Abstract
A new nucleic acid amplification-based rapid test for diagnosis of pandemic influenza (H1N1) 2009 virus was developed. The molecular test for pandemic H1N1, SAMBA (simple amplification-based assay), is based on isothermal amplification and visual detection on a dipstick characterized by high sensitivity, high specificity, a short turnaround time, and minimal technical requirements. The amplification step is monitored with an internal control to ensure correct interpretation of test results. The clinical performance of this assay was evaluated using blinded RNA samples extracted from nasal/throat swab specimens from 262 patients exhibiting influenza-like illness. Compared with the United Kingdom National Standard Method, based on quantitative reverse transcription-PCR, the sensitivity, specificity, positive predictive value, and negative predictive value of the new assay were 95.3% (95% confidence interval, 88.5 to 98.7%), 99.4% (95% confidence interval, 96.9 to 99.9%), 98.8% (95% confidence interval, 93.5 to 99.9%), and 97.8% (95% confidence interval, 94.4 to 99.4%), respectively. The SAMBA for pandemic H1N1 provides a new technology that could potentially facilitate timely diagnosis and management of infected individuals, thereby informing decision making with regard to patient isolation during a pandemic outbreak.
Since the identification of the pandemic influenza (H1N1) 2009 virus and its subsequent antigenic and genetic characterization, this new influenza virus strain has rapidly spread worldwide (1, 10). As of December 2009, >600,000 cases and at least 8,768 deaths were reported (27). In June 2009, the outbreak was officially declared a pandemic by the World Health Organization (WHO). The pandemic (H1N1) 2009 strain evolved from the family of swine triple-reassortant viruses, which contain genes derived from avian, swine, and human influenza viruses. The swine triple-reassortant viruses are mainly enzootic, with only sporadic cases of human infection having been reported (25). The pandemic (H1N1) 2009 strain acquired the hemagglutinin (H) gene from a swine H1N2 virus and the neuraminidase (N) and matrix protein genes from the Eurasian swine lineage, and it evolved into a pathogen capable of sustaining efficient human-to-human transmission (4).
Despite the demonstration of a high viral titer in the lungs as well as viral shedding and replication in the lower respiratory tract (trachea, bronchi, and bronchioles) in ferret models inoculated with the virus, laboratory-confirmed cases of pandemic influenza (H1N1) 2009 virus in humans have so far been suggestive of a generally mild course of disease (19, 20). The clinical symptoms are similar to those associated with seasonal influenza, including fever, cough, and sore throat, with a high proportion of gastrointestinal symptoms (diarrhea, vomiting) being perhaps the only atypical clinical manifestation specific to pandemic (H1N1) 2009 virus. Most individuals experience an uncomplicated influenza virus infection and recover without the need for medical attention. However, pregnant women, young children, and people with underlying chronic diseases are at a higher risk of developing severe illness after infection, which might be associated with reduced IgG2 levels (12, 27). With regard to antiviral treatment, pandemic (H1N1) 2009 virus is sensitive to both oseltamivir and zanamivir (28). A pandemic vaccine has recently become available, and large immunization schemes have been implemented in many countries since November 2009 (29).
Frontline pandemic surveillance relies on rapid diagnosis of suspected cases and timely treatment of infected individuals. The current diagnostic tests for pandemic (H1N1) 2009 virus include nucleic acid amplification tests (NAATs) and antigen-based assays. The NAATs are based on quantitative reverse transcription-PCR (qRT-PCR) analysis with primers specific for the hemagglutinin or neuraminidase gene (or both) of the pandemic virus. Despite the high sensitivity and specificity offered by qRT-PCR, this diagnostic platform is complex and expensive and therefore suitable for use only in centralized settings with highly trained staff. In contrast, the antigen-based assays provide rapid diagnosis (within 15 min) but with a sensitivity of only 56 to 74% compared with the results of NAATs (14, 22). Mathematical modeling has estimated that the populations of developing countries with poor medical resources and infrastructure would account for 96% of the total mortality if a pandemic similar to the 1918 Spanish flu should occur (2, 21). Given that NAATs are not readily accessible in resource-limited settings, the development of diagnostic assays that encompass the precision of nucleic acid-based tests and the point-of-care characteristics of rapid immunoassays at an affordable cost is critically needed for pandemic surveillance. We now describe a molecular diagnostic assay for specific detection of pandemic (H1N1) 2009 virus based on isothermal amplification of nucleic acid and visual detection on a dipstick. The test for pandemic H1N1, designated SAMBA (simple amplification-based assay), was evaluated against the United Kingdom National Standard Method (www.hpa-standardmethods.org.uk) as the “gold standard.”
MATERIALS AND METHODS
Control virus cultures.
Cultured A/England/195/2009 pandemic H1N1 virus was provided by the Centre for Infections (CfI), Health Protection Agency (HPA), London, United Kingdom. The cultured virus was heat inactivated (56°C for 90 min) and diluted in Copan universal transport medium (UTM). Quality Control for Molecular Diagnostics (QCMD) influenza virus hemagglutinin typing panels 2007 and 2008 and cultures of 10 common respiratory pathogens were obtained from Qnostics Ltd. (Glasgow, United Kingdom). RNA extracts from an external quality assessment (EQA) panel were provided by CfI, HPA.
Clinical samples.
A total of 265 mixed nasal/throat swab specimens submitted to the Clinical Microbiology and Public Health Laboratory at Addenbrooke's Hospital, Cambridge, United Kingdom, for pandemic (H1N1) 2009 virus testing between 12 and 27 November 2009 were analyzed in a double-blinded manner by both the United Kingdom National Standard Method and the SAMBA for pandemic H1N1. All of these specimens were randomly selected from individuals who presented with influenza-like symptoms. Three postmortem samples were excluded, leaving 262 valid samples. Patient ages ranged from 19 days to 91 years, with a mean of 22.4 years, and the male-to-female ratio was 1.2 to 1. The specimens were decoded, and the results were compared at the end of each working day. Samples that gave discordant results with qRT-PCR and the SAMBA for pandemic H1N1 were tested again by the SAMBA as well as sent to CfI for confirmation by qRT-PCR and sequencing.
Nucleic acid extraction.
RNA from cultured virus was extracted using a QIAamp viral RNA minikit (Qiagen, Crawley, United Kingdom). Clinical samples (500 μl) were processed with the NucliSENS easyMAG system (bioMérieux, Lyon, France), taking 40 min. As an internal control, bacteriophage MS2 (ATCC 15597-B1) was included (at ∼4,600 PFU) in each sample prior to extraction (24). Viral nucleic acid (all from RNA viruses except two adenovirus isolates, which are DNA viruses) for the specificity study was extracted with the use of a QIAamp DNA minikit (Qiagen). Extracted nucleic acid was used as the template for the SAMBA and the qRT-PCR assay.
Detection of pandemic (H1N1) 2009 virus by the United Kingdom National Standard Method.
Clinical specimens were screened for influenza virus with qRT-PCR assays by biomedical scientists at the regional clinical microbiology laboratory in the Cambridge Health District, United Kingdom. A generic quadriplex assay capable of detecting all influenza A virus subtypes, influenza B virus, the hemagglutinin H5 subtype, and bacteriophage MS2 was performed as described previously (15). A minor modification was made to the specific minor grove binding (MGB) TaqMan probe for the matrix protein gene (at the third position) to improve its similarity to the pandemic H1N1 sequence (VIC-TCYTGTCACCTCTGAC-MGBNFQ [where MGBNFQ is the minor-groove binder nonfluorescence quencher]; Applied Biosystems, Warrington, United Kingdom). The second assay was specific for pandemic (H1N1) 2009 virus and was originally designed and developed at CfI but was modified into a one-step quantitative assay for the Rotor-Gene 3000 or 6000 real-time thermal cycler (Corbett Research, Qiagen). The primers (Metabion, Martinsried, Germany) H1 Sw Forward (5′-TTACCAGATTTTGGCGATCTAYT-3′) and H1 Sw Reverse (5′-CCAGGGAGACTASCARTACCA-3′) target the 5′ terminus of RNA segment 4 (hemagglutinin gene) and yield a 62-bp amplicon. The MGB probe (5′-ACWGTCGCCAGTTC-3′) contains a 6-carboxyfluorescein (FAM) label at its 5′ end. Degeneracies were introduced into the primers and probe to facilitate the additional amplification of positive-control material (A/Aragon/RR3218/2008 virus) supplied by CfI to laboratories in England at the start of the influenza pandemic. Both assays were performed with the use of a SuperScript III Platinum one-step qRT-PCR kit (Invitrogen, Paisley, United Kingdom) in a reaction volume of 25 μl (containing 3 mM MgSO4) using the Rotor-Gene 3000 or 6000 instrument. The same amplification conditions were applied for each assay: incubation at 50°C for 30 min and at 95°C for 2 min, followed by 45 cycles of denaturation at 95°C for 15 s and annealing and extension at 60°C for 1 min. The fluorescence was measured at the end of each cycle. For the pandemic (H1N1) 2009 virus-specific assay, the primer (forward or reverse) and probe concentrations were 600 and 250 nM, respectively. Excluding sample extraction, the assay took 125 min.
Design of primers and probes for SAMBA for pandemic H1N1.
Primer and probe sets were designed to target conserved sequences specific for the pandemic (H1N1) influenza virus. Virus sequences were obtained from the Influenza Virus Resource (National Center for Biotechnology Information; http://www.ncbi.nlm.nih.gov/genomes/flu/flu.html). The sequences of the hemagglutinin genes of A/California/07/2009, A/Brisbane/59/2007 (H1N1), and A/Brisbane/10/2007 (H3N2) viruses were first aligned with the use of the multiple alignment using fast Fourier transform (MAFFT) program, available from the European Molecular Biology Laboratory-European Bioinformatics Institute (EMBL-EBI; http://www.ebi.ac.uk/tools/mafft), and were then analyzed with the Jalview program (version 2.3; University of Dundee, Dundee, United Kingdom). Regions that were conserved among pandemic H1N1 strains but that contained mismatched sequences relative to the sequences of seasonal influenza A viruses were selected as targets for the primers. Detector and capture probes were also designed to target similarly mismatched nucleotides. The primers and probes were compared with the sequences in the Nucleotide Collection database at NCBI with the use of the Basic Local Alignment Search Tool (BLAST) in order to determine their specificities. The primer set was also aligned with all H1, H3, and H5 sequences to confirm subtype specificity. The oligonucleotides were analyzed for predicted secondary structure, GC content, and potential hetero- and homodimer formation with the use of an OligoAnalyzer (version 3.1; IDT Integrated DNA Technologies).
Detection of pandemic (H1N1) 2009 virus by SAMBA for pandemic H1N1.
Amplification of RNA extracted from pandemic (H1N1) 2009 virus was performed by isothermal amplification (18). The amplified products were then detected with a visual detection system on a dipstick, as described previously (5, 17). In brief, after the amplification step, the amplification product was incubated in a 2-ml microcentrifuge tube at 41°C on a heating block. Twenty microliters of amplification product was added to our proprietary detection mixture, and the dipstick was inserted in the reaction mixture. The test results were examined after 25 min of incubation, and the signal on the dipstick was scored by at least two operators, according to the in-house scoring chart (Fig. 1). The total time for amplification and detection was 85 min. The procedure was performed by a research student and a research assistant in the University of Cambridge.
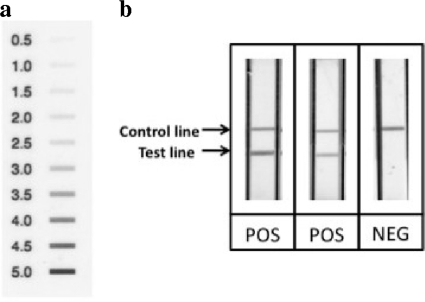
FIG. 1.
Detection of pandemic (H1N1) 2009 virus on the dipstick. After amplification of the influenza viral genome, the amplification product was visually detected on the dipstick by naked eye and the signal strength can be scored according to the scoring chart. (a) Reference scoring chart showing signal strength from 0.5 to 5 in 0.5-point increment. (b) Samples of test results on the dipsticks showing strong positive, intermediate positive, and negative (from left to right) samples. The upper line is the internal control line, which should be present in all tests to validate the test run; the lower line is the test line indicating the presence of the pandemic (H1N1) 2009 viral genome in the samples.
Statistical analysis.
Pearson's correlation coefficient was used to compare the threshold cycle (CT) value of the qRT-PCR and the signal strength of the SAMBA for pandemic H1N1 test. A P value of <0.05 was considered statistically significant.
RESULTS
Primers and probes for SAMBA for pandemic H1N1.
Primer and probe design for the SAMBA for pandemic H1N1 took advantage of the limited number of mismatched nucleotides in the hemagglutinin gene between the pandemic (H1N1) 2009 and seasonal H1N1 strains. A 199-bp target region was amplified by the forward primer (5′-ACTTTGTTGGTCAGCACTAGTAGAT-3′) and reverse primer (5′-CAATCATGACTCGAACAAAGGT-3′), which was attached to a T7 promoter sequence and a linker sequence (5′-AATTCTAATACGACTCACTATAGGGAGAAGG-3′, at the 5′ end). The detector probe (5′-TTCCTTTTTTAACTAGCCAT-3′), which was labeled with multiple hapten moieties, and the capture probe (5′-CTTTCCCTTTATCATTAATG-3′) were designed to anneal to sequences within the single-stranded amplification product. The underlined nucleotides are specific to pandemic (H1N1) 2009 virus. Comparison of the primer sequences with the sequences in the Nucleotide Collection database at NCBI predicted no cross-reaction of the primer set with common H1, H3, H5, H7, or H9 influenza A viruses, influenza B viruses, or other common human pathogens. A panel of common H1, H3, H5, H7, and H9 influenza A viruses, influenza B viruses, and four swine influenza viruses as well as other common respiratory pathogens was used to test the specificity of the SAMBA for pandemic H1N1 (Table 1). All virus strains in the panel, including the two H1N1 and two H1N2 swine viruses, tested negative.
TABLE 1.
Specificity panel used to evaluate the analytical specificity of SAMBA for pandemic H1N1a
| Virus | Type, subtype, or strain |
|---|---|
| Influenza viruses | |
| A/Brisbane/59/2007 | H1N1 |
| A/New Caledonia/20/1999 | H1N1 |
| A/Ned/361/06 | H1 |
| A/Netherlands/565/05 | H3N2 |
| A/Wisconsin/67/2005 | H3N2 |
| A/Wyoming/3/2003 | H3N2 |
| A/Ned/344/06 | H3 |
| A/Hong Kong/213/03 | H5N1 |
| A/Mallard/Netherlands/12/00 | H7N3 |
| A/Mallard/NL/1/05 | H9 |
| A/Swine/IV/1999 | H1N1 |
| A/Swine/Switzerland/2005 | H1N1 |
| A/Swine/CA/790/97 | H1N2 |
| A/Swine/CA/144/2005 | H1N2 |
| B/Malaysia/2506/2004 | B |
| B/Jiangsu/10/2003 | B |
| B Y lineage | B |
| Adenovirus | 5 |
| Adenovirus | 7 |
| Respiratory syncytial virus | A |
| Respiratory syncytial virus | B |
| Parainfluenza virus | 1 |
| Parainfluenza virus | 3 |
| Parainfluenza virus | 4 |
| Rhinovirus | 72 |
| Coronavirus | QC43 |
| Coronavirus | 229E |
The titers of the viruses are 104 TCID50s/ml for A/Brisbane/59/2007, 103.89 TCID50s/ml for A/New Caledonia/20/1999, 103.3 TCID50s/ml for A/Wisconsin/67/2005, 103.96 TCID50s/ml for A/Wyoming/3/2003, 103.8 TCID50s/ml for B/Malaysia/2506/2004, and 103.42 TCID50s/ml for B/Jiangsu/10/2003. Influenza Y-lineage virus was used as the positive control at the Scientific Institute of Public Health, Belgium. A/Swine/IV/1999, A/Swine/Switzerland/2005, A/Swine/CA/790/97, and A/Swine/CA/144/2005 were RNA extracts used by the Centre for Infection, Health Protection Agency, for evaluating the qRT-PCR assay. The rest of the cultured viruses were purchased from Qnostics Ltd.
Analytical sensitivity.
The limit of detection of the SAMBA for pandemic H1N1 was determined by testing diluted RNA extracts from a titrated stock of reference virus (A/England/195/2009). The limit of detection was defined as the lowest concentration of extract at which 95% of replicates tested positive (signal, ≥0.5). Testing of 20 replicates of 5, 1, 0.5, 0.25, 0.125, 0.05, and 0.01 PFU of cultured virus per test by three operators independently yielded an analytical sensitivity of 0.25 PFU per test.
Evaluation of SAMBA for pandemic H1N1 with clinical samples.
Among 262 nose/throat swab samples tested, 82 initially yielded positive results with both the SAMBA pandemic H1N1 test and the comparator method. Seven samples were reactive only by qRT-PCR, and one sample was reactive only by the SAMBA. These eight discordant samples were tested again by the SAMBA for pandemic H1N1, and two of the seven that initially tested negative by this test were found to be positive. The remaining six discordant samples were sent to CfI for further testing. Four samples were not confirmed to harbor pandemic (H1N1) virus, and the remaining two samples were reactive by qRT-PCR, although sequencing failed as a result of a low viral load. In summary, among 86 true-positive and 176 true-negative samples, the SAMBA for pandemic H1N1 detected 82 positive and 175 negative samples but gave one false-positive and four false-negative results. Among the four samples with false-negative results, two yielded a positive result on retesting with a lower volume of extract (5 μl instead of 15 μl), possibly as a result of dilution of residual inhibitor in the extract. The proportion of positive samples detected was 31.3% (82/262), which was similar to the prevalence (34.5%) of pandemic H1N1 between week 46 (week ending 15 November 2009) and week 48 (ending 29 November 2009) in England (13).
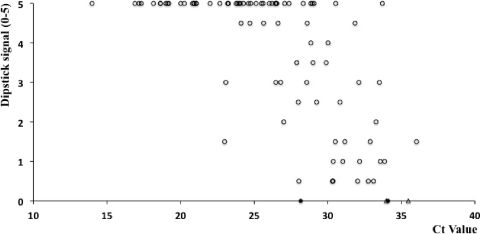
To address the reason for the apparent deficit in sensitivity of the SAMBA for pandemic H1N1, we examined the correlation between the SAMBA signal strength and the CT value for qRT-PCR testing (Fig. 2). The visual signal of the SAMBA pandemic H1N1 test showed a significant inverse correlation with the CT value of qRT-PCR (r = −0.68, P < 0.0001). The sensitivity deficit of the SAMBA appeared to be related to the low H1N1 RNA load in several samples. Compared with that of the gold standard method, the performance of the SAMBA test was thus characterized by a sensitivity of 95.3%, specificity of 99.4%, positive predictive value (PPV) of 98.8%, and negative predictive value (NPV) of 97.8% (Table 2).
FIG. 2.
Correlation of the dipstick signal for the SAMBA for pandemic H1N1 and the CT value for qRT-PCR with clinical samples. Samples that tested positive by both the SAMBA for pandemic H1N1 and qRT-PCR (○), those that tested initially negative but subsequently positive by the SAMBA (•), and those that tested negative by the SAMBA but positive by qRT-PCR (▵) are indicated. The CT value is inversely proportional to the viral load. A significant inverse correlation was apparent between the two sets of results (r = −0.68, P < 0.0001).
TABLE 2.
Overall clinical evaluation of SAMBA for pandemic H1N1 with qRT-PCR as the comparator method
| Value | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| % (no. of samples positive/total no. of samples tested) | 95.3 (82/86) | 99.4 (175/176) | 98.8 (82/83) | 97.8 (175/179) |
| 95% exact confidence interval binomial | 88.5-98.7 | 96.9-99.9 | 93.5-99.9 | 94.4-99.4 |
DISCUSSION
Efficient and accurate diagnosis of infections in individuals is critical for monitoring of a constantly evolving pandemic. A rapid result is also important because timely treatment with neuraminidase inhibitors (oseltamivir or zanamivir) can reduce disease severity and duration (3). Rapid antigen tests were among the first-line diagnostic tools for the detection of pandemic (H1N1) 2009 virus infection during the initial outbreak. However, commercial antigen-based tests show variable performance, with the sensitivities of tests from different manufacturers ranging from 9 to 77% (6, 8, 9, 11). A negative result with an antigen-based rapid test thus does not rule out infection and still requires confirmation by a NAAT. After the emergence of pandemic (H1N1) 2009 virus, qRT-PCR protocols for H1N1 were immediately made available by academic institutions and health agencies around the world (30). Most of these assays require detection of multiple targets (including H1, N1, and matrix protein genes) to confirm a positive case. In particular, the U.S. Centers for Disease Control and Prevention (CDC) protocol recommended by the WHO entails four separate amplifications, including those for the hemagglutinin (H1) and matrix protein genes of influenza A virus, a universal swine target, and an internal positive control (31). Other assays involve detection of the H1 gene, followed by detection of the N1 gene for confirmation. Such assay formats, with prolonged turnaround times and low throughputs, are more labor-intensive and potentially more expensive than a single assay. The SAMBA for pandemic H1N1 bypasses the need for multiple testing by targeting a region of the hemagglutinin gene specific to the pandemic (H1N1) 2009 virus, thus allowing direct identification of the pandemic strain in one reaction with sensitivity comparable to that of the qRT-PCR assay (0.25 PFU versus 0.2 PFU per test). In terms of the turnaround time (from extraction to detection), the SAMBA reduces 25% of the testing time compared to that required for qRT-PCR (125 min versus 165 min). However, the use of a single target by the SAMBA carries the risk of compromising sensitivity if mutation in the target sequence occurs. The development of the SAMBA for pandemic H1N1 was designed as a proof-of-principle study to demonstrate the specific application of the technology in the context of the recent pandemic outbreak with pandemic (H1N1) 2009 virus as the predominant circulating strain. It should be noted that to detect other seasonal/subtypes of influenza viruses, an assay targeting conserved sequences of influenza A and B viruses would be more appropriate.
The SAMBA for pandemic H1N1 showed an overall sensitivity of 95.3% and specificity of 99.4%. The sensitivity deficit of the SAMBA appeared to be related to the low H1N1 RNA load in several samples. By targeting only the hemagglutinin gene, however, the SAMBA showed a high specificity compared with the results of the gold standard assay. The signal strength of the SAMBA dipstick showed a significant inverse correlation with the CT value of qRT-PCR, although the correlation was less apparent for samples with CT values of >32. The overall performance of the SAMBA was similar to the overall performances of the HPA (H1)v (95.5% sensitivity) and CDC (H1)v (90.7% sensitivity) assays, both of which are based on qRT-PCR (7).
Although the occurrence of oseltamivir-resistant pandemic influenza (H1N1) 2009 virus remains sporadic and is usually manifest in immunocompromised individuals, nonselective prescription of antiviral drugs to uninfected individuals results in unnecessary exposure to the side effects of these drugs (26). Furthermore, despite stockpiling of neuraminidase inhibitors in many countries as a pandemic control measure, the stock of anti-influenza antiviral drugs is insufficient globally (32). Rapid and accurate diagnosis of infection with the pandemic (H1N1) 2009 virus may thus be helpful not only for treatment-related decision making but also for prevention of person-to-person transmission in both hospitals and communities. For example, timely decisions on patient isolation and school closure are critical public health issues. Furthermore, long delays of up to 2 weeks in obtaining confirmatory testing results from state health department reference laboratories, where qRT-PCR testing was performed, made contact tracing and appropriate control and treatment of the infected cases ineffective (23). Given the limited number of isolation wards in hospitals, accommodation of a large number of suspected infected individuals would be difficult in practice even in developed countries. Moreover, at the community level, timely closure of schools must be implemented before 1% of the population falls ill to achieve a significant reduction in transmission (33). In these particular settings, the SAMBA for pandemic H1N1 might be a useful tool in giving direct diagnostic information to aid with disease management.
During the preparation of the manuscript, Kubo et al. reported the development of a pandemic H1N1-specific assay based on reverse transcription-loop-medicated isothermal amplification (RT-LAMP) (16). The assay was intended for use in resource-limited settings. The SAMBA differs in that the detection step is achieved on a dipstick, which is very similar to most rapid antigen tests. The interpretation of test results for clinical samples reported here was solely done by the naked eyes of the operators. The SAMBA for pandemic H1N1 also includes an internal control line to monitor the validity of test results (Fig. 1b). The current SAMBA provides an alternative approach to the amplification step with a simple visual detection system. For diagnosis of influenza in emergency rooms, doctor's offices, or resource-limited settings, development of a simple, rapid, inexpensive, and robust sample extraction method is needed.
In conclusion, we report on the development of a NAAT based on isothermal amplification and visual dipstick detection for diagnosis of pandemic influenza (H1N1) 2009 virus infection. This assay provides high sensitivity and specificity through detection of sequences specific to the hemagglutinin gene of the pandemic (H1N1) 2009 virus without the need for a secondary confirmation step. Furthermore, the use of isothermal amplification and dipstick detection eliminates the requirements for expensive equipment and trained technicians. This assay has proven effective in the detection of pandemic (H1N1) 2009 virus infections in individuals presenting with influenza-like illness in November 2009 in the United Kingdom.
Acknowledgments
We thank I. Thomas (Scientific Institute of Public Health, Belgium) for providing cultured influenza viruses for specificity studies, S. Wang and H. Joshi (University of Cambridge) for production of the internal positive control, N. Goel (University of Cambridge) for production of the detector probe, L. Mahilum-Tapay for critical review of the manuscript, and staff at the clinical site of the study.
Footnotes
Published ahead of print on 28 July 2010.
REFERENCES
- 1.Anonymous. 2009. Swine influenza A (H1N1) infection in two children—Southern California, March-April 2009. MMWR Morb. Mortal. Wkly. Rep. 58:400-402. [PubMed] [Google Scholar]
- 2.Anonymous. 2009. Swine influenza: how much of a global threat? Lancet 373:1495. [DOI] [PubMed] [Google Scholar]
- 3.Burch, J., M. Corbett, C. Stock, K. Nicholson, A. J. Elliot, and S. Duffy. 2009. Prescription of anti-influenza drugs for healthy adults: a systemic review and meta-analysis. Lancet Infect. Dis. 9:537-545. [DOI] [PubMed] [Google Scholar]
- 4.Cohan, J. 2009. Straight from the pig's mouth: swine research with swine influenzas. Science 325:140-141. [DOI] [PubMed] [Google Scholar]
- 5.Dineva, M. A., D. Candotti, F. Fletcher-Brown, J. P. Allain, and H. Lee. 2005. Simultaneous visual detection of multiple viral amplicons by dipstick assay. J. Clin. Microbiol. 43:4015-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drexler, J. F., A. Helmer, H. Kirberg, U. Reber, M. Panning, M. Müller, K. Höfling, B. Matz, C. Drosten, and A. M. Eis-Hübinger. 2009. Poor clinical sensitivity of rapid antigen test for influenza A pandemic (H1N1) 2009 virus. Emerg. Infect. Dis. 15:1662-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis, J., M. Iturriza, R. Allen, A. Bermingham, K. Brown, J. Gray, and D. Brown. 2009. Evaluation of four real-time PCR assays for detection of influenza A(H1N1)v viruses. Euro Surveill. 14:pii=19230. [DOI] [PubMed] [Google Scholar]
- 8.Faix, D. J., S. S. Sherman, and S. H. Waterman. 2009. Rapid-test sensitivity for novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 361:728-729. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez, C., M. Cataletto, P. Lee, M. Feuerman, and L. Krilov. 2010. Rapid influenza A testing for novel H1N1: point-of-care performance. J. Postgrad. Med. 122:28-33. [DOI] [PubMed] [Google Scholar]
- 10.Garten, R. J., C. T. Davis, C. A. Russell, B. Shu, S. Lindstrom, A. Balish, W. M. Sessions, X. Xu, E. Skepner, V. Deyde, M. Okomo-Adhiambo, L. Gubareva, J. Barnes, C. B. Smith, S. L. Emery, M. J. Hillman, P. Rivailler, J. Smagala, M. de Graaf, D. F. Burke, R. A. Fouchier, C. Pappas, C. M. Alpuche-Aranda, H. Lopez-Gatell, H. Olivera, I. Lopez, C. A. Myers, D. Faix, P. J. Blair, C. Yu, K. M. Keene, P. D. Dotson, Jr., D. Boxrud, A. R. Sambol, S. H. Abid, K. St. George, T. Bannerman, A. L. Moore, D. J. Stringer, P. Blevins, G. J. Demmler-Harrison, M. Ginsberg, P. Kriner, S. Waterman, S. Smole, H. F. Guevara, E. A. Belongia, P. A. Clark, S. T. Beatrice, R. Donis, J. Katz, L. Finelli, C. B. Bridges, M. Shaw, D. B. Jernigan, T. M. Uyeki, D. J. Smith, A. I. Klimov, and N. J. Cox. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginocchio, C. C., F. Zhang, R. Manji, S. Arora, M. Bornfreund, and L. Falk. 2009. Evaluation of multiple test methods for the detection of the novel 2009 influenza A(H1N1) during the New York City outbreak. J. Clin. Microbiol. 45:191-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon, C. L., P. D. Johnson, M. Permezel, N. E. Holmes, G. Gutteridge, C. F. McDonald, D. P. Eisen, A. J. Stewardson, J. Edington, P. G. Charles, N. Crinis, M. J. Black, J. Torresi, and M. L. Grayson. 2010. Association between severe pandemic 2009 influenza A (H1N1) virus infection and immunoglobulin G2 subclass deficiency. Clin. Infect. Dis. 50:672-678. [DOI] [PubMed] [Google Scholar]
- 13.Health Protection Agency. 24 December 2009, posting date. HPA Weekly National Influenza Report. Health Protection Agency, United Kingdom. http://www.hpa.org.uk/web/hpawebfile/hpaweb_c/1259152416075.
- 14.Hermann, B., C. Larsson, and B. W. Zweygberg. 2001. Simultaneous detection and typing of influenza viruses A and B by a nested reverse transcription-PCR: comparison to virus isolation and antigen detection by immunofluorescence and optical immunoassay (FLU OIA). J. Clin. Microbiol. 39:134-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutchinson, E. C., M. D. Curran, E. K. Read, J. R. Gog, and P. Digard. 2008. Mutational analysis of cis-acting RNA signals in segment 7 of influenza A virus. J. Virol. 82:11869-11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubo, T., M. Agoh, L. Q. Mai, K. Fukushima, H. Nishimura, A. Yamaguchi, M. Hirano, A. Yoshikawa, F. Hasebe, S. Kohno, and K. Morita. 2010. Development of a reverse transcription-loop-mediated isothermal amplification assay for detection of pandemic (H1N1) 2009 virus as a novel molecular method for diagnosis of pandemic influenza in resource-limited settings. J. Clin. Microbiol. 48:728-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, H. H., and M. A. Dineva. January 2002. Improved detection signal and capture in dipstick assays. International Patent Publication WO 02/04667.
- 18.Lee, H. H., M. A. Dineva, and F. Fletcher-Brown. July 2008. Nucleic acid amplification and testing. International Patent Publication WO 2008090340.
- 19.Maines, T. R., A. Jayaraman, J. A. Belser, D. A. Wadford, C. Pappas, H. Zeng, K. M. Gustin, M. B. Pearce, K. Viswanathan, Z. H. Shriver, R. Raman, N. J. Cox, R. Sasisekharan, J. M. Katz, and T. M. Tumpey. 2009. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science 325:484-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munster, V. J., E. de Wit, J. M. van den Brand, S. Herfst, E. J. Schrauwen, T. M. Bestebroer, D. van de Vijver, C. A. Boucher, M. Koopmans, G. F. Rimmelzwaan, T. Kuiken, A. D. Osterhaus, and R. A. Fouchier. 2009. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science 325:481-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray, C. J. L., A. D. Lopez, B. Chin, D. Feehan, and K. H. Hill. 2006. Estimation of potential global pandemic influenza mortality on the basis of viral registry data from the 1918-20 pandemic: a quantitative analysis. Lancet 368:2211-2218. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson, K. G., J. M. Wood, and M. Zambon. 2003. Influenza. Lancet 362:1733-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rebmann, T., and W. Wagner. 2009. Infection preventionists' experience during the first month of the 2009 novel H1N1 influenza A pandemic. Am. J. Infect. Control 37:e5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolfe, K. J., S. Parmar, D. Mururi, T. G. Wreghitt, H. Jalal, H. Zhang, and M. D. Curran. 2007. An internally controlled, one-step, real-time RT-PCR assay for norovirus detection and genotyping. J. Clin. Virol. 39:318-321. [DOI] [PubMed] [Google Scholar]
- 25.Shinde, V., C. B. Bridges, T. M. Uyeki, B. Shu, A. Balish, X. Xu, S. Lindstrom, L. V. Gubareva, V. Deyde, R. J. Garten, M. Harris, S. Gerber, S. Vagasky, F. Smith, N. Pascoe, K. Martin, D. Dufficy, K. Ritger, C. Conover, P. Quinlisk, A. Klimov, J. S. Bresee, and L. Finelli. 2009. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005-2009. N. Engl. J. Med. 360:2616-2625. [DOI] [PubMed] [Google Scholar]
- 26.Tullu, M. S. 2009. Oseltamivir. J. Postgrad. Med. 55:225-230. [DOI] [PubMed] [Google Scholar]
- 27.WHO. 11 September 2009, posting date. Clinical features of severe cases of pandemic influenza.WHO, Geneva, Switzerland. http://www.who.int/csr/don/2009_09_11/en/index.html.
- 28.WHO. 22 December 2009, posting date. Antiviral drugs and pandemic (H1N1) 2009: definitions and use. WHO, Geneva, Switzerland. http://www.who.int/csr/disease/swineflu/frequently_asked_questions/antivirals/definitions_use/en/index.html.
- 29.WHO. 19 November 2009, posting date. Safety of pandemic vaccines. WHO, Geneva, Switzerland. http://www.who.int/csr/disease/swineflu/notes/briefing_20091119/en/index.html.
- 30.WHO. 21 May 2009, posting date. WHO information for laboratory diagnosis of pandemic (H1N1) 2009 virus in humans—revised. WHO, Geneva, Switzerland. http://www.who.int/csr/resources/publications/swineflu/who_diagnostic_recommendationsh1n1_20090521.pdf.
- 31.WHO. 30 April 2009, posting date. CDC protocol of realtime RTPCR for influenza A (H1N1). WHO, Geneva, Switzerland. http://www.who.int/csr/resources/publications/swineflu/cdcrealtimertpcr_swineh1assay-2009_20090430.pdf.
- 32.WHO. 4 November 2005, posting date. Avian influenza: frequently asked questions. Wkly. Epidemiol. Rec. 80:377-388. http://www.who.int/wer/2005/wer8044.pdf. [PubMed] [Google Scholar]
- 33.WHO. 11 September 2009, posting date. Measures in school settings. WHO, Geneva, Switzerland. http://www.who.int/csr/disease/swineflu/notes/h1n1_school_measures_20090911/en/index.html.