Abstract
After a meal, insulin suppresses lipolysis through the activation of its downstream kinase, Akt, resulting in the inhibition of protein kinase A (PKA), the main positive effector of lipolysis. During insulin resistance, this process is ineffective, leading to a characteristic dyslipidemia and the worsening of impaired insulin action and obesity. Here, we describe a noncanonical Akt-independent, phosphoinositide-3 kinase (PI3K)-dependent pathway that regulates adipocyte lipolysis using restricted subcellular signaling. This pathway selectively alters the PKA phosphorylation of its major lipid droplet-associated substrate, perilipin. In contrast, the phosphorylation of another PKA substrate, hormone-sensitive lipase (HSL), remains Akt dependent. Furthermore, insulin regulates total PKA activity in an Akt-dependent manner. These findings indicate that localized changes in insulin action are responsible for the differential phosphorylation of PKA substrates. Thus, we identify a pathway by which insulin regulates lipolysis through the spatially compartmentalized modulation of PKA.
The storage and mobilization of nutrients from specialized tissues requires the spatial organization of both signaling functions and energy stores. Nowhere is this more evident than in mammalian adipose tissue, which maintains the most efficient repository for readily available energy. Here, fuel is segregated into lipid droplets, once thought to be inert storehouses but now recognized as complex structures that represent a regulatable adaptation of a ubiquitous organelle (5, 40). The synthesis and maintenance of functional lipid droplets requires numerous proteins, not only fatty acid binding proteins and enzymes of lipid synthesis but also molecules critical to constitutive and specialized membrane protein trafficking (23).
During times of nutritional need, triglycerides within the adipocyte lipid droplet are hydrolyzed into their components, fatty acids, acyl-glycerides, and, ultimately, glycerol. This process, termed lipolysis, is controlled dynamically by multiple hormonal signals that respond to the nutrient status of the organism. During fasting, catecholamines such as norepinephrine stimulate lipolysis via beta-adrenergic receptor activation, promoting adenylyl cyclase activity and the production of cyclic AMP (cAMP) (17). cAMP binds to the regulatory subunits of its major effector, protein kinase A (PKA), triggering the dissociation of these subunits and the subsequent activation of the catalytic subunits (62, 63). PKA is frequently sequestered into multiple parallel, intracellular signaling complexes, though such structures have not been studied in hormone-responsive adipocytes (68). Two targets of activated PKA important for lipolysis are hormone-sensitive lipase (HSL) and perilipin, the major lipid droplet coat protein (17). The phosphorylation of HSL on Ser 559/660 is crucial for its activation and translocation to the lipid droplet, where HSL catalyzes the hydrolysis of diglycerides to monoglycerides (26, 55). Another lipase, adipose triglyceride lipase (ATGL), carries out the initial cleavage of triglycerides to diglycerides and most likely is rate limiting for lipolysis, but it does not appear to be regulated directly via PKA phosphorylation (24, 73). Perilipin under basal conditions acts as a protective barrier against lipase activity; upon stimulation, the phosphorylation of least six PKA consensus sites triggers a conformational change in perilipin, permitting access to the lipid substrates in the droplet, the recruitment of HSL, and possibly the activation of ATGL (7, 8, 21, 41, 46, 58, 60, 61). Perilipin, therefore, possesses dual functions, both blocking lipolysis in the basal state as well as promoting lipolysis upon its phosphorylation (5, 58, 60).
Following the ingestion of a meal, insulin stimulates the uptake of nutrients such as glucose into specialized tissues and also potently inhibits lipolysis in adipocytes (17). Insulin signaling in the adipocyte involves the activation of the insulin receptor tyrosine kinase, the phosphorylation of insulin receptor substrates, the activation of PI3K, and the subsequent production of specific phosphoinositides at the plasma membrane (59). These phosphoinositides then recruit Akt, via its pleckstrin homology domain, to the plasma membrane, where Akt becomes phosphorylated and activated by two upstream kinases. Akt stimulates the translocation of the glucose transporter GLUT4 to the plasma membrane, thereby promoting the uptake of glucose into the cell (2). The mechanism by which insulin inhibits lipolysis has been proposed to involve the reduction of cAMP levels and thus PKA activity. In this model, insulin signaling activates phosphodiesterase 3b (PDE3b) via the Akt-mediated phosphorylation of Ser273 (14, 32). Upon activation by Akt, PDE3b catalyzes the hydrolysis of cAMP to 5′AMP, thereby attenuating PKA activity and lipolysis. Recent studies of PDE3b knockout mice have highlighted the importance of PDE3b activity in the regulation of lipolysis but were uninformative regarding the mechanism of insulin action (12). Adipocytes isolated from these mice exhibit reduced responses to insulin with respect to lipolysis, but it is not clear whether this is due to the loss of the critical target enzyme or a normal mechanism being overwhelmed by supraphysiological concentrations of cAMP (12). Biochemical studies using dominant-inhibitory Akt have demonstrated that Akt can regulate PDE3b activity, and other studies also have suggested that Akt interacts directly with PDE3b, implying a direct connection to lipolysis regulation (1, 32). Nevertheless, the actual requirement for Akt in insulin action with regard to the lipolysis itself has not been demonstrated directly in, for example, genetic loss-of-function experiments.
There now is substantial evidence implicating elevated free fatty acid levels as a consequence of inappropriate lipolysis as a major etiological factor for insulin resistance and type 2 diabetes mellitus (T2DM) (51). Conditions such as obesity and diabetes are characterized by a pathophysiological state in which these tissues become unresponsive to insulin, which contribute to the adverse long-term sequelae of diseases such as T2DM and the metabolic syndrome (4, 44). Thus, understanding in detail the mechanism by which insulin suppresses fat cell lipolysis is critical to identifying the underlying defect in resistant adipose tissue and ultimately developing effective therapeutics. In the present study, we investigated both Akt-dependent and -independent modes of insulin action toward lipolysis. We found the latter to predominate at low, physiological levels of adrenergic stimulation, acting via a pathway dependent on the preferential phosphorylation of downstream PKA substrates.
MATERIALS AND METHODS
Cell culture.
Dulbecco's modified Eagle's medium (DMEM), bovine serum, penicillin-streptomycin solution, sodium pyruvate solution, and l-glutamine were obtained from Invitrogen (Carlsbad, CA). Fetal bovine serum was obtained from Gemini Bioproducts (West Sacramento, CA).
Antibodies.
The pan-Akt, Akt1, phospho-HSL Ser660, phospho-Akt Thr308 and Ser473, phospho-PKA substrate, phospho-Akt substrate, and PKA-C antibodies were obtained from Cell Signaling Technology (Beverly, MA). Akt2 antibody was generated as previously described (11). Tubulin antibody was obtained from Sigma (St. Louis, MO). HSL antibody was obtained from Novus Biologicals (Littleton, CO). Caveolin antibody was obtained from BD Biosciences (San Jose, CA). The perilipin antibody was a gift from Constantine Londos (NIDDK, NIH). AS160 antibody was generated in rabbits using a synthetic peptide sequence (PTNDKAKAGNKP; described in reference 28) through Quality Controlled Biochemicals (Hopkington, MA). Infrared-labeled secondary antibodies were obtained from Rockland Inc. (Gilbertsville, PA). We used the Odyssey infrared imaging system (LICOR Biosciences, Lincoln, NE) for all immunoblotting and quantification.
Buffers.
Lysis buffer contained 25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 1× complete protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany), and 1× phosphatase inhibitor cocktails 1 and 2 (Sigma, St. Louis, MO). Kreb's Ringer phosphate (KRP) contained 136 mM NaCl, 4.7 mM KCl, 10 mM NaPO4, pH 7.4, 0.9 mM MgSO4, and 0.9 mM CaCl2.
Inhibitors.
Akt inhibitor VIII and LY294002 were obtained from EMD Biosciences (San Diego, CA). Wortmannin was obtained from Sigma.
Cell culture.
3T3-L1 fibroblasts and derived cell lines were cultured and differentiated as described previously (70). Akt2lox/lox mice were generated as described previously (11 and R. M. Easton and M. J. Birnbaum, unpublished observations). Akt2lox/lox mouse embryonic fibroblasts (MEFs) were derived from Akt2lox/lox embryos and immortalized using a 3T6 protocol as described previously (2, 64). Cells typically were used 9 to 11 days after differentiation. In all experiments, cells were first serum starved in DMEM-0.2% bovine serum albumin (BSA) for 2 h. Where pretreatment was necessary, cells were pretreated for 30 min prior to the assay.
Generation of recombinant shRNA lentivirus and stable Akt2 knockdown cells.
Oligonucleotides (sense, 5′-phos-GATCCGCCAACCTTGGCTGTTACATTCAAGAGATGTAACAGCCAAGGTTGGCTTTTTG-3′; antisense, 5′-phos-AATTCAAAAAGCCAACCTTGGCTGTTACATCTCTTGAATGTAACAGCCAAGGTTGGCG-3′) were hybridized to yield an short hairpin RNA (shRNA) template targeting the 3′ untranslated region (3′UTR) of murine Akt2 mRNA (67). This template was cloned into the BamHI-EcoRI site of the lentiviral expression vector pSIH-H1-copGFP (System Biosciences, Mountain View, CA). To generate recombinant Akt2 shRNA lentivirus, pSIH-HA-copGFP, pVSV-G, and pCMV delta R8.2 were transiently transfected into HEK-293T cells. Cell-free viral supernatants were harvested 48 and 72 h posttransfection and used to transduce 3T3-L1 preadipocytes in the presence of polybrene (8 μg/ml). Infected preadipocytes were sorted for low and high green fluorescent protein (GFP) fluorescence as a surrogate for Akt2 shRNA expression using a BD FACSvantage cell sorter (BD Biosciences, San Jose, CA). Cell sorting was performed by the Flow Cytometry and Cell Sorting Facility of the University of Pennsylvania.
Retroviral infection of cells.
High-titer retrovirus was generated using BOSC23 cells as described previously (49). Briefly, BOSC23 cells were transfected with pMSCV-PPARγ using Fugene transfection reagent according to the manufacturer's protocol (Roche). Peroxisome proliferator-activated receptor γ (PPARγ) is necessary to allow the differentiation of MEFs into adipocytes. The medium then was changed after overnight incubation. After 24 h, viral supernatants were filtered through a 0.45-μm Whatman filter and used to infect the target cells. The target cells were subjected to two to three rounds of infection and then underwent selection using puromycin.
Adenoviral infection of cells.
Ad-GFP and Ad-Cre viruses were prepared at the University of Pennsylvania Gene Therapy Vector Core. Adenovirus was added to 2.5 ml DMEM at a multiplicity of infection (MOI) of 1,000 for 15 min. Separately, 18 μl of Lipofectamine 2000 reagent (Invitrogen) was added to 2.5 ml of DMEM. The preparations then were mixed together and incubated for an additional 15 min, after which the mixture was added to the target cells for a 3-h incubation. The medium then was changed to 10% FBS-DMEM. Cells were immediately plated to undergo the differentiation protocol.
Glycerol release assay.
Serum-starved cells were washed in KRP and then incubated for 30 min at 37°C in KRP-4% fatty acid-free BSA plus treatment additions. Each treatment condition was performed in duplicate. Aliquots of media were taken to assay for glycerol content using Sigma glycerol reagent according to the manufacturer's protocol. The cells then were washed in cold phosphate-buffered saline (PBS), lysed, and assessed for protein content using a bicinchoninic acid (BCA) kit from Pierce (Rockland, IL). Glycerol release was normalized to cellular protein content. Lysates then were used for immunoblot analysis via the Licor Odyssey system according to the manufacturer's protocol. The quantification of the images was done using the Licor software with median background subtraction. Basal values were normalized to 1.
Fatty acid release assay.
Serum-starved cells were washed in KRP and then incubated for 30 min at 37°C in KRP-4% fatty acid-free BSA plus treatment additions. Each treatment condition was performed in duplicate. Aliquots of media were taken to assay for fatty acids using the Wako NEFA-C kit (Richmond, VA) according to the manufacturer's protocol. The cells then were washed in cold PBS, lysed, and assessed for protein content using a BCA kit from Pierce (Rockland, IL). Fatty acid release was normalized to protein content in each case, and basal values were normalized to 1. Lysates then were used for immunoblot analysis with the Licor Odyssey system. The quantification of the images was done using the Licor software with median background subtraction. Basal values were normalized to 1.
Glucose uptake assay.
For glucose uptake, serum-starved cells were washed in KRP and assayed as described previously, with the following modifications (2). Serum-starved cells were washed in KRP and then incubated for 30 min at 37°C in KRP-2% BSA plus 5 mM glucose and 0.5 μCi/ml [14C]-U-glucose plus treatment additions. Each condition was performed in duplicate. After incubation, cells were washed in cold PBS and lysed in PBS-0.05% SDS. Aliquots of lysates then were counted on a scintillation counter or used for BCA protein assay. Final analysis was normalized to protein content, and the basal value was set to 1.
Immunoprecipitation.
Following glycerol release, cells were lysed and assayed for protein content using a BCA kit. For each condition, 500 μg of protein was incubated with 4 μl of perilipin antibody and 50 μl protein G agarose beads (Invitrogen) for 3 h at 4°C. As a negative control, an equal amount of nonimmune goat serum was used. The beads were spun down and washed three times in lysis buffer, resuspended in Laemmli sample buffer, boiled, loaded onto an SDS-PAGE gel, and subjected to immunoblot analysis using the Licor Odyssey system.
Kinase assay.
3T3-L1 adipocytes were washed in KRP and incubated for 5 min at 37°C in KRP-4% fatty acid-free BSA plus treatment additions. The cells then were rinsed with ice-cold PBS and lysed prior to assay using the PKA activity assay kit from Upstate USA, Inc. (Lake Placid, NY). For each sample, the assay was conducted with three conditions: no Kemptide (to assess endogenous phosphorylation events), with Kemptide (to assess PKA phosphorylation events), and with Kemptide and the inhibitor peptide, PKI (to control for the specificity of the PKA phosphorylation of Kemptide). The samples were spotted onto P81 paper and then counted in a liquid scintillation counter. The final values were calculated by subtracting Kemptide-plus-PKI counts from Kemptide-alone counts. The values also were normalized to protein content as determined by a BCA assay, and the basal value was set to 1.
Primary adipocytes.
Epididymal fat pads were isolated from male Sprague-Dawley rats (200 to 250 g). Fat pads were dissected and placed in modified Krebs Ringer bicarbonate buffer (120 mM NaCl, 4 mM KH2PO4, 1 mM MgSO4, 1 mM CaCl2, 10 mM NaHCO3, 30 mM HEPES) containing 1% bovine serum albumin. Fat pads were minced in 5 ml of Krebs Ringer buffer (two fat pads per vial) and then digested with 20 mg of type I collagenase from Worthington Biochemical Corporation (Lakewood, NJ) for 60 to 75 min at 37°C with gentle agitation. Adipocytes were isolated by filtration through nylon mesh and then washed three times with modified Krebs Ringer buffer and mild centrifugation. Equal volumes of adipocytes were treated with vehicle, 10 μM Akti, or 100 nM wortmannin for 20 min, followed by incubation with 20 nM insulin (or vehicle) for 20 to 25 min at 37°C with mild agitation. Each of these tubes was further divided and stimulated with isoproterenol (at 0 and 10 nM) for 60 min at 37°C in a final volume of 900 μl. Following incubation, the infranatant was removed and glycerol content was analyzed using Sigma glycerol reagent according to the manufacturer's protocol.
Statistical analysis.
Triplicate experiments were analyzed using the two-tailed t test or one-way analysis of variance (ANOVA) with a Newman-Keuls post test. Statistical significance was defined as P < 0.05.
RESULTS
Differential effects of Akt inhibition depend on the strength of lipolytic stimuli.
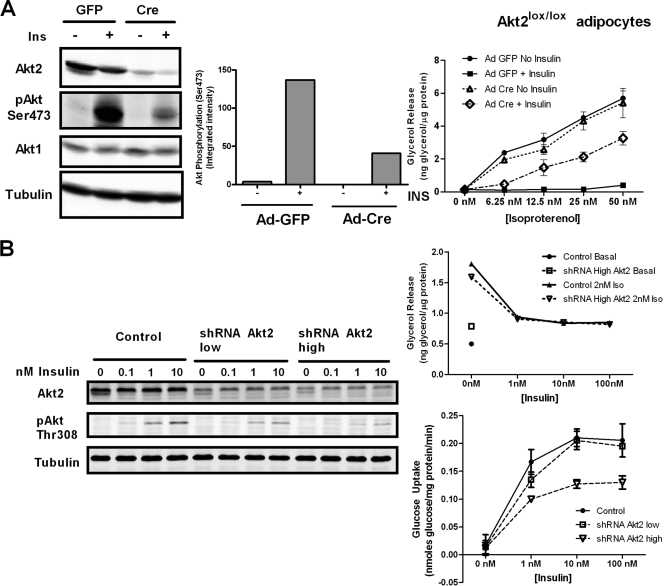
We hypothesized that if Akt were required for insulin action on lipolysis, the inactivation of Akt would reverse the effect of insulin. Using both a genetic approach and small-molecule inhibitors of Akt, we assessed the ability of insulin to inhibit lipolysis when Akt was inactive. To genetically ablate Akt activity, we used peroxisome proliferator-activated receptor γ (PPARγ) to generate adipocytes from spontaneously immortalized mouse fibroblasts from an Akt2 lox/lox embryo. These fibroblasts were infected with adenovirus expressing Cre recombinase (Ad-Cre) to eliminate Akt2, and as a control, the same cells were infected with adenovirus expressing GFP (Ad-GFP). The cells then were immediately stimulated to differentiate and assayed for lipolysis, using glycerol release as an indicator. Akt2 is the predominant isoform of Akt in adipocytes, and thus, after excision by Cre, we expected that most of the Akt in the cell would be absent. We observed a near-complete ablation of Akt2 expression in the adipocytes as well as a substantial reduction in the levels of Akt phosphorylation at Ser473, which is indicative of a strong decrease in the overall Akt activity in the cell (Fig. 1 A). In Ad-GFP-treated cells, insulin inhibited glycerol release at all doses of isoproterenol tested. However, in Ad-Cre-treated cells, the excision of Akt2 partially reversed the effects of insulin on glycerol release in response to isoproterenol at high concentrations (12.5 to 50 nM) but had less effect on inhibition by insulin at a low concentration (6.25 nM) (Fig. 1A). As an independent assessment of independence from Akt, we recapitulated these results using a knockdown model of Akt reduction, in which a lentiviral shRNA construct targeting Akt2 was expressed in 3T3-L1 cells (Fig. 1B). Two levels of shRNA were selected by flow cytometry, using vector-expressed GFP as a surrogate marker, each producing a corresponding knockdown of Akt2 (Fig. 1B). In both cell lines, in spite of a substantial decrease in Akt phosphorylation as well as insulin-stimulated glucose uptake, there was no reduction in the effect of insulin on lipolysis at low concentrations of isoproterenol (Fig. 1B).
FIG. 1.
Differential effects of Akt inhibition at low and high concentrations of isoproterenol. (A) Floxed Akt2 fibroblasts stably expressing PPARγ were infected with either Adeno-GFP or Adeno-Cre and then differentiated into adipocytes. These cells were serum starved and treated with 1 nM insulin and analyzed by immunoblotting using Licor Odyssey for the excision of Akt2 and the loss of phospho-Akt Ser473 signal (left panel; representative of two experiments). The quantification of immunoblot analysis of phospho-Akt Ser473 of Akt2 lox/lox adipocytes infected with Ad-GFP or Ad-Cre is shown (middle panel). A glycerol release assay (right panel) was performed with increasing doses of isoproterenol in the presence and absence of 1 nM insulin for 2 h. Data are expressed as means ± standard deviations (SD) from two experiments performed in duplicate. (B) RNA inteference-mediated reduction in Akt2 does not affect insulin inhibition of glycerol release. 3T3-L1 preadipocytes were infected with an shRNA lentiviral construct that targets Akt2 and sorted for the low and high expression of GFP. Adipocytes were treated with the indicated concentrations of insulin and subjected to the immunoblot analysis of Akt2 and phospho-Akt Thr308, confirming the efficiency of knockdown (left). Highly expressing cells were differentiated into adipocytes, and a glycerol release assay was performed using 2 nM isoproterenol with increasing doses of insulin (upper right). Data are expressed as means ± SD from two experiments performed in duplicate. A glucose uptake assay also was performed on differentiated control, highly, and lowly expressing cells (bottom right).
Akt is not required for insulin-mediated inhibition of lipolysis.
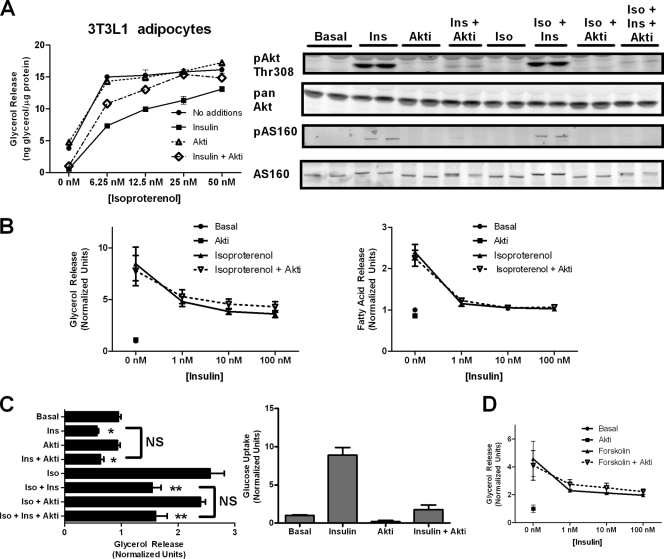
Because of the concern that residual Akt activity remaining in the cell still could mediate the effect of insulin on lipolysis, we also used a small-molecule inhibitor of Akt (Akt VIII; Calbiochem) to produce an ablation in Akt activity in 3T3-L1 adipocytes (Fig. 2 A). As observed with a genetic reduction of Akt2, the pharmacological inhibition of Akt1, Akt2, and, at the concentrations used, Akt3 had differential effects on insulin's ability to suppress lipolysis at high versus low concentrations of isoproterenol (Fig. 2A). Akt inhibitor (Akti) completely reversed insulin's inhibition of lipolysis as stimulated by 25 or 50 nM isoproterenol, partially blocked insulin action at 6.26 or 12.5 nM isoproterenol, and was without effect on basal glycerol release. Under these conditions, Akt inhibitor nearly completely blocked insulin-dependent Akt phosphorylation at Thr308 and reduced to undetectable levels the phosphorylation of its major metabolic substrate, AS160/TBC1D4 (Fig. 2A). Thus, using both genetic and pharmacological approaches, our data suggest that the requirement for Akt in insulin action depends on the level of beta-adrenergic stimulation.
FIG. 2.
Akt inhibition blocks the effect of insulin on glucose transport uptake but not lipolysis. (A) 3T3L1 adipocytes were subjected to a glycerol release assay with increasing doses of isoproterenol in the absence or presence of insulin (1 nM) or Akt inhibitor (5 μM) for 2 h. Data are expressed as means ± standard deviations (SD) from two experiments performed in duplicate. Immunoblots for phospho-Akt Thr308 and phospho-AS160/TBC1D4 using phospho-Akt substrate antibody were performed on cell lysates treated with the indicated conditions, including isoproterenol (2 nM), insulin (25 nM), and Akt inhibitor (20 μM). (B) 3T3-L1 adipocytes were used to generate an insulin dose-response curve of glycerol release (left) and fatty acid release (right) at a low concentration of isoproterenol (2 nM) in the presence and absence of Akt inhibitor (10 μM). Data are expressed as means ± SD from two experiments. (C) Simultaneous glycerol release and glucose uptake assays were performed on cells plated on the same day and cultured side by side with the indicated additions at the following concentrations: isoproterenol (2 nM), insulin (25 nM), and Akt inhibitor (20 μM). Data are expressed as means ± standard errors of the means from three experiments for glycerol release and means ± SD from two experiments for glucose uptake. *, P < 0.05 versus basal levels alone; **, P < 0.05 versus isoproterenol alone; ns, not significant. (D) Glycerol release assay using 1 μM forskolin was performed as described for panel A. Data are expressed as means ± SD from two experiments.
To further address this observation, we examined the dose dependency of insulin action at low concentrations of isoproterenol. At a single submaximal dose of isoproterenol (2 nM), insulin inhibited lipolysis in a concentration-dependent manner, as assayed by either glycerol or fatty acid release (Fig. 2B). Akt inhibitor did not alter the effects of insulin at any of its concentrations (Fig. 2B). As an additional control to ascertain the effectiveness of Akt inhibition, we measured glucose uptake and glycerol release under identical conditions (Fig. 2C). Because Akt is required for insulin-stimulated glucose uptake, we expected that the presence of Akt inhibitor would abrogate the effects of insulin on glucose uptake (2, 11, 20). Indeed, Akt inhibitor blocked insulin-stimulated glucose uptake but had no effect on the inhibition of lipolysis under identical conditions (Fig. 2C). Moreover, insulin reduced both basal- and isoproterenol-stimulated glycerol release in an Akt-independent manner. Insulin also affects PKA activity at the level of the beta-adrenergic receptor by modulating the binding of regulatory proteins (72). To ask whether this was the mechanism of insulin action in these experiments, we treated cells with forskolin, a direct activator of adenylyl cyclase, and observed similar Akt-independent regulation of lipolysis (Fig. 2D). These data indicate that the Akt-independent pathway acts downstream of the beta-adrenergic receptor (Fig. 2D).
Insulin inhibition of lipolysis occurs via a PI3K-dependent signaling pathway.
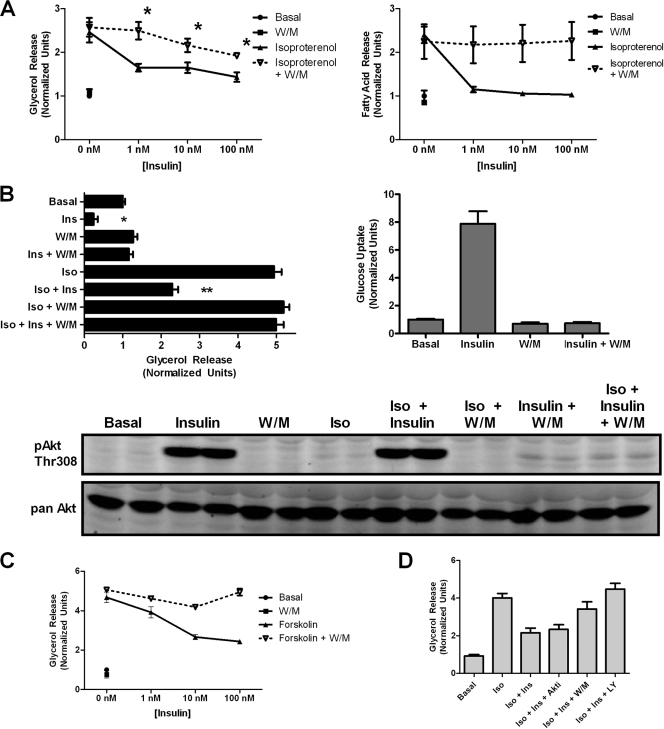
Since PI3K lies upstream of multiple insulin signaling pathways, we asked whether PI3K was required for insulin action toward lipolysis. In contrast to Akt, the PI3K inhibitor wortmannin blocked the effects of insulin on lipolysis as assayed either by glycerol or fatty acid release (Fig. 3 A). Insulin action was PI3K dependent under both basal and isoproterenol-stimulated conditions. The effectiveness of wortmannin as an inhibitor of PI3K was confirmed both by the complete abrogation of insulin-stimulated hexose uptake as well as by the immunoblotting of Akt phosphorylation on Thr308 (Fig. 3B). Note that the level of residual Akt phosphorylation in the presence of wortmannin was comparable to that with Akt inhibitor, even though only the former blocked insulin action on antilipolysis (compare Fig. 2A and 3B). This comparable residual phosphorylation suggests that the minimal Akt activity is unlikely to be responsible for insulin's suppression of lipolysis. Wortmannin blocked insulin's effect on forskolin-stimulated lipolysis as well, ruling out an inhibitory effect at the level of the adrenergic receptor (Fig. 3C). Furthermore, the effect of insulin also was reduced by using another PI3K inhibitor, LY294002 (Fig. 3D). Rapamycin, however, did not have any effect on insulin action (data not shown).
FIG. 3.
PI3K dependence of insulin action on lipolysis and glucose transport. (A) 3T3-L1 adipocytes were serum starved for 2 h and pretreated for 30 min with 100 nM wortmannin (W/M) where indicated. Insulin dose-response curves of glycerol release (left) and fatty acid release (right) were generated in the absence and presence of wortmannin using 2 nM isoproterenol stimulation. Data are expressed as means ± standard errors of the means (SEM) from four experiments for glycerol release and means ± standard deviations (SD) from two experiments for fatty acid release. *, P < 0.05 versus the corresponding point on the isoproterenol-alone graph. (B) Parallel glycerol release and glucose uptake assays were performed on cells plated identically with the indicated additions: isoproterenol (Iso; 2 nM), insulin (Ins; 25 nM), and wortmannin (W/M; 200 nM). Data are expressed as means ± SD from two experiments. Immunoblotting was performed using phospho-Akt Thr308 antibody on cell lysates after a glycerol release assay to confirm the efficacy of wortmannin treatment. (C) Glycerol release assay using 1 μM forskolin stimulation was performed as described for panel A. Data are expressed as means ± SEM from three experiments. (D) Glycerol release assay was performed using the indicated additions: isoproterenol (Iso; 2 nM), insulin (Ins; 25 nM), Akt inhibitor (Akti; 10 μM), wortmannin (W/M; 200 nM), and LY294002 (LY; 25 μM). Data are expressed as means ± SD from two experiments performed in duplicate.
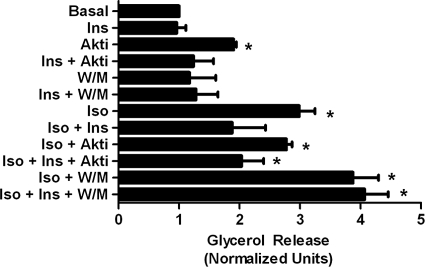
To test the relative potency of PI3K versus Akt inhibitors on blocking insulin's effect on lipolysis more directly, side-by-side comparisons of Akt and PI3K inhibition were performed. As shown in Fig. 4, sufficient Akti or LY294002 was added to 3T3-L1 adipocytes to inhibit Akt, as ascertained by Akt phosphorylation or activity measured in the immune complex. Under conditions in which Akti was as effective or more effective than LY294002 at blocking Akt activity, only the PI3K inhibitor reversed the action of insulin on glycerol release (Fig. 4).
FIG. 4.
Comparison of Akt and PI3K inhibition demonstrates that insulin regulates lipolysis independently of Akt kinase activity. Glycerol release was performed on 3T3-L1 adipocytes using the indicated additions: isoproterenol (Iso; 2 nM), insulin (Ins; 25 nM), Akt inhibitor (Akti; 10 μM), and LY294002 (LY; 50 and 100 μM). Western blotting was performed on whole-cell lysates as shown. Immunoprecipitation was performed using pan-Akt antibody. The immunoprecipitates were subjected to Western blot analysis, and Akt kinase activity was measured using GSK fusion protein. The degree of phosphorylation was assessed using the Licor Odyssey system and graphed as indicated.
Lastly, we ascertained whether the novel resistance of insulin action to Akt inhibition was specific to cultured murine adipocytes or was more generalized. In freshly isolated rat adipocytes, Akt inhibitor alone increased glycerol release from untreated adipocytes or those exposed to isoproterenol (Fig. 5). However, Akt inhibitor was unable to reverse the effects of insulin, as shown above for 3T3-L1 adipocytes. Also consistent with the results in murine cells, wortmannin completely blocked the effects of insulin on isoproterenol-stimulated lipolysis in rat adipocytes.
FIG. 5.
Primary adipocyte analysis demonstrates Akt-independent and PI3K-dependent regulation of lipolysis by insulin. Primary rat adipocytes were isolated and a glycerol release assay was performed using the following conditions: isoproterenol (Iso; 10 nM), insulin (Ins; 20 nM), Akt inhibitor (Akti; 10 μM), and wortmannin (W/M; 100 nM). *, P < 0.05 versus basal values.
Differential regulation of phosphorylation of PKA substrates in response to insulin.
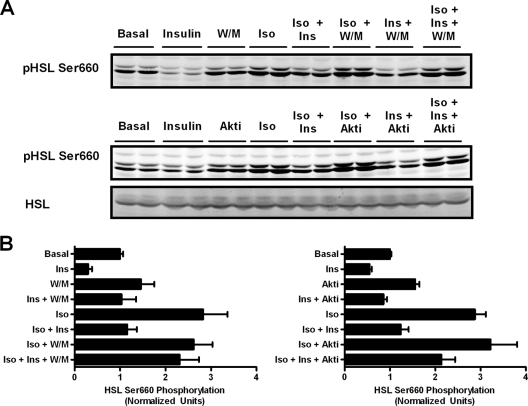
Because the current view holds that insulin signaling inhibits lipolysis by reducing PKA activity, we assessed how treatment with Akt or PI3K inhibitors affected the phosphorylation of known PKA substrates. We first analyzed the phosphorylation of HSL at its major PKA site and observed that wortmannin blocked the inhibitory effect of insulin on isoproterenol-stimulated phosphorylation at Ser660 (Fig. 6 A). In contrast to its lack of effect on glycerol release, the Akt inhibitor partially reversed the inhibition of Ser660 phosphorylation by insulin treatment (Fig. 6A). Data from a series of experiments were quantified and are presented in Fig. 6B.
FIG. 6.
Phosphorylation of HSL is partially dependent on Akt and does not correlate with glycerol release. (A) Following a glycerol release assay as illustrated in Fig. 2 and 3, cells were lysed and lysates were immunoblotted for phospho-HSL Ser660, HSL, and tubulin using the Licor Odyssey system. The doublet represents the two isoforms of HSL expressed in adipocytes (34). (B) The intensities of the lower bands in each lane were quantified and normalized to tubulin and then further normalized to basal values for each experiment. Data are expressed as means ± standard errors of the means from three experiments for Akt inhibitor experiments and means ± standard deviations from two experiments for W/M experiments.
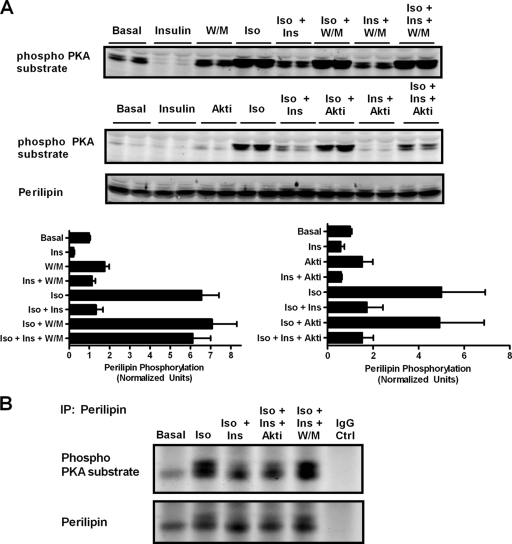
We also assessed the phosphorylation of PKA substrates using an antibody reactive against the conserved PKA phosphorylation site. We observed a prominent, isoproterenol-dependent immunoreactive species with an apparent molecular mass of about 60 kDa (Fig. 7 A). Wortmannin blocked the effect of insulin on the phosphorylation of this protein, whereas the Akt inhibitor was only minimally effective. We suspected that this protein was perilipin, as it has been reported to be the major phosphorylated protein in adipocytes exposed to increases in cAMP (13, 22, 29). To confirm the identity of the protein recognized by the phospho-PKA substrate antibody, we immunoprecipitated perilipin from cell lysates and blotted them with the phospho-PKA substrate antibody. Immunoprecipitated perilipin showed the same response to the various treatments seen in Fig. 7A (Fig. 7B). Thus, these data demonstrate that the inhibition of perilipin phosphorylation by insulin persists in the absence of Akt, but not PI3K, activity, paralleling glycerol release. This contrasts with HSL phosphorylation, which is at least partially sensitive to the inhibition of Akt (Fig. 6A and B).
FIG. 7.
Insulin regulates perilipin phosphorylation independently of Akt and correlates with glycerol release. (A) Following a glycerol release assay as shown in Fig. 2 and 3, lysates were immunoblotted for perilipin, tubulin, and the PKA phospho-substrate antibody; the dominant ∼60-kDa protein (identified as perilipin) is shown. Immunoblots were quantified using Licor Odyssey and normalized to tubulin. Data are expressed as means ± standard errors of the means from three experiments for Akt inhibitor experiments and means ± standard deviations from two experiments for W/M experiments. (B) Perilipin immunoprecipitates from 3T3-L1 adipocytes treated as shown (top) were blotted with phospho-PKA substrate or perilipin antibody as indicated. Additions were isoproterenol (Iso; 2 nM), insulin (Ins; 25 nM), Akt inhibitor (Akti; 10 μM), and wortmannin (W/M; 200 nM).
Regulation of PKA activity in the cytosol and at the lipid droplet by insulin.
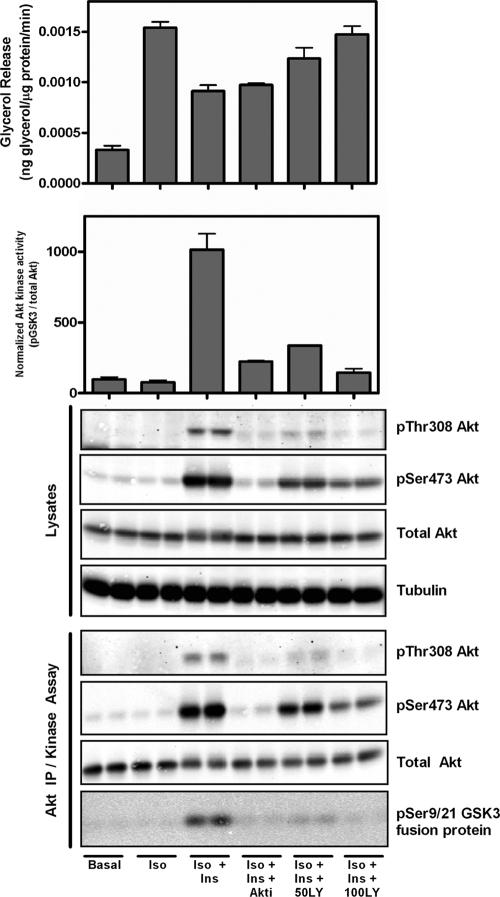
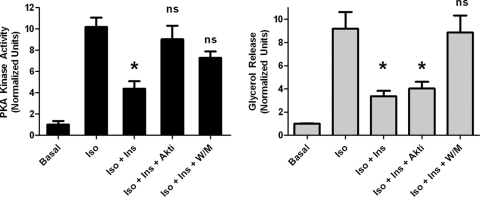
Because the inhibitors of insulin signaling differentially affected PKA substrates, we measured PKA activity in cellular homogenates using an in vitro kinase assay. Treatment with an inhibitor of Akt or PI3K reversed the effect of insulin on PKA activity, but as described above, only wortmannin blocked the effect of insulin on glycerol release (Fig. 8). These results suggest that the effect of insulin on perilipin phosphorylation and lipolysis have occurred in a manner distinct from that on total cellular PKA activity, likely via signaling localized to a distinct compartment, such as the lipid droplet.
FIG. 8.
Lack of correlation between total cellular PKA activity and glycerol release. Duplicate sets of cells were assessed in parallel, one for glycerol release and another for kinase activity under the indicated conditions: isoproterenol (Iso; 6 nM), insulin (Ins; 25 nM), Akt inhibitor (Akti; 10 μM), and wortmannin (W/M; 100 nM) for 5 min. PKA activity was assessed from lysates incubated with and without Kemptide in the presence and absence of PKI. Data are expressed as means ± standard errors of the means from three experiments. *, P < 0.05 versus isoproterenol alone. ns, no significance versus isoproterenol alone.
DISCUSSION
In this study, we have explored the signaling pathways by which insulin suppresses lipolysis in adipocytes, a process critical to the metabolic transition from the fasting to the fed state. There are considerable data implicating a defect in antilipolysis as a critical etiological abnormality initiating the positive amplifying circuit that characterizes insulin resistance (18, 30, 51). Thus, according to this prevailing model, resistance to the suppression of lipolysis by insulin increases extracellular fatty acids and indirectly increases triglycerides, which deposit in tissue, exacerbating the insulin resistance (15, 51). In spite of its importance, the mechanism by which insulin antagonizes adipocyte lipid mobilization has not been established unequivocally, though an attractive model has emerged. There is experimental support for the idea that insulin activates Akt, which phosphorylates PDE3b, thus stimulating the enzyme responsible for the degradation of cAMP (14, 32). The data presented in this report refine and, to some degree, contradict this model, presenting two important conclusions regarding the regulation of lipolysis by insulin. First, under conditions of the submaximal stimulation of lipolysis, insulin antagonizes triglyceride hydrolysis by utilizing a mechanism independent of Akt and thus different from the generally accepted pathway referred to above. This contrasts with the requirement of Akt as an obligate intermediate in the control of most metabolic processes regulated by insulin, most notably glucose transport (16, 50, 66). Second, the insulin-dependent suppression of adipocyte lipolysis occurs independently of the regulation of whole-cell PKA activity while preferentially affecting perilipin phosphorylation, probably through the spatial compartmentalization of signaling pathways. Spatial compartmentalization is a widely used strategy for conferring biological specificity, and the assembly of regulatory complexes by anchoring proteins has been characterized in regard to signaling by cyclic nucleotides (3, 43, 45, 68). However, this is the first indication of such a system for the control of lipolysis and is particularly intriguing as a novel target of insulin action.
Though insulin inhibited lipolysis at all concentrations of isoproterenol tested, the requirement for Akt depended on the degree of beta-adrenergic activation. Submaximal stimulation may more closely approximate conditions that occur within an organism during fasting and feeding. The circulating concentration of norepinephrine is approximately 2 to 10 nM during fasting (25, 27, 57). In rat adipocytes, glycerol release at ∼1 nM isoproterenol is equivalent to that at ∼5 nM norepinephrine (42). Therefore, assuming similar conditions in 3T3-L1 adipocytes, the concentration we used in our analyses (2 nM isoproterenol) would be a close approximation to physiological levels of catecholamine during the fasting state, though admittedly the local concentrations might be considerably higher. Nonetheless, we propose that this Akt-independent pathway is predominant under typical fasting conditions. It is likely that the difference in insulin inhibition at low versus high doses of isoproterenol derives from the nature of the intracellular sequestration of signaling proteins. For example, at higher doses of isoproterenol, the response to insulin appears to be completely Akt dependent, suggesting that a shift from compartmentalized to total cellular signaling pathways confers dependence on the control of cytosolic cAMP by PDE3b. It is likely that at high concentrations of catecholamines, cytosolic cAMP rises to sufficient levels to overwhelm signaling normally restricted to the lipid droplet, and the Akt phosphorylation of PDE3b becomes the dominant means of insulin action. Possibly this pathway comes into play during conditions of extreme stress, when adrenergic stimulation rises to higher levels than typically occur during the fast. Nevertheless, at lower, submaximal levels of stimulation, our data suggest that insulin acts mainly through an Akt-independent pathway.
Our data do not exclude a role for PDE3b in this pathway. Previous studies suggest that PDE3b is required for insulin action under certain conditions. Insulin was not able to inhibit beta-adrenergic-stimulated glycerol release in PDE3b null mice or adipocytes isolated from these animals (12). However, PDE3b-deficient adipocytes exhibit increased levels of glycerol release in response to beta-adrenergic stimulation, and it is likely that supraphysiological levels of cAMP can overwhelm any insulin response dependent on the reduction of PKA-catalyzed phosphorylation, whether or not PDE3b functions as a downstream signaling target. Thus, the phenotype of the PDE3b knockout mice does not exclude a PDE3b-independent pathway in the regulation of adipocyte antilipolysis, nor do our data rule out an Akt-independent modulation of PDE3b. Potential downstream effectors of insulin other than Akt that also may depend on PI3K include atypical protein kinase C (PKC) and serum glucocorticoid kinase (SGK). PKCs have been implicated in insulin-stimulated glucose transport in adipocytes, and perhaps they have additional functional roles in lipolysis regulation (19). The SGK family of kinases is similar in structure to Akt, is also activated by phosphoinositide-dependent kinase-1 (PDK1), and shares common substrates, such as B-raf and FKHR (10, 48, 71). However, the role of SGKs in adipocyte metabolism has not been thoroughly studied.
Another pathway by which PI3K could suppress lipolysis independently of Akt is through the regulation of lipid droplet trafficking by Rab proteins. PI3 kinases have been proposed to interact with Rab proteins and have been implicated in membrane trafficking (31, 53, 54, 65). The proteomic analysis of lipid droplets has identified associated small GTP-binding proteins such as Rab5 and Rab18 (6). In particular, Rab18 is recruited to a subset of lipid droplets in response to beta-adrenergic stimulation, although its role in regulating lipolysis currently is undetermined (38, 39). One possibility is that Rab proteins mediate the interaction between the lipid droplet and other membranes and thus potentially regulates lipid trafficking in the cell (35, 36). Thus, PI3 kinases might also act downstream of the insulin receptor to regulate lipolysis via changes in lipid droplet trafficking.
The stimulation of lipolysis is associated with the PKA-dependent phosphorylation of two critical substrates, HSL and perilipin. HSL phosphorylation in the cytosol leads to its translocation from the cytosol to the lipid droplet, where it acts mainly as a diglyceride lipase. Our data support the notion that HSL phosphorylation is not the sole determinant of lipolysis, as insulin inhibited glycerol release under conditions in which HSL remained phosphorylated at Ser660. A second lipase, ATGL, is responsible for most of the triglyceride lipase activity in adipocytes and is a rate-determining enzyme for lipolysis (24, 73). Although ATGL is not regulated directly by PKA phosphorylation, its activity depends on the phosphorylation state of perilipin at Ser517 (46). The precise mechanism by which phosphorylation triggers ATGL activity is unknown, though it probably involves CGI-58, which can increase ATGL activity by 20-fold (33). CGI-58 binds to perilipin in the basal state and is released upon beta-adrenergic stimulation, presumably allowing it to activate ATGL (21, 56, 69). The PKA phosphorylation of perilipin Ser492 also is critical for lipid droplet dispersion following beta-adrenergic stimulation (37). Other phosphorylation sites of perilipin also may be necessary for achieving maximal lipolysis (47, 52, 58). The data presented herein support an essential role for perilipin phosphorylation in regulating lipolysis, as in all of the experimental manipulations it remains the best correlate of glycerol release. Taken together, these data support a model in which perilipin is the central regulatory hub for lipolytic events in the fat cell.
In conclusion, our data demonstrate a novel, noncanonical insulin signaling pathway that inhibits adipocyte lipolysis. An important implication of this work is that distinct signaling pathways downstream of insulin mediate the control of different metabolic processes, e.g., antilipolysis versus glucose transport. This makes possible in adipose tissue the development of selective insulin resistance during pathological states in which some insulin actions are preserved. Recently, evidence has accumulated for such a phenomenon in the insulin-resistant liver, where function is blunted toward glucose metabolism but preserved toward lipid metabolism (9). Perhaps a similar state occurs within adipose tissue as well during type 2 diabetes mellitus or the metabolic syndrome. The existence of these distinct pathways will undoubtedly influence the approach to the development of treatments that target specific components of the insulin signaling pathway.
Acknowledgments
S.M.C. designed, performed, analyzed, and interpreted all experiments, except for those performed by D.F.T. and D.N.G., and wrote the manuscript. D.F.T. generated the Akt2 shRNA 3T3-L1 cells and designed, performed, and analyzed the glucose uptake assay using these cells. D.F.T. also performed side-by-side comparisons of Akt and PI3K inhibitor with Akt kinase assays and also helped write the manuscript. R.M.E. generated the Akt2lox/lox mice. D.N.G. generated the Akt2lox/lox immortalized cell line, performed the assays in primary adipocytes, and helped write the manuscript. B.R.M. generated the Akt2lox/lox primary cell line. L.M.D. and A.S.D. performed lipid droplet isolations. M.J.B. designed, analyzed, and interpreted the experiments and wrote the manuscript.
We thank Michelle Bland, Russell Miller, and Min Wan for the critical reading of the manuscript and Harry Subramanian for assistance with the lipolysis assays. We also thank Constantine Londos (NIH, NIDDK) for the generous gift of perilipin antibody.
This work was supported by NIH grant R01-DK56886 to M.J.B. and the Penn DERC (NIH grant P30 DK19525), which provides partial support to the Vector Core at the University of Pennsylvania.
Footnotes
Published ahead of print on 23 August 2010.
REFERENCES
- 1.Ahmad, F., R. Lindh, Y. Tang, M. Weston, E. Degerman, and V. C. Manganiello. 2007. Insulin-induced formation of macromolecular complexes involved in activation of cyclic nucleotide phosphodiesterase 3B (PDE3B) and its interaction with PKB. Biochem. J. 404:257-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae, S. S., H. Cho, J. Mu, and M. J. Birnbaum. 2003. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J. Biol. Chem. 278:49530-49536. [DOI] [PubMed] [Google Scholar]
- 3.Beene, D. L., and J. D. Scott. 2007. A-kinase anchoring proteins take shape. Curr. Opin. Cell Biol. 19:192-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouché, C., S. Serdy, C. R. Kahn, and A. B. Goldfine. 2004. The cellular fate of glucose and its relevance in type 2 diabetes. Endocr. Rev. 25:807-830. [DOI] [PubMed] [Google Scholar]
- 5.Brasaemle, D. L. 2007. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 48:2547-2559. [DOI] [PubMed] [Google Scholar]
- 6.Brasaemle, D. L., G. Dolios, L. Shapiro, and R. Wang. 2004. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J. Biol. Chem. 279:46835-46842. [DOI] [PubMed] [Google Scholar]
- 7.Brasaemle, D. L., D. M. Levin, D. C. Adler-Wailes, and C. Londos. 2000. The lipolytic stimulation of 3T3-L1 adipocytes promotes the translocation of hormone-sensitive lipase to the surfaces of lipid storage droplets. Biochim. Biophys. Acta 1483:251-262. [DOI] [PubMed] [Google Scholar]
- 8.Brasaemle, D. L., B. Rubin, I. A. Harten, J. Gruia-Gray, A. R. Kimmel, and C. Londos. 2000. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J. Biol. Chem. 275:38486-38493. [DOI] [PubMed] [Google Scholar]
- 9.Brown, M. S., and J. L. Goldstein. 2008. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7:95-96. [DOI] [PubMed] [Google Scholar]
- 10.Brunet, A., J. Park, H. Tran, L. S. Hu, B. A. Hemmings, and M. E. Greenberg. 2001. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol. Cell. Biol. 21:952-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho, H., J. Mu, J. K. Kim, J. L. Thorvaldsen, Q. Chu, E. B. Crenshaw III, K. H. Kaestner, M. S. Bartolomei, G. I. Shulman, and M. J. Birnbaum. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292:1728-1731. [DOI] [PubMed] [Google Scholar]
- 12.Choi, Y. H., S. Park, S. Hockman, E. Zmuda-Trzebiatowska, F. Svennelid, M. Haluzik, O. Gavrilova, F. Ahmad, L. Pepin, M. Napolitano, M. Taira, F. Sundler, L. Stenson Holst, E. Degerman, and V. C. Manganiello. 2006. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice. J. Clin. Investig. 116:3240-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen, A. W., B. Razani, W. Schubert, T. M. Williams, X. B. Wang, P. Iyengar, D. L. Brasaemle, P. E. Scherer, and M. P. Lisanti. 2004. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes 53:1261-1270. [DOI] [PubMed] [Google Scholar]
- 14.Degerman, E., T. R. Landstrom, J. Wijkander, L. S. Holst, F. Ahmad, P. Belfrage, and V. Manganiello. 1998. Phosphorylation and activation of hormone-sensitive adipocyte phosphodiesterase type 3B. Methods 14:43-53. [DOI] [PubMed] [Google Scholar]
- 15.Delarue, J., and C. Magnan. 2007. Free fatty acids and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care 10:142-148. [DOI] [PubMed] [Google Scholar]
- 16.Dummler, B., and B. A. Hemmings. 2007. Physiological roles of PKB/Akt isoforms in development and disease. Biochem. Soc Trans. 35:231-235. [DOI] [PubMed] [Google Scholar]
- 17.Duncan, R. E., M. Ahmadian, K. Jaworski, E. Sarkadi-Nagy, and H. S. Sul. 2007. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 27:79-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eriksson, J. W., U. Smith, F. Waagstein, M. Wysocki, and P. A. Jansson. 1999. Glucose turnover and adipose tissue lipolysis are insulin-resistant in healthy relatives of type 2 diabetes patients: is cellular insulin resistance a secondary phenomenon? Diabetes 48:1572-1578. [DOI] [PubMed] [Google Scholar]
- 19.Farese, R. V., M. P. Sajan, and M. L. Standaert. 2005. Atypical protein kinase C in insulin action and insulin resistance. Biochem. Soc. Trans. 33:350-353. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez, E., and T. E. McGraw. 2006. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol. Biol. Cell 17:4484-4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Granneman, J. G., H. P. Moore, R. L. Granneman, A. S. Greenberg, M. S. Obin, and Z. Zhu. 2007. Analysis of lipolytic protein trafficking and interactions in adipocytes. J. Biol. Chem. 282:5726-5735. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg, A. S., J. J. Egan, S. A. Wek, N. B. Garty, E. J. Blanchette-Mackie, and C. Londos. 1991. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J. Biol. Chem. 266:11341-11346. [PubMed] [Google Scholar]
- 23.Guo, Y., T. C. Walther, M. Rao, N. Stuurman, G. Goshima, K. Terayama, J. S. Wong, R. D. Vale, P. Walter, and R. V. Farese. 2008. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature 453:657-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haemmerle, G., A. Lass, R. Zimmermann, G. Gorkiewicz, C. Meyer, J. Rozman, G. Heldmaier, R. Maier, C. Theussl, S. Eder, D. Kratky, E. F. Wagner, M. Klingenspor, G. Hoefler, and R. Zechner. 2006. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312:734-737. [DOI] [PubMed] [Google Scholar]
- 25.Higashi, Y., T. Oshima, S. Sasaki, Y. Nakano, M. Kambe, H. Matsuura, and G. Kajiyama. 1998. Angiotensin-converting enzyme inhibition, but not calcium antagonism, improves a response of the renal vasculature to L-arginine in patients with essential hypertension. Hypertension 32:16-24. [DOI] [PubMed] [Google Scholar]
- 26.Holm, C. 2003. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem. Soc. Trans. 31:1120-1124. [DOI] [PubMed] [Google Scholar]
- 27.Hoogenberg, K., and R. P. Dullaart. 1992. Abnormal plasma noradrenaline response and exercise induced albuminuria in type 1 (insulin-dependent) diabetes mellitus. Scand. J. Clin. Lab. Investig. 52:803-811. [DOI] [PubMed] [Google Scholar]
- 28.Kane, S., H. Sano, S. C. Liu, J. M. Asara, W. S. Lane, C. C. Garner, and G. E. Lienhard. 2002. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J. Biol. Chem. 277:22115-22118. [DOI] [PubMed] [Google Scholar]
- 29.Kang, L., and L. E. Nagy. 2006. Chronic ethanol feeding suppresses beta-adrenergic receptor-stimulated lipolysis in adipocytes isolated from epididymal fat. Endocrinology 147:4330-4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelley, D. E., M. Mokan, J. A. Simoneau, and L. J. Mandarino. 1993. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J. Clin. Investig. 92:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinchen, J. M., K. Doukoumetzidis, J. Almendinger, L. Stergiou, A. Tosello-Trampont, C. D. Sifri, M. O. Hengartner, and K. S. Ravichandran. 2008. A pathway for phagosome maturation during engulfment of apoptotic cells. Nat. Cell Biol. 10:556-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitamura, T., Y. Kitamura, S. Kuroda, Y. Hino, M. Ando, K. Kotani, H. Konishi, H. Matsuzaki, U. Kikkawa, W. Ogawa, and M. Kasuga. 1999. Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase Akt. Mol. Cell. Biol. 19:6286-6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lass, A., R. Zimmermann, G. Haemmerle, M. Riederer, G. Schoiswohl, M. Schweiger, P. Kienesberger, J. G. Strauss, G. Gorkiewicz, and R. Zechner. 2006. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 3:309-319. [DOI] [PubMed] [Google Scholar]
- 34.Lindvall, H., P. Nevsten, K. Strom, R. Wallenberg, F. Sundler, D. Langin, M. S. Winzell, and C. Holm. 2004. A novel hormone-sensitive lipase isoform expressed in pancreatic beta-cells. J. Biol. Chem. 279:3828-3836. [DOI] [PubMed] [Google Scholar]
- 35.Liu, P., R. Bartz, J. K. Zehmer, Y. Ying, and R. G. Anderson. 2008. Rab-regulated membrane traffic between adiposomes and multiple endomembrane systems. Methods Enzymol. 439:327-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, P., R. Bartz, J. K. Zehmer, Y. S. Ying, M. Zhu, G. Serrero, and R. G. Anderson. 2007. Rab-regulated interaction of early endosomes with lipid droplets. Biochim. Biophys. Acta 1773:784-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcinkiewicz, A., D. Gauthier, A. Garcia, and D. L. Brasaemle. 2006. The phosphorylation of serine 492 of perilipin a directs lipid droplet fragmentation and dispersion. J. Biol. Chem. 281:11901-11909. [DOI] [PubMed] [Google Scholar]
- 38.Martin, S., K. Driessen, S. J. Nixon, M. Zerial, and R. G. Parton. 2005. Regulated localization of Rab18 to lipid droplets: effects of lipolytic stimulation and inhibition of lipid droplet catabolism. J. Biol. Chem. 280:42325-42335. [DOI] [PubMed] [Google Scholar]
- 39.Martin, S., and R. G. Parton. 2008. Characterization of Rab18, a lipid droplet-associated small GTPase. Methods Enzymol. 438:109-129. [DOI] [PubMed] [Google Scholar]
- 40.Martin, S., and R. G. Parton. 2006. Lipid droplets: a unified view of a dynamic organelle. Nat. Rev. Mol. Cell Biol. 7:373-378. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Botas, J., J. B. Anderson, D. Tessier, A. Lapillonne, B. H. Chang, M. J. Quast, D. Gorenstein, K. H. Chen, and L. Chan. 2000. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat. Genet. 26:474-479. [DOI] [PubMed] [Google Scholar]
- 42.Martins-Afférri, M. P., W. T. Festuccia, L. C. Navegantes, M. A. Garofalo, L. M. Botion, I. C. Kettelhut, and R. H. Migliorini. 2004. Response to intra- and extracellular lipolytic agents and hormone-sensitive lipase translocation are impaired in adipocytes from rats adapted to a high-protein, carbohydrate-free diet. J. Nutr. 134:2919-2923. [DOI] [PubMed] [Google Scholar]
- 43.McConnachie, G., L. K. Langeberg, and J. D. Scott. 2006. AKAP signaling complexes: getting to the heart of the matter. Trends Mol. Med. 12:317-323. [DOI] [PubMed] [Google Scholar]
- 44.McGarry, J. D. 2002. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51:7-18. [DOI] [PubMed] [Google Scholar]
- 45.Michel, J. J., and J. D. Scott. 2002. AKAP mediated signal transduction. Annu. Rev. Pharmacol. Toxicol. 42:235-257. [DOI] [PubMed] [Google Scholar]
- 46.Miyoshi, H., J. W. Perfield, Jr., S. C. Souza, W. J. Shen, H. H. Zhang, Z. S. Stancheva, F. B. Kraemer, M. S. Obin, and A. S. Greenberg. 2007. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J. Biol. Chem. 282:996-1002. [DOI] [PubMed] [Google Scholar]
- 47.Miyoshi, H., S. C. Souza, H. H. Zhang, K. J. Strissel, M. A. Christoffolete, J. Kovsan, A. Rudich, F. B. Kraemer, A. C. Bianco, M. S. Obin, and A. S. Greenberg. 2006. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J. Biol. Chem. 281:15837-15844. [DOI] [PubMed] [Google Scholar]
- 48.Park, J., M. L. Leong, P. Buse, A. C. Maiyar, G. L. Firestone, and B. A. Hemmings. 1999. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J. 18:3024-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. U. S. A. 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sale, E. M., and G. J. Sale. 2008. Protein kinase B: signalling roles and therapeutic targeting. Cell Mol. Life Sci. 65:113-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savage, D. B., K. F. Petersen, and G. I. Shulman. 2007. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol. Rev. 87:507-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Souza, S. C., K. V. Muliro, L. Liscum, P. Lien, M. T. Yamamoto, J. E. Schaffer, G. E. Dallal, X. Wang, F. B. Kraemer, M. Obin, and A. S. Greenberg. 2002. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J. Biol. Chem. 277:8267-8272. [DOI] [PubMed] [Google Scholar]
- 53.Stack, J. H., B. Horazdovsky, and S. D. Emr. 1995. Receptor-mediated protein sorting to the vacuole in yeast: roles for a protein kinase, a lipid kinase and GTP-binding proteins. Annu. Rev. Cell Dev. Biol. 11:1-33. [DOI] [PubMed] [Google Scholar]
- 54.Stein, M. P., C. Cao, M. Tessema, Y. Feng, E. Romero, A. Welford, and A. Wandinger-Ness. 2005. Interaction and functional analyses of human VPS34/p150 phosphatidylinositol 3-kinase complex with Rab7. Methods Enzymol. 403:628-649. [DOI] [PubMed] [Google Scholar]
- 55.Su, C. L., C. Sztalryd, J. A. Contreras, C. Holm, A. R. Kimmel, and C. Londos. 2003. Mutational analysis of the hormone-sensitive lipase translocation reaction in adipocytes. J. Biol. Chem. 278:43615-43619. [DOI] [PubMed] [Google Scholar]
- 56.Subramanian, V., A. Rothenberg, C. Gomez, A. W. Cohen, A. Garcia, S. Bhattacharyya, L. Shapiro, G. Dolios, R. Wang, M. P. Lisanti, and D. L. Brasaemle. 2004. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J. Biol. Chem. 279:42062-42071. [DOI] [PubMed] [Google Scholar]
- 57.Sundell, J., H. Laine, M. Luotolahti, P. Nuutila, and J. Knuuti. 2002. Increased coronary vascular resistance cannot be reduced by inhibiting sympathetic overactivity in hypertension. J. Vasc. Res. 39:456-462. [DOI] [PubMed] [Google Scholar]
- 58.Sztalryd, C., G. Xu, H. Dorward, J. T. Tansey, J. A. Contreras, A. R. Kimmel, and C. Londos. 2003. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J. Cell Biol. 161:1093-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taniguchi, C. M., B. Emanuelli, and C. R. Kahn. 2006. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7:85-96. [DOI] [PubMed] [Google Scholar]
- 60.Tansey, J. T., C. Sztalryd, J. Gruia-Gray, D. L. Roush, J. V. Zee, O. Gavrilova, M. L. Reitman, C. X. Deng, C. Li, A. R. Kimmel, and C. Londos. 2001. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc. Natl. Acad. Sci. U. S. A. 98:6494-6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tansey, J. T., C. Sztalryd, E. M. Hlavin, A. R. Kimmel, and C. Londos. 2004. The central role of perilipin a in lipid metabolism and adipocyte lipolysis. IUBMB Life 56:379-385. [DOI] [PubMed] [Google Scholar]
- 62.Taylor, S. S., C. Kim, D. Vigil, N. M. Haste, J. Yang, J. Wu, and G. S. Anand. 2005. Dynamics of signaling by PKA. Biochim. Biophys. Acta 1754:25-37. [DOI] [PubMed] [Google Scholar]
- 63.Taylor, S. S., J. Yang, J. Wu, N. M. Haste, E. Radzio-Andzelm, and G. Anand. 2004. PKA: a portrait of protein kinase dynamics. Biochim. Biophys. Acta 1697:259-269. [DOI] [PubMed] [Google Scholar]
- 64.Todaro, G. J., and H. Green. 1963. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17:299-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vieira, O. V., C. Bucci, R. E. Harrison, W. S. Trimble, L. Lanzetti, J. Gruenberg, A. D. Schreiber, P. D. Stahl, and S. Grinstein. 2003. Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol. Cell. Biol. 23:2501-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whiteman, E. L., H. Cho, and M. J. Birnbaum. 2002. Role of Akt/protein kinase B in metabolism. Trends Endocrinol. Metab. 13:444-451. [DOI] [PubMed] [Google Scholar]
- 67.Wilson, E. M., and P. Rotwein. 2007. Selective control of skeletal muscle differentiation by Akt1. J. Biol. Chem. 282:5106-5110. [DOI] [PubMed] [Google Scholar]
- 68.Wong, W., and J. D. Scott. 2004. AKAP signalling complexes: focal points in space and time. Nat. Rev. Mol. Cell Biol. 5:959-970. [DOI] [PubMed] [Google Scholar]
- 69.Yamaguchi, T., N. Omatsu, S. Matsushita, and T. Osumi. 2004. CGI-58 interacts with perilipin and is localized to lipid droplets. Possible involvement of CGI-58 mislocalization in Chanarin-Dorfman syndrome. J. Biol. Chem. 279:30490-30497. [DOI] [PubMed] [Google Scholar]
- 70.Yin, W., J. Mu, and M. J. Birnbaum. 2003. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis In 3T3-L1 adipocytes. J. Biol. Chem. 278:43074-43080. [DOI] [PubMed] [Google Scholar]
- 71.Zhang, B. H., E. D. Tang, T. Zhu, M. E. Greenberg, A. B. Vojtek, and K. L. Guan. 2001. Serum- and glucocorticoid-inducible kinase SGK phosphorylates and negatively regulates B-Raf. J. Biol. Chem. 276:31620-31626. [DOI] [PubMed] [Google Scholar]
- 72.Zhang, J., C. J. Hupfeld, S. S. Taylor, J. M. Olefsky, and R. Y. Tsien. 2005. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature 437:569-573. [DOI] [PubMed] [Google Scholar]
- 73.Zimmermann, R., J. G. Strauss, G. Haemmerle, G. Schoiswohl, R. Birner-Gruenberger, M. Riederer, A. Lass, G. Neuberger, F. Eisenhaber, A. Hermetter, and R. Zechner. 2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306:1383-1386. [DOI] [PubMed] [Google Scholar]