Abstract
Induction of potent T-cell memory is the goal of vaccinations, but the molecular mechanisms that regulate the formation of memory CD8 T cells are not well understood. Despite the recognition that controls of cellular proliferation and apoptosis govern the number of memory T cells, the cell cycle regulatory mechanisms that control these key cellular processes in CD8 T cells during an immune response are poorly defined. Here, we have identified the cyclin-dependent kinase inhibitor p27Kip1 as a critical regulator of the CD8 T-cell homeostasis at all phases of the T-cell response to an acute viral infection in mice. By acting as a timer for cell cycle exit, p27Kip1 curtailed the programmed expansion of interleukin-2-producing memory precursors and markedly limited the magnitude and quality of CD8 T-cell memory. In the absence of p27Kip1, CD8 T cells showed superior recall responses shortly after vaccination with recombinant Listeria monocytogenes. Additionally, we show that p27Kip1 constrains proliferative renewal of memory CD8 T cells, especially of the effector memory subset. These findings provide critical insights into the cell cycle regulation of CD8 T-cell homeostasis and suggest that modulation of p27Kip1 could bolster vaccine-induced T-cell memory and protective immunity.
CD8 T cells play an essential role in defense against viral, intracellular bacterial, and protozoan infections. Antigen recognition, in conjunction with appropriate costimulatory and proinflammatory signals, triggers CD8 T cells to clonally expand and differentiate into effector cells (44). Acute viral infections, such as lymphocytic choriomeningitis virus (LCMV), stimulate dramatic expansion of virus-specific CD8 T cells, which account for >70% of activated CD8 T cells in the spleen at the peak of the primary immune response (17, 19). Following viral clearance, the majority of effector CD8 T cells undergo apoptosis at the end of an immune response, but a subset of effector cells, termed memory precursor effector cells (MPECs), exit the cell cycle and differentiate into long-lived memory CD8 T cells (12, 14, 18, 22, 28, 34). Although it is well recognized that this elegantly coordinated process of differentiation from naïve to effector and from effector to memory is linked to cell cycle controls that govern cellular proliferation and apoptosis, the underlying mechanisms are not well defined. Specifically, we do not yet fully understand the cellular controls that regulate the timing of cell cycle entry and exit of antigen-specific effector CD8 T cells following antigenic stimulation. The mechanisms that stop the cell cycle of antigen-stimulated effector CD8 T cells are important because they not only dictate the timing of the transition of effector to memory but also determine the number of fully differentiated memory CD8 T cells.
In mammalian cells, cell cycle traversal is regulated by cyclins and cyclin-dependent kinases (CDKs) that are in turn controlled by CDK inhibitors (CKIs) (31). Antigen receptor signaling or exposure to growth factors triggers de novo synthesis of D-type cyclins, which then associate with their catalytic partners CDK4 or CDK6. These cyclin-CDK complexes, as well as cyclin E-CDK2 complexes later in the cell cycle, phosphorylate retinoblastoma protein (Rb). Hyperphosphorylated Rb can no longer repress the transcription factor E2F, and this leads to activation of genes required for S-phase entry (9). CKIs act as brakes for the cell cycle, restraining the activity of cyclin-CDK complexes to maintain cells in the quiescent G0/G1 phase or to induce cell cycle exit in proliferating cells. There are two classes of CKI: the INK4 inhibitors and the Cip/Kip inhibitors (31, 41). The INK4 proteins include p16INK4a, p15INK4b, p18INK4c, and p19INK4d, which are specific inhibitors of CDK4 and CDK6 (3). The CKIs of the Cip/Kip family are more broadly reactive and include p21Cip1, p27Kip1, and p57Kip2 (32). Induction of p18INK4c appears to be necessary for B cells to exit cell cycle and terminally differentiate into functional plasma cells (37). However, the role of CKIs in controlling cell cycle exit of effector CD8 T cells or generation of CD8 T-cell memory is yet to be studied.
One member of the Cip/Kip family of CKIs, p27Kip1, was originally identified as a cyclin-CDK complex inhibitor in cells arrested by transforming growth factor β (TGF-β) (25). In the CD8 T-cell lineage, p27Kip1 is highly expressed in thymocytes and in mature naïve T cells in the periphery (21, 23). Meanwhile, in peripheral T cells, mitogenic stimulation is known to downregulate p27Kip1 activity (21, 45), and it has been reported that interleukin-2 (IL-2)-induced cell cycle entry of quiescent CD8 T cells occurs by p27Kip1 elimination (24, 35). Other in vitro studies have shown that low levels of p27Kip1 can promote cell division early after activation, but later in the immune response p27Kip1 may oppose CD4 T-cell proliferation and effector function (27). Notably, studies in p27Kip1-transgenic mice have suggested that p27Kip1 acts as an integral brake for the cell cycle in T cells and that downregulation of its activity is necessary for cell cycle entry in response to mitogenic signals (38).
Following an acute viral infection, differentiation into effector CD8 T cells and loss of proliferative potential at the culmination of clonal expansion have been associated with strong p27Kip1 induction (7, 12). It has yet to be determined whether p27Kip1 induction specifically regulates the cell cycle exit and apoptosis of short-lived effector cells (SLECs), the number of MPECs, effector-to-memory cell differentiation, or the proliferative renewal of memory CD8 T cells. Here, we tested the hypothesis that the CDK inhibitor p27Kip1 acts as a molecular timer that limits the number of cell divisions by enforcing cell cycle exit of antigen-stimulated effector CD8 T cells. We document that p27Kip1 is a critical regulator of CD8 T-cell homeostasis during an acute LCMV infection and show that p27Kip1 curtails the duration and magnitude of primary T-cell expansion by acting as a timer controlling the cell cycle exit of effector CD8 T cells. Under conditions of p27Kip1 deficiency, MPECs expressing low levels of KLRG-1 and high levels of IL-7 receptor alpha (KLRG-1low IL-7Rαhigh) and producing IL-2 underwent additional rounds of programmed expansion in an antigen-independent fashion. This markedly augmented the quantity and quality of antigen-specific memory CD8 T cells. Notably, loss of p27Kip1 activity enhanced the primary and recall CD8 T-cell responses following administration of live vaccines. Moreover, we have identified p27Kip1 as a factor that limits the rate of proliferative renewal of effector memory CD8 T cells. Taken together, these data provide new insights into the process of memory CD8 T-cell differentiation and suggest that timed downregulation of p27Kip1 may be a fruitful strategy for augmenting the CD8 T-cell immunity induced by infection or vaccines.
MATERIALS AND METHODS
Mice.
p27Kip1-deficient (−/−) mice on a C57BL/6 background were purchased from Jackson Laboratories (4) (Bar Harbor, ME) and bred at the University of Wisconsin-Madison (WI). Wild-type C57BL/6 (+/+) mice were either littermates or purchased from the National Cancer Institute (Frederick, MD). Congenic B6/Ly5.1 mice were also purchased from Jackson Laboratories. P14 T-cell receptor (TCR)-transgenic mice were provided by K. Murali-Krishna (University of Washington, Seattle, WA). All mice were used at 6 to 8 weeks of age, and experiments were conducted as per the protocols approved by the institutional animal care committee.
Infections.
Mice were infected with 2 × 105 PFU of LCMV Armstrong by intraperitoneal (i.p.) injection, and viral titers in the tissue were quantitated by a plaque assay using Vero cell monolayers (2). Recombinant vaccinia virus that expresses the LCMV glycoprotein (VV-GP) was injected i.p. at a dose of 2 × 106 PFU/mouse (43). Mice were infected intravenously with 5 × 104 CFU of recombinant Listeria monocytogenes that expresses the LCMV GP33 epitope (rGP33 strain) (30). Mice immunized with the rGP33 strain were challenged intravenously with 2 × 106 PFU of LCMV clone 13 (2).
Direct ex vivo CTL assay.
Major histocompatibility complex class I (MHC-I)-restricted, LCMV-specific cytotoxic T lymphocyte (CTL) activity in the murine spleens was assessed ex vivo using a standard 51Cr release assay with syngeneic MC57 cells pulsed with LCMV epitope peptides as target cells (2).
Quantitation of LCMV-specific CD8 T cells by flow cytometry.
MHC-I (Db) tetramers, which are specific to the LCMV CTL epitopes NP396-404 (NP396), GP33-41 (GP33), and GP276-285 (GP276), were prepared as described elsewhere (22). Briefly, single-cell suspensions of splenocytes were stained with MHC-I tetramers and anti-CD8. In some experiments, cells were costained with anti-CD44, anti-CD43, anti-CD62L, anti-CD122, anti-KLRG-1, and anti-CD127 in combination with the MHC-I tetramers (Db/NP396, Db/GP33, or DbGP276). All antibodies were purchased from BD Pharmingen except anti-KLRG-1 (Southern Biotech, Birmingham, AL) and anti-CD127 (eBioscience, San Diego, CA). For intracellular cytokine staining, splenocytes were stimulated in vitro with LCMV epitope peptides in the presence of brefeldin A for 5 h. After culture, cells were stained for surface CD8 and intracellular gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and IL-2 using a Cytofix/Cytoperm intracellular staining kit (BD Pharmingen).
LAMP-1 staining.
At day 8 postinfection (p.i.), splenocytes were incubated with LCMV epitope peptide in the presence of anti-CD107a (antibody specific to lysosomal-associated membrane protein 1 [LAMP-1]; BD Pharmingen) and brefeldin A for 5 h. Following stimulation, cells were surface stained with anti-CD8, followed by intracellular staining for IFN-γ.
Assessment of in vivo proliferation of LCMV-specific CD8 T cells.
Mice were administered bromodeoxyuridine (BrdU; Sigma) in drinking water (0.8 mg/ml) for 8 days. On the eighth day of treatment, splenocytes were stained with anti-CD8, anti-CD127, anti-KLRG-1, anti-CD62L, and MHC-I tetramers. Following surface staining, cells were permeabilized and stained with anti-BrdU antibody using a BrdU staining kit (BD Pharmingen). Intracellular staining for Ki-67 was performed using a Ki-67 staining kit (BD Pharmingen).
Cell cycle analysis.
After being stained with MHC-I tetramers and anti-CD8, splenocytes were permeabilized and stained with Hoechst 33342 (Molecular Probes) for 30 min at 37°C. Samples were acquired using an LSR II flow cytometer (Becton Dickinson), and cell cycle data were analyzed using ModFit LT software.
Cell sorting, CFSE labeling, and adoptive transfer of T cells.
Splenocytes from +/+ or −/− mice were stained with anti-CD8 and anti-CD44. CD44hi CD8 T cells were purified to >98% purity using a FACSVantage cell sorter (Becton Dickinson) and adoptively transferred into naïve congenic B6/Ly5.1 mice after carboxyfluorescein succinimidyl ester (CFSE) labeling. Six to eight million CD44high CD8 T cells were transferred from day 8 infected mice, and 2 to 3 million CD44high CD8 T cells were transferred from day 15 infected mice. For assessment of proliferative renewal of memory CD8 T cells, total T cells from +/+ or −/− mice at day 80 p.i. were purified using negative-selection columns (R&D Systems). After CFSE labeling, 6 × 106 to 8 × 106 T cells were transferred into naïve B6/Ly5.1 mice. CFSE dilution in LCMV-specific memory CD8 T cells was assessed 1 month after cell transfer.
Quantitation of apoptotic cells.
In some experiments, splenocytes were stained with anti-CD8, MHC-I tetramers, and annexin V, as described elsewhere (33). The percentage of annexin V tetramer-binding CD8 T cells was determined by flow cytometry. Apoptotic CD8 T cells were also visualized by staining for activated caspase-3 (BD Pharmingen). Splenocytes were stained with MHC-I tetramers and anti-CD8. Subsequently, cells were permeabilized using a Cytofix/Cytoperm kit and stained for activated caspase-3. To stain for activated caspase-3 after in vitro culture, splenocytes were cultured for 4 h at 37°C. Subsequently, splenocytes were surface stained as described above and stained for activated caspase-3.
In vitro proliferation assay.
CD8 T cells were purified from spleens of +/+ or −/− mice by a negative-selection procedure using Miltenyi beads as per the manufacturer's recommendations. A total of 105 purified CD8 T cells were cultured in triplicates in 96-well round-bottomed plates that were precoated with anti-CD3 (10 μg/ml) and anti-CD28 (5 μg/ml). Cultured cells were pulsed with [3H]thymidine between 0 and 24 h, 24 and 48 h, or 48 and 72 h after stimulation. Cells were harvested onto Unifilter plates (PerkinElmer Life Sciences, Boston, MA) using Filtermate cell harvester (Packard Bioscience Company, Meriden, CT), and radioactivity was measured using a TopCount Microplate Scintillation and Luminescence Counter (Packard Bioscience Company).
RESULTS
CDK inhibitor p27Kip1 controls the duration of expansion and limits the magnitude of clonal burst size of effector CD8 T cells.
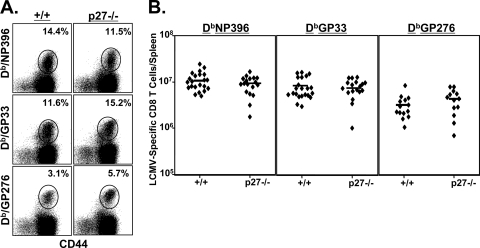
An acute infection of mice with the Armstrong strain of LCMV leads to massive clonal expansion of virus-specific CD8 T cells and differentiation into potent effectors during the first 8 days after infection. To determine the role of p27Kip1 in regulating this expansion and differentiation, we infected groups of +/+ and −/− mice with LCMV. We then assessed the effect of p27Kip1 deficiency on the resultant phenotype of CD8 T cells. On day 8 p.i., at the peak of the T-cell response, LCMV-specific CD8 T cells in the spleens of the +/+ and −/− mice were quantitated using MHC-I tetramers. Data in Fig. 1 show that p27Kip1 deficiency did not significantly affect either the frequency or the absolute number of virus-specific CD8 T cells in LCMV-infected mice. The phenotypes of LCMV-specific effector CD8 T cells (CD44high CD43high CD62Llow CD122high) in +/+ and −/− mice were also largely similar (Fig. 2A and B). Moreover, p27Kip1 deficiency affected neither the expression of IL-7Rα nor the relative proportion of IL-7Rαhigh cells among virus-specific CD8 T cells. Meanwhile, the percentages of effector CD8 T cells (GP33-specific CD8 T cells in particular) that expressed the senescence marker KLRG-1 were significantly lower in −/− mice than in wild-type mice (P = 0.006).
FIG. 1.
Primary CD8 T-cell response to LCMV in p27Kip1-deficient mice. Wild-type and −/− mice were infected with LCMV, and LCMV-specific CD8 T cells were quantitated in the spleens at day 8 p.i. by staining with MHC-I tetramers, anti-CD8, and anti-CD44. The dot plots in panel A are gated on the total CD8 T-cell population, and the numbers indicate percentages of LCMV-specific CD8 T cells among the total CD8 T-cell population. In panel B, each symbol represents an individual mouse. The data in panel A are from one of six independent experiments, and the data in panel B are pooled from six independent experiments.
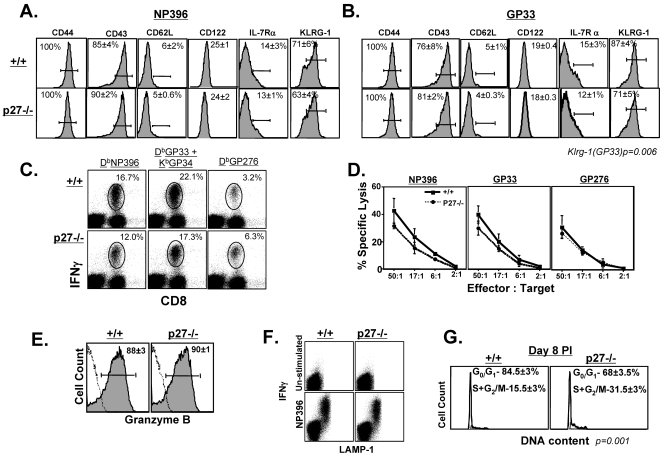
FIG. 2.
Phenotypic, functional, and cell cycle analysis of effector CD8 T cells in −/− mice. (A and B) At day 8 p.i. the cell surface phenotype of effector CD8 T cells was analyzed by flow cytometry. The histograms are gated on NP396 and GP33 tetramer-binding CD8 T cells. (C) Cytokine production by LCMV-specific effector CD8 T cells was assessed by intracellular cytokine staining. The dot plots are gated on total lymphocytes, and the numbers indicate percentages of IFN-γ-producing, LCMV-specific CD8 T cells among the total CD8 T-cell population. (D) MHC-I-restricted cytotoxic activity. CTL activity in the spleens of +/+ and −/− mice was quantitated by a 51Cr release assay. There was no significant lysis of control unpulsed target cells. (E) Intracellular granzyme B expression in NP396-specific CD8 T cells. (F) Antigen-induced degranulation. The dot plots show staining for LAMP-1 and IFN-γ and are gated on the total CD8 T-cell population. (G) Cell cycle status of LCMV-specific effector CD8 T cells. At day 8 p.i., the DNA content of NP396-specific CD8 T cells was analyzed by staining with Hoechst. The flow cytometry plots are gated on NP396 tetramer-binding CD8 T cells, and the data are representative of three independent experiments.
Next, we assessed the effect of p27Kip1 deficiency on the effector function of LCMV-specific CD8 T cells. As illustrated in Fig. 2C, both +/+ and −/− CD8 T cells specific to the various LCMV epitopes produced high levels of IFN-γ upon antigenic stimulation ex vivo. The MHC-I-restricted cytotoxicity of epitope-specific CD8 T cells from −/− mice was comparable to that of +/+ mice (Fig. 2D). Additionally, the granzyme content of virus-specific CD8 T cells (Fig. 2E) and the ability of effector cells to degranulate (based on LAMP-1 expression) (Fig. 2F) were not significantly affected by p27Kip1 deficiency. Taken together, these data suggest that, except for KLRG-1 expression, p27Kip1 deficiency did not affect the phenotype or function of LCMV-specific CD8 T cells at day 8 p.i. Consistent with the normal expansion and effector function of virus-specific CD8 T cells, LCMV titers were below the level of detection in various tissues of +/+ and −/− mice (data not shown).
Concomitant with LCMV clearance and cessation of antigenic stimulation, effector CD8 T cells exit the cell cycle, thereby terminating clonal expansion by day 8 p.i. (16). To determine whether p27Kip1 regulated the cell cycle exit of effector CD8 T cells following LCMV clearance, we assessed the cell cycle status of these T cells in +/+ and −/− mice at day 8 p.i. by analyzing their DNA content. Figure 2G illustrates that the majority of LCMV-specific effector CD8 T cells in +/+ mice were in the G0/G1 phase, which is indicative of cell cycle exit, and ∼15% of T cells were still in the active cycle. In contrast, >30% of virus-specific CD8 T cells were still in the active cycle in −/− mice at day 8 p.i. (P = 0.001). These data suggest that p27Kip1 regulates the cell cycle exit of LCMV-specific effector CD8 T cells.
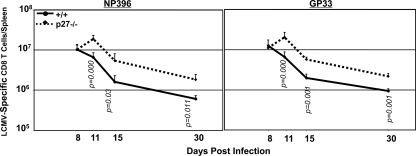
The altered cell cycle status of p27Kip1-deficient, LCMV-specific effector CD8 T cells suggested that p27Kip1 deficiency might alter the dynamics of CD8 T-cell expansion and/or contraction. To investigate this possibility, we carefully analyzed the kinetics of CD8 T-cell responses in +/+ and −/− mice. Consistent with previous work, the number of LCMV-specific CD8 T cells in the +/+ mice peaked at day 8 p.i. and then promptly declined (Fig. 3). Notably, in −/− mice, the number of virus-specific CD8 T cells continued to increase beyond day 8 p.i., with a 200% increase between days 8 and 11 p.i. After day 11, the number of LCMV-specific CD8 T cells in −/− mice declined at a rate similar to that in +/+ mice. However, it should be noted that continued expansion of effector cells beyond day 8 p.i. resulted in a net increase in the absolute number of LCMV-specific CD8 T cells at days 15 and 30 p.i. in −/− mice. These data demonstrate that p27Kip1 not only curtailed the duration of the expansion phase but also limited the clonal burst size of virus-specific effector CD8 T cells during the primary T-cell response to LCMV.
FIG. 3.
p27Kip1 limits the duration and magnitude of the primary expansion of effector CD8 T cells. Groups of +/+ and −/− mice were infected with LCMV, and at the indicated number of days p.i., NP36- or GP33-specific splenic CD8 T cells were quantitated by intracellular cytokine staining for IFN-γ. Data are representative of three to seven independent experiments for each time point.
p27Kip1 limits antigen-independent programmed expansion of effector CD8 T cells following viral clearance.
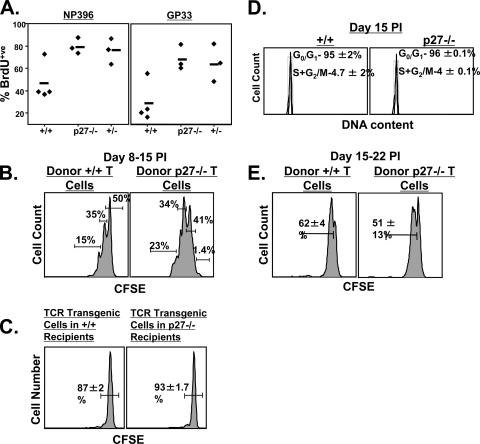
The observed increase in the number of LCMV-specific CD8 T cells at days 11, 15, and 30 p.i. in −/− mice (Fig. 3) may result from altered rates of proliferation and apoptosis. To test this hypothesis, we measured proliferation of virus-specific CD8 T cells in vivo after treating +/+ and −/− mice with BrdU between days 8 and 15 p.i. As shown in Fig. 4A, twice as many −/− LCMV-specific CD8 T cells as wild-type cells incorporated BrdU during this 7-day period. These data suggest that p27Kip1 suppressed proliferation of virus-specific CD8 T cells in +/+ mice. Although LCMV titers were below the detection limit in both +/+ and −/− mice at day 8 p.i., it is possible that continued antigenic stimulation drove the proliferation of virus-specific CD8 T cells in −/− mice. To investigate this possibility, we performed two experiments. First, CD44high CD8 T cells sorted from the spleens of +/+ and −/− mice at day 8 p.i. were labeled with CFSE and adoptively transferred into the antigen-free environment of a naïve, uninfected congenic mouse. One week after adoptive transfer, we assessed the proliferation of donor LCMV-specific CD8 T cells by quantitating cell division-induced loss of CFSE fluorescence. Following transfer into the uninfected congenic mice, 35% of wild-type LCMV-specific CD8 T cells divided once, and only 15% had undergone two divisions (Fig. 4B). In striking contrast, 99% of the adoptively transferred −/− LCMV-specific CD8 T cells had divided at least once, and 57% had divided two to four times in the same 7-day interval (Fig. 4B). These data demonstrate that −/− LCMV-specific effector CD8 T cells continued to proliferate in an antigen-free environment.
FIG. 4.
p27Kip1 limits programmed expansion of effector CD8 T cells. (A) BrdU incorporation by LCMV-specific CD8 T cells. Groups of +/+, p27 heterozygous (+/−), and −/− mice were infected with LCMV and administered BrdU in drinking water between days 8 and 15 p.i. At the end of the BrdU pulse (day 15 p.i), splenocytes were stained with MHC-I tetramers, anti-CD8, and anti-BrdU. The percentage of BrdU-positive cells among tetramer-binding, NP396- or GP33-specific CD8 T cells was assessed by flow cytometry. Each symbol represents an individual mouse. (B) Programmed proliferation of LCMV-specific effector CD8 T cells in an antigen-free environment. At day 8 p.i., CD44high CD8 T cells were sorted from the spleens of +/+ and −/− mice. Six to eight million sorted, CFSE-labeled CD8 T cells were adoptively transferred into naïve congenic B6/Ly5.1 mice. The mice were euthanized 1 week after cell transfer, and CFSE dilution in LCMV-specific CD8 T cells was analyzed by flow cytometry. The histograms are gated on Ly5.2-positive NP396 tetramer-binding CD8 T cells. The results are representative of two independent experiments. (C) Proliferation of naïve TCR-transgenic Ly5.1/P14 CD8 T cells in LCMV-infected +/+ and −/− mice. Naïve CFSE-labeled, LCMV GP33-specific P14 CD8 T cells were transferred into LCMV-infected +/+ or −/− mice at day 8 p.i. CFSE fluorescence of the donor transgenic T cells was analyzed 1 week after cell transfer. Histograms are gated on Ly5.1-positive P14 CD8 T cells. (D) Cell cycle status of LCMV-specific CD8 T cells at day 15 p.i. The DNA content of NP396-specific CD8 T cells was analyzed by staining with Hoechst. The flow cytometry plots are gated on NP396 tetramer-binding CD8 T cells. The data are representative of two independent experiments. (E) Proliferation of LCMV-specific CD8 T cells in an antigen-free environment. At day 15 p.i, 2 × 106 to 3 × 106 CD44high CD8 T cells sorted from the spleens of +/+ or −/− mice were adoptively transferred into naïve B6/Ly5.1 congenic mice after CFSE labeling. The mice were euthanized 1 week after cell transfer, and CFSE dilution was analyzed by flow cytometry. The histograms are gated on Ly5.2-positive NP396 tetramer-binding CD8 T cells. The results are representative of two independent experiments.
In a second experiment, we determined whether continued proliferation of LCMV-specific effector CD8 T cells in −/− mice (Fig. 4A) was related to persistent antigenic stimulation. To test for the presence of persisting LCMV antigen, we adoptively transferred naïve CFSE-labeled GP33-specific P14 naïve TCR-transgenic cells into LCMV-infected +/+ and −/− mice at day 8 p.i., hypothesizing that antigenic stimulation in the infected mice would stimulate the donor CD8 T cells to proliferate. As shown in Fig. 4C, 7 days after adoptive transfer, ∼90% of the donor TCR-transgenic cells in both +/+ and −/− recipients did not exhibit detectable proliferation. These findings (Fig. 4B and C) suggest that protracted proliferation of LCMV-specific effector CD8 T cells in −/− mice was probably not due to prolonged antigenic stimulation. In fact, previous studies have shown that CD8 T cells divide several times in an antigen-free environment, after a brief encounter with the cognate antigen, termed programmed expansion (13, 20, 39). Therefore, based on the data presented in Fig. 4, we infer that p27Kip1 limited the antigen-independent, programmed expansion of effector CD8 T cells following viral clearance in LCMV-infected mice.
We next examined whether LCMV-specific CD8 T cells in p27-deficient mice proliferate indefinitely after cessation of antigenic stimulation. Cell cycle analysis at day 15 p.i. (Fig. 4D) revealed that the majority of −/− LCMV-specific CD8 T cells had exited the cell cycle and that the number remaining in the active cycle at day 15 p.i. was comparable to that of wild-type mice. In addition, we adoptively transferred CFSE-labeled CD44high CD8 T cells sorted from +/+ or −/− mice at day 15 p.i. into naïve congenic mice. Analysis of CFSE dilution 1 week after transfer revealed that 50 to 60% of the +/+ and −/− LCMV-specific CD8 T cells had divided only once in naïve congenic mice, suggesting that p27-deficient CD8 T cells do not divide indefinitely after antigen withdrawal (Fig. 4E). Taken together, the data in Fig. 4 strongly suggest that p27Kip1 acts as an intrinsic timer regulating the cell cycle exit of effector CD8 T cells following antigen withdrawal and that its deficiency leads to additional rounds of programmed antigen-independent cell division in LCMV-infected mice.
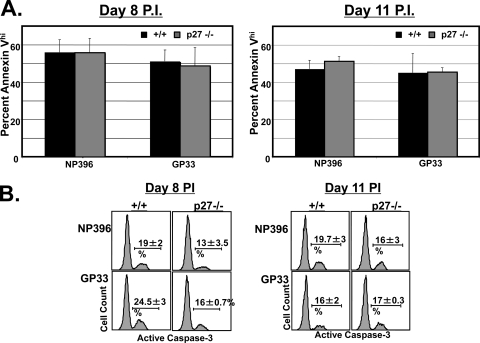
Next, we investigated whether p27Kip1 deficiency also affects the apoptosis of LCMV-specific effector CD8 T cells. We along with others have previously reported (33, 42) that proapoptotic, LCMV-specific effector CD8 T cells may be visualized ex vivo by staining with annexin V. At days 8 and 11 p.i., the percentage of annexin Vhigh cells among LCMV-specific effector CD8 T cells in the spleens of −/− mice was comparable to that of wild-type mice (Fig. 5A). We also quantitated LCMV-specific effector CD8 T-cell apoptosis by staining for active caspase-3 directly ex vivo or after in vitro culture for several hours. At days 8 and 11 p.i., the percentage of LCMV-specific CD8 T cells expressing activated caspase-3 directly ex vivo ranged from 0.4 to 0.7% in both +/+ and −/− mice (data not shown). Upon in vitro culture, the percentage of caspase-3-positive, LCMV-specific CD8 T cells from the spleens of −/− mice was slightly lower than that of +/+ mice at day 8 p.i. but similar at day 11 p.i. (Fig. 5B). These findings, along with the data shown in Fig. 4, indicate that the inflation of LCMV-specific effector CD8 T cells in −/− mice is likely due to higher cellular proliferation rather than to impairment in apoptosis.
FIG. 5.
Effect of p27Kip1 deficiency on apoptosis of effector CD8 T cells. (A) At days 8 and 11 p.i, splenocytes from +/+ and −/− mice were stained with anti-CD8, MHC-I tetramers, and annexin V to quantitate proapoptotic cells directly ex vivo. The percentage of annexin Vhigh tetramer-binding CD8 T cells was analyzed by flow cytometry. (B) At days 8 and 11 p.i, splenocytes were cultured for 4 h in vitro. After culture, the cells were stained with MHC-I tetramers, anti-CD8, and anti-caspase-3. Histograms are gated on Db/NP396 or Db/GP33 tetramer-binding CD8 T cells.
p27Kip1 preferentially restricts the expansion and accumulation of memory precursors during the primary CD8 T-cell response.
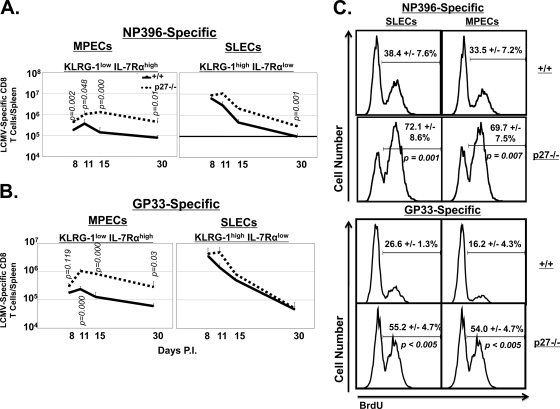
The data shown in Fig. 2 to 4 demonstrate that p27Kip1 deficiency delayed the cell cycle exit of effector CD8 T cells and extended the expansion phase of a T-cell response to acute LCMV infection. It was of interest to determine whether p27Kip1 deficiency triggered the preferential expansion of a subset of effector CD8 T cells. The differential expression pattern of CD127/IL-7Rα and KLRG-1 has been used to identify potential memory precursor cells among effector cell populations (12, 15, 28). MPECs have been reported to exhibit the IL-7Rαhigh KLRG-1low phenotype, and short-lived effector CD8 T cells (SLECs) slated for deletion exhibit the IL-7Rαlow KLRG-1high phenotype (12, 28). Here, we determined the role of p27Kip1 in regulating homeostasis of SLECs and MPECs during the T-cell response to acute LCMV infection. In agreement with published findings (12), LCMV-specific MPECs and SLECs could be distinguished based on IL-7Rα and KLRG-1 expression at day 8 p.i. (Fig. 6). In the +/+ and the −/− mice, (Fig. 6), the decline in the number of NP396- and GP33-specific SLECs between days 8 and 30 p.i. was markedly greater than the decrease in MPECs. In the −/− mice, there was a significant increase in the number of LCMV-specific MPECs (more pronounced for GP33-specific CD8 T cells) (Fig. 6B) between days 8 and 11 p.i., followed by a period of decline in the ensuing 19 days. Primarily by virtue of increased cellular expansion between days 8 and 11 p.i., the −/− spleens contained 5- to 6-fold more LCMV-specific MPECs at day 30 p.i. than the +/+ spleens. It should be noted that, in comparison with the marked effect on MPEC expansion, the effect of p27Kip1 deficiency on SLEC homeostasis was less pronounced and modest at best. In summary, these data provide strong evidence for a nonredundant role for p27Kip1 in limiting the clonal burst size of IL-7Rαhigh KLRG-1low MPECs during acute viral infection.
FIG. 6.
Preferential expansion and maintenance of KLRG-1low IL-7Rαhigh MPEC CD8 T cells in p27Kip1-deficient mice. (A and B) At the indicated number of days after LCMV infection, splenocytes were stained with anti-CD8, anti-KLRG-1, anti-IL-7Rα, and Db/NP396 tetramers (A) or Db/GP33 tetramers (B). The absolute numbers of KLRG-1low IL-7Rαhigh (MPECs) and KLRG-1high IL-7Rαlow (SLECs) NP396 or GP33 tetramer-binding CD8 T cells were calculated. Data are representative of three to seven independent experiments for each time point. (C) Proliferation of MPECs and SLECs in −/− mice. LCMV-infected +/+ and −/− mice were treated with BrdU between days 8 and 15 p.i., and the percentages of NP396- or GP33-specific BrdU-positive MPECs and SLECs were determined at day 15 p.i. The histograms are gated on NP396- or GP33-specific MPECs or SLECs, and the numbers are the percentages of BrdU-positive cells among the gated cell population; data are from three mice/group.
Next, we explored whether preferential expansion of MPECs in −/− mice was linked to higher proliferation of MPECs than SLECs. LCMV-infected +/+ and −/− mice were administered BrdU between days 8 and 15 p.i., and BrdU incorporation by MPECs and SLECs was assessed at day 15 p.i. Data in Fig. 6C show that the percentages of BrdU-positive MPECs and SLECs were similar in +/+ mice, regardless of antigenic specificity. The proliferation of both MPECs and SLECs in −/− mice was significantly higher than that of their respective counterparts in +/+ mice. However, the percentages of proliferating MPECs were similar to those of SLECs in the −/− mice. These data suggested that p27Kip1 deficiency enhances the proliferation of MPECs and SLECs to the same magnitudes. These results also indicated that differences in proliferation between MPECs and SLECs could not explain selective accumulation of MPECs in LCMV-infected −/− mice. This was a surprising result because the levels of p27Kip1 protein have been reportedly higher in SLECs than in MPECs (8).
Preferential expansion and maintenance of high-quality IL-2-producing CD8 T cells in p27Kip1-deficient mice.
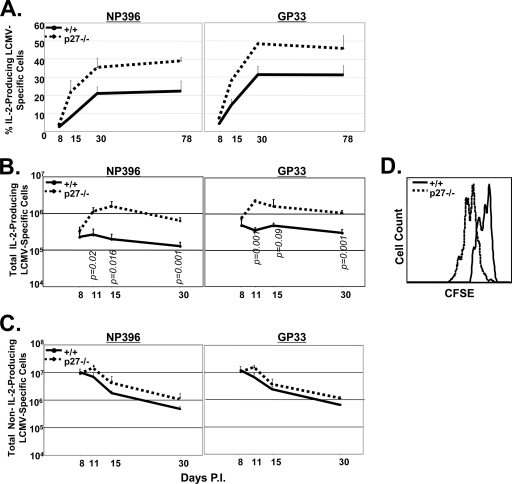
At the peak of the primary T-cell response to LCMV, only a small fraction of effector CD8 T cells is able to produce IL-2, but the relative proportion of IL-2-producing cells does increase during the effector-to-memory transition. Moreover, IL-2 production appears heightened in memory precursors present within the effector CD8 T-cell population (12, 28). Here, we determined the effect of p27Kip1 deficiency on the dynamics of IL-2-producing, LCMV-specific CD8 T cells following LCMV infection. As shown in Fig. 7A, the relative proportion of IL-2-producing CD8 T cells among all IFN-γ-producing CD8 T cells was low at day 8 p.i. in +/+ mice but progressively increased with time. Remarkably, although the proportion of IL-2-producing CD8 T cells was similar to that of wild-type mice at day 8 p.i., the kinetics and magnitude of the increase in IL-2 producers were substantially augmented in −/− mice (Fig. 7A).
FIG. 7.
Preferential expansion and maintenance of IL-2-producing CD8 T cells in p27Kip1-deficient mice. Groups of +/+ and −/− mice were infected with LCMV, and at the indicated number of days after infection, splenocytes were stimulated in vitro with NP396 or GP33 peptides for 5 h. After stimulation, cells were stained for surface CD8 and intracellular IFN-γ and IL-2. Panel A shows the percentage of IL-2-producing cells among NP396- and GP33-specific IFN-γ-producing cells at the indicated days p.i. (B) The line graph shows the total number of IL-2-producing NP396- or GP33-specific CD8 T cells. (C) Line graphs depicting the total number of non-IL-2-producing NP396- and GP33-specific CD8 T cells. (D) Proliferative expansion of IL-2-producing NP396-specific CD8 T cells in an antigen-free environment. At day 8 p.i., CD44high CD8 T cells sorted from the spleens of +/+ or −/− mice were labeled with CFSE and adoptively transferred into naïve congenic B6/Ly5.1 mice. Eight days after cell transfer, splenocytes from the recipient mice were stimulated with NP396 peptide to induce cytokine production. After culture, cells were stained for cell surface Ly5.2 and CD8 and intracellular IL-2. The histograms show CFSE fluorescence and are gated on IL-2-producing, donor NP396-specific CD8 T cells.
The temporal increase in the percentage of IL-2-producing cells among total epitope-specific CD8 T cells in +/+ or −/− mice may be due to a gain of IL-2-producing ability or a selective loss of non-IL-2-producing cells. To determine which mechanism was causing the increase in IL-2 producers, we compared the absolute numbers of NP396- and GP33-specific IL-2-producing CD8 T cells in +/+ and −/− mice. In the wild-type mice, the number of IL-2-producing CD8 T cells remained remarkably stable, but the number of non-IL-2-producing cells declined rapidly between days 8 and 30 p.i. (Fig. 7B and C). Meanwhile, in the −/− mice, the number of IL-2-producing CD8 T cells continued to increase after day 8 p.i. until at least day 11 to 15 p.i., with a 4- to 6-fold increase between days 8 and 11 p.i. (Fig. 7B). In contrast, the number of p27Kip1-deficient non-IL-2-producing CD8 T cells showed only modest expansion after day 8 p.i. and declined rapidly thereafter, similar to the findings in +/+ mice (Fig. 7C). These data demonstrate that p27Kip1 deficiency led to selective accumulation of IL-2-producing, virus-specific CD8 T cells during acute LCMV infection (Fig. 7B and C).
To ascertain whether p27Kip1 deficiency resulted in the increased proliferation of IL-2-producing, LCMV-specific CD8 T cells, we purified and CFSE-labeled CD44high CD8 T cells from the spleens of +/+ and −/− mice at day 8 p.i. These cells were then adoptively transferred into congenic, uninfected wild-type mice. One week after adoptive transfer, we compared the proliferation of donor LCMV-specific +/+ and −/− IL-2-producing CD8 T cells in the recipient mice. As shown in Fig. 7D, −/− IL-2-producing, LCMV-specific CD8 T cells proliferated more than the wild-type cells. Based on these findings, we propose that the cell cycle inhibition of p27Kip1 plays an essential role in restraining the expansion of IL-2-producing memory precursor CD8 T cells during acute viral infection.
Sustained proliferation of p27Kip1-deficient CD8 T cells in vitro.
Data in Fig. 3, 6, and 7 showed that global deficiency of p27Kip1 led to sustained proliferation of LCMV-specific effector CD8 T cells in vivo. However, these studies were unable to determine whether continued proliferation of LCMV-specific CD8 T cells in vivo in −/− mice was linked to cell-intrinsic loss of p27Kip1 activity. Here, using a simple in vitro assay, we compared the dynamics of proliferation of purified p27Kip1-deficient and +/+ CD8 T cells upon stimulation with anti-CD3 and anti-CD28 antibodies (see Fig. S1 in the supplemental material). Data in Fig. S1 show that CD8 T cells from +/+ mice showed peak proliferation between 24 to 48 h after stimulation but declined precipitously thereafter. CD8 T cells from −/− mice also attained peak proliferation between 24 and 48 h after stimulation. However, unlike +/+ CD8 T cells that showed proliferative decline after 48 h, p27Kip1-deficient CD8 T cells continued to proliferate at peak levels between 48 and 72 h after stimulation. These data showed that CD8 T cells from −/− mice undergo protracted proliferation in vitro upon stimulation with anti-CD3 and anti-CD28 antibodies.
Enhanced CD8 T-cell responses to vaccination with recombinant L. monocytogenes or vaccinia virus in the absence of p27Kip1.
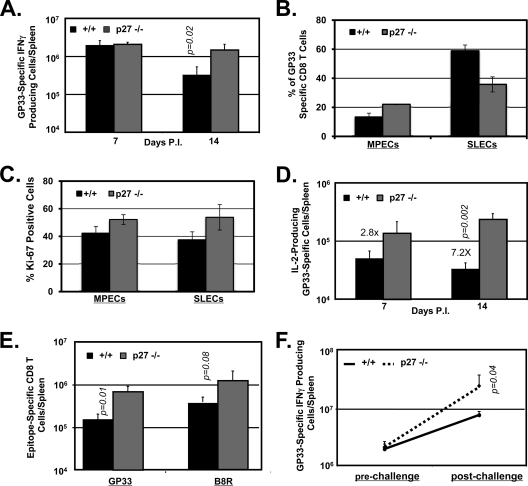
The results thus far showed that p27Kip1 constrained CD8 T-cell responses to an acute LCMV infection. Next, we determined whether findings from LCMV infection are also applicable to live vaccines. Groups of +/+ or −/− mice were immunized with a recombinant L. monocytogenes strain (rGP33) that expresses the MHC-I Db-restricted GP33 epitope of LCMV. As illustrated in Fig. 8, rGP33 elicited strong GP33-specific CD8 T-cell responses in both +/+ and −/− mice, and the numbers of GP33-specific CD8 T cells in the spleen were comparable between the two groups at day 7 p.i. However, at day 7 p.i., there was a striking difference in the relative proportions of GP33-specific MPECs and SLECs between +/+ and −/− mice (Fig. 8B). Compared to percentages in +/+ mice, at day 7 p.i., the percentages of GP33-specific MPECs in the −/− mice exhibited a ∼2-fold increase, with a concomitant 50% reduction in the percentages of SLECs. The percentages of the subset of GP33-specific effector CD8 T cells expressing low levels of KLRG-1 and intermediate levels of CD127 (KLRG-1low CD127int) in +/+ and −/− mice were 13.2% and 29.4%, respectively. These data suggested that p27Kip1 regulates the relative proportions of MPECs and SLECs following rGP33 infection. We also assessed the proliferative status of MPECs and SLECs in rGP33-infected +/+ and −/− mice by staining for Ki-67, a cellular marker for proliferating cells. Data in Fig. 8C show that the percentages of Ki-67-positive MPECs and SLECs in −/− mice were higher than in +/+ mice, which suggested that p27Kip1 deficiency enhanced the proliferation of both MPECs and SLECs following infection with the L. monocytogenes rGP33 strain. At day 14 after immunization with rGP33, spleens of −/− mice contained 5-fold greater numbers of GP33-specific CD8 T cells than the spleens of +/+ mice (Fig. 8A). Likewise, the number of IL-2-producing GP33-specific CD8 T cells was markedly elevated (∼7-fold) in spleens of −/− mice at day 14 p.i. compared to the levels in +/+ mice (Fig. 8D). Notably, the numbers of IL-2-producing GP33-specific CD8 T cells remained relatively stable between days 7 and 14 p.i. in +/+ mice. In contrast, in −/− mice, the number of IL-2-producing GP33-specific CD8 T cells doubled within the same 7-day interval. Next, we immunized +/+ or −/− mice with recombinant vaccinia virus that expresses the LCMV glycoprotein (VV-GP). At day 14 after immunization, the numbers of CD8 T cells that are specific to the VV epitope B8R and GP33 were quantified in the spleen. Data in Fig. 8E show that spleens of −/− mice contained substantially greater numbers of B8R- and GP33-specific CD8 T cells than spleens of +/+ mice. Taken together, these data showed that p27Kip1 suppresses CD8 T-cell responses to live viral or intracellular bacterial vaccines.
FIG. 8.
CD8 T-cell responses to vaccination with recombinant L. monocytogenes or vaccinia virus. (A to D) CD8 T-cell response to L. monocytogenes infection. Groups of wild type (+/+) and −/− mice were infected with the recombinant L. monocytogenes rGP33 strain. At days 7 and 14 after infection, the number of cytokine-producing GP33-specific CD8 T cells was assessed by intracellular staining for IFN-γ and IL-2. Panel A shows the total number of GP33-specific IFN-γ-producing CD8 T cells. At day 7 p.i., splenocytes were stained with anti-CD8, anti-CD127, anti-KLRG-1, and Db/GP33 MHC-I tetramers (B). The percentages of MPECs or SLECs among tetramer-binding CD8 T cells were assessed by flow cytometry. (C) Proliferation of MPECs and SLECs in −/− mice. At day 7 p.i., splenocytes were stained with anti-CD8, anti-CD127, anti-KLRG-1, Db/GP33 MHC-I tetramers, and anti-Ki-67 antibodies. The percentages of Ki-67-positive cells among GP33-specific MPECs and SLECs were determined by flow cytometry. Panel D shows the total number of GP33-specific IL-2-producing CD8 T cells at days 7 and 14 p.i. (E) CD8 T-cell response to vaccinia virus. Both +/+ and −/− mice were infected with recombinant vaccinia virus, VV-GP. At day 14 after infection, the numbers of B8R- and GP33-specific CD8 T cells were quantitated by intracellular staining for IFN-γ. (F) Recall responses of CD8 T cells in L. monocytogenes-immunized mice. Both +/+ and −/− mice were immunized with the rGP33 strain and 7 days later challenged with LCMV clone 13. The numbers of GP33-specific CD8 T cells at days 7 (prechallenge) and 12 (5 days after LCMV clone 13 challenge) were quantitated by intracellular staining for IFN-γ. Data are the mean values from three to four mice/group.
Next, we determined whether p27Kip1 regulates the secondary responses of GP33-specific effector CD8 T cells in rGP33-infected mice. Groups of +/+ and −/− mice were immunized with the L. monocytogenes rGP33 strain and 7 days later challenged with a virulent strain of LCMV, LCMV clone 13. The number of GP33-specific CD8 T cells in the spleen was quantitated prechallenge (day 7 p.i.) and 5 days after LCMV clone 13 challenge. As shown in Fig. 8F, the numbers of GP33-specific CD8 T cells prechallenge were similar in +/+ and −/− mice. Strikingly, however, after challenge, there were marked differences in the secondary expansion of GP33-specific CD8 T cells (Fig. 8F) between +/+ and −/− mice. While GP33-specific CD8 T cells in +/+ mice showed <4-fold expansion, −/− GP33-specific CD8 T cells underwent >10-fold secondary expansion after rechallenge. These data suggested that p27Kip1 negatively regulates the proliferative responses of effector CD8 T cells to antigen recall following immunization with rGP33.
p27Kip1 regulates the quantity and quality of long-term CD8 T-cell memory.
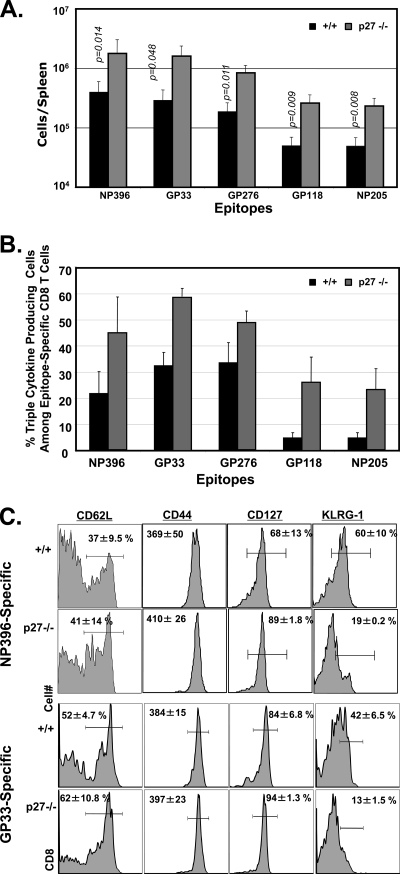
Mice that have recovered from acute LCMV infection develop potent CD8 T-cell memory and life-long immunity to reinfection (36). In LCMV-immune mice, a relatively stable number of memory CD8 T cells is maintained indefinitely in both lymphoid and nonlymphoid organs (18). The number of memory CD8 T cells generated following an acute LCMV infection is influenced by the extent of expansion, or clonal burst size, and the magnitude of cellular apoptosis during the primary T-cell response (1, 11, 14, 22). Data presented in Fig. 6 and 7 document the selective expansion of IL-2-producing, IL-7Rαhigh KLRG-1low MPECs in −/− mice after day 8 p.i. We then investigated whether extended MPEC expansion between days 8 and 15 p.i. augmented the number of long-lived LCMV-specific memory CD8 T cells in −/− mice. As shown in Fig. 9A, the spleens of −/− mice contained 5-fold more memory CD8 T cells than the spleens of +/+ mice. Similar augmentation in the number of LCMV-specific memory CD8 T cells was observed in the nonlymphoid organs of LCMV-immune −/− mice (data not shown). Therefore, the increased number of memory CD8 T cells in the spleens of −/− mice was probably not a result of the anatomic redistribution of memory CD8 T cells, reflecting a genuine systemic increase in CD8 T-cell memory size. These data indicate that p27Kip1 plays a key role in regulating the size of the memory CD8 T-cell compartment.
FIG. 9.
p27Kip1 regulates the quantity and quality of CD8 T-cell memory. Wild-type and −/− mice were infected with LCMV. (A and B) At day 112 p.i., splenocytes were stimulated with LCMV epitope peptides, and the percentages of IFN-γ-, TNF-α-, and IL-2-producing CD8 T cells were assessed by intracellular cytokine staining. (A) Total number of epitope-specific IFN-γ-producing CD8 T cells in +/+ and −/− mice. (B) Antigen-induced cytokine production by LCMV-specific CD8 T cells. The data are percentages of epitope-specific CD8 T cells producing IFN-γ, TNF-α, and IL-2. (C) At day 78 p.i., the cell surface phenotype of virus-specific CD8 T cells was assessed by flow cytometry. Plots are gated on tetramer-binding CD8 T cells. The numbers are the percentages among tetramer-binding CD8 T cells, or the mean fluorescent intensity of staining for the indicated marker.
Next, we examined whether p27Kip1 deficiency altered the qualitative attributes of LCMV-specific memory CD8 T cells. All LCMV-specific memory CD8 T cells produce IFN-γ, but only a subset also produces TNF-α and IL-2. We determined the ability of LCMV-specific memory CD8 T cells to produce the three cytokines, IFN-γ, TNF-α, and IL-2, upon ex vivo stimulation with their cognate antigen. As shown in Fig. 9B, the percentage of LCMV-specific memory CD8 T cells producing all three cytokines in −/− mice was substantially higher than that in wild-type mice. Comparison of the cell surface markers of memory CD8 T cells in +/+ and −/− mice also showed that p27Kip1 deficiency did not significantly affect the relative proportions of central (CD62Lhigh) and effector (CD62Llow) memory CD8 T cells or the expression of CD44 and CD127 (Fig. 9C). Interestingly, the percentage of KLRG-1high memory CD8 T cells was markedly lower in −/− spleens than in wild-type spleens. Taken together, the data shown in Fig. 9A to C demonstrate that p27Kip1 deficiency enhanced the quantity and quality of CD8 T-cell memory.
Regulation of the proliferative renewal of memory CD8 T cells by p27Kip1.
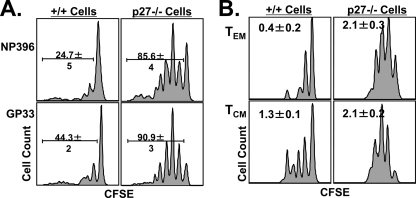
The maintenance of CD8 T-cell memory occurs by proliferative renewal, which is characterized by slow cytokine-driven proliferation (36, 44). Proliferative renewal does not result in a net increase in memory CD8 T cells because the cellular proliferation rate is presumably equivalent to the apoptosis rate. To examine the role of p27Kip1 in regulating LCMV-specific memory CD8 T-cell proliferative renewal, we adoptively transferred CFSE-labeled CD8 T cells from LCMV-immune +/+ or −/− mice into congenic Ly5.1 mice. Thirty days after cell transfer, we assessed the proliferative renewal of LCMV-specific memory CD8 T cells by flow cytometry. As expected, 24 to 44% of the wild-type T cells had divided after adoptive transfer, while a strikingly higher proportion of −/− memory CD8 T cells, 85 to 90%, had divided at least once (Fig. 10A). These findings suggest that p27Kip1 downregulates the proliferative renewal of memory CD8 T cells in LCMV-immune mice.
FIG. 10.
p27Kip1 regulates the proliferative renewal of memory CD8 T cells. At day 80 p.i., T cells were purified from the spleens of +/+ and −/− mice, labeled with CFSE, and adoptively transferred into naïve congenic B6/Ly5.1 mice. Four weeks after cell transfer, CFSE dilution in donor LCMV-specific CD8 T cells was assessed by flow cytometry. (A) Proliferative renewal of NP396- and GP33-specific CD8 T cells. The histograms are gated on Db/NP396 or Db/GP33 tetramer-binding Ly5.2-positive CD8 T cells. (B) Proliferative renewal of central memory (CM) and effector memory (EM) CD8 T cells. The histograms are gated on Db/NP396 tetramer-binding Ly5.2-positive CD8 T cells. Effector memory (CD62Llow) and central memory (CD62Lhigh) subsets were identified based on cell surface expression of CD62L.
The population of memory CD8 T cells is currently classified into central and effector memory cells based on their differential expression of CD62L (18). Although both central (CD62Lhigh) and effector (CD62Llow) memory T cells are maintained by proliferative renewal, the turnover rate for effector cells is lower (18) for unknown reasons. Therefore, we investigated whether p27Kip1 deficiency affected the turnover of the central and effector memory T-cell subsets in LCMV-immune mice. In agreement with published findings (18), the data shown in Fig. 10B indicated that the proliferation of effector memory cells was lower than that of central memory cells in both wild-type and −/− mice. However, the proliferation of both memory CD8 T-cell subsets in −/− mice was higher than that of the subsets in the +/+ mice. Calculations assessing the average number of cell divisions for effector and central memory cells in +/+ and −/− mice (Fig. 10B) revealed that p27Kip1 deficiency had a more prominent effect on effector memory proliferation (0.4 cell divisions for +/+ versus 2.1 divisions for −/− mice) than on central memory cell proliferation (1.3 divisions for +/+ versus 2.0 divisions for −/− mice). Thus, p27Kip1 deficiency increased the proliferation of effector and central memory CD8 T cells by 4- and <2-fold, respectively. These data implicated p27Kip1 as a factor that restrains proliferative renewal of memory CD8 T cells. Although p27Kip1 deficiency augmented the proliferative responses of effector memory CD8 T cells to homeostatic cytokines in vivo, the proliferation rate of these cells was still lower than that for the central memory CD8 T cells in the −/− mice. These data suggested a role for other cell cycle regulatory mechanism(s) in inhibiting the proliferative renewal of effector memory CD8 T cells.
DISCUSSION
Typically, CD8 T-cell responses have been divided into three distinct phases: (i) expansion phase (days 0 to 8 p.i.), when naïve CD8 T cells are activated to undergo clonal expansion and differentiation into effector cells; (ii) the contraction phase (days 8 to 30 p.i.), when ∼90% of the effector T cells are eliminated by apoptosis; and (iii) the memory phase, during which a relatively stable number of memory CD8 T cells is maintained indefinitely (1, 11, 22). One of the intensely investigated areas of research on CD8 T-cell memory is effector CD8 T cells' decision between apoptosis and memory differentiation. It is currently believed that effector CD8 T cells are subdivided into SLECs and MPECs based on their KLRG-1 and CD127 expression levels (12, 28). The majority of SLECs are considered terminally differentiated and destined for apoptosis while MPECs are not terminally differentiated, retaining the potential to survive and differentiate into memory CD8 T cells. However, we lack critical information about the molecular mechanisms that regulate MPEC number and differentiation into long-lived memory CD8 T cells. Here, we provide genetic evidence that the CKI p27Kip1 plays a nonredundant role in regulating various facets of the CD8 T-cell response in order to downregulate the quantity and quality of CD8 T-cell memory. We demonstrate that p27Kip1 (i) induces cell cycle exit and curtails the programmed antigen-independent proliferation of MPECs, (ii) limits the size of the memory CD8 T-cell population, (iii) inhibits the development of memory CD8 T cells that can produce multiple cytokines, and (iv) negatively regulates the proliferative renewal of effector and central memory CD8 T cells. These findings provide critical insight into the cell cycle regulatory mechanisms that influence CD8 T-cell homeostasis and memory.
The CD8 T-cell response to infections involves an exquisitely programmed sequence of events, including the timely entry of antigen-specific CD8 T cells into the active cell cycle following antigenic stimulation and prompt cell cycle exit following antigen withdrawal. At the conclusion of the clonal expansion phase, effector CD8 T cells exit the cell cycle and either undergo apoptosis (SLECs) or begin to differentiate into long-lived memory cells (MPECs). MPEC differentiation into conventional memory T cells includes reversal of cell cycle arrest and reacquisition of a proliferative response to antigen or homeostatic cytokines (14). As in other models of cell differentiation, it is likely that cell cycle control plays a key role in regulating the terminal differentiation and apoptosis of SLECs as well as MPEC differentiation into memory cells. In vitro studies have demonstrated that p27Kip1 plays an integral role in regulating S-phase entry of T cells in response to signaling via the TCR or cytokine receptors (21, 27, 46), and other studies have demonstrated dynamic changes in p27Kip1 expression during the CD8 T-cell response to LCMV (7).
In the current study, we found that p27Kip1 plays a nonredundant role in controlling the cell cycle exit of effector CD8 T cells at the termination of the expansion phase of the primary T-cell response to LCMV. As expected, p27Kip1 expression was downregulated in CD8 T cells during antigen-driven proliferation although p27Kip1 levels were strongly induced at the termination of the expansion phase (7). The number of LCMV-specific CD8 T cells peaked at day 8 p.i. in the wild-type mice but declined precipitously thereafter, and the CD8 T-cell count continued to increase in the −/− mice after day 8 p.i. until at least day 11 p.i. This continued expansion was probably not due to protracted antigenic stimulation for the following two reasons. First, in both +/+ and −/− mice, infectious LCMV titers were below the detection limit in all tissues tested at day 8 p.i. Second, virus-specific effector CD8 T cells from −/− mice exhibited enhanced proliferation after adoptive transfer into an antigen-free environment. These findings strongly suggest that p27Kip1 constrains the programmed expansion of effector CD8 T cells even after cessation of antigenic stimulation. Based on these observations, we propose that p27Kip1 acts as a timer of cell cycle exit for CD8 T cells after antigen withdrawal, which is consistent with the reported role for p27Kip1 in oligodendrocytes (5, 6). It should be noted that that p27Kip1-deficient effector T cells do not proliferate indefinitely after viral clearance; the majority of effector CD8 T cells exited the cell cycle by day 15 p.i. It is possible that effector CD8 T cells become refractory to regulation by p27Kip1 beyond day 11 p.i. or that they switch to cell cycle regulation by other proteins such as p21Cip1, which is also abundant in T cells (45). In the future, it would be interesting to determine whether p21Cip1 and p27Kip1 play redundant or cooperative roles in regulating CD8 T-cell homeostasis.
Although there was a clear increase in the total number of LCMV epitope-specific CD8 T cells in −/− mice beyond day 8 p.i. compared with the number in wild-type mice, the difference is even more striking when the numbers of MPECs and SLECs are compared between the +/+ and −/− conditions. Our studies show that p27Kip1 deficiency preferentially promoted the expansion of IL-2-producing IL-7Rαhigh KLRG-1low MPECs. This preferential MPEC accumulation was not linked to differences in proliferation between SLECs and MPECs because p27Kip1 deficiency led to increased proliferation of both cell types to the same degree. This result was unexpected because it has been reported that SLECs express higher levels of p27Kip1 protein than MPECs (8), and p27Kip1 deficiency would be expected to have a more prominent effect on SLEC proliferation. Similar enhancement of proliferation of MPECs and SLECs induced by p27Kip1 deficiency suggests that the level of p27Kip1 activity in MPECs might be sufficient to suppress cellular proliferation, and increased abundance of p27Kip1 protein in SLECs might be superfluous to function as a cell cycle inhibitor. Taken together, these findings are suggestive of a more complicated scenario, and the implications for higher p27Kip1 protein expression in SLECs in relationship to regulation of memory CD8 T-cell differentiation by proliferation-independent or -dependent mechanisms warrant further investigation. We hypothesize that SLECs are terminally differentiated and programmed to undergo apoptosis, so it is possible that their increased proliferation will not result in a net increase in SLEC number in −/− mice. In striking contrast, MPECs are programmed for cell survival and exhibit less contraction in wild-type mice, so their proliferation would be expected to inflate the absolute number of MPECs and their descendant memory cells. Consequently, the number of memory CD8 T cells in −/− mice was 5- to 6-fold higher than in the wild-type mice. In summary, identification of cellular factors that (i) mitigate survival of proliferating −/− SLECs and (ii) allow survival and accumulation of −/− MPECs is expected to provide insights into the mechanisms that affect preferential enhancement of MPECs in −/− mice.
Compared to fully differentiated memory CD8 T cells, MPECs that are present among effector CD8 T cells at the peak of the T-cell response possess poor proliferative potential (14). Therefore, MPECs are less effective than differentiated memory CD8 T cells in conferring protection against reinfection (14). Although these memory precursors would eventually regain proliferative potential (16), the time lapse during effector-to-memory transition constitutes a rate-limiting step in the development of effective protective immunity after vaccination. Expression of p27Kip1 appears to be higher in effectors than in memory CD8 T cells (7), and downregulation of p27Kip1 activity could be a critical step in the effector-to-memory transition. Therefore, we hypothesized that the lower proliferative potential of effector cells might be linked to p27Kip1 induction. Our studies show that within 7 days after vaccination with the L. monocytogenes rGP33 strain, antigen-specific −/− effector CD8 T cells exhibited markedly greater secondary expansion than their wild-type counterparts. The increased secondary expansion of −/− GP33-specific effector CD8 T cells in rGP33-immunized mice might be related to (i) larger numbers of MPECs that were present in −/− mice prechallenge or (ii) increased proliferative responses of MPECs and/or SLECs to antigen recall. Further studies are warranted to examine the role of the above-listed mechanisms underlying the enhanced secondary responses in −/− mice. Nevertheless, data presented in this report suggest that p27Kip1 might be responsible, at least in part, for suppressing the proliferative expansion of effector CD8 T cells in response to antigen recall.
Protective immunity depends on both the quantity and the quality of CD8 T-cell memory. Memory CD8 T cells are heterogeneous in their cytokine-producing abilities; for example, all LCMV-specific memory CD8 T cells are known to produce IFN-γ;most also secrete TNF-α, but only ∼20 to 30% secrete IL-2 in addition to these two cytokines. Memory CD8 T cells capable of producing multiple cytokines seem more effective at conferring immunity against reinfection than CD8 T cells producing fewer cytokines (29). Our studies demonstrate that the proportion of triple-cytokine-producing memory CD8 T cells was markedly increased in −/− mice, potentially conferring better protection against reinfection than wild-type memory cells. It is currently unknown how p27Kip1 controls the cytokine-producing ability of memory CD8 T cells. Since the relative proportions of KLRG-1high cells among memory CD8 T cells is reduced in −/− mice, a possibility exists that p27Kip1 might promote the terminal differentiation/senescence of antigen-activated CD8 T cells. Alternatively, p27Kip1 might regulate cytokine production by mechanisms independent of cellular proliferation.
Long-term maintenance of CD8 T-cell memory occurs by IL-7- and/or IL-15-driven proliferation, termed proliferative renewal (36, 44). Central and effector memory CD8 T cells express comparable levels of cell surface receptors for IL-7 and IL-15 but differ in their proliferation rates; that is, the turnover of effector memory cells is lower than that of central memory cells (18). Therefore, it appears that one or more cell-intrinsic mechanisms may suppress the proliferative responses of effector memory T cells to homeostatic cytokines. It has been reported that FOXO3a, a transcriptional factor driving p27Kip1 expression, is more active in effector memory T cells than in central memory T cells (26). We also find that LCMV-specific central memory CD8 T cells contain higher levels of the inactive phosphorylated form of FOXO1/O3 than effector memory CD8 T cells (J. Sullivan and M. Suresh, unpublished data). Therefore, we explored whether the difference in proliferative rates between effector and central memory CD8 T cells is linked to p27Kip1. We found that the turnover rate of both central and effector memory cells in −/− mice was higher than that of the respective subsets in +/+ mice, suggesting a role for p27Kip1 in regulating the proliferative renewal of memory CD8 T cells. Interestingly, the effect of p27Kip1 deficiency on cellular proliferation was more prominent for the effector memory subset than for the central memory cells, which suggests that p27Kip1 is at least partly responsible for the lower turnover rate of effector memory T cells. The differential effect of p27Kip1 deficiency on the proliferation of effector and central memory CD8 T cells might be related to differences in the levels and/or subcellular localization (40) of p27Kip1 protein in central and effector memory CD8 T cells. It should be noted that loss of p27Kip1 alone is insufficient to fully restore the proliferation of effector memory CD8 T cells to levels that are comparable to those of central memory CD8 T cells. Increased expression of mRNA for cell cycle-inhibitory molecules including p19ARF, p21Cip1, and p57Kip2 might contribute to lower proliferative responses of effector memory CD8 T cells to homeostatic cytokines IL-7 and IL-15.
Data presented in this report clearly show that p27Kip1 regulates the homeostasis of antigen-activated CD8 T cells. Preliminary analysis of naïve (CD8+ CD44low)-phenotype CD8 T cells in uninfected and LCMV-infected −/− mice indicates that p27Kip1 might also regulate naïve CD8 T-cell homeostasis, albeit to a lesser degree than antigen-activated CD8 T cells. The percentages of naïve-phenotype CD8 T cells among total CD8 T cells in uninfected −/− mice was comparable to those in uninfected +/+ mice (data not shown). Associated with slightly increased spleen size, the total number of naïve-phenotype CD8 T cells in uninfected −/− mice was ∼1.7-fold higher than in +/+ mice. Likewise, in LCMV-infected mice at days 30 to 34 p.i., the percentages of naïve CD8 T cells among all CD8 T cells were unaffected in −/− mice, but the absolute numbers of these cells were ∼2-fold higher in −/− mice than in +/+ mice. In vivo BrdU incorporation studies between days 8 and 15 or 16 and 22 after LCMV infection showed that the percentages of BrdU-positive naïve-phenotype CD8 T cells in −/− mice were ∼2.4-fold higher than in +/+ mice. This preliminary evidence suggests that p27Kip1 constrains proliferation of naïve CD8 T cells, but more detailed experiments are warranted to decipher the role of p27Kip1 in regulating naïve CD8 T-cell homeostasis.
Does p27Kip1 regulate the homeostasis of effector and memory CD8 T cells by cell-intrinsic mechanisms? Our studies show that effector CD8 T cells purified from spleen of LCMV-infected −/− mice exhibit sustained proliferation following transfer into LCMV-free wild-type hosts. The protracted proliferation of −/− effector CD8 T cells might be due to intrinsic deficiency for p27Kip1 in CD8 T cells and/or be a consequence of conditioning of these cells in a p27Kip1-deficient environment during activation and differentiation into effector cells. Although purified −/− CD8 T cells from uninfected mice proliferate for a longer period than +/+ CD8 T cells following stimulation with anti-CD3 and anti-CD28 antibodies in vitro, more definitive experiments will be necessary to establish whether p27Kip1 controls CD8 T-cell proliferation by cell-intrinsic mechanisms in vivo. Studies of LCMV infection using TCR-transgenic p27Kip1-deficient CD8 T cells and bone marrow-chimeric mice should shed light on this very important issue.
Although significant strides have been made in elucidating the molecular basis of T-cell memory, we do not yet fully understand the mechanisms regulating CD8 T-cell memory size. Both intrinsic and extrinsic pathways of apoptosis appear to regulate SLEC elimination at the end of an immune response. However, the mechanisms controlling the number of MPECs generated during a T-cell response are largely obscure. In this study we provide strong evidence that the CKI p27Kip1 plays a critical role in limiting the size of CD8 T-cell memory by downregulating the clonal burst size of MPECs during a primary T-cell response to LCMV. Moreover, we report that apart from its role in limiting CD8 T-cell memory size, p27Kip1 also negatively regulates qualitative attributes of memory CD8 T cells, including cytokine production and proliferative renewal. The present study has improved our understanding of the mechanisms that regulate CD8 T-cell homeostasis and identifies p27Kip1 as a molecular target for development of strategies to augment vaccine-induced CD8 T-cell memory and protective immunity.
Supplementary Material
Acknowledgments
We thank the flow cytometry facility for sorting cells.
This work was supported by PHS grants AI48785, AI59804, and AI68841 to M. Suresh.
Footnotes
Published ahead of print on 30 August 2010.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science 272:54-60. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canepa, E. T., M. E. Scassa, J. M. Ceruti, M. C. Marazita, A. L. Carcagno, P. F. Sirkin, and M. F. Ogara. 2007. INK4 proteins, a family of mammalian CDK inhibitors with novel biological functions. IUBMB Life 59:419-426. [DOI] [PubMed] [Google Scholar]
- 4.Chien, W. M., S. Rabin, E. Macias, P. L. Miliani de Marval, K. Garrison, J. Orthel, M. Rodriguez-Puebla, and M. L. Fero. 2006. Genetic mosaics reveal both cell-autonomous and cell-nonautonomous function of murine p27Kip1. Proc. Natl. Acad. Sci. U. S. A. 103:4122-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durand, B., M. L. Fero, J. M. Roberts, and M. C. Raff. 1998. p27Kip1 alters the response of cells to mitogen and is part of a cell-intrinsic timer that arrests the cell cycle and initiates differentiation. Curr. Biol. 8:431-440. [DOI] [PubMed] [Google Scholar]
- 6.Durand, B., and M. Raff. 2000. A cell-intrinsic timer that operates during oligodendrocyte development. Bioessays 22:64-71. [DOI] [PubMed] [Google Scholar]
- 7.Grayson, J. M., K. Murali-Krishna, J. D. Altman, and R. Ahmed. 2001. Gene expression in antigen-specific CD8+ T cells during viral infection. J. Immunol. 166:795-799. [DOI] [PubMed] [Google Scholar]
- 8.Hand, T. W., M. Morre, and S. M. Kaech. 2007. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc. Natl. Acad. Sci. U. S. A. 104:11730-11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harbour, J. W., R. X. Luo, A. Dei Santi, A. A. Postigo, and D. C. Dean. 1999. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell 98:859-869. [DOI] [PubMed] [Google Scholar]
- 10.Reference deleted.
- 11.Hou, S., L. Hyland, K. W. Ryan, A. Portner, and P. C. Doherty. 1994. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature 369:652-654. [DOI] [PubMed] [Google Scholar]
- 12.Joshi, N. S., W. Cui, A. Chandele, H. K. Lee, D. R. Urso, J. Hagman, L. Gapin, and S. M. Kaech. 2007. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 27:281-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaech, S. M., and R. Ahmed. 2001. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaech, S. M., S. Hemby, E. Kersh, and R. Ahmed. 2002. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111:837-851. [DOI] [PubMed] [Google Scholar]
- 15.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191-1198. [DOI] [PubMed] [Google Scholar]
- 16.Kaech, S. M., E. J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251-262. [DOI] [PubMed] [Google Scholar]
- 17.Kotturi, M. F., B. Peters, F. Buendia-Laysa, Jr., J. Sidney, C. Oseroff, J. Botten, H. Grey, M. J. Buchmeier, and A. Sette. 2007. The CD8+ T-cell response to lymphocytic choriomeningitis virus involves the L antigen: uncovering new tricks for an old virus. J. Virol. 81:4928-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefrancois, L. 2006. Development, trafficking, and function of memory T-cell subsets. Immunol. Rev. 211:93-103. [DOI] [PubMed] [Google Scholar]
- 19.Masopust, D., K. Murali-Krishna, and R. Ahmed. 2007. Quantitating the magnitude of the lymphocytic choriomeningitis virus-specific CD8 T-cell response: it is even bigger than we thought. J. Virol. 81:2002-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercado, R., S. Vijh, S. E. Allen, K. Kerksiek, I. M. Pilip, and E. G. Pamer. 2000. Early programming of T cell populations responding to bacterial infection. J. Immunol. 165:6833-6839. [DOI] [PubMed] [Google Scholar]
- 21.Mohapatra, S., D. Agrawal, and W. J. Pledger. 2001. p27Kip1 regulates T cell proliferation. J. Biol. Chem. 276:21976-21983. [DOI] [PubMed] [Google Scholar]
- 22.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 23.Nakayama, K., N. Ishida, M. Shirane, A. Inomata, T. Inoue, N. Shishido, I. Horii, D. Y. Loh, and K. Nakayama. 1996. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85:707-720. [DOI] [PubMed] [Google Scholar]
- 24.Nourse, J., E. Firpo, W. M. Flanagan, S. Coats, K. Polyak, M. H. Lee, J. Massague, G. R. Crabtree, and J. M. Roberts. 1994. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature 372:570-573. [DOI] [PubMed] [Google Scholar]
- 25.Polyak, K., J. Y. Kato, M. J. Solomon, C. J. Sherr, J. Massague, J. M. Roberts, and A. Koff. 1994. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 8:9-22. [DOI] [PubMed] [Google Scholar]
- 26.Riou, C., B. Yassine-Diab, J. Van Grevenynghe, R. Somogyi, L. D. Greller, D. Gagnon, S. Gimmig, P. Wilkinson, Y. Shi, M. J. Cameron, R. Campos-Gonzalez, R. S. Balderas, D. Kelvin, R. P. Sekaly, and E. K. Haddad. 2007. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J. Exp. Med. 204:79-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowell, E. A., M. C. Walsh, and A. D. Wells. 2005. Opposing roles for the cyclin-dependent kinase inhibitor p27kip1 in the control of CD4+ T cell proliferation and effector function. J. Immunol. 174:3359-3368. [DOI] [PubMed] [Google Scholar]
- 28.Sarkar, S., V. Kalia, W. N. Haining, B. T. Konieczny, S. Subramaniam, and R. Ahmed. 2008. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J. Exp. Med. 205:625-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seder, R. A., P. A. Darrah, and M. Roederer. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8:247-258. [DOI] [PubMed] [Google Scholar]
- 30.Shen, H., M. K. Slifka, M. Matloubian, E. R. Jensen, R. Ahmed, and J. F. Miller. 1995. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc. Natl. Acad. Sci. U. S. A. 92:3987-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 32.Sherr, C. J., and J. M. Roberts. 1995. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 9:1149-1163. [DOI] [PubMed] [Google Scholar]
- 33.Singh, A., and M. Suresh. 2007. A role for TNF in limiting the duration of CTL effector phase and magnitude of CD8 T cell memory. J. Leukoc. Biol. 82:1201-1211. [DOI] [PubMed] [Google Scholar]
- 34.Sprent, J., and D. F. Tough. 2001. T cell death and memory. Science 293:245-248. [DOI] [PubMed] [Google Scholar]
- 35.Stahl, M., P. F. Dijkers, G. J. Kops, S. M. Lens, P. J. Coffer, B. M. Burgering, and R. H. Medema. 2002. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J. Immunol. 168:5024-5031. [DOI] [PubMed] [Google Scholar]
- 36.Surh, C. D., O. Boyman, J. F. Purton, and J. Sprent. 2006. Homeostasis of memory T cells. Immunol. Rev. 211:154-163. [DOI] [PubMed] [Google Scholar]
- 37.Tourigny, M. R., J. Ursini-Siegel, H. Lee, K. M. Toellner, A. F. Cunningham, D. S. Franklin, S. Ely, M. Chen, X. F. Qin, Y. Xiong, I. C. MacLennan, and S. Chen-Kiang. 2002. CDK inhibitor p18INK4c is required for the generation of functional plasma cells. Immunity 17:179-189. [DOI] [PubMed] [Google Scholar]
- 38.Tsukiyama, T., N. Ishida, M. Shirane, Y. A. Minamishima, S. Hatakeyama, M. Kitagawa, K. Nakayama, and K. Nakayama. 2001. Down-regulation of p27Kip1 expression is required for development and function of T cells. J. Immunol. 166:304-312. [DOI] [PubMed] [Google Scholar]
- 39.van Stipdonk, M. J., E. E. Lemmens, and S. P. Schoenberger. 2001. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2:423-429. [DOI] [PubMed] [Google Scholar]
- 40.Veiga-Fernandes, H., and B. Rocha. 2004. High expression of active CDK6 in the cytoplasm of CD8 memory cells favors rapid division. Nat. Immunol. 5:31-37. [DOI] [PubMed] [Google Scholar]
- 41.Vidal, A., and A. Koff. 2000. Cell-cycle inhibitors: three families united by a common cause. Gene 247:1-15. [DOI] [PubMed] [Google Scholar]
- 42.Wang, X. Z., S. E. Stepp, M. A. Brehm, H. D. Chen, L. K. Selin, and R. M. Welsh. 2003. Virus-specific CD8 T cells in peripheral tissues are more resistant to apoptosis than those in lymphoid organs. Immunity 18:631-642. [DOI] [PubMed] [Google Scholar]
- 43.Whitton, J. L., P. J. Southern, and M. B. Oldstone. 1988. Analyses of the cytotoxic T lymphocyte responses to glycoprotein and nucleoprotein components of lymphocytic choriomeningitis virus. Virology 162:321-327. [DOI] [PubMed] [Google Scholar]
- 44.Williams, M. A., and M. J. Bevan. 2007. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25:171-192. [DOI] [PubMed] [Google Scholar]
- 45.Wolfraim, L. A., and J. J. Letterio. 2005. Cutting edge: p27Kip1 deficiency reduces the requirement for CD28-mediated costimulation in naive CD8+ but not CD4+ T lymphocytes. J. Immunol. 174:2481-2484. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, S., V. A. Lawless, and M. H. Kaplan. 2000. Cytokine-stimulated T lymphocyte proliferation is regulated by p27Kip1. J. Immunol. 165:6270-6277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.