Abstract
We have recently demonstrated that human apolipoprotein E (apoE) is required for the infectivity and assembly of hepatitis C virus (HCV) (K. S. Chang, J. Jiang, Z. Cai, and G. Luo, J. Virol. 81:13783-13793, 2007; J. Jiang and G. Luo, J. Virol. 83:12680-12691, 2009). In the present study, we have determined the molecular basis underlying the importance of apoE in HCV assembly. Results derived from mammalian two-hybrid studies demonstrate a specific interaction between apoE and HCV nonstructural protein 5A (NS5A). The C-terminal third of apoE per se is sufficient for interaction with NS5A. Progressive deletion mutagenesis analysis identified that the C-terminal α-helix domain of apoE is important for NS5A binding. The N-terminal receptor-binding domain and the C-terminal 20 amino acids of apoE are dispensable for the apoE-NS5A interaction. The NS5A-binding domain of apoE was mapped to the middle of the C-terminal α-helix domain between amino acids 205 and 280. Likewise, deletion mutations disrupting the apoE-NS5A interaction resulted in blockade of HCV production. These findings demonstrate that the specific apoE-NS5A interaction is required for assembly of infectious HCV. Additionally, we have determined that using different major isoforms of apoE (E2, E3, and E4) made no significant difference in the apoE-NS5A interaction. Likewise, these three major isoforms of apoE are equally compatible with infectivity and assembly of infectious HCV, suggesting that apoE isoforms do not differentially modulate the infectivity and/or assembly of HCV in cell culture.
Hepatitis C virus (HCV) remains a major global health problem, chronically infecting approximately 170 million people worldwide, with severe consequences such as hepatitis, fibrosis/cirrhosis, and hepatocellular carcinoma (HCC) (2, 57). The current standard therapy for hepatitis C is pegylated alpha interferon in combination with ribavirin. However, this anti-HCV regimen has limited efficacy (<50% sustained antiviral response for the dominant genotype 1 HCV) and causes severe side effects (17, 39). Recent clinical studies on the HCV protease- and polymerase-specific inhibitors showed promising results but also found that drug-resistant HCV mutants emerged rapidly (3, 27), undermining the efficacy of specific antiviral therapy for hepatitis C. Therefore, future antiviral therapies for hepatitis C likely require a combination of several safer and more efficacious antiviral drugs that target different steps of the HCV life cycle. The lack of knowledge about the molecular details of the HCV life cycle has significantly impeded the discovery of antiviral drugs and development of HCV vaccines.
HCV is a small enveloped RNA virus classified as a member of the Hepacivirus genus in the family Flaviviridae (46, 47). It contains a single positive-sense RNA genome that encodes a large viral polypeptide, which is proteolytically processed by cellular peptidases and viral proteases into different structural and nonstructural proteins in the order of C, E1, E2, p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B (30, 31). Other novel viral proteins derived from the C-coding region have also been discovered (11, 13, 55, 59). The nucleotides at both the 5′ and 3′ untranslated regions (UTR) are highly conserved and contain cis-acting RNA elements important for internal ribosome entry site (IRES)-mediated initiation of protein translation and viral RNA replication (15, 16, 33, 56, 60).
The success in the development of HCV replicon replication systems has made enormous contributions to the determination of the roles of the conserved RNA sequences/structures and viral NS proteins in HCV RNA replication (4, 5, 7, 32). However, the molecular mechanisms of HCV assembly, morphogenesis, and egression have not been well understood. A breakthrough advance has been the development of robust cell culture systems for HCV infection and propagation, which allow us to determine the roles of viral and cellular proteins in the HCV infectious cycle (9, 29, 54, 63). We have recently demonstrated that infectious HCV particles are enriched in apolipoprotein E (apoE) and that apoE is required for HCV infection and assembly (10, 23). apoE-specific monoclonal antibodies efficiently neutralized HCV infectivity. The knockdown of endogenous apoE expression by a specific small interfering RNA (siRNA) and the blockade of apoE secretion by microsomal triglyceride transfer protein (MTP) inhibitors remarkably suppressed HCV assembly (10, 23). More importantly, apoE was found to interact with the HCV NS5A in the cell and purified HCV particles, as determined by yeast two-hybrid and coimmunoprecipitation (co-IP) studies (6, 23). These findings suggest that apoE has dual functions in HCV infection and assembly via distinct interactions with cell surface receptors and HCV NS5A. To further understand the molecular mechanism of apoE in HCV assembly, we carried out a mutagenesis analysis of apoE and determined the importance of the apoE-NS5A interaction in HCV assembly. Progressive deletion mutagenesis analysis has mapped the NS5A-binding domain of apoE to the C-terminal α-helix region between amino acid residues 205 and 280. Mutations disrupting the apoE-NS5A interaction also blocked HCV production. Additionally, we have determined the effects of three major isoforms of apoE on HCV infection and assembly. Our results demonstrate that apoE isoforms do not determine the infectivity and assembly of infectious HCV in cell culture.
MATERIALS AND METHODS
Cell culture and antibodies.
The Huh-7.5 cell line was kindly provided by Charles M. Rice (Rockefeller University) and was maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), nonessential amino acids, 100 U/ml penicillin, and 100 μg/ml streptomycin. An HCV core-specific monoclonal antibody was purchased from ViroStat (Portland, ME). The HCV NS5A monoclonal antibody 9E10 was generously provided by Charles M. Rice. A rabbit polyclonal antibody against HCV NS5A (276-A) was from ViroGen (Watertown, MA). apoE monoclonal antibodies mAb23 for IP and Wu-E4 for Western blotting were produced in the lab as previously described (10). The human β-actin monoclonal antibody (AC15) and normal mouse immunoglobulin G (IgG) were obtained from Sigma. Horseradish peroxidase-conjugated goat anti-mouse IgG was purchased from Pierce.
DNA construction.
A mammalian two-hybrid system (Clontech) was used for the determination of protein-protein interaction in the cell. Each of the HCV proteins was fused with the Saccharomyces cerevisiae Gal4 DNA-binding domain (Gal4-BD). The cDNA of each HCV protein coding region was amplified by PCR using the JFH1 HCV cDNA as a template and synthetic oligonucleotides as primers (data not shown). PCR DNA fragments were digested with restriction enzymes EcoRI and XbaI and inserted into the pM vector, which was also cut by both EcoRI and XbaI. Human apoE (E2, E3, and E4) was fused with the activation domain of herpes simplex virus (HSV) VP16. The human apoE3 and apoE4 cDNAs were amplified from pcDNA3.1/hApoE3 (a gift of Theodore Mazzone, University of Illinois at Chicago) and pCMV-XL5-hApoE4 (Origene, Rockville, MD), respectively, by PCR using the primer set apoE-EcoRI (5′-GGAATTCATGAAGGTTCTGTGGGCT-3′) and apoE-XbaI (5′-GCTCTAGAAGTGATTGTCGCTGGGC-3′). PCR DNA fragments were digested with EcoRI and XbaI and cloned into the pVP16 vector between EcoRI and XbaI sites, resulting in plasmid DNA constructs designated pVP16-apoE3 and pVP16-apoE4. pVP16-apoE2 was derived from pVP16-apoE3 by replacing the arginine (R) residue at amino acid 158 with a cysteine (C) using a PCR-based site-directed mutagenesis method. The DNA fragment between PstI and SfiI sites in pVP16-apoE3 was replaced with a PCR DNA fragment amplified with two synthetic primers containing a C to T mutation, apoE2-PstI (5′-GCCGATGACCTGCAGAAGTGCCTGGCAGTGTACCAGG-3′) and apoE/SfiI-mPstI (5′-GCGGGCCTGGAAGGCCTCGGCCTGTAGGCGTATCTG-3′). Deletion mutagenesis analysis of apoE3 was carried out by PCR using synthetic oligonucleotides as primers (data not shown). PCR DNA fragments with specific deletions were cloned into pVP16-ApoE3. For ectopic expression of wild-type and mutant apoE proteins, five silent nucleotide mutations, which evade the RNA interference (RNAi) effect but do not change amino acids, were introduced into the siRNA-targeting region of the apoE4 gene using a site-directed mutagenesis kit (Stratagene, La Jolla, CA) and two synthetic oligonucleotide primers, mApoE/Up (5′-GCAAGCGGTGGAGACGGAACCCGAACCGGAGCTGCGCCAGCAG-3′) and mApoE/Bt (5′-CTGCTGGCGCAGCTCCGGTTCGGGTTCCGTCTCCACCGCTTGC-3′). The resulting DNA construct was designated pCMV6XL5/mApoE4. The DNA fragment between two Tth111I sites of pCMV6XL5/mApoE4 was replaced with the corresponding DNA fragment of pcDNA3.1-hApoE3, resulting in pCMV-XL5-mApoE3. To express apoE3 proteins with various deletions, PCR DNA fragments with specific apoE3 deletions in pVP16-apoE3 were excised and subcloned into the apoE-expressing vector pCMV-XL5-mApoE3 between NotI and XbaI restriction enzyme sites. pCMV-XL5-mApoE2 was made by replacing the DNA fragment between NotI and SfiI sites of pCMV-XL5-mApoE3 with the corresponding DNA fragment of pVp16-ApoE2. pCMV-XL5/apoE-Del26-148 was made by removing the DNA fragment between AfeI and FspI sites of the apoE3 gene. The sequences of all DNA constructs were confirmed by DNA sequence analysis at the Northwestern University Biotechnology Laboratory (NUBL; Chicago, IL).
Mammalian two-hybrid assay.
Huh-7.5 cells were seeded at 2 × 105 cells/well in 12-well cell culture plates. Then, 0.25 μg of each pM vector expressing a fusion protein of Gal4-BD and individual HCV protein and plasmid pVP16-apoE DNA were cotransfected with 0.5 μg of the reporter plasmid pFR-Luc (Stratagene) and 0.05 μg of phRL-SV40 (Promega) into Huh-7.5 cells using DMRIE-C reagent according to the manufacturer's instruction (Invitrogen). At 48 h posttransfection, firefly and Renilla luciferase activities were determined using dual luciferase assay kits (Promega). Appropriate controls included pM and pVP16 vector alone, pVP16-apoE, and pM vectors expressing each fusion protein between Gal-BD and the individual HCV protein. phRL-SV40 expressing Renilla luciferase was used as an internal control to normalize DNA transfection efficiency.
Silencing of endogenous apoE expression and ectopic expression of wild-type and mutant apoE.
An apoE-specific siRNA and a nonspecific control (NSC) siRNA were the same as those used for our earlier studies (10, 23). Huh-7.5 cells were seeded in 12-well cell culture plate or 100-mm dishes (for co-IP studies). The expression of endogenous apoE was silenced by an apoE-specific siRNA transfected at 50 nM. In the meantime, wild-type and mutant apoE proteins were expressed ectopically by vector DNAs transfected into Huh-7.5 cells using DMRIE-C reagent (Invitrogen). The methods for DNA and siRNA transfection were described previously (10, 23). At 16 h posttransfection, Huh-7.5 cells were infected with HCV at a multiplicity of infection (MOI) of 2 for 4 h at 37°C. After an additional 20-h incubation (single-cycle HCV growth), cells were lysed for measuring HCV proteins by Western blotting, whereas the media were collected for quantifying the levels of apoE expression, HCV virion RNA (vRNA), and infectivity by Western blotting, quantitative reverse transcription-PCR (qRT-PCR), and infectivity assays, respectively.
Co-IP.
Co-IP of apoE and NS5A was performed using lysate of the HCV-infected Huh-7.5 cells, which were transfected with an apoE-specific or NSC siRNA and DNA vectors expressing wild-type and mutant apoE proteins. The apoE-NS5A complex was pulled down by co-IP using an apoE-specific monoclonal antibody (mAb23) with an isotype-matched normal mouse IgG as a negative control, as previously described (23). NS5A in the precipitate was analyzed by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and was detected by Western blotting using an NS5A-specific monoclonal antibody (9E10).
Western blot analysis.
Protein concentration of cell lysate was determined using a protein assay reagent (Bio-Rad). Twenty-five micrograms of total protein for each sample was separated by 8 to 10% SDS-PAGE and then transferred onto a polyvinylidene difluoride (PVDF) membrane using a semidry blotter (Bio-Rad). Immunoblot analysis was done using an enhanced chemiluminescence kit (Pierce) and monoclonal antibodies specific to the HCV NS5A, apoE, and β-actin.
HCV vRNA extraction and quantification by qRT-PCR.
HCV vRNA in the medium was extracted using Trizol LS reagent (Invitrogen). The level of HCV vRNA was quantified by a real-time RT-PCR method using a Superscript III Platinum one-step qRT-PCR kit (Invitrogen). The oligonucleotide primers 2aF (5′-AGCCATGGCGTTAGTATGAGTGTC-3′) and 2aR (5′-ACAAGGCCTTTCGCAACCCAA-3′) are complementary to nucleotides within the HCV 5′ UTR. The probe (5′-AAACCCACTCTATGCCCGGCCATTT-3′) was conjugated with 6-carboxyfluorescein at the 5′ end and 6-carboxytetramethylrhodamine at the 3′ end (IDT).
Determination of infectious HCV titers by limiting dilution.
The titers of infectious HCV in cell culture supernatants were determined by immunohistochemistry (IHC) staining in conjunction with an endpoint dilution assay as previously described (29). Briefly, HCV in the media was serially diluted and was then used to infect Huh-7.5 cells in multiple wells of 96-well plates. At 4 days postinfection (p.i.), cells were washed and fixed with ice-cold methanol. The HCV-infected cells were subsequently stained using an HCV C-specific monoclonal antibody and ImmPRESS anti-rabbit Ig (peroxidase) polymer detection reagents (Vector Labs, Burlingame, CA). The 50% tissue culture infectious dose (TCID50) per milliliter was calculated as the infectious HCV titer (29).
RESULTS
Molecular interaction of apoE with the HCV NS5A.
In our earlier studies, we have found that apoE interacted with NS5A in the HCV-infected cells and in the affinity-purified HCV particles, as determined by co-IP. In the present study, we used a mammalian two-hybrid system to further understand the molecular interaction between apoE and HCV proteins. Each of the 10 HCV proteins was expressed as a fusion protein with the DNA-binding domain of the yeast protein Gal4 (Gal4-BD), while apoE was fused to the transcriptional activation domain of the herpes simplex virus VP16. pFR-Luc was used as a reporter vector; it expresses a firefly luciferase (fluc) under the control of a synthetic promoter with five tandem repeats of the yeast Gal4 binding sites. Interaction between two-hybrid proteins results in activation of luciferase reporter gene expression (Fig. 1A). To determine the interaction between apoE and HCV protein(s), Huh-7.5 cells were cotransfected with pFR-Luc, pVP16 vector, or pVP16/ApoE, together with pM vector (negative control) or pM/HCV C, E1, E2, p7, NS2, NS3, 4A, 4B, 5A, or 5B. The Renilla luciferase-expressing vector phRL-SV40 (Promega) was used as an internal control to normalize DNA transfection efficiency. At 48 h posttransfection, the levels of luciferase expression were determined using luciferase assay kits (Promega). Results derived from the mammalian two-hybrid experiments show that apoE specifically interacted with NS5A but not other HCV proteins in the cell (Fig. 1B), confirming the previous finding that apoE specifically interacted with the HCV NS5A, as determined by a yeast two-hybrid assay and co-IP studies (6, 23). This mammalian two-hybrid system made it possible to determine the region(s) of apoE important for the apoE-NS5A interaction by mutagenesis analysis.
FIG. 1.
Determination of protein-protein interaction between apoE and HCV proteins by the mammalian two-hybrid system. (A) Diagram of the mammalian two-hybrid system. The reporter vector pFR-Luc contains a synthetic promoter and five yeast Gal4 binding site repeats, which control the expression of firefly luciferase. Each of 10 HCV proteins was fused to the yeast Gal4 DNA-binding domain (G4-BD), while apoE was fused to the HSV VP16 activation domain (AD). Interaction of apoE with HCV protein(s) brings the VP16 AD and the promoter into proximity and therefore activates the transcription of the firefly luciferase gene. (B) Protein-protein interaction determined by the mammalian two-hybrid system. Huh-7.5 cells in 12-well plates were cotransfected with 0.5 μg of pFR-Luc, 0.05 μg of phRL-SV40 (Renilla luciferase as an internal control), 0.25 μg of the vector pM expressing the fusion protein between Gal4-BD and each HCV protein, and 0.25 μg of the vector pVP16-ApoE3 in Opti-MEM containing 2.5 μl of DMIRE-C reagent (Invitrogen). At 48 h posttransfection, the levels of luciferase activity were determined using luciferase assay kits (Promega). The firefly luciferase activity is shown as relative light units (RLU) after normalization with Renilla luciferase activity. Numbers are mean values derived from three independent assays. Vector stands for the negative control using DNA vectors pM and pVP16 only.
The C-terminal α-helix domain of apoE is required for interaction with NS5A.
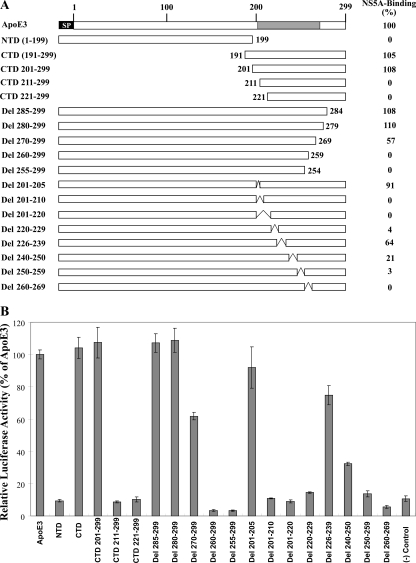
apoE is composed of 317 amino acids with an N-terminal signal peptide of 18 amino acids. The mature apoE is a 299-amino-acid apoprotein with a molecular mass of 34.2 kDa, consisting of two structural and functional domains (Fig. 2A). The 22-kDa N-terminal domain (NTD; 1 to 191) of apoE interacts with members of the low-density lipoprotein receptor (LDLr) family, while the 10-kDa C-terminal α-helical domain (216 to 299) binds to phospholipids (19, 21). To map the NS5A-binding domain of apoE, we initially fused the transcriptional activation domain of the HSV VP16 with the apoE NTD (amino acids 1 to 199) and the C-terminal domain (CTD; amino acids 191 to 299), respectively (Fig. 2A). When tested in the above-described mammalian two-hybrid system, the C-terminal domain of apoE was found to interact with NS5A as efficiently as the full-length apoE3, as indicated by similar levels of luciferase activity (Fig. 2B, CTD 191-299). In contrast, the N-terminal domain of apoE did not produce activation of luciferase reporter gene expression (Fig. 2A, NTD 1-199), suggesting that the apoE N-terminal domain is dispensable for NS5A interaction. These results demonstrate that the NS5A-binding domain is located within the C-terminal third of apoE. To further determine the region(s) of apoE important for NS5A binding, we carried out a progressive deletion mutagenesis analysis of the apoE C-terminal domain (Fig. 2A). The apoE C-terminal domain consisting of amino acids 201 to 299 was still able to interact with NS5A similarly to the full-length CTD (191 to 299) (Fig. 2B). However, further deletions beyond amino acid 211 resulted in a complete inactivation of the apoE CTD for interaction with NS5A (Fig. 2, CTD 211-299 and CTD 221-299), suggesting that the N-terminal border of the NS5A-binding domain is located between amino acids 201 and 211. To map the C-terminal border of the NS5A-binding domain, we constructed several mutants with progressive deletions from the C terminus of apoE (Fig. 2A). Deletions of 15 and 20 amino acids from the C terminus of apoE did not affect the levels of luciferase expression (Fig. 2, Del 285-299 and Del 280-299), suggesting that the C-terminal 20 amino acids are not required for NS5A binding. However, a deletion of 30 amino acid residues from the C terminus of apoE reduced the NS5A-binding ability by 43% (Fig. 2, Del 270-299). Further deletions of 40 and more amino acid residues from the C terminus of apoE completely inactivated its NS5A binding (Fig. 2, Del 260-299 and Del 255-299). These results suggest that the C-terminal border of the NS5A-binding domain of apoE lies between amino acids 269 and 279. Results of the progressive deletion mutagenesis analysis suggested that the NS5A-binding domain resides within the predicted amphipathic α-helix at the C-terminal third of apoE (19).
FIG. 2.
Mutagenesis analysis of apoE. (A) Diagram of apoE deletion mutations. SP, signal peptide; NTD, N-terminal domain; CTD, C-terminal domain. Numbers at the left and in the middle indicate amino acid positions from the N terminus of mature apoE. Numbers at the right show the NS5A-binding activity as percentages of wild-type apoE3 activity (100%); values were derived from the data in panel B. The C-terminal α-helical region important for NS5A binding is highlighted in gray. (B) apoE-NS5A interaction determined by the mammalian two-hybrid system. Huh-7.5 cells in 12-well plates were cotransfected with pFR-Luc, phRL-SV40, the vector pM expressing the Gal4-BD and NS5A fusion protein, and the vector pVP16-ApoE3 as described for Fig. 1B. At 48 h posttransfection, luciferase activity was determined. The relative reporter luciferase activities (%) were calculated and compared with that of apoE3 (wild type), which was considered 100%. Mean values from triplicate experiments are shown.
To further determine whether the entire C-terminal α-helical region of apoE is required for NS5A binding, we created eight mutants with internal deletions within the C-terminal α-helix of apoE (Fig. 2A). Most of the internal deletion mutations completely inactivated the ability of apoE to interact with NS5A (Fig. 2, Del 201-210, Del 201-220, Del 220-229, Del 250-259, and Del 260-269). The Del 240-250 mutation decreased the activity of apoE in NS5A binding by 79%, whereas the Del 226-239 mutatation reduced the NS5A-binding activity of apoE by 36% (Fig. 2). Interestingly, the Del 201-205 mutation did not significantly affect the apoE NS5A-binding activity, consistent with the findings that the apoE CTD consisting of amino acids 201 to 299 is fully functional in NS5A binding but that the CTD consisting of amino acids 211 to 299 completely lost NS5A-binding activity. Collectively, these results demonstrate that the C-terminal α-helix of apoE is critical for interaction with NS5A.
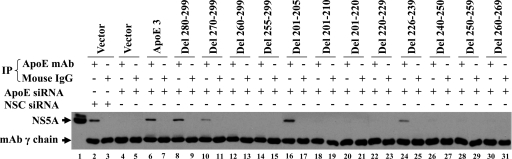
To confirm the specific apoE-NS5A interaction, we performed co-IP experiments. Huh-7.5 cells were infected with HCV and cotransfected with an apoE-specific siRNA together with DNA vectors expressing wild-type apoE and deletion apoE mutants. The apoE-NS5A complex was precipitated with an apoE-specific monoclonal antibody, while NS5A in the precipitate was determined by Western blotting using an anti-NS5A monoclonal antibody. Results are shown in Fig. 3. The endogenous apoE was knocked down to an undetectable level by the apoE-specific siRNA (Fig. 4, lane 2), and consequently no NS5A was detected in the precipitate by an apoE-specific monoclonal antibody (Fig. 3, lane 4). However, ectopic expression of the siRNA-resistant wild-type apoE3 brought down the NS5A level (Fig. 3, lane 6). Deletion mutations of apoE, which resulted in disruption of the apoE-NS5A interaction, all failed to bring down the NS5A level (Fig. 3, lanes 12, 14, 18, 20, 22, 28, and 30), consistent with the data derived from the two-hybrid analysis (Fig. 2). However, those mutations that did not affect the apoE-NS5A interaction were able to bring down the NS5A level, including the apoE Del 201-205 and Del 280-299 mutations (Fig. 3, lanes 8 and 16). Similarly, two deletion mutations, Del 226-239 and Del 270-299, which decreased the levels of luciferase expression by 34% and 43%, respectively, also reduced precipitation of NS5A (Fig. 3, lanes 10 and 24). As negative controls, an isotype-matched normal mouse IgG did not precipitate NS5A. Collectively, these findings demonstrate that the C-terminal α-helix domain of apoE is required for the specific apoE-NS5A interaction.
FIG. 3.
Effects of apoE deletion mutations on the apoE-NS5A interaction, as determined by co-IP. Huh-7.5 cells in 10-cm cell culture dishes were transfected with an apoE-specific siRNA or an NSC siRNA at 50 nM. In the meantime, wild-type apoE3 and individual deletion mutants were ectopically expressed by transfecting cDNA vectors into the HCV-infected cells. After 48 h, cell lysates were subjected to immunoprecipitation using a monoclonal anti-apoE antibody (mAb23) or an isotype-matched normal mouse IgG as a negative control. NS5A in the precipitate was detected by Western blotting using an NS5A monoclonal antibody.
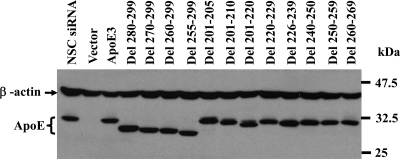
FIG. 4.
Determination of ectopic expression of wild type and mutant apoE proteins by Western blotting. Experiments were done as described in Materials and Methods. Wild-type and mutant apoE proteins in the cell were detected by Western blotting using apoE mAb23.
Mutations disrupting the apoE-NS5A interaction suppressed HCV production.
To determine the effects of apoE mutations on HCV assembly, all deletion mutations were introduced into apoE3 cDNA vector pCMV-XL5-mApoE3, which contained five silent mutations in the siRNA-targeting region (23). Thus, the ectopic expression of wild-type and mutant apoE3 would be unaffected by the apoE-specific siRNA, which was used to silence endogenous apoE expression. DNA vectors expressing wild-type and mutant apoE proteins were transfected into HCV-infected Huh-7.5 cells, which were cotransfected with an apoE siRNA. The levels of apoE expression in the cell were determined by Western blotting. As shown in Fig. 4, wild-type and deletion mutant apoE proteins were all ectopically expressed to similar levels, demonstrating that deletion mutations did not significantly affect apoE expression. It should be noted that the apoE-specific siRNA knocked out endogenous apoE expression to an undetectable level (Fig. 4, vector). The effects of ectopic expression of apoE on HCV replication were determined by measuring the levels of HCV proteins in the transfected and HCV-infected cells. Similar to our previous findings, ectopic expression of wild-type and mutant apoE proteins did not influence HCV replication, as similar levels of NS5A protein (Fig. 5) and positive-stranded HCV RNA (data not shown) were detected by Western blotting and a real-time RT-PCR method, respectively. To determine the effects of apoE deletion mutations on HCV production, we quantified the levels of HCV vRNA in the culture media by a real-time RT-PCR method and determined infectious HCV titers (TCID50/ml) in the media using a serial dilution and IHC staining method, as described previously (29). Strikingly, all apoE deletion mutations disrupting the apoE-NS5A interaction reduced HCV vRNA levels by 90% (Fig. 6A) and decreased the titers of infectious HCV by nearly 10-fold (Fig. 6B) in a single-cycle (24-h) virus growth assay. apoE deletion mutations Del 280-299 and Del 201-205, which had no effect on the apoE-NS5A interaction, did not significantly reduce HCV production. However, deletion mutations Del 270-299 and Del 226-239, which reduced the NS5A-binding activity, also resulted in a decrease of HCV vRNA by 50 to 60% (Fig. 6A) and infectious HCV by about 3-fold (Fig. 6B), respectively. These results are consistent with those obtained from the mammalian two-hybrid and co-IP studies (Fig. 2 and 3). Overall, these findings demonstrate that the apoE-NS5A interaction is important for production of infectious HCV and that disruption of the apoE-NS5A interaction by mutations of apoE suppressed HCV production.
FIG. 5.
Effect of ectopic expression of apoE mutations on HCV replication. Huh-7.5 cells in 12-well plates were cotransfected with 50 nM apoE or NSC siRNA and pCMV-XL5-mApoE3 vector expressing wild-type or deletion mutant apoE. After 16 h of incubation, Huh7.5 cells were infected with cell culture-derived HCV. At 24 h p.i., the media were collected and cells were lysed in a radioimmunoprecipitation assay buffer. The levels of NS5A in cell lysate were determined by Western blotting using an NS5A monoclonal antibody along with β-actin as a control.
FIG. 6.
Determination of the effects of apoE deletion mutations on HCV production. (A) Quantification of HCV vRNA in the media by a real-time RT-PCR method. HCV vRNA in the media was extracted with Trizol LS reagent (Invitrogen). The levels of HCV vRNA were determined by a quantitative RT-PCR as described in Materials and Methods. (B) Determination of infectious HCV titers. Infectious HCV titers in the supernatants of HCV-infected and apoE cDNA-transfected cells were determined by serial dilutions in 96-well plates of Huh-7.5 cells. The HCV-infected cells were stained with an HCV C-specific monoclonal antibody. TCID50 was calculated as previously described (29).
Three major isoforms of apoE are all compatible with NS5A binding and HCV production.
In humans, there are three major isoforms of apoE (E2, E3, and E4), which differ from one another by single amino acid substitutions at residues 112 and 158 (Fig. 7A). Previous genetic studies suggested that apoE polymorphism is associated with the outcomes of HCV infection and antiviral therapy for hepatitis C (42, 45, 49, 58). Initially, we tested these three common isoforms of apoE for their interactions with NS5A using the mammalian two-hybrid assay. Results show that all three apoE isoforms bind NS5A equally well in the cell based on similar levels of luciferase activity (Fig. 7B). When the three apoE isoforms were expressed ectopically (Fig. 7C), they interacted with NS5A at similar efficiencies, as demonstrated by the co-IP assay (Fig. 7D). The apoE-NS5A interactions are similar among different isoforms, consistent with the fact that the apoE C-terminal α-helix domains responsible for NS5A-binding are identical among the different isoforms of apoE. In the HCV-infected Huh-7.5 cells, ectopic expression of all three apoE isoforms did not significantly influence HCV production, as indicated by similar levels of HCV vRNA present in the media (Fig. 7E) as well as similar titers of infectious HCV (Fig. 7E), suggesting that apoE isoforms do not differ in HCV infectivity. Collectively, our findings demonstrate that each of the three major apoE isoforms is equally compatible with HCV infection and assembly, at least in cell culture.
FIG. 7.
Effects of apoE isoforms on the apoE-NS5A interaction and HCV production. (A) Difference in amino acid residues among three common isoforms of apoE. (B) Comparison of protein-protein interactions between NS5A and apoE isoforms. The interaction of apoE isoforms with NS5A was determined as for Fig. 1B and 2B except that pM vectors expressed different apoE isoforms. (C) Ectopic expression of apoE isoforms in the cell. Experiments were done similarly to Fig. 4. (D) Interaction of apoE isoforms with NS5A determined by co-IP. Upon ectopic expression of apoE isoforms, co-IP of NS5A by apoE-specific mAB23 and detection of NS5A by Western blotting were the same as for Fig. 3. (E) Effects of apoE isoforms on HCV production. Experimental procedures were the same as for Fig. 5 and 6 except that different apoE isoforms were expressed ectopically. (F) Effects of apoE isoforms on infectious HCV titers. TCID50 of infectious HCV in the supernatants was determined based on the limiting dilution method described previously by others (29).
DISCUSSION
Human apoE is a major protein constituent of several cholesterol-enriched lipoproteins such as chylomicron, very-low-density lipoprotein (VLDL), and high-density lipoprotein (HDL). It is a major determinant in normal homeostasis of cholesterol and other lipids and in cardiovascular disease (21, 34, 38). Additionally, apoE plays an important role in many other biological processes independent of lipoprotein transport and metabolism, including Alzheimer's disease, immunoregulation, and infectious diseases (37, 38). Recently, we have demonstrated that apoE has dual functions in infection and assembly of infectious HCV (6, 10, 23). apoE was found to be enriched in infectious HCV particles, whose infectivity could be potently neutralized with apoE-specific monoclonal antibodies (10). We believe that apoE is a structural component of the HCV particle, as suggested by our findings obtained from studies with affinity precipitation and protease sensitivity assays (23). The structural nature of apoE in HCV particles was confirmed by immunogold electronic microscopy studies by (Ralf Bartenschlager, presented at the 16th International Symposium on HCV and Related Viruses). More importantly, apoE was found to interact with the HCV NS5A in HCV-infected cells as well as in purified HCV particles (6, 23). NS5A mutations which impaired HCV production were also found to disrupt or reduce the apoE-NS5A interaction (6). In this report, we provide substantial evidence to show that the carboxyl-terminal α-helix domain of apoE is important for its interaction with NS5A, as demonstrated by both mammalian two-hybrid studies and co-IP experiments (Fig. 2 and 3). The α-helical domain itself, when fused with the HSV VP16 activation domain, was sufficient to interact with NS5A, as determined by the mammalian two-hybrid system (Fig. 2). Progressive deletion mutagenesis analysis has mapped the NS5A-binding domain to the middle of the C-terminal α-helical region between amino acid residues 205 and 270 (Fig. 2 [highlighted in gray in panel A] and 3). More significantly, the findings derived from ectopic expression of apoE mutants demonstrate that the apoE-NS5A interaction is important for HCV production in cell culture. The reduction of HCV production was closely correlated with the disruption of the apoE-NS5A interaction by deletion mutations of apoE (Fig. 2, 3, and 6). For instance, mutations disrupting the apoE-NS5A interaction failed to result in HCV production. Two mutations that reduced the apoE-NS5A interaction also decreased the levels of HCV production (Fig. 3 and 6). However, deletion mutations did not significantly affect the levels of apoE expression (Fig. 5) or intracellular distribution (data not shown), suggesting that short deletions did not influence stability or interfere with the lipid-binding activity of apoE. We do not know whether deletion mutations caused any change to the structure of apoE since the structure of the C-terminal α-helix domain of apoE in association with lipids or lipoproteins has not been determined due to technical difficulty. Studies with lipid-free apoE proteins suggested that there are interactions between the N- and C-terminal domains only in apoE4, not in apoE2 or apoE3 (19, 21). Although the N-terminal domain of apoE3 is dispensable for the apoE-NS5A interaction (Fig. 2), it is required for the function of apoE in HCV assembly/production, as the C-terminal domain by itself failed to restore HCV production (data not shown). Further investigations are warranted to determine how the N-terminal domain of apoE determines the functional importance of apoE in HCV assembly/production. Nevertheless, the findings described here support our previous model that the apoE N-terminal domain containing the receptor-binding region mediates HCV infection, while the NS5A-binding domain within the C-terminal lipid- or lipoprotein-binding region is important for HCV assembly (23). Thus, dual functions of apoE in HCV infection and assembly are carried out by two structurally and functionally distinct domains of apoE. It is likely that the apoE-NS5A interaction will be an ideal and novel target for identification of inhibitors to block HCV assembly.
The question of how apoE is assembled to form infectious HCV particles in the cell remains unanswered. It has been postulated that HCV may use the same assembly and secretory pathway as VLDL (18, 20, 44, 48). This model of HCV assembly, morphogenesis, and egression was mainly based on the findings that some of the VLDL-associated components were also found in the membranous complexes isolated from HCV-replicating cells and that apoB was shown to facilitate HCV production (18, 20). There was also a report that NS5A interacted with apoB100, whose secretion was inhibited by HCV RNA replication (12). However, the findings derived from our studies point to a different pathway unrelated to the assembly and secretion of VLDL. Our previous studies have demonstrated that inhibitors of microsomal triglyceride transfer protein (MTP) completely blocked the secretion of apoB-containing lipoproteins but did not affect HCV production (23). Another independent study also found that an MTP inhibitor completely suppressed apoB100 secretion without affecting apoE secretion from hepatocytes (14). More significantly, knockdown of apoB by specific siRNAs had no significant effect on HCV production. However, suppression of HCV production was directly correlated with the knockdown or inhibition of apoE expression in the cell (23). It was thought that HCV assembly takes place in the cell in association with lipid droplets (LD) together with HCV C and NS5A proteins. NS5A mutations disrupting the NS5A-LD interaction resulted in blockade of HCV production (41). Interestingly, these same NS5A mutations were also found to disrupt the apoE-NS5A interaction (6). Additionally, a recent study suggested that the interaction of HCV C with NS5A is critical for HCV assembly and production (40). Based on these findings, we offer an alternative model for the assembly of the apoE-containing HCV particles. apoE binds phospholipids in LD as a discoidal particle independent of MTP and VLDL, as demonstrated by a number of studies (25, 26, 28, 52, 53). The LD-bound apoE interacts with NS5A through its C-terminal NS5A-binding domain, as determined in the present study. Finally, NS5A mediates an interaction with HCV nucleocapsid via binding to the HCV C protein. This HCV assembly model is supported by the findings derived from our recent studies that NS5A was detected in affinity-purified HCV particles (23). The relevance of this alternative pathway for apoE-containing lipoproteins distinct from VLDL to HCV assembly remains to be determined.
apoE is a polymorphic protein with three major isoforms, referred to as apoE2, apoE3, and apoE4 (50, 51, 62). apoE3 is the most common form, accounting for nearly two-thirds of the apoE phenotype. apoE2 is defective in binding to low-density lipoprotein (LDL) receptor and is associated with the genetic disorder type III hyperlipoproteinemia or familial dysbetalipoproteinemia. apoE4 is a major risk factor for Alzheimer's disease (37, 38). It was recently found that apoE4 is also associated with the enhancement of HIV infection and disease progression (8, 61). In the case of HCV, several genetic studies suggested that apoE polymorphism plays a role in the outcomes of HCV infection and anti-HCV therapy (22, 42, 45, 49, 58). In this study, we have examined the role of apoE isoforms in the apoE-NS5A interaction and HCV production in cell culture. We did not observe significant difference in the apoE-NS5A interaction and HCV production among apoE2, apoE3, and apoE4. All three common isoforms of apoE bound to NS5A equally well, as determined by the mammalian two-hybrid and co-IP experiments (Fig. 7). When expressed ectopically, all three isoforms of apoE produced similar levels of HCV production and infectivity. Similar levels of HCV vRNA were detected in the media, and the levels of HCV NS5A protein did not significantly vary in the naive Huh-7.5 cells infected with HCV containing different isoforms of apoE (Fig. 7). Collectively, these findings demonstrate that the three common isoforms of apoE do not significantly differ in the apoE-NS5A interaction and HCV production, at least in cell culture. It is possible that apoE polymorphism may play a role in recovery or persistence of HCV infection due to differences between the isoforms in other biological functions in vivo (21, 37). To our surprise, HCV with apoE2 was as infectious as viruses containing apoE3 and apoE4 (Fig. 7). apoE2 is known to be severely defective in binding to LDL receptor, which was speculated to be an HCV receptor/coreceptor (1). Similarly, a recent study suggested that apoE on HCV particles facilitated HCV infection through interaction with LDL receptor (43). HCV containing apoE2 would be less infectious than apoE3 and apoE4 if apoE mediates HCV infection via binding to the LDL receptor. Alternatively, we speculate that apoE on HCV virions may bind heparin sulfate proteoglycans (HSPG) on the cell surface. HSPG is known to play an important role in apoE-containing lipoprotein metabolism in hepatocytes and other cells (35, 36). In fact, heparin was found to efficiently block HCV infection in cell culture. Similarly, cells treated with heparinase became less susceptible to HCV infection (24), suggesting that HSPG on the cell surface is needed for HCV attachment, probably through apoE. However, it is not clear how HCV infection is mediated by numerous cell surface receptors/coreceptors, which may act at different steps of the viral entry process in a orchestrated manner. Future studies are warranted to determine the underlying molecular mechanism of apoE in HCV cell entry through binding to cell surface receptors/coreceptors and/or other steps after virus attachment.
Acknowledgments
We thank Charles M. Rice (Rockefeller University) for providing both the Huh7.5 cell line and NS5A monoclonal antibody, Takaji Wakita (National Institute of Health, Japan) for the pSGR/JFH1 replicon cDNA, and Theodore Mazzone (University of Illinois at Chicago) for the apoE3 cDNA pcDNA3.1/hApoE3.
This work was supported by NIH grants AI070769 and DK079293.
Footnotes
Published ahead of print on 18 August 2010.
REFERENCES
- 1.Agnello, V., G. Abel, M. Elfahal, G. B. Knight, and Q. X. Zhang. 1999. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc. Natl. Acad. Sci. U. S. A. 96:12766-12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alter, M. J. 2006. Epidemiology of viral hepatitis and HIV co-infection. J. Hepatol. 44:S6-S9. [DOI] [PubMed] [Google Scholar]
- 3.Bartels, D. J., Y. Zhou, E. Z. Zhang, M. Marcial, R. A. Byrn, T. Pfeiffer, A. M. Tigges, B. S. Adiwijaya, C. Lin, A. D. Kwong, and T. L. Kieffer. 2008. Natural prevalence of hepatitis C virus variants with decreased sensitivity to NS3.4A protease inhibitors in treatment-naive subjects. J. Infect. Dis. 198:800-807. [DOI] [PubMed] [Google Scholar]
- 4.Bartenschlager, R., M. Frese, and T. Pietschmann. 2004. Novel insights into hepatitis C virus replication and persistence. Adv. Virus Res. 63:71-180. [DOI] [PubMed] [Google Scholar]
- 5.Bartenschlager, R., and V. Lohmann. 2001. Novel cell culture systems for the hepatitis C virus. Antiviral Res. 52:1-17. [DOI] [PubMed] [Google Scholar]
- 6.Benga, W. J., S. E. Krieger, M. Dimitrova, M. B. Zeisel, M. Parnot, J. Lupberger, E. Hildt, G. Luo, J. McLauchlan, T. F. Baumert, and C. Schuster. 2010. Apolipoprotein E interacts with hepatitis C virus nonstructural protein 5A and determines assembly of infectious particles. Hepatology 51:43-53. [DOI] [PubMed] [Google Scholar]
- 7.Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1975. [DOI] [PubMed] [Google Scholar]
- 8.Burt, T. D., B. K. Agan, V. C. Marconi, W. He, H. Kulkarni, J. E. Mold, M. Cavrois, Y. Huang, R. W. Mahley, M. J. Dolan, J. M. McCune, and S. K. Ahuja. 2008. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proc. Natl. Acad. Sci. U. S. A. 105:8718-8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai, Z., C. Zhang, K. S. Chang, J. Jiang, B. C. Ahn, T. Wakita, T. J. Liang, and G. Luo. 2005. Robust production of infectious hepatitis C virus (HCV) from stably HCV cDNA-transfected human hepatoma cells. J. Virol. 79:13963-13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, K. S., J. Jiang, Z. Cai, and G. Luo. 2007. Human apolipoprotein E is required for infectivity and production of hepatitis C virus in cell culture. J. Virol. 81:13783-13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi, J., Z. Xu, and J. H. Ou. 2003. Triple decoding of hepatitis C virus RNA by programmed translational frameshifting. Mol. Cell. Biol. 23:1489-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domitrovich, A. M., D. J. Felmlee, and A. Siddiqui. 2005. Hepatitis C virus nonstructural proteins inhibit apolipoprotein B100 secretion. J. Biol. Chem. 280:39802-39808. [DOI] [PubMed] [Google Scholar]
- 13.Eng., F. J., J. L. Walewski, A. L. Klepper, S. L. Fishman, S. M. Desai, L. K. McMullan, M. J. Evans, C. M. Rice, and A. D. Branch. 2009. Internal initiation stimulates production of p8 minicore, a member of a newly discovered family of hepatitis C virus core protein isoforms. J. Virol. 83:3104-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan, D., S. Qiu, C. D. Overton, P. G. Yancey, L. L. Swift, W. G. Jerome, M. F. Linton, and S. Fazio. 2007. Impaired secretion of apolipoprotein e2 from macrophages. J. Biol. Chem. 282:13746-13753. [DOI] [PubMed] [Google Scholar]
- 15.Friebe, P., and R. Bartenschlager. 2002. Genetic analysis of sequences in the 3′ nontranslated region of hepatitis C virus that are important for RNA replication. J. Virol. 76:5326-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friebe, P., V. Lohmann, N. Krieger, and R. Bartenschlager. 2001. Sequences in the 5′ nontranslated region of hepatitis C virus required for RNA replication. J. Virol. 75:12047-12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fried, M. W., M. L. Shiffman, K. R. Reddy, C. Smith, G. Marinos, F. L. Goncales, Jr., D. Haussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxi, A. Lin, J. Hoffman, and J. Yu. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975-982. [DOI] [PubMed] [Google Scholar]
- 18.Gastaminza, P., G. Cheng, S. Wieland, J. Zhong, W. Liao, and F. V. Chisari. 2008. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J. Virol. 82:2120-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatters, D. M., C. A. Peters-Libeu, and K. H. Weisgraber. 2006. Apolipoprotein E structure: insights into function. Trends Biochem. Sci. 31:445-454. [DOI] [PubMed] [Google Scholar]
- 20.Huang, H., F. Sun, D. M. Owen, W. Li, Y. Chen, M. Gale, Jr., and J. Ye. 2007. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc. Natl. Acad. Sci. U. S. A. 104:5848-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, Y., K. H. Weisgraber, L. Mucke, and R. W. Mahley. 2004. Apolipoprotein E: diversity of cellular origins, structural and biophysical properties, and effects in Alzheimer's disease. J. Mol. Neurosci. 23:189-204. [DOI] [PubMed] [Google Scholar]
- 22.Itzhaki, R. F., W. L. Irving, and M. A. Wozniak. 2003. Apolipoprotein E and hepatitis C virus. Hepatology 38:1060. [DOI] [PubMed] [Google Scholar]
- 23.Jiang, J., and G. Luo. 2009. Apolipoprotein E but not B is required for the formation of infectious hepatitis C virus particles. J. Virol. 83:12680-12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koutsoudakis, G., A. Kaul, E. Steinmann, S. Kallis, V. Lohmann, T. Pietschmann, and R. Bartenschlager. 2006. Characterization of the early steps of hepatitis C virus infection by using luciferase reporter viruses. J. Virol. 80:5308-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krimbou, L., M. Denis, B. Haidar, M. Carrier, M. Marcil, and J. Genest, Jr. 2004. Molecular interactions between apoE and ABCA1: impact on apoE lipidation. J. Lipid Res. 45:839-848. [DOI] [PubMed] [Google Scholar]
- 26.Krimbou, L., M. Marcil, H. Chiba, and J. Genest, Jr. 2003. Structural and functional properties of human plasma high density-sized lipoprotein containing only apoE particles. J. Lipid Res. 44:884-892. [DOI] [PubMed] [Google Scholar]
- 27.Kuntzen, T., J. Timm, A. Berical, N. Lennon, A. M. Berlin, S. K. Young, B. Lee, D. Heckerman, J. Carlson, L. L. Reyor, M. Kleyman, C. M. McMahon, C. Birch, J. Schulze Zur Wiesch, T. Ledlie, M. Koehrsen, C. Kodira, A. D. Roberts, G. M. Lauer, H. R. Rosen, F. Bihl, A. Cerny, U. Spengler, Z. Liu, A. Y. Kim, Y. Xing, A. Schneidewind, M. A. Madey, J. F. Fleckenstein, V. M. Park, J. E. Galagan, C. Nusbaum, B. D. Walker, G. V. Lake-Bakaar, E. S. Daar, I. M. Jacobson, E. D. Gomperts, B. R. Edlin, S. M. Donfield, R. T. Chung, A. H. Talal, T. Marion, B. W. Birren, M. R. Henn, and T. M. Allen. 2008. Naturally occurring dominant resistance mutations to hepatitis C virus protease and polymerase inhibitors in treatment-naive patients. Hepatology 48:1769-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kypreos, K. E., and V. I. Zannis. 2007. Pathway of biogenesis of apolipoprotein E-containing HDL in vivo with the participation of ABCA1 and LCAT. Biochem. J. 403:359-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindenbach, B. D., M. J. Evans, A. J. Syder, B. Wolk, T. L. Tellinghuisen, C. C. Liu, T. Maruyama, R. O. Hynes, D. R. Burton, J. A. McKeating, and C. M. Rice. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623-626. [DOI] [PubMed] [Google Scholar]
- 30.Lindenbach, B. D., and C. M. Rice. 2007. Flaviviridae: the viruses and their replication. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed., vol. 1. Lippincott-Raven, Philadelphia, PA.
- 31.Lindenbach, B. D., and C. M. Rice. 2005. Unravelling hepatitis C virus replication from genome to function. Nature 436:933-938. [DOI] [PubMed] [Google Scholar]
- 32.Lohmann, V., F. Korner, J. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 33.Luo, G., S. Xin, and Z. Cai. 2003. Role of the 5′-proximal stem-loop structure of the 5′ untranslated region in replication and translation of hepatitis C virus RNA. J. Virol. 77:3312-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahley, R. W. 1988. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240:622-630. [DOI] [PubMed] [Google Scholar]
- 35.Mahley, R. W. 1996. Heparan sulfate proteoglycan/low density lipoprotein receptor-related protein pathway involved in type III hyperlipoproteinemia and Alzheimer's disease. Isr. J. Med. Sci. 32:414-429. [PubMed] [Google Scholar]
- 36.Mahley, R. W., and Z. S. Ji. 1999. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J. Lipid Res. 40:1-16. [PubMed] [Google Scholar]
- 37.Mahley, R. W. and S. C. Rall, Jr. 2000. Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 1:507-537. [DOI] [PubMed] [Google Scholar]
- 38.Mahley, R. W., K. H. Weisgraber, and Y. Huang. 2009. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer's disease to AIDS. J. Lipid Res. 50(Suppl.):S183-S188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 40.Masaki, T., R. Suzuki, K. Murakami, H. Aizaki, K. Ishii, A. Murayama, T. Date, Y. Matsuura, T. Miyamura, T. Wakita, and T. Suzuki. 2008. Interaction of hepatitis C virus nonstructural protein 5A with core protein is critical for the production of infectious virus particles. J. Virol. 82:7964-7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyanari, Y., K. Atsuzawa, N. Usuda, K. Watashi, T. Hishiki, M. Zayas, R. Bartenschlager, T. Wakita, M. Hijikata, and K. Shimotohno. 2007. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 9:1089-1097. [DOI] [PubMed] [Google Scholar]
- 42.Mueller, T., R. Gessner, C. Sarrazin, C. Graf, J. Halangk, H. Witt, E. Kottgen, B. Wiedenmann, and T. Berg. 2003. Apolipoprotein E4 allele is associated with poor treatment response in hepatitis C virus (HCV) genotype 1. Hepatology 38:1592-1593. [DOI] [PubMed] [Google Scholar]
- 43.Owen, D. M., H. Huang, J. Ye, and M. Gale, Jr. 2009. Apolipoprotein E on hepatitis C virion facilitates infection through interaction with low-density lipoprotein receptor. Virology 394:99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Popescu, C. I., and J. Dubuisson. 2010. Role of lipid metabolism in hepatitis C virus assembly and entry. Biol. Cell 102:63-74. [DOI] [PubMed] [Google Scholar]
- 45.Price, D. A., M. F. Bassendine, S. M. Norris, C. Golding, G. L. Toms, M. L. Schmid, C. M. Morris, A. D. Burt, and P. T. Donaldson. 2006. Apolipoprotein epsilon3 allele is associated with persistent hepatitis C virus infection. Gut 55:715-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pringle, C. R. 1999. Virus taxonomy—1999. The universal system of virus taxonomy, updated to include the new proposals ratified by the International Committee on Taxonomy of Viruses during 1998. Arch. Virol. 144:421-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robertson, B., G. Myers, C. Howard, T. Brettin, J. Bukh, B. Gaschen, T. Gojobori, G. Maertens, M. Mizokami, O. Nainan, S. Netesov, K. Nishioka, T. Shin-I, P. Simmonds, D. Smith, L. Stuyver, and A. Weiner. 1998. Classification, nomenclature, and database development for hepatitis C virus (HCV) and related viruses: proposals for standardization. Arch. Virol. 143:2493-2503. [DOI] [PubMed] [Google Scholar]
- 48.Syed, G. H., Y. Amako, and A. Siddiqui. 2010. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol. Metab. 21:33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toniutto, P., C. Fabris, E. Fumo, L. Apollonio, M. Caldato, L. Mariuzzi, C. Avellini, R. Minisini, and M. Pirisi. 2004. Carriage of the apolipoprotein E-epsilon4 allele and histologic outcome of recurrent hepatitis C after antiviral treatment. Am. J. Clin. Pathol. 122:428-433. [DOI] [PubMed] [Google Scholar]
- 50.Utermann, G., U. Langenbeck, U. Beisiegel, and W. Weber. 1980. Genetics of the apolipoprotein E system in man. Am. J. Hum. Genet. 32:339-347. [PMC free article] [PubMed] [Google Scholar]
- 51.Utermann, G., A. Steinmetz, and W. Weber. 1982. Genetic control of human apolipoprotein E polymorphism: comparison of one- and two-dimensional techniques of isoprotein analysis. Hum. Genet. 60:344-351. [DOI] [PubMed] [Google Scholar]
- 52.Vedhachalam, C., V. Narayanaswami, N. Neto, T. M. Forte, M. C. Phillips, S. Lund-Katz, and J. K. Bielicki. 2007. The C-terminal lipid-binding domain of apolipoprotein E is a highly efficient mediator of ABCA1-dependent cholesterol efflux that promotes the assembly of high-density lipoproteins. Biochemistry 46:2583-2593. [DOI] [PubMed] [Google Scholar]
- 53.Wahrle, S. E., H. Jiang, M. Parsadanian, J. Legleiter, X. Han, J. D. Fryer, T. Kowalewski, and D. M. Holtzman. 2004. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J. Biol. Chem. 279:40987-40993. [DOI] [PubMed] [Google Scholar]
- 54.Wakita, T., T. Pietschmann, T. Kato, T. Date, M. Miyamoto, Z. Zhao, K. Murthy, A. Habermann, H. G. Krausslich, M. Mizokami, R. Bartenschlager, and T. J. Liang. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11:791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walewski, J. L., T. R. Keller, D. D. Stump, and A. D. Branch. 2001. Evidence for a new hepatitis C virus antigen encoded in an overlapping reading frame. RNA 7:710-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, C., P. Sarnow, and A. Siddiqui. 1993. Translation of human hepatitis C virus RNA in cultured cells is mediated by an internal ribosome-binding mechanism. J. Virol. 67:3338-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.WHO. 1998. WHO concerns hepatitis C. Lancet 351:1415. [Google Scholar]
- 58.Wozniak, M. A., R. F. Itzhaki, E. B. Faragher, M. W. James, S. D. Ryder, and W. L. Irving. 2002. Apolipoprotein E-epsilon 4 protects against severe liver disease caused by hepatitis C virus. Hepatology 36:456-463. [DOI] [PubMed] [Google Scholar]
- 59.Xu, Z., J. Choi, T. S. Yen, W. Lu, A. Strohecker, S. Govindarajan, D. Chien, M. J. Selby, and J. Ou. 2001. Synthesis of a novel hepatitis C virus protein by ribosomal frameshift. EMBO J. 20:3840-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.You, S., and C. M. Rice. 2008. 3′ RNA elements in hepatitis C virus replication: kissing partners and long poly(U). J. Virol. 82:184-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Youngsteadt, E. 2008. Virology Alzheimer's risk factor also aids HIV. Science 320:1577. [DOI] [PubMed] [Google Scholar]
- 62.Zannis, V. I., and J. L. Breslow. 1981. Human very low density lipoprotein apolipoprotein E isoprotein polymorphism is explained by genetic variation and posttranslational modification. Biochemistry 20:1033-1041. [DOI] [PubMed] [Google Scholar]
- 63.Zhong, J., P. Gastaminza, G. Cheng, S. Kapadia, T. Kato, D. R. Burton, S. F. Wieland, S. L. Uprichard, T. Wakita, and F. V. Chisari. 2005. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. U. S. A. 102:9294-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]