter− tryptone, 3 g liter−1 soybean peptone, 2.5 g liter−1 glucose, 5 g liter−1 NaCl, 5 g liter−1 K2HPO4, and 15 g liter−1 agar) supplemented with rifamycin (Rif). Antibiotics were used at the following final concentrations: Rif at 50 mg liter−1 and kanamycin (Km) at 25 mg liter−1. For in planta studies, media were supplemented with 50 mg liter−1 cycloheximide and 10 mg liter−1 propiconazole to inhibit fungal growth. To prepare inocula, strains were grown for 48 h on appropriate media supplemented with appropriate antibiotics. Bacterial cells were scraped from agar plates and suspended in sterile distilled H2O. Suspensions were turbidimetrically calibrated to 1 × 108 CFU ml−1 and adjusted to the desired final concentrations with sterile distilled H2O.
TABLE 1.
Strains used in this study
| Strain | Relevant genotype or characteristic(s) | Reference or source |
|---|---|---|
| CFBP4834-R | X. citri pv. phaseoli var. fuscans wild-type strain; Rifr | 25 |
| ATCC 33913 | X. campestris pv. campestris wild-type strain; Rifr | 13 |
| C600 | E. coli wild-type strain; Rifr | 42 |
| 4834HRPG | CFBP4834-R hrpG::pVO155; Rifr Kmr | 12 |
| 4834HRCR | CFBP4834-R hrcR::pVO155; Rifr Kmr | 12 |
| 4834YAPH2 | CFBP4834-R yapH2::pVO155; Rifr Kmr | 11 |
| 33913HRCU | ATCC 33913 hrcU::pVO155; Rifr Kmr | Gift from M. Arlat |
Plant material.
In planta experiments were conducted with a susceptible cultivar of dry bean (Phaseolus vulgaris cv. Flavert). Seeds were kindly provided by Vilmorin (La Ménitré, France) and were considered free of X. citri pv. phaseoli var. fuscans following an analysis of about 100,000 seeds per lot with standard tests (44). When necessary, seeds were incubated in sterile germination boxes (17.5 by 11.5 by 15.5 cm) on moistened sterile paper in growth chambers at 25°C for 48 h. Seedlings were transplanted in 10- by 10- by 18-cm pots (1 seed per pot) containing soil substrate (Neuhaus humin substrat S NF 11-44-551; Proveg, La Rochelle, France). Beans were grown in growth chambers as previously described (12) under 16 h of light at 25°C and 8 h of darkness at 22°C and under high (95%) relative humidity. Plants were watered three times per week, and once a week, water was supplemented with 0.3 g liter−1 nitrogen-phosphorous-potassium fertilizer (18:14:18).
Bean inoculations.
Seeds were contaminated by two means. First, the contamination of seeds on mother plants was performed by spray inoculation of beans at the flower bud stage with 1 × 107 CFU ml−1 inoculum as previously reported (12). Mature pods were harvested 5 weeks later, and seeds were manually excised from pods. Seeds were stored at room temperature. Second, seeds were directly inoculated with an inoculum of 1 × 106 CFU ml−1 by soaking in 2 ml inoculum per seed during 30 min and vacuum infiltrated for 3 min. Seeds were dried for 2 h in a sterile environment at room temperature and directly used.
Leaves and seedlings were vacuum infiltrated with an inoculum of 1 × 106 CFU ml−1 for 3 min by soaking in 500 ml of inoculum per plant. Plants were dried for 2 h at room temperature. Plant inoculations were carried out under quarantine at UMR PaVé, Site INRA, Angers, France.
Monitoring of bacterial population sizes.
To quantify bacterial population sizes, seeds were soaked overnight at 4°C in 2 ml of sterile distilled H2O per seed. The samples were then shaken with a vortex for 30 s at maximal speed three times. Seedlings and leaves were weighed and ground (Stomacher 80; Seward, London, United Kingdom) individually for 2 min at maximum power in 5 ml of sterile distilled H2O without any preliminary treatments. Every sample and appropriate dilutions were spiral plated (Spiral Biotech, Bethesda, MD) onto selective medium to quantify the inoculated strain. Samples from control plants were plated onto 100% TSA to quantify bacterial indigenous population sizes. Five samples were analyzed per treatment and per sampling date. Experiments were repeated at least twice.
To quantify the contamination rate of a seed lot, seed bulks were soaked overnight at 4°C in 2 ml of sterile distilled H2O per g of seeds. Samples were analyzed as indicated above. For seedling lots, bulks of 4-day-old seedlings without cotyledons were ground in 5 to 20 ml of sterile distilled H2O and analyzed as indicated above. The contamination rate (p) of a lot was calculated from the analysis of a sample divided into N groups according to the formula pi = 1 − (Y/Ni)1/ni (31), where n is the number of individuals in each group (bulked for analysis) and Y is the number of healthy groups. Preliminary experiments allowed the approximation of the contamination rate of a seed lot to define the adequate group sizes using most-probable-number tables (54). It was expected that each contaminated group contained at most one contaminated individual. To calculate the efficiency of bacterial transmission from seeds to seedlings, groups contained the same number of individuals. The efficiency of transmission was expressed as a ratio of the number of contaminated seeds to the number of contaminated seedlings (55). Experiments were repeated twice (i = 2) with the following sampling: N1 = 15, n1 = 100, N2 = 10, and n2 = 40.
In experiments of X. campestris pv. campestris transmission to and by bean seeds, the identity of X. campestris pv. campestris colonies was confirmed on 3 colonies per sample by PCR using specific primers for X. campestris pv. campestris (45). PCRs were done with 20-μl volumes containing 200 μM deoxynucleoside triphosphates (dNTPs), 1.5 mM MgCl2, 0.5 μM each primer, 0.4 U μl−1 GoTaq polymerase (Promega), and 4 μl of a boiled bacterial suspension (1 × 107 CFU ml−1). PCR conditions were 5 min at 94°C; 35 cycles of 20 s at 94°C, 20 s at 60°C, and 20 s at 72°C; and 7 min at 72°C.
Bacterial adhesion to bean seeds.
Bacterial adhesion to bean seeds was tested as previously described (11). Briefly, columns were filled with 6 g of bean seeds and fully saturated with 12 ml of the inoculum at 1 × 105 CFU ml−1. The inoculum was removed after a 2-h contact time between the culture and the seeds. Seeds in columns were washed twice with 12 ml of sterile distilled H2O. Bacterial population sizes adherent on seeds were monitored by seed maceration. The ratio of the number of attached cells to the number of inoculated cells indicated irreversible adhesion capacities (15). Experiments were repeated at least twice with three samples per treatment.
Bacterial adhesion to polypropylene.
Bacterial adhesion under static conditions was quantified by a crystal violet (CV) incorporation assay with 96-well polypropylene microtiter plates (Microwell; Nunc, Denmark) using a method described previously by O'Toole and Kolter (43), modified as described previously by Darsonval et al. (11). Three wells per strain were filled with 200 μl MME minimal medium [K2HPO4 at 10.5 g liter−1, KH2PO4 at 5.4 g liter−1, (NH4)2SO4 supplemented with 20 mM glutamate at 1 g liter−1, 1 mM MgSO4, and Casamino Acids at 0.15 g liter−1] (2) and inoculated with 1:20 dilutions of the inoculum calibrated at 1 × 107 CFU ml−1. Plates were incubated without shaking at 28°C for up to 3 days. Bacterial growth was confirmed by measuring the optical density at 600 nm (OD600) of the supernatants by using a μQuant Platewell reader (Bio-Tek Instruments, Inc.). To quantify surface-attached bacteria, surface-attached cells in wells were stained for 15 min at room temperature with 200 μl per well of a 1% (wt/vol) CV solution. The CV solution was then discarded, and the wells were thoroughly washed with sterile distilled H2O. Finally, the dye incorporated into attached cells was solubilized in 200 μl of 95% ethanol per well, and the OD600 was determined by using the microplate reader. These tests were repeated at least twice for every bacterial strain.
RT-qPCR.
RNA was extracted from 50 to 250 mg of plant tissue according to a method described previously by Venisse et al. (61) and treated for 1 h at 37°C with DNase according to the manufacturer's recommendations (Ambion; Applied Biosystems, Courtaboeuf, France) to remove DNA. Samples were incubated for 10 min at 75°C to inactivate DNase, and RNA was ethanol precipitated, dissolved in double-distilled H2O, and calibrated at 0.5 μg μl−1. The quality (A260/A280 and A260/A230 ratios) and quantity of RNA were checked with a Nanodrop instrument (Thermo Scientific). The absence of contamination by DNA was checked by quantitative PCR (qPCR) with EF1-α primers (Table 2) using 1/80 dilutions of calibrated RNA. Samples were duplicated, and cDNA was added in a copy of each sample to check the absence of PCR inhibitors. Reverse transcription (RT) was performed with 2 μg of RNA according to a protocol described previously by Venisse et al. (61), final cDNA was dissolved in 30 μl of double-distilled H2O, and 1/16 dilutions were used for qPCR experiments. Quantitative amplifications were performed in a 25-μl volume with 6.25 μl of SYBR green (qPCR MasterMix Plus; Eurogentec, Angers, France) and 5 to 10 pmol of primers (Table 2). The concentration of primers was optimized to obtain a PCR efficiency ranging between 90% and 100%. Amplification conditions were 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 s, and 60°C for 1 min. An additional melt curve was realized in each experiment to check the specificity of the amplification. Relative changes in gene expression were calculated by using the ΔΔCT method (29), normalized to the two internal controls UBI and EF1-α (Table 2) according to a method described previously by Vandesompele et al. (59) and relative to a calibrator (control samples treated with H2O at every sampling date and in every RT-qPCR). The three main signaling pathways of defense responses were investigated through PR-3 (chitinase), representing the salicylic acid pathway (48); ACO (ACC oxidase) for the ethylene pathway (8); and JAR-1 (jasmonate resistant 1 in Arabidopsis thaliana) for the jasmonate pathway (53). When available, annotated P. vulgaris cDNA sequences were used to design primers (22); in the other cases, annotated A. thaliana sequences were blasted against expressed sequence tag (EST) libraries to find homologous P. vulgaris sequences (Table 2). Experiments were performed with three independent duplicated samples of plant material per treatment. Each sample was made of the aerial part of one seedling without cotyledons or primary leaf pieces from one plant. The mean of values from three water-treated samples was used for calibration at each sampling date, and the geometric mean of values obtained for internal controls was used to normalize each sample.
TABLE 2.
Primers used for qPCR experiments
| cDNA | Gene reference no. | Function | Primer sequence | Fragment size (bp) |
|---|---|---|---|---|
| UBI | CV543388a | Ubiquitin | GAGGATGGTCGCACCCTGGCT | 184 |
| CCCTCCTTGTCCTGAATCTTA | ||||
| EF1-α | GI151368189b | Elongation factor 1α | CAAGGATCTCAAGCGTGGTTTCG | 150 |
| TGGGAGGTGTGGCAATCAAGC | ||||
| JAR1 | GI171655005b | Jasmonate-amino synthetase | AAGAACCGGGACACTATGTGA | 174 |
| AATGTTCCCCTCCGAACAAC | ||||
| PR3 | GI62703651b | PR3 protein (chitinase) | TAGGAGTGGTGTGGATGCTG | 192 |
| CGCTGAGATCAGTAGGAGCA | ||||
| ACC | CV541144a | ACC oxidase | TGGCACCAAAGTTACAACTA | 270 |
| ATTCTGGTGCCATCCGTTTGA |
cDNA library of Phaseolus vulgaris (22).
Reference numbers in NCBI, Phaseolus vulgaris cDNA.
Assays for POX and GLU activities.
Enzymes were extracted as described previously by Venisse et al. (61), by grinding 0.8 to 1 g of plant material into 5 ml of 50 mM sodium acetate buffer (pH 5.5) containing 1 mM polyethylene glycol, 1 mM phenylmethylsulfonyl fluoride, 8% (wt/vol) polyvinylpolypyrrolidone, and 0.01% (vol/vol) Triton X-100. Homogenates were centrifuged at 16,000 × g at 4°C for 20 min, and supernatants were centrifuged at 10,000 × g at 4°C for 10 min. Peroxidase (POX) activity was determined according to the method of Chance and Maehly (9) and were expressed in μmol tetraguaiacol mg−1 protein−1 min−1. β-1,3-Glucanase (GLU) activity was assayed according to a method described previously by Wirth and Wolf (64), modified as described previously by Venisse et al. (61), and calculated from the differences in the absorbances at 600 nm between the sample incubated 45 min at 37°C and the sample not incubated. The protein content in crude enzyme extracts was determined according to the method of Bradford (4), using Coomassie protein assay reagent (Pierce, Rockford, IL). Experiments were performed with at least two independent duplicated samples. Each sample was made of the bulk of three to eight seedlings (aerial part without cotyledons) or the bulk of primary leaf pieces from three plants.
Statistical analyses.
Statistical analyses were performed by using Statview 5.0 (SAS Institute Incorporated). Data expressed as means of log10-transformed population sizes were analyzed with Mann-Whitney U tests (51).
RESULTS
Bacterial seed transmission in compatible and incompatible interactions.
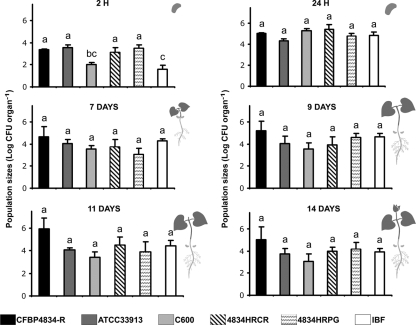
Seed contamination by an incompatible pathogen could represent an epidemiological concern if the bacterium is able to multiply on germinating seeds, colonize seedlings, and disperse to neighboring host plants. To evaluate the risk at emergence of the generation of a primary inoculum associated with nonhost carriage, we measured the efficiency of transmission of pathogenic bacteria from seeds to seedlings of a nonhost plant. Bean seeds contaminated by the incompatible pathogen X. campestris pv. campestris ATCC 33913 were produced following spray inoculation of beans at the flower bud stage. A 100- to 1,000-fold increase in mean population sizes in 4-day-old seedlings compared to seeds indicated that X. campestris pv. campestris ATCC 33913 multiplied in this environment (mean X. campestris pv. campestris ATCC 33913 population sizes of 5.28 log10 CFU [standard error of the mean {SEM} = 0.43] and 4.13 log10 CFU [SEM = 0.34] in seedlings and 2.49 log10 CFU [SEM = 0.35] and 2.18 log10 CFU [SEM = 0.16] in seeds per contaminated individual in two independent experiments, respectively). Population sizes reached up to 1.8 × 104 CFU per contaminated seed and 2.4 × 106 CFU per contaminated seedling. The two harvested seed lots showed contamination rates of 0.6% and 1.4%, and 4-day-old seedlings showed corresponding contamination rates of 0.4% and 3%, respectively. In theses experiments transmission efficiencies ranged from 1.5:1 to 0.5:1 (number of contaminated seeds needed to obtain one contaminated seedling), evidencing an efficient transmission of a plant-pathogenic bacterium from seeds to seedlings of a nonhost plant.
To characterize the behavior of bacteria representing incompatible versus compatible and null interactions with a plant at emergence, we analyzed their dynamics of population sizes during seedling development. Bean seeds were inoculated by vacuum infiltration with X. campestris pv. campestris (incompatible interaction), X. citri pv. phaseoli var. fuscans (compatible interaction), and E. coli (null interaction). Two hours after inoculation, population sizes of the different strains were similar on seeds, except for those of E. coli C600, which were significantly (P < 0.01) lower than the others (Fig. 1). As early as 24 h after inoculation, the multiplication of E. coli C600 allowed it to reach population sizes similar to those of X. citri pv. phaseoli var. fuscans and X. campestris pv. campestris. Until 14 days after germination, X. citri pv. phaseoli var. fuscans strain CFBP4834-R did not multiply significantly better than X. campestris pv. campestris strain ATCC 33913 and E. coli C600 on bean seedlings (P > 0.05) (Fig. 1). Therefore, whatever the type of interaction, bacteria behaved similarly at emergence. Similar results were repeatedly monitored and were also obtained with higher inoculum concentrations (data not shown). During the experiments, no symptoms were ever observed on seeds and seedlings following seed contamination with X. citri pv. phaseoli var. fuscans strain CFBP4834-R, X. campestris pv. campestris, or E. coli. Germination rates and seedling development were not affected by any treatment (data not shown).
FIG. 1.
Colonization of bean seedlings by X. citri pv. phaseoli var. fuscans wild-type strain CFBP4834-R and mutant strains 4834HRCR and 4834HRPG, X. campestris pv. campestris ATCC 33913, and E. coli C600. Bacterial population sizes were quantified on bean seeds 2 h and 24 h after vacuum infiltration (1 × 106 CFU ml−1) of seeds and on primary leaves at 7, 9, 11, and 14 days. The physiological stage of the plant at each sampling time is illustrated on the right of the graphs. Population sizes of indigenous bacterial flora (IBF) were quantified at each sampling date. Means and SEM were calculated for data from four independent experiments with five individuals per sampling date and per treatment. Mean population sizes followed by different letters are significantly (P < 0.05) different on the basis of a Mann-Whitney U test.
The ability of both compatible (X. citri pv. phaseoli var. fuscans) and noncompatible (X. campestris pv. campestris and E. coli) strains to multiply on bean seedlings implies that seedling colonization may not require bacterial pathogenicity determinants. Thus, we tested the impact of mutations in the T3SS on seedling colonization by X. citri pv. phaseoli var. fuscans. The behaviors of a mutant in a structural gene of the T3SS (secretion mutant strain 4834HRCR) and a mutant in a master regulator gene of the T3SS (regulatory mutant strain 4834HRPG) of X. citri pv. phaseoli var. fuscans were compared to that of the wild-type strain. Until 14 days after germination, population sizes of both mutants were not significantly different (P > 0.05) from those of wild-type strain CFBP4834-R on bean seedlings (Fig. 1) and were similar to those of the total indigenous bacterial flora, indicating that each bacterial population reached the carrying capacity of the seedling and that this carrying capacity is similar whatever the bacterium (Fig. 1).
Adhesion capacities on bean seeds and polypropylene.
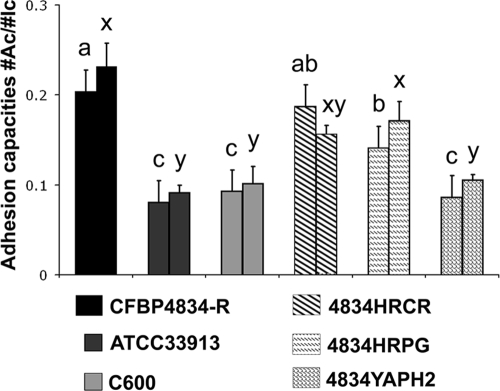
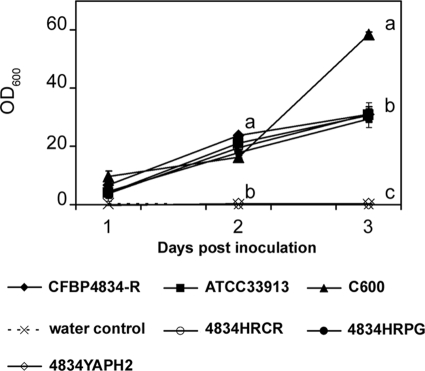
Despite the same initial inoculum concentrations (1 × 106 CFU ml−1), E. coli C600 population densities quantified in seeds 2 h after vacuum inoculation were about 0.02-fold of those of xanthomonads, suggesting an E. coli adaptation defect in this environment. This adaptation defect could involve either an impairment in the capacities of adhesion to bean seeds or the death of a part of the population. Adhesion capacities tested in seed columns were not significantly different (P > 0.05) among a set of strains composed of E. coli C600, X. campestris pv. campestris ATCC 33913, and 4834YAPH2, an adhesin mutant of X. citri pv. phaseoli var. fuscans in the yapH2 gene (Fig. 2). However, their adhesion capacities were significantly lower (P < 0.05) than that of X. citri pv. phaseoli var. fuscans CFBP4834-R. Mutants of X. citri pv. phaseoli var. fuscans in T3SS genes (4834HRCR and 4834HRPG) were slightly impaired in their adhesion capacities on seeds compared to the wild-type strain (Fig. 2). Similar results were obtained in two independent experiments with three samples per treatment and experiment (Fig. 2). To confirm that the alteration in X. campestris pv. campestris ATCC 33913 and E. coli C600 adhesion capacities on bean seeds was not linked to a general lack of adhesion capacities of these strains, we compared the adhesion capacities of the set of strains on a polypropylene surface with a crystal violet incorporation assay in a time course experiment (Fig. 3). E. coli strain C600 adhered significantly (P > 0.05) more on this material than all other tested strains: X. citri pv. phaseoli var. fuscans CFBP4834-R and its hrpG, hrcR, and yapH2 mutants (4834HRPG, 4834HRCR, and 4834YAPH2, respectively) and X. campestris pv. campestris ATCC 33913 (Fig. 3). This test confirmed that no strain was deficient in its adhesion capacities and that X. citri pv. phaseoli var. fuscans and X. campestris pv. campestris had similar (although weaker than E. coli) properties. Thus, both E. coli C600 and X. campestris pv. campestris ATCC 33913 shared an adhesion defect on bean seeds. It did not affect X. campestris pv. campestris ATCC 33913 settlement on bean seeds, as X. campestris pv. campestris ATCC 33913 population sizes were similar to X. citri pv. phaseoli var. fuscans CFBP4834-R population sizes 2 h after inoculation (Fig. 1).
FIG. 2.
Adhesion capacities of bacterial strains on bean seeds. X. citri pv. phaseoli var. fuscans strains CFBP4834-R, 4834HRCR, 4834HRPG, and 4834YAPH2; X. campestris pv. campestris ATCC 33913; and E. coli C600 suspensions at 1 × 105 CFU ml−1 were incubated during 2 h with bean seeds. Bars represent the ratios of numbers of attached cells on seeds (#Ac) versus inoculated cells (#Ic). Means and SEM were calculated for three samples per treatment and per experiment. For each treatment double bars stand for data from two independent experiments. Within an experiment, mean population sizes followed by different letters are significantly different (P < 0.05) on the basis of a Mann-Whitney U test.
FIG. 3.
Kinetics of the adhesion capacities of bacterial strains on a polypropylene surface. X. citri pv. phaseoli var. fuscans strains CFBP4834, 4834HRCR, 4834HRPG, and 4834YAPH2; X. campestris pv. campestris ATCC 33913; and E. coli C600 were cultured during 3 days at 28°C under static conditions in polypropylene microtiter plates from an inoculum at 5 × 105 CFU ml−1. Crystal violet-stained surface-attached cells were quantified by solubilizing the dye absorbed by adherent cells, after the removal of suspensions, in ethanol and determining the optical density at 600 nm. Means and SEM were calculated for data from two independent experiments, each containing three replicates per treatment and per sampling date. For a given sampling date different letters refer to significantly (P < 0.05) different values based on a Mann-Whitney U test.
Plant defense responses.
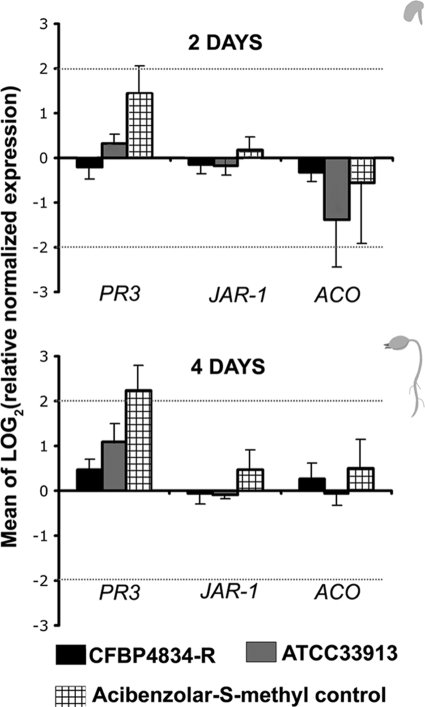
The similar multiplication of bacteria on seedlings, whatever the type of interaction, and the presence of a functional T3SS or not suggested an absence of plant defense reactions. To test this hypothesis, we assayed the three main pathways of plant defense signaling responses in seedlings during the first steps of germination using an RT-qPCR approach. Seeds were inoculated with X. citri pv. phaseoli var. fuscans CFBP4834-R and X. campestris pv. campestris ATCC 33913. We controlled that plant defenses were inducible using acibenzolar-S-methyl (Bion/WG 50, 50% acibenzolar-S-methyl in water dispersible granules; Syngenta), which is known to be a plant defense activator (28). Relative quantifications of salicylic acid, jasmonate, and ethylene pathway inductions were tested with the PR-3, JAR-1, and ACO genes, respectively, by using water as a calibrator and the EF1-α and UBI genes as internal controls. Seed infiltration leading to seedling colonization was used to mimic the natural behavior of bacteria originating from contaminated seeds, while seedling or leaf infiltration was used to force the bacteria into the intercellular spaces of the parenchyma. Two days after seed infiltration, almost a 3-time induction [log2(3) = 1.58] of the PR-3 gene was monitored in seedlings for acibenzolar-S-methyl (positive control), while no induction could be recorded following seed inoculation with the incompatible bacterium X. campestris pv. campestris ATCC 33913 (Fig. 4). However, PR-3 gene expression was induced in the 2 days following the infiltration of 2-day-old seedlings with the same strain and with acibenzolar-S-methyl (Fig. 5a). Furthermore, a 32-time increase [log2(32) = 5] and an 8-time increase [log2(8) = 3] of PR-3 expression were observed 2 days after leaf infiltration with X. campestris pv. campestris ATCC 33913 and with the positive control, respectively, while an 8-time decrease [log2(1/8) = −3] in the expression of this gene was observed for leaves infiltrated with X. citri pv. phaseoli var. fuscans CFBP4834-R (Fig. 5b). This modification of PR-3 gene expression did not last, as it was no longer observed 4 days after leaf infiltration (data not shown). Conversely, the PR-3 gene expression level increased slightly in seedlings 4 days after seed infiltration with X. campestris pv. campestris ATCC 33913 (Fig. 4). These results showed that plant defenses (salicylic acid pathway) were highly induced in leaves following the infiltration of the incompatible bacterium X. campestris pv. campestris while poorly induced during the colonization of bean seedlings after seed inoculation. The expression levels of JAR-1 and ACO varied only slightly and mainly in response to acibenzolar-S-methyl. These results confirmed that these pathways were not involved in the response of bean to bacterial colonization. For this reason, proteins involved in the salicylic acid pathway were assayed to confirm RT-qPCR results by a biochemical approach.
FIG. 4.
Relative normalized expressions of the PR-3, JAR-1, and ACO genes in seedlings following seed inoculation. Seeds were inoculated with 1 × 106 CFU ml−1 of X. citri pv. phaseoli var. fuscans strain CFBP4834-R, X. campestris pv. campestris strain ATCC 33913, acibenzolar-S-methyl (Bion/WG 50; 400 mg liter−1) as a positive control, and water as negative control (calibrator). Dotted bars represent a 4-time [log2(4) = 2] induction or repression relative to the negative control. The mean of water treatment values was used for calibration at each sampling date, and the geometric mean of values obtained for internal controls (UBI and EF1-α) was used to normalize each sample. The physiological stage of plants at the sampling time is illustrated on the right of the graphs. Means and SEM were calculated for data from three independent experiments, each containing three independent samples of plant material per treatment and two replicates per sample.
FIG. 5.
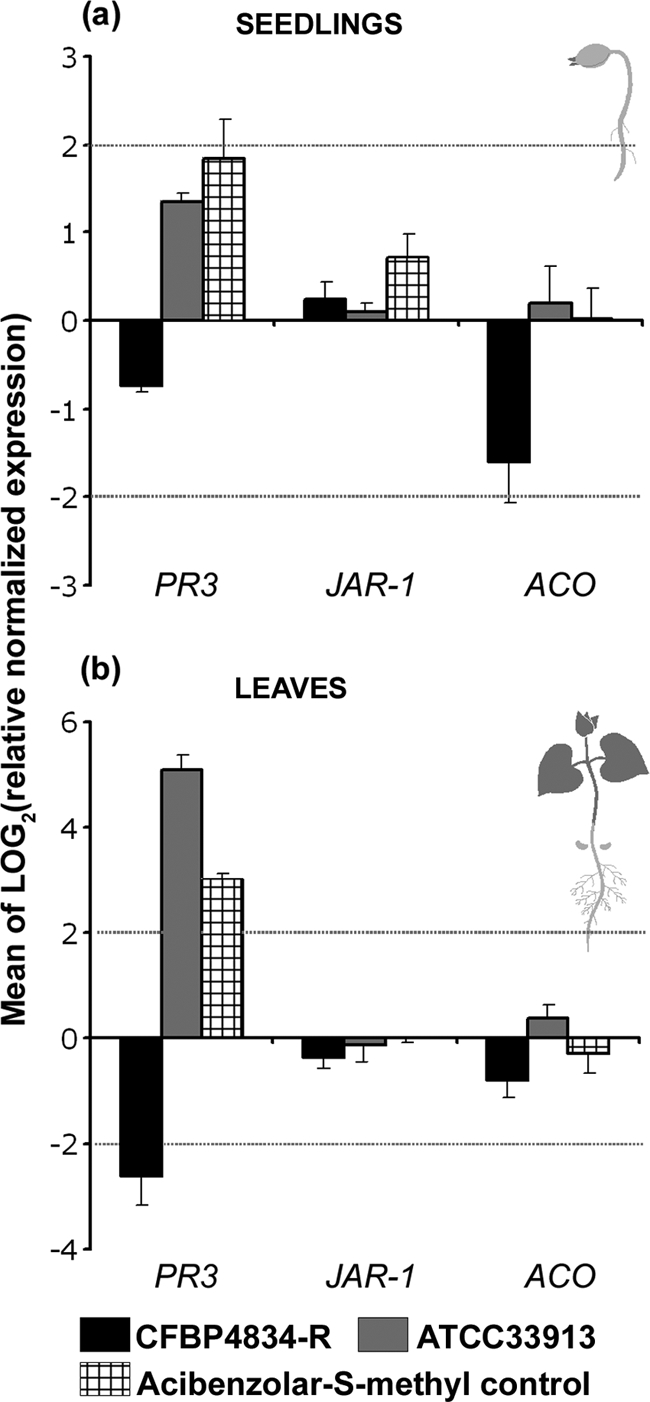
Relative expressions of the PR-3, JAR-1, and ACO genes following infiltration of 2-day-old seedlings (a) and leaves (b). Plants were inoculated with 1 × 106 CFU ml−1 of X. citri pv. phaseoli var. fuscans strain CFBP4834-R, X. campestris pv. campestris strain ATCC 33913, acibenzolar-S-methyl methyl (Bion/WG 50; 400 mg liter−1) as a positive control, and water as a negative control (calibrator). Dotted bars represent a 4-time [log2(4) = 2] induction or repression relative to the negative control. The mean of water treatment values was used for calibration at each sampling date, and the geometric mean of values obtained for internal controls (UBI and EF1-α) was used to normalize each sample. The physiological stage of plants at the sampling time is illustrated at the right of the graphs. Means and SEM were calculated for three independent samples of plant material per treatment and two replicates per sample.
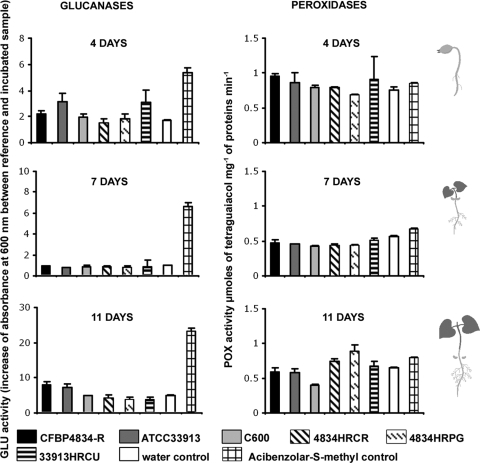
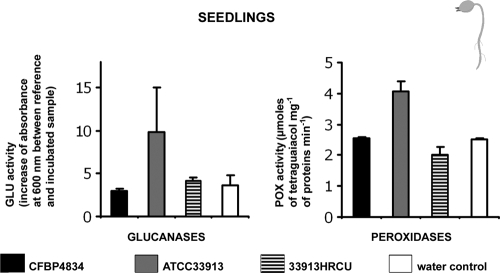
To confirm the low level of defense response induction in the early steps of plant development following bacterial colonization originating from seeds, we measured the enzymatic activities of two other pathogenesis-related (PR) proteins, peroxidase (POX) (EC 1.11.1.7) and β-1,3-glucanase (GLU) (EC 3.2.1.6), in seedlings following seed inoculation versus seedling infiltration. Seeds were inoculated with X. citri pv. phaseoli var. fuscans CFBP4834-R, X. campestris pv. campestris ATCC 33913, and E. coli C600 at 1 × 106 CFU ml−1. Mutants (4834HRCR, 4834HRPG, and 33913HRCU) of X. citri pv. phaseoli var. fuscans CFBP4834-R and X. campestris pv. campestris ATCC 33913 in the T3SS regulatory (hrpG) gene and structural (hrcR and hrcU) genes were tested, as was the positive control acibenzolar-S-methyl. No POX activities were induced in any seedlings treated with the different strains until 11 days after germination. X. campestris pv. campestris ATCC 33913, 33913HRCU, 4834HRCR, and 4834HRPG did not induce more of a defense response in seedlings than X. citri pv. phaseoli var. fuscans CFBP4834-R. GLU activity increased progressively in the positive control (Fig. 6). Similar results were obtained for two independent experiments and were also obtained for a higher-inoculum dose (1 × 107 CFU ml−1) (data not shown). However, vacuum infiltration of 2-day-old seedlings with X. campestris pv. campestris ATCC 33913 induced a 2-fold increase of POX and GLU activities. The levels of these POX and GLU activities were higher than those in the negative control and higher than those in seedlings infiltrated with X. citri pv. phaseoli var. fuscans CFBP4834-R and mutant strain 33913HRCU, indicating that defense responses were inducible in seedlings upon the recognition of an incompatible bacterium (Fig. 7). Similarly, an increase in levels of POX and GLU activities was obtained for primary leaves of 2-week-old plants following vacuum infiltration with X. campestris pv. campestris ATCC 33913 compared to the controls (data not shown), confirming the defense response induction in this bean cultivar faced with X. campestris pv. campestris ATCC 33913. These results showed that plant defenses were inducible in seedlings following the infiltration of the incompatible bacterium X. campestris pv. campestris but did not naturally occur during the colonization of bean seedlings when the inoculum originated from the seed independent of its level.
FIG. 6.
β-1,3-Glucanase (GLU) and peroxidase (POX) activities in protein extracts from bean seedlings after seed infiltration (1 × 106 CFU ml−1) with X. citri pv. phaseoli var. fuscans strains CFBP4834-R, 4834HRCR, and 4834HRPG; X. campestris pv. campestris strains ATCC 33913 and 33913HRCU; E. coli C600; acibenzolar-S-methyl (Bion/WG 50; 400 mg liter−1); and distilled water (control). The physiological stage of plants at the sampling time is illustrated at the right of the graphs. Means and SEM were calculated for two independent duplicated samples. Each sample was made of the bulk of three to eight seedlings (aerial part without cotyledons) or the bulk of primary leaf pieces from three plants.
FIG. 7.
β-1,3-Glucanase (GLU) and peroxidase (POX) activities in protein extracts from infiltrated bean seedlings. Enzymatic activities were assayed 2 days after the infiltration of 2-day-old bean seedlings with 1 × 106 CFU ml−1 of X. citri pv. phaseoli var. fuscans strain CFBP4834-R, X. campestris pv. campestris strains ATCC 33913 and 33913HRCU, and water (negative control). Means and SEM were calculated for two independent duplicated samples. Each sample was made of the bulk of three seedlings (aerial part without cotyledons).
DISCUSSION
Associations developing on germinating seeds mark the first point of contact between plants, pathogens, and microorganisms, with beneficial or harmful results for plant health, growth, and development. In an attempt to evaluate the potential efficiency of control strategies against seed-borne pathogenic bacteria, we designed experiments aimed at quantifying the temporal dynamics of bacterial population sizes and associated defense responses in plant. We demonstrated that bacterial multiplication occurred in seedlings with similar dynamics of population sizes whatever the type of interactions that bacteria develop with the plant. No significant defense responses were induced in the plant for the incompatible interaction until bacteria were introduced within plant tissues. These results indicate that bacterial colonization of seedlings relies mostly on surface colonization and not on parasitic relationships. The transmission of incompatible bacteria to seedlings from contaminated seeds suggests a probable role of seeds of nonhost plant as an inoculum source for seed-borne pathogenic bacteria. Taken together, these results suggest that plant defense responses are not naturally induced by seed-associated bacteria in early stages of plant development.
Interactions occurring in seeds and seedlings between bacterial and plant partners allowing bacteria to multiply and having no negative impact on plants defined a commensal interaction. For compatible situations, it has always been argued that seed-borne bacteria and especially xanthomonads colonize the surface of seedlings and have no early endophytic development (19). With nutrients being available in sufficient amounts on germinating seeds and seedlings (38), bacteria do not need to set up a molecular dialogue with the plant to multiply efficiently in this environment. Here, we show that bacteria colonize germinating seeds and seedlings with similar population sizes whatever the type of interaction that they develop with the plant. Indeed, similar trends for X. citri pv. phaseoli var. fuscans, X. campestris pv. campestris, and E. coli, representing compatible, incompatible, and null interactions, respectively, were monitored on beans from seed imbibition until they were 14-day-old seedlings. In addition, bacterial multiplication did not require a functional T3SS, suggesting that nutrients are not a limiting factor in the spermosphere. Indeed, T3SS genes are not induced in nutrient-rich medium (58). This contrasts with the phyllosphere, where nutrients are in limiting quantities (33). To multiply efficiently in this environment, bacteria have to rely on the expression of T3SS (12). Taken together, these results indicate that seedling colonization requires different bacterial determinants than phyllosphere colonization and that environmental parameters to which bacteria have to adapt are different in these two environments. Specific interactions between X. citri pv. phaseoli var. fuscans and bean take place later on during the colonization of the phyllosphere (11, 12), when nutrient availability is limiting for bacterial multiplication. In addition, our present work indicates that in leaves, X. citri pv. phaseoli var. fuscans negatively regulated the PR-3 gene, suggesting a suppression of host defenses. This is coherent with the ability of T3SS effectors to suppress defense responses induced by PAMPs in compatible interactions (34, 40).
Regarding the incompatible situation studied in the present work, the absence of elevated defense reaction induction by X. campestris pv. campestris in bean seedlings after seed inoculation could result from a similar commensal interaction. Indeed, we verified that defense responses are inducible in juvenile plantlets following the infiltration of seedlings and leaves with X. campestris pv. campestris and acibenzolar-S-methyl. The induction of defense responses was previously observed for other plant seedlings such as cowpea and melon (6, 28). These plant defense responses are specifically induced by bacterial T3SS effectors upon recognition by the corresponding resistance genes (26). Indeed, while the X. campestris pv. campestris wild-type strain induces a defense reaction following the infiltration of seedlings, an X. campestris pv. campestris mutant in the T3SS does not. Thus, the lack of defense response induction in naturally colonized seedlings by incompatible bacteria is consecutive to a nondetection of the bacteria by the plant cells.
Attachment to seeds represents a key step for the introduction of bacteria into the rhizosphere (10) and would represent an advantage for seed-borne pathogens. We report contrasting adhesion capacities for X. citri pv. phaseoli var. fuscans, X. campestris pv. campestris, and E. coli. X. citri pv. phaseoli var. fuscans has strong capacities of attachment to bean seeds compared to those of X. campestris pv. campestris and E. coli. We previously showed that X. citri pv. phaseoli var. fuscans CFBP4834-R produces at least five adhesins, some of which are involved in attachment to seeds (11). E. coli adhesion to bean seeds strikingly contrasts with its high capacities for adhesion to polypropylene. Polypropylene is an apolar surface shown to be particularly well suited to show slight differences in adhesion capacities among Pseudomonas fluorescens mutants (43). However, this material does not adequately reflect the properties of adherence to root surfaces. This is not entirely surprising, since it was previously shown for several bacteria, such as E. coli O157:H7 and Vibrio cholerae, that bacteria rely on disparate sets of genes to adhere onto both biotic and abiotic surface (36, 56). The contrasting adhesion behaviors of E. coli on biotic and abiotic surfaces indicate that in vitro adhesion assays do not simulate an in planta bioassay. On biotic surfaces, adhesion may depend on bacterial species, strains, and seed coat characteristics. For example, it was shown previously that E. coli O157:H7 and Salmonella enterica firmly adhere to alfalfa sprouts and seed coats (10, 18, 50, 56). Altogether, this is useful information in the context of the emergence of E. coli strains spreading in agricultural environments.
Could nonhost seeds serve as an inoculum source for seed-borne pathogenic bacteria? The transmission of a plant-pathogenic bacterium to seeds of a nonhost plant was demonstrated for X. campestris pv. campestris on bean (12). It was suggested previously that this transmission results from the lack of inducible defense responses in flowers (39). Here, we show that the subsequent epidemiological step is also feasible. A plant-pathogenic bacterium can efficiently transmit from seeds to nonhost seedlings as a consequence of saprophytic multiplication, resulting in the establishment of a primary inoculum focus. It was previously demonstrated that plant-pathogenic bacteria can survive in the phyllosphere of nonhost plants (7, 12, 49). The next epidemiological step is the dispersal of these pathogenic bacteria from this inoculum focus situated in a nonhost crop to a neighboring susceptible host crop. This remains to be demonstrated in order to evidence that nonhost carriage could serve to establish reservoirs of bacterial pathogens in the field.
Acknowledgments
This work was supported by a grant from the Conseil Régional des Pays de la Loire.
We acknowledge E. Lauber for critically reviewing the manuscript and M. Arlat for the gift of strains. We thank J. Benard and S. Hanteville for plant production.
Footnotes
Published ahead of print on 20 August 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Ah-You, N., L. Gagnevin, P. A. D. Grimont, S. Brisse, X. Nesme, F. Chiroleu, L. B. T. Ngoc, E. Jouen, P. Lefeuvre, C. Vernière, and O. Pruvost. 2009. Polyphasic characterization of xanthomonads pathogenic to members of the Anacardiacae and their relatedness to the species of Xanthomonas. Int. J. Syst. Evol. Microbiol. 59:306-318. [DOI] [PubMed] [Google Scholar]
- 2.Arlat, M., C. L. Gough, C. E. Barber, C. Boucher, and M. J. Daniels. 1991. Xanthomonas campestris contains a cluster of hrp genes related to the larger hrp cluster of Pseudomonas solanacearum. Mol. Plant Microbe Interact. 4:593-601. [DOI] [PubMed] [Google Scholar]
- 3.Boureau, T., H. ElMaarouf-Bouteau, A. Garnier, M.-N. Brisset, C. Perino, I. Pucheu, and M.-A. Barny. 2006. DspA/E, a type III effector essential for Erwinia amylovora pathogenicity and growth in planta, induces cell death in host apple and non-host tobacco plants. Mol. Plant Microbe Interact. 19:16-24. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Brunings, A. M., and D. W. Gabriel. 2003. Xanthomonas citri: breaking the surface. Mol. Plant Pathol. 4:141-157. [DOI] [PubMed] [Google Scholar]
- 6.Buzi, A., G. Chilosi, D. de Sillo, and P. Magro. 2004. Induction of resistance in melon to Didymella bryoniae and Sclerotinia sclerotiorum by seed treatments with acibenzolar-S-methyl and methyl jasmonate but not with salicylic acid. J. Phytopathol. 152:34-42. [Google Scholar]
- 7.Cafati, C. R., and A. W. Saettler. 1980. Role of nonhost species as alternate inoculum sources of Xanthomonas phaseoli. Plant Dis. 64:194-196. [Google Scholar]
- 8.Chae, H. S., and J. J. Kieber. 2005. Eto Brute? Role of ACS turnover in regulating ethylene biosynthesis. Trends Plant Sci. 10:291-296. [DOI] [PubMed] [Google Scholar]
- 9.Chance, B., and A. C. Maehly. 1955. Assay of catalases and peroxidases. Methods Enzymol. 2:764-775. [DOI] [PubMed] [Google Scholar]
- 10.Danhorn, T., and C. Fuqua. 2007. Biofilm formation by plant-associated bacteria. Annu. Rev. Microbiol. 61:401-422. [DOI] [PubMed] [Google Scholar]
- 11.Darsonval, A., A. Darrasse, K. Durand, C. Bureau, S. Cesbron, and M.-A. Jacques. 2009. Adhesion and fitness in the bean phyllosphere and transmission to seed of Xanthomonas fuscans subsp. fuscans. Mol. Plant Microbe Interact. 22:747-757. [DOI] [PubMed] [Google Scholar]
- 12.Darsonval, A., A. Darrasse, D. Meyer, M. Demarty, K. Durand, C. Bureau, C. Manceau, and M.-A. Jacques. 2008. Type III secretion system of Xanthomonas fuscans subsp. fuscans is involved in the phyllosphere colonization process and in transmission to seeds of susceptible beans. Appl. Environ. Microbiol. 74:2669-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.da Silva, A. C. R., J. A. Ferro, C. S. Farah, L. R. Furlan, R. B. Quaggio, C. B. Monteiro-Vitorello, M. A. van Sluis, N. F. Almeida, L. M. C. Alves, A. M. do Amaral, M. C. Bertolini, L. E. A. Camargo, G. Camarotte, F. Cannavan, J. Cardozo, F. Chambergo, L. P. Ciapina, R. M. B. Cicarelli, L. L. Coutinho, J. R. Cursino-Santos, H. El-Dorry, J. B. Faria, R. C. C. Ferreira, M. I. T. Ferro, E. F. Formighieri, M. C. Franco, C. C. Greggio, A. Gruber, A. M. Katsuyama, L. T. Kishi, R. P. Leite, E. G. M. Lemos, M. V. F. Lemos, E. C. Locali, M. A. Machado, A. M. B. N. Madeira, N. M. Martinez-Rossi, E. C. Martins, J. Meidanis, C. F. M. Menck, C. Y. Miyaki, D. H. Moon, L. M. Moreira, M. T. M. Novo, V. K. Okura, M. C. Oliveira, V. R. Oliveira, H. A. Pereira, A. Rossi, J. A. D. Sena, C. Silva, R. F. de Souza, L. A. F. Spinola, M. A. Takita, R. E. Tamura, E. C. Teixeira, R. I. D. Tezza, M. Trinlade dos Santos, D. Truffi, S. M. Tsai, F. F. White, J. C. Setubal, and J. P. Kitajima. 2002. Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417:459-463. [DOI] [PubMed] [Google Scholar]
- 14.DebRoy, S., R. Thilmony, Y. B. Kwack, K. Nomura, and S. Y. He. 2004. A family of conserved bacterial effectors inhibits salicylic acid-mediated basal immunity and promotes disease necrosis in plants. Proc. Natl. Acad. Sci. U. S. A. 101:9927-9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFlaun, M. F., B. M. Marshall, E. P. Kulle, and S. B. Levy. 1994. Tn5 insertion mutants of Pseudomonas fluorescens defective in adhesion to soil and seeds. Appl. Environ. Microbiol. 60:2637-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenton, A., J. P. Fairbairn, R. Norman, and P. J. Hudson. 2002. Parasite transmission: reconciling theory and reality. J. Anim. Ecol. 71:893-905. [Google Scholar]
- 17.Fessehaie, A., and R. R. Walcott. 2005. Biological control to protect watermelon blossoms and seed from infection by Acidovorax avenae subsp. citrulli. Phytopathology 95:413-419. [DOI] [PubMed] [Google Scholar]
- 18.Fett, W. F., and P. H. Cooke. 2005. A survey of native microbial aggregates on alfalfa, clover and mung bean sprout cotyledons for thickness as determined by confocal scanning laser microscopy. Food Microbiol. 22:253-259. [Google Scholar]
- 19.Gilbertson, R. L., and D. P. Maxwell. 1992. Common bacterial blight of bean, p. 18-39. In H. S. Chaube, U. S. Singh, and A. N. Mukhopadhay (ed.), Diseases of international importance, vol. 2. Prentice Hall, Englewood Cliffs, NJ. [Google Scholar]
- 20.Gitaitis, R., and R. Walcott. 2007. The epidemiology and management of seedborne bacterial diseases. Annu. Rev. Phytopathol. 45:371-397. [DOI] [PubMed] [Google Scholar]
- 21.Gürlebeck, D., F. Thieme, and U. Bonas. 2006. Type III effector proteins from the plant pathogen Xanthomonas and their role in the interaction with the host plant. J. Plant Physiol. 163:233-255. [DOI] [PubMed] [Google Scholar]
- 22.Hernández, G., M. Ramírez, O. Valdés-López, M. Tesfaye, M. A. Graham, T. Czechowski, A. Schlereth, M. Wandrey, A. Erban, F. Cheung, H. C. Wu, M. Lara, C. D. Town, J. Kopka, M. K. Udvardi, and C. P. Vance. 2007. Phosphorus stress in common bean: root transcript and metabolic responses. Plant Physiol. 144:752-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirano, S. S., A. O. Charkowski, A. Collmer, D. K. Willis, and C. D. Upper. 1999. Role of the Hrp type III protein secretion system in growth of Pseudomonas syringae pv. syringae B728a on host plants in the field. Proc. Natl. Acad. Sci. U. S. A. 96:9851-9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu, H., J. J. Churey, and R. W. Worobo. 2004. Heat treatments to enhance the safety of mung bean seeds. J. Food Prot. 67:1257-1260. [DOI] [PubMed] [Google Scholar]
- 25.Jacques, M.-A., K. Josi, R. Samson, and A. Darrasse. 2005. Xanthomonas axonopodis pv. phaseoli var. fuscans is aggregated in stable biofilm population sizes in the phyllosphere of field-grown beans. Appl. Environ. Microbiol. 71:2008-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones, J. D. G., and J. L. Dangl. 2006. The plant immune system. Proc. Natl. Acad. Sci. U. S. A. 444:323-329. [DOI] [PubMed] [Google Scholar]
- 27.Koornneef, A., and C. M. Pieterse. 2008. Cross talk in defense signaling. Plant Physiol. 146:839-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latunde-Dada, A. O., and J. A. Lucas. 2001. The plant defence activator acibenzolar-S methyl primes cowpea [Vigna unguiculata (L.) Walp.] seedlings for rapid induction of resistance. Physiol. Mol. Plant Pathol. 58:199-208. [Google Scholar]
- 29.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 30.Maude, R. B. 1996. Seedborne diseases and their control: principles and practice. CAB International, Oxon, United Kingdom.
- 31.Maury, Y., C. Duby, J.-M. Bossenec, and G. Boudazin. 1985. Group analysis using ELISA: determination of the level of transmission of soybean mosaic virus in soybean seed. Agronomie 5:405-415. [Google Scholar]
- 32.McCallum, H., N. Barlow, and J. Hone. 2001. How should pathogen transmission be modelled? Trends Ecol. Evol. 16:295-300. [DOI] [PubMed] [Google Scholar]
- 33.Mercier, J., and S. E. Lindow. 2000. Role of leaf surface sugars in colonization of plants by bacterial epiphytes. Appl. Environ. Microbiol. 66:369-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mishina, T. E., and J. Zeier. 2007. Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. Plant J. 50:500-513. [DOI] [PubMed] [Google Scholar]
- 35.Mudgett, M. B. 2005. New insights to function of phytopathogenic bacterial type III effectors in plants. Annu. Rev. Plant Biol. 56:509-531. [DOI] [PubMed] [Google Scholar]
- 36.Mueller, R. S., D. McDougald, D. Cusumano, N. Sodhi, S. Kjelleberg, F. Azam, and D. H. Bartlett. 2007. Vibrio cholerae strains possess multiple strategies for abiotic and biotic surface colonization. J. Bacteriol. 189:5348-5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Advisory Committee on Microbiological Criteria for Foods. 1999. Microbiological safety evaluations and recommendations on sprouted seeds. Int. J. Food Microbiol. 52:123-153. [DOI] [PubMed] [Google Scholar]
- 38.Nelson, E. B. 2004. Microbial dynamics and interactions in the spermosphere. Annu. Rev. Phytopathol. 42:271-309. [DOI] [PubMed] [Google Scholar]
- 39.Ngugi, H. K., and H. Scherm. 2006. Biology of flower-infecting fungi. Annu. Rev. Phytopathol. 44:261-282. [DOI] [PubMed] [Google Scholar]
- 40.Nomura, K., M. Melotto, and S.-Y. He. 2005. Suppression of host defense in compatible plant-Pseudomonas syringae interactions. Curr. Opin. Plant Biol. 8:361-368. [DOI] [PubMed] [Google Scholar]
- 41.Nürnberger, T., and V. Lipka. 2005. Non-host resistance in plants: new insights into an old phenomenon. Mol. Plant Pathol. 6:335-345. [DOI] [PubMed] [Google Scholar]
- 42.O'Brien, A. D., J. W. Newland, S. F. Miller, R. K. Holmes, H. W. Smith, and S. B. Formal. 1984. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 226:694-696. [DOI] [PubMed] [Google Scholar]
- 43.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 44.Remeeus, P. M., and J. W. Sheppard. 2006. Proposal for a new method for detecting Xanthomonas axonopodis pv. phaseoli on bean seeds. ISTA Method Validation Rep. 3:1-11. [Google Scholar]
- 45.Rijslaarsdam, A., B. Woudt, G. Simons, H. Koenraadt, J. Oosterhof, M. Asma, P. Buddiger, P. Roorda, V. Grimault, and J. de Koning. 2004. Development of specific primers for the molecular detection of Xanthomonas campestris pv. campestris. http://archives.eppo.org/MEETINGS/2004_meetings/diag_posters/poster_deKoning2.pdf.
- 46.Schaad, N. W., and A. Alvarez. 1993. Xanthomonas campestris pv. campestris: cause of the black rot of crucifers, p. 51-55. In J. G. Swings and E. L. Civerolo (ed.), Xanthomonas. Chapman & Hall, London, United Kingdom.
- 47.Schaad, N. W., E. Postnikova, G. Lacy, A. Sechler, I. Agarkova, P. E. Stromberg, V. K. Stromberg, and A. K. Vidaver. 2006. Emended classification of xanthomonad pathogens on citrus. Syst. Appl. Microbiol. 28:494-518. [DOI] [PubMed] [Google Scholar]
- 48.Sels, J., J. Mathys, B. M. A. De Coninck, B. P. A. Cammue, and M. F. C. De Bolle. 2008. Plant pathogenesis-related (PR) proteins: a focus on PR peptides. Plant Physiol. Biochem. 46:941-950. [DOI] [PubMed] [Google Scholar]
- 49.Sikirou, R., and K. Wydra. 2004. Persistence of Xanthomonas axonopodis pv. vignicola in weeds and crop debris and identification of Sphenostylis stenocarpa as a potential new host. Eur. J. Plant Pathol. 110:939-947. [Google Scholar]
- 50.Singh, B. R., M. Chandra, R. Agarwal, and N. Babu. 2005. Curing of Salmonella enterica, serovar typhimurium-contaminated cowpea seeds and sprouts with vinegar and chlorination. J. Food Process. Preserv. 29:268-277. [Google Scholar]
- 51.Sokal, R. R., and F. J. Rohlf. 1969. Biometry. The principles and practice of statistics in biological research. W. H. Freeman & Co., San Francisco, CA.
- 52.Spinelli, F., F. Ciampolini, M. Cresti, K. Geider, and G. Costa. 2005. Influence of stigmatic morphology on flower colonization by Erwinia amylovora and Pantoea agglomerans. Eur. J. Plant Pathol. 113:395-405. [Google Scholar]
- 53.Suza, W. P., and P. E. Staswick. 2008. The role of JAR1 in jasmonoyl-L-isoleucine production during Arabidopsis wound response. Planta 227:1221-1232. [DOI] [PubMed] [Google Scholar]
- 54.Swaroop, S. 1951. The range of variation of the most probable number of organisms estimated by the dilution method. Ind. J. Med. Res. 39:107-134. [PubMed] [Google Scholar]
- 55.Taylor, J. D., C. L. Dudley, and L. Presly. 1979. Study of halo-blight seed infection and disease transmission in dwarf beans. Ann. Appl. Biol. 93:267-277. [Google Scholar]
- 56.Torres, A. G., C. Jeter, W. Langley, and A. G. Matthysse. 2005. Differential binding of Escherichia coli O157:H7 to alfalfa, human epithelial cells, and plastic is mediated by a variety of surface structures. Appl. Environ. Microbiol. 71:8008-8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tyler, H. L., and E. W. Triplett. 2008. Plants as a habitat for beneficial and/or human pathogenic bacteria. Annu. Rev. Phytopathol. 46:53-73. [DOI] [PubMed] [Google Scholar]
- 58.Valls, M., S. Genin, and C. Boucher. 2006. Integrated regulation of the type III secretion system and other virulence determinants in Ralstonia solanacearum. PLoS Pathog. 2:e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vandesompele, J., K. De Preter, F. Pattyn, B. Poppe, N. Van Roy, A. De Paepe, and F. Speleman. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:research0034.1-research0034.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vauterin, L., B. Hoste, K. Kersters, and J. Swings. 1995. Reclassification of Xanthomonas. Int. J. Syst. Bacteriol. 45:472-489. [Google Scholar]
- 61.Venisse, J.-S., M. Malnoy, M. Faize, J.-P. Paulin, and M.-N. Brisset. 2002. Modulation of defense responses of Malus spp. during compatible and incompatible interactions with Erwinia amylovora. Mol. Plant Microbe Interact. 15:1204-1212. [DOI] [PubMed] [Google Scholar]
- 62.Vidaver, A. K. 1993. Xanthomonas campestris pv. phaseoli: cause of common bacterial blight of bean, p. 40-44. In J. G. Swings and E. L. Civerolo (ed.), Xanthomonas. Chapman & Hall, London, United Kingdom.
- 63.Wichmann, G., and J. Bergelson. 2004. Effector genes of Xanthomonas axonopodis pv. vesicatoria promote transmission and enhance other fitness traits in the field. Genetics 166:693-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wirth, S. J., and G. A. Wolf. 1992. Microplate colorimetric assay for endoacting cellulase, xylanase chitinase, 1,3-β-glucanase and amylase extracted from forest horizons. Soil Biol. Biochem. 24:511-519. [Google Scholar]