Abstract
The vaccinia virus (VACV) complement control protein (VCP) is an immunomodulatory protein that is both secreted from and expressed on the surface of infected cells. Surface expression of VCP occurs though an interaction with the viral transmembrane protein A56 and is dependent on a free N-terminal cysteine of VCP. Although A56 and VCP have been shown to interact in infected cells, the mechanism remains unclear. To investigate if A56 is sufficient for surface expression, we transiently expressed VCP and A56 in eukaryotic cell lines and found that they interact on the cell surface in the absence of other viral proteins. Since A56 contains three extracellular cysteines, we hypothesized that one of the cysteines may be unpaired and could therefore form a disulfide bridge with VCP. To test this, we generated a series of A56 mutants in which each cysteine was mutated to a serine, and we found that mutation of cysteine 162 abrogated VCP cell surface expression. We also tested the ability of other poxvirus complement control proteins to bind to VACV A56. While the smallpox homolog of VCP is able to bind VACV A56, the ectromelia virus (ECTV) VCP homolog is only able to bind the ECTV homolog of A56, indicating that these proteins may have coevolved. Surface expression of poxvirus complement control proteins may have important implications in viral pathogenesis, as a virus that does not express cell surface VCP is attenuated in vivo. This suggests that surface expression of VCP may contribute to poxvirus pathogenesis.
Poxviruses, including vaccinia virus (VACV), encode large numbers of immunomodulatory proteins that help them establish an infection and combat the host's immune response (10, 32). One of these is the vaccinia virus complement control protein (VCP), which is both secreted from and expressed on the surface of infected cells (9, 14, 16, 17). VCP acts against the complement system, a series of soluble proteins that is an important early component of the innate immune system and also shapes adaptive immune responses (15, 42, 43). In response to viral infection, complement can opsonize or inactivate virions and can lyse enveloped virus or infected cells (1, 3, 7, 12). Because of these pressures, a number of viruses, including herpes simplex virus, flaviviruses, and poxviruses, encode novel or host-derived regulators of complement, while others, including HIV and poxviruses, incorporate host complement regulatory proteins into virus particles (7, 11, 31, 39). Many orthopoxviruses encode a complement regulator (8, 20, 23, 29), and the most studied of these is VCP. Structurally, VCP is made up of four short consensus repeats (SCR) that are the basic units of mammalian complement regulators (17, 25), and VCP has been shown to interfere with the complement cascade at multiple steps (2, 16, 20-22, 25, 28-30, 33). Additionally, a VCP knockout virus generates smaller lesions in animal models (14, 16). While some host complement control proteins (CCPs) are secreted, many contain transmembrane domains (or a glycophosphatidylinositol anchor) and are thus expressed on the cell surface (42, 43). Thus, when we found that VCP is also expressed on the infected cell surface and protects infected cells from complement-mediated lysis in vitro (9), we believed this to be an important interaction that required further investigation. We previously found that the N-terminal cysteine on VCP was needed for surface expression and that the VACV transmembrane protein A56 was also required (9). The vaccinia virus A56 protein is a type 1 transmembrane glycoprotein that is found on the surface of infected cells and on extracellular virus particles (4, 18, 26, 27, 36). It interacts with another viral protein, K2 (19, 37, 45), which lacks a transmembrane domain and binds to A56 noncovalently (36). The A56/K2 complex prevents syncytium formation between infected cells and superinfection by interacting with the vaccinia virus entry/fusion complex on virions (24, 38, 40, 41). Here we provide evidence that the N-terminal cysteine on VCP forms an intermolecular disulfide bond with cysteine 162 on the ectodomain of A56. We also demonstrate that similar interactions can occur with other poxvirus CCPs, as the smallpox virus and ectromelia virus homologs of VCP also exhibit A56-dependent surface expression.
MATERIALS AND METHODS
Cells and viruses.
BSC-1, 293T, and RK-13 cells were grown and maintained in minimum essential media (MEM) supplemented with 10% fetal bovine serum (FBS). Viruses were grown and titers were determined in BSC-1 in MEM with 2.5% FBS. The generation and isolation of vaccinia virus VCP knockout (vv-VCPko), vaccinia virus with a mutated VCP lacking the free N-terminal cysteine (vv-VCPmut), and vaccinia virus-VCP wild type (vv-VCPwt) from the parental stain WR has been described previously (9). The VCP rescue virus (VCPrescue) was made by reinserting the VCP open reading frame (ORF) under its native promoter into the VCPko virus. After the initial infection of cells with VCPko and transfection with a plasmid containing the wild-type VCP ORF, the progeny virus was amplified on BSC-1 cells, and the cell lysate was used to infect fresh RK-13 cells. After 24 h of infection these cells were stained for VCP with the anti-VCP monoclonal antibody (MAb) 3F11 and an anti-mouse APC secondary Ab under nonpermeabilized conditions, and positive cells were collected through live cell sorting. The resulting cells were lysed, the virus was plaque purified three times on BSC-1 cells, and a stock of virus was grown and purified. PCR confirmed proper insertion of the gene back into the C3L position and that no mutations occurred during PCR amplification or virus isolation. Expression of VCP in the rescued virus was confirmed by Western blotting and fluorescence-activated cell sorting (FACS) analysis.
Cloning of poxvirus genes into expression plasmids.
The previously generated plasmids containing the ORF of VCP and VCPmut (9), as well as the CCP proteins expressed by variola virus (smallpox virus inhibitor of complement enzymes [SPICE]), ectromelia virus (ECTV; ectromelia virus inhibitor of complement enzymes [EMICE]), and monkeypox virus (MPXV; monkeypox virus inhibitor of complement enzymes [MoPICE]) (20), were used to insert the ORFs into pCAGGS. The VACV A56 ORF was PCR amplified from VACV (strain WR) and initially cloned into TOPO 2.1 by using the primers 5′-CGG GGT ACC ATG ACA CGA TTA CCA ATA CTT TTG-3′ and 5′-CGC GCG GCT ACG CTA GAC TTT GTT CT-3′. This plasmid was then used to generate A56mut1, -mut2, and -mut3 by site-directed mutagenesis using overlapping primers. A56mut1 primers were 5′-GCA ACT CTA TCA TCT AAT CGA AAT AAT ACA AAT G-3′ and 5′-CAT TTG TAT TAT TTC GAT TAG ATG ATA GAG TTG C-3′; A56mut2 primers were 5′-GCC GGT ACT TAT GTA TCT GCA TTC TTT ATG ACA TC-3′ and 5′-GAT GTC ATA AAG AAT GCA GAT ACA TAA GTA CCG GC-3′; A56mut3 primers were 5′-GAT TAT ATA GAT AAT TCT AAT TCC TCG TCG GTA TTC G-3′ and 5′-CGA ATA CCG ACG AGG AAT TAG AAT TAT CTA TAT AAT C-3′. A56mut1+2 was created by site-directed mutagenesis of A56mut1 using the primers for mut2. All four mutated ORFS were then inserted into pCAGGS. The ectromelia virus A56 homolog was PCR amplified from the ECTV Moscow strain (primers 5′-CGG GGT ACC ATG GCA CGA TTG TCA ATA CTT TTG-3′ and 5′-CGC GCG GCT AGC CTA GAC TTT GTT CTC TGT TTT G-3′) and the monkeypox virus A56 homolog PCR amplified from MPXV Zaire and cloned into TOPO2.1 prior to insertion into pCAGGS (forward primer, 5′-CGG GGT ACC ATG ACA CAA TTA CCA ATA CTT TTG-3′; the reverse primer was the same as that for ECTV A56). K2 was cloned from VACV (strain WR) with the addition of a C-terminal hemagglutinin (HA) tag using the primers 5′-CGG GGT ACC ATG ATT GCG TTA TTG ATA CTA TCG-3′ and 5′-CGC CGA TCG TTA GGC ATA ATC GGG AAC ATC GTA GGG GTA AGA GCC ACC GCC ACC AGG AGA TTC CAC CTT ACC CAT AAA C-3′. The ORFs in all final plasmids were sequenced to confirm that sequences (or site-directed point mutations) were correct.
Transfection of poxvirus genes and flow cytometry.
Plasmid transfections were performed in six-well plates of 95% confluent 293T cells by using Lipofectamine and serum-free medium. Two micrograms of each plasmid was used per well, along with 10 μl of Lipofectamine. Forty-eight hours later, the cells were lifted in FACS buffer (phosphate-buffered saline [PBS] without Ca2+/Mg2+ and with 1% FBS and 0.04% sodium azide) and transferred to FACS tubes. Cells were then incubated with anti-VCP Ab (either mouse MAb 3F11 [13, 40] or a rabbit polyclonal antibody [20]) and anti-A56 Ab (either MAb LC10 or a previously described peptide-raised rabbit polyclonal antibody [40]) at 1:1,000 dilutions. After washing, the cells were incubated with appropriate fluorescein isothiocyanate-conjugated or allophycocyanin (APC)-conjugated anti-mouse or anti-rabbit antibodies at a 1:40 dilution and then fixed using 3% paraformaldehyde (PFA). The cells were then read on a FACScalibur and analyzed using FlowJo. Scatter plots were gated on live cells using forward and side scatter. Histograms were created by gating on cells that were A56 positive. For the K2 FACS, collected cells were incubated with the rabbit anti-A56 Ab and a previously described K2 MAb, 4A11-4A3 (5).
Affinity isolation of tagged A56 from infected cells.
To determine how much VCP associates with A56, we infected cells with a previously described recombinant VACV expressing a tagged A56 (40). BSC-1 cells were infected with vA56TAP or a virus expressing an untagged A56 (vv-VCPwt). After 48 h, cell lysates were harvested in radioimmunoprecipitation assay buffer and incubated with streptavidin-Sepharose beads for 1 h at 4°C. After washing and eluting the protein, Western blots were performed using a rabbit polyclonal anti-A56 antibody and the mouse monoclonal anti-VCP antibody 3D1 (13).
Infection of cells for FACS or immunofluorescence.
For VACV and ECTV infection of cells for cell sorting, RK-13 cells were infected in wells of a six-well plate for 24 h with ectromelia virus, vv-VCPwt, or vv-VCPko. The cells were then collected, transferred to FACS tubes, and stained with a rabbit anti-VCP Ab and the mouse anti-A56 MAb as described above. For immunofluorescent images of MPXV-infected cells, BSC-40 cells in eight-well chamber glass slide (Nunc) at ∼50% confluence were infected with 1 PFU/cell of either VACV, MPXV USA, or MPXV Congo and incubated for 18 h in a 6% CO2 incubator at 35.5°C. After a PBS wash, the cells were fixed for 30 min with 2% PFA in PBS, followed by washes and blocking with 5% bovine serum albumin (BSA) in PBS for 1 h 30 min at room temperature (RT). Cells were then stained for 2 h at RT with anti-VCP mouse monoclonal antibody 2E5 at a 1:40 dilution in 5% BSA. After PBS washes, wells were incubated in the dark for 2 h at RT with Alexa Fluor 488 goat anti-mouse IgG (Invitrogen) at 1:200 in 5% BSA. Wells were washed and mounted in VectaShield hard-set mounting medium containing 4′,6-diamidino-2-phenylindole, and images were taken at 400× magnification under oil immersion.
Mice for intranasal and intradermal infections.
For all mouse experiments, 6- to 8-week-old female C57BL/6 mice were purchased from the Jackson Laboratory and housed in a specific-pathogen-free facility at the University of Pennsylvania. For intranasal infections, mice (five per group) were infected with 103, 104, or 105 PFU of the indicated viruses, and weight loss was measured as has been previously described (44). For intradermal infections, groups of mice were anesthetized and inoculated in both ear pinnae with 10 μl of the indicated virus diluted to 2 × 103 PFU/μl in PBS (2 × 104 PFU/ear) using a 29-gauge insulin syringe (BD). Mice were monitored for lesion development, and lesions greater than 1 mm in diameter were measured using digital calipers. Experiments were conducted in accordance with the guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee.
Statistical analysis.
Statistical analysis was performed using the Prism program (GraphPad). Unpaired Student's t tests were used to compare differences in weight or lesion size between two groups, and a two-way analysis of variance (ANOVA) was used to compare differences in lesion sizes between three groups.
RESULTS
VCP cell surface expression is dependent on A56 and does not require other viral proteins.
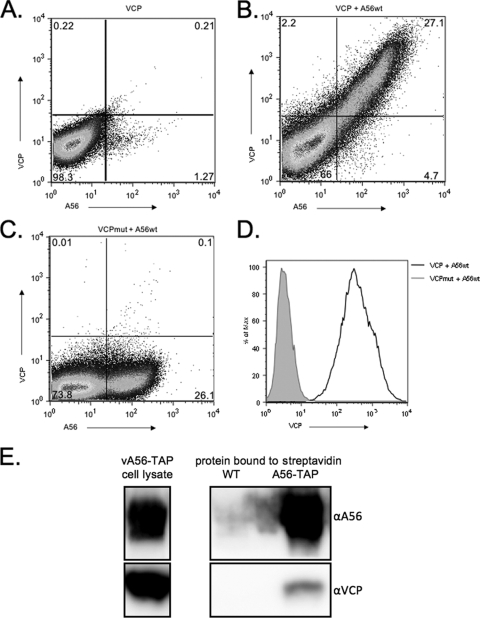
Previously, our lab showed that expression of VCP on the infected cell surface is mediated by an interaction with the viral A56 protein and requires a free N-terminal cysteine on VCP (9). To investigate if A56 is sufficient for VCP surface expression, we transiently transfected cells with plasmids expressing VCPwt, VCPmut, or wild-type A56 (A56wt). We found that when VCPwt is transfected into 293T cells, it is not expressed on the cell surface (Fig. 1 A). However, when VCPwt and A56wt are cotransected, VCP is expressed on the surface of cells that are also A56 positive (Fig. 1B). Importantly, cell surface expression is lost when VCPmut is transfected with A56wt (Fig. 1C), indicating that the transient-transfection system reproduces what we saw in cells that were infected with virus expressing either VCPwt or VCPmut (9). In transfected cells that are A56 positive, a 2-log10 shift in mean fluorescence intensity (MFI) was seen between VCPwt and VCPmut (Fig. 1D).
FIG. 1.
VCP and A56 interact on transfected and infected cells. VCP, VCPmut, and A56wt were transfected into 293T cells either alone or in combination. After 48 h, the cells were collected and stained under nonpermeabilized conditions for FACS analysis with an anti-VCP polyclonal antibody and anti-56 MAb LC10. (A) VCP is not expressed on the cell surface when transfected alone. (B and C) VCP, but not VCPmut, is expressed on the cell surface in the presence of A56. (D) Levels of VCP expression on A56-positive cells. The black solid line represents cells transfected with VCP and A56wt; the gray shaded area represents cells transfected with VCPmut and A56wt. (E) VCP is present after pulling down A56-TAP with streptavidin from cells infected with virus expressing TAP-tagged A56 (A56-TAP). Western blots were probed with anti-A56 and anti-VCP antibodies to show the presence of the indicated proteins. Boxed areas on the left represent blots of the input cell lysate from cells infected with virus expressing A56-TAP. Boxed areas on the right represent blots of the proteins pulled down with streptavidin from cells infected with virus expressing untagged A56 (WT) or from cells infected with virus expressing TAP-tagged A56.
In native Western blot assays, A56 is found as a monomer and a higher-molecular-weight band (40) (data not shown). The existence of a strong monomer band suggests that a surplus of A56 exists relative to the amount of K2 and VCP on the surface of cells. To begin to assess how much VCP is bound to A56, a recombinant VACV expressing a TAP-tagged version of A56 (40) was used to coprecipitate VCP. We found that when tagged A56 was pulled down with streptavidin-Sepharose beads, VCP coprecipitated (Fig. 1E). However, only a small amount of VCP was present relative to the amount of A56-TAP and to the proteins from the input infected cell lysates. This suggests that a surplus of A56 exists relative to the amount of VCP on the surface of cells.
Cysteine 162 of A56 is required for VCP cell surface expression.
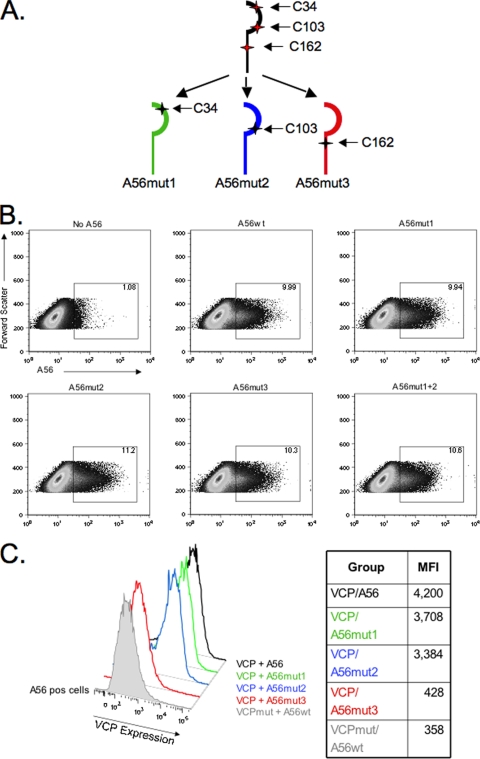
The fact that VCP required its N-terminal cysteine to bind A56 suggested to us that either a disulfide-bonded VCP homodimer (20) interacted with A56 to allow surface expression or that the free cysteine on monomeric VCP formed a disulfide bridge with an unpaired cysteine in the A56 ectodomain. To investigate which model best explained the interaction of VCP with A56, we mutated each of the three cysteine residues in the ectodomain of A56. Each cysteine was mutated to a serine residue, and the resulting expression plasmids were named A56mut1, A56mut2, and A56mut3, respectively (Fig. 2 A). If an intermolecular bridge were required for surface expression of VCP, the loss of VCP's cysteine partner on A56 would prevent VCP expression on the cell surface. While the anti-A56 MAb LC10 used in Fig. 1 could detect A56mut3, it no longer recognized A56mut1 or A56mut2 (data not shown), indicating that the MAb LC10 recognizes a conformation epitope that is lost when either Cys34 or Cys103 is mutated. However, when we used a rabbit polyclonal Ab raised to a peptide in the A56 ectodomain (40), all three mutants were recognized and were expressed at the cell surface at levels similar to A56wt (Fig. 2B). This indicates that the conformational epitope lost in A56mut1 and A56mut2 did not result in drastic misfolding and loss of the trafficking of A56 mutants to the cell surface. Each of the mutants was examined for the ability to interact with VCP and result in surface expression of VCP. When VCP was cotransfected with either A56mut1 or A56mut2, the level of VCP cell surface expression was similar to cells cotransfected with A56wt (Fig. 2C). A double mutant (A56mut1+2) is also able to interact with VCP and result in surface expression at levels similar to A56wt (data not shown). These results indicate that Cys34 and Cys103 are not required for VCP to interact with A56. However, when cells are cotransfected with A56mut3, VCP is not found on the cell surface and has an expression profile similar to VCPmut plus A56wt (Fig. 2C). This demonstrates that surface expression is lost even when the free N-terminal cysteine on VCP is present and homodimers can form. Taken together, these experimental results support a model in which cell surface expression of VCP is dependent on formation of a disulfide bridge between VCP and A56.
FIG. 2.
A56 mutagenesis and VCP surface expression. (A) Schematic of the cysteine pattern in the ectodomain of A56 and the three cysteine mutant A56 proteins. (B) Expression levels of A56wt versus A56mut1, A56mut2, A56mut3, and A56mut1+2. (C) Cells positive for A56 expression were gated and analyzed for VCP expression. Mean fluorescence intensity (MFI) levels are listed in the table to the right of the figure. VCP was cotransfected with the indicated A56 construct and stained with anti-VCP MAb 3F11, anti-A56 rabbit Ab, and appropriate secondary Abs and analyzed.
A56mut3 retains the ability to bind K2.
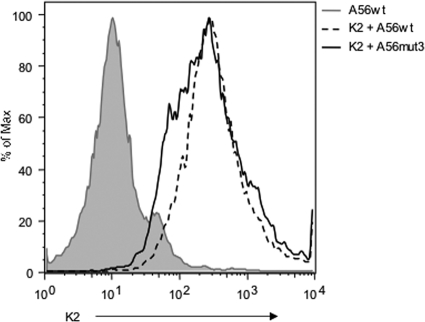
It is possible that mutating Cys162 of A56 could cause misfolding of the protein, resulting in the lack of VCP interaction with A56mut3. Therefore, we examined the ability of A56mut3 to interact with the viral K2 protein, which binds to A56 noncovalently. As was previously shown by the Moyer group (36), transfecting K2 alone does not result in cell surface expression of K2 (data not shown). Also as previously reported (36, 41), we found that K2 is expressed on the surface of cells in the presence of A56wt (Fig. 3). We also found that in the presence of A56mut3, K2 is still expressed on the surface of cells at the same level as with A56wt. These data suggest that mutating Cys162 specifically abrogates the A56-VCP interaction without affecting the interaction between A56 and K2.
FIG. 3.
A56mut3 is able to bind K2. 293T cells were transfected with A56wt alone or with K2 plus A56wt and A56mut3. The cells were then stained with anti-K2 MAb 4A11-4A3 and the rabbit anti-A56Ab. A56-positive cells were gated and used to create the histogram.
The variola virus VCP ortholog binds the vaccinia virus A56 protein.
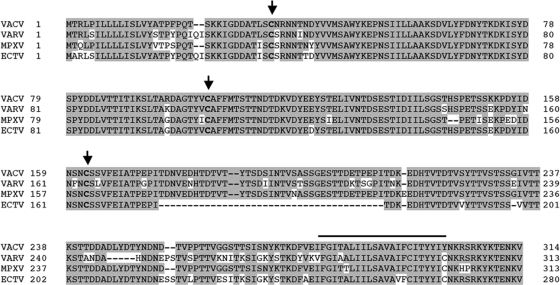
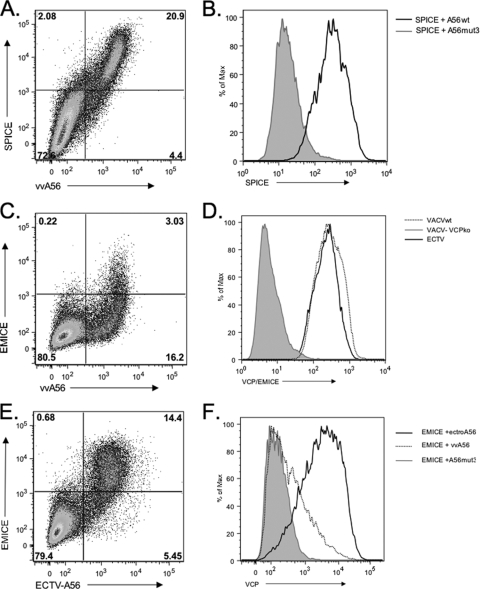
The unpaired N-terminal cysteine in VCP is conserved in many VCP orthologs, including the CCP proteins expressed by variola virus (SPICE) and ectromelia virus (EMICE). MoPICE has lost this N-terminal cysteine, but due to a truncation right after the start of the fourth SCR it has an unpaired cysteine near its C terminus (6, 20). Additionally, all of these viruses contain a homolog to the vaccinia virus A56 protein, and interestingly, all of these A56 orthologs retain the three extracellular cysteines (Fig. 4). Therefore, we hypothesized that the interaction between A56 and poxvirus CCPs may not be limited to VACV. To test this, we first studied the ability of SPICE to interact with VACV A56. We found that SPICE bound to vaccinia virus A56 (Fig. 5 A). We also found that SPICE, like VCP, is not expressed on the cell surface when cotransfected with A56mut3 (Fig. 5B). This suggests that SPICE and A56 can interact on the cell surface through a disulfide bridge at Cys162, similar to VCP and A56.
FIG. 4.
Comparison of orthopoxvirus A56 genes. The protein sequences of vaccinia virus (VACV-WR) A56 and the homologs in variola virus (VARV-Bangladesh), monkeypox virus (MPXV-Zaire), and ectromelia virus (ECTV-Moscow) were downloaded from poxvirus.org and aligned using BLAST. Shaded residues indicate identical amino acids to vaccinia virus, and dashes represent missing amino acids. Conserved cysteines in the ectodomain are indicated in bold and with an arrow. The putative transmembrane domain is marked with the black line above the residues.
FIG. 5.
SPICE and EMICE are also expressed on the cell surface in the presence of A56. SPICE is expressed on cells cotransfected with vaccinia virus A56wt. (A) Scatter plot of SPICE cotransfected with A56wt. (B) Histogram of SPICE expression on A56-positive cells. The black line is SPICE transfected with vaccinia virus A56wt, and the shaded gray area is SPICE transfected with A56mut3. (C) Scatter plot of EMICE transfected with VACV A56. (D) Histogram of CCP expression on virus-infected cells. RK-13 cells were infected with ECTV, VACV, or vv-VCPko and then stained with a polyclonal anti-VCP antibody. Shown is a histogram of surface staining due to EMICE (black line), VACV (dotted line), or vv-VCPko (gray shaded area). (E) Scatter plot of EMICE cotransfected with ECTV A56. (F) Histogram of EMICE expression of cells cotransfected with ECTV A56 (black line), VACV A56 (dotted line), or VACV A56mut3 (gray shaded area).
EMICE, but not MoPICE, binds to its cognate A56 homolog efficiently.
While VCP and SPICE have identical first SCR domains, EMICE has 12 amino acid differences in its first SCR. Cotransfection of EMICE with vaccinia virus A56 produced weak EMICE surface staining (Fig. 5C). However, staining of cells infected with ECTV showed that surface staining of EMICE was clearly evident (Fig. 5D) and that EMICE expression was similar to VCP expression on vaccinia virus-infected cells (Fig. 5D). This suggested to us that EMICE can interact with its A56 cognate, but not vaccinia virus A56. To address this possibility, we cloned the ECTV homolog of A56 (ECTV-A56) and cotransfected it with EMICE. We found that while VACV-A56 and ECTV-A56 were expressed on approximately the same number of cells (19.2% versus 19.8%), EMICE was expressed to a much higher level at the cell surface when cotransfected with ECTV-A56 (Fig. 5E). The peak level of EMICE staining on cells transfected with ECTV-A56 was 2 logs higher than EMICE transfected into cells with VACV-A56 or VACV-A56mut3 (Fig. 5F). This shift in staining mirrors the expression levels of VCP when it is transfected with vaccinia virus A56.
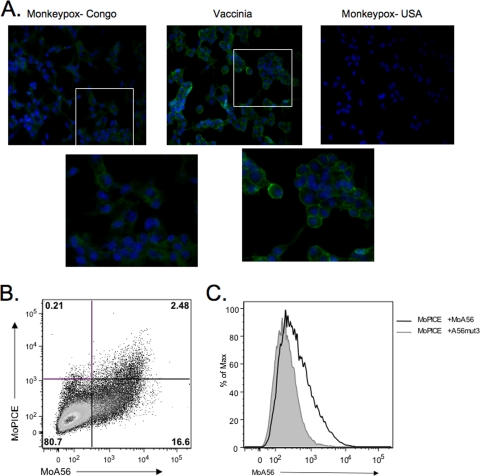
The monkeypox homolog of VCP, MoPICE, differs from these other poxvirus CCPs in several respects. As has been reported elsewhere, MoPICE does not have a free N-terminal cysteine, as this initial residue is a tyrosine (20). Also, MoPICE only contains three SCRs due to a point mutation that results in a truncation after the start of the fourth SCR. Interestingly, this truncation results in an unpaired cysteine near the C terminus, through which MoPICE can form homodimers (20). Immunofluorescence of cells infected with MPXV Congo shows weak cell surface staining of MoPICE compared to vaccinia virus-infected cells (Fig. 6 A). Consistent with the lower level of surface expression compared to VCP expression on vaccinia virus-infected cells, cotransfection of MPXV A56 with MoPICE also resulted in low-level surface expression of MoPICE (Fig. 6B and C), with only 2.48% of cells double positive. These data suggest that MoPICE is not expressed at high levels on infected or transfected cells. Taken with the EMICE data, it appears that the first SCR domain with an N-terminal free cysteine is crucial for optimal binding to A56.
FIG. 6.
MoPICE is expressed on the cell surface at low levels. (A) Immunofluorescence of infected cells. BSC-40 cells were infected for 18 h with MPXV-Congo, VACV, or a CCP-minus strain of MPXV, MPXV-USA, and fixed and stained using anti-VCP MAb 2E5 and fluorescent secondary Ab. To better visualize the cell distribution of VCP or MoPICE, the white box indicates the area of the image that was enlarged (shown in the second row of images), but unaltered. (B) Scatter plot of MoPICE cotransfected with MPXV A56. (C) Histogram of MoPICE expression on A56-positive cells. MoPICE was cotransfected with MPXV-A56 (black line) or VACV A56mut3 (gray shaded area), and cells were then stained for FACS with polyclonal rabbit anti-VCP and anti-A56 MAb.
Vaccinia virus expressing VCPmut is attenuated in vivo.
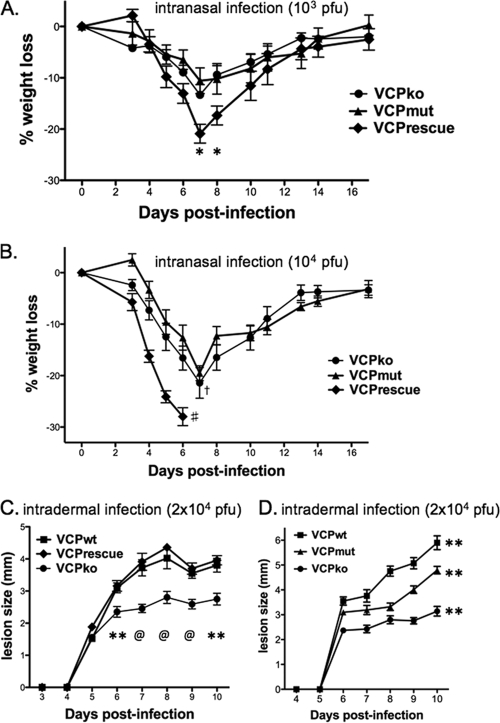
The N-terminal cysteine residue in VCP is conserved among other PICEs and has been shown to mediate both cell surface expression and dimerization of VCP (9, 20). Both cell surface expression and dimerization of recombinant VCP have been shown to enhance its complement regulatory function in vitro (20). In order to determine if cell surface expression and/or dimerization contribute to VCP's function as a virulence factor, we assessed the virulence of our recombinant VACVs using two mouse models of VACV pathogenesis (Fig. 7). We first compared vv-VCPmut to vv-VCPko and vv-VCPrescue after intranasal inoculation. At the lowest dose (103 PFU), all of the mice survived, but mice infected with vv-VCPrescue lost slightly more weight (Fig. 7A). The difference in weight loss between vv-VCPrescue and the other two viruses was significant at days 7 and 8. At the intermediate dose (104 PFU), all of the mice infected with the VCP rescue virus died, while mice infected with VCPko or VCPmut lost approximately the same amount of weight (Fig. 7B). At a high dose (105 PFU), all of the animals lost weight at a similar rate and died (data not shown). These experiments show that after intranasal inoculation a virus expressing wild-type VCP is more pathogenic than viruses with VCP deleted or ones lacking the N-terminal cysteine on VCP (and thus unable to form homodimers and/or be expressed on the cell surface). Interestingly, by this route of infection, vv-VCPko and vv-VCPmut had similar virulence. We also examined pathogenesis of vv-VCPko and vv-VCPmut by using an intradermal model of infection (34, 35). We initially showed that vv-VCPko made smaller ear lesions than vv-VCPwt (and vv-VCPrescue) (Fig. 7C). We then infected mice intradermally with a recombinant VACV encoding VCP lacking the free N-terminal cysteine (vv-VCPmut) and compared lesion sizes formed by this virus to those formed by a virus encoding wild-type VCP or a virus that lacked VCP (vv-VCPko) (Fig. 7D). The lesions formed following infection with vv-VCPmut were larger than those formed by vv-VCPko but smaller than those formed by vv-VCPwt. These differences in lesion sizes were statistically significant. The finding that vv-VCPko is attenuated following intradermal infection in mice confirms that VCP contributes to pathogenesis and is similar to what was reported in the past in rabbits and guinea pigs (14). Furthermore, as opposed to the intranasal route of inoculation, by which VCPko and VCPmut were similarly attenuated, by the intradermal route vv-VCPmut formed lesions of an intermediate size between vv-VCPko and vv-VCPwt. This suggests that the ability of VCP to bind to the cell surface and/or dimerize contributes to pathogenesis.
FIG. 7.
Wild-type VCP is needed for full virulence in mice. Our panel of recombinant viruses was studied in 6- to 8-week-old female C57BL/6 mice either after intranasal (A and B) or intradermal (C and D) inoculation. (A and B) Average weight loss of 6-week-old mice (n = 5 for all groups) infected with vaccinia virus encoding VCPrescue, VCPmut, or VCPko at 103 PFU (A) or 104 PFU (B). #, indicates that all mice infected with vv-VCPrescue died; †, a single mouse in the group infected with vv-VCPko died; *, significant difference (P < 0.05) on the days indicated between VCPrescue and VCPko and between VCPrescue and VCPmut (unpaired Student's t test). (C and D) Lesion diameters in 6- to 8-week-old mice inoculated with 2 × 104 PFU of VCPrescue, VCPwt, or VCPko (C) or VCPwt, VCPko, or VCPmut (D). For panel C, data points represent the mean lesion diameter ± standard error of the mean of 10 infected ears per group (two ears per mouse). P values for the difference between VCPko and VCPwt and between VCPko and VCPrescue for each day are as follows: *, P < 0.05; **, P < 0.01; @, P < 0.001 (unpaired Student's t test). For panel D, data points represent the mean lesion diameter ± standard error of the mean of 20 (VCPko), 14 (VCPwt), or 10 (VCPmut) infected ears per group (two ears per mouse). Differences in lesion size between the groups of mice were statistically significant (P < 0.01; two-way ANOVA).
DISCUSSION
VCP has been previously characterized as the major protein secreted from VACV-infected cells (16, 17) and has only recently found to also be expressed on the infected cell surface (9). The N-terminal cysteine of VCP is necessary for surface expression; however, the precise mechanism by which VCP interacted with A56 was unknown. We found that the primary method for VCP surface expression is through a disulfide bridge with Cys162 of the viral A56 protein. Mutation of A56 Cys34 and/or Cys103 results in loss of recognition by an anti-A56 monoclonal antibody, LC10. However, despite the loss of recognition by this antibody, these mutated proteins can still traffic to the cell surface and interact with VCP. Our work therefore also provides important information about A56. While cysteines 34 and 103 of A56 are unnecessary to bind VCP, they may play a role in the folding of A56. The N-terminal domain of A56 has homology to the Ig superfamily, which is heavily disulfide bonded. It is possible that the first two cysteines in A56 form an intramolecular bridge that, when mutated, alters an epitope recognized by MAb LC10, but the mutated protein still maintains sufficient folding to allow the protein to properly traffic to the cell surface.
VCP forms a disulfide bond with Cys162 of A56, and we have shown that this specific interaction also occurs with other poxviruses proteins, like SPICE. Soluble SPICE is capable of the same catalytic activity as VCP, but with a 100- to 1,000-fold increase in activity against human complement (21, 29, 33). Therefore, it is interesting to speculate that expression of SPICE on the infected cell surface may contribute to its significant pathogenesis in humans. Another significant finding is the interaction between the ectromelia virus homolog of VCP and A56. EMICE, in contrast to SPICE, is only able to interact efficiently with its own homolog of A56. This raises the intriguing possibility that coevolution occurred for these two proteins, as EMICE and ectromelia virus A56 are less similar to the vaccinia virus proteins than their variola virus counterparts. Conversely, the lack of high surface expression of MoPICE may mean that efficient cell surface expression of MoPICE is not possible without an N-terminal cysteine, even in the presence a C-terminal free cysteine. This is interesting, because it has been shown that this free C-terminal cysteine can form homodimers in a similar fashion as the orthologs with a free N-terminal cysteine (20).
We have also shown that a virus that cannot express cell surface VCP is modestly attenuated in vivo. This finding, combined with earlier in vitro data showing that, compared to cells infected with a VCP deletion virus, wild-type vaccinia virus-infected cells are resistant to complement-mediated lysis (9), suggests that the ability of VCP to interact with A56 and be expressed on the cell surface may have important implications for poxvirus pathogenesis. After ear pinnae infection, VCPmut formed larger lesions than VCPko. This was not entirely unexpected, since the majority of VCP is a secreted monomer. Therefore, when VCP is mutated so that it cannot form homodimers and/or be expressed on the cell surface, VCP still has complement regulatory activity. However, since mutating the N-terminal cysteine also abrogates dimerization of VCP, the contribution of secreted VCP homodimers to the attenuated lesion size we observed is not known. Our biochemical analysis of the interaction between VCP and A56 has identified a mutation in A56 that disrupts the A56-VCP interaction without disrupting the A56-K2 complex. Comparing pathogenesis of vv-VCPmut with a recombinant virus expressing A56mut3 will allow us to define the roles of surface-bound VCP and dimeric VCP in poxvirus pathogenesis. As opposed to the intradermal route of infection, where we found statistically significant differences between lesions formed by VCPko and VCPmut, these viruses appeared to be similarly attenuated after intranasal inoculation of mice. This may indicate that in a lung pneumonia model, the contribution of cell surface expression and/or the formation of the VCP homodimer is important for VCP's contribution to pathogenesis. Again, work with a recombinant virus expressing A56mut3 will allow us to define the roles of surface-bound VCP and dimeric VCP in poxvirus pathogenesis.
Acknowledgments
We thank Bernie Moss (NIH) for providing the rabbit anti-A56 antibody and vA56TAP, Dick Moyer (University of Florida, Gainesville, FL) for the anti-K2 MAb 4A11-4A3, Yan Xiang and Guangming Zhong (University of Texas Health Science Center, San Antonio, TX) for anti-A56 MAb LC10, John Atkinson and Kathy Liszewski (Washington University, St. Louis, MO) for rabbit anti-VCP antibody and plasmids containing SPICE and EMICE, Paul Bates (University of Pennsylvania) for pCAGGS, and Edward Alexander for technical assistance.
This work was supported by Public Health Service grant AI-066333 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 18 August 2010.
REFERENCES
- 1.Benhnia, M. R., M. M. McCausland, J. Moyron, J. Laudenslager, S. Granger, S. Rickert, L. Koriazova, R. Kubo, S. Kato, and S. Crotty. 2009. Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. J. Virol. 83:1201-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernet, J., J. Mullick, Y. Panse, P. B. Parab, and A. Sahu. 2004. Kinetic analysis of the interactions between vaccinia virus complement control protein and human complement proteins C3b and C4b. J. Virol. 78:9446-9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blue, C. E., O. B. Spiller, and D. J. Blackbourn. 2004. The relevance of complement to virus biology. Virology 319:176-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, C. K., P. C. Turner, and R. W. Moyer. 1991. Molecular characterization of the vaccinia virus hemagglutinin gene. J. Virol. 65:3598-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brum, L. M., P. C. Turner, H. Devick, M. T. Baquero, and R. W. Moyer. 2003. Plasma membrane localization and fusion inhibitory activity of the cowpox virus serpin SPI-3 require a functional signal sequence and the virus encoded hemagglutinin. Virology 306:289-302. [DOI] [PubMed] [Google Scholar]
- 6.Chen, N., G. Li, M. K. Liszewski, J. P. Atkinson, P. B. Jahrling, Z. Feng, J. Schriewer, C. Buck, C. Wang, E. J. Lefkowitz, J. J. Esposito, T. Harms, I. K. Damon, R. L. Roper, C. Upton, and R. M. Buller. 2005. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 340:46-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung, K. M., M. K. Liszewski, G. Nybakken, A. E. Davis, R. R. Townsend, D. H. Fremont, J. P. Atkinson, and M. S. Diamond. 2006. West Nile virus nonstructural protein NS1 inhibits complement activation by binding the regulatory protein factor H. Proc. Natl. Acad. Sci. U. S. A. 103:19111-19116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciulla, E., A. Emery, D. Konz, and J. Krushkal. 2005. Evolutionary history of orthopoxvirus proteins similar to human complement regulators. Gene 355:40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girgis, N. M., B. C. Dehaven, X. Fan, K. M. Viner, M. Shamim, and S. N. Isaacs. 2008. Cell surface expression of the vaccinia virus complement control protein is mediated by interaction with the viral A56 protein and protects infected cells from complement attack. J. Virol. 82:4205-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haga, I. R., and A. G. Bowie. 2005. Evasion of innate immunity by vaccinia virus. Parasitology 130(Suppl.):S11-S25. [DOI] [PubMed] [Google Scholar]
- 11.Harris, S. L., I. Frank, A. Yee, G. H. Cohen, R. J. Eisenberg, and H. M. Friedman. 1990. Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J. Infect. Dis. 162:331-337. [DOI] [PubMed] [Google Scholar]
- 12.Hook, L. M., J. M. Lubinski, M. Jiang, M. K. Pangburn, and H. M. Friedman. 2006. Herpes simplex virus type 1 and 2 glycoprotein C prevents complement-mediated neutralization induced by natural immunoglobulin M antibody. J. Virol. 80:4038-4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isaacs, S. N., E. Argyropoulos, G. Sfyroera, S. Mohammad, and J. D. Lambris. 2003. Restoration of complement-enhanced neutralization of vaccinia virus virions by novel monoclonal antibodies raised against the vaccinia virus complement control protein. J. Virol. 77:8256-8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isaacs, S. N., G. J. Kotwal, and B. Moss. 1992. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc. Natl. Acad. Sci. U. S. A. 89:628-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, A. H., I. D. Dimitriou, M. C. Holland, D. Mastellos, Y. M. Mueller, J. D. Altman, J. D. Lambris, and P. D. Katsikis. 2004. Complement C5a receptor is essential for the optimal generation of antiviral CD8+ T cell responses. J. Immunol. 173:2524-2529. [DOI] [PubMed] [Google Scholar]
- 16.Kotwal, G. J., S. N. Isaacs, R. McKenzie, M. M. Frank, and B. Moss. 1990. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science 250:827-830. [DOI] [PubMed] [Google Scholar]
- 17.Kotwal, G. J., and B. Moss. 1988. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature 335:176-178. [DOI] [PubMed] [Google Scholar]
- 18.Krauss, O., R. Hollinshead, M. Hollinshead, and G. L. Smith. 2002. An investigation of incorporation of cellular antigens into vaccinia virus particles. J. Gen. Virol. 83:2347-2359. [DOI] [PubMed] [Google Scholar]
- 19.Law, K. M., and G. L. Smith. 1992. A vaccinia serine protease inhibitor which prevents virus-induced cell fusion. J. Gen. Virol. 73:549-557. [DOI] [PubMed] [Google Scholar]
- 20.Liszewski, M. K., M. K. Leung, R. Hauhart, R. M. Buller, P. Bertram, X. Wang, A. M. Rosengard, G. J. Kotwal, and J. P. Atkinson. 2006. Structure and regulatory profile of the monkeypox inhibitor of complement: comparison to homologs in vaccinia and variola and evidence for dimer formation. J. Immunol. 176:3725-3734. [DOI] [PubMed] [Google Scholar]
- 21.Liszewski, M. K., M. K. Leung, R. Hauhart, C. J. Fang, P. Bertram, and J. P. Atkinson. 2009. Smallpox inhibitor of complement enzymes (SPICE): dissecting functional sites and abrogating activity. J. Immunol. 183:3150-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenzie, R., G. J. Kotwal, B. Moss, C. H. Hammer, and M. M. Frank. 1992. Regulation of complement activity by vaccinia virus complement-control protein. J. Infect. Dis. 166:1245-1250. [DOI] [PubMed] [Google Scholar]
- 23.Miller, C. G., S. N. Shchelkunov, and G. J. Kotwal. 1997. The cowpox virus-encoded homolog of the vaccinia virus complement control protein is an inflammation modulatory protein. Virology 229:126-133. [DOI] [PubMed] [Google Scholar]
- 24.Moss, B. 2006. Poxvirus entry and membrane fusion. Virology 344:48-54. [DOI] [PubMed] [Google Scholar]
- 25.Mullick, J., J. Bernet, Y. Panse, S. Hallihosur, A. K. Singh, and A. Sahu. 2005. Identification of complement regulatory domains in vaccinia virus complement control protein. J. Virol. 79:12382-12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Payne, L. G. 1979. Identification of the vaccinia hemagglutinin polypeptide from a cell system yielding large amounts of extracellular enveloped virus. J. Virol. 31:147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Payne, L. G., and K. Kristensson. 1985. Extracellular release of enveloped vaccinia virus from mouse nasal epithelial cells in vivo. J. Gen. Virol. 66:643-646. [DOI] [PubMed] [Google Scholar]
- 28.Rosengard, A. M., L. C. Alonso, L. C. Korb, W. M. Baldwin, 3rd, F. Sanfilippo, L. A. Turka, and J. M. Ahearn. 1999. Functional characterization of soluble and membrane-bound forms of vaccinia virus complement control protein (VCP). Mol. Immunol. 36:685-697. [DOI] [PubMed] [Google Scholar]
- 29.Rosengard, A. M., Y. Liu, Z. Nie, and R. Jimenez. 2002. Variola virus immune evasion design: expression of a highly efficient inhibitor of human complement. Proc. Natl. Acad. Sci. U. S. A. 99:8808-8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahu, A., S. N. Isaacs, A. M. Soulika, and J. D. Lambris. 1998. Interaction of vaccinia virus complement control protein with human complement proteins: factor I-mediated degradation of C3b to iC3b1 inactivates the alternative complement pathway. J. Immunol. 160:5596-5604. [PubMed] [Google Scholar]
- 31.Saifuddin, M., T. Hedayati, J. P. Atkinson, M. H. Holguin, C. J. Parker, and G. T. Spear. 1997. Human immunodeficiency virus type 1 incorporates both glycosyl phosphatidylinositol-anchored CD55 and CD59 and integral membrane CD46 at levels that protect from complement-mediated destruction. J. Gen. Virol. 78:1907-1911. [DOI] [PubMed] [Google Scholar]
- 32.Seet, B. T., J. B. Johnston, C. R. Brunetti, J. W. Barrett, H. Everett, C. Cameron, J. Sypula, S. H. Nazarian, A. Lucas, and G. McFadden. 2003. Poxviruses and immune evasion. Annu. Rev. Immunol. 21:377-423. [DOI] [PubMed] [Google Scholar]
- 33.Sfyroera, G., M. Katragadda, D. Morikis, S. N. Isaacs, and J. D. Lambris. 2005. Electrostatic modeling predicts the activities of orthopoxvirus complement control proteins. J. Immunol. 174:2143-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tscharke, D. C., P. C. Reading, and G. L. Smith. 2002. Dermal infection with vaccinia virus reveals roles for virus proteins not seen using other inoculation routes. J. Gen. Virol. 83:1977-1986. [DOI] [PubMed] [Google Scholar]
- 35.Tscharke, D. C., and G. L. Smith. 1999. A model for vaccinia virus pathogenesis and immunity based on intradermal injection of mouse ear pinnae. J. Gen. Virol. 80:2751-2755. [DOI] [PubMed] [Google Scholar]
- 36.Turner, P. C., and R. W. Moyer. 2006. The cowpox virus fusion regulator proteins SPI-3 and hemagglutinin interact in infected and uninfected cells. Virology 347:88-99. [DOI] [PubMed] [Google Scholar]
- 37.Turner, P. C., and R. W. Moyer. 1992. An orthopoxvirus serpinlike gene controls the ability of infected cells to fuse. J. Virol. 66:2076-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner, P. C., and R. W. Moyer. 2008. The vaccinia virus fusion inhibitor proteins SPI-3 (K2) and HA (A56) expressed by infected cells reduce the entry of superinfecting virus. Virology 380:226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanderplasschen, A., E. Mathew, M. Hollinshead, R. B. Sim, and G. L. Smith. 1998. Extracellular enveloped vaccinia virus is resistant to complement because of incorporation of host complement control proteins into its envelope. Proc. Natl. Acad. Sci. U. S. A. 95:7544-7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagenaar, T. R., and B. Moss. 2007. Association of vaccinia virus fusion regulatory proteins with the multicomponent entry/fusion complex. J. Virol. 81:6286-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagenaar, T. R., and B. Moss. 2009. Expression of the A56 and K2 proteins is sufficient to inhibit vaccinia virus entry and cell fusion. J. Virol. 83:1546-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walport, M. J. 2001. Complement. First of two parts. N. Engl. J. Med. 344:1058-1066. [DOI] [PubMed] [Google Scholar]
- 43.Walport, M. J. 2001. Complement. Second of two parts. N. Engl. J. Med. 344:1140-1144. [DOI] [PubMed] [Google Scholar]
- 44.Xiao, Y., L. Aldaz-Carroll, A. M. Ortiz, J. C. Whitbeck, E. Alexander, H. Lou, H. L. Davis, T. J. Braciale, R. J. Eisenberg, G. H. Cohen, and S. N. Isaacs. 2007. A protein-based smallpox vaccine protects mice from vaccinia and ectromelia virus challenges when given as a prime and single boost. Vaccine 25:1214-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou, J., X. Y. Sun, G. J. Fernando, and I. H. Frazer. 1992. The vaccinia virus K2L gene encodes a serine protease inhibitor which inhibits cell-cell fusion. Virology 189:678-686. [DOI] [PubMed] [Google Scholar]