Abstract
Loop-mediated isothermal amplification (LAMP), a rapid nucleic acid amplification method, was developed for the clinical diagnosis of toxoplasmosis. Three LAMP assays based on the SAG1, SAG2, and B1 genes of Toxoplasma gondii were developed. The sensitivities and specificities of the LAMP assays were evaluated by comparison with the results of conventional nested PCR. The LAMP assays were highly sensitive and had a detection limit of 0.1 tachyzoite, and no cross-reactivity with the DNA of other parasites was observed. Blood was collected from 105 individuals to test the LAMP assays: 40 patients with active toxoplasmosis, 40 negative controls, and 25 patients with other parasitic infections. The SAG2-based LAMP (SAG2-LAMP) had a greater sensitivity (87.5%) than the SAG1-LAMP (80%), B1-LAMP (80%), and nested PCR (62.5%). All the LAMP assays and nested PCR were 100% specific. This is the first report of a study which applied the LAMP method to diagnose toxoplasmosis from human blood samples. Due to its simplicity, sensitivity, and specificity, LAMP is suggested as an appropriate method for routine diagnosis of active toxoplasmosis in humans.
Toxoplasmosis is a common parasitic infection caused by an obligate intracellular parasite, Toxoplasma gondii, which is widespread throughout the world (4). The main host of the parasite is cats, but it can also be carried by the vast majority of warm-blooded animals and humans. Modes of T. gondii transmission include ingestion of oocytes from feline feces, ingestion of cysts from uncooked meat, and waterborne transmission of the oocysts (11). Although it is generally asymptomatic in healthy adults, it may provoke severe conditions in immunosuppressed individuals, including patients with AIDS. In this type of patient, T. gondii infection causes encephalitis, pneumonia, and disseminated infection (35). Transplacental transmission of the parasite occurs during the first two trimesters of pregnancy and can severely damage the fetus, which eventually causes neonatal malformations, neurological damage, blindness, or stillbirth (13).
Diagnosis of toxoplasmosis constitutes an important measure for the control of the disease. It can be achieved by a number of different methods, including the indirect fluorescent-antibody test (IFAT) (16), enzyme-linked immunosorbent assays (ELISA) (26), tissue culture, and PCR (18). Routine diagnosis of T. gondii infection based on detection and measurement of parasite-specific immunoglobulin M (IgM) in sera from patients by IFAT and ELISA is laborious, time-consuming, and expensive. Antigens used in these serological assays are usually prepared from T. gondii cells which are propagated in the mouse or in vitro culture. Such a preparation usually contains extraparasitic materials and may result in interassay variability (27). Moreover, serological methods have some limitations because specific antibody and T. gondii antigens may not be present early in the infection, especially in patients with immunodeficiencies; moreover, antibodies, particularly IgM, may not arise during reactivation of the encysted form of the parasite (3). Alternatively, the identification of acute cases of toxoplasmosis in humans could be done on the basis of the detection of T. gondii DNA in amniotic fluids, tissues, or blood. Several PCR-based techniques have periodically been developed for the diagnosis of toxoplasmosis using various clinical specimens (22, 25). Among these techniques, nested PCR followed by hybridization has been reported to be the most sensitive method (32). However, they are not widely used due to the expensive cost of the apparatus and long reaction time (18, 19). Therefore, the development of cost-effective, simple, and rapid detection methods is still needed for direct detection of T. gondii.
Loop-mediated isothermal amplification (LAMP), which was originally developed by Notomi et al. (30), is a very sensitive, easy, and less time-consuming method. The LAMP method can amplify up to 109 copies in less than an hour under isothermal conditions (65°C). A simple incubator, such as a water bath or block heater, is sufficient for the DNA amplification, making use of incubators under field conditions feasible (5, 31). The method uses four to six primers that recognize six to eight regions of the target DNA, which eliminates nonspecific binding, thereby ensuring the specificity of LAMP (30). Recently, this method was found to be a powerful diagnostic tool, and LAMP targeting the SAG1 gene (SAG1-LAMP) and the 200- to 300-fold repetitive 529-bp gene of T. gondii was successfully used for the diagnosis of toxoplasmosis in animal models, especially mice (24, 37). The SAG1-LAMP was applied to detect the presence of T. gondii in infected mouse organs. This makes the method attractive for T. gondii detection in biopsy specimens (24). LAMP based on the 529-bp repetitive gene has also been shown to be useful for detection of T. gondii DNA extracted from lymph nodes of veterinary samples (37).
In the present study, we developed three LAMP assays targeting the B1, SAG1, and SAG2 genes for the detection of T. gondii genetic material. Although most of the previous work used ocular fluid as the source of template for DNA amplification, Bou et al. (6) showed that PCR produced the same results with aqueous humor and blood samples. Thus, blood samples were used to evaluate the sensitivities of the LAMP assays. This is the first report of using LAMP for rapid diagnosis of active toxoplasmosis in human blood samples.
MATERIALS AND METHODS
Parasite culture.
Tachyzoites of the T. gondii RH strain were obtained from the Institute for Medical Research (IMR), Kuala Lumpur, Malaysia. The tachyzoites were grown in MBDK cell monolayers in RPMI medium supplemented with 10% fetal calf serum at 37°C in a 5% CO2 environment.
Clinical samples.
A total of 105 individuals were studied. The first group consisted of 40 clinically suspected toxoplasmosis patients with acute toxoplasmosis (high Toxoplasma IgM antibody titers) serologically confirmed by Captia Toxoplasma gondii IgG and IgM commercial diagnostic kits (Trinity Biotech, Ireland) and Toxoplasma IgG avidity test (NovaLisa, Dietzenbach, Germany). The second group consisted of 40 healthy volunteers who did not show antibodies against Toxoplasma antigens on the basis of the results of the same tests. The third group consisted of 25 patients with other parasitic infections (confirmed by PCR or serology tests); among these patients, 15 were positive for malaria (Plasmodium spp.) and 10 were positive for filariasis (Brugia malayi).
Analytical sensitivity and specificity of LAMP.
To determine the analytical sensitivity of the LAMP assay, T. gondii RH tachyzoites harvested from the cell culture were counted on a hemocytometer and serially diluted 10-fold to contain from 1 × 106 tachyzoites to 1 tachyzoite in 200 μl of fresh human blood. The genomic DNA was extracted from the 200 μl of solution using a DNeasy blood and tissue kit (Qiagen, Germany), in accordance with the manufacturer's instructions. Genomic DNA was eluted with 200 μl of elution buffer. The reactions were done in triplicate. Genomic DNA of Cryptosporidium parvum (oocyst obtained from human feces), Neospora caninum (oocyst obtained from dog feces), and Toxocara canis (eggs obtained from dog feces) were also extracted using the same method to determine the specificity of the Toxoplasma LAMP assay.
LAMP assay.
Three Toxoplasma-specific LAMP primer sets were designed, using the Primer Explorer program (http://primerexplorer.jp/elamp4.0.0/index.html), to amplify the SAG1, SAG2, and B1 genes (Table 1). LAMP was optimized in 25 μl of a mixture containing 2 μl of the extracted DNA, 40 pmol (each) of primers FIP and BIP, 5 pmol (each) of primers B3 and F3, and 1 μl of Bst DNA polymerase (NEB) in 2.5 μl of buffer [20 mM Tris-HCl (pH 8.8), 10 mM KCl, 8 mM MgSO4, 10 mM (NH4)2SO4, 0.1% Tween 20], 0.8 M betaine (Sigma-Aldrich), and 1.4 mM deoxynucleoside triphosphate (dNTP). The LAMP reaction was performed for 30 to 60 min at 65°C and was inactivated for 2 min at 80°C. The resulting amplicons were detected in real time by the fluorescence of SYTO-9 dye and visual observation after addition of SYBR green I. Twenty microliters of each of the LAMP products was analyzed by electrophoresis with 1.5% agarose gels, followed by ethidium bromide staining and photography.
TABLE 1.
Nucleotide sequences of LAMP primers designed in the present study
| Assay and primer | Primer sequence (5′-3′) | Target |
|---|---|---|
| SAG1-LAMP | ||
| B3 | CGAGGGTCATTGTAGTGGGT | T. gondii SAG1 gene (GenBank |
| F3 | GACAACGCAGACGTTTGTG | accession no. X14080) |
| BIP | AATGTCGCAAGGTGCTCCTACGCTTCCGCAGACAACTTGACA | |
| FIP | ACCGATGAGGCTCTGGCTTGTAGTCGGTTGCATCAAGGGAG | |
| SAG2-LAMP | ||
| B3 | GTAGCAGGACCTTTCGCG | T. gondii SAG2 gene (GenBank |
| F3 | GCGCAACGAAGACTGTTGA | accession no. M33572) |
| BIP | TGGCAAGGAATGCACAGACTCGGACCTTAGCTGTCAAGACCG | |
| FIP | CGCCACTGGGACTGATGGTTAGTGCACCCTCCAGTGGTTC | |
| B1-LAMP | ||
| B3 | ACGTGACAGTGAAGAGAGGA | T. gondii B1 gene (GenBank |
| F3 | CAGATGTGCTAAAGGCGTCA | accession no. AF179871) |
| BIP | TGTTCGCTGTCTGTCTAGGGCAGGTGGTCGACTTCATGGGA | |
| FIP | AGGCGGAACCAACGGAAATCCTTGCTGTTCTGTCCTATCGC | |
| Nested PCR | ||
| B1F1 | CCGTTGGTTCCGCCTCCTTC | T. gondii B1 gene (GenBank |
| B1R1 | GCAAAACAGCGGCAGCGTCT | accession no. AF179871) |
| B1F2 | CCGCCTCCTTCGTCCGTCGT | |
| B1R2 | GTGGGGGCGGACCTCTCTTG |
DNA sequencing of LAMP products.
To confirm the specificity of the LAMP assay for Toxoplasma detection, the PCR method was carried out with the outer primers B3 and F3. PCR amplification was performed in 25 μl of reaction mixtures containing 5 μl of PCR buffer (10×), 5 μl (200-μmol concentration) of each dNTP, 4 μl (200 nmol concentration) (each) of primers B3 and F3, and 0.25 μl (2.5 U) of AmpliTaq Gold polymerase (Applied Biosystems, Darmstadt, Germany). Initial denaturation was done at 95°C for 10 min, followed by 35 cycles of denaturation (45 s at 95°C), annealing (60 s at 60°C), and extension (90 s at 72°C). Five-microliter aliquots of the PCR products were subjected to electrophoresis on a 1.5% agarose gel and visualized under UV light. Purification of the PCR products was performed by a QIAquick PCR purification kit (Qiagen), using the microcentrifuge protocol, according to the manufacturer's instructions. PCR products were sent for sequencing (NHK Bioscience Solution, Malaysia).
Clinical sensitivity and specificity.
The sensitivities and specificities of the LAMP assays in comparison to the results of the nested PCR were determined using 105 blood samples from the three groups of individuals. Genomic DNA was extracted from 100 μl of blood samples using a Qiagen blood and tissue purification kit (Qiagen), in accordance with the manufacturer's instructions. The purified DNA was dissolved in 30 μl of double-distilled water for subsequent LAMP and PCRs. Two microliters of the resulting supernatant was used as the template for nested PCR and the LAMP assay.
On the other hand, genomic DNA was also isolated by using an FTA card, which is a much cheaper and simpler method. For this approach, 100 μl of blood from the three groups of individuals was blotted and dried on an FTA card (Whatman, United Kingdom). The blotted blood was cut out with a 2-mm hole puncher (2.0-mm Harris micropunch; Whatman). A portion of the blotted blood was then washed three times with 100 μl of FTA purification reagent (Whatman) and eluted in 30 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0). Two microliters of the resulting supernatant was used as a template for the LAMP assay.
Sensitivity was calculated as (number of true positives)/(number of true positives + number of false negatives), and specificity was calculated as (number of true negatives)/(number of true negatives + number of false positives).
Nested PCR.
Two pairs of oligonucleotide primers directed against the B1 gene of T. gondii (8) were used to perform a nested PCR. The first round of PCR amplification contained 10 mM Tris-HCl, pH 8.3 (at 25°C), 50 mM KCl, 1.5 mM MgCl2, 0.1 μM each primer (Table 1), 0.2 mM each dNTP, 2.5 U Taq DNA polymerase, and 2 μl of extracted DNA. Reactions were cycled 35 times with denaturation at 94°C for 1 min, followed by annealing at 54°C for 30 s and finally an extension step at 72°C for 1 min. A PCR negative-control sample omitted template DNA, which was replaced by sterile water. Nested PCR mixtures contained 2 μl first-round product, 10 mM Tris-HCl, pH 8.3 (at 25°C), 50 mM KCl, 3 mM MgCl2, 0.5 μM each primer (Table 1), 0.2 mM each dNTP, and 1 U Taq DNA polymerase. Nested PCR was cycled 35 times using a denaturation step of 94°C for 10 s, followed by annealing at 60°C for 10 s and extension at 72°C for 15 s. Negative-control samples from first-round amplification and an additional second-round negative control of sterile water were included in the nested PCRs. The PCR products were electrophoresed in a 1% TAE (Tris-acetate-EDTA) agarose gel and stained with ethidium bromide solution (1 μg/ml). The PCR amplification is expected to yield a product of 213 bp for the positive reaction.
RESULTS
Detection and confirmation of LAMP product.
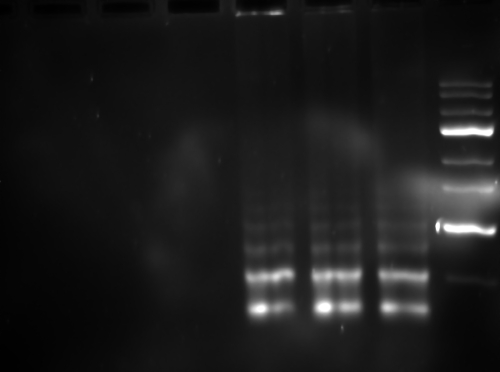
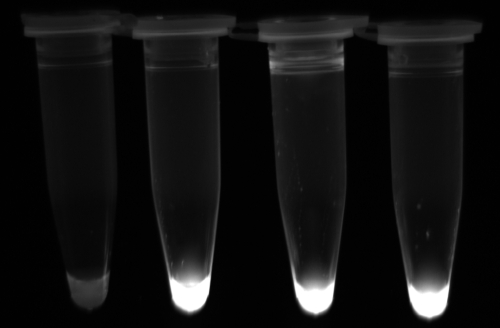
Three LAMP assays based on three genes, SAG1, SAG2, and B1, were developed (Table 1). All the positive LAMP reactions produced a typical ladder of multiple bands on the agarose gel (Fig. 1), indicating that stem-loop DNA with inverted repeats of the target sequence was produced. Moreover, positive reactions turned green on addition of SYBR green I to the PCR tube, while the negative ones remained orange (Fig. 2). PCR was used to amplify these LAMP products, which showed 100% identity with the target sequences after sequencing (data not shown).
FIG. 1.
Electrophoresis of LAMP products amplified from T. gondii tachyzoite genomic DNA. Lanes, from left to right, respectively: no template (negative control of SAG1-LAMP, SAG2-LAMP, and B1-LAMP); SAG1-LAMP; SAG2-LAMP; B1-LAMP, and 100-bp DNA ladder.
FIG. 2.
Detection of T. gondii in positive controls and clinical samples by LAMP. Turbidities of the LAMP reactions were inspected visually. Positive reactions turned green under UV light after the addition of SYBR green I. Tubes, from left to right, respectively: negative control, SAG1-LAMP, SAG2-LAMP, and B1-LAMP.
Optimization of LAMP assay.
The best results were obtained when the reaction temperature was maintained at 65°C. The results of the LAMP assay were identical with either a water bath, a heater block, or a PCR machine, demonstrating its robustness.
Analytical sensitivities and specificities of LAMP assays versus results of nested PCR.
The analytical sensitivities of all the LAMP assays were evaluated against a 10-fold serial dilution of T. gondii tachyzoite DNA. The detection limit of LAMP assays targeting SAG1, SAG2, and B1 is 0.1 tacyhzoite, whereas the detection limit of nested PCR targeted at the B1 gene is 1 tachyzoite (data not shown).
The LAMP assays were tested for their specificities using heterogeneous DNA samples as controls. No amplification was observed with DNA samples from C. parvum, N. caninum, and T. canis (data not shown).
Clinical sensitivities and specificities of LAMP assays versus results of nested PCR.
The two DNA extraction methods are equally efficient in extracting DNA from T. gondii tachyzoites. Analysis of the samples by the LAMP assays and nested PCR revealed no difference in sensitivity between the two DNA extraction methods. The SAG2-LAMP produced positive results for 35 of the 40 blood samples from patients with active toxoplasmosis, while SAG1-LAMP and B1-LAMP produced positive results only for 32 of the cases. DNA fragments of the correct size were amplified by nested PCR from 25 of the 40 blood samples (Table 2). No amplification of DNA fragments by the LAMP assays and nested PCR was seen for all the negative controls, which included 40 blood samples from healthy volunteers and 25 blood samples from patients with other parasitic infections.
TABLE 2.
Detection of T. gondii by conventional nested PCR and LAMP
| Assay and result | No. (%) of samples with the following serology test result: |
|
|---|---|---|
| Positive | Negative | |
| SAG1-LAMP | ||
| Positive | 32 (80) | 0 |
| Negative | 8 (20) | 65 (100) |
| SAG2-LAMP | ||
| Positive | 35 (87.5) | 0 |
| Negative | 5 (12.5) | 65 (100) |
| B1-LAMP | ||
| Positive | 32 (80) | 0 |
| Negative | 8 (20) | 65 (100) |
| B1 nested PCR | ||
| Positive | 25 (62.5) | 0 |
| Negative | 15 (37.5) | 65 (100) |
| Total no. (%) | 40 (100) | 65 (100) |
DISCUSSION
In this study, three LAMP assays based on the SAG1, SAG2, and B1 genes were tested for their sensitivities in detecting T. gondii. These genes were selected because of their frequent use for the detection of T. gondii in clinical specimens (9, 10). To our knowledge, this is the first report of using a LAMP assay for the diagnosis of T. gondii infection using human blood samples. We were able to demonstrate the successful amplification of T. gondii DNA within 60 min at 65°C using the LAMP assay (Fig. 1).
When pure tachyzoite DNA was used, the LAMP assays (detection limit, 0.1 tachyzoite) were 10 times more sensitive than the nested PCR (detection limit, 1 tachyzoite) for detection of T. gondii DNA. The high sensitivity of LAMP is due to the four primers used in the reaction, which target six distinct internal regions on the target DNA, and the autocycling amplification can produce very large amounts of DNA of various sizes (29). These findings are comparable to those presented in previous reports that the sensitivity of the LAMP method is higher than that of the nested PCR for detection of protozoan parasites such as Trypanosoma spp., Theileria spp., Babesia spp., and T. gondii (1, 21, 34, 37). The LAMP assays and nested PCR demonstrated sensitivities slightly better than those shown by Zhang et al. (37), where the detection limits of PCR and LAMP assay (targeted at the 200- to 300-fold repetitive 529 bp of T. gondii) were 10 tachyzoites and 1 tachyzoite, respectively.
LAMP requires four primers, which amplify the target DNA with high specificity. We confirmed the specificity of the LAMP assays by screening different DNAs derived from parasites which could be found in water samples (C. parvum) and blood samples (T. brucei and T. canis) and from parasites phylogenetically close to Toxoplasma, like N. caninum. No cross-reactivity with DNA samples of C. parvum, N. caninum, and T. canis was seen.
Another advantage of LAMP is that the positive amplifications can be easily detected and distinguished from the negative amplifications without the need for gel electrophoresis. This is done simply by observing the turbidity of the end product in the tube. This is because LAMP assay produces magnesium pyrophosphate as a by-product which is visible as white precipitation (28). LAMP products could be visualized with or without addition of a DNA intercalating dye, SYBR green I, to the product in the PCR tube (Fig. 2); this was done without the need for gel electrophoresis, which greatly reduces the time taken for result analysis. However, there is a high risk of contamination, since the tubes have to be opened to add the dye. To reduce the chances of contamination, gloves should be changed regularly and sterile pipetting techniques should be applied. In the present study, amplification of the target sequence was double confirmed by sequence analysis.
Nested PCR, mostly targeted at the B1 gene, was used to detect T. gondii DNA in various biological samples with sensitivities of between 6 and 60%, but specificities ranged from 75 to 100% (7, 15, 17, 33, 36). Cerebrospinal and ocular fluids are the most common biological samples used in PCR (6, 12, 20, 23). However, Bou et al. (6) showed that PCR using blood samples from patients with ocular toxoplasmosis produced results identical to those obtained with aqueous humor samples; this can avoid all the complications resulting from the ocular puncture. Thus, in this study, we evaluate the clinical sensitivities and specificities of the LAMP assays based on blood samples and compare the results with those of conventional nested PCR. The results of the LAMP assays show that they have high sensitivities (80 to 87.5%) for detection of T. gondii in blood samples. No false-positive results were obtained with blood samples from healthy volunteers and patients with other parasitic infections, so the specificity was 100%. Nested PCR amplified DNA from 25 of the 40 positive blood samples, representing 62.5% sensitivity (Table 2). Our results by nested PCR were similar to those found by Angel et al. (58.8%) using the same method with blood samples from patients with acute toxoplasmic lymphadenopathy (ATL) (2). According to the authors, the rate of detection increased to 76.4% by combining the dot blot test and nested PCR for ATL patients (2). However, the diagnostic values of the LAMP assays were still superior among all these tests for the detection of T. gondii DNA (80 to 87.5%), at least in blood samples.
In conclusion, the LAMP assay is a promising new technique which is simple, cheap, and rapid for diagnosis of active Toxoplasma infection using blood samples and which has a detection limit of the equivalent of 0.1 tachyzoite. Since the LAMP assay and reading of results require minimum equipment, the technique has great potential for use in countries where toxoplasmosis is endemic, like Malaysia, as an alternative to the existing tests. This study also showed that preparation of DNA template using FTA cards can be a better option for DNA extraction in terms of the cheaper cost and shortening the duration for preparation.
Acknowledgments
This work was funded by the University of Malaya, UMRG grant.
We thank the University of Malaya Medical Centre (UMMC) and Sarawak General Hospital, Malaysia, for providing the patient samples.
Footnotes
Published ahead of print on 21 July 2010.
REFERENCES
- 1.Alhassan, A., Y. Govind, N. T. Tam, O. M. Thekisoe, N. Yokoyama, N. Inoue, and I. Igarashi. 2007. Comparative evaluation of the sensitivity of LAMP, PCR and in vitro culture methods for the diagnosis of equine piroplasmosis. Parasitol. Res. 100:1165-1168. [DOI] [PubMed] [Google Scholar]
- 2.Angel, S. O., M. Matrajt, J. Margarit, M. Nigro, E. Illescas, V. Pszenny, M. R. Amendoeira, E. Guarnera, and J. C. Garberi. 1997. Screening for active toxoplasmosis in patients by DNA hybridization with the ABGTg7 probe in blood samples. J. Clin. Microbiol. 35:591-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashburn, D., M. M. Davidson, A. W. Joss, T. H. Pennington, and D. O. Ho-Yen. 1998. Improved diagnosis of reactivated toxoplasmosis. Mol. Pathol. 51:105-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhopale, G. M. 2003. Development of a vaccine for toxoplasmosis: current status. Microbes Infect. 5:457-462. [DOI] [PubMed] [Google Scholar]
- 5.Boehme, C. C., P. Nabeta, G. Henastroza, R. Rubhana, Z. Rahim, M. Gerhardt, E. Sanga, M. Hoelscher, T. Notomi, T. Hase, and M. D. Perkins. 2007. Operational feasibility of using loop-mediated isothermal amplification (LAMP) for the diagnosis of pulmonary TB in microscopy centers of developing countries. J. Clin. Microbiol. 45:1936-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bou, G., M. S. Figueroa, P. Marti-Belda, E. Navas, and A. Guerrero. 1999. Value of PCR for detection of Toxoplasma gondii in aqueous humor and blood samples from immunocompetent patients with ocular toxoplasmosis. J. Clin. Microbiol. 37:3465-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brezin, A. P., L. Kasner, P. Thulliez, Q. Li, F. Daffos, R. B. Nusseblatt, and C. Chan. 1994. Ocular toxoplasmosis in the fetus: immunohistochemistry analysis and DNA amplification. Retina 14:19-26. [PubMed] [Google Scholar]
- 8.Burg, J. L., C. M. Grover, P. Pouletty, and J. C. Boothroyd. 1989. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J. Clin. Microbiol. 27:1787-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calderaro, A., G. Piccolo, C. Gorrini, S. Peruzzi, L. Zrebini, S. Bommezzadri, G. Dettori, and C. Chezzi. 2006. Comparison between two real-time PCR assays and a nested-PCR for the detection of Toxoplasma gondii. Acta Biomed. 77:75-80. [PubMed] [Google Scholar]
- 10.Dorota, N., C. Iris, S. R. Jack, G. Michael, G. Elzbieta, J. Wilczynski, and L. S. David. 2006. Genotyping of Toxoplasma gondii by multiplex PCR and peptide-based serological testing of samples from infants in Poland diagnosed with congenital toxoplasmosis. J. Clin. Microbiol. 44:1382-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubey, J. P. 2004. Toxoplasmosis—a waterborne zoonosis. Vet. Parasitol. 126:57-72. [DOI] [PubMed] [Google Scholar]
- 12.Dupon, M., J. Cazenave, J. L. Pellegrin, J. M. Ragnaud, A. Cheyrou, I. Fischer, B. Leng, and J. Y. Lacut. 1995. Detection of Toxoplasma gondii by PCR and tissue culture in cerebrospinal fluid and blood of human immunodeficiency virus-seropositive patients. J. Clin. Microbiol. 33:2421-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fatoohi, A. F., G. J. Cozon, T. Greenland, J. Ferrandiz, J. Bienvenu, S. Picot, and F. Peyron. 2002. Cellular immune responses to recombinant antigens in pregnant women chronically infected with Toxoplasma gondii. Clin. Diagn. Lab. Immunol. 9:704-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reference deleted.
- 15.Franzen, C., M. Altfeld, P. Hegener, P. Hartmann, G. Arendt, H. Jablonowski, J. Rockstroh, V. Diehl, B. Salzberger, and G. Fatkenheuer. 1997. Limited value of PCR for detection of Toxoplasma gondii in blood from human immunodeficiency virus-infected patients. J. Clin. Microbiol. 35:2639-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia, J. L., I. T. Navarro, O. Vidotto, S. M. Gennari, R. Z. Machado, A. B. da Luz Pereira, and I. L. Sinhorini. 2006. Toxoplasma gondii: comparison of a rhoptry-ELISA with IFAT and MAT for antibody detection in sera of experimentally infected pigs. Exp. Parasitol. 113:100-105. [DOI] [PubMed] [Google Scholar]
- 17.Garweg, J., M. Boehnke, and F. Koerner. 1996. Restricted applicability of the polymerase chain reaction for the diagnosis of ocular toxoplasmosis. Ger. J. Ophthalmol. 5:104-108. [PubMed] [Google Scholar]
- 18.Gross, U., A. Roggenkamp, K. Janitschke, and J. Heesemann. 1992. Improved sensitivity of the polymerase chain reaction for detection of Toxoplasma gondii in biological and human clinical specimens. Eur. J. Clin. Microbiol. Infect. Dis. 11:33-39. [DOI] [PubMed] [Google Scholar]
- 19.Guy, E. C., H. Pelloux, M. Lapppalainen, H. Aspock, A. Habl, K. K. Melby, M. Holberg-Pettersen, E. Petersen, J. Simon, and P. Ambroise-Thomas. 1996. Interlaboratory comparison of polymerase chain reaction for the detection of Toxoplasma gondii DNA added to samples of amniotic fluid. Eur. J. Clin. Microbiol. Infect. Dis. 15:836-839. [DOI] [PubMed] [Google Scholar]
- 20.Holliman, R. E., J. D. Johnson, S. H. Gillespie, M. A. Johnson, S. B. Squire, and D. Savva. 1991. New methods in the diagnosis and management of cerebral toxoplasmosis associated with the acquired immune deficiency syndrome. J. Infect. 22:281-285. [DOI] [PubMed] [Google Scholar]
- 21.Iseki, H., A. Alhassan, N. Ohta, O. M. Thekisoe, N. Yokoyama, N. Inoue, A. Nambota, J. Yasuda, and I. Igarashi. 2007. Development of a multiplex loop-mediated isothermal amplification (mLAMP) method for the simultaneous detection of bovine Babesia parasites. J. Microbiol. Methods 71:281-287. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, J. D., P. D. Butcher, D. Savva, and R. E. Holliman. 1993. Application of the polymerase chain reaction to the diagnosis of human toxoplasmosis. J. Infect. 26:147-158. [DOI] [PubMed] [Google Scholar]
- 23.Jones, C. D., N. Okharavi, P. Adamson, S. Tasker, and S. Lightman. 2000. Comparison of PCR detection methods for B1, P30 and 18S rDNA genes of Toxoplasma gondii in aqueous humor. Invest. Ophthalmol. Vis. Sci. 41:634-644. [PubMed] [Google Scholar]
- 24.Krasteva, D., M. Toubiana, S. Hartati, A. Kusumawati, J. F. Dubremetz, and J. S. Widada. 2009. Development of loop-mediated isothermal amplification (LAMP) as a diagnostic tool of toxoplasmosis. Vet. Parasitol. 162:327-331. [DOI] [PubMed] [Google Scholar]
- 25.Lee, P. Y. C., J. Mangan, R. E. Holliman, and P. D. Butcher. 1999. Quantitation of Toxoplasma gondii DNA in a competitive nested polymerase chain reaction. J. Clin. Pathol. 52:61-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meek, B., R. Jan Diepersloot, T. van Gool, D. Speijer, and R. Peek. 2003. IgM recognition of recombinant Toxoplasma gondii antigens by sera of acutely or latently infected humans. J. Diagn. Microbiol. Infect. Dis. 45:45-52. [DOI] [PubMed] [Google Scholar]
- 27.Montoya, J. G. 2002. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J. Infect. Dis. 185:S73-S82. [DOI] [PubMed] [Google Scholar]
- 28.Mori, Y., K. Nagamine, N. Tomita, and T. Notori. 2001. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 289:150-154. [DOI] [PubMed] [Google Scholar]
- 29.Nagamine, K., T. Hase, and T. Notomi. 2002. Accelerated reaction by loop mediated isothermal amplification using loop primers. Mol. Cell. Probes 16:223-229. [DOI] [PubMed] [Google Scholar]
- 30.Notomi, T., O. Hiroto, M. Harumi, Y. Toshihiro, K. Watanabe, A. Nobuyuki, and T. Hase. 2000. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28:E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poon, L. L., B. W. Wong, E. H. Ma, K. H. Chan, L. M. Chow, W. Abeyewickreme, N. Tangpukdee, K. Y. Yuen, Y. Guan, S. Looareesuwan, and J. S. Peiris. 2006. Sensitive and inexpensive molecular test for falciparum malaria: detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin. Chem. 52:303-306. [DOI] [PubMed] [Google Scholar]
- 32.Pujol-Rique, M., F. Derouin, A. Garcia-Quintanilla, M. E. Valls, J. M. Miro, and M. T. Jimenez de Anta. 1999. Design of a one tube hemi-nested PCR for the detection of Toxoplasma gondii and comparison of three DNA purification methods. J. Med. Microbiol. 48:857-862. [DOI] [PubMed] [Google Scholar]
- 33.Robert, F., T. Ouatas, P. Blanche, C. Tourte-Schaefer, D. Sicard, and J. Dupouy-Camet. 1996. Evaluation re′trospective de la détection de Toxoplasma gondii par réaction de polymérisation en chaıne chez des patients sidéns. Presse Med. 25:541-545. [PubMed] [Google Scholar]
- 34.Thekisoe, O. M., N. Inoue, N. Kuboki, D. Tuntasuvan, W. Bunnoy, S. Borisutsuwan, I. Igarashi, and C. Sugimoto. 2005. Evaluation of loop-mediated isothermal amplification (LAMP), PCR and parasitological tests for detection of Trypanosoma evansi in experimentally infected pigs. Vet. Parasitol. 30:327-330. [DOI] [PubMed] [Google Scholar]
- 35.Thomas, P. A. 2001. Parasitic diseases and immunodeficiencies. Parasitology 122:S65-S71. [DOI] [PubMed] [Google Scholar]
- 36.Verbraak, F. D., M. Galema, G. H. Van den Horn, M. Bruinenberg, L. Luyendijk, S. A. Danner, and A. Kijlstra. 1996. Serological and polymerase chain reaction-based analysis of aqueous humor samples in patients with AIDS and necrotizing retinitis. AIDS 10:1091-1099. [PubMed] [Google Scholar]
- 37.Zhang, H., O. M. Thekisoe, G. O. Aboge, H. Kyan, J. Yamagishi, N. Inoue, Y. Nishikawa, S. Zakimi, and X. Xuan. 2009. Toxoplasma gondii: sensitive and rapid detection of infection by loop-mediated isothermal amplification (LAMP) method. Exp. Parasitol. 122:47-50. [DOI] [PubMed] [Google Scholar]