Abstract
Integrin signaling plays critical roles in cell adhesion, spreading, and migration, and it is generally accepted that to regulate these integrin functions accurately, localized actin remodeling is required. However, the molecular mechanisms that control the targeting of actin regulation molecules to the proper sites are unknown. We previously demonstrated that integrin-mediated cell spreading and migration on fibronectin are dependent on the localized activation of phospholipase D (PLD). However, the mechanism underlying PLD activation by integrin is largely unknown. Here we demonstrate that protein kinase Cδ (PKCδ) is required for integrin-mediated PLD signaling. After integrin stimulation, PKCδ is activated and translocated to the edges of lamellipodia, where it colocalizes with PLD2. The abrogation of PKCδ activity inhibited integrin-induced PLD activation and cell spreading. Finally, we show that Thr566 of PLD2 is directly phosphorylated by PKCδ and that PLD2 mutation in this region prevents PLD2 activation, PLD2 translocation to the edge of lamellipodia, Rac translocation, and cell spreading after integrin activation. Together, these results suggest that PKCδ is a primary regulator of integrin-mediated PLD activation via the direct phosphorylation of PLD, which is essential for directing integrin-induced cell spreading.
Integrin-mediated cell adhesion, spreading, and migration, which are essential for cellular differentiation, proliferation, survival, chemotaxis, and wound healing, require cell polarization with an environmental stimulus (32). To regulate these integrin-mediated functions accurately, coordinated and spatial control of localized cytoskeletal rearrangement is required. The key downstream signaling molecules of integrin-mediated actin cytoskeletal rearrangements include small GTPases of the Rho family, such as Rho, Cdc42, and Rac (57, 58). Recently it was suggested that integrin indirectly regulates the recruitment of small G proteins and their localized activation at a specific plasma membrane region called the cholesterol-enriched membrane microdomain. Furthermore, the membrane targeting of these molecules appears to be required for the activation of downstream effectors that induce actin reorganization (8, 9, 48). However, in the absence of integrin signaling, despite the GTP loading status, activated Rac and Cdc42 remain in the cytosol and cannot activate downstream effectors, such as p21-activated kinase (PAK) (8). This regulation of the localization of small GTPases to a specific site is supported by the observation that Rac1 is localized and activated at the leading edges of migrating cells, while Cdc42 is also activated in cellular protrusions and in the peripheral region (33, 51). The differentially localized activation of small GTPases results in coordinated spatially confined signaling leading to cytoskeletal rearrangement, which is critical for the regulation of integrin-mediated cell spreading and directional cell migration.
The hydrolysis of phosphatidylcholine by phospholipase D1 (PLD1) and PLD2 generates the messenger lipid phosphatidic acid (PA) in response to a variety of signals, which include hormones, neurotransmitters, and growth factors (17). It has been shown that PA affects actin cytoskeletal rearrangement and hence lamellipodium extension and integrin-mediated cell spreading as well as migration. PLD activity has been found in detergent-insoluble membrane fractions in which a wide variety of cytoskeletal proteins, such as F-actin, α-actinin, vinculin, paxillin, and talin, were enriched (34). Furthermore, the stimulation of PLD with physiologic and pharmacologic agonists results in its association with actin filaments (34). In addition, actin polymerization and stress fiber formation are tightly coupled to the activation of PLD (14). The formation of lamellipodium structures and membrane ruffles is blocked by PLD inhibition (53, 60), and PLD activity is critical for epithelial cell, leukocyte, and neutrophil adhesion and migration (41, 43, 52). Furthermore, we have previously shown that the activity of PLD is upregulated, and that the activated PLD is translocated to lamellipodia, after integrin activation (3). The PLD product PA acts as a lipid anchor for the membrane translocation of Rac, and this PA-mediated localized activation of Rac is critical for integrin-mediated cell spreading and migration through Rac downstream signaling activation and actin cytoskeleton rearrangement (3). However, the mechanisms that regulate the activation and localization of PLD, which induce the localized downstream activation of integrin signaling, have not been elucidated.
Members of the protein kinase C (PKC) family of serine-threonine kinases are known to play important roles in the transduction of signals from the activation of integrin to cell adhesion and spreading, as well as in cell migration via actin reorganization (11, 25, 61, 66). Several studies have shown that the activities of several PKC isozymes are modulated and are crucially required for integrin-mediated cell spreading and migration. The PKCα, -δ, and -ɛ isotypes were activated and then translocated from the cytosol to the membrane after integrin activation, and inhibition of these PKC isozymes prevented cell spreading (10, 47, 66). In addition, the activation of PKCα, -δ, and -ɛ rescued the spreading of α5 integrin-deficient cells on fibronectin (10), and PKCβΙ mediated platelet cell spreading and migration on fibrinogen (2). It has also been demonstrated that PKCθ activity is involved in endothelial cell migration (65). These results suggest that the kinase activities of diverse members of PKC are involved in the integrin-mediated signaling pathway leading to the actin cytoskeletal rearrangement required for cell spreading and migration. Several PKC substrates are known to influence the actin cytoskeleton directly (42). However, the natures of the isoform-specific functions of PKC members and of their specific downstream effectors for actin cytoskeletal rearrangement induction by integrin signaling remain to be elucidated.
In this study, we found that PKCδ is an upstream modulator of localized PLD activation in the integrin signaling pathway. We demonstrate for the first time that PKCδ activity (not PKCα or PKCɛ activity) is critical for integrin-mediated PLD activation, and we found that PLD2 is phosphorylated at Thr566 by PKCδ in the integrin signaling pathway. Furthermore, we show that this phosphorylation is critical for integrin-mediated targeting of PLD to membrane ruffles, Rac translocation to the membrane, and lamellipodium formation during cell spreading. These findings strongly suggest a bridge between PKCδ and the signaling of actin cytoskeletal rearrangement by the integrin signaling pathway via PLD activation, and they provide a novel molecular mechanism for localized PLD activation via PKCδ phosphorylation, which is critical for the actin cytoskeletal rearrangements required for integrin-mediated cell spreading.
MATERIALS AND METHODS
Materials.
Monoclonal antibodies against PKCδ, PKCα, PKCɛ, and β1 integrin were purchased from BD Transduction Laboratories (Lexington, KY). Monoclonal anti-β-actin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antihemagglutinin (anti-HA), fibronectin (FN), type I collagen, poly-l-lysine (PLL), tetramethyl rhodamine isothiocyanate (TRITC)-conjugated phalloidin, myelin basic protein (MBP), and protein A-Sepharose were purchased from Sigma-Aldrich. Rottlerin and purified PKCδ were purchased from Calbiochem (San Diego, CA). Lipofectamine and Lipofectamine 2000 were purchased from Invitrogen Life Technology (Grand Island, NY). [γ-32P]ATP, 32Pi, and [3H]myristic acid were purchased from Perkin-Elmer Life Science (Boston, MA). A polyclonal anti-pan-PLD antibody was generated as described previously (3).
Cell culture and transfection.
COS-7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum at 37°C in a humidified, CO2-controlled (5%) incubator. For transfection and the transient expression of proteins, cells were transfected using Lipofectamine, as described previously (4). The following plasmids were used to induce overexpression: pcDNA3.1-HA-PKCα, pcDNA3.1-HA-PKCδ, pcDNA3.1-HA-PKCɛ, pcDNA3.1-HA-DN-PKCα, pcDNA3.1-HA-DN-PKCδ, pcDNA3.1-HA-DN-PKCɛ, pEGFP-C1-PLD2, and pcDNA3.1-FLAG-V12-Rac1. To silence PLDs, cells were transfected with synthetic small interfering RNA (siRNA) (50 nM duplex) specific for human PLD2 sequences (nucleotides 704 to 723; 5′-AAGAGGUGGCUGGUGGUGAAG-3′). In order to restore PLD2 expression, three residues of human PLD2 cDNA were replaced (AAGAGATGGCTAGTAGTGAAG) to produce addback mutants of PLD2 that resist siRNA silencing (4). To silence PKCα, PKCδ, or PKCɛ, cells were transfected with synthetic siRNAs (50 nM duplex) specific for human PKCα (5′-UUAUAGGGAUCUGAAGUUA-3′), human PKCδ (5′-CCAUGUAUCCUGAGUGGAA-3′), or human PKCɛ (5′-GUGGAGACCUCAUGUUUCA-3′) (Dharmacon, IL). COS-7 cells were transfected with siRNA by use of the Lipofectamine 2000 transfection reagent (Invitrogen, CA) and were then cultured for an additional 72 h in order to silence PKCα, PKCδ, or PKCɛ. The luciferase GL2 duplex (Dharmacon, IL) was used as a negative control.
Cell suspension and replating assays.
Cell suspension and replating assays were performed as described previously (8). To coat petri dishes with fibronectin or polylysine, petri dishes were first rinsed with a 0.25% gelatin solution and were then coated by drying a 25-μg/ml fibronectin solution or 100 μg/ml polylysine. For replating assays, cells were detached with 0.5% trypsin and 5.4 mM EDTA. Cells were then diluted into a suspension medium (DMEM, 10 mM HEPES [pH 7.4], 0.5 mg/ml fatty-acid-free bovine serum albumin [BSA], 0.5 mg/ml soybean trypsin inhibitor type II), collected by centrifugation (1,000 × g for 3 min), and resuspended in suspension medium. Cells were then incubated in 15-ml or 50-ml conical polypropylene tubes for 3 h at 37°C, with inversion every 5 min to prevent cell clumping. Cells were replated by plating suspended cells on fibronectin- or polylysine-coated plastic petri dishes.
Quantification of cell spreading.
To quantify cell spreading, cells were washed 3 times with phosphate-buffered saline (PBS) and were fixed in 4% formaldehyde. To visualize membrane ruffles, actin filaments were stained with TRITC-labeled phalloidin (0.1 μg/ml) for 30 min. The ratios of spread cells to adherent cells were determined by confocal microscopy. The mean numbers of adherent cells ± standard deviations (SD) per 1.1 mm2 were determined from 10 different images taken from at least two separate experiments in three independent assays. Spreading cells were defined as those cells with a diameter more than twice that of adherent cells on PLL and with lamellipodium structures. The percentage of spreading was calculated as (number of spreading cells/total number of adherent cells) × 100.
In vivo PLD activity assay.
In vivo PLD activities were assayed as described previously (40). In brief, COS-7 cells were first serum starved for 24 h and then loaded with [3H]myristic acid (10 μCi/ml) for 4 h, after which unincorporated [3H]myristic acid was removed by two washes with serum-free DMEM. Cells were detached from culture dishes, maintained in suspension for 3 h, and then replated on fibronectin- or polylysine-coated plastic petri dishes in the presence of 0.4% 1-butanol. Reactions were stopped by removing the medium and adding 0.4 ml of ice-cold methanol to each well. Cells were then scraped into tubes, and 0.4 ml of 1 M NaCl and 0.4 ml of chloroform were added. After vortexing, tubes were centrifuged at 15,000 × g for 1 min; solvents were removed; and residual lipids were separated by thin-layer chromatography on silica gel 60 plates developed with chloroform-methanol-acetic acid (82:9:9 [vol/vol/vol]). The amounts of labeled phosphatidylbutanol (PBt) and total lipids were determined using a Fuji (Tokyo, Japan) BAS 4000 image analyzer. The amounts of PBt formed were expressed as percentages of total 3H-labeled lipids in order to account for differences in cell labeling efficiency.
Purification of recombinant PLD2 from baculovirus-transfected Sf9 cells.
His6-tagged human PLD2 was purified from the detergent extracts of baculovirus-infected Sf9 cells by chelating Sepharose affinity column chromatography, as described previously (40).
Immunoprecipitation.
Cells were harvested and lysed with PLD assay buffer (50 mM HEPES-NaOH [pH 7.3], 3 mM EGTA, 3 mM CaCl2. 3 mM MgCl2, 80 mM KCl) containing 0.5% Triton X-100 (TX-100), 1% cholic acid, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 μg/ml leupeptin, and 5 μg/ml aprotinin. After a brief sonication, lysates were centrifuged at 15,000 × g for 30 min, and cell extracts were incubated with an immobilized anti-pan-PLD antibody overnight. The precipitates were washed five times and were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted.
PKCδ activity assays.
PKCδ activities were determined by measuring the phosphorylation of MBP as a substrate. HA-tagged human PKCδ was transfected into COS-7 cells. Two days later, cells were lysed with lysis buffer containing 1% Triton X-100, immunoprecipitated using a primary antibody against HA at 4°C for 1 h, and precipitated with protein A-agarose at 4°C for 1 h. Immunocomplexes were washed three times with lysis buffer and once with phosphorylation buffer (30 mM Tris-HCl [pH 7.5], 6 mM MgCl2, 0.25 mM EGTA, 0.4 mM CaCl2, 0.1% Triton X-100, 0.12 mM ATP). PKCδ activities in immunoprecipitated fractions were assayed by addition of 20 μl of phosphorylation buffer containing 2 μCi [γ-32P]ATP (5,000 Ci/mmol) and 10 μg MBP. After a 15-min incubation at 37°C, assays were terminated by the addition of sample buffer. The immunoprecipitated proteins were then separated by 10% SDS-PAGE, transferred to nitrocellulose membranes, and exposed to photographic film. The incorporation of radiolabeled phosphate was measured using a Fuji (Tokyo, Japan) BAS 4000 image analyzer.
Phosphorylation of PLD2 in vitro.
One hundred nanograms of purified PLD2 coupled to an anti-PLD antibody on protein A-agarose and 50 ng of purified active PKCδ were incubated in phosphorylation buffer (30 mM Tris-HCl [pH 7.0], 6 mM MgCl2, 0.25 mM EGTA, 0.4 mM CaCl2, 0.1% Triton X-100, 0.12 mM ATP, and 2 μCi of [γ-32P]ATP) for 15 min at 37°C. The reaction was stopped by the addition of sample buffer, and the reaction mixture was electrophoresed through an SDS-polyacrylamide gel, which was then exposed to photographic film. The incorporation of radiolabeled phosphate was measured using a Fuji (Tokyo, Japan) BAS 4000 image analyzer.
Phosphorylation of PLD2 in COS-7 cells.
COS-7 cells were incubated with 5 mCi of 32Pi in 5 ml of phosphate-free DMEM for 5 h at 37°C. After the cells had been washed twice with serum-free medium, they were detached from culture dishes, maintained in suspension for 1 h, and replated on fibronectin- or polylysine-coated plastic. The cells were then washed with ice-cold hypotonic buffer containing phosphatase inhibitors (30 mM NaF, 1 mM Na3VO4, and 30 mM Na4O7P2) and were lysed in 500 μl of hypotonic buffer containing 1% Triton X-100 and phosphatase inhibitors (30 mM NaF, 1 mM Na3VO4, and 30 mM Na4O7P2). After centrifugation (15,000 × g for 15 min), equal amounts of the soluble extract were incubated with 2 μg of an anti-pan-PLD antibody and 30 μl of immobilized protein A. Immunoprecipitated proteins were then separated by 10% SDS-PAGE, transferred to nitrocellulose membranes, and exposed to photographic film. The incorporation of radiolabeled phosphate was measured using a Fuji (Tokyo, Japan) BAS 4000 image analyzer.
Immunocytochemistry.
Immunocytochemistry was performed using a previously reported procedure (4). COS-7 cells were rinsed three times with PBS prewarmed at 37°C and were fixed with 4% (wt/vol) paraformaldehyde for 30 min at 37°C. After a rinse with PBS, cells were blocked with PBS containing 1% goat serum and 0.1% TX-100 for 30 min at room temperature. For the visualization of actin filaments, cells were incubated with TRITC-labeled phalloidin for 40 min at room temperature. For the visualization of HA-PKCδ, cells were incubated with 2 μg/ml of mouse monoclonal antibodies against HA for 2 h at room temperature. After six washes with PBS, cells were incubated with rhodamine-conjugated goat anti-mouse antibodies. After a further six washes with PBS, slides were examined under a confocal microscope (LSM-510 Meta; Zeiss) driven by LSM5 software (Zeiss). The pictures shown were taken with an Apochromat 40×/1.2 W Corr objective (Zeiss).
Measurement of Rac-GTP loading (PBD binding assay).
Cells were washed with PBS and lysed in ice-cold buffer (50 mM Tris [pH 7.6], 0.5 M NaCl, 0.1% SDS, 0.5% deoxycholate, 1% Triton X-100, 0.5 mM MgCl2 0.2 mM sodium vanadate, 1 mM PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin). Lysates were centrifuged at 14,000 × g for 10 min at 4°C. Supernatants were incubated for 30 min at 4°C with 20 μg glutathione-agarose beads coupled to glutathione S-transferase (GST)-p21-binding domain (PBD). Beads were sedimented and were washed four times with 50 mM Tris-HCl (pH 7.6) containing 150 mM NaCl, 1% Triton X-100, and 0.5 mM MgCl2. Bound Rac proteins were detected by Western blotting, and whole-cell lysates were also analyzed for Rac for normalization purposes.
Statistical analysis.
Statistical significance was determined using the Student t test.
RESULTS
PKCδ activity is required for PLD activation and integrin-mediated cell spreading.
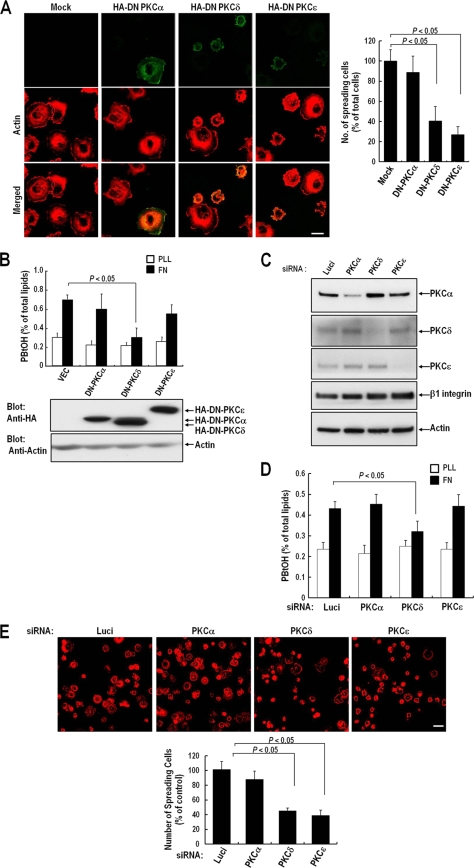
We previously reported that PLD activation is required for integrin-induced cell spreading (3). However, the mechanism of PLD activation remained unclear. PKC is a well-known activator of PLD in various cell types (18, 28, 44, 46). It is also known that PKC isoforms, such as PKCα, -δ, and -ɛ, are involved in the integrin-mediated cell spreading and migration process. To determine whether integrin-mediated PLD activation and cell spreading are PKC dependent and to identify the PKC isoforms involved in integrin-mediated PLD activation, we examined the effects of dominant negative mutants of PKC (DN-PKCs) on fibronectin-induced cell spreading. When detached COS-7 cells were replated onto fibronectin-coated dishes, most adherent cells spread rapidly and adopted a flat morphology with a lamellipodium structure. Detached and adherent COS-7 cells on PLL generally have similar sizes, i.e., a diameter of about 20 μm, but spreading cells on FN have an average diameter of 50 μm (data not shown). When cells were in this state, the expression of DN-PKCδ and DN-PKCɛ significantly reduced integrin-mediated cell spreading, whereas DN-PKCα expression did not (Fig. 1A). Furthermore, we found that only DN-PKCδ expression significantly reduced integrin-mediated PLD activity; this was not observed for DN-PKCα- or DN-PKCɛ-overexpressing cells (Fig. 1B). To confirm the role of PKCδ in integrin-mediated PLD activation and cell spreading, we used siRNA for PKCδ knockdown (Fig. 1C). RNA interference (RNAi) targeting PKCδ reduced integrin-mediated PLD activation (Fig. 1D). In correlation with the generation of PA, PKCδ knockdown reduced cell spreading, but PKCα had no effect on PLD activation and cell spreading. Moreover, PKCɛ knockdown had no effect on PLD activation but significantly reduced integrin-mediated cell spreading (Fig. 1D and E). These results suggest that PKCδ is an upstream regulator of integrin-mediated PLD activation and PA generation and that the kinase activity of PKCδ is critically required for integrin-mediated cell spreading.
FIG. 1.
Effect of PKC inhibition on integrin-induced PLD activation and cell spreading. (A) Cells were transfected as indicated with either a pcDNA vector, dominant negative HA-PKCα (HA-DN PKCα), HA-DN PKCδ, or HA-DN PKCɛ, as indicated. One day after cDNA transfection, cells were serum starved for 24 h, detached from culture dishes, maintained in suspension for 3 h, and replated on FN for 20 min. (Left) After removal of unbound cells, the cells were fixed and stained with TRITC-labeled phalloidin or with an anti-HA monoclonal antibody in order to detect either actin or DN-PKC expression. Bars, 20 μm. (Right) Graph showing the mean numbers of spreading cells among 200 to 250 transfected cells per group. Data are means; error bars, SD (n = 3). (B) Cells were treated as for panel A. PLD activities were measured in cells replated on FN or PLL for 20 min. Data are means; error bars, SD (n = 3). (C) Cells were transfected as indicated with siRNA for PKCα, PKCδ, or PKCɛ, or with luciferase siRNA as a control. Two days after transfection, cells were harvested, and the expression levels of PKCα, PKCδ, PKCɛ, and β1 integrin were analyzed by Western blotting using antibodies directed against each protein. Actin was used as a loading control for Western blotting. The results shown are representative of three independent experiments. (D) Cells were treated as for panel C. PLD activities were measured in cells replated on FN or PLL for 20 min. Data are means; error bars, SD (n = 3). (E) Cells were treated as for panel C. Cells were detached from culture dishes, maintained in suspension for 3 h, and replated on FN for 20 min. (Top) After removal of unbound cells, cells were fixed and stained with TRITC-labeled phalloidin. Bars, 50 μm. (Bottom) Graph showing the quantification of spreading cells. Data are means; error bars, SD (n = 3).
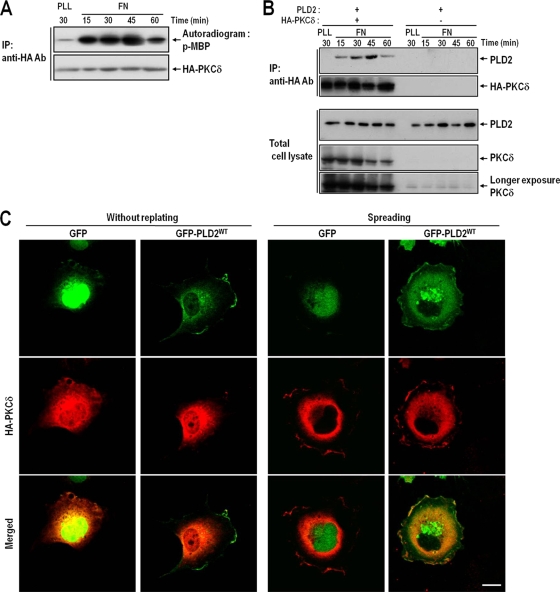
PKCδ is activated and interacts with PLD after integrin activation.
To determine whether PKCδ was activated during integrin-mediated signaling, we performed kinase assays with immunoprecipitated PKCδ at various times after cells were replated onto fibronectin-coated dishes. The results showed that PKCδ was rapidly activated by integrin activation, within 15 min of plating. This coincided with cell spreading, which reached a maximum at 30 to 45 min and slowly decreased from 1 h after integrin activation (Fig. 2A). One important aspect of PLD activation by PKCδ is their interaction and the phosphorylation of PLD, and it has been reported that the association between PLD and PKCδ is enhanced in the presence of phorbol 12-myristate-13 acetate (PMA) (28). To examine the potential involvement of PKCδ in the control of PLD activity, the interaction between PKCδ and PLD2 was examined during integrin-mediated cell spreading, because PLD2 is the major PLD isoform in COS-7 cells (3). We found that the temporal pattern of the association between PKCδ and PLD2 paralleled the activity of PKCδ (Fig. 2Β). Furthermore, the association between PKCδ and PLD increased after the start of cell spreading and peaked at 30 to 45 min after integrin activation. We previously showed that when detached cells are replated onto fibronectin-coated dishes, PLD activity increases from 15 to 45 min later (3), and this activation kinetics of PLD parallels PKCδ activity and the interaction between PKCδ and PLD. Furthermore, this activation and the interaction between these two proteins were confirmed by immunocytochemistry in cells exhibiting integrin-mediated spreading. In resting cells, PLD2 was localized in portions of the plasma membrane and perinuclear regions, whereas PKCδ was localized predominantly in the perinuclear region. However, in integrin-mediated spreading cells, both PLD2 and PKCδ translocated and colocalized at the peripheral regions of the lamellipodia 30 min after cell replating (Fig. 2C). Although in the resting state, PLD2 localized in the plasma membrane and perinuclear region while PKCδ localized in the perinuclear region, our pulldown assays indicated that PKCδ does not interact with PLD in the resting state. However, in spreading cells, PKCδ and PLD interacted and colocalized at the edges of lamellipodia (Fig. 2). These results suggested that activated PKCδ interacts with PLD and participates in PLD activation in lamellipodia during integrin-mediated cell spreading.
FIG. 2.
The interaction between PKCδ and PLD2 parallels increases in PKCδ activity. (A) COS-7 cells were transfected with HA-tagged PKCδ. One day after transfection, cells were serum starved for 24 h, detached from culture dishes, maintained in suspension for 3 h, and replated on FN for the indicated times (15, 30, 45, or 60 min). “PLL” represents the control and indicates the PKCδ activities of adherent cells on PLL for 30 min. Cells were then harvested; HA-PKCδ was immunoprecipitated (IP); and kinase activities were assayed as described in Materials and Methods. The results shown are representative of three independent experiments. Ab, antibody. (B) COS-7 cells were cotransfected with PLD2 and either HA-PKCδ or a vector control as indicated. After 24 h of transfection, cells were serum starved for 24 h, detached from culture dishes, maintained in suspension for 3 h, and replated on FN for the indicated times (15, 30, 45, or 60 min). “PLL” represents the control and indicates the amount of interaction between PLD2 and PKCδ in adherent cells on PLL at 30 min. Cells were then harvested, and HA-PKCδ was immunoprecipitated with an anti-HA antibody. The immune complex thus obtained was subjected to SDS-PAGE and immunoblotted with an anti-pan-PLD or anti-PKCδ antibody. The results shown are representative of three independent experiments. (C) COS-7 cells were cotransfected with HA-PKCδ and either a GFP control vector or wild-type GFP-PLD2 (GFP-PLD2WT), as indicated. Cells were serum starved for 24 h, detached from culture dishes, maintained in suspension for 3 h, and then replated on FN for 30 min. The cells were then fixed and stained with an anti-HA monoclonal antibody in order to detect PKCδ expression. Images are single confocal sections (n = 3). Bars, 20 μm.
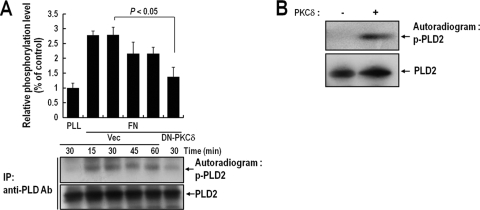
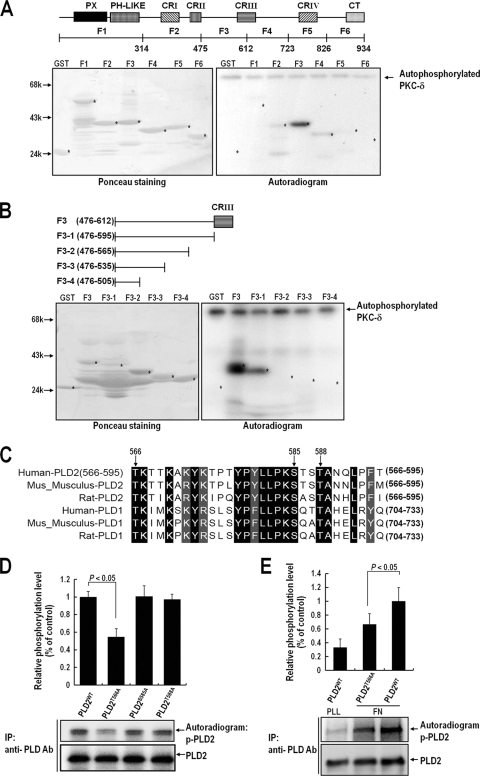
PKCδ phosphorylates Thr566 of PLD2 during integrin-mediated cell spreading.
Because the results concerning the activation status of PKCδ correlated with PLD activation and its subsequent interaction with PLD, we considered the possibility that PKCδ phosphorylates PLD during integrin-mediated signaling. Accordingly, we tested PLD phosphorylation levels during integrin-mediated signaling. As shown in Fig. 3A, we found that PLD2 was significantly phosphorylated when detached cells readhered to FN- but not to PLL-coated plates. Furthermore, the phosphorylation level of PLD2 was inhibited by DN-PKCδ expression. To determine whether PLD2 was directly phosphorylated by PKCδ, we performed an in vitro phosphorylation assay using purified PLD2 and PKCδ in the presence of [γ-32P]ATP. We found that in the presence of active PKCδ, PLD2 was directly phosphorylated (Fig. 3B). To identify the phosphorylation site in PLD, we expressed GST fusion proteins, as shown in Fig. 4A, and performed in vitro phosphorylation assays. We found that the region of PLD2 from amino acid 476 to amino acid 612 was most potently phosphorylated by PKCδ. To locate the phosphorylation site more precisely, we generated serial deletion constructs of the amino acid 476-to-612 region. As shown in Fig. 4B, the region from amino acid 566 to amino acid 595 was found to be essential for the phosphorylation of PLD by PKCδ. Previously, we showed that both PLD1 and PLD2 are upregulated and translocated to the peripheries of lamellipodia during integrin-mediated cell spreading (3). In this integrin-mediated pathway, the interactions between PLD1 and PKCδ and between PLD2 and PKCδ were enhanced (data not shown), suggesting that PLD1 and PLD2 are commonly regulated by PKCδ during integrin-mediated signaling. There are three serine/threonine residues between amino acids 566 and 595 of PLD2, as shown by alignments of human, rat, and mouse sequences of PLD1 and PLD2 (Fig. 4C). To identify the sites of PKCδ-induced phosphorylation, three PLD2 residues were replaced with alanine (T566A, S585A, and T588A). In vitro kinase analysis with purified PLD2 mutants and PKCδ revealed that the Thr566 residue of PLD2 is important for phosphorylation by PKCδ (Fig. 4D). Furthermore, the T566A mutant showed significantly reduced integrin-mediated PLD2 phosphorylation (Fig. 4E). Based on these results, we concluded that PKCδ directly phosphorylates Thr566 of PLD2 during integrin signaling.
FIG. 3.
PKCδ phosphorylates PLD2 during integrin-mediated cell spreading. (A) COS-7 cells were cotransfected with PLD2 and either a vector control (Vec) or DN-PKCδ. One day after transfection, cells were serum starved for 20 h; then they were loaded with 32Pi (1 mCi/ml) for 4 h. Cells were then detached from culture dishes and were maintained in suspension for 3 h. They were then replated on FN or PLL for the indicated times (15, 30, 45, or 60 min). “PLL” represents the control and indicates the phosphorylation level of PLD2 in adherent cells on PLL at 30 min. Cells were harvested, and PLD was immunoprecipitated (IP) with an anti-pan-PLD antibody (Ab). Immunoprecipitated proteins were separated by 8% SDS-PAGE, transferred to nitrocellulose membranes, and exposed to photographic film. The amounts of PLD2 bound to the immune complex were analyzed by immunoblotting with an anti-pan-PLD antibody. The results shown are representative of three independent experiments. (B) A 200 pM concentration of purified PLD2 protein was immobilized with an anti-pan-PLD antibody coupled to protein A resin, and this complex was incubated with 100 pM PKCδ in phosphorylation buffer for 15 min. Reaction mixtures were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and exposed to photographic film. The amounts of PLD2 bound to the immune complex were analyzed by immunoblotting with an anti-pan-PLD antibody. The results shown are representative of three separate experiments.
FIG. 4.
Thr566 of PLD2 is phosphorylated by PKCδ in vivo and in vitro. (A) Equal amounts (1 μg) of purified GST or GST fragments of PLD2 were immobilized on glutathione-Sepharose and incubated with 100 pM PKCδ in phosphorylation buffer for 15 min. (Right) Reaction mixtures were then subjected to SDS-PAGE, transferred to nitrocellulose membranes, and exposed to photographic film. (Left) The amounts of GST fusion proteins on nitrocellulose membranes were quantified by Ponceau staining. The data shown are representative of two independent experiments. (B) Equal amounts (1 μg) of purified GST or GST fragments of the F3 region of PLD2 (amino acids 476 to 612) were immobilized on glutathione-Sepharose and incubated with 100 pM PKCδ in phosphorylation buffer for 15 min. (Right) Reaction mixtures were then subjected to SDS-PAGE, transferred to nitrocellulose membranes, and exposed to photographic film. (Left) The amounts of GST fusion proteins on nitrocellulose membranes were quantified by Ponceau staining. The data shown are representative of two independent experiments. (C) Sequences of human, rat, and mouse PLD1 and PLD2 (all homologous with respect to amino acids 566 to 595 of human PLD2) were aligned using Clustal W software. Identical amino acids are indicated by dark shaded boxes, and homologous amino acids are indicated by light shaded boxes. PLD2 and the other isoforms were found to be identical with respect to the serine/threonine sequence consisting of Thr566, Ser585, and Thr588 between amino acids 566 and 595, as indicated by the arrows. (D) COS-7 cells were transfected with either PLD2WT, PLD2T566A, PLD2S585A, or PLD2T588A. One day after transfection, cells were serum starved for 24 h and were then harvested. PLD was immunoprecipitated (IP) with an anti-pan-PLD antibody (Ab). The immune complex obtained was incubated with purified PKCδ (100 pM) in phosphorylation buffer, and the reaction mixture was then subjected to SDS-PAGE. Blots were transferred to nitrocellulose membranes and were exposed to photographic film. The amounts of PLD2WT and PLD2 mutants bound to the immune complex were quantified by immunoblotting with an anti-pan-PLD antibody. The levels of phosphorylated PLD determined by densitometry were normalized to the levels of precipitated PLD protein. Data are means; error bars, SD (n = 3). (E) COS-7 cells were transfected with PLDWT or PLD2T566A. After 24 h of transfection, cells were serum starved for 20 h, after which they were loaded with 32Pi (1 mCi/ml) for 4 h. After cells were detached from culture dishes, they were maintained in suspension for 3 h. Cells were then replated on FN or PLL for 20 min and were harvested. PLD was immunoprecipitated using an anti-pan-PLD antibody. Immunoprecipitated proteins were subjected to 8% SDS-PAGE, transferred to nitrocellulose membranes, exposed to a phosphorimager plate, and read with an autoradiography imaging system (FLA-5000; Fujifilm). The amounts of PLD2 bound to the immune complex were determined by immunoblotting with an anti-pan-PLD antibody. The levels of phosphorylated PLD determined by densitometry were normalized to the levels of precipitated PLD protein. Data are means; error bars, SD (n = 3).
The phosphorylation of PLD by PKCδ is essential for integrin-mediated cell spreading.
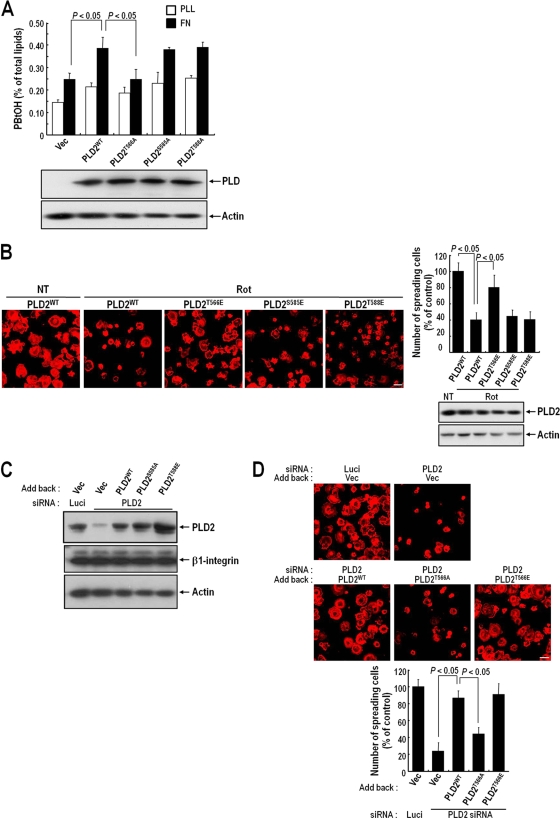
To examine whether phosphorylation at Thr566 of PLD2 by PKCδ affects integrin-mediated PLD activation, we measured the activities of PLD2 mutants in integrin-mediated spreading cells. We found that overexpression of wild-type (WT) PLD2 or its S585A or T588A mutant increased integrin-dependent PLD activity, while overexpression of the T566A mutant did not (Fig. 5A). To examine the significance of PKCδ-mediated PLD2 phosphorylation for integrin-mediated cell spreading, we produced a PLD2 mutant with T566E (the threonine was replaced with glutamic acid), which was expected to mimic the phosphorylated form of PLD2, and then examined the role of phosphorylation in integrin-mediated cell spreading by overexpressing WT PLD2 or the T566E, S585E, or T588E mutant and treating cells with rottlerin. Under these conditions, integrin-mediated cell spreading in the presence of cells overexpressing WT PLD2 or the S585E or T588E mutant was reduced by rottlerin. However, integrin-mediated cell spreading in the presence of T566E overexpression was not affected by rottlerin (Fig. 5B). In addition, PLD RNAi experiments showed that the Thr566 site of PLD2 is important for integrin-mediated cell spreading. In COS-7 cells, RNAi targeting PLD2 reduced cell spreading by >70%. Furthermore, under these conditions, the addition of WT PLD2 or the T566E mutant, but not the T566A mutant, restored integrin-mediated cell spreading (Fig. 5C and D). Taken together, these results indicate that PLD phosphorylation by PKCδ is critically required for integrin-mediated PLD activation and cell spreading.
FIG. 5.
PLD2 activation and cell spreading require the PKCδ-mediated phosphorylation of PLD2 at Thr566. (A) COS-7 cells were transfected with either a vector control (Vec), PLD2WT, PLD2T566A, PLD2S585A, or PLD2T588A. PLD activities were measured in cells replated on FN or PLL for 20 min. Data are means; error bars, SD (n = 3). (B) COS-7 cells were transfected with either PLD2WT, PLD2T566E, PLD2S585E, or PLD2T588E. Cells were serum starved for 24 h, detached from culture dishes, maintained in suspension for 3 h, and pretreated with dimethyl sulfoxide or rottlerin (Rot) (10 μM) for 30 min. (Left) Cells were then replated on FN for 20 min, and after unbounded cells were removed, cells were fixed and stained with TRITC-labeled phalloidin. Bars, 50 μm. (Right) Graph showing the quantification of spreading cells. Data are means; error bars, SD (n = 3). (C) COS-7 cells were transfected as indicated with PLD2 siRNA or with luciferase siRNA as a control. After 24 h of siRNA transfection, cells were transfected with either an empty vector, PLD2WT, PLD2T566A, or PLD2T566E, as indicated. They were then harvested, and the expression levels of PLD2 and β1 integrin were quantified by Western blotting using an anti-pan PLD or anti-β1 integrin antibody. Actin was used as a loading control for Western blotting. The results shown are representative of three independent experiments. (D) Cells treated as described for panel C were serum starved for 24 h, detached from culture dishes, maintained in suspension for 3 h, and then replated on FN for 20 min. (Top) After unbounded cells were removed, cells were fixed and stained with TRITC-labeled phalloidin. Bars, 50 μm. (Bottom) Graph showing the numbers of spreading cells. Data are means; error bars, SD (n = 3).
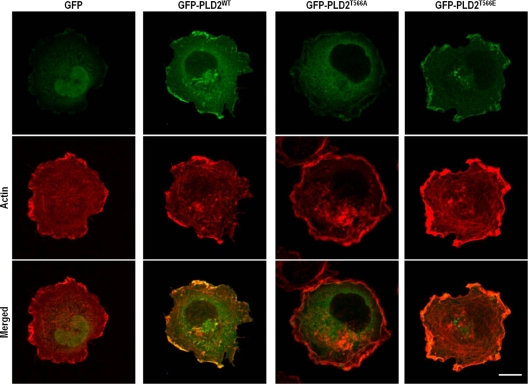
Phosphorylation of PLD by PKCδ is required for PLD localization at lamellipodia in spreading cells.
It has been suggested that integrin activation recruits actin-related molecules that are required for localized actin cytoskeletal rearrangement. Our observations that integrin activation enhanced the association between PKCδ and PLD and the phosphorylation of PLD by PKCδ (Fig. 2 and 4) and that PLD and PKCδ translocated to colocalize at lamellipodia in spreading cells (Fig. 2) suggested that the PKCδ-mediated interaction and phosphorylation of PLD might regulate PLD localization to lamellipodia for localized PLD activation. To examine this, WT green fluorescent protein (GFP)-PLD2 or the GFP-PLD2 T566A or T566E mutant was expressed in COS-7 cells. As shown in Fig. 6, WT GFP-PLD2 or the GFP-PLD2 T566E mutant localized predominantly at the periphery of the lamellipodial region of spreading cells. However, the GFP-PLD2 T566A mutant was not localized to the lamellipodial membrane but was distributed throughout the cytoplasm (Fig. 6). These results suggest that the phosphorylation of PLD by PKCδ is essentially required for PLD localization to the periphery of the lamellipodial region during integrin-mediated spreading.
FIG. 6.
Phosphorylation of PLD2 at Thr566 requires PLD2 localization to the lamellipodia of spreading cells. COS-7 cells were transfected with either a GFP control vector, GFP-PLD2WT, or the GFP-PLD2T566A or GFP-PLD2T566E mutant, as indicated. Cells were then serum starved for 24 h, detached from culture dishes, maintained in suspension for 3 h, and replated on FN. They were then fixed and stained with TRITC-labeled phalloidin. Images are single confocal sections. Bar, 20 μm.
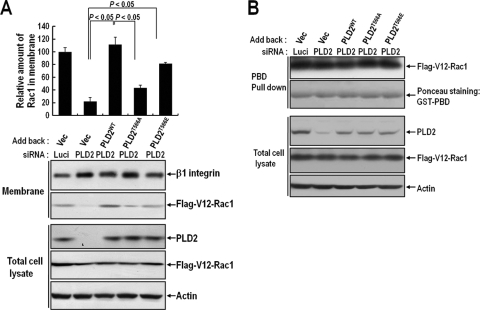
PKCδ is involved in GTP-Rac translocation.
The small G protein Rac is crucial for generating actin-rich lamellipodial protrusions, a key component of cell movement and cell spreading (16). We previously reported that PLD activity is critical for Rac translocation to the membrane in spreading cells (3). In this report, we show that PLD activation in integrin signaling is regulated by PKCδ. To test whether PKCδ is involved in Rac1 membrane targeting through regulation of PLD activity, we examined whether PLD knockdown and phosphorylation mutant expression could regulate Rac1 membrane translocation during the integrin-mediated cell spreading process. We found that an integrin stimulus induced constitutively GTP loaded V12-Rac1 membrane translocation, but PLD knockdown decreased Rac1 membrane translocation. Moreover, the reduced V12-Rac1 membrane translocation due to PLD2 silencing was restored by the addition of WT PLD or the 566E mutant, but not by the PKCδ-mediated phosphorylation-deficient form of the 566A mutant (Fig. 7A). However, PLD phosphorylation did not affect integrin-induced Rac1-GTP loading (Fig. 7B). Together, these results indicate that integrin-mediated PKCδ-PLD signaling, not GTP loading alone, is involved in Rac1 membrane translocation.
FIG. 7.
PKCδ activity regulates GTP-Rac translocation to the membrane. (A) COS-7 cells were transfected as indicated with PLD2 siRNA or with luciferase siRNA as a control. After 24 h of siRNA transfection, cells were cotransfected with Flag-V12-Rac1 and either an empty vector, PLD2WT, PLD2T566A, or PLD2T566E, as indicated. After serum starvation for 24 h, cells were detached from culture dishes, maintained in suspension for 3 h, and replated on PLL or FN for 30 min. Adherent cells were harvested, and particulate membrane fractions were isolated. Samples were analyzed by Western blotting using an anti-pan-PLD or anti-Flag antibody. β1 integrin was used as a membrane fraction marker. The amounts of Rac1 in the membrane determined by densitometry were normalized to the amount of β1 integrin in the membrane. The results shown are representative of three independent experiments. (B) COS-7 cells treated as described for panel A were harvested, and Rac GTP loading was quantified by PBD pulldown assays as described in Materials and Methods.
DISCUSSION
PKCδ has been implicated in the control of cellular spreading and migration. Smooth muscle cells from PKCδ knockout mice have been shown to exhibit reduced actin organization and cell migration (12), and elevated PKCδ expression has been found to increase rat mammary tumor cell spreading and motility (4, 35, 39). Moreover, PKCδ activation promotes muscle cell spreading on fibronectin and rescues cell spreading on fibronectin in α5 integrin-deficient mice (10). However, the detailed mechanism and downstream targets of PKCδ in the process that leads to actin cytoskeletal rearrangement, cell spreading, and migration after integrin signaling are poorly understood. Our results suggest that PLD is a key downstream target of PKCδ during integrin-mediated spreading and demonstrate that the activity and localization of PLD are regulated by PKCδ via direct phosphorylation. In the present study, we found that the abrogation of PKCδ activity inhibited integrin-induced PLD activation (Fig. 1). Furthermore, PKCδ was found to interact transiently with PLD and to colocalize with PLD at membrane peripheries during spreading (Fig. 2 and 6). Finally, PKCδ was found to phosphorylate PLD at Thr566 (Fig. 3), and this phosphorylation was shown to be required for PLD targeting to lamellipodia in the integrin signaling pathway.
Several PKC isozymes have long been considered major activators of PLD, and PKCα-mediated PLD activation is well established. In an in vitro study, PKCα was found to directly activate purified PLD1 (27), and it has also been shown that PKCα activates PLD by interacting with and phosphorylating PLD when induced by several stimuli, including endothelin, thrombin, epidermal growth factor (EGF), insulin, and platelet-derived growth factor (PDGF) (6, 14, 40, 54, 55). PKCδ is also considered a positive regulator of PLD activation. We previously reported that PMA-induced PLD activation and phosphorylation are blocked by inhibition of PKCδ (28). Furthermore, it has been reported that PLD is phosphorylated and activated by PKCδ after stimulation with sphingosine-1-phosphate or bradykinin (20, 45). It was previously reported that PKCα, PKCδ, and PKCɛ are activated during integrin-mediated spreading and migration (10), and in our studies we have observed that integrin also induces PKCα activation in COS-7 cells (data not shown). However, in the present study, only PKCδ, not PKCα or PKCɛ, was found to be involved in integrin-mediated PLD activation (Fig. 1 and 2). This notion was supported by other results. EGF receptor (EGFR) is a well-known, strong signal for PLD activation via a process mediated by PKCα (29, 62). Furthermore, integrin receptor activation has been reported to induce EGFR transactivation without the addition of growth factor (7, 21, 49). However, we found that blockade of EGFR transactivation by AG1478 did not influence PLD activation or cell spreading (data not shown). These results could be explained by different localizations of PKC isotypes during the spreading process. It has also been reported that the specific functions of homologous PKC isozymes are determined by subcellular localization and that upon activation, each PKC isozyme translocates to specific sites. Furthermore, it has been demonstrated that PKCα localizes to focal adhesion sites at the commencement of spreading and that PKCδ localizes at membrane ruffle regions during spreading. Moreover, PKCɛ has been shown to localize to the nucleus, perinuclear regions, and focal adhesion sites (5, 10). In the present study, we found that PLD localized at the periphery of the lamellipodial region and colocalized with PKCδ during spreading (Fig. 3 and 6). These results suggest that different PKC isozymes localize to different sites and that although PKCα, PKCδ, and PKCɛ are activated by integrin signaling and are involved in integrin-mediated cell spreading, only PKCδ is involved in PLD activation.
It has been shown that PLD is phosphorylated by several stimuli through PKCδ and that PKCδ mediation of the threonine phosphorylation level of PLD, not of its serine phosphorylation level, is increased by a sphingosine-1-phosphate stimulus (20, 28). However, no detailed activation mechanism of PLD via PKCδ-dependent phosphorylation has been elucidated, and the location of the phosphorylation site on PLD has not been determined previously. In the present study, we found that threonine at position 566 of PLD2 is phosphorylated by PKCδ during integrin signaling. It is possible that some other phosphorylation regions exist in PLD in the integrin signaling pathway, since the T566A mutant showed only a 60% reduction in phosphorylation, and amino acids 315 to 475 and 613 to 723 of PLD2 were slightly phosphorylated by purified active PKCδ (Fig. 4A). However, it is obvious that Thr566 phosphorylation of PLD is critical for integrin-mediated PLD activation and cell spreading (Fig. 4 and 5). Despite this, PLD2 was not found to be activated by PKCδ-dependent phosphorylation in a previous in vitro study (28). This result suggests that the activation of PLD by PKCδ is conducted in an indirect manner, by a mechanism such as an association between PLD and other activators and/or a localization change. In this study, we demonstrated that PKCδ-dependent phosphorylation influences the localization of PLD to the peripheral regions of lamellipodia (Fig. 6). PLD1 and PLD2 are localized at several cellular sites, including the endoplasmic reticulum (ER), Golgi complex, and plasma membrane (1, 38, 40, 59). Furthermore, it is known that under stimulatory conditions, such as those induced by PMA or EGF, PLD1 and -2 can translocate to the restricted plasma membrane, especially in the caveolin-enriched membrane region, and that these PLD activations are restricted in this region (12, 15, 29). Translocation to the peripheries of lamellipodia could stimulate PLD activity by allowing access to its cofactors, such as phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] and ADP-ribosylation factor (ARF). These proteins are well-known direct activators of both PLD1 and PLD2 (18, 26, 27). Moreover, these proteins are involved in lamellipodium formation and in actin cytoskeleton regulation downstream of integrin signaling (23, 50). PLD has been reported to cotranslocate with phosphatidylinositol 4-phosphate 5-kinase α [PI(4)P5Kα] and ARF to ruffling membranes (31). Cytohesin-2/ARF nucleotide-binding-site opener (ARNO) induces the activation of ARF6, resulting in increased activation of PLD for lamellipodium formation and cell migration (60). Moreover, it has been reported that PMA-mediated PLD activation is blocked by ARF inhibition and is synergistically increased by ARF (63). These results support the notion that PKCδ-mediated PLD phosphorylation may control PLD activity by regulating PLD localization to the lamellipodia, where PLD can be accessed by direct activators such as PI(4,5)P2 and ARF.
The major signaling pathways triggered by integrin activation with respect to the control of actin cytoskeleton organization are those mediated by phospholipids such as PI(4,5)P2 and PI(3,4,5)P3 and by the Rho family of monomeric GTPases (19, 22). In particular, Rac is crucial for generating actin-rich lamellipodial protrusions during cell spreading (16, 36). However, little is known of the detailed mechanism by which PKCδ regulates the actin cytoskeletal structure or of the relationship between PKCδ and Rac or phospholipid in terms of integrin-mediated lamellipodium formation. Our results suggest a link between PKCδ activity and actin rearrangement through the regulation of PLD. In this study, we found that PLD2 silencing and addition of a PLD2 mutant deficient in PKCδ-mediated phosphorylation did not restore integrin-mediated Rac1 translocation to the membrane (Fig. 7). In addition to this relationship with Rac1 regulation, PLD can also control actin dynamics by regulating the local availability of PI(4,5)P2. It has been reported that PA directly interacts with and activates PI(4)P5K to increase the local production of PI(4,5)P2 and that PLD activation is critical to integrin-mediated cell adhesion via PI(4)P5K activation (37, 56). Furthermore, PLD activity-mediated PI(4,5)P2 generation is important for ARNO-induced membrane ruffle formation and cell migration (31, 60). These findings suggest that PKCδ regulates actin cytoskeletal rearrangement through regulation of PLD during integrin signaling. However, for this process to occur, other signaling molecules and actin-related proteins, in addition to PLD, may also be involved. Previously, the possibility that PKCδ activation could directly regulate cytoskeletal organization was supported by the finding that several actin binding and modulating proteins, such as adducin, are PKCδ substrates (5). Furthermore, PKCδ colocalizes with cytoskeletal components in the lamellipodia, suggesting a direct actin regulatory function (24). We found that expression of the 566E mutant rescued the inhibitory effect of rottlerin treatment during cell spreading but that the cell morphology differed from that of normal spreading cells (Fig. 5B). When most COS-7 cells readhered on fibronectin, or upon PLD restoration to PLD knockdown cells, the cells mainly formed lamellipodial structures; however, T566E-expressing cells treated with rottlerin showed lamellipodium- and filopodium-like structures. These results suggest that PKCδ mediated other actin-related downstream molecules and worked cooperatively with PLD in actin cytoskeleton rearrangement during integrin signaling.
Here we demonstrate that PLD activity is regulated by PKCδ during integrin signaling and that this regulation is important for integrin-mediated lamellipodium formation and cell spreading. Our results suggest that PKCδ activity regulates PLD downstream effectors involved in actin cytoskeleton rearrangement. However, PLD has also been reported to be downregulated in integrin signaling, and it has been proposed that PLD plays a negative role in cell spreading and membrane ruffle formation (13, 30, 64). In order to advance understanding of the regulatory mechanism and the roles of integrin-mediated PLD activity, future studies should focus on the signaling pathway between integrin and PLD and should investigate how these regulations are affected and orchestrated by signaling pathways to achieve precise regulation of the actin cytoskeleton during cell spreading and migration.
Acknowledgments
This work was supported by the 21st Frontier Functional Proteomics Project (FPR08B1-300) and the Korean Ministry of Education, Science and Technology. This research was also supported by the World Class University program through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (R31-2008-000-10105-0).
Footnotes
Published ahead of print on 23 August 2010.
REFERENCES
- 1.Brown, F. D., N. Thompson, K. M. Saqib, J. M. Clark, D. Powner, N. T. Thompson, R. Solari, and M. J. Wakelam. 1998. Phospholipase D1 localises to secretory granules and lysosomes and is plasma-membrane translocated on cellular stimulation. Curr. Biol. 8:835-838. [DOI] [PubMed] [Google Scholar]
- 2.Buensuceso, C. S., A. Obergfell, A. Soriani, K. Eto, W. B. Kiosses, E. G. Arias-Salgado, T. Kawakami, and S. J. Shattil. 2005. Regulation of outside-in signaling in platelets by integrin-associated protein kinase Cβ. J. Biol. Chem. 280:644-653. [DOI] [PubMed] [Google Scholar]
- 3.Chae, Y. C., J. H. Kim, K. L. Kim, H. W. Kim, H. Y. Lee, W. D. Heo, T. Meyer, P. G. Suh, and S. H. Ryu. 2008. Phospholipase D activity regulates integrin-mediated cell spreading and migration by inducing GTP-Rac translocation to the plasma membrane. Mol. Biol. Cell 19:3111-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chae, Y. C., S. Lee, H. Y. Lee, K. Heo, J. H. Kim, J. H. Kim, P. G. Suh, and S. H. Ryu. 2005. Inhibition of muscarinic receptor-linked phospholipase D activation by association with tubulin. J. Biol. Chem. 280:3723-3730. [DOI] [PubMed] [Google Scholar]
- 5.Chen, C. L., Y. T. Hsieh, and H. C. Chen. 2007. Phosphorylation of adducin by protein kinase Cδ promotes cell motility. J. Cell Sci. 120:1157-1167. [DOI] [PubMed] [Google Scholar]
- 6.Chen, J. S., and J. H. Exton. 2004. Regulation of phospholipase D2 activity by protein kinase Cα. J. Biol. Chem. 279:22076-22083. [DOI] [PubMed] [Google Scholar]
- 7.Cybulsky, A. V., A. J. McTavish, and M. D. Cyr. 1994. Extracellular matrix modulates epidermal growth factor receptor activation in rat glomerular epithelial cells. J. Clin. Invest. 94:68-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.del Pozo, M. A., N. B. Alderson, W. B. Kiosses, H. H. Chiang, R. G. Anderson, and M. A. Schwartz. 2004. Integrins regulate Rac targeting by internalization of membrane domains. Science 303:839-842. [DOI] [PubMed] [Google Scholar]
- 9.Del Pozo, M. A., W. B. Kiosses, N. B. Alderson, N. Meller, K. M. Hahn, and M. A. Schwartz. 2002. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat. Cell Biol. 4:232-239. [DOI] [PubMed] [Google Scholar]
- 10.Disatnik, M. H., S. C. Boutet, C. H. Lee, D. Mochly-Rosen, and T. A. Rando. 2002. Sequential activation of individual PKC isozymes in integrin-mediated muscle cell spreading: a role for MARCKS in an integrin signaling pathway. J. Cell Sci. 115:2151-2163. [DOI] [PubMed] [Google Scholar]
- 11.Disatnik, M. H., and T. A. Rando. 1999. Integrin-mediated muscle cell spreading. The role of protein kinase C in outside-in and inside-out signaling and evidence of integrin cross-talk. J. Biol. Chem. 274:32486-32492. [DOI] [PubMed] [Google Scholar]
- 12.Du, G., Y. M. Altshuller, N. Vitale, P. Huang, S. Chasserot-Golaz, A. J. Morris, M. F. Bader, and M. A. Frohman. 2003. Regulation of phospholipase D1 subcellular cycling through coordination of multiple membrane association motifs. J. Cell Biol. 162:305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du, G., and M. A. Frohman. 2009. A lipid-signaled myosin phosphatase surge disperses cortical contractile force early in cell spreading. Mol. Biol. Cell 20:200-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eldar, H., P. Ben-Av, U. S. Schmidt, E. Livneh, and M. Liscovitch. 1993. Up-regulation of phospholipase D activity induced by overexpression of protein kinase C-α. Studies in intact Swiss/3T3 cells and in detergent-solubilized membranes in vitro. J. Biol. Chem. 268:12560-12564. [PubMed] [Google Scholar]
- 15.Emoto, M., J. K. Klarlund, S. B. Waters, V. Hu, J. M. Buxton, A. Chawla, and M. P. Czech. 2000. A role for phospholipase D in GLUT4 glucose transporter translocation. J. Biol. Chem. 275:7144-7151. [DOI] [PubMed] [Google Scholar]
- 16.Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature 420:629-635. [DOI] [PubMed] [Google Scholar]
- 17.Exton, J. H. 1999. Regulation of phospholipase D. Biochim. Biophys. Acta 1439:121-133. [DOI] [PubMed] [Google Scholar]
- 18.Frohman, M. A., T. C. Sung, and A. J. Morris. 1999. Mammalian phospholipase D structure and regulation. Biochim. Biophys. Acta 1439:175-186. [DOI] [PubMed] [Google Scholar]
- 19.Fukami, K., K. Furuhashi, M. Inagaki, T. Endo, S. Hatano, and T. Takenawa. 1992. Requirement of phosphatidylinositol 4,5-bisphosphate for α-actinin function. Nature 359:150-152. [DOI] [PubMed] [Google Scholar]
- 20.Ghelli, A., A. M. Porcelli, A. Facchini, S. Hrelia, F. Flamigni, and M. Rugolo. 2002. Phospholipase D1 is threonine-phosphorylated in human-airway epithelial cells stimulated by sphingosine-1-phosphate by a mechanism involving Src tyrosine kinase and protein kinase Cδ. Biochem. J. 366:187-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giancotti, F. G., and G. Tarone. 2003. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell Dev. Biol. 19:173-206. [DOI] [PubMed] [Google Scholar]
- 22.Gilmore, A. P., and K. Burridge. 1996. Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4-5-bisphosphate. Nature 381:531-535. [DOI] [PubMed] [Google Scholar]
- 23.Golub, T., and C. Pico. 2005. Spatial control of actin-based motility through plasmalemmal PtdIns(4,5)P2-rich raft assemblies. Biochem. Soc. Symp. 2005:119-127. [DOI] [PubMed] [Google Scholar]
- 24.Goodnight, J. A., H. Mischak, W. Kolch, and J. F. Mushinski. 1995. Immunocytochemical localization of eight protein kinase C isozymes overexpressed in NIH 3T3 fibroblasts. Isoform-specific association with microfilaments, Golgi, endoplasmic reticulum, and nuclear and cell membranes. J. Biol. Chem. 270:9991-10001. [DOI] [PubMed] [Google Scholar]
- 25.Haimovich, B., N. Kaneshiki, and P. Ji. 1996. Protein kinase C regulates tyrosine phosphorylation of pp125FAK in platelets adherent to fibrinogen. Blood 87:152-161. [PubMed] [Google Scholar]
- 26.Hammond, S. M., Y. M. Altshuller, T. C. Sung, S. A. Rudge, K. Rose, J. Engebrecht, A. J. Morris, and M. A. Frohman. 1995. Human ADP-ribosylation factor-activated phosphatidylcholine-specific phospholipase D defines a new and highly conserved gene family. J. Biol. Chem. 270:29640-29643. [DOI] [PubMed] [Google Scholar]
- 27.Hammond, S. M., J. M. Jenco, S. Nakashima, K. Cadwallader, Q. Gu, S. Cook, Y. Nozawa, G. D. Prestwich, M. A. Frohman, and A. J. Morris. 1997. Characterization of two alternately spliced forms of phospholipase D1. Activation of the purified enzymes by phosphatidylinositol 4,5-bisphosphate, ADP-ribosylation factor, and Rho family monomeric GTP-binding proteins and protein kinase C-α. J. Biol. Chem. 272:3860-3868. [DOI] [PubMed] [Google Scholar]
- 28.Han, J. M., J. H. Kim, B. D. Lee, S. D. Lee, Y. Kim, Y. W. Jung, S. Lee, W. Cho, M. Ohba, T. Kuroki, P. G. Suh, and S. H. Ryu. 2002. Phosphorylation-dependent regulation of phospholipase D2 by protein kinase Cδ in rat pheochromocytoma PC12 cells. J. Biol. Chem. 277:8290-8297. [DOI] [PubMed] [Google Scholar]
- 29.Han, J. M., Y. Kim, J. S. Lee, C. S. Lee, B. D. Lee, M. Ohba, T. Kuroki, P. G. Suh, and S. H. Ryu. 2002. Localization of phospholipase D1 to caveolin-enriched membrane via palmitoylation: implications for epidermal growth factor signaling. Mol. Biol. Cell 13:3976-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiroyama, M., and J. H. Exton. 2005. Studies of the roles of ADP-ribosylation factors and phospholipase D in phorbol ester-induced membrane ruffling. J. Cell. Physiol. 202:608-622. [DOI] [PubMed] [Google Scholar]
- 31.Honda, A., M. Nogami, T. Yokozeki, M. Yamazaki, H. Nakamura, H. Watanabe, K. Kawamoto, K. Nakayama, A. J. Morris, M. A. Frohman, and Y. Kanaho. 1999. Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell 99:521-532. [DOI] [PubMed] [Google Scholar]
- 32.Hynes, R. O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110:673-687. [DOI] [PubMed] [Google Scholar]
- 33.Itoh, R. E., K. Kurokawa, Y. Ohba, H. Yoshizaki, N. Mochizuki, and M. Matsuda. 2002. Activation of Rac and Cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol. Cell. Biol. 22:6582-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iyer, S. S., and D. J. Kusner. 1999. Association of phospholipase D activity with the detergent-insoluble cytoskeleton of U937 promonocytic leukocytes. J. Biol. Chem. 274:2350-2359. [DOI] [PubMed] [Google Scholar]
- 35.Jackson, D., Y. Zheng, D. Lyo, Y. Shen, K. Nakayama, K. I. Nakayama, M. J. Humphries, M. E. Reyland, and D. A. Foster. 2005. Suppression of cell migration by protein kinase Cδ. Oncogene 24:3067-3072. [DOI] [PubMed] [Google Scholar]
- 36.Janmey, P. A., and U. Lindberg. 2004. Cytoskeletal regulation: rich in lipids. Nat. Rev. Mol. Cell Biol. 5:658-666. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins, G. H., P. L. Fisette, and R. A. Anderson. 1994. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J. Biol. Chem. 269:11547-11554. [PubMed] [Google Scholar]
- 38.Jones, D., C. Morgan, and S. Cockcroft. 1999. Phospholipase D and membrane traffic. Potential roles in regulated exocytosis, membrane delivery and vesicle budding. Biochim. Biophys. Acta 1439:229-244. [DOI] [PubMed] [Google Scholar]
- 39.Kiley, S. C., K. J. Clark, S. K. Duddy, D. R. Welch, and S. Jaken. 1999. Increased protein kinase Cδ in mammary tumor cells: relationship to transformation and metastatic progression. Oncogene 18:6748-6757. [DOI] [PubMed] [Google Scholar]
- 40.Kim, J. H., J. M. Han, S. Lee, Y. Kim, T. G. Lee, J. B. Park, S. D. Lee, P. G. Suh, and S. H. Ryu. 1999. Phospholipase D1 in caveolae: regulation by protein kinase Cα and caveolin-1. Biochemistry 38:3763-3769. [DOI] [PubMed] [Google Scholar]
- 41.Knoepp, S. M., M. S. Chahal, Y. Xie, Z. Zhang, D. J. Brauner, M. A. Hallman, S. A. Robinson, S. Han, M. Imai, S. Tomlinson, and K. E. Meier. 2008. Effects of active and inactive phospholipase D2 on signal transduction, adhesion, migration, invasion, and metastasis in EL4 lymphoma cells. Mol. Pharmacol. 74:574-584. [DOI] [PubMed] [Google Scholar]
- 42.Larsson, C. 2006. Protein kinase C and the regulation of the actin cytoskeleton. Cell. Signal. 18:276-284. [DOI] [PubMed] [Google Scholar]
- 43.Lehman, N., M. Di Fulvio, N. McCray, I. Campos, F. Tabatabaian, and J. Gomez-Cambronero. 2006. Phagocyte cell migration is mediated by phospholipases PLD1 and PLD2. Blood 108:3564-3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDermott, M., M. J. Wakelam, and A. J. Morris. 2004. Phospholipase D. Biochem. Cell Biol. 82:225-253. [DOI] [PubMed] [Google Scholar]
- 45.Meacci, E., F. Nuti, S. Catarzi, V. Vasta, C. Donati, S. Bourgoin, P. Bruni, J. Moss, and M. Vaughan. 2003. Activation of phospholipase D by bradykinin and sphingosine 1-phosphate in A549 human lung adenocarcinoma cells via different GTP-binding proteins and protein kinase Cδ signaling pathways. Biochemistry 42:284-292. [DOI] [PubMed] [Google Scholar]
- 46.Min, D. S., S. K. Park, and J. H. Exton. 1998. Characterization of a rat brain phospholipase D isozyme. J. Biol. Chem. 273:7044-7051. [DOI] [PubMed] [Google Scholar]
- 47.Miranti, C. K., S. Ohno, and J. S. Brugge. 1999. Protein kinase C regulates integrin-induced activation of the extracellular regulated kinase pathway upstream of Shc. J. Biol. Chem. 274:10571-10581. [DOI] [PubMed] [Google Scholar]
- 48.Moissoglu, K., B. M. Slepchenko, N. Meller, A. F. Horwitz, and M. A. Schwartz. 2006. In vivo dynamics of Rac-membrane interactions. Mol. Biol. Cell 17:2770-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moro, L., M. Venturino, C. Bozzo, L. Silengo, F. Altruda, L. Beguinot, G. Tarone, and P. Defilippi. 1998. Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 17:6622-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Myers, K. R., and J. E. Casanova. 2008. Regulation of actin cytoskeleton dynamics by Arf-family GTPases. Trends Cell Biol. 18:184-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nalbant, P., L. Hodgson, V. Kraynov, A. Toutchkine, and K. M. Hahn. 2004. Activation of endogenous Cdc42 visualized in living cells. Science 305:1615-1619. [DOI] [PubMed] [Google Scholar]
- 52.Nishikimi, A., H. Fukuhara, W. Su, T. Hongu, S. Takasuga, H. Mihara, Q. Cao, F. Sanematsu, M. Kanai, H. Hasegawa, Y. Tanaka, M. Shibasaki, Y. Kanaho, T. Sasaki, M. A. Frohman, and Y. Fukui. 2009. Sequential regulation of DOCK2 dynamics by two phospholipids during neutrophil chemotaxis. Science 324:384-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Luanaigh, N., R. Pardo, A. Fensome, V. Allen-Baume, D. Jones, M. R. Holt, and S. Cockcroft. 2002. Continual production of phosphatidic acid by phospholipase D is essential for antigen-stimulated membrane ruffling in cultured mast cells. Mol. Biol. Cell 13:3730-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pachter, J. A., J. K. Pai, R. Mayer-Ezell, J. M. Petrin, E. Dobek, and W. R. Bishop. 1992. Differential regulation of phosphoinositide and phosphatidylcholine hydrolysis by protein kinase C-β1 overexpression. Effects on stimulation by α-thrombin, guanosine 5′-O-(thiotriphosphate), and calcium. J. Biol. Chem. 267:9826-9830. [PubMed] [Google Scholar]
- 55.Pai, J. K., E. A. Dobek, and W. R. Bishop. 1991. Endothelin-1 activates phospholipase D and thymidine incorporation in fibroblasts overexpressing protein kinase Cβ1. Cell Regul. 2:897-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Powner, D. J., R. M. Payne, T. R. Pettitt, M. L. Giudici, R. F. Irvine, and M. J. Wakelam. 2005. Phospholipase D2 stimulates integrin-mediated adhesion via phosphatidylinositol 4-phosphate 5-kinase Iγb. J. Cell Sci. 118:2975-2986. [DOI] [PubMed] [Google Scholar]
- 57.Price, L. S., J. Leng, M. A. Schwartz, and G. M. Bokoch. 1998. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell 9:1863-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ridley, A. J., M. A. Schwartz, K. Burridge, R. A. Firtel, M. H. Ginsberg, G. Borisy, J. T. Parsons, and A. R. Horwitz. 2003. Cell migration: integrating signals from front to back. Science 302:1704-1709. [DOI] [PubMed] [Google Scholar]
- 59.Roth, M. G., K. Bi, N. T. Ktistakis, and S. Yu. 1999. Phospholipase D as an effector for ADP-ribosylation factor in the regulation of vesicular traffic. Chem. Phys. Lipids 98:141-152. [DOI] [PubMed] [Google Scholar]
- 60.Santy, L. C., and J. E. Casanova. 2001. Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J. Cell Biol. 154:599-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schlaepfer, D. D., S. K. Hanks, T. Hunter, and P. van der Geer. 1994. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature 372:786-791. [DOI] [PubMed] [Google Scholar]
- 62.Shen, Y., L. Xu, and D. A. Foster. 2001. Role for phospholipase D in receptor-mediated endocytosis. Mol. Cell. Biol. 21:595-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shome, K., Y. Nie, and G. Romero. 1998. ADP-ribosylation factor proteins mediate agonist-induced activation of phospholipase D. J. Biol. Chem. 273:30836-30841. [DOI] [PubMed] [Google Scholar]
- 64.Su, W., O. Yeku, S. Olepu, A. Genna, J. S. Park, H. Ren, G. Du, M. H. Gelb, A. J. Morris, and M. A. Frohman. 2009. 5-Fluoro-2-indolyl des-chlorohalopemide (FIPI), a phospholipase D pharmacological inhibitor that alters cell spreading and inhibits chemotaxis. Mol. Pharmacol. 75:437-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang, S., K. G. Morgan, C. Parker, and J. A. Ware. 1997. Requirement for protein kinase C θ for cell cycle progression and formation of actin stress fibers and filopodia in vascular endothelial cells. J. Biol. Chem. 272:28704-28711. [DOI] [PubMed] [Google Scholar]
- 66.Vuori, K., and E. Ruoslahti. 1993. Activation of protein kinase C precedes α5β1 integrin-mediated cell spreading on fibronectin. J. Biol. Chem. 268:21459-21462. [PubMed] [Google Scholar]