Abstract
We report an exceptional case of life-threatening Escherichia coli-induced necrotizing fasciitis. A combined host-pathogen genetic analysis explained the phenotype: the host displayed a susceptibility to intravascular coagulation, and the strain was capable of producing a necrotic toxin (cytotoxic necrotizing factor 1), showing how E. coli can be a dermonecrotic pathogen.
CASE REPORT
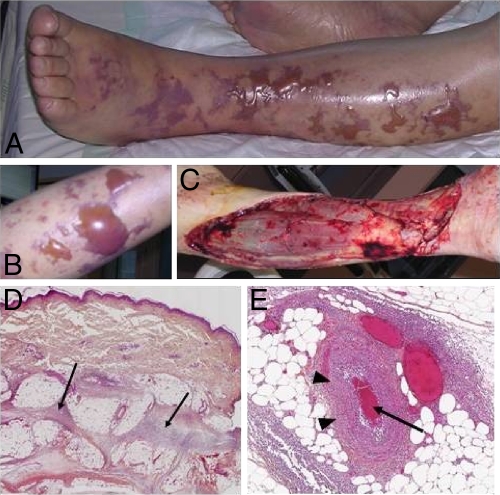
An 83-year-old man was referred to our intensive care unit (ICU) for severe sepsis. His past medical history was significant for aplastic anemia treated by iterative red cell transfusion, chronic atrial fibrillation, and an aortic valvular replacement. He received daily bisoprolol, fluindione, and furosemide. He suffered from fever and left leg pain during 4 days at home and became progressively asthenic and comatose. The patient was then transferred to the ICU. At examination, his central temperature was 39.2°C, heart rate was 150 beats/min, blood pressure was 75/43 mmHg, and Glasgow coma score was 13, without stiff neck. His left leg was erythematous and tender to palpation. The rest of the examination was uninformative. Biological exams revealed severe anemia (hemoglobin, 72 g/liter), acute renal failure (creatinemia, 220 μmol/liter), and a normal creatinine phosphokinase level (52 IU/liter). Initial treatment consisted of broad-spectrum antibiotic therapy with piperacillin-tazobactam, clindamycin, and gentamicin. The patient rapidly developed septic shock that was treated according to the international guidelines (large volume expansion, mechanical ventilation, norepinephrine up to 1.1 μg/kg body weight/min, and low-dose steroids). Rapidly, swelling, bullae, and extensive necrosis appeared (Fig. 1 A and B), leading to an emergency extensive surgical debridement (Fig. 1C). The perioperative examination confirmed the diagnosis of necrotizing fasciitis (NF). Histological examination confirmed the diagnosis and showed dermal-epidermal separation with massive neutrophil infiltrate and multiple thrombi in blood vessels (Fig. 1D and E). Severe disseminated intravascular coagulation (DIC) (platelets, 47 × 109/liter; increased D-dimer level; prothrombin ratio, 16%) occurred in the immediate postoperative hours. However, after surgery, the patient's status improved, norepinephrine was stopped at day 4, and renal function recovered after 6 days. The patient was extubated at day 9. Urinalysis results were within normal limits, and the blood culture was sterile. Direct Gram staining of preoperative samples revealed Gram-negative bacillus. All the samples of necrotic tissues and fascia grew an amoxicillin-susceptible Escherichia coli strain identified by the API 20E system (bioMerieux, France). Anaerobic cultures were negative. Because of the unusual clinical phenotype, pathogen and host genotypes were further determined. The E. coli strain was analyzed as described previously (3). It harbored the serogroup O2 and belonged to the major phylogenetic group B2. The search for 21 virulence factors (VFs) was performed by PCR using previously published primers and amplification conditions (3, 10, 13). The strain carried genes encoding adhesins (P fimbriae with adhesin PapGIII but not PapGII, Sfa/F1C fimbriae, and heat-resistant agglutinin [hra]), iron-acquisition systems (including yersiniabactin, salmochelin, and the Sit system but not aerobactin), and toxins (such as alpha-hemolysin [hly], cytotoxic necrotizing factor 1 [cnf1], vacuolating toxin, and colibactin but not the secreted autotransporter toxin nor the cytolethal distending toxin). Finally the strain had no other genetic determinants (colicin V, colicin Ia, iss, etsABC, OmpT, hlyF) that were specific to a large plasmid carried by the highly virulent E. coli strain S88 recently sequenced (13). Twenty well-characterized human genetic polymorphisms that have been associated with severe sepsis susceptibility or severity, multiple organ dysfunction syndrome, and coagulation disorders (7) were searched. Genomic DNA was extracted from mononuclear cells using MagNA Pure Compact automate (Roche Diagnostics). Real-time PCR allelic discrimination assays were realized by the TaqMan method on ABI 7900 (Applied Biosystems). Polymorphisms associated with severe sepsis susceptibility involve pathogen recognition receptor genes, which detect different molecular patterns of Gram-negative bacteria (lipopeptides by TLR2, lipopolysaccharide by the complex TLR4/CD14, and mannose by mannose binding lectin) to immediately stop bacterial invasion, decrease bacterial load, and initiate the adaptive immune response. Functional variants have been reported in all of these genes, which decrease bacterial clearance and delay immune responses. However, the host genotyping did not reveal any variant of the TLR2, TLR4, CD14, and MBL2 genes. The patient carried common genotypes for genes associated with severity of sepsis (TNFA, IL-10, IL-6) and for the factor V gene. However, the patient was carrying the PAI-1 4G/4G phenotype, a strong susceptibility factor for DIC during sepsis (7).
FIG. 1.
Leg necrotizing fasciitis. (A and B) Preoperative exam showed large bullae and skin necrosis; (C) extensive debridement was performed; (D and E) a histopathological exam showed massive dermic and hypodermic neutrophilic infiltration (arrows) (D) with vasculitis (arrowheads) and small-vessel thrombi (arrow) (E).
We report an exceptional case of monomicrobial E. coli NF with life-threatening septic shock. NF is the most severe soft tissue infection, characterized by widespread necrosis of the skin, subcutaneous tissues, and superficial fascia. It frequently localizes on limbs or pelvises and is generally due to local wounding. Treatment relies on antibiotic therapy and surgical resection of necrotic tissues. However, even with adequate treatment, mortality of NF remains greater than 30% (1). The causal bacteria typically are Streptococcus pyogenes and Staphylococcus aureus. E. coli has been isolated in polymicrobial or pelvic NF in up to 6.5% of cultures from necrotic tissues (2), but it has seldom been reported in monomicrobial lower-limb NF. Nine cases have been published in the English literature, mostly in chronically ill patients (6, 9, 11, 16). Interestingly, all these patients had large bullae, although this clinical manifestation is present in only 15% of NF cases (2). Unfortunately, none of the previously reported cases analyzed the VFs of the isolated strains or the host susceptibility. In this setting of an extremely rare and severe phenotype, we hypothesized that this life-threatening syndrome may be due either to pathogen virulence factors or to host susceptibility factors. Hence, we performed a combined analysis, which highlights the resulting phenotype. The strain belonged to the phylogenetic B2 group, which is the main group of extra-intestinal pathogenic E. coli (ExPEC) (15) and was extremely virulent, as it exhibited 10 VFs shared by ExPEC. In a large study of E. coli-associated skin and soft tissue infections, only 4% of the strains shared 8 or more VFs (14). Of particular interest, we noted the presence of the Cnf1 toxin, which causes cell necrosis through activation of the Rho GTPases (8). In vivo, Cnf1 has been reported to induce dermal necrosis in rabbits, and strains harboring this virulence factor have been designated necrotoxic E. coli (5). In our strain, the codetection of hra, hly, and papGIII with cnf1 indicated the presence of a pathogenicity island IIJ96-like domain, a typical trait of urinary tract infection and neonatal meningitis isolates (4). We demonstrated the presence of vat, a gene encoding a protein with a vacuolating cytotoxic activity, which plays a key role in cellulitis in broiler chickens and is associated in this model with bacteremia and lethality (12). The presence of these VFs could explain tissue necrosis and dermal-epidermal separation in E. coli-associated NF. Thus, our patient was infected with a highly virulent E. coli harboring dermonecrotic toxins. Furthermore, the patient genotyping revealed strong susceptibility for vascular thrombosis, which explains the DIC and the intense thrombi at the histological exam and may have worsened the pathogen effect.
Our report highlights the fact that a patient's disease outcome results from an interaction between the genotypes of both the host and the pathogen. We include for the first time NF in the spectrum of extra-intestinal infections caused by typical ExPEC harboring dermonecrotic toxins. Physicians should be aware that E. coli may be responsible for this life-threatening infection, especially when bullae are present in skin lesions.
Acknowledgments
We have no conflicts of interest.
Footnotes
Published ahead of print on 4 August 2010.
REFERENCES
- 1.Anaya, D. A., and E. P. Dellinger. 2007. Necrotizing soft-tissue infection: diagnosis and management. Clin. Infect. Dis. 44:705-710. [DOI] [PubMed] [Google Scholar]
- 2.Angoules, A. G., G. Kontakis, E. Drakoulakis, G. Vrentzos, M. S. Granick, and P. V. Giannoudis. 2007. Necrotising fasciitis of upper and lower limb: a systematic review. Injury 38(Suppl. 5):S19-S26. [DOI] [PubMed] [Google Scholar]
- 3.Bidet, P., F. Mahjoub-Messai, J. Blanco, M. Dehem, Y. Aujard, E. Bingen, and S. Bonacorsi. 2007. Combined multilocus sequence typing and O serogrouping distinguishes Escherichia coli subtypes associated with infant urosepsis and/or meningitis. J. Infect. Dis. 196:297-303. [DOI] [PubMed] [Google Scholar]
- 4.Bingen-Bidois, M., O. Clermont, S. Bonacorsi, M. Terki, N. Brahimi, C. Loukil, D. Barraud, and E. Bingen. 2002. Phylogenetic analysis and prevalence of urosepsis strains of Escherichia coli bearing pathogenicity island-like domains. Infect. Immun. 70:3216-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caprioli, A., V. Falbo, L. G. Roda, F. M. Ruggeri, and C. Zona. 1983. Partial purification and characterization of an Escherichia coli toxic factor that induces morphological cell alterations. Infect. Immun. 39:1300-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castanet, J., J. P. Lacour, C. Perrin, I. Bodokh, J. F. Dor, and J. P. Ortonne. 1992. Escherichia coli cellulitis: two cases. Acta Derm. Venereol. 72:310-311. [PubMed] [Google Scholar]
- 7.Descheemaeker, P. N., J. P. Mira, F. Bruneel, S. Houzé, M. Tanguy, J. P. Gangneux, E. Flecher, C. Rousseau, J. Le Bras, and Y. Mallédant. 2009. Near-fatal multiple organ dysfunction syndrome induced by Plasmodium malariae. Emerg. Infect. Dis. 15:832-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horiguchi, Y. 2001. Escherichia coli cytotoxic necrotizing factors and Bordetella dermonecrotic toxin: the dermonecrosis-inducing toxins activating Rho small GTPases. Toxicon 39:1619-1627. [DOI] [PubMed] [Google Scholar]
- 9.Horowitz, Y., A. D. Sperber, and Y. Almog. 2004. Gram-negative cellulitis complicating cirrhosis. Mayo Clin. Proc. 79:247-250. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, J. R., B. Johnston, M. A. Kuskowski, J. P. Nougayrede, and E. Oswald. 2008. Molecular epidemiology and phylogenetic distribution of the Escherichia coli pks genomic island. J. Clin. Microbiol. 46:3906-3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, D. M., L. D. Lun, and X. R. Chen. 2006. Necrotising fasciitis with Escherichia coli. Lancet Infect. Dis. 6:456. [DOI] [PubMed] [Google Scholar]
- 12.Parreira, V. R., and C. L. Gyles. 2003. A novel pathogenicity island integrated adjacent to the thrW tRNA gene of avian pathogenic Escherichia coli encodes a vacuolating autotransporter toxin. Infect. Immun. 71:5087-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peigne, C., P. Bidet, F. Mahjoub-Messai, C. Plainvert, V. Barbe, C. Médigue, E. Frapy, X. Nassif, E. Denamur, E. Bingen, and S. Bonacorsi. 2009. The plasmid of Escherichia coli strain S88 (O45:K1:H7) that causes neonatal meningitis is closely related to avian pathogenic E. coli plasmids and is associated with high-level bacteremia in a neonatal rat meningitis model. Infect. Immun. 77:2272-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petkovsek, Z., K. Elersic, M. Gubina, D. Zgur-Bertok, and M. Starcic Erjavec. 2009. Virulence potential of Escherichia coli isolates from skin and soft tissue infections. J. Clin. Microbiol. 47:1811-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russo, T. A., and J. R. Johnson. 2000. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J. Infect. Dis. 181:1753-1754. [DOI] [PubMed] [Google Scholar]
- 16.Yoon, T. Y., S. K. Jung, and S. H. Chang. 1998. Cellulitis due to Escherichia coli in three immunocompromised subjects. Br. J. Dermatol. 139:885-888. [DOI] [PubMed] [Google Scholar]