Abstract
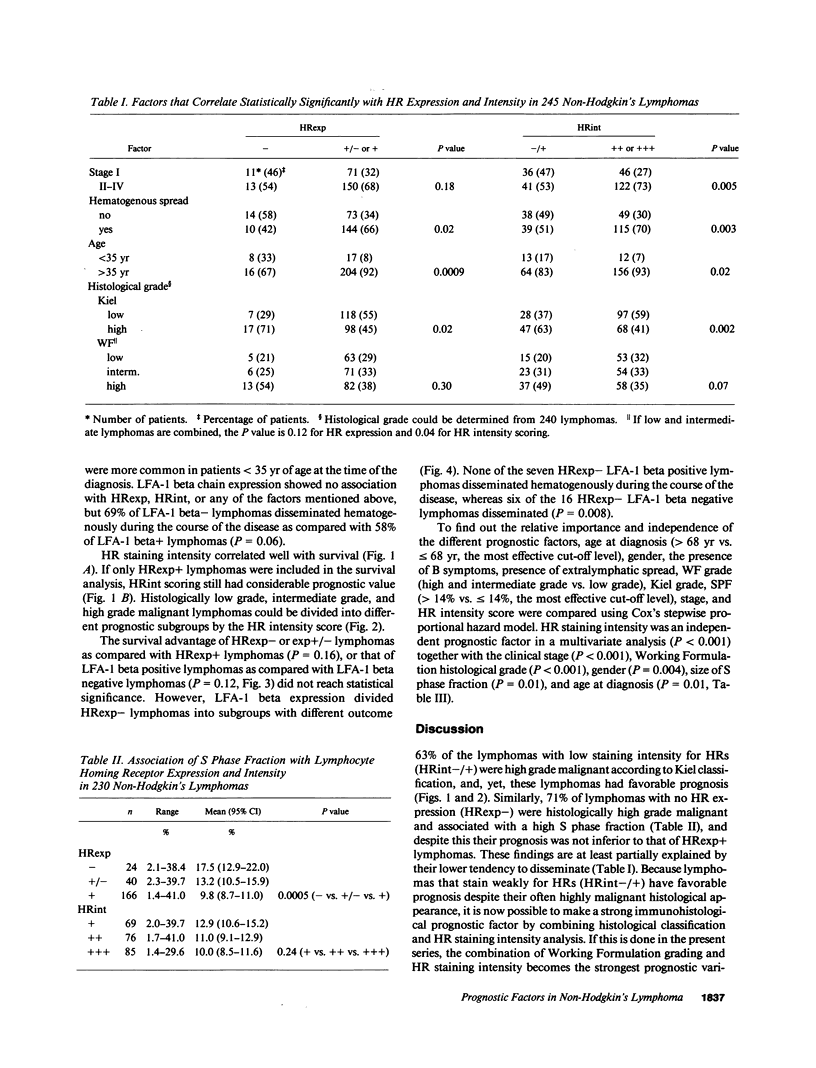
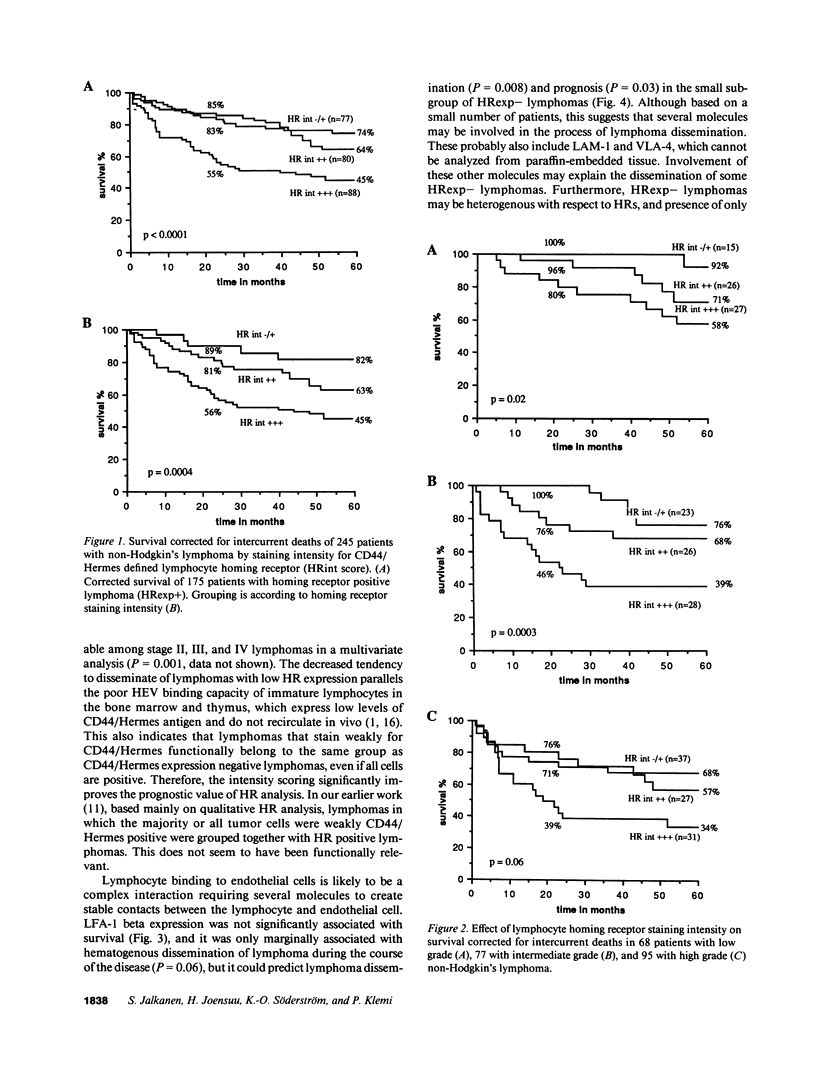
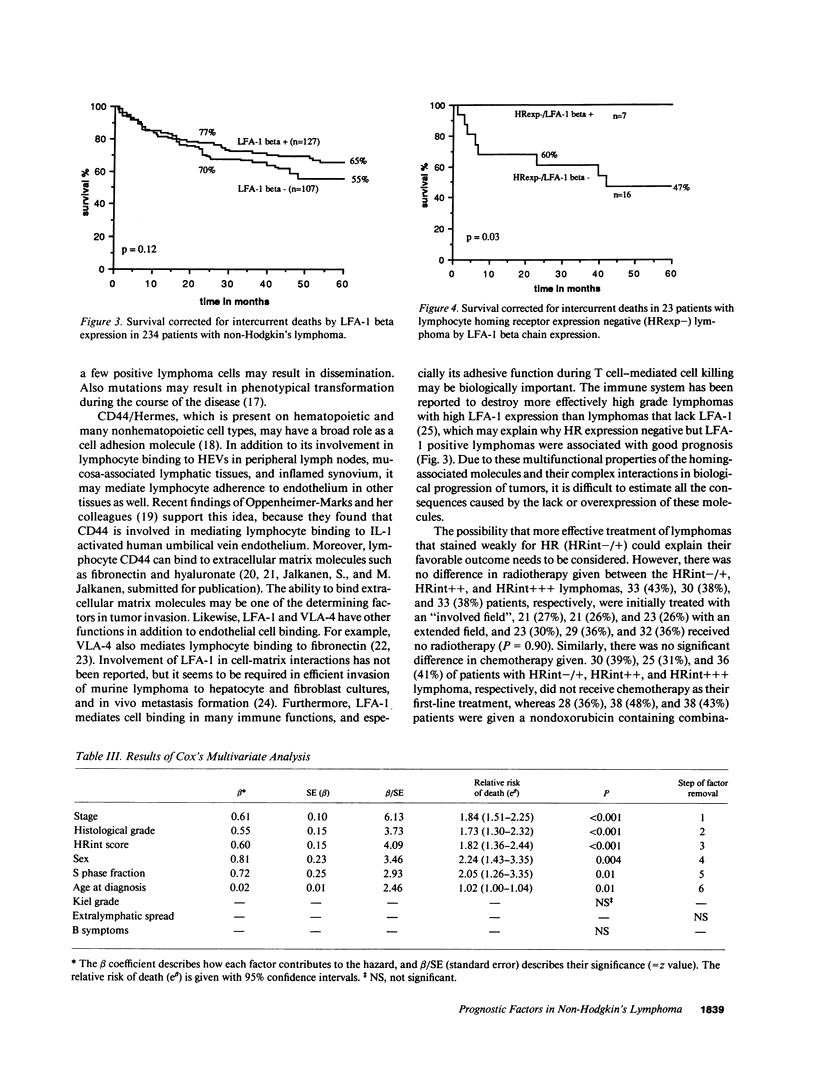
Lymphocyte homing receptors (HRs) defined by Hermes antibodies (anti-CD44) and lymphocyte function associated antigen-1 (LFA-1, CD11a/CD18) are involved in lymphocyte binding to endothelial cells of high endothelial venules (HEVs) at sites where lymphocytes exit the blood. Their expression was correlated to the clinical behavior of 245 non-Hodgkin's lymphomas followed up for the median of 87 mo after the diagnosis. Lymphomas that showed no or weak staining intensity for HRs were more often of stage I (P = 0.005), disseminated less frequently hematogenously (P = 0.003), and had more favorable prognosis than lymphomas with intensive staining for HRs (P less than 0.0001) despite that they were more often histologically of high grade malignancy (P = 0.002). Expression of LFA-1 beta chain (CD18) did not correlate significantly with stage or survival, but had prognostic value in a subgroup of HR expression negative lymphomas (P = 0.03). HR staining intensity was an independent prognostic factor in a multivariate analysis. These findings indicate that Hermes/CD44 molecule is associated to the determination of the metastatic potential and prognosis of non-Hodgkin's lymphomas. They also reveal a new entity among non-Hodgkin's lymphomas, because lymphomas that express low levels of HR have favorable prognosis despite their often highly malignant histological appearance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Bargatze R. F., Wu N. W., Weissman I. L., Butcher E. C. High endothelial venule binding as a predictor of the dissemination of passaged murine lymphomas. J Exp Med. 1987 Oct 1;166(4):1125–1131. doi: 10.1084/jem.166.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. The regulation of lymphocyte traffic. Curr Top Microbiol Immunol. 1986;128:85–122. doi: 10.1007/978-3-642-71272-2_3. [DOI] [PubMed] [Google Scholar]
- Camplejohn R. S., Macartney J. C., Morris R. W. Measurement of S-phase fractions in lymphoid tissue comparing fresh versus paraffin-embedded tissue and 4',6'-diamidino-2 phenylindole dihydrochloride versus propidium iodide staining. Cytometry. 1989 Jul;10(4):410–416. doi: 10.1002/cyto.990100408. [DOI] [PubMed] [Google Scholar]
- Carbone P. P., Kaplan H. S., Musshoff K., Smithers D. W., Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971 Nov;31(11):1860–1861. [PubMed] [Google Scholar]
- Clayberger C., Wright A., Medeiros L. J., Koller T. D., Link M. P., Smith S. D., Warnke R. A., Krensky A. M. Absence of cell surface LFA-1 as a mechanism of escape from immunosurveillance. Lancet. 1987 Sep 5;2(8558):533–536. doi: 10.1016/s0140-6736(87)92924-2. [DOI] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Hynes R. O. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990 Jan 12;60(1):53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- Hamann A., Jablonski-Westrich D., Duijvestijn A., Butcher E. C., Baisch H., Harder R., Thiele H. G. Evidence for an accessory role of LFA-1 in lymphocyte-high endothelium interaction during homing. J Immunol. 1988 Feb 1;140(3):693–699. [PubMed] [Google Scholar]
- Holzmann B., Weissman I. L. Integrin molecules involved in lymphocyte homing to Peyer's patches. Immunol Rev. 1989 Apr;108:45–61. doi: 10.1111/j.1600-065x.1989.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Jalkanen S. T., Bargatze R. F., Herron L. R., Butcher E. C. A lymphoid cell surface glycoprotein involved in endothelial cell recognition and lymphocyte homing in man. Eur J Immunol. 1986 Oct;16(10):1195–1202. doi: 10.1002/eji.1830161003. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Aho R., Kallajoki M., Ekfors T., Nortamo P., Gahmberg C., Duijvestijn A., Kalimo H. Lymphocyte homing receptors and adhesion molecules in intravascular malignant lymphomatosis. Int J Cancer. 1989 Nov 15;44(5):777–782. doi: 10.1002/ijc.2910440505. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Bargatze R. F., de los Toyos J., Butcher E. C. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85-95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol. 1987 Aug;105(2):983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S., Joensuu H., Klemi P. Prognostic value of lymphocyte homing receptor and S phase fraction in non-Hodgkin's lymphoma. Blood. 1990 Apr 1;75(7):1549–1556. [PubMed] [Google Scholar]
- Joensuu H., Klemi P. J., Jalkanen S. Biologic progression in non-Hodgkin's lymphoma. A flow cytometric study. Cancer. 1990 Jun 1;65(11):2564–2571. doi: 10.1002/1097-0142(19900601)65:11<2564::aid-cncr2820651128>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Miyake K., Underhill C. B., Lesley J., Kincade P. W. Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med. 1990 Jul 1;172(1):69–75. doi: 10.1084/jem.172.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nortamo P., Patarroyo M., Kantor C., Suopanki J., Gahmberg C. G. Immunological mapping of the human leucocyte adhesion glycoprotein gp90 (CD18) by monoclonal antibodies. Scand J Immunol. 1988 Nov;28(5):537–546. doi: 10.1111/j.1365-3083.1988.tb01485.x. [DOI] [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Davis L. S., Lipsky P. E. Human T lymphocyte adhesion to endothelial cells and transendothelial migration. Alteration of receptor use relates to the activation status of both the T cell and the endothelial cell. J Immunol. 1990 Jul 1;145(1):140–148. [PubMed] [Google Scholar]
- Pals S. T., Horst E., Ossekoppele G. J., Figdor C. G., Scheper R. J., Meijer C. J. Expression of lymphocyte homing receptor as a mechanism of dissemination in non-Hodgkin's lymphoma. Blood. 1989 Mar;73(4):885–888. [PubMed] [Google Scholar]
- Pals S. T., den Otter A., Miedema F., Kabel P., Keizer G. D., Scheper R. J., Meijer C. J. Evidence that leukocyte function-associated antigen-1 is involved in recirculation and homing of human lymphocytes via high endothelial venules. J Immunol. 1988 Mar 15;140(6):1851–1853. [PubMed] [Google Scholar]
- Picker L. J., Medeiros L. J., Weiss L. M., Warnke R. A., Butcher E. C. Expression of lymphocyte homing receptor antigen in non-Hodgkin's lymphoma. Am J Pathol. 1988 Mar;130(3):496–504. [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Nakache M., Butcher E. C. Monoclonal antibodies to human lymphocyte homing receptors define a novel class of adhesion molecules on diverse cell types. J Cell Biol. 1989 Aug;109(2):927–937. doi: 10.1083/jcb.109.2.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossien F. F., de Rijk D., Bikker A., Roos E. Involvement of LFA-1 in lymphoma invasion and metastasis demonstrated with LFA-1-deficient mutants. J Cell Biol. 1989 May;108(5):1979–1985. doi: 10.1083/jcb.108.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder T. F., Isaacs C. M., Ernst T. J., Demetri G. D., Adler D. A., Disteche C. M. Isolation and chromosomal localization of cDNAs encoding a novel human lymphocyte cell surface molecule, LAM-1. Homology with the mouse lymphocyte homing receptor and other human adhesion proteins. J Exp Med. 1989 Jul 1;170(1):123–133. doi: 10.1084/jem.170.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]



