Abstract
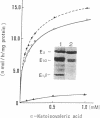
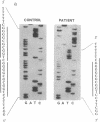
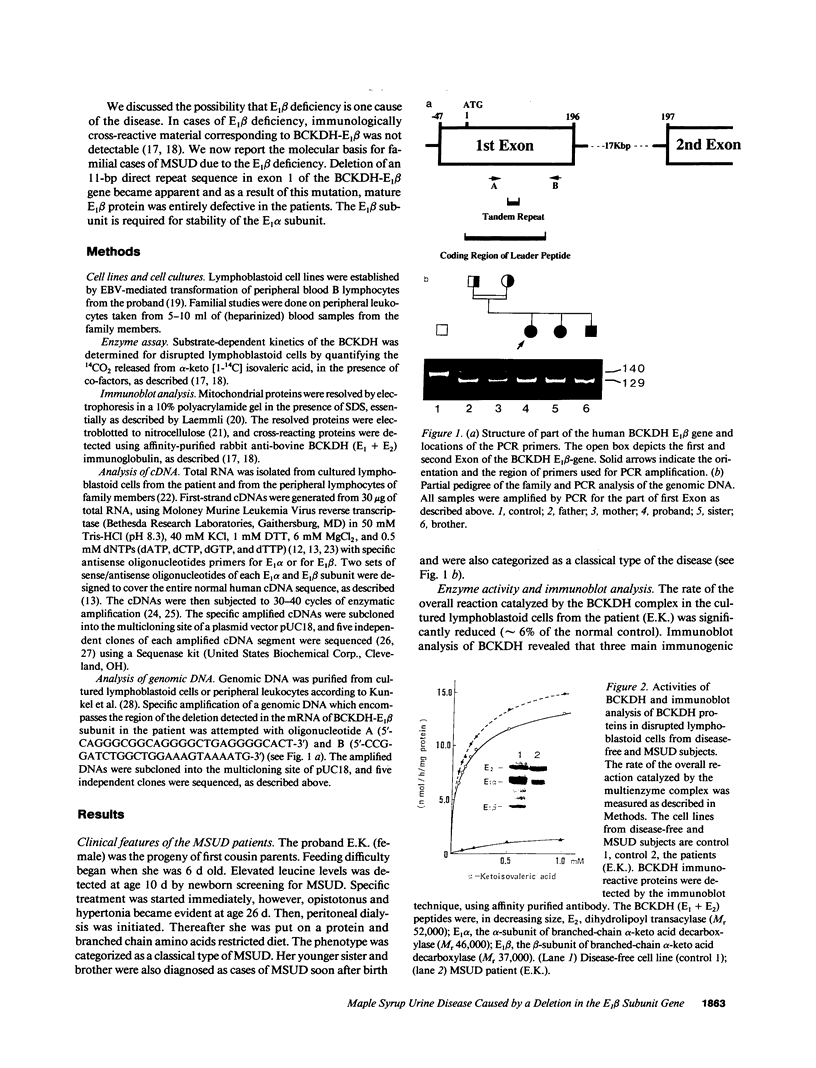
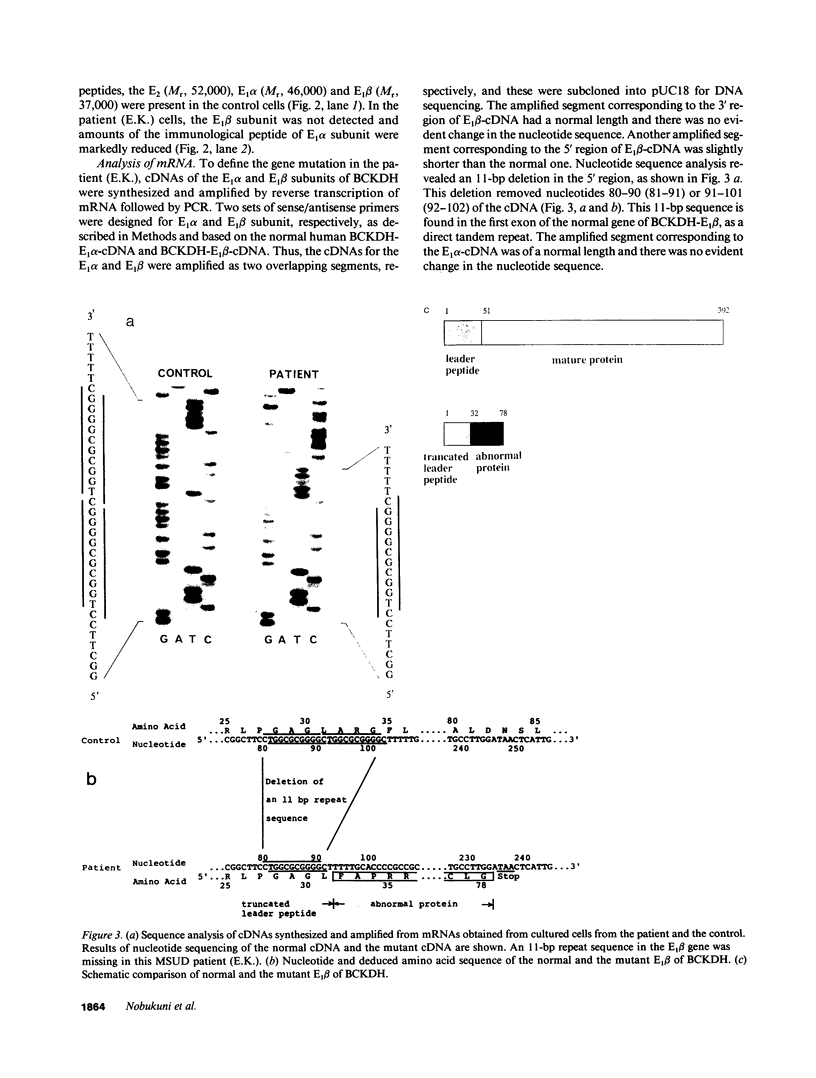
Branched chain alpha-ketoacid dehydrogenase (BCKDH) deficiency results in maple syrup urine disease (MSUD). We examined the molecular basis of familial cases of MSUD by analyzing the activity, subunit structure, mRNA sequence, and genome structure of the affected enzyme. The BCKDH activity in the proband with MSUD was approximately 6% of the normal control level. Immunoblot analysis revealed that the E1 beta subunit of BCKDH was absent and that the E1 alpha subunit of BCKDH was markedly reduced. We amplified the cDNAs of the E1 alpha subunit and the E1 beta subunit of the BCKDH complex obtained from cells of the patient, using the polymerase chain reaction method, then sequenced the amplified cDNAs. The deduced amino acid sequence for the E1 alpha subunit of the patient's cell was normal. An 11-bp deletion was identified in the region that encoded the mitochondrial targeting leader peptide in the E1 beta cDNA. This 11-bp sequence is found in the first exon of the BCKDH-E1 beta gene, as a direct tandem repeat. Amplification of genomic DNA revealed that the consanguineous parents were heterozygous for this mutant allele, and sister and brother of the patient with the disease were homozygous for this mutant allele. This 11-bp deletion mutation caused a change in the reading frame and the mature E1 beta protein was defective. These observations show the biological importance of the E1 beta subunit of BCKDH to maintain normal function of the enzyme activity. The absence of the E1 beta subunit results in instability of the E1 alpha subunit.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandley A. C. Asymmetry in chromosome pairing: a major factor in de novo mutation and the production of genetic disease in man. J Med Genet. 1989 Sep;26(9):546–552. doi: 10.1136/jmg.26.9.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dancis J., Hutzler J., Snyderman S. E., Cox R. P. Enzyme activity in classical and variant forms of maple syrup urine disease. J Pediatr. 1972 Aug;81(2):312–320. doi: 10.1016/s0022-3476(72)80301-9. [DOI] [PubMed] [Google Scholar]
- Danner D. J., Armstrong N., Heffelfinger S. C., Sewell E. T., Priest J. H., Elsas L. J. Absence of branched chain acyl-transferase as a cause of maple syrup urine disease. J Clin Invest. 1985 Mar;75(3):858–860. doi: 10.1172/JCI111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. J., Litwer S., Herring W. J., Pruckler J. Construction and nucleotide sequence of a cDNA encoding the full-length preprotein for human branched chain acyltransferase. J Biol Chem. 1989 May 5;264(13):7742–7746. [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Fisher C. W., Chuang J. L., Griffin T. A., Lau K. S., Cox R. P., Chuang D. T. Molecular phenotypes in cultured maple syrup urine disease cells. Complete E1 alpha cDNA sequence and mRNA and subunit contents of the human branched chain alpha-keto acid dehydrogenase complex. J Biol Chem. 1989 Feb 25;264(6):3448–3453. [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hummel K. B., Litwer S., Bradford A. P., Aitken A., Danner D. J., Yeaman S. J. Nucleotide sequence of a cDNA for branched chain acyltransferase with analysis of the deduced protein structure. J Biol Chem. 1988 May 5;263(13):6165–6168. [PubMed] [Google Scholar]
- Indo Y., Akaboshi I., Nobukuni Y., Endo F., Matsuda I. Maple syrup urine disease: a possible biochemical basis for the clinical heterogeneity. Hum Genet. 1988 Sep;80(1):6–10. doi: 10.1007/BF00451447. [DOI] [PubMed] [Google Scholar]
- Indo Y., Kitano A., Endo F., Akaboshi I., Matsuda I. Altered kinetic properties of the branched-chain alpha-keto acid dehydrogenase complex due to mutation of the beta-subunit of the branched-chain alpha-keto acid decarboxylase (E1) component in lymphoblastoid cells derived from patients with maple syrup urine disease. J Clin Invest. 1987 Jul;80(1):63–70. doi: 10.1172/JCI113064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno Y., Akaboshi I., Katsuki T., Matsuda I. Study on established lymphoid cells in maple syrup urine disease. Correlation with clinical heterogeneity. Hum Genet. 1984;65(4):358–361. doi: 10.1007/BF00291560. [DOI] [PubMed] [Google Scholar]
- Jinno Y., Akaboshi I., Matsuda I. Complementation analysis in lymphoid cells from five patients with different forms of maple syrup urine disease. Hum Genet. 1984;68(1):54–56. doi: 10.1007/BF00293872. [DOI] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau K. S., Griffin T. A., Hu C. W., Chuang D. T. Conservation of primary structure in the lipoyl-bearing and dihydrolipoyl dehydrogenase binding domains of mammalian branched-chain alpha-keto acid dehydrogenase complex: molecular cloning of human and bovine transacylase (E2) cDNAs. Biochemistry. 1988 Mar 22;27(6):1972–1981. doi: 10.1021/bi00406a025. [DOI] [PubMed] [Google Scholar]
- Lyons L. B., Cox R. P., Dancis J. Complementation analysis of maple syrup urine disease in heterokaryons derived from cultured human fibroblasts. Nature. 1973 Jun 29;243(5409):533–535. doi: 10.1038/243533a0. [DOI] [PubMed] [Google Scholar]
- Matsuda I., Nobukuni Y., Mitsubuchi H., Indo Y., Endo F., Asaka J., Harada A. A T-to-A substitution in the E1 alpha subunit gene of the branched-chain alpha-ketoacid dehydrogenase complex in two cell lines derived from Menonite maple syrup urine disease patients. Biochem Biophys Res Commun. 1990 Oct 30;172(2):646–651. doi: 10.1016/0006-291x(90)90723-z. [DOI] [PubMed] [Google Scholar]
- Matsuda I., Yamamoto J., Nagata N., Ninomiya N., Akaboshi I. Lysosomal enzyme activities in cultured lymphoid cell lines. Clin Chim Acta. 1977 Nov 1;80(3):483–486. doi: 10.1016/0009-8981(77)90141-3. [DOI] [PubMed] [Google Scholar]
- McConlogue L., Brow M. A., Innis M. A. Structure-independent DNA amplification by PCR using 7-deaza-2'-deoxyguanosine. Nucleic Acids Res. 1988 Oct 25;16(20):9869–9869. doi: 10.1093/nar/16.20.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsubuchi H., Nobukuni Y., Akaboshi I., Indo Y., Endo F., Matsuda I. Maple syrup urine disease caused by a partial deletion in the inner E2 core domain of the branched chain alpha-keto acid dehydrogenase complex due to aberrant splicing. A single base deletion at a 5'-splice donor site of an intron of the E2 gene disrupts the consensus sequence in this region. J Clin Invest. 1991 Apr;87(4):1207–1211. doi: 10.1172/JCI115120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P. J., Gorski J., White G. C., 2nd, Gidwitz S., Cretney C. J., Aster R. H. Enzymatic amplification of platelet-specific messenger RNA using the polymerase chain reaction. J Clin Invest. 1988 Aug;82(2):739–743. doi: 10.1172/JCI113656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobukuni Y., Mitsubuchi H., Endo F., Akaboshi I., Asaka J., Matsuda I. Maple syrup urine disease. Complete primary structure of the E1 beta subunit of human branched chain alpha-ketoacid dehydrogenase complex deduced from the nucleotide sequence and a gene analysis of patients with this disease. J Clin Invest. 1990 Jul;86(1):242–247. doi: 10.1172/JCI114690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobukuni Y., Mitsubuchi H., Endo F., Asaka J., Oyama R., Titani K., Matsuda I. Isolation and characterization of a complementary DNA clone coding for the E1 beta subunit of the bovine branched-chain alpha-ketoacid dehydrogenase complex: complete amino acid sequence of the precursor protein and its proteolytic processing. Biochemistry. 1990 Feb 6;29(5):1154–1160. doi: 10.1021/bi00457a009. [DOI] [PubMed] [Google Scholar]
- Nobukuni Y., Mitsubuchi H., Endo F., Matsuda I. Complete primary structure of the transacylase (E2b) subunit of the human branched chain alpha-keto acid dehydrogenase complex. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1035–1041. doi: 10.1016/0006-291x(89)91347-8. [DOI] [PubMed] [Google Scholar]
- Otulakowski G., Robinson B. H. Isolation and sequence determination of cDNA clones for porcine and human lipoamide dehydrogenase. Homology to other disulfide oxidoreductases. J Biol Chem. 1987 Dec 25;262(36):17313–17318. [PubMed] [Google Scholar]
- Pons G., Raefsky-Estrin C., Carothers D. J., Pepin R. A., Javed A. A., Jesse B. W., Ganapathi M. K., Samols D., Patel M. S. Cloning and cDNA sequence of the dihydrolipoamide dehydrogenase component human alpha-ketoacid dehydrogenase complexes. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1422–1426. doi: 10.1073/pnas.85.5.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman S. J. The 2-oxo acid dehydrogenase complexes: recent advances. Biochem J. 1989 Feb 1;257(3):625–632. doi: 10.1042/bj2570625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Crabb D. W., Harris R. A. Nucleotide and deduced amino acid sequence of the E1 alpha subunit of human liver branched-chain alpha-ketoacid dehydrogenase. Gene. 1988 Sep 15;69(1):159–164. doi: 10.1016/0378-1119(88)90390-3. [DOI] [PubMed] [Google Scholar]
- Zhang B., Edenberg H. J., Crabb D. W., Harris R. A. Evidence for both a regulatory mutation and a structural mutation in a family with maple syrup urine disease. J Clin Invest. 1989 Apr;83(4):1425–1429. doi: 10.1172/JCI114033. [DOI] [PMC free article] [PubMed] [Google Scholar]