Abstract
Latent Epstein-Barr virus (EBV) infection is an important causative factor in the development of several cancers, including nasopharyngeal carcinoma (NPC). The one EBV protein expressed in the nucleus of NPC cells, EBNA1, has been shown to disrupt promyelocitic leukemia (PML) nuclear bodies (NBs) by inducing the degradation of PML proteins, leading to impaired DNA repair and increased cell survival. Although EBNA1-mediated PML disruption is likely to be an important factor in the development of NPC, little is known about its mechanism. We now show that an interaction between EBNA1 and the host CK2 kinase is crucial for EBNA1 to disrupt PML bodies and degrade PML proteins. EBNA1 increases the association of CK2 with PML proteins, thereby increasing the phosphorylation of PML proteins by CK2, a modification that is known to trigger the polyubiquitylation and degradation of PML. The interaction between EBNA1 and CK2 is direct and occurs through the β regulatory subunit of CK2 and EBNA1 amino acids 387 to 394. The binding of EBNA1 to the host ubiquitin specific protease USP7 has also been shown to be important for EBNA1-mediated PML disruption. We show that EBNA1 also increases the occupancy of USP7 at PML NBs and that CK2 and USP7 bind independently and simultaneously to EBNA1 to form a ternary complex. The combined results indicate that EBNA1 usurps two independent cellular pathways to trigger the loss of PML NBs.
PML protein and its role as a tumor suppressor was first identified in the oncogenesis of acute promyelocytic leukemia (APL). PML forms distinct nuclear structures called promyelocitic leukemia (PML) nuclear bodies (NBs), also known as PODs or ND10s, and the formation of these bodies are essential to mediate the known functions of PML (17, 34). PML exists as six nuclear isoforms and one cytoplasmic isoform that are generated by alternative splicing (6, 25, 34). The six nuclear isoforms are modified by the addition of the small ubiquitinlike modifier SUMO, which enables their interaction to form the structural basis of the PML NB, with which many additional proteins then interact (6). PML NBs can be regarded as nuclear organizing centers that govern many important cellular events, including cell cycle progression, DNA damage response/repair, transcriptional regulation, apoptosis, and activation of p53 (6, 16, 17, 34, 42). In APL, the translocation of the PML gene results in the expression of a fusion protein, PML-RARα, that disrupts the function of PML NBs and is the driving force for the development of APL (34). The loss of PML NBs is also associated with the development or progression of several additional types of tumors (21, 34).
In addition to these cellular functions, PML NBs are part of the innate immune response to suppress lytic viral replication and transcription (17, 19). As a result, many viruses encode proteins that disrupt PML NBs, thereby enabling lytic infection (2, 24, 35, 44). These include the ICP0 protein of herpes simplex virus, which triggers the degradation of PML proteins (11, 18), and the BZLF1 protein of Epstein-Barr virus (EBV) that interferes with the interaction of the PML proteins to form NBs (1). In addition, viral proteins may disrupt PML NBs in order to promote cell survival by inhibiting apoptosis, as appears to be the case with Epstein-Barr nuclear antigen 1 (EBNA1) during EBV latent infection in nasopharyngeal carcinoma cells (40).
EBV is a widespread herpesvirus that induces cell proliferation and survival as part of its normal latent infection. As a result, EBV is strongly associated with a growing list of cancers, including nasopharyngeal carcinoma (NPC), a tumor that is endemic in several parts of the world (32). Latent EBV infection of NPC cells involves expression of one viral nuclear protein, EBNA1 (32). EBNA1 is required for the replication and stable persistence of EBV episomes in proliferating cells and is the only EBV protein that is expressed in all EBV-associated tumors (32). In addition, increasing evidence suggests a role for EBNA1 in the development and/or progression of EBV-associated tumors. For example, downregulation of EBNA1 by RNA interference in several types of EBV-positive cells has been shown to decrease cell proliferation and survival (23, 46). Consistent with these observations, overexpression of a dominant-negative EBNA1 mutant increased cell death in EBV-positive Burkitt's lymphoma cells, indicating an antiapoptotic role for EBNA1 (27). In addition, the expression of EBNA1 in breast carcinoma cells increased metastasis in nude mice via inhibition of a known suppressor of cell migration and tumor metastasis, Nm23-H1 (26, 31).
The role of EBNA1 in inhibiting apoptosis can be partly explained by its interaction with the host ubiquitin specific protease 7 (USP7), also called HAUSP (22, 36). In response to genotoxic stress, USP7 binds and stabilize p53 by removing polyubiquitin chains (12, 28). EBNA1 was found to interfere with USP7 binding to p53, thereby resulting in the destabilization and degradation of p53 (36). However, the effects of EBNA1 on apoptosis are not limited to alteration of p53 levels, since we have found that EBNA1 disrupts PML NBs in NPC cells (40). More specifically, EBV-positive NPC cells were observed to have fewer PML NBs than their EBV-negative counterparts, and silencing EBNA1 restored the PML NBs to the level of EBV-negative cells. Furthermore, expression of EBNA1 on its own in EBV-negative NPC cells dramatically decreased the number of PML NB and the cellular level of PML proteins without affecting PML transcript levels, indicating that EBNA1 induced the degradation of the PML proteins. In keeping with the known functions of PML NBs, EBNA1 expression in this system lead to impaired DNA repair, decreased p53 activation and apoptosis, and increased cell survival after treatment with DNA-damaging agents. Unexpectedly, the ability of EBNA1 to disrupt PML NBs was found to require its interaction with USP7, in that PML NBs were not disrupted by an EBNA1 USP7-binding mutant, nor were they disrupted by wild-type EBNA1 when USP7 was silenced (40). These observations suggested that USP7 itself is a regulator of PML NBs that is utilized by EBNA1, a hypothesis that was further substantiated by showing that PML NB numbers are increased when USP7 is silenced and decreased upon USP7 overexpression (F. Sarkari, unpublished data).
The proteomics approaches that identified USP7 as a binding partner of EBNA1 also showed that EBNA1 stably interacted with protein kinase CK2 (formerly known as casein kinase 2) (22, 36). CK2 is a tetramer consisting of two catalytic subunits (CK2α and/or CK2 α′) and two regulatory subunits of CK2β (29). All three of these subunits were recovered in complex with EBNA1 showing that EBNA1 sequesters or recruits the holoenzyme (22). CK2 is known to regulate many cellular processes through phosphorylation of a variety of cellular targets, including PML proteins. Scaglioni et al. (37, 38) have shown that CK2 phosphorylates PML at serine 517, which primes PML for polyubiquitylation and degradation by the proteasome. The loss of this CK2 phosphorylation site in PML results in stabilization of PML and increases PML-induced apoptosis and senescence. The importance of CK2-mediated PML degradation in cell transformation is reflected in the observations that there is an inverse relationship between CK2 activity and PML expression in several human tumors (14).
We have examined here the significance of the EBNA1-CK2 interaction for PML disruption, the mechanism of EBNA1-CK2 interaction, and the relationship between the CK2 and USP7 interactions with EBNA1. We show that EBNA1 interacts directly with CK2β through EBNA1 residues 387 to 394, thereby increasing CK2 association with and phosphorylation of PML. Furthermore, we show that EBNA1-CK2 interaction occurs independently of the EBNA1-USP7 interaction, which is also required for PML disruption.
MATERIALS AND METHODS
Cell lines.
The EBV-negative nasopharyngeal carcinoma cells CNE2 (also called CNE2Z) and the CNE2E cells that stably express EBNA1 have been previously described (40, 41). Both cell lines were grown at 37°C in alpha minimal essential media (αMEM; Gibco) supplemented with 10% fetal calf serum (Sigma), and 0.5 mg of hygromycin B/ml was added to CNE2E to stably maintain EBNA1 expression.
EBNA1 expression plasmids.
C-terminally SPA-tagged EBNA1 (lacking most of the Gly-Ala repeat) and the EBNA1 mutants Δ41-376, Δ395-450, 31-641, and 452-641 were generated by PCR amplification of these EBNA1 sequences from pc3oriPE or pc3oriPEΔ41-376 (39) or pc3oriPEΔ395-450 (22) and insertion between the NotI and XhoI sites of pMZS3F (47). EBNA1 Δ387-394 in pMZS3F was generated by QuikChange site-directed mutagenesis (Stratagene, La Jolla, CA) of pMZS3F EBNA1. In experiments studying the effect of EBNA1 on PML NBs, EBNA1 was expressed (without tags) from the pc3oriP plasmid (referred to as pc3oriPE), which contains the EBV oriP element. Pc3oriP and pc3oripE are described in Shire et al. in 1999 (39), while pc3oriP expressing EBNA1 Δ395-450 is described in Holowaty et al. in 2003 (22). Pc3oriP expressing Δ387-394 was generated by PCR amplification of EBNA1 Δ387-394 coding sequences in pMZS3F and insertion between the HindIII and BamHI sites of pc3oriP. The sequences of all of the EBNA1 mutants were verified by DNA sequencing.
Transfections and RNA interference.
To generate CNE2 cells transiently expressing EBNA1, 1.5 × 105 cells were transfected with 2 or 8 μg of pc3OriPE using Lipofectamine 2000 (Invitrogen), and pc3OriP was used as a negative control. Where indicated, the same plasmid expressing EBNA1 Δ395-450 or EBNA1 Δ387-394 was used in place of pc3OriPE. Cells were fixed 48 h later for immunofluorescence microscopy as described below, except for experiments designed to determine the ability of EBNA1 proteins and USP7 to localize to PML NBs in which cells were fixed 12 h posttransfection. For RNA interference experiments, 106 CNE2 or CNE2E cells in 10-cm dishes were transfected with 100 pmol of small interfering RNA (siRNA) against green fluorescent protein (GFP) (GCAAGCUGACCCUGAAGUUCAU) or against CK2α (UAGAUGAACCCAUUCGAGCCUGGUC) using 2 μl of Lipofectamine 2000. Both cell lines were treated with an identical second and third rounds of siRNA transfection 24 h after the previous transfection. Samples were harvested 24 h later and processed for microscopy or Western blotting as described below.
Immunofluorescence microscopy.
Cells grown on coverslips were fixed with 3.7% formaldehyde in phosphate-buffered saline (PBS) for 20 min, rinsed twice in PBS, and permeabilized with 1% Triton X-100 in PBS for 5 min. Samples were blocked with 4% bovine serum albumin (BSA) in PBS, followed by incubation with primary antibodies against either EBNA1 (R4 rabbit serum at a 1:300 dilution [22]), PML (Santa Cruz PG-M3 at a 1:50 dilution), phosphoserine 517 in PML (a generous gift from P. P. Pandolfi) or CK2α (Abcam catalog no. 2), followed by incubation with the secondary antibodies goat anti-rabbit Alexa Fluor 555 (Molecular Probes) and goat anti-mouse Alexa Fluor 488 (Molecular Probes) in 4% BSA. Coverslips were mounted onto slides using ProLong Gold antifade medium containing DAPI (4′,6′-diamidino-2-phenylindole; Invitrogen). For triple labeling experiments, PML was conjugated to goat anti-rabbit Alexa Fluor 488, EBNA1 to goat anti-rabbit Alexa Fluor 555, and USP7 to goat anti-rabbit Alexa Fluor 647 according to the manufacturer's instructions (Molecular Probes, catalog no. Z25360). Coverslips were mounted onto slides as described above. Images were obtained by using a ×40 oil objective lens on a Leica inverted fluorescence microscope and processed using OpenLAB (ver.X.0) software. PML NBs were quantified by counting all visible PML foci in 100 cells.
Western blots.
Cells were lysed in 9 M urea-5 mM Tris-HCl (pH 6.8) and briefly sonicated. Then, 50 μg of total protein was subjected to SDS-10% PAGE and transferred to nitrocellulose. Where indicated, CNE2 and CNE2E cells were treated with 10 μM emodin (Sigma-Aldrich) for 3 days prior to lysis. Membranes were blocked in 5% nonfat dry milk in PBS and then incubated with antibodies against PML (Bethyl A301-167A; 1:2,000 dilution), phosphoserine 517 in PML (1:2,000, kindly supplied by P. P. Pandolfi), EBNA1 (OT1X at 1:2,000, kindly supplied by Jaap Middeldorp, or R4 at 1:2,000 [13]), actin (Ab-1, Oncogene Research Products; 1:20,000), CK2α (Abcam ab10466-50, 1:5,000 dilution), CK2β (Bethyl A301-984A, 1:2,500), or USP7 (rabbit serum against full-length USP7) (40). After washing, blots were probed with goat anti-mouse peroxidase (1:3,000) or goat anti-rabbit peroxidase (1:5,000) from Santa Cruz and developed using chemiluminescence reagents (ECL, Perkin-Elmer). Membranes were stripped in 0.1 M glycine (pH 2.9) for 30 min, washed in PBS-Tween, blocked, and reprobed with the next antibody as described above.
Coimmunoprecipitation of endogenous proteins.
CNE2 cells were transfected with either 10 μg of OriP or OriPE using Lipofectamine 2000 (Invitrogen). Cells were harvested 12 to 14 h posttransfection, and nuclei were isolated by using hypotonic lysis buffer (50 mM sodium bisulfate, 8.6% sucrose, 20 mM Tris (pH 8.0), 2 mM MgCl2, 0.1% Triton, and protease inhibitors), followed by Dounce homogenization. Nuclei were lysed in immunoprecipitation buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM MgCl2, 10% glycerol, 1% Triton X-100, and protease inhibitors) on ice for 30 min. After centrifugation, 1.5 mg of the supernatant was added to rabbit ExactaCruz beads (Santa Cruz) that were precoupled to either PML (0.5 μg) or USP7 (1.0 μg) antibody (Bethyl, A300-033A) and mixed for 4 h at 4°C. Beads were collected by centrifugation, washed in immunoprecipitation buffer, and then boiled in SDS loading buffer. Immunoprecipitated proteins were separated by SDS-PAGE and Western blotted as described above.
Protein purification.
Glutathione S-transferase (GST) fusion plasmids expressing C-terminally tagged CK2α or CK2β were kindly provided by David Litchfield. GST-CK2α or CK2β proteins were expressed in Escherichia coli BL21(pLysS) cells and purified as described previously (10). EBNA1 (lacking most of the Gly/Ala repeat) and USP7 was expressed in SF9 insect cells and purified as described previously (22). Prior to glycerol gradient sedimentation, GST-CK2α or CK2β fusion proteins were incubated with 1 μg of factor Xa protease (New England Biolabs) for 50 μg of protein at room temperature for 6 h to remove the GST fusion. Dansyl-Glu-Gly-Arg-chloromethyl ketone (Calbiochem) was then added to a final concentration of 5 μM to inactivate Factor Xa protease, and the mixture was passed through a glutathione-Sepharose column to remove the GST fragment.
GST pull-down assay.
Purified EBNA1 (55 μg) was incubated with GST (27 μg), GST-CK2α (60 μg), or GST-CK2β (48 μg) at a 1:1 molar ratio at 37°C for 1 h in a 300 μl of assay buffer (50 mM Tris-HCl [pH 7.5], 200 mM NaCl, 0.5 mM EDTA, 1 mM dithiothreitol, 5% glycerol). The mixture was passed through a 0.2-ml glutathione-Sepharose column. After extensive washing with assay buffer minus glycerol, bound proteins were eluted with 20 mM reduced glutathione and detected by SDS-PAGE and Coomassie staining.
Glycerol gradient sedimentation assays.
Purified EBNA1 (55 μg), CK2α (45 μg), CK2β (27 μg), and USP7(100 μg) were incubated alone or in the indicated combinations at a 1:1 molar ratio at room temperature for 1 h in assay buffer with 10% glycerol in a final volume of 300 μl. A 12-ml 10 to 20% glycerol gradient in assay buffer was generated by using a Gradient master apparatus (Biocomp), and the protein mixture was layered onto the gradient. After centrifugation at 34,000 rpm in a SW41 rotor (Beckman) for 20 h at 4°C, 24 500-μl fractions were collected from the top of the gradient. Equal volumes of each fraction (5 μl for individual proteins and 10 μl for protein combinations) were then subjected to SDS-PAGE and analyzed by Western blotting.
Mapping of CK2-binding region on EBNA1.
SPA-tagged EBNA1, EBNA1 mutants, or LacZ were expressed in 293T cells by transfecting one 10-cm plate of cells with 4 μg of the corresponding pMZS3F plasmid using 8 μl of 1-μg/μl polyethyleneimine (Polysciences). Afte 48 h, the cells were harvested, washed in PBS, and lysed in 4 volumes of radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.5% NP-40, 0.1% sodium deoxycholate, and protease inhibitors) on ice for 15 min, followed by sonication and centrifugation. Equal amounts of clarified lysate (1.5 mg) were incubated with 30 μl of M2 Flag resin (Sigma) for 4 h at 4°C with mixing. Flag resin was harvested by centrifugation, washed in RIPA buffer, and then boiled in SDS loading buffer. Immunoprecipitated proteins were separated by SDS-PAGE and analyzed by Western blotted as described above.
RESULTS
CK2 plays an important role in EBNA1-mediated disruption of PML NBs.
Since EBNA1 interacts with CK2 and both proteins negatively regulate PML NBs, we investigated the possible role of CK2 in EBNA1-mediated PML NB disruption. This was done initially using siRNA targeted to the catalytic subunit of CK2 to silence CK2α in NPC cells that stably express EBNA1 (CNE2E) or in the parental NPC cells that lack EBNA1 expression (CNE2). As previously shown (40), as a result of EBNA1 expression, CNE2E cells treated with negative-control siRNA (siGFP) have fewer PML NBs (Fig. 1A, rows 1 and 3, and Fig. 1B) and less PML protein (Fig. 1C, compare lanes 1 and 3) compared to CNE2 with the same treatment. siRNA treatment against CK2α greatly reduced the levels of CK2α (Fig. 1A, middle column, and Fig. 1C, bottom panel) and resulted in increased levels of PML NBs (Fig. 1A, right panels, and Fig. 1B) and PML proteins (Fig. 1C, top panel) relative to siGFP treatment in both CNE2 and CNE2E cell lines. The difference in numbers of PML NBs in siGFP- and siCK2α-treated cells is statistically significant for both CNE2 (P < 0.01) and CNE2E cells (P < 0.001). The data suggest that EBNA1 is less able to disrupt PML NBs when CK2 is lacking and that EBNA1 does not interfere with the ability of CK2 to negatively regulate PML NBs. The observation that CK2α silencing in CNE2 cells increased PML NBs and protein levels is consistent with the known mechanism for CK2-mediated disruption of PML NBs.
FIG. 1.
EBNA1-mediated PML degradation requires CK2 and involves increased PML phosphorylation. (A) CNE2 cells and CNE2E cells were transfected with siRNA against CK2α (siCK2α) or GFP (siGFP; negative control) and then stained for CK2α and PML. Images were captured using the same exposure time for both cell lines. (B) Quantification of the number of PML NBs per cell in each of the samples in A. P values are <0.01 for siGFP and siCK2 in CNE2 and <0.001 for siGFP and siCK2 in CNE2E. (C) Equal amounts of cell lysates from panel A were analyzed by Western blotting with the indicated antibodies, where “−” samples are treated with siGFP and “+” samples are treated with siCK2α. (D) CNE2 and CNE2E were treated with 10 μM emodin (in dimethyl sulfoxide [DMSO]; +) or DMSO alone (−) or for 3 days, and then equal amount of cell lysates were analyzed by Western blotting with the indicated antibodies, including one that recognizes only PML phosphorylated at S517 (p517). (E) Equal amount of CNE2 and CNE2E cell lysates were analyzed by Western blotting with the indicated antibodies. (F) CNE2 and CNE2E cells were stained with antibodies against total PML and PML phosphorylated at serine 517 (p517). Images were captured using the same exposure time for both cell lines.
To further verify the importance of CK2 in EBNA1-mediated PML degradation and to determine whether the catalytic activity of CK2 is required for this contribution (as opposed to the presence of the CK2α subunit), emodin was used to inhibit the kinase activity of CK2. Emodin treatment of CNE2 and CNE2E cells decreased phosphorylation of a known CK2 target, serine 517 of PML (37), without affecting the level of CK2α (Fig. 1D), showing that this inhibitor was functioning as expected. In addition, emodin treatment of CNE2 increased PML protein levels (Fig. 1D, top left panel), demonstrating the role for CK2 is regulating PML. Interestingly, emodin treatment of CNE2E also resulted in increased PML levels despite the presence of EBNA1 in these cells (Fig. 1D, top right panel). This supports the CK2α silencing results and further indicates that EBNA1-mediated PML degradation requires CK2 kinase activity.
CK2 has been shown to trigger PML degradation through the phosphorylation of PML proteins at serine 517 (p517) (37). Since EBNA1 induces PML degradation by a mechanism that requires CK2, we sought to determine whether EBNA1 increased CK2-mediated phosphorylation of PML S517, using an antibody specific to the phosphoserine 517 of PML (37). Western blots of whole-cell extracts of CNE2 and CNE2E cells showed that, although CNE2E had considerably less PML protein, it had increased levels of p517 compared to CNE2 (Fig. 1E). This trend was also apparent in the experiment in Fig. 1D (compare lanes 1 and 3). We also examined changes in p517 by immunofluorescence microscopy and observed an increase in the intensity of p517 staining in the EBNA1-expressing CNE2E cells compared to the CNE2 cells (Fig. 1F). In CNE2E cells, the p517 staining also appeared to be more dispersed throughout the nucleoplasm, compared to CNE2 where p517 was mostly concentrated at the PML NBs, a pattern that is likely the result of disruption of the PML NBs. Taken as a whole, the data strongly suggest that EBNA1 triggers the degradation of PML proteins by increasing their phosphorylation by CK2.
EBNA1 directly interacts with CK2β.
CK2 was found to interact with EBNA1 in both in vivo TAP-tagging experiments and in vitro affinity column experiments using human cell lysates (22). However, since several other interactions were also identified in these assays, it was not clear whether EBNA1 interacted directly with CK2 or indirectly through another cellular protein. We tested whether EBNA1 could bind directly to CK2 subunits using purified proteins in both GST pull-down assays and glycerol gradient sedimentation analyses. For the GST pull-down assays, GST-CK2α, GST-CK2β, or GST alone was mixed with equimolar amounts of EBNA1 and then bound to glutathione resin and, after washing, retained proteins were eluted with glutathione. Samples of the input protein, resin flowthrough, and eluted proteins were then analyzed by SDS-PAGE, followed by Coomassie staining (Fig. 2A). Little to no EBNA1 was retained on the resin by GST-CK2α or GST alone relative to the amount of the eluted GST proteins, whereas a considerably higher ratio of EBNA1 to GST-CK2β was recovered in the eluates of the resin containing GST-CK2β, suggesting that EBNA1 directly interacts with the regulatory subunit of CK2.
FIG. 2.
EBNA1 directly interacts with CK2β. (A) Purified EBNA1 was incubated with purified GST-tagged CK2α or CK2β or with GST alone at equimolar ratios and then mixed with glutathione-Sepharose. After washing, proteins were eluted with glutathione and analyzed by SDS-PAGE and colloidal Coomassie blue staining. The samples shown are the input protein mixture (I; 3% of the total), the flowthrough that was not retained on the resin (F; 3% of the total), and protein that was retained by the resin and eluted (E; 50% of the total). The positions of EBNA1, CK2α-GST, CK2β-GST, and molecular weight markers (M) in kilodaltons are indicated. (B) Purified EBNA1, CK2α, or CK2β proteins were analyzed by glycerol gradient sedimentation individually (top panels) or after preincubation of equal molar ratios of EBNA1 and CK2β or CK2α (middle or bottom panels). Equal volume fractions were collected from top of each gradient, and equal volumes of each fractions were analyzed by Western blotting with antibodies against EBNA1, CK2α, or CK2β.
The interaction of EBNA1 with CK2α or CK2β (lacking tags) was also assessed using equimolar amounts of purified protein by glycerol gradient sedimentation. To this end, EBNA1, CK2β, or CK2α was subjected to glycerol gradient sedimentation either individually or in combination; protein fractions were then collected, and aliquots were analyzed by Western blotting (Fig. 2B). When run individually on the gradient, EBNA1 peaked at fractions 5 to 7, CK2α at fraction 4, and CK2β at fraction 5 (Fig. 2B, top three panels). However, when EBNA1 and CK2β were mixed, both proteins shifted to the position of a larger complex and comigrated, peaking at fraction 8 (Fig. 2B, middle panels). The formation of a larger complex was not seen when EBNA1 was mixed with CK2α, since neither protein shifted toward the bottom of the gradient, nor did the two protein peaks correspond closely to each other (Fig. 2B, bottom panels). These results support those of the GST pull-down assays and indicate that EBNA1 binds directly to the CK2β subunit of CK2.
Identification of the CK2-binding site of EBNA1.
To further investigate the mechanism of the EBNA1-CK2 interaction and its functional significance, we tested the ability of a series of EBNA1 mutants (Fig. 3A) to interact with CK2 in human cells. These EBNA1 proteins were expressed by transient transfection of CNE2 cells, fused to a C-terminal SPA tag that includes a triple FLAG epitope (47). Initially, EBNA1 and three EBNA1 mutants—452-641, Δ41-376, and 31-641—were expressed in CNE2, immunoprecipitated with anti-FLAG antibody, and examined for recovery of endogenous CK2 by Western blotting for CK2α. CK2α coimmunoprecipitated with all of these EBNA1 proteins except for 452-641, even though all of the EBNA1 proteins were expressed and recovered at similar levels (Fig. 3B). In addition, the negative control of SPA-tagged LacZ failed to recover detectable levels of CK2α. These results indicated that EBNA1 binds CK2 through internal sequences located between residues 376 and 451.
FIG. 3.
Mapping of CK2-binding region of EBNA1 by coimmunoprecipitation. (A) Schematic representation of EBNA1 and the EBNA1 mutants used in the present study, showing the glycine and arginine-rich regions (G/R), the glycine and alanine-rich region (G/A), the nuclear localization signal (NLS), and the DNA-binding domain (DNA BD). Note that the EBNA1 used in the present study contains a short version of the G/A repeat. The ability of the EBNA1 proteins to bind endogenous CK2 by coimmunoprecipitation is also indicated (as determined in panels B and C). (B and C) 293T cells were transfected with plasmids expressing the indicated FLAG-tagged EBNA1 protein or FLAG-tagged LacZ, and tagged proteins were immunoprecipitated from cell lysates using anti-FLAG resin. Proteins recovered from the FLAG resin were analyzed by Western blotting with antibodies against EBNA1 (B) or the FLAG epitope (C), CK2α (B and C), CK2β (C), and USP7 (C). Western blots are also shown for 10% of the starting cell lysates (Input).
The 376-451 region of EBNA1 includes the nuclear localization sequence, a serine-rich region, and a region known to bind USP7. We have previously shown that the EBNA1 mutant Δ395-450 fails to recover USP7 in TAP-tagging experiments but still recovers CK2 (22). In keeping with this observation, Δ395-450 coimmunoprecipitated both CK2α and CK2β, as did EBNA1 but, unlike EBNA1, failed to recover USP7 (Fig. 3C). This indicated that the CK2 binding site was between residues 376 and 395, prompting the generation of EBNA1 mutant Δ387-394, which lacks a serine-rich sequence but retains the nuclear localization sequence. Δ387-394 was expressed and recovered at levels similar to those seen with EBNA1 and Δ395-450 in FLAG immunoprecipitations (Fig. 3C, bottom panel), and yet failed to coimmunoprecipitate either CK2α and CK2β (Fig. 3C, top two panels). However, Δ387-394 bound USP7 as efficiently as EBNA1, indicating that this mutation did not disrupt the structure of the EBNA1 internal region or EBNA1 nuclear localization. Furthermore, the results indicate that CK2 and USP7 bind independently to EBNA1 and that the Δ387-394 and Δ395-450 mutants are useful for functional studies to specifically address roles EBNA1-CK2 and EBNA1-USP7 interactions, respectively.
An EBNA1 CK2-binding mutant does not disrupt PML NBs.
We have shown that the mechanism by which EBNA1 disrupts PML NBs involves CK2 and results in increased CK2-mediated phosphorylation of PML; however, it was not clear whether these effects were due to EBNA1 binding to CK2 or involve a less direct mechanism. To address this question, we sought to determine whether the EBNA1 mutant that is defective in CK2 binding, Δ387-394, was able to disrupt PML NBs. To this end, EBNA1 and Δ387-394 (lacking any tags) were expressed in CNE2 cells by transient transfection of oriP-based expression plasmids and compared to transfection with the oriP plasmid lacking EBNA1. Cells were then stained with anti-EBNA1 and anti-PML antibodies to identify EBNA1-expressing cells, as well as any differences in PML NBs in neighboring cells expressing or not expressing EBNA1 (Fig. 4A). As previously reported (40), cells expressing wild-type EBNA1 had fewer PML NBs than their neighbors lacking EBNA1 expression (Fig. 4A, top panel), and the number of PML NBs in these EBNA1-expressing cells was on average 3-fold lower than the same cells transfected with the oriP control plasmid with a P value of <0.005 (Fig. 4B). However, the expression of Δ387-394 did not result in any noticeable differences in the abundance, size, or shape of the PML NBs compared to neighboring cells lacking any EBNA1 expression (Fig. 4A, bottom panels) or to cells transfected with the negative control plasmid (Fig. 4B).
FIG. 4.

The EBNA1 CK2-binding mutant fails to disrupt PML NBs. (A) CNE2 cells were transiently transfected with a plasmid expressing EBNA1 or EBNA1 mutant Δ387-394, then stained for EBNA1 and PML 24 h posttransfection. Both EBNA1-expressing (red) cells and nonexpressing cells are shown within the same image. (B) The numbers of PML NBs per cell were counted 24 h after expression of EBNA1 or Δ387-394 and compared to control cells transfected with the empty expression plasmid (OriP). (C) CNE2 cells were transiently transfected with a plasmid expressing EBNA1, Δ387-394, Δ395-450, or no protein (OriP) and, 48 h later, equal amounts of cell lysates were analyzed by Western blotting with the indicated antibodies.
We also examined the effect of Δ387-394 expression on PML protein levels by performing Western blots on lysates of the above cells. As expected, transient expression of wild-type EBNA1 resulted in a large decrease in the amount of PML proteins compared to control cells transfected with the empty oriP plasmid (Fig. 4C, compare lanes 1 and 2). However, despite being expressed at the same level as EBNA1, Δ387-394 did not induce the loss of PML proteins (Fig. 4C, lane 3). This result is similar to that with EBNA1 mutant Δ395-450 (Fig. 4C, lane 4), which does not bind USP7 and which we previously showed does not disrupt PML NBs. The results indicate that EBNA1 must bind CK2 in order to disrupt PML NBs and induces the degradation of PML proteins. In addition, this finding, in conjunction with our previous work, indicates that EBNA1 must bind to both CK2 and USP7 to disrupt PML NBs.
EBNA1 increases the interactions of CK2β and USP7 with PML.
We imagined two different scenarios in which the interaction of EBNA1 with CK2 and USP7 might be important for EBNA1-mediated disruption of PML NBs. First, since a proportion of USP7 and CK2 is associated with PML NBs, EBNA1 binding to one or both of these proteins might be required for EBNA1 to localize to PML NBs. Second, EBNA1 might associate with PML NBs independently of CK2 or USP7 and bring additional CK2 and USP7 to the PML NBs, where they can trigger PML degradation. To test the first possibility, we examined the nuclear localization of the Δ387-394 and Δ395-450 EBNA1 mutants that fail to bind CK2 and USP7, respectively, soon after their expression when PML NBs are largely intact. As shown in Fig. 5A, both EBNA1 mutants were observed to form foci that localize to PML NBs in addition to giving more diffuse nuclear staining, a pattern that is typical of wild-type EBNA1 (40) (Fig. 5B, rows 2 and 6). Therefore, EBNA1 binding to CK2 or USP7 does not appear to be required for EBNA1 to associate with PML NBs.
FIG. 5.
EBNA1 increases association of CK2β and USP7 with PML NBs. (A) EBNA1 mutants Δ387-394 or Δ395-450 were expressed in CNE2 by transient transfection and, 12 h later, the cells were stained for EBNA1 and PML. (B) CNE2 cells were transiently transfected with the EBNA1 expression plasmid and, 12 h later, the cells were stained for EBNA1, USP7, and PML with specific antibodies directly conjugated to Alexa Fluor dyes. Overlays of USP7 with PML and EBNA1 with PML are also shown in which USP7 and EBNA1 have been colorized red and PML is green. (C) Quantification of the percentage of PML NBs that have associated USP7 foci with or without EBNA1 expression (P < 0.001). (D to F) CNE2 cells were transfected with a plasmid expressing EBNA1 (+) or the empty plasmid (−) and, 14 h later, immunoprecipitations were performed from nuclear lysates using anti-USP7 (D) or anti-PML antibody (E and F). IgG is a negative control in which immunoprecipitations were performed with rabbit IgG beads. Samples were Western blotted with the indicated antibodies. One-fifteenth of the starting nuclear extracts (input) is also shown. In panel E, the blot was initially probed for USP7 (bottom panel) and then stripped and reprobed for PML (top panel). Due to incomplete stripping, some residual USP7 is still visible, as indicated in the top panel.
We then examined whether EBNA1 affected the degree to which USP7 and CK2 were associated with PML NBs. These experiments were performed by expressing EBNA1 in CNE2 cells by transient transfection and imaging or harvesting cells soon after EBNA1 expression, at which time the PML NBs are still largely intact (Fig. 5B to D). Immunofluorescence imaging of these cells for EBNA1, USP7, and PML, showed that, in cells lacking EBNA1 expression, USP7 gave mostly diffuse nuclear staining and formed a small number of foci that were associated with PML NBs (Fig. 5B). This is consistent with previous reports of partial association of USP7 with PML NBs (15). However, in EBNA1-expressing cells present on the same slides, there was a noticeable increase in the number of USP7 foci (Fig. 5B, row 4, compare neighboring cells in the same panel), most of which were closely associated with PML NBs (Fig. 5B, row 5). Quantification of these results indicated that EBNA1 caused a 2- to 3-fold increase in the percentage of PML bodies with associated USP7 with a P value of <0.001 (Fig. 5C). In addition, more PML protein was found to coimmunoprecipitate with USP7 from CNE2 cells after transfection with the EBNA1 expression plasmid than after transfection with the empty plasmid (Fig. 5D), further indicating that EBNA1 increases the association of USP7 with PML NBs. The finding that USP7 preferentially immunoprecipitates one PML isoform is consistent with our previous observations (Sarkari, unpublished). As expected, a fraction of the EBNA1 also coimmunoprecipitated with USP7 (Fig. 5D, bottom panel). Coimmunoprecipitations were also done in reverse, where total PML was immunoprecipitated and the amount of associated USP7 was compared in the presence or absence of EBNA1 (Fig. 5E). In keeping with the above experiments, more USP7 was recovered with PML when EBNA1 was present.
We also examined the effect of EBNA1 expression on the association of CK2 with PML NBs. Antibodies against either CK2α or CK2β both result in bright pan-nuclear staining, making it difficult to assess the degree of their association with PML NBs by immunofluorescence imaging (data not shown). However, the effect of EBNA1 on the association of CK2 with PML NBs could be assessed by the amount of CK2 that coimmunoprecipitated with PML in the presence or absence of EBNA1. We consistently observed that more CK2β was recovered with PML in the presence of EBNA1 than in its absence (Fig. 5F, compare lanes 5 and 6). Taken together, the data suggest that EBNA1 is able to increase the occupancy of both CK2 and USP7 at PML NBs.
EBNA1, USP7, and CK2β form a ternary complex.
Our data indicate that EBNA1 can directly bind USP7 and CK2β and recruit them to PML NBs. There are two scenarios by which this may occur. First, since multiple EBNA1 molecules are likely to associate with any given PML NB, it could be that some of these EBNA1 molecules bind and recruit USP7, while other EBNA1 molecules bind and recruit CK2 to the same PML NB. In this scenario, USP7 and CK2 would not be bound to the same EBNA1 protein. Second, a single EBNA1 molecule might bind to both USP7 and CK2, bringing both of these enzymes to the PML NB. If this is the case then we should be able to detect EBNA1, USP7, and CK2 in a ternary complex.
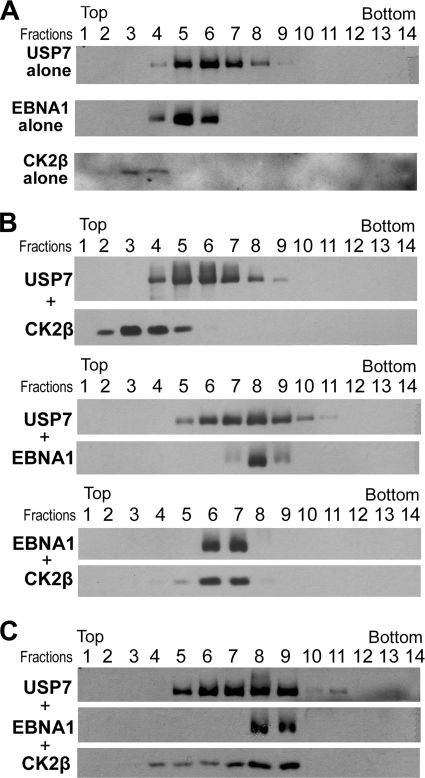
We examined whether EBNA1, USP7, and CK2β could form a ternary complex by combining these purified proteins in various combinations and subjecting them to glycerol gradient sedimentation analyses. When analyzed individually, USP7, EBNA1, and CK2β peaked at fractions 6, 5, and 3, respectively (Fig. 6A). When USP7 and EBNA1 were preincubated prior to analyses, the proteins comigrated on the glycerol gradient and shifted to the position of a larger complex, peaking at fraction 8 (Fig. 6B, middle panel), confirming that USP7 forms a stable complex with EBNA1. Similarly, when combined, EBNA1 and CK2β comigrated and shifted to a peak at fraction 6 and 7 (Fig. 6B, bottom panel), confirming their interaction. However, no direct interaction was detected between USP7 and CK2β, as when combined, the migrations of these proteins were unchanged from their migration when analyzed individually and comigration was not observed (Fig. 6B, top panel). Finally, when USP7, EBNA1, and CK2β were combined in equimolar amounts, a significant proportion of each protein was observed to comigrate at a position of a complex larger than that of EBNA1-USP7 or EBNA1-CKβ, peaking at fractions 8 and 9 (Fig. 6C). Most notably, most of the CK2β shifted two fraction from its position as an EBNA1-CK2β complex (fractions 6 and 7) to a larger complex including USP7 (fractions 8 and 9). These results indicate that EBNA1 can bind USP7 and CK2β simultaneously forming a ternary complex.
FIG. 6.
EBNA1, USP7, and CK2β form a ternary complex. Glycerol gradient sedimentation was performed with purified EBNA1, CK2β, and USP7 on 10 to 20% glycerol gradients. Equal volume fractions were collected from top of each gradient and equal volumes of each fraction were analyzed by Western blotting with antibodies against each protein (indicate on the left of each panel). Fraction 14 is the pellet fraction from the bottom of the tube. (A) The sedimentation of USP7, EBNA1 or CK2β proteins was analyzed individually (top, middle, and bottom panels, respectively). (B) Equal molar ratios of USP7 and CK2β (top panel), USP7 and EBNA1 (middle panel), or EBNA1 and CK2β (bottom panel) were preincubated then analyzed by glycerol gradient sedimentation. (C) Equal molar ratios of USP7, CK2β, and EBNA1 were preincubated and then analyzed by glycerol gradient sedimentation.
DISCUSSION
We have previously shown that EBNA1 disrupts PML NBs in NPC cells by inducing the degradation of PML proteins (40). PML NB play critical roles in p53 activation, DNA repair, and apoptosis and, accordingly, EBNA1 was found to disrupt all of these processes (36, 40). The combination of these EBNA1 effects would be expected to contribute to the development of this tumor, suggesting a direct role for EBNA1 in oncogenesis. In addition, it is interesting that PML disruption by EBNA1 occurs in epithelial cells, which are the main sites of lytic infection, but has not been observed in B lymphocytes (5), which are predominantly responsible for latent infection. Since PML NBs are known to be repressive for lytic infection of at least some herpesviruses (19, 43), these observations raise the possibility that EBNA1 might promote the switch to lytic infection in epithelial cells through PML disruption. We have extended here our initial studies on EBV infection and PML NBs by providing the mechanism of EBNA1-induced PML disruption.
We have shown that the ability of EBNA1 to induce the degradation of PML proteins depends on the presence of the host CK2, on the catalytic activity of CK2, and on the direct interaction of CK2 with EBNA1. CK2 has been previously shown to phosphorylate PML at S517, which enables the polyubiquitylation of PML and its subsequent degradation by the proteasome (37, 38). Our studies support this important role of CK2 in regulating PML levels and show that this pathway is being usurped by EBNA1. In particular, EBNA1 was shown to increase the occupancy of CK2 at PML NBs and the subsequent phosphorylation of S517. Experiments performed with EBNA1 mutants showed that the EBNA1-CK2 interaction was critical for PML disruption but was not sufficient for this effect since the EBNA1 interaction with USP7 was also required to trigger PML degradation through a mechanism that is not yet well understood (40).
Our data indicate that the EBNA1-CK2 interaction is mediated by EBNA1 residues between positions 387 and 394. The region from 387 to 394 of EBNA1 is a serine-rich stretch that includes a phosphoserine at position 393 (13) and that has no previously assigned function. One reason that EBNA1 might interact with CK2 is that EBNA1 may be a target of CK2 phosphorylation. This possibility is supported by the finding that EBNA1 is phosphorylated at serine 21 (13), which is a predicted CK2 phosphorylation site. However, neither deletion of the 387-394 CK2 binding site nor deletion of N-terminal sequences spanning S21 disrupted the ability of EBNA1 to function in EBV replication, plasmid maintenance or transcriptional activation (30, 45; our unpublished data), nor is the S21 site conserved in EBNA1 homologous in the EBV-like cynomolgus monkey virus and herpesvirus papio (13). These findings suggest that EBNA1 binding to CK2 may be for the purpose of altering the host cell environment rather than for modifying EBNA1 to affect its functions at the EBV genome.
CK2 is a tetramer comprised of two catalytic isoforms (either CK2α, CK2α′, or one of each) and two regulatory subunits of CK2β (reviewed in reference 29). CK2β is important for enhancing the catalytic activity and stability of the enzyme and can mediate interactions with CK2 substrates, as has been shown for p53, DNA topoisomerase II, and the CD5 receptor (4, 8, 33). In addition, protein interactions with CK2β can redirect CK2 to increase CK2 activity toward specific substrates. For example, the interaction of CK2β with fibroblast growth factor-2 has been shown to increase CK2 phosphorylation of nucleolin (9). Similarly, we have clearly shown that CK2β mediates the interaction of CK2 with EBNA1 and that this interaction results in increased phosphorylation of PML proteins by CK2.
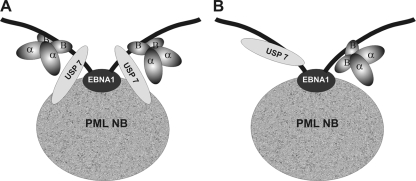
Our data point to a model of PML disruption by EBNA1 in which an EBNA1 protein associates with PML NBs by a mechanism that is independent of its interaction with USP7 or CK2. We have previously shown that EBNA1 preferentially associates with a single PML isoform that appears to be PML IV; however, it is not clear whether this interaction is direct or mediated by another protein (40). Interestingly, USP7 also preferentially interacts with what appears to be the same PML isoform as EBNA1. We have shown that a single EBNA1 protein can interact with both CK2 and USP7 to form a ternary complex and that the interaction with CK2 occurs through the β regulatory subunit. EBNA1 is known to form stable dimers via a β-barrel structure formed by its C-terminal DNA-binding domain and has never been detected in the monomeric form (3, 7, 20). Therefore, the USP7-EBNA1-CK2 ternary complex could either result from each EBNA1 monomer in the dimer binding to both USP7 and CK2 (Fig. 7A) or from one monomer within the dimer binding to USP7 and the other monomer binding to CK2 (Fig. 7B). The former would result in a larger complex than the later but, due to limitations in resolution on the glycerol gradient and the contribution of shape to a molecule's migration, we cannot differentiate these two possibilities by the sedimentation rate of the ternary complex alone. We have previously shown that EBNA1 binds USP7 through EBNA1 residues 442 to 448 (36), and here we show that EBNA1 binds CK2 through residues 387 to 394. The proximity of these sites raises the possibility that the binding of CK2 or USP7 to one EBNA1 monomer might sterically hinder the interaction of the second protein with the same monomer (as shown in Fig. 7B). However, depending on the orientation of the bound proteins, it is also possible that interactions with both proteins could occur simultaneously on the same monomer. Indeed, comparison of the glycerol gradient sedimentation of the EBNA1-USP7 complex (Fig. 6B) to that of the ternary complex (Fig. 6C) supports the model in which USP7 and CK2β both bind to each monomer within the dimer (Fig. 7A) as opposed to separate monomers, since the EBNA1-USP7 complex appears to get bigger in the presence of CK2β, shifting toward the bottom of the gradient. If the model in Fig. 7B was correct, we would expect the ternary complex to be smaller than the EBNA1-USP7 complex, since the 135-kDa USP7 protein bound to one monomer would be replaced by the smaller (25 kDa) CK2β protein.
FIG. 7.
Models of the CK2-EBNA1-USP7 ternary complex. An EBNA1 dimer (black) associated with a PML NB is shown where the black oval represents the dimerized dimerization/DNA-binding domain. CK2 (α and β) and USP7 proteins are shown bound to the extended EBNA1 sequences outside of the dimerization domain. (A) CK2 and USP7 associate with the same monomer within the EBNA1 dimer. (B) CK2 and USP7 interact with opposite monomers within the EBNA1 dimer due to steric hindrance.
The increased recruitment of CK2 to PML NBs by EBNA1 results in increased phosphorylation of PML by CK2, as evidenced by the EBNA1-induced increase in phosphoserine 517. CK2 is known to phosphorylate this site on PML triggering the polyubiquitylation and degradation of PML by an as-yet-unidentified ubiquitin ligase (37, 38). Therefore, it is likely that the increase in p517 in the presence of EBNA1 results in increased degradation of PML. CK2 is not strictly localized to PML NBs but rather is found throughout the nucleus, suggesting that it transiently associates with PML NB is order to maintain these structures at a specific level. By increasing the occupancy of CK2 at PML NBs, EBNA1 would shift this equilibrium resulting in lower levels of PML NB and PML proteins.
A similar scenario appears to be occurring with USP7, another protein that is only partly associated with PML NBs, in that EBNA1 increases its occupancy at PML NBs. Although USP7 is normally associated with the stabilization of specific proteins through its ability to cleave ubiquitin from them, we have shown that USP7 promotes the degradation of PML proteins by a mechanism that does not involve the catalytic activity of USP7 (Sarkari, unpublished). Rather, this involves the protein interaction domains of USP7, suggesting that USP7 recruits another protein(s) to PML NBs that may polyubiquitylate PML (Sarkari, unpublished). In addition, the ability of USP7 to trigger PML degradation was shown to be independent of CK2 (Sarkari, unpublished). Taken together, the results indicate that EBNA1 uses two independent pathways to trigger the degradation of PML proteins: one that involves CK2 and another that involves USP7.
EBNA1 forms a stable complex with CK2, and we have now shown that this interaction is significant for the EBNA1-mediated disruption of PML NBs. However, given the numerous roles reported for CK2 in cellular processes, this is unlikely to be the only CK2 function affected by EBNA1. For example CK2 has been shown to be important for transitions through several stages of the cell cycle and to play multiple roles in regulating apoptosis (14, 29). In addition, CK2 regulates both tumor suppressor proteins and oncogenes in ways that promote tumorigenesis and, as a result, elevated levels of CK2 are associated with malignant transformation of many cell types and aggressive tumor behavior (reviewed in reference 14). The possibility that EBNA1 affects additional CK2 functions to promote the cell survival critical for latent infection and/or tumorigenesis associated with EBV infections merits further investigation.
Acknowledgments
We are grateful to Pier P. Pandolfi for providing antibody against PML phosphoserine 517 and to David Litchfield for plasmids expressing GST-CK2α and GST-CK2β. We also thank Kathy Shire for generating plasmid pc3oriPEΔ387-394.
This study was funded by an operating grant to L.F. from the Canadian Cancer Society. N.S. was supported by a Canadian Institutes for Health Research Doctoral award. L.F. is a tier 1 Canada Research Chair in Molecular Virology.
Footnotes
Published ahead of print on 18 August 2010.
REFERENCES
- 1.Adamson, A. L., and S. Kenney. 2001. Epstein-Barr virus immediate-early protein BZLF1 is SUMO-1 modified and disrupts promyelocytic leukemia bodies. J. Virol. 75:2388-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn, J. H., and G. S. Hayward. 1997. The major immediate-early proteins IE1 and IE2 of human cytomegalovirus colocalize with and disrupt PML-associated nuclear bodies at very early times in infected permissive cells. J. Virol. 71:4599-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambinder, R. F., M. Mullen, Y. Chang, G. S. Hayward, and S. D. Hayward. 1991. Functional domains of Epstein-Barr nuclear antigen EBNA-1. J. Virol. 65:1466-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appel, K., P. Wagner, B. Boldyreff, O. G. Issinger, and M. Montenarh. 1995. Mapping of the interaction sites of the growth suppressor protein p53 with the regulatory beta-subunit of protein kinase CK2. Oncogene 11:1971-1978. [PubMed] [Google Scholar]
- 5.Bell, P., P. M. Lieberman, and G. G. Maul. 2000. Lytic but not latent replication of Epstein-Barr virus is associated with PML and induces sequential release of nuclear domain 10 proteins. J. Virol. 74:11800-11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernardi, R., and P. P. Pandolfi. 2007. Structure, dynamics, and functions of promyelocytic leukaemia nuclear bodies. Nat. Rev. Mol. Cell. Biol. 8:1006-1016. [DOI] [PubMed] [Google Scholar]
- 7.Bochkarev, A., J. Barwell, R. Pfuetzner, W. Furey, A. Edwards, and L. Frappier. 1995. Crystal structure of the DNA binding domain of the Epstein-Barr virus origin binding protein EBNA1. Cell 83:39-46. [DOI] [PubMed] [Google Scholar]
- 8.Bojanowski, K., O. Filhol, C. Cochet, E. M. Chambaz, and A. K. Larsen. 1993. DNA topoisomerase II and casein kinase II associate in a molecular complex that is catalytically active. J. Biol. Chem. 268:22920-22926. [PubMed] [Google Scholar]
- 9.Bonnet, H., O. Filhol, I. Truchet, P. Brethenou, C. Cochet, F. Amalric, and G. Bouche. 1996. Fibroblast growth factor-2 binds to the regulatory beta subunit of CK2 and directly stimulates CK2 activity toward nucleolin. J. Biol. Chem. 271:24781-24787. [DOI] [PubMed] [Google Scholar]
- 10.Bosc, D. G., E. Slominski, C. Sichler, and D. W. Litchfield. 1995. Phosphorylation of casein kinase II by p34cdc2. Identification of phosphorylation sites using phosphorylation site mutants in vitro. J. Biol. Chem. 270:25872-25878. [DOI] [PubMed] [Google Scholar]
- 11.Chelbi-Alix, M. K., and H. de The. 1999. Herpesvirus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18:935-941. [DOI] [PubMed] [Google Scholar]
- 12.Cummins, J. M., C. Rago, M. Kohli, K. W. Kinzler, C. Lengauer, and Vogelstein. 2004. Tumour suppression: disruption of HAUSP gene stabilizes p53. Nature 428:486-487. [DOI] [PubMed] [Google Scholar]
- 13.Duellman, S. J., K. L. Thompson, J. J. Coon, and R. R. Burgess. 2009. Phosphorylation sites of Epstein-Barr virus EBNA1 regulate its function. J. Gen. Virol. 90:2251-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan, J. S., and D. W. Litchfield. 2008. Too much of a good thing: the role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochim. Biophys. Acta 1784:33-47. [DOI] [PubMed] [Google Scholar]
- 15.Everett, R., M. Meredith, A. Orr, A. Cross, M. Kathoria, and J. Parkinson. 1997. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 16:1519-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everett, R. D. 2006. Interactions between DNA viruses, ND10 and the DNA damage response. Cell Microbiol. 8:365-374. [DOI] [PubMed] [Google Scholar]
- 17.Everett, R. D., and M. K. Chelbi-Alix. 2007. PML and PML nuclear bodies: implications in antiviral defense. Biochimie 89:819-830. [DOI] [PubMed] [Google Scholar]
- 18.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72:6581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everett, R. D., S. Rechter, P. Papior, N. Tavalai, T. Stamminger, and A. Orr. 2006. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J. Virol. 80:7995-8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frappier, L., and M. O'Donnell. 1991. Overproduction, purification and characterization of EBNA1, the origin binding protein of Epstein-Barr virus. J. Biol. Chem. 266:7819-7826. [PubMed] [Google Scholar]
- 21.Gurrieri, C., P. Capodieci, R. Bernardi, P. P. Scaglioni, K. Nafa, L. J. Rush, D. A. Verbel, C. Cordon-Cardo, and P. P. Pandolfi. 2004. Loss of the tumor suppressor PML in human cancers of multiple histologic origins. J. Natl. Cancer Inst. 96:269-279. [DOI] [PubMed] [Google Scholar]
- 22.Holowaty, M. N., M. Zeghouf, H. Wu, J. Tellam, V. Athanasopoulos, J. Greenblatt, and L. Frappier. 2003. Protein profiling with Epstein-Barr nuclear antigen 1 reveals an interaction with the herpesvirus-associated ubiquitin-specific protease HAUSP/USP7. J. Biol. Chem. 278:29987-29994. [DOI] [PubMed] [Google Scholar]
- 23.Hong, M., Y. Murai, T. Kutsuna, H. Takahashi, K. Nomoto, C. M. Cheng, S. Ishizawa, Q. L. Zhao, R. Ogawa, B. V. Harmon, K. Tsuneyama, and Y. Takano. 2006. Suppression of Epstein-Barr nuclear antigen 1 (EBNA1) by RNA interference inhibits proliferation of EBV-positive Burkitt's lymphoma cells. J. Cancer Res. Clin. Oncol. 132:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoppe, A., S. J. Beech, J. Dimmock, and K. N. Leppard. 2006. Interaction of the adenovirus type 5 E4 Orf3 protein with promyelocytic leukemia protein isoform II is required for ND10 disruption. J. Virol. 80:3042-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen, K., C. Shiels, and P. S. Freemont. 2001. PML protein isoforms and the RBCC/TRIM motif. Oncogene 20:7223-7233. [DOI] [PubMed] [Google Scholar]
- 26.Kaul, R., M. Murakami, T. Choudhuri, and E. S. Robertson. 2007. Epstein-Barr virus latent nuclear antigens can induce metastasis in a nude mouse model. J. Virol. 81:10352-10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy, G., J. Komano, and B. Sugden. 2003. Epstein-Barr virus provide a survival factor to Burkitt's lymphomas. Proc. Natl. Acad. Sci. U. S. A. 100:14269-14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, M., D. Chen, A. Shiloh, J. Luo, A. Y. Nikolaev, J. Qin, and W. Gu. 2002. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416:648-653. [DOI] [PubMed] [Google Scholar]
- 29.Litchfield, D. W. 2003. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem. J. 369:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackey, D., and B. Sugden. 1999. The linking regions of EBNA1 are essential for its support of replication and transcription. Mol. Cell. Biol. 19:3349-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami, M., K. Lan, C. Subramanian, and E. S. Robertson. 2005. Epstein-Barr virus nuclear antigen 1 interacts with Nm23-H1 in lymphoblastoid cell lines and inhibits its ability to suppress cell migration. J. Virol. 79:1559-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raab-Traub, N. 2002. Epstein-Barr virus in the pathogenesis of NPC. Semin. Cancer Biol. 12:431-441. [DOI] [PubMed] [Google Scholar]
- 33.Raman, C., A. Kuo, J. Deshane, D. W. Litchfield, and R. P. Kimberly. 1998. Regulation of casein kinase 2 by direct interaction with cell surface receptor CD5. J. Biol. Chem. 273:19183-19189. [DOI] [PubMed] [Google Scholar]
- 34.Salomoni, P., B. J. Ferguson, A. H. Wyllie, and T. Rich. 2008. New insights into the role of PML in tumour suppression. Cell Res. 18:622-640. [DOI] [PubMed] [Google Scholar]
- 35.Salsman, J., N. Zimmerman, T. Chen, M. Domagala, and L. Frappier. 2008. Genome-wide screen of three herpesviruses for protein subcellular localization and alteration of PML nuclear bodies. PLoS Pathog. 4:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saridakis, V., Y. Sheng, F. Sarkari, M. N. Holowaty, K. Shire, T. Nguyen, R. G. Zhang, J. Liao, W. Lee, A. M. Edwards, C. H. Arrowsmith, and L. Frappier. 2005. Structure of the p53 binding domain of HAUSP/USP7 bound to Epstein-Barr nuclear antigen 1 implications for EBV-mediated immortalization. Mol. Cell 18:25-36. [DOI] [PubMed] [Google Scholar]
- 37.Scaglioni, P. P., T. M. Yung, L. F. Cai, H. Erdjument-Bromage, A. J. Kaufman, B. Singh, J. Teruya-Feldstein, P. Tempst, and P. P. Pandolfi. 2006. A CK2-dependent mechanism for degradation of the PML tumor suppressor. Cell 126:269-283. [DOI] [PubMed] [Google Scholar]
- 38.Scaglioni, P. P., T. M. Yung, S. C. Choi, C. Baldini, G. Konstantinidou, and P. P. Pandolfi. 2008. CK2 mediates phosphorylation and ubiquitin-mediated degradation of the PML tumor suppressor. Mol. Cell Biochem. 316:149-154. [DOI] [PubMed] [Google Scholar]
- 39.Shire, K., D. F. J. Ceccarelli, T. M. Avolio-Hunter, and L. Frappier. 1999. EBP2, a human protein that interacts with sequences of the Epstein-Barr nuclear antigen 1 important for plasmid maintenance. J. Virol. 73:2587-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sivachandran, N., F. Sarkari, and L. Frappier. 2008. Epstein-Barr Nuclear antigen 1 contributes to nasopharyngeal carcinoma through the disruption of PML nuclear bodies. PLoS Pathog. 4:e1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun, Y., G. Hegamyer, Y. J. Cheng, A. Hildesheim, J. Y. Chen, I. H. Chen, Y. Cao, K. T. Yao, and N. H. Colburn. 1992. An infrequent point mutation of the p53 gene in human nasopharyngeal carcinoma. Proc. Natl. Acad. Sci. U. S. A. 89:6516-6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi, Y., V. Lallemand-Breitenbach, J. Zhu, and H. de The. 2004. PML nuclear bodies and apoptosis. Oncogene 23:2819-2824. [DOI] [PubMed] [Google Scholar]
- 43.Tavalai, N., P. Papior, S. Rechter, M. Leis, and T. Stamminger. 2006. Evidence for a role of the cellular ND10 protein PML in mediating intrinsic immunity against human cytomegalovirus infections. J. Virol. 80:8006-8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ullman, A. J., and P. Hearing. 2008. Cellular proteins PML and Daxx mediate an innate antiviral defense antagonized by the adenovirus E4 ORF3 protein. J. Virol. 82:7325-7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yates, J. L., and S. M. Camiolo. 1988. Dissection of DNA replication and enhancer activation functions of Epstein-Barr virus nuclear antigen 1. Cancer Cells 6:197-205. [Google Scholar]
- 46.Yin, Q., and E. K. Flemington. 2006. siRNAs against the Epstein Barr virus latency replication factor, EBNA1, inhibit its function and growth of EBV-dependent tumor cells. Virology 346:385-393. [DOI] [PubMed] [Google Scholar]
- 47.Zeghouf, M., J. Li, G. Butland, A. Borowska, V. Canadien, D. Richards, B. Beattie, A. Emili, and J. F. Greenblatt. 2004. Sequential peptide affinity (SPA) system for the identification of mammalian and bacterial protein complexes. J. Proteome Res. 3:463-468. [DOI] [PubMed] [Google Scholar]