Abstract
Two distinct envelope fusion proteins (EFPs) (GP64 and F) have been identified in members of the Baculoviridae family of viruses. F proteins are found in group II nucleopolyhedroviruses (NPVs) of alphabaculoviruses and in beta- and deltabaculoviruses, while GP64 occurs only in group I NPVs of alphabaculoviruses. It was proposed that an ancestral baculovirus acquired the gp64 gene that conferred a selective advantage and allowed it to evolve into group I NPVs. The F protein is a functional analogue of GP64, as evidenced from the rescue of gp64-null Autographa californica multicapsid nucleopolyhedrovirus (MNPV) (AcMNPV) by F proteins from group II NPVs or from betabaculoviruses. However, GP64 failed to rescue an F-null Spodoptera exigua MNPV (SeMNPV) (group II NPV). Here, we report the successful generation of an infectious gp64-rescued group II NPV of Helicoverpa armigera (vHaBacΔF-gp64). Viral growth curve assays and quantitative real-time PCR (Q-PCR), however, showed substantially decreased infectivity of vHaBacΔF-gp64 compared to the HaF rescue control virus vHaBacΔF-HaF. Electron microscopy further showed that most vHaBacΔF-gp64 budded viruses (BV) in the cell culture supernatant lacked envelope components and contained morphologically aberrant nucleocapsids, suggesting the improper BV envelopment or budding of vHaBacΔF-gp64. Bioassays using pseudotyped viruses with a reintroduced polyhedrin gene showed that GP64-pseudotyped Helicoverpa armigera single nucleocapsid nucleopolyhedrovirus (HearNPV) significantly delayed the mortality of infected H. armigera larvae.
The envelope fusion protein (EFP) of budded viruses (BV) (30) of baculoviruses is critical for virus entry (attachment and fusion) and egress (assembly and budding) (7, 13, 21). Two types of BV EFPs have been identified in the Baculoviridae family of viruses. The F proteins are similar in structure, but they are very diverse in their amino acid sequences (20 to 40% identity). They are widespread within the baculovirus family (group II NPVs of the alphabaculoviruses and in beta- and deltabaculoviruses) (23) and are thought to be carried by ancestral members (26). In contrast, the baculovirus GP64 homologs are all closely related EFPs (>74% sequence identity) and found only in group I NPVs of the alphabaculoviruses (23). It has been suggested that a gp64 gene was acquired relatively recently by an ancestral virus of the group II NPV, thereby giving these viruses a selective advantage and obviating the need of the envelope fusion function of the F protein (23). A nonfusogenic F homolog (F-like protein), however, is maintained in the genome of group I NPVs, functioning as a virulence factor (9, 17, 24, 32).
GP64 and F proteins play similar roles during the baculovirus infection processes, such as virus-cell receptor attachment, membrane fusion, and efficient budding. However, there are striking differences between the receptor usage of GP64 and F proteins as well. These two types of proteins are very different in structure, mode of action, and receptor exploitation. The crystal structure reveals that GP64 belongs to class III viral fusion proteins, with its fusion loop located in the internal region of the protein, and proteolytic cleavage is not required for activation of fusion activity (10). F proteins by contrast share common features of class I viral fusion proteins (12). The proteolytic cleavage of the F precursor (F0) by a furin-like protease generates an N-terminal F2 fragment and a C-teminal F1 fragment. This cleavage is essential for exposing the N-terminal fusion peptide of F1 and for activating F fusogenicity (8, 36). Although the nature of the baculovirus host cell receptors is still enigmatic, it has been reported that Autographa californica multicapsid nucleopolyhedrovirus (MNPV) (AcMNPV)) and Orgyia pseudotsugata MNPV (OpMNPV), both using GP64 as their EFPs, exploit the same insect cell receptor, while Lymantria dispar MNPV (LdMNPV) with an F protein as the EFP utilizes a cell receptor different from that used by AcMNPV (7, 37). Additionally, in the case of SeMNPV, using competition assays, it was confirmed that the baculovirus F protein and GP64 recognized distinct receptors to gain entry into cultured insect cells (34).
Pseudotyping viral nucleocapsid with heterologous EFPs to form pseudotype virions is a valuable approach to studying the structure, function, and specificity of heterologous EFPs. It has been a successful strategy to expand or alter viral host range, i.e., in gene delivery (3). For example, vesicular stomatitis virus G (VSV-G)-pseudotyped lentivirus and AcMNPV gp64-pseudotyped HIV-1 exhibit high virus titers and wider tropism (5, 14, 38); the gp64-pseudotyped human respiratory syncytial virus (HRSV) lacking its own glycoproteins is of high and stable infectivity (22); furthermore, pseudotyped lentiviruses with modified fusion proteins of GP64 with targeting peptides (i.e., hepatitis B virus PreS1 peptide, involved in viral attachment) or with the decay accelerating factor (DAF) facilitate the targeting to specific cell types or confer stability against serum inactivation, respectively (6, 19). For the Baculoviridae, a series of pseudotyping studies have investigated the functional analogy between GP64 and F proteins. F proteins of group II NPVs (SeMNPV, LdMNPV, and Helicoverpa armigera single nucleocapsid nucleopolyhedrovirus [HearNPV]) can substitute for GP64 in gp64-null AcMNPV viruses (15, 16). Recent studies indicated that many granulovirus (GV) F proteins, but not F protein from Plutella xylostella GV (PxGV), can rescue a gp64-null AcMNPV (16, 39). These results demonstrated that baculovirus F proteins are functional analogues to GP64. Since it was postulated that GP64 was captured by a baculovirus during evolution (24), one would expect the functional incorporation of GP64 into the BV of an F-null group II NPV. However, the reverse substitution of a group II NPV (SeMNPV) F protein by GP64 failed to produce infectious progeny viruses (35).
In this paper, we show that AcMNPV gp64 could be inserted into an F-null HearNPV genome and produce infectious progeny virus upon transfection of insect cells. The infectivity of the pseudotyped virus, however, was greatly impaired, and large amounts of morphologically defective BV were produced. Bioassay experiments indicated that the infectivity of GP64-pseudotyped F-null HearNPV for insect larvae was not reduced, but that the time to death was significantly delayed. These results demonstrate that GP64 alone can only partially complement HearNPV F protein function.
MATERIALS AND METHODS
Insect cells and viruses.
HzAM1 cells were cultured at 27°C in Grace's insect medium (Gibco-BRL), pH 6.0, supplemented with 10% fetal bovine serum (FBS). The F-null HearNPV bacmid (HaBacΔF) and the HaF-repaired virus vHaBacΔF-HaF used in this study as positive and negative controls, respectively, were constructed and described previously (33).
Construction of recombinant bacmids.
The AcMNPV gp64 gene was amplified from AcBacmid (bMON14272) with primers gp64-For, 5′-AAGCTTGCCTCAATGCTACTAGTAAATC-3 (HindIII site underlined), and gp64-Rev, 5′-AAGCTTGTGAGTTCAAGTCTCGCC-3 (HindIII site underlined), using Pyrobest DNA polymerase (Takara Bio, Inc., Japan). The PCR product was extended by an A tail by Taq DNA polymerase (Biostar, China), then cloned into a pGEM-T easy vector (Promega), and sequenced. The gp64 gene was cloned downstream of the Op166 promoter of pFB-Op166 (33) into the HindIII site to obtain the transfer vector pFB-Op166-gp64. To reintroduce polyhedrin (ph) into the recombinant bacmids, the Op166-HaF and Op166-gp64 cassettes were digested from pFB-Op166-HaF (33) or pFB-Op166-gp64 and further cloned into the polyhedrin gene-containing pFBD-ph vector (27), generating pFB-Op166-HaF-ph and pFB-Op166-gp64-ph, respectively. Each individual transfer vector was transposed into the attTn7 integration site of HaBacΔF, according to the Bac-to-Bac manual (Invitrogen). Recombinant bacmids were selected by gentamicin resistance and their sequences confirmed at the insertion loci by PCR as described previously (33).
Transfection-infection assay of recombinant viruses.
One microgram of each recombinant bacmid was transfected into 1 × 106 HzAM1 cells using 10 μl Lipofectin (Invitrogen), according to the Bac-to-Bac manual (Invitrogen). At 6 days posttransfection (p.t.), 1 ml of the supernatant from HaBacΔF-HaF-transfected cells (noted as passage 0 [P0]) was clarified for 10 min at 3,000 × g and used to infect a new batch of HzAM1 cells (noted as P1). Since the titer of the progeny viruses produced from HaBacΔF-gp64-transfected cells was very low, the transfected cells plus supernatant (P0) were transferred to a new flask at 6 days p.t. and passaged two more times to accumulate and achieve a relatively high virus titer. After these 2 passages, 1 ml of the supernatant (noted as P2) was used to infect a new batch of HzAM1 cells (P3) and so on. The supernatants collected after passage 5 were used for experiments. At 72 h p.t. or postinfection (p.i.), transfected or infected cells were inspected by fluorescence microscopy for the presence of green fluorescent protein (GFP).
Western blot analysis of recombinant viruses.
The expression of GP64 or F proteins and their incorporation into BV were examined by Western analysis. For generating anti-GP64 antibody, a fragment (218 amino acids [aa] to 457 aa) of the gp64 gene was amplified by PCR with primers GP64C-for, 5′-CCCGAATTCGCTTCACCACGCGCCAAATAAAA-3′ (EcoRI site underlined), and GP64C-rev, 5′-CCCAAGCTTTTACTTGGTGTTCTCCATGGTGG-3′ (HindIII site underlined). The PCR products were cloned into a pGEX-KG expression vector. The proteins were purified and then used to immunize a rabbit for generating polyclonal antiserum against GP64 as described previously (33).
Western analysis was performed using polyclonal antibodies against HaF1 (33), against GP64, or against VP80 (4) to probe sediment BV from supernatants of infected cells. Briefly, BV were disrupted in 6× SDS-PAGE sample buffer, and approximately 10 μg of BV proteins were separated by 12% SDS-PAGE and subjected to Western blot analysis. The antisera were used at a 1:1,000 dilution, and the proteins were detected by treatment with alkaline phosphatase conjugated with goat anti-rabbit immunoglobulin (Sigma). The final signal was detected by nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolylphosphate (BCIP) (Sino-American Biotechnology Company).
Syncytium formation assay.
To generate transient expression vectors containing EFP genes, a phsp70-egfp-SV40 cassette was inserted into pIZ/V5-His (Invitrogen) by XhoI and HindIII, generating control plasmid pIZ/V5-egfp to monitor transfection. Then, the HaF and the AcMNPV gp64 genes were digested from the pGEM-T easy vector (Promega) by HindIII, and each was subcloned into pIZ/V5-egfp. The resulting plasmids were named pIZ/V5-HaF-egfp and pIZ/V5-gp64-egfp, respectively.
Syncytium formation (HzAM1-HzAM1 fusion) assays were performed by transfection with either pIZ/V5-HaF-egfp or pIZ/V5-gp64-egfp or infection with pseudotyped virus vHaBacΔF-gp64 or control virus vHaBacΔF-HaF at a dose of 5 50% tissue culture infectious dose (TCID50) units/cell. At 48 h p.t. or p.i., cells were washed twice with 1 ml Grace's medium (pH 6.0) and treated for 5 min with acidified Grace's medium (pH 5.0). The acidic medium was removed and replaced with 2 ml Grace's medium (pH 6.0) with 10% fetal bovine serum (FBS). Syncytium formation was observed by fluorescence microscopy 24 h after treatment with low-pH Grace's medium.
Comparison of one-step growth curves of vHaBacΔF-gp64 and vHaBacΔF-HaF.
Infectious BV production was assessed by a one-step growth curve. HzAM1 cells (2 × 105) were infected with 5 TCID50 units/cell. At 0, 24, 48, 72, 96, and 120 h p.i., supernatants were harvested and titrated by an endpoint dilution assay. Each virus infection was performed in triplicate. The average of the triple log-transformed TCID50 values, and the standard deviations of the mean were determined by using Microsoft Excel software (version 2003).
Q-PCR of recombinant viruses.
Quantitative real-time PCR (Q-PCR) was performed using Eva Green dye (Biotium, Inc.) to determine the number of genomic DNA copies compared to viral titers, using primers lef8-For, 5′-GGCCATCCATTCGGTGAGGTC-3′, and lef8-Rev, 5′-CATCGTGGGCGTGGTAGTGTC-3′, for amplification of a 301-bp region of the HearNPV lef-8 gene (GeneID 919958) using a real-time PCR detection system (Bio-Rad Opticon 2). Supernatant (500 μl) from infected cells (120 h p.i.) was incubated with 500 μl 20% PEG8000 in 1 M NaCl for 30 min at room temperature (RT), and then the virions were spun down at 12,000 × g for 15 min and resuspended in 20 μl H2O. Virions were lysed by adding 80 μl virus disruption buffer (10 mM Tris-HCl [pH 7.6], 10 mM EDTA, 0.25% SDS) and 5 μl proteinase K (20 mg/ml). After incubation at 50°C for 1 h, viral DNA was phenol extracted, ethanol precipitated, and dissolved in 20 μl H2O. One microliter of BV DNA was used for Q-PCR analysis. The Q-PCR was carried out by 38 cycles at 95°C for denaturation and at 63°C and 72°C for annealing/extension.
Electron microscopy of budded virions and polyhedron of recombinant viruses.
Fresh BV from vHaBacΔF-HaF- or vHaBacΔF-gp64-infected cell supernatants were adsorbed onto carbon-coated copper 200-mesh grids for 30 min at room temperature. Grids were washed briefly on a large drop of ultrapure distilled water and transferred onto a drop of negative stain (2% phosphomolybdic acid, pH 6.5) for 20 s.
Polyhedra were purified 6 days p.i. from fourth-instar H. armigera larvae infected with vHaBacΔF-HaF-polh or HaBacΔF-gp64-polh. All samples were processed for electron microscopy examination as described previously (31). Specimens were examined by using an electron microscope (FEI Tecnai G2) operated at 200 kV.
Virus competition assay.
Recombinant HearNPV expressing a red fluorescent protein (RFP) gene was constructed as a competitor virus. The RFP gene was digested with EcoRI and NotI from pDsRed2-N1 (Clontech) and subcloned into pFB-Op166, generating transfer vector pFB-Op166-RFP. Then the RFP gene was transposed into HaBacHz8 to generate recombinant bacmid Ha-RFP. Finally, to produce the recombinant baculovirus vHa-RFP, the bacmid was transfected according to the Bac-to-Bac manual (Invitrogen) into HzAM1 cells, and the BV from the supernatants were stored at 4°C until use. BV of vHa-RFP were inactivated by UV light.
HzAM1 cells (5 × 104) were inoculated into 24-well plates and preincubated with increasing concentrations of UV-inactivated vHa-RFP for 1 h at 4°C. vHaBacΔF-gp64 and vHaBacΔF-HaF (5 TCID50 units/cell) were then added to monolayers to allow virus attachment at 4°C for 2 h. The BV-containing medium was removed and replaced by 500 μl normal medium. The infection ratios were quantified at 24 h p.i. using a Beckman Coulter (EPICS XL) flow cytometer by quantifying the GFP expression of infected cells at 480 nm for excitation and 520 nm for emission.
Bioassays of polyhedrin-repaired recombinant viruses vHaBacΔF-gp64-polh and vHaBacΔF-HaF-polh.
The oral infectivity of the recombinant viruses was measured by the droplet feeding method with early third-instar H. armigera larvae. The occlusion bodies (OBs) used for the bioassay were harvested and purified from diseased larvae as described previously (29). Larvae were exposed to eight concentrations: 3 × 106, 1 × 106, 3 × 105, 1 ×105, 3 × 104, 1 ×104, 3 × 103, and 1 × 103 OBs ml−1 of each. Forty-eight insects were used per each virus concentration, and experiments were repeated twice. Median lethal concentration (LC50) values (OBs ml−1) were calculated by POLO probit analysis and compared by standard lethal dose ratio comparison (25). Median survival time (ST50) values of the viruses (3 × 106 OBs ml−1) were calculated using the Kaplan-Meier estimator and further compared using a log rank test (11).
RESULTS
AcMNPV GP64 protein is able to rescue an F-null HearNPV bacmid.
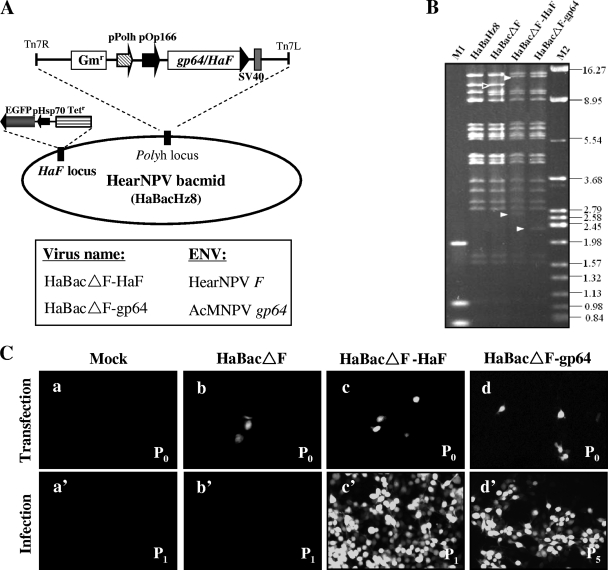
An F-null HearNPV bacmid (HaBacΔF) and a bacmid rescued for F (HaBacΔF-HaF) were previously constructed (33) and used to generate negative- and positive-control viruses. The AcMNPV gp64 gene under the control of the OpMNPV gp64 promoter (Op166) was inserted into HaBacΔF, generating gp64-pseudotyped bacmid HaBacΔF-gp64 (Fig. 1 A). The correct insertions in the recombinant bacmids were confirmed by PCR and EcoRI digestion. In HaBacΔF, a 12.2-kb EcoRI band (arrow) containing the polyhedrin locus is present, while two new 14.4-kb and 2.7-kb EcoRI bands were generated due to insertion of the HaF gene (Fig. 1B), which contains one EcoRI site (33). In HaBacΔF-gp64, due to insertion of the gp64 gene, the 12.2-kb EcoRI band changed to two bands of 14.4 kb and 2.3 kb (Fig. 1B). The restriction profile showed that all of the bands in the recombinant bacmids were shifted as expected (indicated by arrowheads, Fig. 1B), confirming the correct insertion of the EFP genes.
FIG. 1.
Construction and transfection-infection assay of recombinant bacmids. (A) Schematic representative genomes of F-null bacmid HaBacΔF, HaF-rescued bacmid HaBacΔF-HaF, or gp64-pseudotyped bacmid HaBacΔF-gp64. pOp166, OpMNPV fusion protein (ORF126) promoter; pPolh, AcMNPV polyhedrin promoter; SV40, simian virus 40 terminator. (B) Identification of the parental (HaBacHZ8) and recombinant bacmids by EcoRI digestion. M1, DL2000 DNA ladder; M2, λ DNA digested by HindIII plus BamHI plus EcoRI. Shifted bands are indicated by arrowheads (original bands with open arrowheads and shifted bands with white solid arrowheads). The sizes of the molecular weight markers (in thousands) are indicated on the right. (C) Transfection-infection assay. HzAM1 cells were transfected with the indicated bacmids (b, c, and d) or mock treated (a). At 6 days p.t., supernatants from the mock-, HaBacΔF-, and HaBacΔF-HaF-transfected cells (P0) were used to infect healthy HzAM1 cells (P1) (panels a', b', and c'). For vHaBacΔF-gp64, supernatant from P5 was used to infect healthy HzAM1 cells (d'). The cells were observed by fluorescence microscopy at 3 days p.t. or p.i.
HzAm1 cells were transfected with HaBacΔF, HaBacΔF-HaF, or HaBacΔF-gp64 or mock transfected. At 72 h p.t., GFP-positive cells were observed in a fluorescence microscope in all plates except those that were mock transfected (Fig. 1C). Although the HaBacΔF bacmid entered cells by transfection, as evidenced from the presence of GFP-positive cells (Fig. 1C-b), infectious BV were not produced, as secondary infection could not be established and not even a single GFP-positive cell was observed (Fig. 1C-b'). This result is in agreement with previous observations that HaF is an essential gene for HearNPV infection (33). In parallel experiments using HaF-rescued bacmid HaBacΔF-HaF, fluorescence spread in both transfected and infected cells (Fig. 1C-c and C-c'), indicating that the defect in infectivity can be rescued by reintroducing an HaF gene into HaBacΔF. Transfection with pseudotyped bacmid HaBacΔF-gp64 also resulted in primary as well as in secondary infections (Fig. 1C-d), as evidenced by infecting a new batch of HzAM1 cells using supernatants from passage 5 (Fig. 1C-d'). Thus, gp64 is able to rescue an F-null HearNPV bacmid. It is necessary to point out that the infectious progeny BV of pseudotyped virus vHaBacΔF-gp64 were not always produced in each independent transfection assay (∼1 positive per 6 transfections). Successful transfection and BV production seemed to depend on both optimal cell conditions and high transfection efficiency and require further optimization of the system.
The expression and fusogenic ability of GP64 in pseudotyped virus.
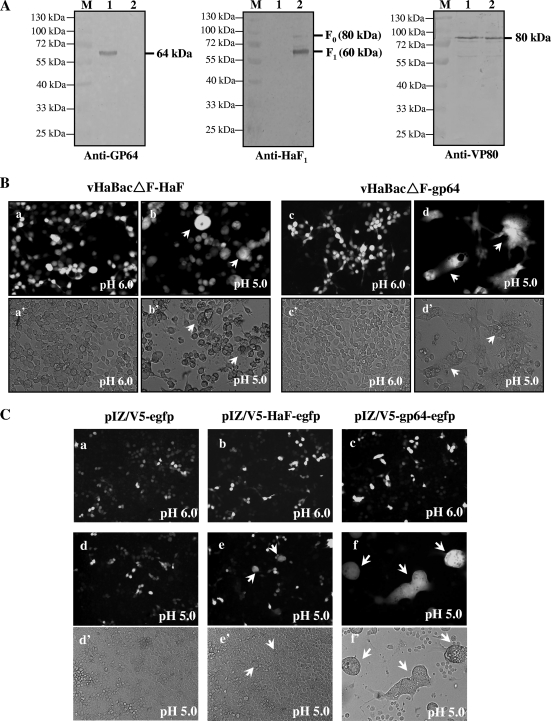
Diagnostic PCR (data not shown) and Western blot analyses of purified virions were conducted to confirm the correct assembly of recombinant BV. Anti-GP64 antiserum detected a band corresponding to the predicted molecular mass of GP64 (∼64 kDa) in vHaBacΔF-gp64 BV (Fig. 2 A, left, lane 1), but not in vHaBacΔF-HaF BV (Fig. 2A, left, lane 2). Anti-HaF1 antiserum detected a major band (∼59 kDa) of the cleaved HaF1 subunit and a minor band (∼80 kDa) of uncleaved F0 precursor only in vHaBacΔF-HaF BV (Fig. 2A, middle, lane 2) (15, 33) but not in vHaBacΔF-gp64 BV (lane 1). The antibody against nucleocapsid protein VP80 (∼80 kDa) was used as an internal control for the presence of equal amounts of BV proteins in each lane (Fig. 2A, right). Thus, Western analysis confirmed the correct expression and incorporation of GP64 into pseudotyped HearNPV BV.
FIG. 2.
Detection of GP64 expression and fusogenicity. (A) Western blot analyses of envelope protein in recombinant BV. Virions were harvested and purified from the supernatants of infected HzAM1 cells at 6 days p.i., and the proteins were separated by SDS-PAGE. The blots were probed with antibodies against GP64 (left), HaF1 (middle), and VP80 (right). Lanes: 1, HaBacΔF-gp64 BV; 2, vHaBacΔF -HaF BV; M, the protein marker with indicated molecular mass. (B) Syncytium formation assay of HzAM1 cells infected by recombinant viruses. HzAM1 cells were infected with vHaBacΔF-HaF or vHaBacΔF-gp64 at 5 TCID50 units/cell. At 48 h p.i., cells were incubated with Grace's insect medium at pH 6.0 (a and c) or 5.0 (b and d) for 5 min. Syncytium formation was examined by fluorescence microscopy 24 h after dropping the pH. Images in panels a to d were taken under UV light, and panels a' to d' show the same fields as those shown in a to d under white light, respectively. Multinuclear cells are indicated by arrows. (C) Cell-to-cell fusion of HzAM1 cells transfected with plasmids containing the gp64 or HaF gene. Cells were transfected with 10 μg of plasmid pIZ/V5-HaF-egfp or pIZ/V5-gp64-egfp or a control plasmid, pIZ/V5-egfp. At 48 h p.t., cells were treated for 5 min with Grace's insect medium at pH 6.0 (a, b, and c) or 5.0 (d, e, and f) for 5 min. Syncytium formation were scored by fluorescence microscopy 24 h after dropping the pH. Images shown in panels a to f were taken under UV light, and panels d' to f' show the same fields as those shown in panels d to f under white light, respectively. Multinuclear cells are indicated by arrows.
Viral-cell membrane fusion is a key step for delivering the viral genetic material into the cytoplasm of the host cell. Both baculovirus GP64 and F protein are low-pH-triggered membrane fusion proteins (1, 23, 36). In order to examine the fusogenic ability of GP64 for HzAM1 cells, syncytium formation assays were conducted as described in Materials and Methods. When vHaBacΔF-HaF-infected cells were treated with low pH, multinuclear cells were observed (Fig. 2B-b and B-b'), which was consistent with the previous findings that HaF can cause cell fusion (33). Meanwhile, vHaBacΔF-gp64-infected cells exposed to low-pH syncytium formation was immediately (<5 min) detected (data not shown). Twenty-four hours later, stable syncytia were observed, and both the scale and size of multinuclear cells resulting from vHaBacΔF-gp64 infection were remarkably larger (Fig. 2B-d and B-d') than those induced by vHaBacΔF-HaF (Fig. 2B-b and B-b'). Similar results were found in the transient transfection assays by using a plasmid expressing GP64 or HaF protein under the control of the immediate early promoter IE1 (Fig. 2C). These results confirmed that the membrane fusion capability of GP64 is higher than that of HaF protein in HzAM1 cells and reflects the high fusogenic ability of GP64 (1).
GP64 pseudotyping leads to less-infectious recombinant HearNPV BV.
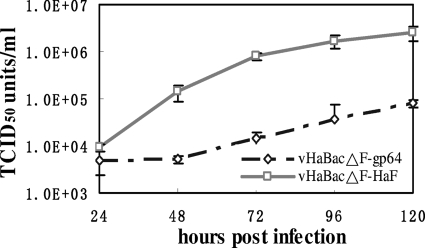
To characterize the effect of gp64 pseudotyping on HearNPV BV production, one-step growth assays were carried out and the curves compared between vHaBacΔF-gp64 and vHaBacΔF-HaF (Fig. 3). HzAM1 cells were infected with vHaBacΔF-HaF or vHaBacΔF-gp64 with 5 TCID50 units/cell, and BV production was measured at different time points p.i. by an endpoint dilution assay. The results showed that the two viruses had very different BV production kinetics. The lag phase of vHaBacΔF-gp64 BV production (0 to 48 h p.i.) was much longer than that of vHaBacΔF-HaF (0 to 12 h p.i.) (33). All through the log phase and the stationary phase, the infectious BV production of vHaBacΔF-HaF was about 1 or 2 log units higher than that of vHaBacΔF-gp64 (Fig. 3). At 120 h p.i., the BV titer of vHaBacΔF-HaF was 2.53 ± 0.92 × 106 TCID50 units ml−1, while the BV titer of vHaBacΔF-gp64 was only 7.90 ± 1.39 × 104 TCID50 units ml−1, almost 32 times lower than that for vHaBacΔF-HaF. Thus, the insertion of gp64 into F-null HearNPV leads to a significant reduction in infectious BV production compared to vHaBacΔF-HaF.
FIG. 3.
One-step growth curve assays of recombinant viruses. HzAm1 cells were infected with vHaBacΔF-HaF or vHaBacΔF-gp64 at 5 TCID50 units/cell. BV titers at different time points (h p.i.) were quantified by endpoint dilution assays. Each data point represents the average value from three separate infections. Error bars, standard deviations.
To further investigate whether the reduced infectious BV production of vHaBacΔF-gp64 was due to a lower BV yield or a lower infectivity, quantitative Q-PCR was performed using BV samples at 120 h p.i. from a one-step growth curve assay. The results showed that for vHaBacΔF-HaF, one TCID50 unit was equivalent to 5.4 × 104 copies of viral genomic DNA (Table 1), whereas for gp64-pseudotyped virus, vHaBacΔF-gp64, one TCID50 unit was equivalent to 2.8 × 105 copies of viral genomic DNA (Table 1). Hence, the infectious virus per particle ratio of vHaBacΔF-gp64 is about five times lower than that of vHaBacΔF-HaF. The infectious BV production of vHaBacΔF-gp64 is about 32 times lower than that of vHaBacΔF-HaF (Fig. 3). If taking into account the TCID50/BV genome ratio, then the total vHaBacΔF-gp64 BV yield in terms of BV genome copies was reduced about 6-fold.
TABLE 1.
Q-PCR to determine viral genomic DNA copies compared to infectious BV titers
| Recombinant virus (120 h p.i.) | DNA copies determined by real-time PCR (no. of copies/ml) | Virus titers (TCID50 U/ml) | Viral infectivity (no. of copies per TCID50 unit) |
|---|---|---|---|
| vHaBacΔF-HaF | 1.37 ± 0.27 × 1011 | 2.53 ± 0.92 × 106 | 5.42 ± 0.41 × 104 |
| vHaBacΔF-gp64 | 2.18 ± 0.36 × 1010 | 7.90 ± 1.39 × 104 | 2.76 ± 0.24 × 105 |
GP64-pseudotyped virus vHaBacΔF-gp64 exhibits markedly defective BV morphology.
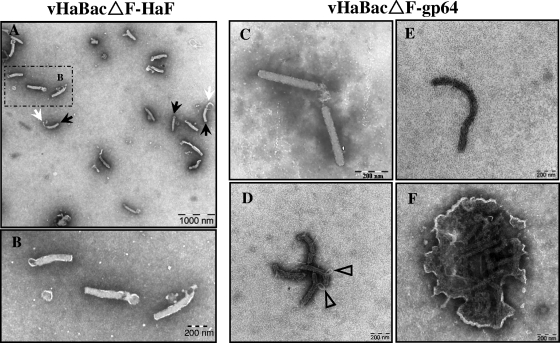
Viral envelope proteins have been proposed to play important roles in the processes of assembly, morphogenesis, budding, and release of progeny virus at the cell surface. To determine whether GP64-pseudotyped HearNPV BV had an altered morphology, transmission electron microscopy was used to compare virions of vHaBacΔF-gp64 with those from the control virus, vHaBacΔF-HaF (Fig. 4). To fully preserve the integrity of the virion structure and avoid influence of ultracentrifugation on BV morphology, supernatants from vHaBacΔF-gp64- or vHaBacΔF-HaF-infected cells were observed directly by electron microscopy. We found a remarkable difference between vHaBacΔF-HaF BV and vHaBacΔF-gp64 BV in virion structure integrity. Most (>90%) of the vHaBacΔF-HaF BV consisted of enveloped rod-shaped particles (Fig. 4A and B), with sometimes a cap-like structure (Fig. 4A, indicated by black arrows) near one end of the rod-shaped nucleocapsid and with an extension in the other end (Fig. 4A, white arrows). These characteristics were typical of wild-type HearNPV BV as well, under these conditions (data not shown). In contrast, very few of the vHaBacΔF-gp64 BV consisted of enveloped rod-shaped particles (Fig. 4C). It was clearly visible that most (>95%) of the vHaBacΔF-gp64 virions lacked an envelope and appeared like nucleocapsids (Fig. 4D to F). These nucleocapsids could be greater (ranging from approximately 800 nm to 1,200 nm) (Fig. 4E) in length than the virions of vHaBacΔF-HaF (around 400 nm) (Fig. 4A and B). Some nucleocapsids appeared distorted or broken, with the ribonucleoprotein complex of DNA plus viral capsid proteins protruding through the breaks of the nucleocapsids (Fig. 4D, triangles). Some aggregates of nucleocapsids surrounded by membrane-like structures were also detected in vHaBacΔF-gp64 preparations (Fig. 4F). These findings suggested that vHaBacΔF-gp64 BV had an aberrant and highly pleiomorphic appearance.
FIG. 4.
Electron micrographs of control vHaBacΔF-HaF BV or pseudotyped vHaBacΔF-gp64 BV. (A) Virions of vHaBacΔF-HaF BV. (B) Higher-magnification image of the boxed region in panel A. (C) Intact virions of vHaBacΔF-gp64 BV. (D to F) Broken, extended, or aggregated nucleocapsids of vHaBacΔF-gp64 BV. Black arrows, cap-like structure; white arrows, extension structure; open triangles, nucleoprotein extrusions.
vHaBacΔF-gp64 utilizes the same entry component as wild-type HearNPV to gain entry into HzAM1 cells.
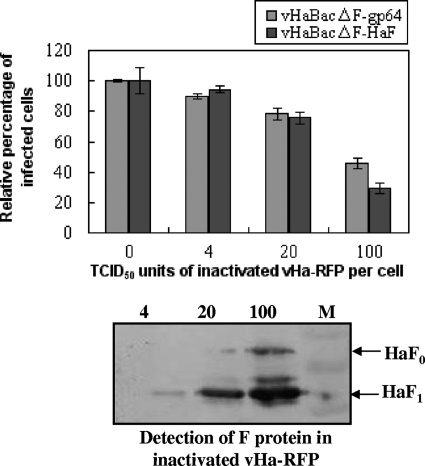
The cell surface receptor is a major determinant restricting the viral host range as well as infectivity. To investigate the receptor usage of vHaBacΔF-gp64 and vHaBacΔF-HaF in HzAM1 cells, we constructed recombinant HearNPV expressing RFP (vHa-RFP) as a competitor virus and conducted competition assays using UV-inactivated vHa-RFP. Since the EGFP marker gene was used by vHaBacΔF-gp64 and vHaBacΔF-HaF, the RFP marker gene was used and cloned to the competitor virus. The UV inactivation of the viruses was confirmed by infection assays showing no residual infectivity (red fluorescence) after UV treatment in HzAM1 cells but abundant intracellular red fluorescence when untreated viruses were used (data not shown). As Fig. 5 indicates, both vHaBacΔF-gp64 and vHaBcΔF-HaF were competed out by inactivated vHa-RFP in a dose-dependent manner. At 100 TCID50 units/cell for vHa-RFP, the infection rates of vHaBcΔF-HaF and HaBacΔF-gp64 were reduced to 28.9 ± 3.2% and 48.6 ± 3.5%, respectively. These data suggest that in HzAM1 cells vHaBacΔF-gp64 binds to (or blocks binding to) the same virus binding sites as HearNPV to gain entry into HzAM1 cells.
FIG. 5.
Competition assay using inactivated vHa-RFP. HzAm1 cells were preincubated with increasing amounts of UV-inactivated vHa-RFP for 1 h, and then infected with vHaBacΔF-HaF or vHaBacΔF-gp64 at 5 TCID50 units/cell. The infection rates were quantified at 24 h p.i. by flow cytometry. Data points represent the average value from triplicate infections. Error bars, standard deviations of the mean. The blot below the figure represents Western analysis of UV-inactivated vHa-RFP, which was used in the competition assay, probed by antibody against HaF1. M, the protein marker.
GP64 delays mortality in Helicoverpa armigera larvae.
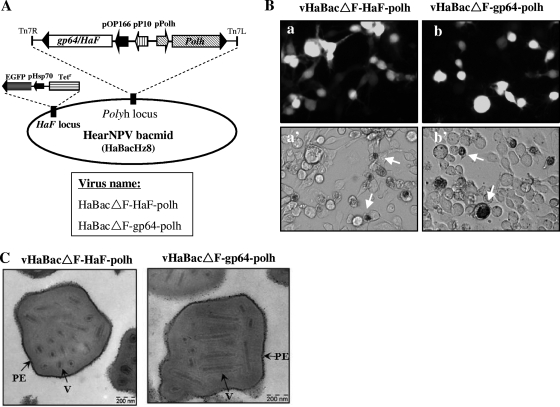
Baculovirus GP64 and F proteins of group II NPVs (HearNPV, SeMNPV, etc.) are essential for cell-to-cell spread in insect cell culture and within infected larvae. In order to determine the effects of GP64 expression on occlusion-derived virus (ODV) morphology and infectivity in vivo, polh and gp64, or polh and HaF, were reintroduced into HaBacΔF by transposition (Fig. 6 A). The resulting bacmids HaBacΔF-gp64-polh and HaBacΔF-HaF-polh were confirmed by PCR (data not shown). Infectious progeny BV were produced after transfection of recombinant bacmids into HzAM1 cells, which were then characterized by detection of GFP and the appearance of OBs in infected cells (Fig. 6B). To study OB morphology of pseudotyped virus, polyhedra were purified from vHaBacΔF-gp64-polh- or vHaBacΔF-HaF-polh-infected larvae and inspected by electron microscopy. OBs of vHaBacΔF-gp64-polh had shapes and sizes similar to those of vHaBacΔF-HaF-polh (Fig. 6C); also, the numbers of ODVs and their distributions and relative proportions per OB were very similar. This indicated that the swapping of BV EFPs had no impact on the formation and morphology of OBs.
FIG. 6.
Construction of polh-reintroduced recombinant viruses. (A) Schematic representation of polh-reintroduced viruses vHaBacΔF-HaF-polh or vHaBacΔF-gp64-polh. (B) Fluorescence (top) and bright-field (bottom) images of HzAM1 cells transfected with HaBacΔF-HaF-polh (a) or HaBacΔF-gp64-polh (b) and analyzed at 5 days p.t. Cells containing OBs are indicated by arrows (a' and b'). (C) Electron microscopy of OB morphology. Purified OBs from H. armigera larvae deceased after infection with vHaBacΔF-HaF-polh or vHaBacΔF-gp64-polh. V, virions; PE, polyhedron envelope.
To study the oral infectivity of recombinant viruses, third-instar H. armigera larvae were infected with eight OB concentrations of vHaBacΔF-gp64-polh or vHaBacΔF-HaF-polh (Table 2). Standard lethal dose ratio comparison (25) indicates that there is no significant difference between the infectivity of these two viruses, as the 95% confidence limits (CL) of the potency ratio, which is the ratio of the relative infectivity of pseudotyped virus to that of the control virus, include the value 1.0 in both assays. Meanwhile, the LC50s of the two recombinant viruses on third-instar larvae are similar to that of wild-type (wt) HearNPV, which is about 3.1 × 104 OBs ml−1 (28). Therefore, GP64 pseudotyping did not affect the LC50 (infectivity per OB) of HearNPV. However, insects fed with pseudotyped virus vHaBacΔF-gp64-polh exhibited a significant delay in mortality (Table 2). Larvae orally infected with a concentration of 3 × 106 OBs ml−1 of vHaBacΔF-gp64-polh showed 50% mortality at approximately 114 h p.i., and the calculated 50% survival time (ST50) value was 132 h, while the control virus vHaBacΔF-HaF-polh showed 50% mortality at about 92 h p.i. and the ST50 value was approximately 108 h (Table 2). The statistical analysis indicated that there was a significant difference (P < 0.01) in the ST50 values between vHaBacΔF-gp64-polh and vHaBacΔF-HaF-polh (Table 2). Thus, GP64-pseudotyped HearNPV delayed the mortality of the host insect H. armigera by about 24 h.
TABLE 2.
Bioassays of the recombinant viruses in third-instar H. armigera larvae
| Test no. | Virus | LC50 (95% CL) (×104) (PIBs ml1)a | Potency ratio (95% CL) | ST50 ± SEM (h) | Significance (P) |
|---|---|---|---|---|---|
| 1 | vHaBacΔF-gp64-polh | 0.53 (0.35-0.78) | 0.42 (0.17-1.02) | 132 ± 3.17 | <0.01 |
| vHaBacΔF-HaF-polh | 1.26 (0.83-1.93) | 108 ± 1.63 | |||
| 2 | vHaBacΔF-gp64-polh | 1.23 (0.73-2.15) | 0.99 (−1.61-3.59) | 132 ± 1.47 | <0.01 |
| vHaBacΔF-HaF-polh | 1.24 (0.89-1.76) | 108 ± 0.00 |
LC50s were calculated using the POLO probit analysis program and reported with 95% confidence limits (CL). For each treatment, LC50s were not significantly different. Significant difference was determined based on whether the 95% CL of the potency ratio included the value 1.0 (25). Potency ratio equal to LC50 of the pseudotyped virus vHaBacΔF-gp64 divided by the LC50 of the control virus vHaBacΔF-HaF (25). PIBs, polyhedral inclusion bodies.
DISCUSSION
Baculovirus F proteins are functional analogues to GP64, as evidenced from the rescue of gp64-null AcMNPV by F proteins from group II NPVs or betabaculoviruses (16, 39). However, GP64 failed to conversely rescue an F-null SeMNPV (group II NPV) in many transfection trials (35). Since the virus backbones (SeMNPV in the previous study and HearNPV in this study) taken for these substitution assays are different, there are many possible reasons that could have led to the different results, including (i) a rate of transfection of the SeMNPV bacmid into Se301 cells (35) that is lower (<0.1%) than that of the HearNPV bacmid into HzAM1 cells (0.1 to 1%), (ii) a lower compatibility of GP64 with SeMNPV viral proteins, or (iii) a lower affinity of the Se301 cellular receptor with GP64. In this study, it is also very difficult to generate infectious pseudotyped virus vHaBacΔF-gp64 since the virus titers in the early passages (P1 to P3) are very low (<103 TCID50 units/ml). The virus titer can finally reach approximately 5 × 105 TCID50 units/ml after 5 cell passages. However, once the infectious pseudotyped virus vHaBacΔF-gp64 is propagated to a high level, it can be passaged continuously in cell culture and can be lethal to H. armigera larvae. Thus, this study is the first successful attempt to pseudotype a group II NPV by GP64 (group I NPV) in the absence of an authentic group II F protein (Fig. 1 and 2) and demonstrate that GP64 can, in principle, partially compensate for essential functions of F protein.
During virus propagation in HzAM1 cells, we observed that the F-null HearNPV pseudotyped with AcMNPV GP64 (vHaBacΔF-gp64) spread from cell to cell but at a lower rate and generated substantially lower virus titers than control virus (vHaBacΔF-HaF) (Fig. 1C). One-step growth curve analysis (Fig. 3) showed that the overall dynamics of vHaBacΔF-gp64 infection (viruses from P5) were much slower than those of vHaBacΔF-HaF (viruses from P2), with an approximately 30- to 50-fold decrease in infectious BV production for the former virus, but in the early passages the reduction could be as high as 104-fold. In most other pseudotyping cases within the Baculoviridae family, the pseudotyped BV titers were reduced but often less (10-fold) than those for the wild-type phenotype (16). The vesicular stomatitis virus G (VSV-G) protein was able to complement the deletion of GP64 in AcMNPV, but the detection of infectious BV was 1 to 10% of that detected from wild-type AcMNPV (18); the SeMNPV F protein was also demonstrated to functionally complement F-null HearNPV, and the titer of pseudotyped virus vHaBacΔF-SeF also decreased up to 10-fold (33). These examples reflect a reduced degree of compatibility between heterologous EFPs with alternate baculovirus vectors and host cells. It remains to be tested whether GP64 can be used to pseudotype other group II NPVs, in particular those of more distantly related species, with a similar reduction in titer and delay in infection characteristics.
Viral EFPs are not only important for BV infectivity, but also for BV morphogenesis and egress. In the case of VSV-G-pseudotyped AcMNPVΔgp64, gp64-pseudotyped HRSV lacking G, F, and SH protein (HRSVΔSH,G,F), and VSV G-pseudotyped HIV, the virion structure seems to be similar to those of wild-type viruses, as they are morphologically indistinguishable (18, 22). In contrast, although gE and gM are not essential for herpesvirus replication, simultaneous deletion of both envelope genes drastically inhibits virion envelopment in the trans-Golgi area (2, 20). Electron microscopy results revealed that gp64-pseudotyped HearNPV exhibits aberrant BV morphology, with abundant extended or broken nucleocapsids lacking a detectable envelope (Fig. 4E and F). This could be the result of improper envelopment of HearNPV nucleocapsids or an unstable envelope directed by GP64 or lack of F. Nevertheless, we did find GP64 in virion preparations (Fig. 2A), but their distribution over the virions may be erratic or clustered in membrane vesicles carrying multiple nucleocapsids (Fig. 4F). We therefore infer that there is a direct correlation between abnormal virion morphology and reduced infectivity, most likely as a result of abortive infections by most of these morphologically defective virions.
The OB morphology of vHaBacΔF-gp64-polh is similar to that of vHaBacΔF-HaF-polh, indicating gp64 pseudotyping did not have any obvious impact on the expression and trafficking of polyhedron-associated proteins in the assembly process (Fig. 6C). This underscores the independence of the ODV occlusion process and BV assembly. The bioassay results confirmed this view, as gp64 pseudotyping did not alter the morphology of OBs and the LC50 of the virus (Table 2). However, the pseudotyping of HearNPV with GP64 did increase the ST50 of the infected larvae significantly (Table 2). Similarly, deletion of the F-like protein (Ac23) in AcMNPV results in not only a significant delay in ST50 but also a 100-fold decrease in BV production of gp64null AcMNPV pseudotyped with SeF without affecting BV formation and infectivity (17, 32). We assume that the delayed killing of infected larvae is due to the decreased infectivity of gp64-pseudotyped HearNPV BV, which may be the consequence of reduced HearNPV intercellular spread from midgut epithelial cells to other cell types and beyond, thereby reducing the overall speed of virus propagation within infected insect larvae.
In summary, in this study we successfully generated a gp64-pseudotyped F-null HearNPV and extensively analyzed its biological properties and morphologies. The fusogenic ability of GP64 for HzAM1 cells is found to be higher than that of the F protein. However, on account of the decreased infectivity and defective virion morphology, we conclude that gp64 can only partially substitute for the F protein in HearNPV, leading to the production of partially defective virions. The evolution hypothesis suggests that an ancestral group II baculovirus captured an ancestral gp64 gene, which allowed higher fusogenicity and led to the rapid divergence and evolution of group I NPVs (24, 26). In this study the functional recruitment of gp64 into an F-null group II NPV provides experimental support for this supposition. Since the substitution of an F-null HearNPV by AcMNPV GP64 is imperfect, we propose that F proteins possess important functions (such as mediating proper virus-cell receptor interaction, directing efficient budding, and conferring envelope stability) over those of GP64 during group II NPV infection and morphogenesis. The preservation of a remnant F (F-like) protein in group I NPVs upon the replacement of the ancestral F with GP64 for fusogenicity indirectly supports the above hypothesis.
Acknowledgments
This work was supported by grants from the National Science Foundation of China (grants 30670078, 30770084, and 90813017), the National Basic Research Program of China program (973 program, no. 2009CB118903 and 2010CB530103), and Programme Strategic Scientific Alliances between China and the Netherlands (2008DFB30220).
Footnotes
Published ahead of print on 25 August 2010.
REFERENCES
- 1.Blissard, G. W., and J. R. Wenz. 1992. Baculovirus gp64 envelope glycoprotein is sufficient to mediate pH-dependent membrane fusion. J. Virol. 66:6829-6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brack, A. R., J. M. Dijkstra, H. Granzow, B. G. Klupp, and T. C. Mettenleiter. 1999. Inhibition of virion maturation by simultaneous deletion of glycoproteins E, I, and M of pseudorabies virus. J. Virol. 73:5364-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cronin, J., X. Y. Zhang, and J. Reiser. 2005. Altering the tropism of lentiviral vectors through pseudotyping. Curr. Gene Ther. 5:387-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng, F., R. Wang, M. Fang, Y. Jiang, X. Xu, H. Wang, X. Chen, B. M. Arif, L. Guo, H. Wang, and Z. Hu. 2007. Proteomics analysis of Helicoverpa armigera single nucleocapsid nucleopolyhedrovirus identified two new occlusion-derived virus-associated proteins, HA44 and HA100. J. Virol. 81:9377-9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DePolo, N. J., J. D. Reed, P. L. Sheridan, K. Townsend, S. L. Sauter, D. J. Jolly, and T. W. Dubensky, Jr. 2000. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol. Ther. 2:218-222. [DOI] [PubMed] [Google Scholar]
- 6.Guibinga, G. H., and T. Friedmann. 2005. Baculovirus GP64-pseudotyped HIV-based lentivirus vectors are stabilized against complement inactivation by codisplay of decay accelerating factor (DAF) or of a GP64-DAF fusion protein. Mol. Ther. 11:645-651. [DOI] [PubMed] [Google Scholar]
- 7.Hefferon, K. L., A. G. Oomens, S. A. Monsma, C. M. Finnerty, and G. W. Blissard. 1999. Host cell receptor binding by baculovirus GP64 and kinetics of virion entry. Virology 258:455-468. [DOI] [PubMed] [Google Scholar]
- 8.IJkel, W. F. J., M. Westenberg, R. W. Goldbach, G. W. Blissard, J. M. Vlak, and D. Zuidema. 2000. A novel baculovirus envelope fusion protein with a proprotein convertase cleavage site. Virology 275:30-41. [DOI] [PubMed] [Google Scholar]
- 9.Jiang, Y., F. Deng, S. Rayner, H. Wang, and Z. Hu. 2009. Evidence of a major role of GP64 in group I alphabaculovirus evolution. Virus Res. 142:85-91. [DOI] [PubMed] [Google Scholar]
- 10.Kadlec, J., S. Loureiro, N. G. Abrescia, D. I. Stuart, and I. M. Jones. 2008. The postfusion structure of baculovirus gp64 supports a unified view of viral fusion machines. Nat. Struct. Mol. Biol. 15:1024-1030. [DOI] [PubMed] [Google Scholar]
- 11.Kalbfleisch, J. D., and R. L. Prentice. 1980. The statistical analysis of failure time data. Wiley, New York, NY.
- 12.Kielian, M., and F. A. Rey. 2006. Virus membrane-fusion proteins: more than one way to make a hairpin. Nat. Rev. Microbiol. 4:67-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kingsley, D. H., A. Behbahani, A. Rashtian, G. W. Blissard, and J. Zimmerberg. 1999. A discrete stage of baculovirus GP64-mediated membrane fusion. Mol. Biol. Cell. 10:4191-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar, M., B. P. Bradow, and J. Zimmerberg. 2003. Large-scale production of pseudotyped lentiviral vectors using baculovirus GP64. Hum. Gene Ther. 14:67-77. [DOI] [PubMed] [Google Scholar]
- 15.Long, G., M. Westenberg, H. Wang, J. M. Vlak, and Z. Hu. 2006. Function, oligomerization and N-linked glycosylation of the Helicoverpa armigera single nucleopolyhedrovirus envelope fusion protein. J. Gen. Virol. 87:839-846. [DOI] [PubMed] [Google Scholar]
- 16.Lung, O., M. Westenberg, J. M. Vlak, D. Zuidema, and G. W. Blissard. 2002. Pseudotyping Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV): F proteins from group II NPVs are functionally analogous to AcMNPV GP64. J. Virol. 76:5729-5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lung, O. Y., M. Cruz-Alvarez, and G. W. Blissard. 2003. Ac23, an envelope fusion protein homolog in the baculovirus Autographa californica multicapsid nucleopolyhedrovirus, is a viral pathogenicity factor. J. Virol. 77:328-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mangor, J. T., S. A. Monsma, M. C. Johnson, and G. W. Blissard. 2001. A GP64-null baculovirus pseudotyped with vesicular stomatitis virus G protein. J. Virol. 75:2544-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markusic, D. M., A. Kanitz, R. P. J. Oude-Elferink, and J. Seppen. 2007. Preferential gene transfer of lentiviral vectors to liver-derived cells, using a hepatitis B peptide displayed on GP64. Hum. Gene Ther. 18:673-679. [DOI] [PubMed] [Google Scholar]
- 20.Mettenleiter, T. C. 2003. Pathogenesis of neurotropic herpesviruses: role of viral glycoproteins in neuroinvasion and transneuronal spread. Virus Res. 92:197-206. [DOI] [PubMed] [Google Scholar]
- 21.Oomens, A. G., and G. W. Blissard. 1999. Requirement for GP64 to drive efficient budding of Autographa californica multicapsid nucleopolyhedrovirus. Virology 254:297-314. [DOI] [PubMed] [Google Scholar]
- 22.Oomens, A. G., and G. W. Wertz. 2004. The baculovirus GP64 protein mediates highly stable infectivity of a human respiratory syncytial virus lacking its homologous transmembrane glycoproteins. J. Virol. 78:124-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearson, M. N., C. Groten, and G. F. Rohrmann. 2000. Identification of the Lymantria dispar nucleopolyhedrovirus envelope fusion protein provides evidence for a phylogenetic division of the Baculoviridae. J. Virol. 74:6126-6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson, M. N., and G. F. Rohrmann. 2002. Transfer, incorporation, and substitution of envelope fusion proteins among members of the Baculoviridae, Orthomyxoviridae, and Metaviridae (insect retrovirus) families. J. Virol. 76:5301-5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson, J. L., and H. K. Preisler. 1992. Pesticide bioassays with arthropods. CRC Press, Baton Rouge, LA.
- 26.Rohrmann, G. F., and P. A. Karplus. 2001. Relatedness of baculovirus and gypsy retrotransposon envelope proteins. BMC Evol. Biol. 1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song, J., R. Wang, F. Deng, H. Wang, and Z. Hu. 2008. Functional studies of per os infectivity factors of Helicoverpa armigera single nucleocapsid nucleopolyhedrovirus. J. Gen. Virol. 89:2331-2338. [DOI] [PubMed] [Google Scholar]
- 28.Sun, X., H. Wang, X. Sun, X. Chen, C. Peng, D. Pan, J. A. Jehle, W. van der Werf, J. M. Vlak, and Z. Hu. 2004. Biological activity and field efficacy of a genetically modified Helicoverpa armigera SNPV expressing an insect-selective toxin from a chimeric promoter. Biol. Control 29:124-137. [Google Scholar]
- 29.Sun, X., G. Zhang, Z. Zhang, Z. Hu, J. M. Vlak, and B. M. Arif. 1998. In vivo cloning of Helicoverpa armigera single nucleocapsid nuclear polyhedrosis virus genotypes. Virol. Sin. 13:83-88. [Google Scholar]
- 30.Volkman, L. E., and M. D. Summers. 1977. Autographa californica nucleopolyhedrosis virus: comparative infectivity of the occluded, alkali-liberated, and nonoccluded forms. J. Invertebr. Pathol. 30:102-103. [DOI] [PubMed] [Google Scholar]
- 31.Wang, H., F. Deng, G. P. Pijlman, X. Chen, X. Sun, J. M. Vlak, and Z. Hu. 2003. Cloning of biologically active genomes from a Helicoverpa armigera single-nucleocapsid nucleopolyhedrovirus isolate by using a bacterial artificial chromosome. Virus Res. 97:57-63. [DOI] [PubMed] [Google Scholar]
- 32.Wang, M., Y. Tan, F. Yin, F. Deng, J. M. Vlak, Z. Hu, and H. Wang. 2008. The F-like protein Ac23 enhances the infectivity of the budded virus of gp64-null Autographa californica multinucleocapsid nucleopolyhedrovirus pseudotyped with baculovirus envelope fusion protein F. J. Virol. 82:9800-9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, M., Y. Tan, F. Yin, F. Deng, J. M. Vlak, Z. Hu, and H. Wang. 2008. The F protein of Helicoverpa armigera single nucleopolyhedrovirus can be substituted functionally with its homologue from Spodoptera exigua multiple nucleopolyhedrovirus. J. Gen. Virol. 89:791-798. [DOI] [PubMed] [Google Scholar]
- 34.Westenberg, M., P. Uijtdewilligen, and J. M. Vlak. 2007. Baculovirus envelope fusion proteins F and GP64 exploit distinct receptors to gain entry into cultured insect cells. J. Gen. Virol. 88:3302-3306. [DOI] [PubMed] [Google Scholar]
- 35.Westenberg, M., and J. M. Vlak. 2008. GP64 of group I nucleopolyhedroviruses cannot readily rescue infectivity of group II f-null nucleopolyhedroviruses. J. Gen. Virol. 89:424-431. [DOI] [PubMed] [Google Scholar]
- 36.Westenberg, M., H. Wang, W. F. J. IJkel, R. W. Goldbach, J. M. Vlak, and D. Zuidema. 2002. Furin is involved in baculovirus envelope fusion protein activation. J. Virol. 76:178-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wickham, T. J., M. L. Shuler, D. A. Hammer, R. R. Granados, and H. A. Wood. 1992. Equilibrium and kinetic analysis of Autographa californica nuclear polyhedrosis virus attachment to different insect cell lines. J. Gen. Virol. 73:3185-3194. [DOI] [PubMed] [Google Scholar]
- 38.Yang, Y., E. F. Vanin, M. A. Whitt, M. Fornerod, R. Zwart, R. D. Schneiderman, G. Grosveld, and A. W. Nienhuis. 1995. Inducible, high-level production of infectious murine leukemia retroviral vector particles pseudotyped with vesicular stomatitis virus G envelope protein. Hum. Gene Ther. 6:1203-1213. [DOI] [PubMed] [Google Scholar]
- 39.Yin, F., M. Wang, Y. Tan, F. Deng, J. M. Vlak, Z. Hu, and H. Wang. 2008. A functional F analogue of Autographa californica nucleopolyhedrovirus GP64 from the Agrotis segetum granulovirus. J. Virol. 82:8922-8926. [DOI] [PMC free article] [PubMed] [Google Scholar]