Abstract
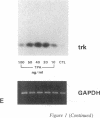
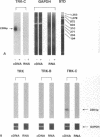
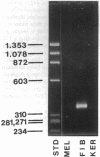
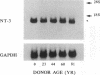
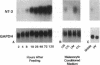
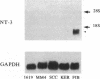
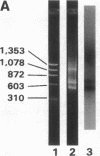
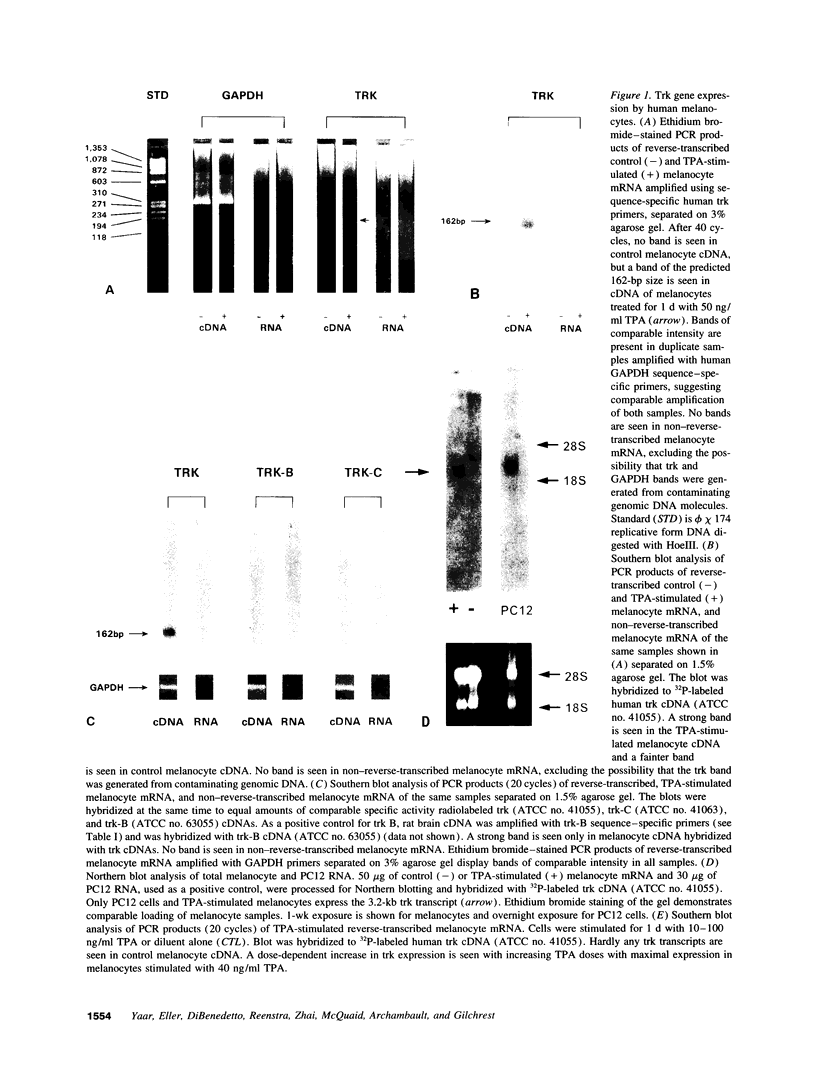
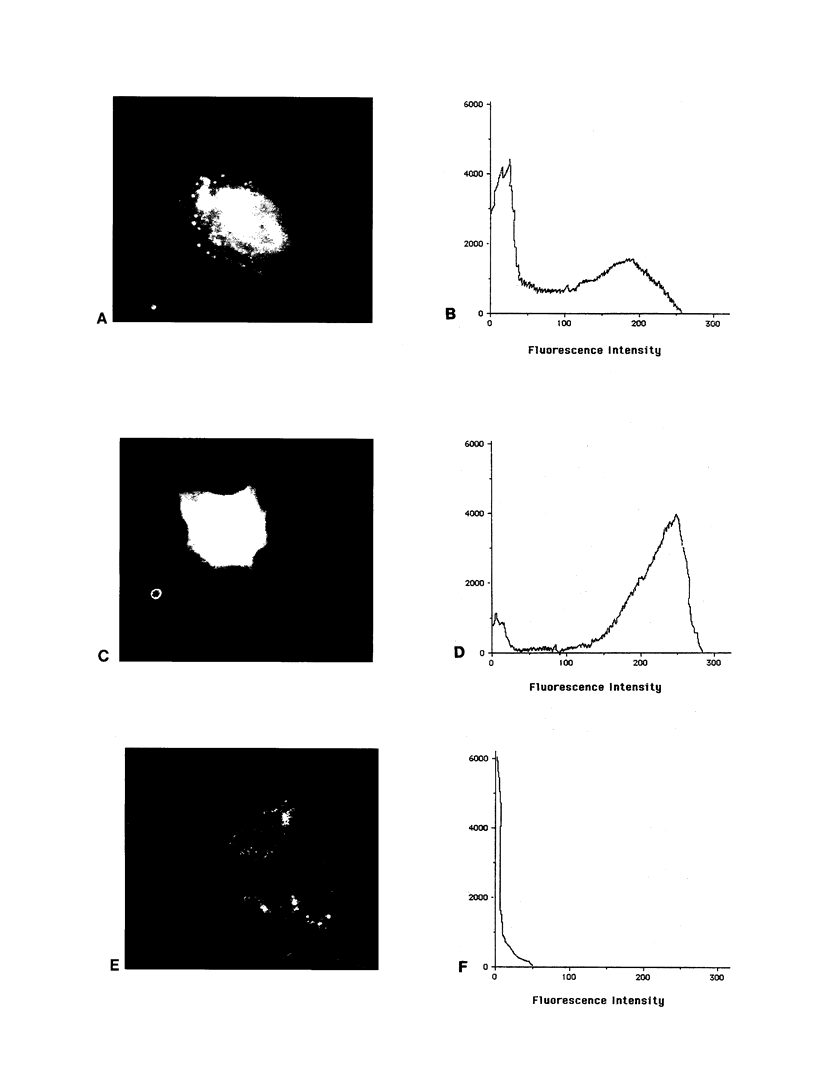
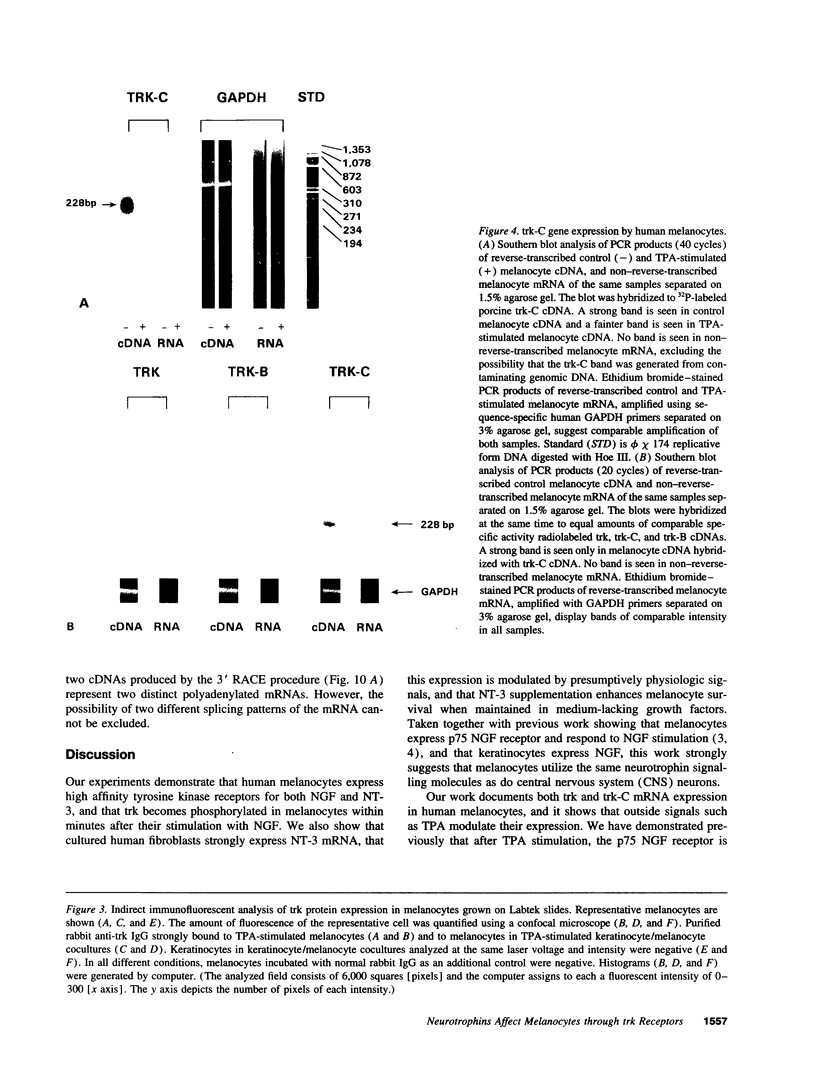
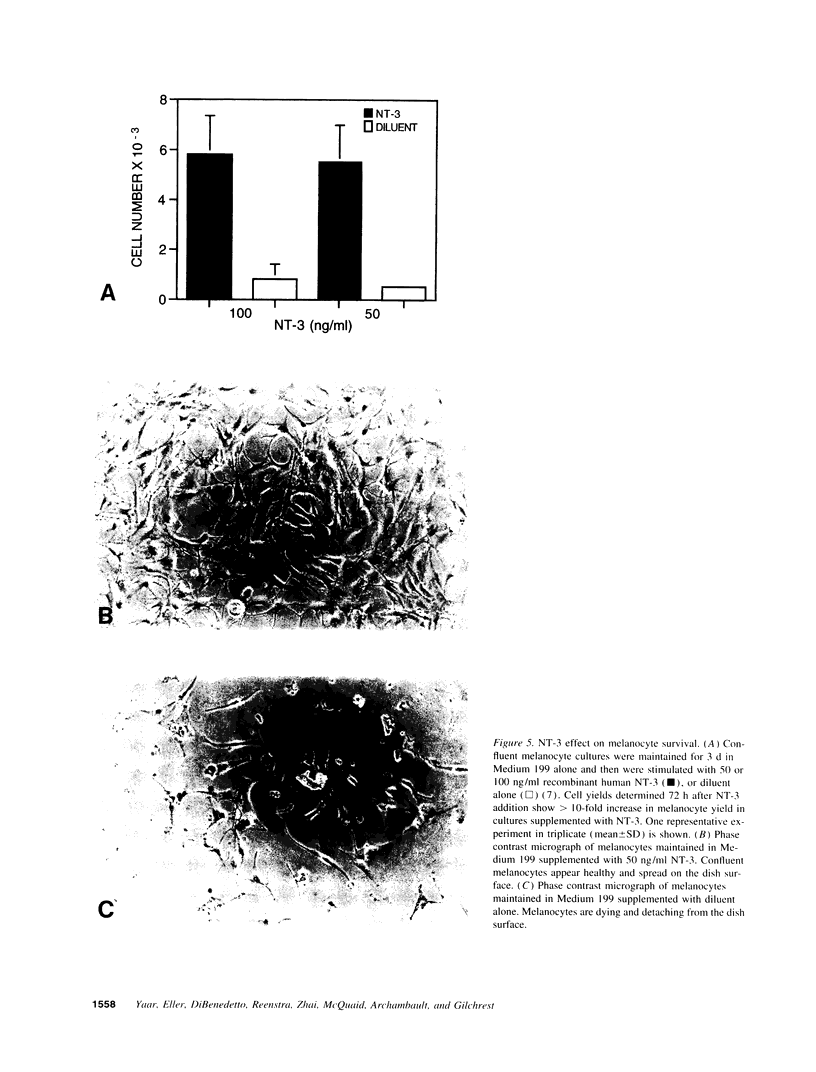
We have recently shown that (a) human melanocytes express the p75 nerve growth factor (NGF) receptor in vitro; (b) that melanocyte dendricity and migration, among other behaviors, are regulated at least in part by NGF; and (c) that cultured human epidermal keratinocytes produce NGF. We now report that melanocyte stimulation with phorbol 12-tetra decanoate 13-acetate (TPA), previously reported to induce p75 NGF receptor, also induces trk in melanocytes, and TPA effect is further potentiated by the presence of keratinocytes in culture. Moreover, trk in melanocytes becomes phosphorylated within minutes after NGF stimulation. As well, cultures of dermal fibroblasts express neurotrophin-3 (NT-3) mRNA; NT-3 mRNA levels in cultured fibroblasts are modulated by mitogenic stimulation, UV irradiation, and exposure to melanocyte-conditioned medium. Moreover, melanocytes constitutively express low levels of trk-C, and its expression is downregulated after TPA stimulation. NT-3 supplementation to cultured melanocytes maintained in Medium 199 alone prevents cell death. These combined data suggest that melanocyte behavior in human skin may be influenced by neurotrophic factors, possibly of keratinocyte and fibroblast origin, which act through high affinity receptors.
Full text
PDF







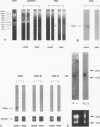
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barde Y. A., Edgar D., Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. M. Protein kinase C activation by diacylglycerol second messengers. Cell. 1986 Jun 6;45(5):631–632. doi: 10.1016/0092-8674(86)90774-9. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J. The MARCKS family of cellular protein kinase C substrates. J Biol Chem. 1993 Jan 25;268(3):1501–1504. [PubMed] [Google Scholar]
- Boissy R. E. The melanocyte. Its structure, function, and subpopulations in skin, eyes, and hair. Dermatol Clin. 1988 Apr;6(2):161–173. [PubMed] [Google Scholar]
- Bollag G. E., Roth R. A., Beaudoin J., Mochly-Rosen D., Koshland D. E., Jr Protein kinase C directly phosphorylates the insulin receptor in vitro and reduces its protein-tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5822–5824. doi: 10.1073/pnas.83.16.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell M. Keeping track of neurotrophin receptors. Cell. 1991 Jun 14;65(6):915–918. doi: 10.1016/0092-8674(91)90540-f. [DOI] [PubMed] [Google Scholar]
- Chao M. V., Bothwell M. A., Ross A. H., Koprowski H., Lanahan A. A., Buck C. R., Sehgal A. Gene transfer and molecular cloning of the human NGF receptor. Science. 1986 Apr 25;232(4749):518–521. doi: 10.1126/science.3008331. [DOI] [PubMed] [Google Scholar]
- Ciesielski-Treska J., Ulrich G., Aunis D. Protein kinase C-induced redistribution of the cytoskeleton and phosphorylation of vimentin in cultured brain macrophages. J Neurosci Res. 1991 Jul;29(3):362–378. doi: 10.1002/jnr.490290312. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., Tapley P., Jing S. Q., Nanduri V., O'Rourke E., Lamballe F., Kovary K., Klein R., Jones K. R., Reichardt L. F. The trk tyrosine protein kinase mediates the mitogenic properties of nerve growth factor and neurotrophin-3. Cell. 1991 Jul 12;66(1):173–183. doi: 10.1016/0092-8674(91)90149-s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M., Kent R. B., Sonenshein G. E. Transcriptional activation of immunoglobulin alpha heavy-chain genes by translocation of the c-myc oncogene. 1983 Sep 29-Oct 5Nature. 305(5933):443–446. doi: 10.1038/305443a0. [DOI] [PubMed] [Google Scholar]
- Di Marco E., Marchisio P. C., Bondanza S., Franzi A. T., Cancedda R., De Luca M. Growth-regulated synthesis and secretion of biologically active nerve growth factor by human keratinocytes. J Biol Chem. 1991 Nov 15;266(32):21718–21722. [PubMed] [Google Scholar]
- Ebendal T. NGF in CNS: experimental data and clinical implications. Prog Growth Factor Res. 1989;1(3):143–159. doi: 10.1016/0955-2235(89)90008-2. [DOI] [PubMed] [Google Scholar]
- Eisinger M., Marko O. Selective proliferation of normal human melanocytes in vitro in the presence of phorbol ester and cholera toxin. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2018–2022. doi: 10.1073/pnas.79.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P., Wetmore C., Olson L., Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990 Oct;5(4):511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- Fisch T. M., Prywes R., Roeder R. G. c-fos sequence necessary for basal expression and induction by epidermal growth factor, 12-O-tetradecanoyl phorbol-13-acetate and the calcium ionophore. Mol Cell Biol. 1987 Oct;7(10):3490–3502. doi: 10.1128/mcb.7.10.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann P. S., Gilchrest B. A. Ultraviolet radiation directly induces pigment production by cultured human melanocytes. J Cell Physiol. 1987 Oct;133(1):88–94. doi: 10.1002/jcp.1041330111. [DOI] [PubMed] [Google Scholar]
- Gilchrest B. A. Prior chronic sun exposure decreases the lifespan of human skin fibroblasts in vitro. J Gerontol. 1980 Jul;35(4):537–541. doi: 10.1093/geronj/35.4.537. [DOI] [PubMed] [Google Scholar]
- Gilchrest B. A. Relationship between actinic damage and chronologic aging in keratinocyte cultures of human skin. J Invest Dermatol. 1979 May;72(5):219–223. doi: 10.1111/1523-1747.ep12530769. [DOI] [PubMed] [Google Scholar]
- Gilchrest B. A., Vrabel M. A., Flynn E., Szabo G. Selective cultivation of human melanocytes from newborn and adult epidermis. J Invest Dermatol. 1984 Nov;83(5):370–376. doi: 10.1111/1523-1747.ep12264638. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hallbök F., Ibáez C. F., Persson H. Evolutionary studies of the nerve growth factor family reveal a novel member abundantly expressed in Xenopus ovary. Neuron. 1991 May;6(5):845–858. doi: 10.1016/0896-6273(91)90180-8. [DOI] [PubMed] [Google Scholar]
- Hempstead B. L., Martin-Zanca D., Kaplan D. R., Parada L. F., Chao M. V. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991 Apr 25;350(6320):678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Hohn A., Leibrock J., Bailey K., Barde Y. A. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990 Mar 22;344(6264):339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- Hory-Lee F., Russell M., Lindsay R. M., Frank E. Neurotrophin 3 supports the survival of developing muscle sensory neurons in culture. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2613–2617. doi: 10.1073/pnas.90.7.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Ling N., Cooper J. A. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature. 1984 Oct 4;311(5985):480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- Jeng A. Y., Srivastava S. K., Lacal J. C., Blumberg P. M. Phosphorylation of ras oncogene product by protein kinase C. Biochem Biophys Res Commun. 1987 Jun 15;145(2):782–788. doi: 10.1016/0006-291x(87)91033-3. [DOI] [PubMed] [Google Scholar]
- Jones K. R., Reichardt L. F. Molecular cloning of a human gene that is a member of the nerve growth factor family. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8060–8064. doi: 10.1073/pnas.87.20.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalcheim C., Carmeli C., Rosenthal A. Neurotrophin 3 is a mitogen for cultured neural crest cells. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1661–1665. doi: 10.1073/pnas.89.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Hempstead B. L., Martin-Zanca D., Chao M. V., Parada L. F. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991 Apr 26;252(5005):554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Martin-Zanca D., Parada L. F. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991 Mar 14;350(6314):158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kiley S. C., Parker P. J., Fabbro D., Jaken S. Hormone- and phorbol ester-activated protein kinase C isozymes mediate a reorganization of the actin cytoskeleton associated with prolactin secretion in GH4C1 cells. Mol Endocrinol. 1992 Jan;6(1):120–131. doi: 10.1210/mend.6.1.1738365. [DOI] [PubMed] [Google Scholar]
- Klein R., Conway D., Parada L. F., Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990 May 18;61(4):647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- Klein R., Martin-Zanca D., Barbacid M., Parada L. F. Expression of the tyrosine kinase receptor gene trkB is confined to the murine embryonic and adult nervous system. Development. 1990 Aug;109(4):845–850. doi: 10.1242/dev.109.4.845. [DOI] [PubMed] [Google Scholar]
- Klein R., Nanduri V., Jing S. A., Lamballe F., Tapley P., Bryant S., Cordon-Cardo C., Jones K. R., Reichardt L. F., Barbacid M. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell. 1991 Jul 26;66(2):395–403. doi: 10.1016/0092-8674(91)90628-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Parada L. F., Coulier F., Barbacid M. trkB, a novel tyrosine protein kinase receptor expressed during mouse neural development. EMBO J. 1989 Dec 1;8(12):3701–3709. doi: 10.1002/j.1460-2075.1989.tb08545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Kochs G., Hummel R., Vahidi H., Mischak H., Finkenzeller G., Marmé D., Rapp U. R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993 Jul 15;364(6434):249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- Lamballe F., Klein R., Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991 Sep 6;66(5):967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- Leibrock J., Lottspeich F., Hohn A., Hofer M., Hengerer B., Masiakowski P., Thoenen H., Barde Y. A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989 Sep 14;341(6238):149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor: thirty-five years later. EMBO J. 1987 May;6(5):1145–1154. doi: 10.1002/j.1460-2075.1987.tb02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O., Ernfors P., Bengzon J., Kokaia Z., Smith M. L., Siesjö B. K., Persson H. Differential regulation of mRNAs for nerve growth factor, brain-derived neurotrophic factor, and neurotrophin 3 in the adult rat brain following cerebral ischemia and hypoglycemic coma. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):648–652. doi: 10.1073/pnas.89.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre P. C., Belluscio L., Friedman B., Alderson R. F., Wiegand S. J., Furth M. E., Lindsay R. M., Yancopoulos G. D. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990 Oct;5(4):501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Maisonpierre P. C., Belluscio L., Squinto S., Ip N. Y., Furth M. E., Lindsay R. M., Yancopoulos G. D. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990 Mar 23;247(4949 Pt 1):1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- Maisonpierre P. C., Le Beau M. M., Espinosa R., 3rd, Ip N. Y., Belluscio L., de la Monte S. M., Squinto S., Furth M. E., Yancopoulos G. D. Human and rat brain-derived neurotrophic factor and neurotrophin-3: gene structures, distributions, and chromosomal localizations. Genomics. 1991 Jul;10(3):558–568. doi: 10.1016/0888-7543(91)90436-i. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Barbacid M., Parada L. F. Expression of the trk proto-oncogene is restricted to the sensory cranial and spinal ganglia of neural crest origin in mouse development. Genes Dev. 1990 May;4(5):683–694. doi: 10.1101/gad.4.5.683. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Hughes S. H., Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. 1986 Feb 27-Mar 5Nature. 319(6056):743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Oskam R., Mitra G., Copeland T., Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol. 1989 Jan;9(1):24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemas D. S., Lindberg R. A., Hunter T. trkB, a neural receptor protein-tyrosine kinase: evidence for a full-length and two truncated receptors. Mol Cell Biol. 1991 Jan;11(1):143–153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeyaert J. M., Eller M., Gordon P. R., Park H. Y., Gilchrest B. A. Pigment content of cultured human melanocytes does not correlate with tyrosinase message level. Br J Dermatol. 1991 Oct;125(4):297–303. doi: 10.1111/j.1365-2133.1991.tb14161.x. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Peacocke M., Yaar M., Mansur C. P., Chao M. V., Gilchrest B. A. Induction of nerve growth factor receptors on cultured human melanocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5282–5286. doi: 10.1073/pnas.85.14.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Tébar A., Dechant G., Barde Y. A. Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990 Apr;4(4):487–492. doi: 10.1016/0896-6273(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Goeddel D. V., Nguyen T., Lewis M., Shih A., Laramee G. R., Nikolics K., Winslow J. W. Primary structure and biological activity of a novel human neurotrophic factor. Neuron. 1990 May;4(5):767–773. doi: 10.1016/0896-6273(90)90203-r. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihag R. K., Jeng A. Y., Nixon R. A. Phosphorylation of neurofilament proteins by protein kinase C. FEBS Lett. 1988 Jun 6;233(1):181–185. doi: 10.1016/0014-5793(88)81380-2. [DOI] [PubMed] [Google Scholar]
- Snyder S. H. Parkinson's disease. Fresh factors to consider. Nature. 1991 Mar 21;350(6315):195–195. doi: 10.1038/350195a0. [DOI] [PubMed] [Google Scholar]
- Soppet D., Escandon E., Maragos J., Middlemas D. S., Reid S. W., Blair J., Burton L. E., Stanton B. R., Kaplan D. R., Hunter T. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991 May 31;65(5):895–903. doi: 10.1016/0092-8674(91)90396-g. [DOI] [PubMed] [Google Scholar]
- Svoboda K. K. Embryonic corneal epithelial actin alters distribution in response to laminin. Invest Ophthalmol Vis Sci. 1992 Feb;33(2):324–333. [PMC free article] [PubMed] [Google Scholar]
- Svoboda K. K. Intracellular localization of types I and II collagen mRNA and endoplasmic reticulum in embryonic corneal epithelia. J Cell Sci. 1991 Sep;100(Pt 1):23–33. doi: 10.1242/jcs.100.1.23. [DOI] [PubMed] [Google Scholar]
- Sözeri O., Vollmer K., Liyanage M., Frith D., Kour G., Mark G. E., 3rd, Stabel S. Activation of the c-Raf protein kinase by protein kinase C phosphorylation. Oncogene. 1992 Nov;7(11):2259–2262. [PubMed] [Google Scholar]
- Thoenen H., Bandtlow C., Heumann R. The physiological function of nerve growth factor in the central nervous system: comparison with the periphery. Rev Physiol Biochem Pharmacol. 1987;109:145–178. doi: 10.1007/BFb0031026. [DOI] [PubMed] [Google Scholar]
- Tron V. A., Coughlin M. D., Jang D. E., Stanisz J., Sauder D. N. Expression and modulation of nerve growth factor in murine keratinocytes (PAM 212). J Clin Invest. 1990 Apr;85(4):1085–1089. doi: 10.1172/JCI114539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter M. L., Martin-Zanca D., Parada L. F., Bishop J. M., Kaplan D. R. Nerve growth factor rapidly stimulates tyrosine phosphorylation of phospholipase C-gamma 1 by a kinase activity associated with the product of the trk protooncogene. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5650–5654. doi: 10.1073/pnas.88.13.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werninghaus K., Handjani R. M., Gilchrest B. A. Protective effect of alpha-tocopherol in carrier liposomes on ultraviolet-mediated human epidermal cell damage in vitro. Photodermatol Photoimmunol Photomed. 1991 Dec;8(6):236–242. [PubMed] [Google Scholar]
- Whitfield J. F., Durkin J. P., Franks D. J., Kleine L. P., Raptis L., Rixon R. H., Sikorska M., Walker P. R. Calcium, cyclic AMP and protein kinase C--partners in mitogenesis. Cancer Metastasis Rev. 1987;5(3):205–250. doi: 10.1007/BF00046999. [DOI] [PubMed] [Google Scholar]
- Whittemore S. R., Seiger A. The expression, localization and functional significance of beta-nerve growth factor in the central nervous system. Brain Res. 1987 Nov;434(4):439–464. doi: 10.1016/0165-0173(87)90008-7. [DOI] [PubMed] [Google Scholar]
- Yaar M., Gilchrest B. A. Human melanocyte growth and differentiation: a decade of new data. J Invest Dermatol. 1991 Oct;97(4):611–617. doi: 10.1111/1523-1747.ep12482985. [DOI] [PubMed] [Google Scholar]
- Yaar M., Grossman K., Eller M., Gilchrest B. A. Evidence for nerve growth factor-mediated paracrine effects in human epidermis. J Cell Biol. 1991 Nov;115(3):821–828. doi: 10.1083/jcb.115.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaar M., Peacocke M., Cohen M. S., Gilchrest B. A. Dissociation of proto-oncogene induction from growth response in normal human fibroblasts. J Cell Physiol. 1990 Oct;145(1):39–45. doi: 10.1002/jcp.1041450107. [DOI] [PubMed] [Google Scholar]