Abstract
We report a case of invasive gastric infection caused by Monascus ruber observed in a patient from French Guiana with gastric adenocarcinoma. The originality of this case is that, first, this invasive mycosis is extremely rare and, second, the probable mode of infection was by the consumption of Monascus ruber-contaminated food.
CASE REPORT
In July 2003, a 66-year-old man was hospitalized in Cayenne Hospital, French Guiana, with a history of chronic cough for 7 months, dyspnea, asthenia, anorexia, gastroesophageal reflux, constipation, intermittent fever, and calf pain. He lived in the vicinity of Grand Santi, a village located on the French side of the Maroni River, in the East of French Guiana. Except for high tobacco consumption for 45 years, he had no notable medical antecedents. At the time of admission, the results of pulmonary, cardiovascular, and abdominal clinical examinations were normal. The patient was in good general condition without fever. Abnormal laboratory findings included normochromic normocytic anemia (hemoglobin, 9.8g/dl) and an increased level of C-reactive protein (97 mg/ml with N <5 mg/ml). Other biologic data were within the normal range.
An abdominopelvic ultrasound revealed celiac and mesenteric lymphadenopathy, without other anomalies. Abdominal tomography showed considerable gastric distension with stasis, associated with antral tissue hypertrophy. Given these results, an endoscopy was carried out. Gastric and duodenal biopsies were performed, and duodenal liquid was aspirated for analysis. Duodenal biopsy specimens showed interstitial duodenitis, and histological analysis of the gastric biopsy specimens revealed moderately differentiated gastric adenocarcinoma associated with chronic gastritis, extensive metaplasia, and massive bacterial superinfection, including Helicobacter pylori infection.
Septated, wide, and banded fungal filaments with right-angle branching were observed in the gastric biopsy specimens with Gomori-Grocott and periodic acid-Schiff (PAS) stains. The pathologist first concluded that there was a fungus from the Mucoraceae family.
Fungal cultures were carried out with fragments of the gastric biopsy specimens and duodenal liquid on gentamicin-chloramphenicol Sabouraud medium with or without cycloheximide at 30°C and 37°C. Two fungi were isolated from the biopsy specimens at 30°C and 37°C: a Mucoraceae fungus without sporulation on the two fungal media and a white-to-purple fungus with a dull reddish pigment on the reverse side of the culture plate on the Sabouraud medium without cycloheximide. The growth of the second fungus was rapid, and the colonies were thinly floccose, spreading from the middle (Fig. 1 A). The microscopic examination of the culture was positive for Monascus ruber (anamorph: Basipetospora rubra), a fungus with the following distinctive characteristics: chains of round and colorless conidia (9 to 10.5 μm by 7 to 9 μm) with flattened bases (Fig. 1B1), and young, round, thin-walled ascoscarps containing oval ascospores with smooth walls (5 to 6 μm by 4 to 5 μm) (Fig. 1B2). The Mucoraceae fungus and M. ruber were also isolated from the duodenal aspiration liquid, associated with Candida glabrata.
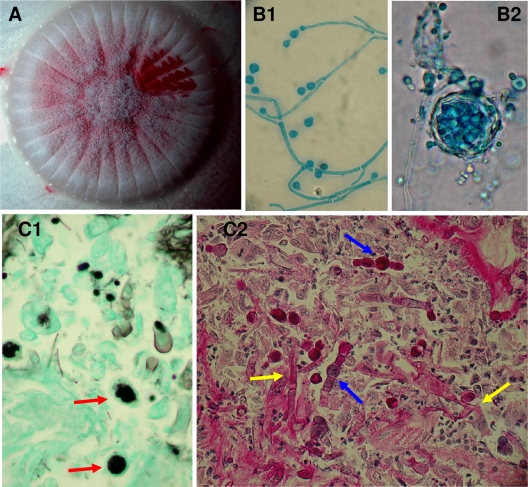
FIG. 1.
Monascus ruber. (A) Macroscopic aspect with red pigment after growth on Sabouraud agar medium for 7 days at 37°C. (B1 and B2) Microscopic characteristics of the M. ruber isolate with two types of reproduction: the asexual form with a chain of conidia (magnification, ×200) (B1), and the sexual form with thin-walled ascoscarps containing ovals ascospores (magnification, ×400) (B2). (C1 and C2) Histological microscopic examination of a gastric biopsy specimen (magnification, ×400): Gomori-Grocott-stained area of tumor necrosis with truncated fungal filaments (red arrows) (C1), and PAS-stained area with banded fungal filaments branched at right angles characteristic of a Mucoraceae fungus (yellow arrows) and wide vesiculated septate filaments (blue arrows) characteristic of Monascus (C2).
The histological slides of the gastric biopsy specimens were then carefully reexamined, and the morphological characteristics of two types of fungi were recognized, confirming the presence of M. ruber in the biopsy specimens (Fig. 1C1 and C2).
Treatment with amphotericin B, 50 mg per day, was initiated. Three days later, because of nephrotoxicity (the creatinine level increased from 88 to 150 μmol/liter), amphotericin B was switched to the liposomal form, 200 mg per day. Five days later, the dose was decreased (200 mg one every other day) because of an increase in kidney failure.
The origin of this atypical infection was researched, and an alimentary cause was suspected. The patient was a high consumer of fish, principally dried and salted. Fungal cultures were carried out on the remains of salted fish (Serrasalmus rhombeus [called pëne]) found in his room at the hospital and consumed by the patient during his hospitalization. The skin, the flesh, and the salt were cultured separately at 30°C and 37°C on the previously used media. The salt and flesh cultures were positive for Monascus ruber on Sabouraud medium without cycloheximide at 30°C and 37°C, and the skin culture was negative.
The surgical treatment of the gastric adenocarcinoma was not possible because of deterioration of the patient's general condition and extensive venal thrombosis of the inferior left member.
A second control gastric biopsy specimen was taken 45 days after the first one (after 5 weeks of treatment). The direct examination showed the same fungal elements, and Monascus ruber was isolated alone by culture at 30°C and 37°C. After this, the patient was released and returned home, where he died shortly after.
Monascus ruber (anamorph: Basipetospora rubra) is a filamentous fungus (family Monascaceae, order Eurotiales) (16). This ascomycete has rarely been implicated in human infection (13). We report here an uncommon case of Monascus ruber invasive gastric infection associated with the consumption of contaminated dried and salted fish.
In Asia, this fungus is traditionally used to produce Monascus-fermented rice and is commonly employed as a food colorant, flavoring agent, or additive for preserving fish and meat (14). In industry, Monascus species are important sources of pigments or bioactive compounds (8), like monacolin K, which is a very effective hypocholesterolemic agent (4).
In humans, Monascus can be pathogenic in several ways. (i) Allergy and anaphylaxis in response to red yeast rice have been described previously (6), due to an immediate sensitivity to Monascus purpureus. (ii) Some Monascus compounds are toxic, such as citrinin, which is a nephrohepatotoxic agent (7). Citrinin permeates the mitochondria, where it alters Ca2+ homeostasis (2) and interferes with the electron transport system (12). Moreover, a toxic drug interaction has also been reported between cyclosporine and monacolin K of the red yeast rice (Monascus purpureus) that led to a rhabdomyolosis in a renal transplant recipient (11). (iii) One study described a direct renal infection after surgery due to Monascus ruber in a 70-year-old patient with multiple kidney stones (13). Our report is the second description worldwide of Monascus ruber infection with tissue invasion and the first case where an alimentary contamination was highlighted. The presence of the fungus in the histological tissue of the gastric biopsy specimens demonstrated the invasive capacity of this mycosis.
Monascus ruber is a salt- and acid-tolerant fungus (10). At 35°C (approximate gastric temperature), the lower pH limit permissive of Monascus growth is 3.7 but the development of the fungus is further promoted when the pH rises to neutral (10). In a healthy stomach, the very low pH of 1 to 2 should not allow the proliferation of the fungus. Functional alterations of the stomach (dyspepsia), associated with an increase in the gastric pH, may be one of the factors favoring this infection. As Monascus ruber is NaCl tolerant, it was not surprising to find Monascus ruber in the dried and salted fish. Monascus had already been isolated from dried and salted fish in Sri Lanka (1), Indonesia (15), and Nigeria (3) and from green table olives (9), but food contamination in humans has never been observed.
After treatment with amphotericin B and the liposomal form, nephrotoxicity developed although the patient's renal function was normal at admission. It is possible that this toxicity was the consequence of not only amphotericin B, but also of the citrinin, produced during the lysis of the fungus (5, 7). Thus, it would be preferable to use an antifungal drug without nephrotoxicity to avoid the possible potentiation of renal failure by citrinin.
Acknowledgments
We gratefully acknowledge John Woodley and Lilia Khodjet El Khil for the English revision of the manuscript and Michel Joubert from the “département des centres de santé de Guyane” for help in gathering information about the patient.
This study was supported by the Laboratoire Hospitalier et Universitaire de Parasitologie-Mycologie, Cayenne Hospital.
The authors declare that they have no conflicts of interest.
Footnotes
Published ahead of print on 4 August 2010.
REFERENCES
- 1.Atapattu, R., and U. Samarajeewa. 1990. Fungi associated with dried fish in Sri Lanka. Mycopathologia 111:55-59. [DOI] [PubMed] [Google Scholar]
- 2.Chagas, G. M., M. A. Oliveira, A. P. Campello, and M. L. Kluppel. 1995. Mechanism of citrinin-induced dysfunction of mitochondria. IV. Effect on Ca2 + transport. Cell Biochem. Funct. 13:53-59. [DOI] [PubMed] [Google Scholar]
- 3.Diyaolu, S. A., and L. O. Adebajo. 1994. Effects of sodium chloride and relative humidity on growth and sporulation of moulds isolated from cured fish. Nahrung 38:311-317. [DOI] [PubMed] [Google Scholar]
- 4.Endo, A. 1979. Monacolin K, a new hypocholesterolemic agent produced by a Monascus species. J. Antibiot. (Tokyo) 32:852-854. [DOI] [PubMed] [Google Scholar]
- 5.Flajs, D., and M. Peraica. 2009. Toxicological properties of citrinin. Arh. Hig. Rada Toksikol. 60:457-464. [DOI] [PubMed] [Google Scholar]
- 6.Hipler, U. C., W. Wigger-Alberti, A. Bauer, and P. Elsner. 2002. Case report. Monascus purpureus—a new fungus of allergologic relevance. Mycoses 45:58-60. [PubMed] [Google Scholar]
- 7.Krejci, M. E., N. S. Bretz, and D. A. Koechel. 1996. Citrinin produces acute adverse changes in renal function and ultrastructure in pentobarbital-anesthetized dogs without concomitant reductions in [potassium]plasma. Toxicology 106:167-177. [DOI] [PubMed] [Google Scholar]
- 8.Lin, Y. L., T. H. Wang, M. H. Lee, and N. W. Su. 2008. Biologically active components and nutraceuticals in the Monascus-fermented rice: a review. Appl. Microbiol. Biotechnol. 77:965-973. [DOI] [PubMed] [Google Scholar]
- 9.Panagou, E. Z., C. Z. Katsaboxakis, and G. J. Nychas. 2002. Heat resistance of Monascus ruber ascospores isolated from thermally processed green olives of the Conservolea variety. Int. J. Food Microbiol. 76:11-18. [DOI] [PubMed] [Google Scholar]
- 10.Panagou, E. Z., P. N. Skandamis, and G. J. Nychas. 2005. Use of gradient plates to study combined effects of temperature, pH, and NaCl concentration on growth of Monascus ruber van Tieghem, an Ascomycetes fungus isolated from green table olives. Appl. Environ. Microbiol. 71:392-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasad, G. V., T. Wong, G. Meliton, and S. Bhaloo. 2002. Rhabdomyolysis due to red yeast rice (Monascus purpureus) in a renal transplant recipient. Transplantation 74:1200-1201. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro, S. M., G. M. Chagas, A. P. Campello, and M. L. Kluppel. 1997. Mechanism of citrinin-induced dysfunction of mitochondria. V. Effect on the homeostasis of the reactive oxygen species. Cell Biochem. Funct. 15:203-209. [DOI] [PubMed] [Google Scholar]
- 13.Sigler, L., P. C. Kibsey, D. A. Sutton, S. P. Abbott, E. Zilkie, D. I. McCarthy, and A. Fothergill. 1999. Monascus ruber, causing renal infection, abstr. F-8, p. 297. Abstr. 99th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC.
- 14.Wang, T. H., and T. F. Lin. 2007. Monascus rice products. Adv. Food Nutr. Res. 53:123-159. [DOI] [PubMed] [Google Scholar]
- 15.Wheeler, K. A., and A. D. Hocking. 1993. Interactions among xerophilic fungi associated with dried salted fish. J. Appl. Bacteriol. 74:164-169. [DOI] [PubMed] [Google Scholar]
- 16.Young, E. M. 1930. Physiological studies in relation to the taxonomy of Monascus spp., p. 227-244. In C. Juday (ed.), Transactions of the Wisconsin Academy of Sciences, Arts and Letters. Wisconsin Academy of Sciences, Madison, WI.