Abstract
Innate recognition of viruses is mediated by pattern recognition receptors (PRRs) triggering expression of antiviral interferons (IFNs) and proinflammatory cytokines. In mice, Toll-like receptor 2 (TLR2) and TLR9 as well as intracellular nucleotide-sensing pathways have been shown to recognize herpes simplex virus (HSV). Here, we describe how human primary macrophages recognize early HSV infection via intracellular pathways. A number of inflammatory cytokines, IFNs, and IFN-stimulated genes were upregulated after HSV infection. We show that early recognition of HSV and induction of IFNs and inflammatory cytokines are independent of TLR2 and TLR9, since inhibition of TLR2 using TLR2 neutralizing antibodies did not affect virus-induced responses and the macrophages were unresponsive to TLR9 stimulation. Instead, HSV recognition involves intracellular recognition systems, since induction of tumor necrosis factor alpha (TNF-α) and IFNs was dependent on virus entry and replication. Importantly, expression of IFNs was strongly inhibited by small interfering RNA (siRNA) knockdown of MAVS, but this MAVS-dependent IFN induction occurred independently of the recently discovered polymerase III (Pol III)/RIG-I DNA sensing system. In contrast, induction of TNF-α was largely independent of MAVS, suggesting that induction of inflammatory cytokines during HSV infection proceeds via a novel pathway. Transfection with ODN2006, a broad inhibitor of intracellular nucleotide recognition, revealed that nucleotide-sensing systems are employed to induce both IFNs and TNF-α. Finally, using siRNA knockdown, we found that MDA5, but not RIG-I, was the primary mediator of HSV recognition. Thus, innate recognition of HSV by human primary macrophages occurs via two distinct intracellular nucleotide-sensing pathways responsible for induction of IFNs and inflammatory cytokine expression, respectively.
Virus recognition is essential for activation of innate antiviral immune defense and the subsequent induction of acquired immunity. Conserved pathogen motifs, termed pathogen-associated molecular patterns (PAMPs), are recognized by pattern recognition receptors (PRRs). Virus-recognizing PRRs include Toll-like receptors (TLRs), RIG-I-like receptors (RLRs), and a number of intracellular DNA receptors. Several TLRs have been attributed roles in the recognition of virus. TLR2 and TLR4 recognize viral surface structures (3, 6, 18, 31), TLR3 recognizes double-stranded RNA (dsRNA) (2), and TLR7/8 and TLR9 function as signaling receptors for viral single-stranded RNA (ssRNA) (8, 11, 21) and CpG DNA (12, 20), respectively.
Within the cell, cytoplasmic RLRs RIG-I and MDA5 both recognize accumulation of virus-derived dsRNA; in addition, RIG-I recognizes 5′-triphosphated RNA (14, 27, 39, 40). In addition to the RLRs, a number of receptors recognize foreign DNA. Presently, three DNA receptors have been identified: Z-DNA binding protein 1 (ZBP-1, or DAI) (36) and RNA polymerase III (Pol III) (1, 4) both mediate interferon (IFN) and cytokine production, whereas the AIM2 inflammasome is involved in caspase 1 activation in response to cytoplasmic dsDNA (13).
Herpes simplex virus type 1 (HSV-1) and HSV-2 are two closely related human DNA viruses associated with a number of serious diseases, including orofacial infections, encephalitis, and genital infections (34). Macrophages play an important role in the first line of defense against viral infection via production of IFNs, cytokines, and chemokines that regulate the progress of the virus infection and activate and support appropriate defense mechanisms (9, 10, 24).
TLR2, TLR3, and TLR9 have been identified as mediators of proinflammatory cytokine production during HSV infections. TLR2 mediates an overzealous inflammatory cytokine response following HSV-1 infection in mice, promoting mononuclear cell infiltration of the brain and development of encephalitis (18). TLR3 mediates type I and III IFN production in human fibroblasts (41). TLR9 recognizes genomic DNA from HSV-1 and HSV-2 in murine plasmacytoid dendritic cells (DCs) (17, 20) and mediates tumor necrosis factor alpha (TNF-α) and CCL5 production in murine macrophages (22). Both TLR2 and TLR9 mediate recognition of HSV and cytokine production in murine conventional DCs (35). HSV is recognized by an RLR/MAVS-dependent mechanism in murine macrophages and mouse embryonic fibroblasts (MEFs) (5, 29, 30). Recent data suggest that RNA Pol III mediates IFN production following HSV-1 infection and transfection with HSV-1 DNA in macrophage-like RAW 264.7 cells (4). Finally, murine L929 fibroblast-like cells are moderately inhibited in their ability to produce IFN after HSV-1 infection when ZBP-1 is knocked down (19, 36). Thus, several PRRs have been reported to recognize HSV-1 in murine cells and different cell lines, but the pathways responsible for sensing this virus in human primary macrophages and their impact on cytokine expression have not previously been described.
In this work, we investigate the recognition pathways underlying HSV-induced cytokine and chemokine expression in human primary macrophages. We demonstrate that HSV-1-induced IFN and cytokine expression is independent of TLR2 and TLR9 but highly dependent on virus replication and intracellular nucleotide recognition systems. Specifically, induction of IFNs is dependent on MAVS and MDA5, whereas TNF-α is induced by a novel intracellular nucleotide-sensing system.
MATERIALS AND METHODS
Monocyte-derived macrophages.
Leukocyte-rich buffy coats obtained from healthy blood donors were supplied by the Finnish Red Cross blood transfusion service (Helsinki, Finland) or from the Skejby Hospital blood bank. Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation on a Ficoll-Hypaque gradient (Pharmacia Biotech), and mononuclear cells were collected. For generation of macrophages, mononuclear cells were allowed to adhere onto plastic 6-well plates, 12-well plates, or 24-well plates (Falcon Multiwell, BD Biosciences, or Nunc) for 1 h at 37°C in RPMI 1640 medium supplemented with penicillin (0.6 μg/ml) and streptomycin (60 μg/ml) (Invitrogen). For confocal microscopy, cells were allowed to adhere to glass coverslips in 24-well plates. After monocyte adherence, nonadherent cells were removed, and the wells were washed three times with phosphate-buffered saline (PBS), pH 7.4. Adherent cells were then grown for 7 to 8 days in macrophage serum-free medium (SFM) (Invitrogen) supplemented with antibiotics and 10 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) (Nordic Biosite or Invitrogen). Medium was changed every 2 days.
Virus preparations.
The viruses used in this study were the 17+ and KOS strains of HSV-1, an HSV-1 virus deficient in glycoprotein L (gL86), and the MS strain of HSV-2. The viruses were produced essentially as previously described (30). Prior to use, the virus was thawed and used as infectious virus or inactivated by exposure to UV light for 5 min (30).
Stimulation experiments.
The cells were treated with virus at a titer of 5 × 105 PFU/ml (multiplicity of infection [MOI] of 1) or 2.5 × 105 PFU/ml (MOI of 0.5). The TLR2 ligand Pam3CysSerLysLysLysLys (Pam3CSK4; EMC Microcollections) was used at a concentration of 100 ng/ml. Extracellular poly(I:C) (Sigma-Aldrich) was used at a concentration of 30 μg/ml. The TLR7/8 ligand R848 (InVivoGen, San Diego, CA) was used at a final concentration of 1 μg/ml, and the CpG TLR9 ligand ODN2216 (InVivoGen) was used at 2 μg/ml. For transfections, the dsDNA analog poly(dA:dT) (Sigma-Aldrich) was used at 2 μg/ml. Anti-human TLR2 neutralizing antibodies (ImmunoKontact and Imgenex) were added at a final concentration of 8.5 μg/ml 30 min prior to virus stimulation. The RNA polymerase III inhibitor ML60218 (Calbiochem) was added at a concentration of 10 μM 16 h before infection or transfection. Forty-five minutes before further treatments, ODN2006 (InVivoGen) transfections were achieved using Lipofectamine 2000 (Invitrogen) as described by the manufacturer, with 3 μg ODN2006 per ml. Poly(I:C) and poly(dA:dT) transfections (2 μg/ml) were achieved using Lipofectamine 2000 at 2 μg/ml, as recommended by the manufacturer. Harvested supernatants and RNA were stored at −80°C before analysis by enzyme-linked immunosorbent assay (ELISA) or reverse transcription-PCR (RT-PCR).
siRNA assays.
After 5 days of cell culture in 12-well plates, macrophages were transfected with 50 nM nontargeting control small interfering RNA (siRNA) (AllStars negative-control siRNA; Qiagen) and with 25 nM MAVS siRNA (Hs_ VISA_1 and Hs_VISA2; Qiagen), RIG-I siRNA (Hs_DDX58_6 and Hs_ DDX58_10; Qiagen), or MDA5 siRNA (Hs-IFIH1_7 and HsIFIH1_8; Qiagen) by using HiPerFect transfection reagent (Qiagen) according to the manufacturer's instruction. The transfection was repeated the following day, and after 24 h, macrophages were left unstimulated or infected with HSV-1 for 6 h before total cellular RNA was harvested.
RNA isolation and RT.
Cells were washed once with PBS and lysed, and total cellular RNA was recovered using an RNA purification kit (Macherey-Nagel NucleoSpin RNA II or Qiagen Midi kit) as described by the manufacturer. Purified RNA was stored at −80°C until analysis. cDNA synthesis was performed with 1 to 2 μg RNA by using a Moloney murine leukemia virus (MMLV) reverse transcriptase kit (Invitrogen) according to the manufacturer's instructions with an oligo(dT)18 primer (DNA Technology, Aarhus, Denmark) or as described previously (33).
Quantitative real-time PCR.
The cDNA obtained from cells was quantified by real-time PCR as previously described (23), using the following primers (DNA Technology): for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), CGACCACTTTGTCAAGCTCA (forward) and GGTGGTCCAGGGGTCTTACT (reverse); for IFN-β, TGGGAGGATTCTGCATTACC (forward) and AAGCAATTGTCCAGTCCCAG (reverse); and for TNF-α, TGCTTGTTCCTCAGCCTCTT (forward) and AGATGATCTGACTGCCTGGG (reverse). TaqMan PCR analysis was done using primers and probes from Applied Biosystems for IFN-β, IFN-λ1, CXCL10, TNF-α, MAVS, ICP27, RIG-I, and MDA5, as previously described (33).
Cytokine- and chemokine-specific ELISAs.
Cytokine and chemokine levels from cell culture supernatants were analyzed by sandwich ELISA methods essentially as described by the manufacturer. CCL2 (MCP-1) was determined using antibody pairs and standards obtained from BD Pharmingen. CCL3 (MIP-1α) was determined using Duoset antibody pairs and standards from R&D Systems. TNF-α and CXCL10 were determined using antibody pairs and standards obtained from BD Pharmingen or Cytoset ELISAs from Invitrogen.
Luminex assay.
Total cell extracts were measured by a Luminex assay for detection of intracellular phosphorylated proteins from the p38 mitogen-activated protein kinase (MAPK) and Jun N-terminal protein kinase (JNK) signaling pathways. Experiments were performed as specified by the manufacturer (Biosource).
Confocal microscopy.
Cells were stained with rabbit polyclonal anti-human interferon regulatory factor 3 (IRF-3) antibodies (Santa Cruz) according to the manufacturer's instructions. Briefly, cells were fixed with methanol at −20°C for 5 min and stained with primary antibodies at a 1:50 dilution. DAPI (4′,6-diamidino-2-phenylindole) was used to stain the nucleus. Cells were imaged on a Zeiss LSM710 laser scanning microscope. The percentage of cells demonstrating nuclear localization of IRF-3 was quantified for 3 independent donors, and at least 100 cells were scored per condition.
Statistical analyses.
Statistical analyses were performed using a paired Student t test.
RESULTS
Induction of IFNs, IFN-stimulated genes (ISGs), and proinflammatory cytokines following HSV infection and stimulation via virus-associated PRRs.
In order to evaluate how human primary macrophages respond to viral PAMPs and to identify PRRs recognizing viruses, we stimulated cells with agonists for TLRs, RLRs, and intracellular DNA receptors. Human macrophages were infected with HSV-1 or -2 or transfected with the synthetic dsRNA analogue poly(I:C) or the dsDNA analogue poly(dA:dT). RNA and culture supernatants were harvested and assayed using real-time RT-PCR and ELISA, respectively.
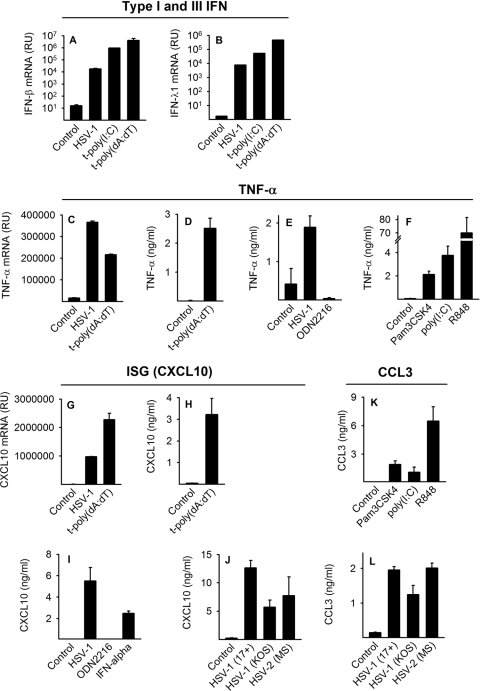
At early time points, HSV and intracellular RNA and DNA induced type I and type III IFNs, TNF-α, and CXCL10 (Fig. 1A to D, G, and H). In addition, proinflammatory cytokines CCL2 and CCL3 were induced by HSV-1 (Fig. 1L and not shown). We found that the induced cytokine response was not strain or type specific, since both HSV-1 strains 17+ and KOS and HSV-2 strain MS were capable of inducing cytokines (Fig. 1J and L).
FIG. 1.
HSV and PAMPs induce IFN, ISGs, and proinflammatory cytokines in human primary macrophages. (A to C and G) Macrophages were infected with HSV-1 (17+, 5 × 105 PFU/ml, MOI of 1) or transfected with poly(I:C) [t-poly(I:C); 2 μg/ml] or poly(dA:dT) [t-poly(dA:dT); 2 μg/ml]. (A to C and G) After 6 h, after which RNA was harvested and mRNA accumulation of IFN-β, IFN-λ1, CXCL10, and TNF-α was assayed by real-time PCR. (D, E, and H) In similar experiments, cell supernatants were harvested after 20 h and assayed for the presence of TNF-α, CXCL10, and CCL3 by ELISA. (E, F, I, and K) Macrophages were infected with HSV or stimulated with IFN-α (100 IU/ml) or the agonist for TLR2 (Pam3CSK4, 100 ng/ml), TLR3 [poly(I:C), 30 μg/ml], TLR7/8 (R848, 1 μg/ml), or TLR9 (ODN2216, 2 μg/ml). After 20 h of stimulation, supernatants were collected and protein levels assayed by ELISA. (J and L) Cells were infected with HSV-1 (17+ or KOS, 5 × 105 PFU/ml, MOI of 1) or HSV-2 (MOI of 1). Supernatants were harvested at 18 h and assayed by ELISA for CXCL10 and CCL3. The real-time PCR data are depicted as relative units (RU), which is a fold change in gene expression normalized to that of the endogenous reference gene 18S rRNA and is relative to the no template control (NTC) calibrator. The results depicted are means ± standard deviations (SD) within the shown experiment. Experiments were done five times (A and B) or two times (C to L), with similar findings.
To evaluate the potential role of TLR pathways in virus recognition, we stimulated cells with Pam3CSK4 (TLR2), poly(I:C) (TLR3), R848 (TLR7/8), and ODN2216 (TLR9). Macrophages responded to TLR2, TLR3, and TLR7/8 agonists (Fig. 1F and K), indicating functional TLR2, TLR3, and TLR7/8 pathways. In contrast, TLR9 stimulation with synthetic CpG DNA (ODN2216) did not induce cytokine production (Fig. 1E and I). Northern blot analysis demonstrated no TLR9 expression in human primary macrophages following IFN-α stimulation or HSV-1 infection (data not shown). This is consistent with previous reports showing that TLR9 is not expressed in human monocyte-derived macrophages (25) but rather only in human plasmacytoid DCs and B cells (15).
In conclusion, human macrophages generate IFN, ISGs, and proinflammatory cytokines following HSV infection and stimulation of known virus-recognizing PRRs TLR2, TLR3, and TLR7/8. Furthermore, intracellular RLR and DNA recognition receptor systems are functional in human primary macrophages.
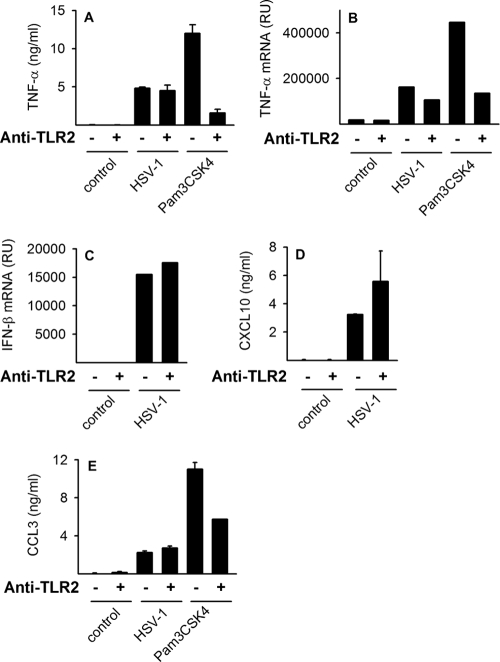
TLR2-independent cytokine expression in response to HSV-1 infection.
Since TLR2 is a functional signaling receptor in human macrophages (Fig. 1F and K) and TLR2-mediated recognition of HSV-1 has been reported in mice (18), TLR2 may play a role in HSV-1-induced cytokine expression. To investigate this, cells were infected with HSV-1 or stimulated with the TLR2 ligand Pam3CSK4, in the presence or absence of anti-TLR2 neutralizing antibodies, for 6 h. TLR2 neutralization markedly inhibited Pam3CSK4-induced accumulation of TNF-α mRNA and protein (Fig. 2A and B) and CCL3 protein (Fig. 2E). However, TLR2 neutralization did not affect HSV-1-induced accumulation of TNF-α mRNA or TNF-α protein in cell culture supernatants (Fig. 2A and B), nor did inhibition of TLR2 affect IFN, CXCL10, or CCL3 induction after HSV infection (Fig. 2C to E). Collectively, these data strongly suggest that TLR2 is dispensable for the HSV-1-elicited cytokine response in human primary macrophages.
FIG. 2.
HSV-1 infection activates cytokine expression independently of TLR2. Cells were infected with HSV-1 (17+, MOI of 1) or stimulated with TLR2 ligand Pam3CSK4 (100 ng/ml) in the presence or absence of TLR2 neutralizing antibodies (8.5 μg/ml). After 6 h, RNA was harvested, and after 18 h, supernatants were collected. Accumulation of TNF-α and IFN-β mRNA was assessed by real-time PCR (B and C), and the amounts of secreted TNF-α, CXCL10, and CCL3 were measured by ELISA (A, D, and E). RU, relative units. The results depicted are means ± SD from one of two experiments showing similar results.
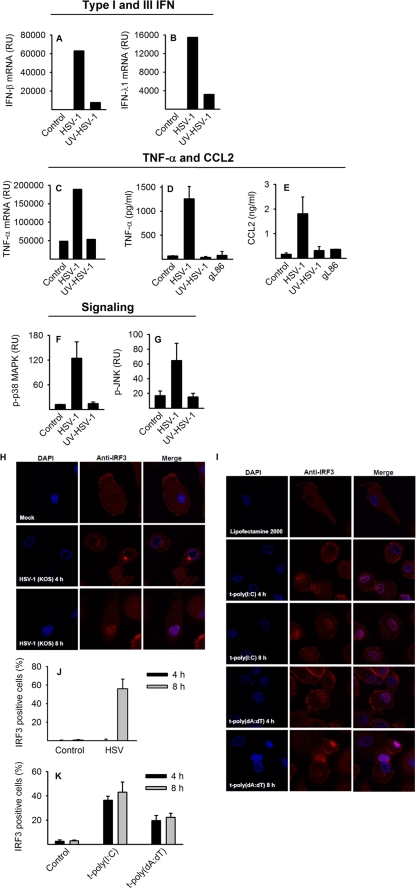
Virus requirements for activation of intracellular signaling and induced IFN, ISG, and cytokine expression.
To further characterize virus-induced cytokine expression, we treated macrophages with infectious HSV-1, UV-inactivated virus, or a virus mutant lacking functional glycoprotein L (gL86), which is essential for virus entry (26, 32). As seen in Fig. 3A to E, virus entry and replication were required for the production of IFNs and inflammatory cytokines. Similarly, activation of MAPK pathways was dependent on replicating virus (Fig. 3F and G). IRF-3, which acts upstream of IFN and ISG expression, translocated to the nucleus following HSV infection (Fig. 3H and I) but with delayed kinetics compared to intracellular delivery of synthetic dsRNA or dsDNA (Fig. 3J and K). In conclusion, HSV-induced innate signaling is delayed compared to direct PRR stimulation, and HSV-induced IFN and cytokine responses rely on cellular entry and virus replication, suggesting that virus replication-derived products are recognized by PRRs.
FIG. 3.
Virus-induced signaling and virus requirements for IFN and cytokine production. Cells were infected with live HSV-1 (KOS, MOI of 1) or an equivalent amount of UV-inactivated HSV-1 or glycoprotein L-deficient virus (gL86). RNA was harvested after 6 h, and IFN-β, IFN-λ1, and TNF-α mRNA accumulation was measured using real-time PCR (A to C). Supernatants were harvested after 20 h, and TNF-α and CCL2 levels were measured using ELISA (D and E). Whole-cell extracts were harvested at 9 h after infection, and levels of phosphorylated p38 MAPK (p-p38 MAPK) and JNK were measured by a Luminex assay (F and G). Cells seeded on coverslips were transfected with poly(I:C) (2 μg/ml) or poly(dA:dT) (2 μg/ml) or infected with HSV-1 (KOS, MOI of 1). After 4 and 8 h, slides were fixed and stained for intracellular IRF-3 or nuclear stained with DAPI and visualized using confocal microscopy (H and I). Percentage of cells demonstrating nuclear localization of IRF3 after HSV-1 infection (J) and intracellular poly(I:C) or poly(dA:dT) (K). For panels A to G, similar results were obtained in two independent experiments, and results are depicted as means ± SD. For panels H and I, confocal images represent one of three donors from one of two experiments showing similar results. For panels J and K, results are shown as means ± SD from the three donors from one experiment.
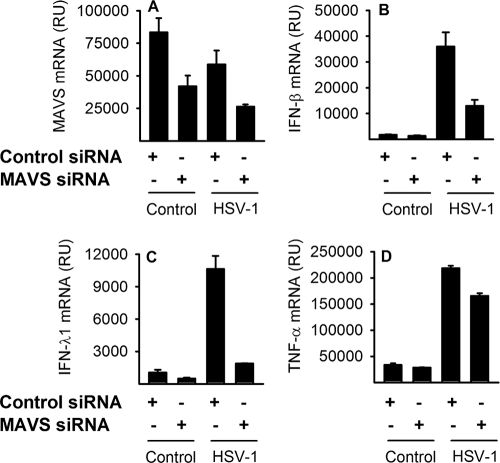
MAVS-dependent and -independent IFN and cytokine expression.
Mouse studies show that HSV-induced IFN is highly dependent on MAVS (30). To evaluate which recognition system is responsible for the early IFN and TNF-α production in human macrophages, we infected macrophages transfected with MAVS siRNA or control siRNAs and harvested RNA for real-time PCR analysis. We found that knockdown of MAVS resulted in strongly reduced levels of IFN-β and IFN-λ1 (Fig. 4B and C). Looking at TNF-α expression, we found a striking difference from type I and III IFN expression, since MAVS siRNA reduced TNF-α mRNA accumulation only slightly (Fig. 4D). Thus, MAVS is involved in type I and III IFN production after HSV infection, whereas the main recognition system triggering TNF-α production in human macrophages is independent of MAVS.
FIG. 4.
MAVS-dependent and MAVS-independent IFN and TNF-α responses. Macrophages transfected either with nontargeting control siRNA (50 nM) or with 25 nM each of two different siRNAs targeting MAVS were infected with HSV-1 (KOS, MOI of 1). RNA was harvested after 6 h and assayed for MAVS, IFN-β, IFN-λ1, and TNF-α mRNA accumulation by real-time PCR. RU, relative units. The results represent means ± SD from one of two experiments with similar results.
RNA polymerase III-independent IFN and TNF-α production after HSV infection.
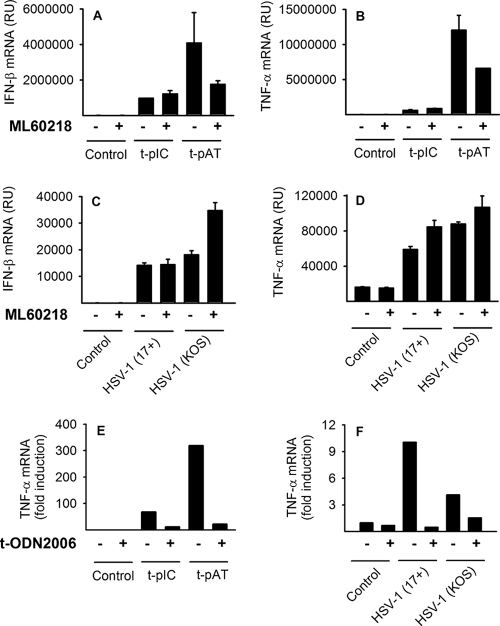
A recent paper suggests that RNA Pol III recognizes HSV-1 infection and HSV-1 DNA in murine macrophage-like RAW 264.7 cells (4). To investigate whether RNA Pol III recognizes HSV-1 infection in human macrophages, we infected cells with HSV-1 or transfected cells with poly(dA:dT) or poly(I:C) in the presence or absence of the RNA Pol III inhibitor ML60218. Pretreatment with ML60218 had no effect on the response of macrophages to poly(I:C); however, pretreatment with ML60218 reduced levels of IFN-β and TNF-α induced following poly(dA:dT) stimulation (Fig. 5A and B). In contrast, HSV-induced TNF-α and IFN-β mRNA levels were not affected by the inhibitor (Fig. 5C and D). Consistent with these results, HSV-1-induced secretion of CXCL10 and CCL3 was also not affected by the inhibitor (data not shown). In summary, the data suggest that the RNA Pol III pathway is capable of recognizing intracellular dsDNA in the form of poly(dA:dT). However, the RNA Pol III pathway does not seem to mediate early innate recognition of HSV-1.
FIG. 5.
RNA Pol III-independent IFN and cytokine production after HSV infection. (A to D) Macrophages were treated with the RNA Pol III inhibitor ML60218 (10 μM) for 16 h before transfection with poly(I:C) (2 μg/ml) or poly(dA:dT) (2 μg/ml) or infection with HSV-1 (KOS, MOI of 0.5, or 17+, MOI of 1). (E and F) Macrophages were transfected with ODN2006 (3 μg/ml) or treated with Lipofectamine 2000 as a control. After 45 min, the cells were transfected with poly(dA:dT) or poly(I:C) (1.5 μg/ml) or infected with HSV-1 (KOS or 17+, MOI of 1). After 6 h, RNA was harvested and analyzed using real-time PCR. RU, relative units. The results represent means ± SD from two experiments (A to D) or three experiments (E and F) showing similar results.
The TLR9 agonist phosphorothioate oligonucleotide ODN2006 (type B ODN) has been shown to antagonize RIG-I signaling (28) and was recently demonstrated to broadly antagonize intracellular nucleotide signaling, at least partly by binding HMGB protein, which is essential for the nucleic acid-mediated innate immune response (38). Interestingly, transfection of macrophages with ODN2006 strongly reduced levels of TNF-α after poly(dA:dT) and poly(I:C) transfection (Fig. 5E) and reduced TNF-α production after HSV infection (Fig. 5F). Overall, these data suggest that early TNF-α production following HSV infection proceeds via intracellular recognition of nucleotides.
MDA5-mediated recognition of HSV infection.
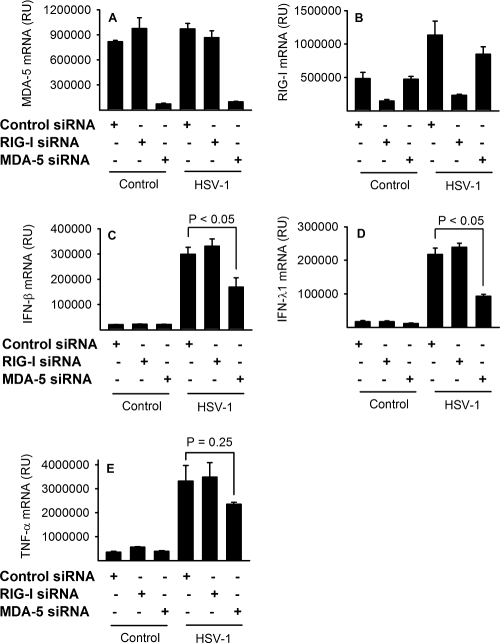
To address whether RIG-I or MDA5 is responsible for the MAVS-dependent IFN and TNF-α production in human macrophages, we infected macrophages transfected with RIG-I or MDA5 siRNA or control siRNAs (Fig. 6A and B). We found that knockdown of MDA5 led to a clear reduction in the levels of virus-induced IFN-β and IFN-λ1 (Fig. 6C and D) but had little effect on TNF-α mRNA accumulation (Fig. 6E). In contrast, knockdown of RIG-I did not affect early HSV-induced IFN or TNF-α (Fig. 6C to E). In conclusion, MAVS-mediated HSV recognition proceeds via MDA5 in human primary macrophages.
FIG. 6.
MDA5-dependent and RIG-I-independent HSV recognition. Macrophages transfected either with nontargeting control siRNA (50 nM) or with 25 nM each of two different siRNAs targeting RIG-I or MDA5 were infected with HSV-1 (KOS, MOI of 1). RNA was harvested after 6 h and assayed for MAVS, IFN-β, IFN-λ1, and TNF-α mRNA accumulation by real-time PCR. RU, relative units. The results represent means ± SD from one of two experiments with similar results.
Virus gene expression coincides with early cytokine production.
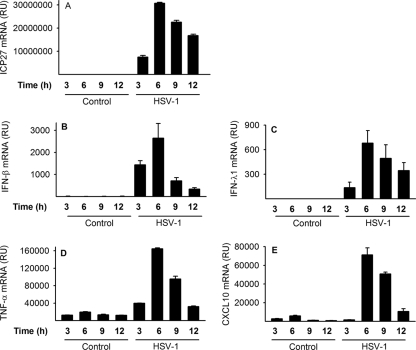
To evaluate virus replication in conjunction with cytokine production, we infected macrophages at different time points and evaluated virus gene expression and cytokine production. We found that immediate early ICP27 was detectable 3 h after infection and peaked at 6 h after infection (Fig. 7A). Virus ICP27 immediate early gene expression coincided with expression of IFN, TNF-α, and CXCL10 (Fig. 7B to E). All cytokines peaked at 6 h after infection. The data suggest that early virus gene expression coincides with the early innate response in human macrophages.
FIG. 7.
Virus gene expression precedes and coincides with the early innate cytokine response. Macrophages were infected with HSV-1 (KOS, MOI of 1), and RNA was harvested at the indicated time points. Accumulation of viral ICP27 mRNA and cellular IFN-β, IFN-λ1, and TNF-α mRNA was investigated using real-time PCR. RU, relative units. The results represent means ± SD from two experiments showing similar results.
DISCUSSION
In the present work, we have investigated the recognition mechanisms underlying HSV-induced cytokine and chemokine expression in human primary macrophages. Two TLRs have been identified as the main mediators of proinflammatory cytokine production following HSV infection. TLR2 stimulates production of proinflammatory cytokines following infection with HSV-1 in mice (18), and TLR9 recognizes genomic DNA from HSV-1 and HSV-2 in murine plasmacytoid DCs (17, 20) and mediates cytokine production in murine macrophages and conventional DCs (22, 35). We found no induction of the investigated cytokines following TLR9 stimulation of human primary macrophages, and no TLR9 expression was seen after IFN-α or HSV treatment (data not shown). Our results are consistent with previous studies demonstrating that only human plasmacytoid DCs and B cells are TLR9 responders (15) and that there is no significant TLR9 expression in human primary macrophages (25). Because TLR9 was excluded as a mediator of HSV-1-induced cytokine expression, we examined the cytokine response generated from other extracellular and intracellular PRRs known to recognize virus. We found that macrophages responded via TLR2, TLR3, and TLR7/8 in addition to intracellular dsRNA and dsDNA pathways (Fig. 1). Since TLR2 stimulates cytokine production in mice following HSV-1 infection (18), we next examined whether TLR2 mediates IFN and cytokine expression in human primary macrophages during HSV infection. No reduction in HSV-induced cytokines was seen when TLR2 was neutralized, suggesting a TLR2-independent production of IFNs, ISGs, and proinflammatory cytokines during early HSV infection (Fig. 2). It should be noted that we used low MOIs throughout the experiments. Furthermore, some variation in TLR2 stimulatory potential has been reported for HSV strains (35). Therefore, we cannot exclude that some TLR2 dependency would be seen at a very high virus dose, with other virus strains, or at later stages of infection. Nevertheless, our results suggest that HSV-induced IFN and cytokine expression does not proceed through TLR2 or TLR9 in human macrophages.
To determine the PRR responsible for the early response to HSV, we further characterized the virus requirements for virus-induced signaling and IFN and cytokine production. We found that early induction of IFN and TNF-α is highly dependent on entry and replication of the virus (Fig. 3A to E). Furthermore, virus-induced signaling was dependent on live virus, again suggesting the need for replication-derived components for innate recognition (Fig. 3F and G). One candidate PAMP is virus-derived dsRNA, known to accumulate in cells infected with HSV (37). RLR-mediated recognition of HSV-derived dsRNA is dependent on the MAVS pathway in murine fibroblasts (5, 30). We found that the early IFN response was highly MAVS dependent, since knockdown of MAVS in the macrophages resulted in a strong reduction in the levels of IFN types I and III (Fig. 4B and C). In contrast, TNF-α production was affected only marginally by MAVS knockdown, despite the expression of TNF-α being highly dependent on virus entry and replication (Fig. 4D). Thus, the data suggest that TNF-α induction proceeds via a novel intracellular pathway that is independent of MAVS but dependent on accumulating RNA or DNA via virus replication.
Independently, two groups have demonstrated that RNA Pol III mediates recognition of dsDNA via RNA intermediates that are subsequently recognized by RIG-I, which signals via the MAVS pathway (1, 4). Having shown that TLR2 or TLR9 does not trigger the early response to HSV-1 and that the production of IFNs is MAVS dependent, we assessed the functionality of the RNA Pol III pathway in macrophages. The RNA Pol III pathway is likely to mediate some of the observed IFN-β and TNF-α expression following poly(dA:dT) stimulation, since the RNA Pol III inhibitor reduced levels of poly(dA:dT)-stimulated IFN and TNF-α (Fig. 5A and B). However, RNA Pol III appears not to mediate early IFN and cytokine production following HSV infection (Fig. 5C and D).
To evaluate the role of MDA5 and RIG-I in recognizing HSV, we used individual siRNA knockdown of both receptors. We found that MDA5, but not RIG-I, was the primary mediator of HSV recognition. The specific use of MDA5 seems rather surprising, since mouse studies using knockout MEFs and dominant negative RIG-I have suggested a dual role for RIG-I and MDA5 in recognition of HSV (5, 29). Our results point at differences between cell types and species and emphasize the great importance of considering the relevance of experimental models and the importance of doing studies in human primary cells.
The MAVS-independent recognition of HSV requires additional characterization. One candidate is TLR3, recognizing dsRNA in endosomes. However, based on two facts, we do not think that TLR3 is the primary candidate for recognition of early HSV infection. First, despite playing a role in production of type I and III IFN in human fibroblasts following HSV infections, human PBMCs deficient in TLR3 are fully capable of producing IFN during HSV infection (41). Second, TLR3 is localized in endosomes, and thus TLR3-mediated signaling likely requires uptake of dsRNA from the extracellular lumen after release by the cells. Another potential candidate is ZBP-1. In the murine fibroblast-like cell line L929, HSV-induced IFN mRNA accumulation was partially dependent on ZBP-1 (36). In addition, human cytomegalovirus-induced IFN proceeds via ZBP-1, independently of MAVS, in human fibroblasts (7). It is possible that the slight UV-inactivated HSV-induced type I and III IFN expression we observed (Fig. 3A and B) is mediated partly via ZBP-1. However, we suggest that the majority of early HSV-induced IFN and cytokine production is ZBP-1 independent, since siRNA knockdown of ZBP-1 significantly reduces poly(dA:dT)-induced IFN in L929 fibroblast-like cells only at later time points (9 h) and not at early time points (6 h) (19). Furthermore, Ishii and colleagues suggest that early innate responses to poly(dA:dT) are independent of ZBP-1 in MEFs (16). In addition, human cells such as lung epithelial A549 and HEK293 cells recognize dsDNA independently of ZBP-1 (19). Whether ZBP-1 plays a role during innate recognition in cells other than fibroblasts, including human primary macrophages, remains to be defined.
Collectively, we demonstrate that innate recognition of HSV by human primary macrophages occurs via two distinct intracellular nucleotide-sensing pathways: the RLR/MAVS pathway, mediating IFN production, and a yet-to-be-identified intracellular receptor responsible for inflammatory cytokine expression. Moreover, we present evidence that MDA5 is the principal mediator of the MAVS-dependent IFN production seen in human primary macrophages.
Acknowledgments
This work was supported by grants from the Aarhus University Research Foundation, the Toyota Foundation Denmark, the Dagmar Marshall Foundation, the Augustinus Foundation, the Scandinavian Society of Antimicrobial Chemotherapy, The Lundbeck Foundation (grant R34-A3855), The Danish Medical Research Council (grant 09-072636), the Medical Research Council of the Academy of Finland, and the Sigrid Juselius Foundation.
We thank Erik Hagen Nielsen and Kirsten Stadel Pedersen for technical assistance.
Footnotes
Published ahead of print on 25 August 2010.
REFERENCES
- 1.Ablasser, A., F. Bauernfeind, G. Hartmann, E. Latz, K. A. Fitzgerald, and V. Hornung. 2009. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat. Immunol. 10:1065-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 3.Bieback, K., E. Lien, I. M. Klagge, E. Avota, J. Schneider-Schaulies, W. P. Duprex, H. Wagner, C. J. Kirschning, V. ter Meulen, and S. Schneider-Schaulies. 2002. Hemagglutinin protein of wild-type measles virus activates Toll-like receptor 2 signaling. J. Virol. 76:8729-8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu, Y. H., J. B. Macmillan, and Z. J. Chen. 2009. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138:576-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi, M. K., Z. Wang, T. Ban, H. Yanai, Y. Lu, R. Koshiba, Y. Nakaima, S. Hangai, D. Savitsky, M. Nakasato, H. Negishi, O. Takeuchi, K. Honda, S. Akira, T. Tamura, and T. Taniguchi. 2009. A selective contribution of the RIG-I-like receptor pathway to type I interferon responses activated by cytosolic DNA. Proc. Natl. Acad. Sci. U. S. A. 106:17870-17875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Compton, T., E. A. Kurt-Jones, K. W. Boehme, J. Belko, E. Latz, D. T. Golenbock, and R. W. Finberg. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J. Virol. 77:4588-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeFilippis, V. R., D. Alvarado, T. Sali, S. Rothenburg, and K. Fruh. 2010. Human cytomegalovirus induces the interferon response via the DNA sensor ZBP1. J. Virol. 84:585-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 9.Duerst, R. J., and L. A. Morrison. 2003. Innate immunity to herpes simplex virus type 2. Viral Immunol. 16:475-490. [DOI] [PubMed] [Google Scholar]
- 10.Ellermann-Eriksen, S. 2005. Macrophages and cytokines in the early defence against herpes simplex virus. Virol. J. 2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303:1526-1529. [DOI] [PubMed] [Google Scholar]
- 12.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 13.Hornung, V., A. Ablasser, M. Charrel-Dennis, F. Bauernfeind, G. Horvath, D. R. Caffrey, E. Latz, and K. A. Fitzgerald. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458:514-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornung, V., J. Ellegast, S. Kim, K. Brzozka, A. Jung, H. Kato, H. Poeck, S. Akira, K. K. Conzelmann, M. Schlee, S. Endres, and G. Hartmann. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994-997. [DOI] [PubMed] [Google Scholar]
- 15.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of Toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531-4537. [DOI] [PubMed] [Google Scholar]
- 16.Ishii, K. J., T. Kawagoe, S. Koyama, K. Matsui, H. Kumar, T. Kawai, S. Uematsu, O. Takeuchi, F. Takeshita, C. Coban, and S. Akira. 2008. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 451:725-729. [DOI] [PubMed] [Google Scholar]
- 17.Krug, A., G. D. Luker, W. Barchet, D. A. Leib, S. Akira, and M. Colonna. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood 103:1433-1437. [DOI] [PubMed] [Google Scholar]
- 18.Kurt-Jones, E. A., M. Chan, S. Zhou, J. Wang, G. Reed, R. Bronson, M. M. Arnold, D. M. Knipe, and R. W. Finberg. 2004. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. U. S. A. 101:1315-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lippmann, J., S. Rothenburg, N. Deigendesch, J. Eitel, K. Meixenberger, V. van Laak, H. Slevogt, P. D. N′guessan, S. Hippenstiel, T. Chakraborty, A. Flieger, N. Suttorp, and B. Opitz. 2008. IFNbeta responses induced by intracellular bacteria or cytosolic DNA in different human cells do not require ZBP1 (DLM-1/DAI). Cell. Microbiol. 10:2579-2588. [DOI] [PubMed] [Google Scholar]
- 20.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. U. S. A. 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malmgaard, L., J. Melchjorsen, A. G. Bowie, S. C. Mogensen, and S. R. Paludan. 2004. Viral activation of macrophages through TLR-dependent and -independent pathways. J. Immunol. 173:6890-6898. [DOI] [PubMed] [Google Scholar]
- 23.Melchjorsen, J., H. Kristiansen, R. Christiansen, J. Rintahaka, S. Matikainen, S. R. Paludan, and R. Hartmann. 2009. Differential regulation of the OASL and OAS1 genes in response to viral infections. J. Interferon Cytokine Res. 29:199-207. [DOI] [PubMed] [Google Scholar]
- 24.Melchjorsen, J., J. Siren, I. Julkunen, S. R. Paludan, and S. Matikainen. 2006. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-kappaB and IRF-3. J. Gen. Virol. 87:1099-1108. [DOI] [PubMed] [Google Scholar]
- 25.Miettinen, M., T. Sareneva, I. Julkunen, and S. Matikainen. 2001. IFNs activate Toll-like receptor gene expression in viral infections. Genes Immun. 2:349-355. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 27.Pichlmair, A., O. Schulz, C. P. Tan, T. I. Naslund, P. Liljestrom, F. Weber, and C. Reis e Sousa. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314:997-1001. [DOI] [PubMed] [Google Scholar]
- 28.Ranjith-Kumar, C. T., A. Murali, W. Dong, D. Srisathiyanarayanan, R. Vaughan, J. Ortiz-Alacantara, K. Bhardwaj, X. Li, P. Li, and C. C. Kao. 2009. Agonist and antagonist recognition by RIG-I, a cytoplasmic innate immunity receptor. J. Biol. Chem. 284:1155-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen, S. B., S. B. Jensen, C. Nielsen, E. Quartin, H. Kato, Z. J. Chen, R. H. Silverman, S. Akira, and S. R. Paludan. 2009. Herpes simplex virus infection is sensed by both Toll-like receptors and retinoic acid-inducible gene-like receptors, which synergize to induce type I interferon production. J. Gen. Virol. 90:74-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasmussen, S. B., L. N. Sorensen, L. Malmgaard, N. Ank, J. D. Baines, Z. J. Chen, and S. R. Paludan. 2007. Type I IFN production during herpes simplex virus infection is controlled by cell-type specific viral recognition through TLR9, the MAVS pathway, and novel recognition systems. J. Virol. 81:13315-13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rassa, J. C., J. L. Meyers, Y. Zhang, R. Kudaravalli, and S. R. Ross. 2002. Murine retroviruses activate B cells via interaction with Toll-like receptor 4. Proc. Natl. Acad. Sci. U. S. A. 99:2281-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reske, A., G. Pollara, C. Krummenacher, B. M. Chain, and D. R. Katz. 2007. Understanding HSV-1 entry glycoproteins. Rev. Med. Virol. 17:205-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rintahaka, J., D. Wiik, P. E. Kovanen, H. Alenius, and S. Matikainen. 2008. Cytosolic antiviral RNA recognition pathway activates caspases 1 and 3. J. Immunol. 180:1749-1757. [DOI] [PubMed] [Google Scholar]
- 34.Roizman, B., D. M. Knipe, and R. J. Whitley. 2007. Herpes simplex virus, p. 2501-2601. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 35.Sato, A., M. M. Linehan, and A. Iwasaki. 2006. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 103:17343-17348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takaoka, A., Z. Wang, M. K. Choi, H. Yanai, H. Negishi, T. Ban, Y. Lu, M. Miyagishi, T. Kodama, K. Honda, Y. Ohba, and T. Taniguchi. 2007. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448:501-505. [DOI] [PubMed] [Google Scholar]
- 37.Weber, F., V. Wagner, S. B. Rasmussen, R. Hartmann, and S. R. Paludan. 2006. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 80:5059-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanai, H., T. Ban, Z. Wang, M. K. Choi, T. Kawamura, H. Negishi, M. Nakasato, Y. Lu, S. Hangai, R. Koshiba, D. Savitsky, L. Ronfani, S. Akira, M. E. Bianchi, K. Honda, T. Tamura, T. Kodama, and T. Taniguchi. 2009. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature 462:99-103. [DOI] [PubMed] [Google Scholar]
- 39.Yoneyama, M., M. Kikuchi, K. Matsumoto, T. Imaizumi, M. Miyagishi, K. Taira, E. Foy, Y. M. Loo, M. Gale, Jr., S. Akira, S. Yonehara, A. Kato, and T. Fujita. 2005. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity J. Immunol. 175:2851-2858. [DOI] [PubMed] [Google Scholar]
- 40.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, S. Y., E. Jouanguy, S. Ugolini, A. Smahi, G. Elain, P. Romero, D. Segal, V. Sancho-Shimizu, L. Lorenzo, A. Puel, C. Picard, A. Chapgier, S. Plancoulaine, M. Titeux, C. Cognet, H. von Bernuth, C. L. Ku, A. Casrouge, X. X. Zhang, L. Barreiro, J. Leonard, C. Hamilton, P. Lebon, B. Heron, L. Vallee, L. Quintana-Murci, A. Hovnanian, F. Rozenberg, E. Vivier, F. Geissmann, M. Tardieu, L. Abel, and J. L. Casanova. 2007. TLR3 deficiency in patients with herpes simplex encephalitis. Science 317:1522-1527. [DOI] [PubMed] [Google Scholar]