Abstract
The type I alpha/beta interferons (IFN-α/β) are known to play an important role in host defense against influenza A virus infection, but we have now discovered that the recently identified type III IFNs (IFN-λ) constitute the major response to intranasal infection with this virus. Type III IFNs were present at much higher levels than type I IFNs in the lungs of infected mice, and the enhanced susceptibility of STAT2−/− animals demonstrated that only signaling through the IFN-α/β or IFN-λ pathways was sufficient to mediate protection. This finding offers a possible explanation for the similar levels of antiviral protection found in wild-type (WT) mice and in animals lacking a functional type I IFN receptor (IFNAR−/−) but also argues that our current understanding of type III IFN induction is incomplete. While murine IFN-λ production is thought to depend on signaling through the type I IFN receptor, we demonstrate that intranasal influenza A virus infection leads to the robust type III IFN induction in the lungs of both WT and IFNAR−/− mice. This is consistent with previous studies showing that IFNAR-mediated protection is redundant for mucosal influenza virus infection and with data showing that the type III IFN receptor is expressed primarily by epithelial cells. However, the overlapping effects of these two cytokine families are limited by their differential receptor expression, with a requirement for IFN-α/β signaling in combating systemic disease.
Type I interferons (IFNs) were first recognized for their ability to interfere with influenza virus replication (31) and are now recognized as an early and powerful host defense against virus infection. In all cell types that have been investigated, virus infection results in the synthesis and secretion of the type I alpha/beta interferons (IFN-α/β). Once secreted, IFN-α/β acts in an autocrine or paracrine manner by binding the ubiquitously expressed IFN-α/β receptor (IFNAR). Receptor binding activates the Jak-STAT signaling cascade leading to transcriptional upregulation of the IFN-stimulated genes which mediate the biological effects of IFN (10, 18).
IFN induction by influenza A virus involves recognition of viral components by both cytoplasmic receptors and TLR7, although the precise mechanism used depends upon the infected cell type. In fibroblasts, epithelial cells, and conventional dendritic cells (cDCs), IFN-β gene expression is largely dependent upon virus activation of the RNA helicase retinoic acid-induced gene I (RIG-I) (26), with the subsequent phosphorylation of IFN regulatory factor 3 (IRF3) by IκB kinase ɛ (IKKɛ)/TANK-binding kinase 1 (TBK1). Once IFN-β (as well as IFN-α4 in mouse) has been synthesized and secreted, signaling through the Jak-STAT pathway upregulates production of IRF7, which then mediates the transcription of additional IFN-α genes (18, 33, 45). In this way, an amplification pathway is established wherein early, IRF3-mediated production of IFN-β promotes the synthesis of multiple IFN-α subtypes.
Type III IFNs were very recently discovered and are designated IFN-λ1, IFN-λ2, and IFN-λ3 (28), also known as interleukin 29 (IL-29), IL-28A, and IL-28B, respectively (49). Although type III IFNs are not highly homologous to the type I IFNs (only 15 to 20% amino acid identity), their IFN definition is now widely accepted because their induction, signaling, and biological activities are very similar to those of type I IFNs. They signal through a distinct IFN-λ receptor complex composed of a unique IFN-λR1 chain and a shared IL-10R2 chain that also serves as the second subunit of the IL-10, IL-22, and IL-26 receptor complexes (29). Despite their differences, activation of either the type I or type III IFN receptor results in phosphorylation of the receptor-associated tyrosine kinases, Jak1 and Tyk2, followed by phosphorylation of STAT1 and STAT2 (14, 28, 42). The phosphorylated STATs interact with the DNA-binding protein interferon regulatory factor 9 (IRF9) to form a complex called the interferon-stimulated gene factor 3 (ISGF3), which then translocates into the nucleus and binds to the interferon-stimulated response element (ISRE), an enhancer sequence present in the promoters of type I and type III IFN-responsive genes. Consequently, ISGF3 activation by either type of IFN leads to the transcriptional upregulation of the same set of ∼300 genes (11, 13, 14, 28, 32, 57), which mediate similar activities (2, 29). Nonetheless, the biology of these cytokines is likely to be quite different based on the differences in receptor expression. Whereas the IFN-α/β receptor is ubiquitously expressed, IFN-λR1 is found primarily on epithelial cells (30, 51). Therefore, although the type I and type III IFNs activate a common signaling pathway, they are likely to play distinct roles in vivo.
In the studies described herein, we examined the roles of IFN-α/β, -γ, and -λ in protecting the respiratory tract from influenza A virus infection and found that activation of ISGF3 by either type I or type III IFN was necessary and sufficient for virus containment. However, and distinct from results generated using in vitro models, we observed that type I and type III IFNs are differently regulated by infection in vivo. While both IFN-α/β and IFN-λ are synthesized by infected epithelium and plasmacytoid dendritic cells (pDCs) in response to influenza infection, IFN-λ is produced at much higher levels than IFN-α or IFN-β for an extended period of time. These results suggest that it is the type III IFNs that are primarily responsible for protection against viral invaders in the respiratory tract and that type I IFN synthesis may be more important for systemic, rather than local, antiviral defense.
MATERIALS AND METHODS
Mice.
Wild-type (WT) BALB/c mice were purchased from Harlan Laboratories (Indianapolis, IN). IFNAR−/− animals, lacking a functional IFN-α/β receptor, were initially obtained from Michel Aguet (37) and backcrossed onto the BALB/c background for nine generations. IFNAR−/− mice on the 129SvEv background were provided by R. Schreiber (Washington University, St. Louis, MO) and originally derived by Michel Aguet. Strain-matched controls were purchased from Taconic Farms. Mice with a targeted disruption of the stat1 gene (15) were backcrossed onto the 129SvEv strain, and animals of the ninth backcross generation were used in these experiments. Mice with a targeted disruption of the stat2 gene (41) on the 129SvEv background were provided by Christian Schindler (Columbia University, New York, NY). These animals were maintained under specific pathogen-free conditions in the vivarium of the Research Institute at Nationwide Children's Hospital. All experiments were approved by the Committee on the Use and Care of Animals at the Research Institute at Nationwide Children's Hospital.
Viruses and virus quantitation.
Influenza A/WSN/33 virus (WSN; H1N1), provided by Adolfo Garcia-Sastre (Mount Sinai School of Medicine), was grown in Madin-Darby bovine kidney cells. Infectivity of WSN was assayed with a fibroblast cell line derived from STAT1−/− mice (34). For WSN, virus titers were determined 24 h after the inoculation of indicator cells by fluorescent focus assays using a polyclonal rabbit serum raised against whole WSN (4, 17) and a secondary fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). In experiments utilizing UV-inactivated virus, the virus was inactivated by exposure to a 30-W germicidal UV bulb for 2 min at a distance of 60 cm from the source.
Influenza virus.
A/HK-X31 virus (H3N2), provided by Emilio Flano (Ohio State University School of Medicine), was grown in 10-day-old embryonated chicken eggs and stored as previously described (9). Infectivity of HK-X31 was assayed in NY3 cells by fluorescent focus assay using a polyclonal goat serum raised against influenza A virus (H3N2) (Biodesign/Meridian, Cincinnati, OH) and a secondary FITC-labeled donkey anti-goat antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA).
Interferon antiviral bioassay.
MLE-15 cells, a transformed distal respiratory murine cell line (56), were treated with interferon, challenged with vesicular stomatitis virus, and stained with crystal violet as described previously (33).
Virus infection.
Mice were infected intranasally (i.n.) under light anesthesia with various doses of influenza A virus in a 50-μl volume. Groups of 3 to 6 animals, all from 6 to 10 weeks of age, were used for each data point. To assess susceptibility of wild-type and knockout mice to HK-X31 infection, animals were checked twice daily for signs of illness, and moribund animals were euthanized immediately as required by our IUCAC.
Bronchoalveolar lavage.
Mice were euthanized, and their tracheas were isolated by blunt dissection. Tracheas were cannulated, and lungs were rinsed with 1 ml of phosphate-buffered saline (PBS). Each bronchoalveolar lavage (BAL) sample was centrifuged, and the supernatants were stored at −80°C until further use.
Cytokines and cytokine quantitation.
Type I murine IFN (L cell or mouse fibroblast IFN) was obtained from Access Biomedical (San Diego, CA). Murine IFN-λ2 was obtained from Peprotech (Rocky Hill, NJ). Murine IFN levels in BAL and bone marrow-derived dendritic cell (BMDC) supernatants were assayed for IFN-α (PBL Biomedical Laboratories, Piscataway, NJ) and IFN-λ protein (R&D Systems, Minneapolis, MN) using commercially available enzyme-linked immunosorbent assay (ELISA) kits. The IFN-λ bioassay was performed using a derivative of the Chinese hamster ovary cell line (CHO-K1) engineered to express a chimeric IFN-λR1/IFN-γR1 receptor that responds to the presence of human or murine IFN-λ by activation of STAT1 homodimers (28). These were measured by electrophoretic mobility shift assay (EMSA) as previously described (6).
Dendritic cell culture and infection.
Bone marrow-derived cells isolated from the femurs and tibiae of WT BALB/c mice were cultured in RPMI 1640 with 10% heat-inactivated fetal bovine serum (FBS), 1 mmol/liter penicillin/streptomycin, 50 μM 2-mercaptoethanol, and 100 ng/ml of murine FLT-3 ligand (R&D). On day 7, individual wells were infected with live or UV-inactivated influenza virus (WSN) at a multiplicity of infection (MOI) of 1, left untreated (mock), or treated with 2 μg/ml CpG ODN 1826 (InvivoGen). The medium was harvested for the determination of IFN-α and IFN-λ levels at the time points after infection indicated in the figures.
RESULTS
IFN-λ is preferentially induced by intranasal influenza A virus infection.
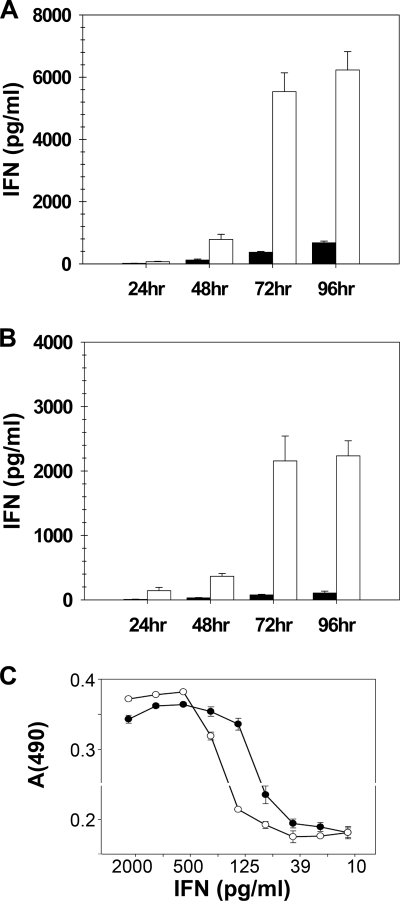
Although synthesis of type I and type III IFN in vitro are coinduced by virus infection of many different cells types (3, 7, 13, 29, 32, 35, 50, 52), production of type I and type III IFNs in vivo has not been comparatively investigated. Because IFN-λ receptor expression is limited primarily to the epithelium, we looked to see whether IFN-λ could be detected in the lung following respiratory virus infection. To answer this question, wild-type BALB/c mice were inoculated intranasally with 104 PFU of the mouse-adapted A/WSN/33 strain of influenza A virus. BAL supernatants collected from infected animals at 24, 48, 72, and 96 h were then assayed by ELISA for the presence of IFN-λ and IFN-α proteins (Fig. 1 A). At this virus dosage, levels of both IFN-λ and IFN-α were barely detectable at 24 h (24) but rose steadily over time. The most striking and unexpected finding was the observation that IFN-λ levels in the BAL fluid exceeded IFN-α levels by more than an order of magnitude at the later time points (Fig. 1A). IFN-β was not measured in this experiment, but in previous work (24) we have found that the type I IFN in similar experiments is dominated by IFN-α. The specific antiviral activity of IFN-λ2 was found to be within a half log of that of IFN-α/β, as measured on mouse lung epithelial cells (MLE-15) (Fig. 1C). Thus, the IFN-λ response appears to be much greater than the IFN-α response, whether measured as biological activity or as mass.
FIG. 1.
Infection with influenza virus induced significantly more IFN-λ than IFN-α. Cohorts of BALB/c wild-type (A) or IFNAR−/− (B) mice (n = 4) were infected i.n. with 104 PFU of influenza A/WSN/33, and BAL samples were harvested 24, 48, 72, or 96 h postinfection. Concentrations of IFN-α (black bars) and IFN-λ (white bars) were determined by ELISA. Data represent the means and standard errors of the means. (C) Results of an assay of IFN-α/β and IFN-λ2 antiviral activity on the mouse lung epithelial cell line MLE-15.
The observation by Price et al. (44) that mice lacking the IFNAR1 chain of the IFN-α/β receptor were no more susceptible than wild-type animals to infection with the HK-X31 strain of influenza A virus suggested that either type I IFNs were not important for virus clearance or that there was an alternative compensatory mechanism in place. The discovery of IFN-λ, which activates the same ISGF3-mediated signaling pathway through a distinct receptor, opened the possibility that type III IFNs could serve as this alternative antiviral mechanism. While this was an attractive hypothesis, it was difficult to reconcile this notion with published data demonstrating that, like induction of IFN-α, induction of IFN-λ2 and -λ3 was dependent on IFNAR signaling (38, 40). In order to protect IFNAR−/− mice against influenza A virus, the production of type III IFNs would have to be independent of signaling through the IFN-α/β receptor. To determine whether type III IFNs could be detected in the lungs of influenza A virus-infected BALB/c IFNAR−/− mice, we instilled virus intranasally, as described above, and analyzed BAL fluids from these animals for the presence of IFN-α and IFN-λ. As has been shown previously (24, 43), there was very little detectable IFN-α in the lungs of the IFNAR−/− mice. In contrast, high levels of IFN-λ, approximately 50% of that found in WT mice, were still produced (Fig. 1B). These data suggest that IFN-λ alone can protect mice from influenza A virus infection, even when nonattenuated mouse-adapted strains are used.
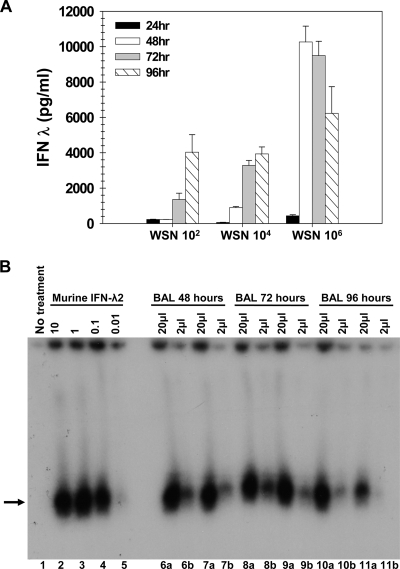
IFN-λ1 is induced by virus infection of human cells, but the murine IFN-λ1 gene is a nonfunctional pseudogene (30). Because currently available ELISA reagents measure only IFN-λ3 protein and not IFN-λ2 protein, we wished to correlate the presence of immunoreactive IFN-λ protein with biological activity. The standard biological assay for type I IFN activity relies on the ability of a given sample to protect a cell monolayer from virus infection. Although similar assays can be used to measure type III IFN biological activity, there is no cell line that responds only to type III IFNs; thus, it is difficult to determine whether antiviral protection is mediated by type III or type I IFNs. Therefore, in order to assess the biological activity of our samples, we utilized a previously created hamster cell line expressing a modified human IFN-λ receptor complex composed of chimeric human IFN-λR1/γR1 with the ligand-binding domain derived from the IFN-λ receptor, the intracellular domain derived from the IFN-γ receptor, and the intact IL-10R2 (28). These hamster cells are responsive to both human and mouse IFN-λs (30), and STAT1 activation in these cells was measured to evaluate the amount of IFN-λs in the samples. STAT1 activation was not detected in parental hamster cells treated with the samples, demonstrating that no murine cytokine present in these samples is able to activate STAT1 via an endogenous receptor. Likewise, STAT1 could not be activated by BAL samples from uninfected mice (data not shown). Using this assay, we were able to show that IFN-λ levels quantitated by ELISA were consistent with bioactivity (Fig. 2 B).
FIG. 2.
Dose dependence of IFN-λ induced by WSN virus infection. (A) Wild-type BALB/c mice (n = 4) were infected i.n. with 102, 104, or 106 PFU of influenza A/WSN/33, and BAL samples from infected animals were harvested 24 (black bars), 48 (white bars), 72 (gray bars), or 96 (hatched bars) hours postinfection. IFN-λ protein levels were determined by ELISA. Data represent the means and standard errors of the means. (B) Chinese hamster cells expressing a chimeric human IFN-λ receptor complex were left untreated (lane 1), treated with recombinant murine IFN-λ2 at 10 ng/150 μl, 1 ng/150 μl, 0.1 ng/150 μl, and 0.01 ng/150 μl (lanes 2 to 5, respectively), or incubated with BAL samples from BALB/c mice infected i.n. with 106 PFU of WSN. Either 20 μl (a) or 2 μl (b) of BAL fluid was added to the samples, for a total volume of 150 μl. Shown are assay results from individual animals at 48 h (lanes 6 and 7), 72 h (lanes 8 and 9), and 96 h (lanes 10 and 11) postinfection. After a 15-min incubation with the sample, the indicator cells were lysed, and nuclear extracts were assayed by EMSA for STAT1 activation. Recombinant murine IFN-λ2 of a known concentration was used as a standard (lanes 2, 3, 4, and 5). The arrow denotes STAT1:STAT1 homodimers. No signal was present in BAL fluid harvested from uninfected mice (data not shown).
In addition, we looked to see how levels of type III IFN produced by infected lungs correlated with the dose of virus instilled. We previously observed that levels of IFN-α/β present in influenza A virus-infected lungs correlated with virus dose at early but not later time points. The same concentration was present 72 h after instillation of 102, 104, or 106 PFU of WSN (24). To determine whether the same correlation existed between viral dose and IFN-λ synthesis, we repeated this experiment and examined BAL supernatants for the presence of IFN-λ. As shown in Fig. 2A, maximal IFN-λ induction does correlate with the infecting dose of virus, unlike IFN-α/β induction. This result suggests that the type I and type III IFN genes respond differently to viral triggers of innate immunity and/or there may be a different cellular source.
Both epithelial cells and pDCs are important sources of IFN-λ.
In a prior study, we assessed the contribution of various cell types to type I IFN production in the lungs of WSN-infected BALB/c mice and found that both the infected epithelial cells and the pDCs were important sources (24). To assess the ability of murine pDCs to produce IFN-λ following infection with the WSN strain of influenza A virus, BMDCs from both WT and IFNAR−/− mice were cultured with FLT-3 ligand (21), and the presence of pDCs (CD11c+, B220+, CD11b−) within these cultures was confirmed by flow cytometry (data not shown). The cultures were then mock infected or infected with live or UV-inactivated WSN at an MOI of 1. CpG oligonucleotides were used as a positive control. Culture supernatants harvested at 24 h postinfection were then assayed by ELISA for the presence of IFN-λ (Fig. 3 A) and IFN-α (Fig. 3B). Similar to the data shown in Fig. 1, IFN-λ levels were much greater than IFN-α levels following influenza A virus infection, and again this difference was on the order of 10-fold. Only minimal levels of IFN-λ could be detected following infection of pDCs derived from the IFNAR−/− mice, and this negative result was consistent over multiple experiments (data not shown).
FIG. 3.
Both IFN-α and IFN-λ are secreted by pDCs following influenza A infection. BMDCs cultured with FLT-3 ligand were mock infected or infected with WSN (live or UV inactivated) at an MOI of 1 or treated with CpG (2 μg/ml). At 24 h postinfection, the DC supernatants were assayed by ELISA for the presence of IFN-λ (A) or IFN-α (B) protein. Data represent the means and standard deviations of results from three replicate samples.
STAT1 and STAT2 are required for resistance to influenza A virus infection in vivo.
Previous work by Price et al. (44) demonstrated that neither IFN-α/β nor IFN-γ is essential for controlling influenza virus replication in vivo. Based on our findings, we hypothesized that the high levels of IFN-λ present in the lungs of infected IFNAR−/− mice were compensating for the loss of the type I IFN response. To test this hypothesis, we looked to see whether the control of virus replication and susceptibility to infection were dependent on both STAT1 and STAT2. Many cytokines can activate STAT1, but only type I and type III IFN signaling leads to tyrosine phosphorylation of STAT2 (28, 46, 47). It was not possible to compare WSN infection of wild-type, IFNAR−/−, STAT1−/−, and STAT2−/− mice, because i.n. infection of IFNAR−/− mice with this strain of influenza A virus results in the spread of the virus to extrapulmonary tissues and mortality (19). The influenza A/HK-X31 strain used by Price et al. (44) lacks the NA mutation present in the WSN strain that allows cleavage of the hemagglutinin (HA) molecule outside the respiratory tract (48, 54), and thus infection remains localized to the respiratory tract. For this reason, the HK-X31 strain of influenza A virus was chosen to study the impact of IFN-λ signaling on resistance to virus infection. Mice on the 129SvEV background were used for this experiment, as STAT2−/− mice are available only on this background.
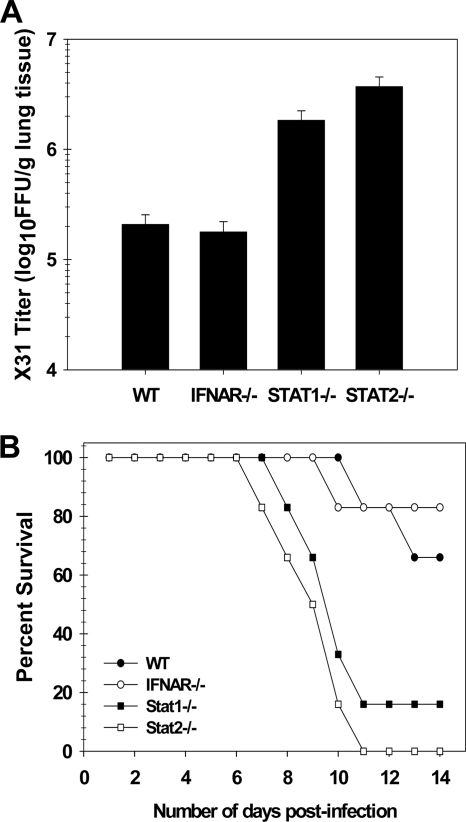
For this study, wild-type, IFNAR−/−, STAT1−/−, and STAT2−/− mice were inoculated i.n. with 100 PFU of HK-X31, and virus titers were determined by fluorescent focus assay of lung homogenates prepared 3 days postinfection. In this experiment (Fig. 4 A), comparable virus titers were seen in wild-type and IFNAR−/− mice, while titers in STAT1−/− and STAT2−/− mice were elevated 10-fold. This result extends the observation of Price et al. (44) and demonstrates that, although neither type I nor type II IFN signaling is necessary, both STAT1 and STAT2 are required to control influenza virus replication in vivo.
FIG. 4.
Control of influenza A virus infection is STAT1 and STAT2 dependent but IFN-α/β independent. (A) Cohorts of 6- to 8-week-old 129Sv mice of the indicated genotypes were infected i.n. with 100 PFU of influenza virus A/HK-X31. Titers (expressed as fluorescent focus units per gram of lung tissue) were determined by fluorescent focus assay of lung homogenates collected on day 3 after infection. (B) Susceptibility of 129SvEv wild-type, IFNAR−/−, STAT1−/−, and STAT2−/− mice to influenza virus was compared following infection with 100 PFU. Five or six 6- to 8-week-old mice of each strain were used. The percent survival for each group is shown.
To determine if susceptibility to HK-X31 influenza infection is increased in the absence of STAT1 and STAT2, wild-type, IFNAR−/−, STAT1−/−, and STAT2−/− mice were infected with 100 PFU of HK-X31 and observed over a period of 14 days. Mice exhibiting signs of severe illness, defined as >20% weight loss, dehydration, labored breathing, and/or decreased movement, were euthanized. As shown in Fig. 4B, 66% of wild-type and 83% of IFNAR−/− mice survived to day 14 postinfection compared with ∼20% of the STAT1−/− animals. All of the STAT2−/− animals were moribund and euthanized by day 11 postinfection. At day 14, all surviving animals appeared healthy and had returned to their preinfection body weight. Taken together, these data demonstrate that signaling through STAT1 and STAT2 is required for early control of influenza virus infection. In contrast, the type I IFN receptor appears to be dispensable. As only IFN-α/β and IFN-λ are known to require both STAT1 and STAT2 for antiviral signaling, this suggests an important role for the latter in antiviral immunity and is consistent with recently published data showing protection of IFNAR−/− mice from challenge with an H7N7 strain of influenza A virus, as well as two attenuated strains, after i.n. treatment with IFN-λ (36).
The presence of IFN-λ in infected lungs is correlated with protection in vivo.
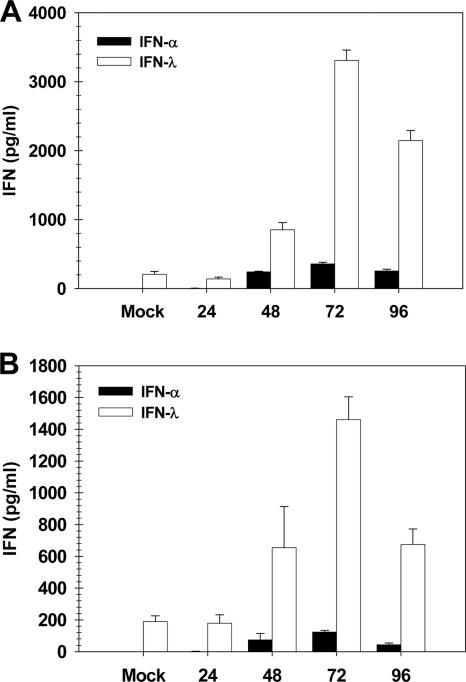
To correlate the STAT1 and STAT2-dependent protection from HK-X31 virus with IFN-λ expression, we measured IFN-λ protein levels in BAL samples collected from infected wild-type and IFNAR−/− 129SvEv animals. As before, mice were infected with 100 PFU of the HK-X31 strain of the influenza A virus, and BAL samples were collected at 24, 48, 72, and 96 h postinfection. IFN-λ protein levels were measured by ELISA, and these data are shown in Fig. 5. Results from this experiment were similar to those shown in Fig. 1. As with the BALB/c animals infected with the WSN strain, an order of magnitude more IFN-λ than IFN-α was induced by infection of the 129SvEv animals (Fig. 5A). Also, as previously noted, IFN-λ levels in the BAL samples of IFNAR−/− mice were approximately 50% of levels measured in those of the wild-type mice (Fig. 5B). So, although the overall IFN production in response to influenza A infection of 129SvEv mice is less (approximately half) of that seen in the BALB/c strain, the results for the two strains are similar and support the conclusion that STAT1 and STAT2 activation by IFN-λ alone is sufficient to mediate the protective effects of IFN signaling.
FIG. 5.
IFN-λ is induced in the airways of wild-type and IFNAR−/− 129SvEv mice by influenza virus A/HK-X31 infection. BAL samples collected from cohorts (n = 5) of HK-X31-infected WT (A) or IFNAR−/− (B) 129Sv mice were assayed by ELISA to determine concentrations of IFN-α and IFN-λ proteins. Data represent the means and standard errors of the means.
DISCUSSION
Type I IFNs have long been known to be an essential defense against virus infection. Only a small number of patients lacking a functional type I IFN pathway have ever been identified, and these have died in infancy with overwhelming infection (5). Nonetheless, we have been puzzled for several years by the observation that the increased susceptibility of STAT1−/− mice to either influenza A virus or respiratory syncytial virus infection was not evident in mice lacking the receptor for IFN-α/β, IFN-γ, or both (25, 44). A possible explanation for these data lay with the ability of many inflammatory cytokines, such as IL-6, to activate STAT1 in an IFN-independent manner. Thus, we hypothesized that STAT1 activation by other cytokines was sufficient to establish an antiviral state. In this study, we tested that hypothesis by including STAT2−/− mice in our influenza A virus challenge experiment and learned that both STAT1 and STAT2 are essential for inhibition of virus replication. These results could be explained only if STAT1 and STAT2 were activated by yet another receptor, and we postulated that the newly discovered type III IFN receptor might function in this manner. This receptor consists of a unique IFN-λR1 chain and a shared IL-10R2 chain that also serves as a subunit of the IL-10, IL-22, and IL-26 receptor complexes (29). Binding of IFN-λs to the IFN-λ receptor leads to the activation of both STAT1 and STAT2 by tyrosine phosphorylation (28).
Although expression of the IFN-λ receptor is limited primarily to epithelial surfaces (51), it should be sufficient to inhibit the growth of respiratory viruses, like influenza virus and RSV, that are restricted to the epithelium of the lung. However, in order to conclude that IFN-λ functioned as an effective antiviral defense in vivo, it was necessary to determine whether this cytokine was present in the airways at early times postinfection. In this study, we have been able to demonstrate that (i) IFN-λ is the predominant IFN produced by both epithelial and dendritic cells in response to influenza A infection and (ii) it is made in quantities sufficient to protect the susceptible cell type.
Additionally, these experiments also demonstrate that current models of IFN-λ gene regulation do not explain the high levels of this cytokine present in the lungs of IFNAR−/− mice. Type I IFN induction by influenza virus infection is well characterized, both in respiratory epithelial cells and in pDCs. In the epithelium, the process is initiated by activation of the RNA helicase RIG-I (31a) and results in phosphorylation of the transcription factor IRF3. Activated IRF3, in concert with NF-κB, binds to the IFN-β promoter and upregulates transcription of the IFN-β gene (22). Secreted IFN-β then binds to the IFN-α/β receptor in an autocrine manner, and signaling through this receptor allows amplification of the type I IFN response via induction of IRF7 protein synthesis. Activated IRF7 binds enhancer elements present in the promoters of the 13 human IFN-α genes (14 in mice), resulting in a wave of IFN-α production. The requirement of IFN-α/β receptor signaling for this response is evident in Fig. 1 and 5, which show minimal IFN-α production in the lungs of the influenza A virus-infected IFNAR−/− mice.
It has been demonstrated that the IFN-λ1 gene, like the IFN-β gene, is regulated primarily by IRF3 in concert with NF-κB and that IFN-λ2 and -λ3 production, like that of IFN-α, is dependent on the presence of activated IRF7 (39). In human cells, early production of IFN-λ1 leads to IRF7 induction via signaling through the IFN-λ receptor; thus, a mechanism for IFN-λ2 and -λ3 production in the absence of type I IFN signaling is known. However, since the murine IFN-λ1 gene is inactive (30), the upregulation of IFN-λ2 and -λ3 in infected IFNAR−/− mice that we have observed in this study clearly demonstrates that the murine IFN-λ2 and -λ3 genes are regulated differently from their human counterparts. Thus, IFN-λs can be induced in an IFNAR−/−-independent manner in mouse and is unlikely to mirror known patterns of type I IFN induction in vivo. Clearly, further experiments are required to comprehensively investigate the regulatory mechanisms governing expression of type III IFN genes in various types of mouse and human cells.
Taken together, our data suggest that IFN-λ is the primary cytokine mediating anti-viral defense in the lung. When influenza A virus is introduced into the airway, infected cells respond with a modest burst of type I IFN and a much larger burst of type III IFN. This is consistent with the work of Wang et al. (55), who reported that detectable levels of IFN-λ but not IFN-β were secreted by influenza A virus-infected, differentiated human airway epithelial cells. Thus, IFN-λ, alone or in concert with IFN-α/β, can control virus infection of the susceptible cell type at the mucosal surface. This idea is consistent with the initial characterization of IFN-λR1 knockout animals (1), as well as mice lacking both IFNAR and the IFN-λR1 (36), wherein it was demonstrated that either type I or type III IFNs alone are sufficient to protect the lung from influenza virus challenge.
Interestingly, we and others have previously shown that IFN-λ alone is unable to control systemic virus infection. When the WSN strain of influenza A virus was used to infect IFNAR−/− mice with an intact IFN-λ pathway, these animals were as susceptible as STAT1−/− mice that lack all IFN signaling (19). That result is very different from the data shown in Fig. 4, where IFNAR−/− mice infected with the HK-X31 strain of influenza A virus are much less susceptible than STAT1−/− or STAT2−/− animals. We propose that this discrepancy arises because of a mutation in the NA gene of the WSN strain that allows the cleavage of the viral HA to occur outside the respiratory tract (48, 54). In IFNAR−/− or STAT1−/− mice, the WSN strain of influenza A virus spread beyond the respiratory tract and grew to high titers in livers, brains, and spleens (19). In wild-type mice, this did not occur. Therefore, type I IFN is essential for limiting the spread of a systemic viral infection, but IFN-λ is sufficient for protection against a respiratory virus infection that is restricted to the epithelial lining of the lung. This conclusion is supported by a recent report from Ank et al. showing that intravenous administration of murine IFN-α, but not IFN-λ, was effective against lymphocytic choriomeningitis virus (LCMV) infection in mice (2).
One caveat to this conclusion is the absence of the Mx1 gene in most laboratory mouse strains. The type I and type III IFN-inducible Mx GTPases have been found to play a major role in the inhibition of influenza virus transcription, and Mx1 is essential for protection of mice against some strains of influenza virus (12). This was true for H5N1 infection of BALB/c mice; those reconstituted for the Mx1 gene were protected by IFN-α/β treatment, while wild-type BALB/c animals lacking Mx1 succumbed to systemic infection (21a). Therefore, in the case of some influenza virus strains, control of systemic infection clearly requires Mx1, while in our model, other interferon-stimulated genes can also inhibit virus replication (19).
Based on these findings, we propose a model wherein the high levels of IFN-λ produced by both epithelial cells and pDCs in the course of influenza A virus infection constitute the primary antiviral defense in the airway. However, the limited expression of the IFN-λ receptor means that, while IFN-λ may have a major role in combating virus replication at mucosal surfaces, type I IFNs have unique as well as overlapping functions in vivo. Studies carried out using cell lines in vitro have clearly shown that type I and type III IFNs activate the same signaling pathway, resulting in the expression of the identical sets of genes (11, 13, 14, 28, 32, 57), but their in vivo effects will be determined by the anatomy of the host. Unless sufficient quantities of a cytokine are present in the same anatomic compartment with cells expressing its receptor, signaling cannot occur, and it is for this reason that IFN-λ is unable to limit the spread of systemic viral pathogens. In addition, receptor expression may also determine whether IFN-λ shares the immunomodulatory functions of IFN-α/β (8, 20, 23, 27). Thus, while many questions regarding IFN-λ remain to be answered, our studies point to a unique role for this newly discovered IFN family in protecting the lung from influenza A virus infection.
Acknowledgments
This work was supported by Public Health Service grants AI51139 to S.V.K. and AI082994 to J.E.D. and S.V.K. and funding from the Research Institute at Nationwide Children's Hospital.
Footnotes
Published ahead of print on 25 August 2010.
REFERENCES
- 1.Ank, N., M. B. Iversen, C. Bartholdy, P. Staeheli, R. Hartmann, U. B. Jensen, F. Dagnaes-Hansen, A. R. Thomsen, Z. Chen, H. Haugen, K. Klucher, and S. R. Paludan. 2008. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J. Immunol. 180:2474-2485. [DOI] [PubMed] [Google Scholar]
- 2.Ank, N., H. West, C. Bartholdy, K. Eriksson, A. R. Thomsen, and S. R. Paludan. 2006. Lambda interferon (IFN-λ), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J. Virol. 80:4501-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berghall, H., J. Siren, D. Sarkar, I. Julkunen, P. B. Fisher, R. Vainionpaa, and S. Matikainen. 2006. The interferon-inducible RNA helicase, mda-5, is involved in measles virus-induced expression of antiviral cytokines. Microbes Infect. 8:2138-2144. [DOI] [PubMed] [Google Scholar]
- 4.Bourmakina, S. V., and A. Garcia-Sastre. 2003. Reverse genetics studies on the filamentous morphology of influenza A virus. J. Gen. Virol. 84:517-527. [DOI] [PubMed] [Google Scholar]
- 5.Bustamante, J., S. Boisson-Dupuis, E. Jouanguy, C. Picard, A. Puel, L. Abel, and J. L. Casanova. 2008. Novel primary immunodeficiencies revealed by the investigation of paediatric infectious diseases. Curr. Opin. Immunol. 20:39-48. [DOI] [PubMed] [Google Scholar]
- 6.Chi, B., H. L. Dickensheets, K. M. Spann, M. A. Alston, C. Luongo, L. Dumoutier, J. Huang, J. C. Renauld, S. V. Kotenko, M. Roederer, J. A. Beeler, R. P. Donnelly, P. L. Collins, and R. L. Rabin. 2006. Alpha and lambda interferon together mediate suppression of CD4 T cells induced by respiratory syncytial virus. J. Virol. 80:5032-5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contoli, M., S. D. Message, V. Laza-Stanca, M. R. Edwards, P. A. Wark, N. W. Bartlett, T. Kebadze, P. Mallia, L. A. Stanciu, H. L. Parker, L. Slater, A. Lewis-Antes, O. M. Kon, S. T. Holgate, D. E. Davies, S. V. Kotenko, A. Papi, and S. L. Johnston. 2006. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat. Med. 12:1023-1026. [DOI] [PubMed] [Google Scholar]
- 8.Curtsinger, J. M., J. O. Valenzuela, P. Agarwal, D. Lins, and M. F. Mescher. 2005. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 174:4465-4469. [DOI] [PubMed] [Google Scholar]
- 9.Daly, K., P. Nguyen, D. L. Woodland, and M. A. Blackman. 1995. Immunodominance of major histocompatibility complex class I-restricted influenza virus epitopes can be influenced by the T-cell receptor repertoire. J. Virol. 69:7416-7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darnell, J. E., Jr. 1997. STATs and gene regulation. Science 277:1630-1635. [DOI] [PubMed] [Google Scholar]
- 11.Der, S. D., A. Zhou, B. R. Williams, and R. H. Silverman. 1998. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. U. S. A. 95:15623-15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dittmann, J., S. Stertz, D. Grimm, J. Steel, A. Garcia-Sastre, O. Haller, and G. Kochs. 2008. Influenza A virus strains differ in sensitivity to the antiviral action of Mx-GTPase. J. Virol. 82:3624-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doyle, S. E., H. Schreckhise, K. Khuu-Duong, K. Henderson, R. Rosler, H. Storey, L. Yao, H. Liu, F. Barahmand-pour, P. Sivakumar, C. Chan, C. Birks, D. Foster, C. H. Clegg, P. Wietzke-Braun, S. Mihm, and K. M. Klucher. 2006. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology 44:896-906. [DOI] [PubMed] [Google Scholar]
- 14.Dumoutier, L., A. Tounsi, T. Michiels, C. Sommereyns, S. V. Kotenko, and J. C. Renauld. 2004. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-lambda 1: similarities with type I interferon signaling. J. Biol. Chem. 279:32269-32274. [DOI] [PubMed] [Google Scholar]
- 15.Durbin, J., R. Hackenmiller, M. C. Simon, and D. E. Levy. 1996. Targeted disruption of the STAT1 gene results in compromised innate immunity to viral disease. Cell 84:443-450. [DOI] [PubMed] [Google Scholar]
- 16.Reference deleted.
- 17.Flandorfer, A., A. Garcia-Sastre, C. F. Basler, and P. Palese. 2003. Chimeric influenza A viruses with a functional influenza B virus neuraminidase or hemagglutinin. J. Virol. 77:9116-9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Sastre, A., and C. A. Biron. 2006. Type 1 interferons and the virus-host relationship: a lesson in detente. Science 312:879-882. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Sastre, A., R. K. Durbin, H. Zheng, P. Palese, R. Gertner, D. E. Levy, and J. E. Durbin. 1998. The role of interferon in influenza virus tissue tropism. J. Virol. 72:8550-8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gil, M. P., R. Salomon, J. Louten, and C. A. Biron. 2006. Modulation of STAT1 protein levels: a mechanism shaping CD8 T-cell responses in vivo. Blood 107:987-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilliet, M., A. Boonstra, C. Paturel, S. Antonenko, X. L. Xu, G. Trinchieri, A. O'Garra, and Y. J. Liu. 2002. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Haller, O., P. Staeheli, and G. Kochs. 2009. Protective role of interferon-indeed Mx GTPases against influenza viruses. Rev. Sci. Tech. 28:219-231. [DOI] [PubMed] [Google Scholar]
- 22.Hiscott, J. 2007. Triggering the innate antiviral response through IRF-3 activation. J. Biol. Chem. 282:15325-15329. [DOI] [PubMed] [Google Scholar]
- 23.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-alpha-beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. U. S. A. 100:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jewell, N. A., N. Vaghefi, S. E. Mertz, P. Akter, R. S. Peebles, Jr., L. O. Bakaletz, R. K. Durbin, E. Flano, and J. E. Durbin. 2007. Differential type I interferon induction by respiratory syncytial virus and influenza A virus in vivo. J. Virol. 81:9790-9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, T. R., S. E. Mertz, N. Gitiban, S. Hammond, R. Legallo, R. K. Durbin, and J. E. Durbin. 2005. Role for innate IFNs in determining respiratory syncytial virus immunopathology. J. Immunol. 174:7234-7241. [DOI] [PubMed] [Google Scholar]
- 26.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23:19-28. [DOI] [PubMed] [Google Scholar]
- 27.Kolumam, G. A., S. Thomas, L. J. Thompson, J. Sprent, and K. Murali-Krishna. 2005. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202:637-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotenko, S. V., G. Gallagher, V. V. Baurin, A. Lewis-Antes, M. Shen, N. K. Shah, J. A. Langer, F. Sheikh, H. Dickensheets, and R. P. Donnelly. 2003. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4:69-77. [DOI] [PubMed] [Google Scholar]
- 29.Kotenko, S. V., and J. A. Langer. 2004. Full house: 12 receptors for 27 cytokines. Int. Immunopharmacol. 4:593-608. [DOI] [PubMed] [Google Scholar]
- 30.Lasfar, A., A. Lewis-Antes, S. V. Smirnov, S. Anantha, W. Abushahba, B. Tian, K. Reuhl, H. Dickensheets, F. Sheikh, R. P. Donnelly, E. Raveche, and S. V. Kotenko. 2006. Characterization of the mouse IFN-lambda ligand-receptor system: IFN-lambdas exhibit antitumor activity against B16 melanoma. Cancer Res. 66:4468-4477. [DOI] [PubMed] [Google Scholar]
- 31.Lindenmann, J. 1982. From interference to interferon: a brief historical introduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 299:3-6. [DOI] [PubMed] [Google Scholar]
- 31a.Loo, Y.-M., J. Fornek, N. Crochet, G. Bajwa, O. Perwitasari, L. Martinez-Sobrido, S. Akira, M. A. Gill, A. García-Sastre, M. G. Katze, and M. Gale, Jr. 2008. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 82:335-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcello, T., A. Grakoui, G. Barba-Spaeth, E. S. Machlin, S. V. Kotenko, M. R. MacDonald, and C. M. Rice. 2006. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 131:1887-1898. [DOI] [PubMed] [Google Scholar]
- 33.Marie, I., J. E. Durbin, and D. E. Levy. 1998. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 17:6660-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez-Sobrido, L., N. Gitiban, A. Fernandez-Sesma, J. Cros, S. E. Mertz, N. A. Jewell, S. Hammond, E. Flano, R. K. Durbin, A. Garcia-Sastre, and J. E. Durbin. 2006. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J. Virol. 80:1130-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matikainen, S., J. Siren, J. Tissari, V. Veckman, J. Pirhonen, M. Severa, Q. Sun, R. Lin, S. Meri, G. Uze, J. Hiscott, and I. Julkunen. 2006. Tumor necrosis factor alpha enhances influenza A virus-induced expression of antiviral cytokines by activating RIG-I gene expression. J. Virol. 80:3515-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mordstein, M., G. Kochs, L. Dumoutier, J. C. Renauld, S. R. Paludan, K. Klucher, and P. Staeheli. 2008. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 4:e1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller, U., U. Steinhoff, L. F. L. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional roles of type I and type II interferons in antiviral defense. Science 264:1818-1821. [DOI] [PubMed] [Google Scholar]
- 38.Onoguchi, K., M. Yoneyama, A. Takemura, S. Akira, T. Taniguchi, H. Namiki, and T. Fujita. 2007. Viral infections activate types I and III interferon genes through a common mechanism. J. Biol. Chem. 282:7576-7581. [DOI] [PubMed] [Google Scholar]
- 39.Osterlund, P., T. Pietila, V. Veckman, S. Kotenko, and I. Julkunen. 2007. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. J. Immunol. 179:3434-3442. [DOI] [PubMed] [Google Scholar]
- 40.Osterlund, P., V. Veckman, J. Siren, K. M. Klucher, J. Hiscott, S. Matikainen, and I. Julkunen. 2005. Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells. J. Virol. 79:9608-9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park, C., S. Li, E. Cha, and C. Schindler. 2000. Immune response in Stat2 knockout mice. Immunity 13:795-804. [DOI] [PubMed] [Google Scholar]
- 42.Pestka, S., C. D. Krause, and M. R. Walter. 2004. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202:8-32. [DOI] [PubMed] [Google Scholar]
- 43.Prakash, A., E. Smith, C. K. Lee, and D. E. Levy. 2005. Tissue-specific positive feedback requirements for production of type I interferon following virus infection. J. Biol. Chem. 280:18651-18657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price, G., A. Gaszewska-Mastarlarz, and D. Moskophidis. 2000. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. J. Virol. 74:3996-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539-548. [DOI] [PubMed] [Google Scholar]
- 46.Schindler, C., and J. E. Darnell, Jr. 1995. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu. Rev. Biochem. 64:621-651. [DOI] [PubMed] [Google Scholar]
- 47.Schindler, C., D. E. Levy, and T. Decker. 2007. JAK-STAT signaling: from interferons to cytokines. J. Biol. Chem. 282:20059-20063. [DOI] [PubMed] [Google Scholar]
- 48.Schulman, J. L., and P. Palese. 1977. Virulence factors of influenza A viruses: WSN virus neuraminidase required for plaque production in MDBK cells. J. Virol. 24:170-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheppard, P., W. Kindsvogel, W. Xu, K. Henderson, S. Schlutsmeyer, T. E. Whitmore, R. Kuestner, U. Garrigues, C. Birks, J. Roraback, C. Ostrander, D. Dong, J. Shin, S. Presnell, B. Fox, B. Haldeman, E. Cooper, D. Taft, T. Gilbert, F. J. Grant, M. Tackett, W. Krivan, G. McKnight, C. Clegg, D. Foster, and K. M. Klucher. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 4:63-68. [DOI] [PubMed] [Google Scholar]
- 50.Siren, J., T. Imaizumi, D. Sarkar, T. Pietila, D. L. Noah, R. Lin, J. Hiscott, R. M. Krug, P. B. Fisher, I. Julkunen, and S. Matikainen. 2006. Retinoic acid inducible gene-I and mda-5 are involved in influenza A virus-induced expression of antiviral cytokines. Microbes Infect. 8:2013-2020. [DOI] [PubMed] [Google Scholar]
- 51.Sommereyns, C., S. Paul, P. Staeheli, and T. Michiels. 2008. IFN-lambda (IFN-λ) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 4:e1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spann, K. M., K. C. Tran, B. Chi, R. L. Rabin, and P. L. Collins. 2004. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J. Virol. 78:4363-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reference deleted.
- 54.Sugiura, A., and M. Ueda. 1980. Neurovirulence of influenza virus in mice. I. Neurovirulence of recombinants between virulent and avirulent virus strains. Virology 101:440-449. [DOI] [PubMed] [Google Scholar]
- 55.Wang, J., R. Oberley-Deegan, S. Wang, M. Nikrad, C. Funk, K. Hartshorn, and R. Mason. 2009. Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-λ1) in response to influenza A infection. J. Immunol. 182:1296-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wikenheiser, K. A., D. K. Vorbroker, W. R. Rice, J. C. Clark, C. J. Bachurski, H. K. Oie, and J. A. Whitsett. 1993. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 90:11029-11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou, Z., O. J. Hamming, N. Ank, S. R. Paludan, A. L. Nielsen, and R. Hartmann. 2007. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J. Virol. 81:7749-7758. [DOI] [PMC free article] [PubMed] [Google Scholar]