Abstract
The endoplasmic reticulum (ER) chaperone BiP (immunoglobulin binding protein) plays a major role in the control of the unfolded protein response. We have previously shown that BiP levels are dramatically increased during human cytomegalovirus (HCMV) infection, where BiP performs unique roles in viral assembly and egress. We show that BiP mRNA levels increase during infection due to activation of the BiP promoter by the major immediate-early (MIE) proteins. The BiP promoter, like other ER stress-activated promoters, contains endoplasmic reticulum stress elements (ERSEs), which are activated by unfolded protein response (UPR)-induced transcription factors. However, these elements are not needed for MIE protein-mediated transcriptional activation; thus, a virus-specific transcriptional activation mechanism is used. Transcriptional activation results in only a 3- to 4-fold increase in BiP mRNA, suggesting that additional mechanisms for BiP production are utilized. The BiP mRNA contains an internal ribosome entry site (IRES) which increases the level of BiP mRNA translation. We show that utilization of the BiP IRES is dramatically increased in HCMV-infected cells. Utilization of the BiP IRES can be activated by the La autoantigen, also called Sjögren's syndrome antigen B (SSB). We show that SSB/La levels are significantly increased during HCMV infection, and SSB/La depletion causes the loss of BiP IRES utilization and lowers endogenous BiP levels in infected cells. Our data show that BiP levels increase in HCMV-infected cells through the combination of increased BiP gene transcription mediated by the MIE proteins and increased BiP mRNA translation due to SSB/La-induced utilization of the BiP IRES.
Human cytomegalovirus (HCMV) is the largest human herpesvirus, estimated to encode at least 200 proteins. Despite this large coding capacity, the replication cycle is slow and increasingly stressful to the host cell as the infection progresses. A repertoire of cellular stress responses serve to counteract and alleviate these stresses. In order to maintain conditions optimal for viral replication, HCMV must selectively modulate these stress responses to take advantage of their beneficial effects while inhibiting effects that are deleterious to viral replication.
One stress response that HCMV specifically modulates is the unfolded protein response (UPR) (13, 30), a complex signaling cascade that restores endoplasmic reticulum (ER) homeostasis in response to ER stress (reviewed in references 20 and 24). The UPR is mediated by three transmembrane sensors: protein kinase R (PKR)-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 (IRE1). Under nonstressed conditions, the sensors are bound in an inactive state by the ER chaperone immunoglobulin binding protein (BiP), also called glucose-regulated protein 78 (GRP78). When the ER becomes stressed, BiP detaches from the three sensors to perform its ER chaperone functions. This allows each sensor to become activated and perform its UPR function (2, 25). Analysis of the UPR during an HCMV infection shows that the virus selectively modulates the individual signaling pathways activated by the three sensors, maintaining advantageous aspects of the UPR and inhibiting aspects that would impede infection (13).
In previous studies, we have shown that BiP levels dramatically increase during the course of infection (5, 13). The induction of BiP may help the virus control UPR activation. In addition, the induction is beneficial for viral replication due to BiP's involvement in immune evasion, interacting with the viral proteins US2 and US11 to degrade both major histocompatibility complex (MHC) class I and II (11, 27). The increase in BiP levels is also involved in viral assembly and egress, as infected cells depleted of BiP lose viral cytoplasmic activity and assembly compartment integrity (4, 5). Thus, BiP has several important roles during HCMV infection.
UPR activation in noninfected cells results in increased BiP levels, due, in part, to increased transcription of the BiP gene. The BiP promoter, like other ER stress-activated promoters, contains endoplasmic reticulum stress elements (ERSEs), which are activated by UPR-induced transcription factors, ATF6, XBP1, and ATF4 (18, 32, 33). During HCMV infection, the increase in BiP levels is precisely regulated in a temporal manner. BiP begins to increase early in infection, attains very high levels by 60 to 72 h postinfection (hpi), and drops off gradually at later time points (5). However, during HCMV infection the transcriptionally active forms of XBP1 and ATF6 are not produced, and the increase in BiP precedes the increase in ATF4 (13). Therefore, it appears that the great increase in BiP levels in HCMV-infected cells may occur independent of the UPR-induced transcription factors and the ERSEs.
In the following experiments, we show that BiP mRNA levels increase during infection as a result of transcriptional activation of the BiP promoter by the major immediate-early (MIE) proteins. The ERSEs are not needed for MIE protein-mediated transcriptional activation; thus, the virus utilizes a unique transcriptional activation mechanism. Despite this transcriptional activation, the increase in BiP mRNA is only 3- to 4-fold, suggesting that additional mechanisms for BiP production are utilized. The BiP mRNA contains an internal ribosome entry site (IRES), which can affect the level of translation of the BiP mRNA, especially during times of stress (31). We show that utilization of the BiP IRES is dramatically increased in HCMV-infected cells. Utilization of the BiP IRES can be activated by the La autoantigen, also called Sjögren's syndrome antigen B (SSB) (14). We show that SSB/La levels are significantly increased during HCMV infection, and SSB/La depletion by using small interfering RNAs (siRNAs) causes the loss of BiP IRES utilization and lowering of endogenous BiP levels in infected cells. Thus, our data show that BiP levels are increased in HCMV-infected cells by the combination of increased BiP gene transcription mediated by the MIE proteins, using an ERSE-independent mechanism, and increased BiP mRNA translation due, at least in part, to SSB/La-induced utilization of the BiP IRES.
MATERIALS AND METHODS
Tissue culture and reagents.
Low-passage, life-extended human foreskin fibroblasts (HFs) (3) were cultured at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) (supplemented with 10% fetal calf serum [FCS], 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM GlutaMAX). Thapsigargin was purchased from Calbiochem and was used at 2 μg/ml. The control siRNA against green fluorescent protein (GFP) was purchased from Qiagen (1022064). The sense (GGUUAUCAGUGUAAUGAAG) and antisense (CUUCAUUACACUGAUAACC) siRNA sequences that have been previously shown to target the HCMV MIE protein IEP72 (12) were custom generated by Thermo Scientific (formerly Dharmacon, Lafayette, CO). The siRNA against La autoantigen (D-006877-01) and the scrambled control (D-001210-03) were also purchased from Thermo Scientific. The antibody that detects exons 2 and 3 of the immediate-early proteins was described previously (10). Antibodies that detect pp65 and actin were purchased from U.S. Biologicals and Chemicon, respectively.
Virus preparation, titration, and growth curves.
HCMV Towne strain virus stocks were prepared as previously described (2), and titers were determined by the 50% tissue culture infective dose (TCID50) method. HCMV was inactivated by 254-nm UV light administered by a UV Stratalinker 1800 for 5 min. All infections were done at a multiplicity of infection (MOI) of 3.
Quantitative PCR.
RNA was isolated from fibroblasts using the RNeasy minikit (Qiagen) according to manufacturer's instructions. cDNA was generated from RNA with cDNA Superscript first-strand (Invitrogen). Quantitative PCR was performed on a 7900 HT system (Applied Biosystems), using Applied Biosystems TaqMan gene expression master mix and gene expression assays for BiP (Hs00607129_gH) and GAPDH (glyceraldehyde-3-phosphate dehydrogenase; Hs00266705_g1). PCR results were analyzed using SDS 2.3 software.
Plasmids and luciferase assay.
All plasmids were grown in Escherichia coli DH5α. Plasmid DNA was prepared using Qiagen plasmid maxikits. Plasmids pRL43a (22) and pSVH (7), containing major immediate-early protein genomic DNA, were obtained from Gary Hayward (Johns Hopkins University) and Richard Stenberg (Eastern Virginia Medical School), respectively. The major immediate-early protein genomic DNA expressing plasmid pCD-MIE was made in a two-step cloning process in which the genomic region of pRL43a was inserted into pcDNA3. First, a 1.4-kb XhoI/SalI blunt fragment from pRL43a replaced the XhoI/BsmI segment in pCDNA3, and then, a 3.8-kb NdeI/XhoI fragment from pRL43a replaced the NdeI/XhoI segment in the plasmid made in the first step. The IE1 and IE2 cDNA-expressing plasmids pRSV72 and pRSV86 (8) and pIEP72 and pIEP86 (26) were all obtained from Richard Stenberg. The GFP-expressing plasmid pMAX-GFP is from Lonza and was provided as part of the basic Nucleofector kit for primary mammalian fibroblasts. The wild-type (WT) plasmid WT-BiP-Luc and mutant plasmid mut-BiP-Luc were a gift from Kazutoshi Mori (Kyoto University) (32).
For stable cell line generation, promoter sequences were cut from the pGL3 backbone using the restriction enzymes KpnI and HindIII and inserted into pGL4.17 (Promega). The plasmids were then electroporated into HFs using program U-023 for the Amaxa Nucleofector (Lonza). After electroporation, the cells were serially passaged in the presence of 1 mg/ml G418. After selection, cells were propagated in medium containing 200 μg/ml G418 and then maintained in normal medium. The pDL-N dual-luciferase plasmid has been described previously (29). The BiP 5′ noncoding region (NCR) containing the BiP IRES was PCR amplified from the pSVCAT/BiP/Luc plasmid (19), provided by Maria Hatzoglou (Case Western Reserve University), using the following forward (CGCTGCAGAGGTCGACGCCGG) and reverse (CGGGTACCCTTGCCAGCCAGTTGG) primers and cloned into pDL-N using the restriction enzymes KpnI and PstI.
For luciferase assays, plasmid DNA was electroporated (260 V, 950 μF) into HFs using Gene Pulser II (Bio-Rad) or with an Amaxa Nucleofector as described above. HCMV infection was done 24 h postelectroporation, and samples were harvested 24 hpi and processed using the luciferase assay system and the Renilla luciferase assay system (both from Promega) according to the manufacturer's instructions.
RESULTS
HCMV infection increases BiP mRNA levels.
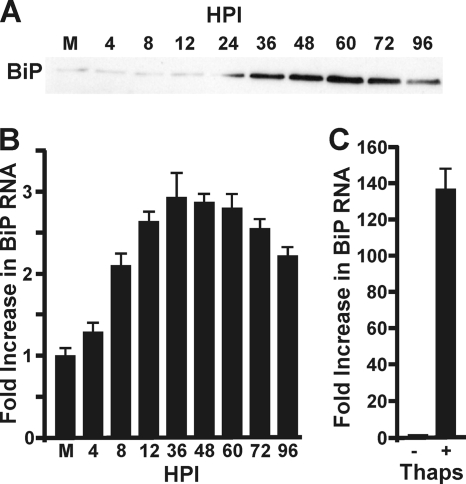
We have previously shown that BiP levels are dramatically increased during HCMV infection (5). This result is reiterated in Fig. 1 A, showing a Western analysis of BiP accumulation during an HCMV infection time course. As previously reported, the levels of BiP begin to increase by 24 h postinfection (hpi) and attained a maximum at 60 and 72 hpi. The highest levels are comparable to the levels attained in uninfected cells after treatment with a known UPR inducer such as thapsigargin for 24 h (5). To determine if the HCMV-induced increase in BiP involves increasing BiP mRNA levels, total mRNA was harvested from mock-infected fibroblasts and fibroblasts infected with HCMV at 4, 8, 12, 36, 48, 60, 72, and 96 hpi. Quantitative PCR (Fig. 1B) showed an increase in BiP mRNA as early as 8 hpi. This increase was sustained throughout infection, peaking at a level three times greater than mock infection between 36 and 72 hpi before decreasing slightly at the later times during infection. While this rise in BiP mRNA generally correlates with the increase in BiP, the maximum fold increase in BiP mRNA is dramatically less than that seen after treatment of mock-infected cells with the UPR inducer thapsigargin for 24 h (Fig. 1C). These data suggest that the increased BiP levels in HCMV-infected cells cannot be completely accounted for by increased BiP mRNA; thus, other mechanisms may be involved in increasing BiP levels specifically in HCMV-infected cells. In the following sections, we examine the effects of HCMV infection on the activation of the BiP promoter as it relates to increased mRNA levels and alterations in the translation of BiP mRNA during infection.
FIG. 1.
HCMV increases BiP protein and mRNA levels. (A) Western analysis probing for BiP accumulation during a HCMV infection time course; this reiterates previously reported results (5). (B) cDNA was generated from total RNA harvested from mock-infected (M) or HCMV-infected HFs at the indicated times postinfection (hpi) and subjected to quantitative PCR analysis using primers specific for BiP and GAPDH. BiP mRNA was normalized to GAPDH mRNA. (C) RNA was harvested from untreated (−) or thapsigargin (Thaps)-treated (+) uninfected HFs and analyzed as described in panel A. Fold activation in panels A and B is relative to the level in the mock sample, which was set to 1.
HCMV activates the BiP gene promoter in an ERSE-independent manner.
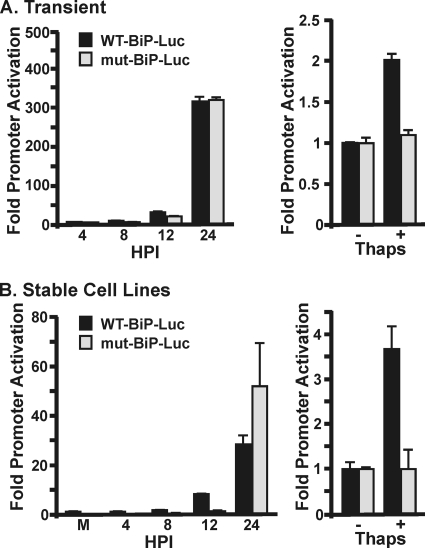
During UPR activation, increased BiP transcription results from activation of the BiP gene's ERSE-containing promoter. Thus, we investigated BiP promoter activation during infection by using luciferase reporter plasmids in which the luciferase gene was under the control of either the wild-type BiP promoter (WT-BiP-Luc) or a mutant BiP promoter (mut-BiP-luc) which lacked the three ERSEs (32) (Fig. 2 A). Twenty-four hours after electroporation of the reporter plasmids, HFs were infected with HCMV. Increased luciferase levels from the WT-BiP-Luc plasmid are detected between 8 and 12 hpi; by 24 hpi, luciferase levels are greatly increased (Fig. 2A). The levels of luciferase produced from the mut-BiP-Luc promoter are similar to those from the WT-BiP-Luc promoter, suggesting that HCMV-induced activation of the BiP promoter does not require the ERSEs. Importantly, treatment of electroporated, uninfected cells with thapsigargin increases luciferase levels in cells expressing the WT-BiP-Luc plasmid, but not the mut-BiP-Luc reporter (Fig. 2A). This confirms that under our transfection conditions in uninfected cells, both reporter plasmids responded as expected to ER stress.
FIG. 2.
BiP promoter activation by HCMV does not require the ERSEs. (A) HFs were electroporated with plasmids expressing luciferase under the control of the WT BiP promoter (WT-BiP-Luc) or a promoter with mutations in the three ERSEs (mut-BiP-Luc). The cells were either infected with HCMV 24 h postelectroporation or treated with thapsigargin (Thaps) for 24 h. Proteins were harvested at the indicated times postinfection for luciferase analysis. Fold activation was determined by normalizing luciferase levels in infected or thapsigargin-treated samples to the levels in mock-infected samples. (B) HFs stably expressing WT-BiP-Luc or mut-BiP-Luc were mock infected, HCMV infected, or treated with thapsigargin and analyzed for luciferase expression as described in panel A.
It is important to note the apparent discrepancy in the thapsigargin-treated, uninfected cell samples between the large fold increase in BiP mRNA (Fig. 1B) and the relatively modest promoter activation, as measured by luciferase assay (Fig. 2A). The promoter activity is determined by the level of luciferase, which is dependent on the level of translation of the reporter gene's mRNA. Since the reporter gene's mRNA is subject to the translational inhibition mediated by thapsigargin-induced activation of the UPR, the production of luciferase is inhibited. Thus, the assay provides a low estimate of promoter activity under these conditions. Overall, these data suggest that during HCMV infection, the BiP gene promoter is activated by a virus-mediated mechanism that does not require the ERSEs.
In the HCMV field, there is a belief that plasmid-borne promoters are readily and promiscuously activated by the HCMV MIE proteins. Although this can be refuted by existing data (17; and see below), we generated HF cell lines that stably express the luciferase reporter from either the wild-type or ERSE mutant BiP promoters (see Materials and Methods). This was done to ensure the specificity of BiP promoter activation by HCMV. The results of these analyses were similar to those using transient transfection. When HFs stably expressing the luciferase plasmids were infected, an increase in luciferase was detected from both the wild-type and mutant promoters (Fig. 2B). Similar to the transient transfection results, activation began by 12 hpi and increased significantly by 24 hpi (Fig. 2B). Treatment of uninfected cells with the UPR inducer thapsigargin confirmed inducibility of the wild-type BiP promoter, but not the mutant promoter, in response to ER stress (Fig. 2B). These data confirm that the BiP promoter is activated during infection and that HCMV-mediated activation does not require the ERSEs.
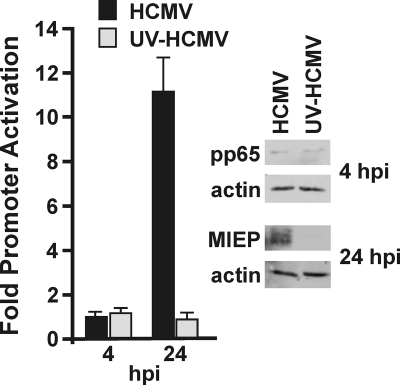
The BiP promoter is not activated by incoming virion proteins.
The increase in BiP RNA levels by 8 hpi suggests that activation may be mediated by either proteins that enter with the virions—for example, tegument proteins—or by the synthesis of immediate-early viral proteins. To test if the BiP promoter is activated by incoming viral proteins, HFs stably expressing luciferase under the control of the BiP promoter were infected with either HCMV or UV-inactivated HCMV (UV-HCMV). UV irradiation damages the HCMV genome such that the virus can enter cells, release tegument proteins, and initiate signaling pathways that prepare the cell for infection; however, no gene expression occurs from the irradiated virions. Proteins were harvested for luciferase assays at 4 and 24 hpi. Figure 3 shows increased luciferase levels in cells infected with normal HCMV by 24 hpi, similar to the BiP promoter activation described above. In contrast, luciferase levels were not increased in cells infected with UV-HCMV. These results indicate that neither viral attachment nor incoming tegument proteins are responsible for transcriptional activation of the BiP promoter during HCMV infection. The Western analysis in Fig. 3 shows equivalent levels of pp65, a prominent tegument protein, in cell extracts from both WT and UV-irradiated virus infections extracted at 4 hpi. This suggests that equivalent viral attachment and entry occurred with WT and UV-irradiated viruses. Despite long exposure, the intensities of the pp65 bands are very low because the only source of pp65 was that which entered with the incoming virions. That the UV inactivation was successful was confirmed by detecting production of the HCMV MIE proteins in the normal HCMV-infected samples but not the UV-irradiated HCMV samples at 24 hpi.
FIG. 3.
The BiP promoter is not activated by incoming virion-associated proteins. HFs stably expressing the WT-BiP-Luc were infected with normal HCMV or HCMV inactivated by UV irradiation (UV-HCMV). Protein samples were harvested for luciferase analysis at the indicated times postinfection. Fold activation was determined by normalizing luciferase levels in infected samples to those in the mock-infected samples. The levels of the HCMV MIE proteins (MIEP) and the tegument protein pp65 were detected by Western analysis in lysates from normal HCMV and UV-HCMV-infected samples. Actin was used as a loading control.
The HCMV MIE proteins activate the BiP promoter.
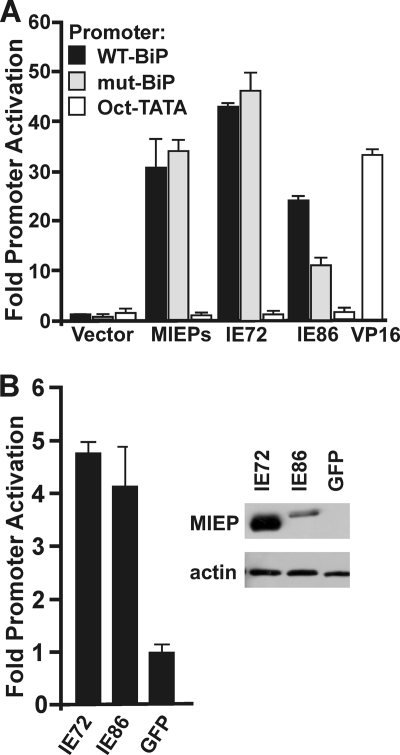
The above data suggest that the BiP promoter is activated prior to 24 hpi by newly synthesized viral immediate-early proteins. The most abundant immediate-early proteins are the MIE proteins, the 72-kDa protein IEP72 (also called IE1) and the 86-kDa protein IEP86 (also called IE2). Because these are well-characterized transcriptional activating proteins, we tested their ability to activate the BiP promoter. Figure 4 A shows that coelectroporation of WT-BiP-Luc or mut-BiP-Luc with pRL43a (22), a plasmid containing the genomic major immediate-early gene and which expresses both IEP72 and IEP86 (MIE proteins), resulted in significant increase in BiP promoter activity from both the WT and ERSE mutant promoters. The same experiment was done using plasmids that expressed IEP72 and IEP86 individually. Figure 4A shows that increased luciferase activity was detected by the introduction of either IEP72 or IEP86. Similar analysis using the mut-BiP-Luc reporter shows that promoter activation by IEP72 and IEP86 does not depend on the ERSEs, although there is some reduction in activation by IEP86 alone. This may be a result of the mutations in the CAAT elements of the ERSEs, since previous analysis has shown that IEP86 has a preference for activating promoters with CAAT sites (17). In sum, the data suggest that the HCMV MIE proteins can activate the BiP promoter by a mechanism that does not require the ERSEs. We have also included a control promoter that cannot be activated by the MIE proteins. This promoter contains six Oct1 binding sites upstream of a TATA element (Oct-TATA). This promoter was constructed using an Oct1-Tef1 element of the simian virus 40 (SV40) late promoter that had been mutated to disable the Tef1 site (17). Our previous studies have shown that this promoter cannot be activated by the MIE proteins but remains activatable by the herpes simplex virus VP16 transactivator (17); these findings are confirmed in Fig. 4A.
FIG. 4.
The HCMV MIE proteins (MIEPs) activate the BiP promoter. (A) A vector control plasmid or plasmids expressing the genomic MIE region or the individual MIE proteins IEP72 and IEP86 were coelectroporated into HFs with WT-BiP-Luc or mut-BiP-Luc and harvested 48 h postelectroporation for luciferase analysis. Fold activation was determined by normalizing luciferase levels to those of the vector control, set at 1. The Oct-TATA promoter control is described in the text. It cannot be activated by the MIE proteins, but it remains activatable by the herpes simplex virus VP16 transactivator (17). (B) HFs stably expressing luciferase under the control of the WT BiP promoter were transfected with plasmids expressing IEP72, IEP86, and GFP. Protein lysates were harvested 48 h postelectroporation for luciferase and Western analysis. Actin was used as a loading control for the Western analysis.
Activation of the BiP promoter by IEP72 and IEP86 was also tested by electroporating the IEP72- and IEP86-expressing plasmids into the cell line stably expressing luciferase under the control of the WT BiP promoter (Fig. 4B). Both IEP72 and IEP86 activated the BiP promoter, as indicated by increased luciferase levels in comparison to the control, which was represented by electroporation of a plasmid expressing GFP (Fig. 4B). Western analysis of the transfected lysates confirms the presence of each MIE protein (Fig. 4B). Electroporation of both the IEP72 and IEP86 plasmids together did not increase the luciferase expression over that of the individual electroporations (not shown). The above data suggest that the HCMV MIE proteins activate the BiP promoter independent of the ERSEs.
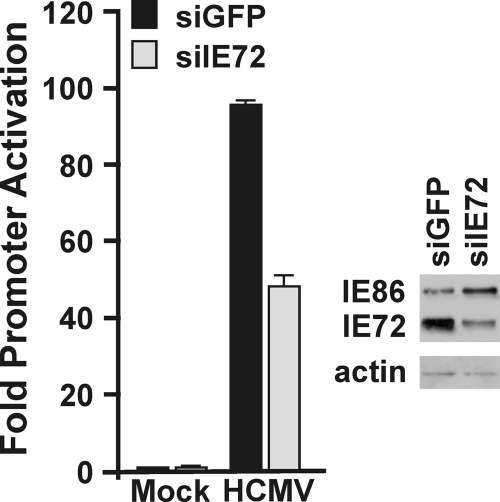
During HCMV infection, the depletion of IEP72, using siRNAs, impairs BiP promoter activation.
The above experiments all employ transfection of uninfected cells to examine MIE protein effects on BiP promoter activation. In order to be more physiologically relevant, the cell line stably expressing luciferase under the control of the WT BiP promoter was infected with HCMV; at 2 hpi, the cells were electroporated with siRNA against IEP72 (siIE72) or GFP (siGFP) (see Materials and Methods). The cells were harvested at 24 hpi. We chose IEP72 because it consistently showed greater BiP promoter activation than IEP86, and as shown in Fig. 4A, the activation by IEP72 is not affected by the BiP promoter mutation. The cell line stably expressing luciferase under the control of the WT BiP promoter was infected with HCMV; at 2 hpi, the cells were electroporated with siRNA against IEP72 (siIE72) or GFP (siGFP). The cells were harvested at 24 hpi. Western analysis shows that IEP72 was significantly depleted in cells electroporated with the siRNA targeting IEP72 (Fig. 5). It is important to note that we have established that only 50 to 60% of the cells are transfected under the electroporation conditions used (not shown); thus, the 50 to 60% depletion of IEP72 is consistent. In agreement with the above results, luciferase levels rose upon infection in cells treated with the control siGFP. However, there was a 50 to 60% reduction in luciferase activity when IEP72 was depleted using siIE72; this reduction in luciferase activity is proportional to the depletion of IEP72. These data confirm that IEP72 has a major role in the induction of BiP transcription during infection.
FIG. 5.
Depletion of IEP72 reduces BiP promoter activation. Cells stably expressing luciferase under the control of the WT BiP promoter were infected with HCMV; at 2 hpi, the cells were electroporated with siRNA against IEP72 (siIE72) or GFP (siGFP). The cells were harvested at 24 hpi for Western analysis of IEP72 and for luciferase assay. Fold activation of luciferase activity was relative to that in mock-infected samples.
HCMV activates translation from the BiP IRES.
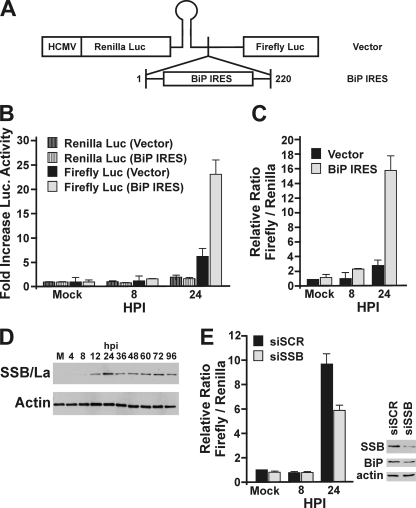
Our data show that HCMV can activate the BiP promoter in an ERSE-independent manner, and this is accomplished, at least in part, by IEP72. However, the 3- to 4-fold increase in mRNA does not correlate with the dramatic increase in BiP protein that we have previously reported (5) and show in Fig. 1A. This suggests that HCMV may utilize additional methods for increasing BiP. BiP mRNA contains an internal ribosome entry site (IRES) in its 5′ UTR (31). Therefore, we tested whether HCMV infection results in increased utilization of the BiP IRES, to increase BiP levels during infection. For this experiment, a dual-luciferase plasmid was generated by using the pDL-N vector (29) (Fig. 6 A), which expresses an mRNA encoding both Renilla luciferase and firefly luciferase. Prior to the firefly luciferase, we inserted the first 221 nucleotides of the BiP transcript, the 5′ UTR, which contains the BiP IRES (Fig. 6A). A stable stem-loop structure, inserted after the Renilla luciferase ORF, reduces activation of firefly luciferase by ribosomal scanning (29). HFs were electroporated with the BiP IRES dual-luciferase plasmid or a vector control plasmid that did not contain the BiP IRES. The cells were mock infected or infected with HCMV 24 h postelectroporation. Protein was harvested for luciferase analysis at 8 and 24 hpi. Figure 6B shows the levels of Renilla and firefly luciferase expressed from either the BiP IRES-containing plasmid or control plasmid under mock-infected and infected conditions. Luciferase levels were normalized to the amount expressed in mock-infected cells, which was set at 1. The levels of Renilla luciferase, representing the transcript level, were comparable between the two plasmid samples at all time points, indicating that transcript levels were equivalent under all conditions. The levels of firefly luciferase were comparable between the control and BiP IRES-containing plasmids at 8 hpi; however, by 24 hpi, firefly luciferase was significantly increased in the presence of the BiP IRES (Fig. 6B). This is further illustrated in Fig. 6C, which shows the ratio of firefly to Renilla luciferase for both plasmids in either mock-infected samples or HCMV-infected samples at 8 and 24 hpi. The data show that utilization of the BiP IRES is significantly increased during HCMV infection. The increased utilization of the BiP IRES by 24 hpi correlates with the time when BiP levels begin to increase in infected cells (5).
FIG. 6.
HCMV activates translation from the BiP IRES through induction of SSB/La. (A) Schematic of dual-luciferase plasmid generated by inserting the 221-nucleotide 5′ UTR of BiP (BiP IRES) into pDL-N (vector). (B and C) HFs were electroporated with the control dual-luciferase plasmid or the dual-luciferase plasmid containing the BiP IRES upstream of the firefly luciferase. Twenty-four hours postelectroporation, the cells were mock infected or HCMV infected. Protein lysates were harvested at 8 and 24 hpi. Results are graphed as fold increase in luciferase activity relative to the level in mock-infected samples (B) or as the relative ratio of firefly to Renilla luciferase for each sample, where the ratio of the mock-infected vector control was set at 1 (C). (D) Proteins from mock-infected (M) and HCMV-infected HFs were harvested at the indicated times postinfection. Western analysis was performed using antibodies against SSB/La autoantigen and actin (loading control). (E) Mock- and HCMV-infected HFs were electroporated with the dual-luciferase BiP IRES plasmid and siRNA against SSB/La (siSSB) or a control scrambled sequence (siSCR). Protein was harvested for luciferase and Western analysis at the indicated times postinfection. Results are graphed as the relative ratio of firefly to Renilla luciferase for each sample, where level of the mock-infected siSCR samples was set at 1. Western analysis was done to detect the levels of SSB/La, BiP, and actin.
The BiP IRES has been shown to be regulated by the La autoantigen, also called SSB (for Sjögren's syndrome antigen B) (14). The Western analysis in Fig. 6D shows that SSB/La is increased during HCMV infection, beginning at 12 hpi and remaining elevated during the course of the infection. The increase in SSB/La from 12 to 24 hpi correlates with both the onset of BiP IRES activity (Fig. 6B and C) and the increase in BiP (Fig. 1A), implicating it as a potential regulator of BiP IRES activity during infection.
To determine the role of SSB/La in regulating BiP IRES activity during HCMV infection, mock- or HCMV-infected HFs were electroporated with the BiP IRES dual-luciferase plasmid and either an siRNA targeting SSB/La (siSSB) or a scrambled nonspecific siRNA (siSCR). Proteins were harvested for luciferase assays from the mock- and HCMV-infected samples at 8 and 24 hpi. The ratios between firefly and Renilla luciferase are comparable between the mock-infected and 8 hpi samples (Fig. 6E). In contrast, the firefly/Renilla luciferase ratio at 24 hpi is significantly lower in the samples where SSB/La was depleted. Western analysis (Fig. 6E) confirms the depletion of SSB/La in the 24-hpi samples. In addition, we probed for endogenous BiP levels in these samples and found that depletion of SSB/La correlated with a lowering of endogenous BiP levels (Fig. 6E). Thus, the endogenous gene expression mirrors the results of the dual-luciferase data. We conclude that the increase in the cellular levels of SSB/La in infected cells is at least one factor contributing to increased utilization of the BiP IRES during infection.
DISCUSSION
We have previously shown that the expression of the ER chaperone BiP is closely regulated during HCMV infection (5). This results in a temporally precise increase in BiP levels beginning at 24 hpi, peaking by 60 to 72 hpi, and decreasing gradually thereafter (5). At the peak of accumulation, the level of BiP is dramatically increased, at least 50-fold, compared to uninfected cells and is comparable to cells exposed to ER stress inducing chemicals (5, 13). Our data suggest that this increase results from a combination of (i) HCMV-induced activation of BiP gene transcription, resulting in a modest (3- to 4-fold) increase in mRNA; and (ii) enhanced translation of the BiP mRNA through increased utilization of the BiP IRES. It has previously been shown that BiP can be controlled by both transcriptional and posttranscriptional mechanisms (16).
The increase in BiP mRNA occurs as early as 4 to 8 hpi and peaks from 12 to 60 hpi. This early activation of the BiP promoter implicates either the involvement of proteins that enter with virions or the synthesis of immediate-early proteins. Our data show that incoming virion-associated proteins do not affect the promoter. However, the production of the HCMV MIE proteins (IEP72 and IEP86) activates the BiP promoter. Furthermore, this MIE protein-mediated mechanism does not require the ERSEs, promoter elements that are normally required for stress-mediated induction of the BiP promoter. Thus, HCMV either utilizes a novel means to transcriptionally activate the BiP promoter; or it significantly increases the basal promoter activity that normally maintains nonstressed levels of BiP.
Although the increase in BiP mRNA is sustained throughout infection, the 3- to 4-fold activation that we have detected is minimal compared to the increase that occurs in response to ER stress. Treatment with thapsigargin resulted in a greater than 100-fold increase in BiP mRNA compared to unstressed cells. This result suggested that HCMV may utilize additional mechanisms to obtain the high levels of BiP protein that have been observed in infected cells (5). BiP protein levels can be increased by altering the stability of the BiP protein or by increasing the translation efficiency of BiP mRNA (9, 15, 28). The translational efficiency of BiP mRNA can be regulated by the presence of an IRES, located in the 5′ UTR (19).
A recent study has shown that a related herpesvirus, herpes simplex virus type 1, activates the BiP IRES during infection (23). Similarly, our studies show that HCMV also activates translation from the BiP IRES. Activation occurs between 8 and 24 hpi, the same time during infection when increased BiP is first detected. Several cellular proteins have been implicated in the translational activity of the BiP IRES; these include NS1-associated protein 1 (NSAP1), SSB/La autoantigen, p50, and p95 (6, 14, 31). SSB/La is of particular interest because it is involved in translational activation from IRESs during both poliovirus and hepatitis C virus infections (1, 21). Analysis of SSB/La protein during HCMV infection revealed that SSB/La is induced early in infection, peaking at 24 hpi, corresponding to the time of activation of the BiP IRES in a reporter plasmid and the beginning of endogenous BiP accumulation in infected cells (5). Accordingly, depletion of SSB/La by using siRNA reduced BiP IRES activity at 24 hpi, confirming a role for SSB/La in activating the BiP IRES during HCMV infection.
Thus, our data show that HCMV utilizes multiple mechanisms to induce the elevated levels of BiP which are needed during infection. These include transcriptional activation of the BiP promoter by the MIE proteins by an ERSE-independent mechanism, as well as induction of the translational utilization of the BiP mRNA IRES by a mechanism that involves SSB/La.
Acknowledgments
We thank all of the members of the Alwine lab for advice, criticism, and help with the performance of these experiments. We thank Sherri Adams for critical analysis of the data and manuscript.
This work was supported by NIH grant R01CA28379-29.
Footnotes
Published ahead of print on 25 August 2010.
REFERENCES
- 1.Ali, N., and A. Siddiqui. 1997. The La antigen binds 5′ noncoding region of the hepatitis C virus RNA in the context of the initiator AUG codon and stimulates internal ribosome entry site-mediated translation. Proc. Natl. Acad. Sci. U. S. A. 94:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertolotti, A., Y. Zhang, L. M. Hendershot, H. P. Harding, and D. Ron. 2000. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2:326-332. [DOI] [PubMed] [Google Scholar]
- 3.Bresnahan, W. A., G. E. Hultman, and T. Shenk. 2000. Replication of wild type and mutant human cytomegalovirus in life-extended human diploid fibroblasts. J. Virol. 74:10816-10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchkovich, N. J., T. G. Maguire, A. W. Paton, J. C. Paton, and J. C. Alwine. 2009. The endoplasmid reticulum chaperone BiP/GRP78 is important in the structure and function of the human cytomegalovirus assembly compartment. J. Virol. 83:11421-11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchkovich, N. J., T. G. Maguire, A. W. Paton, J. C. Paton, and J. C. Alwine. 2008. Human cytomegalovirus specifically controls the levels of the endoplasmic reticulum chaperone BiP/GRP78 which is required for virion assembly. J. Virol. 82:31-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho, S., S. M. Park, T. D. Kim, J. H. Kim, K. T. Kim, and S. K. Jang. 2007. BiP internal ribosomal entry site activity is controlled by heat-induced interaction of NSAP1. Mol. Cell. Biol. 27:368-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Depto, A. S., and R. M. Stenberg. 1989. Regulated expression of the human cytomegalovirus pp65 gene: octamer sequence in the promoter is required for activation by viral gene products. J. Virol. 63:1232-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghazal, P., J. Young, E. Giulietti, C. DeMattei, J. Garcia, R. Gaynor, R. M. Stenberg, and J. A. Nelson. 1991. A discrete cis element in the human immunodeficiency virus long terminal repeat mediates synergistic trans activation by cytomegalovirus immediate-early proteins. J. Virol. 65:6735-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulow, K., D. Bienert, and I. G. Haas. 2002. BiP is feed-back regulated by control of protein translation efficiency. J. Cell Sci. 115:2443-2452. [DOI] [PubMed] [Google Scholar]
- 10.Harel, N. Y., and J. C. Alwine. 1998. Phosphorylation of the human cytomegalovirus 86-kilodalton immediate early protein IE2. J. Virol. 72:5481-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hegde, N. R., M. S. Chevalier, T. W. Wisner, M. C. Denton, K. Shire, L. Frappier, and D. C. Johnson. 2006. The role of BiP in endoplasmic reticulum-associated degradation of major histocompatibility complex class I heavy chain induced by cytomegalovirus proteins. J. Biol. Chem. 281:20910-20919. [DOI] [PubMed] [Google Scholar]
- 12.Hwang, E. S., Z. Zhang, H. Cai, D. Y. Huang, S. M. Huong, C. Y. Cha, and E. S. Huang. 2009. Human cytomegalovirus IE1-72 protein interacts with p53 and inhibits p53-dependent transactivation by a mechanism different from that of IE2-86 protein. J. Virol. 83:12388-12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isler, J. A., A. H. Skalet, and J. C. Alwine. 2005. Human cytomegalovirus infection activates and regulates the unfolded protein response. J. Virol. 79:6890-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, Y. K., S. H. Back, J. Rho, S. H. Lee, and S. K. Jang. 2001. La autoantigen enhances translation of BiP mRNA. Nucleic Acids Res. 29:5009-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leborgne-Castel, N., E. P. Jelitto-Van Dooren, A. J. Crofts, and J. Denecke. 1999. Overexpression of BiP in tobacco alleviates endoplasmic reticulum stress. Plant Cell 11:459-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, E. S., J. H. Ou, and A. S. Lee. 1992. Brefeldin A as a regulator of grp78 gene expression in mammalian cells. J. Biol. Chem. 267:7128-7133. [PubMed] [Google Scholar]
- 17.Lukac, D. M., J. R. Manuppello, and J. C. Alwine. 1994. Transcriptional activation by the human cytomegalovirus immediate early proteins: requirements for simple promoter structures and interactions with multiple components of the transcription complex. J. Virol. 68:5184-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo, S., P. Baumeister, S. Yang, S. F. Abcouwer, and A. S. Lee. 2003. Induction of Grp78/BiP by translational block. J. Biol. Chem. 278:37375-37385. [DOI] [PubMed] [Google Scholar]
- 19.Macejak, D. G., and P. Sarnow. 1991. Internal initiation of translation mediated by the 5′ leader of a cellular mRNA. Nature 353:90-94. [DOI] [PubMed] [Google Scholar]
- 20.Malhotra, J. D., and R. J. Kaufman. 2007. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 18:716-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meerovitch, K., Y. V. Svitkin, H. S. Lee, F. Lejbkowicz, D. J. Kenan, E. K. Chan, V. I. Agol, J. D. Keene, and N. Sonenberg. 1993. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J. Virol. 67:3798-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pizzorno, M. C., P. O'Hare, L. Sha, R. L. LaFemina, and G. S. Hayward. 1988. Transactivation and autoregulation of gene expression by the immediate-early region 2 gene products of human cytomegalovirus. J. Virol. 62:1167-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saffran, H. A., G. S. Read, and J. R. Smiley. 2010. Evidence for translational regulation by the herpes simplex virus virion host shutoff protein. J. Virol. 84:6041-6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroder, M. 2008. Endoplasmic reticulum stress responses. Cell Mol. Life Sci. 65:862-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen, J., X. Chen, L. M. Hendershot, and R. Prywes. 2002. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 3:99-111. [DOI] [PubMed] [Google Scholar]
- 26.Stenberg, R. M., J. Fortney, S. W. Barlow, B. P. Magrane, J. A. Nelson, and P. Ghazal. 1990. Promoter-specific trans activation and repression by human cytomegalovirus immediate-early proteins involves common and unique protein domains. J. Virol. 64:1556-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tirosh, B., N. N. Iwakoshi, B. N. Lilley, A. Lee, L. H. Glimcher, and H. L. Ploegh. 2005. Human cytomegalovirus protein US11 provokes an unfolded protein response that may facilitate the degradation of class1 major histocompatibility complex products. J. Virol. 79:2768-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulatowski, L. M., M. Lam, G. Vanderburg, M. R. Stallcup, and C. W. Distelhorst. 1993. Relationship between defective mouse mammary tumor virus envelope glycoprotein synthesis and GRP78 synthesis in glucocorticoid-treated mouse lymphoma cells. Evidence for translational control of GRP78 synthesis. J. Biol. Chem. 268:7482-7488. [PubMed] [Google Scholar]
- 29.Venkatesan, A., R. Sharma, and A. Dasgupta. 2003. Cell cycle regulation of hepatitis C and encephalomyocarditis virus internal ribosome entry site-mediated translation in human embryonic kidney 293 cells. Virus Res. 94:85-95. [DOI] [PubMed] [Google Scholar]
- 30.Xuan, B., Z. Qian, E. Torigoi, and D. Yu. 2009. Human cytomegalovirus protein pUL38 induces ATF4 expression, inhibits persistent JNK phosphorylation, and suppresses endoplasmic reticulum stress-induced cell death. J. Virol. 83:3463-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang, Q., and P. Sarnow. 1997. Location of the internal ribosome entry site in the 5′ non-coding region of the immunoglobulin heavy-chain binding protein (BiP) mRNA: evidence for specific RNA-protein interactions. Nucleic Acids Res. 25:2800-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida, H., K. Haze, H. Yanagi, T. Yura, and K. Mori. 1998. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. J. Biol. Chem. 273:33741-33749. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida, H., T. Okada, K. Haze, H. Yanagi, T. Yura, M. Negishi, and K. Mori. 2001. Endoplasmic reticulum stress-induced formation of transcription factor complex ERSF including NF-Y (CBF) and activating transcription factors 6 and 6 that activates the mammalian unfolded protein response. Mol. Cell. Biol. 21:1239-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]