Abstract
In accordance with recent WHO recommendations, this study evaluates the sensitivities of PCR and microscopy for fine-needle aspiration (FNA) versus techniques involving swabs and punch biopsy specimens and suggests that FNA can replace punch biopsies for nonulcerative lesions and may serve as an alternative for ulcerative lesions in cases where scarred edges prevent the collection of swabs.
Buruli ulcer disease (BUD), caused by Mycobacterium ulcerans, is an emerging disease predominantly affecting West and Central Africa. BUD initially presents as a painless nodule, papule, and plaque (nonulcerative lesions), evolving into a painless ulcer with characteristically undermined edges (ulcerative lesions). Scarring and contractures may cause severe functional disability (9, 11, 12). Among the currently available diagnostic laboratory methods (microscopy, culture, PCR, and histopathology), PCR provides the highest sensitivity and is therefore regarded as the method of choice for laboratory confirmation. The WHO encourages all countries where BUD is endemic to ensure PCR confirmation of at least 50% of all cases (1, 12, 13). With the introduction of antimycobacterial treatment, laboratory confirmation of suspect cases became crucial for clinical management of the disease (6, 12-15). Swabs, punch biopsy specimens, and surgically excised tissue are suitable diagnostic samples (3, 5-7). Recently, the WHO recommended fine-needle aspiration (FNA) as a minimally invasive method for nonulcerative lesions as well as for ulcerative lesions where scarring of edges prevents collection of swab samples (15).
The present study retrospectively compares the sensitivities of PCR and microscopy for FNA samples, swabs, punch biopsy specimens, and surgically excised tissue.
From February 2008 until December 2008, 173 clinically suspected BUD cases from Ghana (n = 112) and Togo (n = 61) were included in the study. FNA was performed with 21-gauge needles by transdermal aspiration. The needle was inserted into the center of the nonulcerative lesions or the subcutaneous tissue of the ulcer (the maximal distance from the margins was 1 to 2 cm) and was moved back and forth about three times in different directions under suction without withdrawal of the needle. Swabs, 3-mm punch biopsy specimens, and surgically excised tissue were taken according to standardized procedures, and all samples were stored in transport media as previously described and forwarded to the laboratories (5). In Ghana, 68 swabs, 112 FNA samples, 108 punch biopsy specimens, and 14 surgically excised tissue samples were subjected to microscopy and dry-reagent-based IS2404 PCR at the Kumasi Centre for Collaborative Research in Tropical Medicine, Kumasi (5, 10). The samples from Togo (43 swabs, 61 FNA samples, 45 punch biopsy specimens, and 7 surgically excised tissue samples) were analyzed at the Centre National de Référence et de Traitement d'Ulcère de Buruli, Tsévié (microscopy), and the Department of Infectious Diseases and Tropical Medicine, University of Munich, Germany (IS2404 standard PCR), in accordance with standardized procedures (5, 10).
One hundred ten suspects (63.6%) with 37 (33.6%) nonulcerative lesions and 73 (66.4%) ulcerative lesions were confirmed by at least one positive test result. The categories of the lesions according to the WHO definitions were known for 107 of these cases (12) (category I, 49 cases [45.8%]; category II, 44 cases [41.1%]; and category III, 14 cases [13.1%]).
Among the 37 nonulcerative cases, the sensitivities of PCR, defined as the number of positive test results divided by the number of laboratory-confirmed cases (5), were 88.9% (32/36) for FNA samples and 87.5% (28/32) for punch biopsy specimens, and the sensitivities of microscopy were 58.3% (21/36) for FNA samples and 55.6% (15/27) for punch biopsy specimens. For both tests, there was no significant difference in sensitivity between the two types of samples (the P value for PCR [PPCR] was 0.86, and the P value for microscopy [Pmicroscopy] was 0.83) (Table 1).
TABLE 1.
Sensitivities of dry-reagent-based IS2404 PCR and microscopic examinationa
| Type of lesion and diagnostic test | Sensitivity (%) (no. of positive results/no. of tested specimens)b |
Pc |
||||||
|---|---|---|---|---|---|---|---|---|
| Swab | FNA | PB | TS | FNA vs swab | FNA vs PB | FNA vs TS | Swab vs PB | |
| Nonulcerative (n = 37) | ||||||||
| PCR | NA | 88.9 (32/36) | 87.5 (28/32) | NC (3/3) | 0.86 | |||
| Microscopic examination | NA | 58.3 (21/36) | 55.6 (15/27) | NC (1/2) | 0.83 | |||
| Ulcerative (n = 73) | ||||||||
| PCR | 75.0 (51/68) | 55.6 (40/72) | 66.2 (43/65) | 30.0 (3/10) | 0.02* | 0.20 | 0.13 | 0.26 |
| Microscopic examination | 46.4 (32/69) | 22.2 (16/72) | 37.5 (18/48) | 20.0 (1/5) | <.01* | 0.07 | 0.91 | 0.34 |
The diagnostic results for swabs, fine-needle aspirates (FNA), punch biopsy specimens (PB), and surgically excised tissue (TS) from 110 laboratory-confirmed BUD cases presenting with nonulcerative (n = 37) and ulcerative (n = 73) lesions from Ghana (Agogo Presbyterian Hospital, Agroyesum Hospital, Apromase Hospital, and Dunkwa Governmental Hospital) and Togo (Tsévié Regional Hospital) were analyzed. For subgroups with sample sizes of <5, the sensitivity was not calculated (NC). NA, not applicable.
The P values comparing the sensitivities of 2 tests for laboratory diagnosis of BUD (PCR and microscopic examination), stratified into 4 techniques of specimen collection (involving swabs, fine-needle aspirates [FNA], punch biopsy specimens [PB], and surgically excised tissue [TS]), were as follows: for swabs, <0.01 for ulcerative lesions; for FNA, <0.01 for nonulcerative and ulcerative lesions; for PB, <0.01 for nonulcerative and ulcerative lesions; and for TS, 0.68 for ulcerative lesions. P values of <0.05 were considered significant.
The P values comparing the sensitivities of 2 out of 4 techniques of specimen collection (involving swabs, fine-needle aspirates [FNA], punch biopsy specimens [PB], and surgically excised tissue [TS]), stratified into 2 tests for laboratory diagnosis of BUD (PCR and microscopic examination), are given. P values of <0.05 were considered significant (*).
Among the 73 ulcerative cases, the sensitivities of PCR were 75.0% (51/68) for swabs, 55.6% (40/72) for FNA samples, 66.2% (43/65) for punch biopsy specimens, and 30.0% (3/10) for surgically excised tissue. The sensitivities of microscopy were 46.4% (32/69) for swabs, 22.2% (16/72) for FNA samples, 37.5% (18/48) for punch biopsy specimens, and 20.0% (1/5) for surgically excised tissue. For both tests, the sensitivities for FNA samples were significantly lower than the sensitivities for swabs (PPCR = 0.02; Pmicroscopy <0.01) but not significantly different from the sensitivities for punch biopsy specimens (PPCR, 0.20, and Pmicroscopy, 0.07) or surgically excised tissue (PPCR, 0.13, and Pmicroscopy, 0.91) (Table 1).
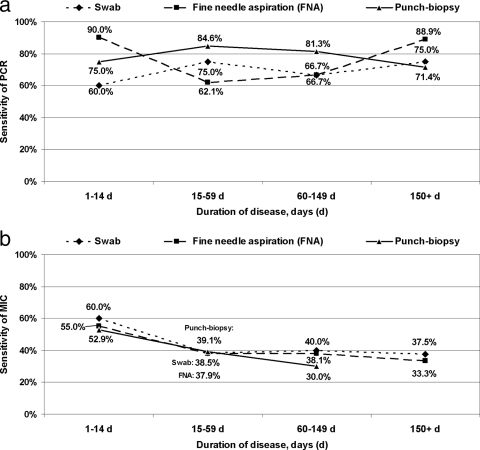
There was no significant correlation between category of lesion and sensitivity of PCR and microscopy for swabs, FNA samples, and punch biopsy specimens (PPCR-swab, 0.25;PPCR-FNA, 0.48; PPCR-punch, 0.15; Pmicroscopy-swab, 0.23; Pmicroscopy-FNA, 0.55; and Pmicroscopy-punch, 0.06). As shown in Fig. 1 a and b, for 79 cases without previous antimycobacterial treatment, sensitivity of PCR was not associated with duration of disease (R2PCR-swab [coefficient of determination], 0.42; R2PCR-FNA, <0.01; and R2PCR-punch, 0.09); however, a negative trend was noted for microscopy (R2microscopy-swab, 0.63; R2microscopy-FNA, 0.77; and R2microscopy-punch, 0.99). Also, after stratification into nonulcerative and ulcerative lesions, no correlation between test sensitivity and the independent variables involving category of lesion and duration of disease was found (data not shown). For 69 ulcerative lesions (44 untreated and 25 treated for ≥28 days), sensitivity of PCR and microscopy for swabs, FNA samples, and punch biopsy specimens were not significantly correlated with duration of treatment (PPCR-swab, 0.42; PPCR-FNA, 0.16; PPCR-punch, 0.40; Pmicroscopy-swab, 0.28; Pmicroscopy-FNA, 0.29; Pmicroscopy-punch, 0.07).
FIG. 1.
Correlation between sensitivity of IS2404 PCR (a) and microscopy (MIC) (b) and duration of disease among 79 laboratory-confirmed BUD patients (35 nonulcerative lesions and 44 ulcerative lesions) without previous antimycobacterial treatment. The analysis includes swabs (from ulcerative lesions), FNA samples, and punch biopsy specimens (from ulcerative and nonulcerative lesions). The patients were divided into four groups according to duration of disease (1 to 14 days, 15 to 59 days, 60 to 149 days, and ≥150 days). Due to a sample size of <5, calculation of the sensitivity of microscopy for punch biopsy specimens was not possible (b). Linear trends in proportions are shown as coefficient of determination (R2) for PCR (R2swab, 0.42; R2FNA, <0.01; R2punch, 0.09) and for microscopy (R2swab, 0.63; R2FNA, 0.77; R2punch, 0.99).
In the past, punch biopsy specimens were considered suitable diagnostic samples for laboratory confirmation of BUD (5, 7). However, due to the invasive character of the sample collection method, a consensus has been reached that in the interest of the patient, the method should be restricted to special diagnostic questions (e.g., differential diagnosis or identification of failure and recurrent cases) and that other methods should be applied for routine laboratory confirmation. Our findings regarding PCR assessment of FNA from nonulcerative lesions are in line with three recent studies from Ghana (8) and Benin (2, 4). All groups report sensitivities around 90%, which equals the sensitivity for punch biopsy specimens according to our data and the findings of Eddyani et al. (4). We also determined equal sensitivities for microscopy of FNA samples (corresponding to 65%, as reported by Eddyani et al. [4]) and punch biopsy specimens from nonulcerative lesions. For ulcerative lesions, available data, including our own, suggest that for both diagnostic tests, swabs are clearly superior to tissue samples (2) and that there are no significant differences in sensitivity between the use of FNA samples and that of punch biopsy specimens (4, 8).
In conclusion, FNA can replace punch biopsies for nonulcerative lesions and may serve as an alternative for ulcerative lesions in cases where scarred edges prevent the collection of swabs.
So far, only two studies report data on the sensitivity of FNA cultures, with values ranging from 10 to 44% (4, 8). As certain diagnostic questions still require assessment of cultures, further studies are needed to resolve the issue if FNA can be considered adequate diagnostic samples for culturing as well.
Acknowledgments
The study was supported by the European Commission (project no. INCO-CT-2005-015476-BURULICO) and the German Leprosy and Tuberculosis Relief Association, Würzburg, Germany.
Footnotes
Published ahead of print on 25 August 2010.
REFERENCES
- 1.Beissner, M., K. H. Herbinger, and G. Bretzel. 2010. Laboratory diagnosis of Buruli ulcer disease. Fut. Microbiol. 5:363-370. [DOI] [PubMed] [Google Scholar]
- 2.Cassisa, V., A. Chauty, E. Marion, M. F. Ardant, S. Eyangoh, J. Cottin, J. Aubry, H. Koussemou, B. Lelievre, S. Ferec, F. Tekaia, C. Johnson, and L. Marsollier. 2010. Use of fine-needle aspiration for diagnosis of Mycobacterium ulcerans infection. J. Clin. Microbiol. 48:2263-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eddyani, M., M. Debacker, A. Martin, J. Aguiar, C. R. Johnson, C. Uwizeye, K. Fissette, and F. Portaels. 2008. Primary culture of Mycobacterium ulcerans from human tissue specimens after storage in semisolid transport medium. J. Clin. Microbiol. 46:69-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eddyani, M., A. G. Fraga, F. Schmitt, C. Uwizeye, K. Fissette, C. Johnson, J. Aguiar, G. Sopoh, Y. Barrogui, W. M. Meyers, J. Pedrosa, and F. Portaels. 2009. Fine-needle aspiration, an efficient sampling technique for bacteriological diagnosis of nonulcerative Buruli ulcer. J. Clin. Microbiol. 47:1700-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbinger, K. H., O. Adjei, N. Y. Awua-Boateng, W. A. Nienhuis, L. Kunaa, V. Siegmund, J. Nitschke, W. Thompson, E. Klutse, P. Agbenorku, A. Schipf, S. Reu, P. Racz, B. Fleischer, M. Beissner, E. Fleischmann, K. Helfrich, T. S. van der Werf, T. Loscher, and G. Bretzel. 2009. Comparative study of the sensitivity of different diagnostic methods for the laboratory diagnosis of Buruli ulcer disease. Clin. Infect. Dis. 48:1055-1064. [DOI] [PubMed] [Google Scholar]
- 6.Nienhuis, W. A., Y. Stienstra, W. A. Thompson, P. C. Awuah, K. M. Abass, W. Tuah, N. Y. Awua-Boateng, E. O. Ampadu, V. Siegmund, J. P. Schouten, O. Adjei, G. Bretzel, and T. S. van der Werf. 2010. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet 375:664-672. [DOI] [PubMed] [Google Scholar]
- 7.Phillips, R., C. Horsfield, S. Kuijper, A. Lartey, I. Tetteh, S. Etuaful, B. Nyamekye, P. Awuah, K. M. Nyarko, F. Osei-Sarpong, S. Lucas, A. H. Kolk, and M. Wansbrough-Jones. 2005. Sensitivity of PCR targeting the IS2404 insertion sequence of Mycobacterium ulcerans in an Assay using punch biopsy specimens for diagnosis of Buruli ulcer. J. Clin. Microbiol. 43:3650-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips, R. O., F. S. Sarfo, F. Osei-Sarpong, A. Boateng, I. Tetteh, A. Lartey, E. Adentwe, W. Opare, K. B. Asiedu, and M. Wansbrough-Jones. 2009. Sensitivity of PCR targeting Mycobacterium ulcerans by use of fine-needle aspirates for diagnosis of Buruli ulcer. J. Clin. Microbiol. 47:924-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schunk, M., W. Thompson, E. Klutse, J. Nitschke, K. Opare-Asamoah, R. Thompson, E. Fleischmann, V. Siegmund, K. H. Herbinger, O. Adjei, B. Fleischer, T. Loscher, and G. Bretzel. 2009. Outcome of patients with buruli ulcer after surgical treatment with or without antimycobacterial treatment in Ghana. Am. J. Trop. Med. Hyg. 81:75-81. [PubMed] [Google Scholar]
- 10.Siegmund, V., O. Adjei, J. Nitschke, W. Thompson, E. Klutse, K. H. Herbinger, R. Thompson, F. van Vloten, P. Racz, B. Fleischer, T. Loescher, and G. Bretzel. 2007. Dry reagent-based polymerase chain reaction compared with other laboratory methods available for the diagnosis of Buruli ulcer disease. Clin. Infect. Dis. 45:68-75. [DOI] [PubMed] [Google Scholar]
- 11.Sizaire, V., F. Nackers, E. Comte, and F. Portaels. 2006. Mycobacterium ulcerans infection: control, diagnosis, and treatment. Lancet Infect. Dis. 6:288-296. [DOI] [PubMed] [Google Scholar]
- 12.WHO. 2008. Buruli ulcer: progress report, 2004-2008. Wkly. Epidemiol. Rec. 17:145-154. [PubMed] [Google Scholar]
- 13.WHO. 2008. Meeting of the WHO Technical Advisory Group on Buruli ulcer, 3 April 2008. WHO, Geneva, Switzerland.
- 14.WHO. 2004. Provisional guidance on the role of specific antibiotics in the management of Mycobacterium ulcerans disease (Buruli ulcer). WHO, Geneva, Switzerland.
- 15.WHO. 2007. Summary report of the WHO annual meeting on Buruli ulcer, 2-4 April 2007, and report of the Technical Advisory Group (TAG) meeting, 5 April 2007. World Health Organization, Geneva, Switzerland.